 Open Access Article
Open Access ArticleRecent overview of nanotechnology based approaches for targeted delivery of nutraceuticals
Jhalak
Mehta
 ,
Khushboo
Pathania
and
Sandip V.
Pawar
,
Khushboo
Pathania
and
Sandip V.
Pawar
 *
*
Pharmaceutical Biotechnology Research Lab, University Institute of Pharmaceutical Sciences, Panjab University, Chandigarh-160014, India. E-mail: pawars@pu.ac.in
First published on 27th May 2025
Abstract
Nutraceuticals and dietary supplements have experienced a remarkable surge in demand over the past decade, driven by growing emphasis on preventive healthcare and heightened consumer preference for bioactive products. Nutraceuticals serve as an interface between pharmaceuticals and bioactives, offering therapeutic potential with minimal adverse effects. However, their clinical applications are often hindered by their inherent physicochemical characteristics, including low bioavailability, susceptibility to environmental degradation, poor aqueous solubility, instability, and post-delivery structural degradation. To address these challenges, nanotechnology has emerged as a promising avenue for enhancing the therapeutic efficacy and bioavailability of nutraceuticals. Nano-sized cargos such as liposomes, nanoparticles, nano-emulsions, and nanogels enable improved encapsulation, stability, bioavailability, cellular internalization, and targeted delivery of nutraceuticals. Furthermore, the sustainable manufacturing of nutraceuticals has undergone substantial technological advancements to enhance the bioavailability, therapeutic effect, and long-term stability. This review provides a comprehensive overview of recently published literature addressing different nano-enabled approaches employed for nutraceuticals, highlighting their targeted applications in disease prevention and management. Additionally, it critically examines the regulatory challenges associated with their production scalability, safety concerns, and environmental impact, while offering insights into existing regulatory frameworks and future considerations for the pervasive use of nanotechnology in the nutraceutical industry.
Sustainability spotlightNutraceuticals are bioactive compounds derived from food sources with purported health benefits and offer great potential for promoting wellness and preventing diseases. However, they face certain limitations including poor bioavailability, high sensitivity to light and oxygen, low water solubility, limited stability, and probable chemical changes after delivery that restrict their applications and health benefits. The integration of advancements in nanotechnology into sustainable nutraceutical manufacturing is driving impactful innovation in industry. Nanotechnology offers a promising avenue for delivery of nutraceuticals to improve bioavailability and targeted delivery. Natural and biodegradable polymers like chitosan, alginate, lignin, zein, casein, etc. are being employed for the development of nanocarriers. These biopolymers are non-toxic, non-irritant, easily available, and biodegradable, making them excellent candidates for the delivery of nutraceutical and dietary supplements. Nanotechnology in the food and pharmaceutical industry holds great potential to transform food and agricultural practices as it presents enormous benefits and sustainable production methods to shape the future of nutraceuticals. This review provides a comprehensive overview of the latest advancements in nanotechnological approaches aimed at improving the encapsulation of bioactive compounds emphasizing their targeted applications in disease management. |
1 Introduction
Nutraceuticals are bioactive compounds that provide therapeutic benefits beyond basic nutrition, contributing to disease prevention and overall health maintenance. The term is a combination of ‘nutrition’ and ‘pharmaceutical’, reflecting their dual role in nourishment and therapeutic efficacy.1 These compounds encompass a wide range of bioactive substances, including vitamins, minerals, antioxidants, prebiotics, probiotics, herbal products, and spices, as well as a range of polyunsaturated fatty acids (PUFA).2 They are commonly incorporated into functional foods, dietary supplements, and fortified food products such as cereals, soups, and beverages.3Dietary supplements, a subset of nutraceuticals, are formulated to augment dietary intake and may contain a combination of essential nutrients, herbs, botanicals, amino acids, metabolites, or bioactive extracts. Regulatory authorities such as the United States Food and Drug Administration (USFDA) mandate that dietary supplements must be appropriately labeled and are permitted to bear specific health claims only when supported by robust scientific evidence.4,5 They provide various benefits, including anti-aging properties, antioxidant properties, promoting good health and preventing diseases, fewer side effects, easy availability, and a holistic approach to wellness.5 The consumption of nutraceuticals and dietary supplements has increased substantially due to growing consumer interest in naturally derived compounds and preventive healthcare strategies.6 Extensive research highlights their therapeutic potential in mitigating oxidative stress-related disorders, including cardiovascular diseases7,8 and cancer,9,10 neurodegenerative conditions such as Alzheimer's disease11 and Parkinson's disease,12,13 metabolic disorders like diabetes mellitus,14,15 and obesity,16,17 as well as immunological18,19 and inflammatory disorders.20,21 The global nutraceutical market is experiencing significant expansion, driven by increasing consumer awareness and advancements in functional food development.22 As of 2022, the market was valued at approximately $317.22 billion and is projected to grow at a compound annual growth rate (CAGR) of 9.6%, reaching nearly $600 billion by 203023 (see Fig. 1). While India accounted for only 2% of the global nutraceutical market in 2017,24 recent estimates suggest it is anticipated to expand at a CAGR of 15% between 2023 and 2028, with the preventive healthcare segment anticipated to reach $195 billion by 2025 at a CAGR of 22%.25 This illustrates both the economic potential and the increasing significance of nutraceuticals in the global health sector.
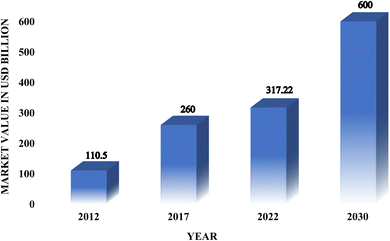 | ||
| Fig. 1 The global market value of nutraceuticals in USD Billion from the year 2012 to 2027.24,26 | ||
Despite their promising therapeutic applications, the efficacy of many nutraceuticals is hindered by inherent physicochemical limitations, including poor bioavailability, low aqueous solubility, high sensitivity to light and oxygen, limited stability, and probable chemical changes after their delivery.27 These challenges necessitate innovative delivery strategies to enhance their absorption, protect them from environmental degradation, and ensure targeted delivery to specific physiological sites.28 Thus, nanotechnology has been seen as a breakthrough invention for the efficient delivery of nutraceuticals and dietary supplements and in activating the positive characteristics of human health, hence improving their efficacy in a variety of ailments.29
This literature review intends to provide a comprehensive analysis of nanotechnology-based delivery systems for nutritional supplements, highlighting their targeted applications in disease prevention and management. It further examines how various nanocarriers address the physicochemical limitations of conventional nutraceutical formulations, thereby advancing their clinical and commercial viability. Additionally, it discusses the limitations of existing regulatory frameworks and provides a comparative analysis of different nanoformulations based on their production costs, scalability, regulatory challenges, and environmental concerns.
2 Strategies to improve the nutritional properties of foods using nanotechnology
Nutraceuticals exhibit several physicochemical limitations that hinder their therapeutic efficacy, including poor bioavailability, low aqueous solubility as a result of their hydrophobic nature, sensitivity to temperature, light, pH, free radicals, or oxygen, limited stability, and potential structural degradation during their delivery.27,28 Many bioactive compounds derived from natural sources, such as curcumin and quercetin, face significant challenges in achieving their full therapeutic potential due to inefficient absorption, short systemic circulation time, and rapid metabolic degradation.30–32 To overcome these limitations, nanotechnology has emerged as a promising approach for effectively delivering nutritional supplements.33,34 Nanoencapsulation offers several advantages, including the ability to protect bioactive compounds from environmental degradation (e.g., pH fluctuations, photodegradation, temperature variations, and oxidative stress), enhance bioavailability, facilitate targeted delivery, and permit controlled and sustained release of the encapsulated compound35,36 (Fig. 2).The encapsulation process must be carefully designed to ensure precise regulation of release kinetics, allowing for controlled dosage at the intended site of action.37 An optimal delivery system should protect against external destabilizing factors such as enzymatic degradation, moisture, and temperature fluctuations, thereby preserving the structural and functional integrity of the encapsulated compound.38 Additionally, once encapsulated within nanocarriers, the physicochemical properties of nutraceuticals are largely influenced by the characteristics of the carrier system rather than the entrapped bioactive compound, thereby enhancing solubility and stability. Moreover, nanocarrier-based systems enable the co-delivery of hydrophilic and lipophilic bioactives, facilitating their synergistic therapeutic effects and improved bioavailability.39,40
It is imperative to ensure that nanotechnology-based delivery systems utilize biocompatible, non-toxic, and non-immunogenic materials that pose no threat to human health.41 The materials commonly employed in nanocarrier fabrication include lipid, polymer, and protein-based systems, all of which must comply with regulatory standards and be categorized as Generally Recognized as Safe (GRAS).41 The selection of an appropriate nanocarrier system is determined by the physicochemical properties of the encapsulated nutraceutical, the intended site of action, and the specific therapeutic application.42,43 Among the key nanocarriers explored in recent research are nanogels, nanoemulsions, nanoparticles (lipidic, polymeric, or protein-based), niosomes (non-ionic surfactant vesicles), liposomes, nanocrystals, and polymeric nanocapsules (Fig. 3). The following sections provide a comprehensive discussion of these nanocarrier systems and their applications in enhancing nutraceutical delivery.
2.1 Nanogels
Nanogels are hydrogel particles with remarkable versatility in nutraceutical delivery due to their nanoscale size (typically <1000 nm), high loading capacity, stability, and ability to protect and control the release of bioactive compounds.44 They are predominantly synthesized through self-assembly, chemical modifications, ionic gelation, and ultrasonication techniques, often incorporating proteins and polysaccharides as core materials. Recent advancements in nanogel formulation have demonstrated significant improvements in the encapsulation efficiency, bioavailability, and functional properties of encapsulated nutraceuticals.He M. et al. developed soy protein isolate (SPI)-based nanogels modified with dextran and succinic acid anhydride to encapsulate curcumin. The Maillard reaction and succinic acid anhydride modification enhanced the functional properties of SPI, such as hydrophobicity and charge distribution, facilitating self-assembly into nanogels. This resulted in nanogels with a particle size of 143 nm, a dispersion index of 0.20, an encapsulation efficiency of 93%, and a loading capacity of 54%.45 These nanogels exhibited excellent stability and antioxidant activity, making them suitable for addressing oxidative stress-related diseases. Similarly, Wang et al. formulated self-assembled nanogels using acylated rapeseed protein isolate (ARPI) through a process of chemical acylation and heat-induced protein denaturation.46 This approach endowed the nanogels with unique secondary and tertiary structures, reduced sulfhydryl groups, and increased hydrophobic surfaces, resulting in spherical particles with a hydro-diameter of 170 nm and a light core-dark shell morphology. ARPI nanogels were effective in enclosing curcumin with an exceptional encapsulation efficiency of 95% which significantly boosted its potential to fight cancer in different cell lines. However, the study lacks data on pH stability and performance in complex food matrices and in vivo studies to prove their safety and efficacy.46 In another approach, Yu et al. formulated acylated kidney bean protein isolate (AcKPI) nanogels via the self-assembly method, achieving uniform particles with particle size of 137 nm and PDI of 0.3. They exhibited excellent encapsulation efficiency of 92%, significant antioxidant properties, and pH and temperature tolerance. The study provides the application scope of AcKPI-nanogels for potential use as an innovative and ideal delivery system for bioactive compounds for controlled release.47
Beyond protein-based nanogels, Xu et al. designed an innovative nanogel encapsulating lutein in an ovomucin and chitosan oligosaccharide blend through the self-assembly technique.48 The encapsulated lutein showed remarkable stability across a wide pH range and ionic concentrations, retained its amorphous state, and achieved controlled release with significant antioxidant activity. The nanogels demonstrated no cytotoxicity toward L929 fibroblast cells at higher concentrations. The study presents the potential application of lutein-loaded ovomucin and chitosan oligosaccharide blend nanogels as the promising and effective oral carrier of lutein. However, the absence of in vivo studies left uncertainties regarding their oral bioavailability and long-term safety.48 Another study encapsulated folic acid (vitamin B9) in soy proteins and polysaccharides using a self-assembling technique to prevent deterioration due to pH, temperature, and light.49 The folic acid-encapsulated nanogels could be used for intestinal release, as folic acid was gradually released under neutral pH. Furthermore, the nano-encapsulated substance remained stable at high temperatures, high light exposure, and high oxygen pressures, making it a feasible option for employing the nanogels in a variety of food and drink formulations without worrying about the risk of deterioration.49 Additionally, Sun et al. utilized a Maillard reaction and self-assembly to fortify orange juice with curcumin.50 The encapsulated curcumin demonstrated superior stability and antioxidant activity, expanding its potential for acidic beverage applications. Despite their advantages, they may pose allergenicity risks and limit their applicability in certain populations. Nevertheless, self-assembled nanogels can effectively encapsulate a variety of nutraceuticals and drugs, improving their stability and bioavailability. The process avoids the use of toxic solvents, aligning with green chemistry principles.
The ultrasonication technique is another widely employed method for nanogel synthesis, offering advantages such as controlled particle size tuning (30–200 nm), crosslink density modulation, and adjustable drug release profiles by varying ultrasonication time, amplitude, and polymer concentration.51 Jin et al. encapsulated vitamin B2 in nanogels made with dextran and thermally denatured soy protein using an ultrasonication technique, having a diameter of 40 nm and maintaining their spherical shape at pH 6.52 Encapsulation efficiency was 65.9%, with gradual intestinal release, making them suitable for specific gastrointestinal applications. The study demonstrated that nanogels made with dextran and thermally denatured soy protein had low dispersion, nanoscale sizes, and fair encapsulation efficiency when riboflavin was delivered in an intact form, supporting their potential as functional nutraceutical carriers.52
Another promising approach in nanogel synthesis is ionic gelation, a simple and mild technique that enables the fabrication of nanogels under physiological pH and ambient temperatures, making it particularly suitable for encapsulating sensitive bioactive compounds.53 Buosi et al. developed a resveratrol-loaded chitosan-sodium tripolyphosphate nanogel targeted for ophthalmic delivery.54 The nanogel demonstrated remarkable protection of the encapsulated bioactive compound from UV light. It exhibited biocompatibility with a human retinal pigment epithelial cell line (ARPE-19). These findings underscore the ability of ionic gelation-derived nanogels to serve as protective carriers for bioactives while maintaining cellular compatibility.
Collectively, these studies showcase innovative methods to prepare nanogels with tailored properties for nutraceutical delivery. The techniques employed, such as chemical modification, self-assembly, ionic gelation, and the Maillard reaction, enhance the functional capabilities of protein-based carriers. However, a significant limitation across most studies is the absence of in vivo validation, which remains crucial for assessing the clinical efficacy, safety, and scalability of these systems. Future research should focus on optimizing nanogel formulations to address allergenicity concerns, improving stability in complex biological environments, and conducting comprehensive in vivo evaluations to establish their commercial viability. Other recent studies related to the entrapment of bioactives into polymeric nanogels are summarised in Table 1.
