 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Applications of soft matter physics in food science: from molecular interactions to macro-scale food structures
Subhash
Pawde
 a and
Jaydeep
Dave
a and
Jaydeep
Dave
 *b
*b
aInnovative Food Science and Technology Program, Unit of Innovative Food Packaging and Biomaterials, School of Agro-Industry, Mae Fah Luang University, Chiang Rai 57100, Thailand
bFaculty of Medical Technology, Mahidol University, Salaya, Phutthamonthon, Nakhon Pathom 73170, Thailand. E-mail: jdavefst@gmail.com; jaydeep.dav@mahidol.ac.th; Tel: +66 0611433695
First published on 6th June 2025
Abstract
Soft matter physics, encompassing materials such as polymers, colloids, emulsions, gels, and foams, provides a powerful framework for understanding the structural and functional complexity of food systems. This review explores the application of soft matter principles in food science, from molecular interactions to macroscopic structuring. The behavior of food materials under various stresses and environmental conditions is governed by key physical principles including thermodynamics, phase transitions, and molecular dynamics. These principles elucidate how protein-polysaccharide networks, colloidal assemblies, and emulsified systems determine food texture, stability, and sensory properties. Rheology, a central tool of soft matter science, enables quantitative analysis of viscoelastic properties, guiding product design, formulation, and processing optimization. Processing techniques such as extrusion, high-pressure processing, and 3D printing are examined through the lens of soft matter behavior, offering precise control over microstructure and texture. Furthermore, the review highlights the emerging integration of artificial intelligence (AI) in modeling and predicting the physicochemical properties of complex food matrices, accelerating innovation and quality control. By bridging molecular–scale interactions with macro-scale material behavior, soft matter physics enables the rational design of functional, sustainable, and consumer-appealing food products. This interdisciplinary perspective not only advances fundamental scientific understanding but also provides practical insights for improving food quality, safety, and personalization. Overall, the review underscores the transformative potential of soft matter physics in shaping the future of food science and engineering.
Sustainability spotlightThis review highlights how principles of soft matter physics can drive the sustainable design of food systems by enabling precise control over structure, functionality, and processing efficiency, thereby reducing resource use, enhancing product quality, and supporting environmentally responsible innovation in the food industry. |
1. Introduction
The study of food science is increasingly intersecting with physics, particularly in the development of soft matter physics, as researchers strive to understand the fundamental physical principles that govern the properties and behaviors of food materials. Soft matter represents a distinct class of materials characterized by their susceptibility to significant structural deformation when subjected to thermal fluctuations or weak external stresses at energy scales comparable to room temperature thermal energy (kBT).1 These materials exhibit an intermediate state between conventional solids and liquids, demonstrating viscoelastic properties, structural complexity, and responsiveness to external stimuli that derive from their mesoscopic structure (1–100 nm scale). The essential defining features of soft matter include weak interparticle interactions dominated by entropic effects rather than enthalpic contributions; structural heterogeneity with characteristic length scales exceeding atomic dimensions; significant thermal fluctuations leading to complex phase behaviors; and pronounced sensitivity to boundary conditions and processing history.2Food systems, comprising proteins, polysaccharides, lipids, and their assemblies, are quintessential examples of soft matter, as they form complex structures including emulsions, foams, gels, and colloidal dispersions through non-covalent interactions and self-assembly processes. These materials are prevalent in food products, where their unique properties contribute to the texture, stability, and overall sensory experience of foods. The ability to manipulate and control these properties through a deep understanding of their physical basis is becoming a key area of focus for food scientists.
Recent advancements in soft matter physics have provided valuable insights into the structuring and behavior of food materials at various scales, from the molecular level to macroscopic structures. For example, the study of colloidal interactions has led to a better understanding of emulsion stability, which is crucial for products like mayonnaise and salad dressings.3 Similarly, the application of rheological principles has been essential in optimizing the texture and flow properties of foods, which directly impact consumer perception and acceptability.4 Moreover, the incorporation of advanced processing techniques, guided by principles from soft matter physics, has enabled the development of novel food structures and textures, enhancing both the functional and sensory attributes of food products.5
Studies on molecular dynamics and self-assembly processes have also contributed significantly to understanding the formation of food structures at the nanoscale. For instance, research into protein and polysaccharide interactions has revealed how these molecules self-organize to form gels and foams, which are vital for the texture of many food products.6 The study of phase transitions and gelation mechanisms further enhances our ability to manipulate food properties during processing and storage.7 Additionally, understanding the role of surface and interfacial tensions in emulsions and foams has enabled the development of more stable and desirable food products.8
The relevance of physics to food science extends beyond traditional boundaries, engaging researchers from diverse fields such as condensed matter physics, colloid science, and rheology. This interdisciplinary approach not only deepens our understanding of food materials but also fosters innovation in food product development and processing technologies. For example, high-pressure processing, informed by the principles of phase behavior and material deformation, has been widely adopted to improve the safety and quality of various food products without compromising their sensory attributes.9 Similarly, advancements in extrusion technology, supported by rheological studies, have enabled the creation of novel textures and shapes in processed foods.10
By leveraging physical principles, food scientists can better predict and manipulate the behavior of complex food systems, leading to more efficient production processes, enhanced product quality, and improved consumer satisfaction. Emerging technologies such as 3D food printing and the application of artificial intelligence in food design are opening new frontiers in the intersection of physics and food science, demonstrating the dynamic and evolving nature of this field.11
The objective of this review is to explore how principles of soft matter physics are applied to food science, highlighting both fundamental and applied aspects. This review will discuss the physical principles underlying food materials, from molecular interactions and self-assembly processes to the macro-scale structuring of food products. Key areas covered will include the role of colloid science in food design, the importance of rheological properties in food texture and consistency, and the impact of advanced processing techniques informed by soft matter physics. Additionally, the review will examine the emerging role of artificial intelligence in modeling and predicting the physical properties of food systems, demonstrating the evolving nature of this interdisciplinary field. By providing a comprehensive overview of these topics, this review aims to bridge the gap between fundamental physics and practical applications in food science, offering valuable insights for researchers and industry professionals alike.
2. Fundamental principles of soft matter physics in food science
Soft matter constitutes a distinct class of condensed matter characterized by: (1) structural organization at intermediate length scales (nanometers to micrometers) between atomic and macroscopic dimensions; (2) high susceptibility to deformation by thermal fluctuations or weak external forces (stresses on the order of 106 to 103 Pa); (3) non-equilibrium behaviors including metastability, path-dependence, and structural relaxation phenomena; and (4) emergent physical properties that arise from collective interactions rather than individual molecular attributes.3 These materials including polymers, colloids, foams, emulsions, gels, and liquid crystals exhibit dual solid-like and liquid-like properties depending on observation timescales and applied stresses, a phenomenon quantified through viscoelastic parameters. This duality stems from the delicate energetic balance between entropic and enthalpic contributions, where thermal energy (kBT) often competes with interparticle interaction potentials, enabling rich phase behaviors and structural transitions. In food systems, soft matter principles govern the hierarchical organization from molecular assemblies to macroscopic structures, directly influencing texture, stability, and functional properties. This intrinsic multiscale character makes soft matter an essential framework for understanding and controlling food material properties across physics, chemistry, biology, and engineering disciplines.Recent advancements in soft matter physics have focused on understanding the structural dynamics and phase transitions of these materials under various conditions. For instance, studies using quantum sensors to probe phase transitions in soft matter systems, such as liquid crystals, have demonstrated distinct phase behaviors near room temperature, highlighting the material's sensitivity to external conditions.12
2.1 Key principles: thermodynamics, phase transitions, and molecular dynamics
Soft matter physics is governed by several key principles, including thermodynamics, phase transitions, and molecular dynamics, which collectively determine the behavior and properties of soft materials. Thermodynamics, the study of energy and its transformations, plays a crucial role in understanding the stability and interactions within soft matter systems. For example, the phase behavior of emulsions and foams can be analyzed through thermodynamic parameters such as free energy, enthalpy, and entropy.3Phase transitions, such as gelation, crystallization, and glass transitions, are critical phenomena in soft matter physics that describe the changes in state or phase of a material under varying conditions of temperature, pressure, or concentration. Understanding phase transitions is vital in food science for manipulating the texture and consistency of food products, such as the transformation of liquid milk into yogurt or cheese through controlled coagulation and gelation processes. Recent studies have employed micro-photonics and optical sensors to monitor these phase transitions in real-time, providing new insights into the molecular mechanisms underlying these changes.13
Molecular dynamics, which involves the simulation of particle motion at the atomic or molecular scale, provides insights into the kinetic behaviors and structural evolution of soft matter systems. These simulations have been instrumental in modeling the self-assembly of proteins, polysaccharides, and lipid molecules in food systems, helping researchers predict the formation of various structures and textures in foods.14 Additionally, the elastocapillary interactions in thermoresponsive microgels have been studied to understand how these materials transition between swollen and collapsed states, which is crucial for designing responsive food textures.15
2.2 Types of soft matter in food systems
Soft matter physics encompasses various types of materials commonly found in food systems, each playing a distinct role in determining the structure, texture, and stability of food products.Proteins undergo self-assembly and aggregation processes to create various soft matter states including gels, foams, and interfacial networks. For instance, globular proteins like β-lactoglobulin and α-lactalbumin form three-dimensional gel networks through controlled denaturation and cross-linking, where the resulting viscoelastic properties emerge from the collective organization of individual protein molecules rather than from the properties of isolated proteins.16 Similarly, fibrillar proteins such as collagen and myofibrillar proteins contribute to meat texture by assembling into networked structures whose rheological behavior characterizes them as soft matter.17 The soft matter characteristics arise from the supramolecular assemblies formed by these proteins, not from the individual protein molecules.
Polysaccharides likewise serve as building blocks for soft matter systems in foods. Starch, upon gelatinization, transforms from granular particles into a continuous viscoelastic matrix exhibiting characteristic soft matter properties including shear-thinning behavior, yield stress, and thixotropy. Other polysaccharides such as cellulose derivatives, pectins, and alginate form soft matter systems through gelation, often triggered by specific conditions (thermal treatment, ion presence, pH changes). For example, low-methoxyl pectins form soft matter gels through calcium-mediated junction zones, where the structural organization across multiple length scales determines the mechanical and functional properties of the resulting system.18
These biopolymer-based soft matter systems derive their physical characteristics from the collective interactions and structural organization of their constituent molecules rather than from the properties of individual biopolymers. The distinction is analogous to that between individual bricks and a completed building: just as bricks themselves are not buildings but rather the components from which buildings are constructed, proteins and polysaccharides are not themselves soft matter but rather the molecular building blocks from which soft matter systems in foods are assembled.
Recent research has provided a comprehensive understanding of how food biopolymers can be manipulated to control the formation and properties of soft matter systems. For instance, controlled aggregation of proteins through precise adjustment of pH, ionic strength, and temperature enables the creation of soft matter structures with tailored rheological and textural properties.19 Similarly, the modification of polysaccharide architecture through enzymatic or chemical means allows for customization of the resulting soft matter behaviors, enhancing functionality in food applications.
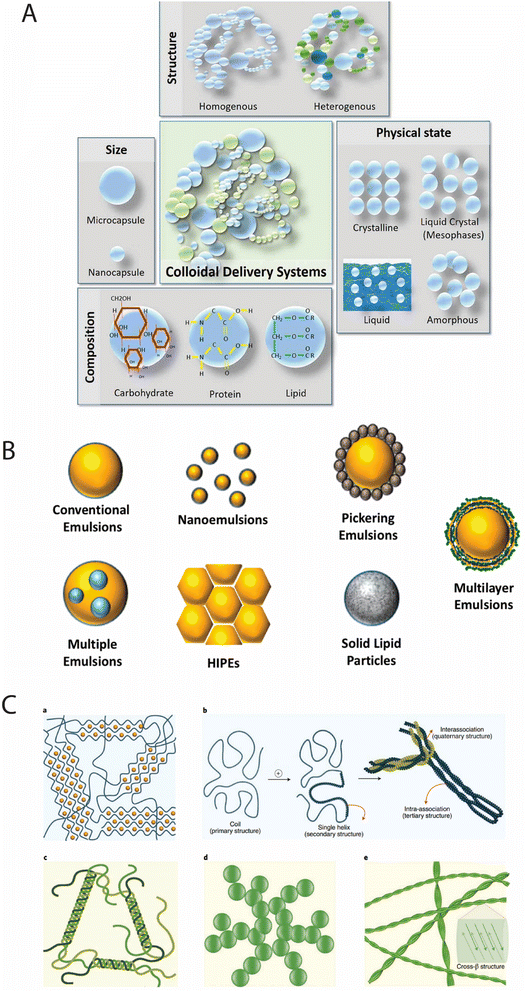 | ||
| Fig. 1 (A) Different categories of colloidal delivery systems;29 (B) different kinds of advanced emulsion systems that can be designed using food-grade ingredients;30 (C) mechanisms of food gel formation and their typical structures. (a) Polysaccharide gels: egg-box model in Ca2+ alginate gels. (b) κ-Carrageenan gels formed by the coil chain-to-single helix transition and intra-/interhelical associations, progressing through primary, secondary, tertiary, and quaternary structures. (c) Protein gels: triple helix formation in gelatin gels. (d) Fractal colloidal network formation resulting from random protein aggregation near the isoelectric point (IEP) or under high salt conditions. (e) Amyloid fibril network with a characteristic cross-β structure, formed at low pH.31 | ||
2.2.2.1. Solid and semi-solid colloidal dispersions. Solid and semi-solid colloidal dispersions in foods include protein aggregates, starch granules, fat crystals, and micelles dispersed in continuous phases. Milk serves as a classic example of a complex colloidal system where casein micelles (colloidal assemblies of approximately 120 nm diameter) coexist with fat globules and whey proteins in an aqueous continuous phase. The stability and rheological characteristics of these dispersions derive from the balance between attractive and repulsive forces including electrostatic interactions, steric hindrance, and van der Waals attractions.16
As shown in Table 1, multicomponent colloidal systems provide elastic structure, enhanced stability, and controlled digestibility in food applications. These systems help maintain structural integrity and modulate lipid digestion rates in the gastrointestinal tract,21 demonstrating how the colloidal nature of food structures directly influences both sensory and nutritional properties.
| Soft matter type | Structure | Effect on structural, functional, and sensorial attributes | Findings/data | References |
|---|---|---|---|---|
| Emulsion gels | 3D networks with emulsion dispersion | Enhances texture by providing a semisolid, creamy structure; improves stability and controlled release of bioactive compounds | Emulsion gels are used for texture modification, fat replacement, and probiotic delivery | 32 |
| Colloidal systems | Multicomponent colloids | Provides elastic structure, enhanced stability, and controlled digestibility | Colloidal systems help maintain structural integrity and affect lipid digestion rates in the GI tract | 28 |
| Protein-stabilized emulsion gels | Protein-based droplet gels | Improves viscoelastic properties, enhancing food texture and stability | The study found power-law behavior in the viscoelasticity of protein gels, improving gel hardness | 33 |
| Polymeric gels | Linear or branched polymer networks | Enhances mechanical strength, contributes to fat replacement, and increases texture hardness | Multi-component organogels showed significant rheological improvement due to synergistic interactions of gelators | 26 |
| Colloidal gels | Fractal colloidal networks | Provides solid-like mechanical properties; reduces elasticity when embedded with active colloids | Active colloids embedded in fractal cluster gels reduced gel elasticity, creating reconfigurable properties | 27 |
| Microgels | Cross-linked polymer networks | Affects texture and stability in food foams and emulsions, enhancing phase behavior | Microgels improve foam and emulsion stability, with complex phase behaviors due to particle deformability | 34 |
| Bigels | Two-phase system with hydrogel and oleogel | Improves hardness and mechanical strength, suitable for fat replacement and texture enhancement | Bigels exhibit synergistic properties, with higher moduli and hardness than mono-component gels | 26 |
| Colloidal foams | Colloidal particles dispersed in gas | Provides aeration and lightness to food textures; improves stability | Colloidal foams were found to stabilize emulsions and improve texture in food products like mousses | 35 |
| Emulsion particulate gels | Network of aggregated emulsion droplets | Enhances texture through droplet-induced gelation, offering fat replacement potential | Active oil droplets increase gel modulus, while inactive droplets reduce gel texture hardness | 28 |
| Responsive microgels | Adsorbed at fluid interfaces | Enhances foam and emulsion stability, providing on-demand texture modification | Responsive microgels deform at interfaces, offering control over foam and emulsion properties | 36 |
2.2.2.2. Emulsions. Emulsions constitute a specific subcategory of liquid–liquid colloidal dispersions where immiscible fluids, typically oil and water, form metastable systems through the action of emulsifiers. Fig. 1B illustrates the diversity of advanced emulsion systems that can be designed using food-grade ingredients, including conventional emulsions, nanoemulsions, pickering emulsions stabilized by particles, multiple emulsions (W/O/W), high internal phase emulsions (HIPEs), solid lipid particles, and multilayer emulsions.22 This systematic progression from simple to complex emulsion architectures demonstrates how structural engineering at the colloidal level enables enhanced functionality.
The stability and rheological behavior of food emulsions depend on interfacial tension, droplet size distribution, and stabilizing mechanisms. Recent research has developed multiscale frameworks to understand how molecular particle architecture affects emulsion behavior at liquid interfaces, providing predictive models for emulsion stability.23Table 1 documents how protein-stabilized emulsion gels improve viscoelastic properties, enhancing food texture and stability, with quantitative studies revealing power-law behavior in the viscoelasticity of protein gels that contributes to improved gel hardness.
Emulsion particulate gels, as noted in Table 1, represent an interesting hybrid system where networks of aggregated emulsion droplets enhance texture through droplet-induced gelation, offering significant potential for fat replacement applications. The active vs. inactive behavior of oil droplets in these systems has been shown to directly influence gel modulus and texture hardness, highlighting the relationship between microscopic structure and macroscopic properties in colloidal food systems.
2.2.2.3. Foams. Foams represent gas-in-liquid or gas-in-solid colloidal dispersions characterized by gas bubbles distributed within continuous phases. In foods, foams contribute to products like whipped cream, bread, meringues, and mousses, where their stability and textural properties derive from interfacial phenomena and structural mechanics.24
As documented in Table 1, colloidal foams—where colloidal particles are dispersed at gas–liquid interfaces provide aeration and lightness to food textures while significantly improving stability compared to conventional surfactant-stabilized foams. These systems have been found to stabilize emulsions and improve texture in food products like mousses,24 demonstrating the advantages of particle stabilization in foam structures.
Research into chemoresponsive soft matter using hydrogen-bonded liquid crystals has expanded the potential for developing food foams with responsive characteristics that change properties in response to specific stimuli.25Table 1 also highlights how responsive microgels adsorbed at fluid interfaces enhance foam and emulsion stability, providing on-demand texture modification capabilities through environmental responsiveness. These microgels deform at interfaces, offering unprecedented control over foam and emulsion properties, representing an advanced application of colloidal principles in food structure design.
2.2.2.4. Gels. Gels represent a distinct category of colloidal systems characterized by three-dimensional networks that immobilize large volumes of liquid, creating viscoelastic structures with solid-like mechanical properties despite high liquid content (often exceeding 90%). Fig. 1C illustrates several gelation mechanisms in food systems: (a) polysaccharide gels formed through the “egg-box” model in Ca2+ alginate gels; (b) κ-carrageenan gels progressing through primary to quaternary structures via coil-to-helix transitions and inter-helical associations; (c) protein gels formed through triple helix structures in gelatin; (d) fractal colloidal networks resulting from protein aggregation; and (e) amyloid fibril networks with characteristic cross-β structures. This systematic illustration of diverse gelation mechanisms demonstrates how different molecular interactions and assembly pathways lead to distinct gel structures with varying functional properties.
Table 1 documents multiple gel-based soft matter systems and their functional contributions to food properties. Polymeric gels formed from linear or branched polymer networks enhance mechanical strength, contribute to fat replacement, and increase texture hardness. Multi-component organogels show significant rheological improvement due to synergistic interactions between gelators,26 demonstrating how combination approaches can enhance gel functionality beyond what is possible with single-component systems.
Colloidal gels, characterized by fractal colloidal networks, provide solid-like mechanical properties that can be modulated through composition and processing.27 As noted in Table 1, embedding active colloids in fractal cluster gels reduces gel elasticity, creating reconfigurable properties that respond to external stimuli. This responsive behavior offers new possibilities for creating adaptive food textures that change during consumption or processing.
Microgels, cross-linked polymer networks at the microscale—affect texture and stability in food foams and emulsions, enhancing phase behavior through their unique deformability characteristics, as documented in Table 1. Bigels, representing two-phase systems combining hydrogel and oleogel components, demonstrate synergistic properties with higher moduli and hardness than mono-component gels,26 offering novel approaches to fat replacement and texture enhancement in food applications.
Emulsion gels, as highlighted in Table 1, combine the properties of emulsions and gels to create 3D networks with emulsion dispersion, enhancing texture through semisolid, creamy structures while improving stability and enabling controlled release of bioactive compounds. These systems have been successfully employed for texture modification, fat replacement, and probiotic delivery applications,28 demonstrating their versatility as functional food ingredients.
3. Molecular interactions in food systems
Molecular interactions including van der Waals forces, hydrogen bonding, ionic interactions, and hydrophobic interactions govern the assembly, stability, and functional behavior of food molecules across multiple scales. Understanding these interactions at a mechanistic level is essential for controlling food structure and properties.3.1 Van der Waals forces
van der Waals forces comprise three distinct contributions Keesom (dipole–dipole), Debye (dipole-induced dipole), and London dispersion (induced dipole-induced dipole) interactions that collectively determine the aggregation and stabilization behavior of food macromolecules.37 In food systems, these relatively weak forces (0.4–4 kJ mol−1) operate at short distances with interaction potentials scaling as r−6, where r represents intermolecular separation. A quantitative analysis of macadamia nut protein-lipid interactions revealed that van der Waals forces contribute approximately 60% of the total binding energy (−7.32 kcal mol−1) when interacting with palmitoleic acid, with interaction distances ranging between 3.8-4.2 Å for optimal stability.38 This effect, summarized in Table 2, demonstrates how these relatively weak interactions can significantly enhance protein stabilization and textural properties in food systems.| Interaction type | Soft matter | Impact on stability/behavior | Findings | References |
|---|---|---|---|---|
| Van der Waals forces | Macadamia nut proteins & palmitoleic acid | Enhances protein stabilization and texture | Van der Waals forces stabilize the macadamia nut proteins when interacting with palmitoleic acid, leading to improved complex stability | 38 |
| Hydrogen bonding | Xanthan gum and mucin complexes | Elevates viscosity and improves stability | Hydrophobic and hydrogen bonding interactions between xanthan gum and mucin increase viscosity significantly (45–70×) and promote the formation of stable, aggregated structures under acidic pH conditions | 47 |
| Ionic interactions | Ionic liquid and amino acid side chains | Stabilizes protein structures through ionic interactions | Ionic liquids interact with aromatic amino acid side chains via stacking interactions, stabilizing the protein structure, which is mediated by a favorable enthalpy contribution compensating for the entropy cost | 48 |
| Hydrophobic interactions | Rice glutelin and resveratrol complex | Improves emulsion stability and structure | Resveratrol interacts with rice glutelin via hydrophobic forces, altering protein conformation and reducing surface hydrophobicity, leading to more stable emulsions with small, highly anionic droplets | 49 |
| Non-covalent interactions | β-Lactoglobulin and chlorogenic acid | Enhances antioxidant activity and thermal stability | Non-covalent and covalent interactions between β-lactoglobulin and chlorogenic acid enhance thermal stability and increase antioxidant capacity of the complex, retaining its functionality under heat | 50 |
| Hydrogen bonding & van der Waals | Lysozyme and naphthol Yellow S (food dye) | Alters enzyme structure and reduces activity | Binding between naphthol Yellow S and lysozyme through hydrogen bonding and van der Waals forces caused conformational changes in lysozyme and led to reduced enzymatic activity | 51 |
| Electrostatic & hydrophobic | β-Lactoglobulin and sugar beet pectin | Improves emulsion stability through complex formation | Electrostatic and hydrophobic interactions between β-lactoglobulin and sugar beet pectin during Maillard-type reactions improved the stability of emulsions by forming conjugates with altered molecular structures and enhanced chemical bond stiffness | 52 |
| Ionic & entropic interactions | Polyethylenimine and silica colloids | Modulates colloid stability through complex entropic interactions | Non-monotonic stability behavior observed in silica colloids with increasing concentrations of polyethylenimine, where low and intermediate concentrations led to aggregation, and high concentrations re-stabilized colloids through depletion forces and entropic interactions | 53 |
In Pickering emulsions, where solid particles stabilize oil–water interfaces, van der Waals forces create a delicate equilibrium with image charge repulsions, maintaining non-adsorbed particles at critical separation distances (h0 ≤ 50 nm) from the interface, as depicted in Fig. 2A.