| Loaded bioactive compounds | Polymers used for encapsulation | Methods of preparation | Outcomes | Targeted applications | Ref. |
|---|---|---|---|---|---|
| Curcumin | Soy protein isolate (SPI) | Heat-induction | • Reported particle size, PDI, and EE% of 143 nm, 0.2 and 93%, respectively | Targeted therapy for numerous ailments like cancer, diabetes, and cardiovascular diseases | 45 |
| • Revealed sustained release of curcumin (approx. 80%) | |||||
| • Showed enhanced stability and antioxidant nature | |||||
| Rapeseed protein isolate | Chemical acylation and heat-induced protein denaturation | • Reported a hydro-diameter of 170 nm and encapsulation efficiency of 95% | Anti-cancer therapy for several cell lines | 46 | |
| • Had significantly different spatial secondary and tertiary structures | |||||
| • Demonstrated enhanced hydrophobic surfaces and reduced levels of free sulfhydryl groups | |||||
| Polyglutamate | Esterification reaction with vitamin B6 | • Exhibited remarkable anti-inflammatory activity in vitro as well as in vivo | Targeted anti-inflammatory effect | 55 | |
| • Macrophages incorporated curcumin-loaded nanogels to release it under acidic conditions | |||||
| Soy protein isolate and dextran | Maillard reaction and self-assembly technique | • Reported EE% and DL% of 89% and 17%, respectively | Fortification of acidic beverages like orange juice | 50 | |
| • Exhibited remarkable bioavailability of 55%, enhanced stability, and excellent antioxidant activity | |||||
| Vitamin B2 (riboflavin) | Dextran and modified soy protein | Ultrasonication | • Reported a spherical shape of 40 nm and EE% of 66% | Targeted delivery system for vitamin B2 | 52 |
| • Exhibited low dispersion, nanoscale sizes, and fair encapsulation efficiency | |||||
| Vitamin B9 (folic acid) | Soy protein isolate | Self-assembly | • The stability of vitamin B9 was independent of the presence of a singlet oxygen atom | Targeted delivery of folic acid | 49 |
| • Stable at high temperatures, light exposure, and oxygen pressures | |||||
| Capsaicin | Poly(vinyl alcohol) and gelatin | Esterification | • Crystallinity was inversely proportional to gelatin concentration | Innovative delivery system for capsaicin | 56 |
| • Exhibited excellent sensitivity to both pH and temperature | |||||
| Resveratrol | Chitosan and sodium tripolyphosphate | Ionic gelation | • Reported particle size, ZP, and EE% of 140 nm, 32 mV, and 59%, respectively | Targeted ophthalmic delivery | 54 |
| • Demonstrated biocompatibility with human pigment epithelial cell line (ARPE-19) and remarkable protection from UV light | |||||
| Tribasic acid and 1,2,5-pentanetriol | Esterification precipitation reaction | • Reported an encapsulation effectiveness of 94.5% and a spherical shape with a particle size of 220 nm | For the enhancement of protective effects in oxidative stress cells | 57 | |
| • In vitro results showed high protective activity of resveratrol against oxidative stress in fibroblast and neuroblastoma cells | |||||
| Quercetin | Chitosan | Ionic-gelation | • Nanogel particle size, PDI, and zeta-potential were reported to be 370 nm, 0.528, and −24.8 mV, respectively | Targeted transdermal delivery of quercetin as an antioxidant | 58 |
| • Reported an entrapment efficiency of 98% | |||||
| • Exhibited sustained release of the drug up to 48 hours | |||||
| Poly-ε-caprolactone (PCL) and poly ethylene glycol (PEG) | Crosslinking with folic acids | • Reported particle size ranges from 44 to 194 nm | Targeted delivery system for a wide range of cancers | 59 | |
| • Revealed remarkable drug loading and entrapment efficiencies | |||||
| • Nanogels with greater crosslinking densities disintegrated in buffer solution too quickly | |||||
| Lutein | Ovomucin and chitosan oligosaccharide | Self-assembly method | • Demonstrated particle size, PDI, ZP, EE%, and DL% of 210 nm, 0.25, −20.71 mV, 90%, and 6.5%, respectively | Novel oral delivery system for lutein and other hydrophobic drugs | 48 |
| • Exhibited remarkable stability over a wide range of pH values | |||||
| • Exhibited significant antioxidant activity and no cytotoxicity toward L929 fibroblast cells | |||||
| β-Carotene | Chitosan and carboxymethyl starch | Chemical cross-linking | • Demonstrated excellent protection from UV light and high temperature | Targeted oral delivery of β-carotene and other bioactives | 60 |
| • Exhibited remarkable stability indicated by enhanced retention (56–69%) of encapsulated β-carotene upon one month storage | |||||
| • Showed improved bioavailability of encapsulated β-carotene |
2.2 Nano-emulsions
Nano-emulsions (NEs) are small-sized colloidal particulate systems utilized for the transportation of numerous drug molecules.61 They differ from traditional emulsions due to their smaller droplet size, typically in the nanometre range. They typically consist of water, oil, and an emulsifier, which plays a crucial role in reducing the interfacial surface tension between water and oil phases, stabilizing the NEs, and preventing coalescence.62 Surfactants, proteins, and lipids are commonly used emulsifiers.63,64 NEs offer multiple advantages such as shielding active ingredients, enhancing their effectiveness, and serving as delivery systems for nutraceuticals and food components.65,66Recent studies have extensively investigated the potential of NEs as advanced nutraceutical delivery systems, focusing on their ability to enhance bioavailability, stability, and functional properties. In one such study, researchers encapsulated co-enzyme Q10 into chitosan-based NEs, demonstrating excellent stability, reducing oxidative stress and inflammation in cardiomyoblast cells and hepatocytes against the damaging effects of the potent anticancer drug doxorubicin.67 They also increased cell viability by 35–40% in hepatocytes and cardiomyocytes and decreased the production of nitric oxide, interleukins, and TNF-α. The chitosan surface provided biocompatibility and enhanced bio-adhesion, improving cellular uptake.67 Similarly, another study encapsulated clove oil in whey-protein NEs utilizing the ultrasonication method, achieving a droplet size of 280 nm, a PDI of <2, and a zeta potential (ZP) of −35 mV.68 These properties contributed to stability across various pH levels, temperatures, and ionic concentrations. These NEs exhibited potent antimicrobial efficacy against E.coli and B. subtilis strains with a minimum inhibitory concentration and minimum bactericidal concentration of 50 and 90 μg mL−1, respectively, suggesting their potential for food safety applications. The small droplet size and negative zeta potential likely enhanced the interaction with microbial membranes, improving efficacy. This study heralds a promising avenue for leveraging clove oil's antimicrobial properties in practical applications within food systems.68 Another study utilizing a NE mixture of whey protein isolate (WPI) and gardenia fruit oil encapsulated three different bioactive compounds, named β-carotene, hesperetin, and naringenin, achieving remarkable encapsulation efficiencies of 80%, 51%, and 46%, respectively.69 Small droplet sizes (<300 nm) achieved through ultrasound application stabilized the NEs under challenging environments, i.e., lower temperatures, alkaline conditions, and reduced cationic concentrations. They impressively curtailed the generation rate and peroxide value (PoV) and amount of thiobarbituric acid reactive substances (TARS) during the 14 day accelerated oxidation experiment, confirming the improved oxidation stability of gardenia fruit oil.69
In terms of functional bioavailability and cellular uptake, albumin-stabilized NEs encapsulating resveratrol exhibited neuroprotective effects by mitigating postoperative cognitive dysfunction in older rats by decreasing hippocampal inflammation.70,71 The smooth surface and bioactive encapsulation protected resveratrol from degradation and improved its interaction with cellular targets. The neuroprotective effects were linked to the activation of the SIRT1 signaling pathway, highlighting the role of surface characteristics in influencing biological pathways.71 Additionally, N. Walia and L. Chen demonstrated that the encapsulation of vitamin D by pea protein-stabilized NEs enhanced its bioavailability and potentially alleviated vitamin insufficiency in older adults. The protein surface enhanced cellular absorption, highlighting the value of protein–emulsifier interactions in optimizing nutrient delivery.72 V. Campani et al. utilized low-energy techniques to prepare vitamin K1 (VK1)-loaded PLGA NEs, which exhibited intriguing droplet size and stability across different storage conditions. The porous surface facilitated skin penetration and transdermal delivery. Nebulization studies confirmed the potential for spray formulations without altering the NE properties, suggesting commercial viability for topical applications.73
From an antioxidant and food system perspective, black rice bran phenolics (ferulic and p-coumaric acids) were successfully entrapped in sunflower oil NEs using homogenization and ultrasonication, exhibiting sustained antioxidant activity across varying thermal and ionic conditions (0.2–1 mol L−1), thereby suggesting their utility in food preservation.74 Similarly, Li J. et al. incorporated lycopene from tomato waste into oil-in-water NEs utilizing isopropyl myristate (oil phase) and Pluronic F-127 (emulsifier) through high-speed homogenization combined with spray-drying technology. The spray-drying technique preserved lycopene's bioactivity, and small droplet sizes enhanced its dispersion and uptake in food systems.75
Despite these promising attributes, several challenges remain in the development and commercialization of NE-based nutraceuticals. While NEs demonstrate superior EE%, payload capacity, and protection from degradation, their stability is highly dependent on the selection of appropriate surfactants and emulsifiers. Expanding in vivo studies to validate their bioavailability and therapeutic efficacy is essential for their clinical and commercial translation. Future innovations should explore hybrid NE systems integrating multiple encapsulation strategies to achieve synergistic effects. Table 2 summarizes studies on nutraceutical delivery via NEs, highlighting natural and synthetic polymers used for encapsulating nutritional supplements and their targeted applications.
| Loaded bioactive compounds | Polymers used for encapsulation | Methods of preparation | Outcomes | Targeted applications | Ref. |
|---|---|---|---|---|---|
| Coenzyme Q10 | Chitosan | High-pressure homogenization | • Cell viability increased by 35–40% in coenzyme Q10-loaded nanoemulsions | Protection of cardiomyocytes and hepatocytes against the damaging effects of doxorubicin and trastuzumab | 67 |
| • Decreased the production of nitric oxide, interleukins and TNF-α | |||||
| • Reduced oxidative stress and inflammation in cardiomyoblast cells (HCF cell line) and hepatocytes (THLE-2 cell line) | |||||
| Eugenol from clove oil | Whey protein | Ultrasonication | • Reported droplet size, PDI, and zeta potential of 280 nm, less than 0.2, and −35 mV, respectively | Antimicrobial agent for various food products | 68 |
| • Exhibited astonishing resistance to different pH values (3–7), temperatures (60–120 °C), and ionic concentrations (0.1–1 M NaCl) | |||||
| • Demonstrated potent antimicrobial efficacy against E.coli and B. subtilis strains after a rigorous contact period of 8 hours | |||||
| β-Carotene, hesperetin, and naringenin | Whey protein isolate (WPI) and gardenia fruit oil | High-speed homogenization and ultrasonication techniques | • Reported small droplet sizes (<300 nm) at 10% v/v concentration of oil | Targeted and controlled delivery of lipid-soluble nutraceuticals | 69 |
| • Demonstrated excellent stability at low temperature, high pH, and low ionic strength | |||||
| • All three NEs exhibited remarkable encapsulation efficiencies | |||||
| Resveratrol | Albumin | Low-energy emulsification | • Reduced the cognitive impairment brought on by surgery and associated hippocampus neuroinflammation | Prevention of cognitive dysfunction | 71 |
| • The concurrent injection of sirtinol decreased the neuroprotective effects of resveratrol's nano-emulsion | |||||
| Vitamin D | Pea protein | High-pressure homogenization | • Revealed excellent stability, reduced droplet sizes (170–350 nm), and remarkable encapsulation efficiency (94–96%) | Alleviation of vitamin insufficiency in elderly people | 72 |
| • Exhibited high cellular uptake in the Caco-2 cell line | |||||
| Vitamin K1 (Phytonadione) | PLGA (polylactic-co-glycolic acid) | Spontaneous emulsification | • Exhibited intriguing droplet size and stability over time at various storage temperatures | Targeted transdermal delivery of VK1 | 73 |
| • Had the potential to be used for the commercial production of an aqueous spray formulation for the topical administration of VK1 | |||||
| Curcumin | Chitosan | Homogenization | • Chitosan improved the spreadability, feel, and consistency of the nano-emulsion | For the transdermal delivery of curcumin | 76 |
| • Showed improved skin permeability | |||||
| Polyacrylic acid | Low-energy emulsification | • Increased the solubility and skin permeability of curcumin | For the management of psoriasis | 77 | |
| • Droplet size, PDI, and zeta potential were 10.57 nm, 0.094, and −18.7 mV, respectively | |||||
| • Results showed fast recovery of psoriasis in rat models | |||||
| Ferulic acid and p-coumaric acid | Polysorbate 80 and soy lecithin | Homogenization and ultrasonication | • All NEs reported droplet sizes within 128–226 nm | Targeted drug delivery system for polyphenols | 74 |
| • Exhibited excellent stability at high temperature (65 °C) over a one-month storage period | |||||
| • Demonstrated remarkable stability and antioxidant activity at different ionic strengths (0.2, 0.5, and 1 mol L−1) | |||||
| Lycopene | Isopropyl myristate and Pluronic F-127 | High-speed homogenization and spray-drying techniques | • Reported droplet sizes in the range of 259–276 nm with uniform spherical shape | Novel drug delivery system for lycopene and other bioactives | 75 |
| • Evaluated the effect of different coating agents, drying temperatures (120–170 °C), and feed flow rates (3–9 ml min−1) on droplet size | |||||
| • Maltodextrin (coating agent) enhanced the stability of NEs |
2.3 Liposomes and Niosomes
Liposomes and niosomes are advanced vesicular nanocarriers used for the efficient delivery of hydrophilic and lipophilic substances. Unlike nanogels and nanoemulsions, these vesicles can encapsulate a wide range of bioactives due to their unique multilamellar structure.78 Both hydrophilic and lipophilic molecules, as well as amphiphilic substances, can be incorporated into their structures (Fig. 3).79 Multilamellar liposomes, for instance, offer additional versatility by hosting substances within their multiple bilayers. Liposomes primarily consist of phospholipids and surfactant molecules, while niosomes are synthesized from non-ionic surfactants, making them cost-effective.80 Numerous techniques are employed for the preparation of these vesicles, including lipid layer hydration, reversed-phase evaporation, transmembrane pH gradient, micro-fluidization, ether injection, and extrusion techniques.81 Critical factors influencing their synthesis include the type of vesicle, phospholipid properties, their interactions with the dispersion medium, and the bioactive molecules being encapsulated.82Liposomes and niosomes have demonstrated remarkable potential in delivering various vitamins (A, C, and E), phytosterols, and other bioactive substances.83 Encapsulation in liposomes improved all-trans retinoic acid's (ATRA's) photo-stability and anti-cancer efficacy, enhancing cellular uptake and reducing degradation.84,85 The anticancer effects of retinoic acid were enhanced on thyroid carcinoma cell lines (FRO, PTC-1, and B-CPAP) when ATRA was delivered via liposomes. The intracellular uptake of the vesicular formulation in the in vitro assays on FRO and B-CPAP cell lines demonstrated a more pronounced anticancer effect when compared with the free drug.86 Similarly, niosomes synthesized using the film hydration technique demonstrated a narrow size distribution (107–190 nm) and high encapsulation efficiency, enhancing stability and reducing lipid peroxidation.87
In a parallel investigation, liposomal formulations of curcumin and α-tocopherol, prepared with the homogenization method, retained the antioxidant activity in fortified cookies without compromising sensory attributes. Their encapsulation efficiencies were reported to be above 90%. DPPH and ferric-reducing antioxidant power assays reported the successful liposomal encapsulation of curcumin and α-tocopherol into the fortified cookies, preserving the antioxidant properties of both.88 Another group of researchers explored niosomal formulations for co-delivering nutraceuticals and dietary supplements. They encapsulated gallic acid with curcumin and ascorbic acid with quercetin in niosomes, which significantly boosted solubility and antioxidant efficacy.89 This enhanced synergistic antioxidant activity, making it more promising for chronic disease management. A separate investigation highlighted the encapsulation of gallic acid extracted from Indian gooseberry and sappan wood heartwood using the ethanol injection method to produce mucus-penetrating niosomes with potent anti-inflammatory activity. The addition of poloxamer 407 influenced the diffusion mechanisms, enabling efficient intestinal absorption.90
Incorporating bioactive lipids into vesicular systems has also demonstrated promising outcomes. Zelikina D. et al. incorporated curcumin and fish oil PUFAs (n − 3 and n − 6) into liposomes made from a WPI-chitosan conjugate via the Maillard reaction. The liposomes exhibited the sustained release, enhanced bioavailability, and mucoadhesiveness of the encapsulated nutraceuticals during gastric and small intestinal stages.91 Semenova M. et al. encapsulated a combination of lipophilic (n − 3 PUFAs, vitamin D3, and eugenol) and hydrophilic (γ-aminobutyric acid) nutraceuticals in phosphatidylcholine liposomes coated with a WPI-chitosan conjugate. These liposomes exhibited small particle size, remarkable encapsulation efficiencies (>80% for lipophilic and >49% for hydrophilic), and improved interaction with bile salts and mucin in the intestine. The study demonstrated the potential of phosphatidylcholine liposomes as novel oral carriers for lipophilic and hydrophilic bioactive compounds.92
Liposomes and niosomes, with their tunable shape, surface properties, and multi-compartment structure, serve as efficient carriers for bioactives, ensuring improved stability, bioavailability, and targeted delivery. These attributes position them as promising candidates for nutraceutical and functional food applications. Despite their significant advantages, further research is needed to optimize these delivery systems by improving their stability in biological environments, ensuring large-scale reproducibility, and conducting extensive in vivo evaluations to confirm their therapeutic potential (Table 3).
| Loaded bioactive compounds | Phospholipids and surfactants used | Methods of preparation | Outcomes | Potential applications | Ref. |
|---|---|---|---|---|---|
| All-trans retinoic acid (ATRA) | Phosphatidylcholine, cholesterol, and polyethylene glycol (PEG) | Reversed-phase evaporation | • Reported particle size and EE% of 200 nm and 82%, respectively | Potential therapy for anaplastic thyroid carcinoma | 86 |
| • Exhibited remarkable stability in 60% FBS | |||||
| • Enhanced anticancer effect on thyroid carcinoma cell lines (FRO, PTC-1, and B-CPAP) | |||||
| α-Tocopherol | Dicetyl phosphate (DCP), cholesterol, Span 60, and Tween 60 | Film hydration technique | • Showed a particle size range, ZP, EE%, and PDI of 107–190 nm, −30 mV, >80%, and 0.34 | Novel oral carrier for α-Tocopherol and other lipophilic bioactives | 87 |
| • Improved stability with DCP and cholesterol | |||||
| • Exhibited initial burst release followed by sustained release | |||||
| Curcumin and α-tocopherol | Cholesterol and soy lecithin | Homogenization method | • Reported a particle size range and EE% of 14–16 μm and more than 90%, respectively | Fortification of cookies and other food products with curcumin and α-tocopherol | 88 |
| • Encapsulated nutraceuticals did not alter the sensory attributes of the cookies | |||||
| • Retained the antioxidant activities of the loaded bioactives | |||||
| Gallic acid/curcumin and quercetin/vitamin C | Tween 60 | Film hydration method | • All niosomes had particle sizes from 500 nm to 700 nm | Alleviation of diseases caused by oxidative stress | 89 |
| • Combination of two nutraceuticals substantially increased the antioxidant activity and aqueous solubility | |||||
| Gallic acid from Indian gooseberry and sappan wood | Poloxamer 407, cholesterol, Span 60, and Tween 80 | Ethanol injection technique | • Reported particle sizes between 96 and 400 nm | Targeted transmucosal delivery system for mitigation of mucositis | 90 |
| • Decrease in mucus penetration caused by incorporation of poloxamer 407 | |||||
| • Enhanced anti-inflammatory activity due to the combination of two extracts | |||||
| Curcumin and fatty acids of fish oil (n − 3 and n − 6 PUFAs) | Phosphatidylcholine and biopolymers (WPI and chitosan) | Maillard reaction | • Exhibited ZP and EE% of 29 mV and >92%, respectively | Novel oral delivery system for lipophilic bioactives | 91 |
| • Enhanced bioavailability, sustained release, and increased mucoadhesiveness of the encapsulated nutraceuticals at pH 1.2 and pH 6.8 | |||||
| • Increased solubility and stability of curcumin | |||||
| Lipophilic: n − 3 PUFAs, vitamin D3, and eugenol | Phosphatidylcholine and biopolymers (WPI and chitosan) | Maillard reaction | • Reported remarkable encapsulation efficiencies for lipophilic (>80%) and hydrophilic bioactives (>49%) | Targeted oral delivery system for lipophilic and hydrophilic nutraceuticals | 92 |
| Hydrophilic: γ-aminobutyric acid | • Reduced microviscosity and particle size leading to enhanced aqueous solubility and excellent stability | ||||
| • Increased bioavailability of the encapsulated nutraceuticals resulting in their interaction with bile salts and mucin in the small intestine |
2.4 Nanoparticles
Nanoparticles (NPs) are extensively employed as drug delivery systems due to their versatility in composition, and include a variety of materials, including proteins,93 lipids,94 and polymers such as poly-D,L-lactide-co-glycolide(PLGA),95 polylactic acid (PLA),96 poly-ε-caprolactone (PCL),97 chitosan,98 alginate,99 and lignin.100 These materials offer unique properties for encapsulating bioactives and ensuring controlled release. The use of food-grade materials is imperative to ensure safety and compliance for applications in the food and nutraceutical industries. Zein,101 chitosan,102 gelatin,103 and lignin104 are a few examples of the food-grade materials employed in NP formulations (Fig. 4). These materials not only provide biocompatibility and biodegradability but also align with the regulatory requirements, making them suitable candidates for delivering bioactive compounds in functional foods and dietary supplements. | ||
| Fig. 4 Classification of nanoparticles based on the material used to prepare them for delivery of nutraceuticals. | ||
Sunflower seed protein isolate (SFPI) NPs encapsulated curcumin, a hydrophobic anti-inflammatory compound, significantly enhancing its solubility, stability, and anti-oxidant and anti-inflammatory properties. The encapsulation efficiency was observed at 83 ± 3%, with improved curcumin solubility (8.1 μg ml−1). Additionally, the encapsulated curcumin demonstrated higher lipoxygenase activity (IC50 = 45.3 μM) compared to free curcumin. However, the study did not explore the long-term stability or in vivo performance of SFPI-encapsulated curcumin, limiting its translational applicability.109 Similarly, Liu L et al. synthesized soy-β-glycinin NPs employing urea-induced disassembly and reassembly techniques to deliver curcumin. These core–shell nanostructures, comprising an aggregated β-subunit core and hydrophilic α- and α′-subunit shell, achieved a remarkable EE% (79%) and improved curcumin bioaccessibility (40%) compared to free curcumin (20%). These soy β-conglycinin nanostructures offer a potential biocompatible delivery mechanism for hydrophobic substances.110 In a parallel investigation, casein NPs, synthesized via coacervation and stabilized with lysine or arginine, demonstrated gastro-resistance and enabled controlled intestinal release of vitamin B9. These NPs, with an average size of 150 nm, contained 25 mg of vitamin B9 per mg and improved oral bioavailability in vitro. However, the absence of in vivo pharmacokinetic data limits the conclusions regarding their efficacy as an oral delivery system for vitamin B9.111
Another study drew attention to the development of plant-based protein NPs encapsulating quercetin that were resistant to gastric digestion, had antioxidant properties, and were stable enough to withstand higher temperatures.112 High-intensity sonication was employed to prepare soybean, rice, and walnut protein-based NPs with particle size < 110 nm and PDI < 0.20. These NPs exhibited remarkable thermal stability, antioxidant activity, and resistance to gastric digestion while maintaining morphology during digestion. This study provided valuable insights into the development of plant protein-based NPs as promising delivery systems for bioactive compounds.112
Beyond nutraceutical delivery, protein-based NPs have emerged as promising candidates for targeted drug delivery in cancer therapy. A novel study synthesized capsaicin-encapsulated lactoferrin-functionalized carboxymethyl dextran-coated egg albumin NPs (Cap-LF-CMD-EA-NPs) for the treatment of colorectal cancer. The preparation involved esterification, Maillard reaction, and gelation, where hydrophobic interactions between capsaicin and protein polymers facilitated nanoparticle formation.113 Spectral analyses indicated the successful synthesis of smooth and spherical NPs with excellent EE% and DL%. Drug release studies revealed sustained release of capsaicin (up to 80% in 24 hours) in pH 5.8 with anomalous transport attributed to the CMD and EA matrix shell. Moreover, enhanced cytotoxicity against HCT116 and LoVo cell lines was observed owing to the overexpression of lactoferrin receptors in colorectal HCT116 cells. This study illustrates the potential of functionalized protein carriers for the treatment of colorectal cancer.113
Overall, protein-based NPs present a versatile and biocompatible platform for the encapsulation and delivery of bioactive compounds. Their tunable physicochemical properties, combined with high encapsulation efficiency and controlled release capabilities, make them promising candidates for applications in nutraceuticals and targeted drug delivery. However, future research is warranted to optimize their stability, large-scale production, and in vivo performance to facilitate clinical translation.