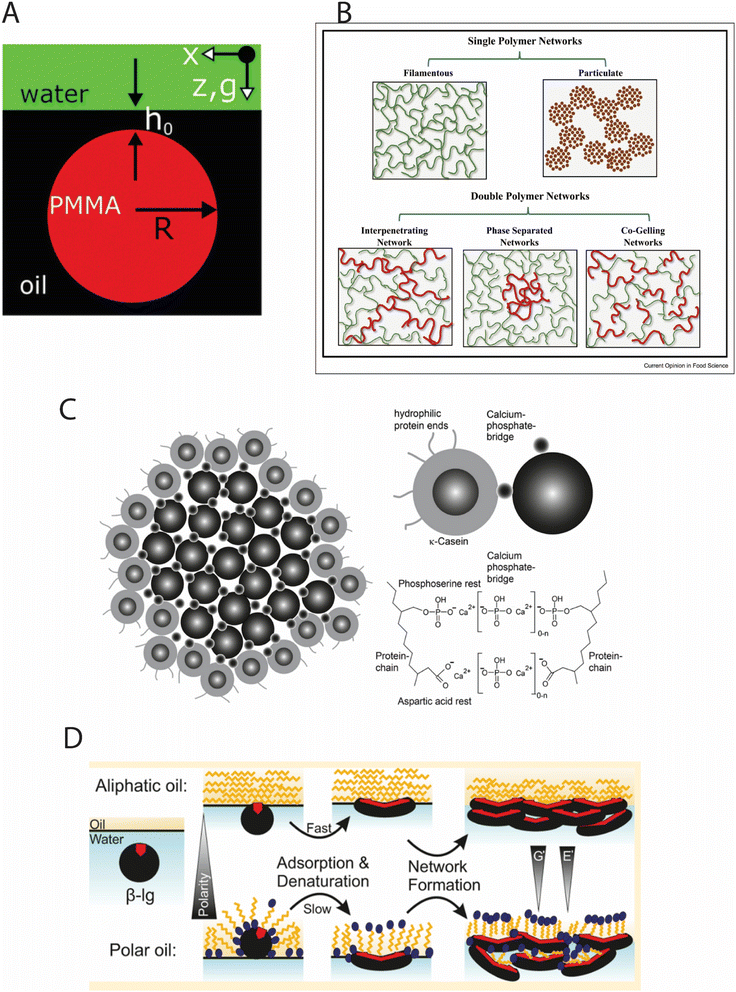 | ||
| Fig. 2 (A) Schematic representation of a non-contacting particle, suspended in the oil phase and maintained at a finite distance (h0 ≤ 50 nm) from the interface due to the equilibrium between image charge attractions and van der Waals repulsions in Pickering emulsions with non-touching colloids;44 (B) Hydrogen bond interaction in biopolymer hydrogel networks in meat analogs;17 (C) Illustrative diagram of the traditional model for casein micelles in raw cow's milk, where casein molecules are linked by calcium phosphate bridges, forming micelles approximately 120 nm in size,45 (D) Influence of oil hydrophobicity on the adsorption behavior and rheological properties of β-lactoglobulin at oil–water interfaces.46 | ||
Mechanistically, van der Waals forces contribute to food texture development through their cooperative effects in multicomponent systems. For instance, in wheat dough systems, the cumulative effect of numerous weak van der Waals interactions between gluten proteins creates sufficient cohesive strength (measured rheologically as storage moduli exceeding 104 Pa) to maintain structural integrity during processing.39 The temperature dependence of these interactions (decreasing by approximately 0.5% per °C) explains the observed textural changes during thermal processing of protein-rich foods.
3.2 Hydrogen bonding
Hydrogen bonds is a directional electrostatic interactions between a hydrogen atom bonded to an electronegative donor (O, N, F) and another electronegative acceptor atom are crucial determinants of food structure, with bond energies ranging from 4–40 kJ mol−1 depending on donor–acceptor geometry and electronic environment.40 A mechanistic case study of xanthan gum–mucin interactions, documented in Table 2, demonstrated that hydrogen bonding significantly increases complex viscosity (by factors of 45–70×) under acidic conditions (pH 3.0–4.5). Spectroscopic analysis revealed that this effect derives from the formation of intermolecular hydrogen bonds between the pyruvate and acetate groups of xanthan and the glycosylated regions of mucin, with bond densities exceeding 0.8 bonds per monosaccharide unit. This hydrogen bonding network creates transient junction zones that dramatically enhance viscoelastic properties, as evidenced by dynamic mechanical analysis showing storage moduli (G′) increasing from 2.3 Pa to 138 Pa upon complex formation.In meat analog systems, hydrogen bonding between plant protein β-sheets creates a hierarchical fibrous structure that mimics muscle tissue organization, as illustrated in Fig. 2B. These biopolymer networks demonstrate how hydrogen bonds can create complex architectural elements in food systems, providing structural integrity and texture. Fourier transform infrared (FTIR) spectroscopy quantitatively demonstrates shifts in amide I bands (1620–1640 cm−1) during thermal processing, corresponding to intermolecular hydrogen bond formation with enthalpic contributions of 12–18 kJ mol−1.17 This mechanistic understanding enables precise control of textural properties through processing parameter optimization.
3.3 Ionic interactions
Ionic interactions in food systems arise from coulombic forces between charged groups, with interaction energies of 20–40 kJ mol−1 in aqueous environments due to dielectric screening. The classic example of ionic interaction-mediated structure formation in food systems is the casein micelle, where phosphoserine residues interact with calcium phosphate nano-clusters to form a colloidal assembly approximately 120 nm in diameter, as depicted in Fig. 2C. Quantitative small-angle X-ray scattering (SAXS) analysis has revealed that these calcium-mediated ionic bridges contribute binding energies of 15–25 kJ mol−1 per interaction, creating a thermodynamically stable structure that resists dissociation under normal food processing conditions until the calcium activity is reduced below critical thresholds (approximately 3 mM free Ca2+).41As shown in Table 2, ionic interactions between amino acid side chains and ionic liquids can stabilize protein structures through favorable enthalpy contributions that compensate for entropic costs. These interactions are particularly important in foods with varying pH and salt concentrations, where they can significantly impact structure formation and stability.
The pH-dependent nature of ionic interactions enables switchable functionality in food systems. For example, studies of whey protein isolate gelation demonstrate that lowering pH from 7.0 to 5.0 (approaching the isoelectric point) reduces electrostatic repulsion between protein molecules, allowing closer approach and enabling other attractive interactions.42 This process increases gel strength by nearly an order of magnitude (G′ increasing from ∼100 Pa to ∼900 Pa) and reduces the critical gelation concentration from 12% to 8% w/w.
3.4 Hydrophobic interactions
Hydrophobic interactions the entropy–driven association of nonpolar moieties in aqueous environments are among the most significant forces driving protein folding, self-assembly, and interfacial phenomena in food systems. A quantitative case study examining β-lactoglobulin adsorption at oil–water interfaces demonstrated that hydrophobicity significantly influences interfacial rheology, with more hydrophobic oils (aliphatic vs. polar) dramatically altering protein adsorption kinetics, denaturation rates, and network formation, as illustrated in Fig. 2D. This visualization clearly demonstrates how the polarity of the oil phase affects both adsorption rates and the resulting interfacial network structure, with more hydrophobic oils inducing faster adsorption and more extensive protein networks.The binding of resveratrol to rice glutelin provides another mechanistic example documented in Table 2, where hydrophobic interactions within binding pockets (measured binding constant Ka = 1.04 × 104 M−1 at 25 °C) alter protein conformation and reduce surface hydrophobicity by 18%. This conformational change enhances emulsification properties, producing smaller droplet sizes (d43 decreasing from 2.8 μm to 1.5 μm) and more anionic interfaces (ζ-potential decreasing from −15 mV to −28 mV), thereby improving emulsion stability against coalescence and flocculation through combined steric and electrostatic repulsion mechanisms.
In fat crystal networks, hydrophobic interactions between triacylglycerol molecules drive crystallization and network formation, with binding energies of 5–15 kJ mol−1 per methylene group.43 Quantitative differential scanning calorimetry (DSC) measurements reveal that these interactions produce crystallization enthalpies of 120–160 J g−1, creating three-dimensional networks with yield stresses reaching 103–105 Pa depending on solid fat content and crystal morphology.
3.5 Impact of molecular interactions on stability and behavior of food molecules
The collective influence of these molecular interactions creates a complex energy landscape that determines food structure and functional properties across multiple length scales. As summarized in Table 2, different interaction types contribute uniquely to food stability and functional behavior, often working synergistically to enhance overall performance.The non-covalent interactions between β-lactoglobulin and chlorogenic acid exemplify this synergistic effect, as documented in Table 2. Isothermal titration calorimetry (ITC) measurements revealed binding constants (Ka) of 2.3 × 104 M−1 at pH 6.0, with enthalpy-driven binding (ΔH = −18.7 kJ mol−1) indicative of hydrogen bonding and van der Waals contributions. This complex exhibits enhanced thermal stability (denaturation temperature increasing from 76.2 °C to 82.5 °C) and antioxidant capacity retention (89% vs. 45% after heating at 80 °C for 30 min), demonstrating how molecular interactions directly translate to macroscopic functional properties.
The combination of hydrogen bonding and van der Waals forces in food dye–enzyme interactions, as shown for lysozyme and Naphthol Yellow S in Table 2, can cause conformational changes that alter enzyme activity. This finding has significant implications for food processing where colorants and bioactive components coexist.
In commercial food systems, these molecular interactions guide ingredient selection and processing optimization. For example, sugar beet pectin-β-lactoglobulin conjugates formed through Maillard-type reactions demonstrate enhanced emulsion stability through combined electrostatic and hydrophobic interaction mechanisms.52 Quantitative analysis shows that these conjugates reduce interfacial tension by 35% (from 15 mN m−1 to 9.7 mN m−1) and increase emulsion stability index by 270% compared to unconjugated protein, with no phase separation observed over 30 days at ambient temperature.
The synergistic integration of multiple interaction types creates robust food structures with targeted functionalities. As highlighted in Table 2, ionic and entropic interactions in polyethylenimine-stabilized silica colloids exhibit non-monotonic stability behavior, where low and intermediate polyelectrolyte concentrations induce aggregation through charge neutralization and bridging, while higher concentrations (>0.2% w/v) re-stabilize the system through steric repulsion and depletion effects. This mechanistic understanding enables precise control of colloidal stability in beverages and emulsified systems through careful manipulation of formulation parameters.
4. Self-assembly and supramolecular structures in food systems
Having established the fundamental molecular interactions that operate in food systems, these forces collectively drive the formation of higher-order structures through self-assembly processes (Fig. 3). The transition from molecular interactions to supramolecular organization represents a critical bridge between nanoscale phenomena and macroscale food properties that affect processing, stability, and consumer perception.4.1 Mechanisms of self-assembly in food systems driven by molecular interactions
Self-assembly in food systems occurs when individual molecules spontaneously organize into ordered structures through non-covalent interactions without external direction. This phenomenon relies on a delicate balance of multiple molecular forces, each contributing uniquely to the resulting supramolecular architectures. Though individually weak, van der Waals forces (0.4–4 kJ mol−1) become collectively powerful when operating across multiple contact points, as seen in lipid crystalline networks where they drive the precise packing of triacylglycerol molecules into lamellar arrangements.43 Similarly, the directional nature of hydrogen bonds (4–40 kJ mol−1) creates specific geometric patterns in protein-based assemblies, yielding remarkable mechanical properties like those observed in β-sheet-rich fibrils with elastic moduli exceeding 2–4 GPa.40 Charge-based ionic interactions (20–40 kJ mol−1) facilitate phase separation phenomena such as complex coacervation between proteins and polysaccharides, functioning optimally at intermediate ionic strengths where attractive forces remain effective without complete charge screening.48 Perhaps most significant in aqueous food systems are hydrophobic interactions, where the unfavorable energy of exposing nonpolar regions to water drives amphiphilic molecules to form micelles and bilayers at specific concentration thresholds. These various forces rarely operate in isolation instead, they create complex self-assembly landscapes with competing and cooperative effects. In protein fibrillation, for instance, hydrophobic associations initiate the process, hydrogen bonds stabilize growing structures, and electrostatic repulsions modulate dimensions, explaining why small environmental changes can dramatically alter final morphologies from straight fibrils to curved filaments or spherical aggregates.4.2 Supramolecular structures in foods: from molecular interactions to functionality
Supramolecular structures such as micelles, vesicles, and fibrils are abundant in food systems and play pivotal roles in determining their functionality. Each structure represents a specific organizational outcome of the molecular interactions detailed in Section 3, with direct consequences for food properties and performance.β-Lactoglobulin forms micelles that encapsulate bioactive compounds like chlorogenic acid, leveraging the same hydrophobic interactions that contribute to protein-polyphenol binding as described in Section 3.5. The micelle structure directly translates molecular-level hydrophobic interactions into functional attributes including enhanced bioavailability, stability, and controlled release of encapsulated compounds.
In a study on supramolecular amphiphiles based on cucurbit[n]uril, vesicle formation resulted from the precise balance between hydrophobic interactions in the tail region and host–guest interactions (a specific type of non-covalent binding) at the headgroup.55 These vesicles demonstrated remarkable stability and encapsulation efficiency, encapsulating hydrophilic compounds in their aqueous core while embedding hydrophobic molecules within the bilayer. This structural arrangement directly translates molecular interactions into functional delivery systems for both hydrophilic and hydrophobic bioactive compounds in foods.
A slow-evolving supramolecular gel studied through time-resolved rheology and microscopy revealed distinct kinetic pathways during its self-assembly. Initial fibril formation occurred through hydrophobic assembly and hydrogen bonding, followed by fiber elongation guided by the same forces, and ultimately network formation through fiber entanglement and junction point formation. These hierarchical processes directly translated molecular interactions into mechanical robustness, with storage moduli (G′) increasing from <1 Pa in the initial state to >1000 Pa in the fully formed network.56 This structure–function relationship illustrates how molecular forces progressively contribute to macroscopic properties through hierarchical assembly.
The functionality of coacervates in food applications stems directly from their structural characteristics. Their dense but fluid nature allows them to effectively encapsulate sensitive ingredients while maintaining adequate diffusion properties for controlled release. For example, protein-polysaccharide coacervates encapsulating flavor compounds demonstrated significantly enhanced retention during processing and storage, with release profiles that could be modulated by adjusting pH to alter the electrostatic interactions holding the coacervate together.57 This direct connection between molecular interactions, resulting structure, and functional performance demonstrates the coherent progression from fundamental forces to practical applications.
These hierarchical structures demonstrate how different molecular interactions can cooperatively direct assembly across multiple length scales. At the smallest scale, specific interactions between chemical groups determine local molecular packing; at an intermediate scale, these packings create defined morphologies like cylinders or lamellae; at the largest scale, these morphologies organize into macroscopic structures with emergent properties not predictable from any single interaction type.58
The functional implications of such hierarchical organization include enhanced mechanical properties through structural reinforcement, sophisticated release mechanisms that respond to multiple stimuli, and unique optical or interfacial behaviors that derive from the multiscale organization. For instance, hierarchically structured protein-polysaccharide complexes used in fat replacement applications provide both the creamy mouthfeel of fat through their mesoscale structure and the appropriate breakdown behavior during oral processing through their response to salivary enzymes and mechanical forces.59
5. Rheological properties of food materials: a soft matter physics perspective
5.1 Rheology as a tool for probing soft matter structure in foods
Rheology serves as a powerful analytical window into the multiscale structure and dynamics of soft matter food systems. Rather than merely characterizing flow properties, rheological measurements probe the underlying physical organization of food materials, revealing how molecular interactions translate into macroscopic behavior. In soft matter physics, rheology quantifies the response of materials that occupy the middle ground between perfect solids and Newtonian liquids—precisely where most structured food systems exist.The rheological signature of a food material directly reflects its hierarchical structure. For instance, in protein-stabilized emulsions, small-amplitude oscillatory shear measurements reveal characteristic frequency-dependent viscoelastic moduli (G′, G′′) that quantitatively map to specific structural features: high-frequency responses (10–100 rad per s) probe protein film properties at the oil–water interface (elastic moduli typically 10–50 mN m−1), while low-frequency behavior (0.01–0.1 rad per s) reflects droplet–droplet interactions and network formation (network strength typically 10–1000 Pa).60 This multiscale rheological fingerprint provides insights into both molecular organization and mesoscale structure simultaneously.
As illustrated in Fig. 4, rheological analysis serves multiple critical functions in food science, with particularly high significance in texture optimization, product formulation, and food engineering innovation. The relative importance of these application domains reflects the central role of rheology in translating fundamental soft matter principles into practical food development outcomes.
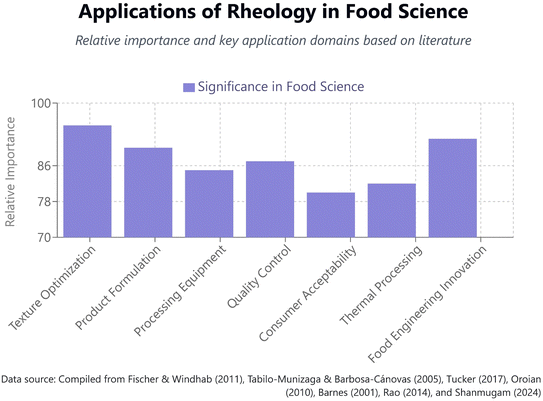 | ||
| Fig. 4 The comparative significance of seven rheological application domains within food science research and development. | ||
Recent advances in rheo-imaging and rheo-scattering techniques have strengthened the connection between rheological measurements and structural understanding. For example, studies combining small-angle neutron scattering with rheometry (rheo-SANS) have demonstrated how shear-induced alignment of caseinate-stabilized emulsions correlates with specific yielding behavior, where structural anisotropy parameters quantitatively predict the transition from solid-like to liquid-like response at critical stress values (typically 2–20 Pa).61 These structure–property relationships enable rational design of food materials with targeted mechanical responses.
5.2 Factors affecting food rheology
The rheological behavior of food soft matter systems depends on multiple factors that operate across different length scales, from molecular composition to processing conditions. Table 3 summarizes key factors influencing food rheology, highlighting the multidimensional nature of structure–property relationships in these complex systems.| Factor | Rheological parameter studied | Food type/system | Key findings | Reference |
|---|---|---|---|---|
| Viscosity and flow behavior | Viscosity, yield stress | General (Newtonian vs. non-Newtonian foods) | Identified differences in flow behavior based on food structure and shear rate | 62 |
| Composition and molecular structure | Viscoelasticity, dynamic moduli | Gel systems, starch–protein blends | Composition determines elastic/viscous balance and affects texture stability | 63 |
| Thickening agents (e.g., hydrocolloids) | Elastic modulus, thixotropy | Hydrocolloid solutions, dysphagia foods | Hydrocolloids modulate mouthfeel and safety in dysphagia formulations | 64 |
| Temperature and shear conditions | Apparent viscosity, shear rate | Yogurt (low-temp) | Simulated oral conditions improved prediction of in-mouth thickness and mouthfeel | 59 |
| Encapsulation techniques | Storage modulus, phase behavior | Nanoencapsulated ingredients | Rheology revealed stability and texture changes in nanoemulsions and solid-lipid particles | 65 |
| Food microstructure and texture | Nonlinear viscoelasticity (Laos) | Processed model foods | LAOS analysis revealed microstructural transitions affecting sensory perception | 66 |
| Processing techniques (3D printing) | Yield stress, storage modulus | Complex formulations with insect flour | Identified optimal starch concentration for balance between flow and shape retention | 67 |
As documented in Table 3, viscosity and flow behavior fundamentally differ between Newtonian and non-Newtonian food systems, with these differences directly linked to underlying structural attributes and shear rate dependencies.62 Molecular composition plays a decisive role in determining the elastic/viscous balance in gel systems and starch–protein blends63 creating the foundation for texture stability in these soft matter systems.
The incorporation of thickening agents, particularly hydrocolloids, strategically modifies rheological properties including elastic modulus and thixotropic behavior. As shown in Table 3, these modifications not only alter sensory perception but can serve critical functional purposes in specialized applications such as dysphagia formulations. Environmental conditions during measurement, particularly temperature and shear conditions, significantly impact rheological parameters, with studies demonstrating that simulated oral conditions provide more accurate predictions of in-mouth thickness and mouthfeel perception in soft matter systems like yogurt.59
Advanced formulation approaches including encapsulation techniques introduce additional complexity to rheological behavior. As noted in Table 3, rheological analysis reveals stability and texture changes in nanoemulsions and solid-lipid particles, providing crucial insights into how nanostructured soft matter responds to environmental stresses. Recent methodological innovations employing nonlinear viscoelasticity analysis (LAOS – Large Amplitude Oscillatory Shear) have revealed microstructural transitions that directly affect sensory perception in processed model foods,16 establishing quantitative relationships between nonlinear rheological signatures and consumer experience.
Processing technologies introduce further variables affecting rheological behavior. Table 3 highlights how 3D printing applications require precise rheological control, with recent research identifying optimal starch concentration ranges that balance flow behavior during extrusion with shape retention after deposition. These findings demonstrate how rheological characterization guides technological innovation in food processing.
5.3 Connecting rheology to food structure and functionality
Rheological principles from soft matter physics provide a unifying framework for understanding structure–function relationships across diverse food systems.At the macroscale (>1 μm), phase relationships and composite structures determine bulk mechanical behavior. Phase-separated protein-polysaccharide systems, for instance, exhibit rheological properties that depend on which phase forms the continuous matrix. When the protein phase is continuous, elastic behavior dominates (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = G′′/G′ < 0.2), while continuous polysaccharide phases often show more pronounced viscous character (tan
δ = G′′/G′ < 0.2), while continuous polysaccharide phases often show more pronounced viscous character (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = 0.3–0.5).69
δ = 0.3–0.5).69
For processed meat analogs, the rheological signature reveals the balance between plant protein gelation, fiber formation, and lipid incorporation. These systems represent protein-stabilized oil-in-protein gel emulsions with anisotropic structural elements—a specific soft matter state rather than a generic “plant-based system.” Small-amplitude oscillatory shear measurements reveal characteristic anisotropic viscoelastic properties, with storage moduli (G′) 3–8× higher when measured parallel versus perpendicular to the fiber direction.70
![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) (n−1)) optimally range from 0.3–0.5, ensuring appropriate bottle flow while maintaining suspension of herbs and particulates.72
(n−1)) optimally range from 0.3–0.5, ensuring appropriate bottle flow while maintaining suspension of herbs and particulates.72
Processing operations directly manipulate the rheological state of food materials. High-pressure homogenization of emulsions not only reduces droplet size but fundamentally alters interfacial composition through competitive adsorption effects. Increasing homogenization pressure from 200 to 600 bar in whey protein-stabilized emulsions progressively shifts interfacial rheology from viscoelastic solid-like behavior (interfacial elastic modulus Gi’ ≈ 0.07 N m−1) to more fluid-like response (Gi’ ≈ 0.02 N m−1) as surface area increases and protein surface density decreases.73
6. Processing and engineering applications of soft matter physics in food
Soft matter physics-based food system engineering provides a fundamental departure from conventional empirical methods in favour of mechanism-based, predictive processing techniques. The term “soft matter” refers to a wide range of food materials with complex rheological and structural characteristics controlled by weak, reversible interactions, including emulsions, gels, foams, and colloidal suspensions. These systems are excellent candidates for customised manipulation via exact process engineering because they often exhibit behaviours that fall in between the traditional solid and liquid phases.74,75New technologies like digital twin systems, cold plasma, and precise fermentation are combined with soft matter principles in recent food processing advancements. These developments help create sustainable, effective, and adaptable food production platforms in addition to improving control over microstructure and functioning. The parts that follow go into further detail on the fundamental soft matter concepts of current food engineering and show how they are used in both established and new processing methods.