Chitosan nanoparticles (CS-NPs) have been extensively studied for their potential in targeted cancer therapy. In one of the studies, liver-targeting nanosystems were developed that utilized trans-resveratrol-loaded CS-NPs to target hepatic carcinoma. The researchers modified the nanoparticle surface with biotin (B-CS-NPs) or biotin and avidin (A-B-CS-NPs) to enhance cellular uptake and adhesion to cancer cell lectins.116 NPs were prepared via ionic gelation, a technique known for its simplicity and ability to encapsulate hydrophilic drugs. In vitro studies on HepG2 cells revealed that modified CS-NPs exhibited superior anticancer activity compared to free trans-resveratrol, showcasing enhanced cellular internalization and sustained drug release. However, chitosan's solubility limitations at physiological pH may restrict systemic applications, necessitating further optimization.116
A separate investigation brought attention to the development of PLGA-NPs encapsulating resveratrol modified with chitosan-folate (RSV-CS-F-PLGA-NPs), prepared using a single emulsion solvent evaporation method, as an innovative targeted delivery system for prostate cancer.117 This technique yields highly stable nanoparticles with remarkable drug-loading efficiency. Biological assays on the PC-3 prostate cancer cell line indicated that these NPs induced oxidative stress and apoptosis more effectively than free resveratrol. This study affirms the potential of RSV-CS-F-PLGA-NPs as an efficacious treatment option for prostate cancer. Despite their advantages, PLGA NPs may suffer from burst release effects, though the chitosan coating helps mitigate this limitation by providing an additional diffusion barrier.117
Another study explored the application of PLA nanoparticles to deliver quercetin, prepared using the solvent evaporation method, as a novel approach. The resulting NPs exhibited a particle size of 130 nm, remarkable EE% of 96.7%, and controlled quercetin release, preserving quercetin's bioactivity.118 Fluorescence quenching studies confirmed the protective effect of PLA NPs on quercetin stability. This study paved the way for encapsulating anti-oxidant nutraceuticals toward the development of better therapeutic compounds.118
Vitamin D3 (VD3)-loaded tyrospheres were developed for topical applications to enhance bioactive stability and skin penetration. These polymeric nanospheres demonstrated high drug loading efficiency and protected VD3 from photodegradation and hydrolysis. The ease of formulation and improved drug retention made tyrospheres attractive for dermatological applications, though their limited penetration depth may require complementary techniques for systemic effects.119 Furthermore, Prabhuraj et al. developed PLGA NPs loaded with curcumin, coated with polyethylene glycol (PEG), and conjugated with various targeting moieties, folic acid, hyaluronic acid, and transferrin. The solvent evaporation method produced homogeneous, well-coated NPs with enhanced circulation time and reduced macrophage uptake. TEM imaging confirmed a particle size increase from 85 nm to 124 nm upon PEGylation, correlating with prolonged drug release and enhanced efficacy against aggressive and metastatic MDA-MB-231 breast cancer cells. Nevertheless, PEGylation can sometimes trigger immune responses, which may limit clinical translation.120
A composite nanoparticle system incorporating hydroxyapatite and PLA NPs encapsulating capsaicin (Cap-HA/PLA-NPs) was developed utilizing the ultrasound-assisted dispersion method. SEM imaging revealed uniform, spherical NPs (approximately 50 nm), while pharmacokinetic studies demonstrated prolonged drug release and significantly enhanced bioavailability.121 The biphasic release profile, influenced by HA concentration, allows for an initial rapid release followed by sustained release. They exhibited remarkable biocompatibility and served as effective long-term controlled release carriers, thereby improving the solubility and bioavailability of lipophilic drugs. However, the complex synthesis process and potential for aggregation may present formulation challenges.121
Various other studies related to biodegradable polymers used to encapsulate nutritional supplements into polymeric nanoparticles along with their targeted applications have been summarized in Table 4. These studies highlight the versatility of polymeric NPs in nutraceutical delivery. While natural polymers offer superior biocompatibility and safety, synthetic polymers provide adjustable drug release kinetics and structural stability. The choice of polymer, surface modification strategy, and fabrication method must be tailored to the desired therapeutic outcome. Future research should focus on optimizing formulation parameters, scaling up production, and conducting comprehensive in vivo studies to facilitate clinical translation. By addressing these challenges, polymeric nanocarriers hold immense potential to revolutionize nutraceutical and drug delivery systems.
| Loaded bioactive compounds | Polymers used for encapsulation | Methods of preparation | Outcomes | Targeted applications | Ref. |
|---|---|---|---|---|---|
| Capsaicin | α-Lactalbumin | Self-assembly | • Enhanced rate of capsaicin endocytosis | Treatment of non-alcoholic fatty liver disease (NAFLD) | 122 |
| • Deep penetration and anti-lipogenesis impact on steatotic HepG2 spheroid model | |||||
| Alkali lignin | Modified nanoprecipitation | • Enhanced cellular internalization of Cap-LNPs in HepG2 cells | NAFLD and other chronic liver-inflammatory conditions | 100 | |
| • Decreased intracellular triglyceride accumulation in an OA-induced HepG2 cell model confirmed by oil red O staining and triglyceride quantification | |||||
| Curcumin | β-Lactoglobulin (BLG) | Desolvation | • Loading efficiency(LE) up to 157% | Targeted delivery of curcumin | 123 |
| • The ideal level of glutaraldehyde dropped by 50% when the curcumin/protein ratio rose | |||||
| PLGA(polylactic-co-glycolic acid) | Solvent displacement | • Hyaluronic acid and folic acid were superior targeting moieties amongst hyaluronic acid, folic acid, and transferrin | Targeted therapy for breast cancer | 120 | |
| • Highly effective against metastatic MDA-MB-231 breast cancer cells | |||||
| Chitosan | Oil-in-water emulsification and ionotropic gelation technique | • Formed NPs demonstrated superior biopharmaceutical and biological activities compared with free curcumin | Targeted therapy for colon cancer | 124 | |
| • Improved mucoadhesion, cytotoxicity, and cellular uptake against Caco-2 cells | |||||
| • Showed improved antioxidant and anti-inflammatory properties in activated RAW264.7 cells | |||||
| Alginate | Self-assembly | • Hydrophilicity and bioavailability of curcumin increased | Ulcerative colitis treatment | 125 | |
| • Alg-Cur effectively reduced inflammation in RAW264.7 cells | |||||
| Albumin | Self-assembly | • Encapsulation efficiency and loading capacity were 83.22% and 8.33% | Targeted drug delivery system for breast cancer | 126 | |
| • Showed glutathione (GSH)-triggered curcumin release | |||||
| • In vitro results showed increased cytotoxicity against MDA-MB-231 triple-negative human breast cancer cells | |||||
| Resveratrol | Chitosan | Conjugation with biotin alone and along with avidin | • Both B-CS-NPs and A-B-CS-NPs had improved anti-cancer activity against HepG2 cells | Targeted resveratrol delivery for hepatic carcinoma | 116 |
| Solvent displacement method | • Conjugation with biotin enhanced the hepatic tissue penetrability | ||||
| • B-CS-NPs were less cytotoxic than A-B-CS-NPs | |||||
| Chitosan/γ-polyglutamic acid (γ-PGA) | Ionic gelation | • Stability and solubility of resveratrol-loaded NPs were affected by their particle size | Targeted delivery system for resveratrol | 127 | |
| • Nanoencapsulation improved resveratrol solubility and stability | |||||
| • Stability rose but solubility dropped with an increase in particle size | |||||
| Alkali lignin | Self-assembly | • Exhibited improved stability and anti-neoplastic activity of RSV | Targeted delivery system for lipophilic drugs | 128 | |
| • Demonstrated remarkable drug loading efficiency (above 20% wt) | |||||
| Silibinin (SIL) | Bovine serum albumin (BSA) | Nano-precipitation | • SIL/BSA NPs reported to have a diameter of 90 nm | Innovative treatment strategy for acute liver injury | 129 |
| • Reduced acute liver damage caused by APAP and LPS/D-GaIN in mice | |||||
| • Exhibited antioxidant benefits against intracellular oxidative stress | |||||
| PLGA/PCL | Double emulsion solvent evaporation technique | • Reported particle size of 284 nm | Targeted delivery system for pulmonary carcinoma | 130 | |
| • Exhibited sustained release up to 48 h | |||||
| • Improved cell inhibition in a human lung cancer cell line | |||||
| Quercetin | Chitosan | Ionic gelation | • NP size (<200 nm) and encapsulation efficiency of 79.78% were reported | For the treatment of lung and breast cancer | 131 |
| • In vitro results showed quercetin release of about 67.28% | |||||
| • IC50 value reduced in invitro cytotoxicity assay | |||||
| PCL | Modified nanoprecipitation technique | • Particle size, ZP, PDI, and EE% of NCs reported to be 230 nm, −17.5 mV, 0.383 and 93%, respectively | Innovative targeted delivery system for anxiety treatment | 132 | |
| • Demonstrated biphasic release i.e., initial burst release followed by sustained release | |||||
| • More effective intranasally as compared with the oral route of administration | |||||
| Prunus armeniaca gum exudate | Ionotropic gelation | • Exhibited remarkable EE% and sustained drug release | Targeted drug-delivery system for quercetin against antibiotic-resistant S. aureus bacteria | 133 | |
| • Demonstrated significant reduction in bacterial load, IL-6 and IL-1β in the kidney tissues | |||||
| • Greatly improved the intestinal permeation and stability of the bioactive compound | |||||
| Eudragit L-100 | Sonication along with emulsification solvent evaporation | • Administered orally to streptozotocin-induced diabetic rats for 21 days | Innovative targeted drug delivery system for diabetes | 134 | |
| • Exhibited significant anti-diabetic effect on rats | |||||
| PLA (polylactic acid) | Solvent evaporation | • Exhibited remarkable antioxidant activity | Innovative delivery system for quercetin and other anti-oxidant bioactives | 118 | |
| • Reported to have a particle size of 130 nm and EE% of 97% | |||||
| • Demonstrated initial burst release followed by sustained release | |||||
| Fucoxanthin | Alginate and chitosan | O/W emulsification and ionic gelation | • Exhibited particle size, ZP, and EE% of 225 nm, 35.3 mV and 81%, respectively | Innovative delivery system for fucoxanthin as well as other bioactives | 135 |
| • Displayed remarkable stability in simulated environmental conditions | |||||
| • Improved bioavailability, anti-oxidant properties, and cytotoxicity in various cancer cells | |||||
| Vitamin B9 (folic acid) | PLA | Nanoprecipitation technique | • NP size, PDI, and EE% were reported to be 180 nm, 0.18, and 89% respectively | Targeted glycoalkaloidic delivery strategy for breast and bladder cancer treatment | 136 |
| • Exhibited higher cellular uptake for breast(MDA-MB-231) and bladder (RT4) cancer cells | |||||
| PLGA | Nanoprecipitation | • Cytotoxicity in normal and tumor cells was reported | Targeted 5-fluorouracil delivery system for treatment of colon and breast cancer | 137 | |
| • IC50 for folic acid was 4 times lower than that of 5-fluorouracil-loaded PLGA NPs | |||||
| Vitamin D3 (VD3) (Cholecalciferol) | Tyrospheres (tyrosine-derived nanospheres) | Centrifugation and dispersion | • Shielded the bioactive compound against photodegradation and hydrolysis | Topical delivery of vitamin D3 | 119 |
| • Enhanced the stability of VD3 | |||||
| Ovalbumin | Water-in-oil-in-water double emulsion solvent evaporation method | • Inhibited the production of ovalbumin-specific-CTLs by intravenous administration of NPs | Anti-specific immune suppression | 138 | |
| Vitamin K1 (VK1) | Alginate | Nanoprecipitation | • Reported to have an average diameter of 211 nm and ZP of −15 mV | Transdermal delivery of VK1 | 139 |
| • Exhibited enhanced stability of VK1 | |||||
| • Demonstrated higher retention of VK1 in the epidermal layer of skin | |||||
| α-Tocopherol | Alginate | Ionotropic gelation | • Reported particle size of 21.9 nm and EE% of 77% | Spinal cord injury treatment | 140 |
| • Exhibited improved cell viability and anti-oxidant activity in human astrocyte spinal cord cells | |||||
| PLGA | Nanoprecipitation | • Reported to have a mean diameter of 165 nm and 172 nm at the lab and pilot scale, respectively | Innovative delivery system for vitamin E | 141 | |
| • Greatly improved antioxidant activity of vitamin E |
One of the significant applications of polymeric nanocapsules is improving the bioavailability of essential nutrients. A study investigated the encapsulation of vitamin K1 (VK1) into sodium alginate nanocapsules using the nanoprecipitation method.139 These nanocapsules reported an average diameter of 211 nm and a negative zeta potential of 15 mV. Franz-type diffusion and tape-stripping assays revealed enhanced VK1 retention in the dermis while reducing unwanted systemic absorption. This highlights nanocapsules' potential in transdermal delivery, though their negative zeta potential may impact long-term stability.139
Similarly, Khayata et al. developed α-tocopherol-loaded nanocapsules (α-T NCs) utilizing the membrane contractor approach, showcasing their promising potential at lab and pilot scales.141 These nanocapsules exhibited particle sizes of 165 and 172 nm at the lab and pilot scales, respectively, with remarkable encapsulation efficiencies of 98% and 97%. A six-month accelerated stability study revealed that α-T NCs were stable without significant changes in mean diameter, zeta potential, and drug encapsulation efficiency. More importantly, the antioxidant activity of vitamin E was significantly improved due to its nano-encapsulation, underscoring the potential of nanocapsules in improving the functionality of dietary antioxidants. Despite these benefits, membrane contractor methods may require precise parameter control for scalability.141
Expanding on the application of natural bioactives, Abbas et al. developed multilayered curcumin-loaded nanocapsules using the self-assembly method.145 The study utilized the ultrasound-assisted NEs as templates, where the polyelectrolytes such as chitosan, partially deacylated chitosan, sodium carboxymethylcellulose (Na-CMC), and purity gum ultra (OSA-modified starch) were employed for the fabrication of stable multilayered curcumin nanocapsules. The outcomes suggested that regulated sonication played a crucial role in producing uniform NE droplets, providing a scalable approach for encapsulating hydrophobic bioactives with enhanced stability.145
Beyond improving bioavailability, polymeric nanocapsules have shown remarkable therapeutic potential in the treatment of colorectal cancer.146 For instance, the co-encapsulation of curcumin and salicylic acid within mucoadhesive copolymer m-PEG-b-PCL NCs demonstrated a sustained release profile and strong adhesion to colonic mucosa. They exhibited a particle size of less than 500 nm and a remarkable EE% of 14% and 91% for curcumin and salicylic acid, respectively. The ability to co-deliver multiple therapeutic agents highlights the versatility of nanocapsules in colorectal cancer treatment.146
For advancing cancer treatment, dual-targeted folic acid-PLGA NCs encapsulating pterostilbene were developed for alleviating hepatocellular carcinoma (HCC).147 They exhibited a particle size of 220 nm and sustained release of pterostilbene for up to 48 hours. More importantly, they demonstrated superior anticancer activity compared to free pterostilbene, with a 20-fold reduction in IC50 against HepG2 cells. In vivo investigations conducted on HCC-induced animals further showcased the superiority of dual-targeted nanocapsules over the free pterostilbene while enhancing apoptotic signaling, reinforcing their potential for targeted cancer therapy.147
In addition to synthetic polymeric carriers, natural nanocarriers have also been explored for bioactive delivery. A study utilizing lotus sporopollenin-based exine capsules for anthocyanin delivery, isolated from red cabbage, demonstrated their potential as an oral delivery system.148 The prolonged acidolysis technique successfully converted microcapsules into nanocapsules with a hydrodynamic size of less than 220 nm and a PDI of less than 0.25, indicating uniform size distribution. HRSEM images confirmed the structural stability of these nanocapsules, even under gastric acid conditions, underscoring their potential as a promising vehicle for various plant-derived bioactive compounds. The natural origin of sporopollenin enhances biocompatibility, though the acidolysis process may affect production efficiency.148
These studies underscore the versatility of polymeric nanocapsules in improving the bioavailability, stability, and targeted delivery of bioactive compounds. The integration of natural and synthetic polymers further expands the potential of nanocapsules, paving the way for innovative advancements in bioactive encapsulation. The choice of polymer, encapsulation method, and surface modification strategy directly impact the efficacy and practicality of the delivery system. Future research should focus on optimizing these parameters, exploring alternative biodegradable polymers, and conducting long-term in vivo studies to ensure clinical translation.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 to 99
30 to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, with surfactant concentrations between 1.5% and 5% (w/v).150 Various techniques, including ultrasonication, solvent evaporation, solvent emulsification-diffusion, and supercritical fluid methods, are employed for their synthesis.152
0.1, with surfactant concentrations between 1.5% and 5% (w/v).150 Various techniques, including ultrasonication, solvent evaporation, solvent emulsification-diffusion, and supercritical fluid methods, are employed for their synthesis.152
Liposoluble nutraceuticals, including carotenoids, vitamins A, D, and E, omega-3 fatty acids, and essential oils, are compatible and miscible with the lipid matrix used in these nanovehicles.153–155 For instance, researchers have improved the physicochemical stability of β-carotene by encapsulating it within WPI-stabilized SLNs containing palmitic acid and corn oil. The palmitic acid crystals developed a protective shell around the oil droplets' surface, enhancing the oxidative stability, while WPI contributed to colloidal stability. This strategy highlights the potential of SLNs in improving the shelf life and bioavailability of carotenoids in functional foods and supplements.156
Another promising nutraceutical, lutein, acts as a free radical scavenger and blue light filter, making it a valuable candidate for skincare applications.157,158 Researchers formulated lutein into SLNs, NLC, and NEs using a high-pressure homogenization technique, with particle sizes ranging from 150 to 350 nm.159 NEs exhibited the highest in vitro release of lutein among the three forms. The study underscored the potential of these nanocarriers as novel antioxidants and photoprotective agents, capable of shielding the skin from blue-light induced oxidative stress and photodamage.159
In another study, ergocalciferol (vitamin D2) was encapsulated in tripalmitin-based SLNs stabilized with Tween 20 using a nozzle-type high-pressure homogenizer and the hot homogenization method. The SLN dispersion, formulated with 5% w/w ergocalciferol, exhibited particle sizes between 65 and 120 nm, depending on the vitamin concentration. The outcomes indicated that the higher vitamin D2 concentrations enhanced the stability of the lipid crystal structure, improving protection against oxygen and light exposure. This approach presents an alternative delivery strategy for vitamin D2 in functional foods, such as fortified milk and margarine, though further studies are needed to ensure sustained bioavailability post-ingestion.160
Beyond nutraceuticals, SLNs have also been explored for their therapeutic applications. One study focused on improving the intestinal permeability and bioavailability of γ-tocotrienol (γ-T3), a member that belongs to the vitamin E family with limited absorption due to its lipophilicity.161,162 Encapsulation of γ-T3 in SLNs via the solvent evaporation technique led to a marked enhancement in oral bioavailability by increasing passive permeability, as confirmed by in vivo investigations. However, the study emphasized the need for further optimization to maximize absorption efficiency and metabolic stability.162
The therapeutic potential of lipid nanocarriers extends to cancer treatment. Researchers developed quercetin-encapsulated SLNs employing stearic acid and tripalmitin, stabilized with Tween 80 and Span 80, and optimized their formulation using the Box–Behnken design approach.163 The resulting SLNs exhibited spherical shapes with a reduced particle size of 132 nm and outstanding EE% of 98%, facilitating gradual release of quercetin for up to two days. Furthermore, the in vitro cytotoxicity investigations conducted on the Caco-2 cell line at IC50 of 49 μM ml−1 demonstrated the therapeutic effectiveness of quercetin-loaded SLNs by inducing apoptosis along with minimal necrosis and oxidative stress in the cancer cells. However, challenges such as large scale production, long-term stability, and in vivo biodistribution remain areas for future research.163
Overall, SLNs and NLCs offer a versatile platform for the delivery of nutraceuticals and therapeutics, with applications ranging from functional foods to cancer therapy. Despite their advantages, challenges such as scale-up feasibility, long-term stability, and precise control over drug release kinetics must be addressed. Future research should focus on optimizing formulation parameters, improving biocompatibility, and conducting extensive in vivo studies to validate their efficacy and safety for clinical applications.