6.1 Governing principles of soft matter in food processing
ΔGmix = kT[n1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(φ1) + n2 ln(φ1) + n2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(φ2) + n1φ2χ] ln(φ2) + n1φ2χ] |
Recent advancements in precision fermentation have added complexity to the issues of phase behaviour. Proteins generated by fermentation have distinct thermodynamic characteristics that diverge from those obtained through standard methods, necessitating the adjustment of processing settings. The regulated production setting of precision fermentation facilitates the synthesis of proteins with customised molecular structures, influencing their phase behaviour and processing attributes.78
The Gibbs–Thomson effect regulates crystallisation in lipid systems, with the chemical potential difference facilitating Ostwald ripening as described by Δμ = 2σVm/r, elucidating the thermodynamic impetus for crystal growth and informing methods for managing crystal size distributions in structured lipid products.79
τ = τ0 + K × ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) n, n, |
This approach is essential for new applications such as 3D food printing, which necessitates yield stress values of 300–800 Pa and flow behaviour indices (n) of 0.3–0.4 for effective shape preservation.81 Advanced digital monitoring systems now provide real-time rheological characterisation during processing. Machine learning systems evaluate rheological signs to forecast product quality and autonomously modify processing settings, marking a substantial progression from conventional empirical optimisation methods.82 These systems amalgamate concepts of soft matter physics with Industry 4.0 technology to attain unparalleled process control.
The time-dependent rheological behaviour leads to thixotropic phenomena, characterised by structural degradation under shear, which adheres to kinetic models expressed as dλ/dt = k1(1 − λ) − k2λ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) , facilitating the optimisation of processing settings to reduce irreversible structural damage.83 Contemporary processing apparatus integrates sensors that continuously observe structural alterations, facilitating adaptive control methodologies.
, facilitating the optimisation of processing settings to reduce irreversible structural damage.83 Contemporary processing apparatus integrates sensors that continuously observe structural alterations, facilitating adaptive control methodologies.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C) delineates surface excess concentrations of 1–5 mg m−2 for food emulsifiers.22 The efficacy of steric stabilisation may be measured using interaction potentials V(h) = kT(δ/h)(−9/4) for h < 2δ, elucidating the enhanced stability of protein-stabilised emulsions in comparison to small-molecule surfactant systems.
C) delineates surface excess concentrations of 1–5 mg m−2 for food emulsifiers.22 The efficacy of steric stabilisation may be measured using interaction potentials V(h) = kT(δ/h)(−9/4) for h < 2δ, elucidating the enhanced stability of protein-stabilised emulsions in comparison to small-molecule surfactant systems.
Recent advancements in precision fermentation have facilitated the creation of innovative emulsifying proteins with tailored interfacial characteristics. Companies like New Culture and Perfect Day have engineered casein proteins via fermentation that have superior emulsification properties relative to dairy-derived proteins, allowing novel food combinations that were previously unattainable with traditional ingredients.84 Pickering stabilisation, in which solid particles confer interfacial stabilisation, necessitates energy E = πr2γ(1 ± cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ)2 for the detachment of particles from surfaces. For food-grade particles with suitable wetting properties, this energy may surpass 1000 kT, ensuring remarkable durability against coalescence.85 Advanced particle engineering by controlled precipitation and spray-drying allows customised particle characteristics for particular applications.
θ)2 for the detachment of particles from surfaces. For food-grade particles with suitable wetting properties, this energy may surpass 1000 kT, ensuring remarkable durability against coalescence.85 Advanced particle engineering by controlled precipitation and spray-drying allows customised particle characteristics for particular applications.
6.2 Integration of soft matter principles in advanced processing technologies
The pressure dependence of chemical equilibria adheres to the Clausius–Clapeyron equation dP/dT = ΔH/(T·ΔV), wherein volume alterations during protein unfolding (−30 to −300 mL mol−1) facilitate pressure-induced gelation at ambient temperatures. Recent advancements merge high-pressure processing (HPP) with intelligent packaging systems that track pressure history and forecast product quality throughout storage, exemplifying the fusion of physics-based processing with digital technologies.87
Starch gelatinisation under pressure transpires at temperatures 10–20 °C lower than those of atmospheric processing, resulting in distinctive swelling features and modified rheological properties.88 Commercial uses have evolved beyond preservation to structural modification for plant-based meat substitutes, wherein pressure-induced protein aggregation produces fibrous textures akin to traditional meat products.
The regulated production environment facilitates the synthesis of molecules with specifically specified structures, influencing their phase behaviour, interfacial properties, and rheological features. For instance, proteins obtained from precision fermentation may be designed with particular amino acid sequences that improve gelation characteristics or alter emulsification qualities beyond the capabilities of traditional protein sources.90
The downstream processing of fermentation broths necessitates a profound comprehension of soft matter physics, as the separation and purification of target molecules entail intricate phase separations, filtration through biological membranes, and concentration processes that must maintain molecular functionality. Forward osmosis and other mild concentration methods maintain protein structure while attaining commercial feasibility.91
The application of cold plasma to food processing leverages soft matter physics principles through controlled modification of surface properties. Plasma treatment can alter surface hydrophobicity, modify protein conformation at interfaces, and create functional groups that enhance binding of bioactive compounds. These surface modifications occur without bulk heating, preserving thermolabile nutrients and sensory properties.
Recent studies demonstrate that cold plasma treatment enhances drying rates by modifying surface moisture transport properties, reducing processing times by 20–40% while maintaining product quality.93 Cold plasma, especially when combined with ozone, ensures significant microbial and aflatoxin B1 reduction (∼85%) while preserving overall quality attributes, making it a promising non-thermal approach for enhancing the safety of high-value products.94,95 The technology shows particular promise for processing heat-sensitive functional foods and nutraceuticals where conventional thermal processing would degrade bioactive compounds.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ above critical thresholds (0.5–1 V) while preserving structural integrity.96
θ above critical thresholds (0.5–1 V) while preserving structural integrity.96
Contemporary PEF systems use artificial intelligence for process enhancement, employing machine learning algorithms to assess variations in electrical conductivity during treatment and autonomously modify field settings for best outcomes. These systems exemplify the effective amalgamation of fundamental physics principles with Industry 4.0 technology.91 Moderate electric fields augment protein gelation via dipole alignment processes, resulting in gel strength enhancements of 30–120% when fields (5–20 V cm−1) are applied during thermal gelation without further heat treatment.97 Recent advancements involve the use of electric fields in precision fermentation to augment protein expression and alter product attributes.
![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ) surpasses one, protein molecules preferentially align with the flow direction, facilitating the production of fibrous structures.98
) surpasses one, protein molecules preferentially align with the flow direction, facilitating the production of fibrous structures.98
High-moisture extrusion illustrates this idea, wherein regulated shear stress (102–103 Pa) coupled with heat treatment facilitates transformations in protein secondary structures from random coil to β-sheet topologies orientated parallel to the flow direction. This molecular alignment propagates over various length scales, resulting in macroscopic fibrous formations that have mechanical anisotropy ratios ranging from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 8
1 to 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.98
1.98
Recent advancements incorporate robots and automated monitoring systems that perpetually modify processing settings depending on real-time assessments of product structure. Computer vision systems assess fibre alignment and autonomously adjust screw configurations, temperature profiles, and moisture levels to provide uniform product quality despite fluctuations in raw materials.99
6.3 Emerging technologies and digital transformation
Recent commercial uses encompass tailored nutrition solutions, wherein 3D printing facilitates individualised vitamin and nutrient delivery systems. Companies like Nourished manufacture personalised supplement gummies with meticulously regulated release profiles, whilst stores like as Kroger implement 3D cake printing technologies that provide real-time customisation.100
The physics of 3D food printing entails intricate rheological factors, as materials must demonstrate suitable flow characteristics during extrusion while preserving structural integrity post-deposition. Advanced formulation methodologies utilise sensitive polymers that experience sol–gel transitions induced by variations in temperature, pH, or ionic strength during the printing process.
Recent advancements feature robotic systems tailored for soft matter manipulation, including force feedback and adaptive grasping mechanisms that respond to product deformation. These systems effectively handle fragile things, like baked goods, fresh produce, and delicate gel structures, without causing harm. Vision-guided robotic systems conduct real-time quality evaluations, detecting faults, foreign substances, and dimensional discrepancies with precision beyond human skills. Integration with process control systems facilitates prompt corrective measures, ensuring uniform product quality during production cycles.
Energy-efficient processing methods integrate many unit functions to reduce total energy usage. Heat integration systems reclaim thermal energy from exothermic processes to facilitate endothermic operations, whereas sophisticated heat exchangers attain over 90% energy recovery in thermal processing activities.104
Hyperlocal production systems signify a transformative move towards decentralised manufacturing, minimising transportation expenses and ecological consequences. Firms such as Relocalize create autonomous micro-factories that may be situated locally, generating food goods in proximity to consumption sites while ensuring uniform quality via automated control systems.
6.4 Process integration and future perspectives
The amalgamation of biotechnology and conventional processing will persist in its expansion, with precision fermentation facilitating the synthesis of more intricate molecules possessing customised functionality. Integrating fermentation-derived components with traditional processing methods will yield innovative product categories with improved nutritional and functional attributes. Sustainability will persist in propelling innovation, focussing on closed-loop processing systems that eradicate waste production and optimise resource use. Incorporating life cycle assessment into process design will guarantee that environmental factors are included throughout technology development. Personalised nutrition systems will provide real-time customisation of food items according to individual health profiles, genetic data, and lifestyle variables. These systems will necessitate unparalleled integration of biology, processing technology, and data analytics to provide personalised products at scale.
7. Artificial intelligence and modeling in food physics
The amalgamation of artificial intelligence (AI) with food physics has transformed our comprehension, forecasting, and regulation of intricate food systems, tackling essential challenges that conventional analytical methods frequently struggle to elucidate due to the nonlinear, multiscale, and dynamic characteristics inherent in soft matter systems. Food materials exhibit intrinsic computational complexity due to hierarchical structures that range across six orders of magnitude, from molecular interactions (∼1 nm) to product–level properties (∼1 cm), compositional heterogeneity comprising hundreds of chemical species, and processing-induced structural evolution through interrelated physical, chemical, and biological phenomena.75The integration of AI with food physics is founded on decades of research in computational physics and materials science, utilising recent advancements in machine learning algorithms, processing capabilities, and data gathering technologies. This shift has generated unparalleled chances for comprehending intricate structure–property interactions, optimising processing conditions, and devising innovative food materials with specific capabilities.107 The worldwide AI industry in food and drinks is anticipated to expand from $8 billion in 2023 to $214.62 billion by 2033, demonstrating the revolutionary potential of these integrated methodologies for developing safer, more sustainable, and higher-quality food systems.108
7.1 Fundamental AI approaches in food soft matter analysis
The automatic feature extraction uncovered previously unrecognised structure–function correlations that contradicted established thinking. Contrary to traditional percolation theory predictions, CNN analysis revealed that strand thickness, rather than pore size, dictates gel strength in mixed protein systems. This discovery facilitated the focused adjustment of gelation conditions by meticulously regulating protein content, pH, and ionic strength to enhance strand production instead of overall porosity.111
Recent advancements in attention processes allow networks to concentrate on relevant structural traits while disregarding extraneous fluctuations, hence enhancing prediction accuracy for heterogeneous systems. Transformer architectures, initially designed for natural language processing, have been modified for the analysis of sequential structural changes in food processing, effectively capturing long-range dependencies in structural evolution and facilitating the prediction of final properties from early-stage measurements.112
The physics-informed element guaranteed compliance with essential mass conservation and nucleation theory: X(t) = 1 − exp(−k·tn), where X(t) represents the proportion crystallised, k denotes a rate constant that includes nucleation density, and n signifies the Avrami exponent associated with growth dimensionality. The neural network components discerned how minor constituents (monoglycerides, phospholipids) influenced these parameters in ways that solely physics-based models cannot anticipate due to intricate chemical interactions.114
The resultant models attained correlation coefficients r2 > 0.95 for forecasting solid fat content changes during non-isothermal crystallisation across various fat blends, encompassing intricate formulations with structured lipids and crystallisation modifiers. Examination of acquired data indicated distinct mechanisms: monoglycerides predominantly impacted nucleation density (k parameter), whereas phospholipids controlled the dimensionality of crystal development (n parameter).115
In protein gelation phenomena, Physics-Informed Neural Networks (PINNs) utilise percolation theory to facilitate network creation while acquiring system-specific interaction characteristics. The essential physics of gelation can be elucidated via percolation theory, wherein gel strength correlates with protein concentration as G′ ∝ (φ − φc)t, with φ representing protein concentration, φc denoting the critical gelation concentration, and t being a critical exponent (generally ranging from 1.3 to 1.8 for protein networks).74
Huang et al. (2024) created LSTM networks that integrate real-time pH, temperature, and dielectric spectroscopic data to forecast fermentation advancement and ultimate gel characteristics. The temporal modelling elucidated intricate links among acidification rate, protein network development, and final texture, allowing predictive control schemes that modified incubation temperature to account for batch-to-batch differences in milk composition.110 Recent advancements in computer vision systems driven by deep learning algorithms allow automated quality inspection and flaw identification, surpassing human inspectors in both speed and consistency. The integration of various imaging modalities, such as visible light, near-infrared, and X-ray imaging, enhances quality assessment capabilities, utilising fusion algorithms to amalgamate data from diverse sensors, thereby improving detection accuracy and minimising false positive rates.117
7.2 Multiscale computational frameworks
Coarse-grained molecular dynamics simulations are particularly advantageous for food systems as they may explore extended time scales (microseconds to milliseconds) while preserving adequate molecular resolution to capture fundamental physical phenomena. The simulations revealed distinct binding patterns defined by complementary charge patches, exhibiting interaction energies between −15 and −40 kJ mol−1, elucidating experimental findings about pH-dependent complex formation and dissociation.119 Electrostatic potential maps derived from molecular dynamics trajectories indicated that binding preferentially occurred in protein areas exhibiting locally high positive charge density, despite the overall negative charge of the protein.
All-atom MD simulations have demonstrated that slight structural differences in triglyceride composition result in significantly variable nucleation rates and polymorphic results during lipid crystallisation. Simulations of triglycerides including palmitic acid indicated that individual fatty acid substitutions might modify nucleation energy barriers by 10–30 kJ mol−1, elucidating the significant impact of fat content on crystal structure and mechanical characteristics reported in experiments.120
The model well delineated the experimentally observed correlation between homogenisation pressure (100–600 bar), resultant droplet size distribution (0.2–2 μm), and long-term stability metrics. Simulations indicated that intermediate pressures can produce more stable emulsions than maximum pressure, attributed to optimal protein surface coverage, while excessive homogenisation results in protein depletion at newly-formed interfaces.122 Phase field modelling offers robust methodologies for monitoring interface evolution in multiphase systems without the need for explicit representation of interface boundaries. The phase field method characterises interfaces using order parameters that transition smoothly across interface areas, governed by evolution equations of the type ∂φ/∂t = −M∇2(δF/δφ), where φ represents the order parameter, M denotes mobility, and F signifies the free energy functional.123
Anisotropic constitutive models have been especially formulated for structured food materials, including extruded plant proteins and laminated dough systems. These models include orientation tensors that encapsulate directional characteristics resulting from processing-induced structure: σ = C: ε, where σ denotes the stress tensor, C represents the fourth-order stiffness tensor that includes anisotropy, and ε signifies the strain tensor.126 The integrated model precisely forecasted instrumental texture profile analysis parameters and sensory fracture patterns during simulated mastication, with prediction errors under 12% for essential texture properties such as hardness, springiness, and fracturability. This capacity enabled the optimisation of extrusion settings to attain specific textural objectives while reducing energy consumption and preserving nutritional quality.127
7.3 Machine learning for inverse design and optimization of food soft matter
The RL agent controlled multiple process variables including barrel temperature profiles (110–170 °C across 6–8 zones), screw speed (300–700 rpm), moisture content (45–65%), and protein blend ratios while receiving feedback on resulting texture attributes, energy consumption, and product quality metrics. The reward function combined multiple objectives weighted according to their importance: texture similarity to target values (+100 to −50 points), energy efficiency (±30 points), and process stability (±20 points).
Through iterative learning over 1500 trials combining physical experimentation with validated simulation models, the RL system identified optimal processing conditions that reduced energy consumption by 28% while maintaining or improving texture attributes compared to conventionally optimized processes. The learned control policies exhibited sophisticated adaptive behavior, automatically adjusting parameters in response to raw material variations and equipment drift.134
7.4 Digital twins and advanced process control
7.5 Recent developments and industry applications
8. Future directions and emerging trends
8.1 Integrating physics with other disciplines in food science
The future of food physics increasingly depends on integration with other scientific disciplines to address complex food-related challenges. Food systems inherently span molecular, structural, microbial, and sensory phenomena, requiring multidisciplinary approaches rather than isolated disciplinary perspectives. The convergence of food physics with chemistry, biology, materials science, and computational engineering is reshaping food science, enabling comprehensive understanding of food behavior under various conditions and tailoring to meet evolving nutritional, sensory, and sustainability needs. The integration of food physics with nutritional biochemistry recognizes physical structure as a key determinant of nutrient digestion and bioavailability. Researchers apply soft matter principles to design protein-polyphenol complexes and emulsified systems that control bioactive compound release and absorption.133 These innovations support personalized nutrition strategies, where food matrices can be engineered for site-specific nutrient delivery along the gastrointestinal tract.133The convergence of food physics with microbiology has uncovered how physical environments influence microbial populations. Beyond water activity and pH, factors including surface tension, spatial confinement, and interfacial mechanics affect microbial gene expression, growth patterns, and stress responses.147 Physics-based modeling of biofilms and fermentation environments enables precise control over microbial dynamics, with applications in food safety, preservation, and fermentation optimization.148 Food physics integration with sensory and oral processing sciences has deepened understanding of texture perception and mastication dynamics. Biomechanical and tribological models now predict mouthfeel and structural breakdown during consumption, connecting measurable physical attributes with subjective sensory experiences. These insights are critical for designing plant-based proteins where mimicking layered, anisotropic structures of animal tissues requires control over chewing and swallowing behavior.149
Sustainability science benefits from physics-based approaches through optimized processing techniques like pulsed electric field treatment, which disrupts plant tissues with minimal thermal input, reducing energy consumption.150 Colloid and polymer science principles help valorize food industry by-products, converting waste streams into valuable ingredients.151 Physics also guides development of smart packaging materials and bio-based films through structural manipulation at nano- and micro-scales. Realizing the full potential of these interdisciplinary efforts requires transdisciplinary research platforms and shared methodological standards. Collaborative teams integrating physicists, chemists, microbiologists, nutritionists, and engineers can develop frameworks that accurately reflect food system complexities. Experimental setups capable of simultaneously characterizing physical, chemical, and biological properties, alongside standardized data ontologies, will facilitate deeper insights and model integration.151 These frameworks will enable rational design of functional, sustainable foods informed by comprehensive, cross-disciplinary understanding.
8.2 Emerging technologies and innovations
Advanced imaging and characterization technologies are fundamentally reshaping food physics by enabling deeper, more precise investigation of food structures across multiple scales. X-ray micro-computed tomography (μ-CT), neutron scattering, microrheology, and super-resolution fluorescence microscopy provide non-destructive, high-resolution visualization of food microstructures and molecular self-assembly processes.152 Time-resolved μ-CT reveals ice crystal growth and recrystallization in frozen emulsions under controlled conditions, while acoustic spectroscopy allows real-time, in-line monitoring of emulsification and crystallization in opaque systems by analyzing sound attenuation and velocity.153 These techniques generate rich datasets facilitating AI and physics-informed modeling for predictive process control. Three-dimensional food printing has emerged as a valuable tool for producing model food systems with precise structural parameters, enabling systematic studies of structure–property relationships and supporting personalized nutrition innovations.154Microfluidic technologies and nonthermal processing methods offer unprecedented control over food formulation and transformation. Droplet-based microfluidic platforms enable production of highly monodisperse double emulsions and encapsulated structures with tunable interfacial properties, facilitating studies of emulsion stability and encapsulation kinetics.155,156 These systems are being scaled for industrial applications such as controlled flavor release. Nonthermal technologies including pulsed electric fields and cold plasma enable microbial inactivation while preserving sensitive structural attributes.156 High-pressure processing, including pressure-shift freezing, modifies protein and polysaccharide structures to achieve novel textures and functional properties. Innovations like microwave volumetric heating (MVH) provide uniform energy delivery in heterogeneous food systems. When integrated with AI-based computational models and high-throughput experimental screening, these technologies form a synergistic platform for rational design and processing of structured food materials.153
8.3 Challenges and opportunities
Despite remarkable advancements in applying soft matter physics and AI to food systems, significant challenges persist each offering potential for transformative innovation. A fundamental issue lies in the multiscale and nonequilibrium nature of food materials, which span molecular to macroscopic scales and are governed by kinetic constraints rather than thermodynamic equilibrium.153 Current analytical methods often operate efficiently at discrete scales but struggle to integrate information across hierarchical structures. This has driven interest in multi-modal characterization techniques combining spectroscopy, scattering, and real-time imaging, alongside computational frameworks that couple molecular dynamics with mesoscale and continuum models.157 The path-dependent behavior of food systems requires novel theoretical approaches rooted in non-equilibrium thermodynamics, applying tools from soft matter physics such as jamming theory and dissipative structures to explain phase separation and texture setting during processing. Food composition complexity challenges conventional modeling, necessitating frameworks from statistical mechanics, percolation theory, and complex systems science to predict emergent properties like stability, mouthfeel, and nutrient delivery.154Regulatory and translational barriers pose additional challenges. Heterogeneity in food data, limited model interoperability, and lack of regulatory recognition for physical structure as opposed to chemical composition alone restrict widespread adoption of physics-informed design in industry.158 AI-based modeling adoption faces concerns regarding transparency, algorithmic accountability, and consumer trust. Regulatory frameworks incorporating physical structure descriptors are urgently needed, particularly for evaluating texture, allergen exposure, or nutrient release profiles. The transition from laboratory innovation to industrial implementation often faces scale-up complexities, economic feasibility challenges, and lack of in-line structural monitoring. This gap presents opportunities to develop robust physical design principles, real-time sensors based on acoustic or optical physics, and data-driven process control systems. Promising future directions include translating structure–function relationships across scales, establishing models for non-equilibrium processing behavior, and applying physical principles to design sustainable, personalized, and circular food systems. These approaches promise to enhance food quality and functionality while aligning food production with broader health, transparency, and environmental resilience goals.
Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Conflicts of interest
There are no conflicts to declare.References
- I. G. Loscertales, Fluid Flows for Engineering Complex Materials, ed. A. Fernandez-nieves and A. M. Puertas, 2016, pp. 29–42, DOI:10.1002/9781119220510.ch3.
- S. R. Nagel, Reviews of modern physics, 2017, 89, 025002 CrossRef.
- S. Assenza and R. Mezzenga, Nat. Rev. Phys., 2019, 1, 551–566 CrossRef.
- R. E. Rudge, E. Scholten and J. A. Dijksman, Curr. Opin. Food Sci., 2019, 27, 90–97 CrossRef.
- M. T. Pedersen and T. A. Vilgis, Int. J. Gastron. Food Sci, 2019, 16, 100135 CrossRef.
- A. Boire, D. Renard, A. Bouchoux, S. Pezennec, T. Croguennec, V. Lechevalier, C. Le Floch-Fouéré, S. Bouhallab and P. Menut, Annu. Rev. Food Sci. Technol., 2019, 10, 521–539 CrossRef CAS PubMed.
- A. Jangizehi, F. Schmid, P. Besenius, K. Kremer and S. Seiffert, Soft Matter, 2020, 16, 10809–10859 RSC.
- Y.-K. Kim, J. Noh, K. Nayani and N. L. Abbott, Soft Matter, 2019, 15, 6913–6929 RSC.
- İ. Tekiner, A. Knoblauch, B. Özatila and M. Ay, Soft Matter Physics Can Set Biological Clock of Industrial Food Science and (Bio) Technology, In 4th International Conference Business Meets Technology 2022, Editorial Universitat Politècnica de València, 2023, pp. 12–21 Search PubMed.
- L. S. Hirst, Fundamentals of Soft Matter Science, CRC press, 2019 Search PubMed.
- A. Z. Nelson, Phys. Fluids, 2022, 34(3), 031801 CrossRef CAS.
- V. K. Kavatamane, D. Duan, S. R. Arumugam, N. Raatz, S. Pezzagna, J. Meijer and G. Balasubramanian, New J. Phys., 2019, 21, 103036 CrossRef CAS.
- L. Garnier, R. Castro-Beltran, A. Saint-Jalmes, H. Lhermite, A.-L. Fameau, V. Vié, E. Gicquel, H. Cormerais and B. Bêche, Opt. Commun., 2020, 468, 125773 CrossRef.