2.5 Nanocrystals
Nanocrystals are nanosized formulations composed of drug particles stabilized using appropriate stabilizers or surfactants, which typically fall within the nanometre size range, often between a few nanometres and 1000 nm.164 These nanocrystals are often formulated as nanosuspensions by dispersing them in an aqueous medium.165 Unlike polymeric or lipidic nanoparticles, nanocrystals consist entirely of the active pharmaceutical ingredient or nutraceutical molecule, offering a pure drug delivery system. Their synthesis involves either the application of high-energy size reduction techniques to macro-sized drug dispersions in the presence of stabilizers or precipitation of the drug from an organic solvent upon the addition of an aqueous solution of surfactant or stabilizer.166Recent studies have demonstrated the potential of nanocrystals in improving the bioactivity and stability of various bioactive compounds. For instance, Akhlagi et al. encapsulated vitamin C (VC) in cellulose nanocrystals grafted with chitosan oligosaccharide via ionic complexation with tripolyphosphate. This formulation significantly improved the stability and free radical scavenging activity of VC. Notably, these nanocrystals exhibited encapsulation efficiencies of 72% and 91% at pH 3 and 5, respectively. These findings underscore the potential of nanocrystals in preserving the functionality of antioxidant molecules; however, further investigation into their long-term stability and release kinetics is warranted.167
Similarly, another notable study investigated the synthesis of quercetin-loaded-cellulose nanocrystals (QCT-CNCs) derived from celery stalks. Morphological analysis using FESEM and TEM revealed spherical nanocrystals with a reduced particle size from 600 nm to 400 nm.168 X-ray diffraction analysis confirmed the successful hydrolysis of the cellulose, and FTIR analysis indicated reduced crystallinity due to quercetin-cellulose interactions. Furthermore, binding affinity studies with human holo transferrin (HHT) demonstrated fluorescence quenching, confirming the interaction between QCT-CNCs and HHT. These findings suggest that QCT-CNCs represent a promising nanocarrier delivery strategy for quercetin and other bioactive compounds. However, challenges such as optimizing drug loading efficiency, stability, and in vivo biodistribution requires further investigation.168
Beyond preserving the natural structure and bioactivity of nutraceuticals, nanocrystals also aid in improving the therapeutic index of various bioactive compounds. One such study by Ndong Ntoutoume et al. explored the application of curcumin-loaded-cyclodextrin/cellulose nanocrystals (Cur-CdxCNs) for the treatment of colorectal and prostate cancer. These nanocrystals were prepared via ionic association with cationic β-cyclodextrin (CD). The in vitro assessments demonstrated promising anticancer activity on colorectal and prostatic cell lines, although the study was limited by the absence of in vivo validation, highlighting the necessity for further preclinical evaluation to assess its pharmacokinetics and therapeutic efficiency.169
Delving further into the therapeutic potential of nanocrystals, Manca et al. synthesized quercetin nanosuspensions via the wet medium milling technique, employing Tween 80 and Poloxamer 188 as stabilizers for treating skin disorders.170 The resulting quercetin nanocrystals, characterized by DSC, FTIR, and X-ray powder diffractometry, exhibited mean particle diameters ranging from 326 to 474 nm with a PDI below 0.30. This nanosizing approach significantly improved the solubility and bioavailability of quercetin, enhancing its potential for treating skin disorders. In vitro evaluations utilizing keratinocytes confirmed its dermatological applications; however, further clinical studies are required to determine its efficacy in human subjects and asses potential cytotoxicity concerns at higher concentrations.170
While nanocrystals offer a versatile platform for enhancing drug solubility, stability, and bioavailability, several limitations must be addressed before their clinical translation. These include the need for comprehensive pharmacokinetic studies, optimization of large-scale production techniques, and assessment of potential toxicity associated with prolonged exposure. Future research should emphasize refining nanocrystal formulations for targeted delivery, controlled release, and enhanced therapeutic outcomes in diverse biomedical applications.
3 Navigating the pros and cons of nanocarriers
Nanoformulations provide significant advantages for the encapsulated nutraceuticals, including enhanced bioavailability, improved physicochemical stability, targeted delivery, controlled release, protection against photodegradation, thermal, and oxidative degradation.171,172 Their nano-size enables superior drug delivery efficiency compared to conventional formulations, facilitating improved therapeutic outcomes. Given these attributes, nanoformulations hold immense potential as preventive and therapeutic alternatives for various diseases such as cancer, diabetes, ocular diseases, neurodegenerative disorders, NAFLD, cardiovascular diseases, obesity, mucositis, and many more.173–181Despite their numerous merits and potential applications, concerns regarding their safety, environmental impact, and biocompatibility are ambiguous. The reduced particle size of the nanoparticles, liposomes, niosomes, and nanocrystals increases their surface reactivity, potentially contributing to undesirable interactions in the biological systems, cytotoxicity, and other adverse health problems.182 The different materials used in nanoformulation development, such as polymers, surfactants, cross-linkers, solvents, drugs, and probes, pose additional risks, including cytotoxicity and hemotoxicity. Furthermore, there is a lack of comprehensive, evidence-based studies addressing the long-term toxicity, absorption, clearance, and biodistribution of nutraceutical-loaded nanoformulations in biological systems, contributing to concerns regarding their safety profile.54,91,92 Another critical challenge is the stability of nanoformulations during storage and transportation. Nanoparticles are prone to aggregation and degradation due to environmental conditions, which can significantly compromise their efficacy.183 Additionally, the high production cost associated with nanoformulations, driven by the requirement for sophisticated technology, specialized equipment, and stringent manufacturing conditions, limits their large-scale commercialization.184 Regulatory challenges further complicate the widespread adoption of nanoformulations. The absence of standardized guidelines and discrepancies among regulatory authorities has created inconsistencies in evaluating the safety and efficacy of these formulations.185 Consequently, extensive preclinical and clinical investigations are necessary to establish their therapeutic potential, prolonging the approval process and delaying market entry. Table 5 illustrates a comparative analysis of different nanoformulations discussed in this review based on their production cost, scalability concerns, regulatory challenges, and environmental impact.
| Nanoformulations | Production cost | Scalability | Regulatory complexity | Environmental impact | Ref. |
|---|---|---|---|---|---|
| Nanogels | High due to the need for specialized polymers and cross-linking agents | Complex synthesis may hinder large-scale manufacturing | Limited clinical data may delay regulatory approval | Potential environmental persistence raises concerns | 186 and 187 |
| Nanoemulsions | Moderate due to the utilization of relatively straightforward manufacturing techniques | Amenable to industrial-scale production due to established emulsification methods | Generally safe, but novel formulations still require comprehensive evaluation | Generally considered eco-friendly depending on constituent materials | 187 and 188 |
| Liposomes | Substantial costs as they incorporate high-purity phospholipids and necessitate stringent production conditions | Feasible but requires meticulous control over size and encapsulation efficiency | Several FDA-approved liposomal drugs exist; however, new formulations must undergo rigorous testing | Biodegradable components minimize environmental risks | 189–191 |
| Niosomes | Cost-effective alternative to liposomes as they employ non-ionic surfactants | Simpler preparation methods facilitate scalability | Face uncertain regulatory pathways as they are emerging carriers | Biocompatibility suggests low ecological impact | 189 and 190 |
| Nanoparticles | Cost is determined by the materials used for production. For instance, gold nanoparticles are notably more expensive than polymeric nanoparticles | Scalability is material-dependent. For instance. Polymeric nanoparticles are generally easier to produce in bulk compared to metallic ones | Concerns about toxicity and long-term effects necessitate extensive safety assessments | Non-biodegradable variants, such as certain metals, may accumulate in ecosystems, leading to potential toxicity | 189, 192 and 193 |
| Nanocrystals | Moderate to high costs due to the involvement of sophisticated crystallization techniques | Challenges in controlling crystal size and purity can impede large-scale production | Regulatory bodies require detailed characterization and proof of stability | Environmental effects are largely material-specific and require thorough investigation | 188, 191 and 194 |
4 Regulatory considerations and limitations
The integration of nanotechnology in nutraceuticals necessitates stringent compliance and safety regulations to protect consumers and maintain public confidence.195,196 However, the current global landscape lacks standardized, mandatory legal frameworks specifically governing nano-encapsulated nutraceutical products.197 Consequently, major global regulatory authorities such as the United States Food and Drug Administration (USFDA), the European Chemical Agency (ECHA), the European Food Safety Authority (EFSA), and the World Health Organization (WHO) have introduced comprehensive guidelines and frameworks to regulate nanotechnology applications in nutraceuticals worldwide.198 In 2021, EFSA established a structured risk assessment approach for nanomaterials in numerous food and feed products, emphasizing physicochemical characterization, scientific evaluation procedures, transparency in regulatory communication practices, and active stakeholder engagement through public consultations.199 Similarly, in 2022, the USFDA issued guidance on key regulatory considerations, including quality assurance, manufacturing protocols, safety assessment, characterization techniques, analytical validation, risk management strategies, and regulatory submission requirements of nano-based drug and biological products, ensuring transparency in procuring new materials, addressing ethical and environmental issues, and promoting international trade and conformity worldwide.200 ECHA governs nanomaterials under the Registration, Evaluation, Authorisation, and Restriction of Chemicals (REACH) regulation, yet its adaptation to nano-specific risks remains a challenge due to the evolving nature of nanotechnology.201 WHO, recognizing the increasing global reliance on nanotechnology, has recommended a risk-based regulatory framework that incorporates both hazard identification and lifecycle assessments of nano-nutraceuticals, addressing potential long-term implications, bioaccumulation risks, and environmental impact in humans.202,203 WHO also emphasizes the need for interdisciplinary research collaborations, promoting the integration of nanotechnology risk management strategies into global public health policies.204International initiatives have been launched to harmonize risk assessment methodologies to enhance regulatory oversight and ensure the development of standardized testing protocols. The ‘Malta Initiative’ fosters collaboration between ECHA, Member States, the European Commission, and industry to refine test guidelines that address nano-specific regulatory requirements.205,206 Similarly, the Horizon 2020-funded NanoHarmony project aims to accelerate the development of harmonized test methods by aligning research institutions with the OECD and ECHA.207,208 The ‘MACRAMÉ Project’, an initiative between the European Green Deal and Chemical Strategy for Sustainability, is intended to ensure the safety and sustainability of advanced materials such as carbon nanofibers, polymeric nanoparticles, etc., by developing standardized methodologies for their detection, characterization, and risk assessment throughout their life cycle.209 Complementing traditional toxicity assessments, computational modeling techniques, including quantitative structure–activity relationships (QSARs) and read-across frameworks, are being increasingly adopted to predict nanomaterial behaviour based on existing datasets. Organizations such as OECD, ECHA, and EFSA are actively supporting research efforts to advance these predictive modeling approaches, thereby improving the efficiency and reliability of safety evaluations. These initiatives collectively contribute to a more robust regulatory landscape, facilitating transparent risk assessments, promoting scientific advancements, and ensuring the safe integration of nanotechnology into nutraceutical applications.210,211
Despite significant advancements in regulatory guidelines, several gaps persist in understanding the long-term safety implications of nano-encapsulated nutraceutical products in biological systems, potentially contributing to ambiguities in risk assessments and insufficient safety measures. The rapid evolution of nanotechnology further complicates regulatory adaptation, rendering guidelines susceptible to obsolescence.212 The absence of a globally unified regulatory environment exacerbates these challenges, as discrepancies between international regulatory bodies result in inconsistencies in the requirements and standards imposed.213 This regulatory fragmentation leads to duplicity, market barriers, and potential discrepancies in product safety and quality across different jurisdictions. Furthermore, while current regulatory frameworks prioritize human and animal health, the potential environmental impact of nanomaterials remains an underexplored domain.214 The fate of nanoformulations in ecosystems, their bioaccumulation potential, and their long-term environmental interactions require systematic evaluation to prevent unforeseen ecological consequences. Addressing these challenges necessitates dynamic and evolving standards of regulatory mechanisms that can adapt to new emerging technologies and scientific enlightenment.
A multifaceted approach must be adopted to enhance the effectiveness of regulatory oversight in nanonutraceuticals. A key priority is harmonizing global standards, which requires a collaborative effort among international regulatory bodies to establish unified guidelines. Furthermore, advancements in risk assessment methodologies are essential to accurately evaluate the interactions of nanomaterials at both molecular and systemic levels. The development of advanced toxicological models and in vitro/in vivo studies is needed to assess nanomaterial interactions at the molecular and systemic levels. Regulatory bodies must integrate continuous scientific advancements to ensure relevance and efficacy in risk management.
5 Conclusion and future perspectives
Nutraceuticals and dietary supplements play a pivotal role in promoting health and preventing various chronic conditions, including cardiovascular diseases, neurodegenerative disorders, dermatological problems, cancer, NAFLD, ocular diseases, and inflammatory conditions. However, their clinical efficacy is often limited by inherent physicochemical drawbacks such as low bioavailability, high susceptibility to environmental degradation (light, oxygen, and temperature), instability during storage and delivery, and poor aqueous solubility. Nanotechnology has emerged as a transformative strategy in the food and pharmaceutical industries, offering solutions that enhance the bioavailability, stability, and controlled release of nutraceuticals encapsulated in nanocarriers enhancing their biological efficacy. Despite many significant advantages of nano-enabled strategies, several challenges hinder their widespread adoption, including scalability constraints, the requirement of sophisticated equipment, potential toxicity concerns, regulatory ambiguities, and environmental impact. This comprehensive review underscores the applications of nanotechnology in nutraceutical delivery, demonstrating how polymeric and lipid-based nanocarriers facilitate targeted and controlled release of bioactives. The utilization of GRAS biopolymers, including chitosan, alginate, lignin, zein, and casein, further ensures safety, biodegradability, and non-toxicity, making nanotechnology a viable approach for nutraceutical delivery. Additionally, this review critically evaluates existing regulatory frameworks, highlighting the need for harmonized guidelines, robust safety assessments, and transparent policies to enable the integration of nanotechnology into the nutraceutical industry.Advancements in nanotechnology continue to open new frontiers in nutraceutical delivery. Future research should focus on the development of sustainable nanocarriers derived from eco-friendly, biodegradable polymers to mitigate environmental concerns. Innovations in biopolymeric nanoparticles present significant potential for improving targeted delivery under conditions such as NAFLD, cardiovascular diseases, and metabolic disorders. Additionally, nanogels and nano-emulsions hold promise for transdermal and ocular delivery of nutraceuticals, enhancing the therapeutic efficacy for eye and skin disorders. Emerging technologies such as artificial intelligence (AI) and machine learning are set to revolutionize nanocarrier design by optimizing formulation parameters, predicting stability and encapsulation efficiency, and improving drug release kinetics. AI-driven models can accelerate the development of next-generation nanocarriers by simulating interactions between encapsulated nutraceuticals and biological systems, thus reducing reliance on extensive in vivo experimentation. The integration of AI and sustainable materials in nanocarrier development not only enhances the efficacy and safety of nutraceutical delivery systems but also paves the way for more cost-effective, scalable, and environmentally responsible solutions. Moreover, the integration of wearable biosensors with nanotechnology could enable real-time monitoring of nutraceutical delivery and metabolic responses, facilitating personalized nutrition and precision medicine approaches. With increasing concerns about nanotoxicity and environmental impact, future research should focus on the development of biodegradable smart nanocarriers composed of bioresorbable polymers to ensure safe degradation and minimal ecological footprint. Expanding beyond conventional drug delivery, stimuli-responsive nanocarriers could be tailored for precise gene editing and RNA-based therapies. These systems could enhance CRISPR-Cas9 and siRNA delivery, enabling targeted genetic interventions with minimized off-target effects. The combination of microfluidic nanocarrier synthesis with organ-on-a-chip platforms could accelerate preclinical validation of nanoformulations, reducing the reliance on animal studies and improving translational research outcomes.
To further advance nanotechnology-based nutraceuticals, interdisciplinary collaboration among materials scientists, biotechnologists, regulatory bodies, and industry stakeholders is imperative. Addressing regulatory challenges, ensuring scalability, and prioritizing safety assessments will be crucial for translating laboratory-scale innovations into commercial products. In conclusion, nanotechnology holds immense potential to revolutionize nutraceutical formulations, paving the way for enhanced therapeutic efficacy, precision-targeted interventions, and sustainable health solutions.
Abbreviations
| PUFA | Polyunsaturated fatty acids |
| CAGR | Compound annual growth rate |
| GRAS | Generally recognized as safe |
| SPI | Soy protein isolate |
| ARPI | Acylated rapeseed protein isolate |
| WPI | Whey protein isolate |
| BSA | Bovine serum albumin |
| PLGA | Polylactic-co-glycolic acid |
| PCL | Poly-ε-caprolactone |
| PLA | Polylactic acid |
| PAMAM | Polyamidoamine |
| γ-PGA | γ-Polyglutamic acid |
| TNF-α | Tumor necrosis factor alpha |
| NE | Nano-emulsion |
| SIRT1 | Sirtuin 1 |
| VK1 | Vitamin K1 |
| VD3 | Vitamin D3 |
| PDI | Polydispersity index |
| EE | Encapsulation efficiency |
| AcKPI | Acylated kidney bean protein isolate |
| PoV | Peroxide value |
| TARS | Thiobarbituric acid reactive substances |
| ATRA | All-trans retinoic acid |
| DCP | Dicetyl phosphate |
| Chol | Cholesterol |
| HLB | Hydrophilic–lipophilic balance |
| ZP | Zeta potential |
| SFPI | Sunflower seed protein isolate |
| NPs | Nanoparticles |
| β-CG | Soy-β-conglycinin |
| IC50 | Half-maximal inhibitory concentration |
| SDS-PAGE | Sodium dodecyl sulphate polyacrylamide gel electrophoresis |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| CS-NPs | Chitosan nanoparticles |
| B-CS-NPs | Biotin chitosan nanoparticles |
| AIA | Anti-inflammatory activity |
| A-B-CS-NPs | Biotin and avidin chitosan nanoparticles |
| BLG | β-Lactoglobulin |
| Cap-LF-CMD-EA-NPs | Capsaicin-encapsulated lactoferrin-functionalized carboxymethyl dextran-coated egg albumin NPs |
| NAFLD | Non-alcoholic fatty liver disease |
| LE | Loading efficiency |
| GSH | Glutathione |
| SIL/BSA NPs | Silibinin bovine serum albumin nanoparticles |
| APAP | Acetaminophen |
| LPS/D-GaIN | Lipopolysaccharide/D-galactosamine-induced acute liver injury |
| CTLs | Cytotoxic T lymphocytes |
| MRT | Mean retention time |
| Cap-HA/PLA-NPs | Capsaicin loaded hydroxyapatite and polylactic acid NPs |
| TEM | Transmission electron microscope |
| PEG | Polyethylene glycol |
| Cur | Curcumin |
| RA | Retinoic acid |
| HCT116 | Human colorectal carcinoma cell line |
| ARPE-19 | Human retinal pigment epithelial cell line dopaminergic |
| RA-NPs | Retinoic acid nanoparticles |
| m-PEG-b-PCL NCs | Copolymer m-PEG-b-PCL nanocapsules |
| MPTP | 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| SN | Substantia Nigra |
| PD | Parkinson's disease |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| ALP | Alkaline phosphatase |
| AFP | α-Fetoprotein |
| Bcl2 | B-cell lymphoma 2 |
| HCC | Hepatocellular carcinoma |
| Na-CMC | Sodium carboxymethylcellulose |
| OSA | Octenyl-succinic-anhydride |
| α-T NCs | α-Tocopherol-loaded nanocapsules |
| SLNs | Solid lipid nanoparticles |
| NLCs | Nanostructured lipid carriers |
| γ-T3 | γ-Tocotrienol |
| VC | Vitamin C |
| Cur-CdxCNs | Curcumin-loaded-cyclodextrin/cellulose nanocrystals |
| CD | β-Cyclodextrin |
| DSC | Differential scanning calorimetry |
| FT-IR | Fourier transform infrared spectroscopy |
| QCT-CNCs | Quercetin-loaded-cellulose nanocrystals |
| HHT | Human holo transferrin |
| USFDA | United States Food and Drug Administration |
| ECHA | European Chemical Agency |
| EFSA | European Food Safety Authority |
| WHO | World Health Organization |
| REACH | Registration Evaluation Authorisation and Restriction of Chemicals |
| OECD | Organization for Economic Cooperation and Development |
| QSARs | Quantitative structure–activity relationships |
| AI | Artificial intelligence |
Data availability
No primary research results have been included and no new data were generated as part of this review.Author contributions
JM: collection of the information, drafting of the manuscript, and writing review; KP: writing review; SVP: data curation; conceptualization, planning, analysis, review, and editing. All authors approved the final submitted version of the manuscript.Conflicts of interest
None.Acknowledgements
The authors thank the University Institute of Pharmaceutical Sciences, Panjab University, Chandigarh, India. KP is grateful to the Council of Scientific & Industrial Research (CSIR) for her Junior Research Fellowship (JRF) [File No. 09/0135(13255)/2022-EMRI].References
- A. S. Chopra, R. Lordan, O. K. Horbańczuk, A. G. Atanasov, I. Chopra, J. O. Horbańczuk, A. Jóźwik, L. Huang, V. Pirgozliev, M. Banach, M. Battino and N. Arkells, The current use and evolving landscape of nutraceuticals, Pharmacol. Res., 2022, 175, 106001, DOI:10.1016/j.phrs.2021.106001.
- H. Nasri, A. Baradaran, H. Shirzad and M. Rafieian-Kopaei, New concepts in nutraceuticals as alternative for pharmaceuticals, Int. J. Prev. Med., 2014,(12), 1487–1499 Search PubMed.
- N. A. Helal, H. A. Eassa, A. M. Amer, M. A. Eltokhy, I. Edafiogho and M. I. Nounou, Nutraceuticals' novel formulations: the good, the bad, the unknown and patents involved, Recent Pat. Drug Deliv. Formul., 2019, 13(2), 105–156, DOI:10.2174/1872211313666190503112040.
- S. Binda, C. Hill, E. Johansen, D. Obis, B. Pot, M. E. Sanders, A. Tremblay and A. C. Ouwehand, Criteria to qualify microorganisms as “probiotic” in foods and dietary supplements, Front. Microbiol., 2020, 11, 1662, DOI:10.3389/fmicb.2020.01662.
- Dietary supplements, U.S Food and Drug Administration, https://www.fda.gov/food/dietary-supplements, accessed on 2024-10-03 Search PubMed.
- J. D. Flores-Félix, A. C. Gonçalves, G. Alves and L. R. Silva, Consumption of phenolic-rich food and dietary supplements as a key tool in SARS-CoV-19 infection, Foods, 2021, 10(9), 2084, DOI:10.3390/foods10092084.
- M. F. McCarty, A. Lerner, J. J. DiNicolantonio and C. Benzvi, Nutraceutical aid for allergies – Strategies for down-regulating mast cell degranulation, J. Asthma Allergy, 2021, 14, 1257–1266, DOI:10.2147/JAA.S332307.
- S. Kaag and A. Lorentz, Effects of dietary components on mast cells: possible use as nutraceuticals for allergies?, Cells, 2023, 12(22), 2602, DOI:10.3390/cells12222602.
- L. A. Minhas, M. Kaleem, H. M. U. Farooqi, F. Kausar, R. Waqar, T. Bhatti, S. Aziz, D. W. Jung and A. S. Mumtaz, Algae-derived bioactive compounds as potential pharmaceuticals for cancer therapy: a comprehensive review, Algal Res., 2024, 78, 103396, DOI:10.1016/j.algal.2024.103396.
- R. Di Napoli, N. Balzano, A. Mascolo, C. Cimmino, A. Vitiello, A. Zovi, A. Capuano and M. Boccellino, What is the role of nutraceutical products in cancer patients? A systematic review of randomized clinical trials, Nutrients, 2023, 15(14), 3249, DOI:10.3390/nu15143249.
- C. Calfio, A. Gonzalez, S. K. Singh, L. E. Rojo and R. B. Maccioni, The emerging role of nutraceuticals and phytochemicals in the prevention and treatment of Alzheimer's disease, J. Alzheim. Dis., 2020, 77(1), 33–51, DOI:10.3233/JAD-200443.
- L. Yao, Y. Yang, X. Yang and M. J. Rezaei, The interaction between nutraceuticals and gut microbiota: a novel therapeutic approach to prevent and treat Parkinson's disease, Mol. Neurobiol., 2024, 61(11), 9078–9109, DOI:10.1007/s12035-024-04151-2.
- R. Sharma, L. Bhate, Y. Agrawal and A. Aspatwar, Advanced nutraceutical approaches to Parkinson's disease: bridging nutrition and neuroprotection, Nutr. Neurosci., 2025, 1–17, DOI:10.1080/1028415X.2025.2469170.
- G. Derosa, A. D'Angelo and P. Maffioli, The role of selected nutraceuticals in management of prediabetes and diabetes: an updated review of the literature, Phytother Res., 2022, 36(10), 3709–3765, DOI:10.1002/ptr.7564.
- G. Davì, F. Santilli and C. Patrono, Nutraceuticals in diabetes and metabolic syndrome, Cardiovasc. Ther., 2010, 28(4), 216–226, DOI:10.1111/j.1755-5922.2010.00179.x.
- H. Shahinfar, A. Jayedi, K. Torabynasab, N. Payandeh, F. Martami, H. Moosavi, E. Bazshahi and S. Shab-Bidar, Comparative effects of nutraceuticals on body weight in adults with overweight or obesity: a systematic review and network meta-analysis of 111 randomized clinical trials, Pharmacol. Res., 2023, 196, 106944, DOI:10.1016/j.phrs.2023.106944.
- Z. Dai, Y. Zhang, Y. Meng, S. Li, Z. Suonan, Y. Sun, J. Ji, Q. Shen, H. Zheng and Y. Xue, Targeted delivery of nutraceuticals derived from food for the treatment of obesity and its related complications, Food Chem., 2023, 418, 135980, DOI:10.1016/j.foodchem.2023.135980.
- S. Basak and J. Gokhale, Immunity-boosting nutraceuticals: current trends and challenges, J. Food Biochem., 2022, 46(3), e13902, DOI:10.1111/jfbc.13902.
- C. Mannucci, M. Casciaro, E. E. Sorbara, F. Calapai, E. Di Salvo, G. Pioggia, M. Navarra, G. Calapai and S. Gangemi, Nutraceuticals against oxidative stress in autoimmune disorders, Antioxidants, 2021, 10(2), 261, DOI:10.3390/antiox10020261.
- A. Dama, K. Shpati, P. Daliu, S. Dumur, E. Gorica and A. Santini, Targeting metabolic diseases: the role of nutraceuticals in modulating oxidative stress and inflammation, Nutrients, 2024, 16(4), 507, DOI:10.3390/nu16040507.
- S. Surma, A. Sahebkar and M. Banach, Nutrition, nutraceuticals, and bioactive compounds in the prevention and fight against inflammation, Nutrients, 2023, 15(11), 2629, DOI:10.3390/nu15112629.
- G. Baltazar-Martins, D. Brito de Souza, M. Aguilar-Navarro, J. Muñoz-Guerra, M. Plata, M. del and J. Del Coso, Prevalence and patterns of dietary supplement use in elite Spanish athletes, J. Int. Soc. Sports Nutr., 2019, 16(1), 30–39, DOI:10.1186/s12970-019-0296-5.
- Global nutraceuticals market size, share & trends analysis report by product (2024-2030). Research and Markets, https://www.researchandmarkets.com/reports/4452005/global-nutraceuticals-market-size-share-and, accessed on 2024-10-10 Search PubMed.
- India’s nutraceutical market growth, Ministry of Food Processing, https://foodprocessingindia.gov.in/newsletter/emailer/two#:%7E:text=Currently%2CtheIndianmarketimports,25%25annuallyduringthepandemic, accessed on 2024-10-10 Search PubMed.
- P. Sood, Nutraceuticals market in India: Outlook for the future, The Times of India, https://timesofindia.indiatimes.com/blogs/voices/nutraceuticals-market-in-india-outlook-for-the-future/, accessed on 2024-10-10 Search PubMed.
- Nutraceuticals Global Market Report 2023. Research and Markets, https://www.researchandmarkets.com/reports/5744368/nutraceuticals-global-market-report, accessed on 2024-10-10 Search PubMed.
- Z. Zhang, X. Li, S. Sang, D. J. McClements, L. Chen, J. Long, A. Jiao, Z. Jin and C. Qiu, Polyphenols as plant-based nutraceuticals: health effects, encapsulation, nano-delivery, and application, Foods, 2022, 11(15), 2189, DOI:10.3390/foods11152189.
- J. Chen and L. Hu, Nanoscale delivery system for nutraceuticals: preparation, application, characterization, safety, and future trends, Food Eng. Rev., 2020, 12(1), 14–31, DOI:10.1007/s12393-019-09208-w.
- D. Paolino, A. Mancuso, M. C. Cristiano, F. Froiio, N. Lammari, C. Celia and M. Fresta, Nanonutraceuticals: the new frontier of supplementary food, Nanomaterials, 2021, 1–20, DOI:10.3390/nano11030792.
- M. Mandal, P. Jaiswal and A. Mishra, Role of curcumin and its nanoformulations in neurotherapeutics: a comprehensive review, J. Biochem. Mol. Toxicol., 2020, 34(6), e22478, DOI:10.1002/jbt.22478.
- S. R. Soofiyani, K. Hosseini, H. Forouhandeh, T. Ghasemnejad, V. Tarhriz, P. Asgharian, Ž. Reiner, J. Sharifi-Rad and W. C. Cho, Quercetin as a novel therapeutic approach for lymphoma, Oxid. Med. Cell. Longev., 2021, 2021, 1–15, DOI:10.1155/2021/3157867.
- A. B. Kunnumakkara, M. Hegde, D. Parama, S. Girisa, A. Kumar, U. D. Daimary, P. Garodia, S. C. Yenisetti, O. V. Oommen and B. B. Aggarwal, Role of Turmeric and Curcumin in Prevention and Treatment of Chronic Diseases: Lessons Learned from Clinical Trials, ACS Pharmacol Transl Sci., 2023, 6(4), 447–518, DOI:10.1021/acsptsci.2c00012.
- Md. Aklakur, R. M. Asharf and N. N. Kumar, An emerging avenue for nutraceuticals and drug delivery, Crit. Rev. Food Sci. Nutr., 2016, 56(14), 2352–2361, DOI:10.1080/10408398.2013.839543.
- M. Kharat and D. J. McClements, Recent advances in colloidal delivery systems for nutraceuticals: a case study – Delivery by design of curcumin, J. Colloid Interface Sci., 2019, 557, 506–518, DOI:10.1016/j.jcis.2019.09.045.
- M. C. Cristiano, F. Froiio, A. Mancuso, D. Cosco, L. Dini, L. Di Marzio, M. Fresta and D. Paolino, Oleuropein-laded ufasomes improve the nutraceutical efficacy, Nanomaterials, 2021, 11(1), 105, DOI:10.3390/nano11010105.
- B. Rasti, A. Erfanian and J. Selamat, Novel nano-liposomal encapsulated omega-3 fatty acids and their applications in food, Food Chem., 2017, 230, 690–696, DOI:10.1016/j.foodchem.2017.03.089.
- R. F. S. Gonçalves, J. T. Martins, C. M. M. Duarte, A. A. Vicente and A. C. Pinheiro, Advances in nutraceutical delivery systems: from formulation design for bioavailability enhancement to efficacy and safety evaluation, Trends Food Sci. Technol., 2018, 78, 270–291, DOI:10.1016/j.tifs.2018.06.011.
- D. J. McClements and Y. Li, Structured emulsion-based delivery systems: controlling the digestion and release of lipophilic food components, Adv. Colloid Interface Sci., 2010, 159(2), 213–228, DOI:10.1016/j.cis.2010.06.010.
- M. Agrawal, D. K. Tripathi, S. Saraf, S. Saraf, S. G. Antimisiaris, S. Mourtas, M. Hammarlund-Udenaes and A. Alexander, Recent advancements in liposomes targeting strategies to cross blood–brain barrier (BBB) for the treatment of Alzheimer's disease, J. Controlled Release, 2017, 260, 61–77, DOI:10.1016/j.jconrel.2017.05.019.
- A. Gumezescu and A. E. Oprea, Nanotechnology Applications in Food: Flavor, Stability, Nutrition and Safety, 1st edn, Academic Press, Cambridge, USA, 2017 Search PubMed.
- S. Manocha, S. Dhiman, A. S. Grewal and K. Guarve, Nanotechnology: an approach to overcome bioavailability challenges of nutraceuticals, J. Drug Deliv. Sci. Technol., 2022, 72, 103418, DOI:10.1016/j.jddst.2022.103418.
- N. P. Aditya, Y. G. Espinosa and I. T. Norton, Encapsulation systems for the delivery of hydrophilic nutraceuticals: food application, Biotechnol. Adv., 2017, 35(4), 450–457, DOI:10.1016/j.biotechadv.2017.03.012.
- A. Ali, U. Ahmad, J. Akhtar and M. M. Khan, Engineered nano-scale formulation strategies to augment efficiency of nutraceuticals, J. Funct. Foods, 2019, 62, 103554, DOI:10.1016/j.jff.2019.103554.
- R. Gheorghita, L. Anchidin-Norocel, R. Filip, M. Dimian and M. Covasa, Applications of biopolymers for drugs and probiotics delivery, Polymers, 2021, 13(16), 2729, DOI:10.3390/polym13162729.
- M. He, F. Teng, H. Chen, C. Wu, Y. Y. Huang and Y. Li, Fabrication of soy protein isolate-succinic anhydride-dextran nanogels: properties, performance, and controlled release of curcumin, Int J Biol Macromol., 2023, 244, 125104, DOI:10.1016/j.ijbiomac.2023.125104.
- Z. Wang, R. X. Zhang, C. Zhang, C. Dai, X. Ju and R. He, Fabrication of stable and self-assembling rapeseed protein nanogel for hydrophobic curcumin delivery, J. Agric. Food Chem., 2019, 67(3), 887–894, DOI:10.1021/acs.jafc.8b05572.
- Z. Yu, Y. Gao, Z. Shang, T. Wang, X. He, J. Lei, F. Tai, L. Zhang and Y. Chen, A stable delivery system for curcumin: fabrication and characterization of self-assembling acylated kidney bean protein isolate nanogels, Food Chem., 2024, 443, 138526, DOI:10.1016/j.foodchem.2024.138526.
- Q. Xu, H. Teng, X. Li, Z. Zhang, Y. Han and H. Sun, Natural biomolecule ovomucin–chitosan oligosaccharide self-assembly nanogel for lutein application enhancement: characterization, environmental stability, and bioavailability, J. Funct. Biomater., 2024, 15(4), 111, DOI:10.3390/jfb15040111.
- A. M. Gazzali, M. Lobry, L. Colombeau, S. Acherar, H. Azaïs, S. Mordon, P. Arnoux, F. Baros, R. Vanderesse and C. Frochot, Stability of folic acid under several parameters, Eur. J. Pharm. Sci., 2016, 93, 419–430, DOI:10.1016/j.ejps.2016.08.045.
- Y. Sun, W. Yue, X. Xiang, Z. Chen, J. Chen, S. Li, S. Liu, A. S. M. Saleh, W. Qin and Q. Zhang, Curcumin-loaded soybean-dextran conjugate nanogels: construction, characterization, and incorporation into orange juice beverage, Food Biosci., 2024, 59, 104140, DOI:10.1016/j.fbio.2024.104140.
- L. Blagojevic and N. Kamaly, Nanogels: a chemically versatile drug delivery platform, Nano Today, 2025, 61, 102645, DOI:10.1016/j.nantod.2025.102645.
- B. Jin, X. Zhou, X. Li, W. Lin, G. Chen and R. Qiu, Self-assembled modified soy protein/dextran nanogel induced by ultrasonication as a delivery vehicle for riboflavin, Molecules, 2016, 21(3), 282, DOI:10.3390/molecules21030282.
- E. Altuntaş, B. Özkan, S. Güngör and Y. Özsoy, Biopolymer-based nanogel approach in drug delivery: basic concept and current developments, Pharmaceutics, 2023, 15(6), 1644, DOI:10.3390/pharmaceutics15061644.
- F. S. Buosi, A. Alaimo, M. C. Di Santo, F. Elías, G. García Liñares, S. L. Acebedo, M. A. Castañeda Cataña, C. C. Spagnuolo, L. Lizarraga, K. D. Martínez and O. E. Pérez, Resveratrol encapsulation in high molecular weight chitosan-based nanogels for applications in ocular treatments: impact on human ARPE-19 culture cells, Int. J. Biol. Macromol., 2020, 165, 804–821, DOI:10.1016/j.ijbiomac.2020.09.234.
- H.-C. Lin, H.-P. Chiang, W.-P. Jiang, Y.-H. Lan, G.-J. Huang, M.-T. Hsieh, S.-C. Kuo, C.-L. Lo and Y.-T. Chiang, Exploitation of a rod-shaped, acid-labile curcumin-loaded polymeric nanogel system in the treatment of systemic inflammation, Biomater. Adv., 2022, 133, 112597, DOI:10.1016/j.msec.2021.112597.
- W. Sukhlaaied and S.-A. Riyajan, Green robust pH–temperature-sensitive maleated poly(vinyl alcohol)-G-gelatin for encapsulated capsaicin, Polym. Bull., 2016, 73(8), 2303–2320, DOI:10.1007/s00289-016-1609-3.
- L. Radeva, D. Stefanova, Y. Yordanov, K. Kamenova, P. D. Petrov, M. K. Marinova, S. P. Simeonov, M. Kondeva-Burdina, V. Tzankova and K. Yoncheva, Incorporation of resveratrol in polymeric nanogel for improvement of its protective effects on cellular and microsomal oxidative stress models, Gels, 2023, 9(6), 450, DOI:10.3390/gels9060450.
- D. K. Chellappan, N. J. Yee, B. J. Kaur Ambar Jeet Singh, J. Panneerselvam, T. Madheswaran, J. Chellian, S. Satija, M. Mehta, M. Gulati, G. Gupta and K. Dua, Formulation and characterization of glibenclamide and quercetin-loaded chitosan nanogels targeting skin permeation, Ther. Deliv., 2019, 10(5), 281–293, DOI:10.4155/tde-2019-0019.
- S. Khoee and A. Kavand, Preparation, co-assembling and interfacial crosslinking of photocurable and folate-conjugated amphiphilic block copolymers for controlled and targeted drug delivery: smart armored nanocarriers, Eur. J. Med. Chem., 2014, 73, 18–29, DOI:10.1016/j.ejmech.2013.11.033.
- X. M. Li, X. Li, Z. Wu, Y. Wang, J. S. Cheng, T. Wang and B. Zhang, Chitosan hydrochloride/carboxymethyl starch complex nanogels stabilized pickering emulsions for oral delivery of β-carotene: protection effect and in vitro digestion study, Food Chem., 2020, 315, 126288, DOI:10.1016/j.foodchem.2020.126288.
- K. S. Kumar, A. Nanoemulsions: Techniques for the preparation and the recent advances in their food applications, Innov. Food Sci. Emerg. Technol., 2022, 76, 102914, DOI:10.1016/j.ifset.2021.102914.
- S. Chaudhary, S. Kumar, V. Kumar and R. Sharma, Chitosan nanoemulsions as advanced edible coatings for fruits and vegetables: composition, fabrication and developments in last decade, Int. J. Biol. Macromol., 2020, 152, 154–170, DOI:10.1016/j.ijbiomac.2020.02.276.
- T. Delmas, H. Piraux, A.-C. Couffin, I. Texier, F. Vinet, P. Poulin, M. E. Cates and J. Bibette, How to prepare and stabilize very small nanoemulsions, Langmuir, 2011, 27(5), 1683–1692, DOI:10.1021/la104221q.
- D. J. McClements and J. Rao, Food-grade nanoemulsions: formulation, fabrication, properties, performance, biological fate, and potential toxicity, Crit. Rev. Food Sci. Nutr., 2011, 51(4), 285–330, DOI:10.1080/10408398.2011.559558.
- M. Kumar, R. S. Bishnoi, A. K. Shukla and C. P. Jain, Techniques for formulation of nanoemulsion drug delivery system: a review, Prev. Nutr. Food Sci., 2019, 24(3), 225–234, DOI:10.3746/pnf.2019.24.3.225.
- S. J. Choi and D. J. McClements, Nanoemulsions as delivery systems for lipophilic nutraceuticals: strategies for improving their formulation, stability, functionality and bioavailability, Food Sci. Biotechnol., 2020, 29(2), 149–168, DOI:10.1007/s10068-019-00731-4.
- V. Quagliariello, R. Vecchione, A. De Capua, E. Lagreca, R. V. Iaffaioli, G. Botti, P. A. Netti and N. Maurea, Nano-encapsulation of coenzyme q10 in secondary and tertiary nano-emulsions for enhanced cardioprotection and hepatoprotection in human cardiomyocytes and hepatocytes during exposure to anthracyclines and trastuzumab, Int. J. Nanomedicine, 2020, 15, 4859–4876, DOI:10.2147/IJN.S245170.
- M. Sharma, B. Mann, R. Pothuraju, R. Sharma and R. Kumar, Physico-chemical characterization of ultrasound assisted clove oil-loaded nanoemulsion as enhanced antimicrobial potential, Biotechnol. Reports, 2022, 34, e00720, DOI:10.1016/j.btre.2022.e00720.
- D. Wang, A. Bao, Y. Yuan, Y. Wang, L. Li, G. Song, T. Yuan and J. Gong, Whey protein isolate-stabilized gardenia fruit oil nanoemulsions: ultrasonic preparation, characterization and applications in nutraceuticals delivery, Ind. Crops Prod., 2024, 212, 118345, DOI:10.1016/j.indcrop.2024.118345.
- A. I. Foudah, S. Devi, A. Alam, M. A. Salkini and S. A. Ross, Anticholinergic effect of resveratrol with vitamin e on scopolamine-induced Alzheimer’s disease in rats: mechanistic approach to prevent inflammation, Front. Pharmacol, 2023, 14, 1115721, DOI:10.3389/fphar.2023.1115721.
- F. M. Locatelli, T. Kawano, H. Iwata, B. Aoyama, S. Eguchi, A. Nishigaki, D. Yamanaka, H. Tateiwa, M. Shigematsu-Locatelli and M. Yokoyama, Resveratrol-loaded nanoemulsion prevents cognitive decline after abdominal surgery in aged rats, J. Pharmacol. Sci., 2018, 137(4), 395–402, DOI:10.1016/j.jphs.2018.08.006.
- N. Walia and L. Chen, Pea protein-based vitamin D nanoemulsions: fabrication, stability and in vitro study using Caco-2 cells, Food Chem., 2020, 305, 125475, DOI:10.1016/j.foodchem.2019.125475.
- V. Campani, M. Biondi, L. Mayol, F. Cilurzo, M. Pitaro and G. De Rosa, Development of nanoemulsions for topical delivery of vitamin K1, Int. J. Pharm., 2016, 511(1), 170–177, DOI:10.1016/j.ijpharm.2016.07.004.
- M. N. Saleh, M. A. Salam and E. Capanoglu, Encapsulation of black rice bran extract in a stable nanoemulsion: effects of thermal treatment, storage conditions, and in vitro digestion, ACS Omega, 2024, 9(11), 12585–12595, DOI:10.1021/acsomega.3c07060.
- J. Li, R. Campardelli, G. Firpo, J. Zhang and P. Perego, Oil-in-water nanoemulsions loaded with lycopene extracts encapsulated by spray drying: formulation, characterization and optimization, Chin. J. Chem. Eng., 2024, 70, 73–81, DOI:10.1016/j.cjche.2024.03.002.
- L. Thomas, F. Zakir, M. A. Mirza, Md. K. Anwer, F. J. Ahmad and Z. Iqbal, Development of curcumin loaded chitosan polymer based nanoemulsion gel: in vitro, ex vivo evaluation and in vivo wound healing studies, Int. J. Biol. Macromol., 2017, 101, 569–579, DOI:10.1016/j.ijbiomac.2017.03.066.
- M. S. Algahtani, M. Z. Ahmad and J. Ahmad, Nanoemulsion loaded polymeric hydrogel for topical delivery of curcumin in psoriasis, J. Drug Deliv. Sci. Technol., 2020, 59, 101847, DOI:10.1016/j.jddst.2020.101847.
- C. Has and P. Sunthar, A comprehensive review on recent preparation techniques of liposomes, J. Liposome Res., 2020, 30(4), 336–365, DOI:10.1080/08982104.2019.1668010.
- R. Kashapov, G. Gaynanova, D. Gabdrakhmanov, D. Kuznetsov, R. Pavlov, K. Petrov, L. Zakharova and O. Sinyashin, Self-assembly of amphiphilic compounds as a versatile tool for construction of nanoscale drug carriers, Int. J. Mol. Sci., 2020, 21(18), 6961, DOI:10.3390/ijms21186961.
- M. G. Umbarkar, Niosome as a novel pharmaceutical drug delivery: a brief review highlighting formulation, types, composition and application, Indian J. Pharm. Educ. Res., 2021, 55(1s), 11–28, DOI:10.5530/ijper.55.1s.34.
- R. Joy, J. George and F. John, Brief outlook on polymeric nanoparticles, micelles, niosomes, hydrogels and liposomes: preparative methods and action, ChemistrySelect, 2022, 7(6), e202104045, DOI:10.1002/slct.202104045.
- Ş. Dima, C. Dima and G. Iordăchescu, Encapsulation of functional lipophilic food and drug biocomponents, Food Eng. Rev., 2015, 417–438, DOI:10.1007/s12393-015-9115-1.
- C. laslau, D. gologan, C. ott, B. balanuca and R. stan, Niosomes loaded with ascorbic acid – Influence of surfactants on stability and morphology, UPB Sci. Bull., 2020, 82(3), 101–112 CAS.
- M. V. Giuli, P. N. Hanieh, E. Giuliani, F. Rinaldi, C. Marianecci, I. Screpanti, S. Checquolo and M. Carafa, Current trends in ATRA delivery for cancer therapy, Pharmaceutics, 2020, 12(8), 707, DOI:10.3390/pharmaceutics12080707.
- D. Bouriez, J. Giraud, C. Gronnier and C. Varon, Efficiency of all-trans retinoic acid on gastric cancer: a narrative literature review, Int. J. Mol. Sci., 2018, 19(11), 3388, DOI:10.3390/ijms19113388.
- M. C. Cristiano, D. Cosco, C. Celia, A. Tudose, R. Mare, D. Paolino and M. Fresta, Anticancer activity of all-trans retinoic acid-loaded liposomes on human thyroid carcinoma cells, Colloids Surf., B Biointerfaces, 2017, 150, 408–416, DOI:10.1016/j.colsurfb.2016.10.052.
- L. Basiri, G. Rajabzadeh and A. Bostan, Physicochemical properties and release behavior of span 60/tween 60 niosomes as vehicle for α-tocopherol delivery, LWT, 2017, 84, 471–478, DOI:10.1016/j.lwt.2017.06.009.
- G. K. Pamunuwa, M. Prasadani, T. U. G. Nanayakkara and S. N. Atapattu, Effect of liposomal encapsulation of curcumin and α-tocopherol on sensory and physicochemical properties, and retention of antioxidant capacity of fortified cookies during baking, Food Chem. Adv., 2023, 3, 100504, DOI:10.1016/j.focha.2023.100504.
- L. Tavano, R. Muzzalupo, N. Picci and B. De Cindio, Co-encapsulation of antioxidants into niosomal carriers: gastrointestinal release studies for nutraceutical applications, Colloids Surf., B Biointerfaces, 2014, 114, 82–88, DOI:10.1016/j.colsurfb.2013.09.058.
- S. Oontawee, C. Phonprapai, A. Itharat, R. Asasutjarit and N. M. Davies, Mucus-penetrating nanovesicles encapsulating a novel anti-inflammatory recipe as a transmucosal remedy: herbal extract preparation, synergistic combination, and niosomal encapsulation, J. Herb. Med., 2023, 40, 100670, DOI:10.1016/j.hermed.2023.100670.
- D. Zelikina, S. Chebotarev, A. Antipova, E. Martirosova, M. Anokhina, N. Palmina, N. Bogdanova and M. Semenova, Efficacy of a Maillard-type conjugate of whey protein isolate with chitosan as a carrier for a liposomal form of a combination of curcumin and balanced amounts of n-3 and n-6 PUFAS. Part II. Carrier behaviour under simulated in vitro digestion, Int. Dairy J., 2024, 154, 105924, DOI:10.1016/j.idairyj.2024.105924.
- M. G. Semenova, A. S. Antipova, E. I. Martirosova, N. P. Palmina, D. V. Zelikina, S. A. Chebotarev, N. G. Bogdanova, M. S. Anokhina and V. V. Kasparov, Key structural factors and intermolecular interactions underlying the formation, functional properties and behaviour in the gastrointestinal tract in vitro of the liposomal form of nutraceuticals coated with whey proteins and chitosan, Food Funct., 2024, 15(4), 2008–2021, 10.1039/D3FO04285E.
- A. Jain, S. K. Singh, S. K. Arya, S. C. Kundu and S. Kapoor, Protein nanoparticles: promising platforms for drug delivery applications, ACS Biomater. Sci. Eng., 2018, 4(12), 3939–3961, DOI:10.1021/acsbiomaterials.8b01098.
- C. Desfrançois, R. Auzély and I. Texier, Lipid nanoparticles and their hydrogel composites for drug delivery: a review, Pharmaceuticals, 2018, 11(4), 118, DOI:10.3390/ph11040118.
- F. da S. Feltrin, T. Agner, C. Sayer and L. M. F. Lona, Curcumin encapsulation in functional PLGA nanoparticles: a promising strategy for cancer therapies, Adv. Colloid Interface Sci., 2022, 300, 102582, DOI:10.1016/j.cis.2021.102582.
- E. Niza, M. Božik, I. Bravo, P. Clemente-Casares, A. Lara-Sanchez, A. Juan, P. Klouček and C. Alonso-Moreno, PEI-coated PLA nanoparticles to enhance the antimicrobial activity of carvacrol, Food Chem., 2020, 328, 127131, DOI:10.1016/j.foodchem.2020.127131.
- S. Łukasiewicz, A. Mikołajczyk, E. Błasiak, E. Fic and M. Dziedzicka-Wasylewska, Polycaprolactone nanoparticles as promising candidates for nanocarriers in novel nanomedicines, Pharmaceutics, 2021, 13(2), 191, DOI:10.3390/pharmaceutics13020191.
- M. Yanat and K. Schroën, Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging, React. Funct. Polym., 2021, 161, 104849, DOI:10.1016/j.reactfunctpolym.2021.104849.
- G. Pamunuwa, N. Anjalee, D. Kukulewa, C. Edirisinghe, F. Shakoor and D. N. Karunaratne, Tailoring of release properties of folic acid encapsulated nanoparticles via changing alginate and pectin composition in the matrix, Carbohydr. Polym. Tech. Appl., 2020, 1, 100008, DOI:10.1016/j.carpta.2020.100008.
- J. Mehta, P. Kumar and S. V. Pawar, Exploration of capsaicin-encapsulated lignin nanoparticles for alleviating non-alcoholic fatty liver disease: in-vitro study, Int. J. Biol. Macromol., 2025, 303, 140616, DOI:10.1016/j.ijbiomac.2025.140616.
- Y. Yuan, H. Li, J. Zhu, C. Liu, X. Sun, D. Wang and Y. Xu, Fabrication and characterization of zein nanoparticles by dextran sulfate coating as vehicles for delivery of curcumin, Int. J. Biol. Macromol., 2020, 151, 1074–1083, DOI:10.1016/j.ijbiomac.2019.10.149.
- K. Xiong, L. Zhou, J. Wang, A. Ma, D. Fang, L. Xiong and Q. Sun, Construction of food-grade pH-sensitive nanoparticles for delivering functional food ingredients, Trends Food Sci. Technol., 2020, 96, 102–113, DOI:10.1016/j.tifs.2019.12.019.
- X. Feng, H. Dai, L. Ma, Y. Fu, Y. Yu, H. Zhou, T. Guo, H. Zhu, H. Wang and Y. Zhang, Properties of pickering emulsion stabilized by food-grade gelatin nanoparticles: influence of the nanoparticles concentration, Colloids Surf., B Biointerfaces, 2020, 196, 111294, DOI:10.1016/j.colsurfb.2020.111294.
- M. C. Biswas, D. Banerjee, K. Saha and S. Anjum, Lignin-based nanoparticles, Biopolymeric Nanomaterials: Fundamentals and Applications, 2021, pp. 203–219, DOI:10.1016/B978-0-12-824364-0.00007-1.
- S. Hong, D. W. Choi, H. N. Kim, C. G. Park, W. Lee and H. H. Park, Protein-based nanoparticles as drug delivery systems, Pharmaceutics, 2020, 12(7), 604, DOI:10.3390/pharmaceutics12070604.
- J. Zhong, R. Qiao and W.-E. Yuan, Protein-based nanocarriers for delivery of nutraceutical, diagnostic and therapeutic agents, Front. Pharmacol, 2022, 13, 991474, DOI:10.3389/fphar.2022.991474.
- Q. Liu, J. Cheng, X. Sun and M. Guo, Preparation, characterization, and antioxidant activity of zein nanoparticles stabilized by whey protein nanofibrils, Int. J. Biol. Macromol., 2021, 167, 862–870, DOI:10.1016/j.ijbiomac.2020.11.043.
- E. Kianfar, Protein nanoparticles in drug delivery: animal protein, plant proteins and protein cages, albumin nanoparticles, J. Nanobiotechnol., 2021, 19(1), 159, DOI:10.1186/s12951-021-00896-3.
- A. H. Sneharani, Curcumin–sunflower protein nanoparticles—A potential anti-inflammatory agent, J. Food Biochem., 2019, 43(8), e12909, DOI:10.1111/jfbc.12909.
- L.-L. Liu, P.-Z. Liu, X.-T. Li, N. Zhang and C.-H. Tang, Novel soy β-conglycinin core–shell nanoparticles as outstanding eco-friendly nanocarriers for curcumin, J. Agric. Food Chem., 2019, 67(22), 6292–6301, DOI:10.1021/acs.jafc.8b05822.
- R. Penalva, I. Esparza, M. Agüeros, C. J. Gonzalez-Navarro, C. Gonzalez-Ferrero and J. M. Irache, Casein nanoparticles as carriers for the oral delivery of folic acid, Food Hydrocoll., 2015, 44, 399–406, DOI:10.1016/j.foodhyd.2014.10.004.
- X. Lin, C. Zhu, M. Chen, P. R. Gonzalez, X. Chen, Z. Zhao, D. Danino and H. Corke, Development of gastric digestion-tolerant plant protein-based nanoparticles: fabrication, characterization, antioxidant activity, and stability, Food Hydrocoll., 2024, 151, 109815, DOI:10.1016/j.foodhyd.2024.109815.
- H. Rajput, S. Nangare, Z. Khan, A. Patil, S. Bari and P. Patil, Design of lactoferrin functionalized carboxymethyl dextran coated egg albumin nanoconjugate for targeted delivery of capsaicin: spectroscopic and cytotoxicity studies, Int. J. Biol. Macromol., 2024, 256, 128392, DOI:10.1016/j.ijbiomac.2023.128392.
- R. Sabra, C. J. Roberts and N. Billa, Courier properties of modified citrus pectinate-chitosan nanoparticles in colon delivery of curcumin, Colloids Interface Sci. Commun., 2019, 32, 100192, DOI:10.1016/j.colcom.2019.100192.
- M. Elmowafy, K. Shalaby, M. H. Elkomy, O. A. Alsaidan, H. A. M. Gomaa, M. A. Abdelgawad and E. M. Mostafa, Polymeric nanoparticles for delivery of natural bioactive agents: recent advances and challenges, Polymers, 2023, 15(5), 1123, DOI:10.3390/polym15051123.
- L. Bu, L. C. Gan, X. Q. Guo, F. Z. Chen, Q. Song, Q. Zhao, X. J. Gou, S. X. Hou and Q. Yao, Trans-resveratrol loaded chitosan nanoparticles modified with biotin and avidin to target hepatic carcinoma, Int. J. Pharm., 2013, 452(1–2), 355–362, DOI:10.1016/j.ijpharm.2013.05.007.
- H. Amiri, H. Javid, E. Einafshar, F. Ghavidel, A. Rajabian, S. I. Hashemy and H. Hosseini, Development and evaluation of PLGA nanoparticles surfaced modified with chitosan-folic acid for improved delivery of resveratrol to prostate cancer cells, Bionanoscience, 2024, 14(2), 988–998, DOI:10.1007/s12668-024-01345-9.
- A. Kumari, S. K. Yadav, Y. B. Pakade, B. Singh and S. C. Yadav, Development of biodegradable nanoparticles for delivery of quercetin, Colloids Surf., B Biointerfaces, 2010, 80(2), 184–192, DOI:10.1016/j.colsurfb.2010.06.002.
- T. Ramezanli, B. E. Kilfoyle, Z. Zhang and B. B. Michniak-Kohn, Polymeric nanospheres for topical delivery of vitamin D3, Int. J. Pharm., 2017, 516(1–2), 196–203, DOI:10.1016/j.ijpharm.2016.10.072.
- R. S. Prabhuraj, K. Bomb, R. Srivastava and R. Bandyopadhyaya, Selection of superior targeting ligands using PEGylated PLGA nanoparticles for delivery of curcumin in the treatment of triple-negative breast cancer cells, J. Drug Deliv. Sci. Technol., 2020, 57, 101722, DOI:10.1016/j.jddst.2020.101722.
- Y. Zhu, H. Wang, M. Adu-Frimpong, Z. Zou, Z. Jin, P. Zhang, Y. Xue, S. Li, Y. Xu, J. Yu and X. Xu, HA/PLA composite nanoparticles for enhanced oral bioavailability of capsaicin: fabrication, characterization and in vitro-in vivo evaluation, ChemistrySelect, 2024, 9(4), e202303080, DOI:10.1002/slct.202303080.
- C. Zuo, H. Zhang, S. Liang, W. Teng, C. Bao, D. Li, Y. Hu, Q. Wang, Z. Li and Y. Li, The alleviation of lipid deposition in steatosis hepatocytes by capsaicin-loaded α-lactalbumin nanomicelles via promoted endocytosis and synergetic multiple signaling pathways, J. Funct. Foods, 2021, 79, 104396, DOI:10.1016/j.jff.2021.104396.
- Z. Teng, Y. Li and Q. Wang, Insight into curcumin-loaded β-lactoglobulin nanoparticles: incorporation, particle disintegration, and releasing profiles, J. Agric. Food Chem., 2014, 62(35), 8837–8847, DOI:10.1021/jf503199g.
- F. N. Sorasitthiyanukarn, C. Muangnoi, W. Thaweesest, P. Rojsitthisak and P. Rojsitthisak, Enhanced cytotoxic, antioxidant and anti-inflammatory activities of curcumin diethyl disuccinate using chitosan-tripolyphosphate nanoparticles, J. Drug Deliv. Sci. Technol., 2019, 53, 101118, DOI:10.1016/j.jddst.2019.06.015.
- Y. Wang, Y. Li, L. He, B. Mao, S. Chen, V. Martinez, X. Guo, X. Shen, B. Liu and C. Li, Commensal flora triggered target anti-inflammation of alginate-curcumin micelle for ulcerative colitis treatment, Colloids Surf., B Biointerfaces, 2021, 203, 111756, DOI:10.1016/j.colsurfb.2021.111756.
- A. C. Egil, H. Kesim, B. Ustunkaya, Ö. Kutlu and G. Ozaydin Ince, Self-assembled albumin nanoparticles for redox responsive release of curcumin, J. Drug Deliv. Sci. Technol., 2022, 76, 103831, DOI:10.1016/j.jddst.2022.103831.
- J. H. Chung, J.-S. Lee and H. G. Lee, Resveratrol-loaded chitosan–γ-poly(glutamic acid) nanoparticles: optimization, solubility, UV stability, and cellular antioxidant activity, Colloids Surf., B Biointerfaces, 2020, 186, 110702, DOI:10.1016/j.colsurfb.2019.110702.
- L. Dai, R. Liu, L.-Q. Hu, Z.-F. Zou and C.-L. Si, Lignin nanoparticle as a novel green carrier for the efficient delivery of resveratrol, ACS Sustain. Chem. Eng., 2017, 5(9), 8241–8249, DOI:10.1021/acssuschemeng.7b01903.
- Y. Ding, S. Zhang, Z. Sun, Z. Tong, Y. Ge, L. Zhou, Q. Xu, H. Zhou and W. Wang, Preclinical validation of silibinin/albumin nanoparticles as an applicable system against acute liver injury, Acta Biomater., 2022, 146, 385–395, DOI:10.1016/j.actbio.2022.04.021.
- M. Raval, P. Patel, V. Airao, V. Bhatt and N. Sheth, Novel silibinin loaded chitosan-coated PLGA/PCL nanoparticles based inhalation formulations with improved cytotoxicity and bioavailability for lung cancer, Bionanoscience, 2021, 11(1), 67–83, DOI:10.1007/s12668-020-00797-z.
- R. Baksi, D. P. Singh, S. P. Borse, R. Rana, V. Sharma and M. Nivsarkar, In vitro and in vivo anticancer efficacy potential of quercetin loaded polymeric nanoparticles, Biomed. Pharmacother., 2018, 106, 1513–1526, DOI:10.1016/j.biopha.2018.07.106.
- K. Y. Mahmoud, N. A. Elhesaisy, A. R. Rashed, E. S. Mikhael, M. I. Fadl, M. S. Elsadek, M. A. Mohamed, M. A. Mostafa, M. A. Hassan, O. M. Halema, Y. H. Elnemer and S. A. Swidan, Exploring the potential of intranasally administered naturally occurring quercetin loaded into polymeric nanocapsules as a novel platform for the treatment of anxiety, Sci. Rep., 2023, 13(1), 510, DOI:10.1038/s41598-023-27665-6.
- E. A. Mazyed, G. Magdy, E. Elekhnawy, M. Yammine, C. Rolando and M. H. ElNaggar, Formulation and characterization of quercetin-loaded prunus armeniaca gum nanoparticles with enhanced anti-bacterial effect, J. Drug Deliv. Sci. Technol., 2024, 105485, DOI:10.1016/j.jddst.2024.105485.
- M. Z. Ahmad, K. Pathak, R. J. Das, R. Saikia, H. Sarma, N. Gogoi, U. Gogoi, A. Das, A. S. Alasiri, B. A. Abdel-Wahab and M. M. Abdullah, Design and optimization of quercetin-loaded polymeric eudragit l-100 nanoparticles for anti-diabetic activity with improved oral delivery: in-vitro and in-vivo evaluation, J. Inorg. Organomet. Polym. Mater., 2023, 33(8), 2411–2428, DOI:10.1007/s10904-023-02694-w.
- F. N. Sorasitthiyanukarn, C. Muangnoi, P. Rojsitthisak and P. Rojsitthisak, Stability and biological activity enhancement of fucoxanthin through encapsulation in alginate/chitosan nanoparticles, Int. J. Biol. Macromol., 2024, 130264, DOI:10.1016/j.ijbiomac.2024.130264.
- M. A. Miranda, L. B. Silva, I. P. S. Carvalho, R. Amaral, M. H. de Paula, K. Swiech, J. K. Bastos, J. A. R. Paschoal, F. S. Emery, R. B. dos Reis, M. V. L. B. Bentley and P. D. Marcato, Targeted uptake of folic acid-functionalized polymeric nanoparticles loading glycoalkaloidic extract: in vitro and in vivo assays, Colloids Surf., B Biointerfaces, 2020, 192, 111106, DOI:10.1016/j.colsurfb.2020.111106.
- M. M. El-Hammadi, Á. V. Delgado, C. Melguizo, J. C. Prados and J. L. Arias, Folic acid-decorated and PEGylated PLGA nanoparticles for improving the antitumour activity of 5-fluorouracil, Int. J. Pharm., 2017, 516(1–2), 61–70, DOI:10.1016/j.ijpharm.2016.11.012.
- H.-H. Jung, S.-H. Kim, J.-H. Moon, S.-U. Jeong, S. Jang, C.-S. Park and C.-K. Lee, Polymeric nanoparticles containing both antigen and vitamin D3 induce antigen-specific immune suppression, Immune Netw., 2019, 19(3), e19, DOI:10.4110/in.2019.19.e19.
- A. L. M. da Silva, R. V. Contri, D. S. Jornada, A. R. Pohlmann and S. S. Guterres, Vitamin K1-loaded lipid-core nanocapsules: physicochemical characterization and in vitro skin permeation, Skin Res. Technol., 2013, 19(1), e223–e230, DOI:10.1111/j.1600-0846.2012.00631.x.
- A. Laliwala, A. Daverey, S. K. Agrawal and A. K. Dash, Alpha tocopherol loaded polymeric nanoparticles: preparation, characterizations, and in vitro assessments against oxidative stress in spinal cord injury treatment, AAPS PharmSciTech, 2022, 23(6), 195, DOI:10.1208/s12249-022-02345-2.
- N. Khayata, W. Abdelwahed, M. F. Chehna, C. Charcosset and H. Fessi, Preparation of vitamin E-loaded nanocapsules by the nanoprecipitation method: from laboratory scale to large scale using a membrane contactor, Int. J. Pharm., 2012, 423(2), 419–427, DOI:10.1016/j.ijpharm.2011.12.016.
- C. Anandharamakrishnan, P. I. S. Techniques for nanoencapsulation of food ingredients, SpringerBriefs in Food, Health, and Nutrition, University of Wisconsin, USA, 2014, pp. 2197–5728, DOI:10.1007/978-1-4614-9387-7.
- S. A. Mahdavi, S. M. Jafari, M. Ghorbani and E. Assadpoor, Spray-drying microencapsulation of anthocyanins by natural biopolymers: a review, Dry. Technol., 2014, 32(5), 509–518, DOI:10.1080/07373937.2013.839562.
- N. Hussein, H. Omer, A. Ismael, M. Albed Alhnan, A. Elhissi and W. Ahmed, Spray-dried alginate microparticles for potential intranasal delivery of ropinirole hydrochloride: development, characterization and histopathological evaluation, Pharm. Dev. Technol., 2020, 25(3), 290–299, DOI:10.1080/10837450.2019.1567762.
- S. Abbas, E. Karangwa, M. Bashari, K. Hayat, X. Hong, H. R. Sharif and X. Zhang, Fabrication of polymeric nanocapsules from curcumin-loaded nanoemulsion templates by self-assembly, Ultrason. Sonochem., 2015, 23, 81–92, DOI:10.1016/j.ultsonch.2014.10.006.
- C. Moreno, J. Forero, Y. Baena and L. Perez, Co-encapsulation of curcumin and salicylic acid in mucoadhesive polymer nanocapsules, J. Appl. Pharm. Sci., 2024, 14(1), 189–196, DOI:10.7324/JAPS.2024.149645.
- M. F. Kabil, S. A. A. Gaber, M. A. Hamzawy, I. M. El-Sherbiny and M. Nasr, Folic/lactobionic acid dual-targeted polymeric nanocapsules for potential treatment of hepatocellular carcinoma, Drug Deliv. Transl. Res., 2023, 14(5), 1338–1351, DOI:10.1007/s13346-023-01467-9.
- U. Priyadharshini, N. Rebeca, S. C. Regi, S. Narayan, S. Priyadharshini and S. Manivannan, Preparation and characterization of anthocyanin-loaded lotus pollen exine nanocapsules for its use in oral drug delivery applications, Proc. Natl. Acad. Sci. India B Biol. Sci., 2024, 94(2), 341–350, DOI:10.1007/s40011-023-01538-4.
- S. Nunes, R. Madureira, D. Campos, B. Sarmento, A. M. Gomes, M. Pintado and F. Reis, Solid lipid nanoparticles as oral delivery systems of phenolic compounds: overcoming pharmacokinetic limitations for nutraceutical applications, Crit. Rev. Food Sci. Nutr., 2017, 57(9), 1863–1873, DOI:10.1080/10408398.2015.1031337.
- F. Tamjidi, M. Shahedi, J. Varshosaz and A. Nasirpour, Nanostructured lipid carriers (NLC): a potential delivery system for bioactive food molecules, Innov. Food Sci. Emerg. Technol., 2013, 19, 29–43, DOI:10.1016/j.ifset.2013.03.002.
- J. Vishal, N. S. Z. Lingayat and S. S. R, Solid lipid nanoparticles: a review, Nanosci. Nanotechnol. Res., 2017, 4(2), 67–72, DOI:10.12691/nnr-4-2-5.
- A. Beloqui, M. Á. Solinís, A. Rodríguez-Gascón, A. J. Almeida and V. Préat, Nanostructured lipid carriers: promising drug delivery systems for future clinics, Nanomedicine, 2016, 12(1), 143–161, DOI:10.1016/j.nano.2015.09.004.
- A. Rehman, Q. Tong, S. M. Jafari, E. Assadpour, Q. Shehzad, R. M. Aadil, M. W. Iqbal, M. M. A. Rashed, B. S. Mushtaq and W. Ashraf, Carotenoid-loaded nanocarriers: a comprehensive review, Adv. Colloid Interface Sci., 2020, 275, 102048, DOI:10.1016/j.cis.2019.102048.
- S. Al Zahabi, O. S. Sakr and A. A. Ramadan, Nanostructured lipid carriers incorporating prickly pear seed oil for the encapsulation of vitamin A, J. Cosmet. Dermatol., 2019, 18(6), 1875–1884, DOI:10.1111/jocd.12891.
- M. M. J. O. Wijekoon, K. Mahmood, F. Ariffin, A. Mohammadi Nafchi and M. Zulkurnain, Recent advances in encapsulation of fat-soluble vitamins using polysaccharides, proteins, and lipids: a review on delivery systems, formulation, and industrial applications, Int. J. Biol. Macromol., 2023, 241, 124539, DOI:10.1016/j.ijbiomac.2023.124539.
- B. Mehrad, R. Ravanfar, J. Licker, J. M. Regenstein and A. Abbaspourrad, Enhancing the physicochemical stability of β-carotene solid lipid nanoparticle (SLNP) using whey protein isolate, Food Res. Int., 2018, 105, 962–969, DOI:10.1016/j.foodres.2017.12.036.
- M. Liu, F. Wang, C. Pu, W. Tang and Q. Sun, Nanoencapsulation of lutein within lipid-based delivery systems: characterization and comparison of zein peptide stabilized nano-emulsion, solid lipid nanoparticle, and nano-structured lipid carrier, Food Chem., 2021, 358, 129840, DOI:10.1016/j.foodchem.2021.129840.
- F. Tan, H. Cui, C. Bai, C. Qin, L. Xu and J. Han, Preparation, optimization, and transcorneal permeability study of lutein-loaded solid lipid nanoparticles, J. Drug Deliv. Sci. Technol., 2021, 62, 102362, DOI:10.1016/j.jddst.2021.102362.
- K. Mitri, R. Shegokar, S. Gohla, C. Anselmi and R. H. Müller, Lipid nanocarriers for dermal delivery of lutein: preparation, characterization, stability and performance, Int. J. Pharm., 2011, 414(1–2), 267–275, DOI:10.1016/j.ijpharm.2011.05.008.
- M. R. Patel and M. F. San Martin-Gonzalez, Characterization of ergocalciferol loaded solid lipid nanoparticles, J. Food Sci., 2012, 77(1), N8–N13, DOI:10.1111/j.1750-3841.2011.02517.x.
- J.-Y. Fu, P. Meganathan, N. Gunasegaran and D. M. Y. Tan, Effect of nano-delivery systems on the bioavailability and tissue biodistribution of vitamin E tocotrienols, Food Res. Int., 2023, 171, 113048, DOI:10.1016/j.foodres.2023.113048.
- B. S. Abuasal, C. Lucas, B. Peyton, A. Alayoubi, S. Nazzal, P. W. Sylvester and A. Kaddoumi, Enhancement of intestinal permeability utilizing solid lipid nanoparticles increases γ-tocotrienol oral bioavailability, Lipids, 2012, 47(5), 461–469, DOI:10.1007/s11745-012-3655-4.
- J. M. M. Mohamed, F. Ahmad, M. El-Sherbiny, M. A. Al Mohaini, K. Venkatesan, Y. B. A. Alrashdi, M. B. Eldesoqui, A. E. Ibrahim, A. F. Dawood, A. M. Ibrahim and S. El Deeb, Optimization and characterization of quercetin-loaded solid lipid nanoparticles for biomedical application in colorectal cancer, Cancer Nanotechnol., 2024, 15, 16, DOI:10.1186/s12645-024-00249-3.
- K. Zhou and Y. Li, Catalysis based on nanocrystals with well-defined facets, Angew. Chem., Int. Ed., 2012, 51(3), 602–613, DOI:10.1002/anie.201102619.
- J. Wang, X. Xue and X. Miao, Antioxidant effects of quercetin nanocrystals in nanosuspension against hydrogen peroxide-induced oxidative stress in a zebrafish model, Pharmaceuticals, 2023, 16(9), 1209, DOI:10.3390/ph16091209.
- M. Mariano, N. El Kissi and A. Dufresne, Cellulose nanocrystals and related nanocomposites: review of some properties and challenges, J. Polym. Sci. B Polym. Phys., 2014, 52(12), 791–806, DOI:10.1002/polb.23490.
- S. P. Akhlaghi, R. M. Berry and K. C. Tam, Modified cellulose nanocrystal for vitamin C delivery, AAPS PharmSciTech, 2015, 16(2), 306–314, DOI:10.1208/s12249-014-0218-4.
- S. Arman, M. Hadavi, A. Rezvani-Noghani, A. Bakhtparvar, M. Fotouhi, A. Farhang, P. Mokaberi, R. Taheri and J. Chamani, Cellulose nanocrystals from celery stalk as quercetin scaffolds: a novel perspective of human holo-transferrin adsorption and digestion behaviours, Luminescence, 2024, 39(1), e4634, DOI:10.1002/bio.4634.
- G. M. A. Ndong Ntoutoume, R. Granet, J. P. Mbakidi, F. Brégier, D. Y. Léger, C. Fidanzi-Dugas, V. Lequart, N. Joly, B. Liagre, V. Chaleix and V. Sol, Development of curcumin–cyclodextrin/cellulose nanocrystals complexes: new anticancer drug delivery systems, Bioorg. Med. Chem. Lett., 2016, 26(3), 941–945, DOI:10.1016/j.bmcl.2015.12.060.
- M. L. Manca, F. Lai, R. Pireddu, D. Valenti, M. Schlich, E. Pini, G. Ailuno, A. M. Fadda and C. Sinico, Impact of nanosizing on dermal delivery and antioxidant activity of quercetin nanocrystals, J. Drug Deliv. Sci. Technol., 2020, 55, 101482, DOI:10.1016/j.jddst.2019.101482.
- M. Bernela, P. Kaur, M. Ahuja and R. Thakur, Nano-based delivery system for nutraceuticals: the potential future, Advances in Animal Biotechnology and its Applications, Springer Singapore, Singapore, 2018, pp. 103–117, DOI:10.1007/978-981-10-4702-2_7.
- A. Kyriakoudi, E. Spanidi, I. Mourtzinos and K. Gardikis, Innovative delivery systems loaded with plant bioactive ingredients: formulation approaches and applications, Plants, 2021, 10(6), 1238, DOI:10.3390/plants10061238.
- F. Din, W. Aman, I. Ullah, O. S. Qureshi, O. Mustapha, S. Shafique and A. Zeb, Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors, Int. J. Nanomed., 2017, 12, 7291–7309, DOI:10.2147/IJN.S146315.
- S. Z. Moradi, S. Momtaz, Z. Bayrami, M. H. Farzaei and M. Abdollahi, Nanoformulations of herbal extracts in treatment of neurodegenerative disorders, Front. Bioeng. Biotechnol., 2020, 8, 238, DOI:10.3389/fbioe.2020.00238.
- S. A. Moosavian, T. Sathyapalan, T. Jamialahmadi and A. Sahebkar, The emerging role of nanomedicine in the management of nonalcoholic fatty liver disease: a state-of-the-art review, Bioinorg. Chem. Appl., 2021, 2021, 1–13, DOI:10.1155/2021/4041415.
- A. F. Illahi, F. Muhammad and B. Akhtar, Nanoformulations of nutraceuticals for cancer treatment, Crit. Rev. Eukaryot. Gene Expr., 2019, 29(5), 449–460, DOI:10.1615/CritRevEukaryotGeneExpr.2019025957.
- S. Fatima and N. Yadav, Nutraceutical and nanonutraceutical in the management of CVD and hypertension, Handbook of Nutraceuticals, Springer International Publishing, Cham, 2024, pp. 1–38, DOI:10.1007/978-3-030-69677-1_5-1.
- D. Khiev, Z. A. Mohamed, R. Vichare, R. Paulson, S. Bhatia, S. Mohapatra, G. P. Lobo, M. Valapala, N. Kerur, C. L. Passaglia, S. S. Mohapatra and M. R. Biswal, Emerging nano-formulations and nanomedicines applications for ocular drug delivery, Nanomaterials, 2021, 11(1), 173, DOI:10.3390/nano11010173.
- Y. Davatgaran Taghipour, M. Hajialyani, R. Naseri, M. Hesari, P. Mohammadi, A. Stefanucci, A. Mollica, M. H. Farzaei and M. Abdollahi, Nanoformulations of natural products for management of metabolic syndrome, Int. J. Nanomed., 2019, 14, 5303–5321, DOI:10.2147/IJN.S213831.
- G. I. Ash, D. Kim and M. Choudhury, Promises of nanotherapeutics in obesity, Trends Endocrinol. Metabol., 2019, 30(6), 369–383, DOI:10.1016/j.tem.2019.04.004.
- C. M. Pinto, L. S. Horta, A. P. Soares, B. A. Carvalho, E. Ferreira, E. B. Lages, L. A. M. Ferreira, A. A. G. Faraco, H. C. Santiago and G. A. C. Goulart, Nanoencapsulated doxorubicin prevents mucositis development in mice, Pharmaceutics, 2021, 13(7), 1021, DOI:10.3390/pharmaceutics13071021.
- S. Z. Alshawwa, A. A. Kassem, R. M. Farid, S. K. Mostafa and G. S. Labib, Nanocarrier drug delivery systems: characterization, limitations, future perspectives and implementation of artificial intelligence, Pharmaceutics, 2022, 14(4), 883, DOI:10.3390/pharmaceutics14040883.
- S. Sultana, N. Alzahrani, R. Alzahrani, W. Alshamrani, W. Aloufi, A. Ali, S. Najib and N. A. Siddiqui, Stability issues and approaches to stabilised nanoparticles based drug delivery system, J. Drug Target., 2020, 28(5), 468–486, DOI:10.1080/1061186X.2020.1722137.
- H. Ahmed, Challenges behind scaling up nanomaterials, AZoNano, 2022, https://www.azonano.com/article.aspx?ArticleID=6126, accessed on 2025-03-04 Search PubMed.
- R. Bawa, Y. Barenholz and A. Owen, The challenge of regulating nanomedicine: Key issues, 2016, ch. 12, pp. 290–314, 10.1039/9781782622536-00290.
- N. Nassiri Koopaei and M. Abdollahi, Opportunities and obstacles to the development of nanopharmaceuticals for human use, Daru, J. Pharm. Sci., 2016, 24(1), 23, DOI:10.1186/s40199-016-0163-8.
- R. Foulkes, E. Man, J. Thind, S. Yeung, A. Joy and C. Hoskins, The regulation of nanomaterials and nanomedicines for clinical application: current and future perspectives, Biomater. Sci., 2020, 8(17), 4653–4664, 10.1039/D0BM00558D.
- C. O. Egwu, C. Aloke, K. T. Onwe, C. I. Umoke, J. Nwafor, R. A. Eyo, J. A. Chukwu, G. O. Ufebe, J. Ladokun, D. T. Audu, A. O. Agwu, D. C. Obasi and C. O. Okoro, Nanomaterials in drug delivery: strengths and opportunities in medicine, Molecules, 2024, 29(11), 2584, DOI:10.3390/molecules29112584.
- R. Joy, J. George and F. John, Brief outlook on polymeric nanoparticles, micelles, niosomes, hydrogels and liposomes: preparative methods and action, ChemistrySelect, 2022, 7(6), e202104045, DOI:10.1002/slct.202104045.
- D. Nagarjuna Reddy, M. Singh, S. Birendra and R. K. Konda, Regulatory compliances in development of commercial-scale process of nanoparticles (liposomes and niosomes) in pharmaceutical industry: a review, Int. J. Res. Pharm. Sci., 2020, 11(4), 7094–7101, DOI:10.26452/ijrps.v11i4.3830.
- F. Farjadian, A. Ghasemi, O. Gohari, A. Roointan, M. Karimi and M. R. Hamblin, Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities, Nanomedicine, 2019, 14(1), 93–126, DOI:10.2217/nnm-2018-0120.
- R. T. Stiepel, E. Duggan, C. J. Batty and K. M. Ainslie, Micro and nanotechnologies: the little formulations that could, Bioeng. Transl. Med., 2023, 8(2), e10421, DOI:10.1002/btm2.10421.
- C. Ferraris, C. Rimicci, S. Garelli, E. Ugazio and L. Battaglia, Nanosystems in cosmetic products: a brief overview of functional, market, regulatory and safety concerns, Pharmaceutics, 2021, 13(9), 1408, DOI:10.3390/pharmaceutics13091408.
- A. Kad, A. Pundir, S. K. Arya, N. Bhardwaj and M. Khatri, An elucidative review to analytically sieve the viability of nanomedicine market, J. Pharm. Innov., 2022, 17(1), 249–265, DOI:10.1007/s12247-020-09495-5.
- M. E. Karim, Nanoproducts and legal aspects of consumer protections: an evaluation, in Handbook of Consumer Nanoproducts, Springer Nature Singapore, Singapore, 2022, pp. 1381–1406, DOI:10.1007/978-981-16-8698-6_79.
- S. A. O. Adeyeye, Food packaging and nanotechnology: safeguarding consumer health and safety, Nutr. Food Sci., 2019, 49(6), 1164–1179, DOI:10.1108/NFS-01-2019-0020.
- N. A. Helal, H. A. Eassa, A. M. Amer, M. A. Eltokhy, I. Edafiogho and M. I. Nounou, Nutraceuticals' novel formulations: the good, the bad, the unknown and patents involved, Recent Pat. Drug Deliv. Formul., 2019, 13(2), 105–156, DOI:10.2174/1872211313666190503112040.
- J. Allan, S. Belz, A. Hoeveler, M. Hugas, H. Okuda, A. Patri, H. Rauscher, P. Silva, W. Slikker, B. Sokull-Kluettgen, W. Tong and E. Anklam, Regulatory landscape of nanotechnology and nanoplastics from a global perspective, Regul. Toxicol. Pharmacol., 2021, 122, 104885, DOI:10.1016/j.yrtph.2021.104885.
- S. More, V. Bampidis, D. Benford, C. Bragard, T. Halldorsson, A. Hernández-Jerez, S. Hougaard Bennekou, K. Koutsoumanis, C. Lambré, K. Machera, H. Naegeli, S. Nielsen, J. Schlatter, D. Schrenk, V. Silano (deceased), D. Turck, M. Younes, J. Castenmiller, Q. Chaudhry, F. Cubadda, R. Franz, D. Gott, J. Mast, A. Mortensen, A. G. Oomen, S. Weigel, E. Barthelemy, A. Rincon, J. Tarazona and R. Schoonjans, Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: human and animal health, EFSA J., 2021, 19(8), e06768, DOI:10.2903/j.efsa.2021.6768.
- Drug Products, Including Biological Products, That Contain Nanomaterials Guidance for Industry Contains Non-binding Recommendations, 2022, https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htmand/or, accessed on 2025-03-04 Search PubMed.
- J. Allan, S. Belz, A. Hoeveler, M. Hugas, H. Okuda, A. Patri, H. Rauscher, P. Silva, W. Slikker, B. Sokull-Kluettgen, W. Tong and E. Anklam, Regulatory landscape of nanotechnology and nanoplastics from a global perspective, Regul. Toxicol. Pharmacol., 2021, 122, 104885, DOI:10.1016/j.yrtph.2021.104885.
- I. C. Festus-Ikhuoria, N. C. Obiuto, R. Adekola Adebayo and O. K. Olajiga, Nanotechnology in consumer products: a review of applications and safety considerations, World J. Adv. Res. Rev., 2023, 21(3), 2050–2059, DOI:10.30574/wjarr.2024.21.3.0923.
- Health Organization W., Office for Europe, R. Nanotechnology and human health: Scientific evidence and risk governance, 2013, http://www.euro.who.int/pubrequest, accessed on 2025-03-04 Search PubMed.
- S. Malik, K. Muhammad and Y. Waheed, Emerging applications of nanotechnology in healthcare and medicine, Molecules, 2023, 28(18), 6624, DOI:10.3390/molecules28186624.
- E. Heunisch, E. Bleeker, S. Kelly, M. Matzke, A. Pohl, T. Serchi, B. Suarez-Merino, T. Buerkli, F. Cassee, R. Cross, M. Dusinksa, E. Fritsche, A. Haase, M. Pink, U. Vogel, N. Latkovic and S. Caglar, Malta Initiative: Priority List for Making OECD Test Guidelines and Guidance Documents Applicable for Nanomaterials and Advanced Materials, 2024, pp. 1–29 Search PubMed.
- Malta Initiative: Support Safe and Sustainable Innovation - Help Overcome Trade - Make Legislation Enforceable, 2024, https://malta-initiative.org/, accessed on 2025-03-04 Search PubMed.
- E. Heunisch, F. Cassee, E. Bleeker, T. Kuhlbusch and M. Gonzalez, Development or revisions of OECD test guideline (TG) and guidance documents (GD) applicable for nanomaterials, 2022, https://testguideline-development.org/revision-tggd, accessed on 2025-03-04 Search PubMed.
- Environment Directorate of Chemicals and Biotechnology Committee, Developments in delegations on the safety of manufactured nanomaterials and advanced materials-tour de table, 2024, http://www.oecd.org/chemicalsafety/, accessed on 2025-03-04 Search PubMed.
- S. Friedrichs, C. Seitz, E. Bleeker, R. Vandebriel, S. Ouhajji, E. Duistermaat, M. Wiemann, A. Vennemann, A. Katsumiti, T. Buerki-Thurnherr, G. Gupta, P. Wick, L. M. Ortiz-Galvez, J. Wu, K.-M. Weltring, D. Haase, E. Heunisch, A. Pohl, K. Reilly, Z. Guo, I. Lynch, T. Exner, B. Hagenhoff and D. Breitenstein, MACRAMÉ – Advanced characterisation methodologies to assess and predict the health and environmental risks of advanced materials, Comput. Struct. Biotechnol. J., 2025, 29, 95–109, DOI:10.1016/j.csbj.2025.03.032.
- J. I. Bueso-Bordils, G. M. Antón-Fos, R. Martín-Algarra and P. A. Alemán-López, Overview of computational toxicology methods applied in drug and green chemical discovery, J. Xenobiot, 2024, 14(4), 1901–1918, DOI:10.3390/jox14040101.
- S. Schmeisser, A. Miccoli, M. von Bergen, E. Berggren, A. Braeuning, W. Busch, C. Desaintes, A. Gourmelon, R. Grafström, J. Harrill, T. Hartung, M. Herzler, G. E. N. Kass, N. Kleinstreuer, M. Leist, M. Luijten, P. Marx-Stoelting, O. Poetz, B. van Ravenzwaay, R. Roggeband, V. Rogiers, A. Roth, P. Sanders, R. S. Thomas, A. Marie Vinggaard, M. Vinken, B. van de Water, A. Luch and T. Tralau, New approach methodologies in human regulatory toxicology – not if, but how and when, Environ. Int., 2023, 178, 108082, DOI:10.1016/j.envint.2023.108082.
- R. Justo-Hanani, Nanosafety regulatory policies: comprehensive and limited approaches, Governing Nanotechnology Safety, Edward Elgar Publishing, 2024, pp. 11–18, DOI:10.4337/9781800372870.00006.
- J. A. Chávez-Hernández, A. J. Velarde-Salcedo, G. Navarro-Tovar and C. Gonzalez, Safe nanomaterials: from their use, application, and disposal to regulations, Nanoscale Adv., 2024, 6(6), 1583–1610, 10.1039/D3NA01097J.
- M. A. Subhan, T. Subhan, K. P. Choudhury and N. Neogi, Safety measures, regulations, ethical, and legal issues for nanomaterials, Handbook of Nanomaterials, Elsevier, 2024, vol. 2, pp. 791–828, DOI:10.1016/B978-0-323-95513-3.00006-X.
| This journal is © The Royal Society of Chemistry 2025 |