- H. Wu, H. Friedrich, J. P. Patterson, N. A. Sommerdijk and N. De Jonge, Adv. Mater., 2020, 32, 2001582 CrossRef CAS PubMed.
- S. Chen and X. Yong, J. Colloid Interface Sci., 2021, 584, 275–280 CrossRef CAS PubMed.
- C. Wang and M. Guo, Whey Protein Production, Chemistry, Functionality, and Applications, 2019, pp. 67–101 Search PubMed.
- D. J. McClements, Curr. Opin. Food Sci., 2024, 55, 101120 CrossRef CAS.
- I. Ventura, J. Jammal and H. Bianco-Peled, Carbohydr. Polym., 2013, 97, 650–658 CrossRef CAS PubMed.
- U. Amin, Y. Lin, X. Zuo and H. Zheng, Food Hydrocolloids, 2024, 151, 109828 CrossRef CAS.
- J. Goodwin, Colloids and Interfaces with Surfactants and Polymers, John Wiley & Sons, 2009 Search PubMed.
- D. J. McClements and Y. Li, Adv. Colloid Interface Sci., 2010, 159, 213–228 CrossRef CAS PubMed.
- D. J. McClements, Food Emulsions: Principles, Practices, and Techniques, CRC press, 2004 Search PubMed.
- J. E. C. C. Jaramillo, L. E. Achenie, O. A. Álvarez, M. P. C. Bautista and A. F. G. Barrios, Curr. Opin. Chem. Eng., 2020, 27, 65–71 CrossRef.
- F. Zhan, M. Youssef, B. R. Shah, J. Li and B. Li, Food Hydrocolloids, 2022, 125, 107435 CrossRef CAS.
- H. Yu, K. Wang, T. Szilvási, K. Nayani, N. Bao, R. J. Twieg, M. Mavrikakis and N. L. Abbott, Materials, 2021, 14, 1055 CrossRef CAS PubMed.
- A. Shakeel, U. Farooq, D. Gabriele, A. G. Marangoni and F. R. Lupi, Food Hydrocolloids, 2021, 111, 106190 CrossRef CAS.
- M. E. Szakasits, K. T. Saud, X. Mao and M. J. Solomon, Soft Matter, 2019, 15, 8012–8021 RSC.
- T. Farjami and A. Madadlou, Trends Food Sci. Technol., 2019, 86, 85–94 CrossRef CAS.
- M. Fathi, M. Vinceković, S. Jurić, M. Viskić, A. Režek Jambrak and F. Donsì, Food Rev. Int., 2021, 37, 1–45 CrossRef CAS.
- C. Tan and D. J. McClements, Foods, 2021, 10, 812 CrossRef CAS PubMed.
- Y. Cao and R. Mezzenga, Nat. Food, 2020, 1, 106–118 CrossRef PubMed.
- Abdullah, L. Liu, H. U. Javed and J. Xiao, Front. Nutr., 2022, 9, 890188 CrossRef CAS PubMed.
- M. Roullet, P. S. Clegg and W. J. Frith, J. Colloid Interface Sci., 2020, 579, 878–887 CrossRef CAS PubMed.
- M. Rey, M. A. Fernandez-Rodriguez, M. Karg, L. Isa and N. Vogel, Acc. Chem. Res., 2020, 53, 414–424 CrossRef CAS PubMed.
- A. Dinache, M.-L. Pascu and A. Smarandache, Molecules, 2021, 26, 7704 CrossRef CAS PubMed.
- S. Bochenek, C. E. McNamee, M. Kappl, H.-J. Butt and W. Richtering, Phys. Chem. Chem. Phys., 2021, 23, 16754–16766 RSC.
- R. G. dos Santos, in Fundamentals of Surface Thermodynamics: Phase Behavior and its Related Properties, Springer, 2024, pp. 33–56 Search PubMed.
- F. Pan, L. Zhao, S. Cai, X. Tang, A. Mehmood, F. Alnadari, T. Tuersuntuoheti, N. Zhou and X. Ai, Food Chem., 2022, 367, 130677 CrossRef CAS PubMed.
- N. Ooms and J. A. Delcour, Curr. Opin. Food Sci., 2019, 25, 88–97 CrossRef.
- P. W. Kenny, J. Chem. Inf. Model., 2009, 49, 1234–1244 CrossRef CAS PubMed.
- M. A. Greenfield, Modulating the forces between self-assembling molecules to control the shape of vesicles and the mechanics and alignment of nanofiber networks, Doctoral dissertation, Northwestern University, 2009.
- Q. Tang, Y. H. Roos and S. Miao, Trends Food Sci. Technol., 2024, 104464 CrossRef CAS.
- D. Yang, Y.-Y. Lee, Y. Lu, Y. Wang and Z. Zhang, Molecules, 2024, 29, 1847 CrossRef CAS PubMed.
- N. A. Elbers, J. E. van der Hoeven, D. M. de Winter, C. T. Schneijdenberg, M. N. van der Linden, L. Filion and A. van Blaaderen, Soft Matter, 2016, 12, 7265–7272 RSC.
- T. A. Vilgis, Rep. Prog. Phys., 2015, 78, 124602 CrossRef PubMed.
- J. Bergfreund, P. Bertsch, S. Kuster and P. Fischer, Langmuir, 2018, 34, 4929–4936 CrossRef CAS PubMed.
- M. Ahmad, C. Ritzoulis, J. Chen, H. Meigui, R. Bushra, Y. Jin and H. Xiao, Food Hydrocolloids, 2021, 114, 106579 CrossRef CAS.
- K. P. Ghanta, S. Mondal, T. Hajari and S. Bandyopadhyay, J. Chem. Inf. Model., 2023, 63, 959–972 CrossRef CAS PubMed.
- T. Dai, R. Li, C. Liu, W. Liu, T. Li, J. Chen, M. Kharat and D. J. McClements, Food Hydrocolloids, 2019, 97, 105234 CrossRef CAS.
- X. Qie, W. Chen, M. Zeng, Z. Wang, J. Chen, H. D. Goff and Z. He, Food Hydrocolloids, 2021, 121, 107059 CrossRef CAS.
- Z. Asemi-Esfahani, B. Shareghi, S. Farhadian and L. Momeni, J. Mol. Liq., 2021, 332, 115846 CrossRef CAS.
- P. X. Qi, H. K. Chau and A. T. Hotchkiss Jr, Food Hydrocolloids, 2019, 91, 10–18 CrossRef CAS.
- S. Mehta, J. Bahadur, D. Sen, V. K. Aswal and J. Kohlbrecher, Phys. Chem. Chem. Phys., 2022, 24, 21740–21749 RSC.
- N. Baccile, C. Seyrig, A. Poirier, S. Alonso-de Castro, S. L. Roelants and S. Abel, Green Chem., 2021, 23, 3842–3944 RSC.
- J. M. Zayed, Self-assembly of synthetic and biological components in water using cucurbit [8] uril, Doctoral dissertation, 2012.
- A. Marczak and A. C. Mendes, Foods, 2024, 13, 1952 CrossRef CAS PubMed.
- F. Späth, C. Donau, A. M. Bergmann, M. Kränzlein, C. V. Synatschke, B. Rieger and J. Boekhoven, J. Am. Chem. Soc., 2021, 143, 4782–4789 CrossRef PubMed.
- Y. Lu, J. Lin, L. Wang, L. Zhang and C. Cai, Chem. Rev., 2020, 120, 4111–4140 CrossRef CAS PubMed.
- M.-A. Peyron, T. Sayd and V. Santé-Lhoutellier, Nutrient bioaccessibility is reduced in Elderly with oral deficiency combining in vitro mastication and digestive approaches, In 5th International Conference on Food Oral Processing, 2018, p. 156 Search PubMed.
- B. Paul, Structure, Rheology, and Phase Behavior of Protein Formulations Under High Pressure, Doctoral dissertation, University of Delaware, 2025.
- A. P. Williams, J. P. King, A. Sokolova and R. F. Tabor, Adv. Colloid Interface Sci., 2024, 103161 CrossRef CAS PubMed.
- L. P. Martínez-Padilla, J. Texture Stud., 2024, 55, e12802 CrossRef PubMed.
- Y. Kumar, M. Bhardwaj, A. Kheto and D. Saxena, in Current Developments in Biotechnology and Bioengineering, Elsevier, 2022, pp. 25–65 Search PubMed.
- D. Raheem, C. Carrascosa, F. Ramos, A. Saraiva and A. Raposo, Int. J. Environ. Res. Public Health, 2021, 18, 5125 CrossRef CAS PubMed.
- M. Liu, F. Wang, C. Pu, W. Tang and Q. Sun, Food Chem., 2021, 358, 129840 CrossRef CAS PubMed.
- Y. Wang and C. Selomulya, Trends Food Sci. Technol., 2022, 127, 221–244 CrossRef CAS.
- N. García-Gutiérrez, A. Salvador, T. Sanz, M. Ferrando, C. Güell, C. Méndez and S. de Lamo-Castellví, Food Bioprocess Technol., 2024, 1–9 Search PubMed.
- N. Lin and X. Y. Liu, Chem. Soc. Rev., 2015, 44, 7881–7915 RSC.
- M. N. M. Yiasmin, S. Al Azad, M. Easdani, M. S. Islam, M. Hussain, W. Cao, N. Chen, A. Uriho, M. Asaduzzaman, H. Xiao, A Review of Polysaccharide-Protein Interactions in Food Systems: Mechanisms, Stability, and Applications, 2024, DOI:10.20944/preprints202412.0091.v1.
- G. Giménez-Ribes, M. Oostendorp, A. J. van der Goot, E. van der Linden and M. Habibi, Food Hydrocolloids, 2024, 149, 109509 CrossRef.
- A. Sun and S. Gunasekaran, Int. J. Food Prop., 2009, 12, 70–101 CrossRef.
- O. Kaltsa, S. Yanniotis, M. Polissiou and I. Mandala, Lwt, 2018, 97, 404–413 CrossRef CAS.
- S. Zhang, J. Han and L. Chen, Food Hydrocolloids, 2023, 144, 109002 CrossRef CAS.
- R. Mezzenga, P. Schurtenberger, A. Burbidge and M. Michel, Nat. Mater., 2005, 4, 729–740 CrossRef CAS PubMed.
- P. G. de Gennes, Rev. Mod. Phys., 1992, 64(3), 645–648 CrossRef.
- V. Tolstoguzov, Food Hydrocolloids, 2003, 17, 1–23 CrossRef CAS.
- B. Zhang, G. Liu, D. Ying, L. Sanguansri and M. A. Augustin, Food Res. Int., 2017, 100, 658–664 CrossRef CAS PubMed.
- S. E. Brune, L. J. Hoppenreijs, T. Kühl, V. Lautenbach, J. Walter, W. Peukert, K. Schwarz, D. Imhof, R. M. Boom and R. Krull, Int. Dairy J., 2023, 147, 105772 CrossRef CAS.
- K. Sato, Chem. Eng. Sci., 2001, 56, 2255–2265 CrossRef CAS.
- M. A. Rao, Rheology of Fluid and Semisolid Foods: Principles and Applications, Springer Science & Business Media, 2010 Search PubMed.
- Z. Liu, M. Zhang, B. Bhandari and Y. Wang, Trends Food Sci. Technol., 2017, 69, 83–94 CrossRef CAS.
- M. A. Schutyser, J. Perdana and R. M. Boom, Trends Food Sci. Technol., 2012, 27, 73–82 CrossRef CAS.
- H. A. Barnes, J. Non-Newtonian Fluid Mech., 1997, 70, 1–33 CrossRef CAS.
- F. B. News, The future is now for precision fermentation, https://www.foodbusinessnews.net/articles/25553-the-future-is-now-for-precision-fermentation, (accessed 14 February 2024).
- E. Dickinson, Trends Food Sci. Technol., 2012, 24, 4–12 CrossRef CAS.
- V. Balasubramaniam, S. I. Martinez-Monteagudo and R. Gupta, Annu. Rev. Food Sci. Technol., 2015, 6, 435–462 CrossRef CAS PubMed.
- A. A. Sojecka, A. Drozd-Rzoska and S. J. Rzoska, Foods, 2024, 13, 3028 CrossRef CAS PubMed.
- J. Douzals, J. Perrier-Cornet, J. Coquille and P. Gervais, J. Agric. Food Chem., 2001, 49, 873–876 CrossRef CAS PubMed.
- M. A. Augustin, C. J. Hartley, G. Maloney and S. Tyndall, Crit. Rev. Food Sci. Nutr., 2024, 64, 6218–6238 CrossRef CAS PubMed.
- L. Zhou, M. Höfte and R. C. Hennessy, Front. Bioeng. Biotechnol., 2024, 12, 1363183 CrossRef PubMed.
- S. Technology, Machine learning and AI in the food industry, https://www.spd.tech/machine-learning/machine-learning-and-ai-in-food-industry/.
- P. Gazda and P. Glibowski, Appl. Sci., 2024, 14, 3617 CrossRef CAS.
- J.-W. Bai, D.-D. Li, R. Abulaiti, M. Wang, X. Wu, Z. Feng, Y. Zhu and J. Cai, Foods, 2025, 14, 84 CrossRef CAS PubMed.
- N. Tanwar, S. N. Mudliar, P. Vasu and S. Debnath, J. Food Eng., 2025, 392, 112494 CrossRef CAS.
- N. Tanwar, B. Bhavana, S. N. Mudliar, P. Bhat, P. Vasu and S. Debnath, Food Control, 2025, 111408 CrossRef.
- S. Toepfl, A. Mathys, V. Heinz and D. Knorr, Food Rev. Int., 2006, 22, 405–423 CrossRef CAS.
- K. Rajeswari, M. T. Anand and R. Mahendran, ACS Food Sci. Technol., 2024, 4, 1979–1996 CrossRef CAS.
- B. L. Dekkers, R. M. Boom and A. J. van der Goot, Trends Food Sci. Technol., 2018, 81, 25–36 CrossRef CAS.
- S. Insights, Top 10 food processing industry trends in 2025, https://www.startus-insights.com/innovators-guide/food-processing-industry-trends/.
- F. I. Tech, Innovative food processing technologies to watch in 2024, https://www.foodinfotech.com/innovative-food-processing-technologies-to-watch-in-2024/.
- R. Kler, G. Elkady, K. Rane, A. Singh, M. S. Hossain, D. Malhotra, S. Ray and K. K. Bhatia, J. Food Qual., 2022, 2022, 8521236 CrossRef.
- H. Onyeaka, P. Tamasiga, U. M. Nwauzoma, T. Miri, U. C. Juliet, O. Nwaiwu and A. A. Akinsemolu, Sustainability, 2023, 15, 10482 CrossRef.
- T. Haesevoets, D. De Cremer, K. Dierckx and A. Van Hiel, Comput. Hum. Behav., 2021, 119, 106730 CrossRef.
- G. Green, 2024food industry trends – future of technology and sustainability, https://www.gulfoodgreen.com/news-insights/2024-food-industry-trends-future-technology-sustainability.
- D. Wang, M. Zhang, A. S. Mujumdar and D. Yu, Food Eng. Rev., 2022, 14, 176–199 CrossRef.
- Z. C. Kennedy, D. E. Stephenson, J. F. Christ, T. R. Pope, B. W. Arey, C. A. Barrett and M. G. Warner, J. Mater. Chem. C, 2017, 5, 9570–9578 RSC.
- R. P. Singh and D. R. Heldman, Introduction to Food Engineering, Gulf Professional Publishing, 2001 Search PubMed.
- P. Research, Artificial intelligence in food and beverages market size, share & trends analysis report, https://www.precedenceresearch.com/ai-in-food-and-beverages-market.
- Y. LeCun, Y. Bengio and G. Hinton, Nature, 2015, 521, 436–444 CrossRef CAS PubMed.
- Z. Cheng, W. Xia, J.-J. Zhub, J. Cao, Z. J. Renb and H. Yuan, A Comprehensive Guideline for Hybrid Modeling of Engineered Microbial Processes, 2025, DOI:10.31219/osf.io/na72w.
- N. Stephanopoulos, R. Freeman and H. Yan, ACS Appl. Bio Mater., 2022, 5(10), 4579–4580 CrossRef PubMed.
- A. Vaswani, N. Shazeer, N. Parmar, J. Uszkoreit, L. Jones, A. N. Gomez, Ł. Kaiser and I. Polosukhin , Attention is all you need, Advances in Neural Information Processing Systems 30 , 2017 Search PubMed.
- M. Raissi, P. Perdikaris and G. E. Karniadakis, J. Comput. Phys., 2019, 378, 686–707 CrossRef.
- N. Fiuza-Maneiro, K. Sun, I. Lopez-Fernandez, S. Gomez-Grana, P. Muller-Buschbaum and L. Polavarapu, ACS Energy Lett., 2023, 8, 1152–1191 CrossRef CAS.
- A. H. Saberi, O.-M. Lai and M. S. Miskandar, Food Bioprocess Technol., 2012, 5, 1674–1685 CrossRef CAS.
- S. Hochreiter and J. Schmidhuber, Neural Comput., 1997, 9, 1735–1780 CrossRef CAS PubMed.
- M. A. A. Faisal, I. Mecheter, Y. Qiblawey, J. H. Fernandez, M. E. Chowdhury and S. Kiranyaz, arXiv, 2025, preprint, arXiv:2502.07826, DOI:10.48550/arXiv.2502.07826.
- D. Frenkel and B. Smit, Understanding Molecular Simulation: from Algorithms to Applications, Elsevier, 2023 Search PubMed.
- E. A. Rifai, M. van Dijk, N. P. Vermeulen, A. Yanuar and D. P. Geerke, J. Chem. Inf. Model., 2019, 59, 4018–4033 CrossRef CAS PubMed.
- K. Sato and S. Ueno, Bailey's Industrial Oil and Fat Products, 2005 Search PubMed.
- S. Succi, The Lattice Boltzmann Equation: for Fluid Dynamics and beyond, Oxford university press, 2001 Search PubMed.
- N. Innocente, M. Biasutti, E. Venir, M. Spaziani and G. Marchesini, J. Dairy Sci., 2009, 92, 1864–1875 CrossRef CAS PubMed.
- D. M. Anderson, G. B. McFadden and A. A. Wheeler, Annu. Rev. Fluid. Mech., 1998, 30, 139–165 CrossRef.
- B. G. Osborne, T. Fearn and P. H. Hindle, Practical NIR Spectroscopy with Applications in Food and Beverage Analysis, 1993 Search PubMed.
- O. C. Zienkiewicz and R. L. Taylor, The Finite Element Method, McGraw-HillLondon, 1997 Search PubMed.
- C. Krauß, J. K. Bauer, J. Mitsch, T. Böhlke and L. Kärger, J. Elasticity, 2024, 156, 279–306 CrossRef.
- K. J. Grabowska, S. Tekidou, R. M. Boom and A.-J. van der Goot, Food Res. Int., 2014, 64, 743–751 CrossRef CAS PubMed.
- M. Karg, A. Pich, T. Hellweg, T. Hoare, L. A. Lyon, J. Crassous, D. Suzuki, R. A. Gumerov, S. Schneider and I. I. Potemkin, Langmuir, 2019, 35, 6231–6255 CrossRef CAS PubMed.
- K. Arab, B. Ghanbarzadeh, S. Karimi, B. Ebrahimi and M. Hosseini, Int. J. Biol. Macromol., 2023, 246, 125603 CrossRef CAS PubMed.
- Y. Akkem, S. K. Biswas and A. Varanasi, Eng. Appl. Artif. Intell., 2024, 131, 107881 CrossRef.
- A. T. Krasley, E. Li, J. M. Galeana, C. Bulumulla, A. G. Beyene and G. S. Demirer, Chem. Rev., 2024, 124, 3085–3185 CrossRef CAS PubMed.
- K. Vrettos, E. E. Vassalou, G. Vamvakerou, A. H. Karantanas and M. E. Klontzas, Acad. Radiol., 2025, 32(6), 3563–3573 CrossRef PubMed.
- J. Y. Zhang, J. K. Pandya, D. J. McClements, J. Lu and A. J. Kinchla, Crit. Rev. Food Sci. Nutr., 2022, 62, 4752–4768 CrossRef PubMed.
- P. Michailidis, I. Michailidis and E. Kosmatopoulos, Energies, 2025, 18, 1724 CrossRef CAS.
- C. K. Williams and C. E. Rasmussen, Gaussian Processes for Machine Learning, MIT pressCambridge, MA, 2006 Search PubMed.
- G. Tom, S. P. Schmid, S. G. Baird, Y. Cao, K. Darvish, H. Hao, S. Lo, S. Pablo-García, E. M. Rajaonson and M. Skreta, Chem. Rev., 2024, 124, 9633–9732 CrossRef CAS PubMed.
- M. Grieves, Digital twin: manufacturing excellence through virtual factory replication, White paper, 2014, vol. 1, pp. 1–7.
- A. Cinar, S. J. Parulekar, C. Undey and G. Birol, Batch Fermentation: Modeling: Monitoring, and Control, CRC press, 2003 Search PubMed.
- A. Lewandowski and K. Wilczyński, Polymers, 2022, 14, 274 CrossRef CAS PubMed.
- CAS, Embracing the future of AI in the food industry, https://www.cas.org/resources/cas-insights/embracing-future-ai-food-industry.
- F. Navigator, AI watch: The latest on artificial intelligence in food, https://www.foodnavigator.com/Article/2024/05/07/AI-watch-The-latest-on-artificial-intelligence-in-food/.
- A. K. Singh, A. A. S. K. Singh and S. Singh, Recent Advances in Computational Intelligence and Cyber Security, 2024 Search PubMed.
- M. Company, The future of food: How technology will transform food production, McKinsey Global Institute, 2023, vol. 8, 3, pp. 112–134, https://ruralhandmade.com/blog/the-future-of-food-how-technology-is-changing-the-way-we Search PubMed.
- M. Raisul Islam, et al., Deep Learning and Computer Vision Techniques for Enhanced Quality Control in Manufacturing Processes, in IEEE Access, 2024, vol. 12, pp. 121449–121479, DOI:10.1109/ACCESS.2024.3453664.
- V. Zatsu, A. E. Shine, J. M. Tharakan, D. Peter, T. V. Ranganathan, S. S. Alotaibi, R. Mugabi, A. B. Muhsinah, M. Waseem and G. A. Nayik, Food Chem.:X, 2024, 101867 CAS.
- A. I. Appinventiv in Food Industry: Transforming Food with AI and Robotics, 2025, https://appinventiv.com/blog/ai-in-food-industry/.
- A. Justé, B. Thomma and B. Lievens, Food Microbiol.:Public Health Spoilage Aspects, 2008, 25, 745–761 CrossRef PubMed.
- H. Masood, F. J. Trujillo, K. Knoerzer and P. Juliano, in Innovative Technologies for Food Preservation, Elsevier, 2018, pp. 187–229 Search PubMed.
- C. Pradal and J. R. Stokes, Curr. Opin. Food Sci., 2016, 9, 34–41 CrossRef.
- T. Chatzimitakos, V. Athanasiadis, D. Kalompatsios, M. Mantiniotou, E. Bozinou and S. I. Lalas, Biomass, 2023, 3, 367–401 CrossRef CAS.
- C. M. Galanakis, in Sustainable Food Systems from Agriculture to Industry, Elsevier, 2018, pp. 401–419 Search PubMed.
- L. Schoeman, P. Williams, A. Du Plessis and M. Manley, Trends Food Sci. Technol., 2016, 47, 10–24 CrossRef CAS.
- I. Pérez Núñez, R. Díaz, J. Quiñones, A. Martínez, L. Velázquez, R. Huaiquipán, D. Tapia, A. Muñoz, M. Valdés and N. Sepúlveda, Molecules, 2024, 29, 5427 CrossRef PubMed.
- A. Dick, S. Prakash and B. Bhandari, Food Formulation: Novel Ingredients and Processing Techniques, 2021, pp. 101–119 Search PubMed.
- P. Dimitriou, Microfluidic construction and operation of artificial cell chassis encapsulating living cells and pharmaceutical compounds towards their controlled interaction, Doctoral dissertation, Cardiff University, 2023.
- R. Mu, N. Bu, J. Pang, L. Wang and Y. Zhang, Foods, 2022, 11, 3727 CrossRef CAS PubMed.
- E. Nocerino, 2025, preprint, arXiv:2503.20266, DOI:10.48550/arXiv.2503.20266.
- H. Feng and W. Yang, Nonthermal Processing Technologies for Food, 2011, pp. 135–154 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |

