Open Access Article
Open Access ArticleExtraction, structural characterization and functional properties of protein fractions from millet bran†
Wenjie
Zhao
,
Xia
Fan
,
Juan
Shen
,
Fanqiang
Meng
,
Fengxia
Lv
,
Zhaoxin
Lu
 and
Haizhen
Zhao
and
Haizhen
Zhao
 *
*
College of Food Science and Technology, Nanjing Agricultural University, Nanjing 210095, Jiangsu Province, China. E-mail: zhaohz@njau.edu.cn; Tel: +86 13451879518
First published on 23rd May 2025
Abstract
To promote the development and high-value application of the millet bran protein from Huangjingu, the Osborne sequential extraction procedure was used to extract protein fractions (albumin, globulin, prolamin and glutelin) from defatted millet bran, and the physicochemical and functional properties of the extracted protein fractions were investigated. The results showed that the yield of albumin, globulin, prolamin and glutelin was 1.22%, 0.98%, 3.25%, and 0.49%, respectively, and their purity was 84.87%, 76.02%, 80.80%, and 56.76%, respectively. Albumin and globulin had lower molecular weights than prolamin and glutelin. The amino acid compositions of the four protein fractions were different. FTIR spectra displayed that β-turns and β-sheets were the principal structures in the four protein fractions; ultraviolet absorption spectra showed that the tertiary structures of the four protein fractions were different, with prolamin having the highest absorbance. The denaturation temperature of the four protein fractions was within the range of 85–95 °C. Prolamin and glutelin showed smaller particle sizes than globulin and albumin. Prolamin exhibited the strongest surface hydrophobicity. pH and temperature could affect the functional characteristics of protein fractions. Albumin exhibited the highest water holding capacity and solubility, while prolamin had the highest oil holding capacity and emulsifying capacity, and globulin displayed the best foaming capacity. These results showed that millet bran protein fractions from Huangjingu have the potential to be applied in the food industry as a functional additive.
Sustainability spotlightMillet has extremely high nutritional value, it can grow in arid land and resist climate change. Therefore, millet is an excellent crop for countries to enhance their self-sufficiency and decrease reliance on imported grains. Their ability to adapt and resist severe climate change offers opportunities to enhance food security and stimulate economic growth, potentially creating sustainable market prospects for both producers and consumers. Millet bran is composed of a millet seed layer, an aleurone layer and the germ. By exploring the functional properties of millet bran protein, we can establish a new approach to developing protein-rich foods or meat substitutes utilizing millet bran. This will promote the broader development and utilization of millet, contributing to sustainable food production. |
1. Introduction
Nowadays, many factors such as climate change, increasing inflation, water shortage, ecological pollution and socio-economic impact lead to the decline of agricultural productivity. As grain supply decreases, while their demand remains constant or increases, food prices will rise. Food insecurity manifests as hunger, malnutrition, and unstable access to food. In extreme cases, food shortages and price surges may trigger social unrest, migration waves, or even conflicts, further threatening global stability.1 The high yield and biological and nutritional values of cereal proteins can ensure global food security. Compared with animal foods containing protein, cereal crops containing protein are easy to grow, renewable and of low cost, thus becoming a regular source of protein for consumption worldwide.2 From the viewpoint of nutrition, cereal crops with enough protein content are very desirable for the feeding of animals and people in developing countries;3,4 cereal proteins also have great biological potential for treating chronic diseases such as obesity, diabetes, heart diseases, inflammatory diseases and cancer.3 In addition, cereal proteins contribute to the sensory attributes of food such as texture, aroma, flavor and safety. For example, water absorption of rice protein will affect the texture of rice; formation of a heat-induced gluten network in wheat flour allows the production and quality of various foods.4 These proteins can also be used as building blocks and as a repository of amino acids for the synthesis of bulk compounds and biopolymers.5 Therefore, the existence of cereal proteins is essential for nutrition, food and pharmaceutical applications. However, the millet bran protein is a new research field, which may be explored as a promising source of high-quality protein.Millet is a diverse group of nutritionally dense cereal crops classified under various subfamilies of the Poaceae family. As a climate-resilient crop, it adapts well to temperate, subtropical, and tropical agroecological zones, including marginal lands.4 Globally, farmers cultivate major millets—such as sorghum (Sorghum bicolor (L.) Moench), pearl millet (Cenchrus americanus (L.) Morrone), finger millet (Eleusine coracana Gaertn.), foxtail millet (Setaria italica (L.) P. Beauv.), kodo millet (Paspalum scrobiculatum L.), barnyard millet (Echinochloa esculenta (A. Braun) H. Scholz), proso millet (Panicum miliaceum L.) and little millet (Panicum sumatrense Roth ex Roem. & Schult.).4 Foxtail millet (Setaria italica (L.) P. Beauv.) is also known as Italian millet and Setaria, which is known worldwide as a relatively stress-tolerant C4 crop species.5 As a dehulled grain product, foxtail millet is primarily cultivated in Asian countries such as China and India, certain regions of Africa, and Southeastern Europe, with classification criteria varying significantly across nations.4 In China, most foxtail millet varieties belong to the Setaria italica species and are predominantly distributed across Shanxi, Shaanxi, Henan, Hebei, Heilongjiang, and Inner Mongolia. These varieties are further classified into distinct regional types, including Jingu (Shanxi Province), Yangu (Shaanxi Province), Jigu (Hebei Province), Yugu (Henan Province), Zhangzagu (hybrid variety), Longgu (Heilongjiang Province), Huangjingu (Inner Mongolia), and Chigu (regional variety).6 Notably, due to variations in cultivation regions, climatic conditions, and topographical features, these millet varieties exhibit marked differences in nutritional composition, particularly in protein content and profile.6 Millet is rich in protein, fatty acids and dietary fiber, and provides key vitamins, minerals and polyphenols. The protein in millet is usually more than that in rice or wheat, and it contains many important amino acids, including sulfur-containing cysteine, methionine, and most other essential amino acids.7 Millet has strong antioxidant and antibacterial abilities and has many physiological functions, such as anti-diabetic, promoting intestinal health, relieving heart attack, preventing coronary artery disease and so on.8 This shows that millet is a stable and healthy crop substitute for traditional grains and helps to meet the growing global demand for protein. Although millet has broad potential in improving food security and dietary diversification, its economic viability is often limited by the reduction of total output caused by current production methods. Therefore, we are committed to expanding its production and processing value and achieving its goal of helping global food achieve sustainable development.
Millet bran consists of a millet seed layer, an aleurone layer and the germ, accounting for about 10% of the total mass of millet bran, and is rich in nutrients. Studies have shown that the protein content of millet bran is about 8.1–19.6 g/100 g, which is higher than that of other grain brans and even comparable to some plant protein content.1 However, in recent years, the utilization of millet bran has been limited to animal feed or boiler fuel, and its nutritional potential is not fully utilized. The development of related products mainly focused on millet bran oil, polysaccharides and other active ingredients, and there is less research on protein extract and its structure and function in food applications. Millet protein is regarded as a healthy substitute for other protein sources (such as nuts, meat and dairy products), and its functions, including solubility, stability, emulsifying ability and foaming ability, have been widely studied.9 At present, there are few studies on millet bran protein, and the majority of them focus on the bioactivities of millet bran peptides. Peptides from the concentrated hydrolysate of millet bran were separated, and the changes in their structure, function, activity and peptide spectrum under different ultrasonication power were studied.10 A new protein with anti-colon cancer activity was extracted and purified from millet bran, which showed selective anti-tumor activity and might be developed as an effective therapeutic agent for colon cancer.11 Peptides with ACE inhibitory activity and Zn chelating ability were purified and identified from millet bran albumin hydrolysates.12 Antioxidant peptides from glutelin-2 hydrolysate of millet bran were identified and characterized.13
Research related to millet bran protein is still relatively lacking, especially a systematic study on the structural characterization and physicochemical properties of different protein fractions. Although there are many reports on foxtail millet proteins, they are limited to non-Chinese varieties (e.g., India and Africa) and domestic cultivars such as Jingu (Shanxi Province) and Longgu (Heilongjiang Province),5 and the systematic investigation of bran protein from Huangjingu—a regionally distinctive variety—is few. Due to its strong lodging resistance, stress resistance, fine grain texture and palatability, Huangjingu is planted widely in northern areas such as Hebei Province, Shanxi Province and Inner Mongolia.14 So, its millet bran is selected as a raw material for protein fractions. To further promote the comprehensive utilization of the defatted millet bran protein (DMBP) from Huangjingu, different fractions of DMBP (albumin, globulin, prolamin and glutelin) were extracted, and their structure and physicochemical properties were investigated. This study will provide a theoretical basis for the development and high-value application of DMBP from Huangjingu in the food industry and make it a promising source of functionally unique protein that has not yet been developed. The research also shows the value of sustainable development of the grain from production to processing.
2. Materials and methods
2.1. Materials
Millet bran from “Huangjingu” processing (Setaria italica (L.) P. Beauv.) was purchased from Xingtai County (Hebei, China). Fig. S1 and S2, ESI,† show millet and millet bran used in this experiment. Pepsin (from porcine gastric mucosa, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units per mg solids) was purchased from Shanghai Macklin Bio-Chem Technology Co., Ltd (Shanghai, China), pancreatin (from porcine pancreas, 250 units per mg of solids) was purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd (Shanghai, China), 8-anilino-1-naphthalene sulfonic acid ammonium salt (ANSA) was obtained from Shanghai Yuan Ye Bio-Technology Co., Ltd (Shanghai, China), and sodium dodecyl sulfate (SDS) was obtained from Shanghai Macklin Bio-Chem Technology Co., Ltd (Shanghai, China). Soybean oil was purchased from Lu Hua Group Co., Ltd (Shandong Province, China). Anhydrous ethanol, sodium chloride, hydrochloric acid, sodium hydroxide and other reagents used were of analytical grade and purchased from National Medicine Group Chemical Reagent Co., Ltd (Nanjing, China).
000 units per mg solids) was purchased from Shanghai Macklin Bio-Chem Technology Co., Ltd (Shanghai, China), pancreatin (from porcine pancreas, 250 units per mg of solids) was purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd (Shanghai, China), 8-anilino-1-naphthalene sulfonic acid ammonium salt (ANSA) was obtained from Shanghai Yuan Ye Bio-Technology Co., Ltd (Shanghai, China), and sodium dodecyl sulfate (SDS) was obtained from Shanghai Macklin Bio-Chem Technology Co., Ltd (Shanghai, China). Soybean oil was purchased from Lu Hua Group Co., Ltd (Shandong Province, China). Anhydrous ethanol, sodium chloride, hydrochloric acid, sodium hydroxide and other reagents used were of analytical grade and purchased from National Medicine Group Chemical Reagent Co., Ltd (Nanjing, China).
2.2. Preparation of defatted foxtail millet bran
Defatting was carried out following a previous method15 without any modifications. The detailed method is described in ESI Method 1.†2.3. Assay of the proximate composition of defatted foxtail millet bran
The moisture content, ash content, fat content, crude fibre content and protein content were determined according to the methods of GB/T 5009.3-2016,16 GB/T 5009.4-2016,17 GB/T 5009.6-2016,18 GB 5009.88-2023,19 and GB 5009.5-2016,20 respectively.2.4. Preparation of millet bran protein fractions
Protein fractions (albumin, globulin, prolamin and glutelin) were prepared from defatted millet bran (DMB) using the method described by Van de Vondel et al.21 Fig. S3† illustrates a schematic diagram of the fractionation protocol. In brief, DMB was dispersed in distilled water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 w/v), and the pH of the mixture was adjusted to 9.0 using 1.0 M NaOH. After stirring for 2 h at room temperature, the mixture was centrifuged at 5000 rpm for 20 min, allowing for the collection of the supernatant and residue separately. Then, the residue was extracted using 70% ethanol (1
10 w/v), and the pH of the mixture was adjusted to 9.0 using 1.0 M NaOH. After stirring for 2 h at room temperature, the mixture was centrifuged at 5000 rpm for 20 min, allowing for the collection of the supernatant and residue separately. Then, the residue was extracted using 70% ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 w/v) and agitated at 45 °C for 2 h. Upon the completion of the extraction, the slurry was centrifuged at 5000 rpm for 20 min to collect the prolamin-containing supernatant. A rotary evaporator (model RE52-99, Yarong Biochemical Instrument Factory, Shanghai, China) was used to evaporate the ethanol, and the precipitated protein was collected. Simultaneously, the supernatant was adjusted to pH 7.0 using 1.0 M NaOH and HCl and stirred for 1.5 h. Then, the mixture was centrifuged at 5000 rpm for 20 min, and the albumin-containing supernatant was collected. The precipitate was redissolved in 1.0 M NaCl solution (1
8 w/v) and agitated at 45 °C for 2 h. Upon the completion of the extraction, the slurry was centrifuged at 5000 rpm for 20 min to collect the prolamin-containing supernatant. A rotary evaporator (model RE52-99, Yarong Biochemical Instrument Factory, Shanghai, China) was used to evaporate the ethanol, and the precipitated protein was collected. Simultaneously, the supernatant was adjusted to pH 7.0 using 1.0 M NaOH and HCl and stirred for 1.5 h. Then, the mixture was centrifuged at 5000 rpm for 20 min, and the albumin-containing supernatant was collected. The precipitate was redissolved in 1.0 M NaCl solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 w/v), agitated at 45 °C for 1 h, and then centrifuged at 5000 rpm for 20 min to collect the supernatant containing the salt-soluble protein. Furthermore, the precipitate was dissolved in 0.1 M NaOH solution (1
10 w/v), agitated at 45 °C for 1 h, and then centrifuged at 5000 rpm for 20 min to collect the supernatant containing the salt-soluble protein. Furthermore, the precipitate was dissolved in 0.1 M NaOH solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 w/v) and agitated at 50 °C for 1 h before centrifuging at 5000 rpm for 20 min to obtain the supernatant containing glutelin fraction. Finally, the pH of supernatants containing albumin, globulin and glutelin was adjusted to 4.0, 4.2 and 4.6, respectively. They were then allowed to precipitate overnight and centrifuged at 8000 rpm for 10 min, with the residues collected afterward. All protein fractions were dialyzed against deionized water at 4 °C for 48 h (molecular weight cutoff, 6000–8000 Da), then lyophilized at −80 °C and under a vacuum of −0.01 mbr (2492 Freeze Dryer, Taishida Company, Spain) for 48 h to dry and stored at −20 °C for further use. Here, the protein content in each supernatant was determined using the Bradford method with bovine serum albumin as the gold standard.22
10 w/v) and agitated at 50 °C for 1 h before centrifuging at 5000 rpm for 20 min to obtain the supernatant containing glutelin fraction. Finally, the pH of supernatants containing albumin, globulin and glutelin was adjusted to 4.0, 4.2 and 4.6, respectively. They were then allowed to precipitate overnight and centrifuged at 8000 rpm for 10 min, with the residues collected afterward. All protein fractions were dialyzed against deionized water at 4 °C for 48 h (molecular weight cutoff, 6000–8000 Da), then lyophilized at −80 °C and under a vacuum of −0.01 mbr (2492 Freeze Dryer, Taishida Company, Spain) for 48 h to dry and stored at −20 °C for further use. Here, the protein content in each supernatant was determined using the Bradford method with bovine serum albumin as the gold standard.22
2.5. Yield and purity of protein fractions
The yield of protein fractions extracted from DMB was determined according to eqn (1).| Yield (%) = Wp/Wmb × 100 | (1) |
The protein purity of each fraction was estimated using the Kjeldahl method (GB 5009.5-2016).20
2.6. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
The detailed method is described in ESI Method 2.†2.7. Amino acid content assay
The amino acid (AA) composition and content were determined according to the method of GB5009.124-2016 with some modifications. The detailed description is provided in ESI Method 3.†2.8. Characterization of structural characteristics of protein fractions from DMB
2.9. Physicochemical properties of protein fractions from DMB
2.10. In vitro protein digestibility
In vitro protein digestibility (IVPD) was determined according to the method of Yang et al.27 with slight modifications. The detailed method is described in ESI Method 14.†2.11. Statistical analyses
The results are presented as mean ± standard deviation of triplicate evaluations. One-way analysis of variance (ANOVA) was performed to check the variation in properties. Origin 2024 was used to analyse experimental data and draw graphs.3. Results and discussion
3.1. Proximate composition of DMB
We measured the proximate composition of DMB. All data were presented on a dry basis (d.b.). It could be found that the dietary fibre was the main chemical composition accounting for 50.96%. The content of fat, moisture and ash was 2.36%, 5.82% and 6.38%, respectively. The crude protein content was 15.54%. Meena et al.28 also reported that millet bran crude protein was 6–9%. Here, the high protein content might be related to the reduction of fat in DMB. This indicated that DMB could be a potential protein resource.3.2. Yield and purity of different protein fractions
The Osborne method was used to extract different protein fractions, and Table 1 shows the yield and purity of the four protein fractions. The yield of prolamin was the highest (3.25%), followed by albumin (1.22%), globulin (0.98%) and glutelin (0.49%). Prolamin and albumin accounted for the most protein fractions, which was different from the reported results that prolamin and glutelin made up the majority of the protein content in most cereals.29 The difference might be attributed to the differences in the extraction and purification methods used. The purity of albumin, globulin and prolamin was more than 75%. Among them, the purity of albumin was the highest (84.87%), while the purity of glutelin was the lowest, and this might be related to the differences in the extraction solvents used.| Protein fractions | Protein yield (%) | Protein purity (%) |
|---|---|---|
| a The data within a column with different superscript letters are significantly different (p < 0.05). | ||
| Albumin | 1.22 ± 0.11b | 84.87 ± 3.71a |
| Globulin | 0.98 ± 0.07b | 76.02 ± 1.75b |
| Prolamin | 3.25 ± 0.39a | 80.80 ± 3.83 ab |
| Glutelin | 0.49 ± 0.02c | 56.76 ± 2.12c |
In this paper, the Osborne method was adopted to extract four proteins from millet bran. However, the protein extraction rate was relatively low. Many research reports have confirmed this result. For instance, He et al.30 used the Osborne method to extract buckwheat proteins, and the yields of the separated albumin, globulin, prolamin, and glutenin were 1.32%, 0.44%, 0.30%, and 1.05%, respectively. Sun et al.31 employed the same method and obtained the yields of 0.48%, 0.25%, 0.19%, and 0.08% for albumin, globulin, prolamin, and glutenin in wheat bran, respectively. The Osborne method is a classical approach for classifying cereal proteins based on their solubility in water, salt solutions, alcoholic solutions, and alkaline solutions. Compared to alkaline extraction and enzymatic extraction, this method provides a more comprehensive separation of protein fractions while preserving their distinct solubility properties. However, its extraction conditions remain suboptimal, as the results are highly sensitive to reagent concentration, stirring intensity, extraction duration, and temperature.32 For albumin, excessive dilution might reduce the protein concentration per unit volume, leading to lower extraction efficiency. The low yields of globulin and prolamin might result from partial protein denaturation under prolonged exposure to salt ions and ethanol, promoting aggregation and subsequent loss during centrifugation. Glutenin exhibits peak solubility only at a specific alkali concentration; insufficient alkali might lead to incomplete dissolution, while excess alkali might induce partial denaturation, aggregation, and precipitation.32
3.3. SDS-PAGE assay
SDS-PAGE can detect the subunit distribution of the four protein fractions. The electropherograms showed the pattern and molecular weight distribution of the four protein fractions (Fig. 1). Albumin fraction (band 1) showed seven polypeptide chains with the major bands at 11, 16, 17 and 75 kDa, indicating that most of the proteins had a relatively low molecular weight. Globulin fraction (band 2) displayed two significant subunit bands at 35 and 36 kDa. Albumin and globulin displayed similar band patterns ranging from 11 and 75 kDa, respectively. This phenomenon might be attributed to the partial solubility of the proteins in water and salt solutions. The faint bands for proso millet were observed at approximately 65 and 48 kDa, which were identified as the 7S globulin fraction.29 A small molecular weight meant better physiological functions such as solubility and foaming. The same results were also found in rice and rice bran. The rice bran protein contained 9 major protein bands; the molecular weight of glutelin was 53–61 kDa.33 And glutelin (57–62 kDa) was identified as the major polypeptide in rice protein.34 The separation results of albumin and globulin subunits were much better than the other two proteins, which might be due to their high solubility.35 The glutelin fraction (band 4) exhibited few bands in the range of 11–75 kDa, and only a major band was observed at 100 kDa. Determination of the molecular weight of prolamin was difficult since its bands were not visible, which might be due to the molecular weight of prolamin being large and it could not enter the separated gel.36 Some bands with the same molecular weight were found in four protein fractions, and these similar bands might indicate the similarity of the protein structure.37 Kumar et al.38 found that the SDS-PAGE bands of albumin/globulin in foxtail millet (TNAU-173) were observed at 54 kDa, 34 kDa, 22 kDa and 12 kDa, the bands of prolamin were distributed within the molecular weight range of 12–27 kDa, and the glutelin revealed many bands throughout the length of the gel. The distinct SDS-PAGE bands between millet protein fractions and millet bran protein fractions indicated divergences in their molecular weight distribution. This showed that differences in the same type of protein might arise either from different varieties or from distinctions between millet bran-derived and millet-derived proteins. | ||
| Fig. 1 SDS-PAGE of the four protein fractions from DMB (bands 1–4: albumin, globulin, prolamin and glutelin). | ||
3.4. Amino acid content analysis
AA profiles of protein play an important role in determining its nutritional quality and properties. AA profiles (mg/100 mg protein) of the four protein fractions included essential AAs and non-essential AAs (Table 2). The main AA compositions of albumin, globulin and glutelin were Glu, Asp, Leu and Arg, while it was Glu, Asp, Leu and Ala for prolamin. This was consistent with the previous results of pearl millet.39 Generally speaking, cereal proteins often contain a high content of acidic AAs,40 and the proportion of acidic AAs in the present study was also high. Among the essential AAs, Leu (5.13–11.26 mg/100 mg protein), Val (3.67–4.60 mg/100 mg protein) and Phe (3.50–4.12 mg/100 mg protein) were the most abundant extracts. Ile, Leu and Val endowed related proteins with strong hydrophobic components; simultaneously, this characteristic increased the protein stability, as these AAs formed a more aggregated and compact protein core.41 Among the non-essential AAs, Asp (4.46–6.11 mg/100 mg protein), Glu (7.74–15.73 mg/100 mg protein) and Ala (4.29–7.65 mg/100 mg protein) were more abundant. Studies have shown that these non-essential AAs are usually high in plant protein extracts,41 and these AAs are also beneficial to the human body, because all of them could react with free radicals, thus preventing oxidative damage.42 In addition, a little difference was observed in the content of Cys; prolamin had the highest Cys content (1.11%), followed by globulin (0.92%), glutelin (0.83%) and albumin (0.80%), which was closely related to the formation of the SS.43 While millet bran protein fractions and conventional millet protein fractions share overlapping AA profiles in terms of compositional categories, their quantitative distributions exhibited marked disparities. For example, the Lys content of albumin, globulin, prolamin and glutenin in the present study was 3.71, 5.29, 0.35 and 4.99 mg/100 mg protein, respectively, while that of albumin–globulin, prolamin and glutenin in Italian millet protein fractions reported in the literature was 7.14, 0.11 and 6.52 mg/100 mg protein, respectively.44 Therefore, millet bran protein had a better AA composition structure, which can improve the condition of limited AA deficiency in the millet protein core.41| Amino acid mg/100 mg protein | Albumin | Globulin | Prolamin | Glutelin | WHO/FAO | |
|---|---|---|---|---|---|---|
| Children | Adult | |||||
| a EAA: Essential amino acid. Requirement by (FAO/WHO, 2007) for 2–5-year-old children or adults. BAA: basic amino acid (Lys, Arg, and His). BCAA: branched-chain amino acid (Val, Ile, and Leu). NEAA: non-essential amino acid. TAA: total Amino Acids. Values are expressed as mean ± SD (n = 3). Means followed by different letter superscripts in the same row are significantly different (p < 0.05). | ||||||
| Essential amino acids | ||||||
| Threonine (Thr) | 2.36 ± 0.12b | 3.20 ± 0.10a | 2.65 ± 0.12b | 3.25 ± 0.24a | 3.4 | 0.90 |
| Methionine (Met) | 1.07 ± 0.14c | 1.64 ± 0.02b | 1.95 ± 0.08a | 1.64 ± 0.06b | ||
| Isoleucine (Ile) | 2.56 ± 0.10b | 3.06 ± 0.10a | 3.08 ± 0.09 ab | 3.01 ± 0.08 ab | 2.8 | 1.3 |
| Leucine (Leu) | 5.13 ± 0.05c | 6.29 ± 0.22b | 11.26 ± 0.10a | 6.25 ± 0.21b | 6.6 | 1.9 |
| Valine (Val) | 4.35 ± 0.05a | 4.60 ± 0.36a | 3.67 ± 0.34b | 4.30 ± 0.04a | 3.5 | 1.3 |
| Phenylalanine (Phe) | 3.78 ± 0.08 ab | 3.84 ± 0.34a | 3.72 ± 0.21 ab | 3.50 ± 0.13b | ||
| Lysine (Lys) | 3.71 ± 0.05c | 5.29 ± 0.08a | 0.35 ± 0.20d | 4.99 ± 0.05b | 5.8 | 0.50 |
| Histidine (His) | 2.58 ± 0.04a | 2.23 ± 0.01b | 1.26 ± 0.14d | 1.98 ± 0.04c | 1.90 | 1.60 |
| Met + Cys | 1.87 ± 0.03c | 2.56 ± 0.18b | 3.06 ± 0.11a | 2.47 ± 0.10b | 2.70 | 1.70 |
| Phe + Tyr | 6.44 ± 0.11a | 6.42 ± 0.17a | 6.54 ± 0.30a | 6.18 ± 0.12a | 6.30 | 1.90 |
| EAA | 25.54 ± 0.14c | 30.15 ± 0.22a | 28.34 ± 0.54b | 28.92 ± 0.53b | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Non-essential amino acids | ||||||
| Aspartic acid (Asp) | 5.50 ± 0.36b | 6.55 ± 0.04a | 4.46 ± 0.52c | 6.11 ± 0.52 ab | ||
| Serine (Ser) | 4.22 ± 0.28a | 3.89 ± 0.12 ab | 3.57 ± 0.01bc | 3.45 ± 0.16c | ||
| Glutamic acid (Glu) | 11.60 ± 1.56b | 9.42 ± 0.19c | 15.73 ± 0.20a | 7.74 ± 0.26d | ||
| Glycine (Gly) | 4.23 ± 0.07a | 4.27 ± 0.27a | 1.32 ± 0.14b | 3.94 ± 0.34a | ||
| Alanine (Ala) | 4.29 ± 0.07c | 5.05 ± 0.12b | 7.65 ± 0.16a | 5.04 ± 0.20b | ||
| Cysteine (Cys) | 0.80 ± 0.02b | 0.92 ± 0.02 ab | 1.11 ± 0.15a | 0.83 ± 0.12b | ||
| Tyrosine (Tyr) | 2.66 ± 0.04b | 2.58 ± 0.02b | 2.82 ± 0.08a | 2.68 ± 0.05b | ||
| Arginine (Arg) | 9.02 ± 0.11a | 7.05 ± 0.28b | 1.43 ± 0.06d | 5.90 ± 0.10c | ||
| NEAA | 42.32 ± 0.40a | 39.73 ± 0.64b | 37.69 ± 0.62c | 35.69 ± 0.27d | ||
| BAA | 15.31 ± 0.74a | 14.57 ± 0.12b | 3.04 ± 0.02d | 12.87 ± 0.13c | ||
| BCAA | 12.04 ± 0.09c | 13.95 ± 0.64b | 18.01 ± 0.11a | 13.56 ± 0.19b | ||
| TAA | 67.86 ± 0.49 ab | 69.88 ± 0.46a | 66.03 ± 0.58b | 64.61 ± 0.61b | ||
According to hydrophilicity, AAs could be categorized into hydrophobic, hydrophilic and neutral AAs, including hydrophobic (Ala, Val, Leu, Ile, Met, Phe and Cys), hydrophilic (Arg, Lys, Asp and Glu) and neutral amino acids (Gly, Ser, Thr and Tyr). Among them, the hydrophobic AA content of the prolamin was the highest accounting for 32.84%. Regarding hydrophilic AAs, albumin had the highest content accounting for 29.83%. Generally, the proportion of acidic AAs was higher than that of alkaline AAs, indicating that the extracted DMBP fractions might have relatively high solubility.
Table 2 also compares the AA content of DMBP with WHO/FAO recommendations for adults and infants aged 2–5. This helped to clarify the benefits of DMBP AAs. It is noteworthy that the protein fractions exhibited higher levels of all EAAs than the FAO/WHO requirements for adults. Only some AA content such as sulfur-containing amino acids, Lys and Thr, was slightly lower than the recommended levels for children. And the contents of Lys of prolamin were much higher than the recommended levels of 5.8 mg/100 mg protein for children. Although the four protein fractions had a comparable AA distribution, globulin had the most EAA (30.15%). Furthermore, prolamin (18.01%) had a greater BCAA content than the others, indicating it as a nutritionally better protein. The AA composition of DMBP fractions was comparable to FAO/WHO reference proteins, indicating that they might be included in a range of food products as potentially nutritional and functional components. According to the FAO/WHO recommended standard, high-quality proteins typically have an EAA/TAA ratio of around 40% and an EAA/NEAA ratio of above 0.6.45 All DMBP fractions analysed in this study exhibited abundant EAA content. Specifically, the EAA/TAA ratios of globulin, prolamin and glutelin were 43.15%, 42.92% and 44.76%, respectively, exceeding the FAO/WHO standard by 3.15, 2.92 and 4.76 percentage points, respectively. The EAA/NEAA ratios of four protein fractions reached 60.35%, 75.89%, 75.19% and 81.03%, respectively, indicating that all DMBP fractions qualify as excellent protein sources. According to reports, the EAA/TAA ratios of albumin–globulin, prolamin and glutelin were 37.49%, 38.26% and 41.85%, respectively. Existing studies report that the EAA/TAA ratio of albumin–globulin, prolamin, and glutelin in Italian millet proteins was 37.49%, 38.26%, and 41.85%, respectively.44 In contrast, the present analysis of millet bran protein fractions revealed a marked disparity. This divergence demonstrates that millet bran proteins exhibited a more balanced AA distribution compared to conventional millet proteins. This suggested that divergence in identical protein types might stem from either inter-varietal differences or contrasts between millet bran-derived and millet-derived proteins.
3.5. Structural characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−1; amide B, 3200–3100
cm−1; amide B, 3200–3100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−1; amide I, 1700–1600
cm−1; amide I, 1700–1600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−1; amide II, 1480–1575
cm−1; amide II, 1480–1575![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−1; amide III, 1220–1330
cm−1; amide III, 1220–1330![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−1).43 The amide I region is the most sensitive spectral region to protein secondary structural components, which was due almost entirely to the C
cm−1).43 The amide I region is the most sensitive spectral region to protein secondary structural components, which was due almost entirely to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of the peptide linkages (approximately 80%).45 The frequencies of the amide I band components are found to be closely correlated to each secondary structural element of the proteins. The amide II band, in contrast, is derived mainly from in-plane NH bending (40–60% of the potential energy) and the CN stretching vibration (18–40%), showing much less protein conformational sensitivity than its amide I counterpart.41 Other amide vibrational bands are very complex, depending on the details of the force field, the nature of side chains and hydrogen bonding, which therefore are of little practical use in protein conformational studies.41 Four peaks in the amide I band could be ascribed to β-sheet (1600–1640 cm−1), random coil (1640–1650 cm−1), α-helix (1650–1660 cm−1) and β-turn (1660–1700 cm−1) structures. Peak separation calculations were performed through curve-fitting of the amide I region ranging from 1600 to 1700
O stretching vibrations of the peptide linkages (approximately 80%).45 The frequencies of the amide I band components are found to be closely correlated to each secondary structural element of the proteins. The amide II band, in contrast, is derived mainly from in-plane NH bending (40–60% of the potential energy) and the CN stretching vibration (18–40%), showing much less protein conformational sensitivity than its amide I counterpart.41 Other amide vibrational bands are very complex, depending on the details of the force field, the nature of side chains and hydrogen bonding, which therefore are of little practical use in protein conformational studies.41 Four peaks in the amide I band could be ascribed to β-sheet (1600–1640 cm−1), random coil (1640–1650 cm−1), α-helix (1650–1660 cm−1) and β-turn (1660–1700 cm−1) structures. Peak separation calculations were performed through curve-fitting of the amide I region ranging from 1600 to 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−1, and the proportion of each estimated secondary structure constituent is shown in Table 3. For the four protein fractions, β-sheets and β-turns were the main structures. Li et al.46 found in their study on selenium-enriched millet that FTIR analysis also indicated β-sheets as the predominant secondary structure of millet protein. For each secondary structure, the highest content of α-helix, β-sheet, β-turn and random coil occurred in albumin (23.16%), glutelin (37.66%), globulin (41.32%) and prolamin (20.78%), respectively. The hydrogen bond in α-helix is the main factor in maintaining its structure, and a lack of it would lead to more hydrophobic fragments exposed on the molecular surface and reduce the solubility.47 It was reported that proteins rich in β-sheet structure had high hydrophobicity and those rich in α-helix had higher digestibility than others.48 In addition, studies had indicated that a high β-sheet content in the secondary structure could improve the thermal stability of proteins.49
cm−1, and the proportion of each estimated secondary structure constituent is shown in Table 3. For the four protein fractions, β-sheets and β-turns were the main structures. Li et al.46 found in their study on selenium-enriched millet that FTIR analysis also indicated β-sheets as the predominant secondary structure of millet protein. For each secondary structure, the highest content of α-helix, β-sheet, β-turn and random coil occurred in albumin (23.16%), glutelin (37.66%), globulin (41.32%) and prolamin (20.78%), respectively. The hydrogen bond in α-helix is the main factor in maintaining its structure, and a lack of it would lead to more hydrophobic fragments exposed on the molecular surface and reduce the solubility.47 It was reported that proteins rich in β-sheet structure had high hydrophobicity and those rich in α-helix had higher digestibility than others.48 In addition, studies had indicated that a high β-sheet content in the secondary structure could improve the thermal stability of proteins.49
 | ||
| Fig. 2 Fourier transform infrared spectra of the four protein fractions from DMB (A); curve-fitting spectra of peaks of albumin (B), globulin (C), prolamin (D) and glutelin (E) from DMB. | ||
| Protein fractions | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random coil (%) |
|---|---|---|---|---|
| a The results are expressed as the average of three repeated experiments with standard deviation. The averages within a column with different superscript letters are significantly different (p < 0.05). | ||||
| Albumin | 23.16 ± 0.03a | 31.25 ± 0.01cb | 34.35 ± 0.05c | 11.24 ± 0.03b |
| Globulin | 12.79 ± 0.05c | 35.62 ± 0.06b | 41.32 ± 0.04a | 10.28 ± 0.04d |
| Prolamin | 12.22 ± 0.01b | 27.64 ± 0.02d | 39.36 ± 0.05b | 20.78 ± 0.01a |
| Glutelin | 18.72 ± 0.05b | 37.66 ± 0.01a | 32.24 ± 0.02d | 10.38 ± 0.03c |
3.6. Physicochemical properties of DMB proteins
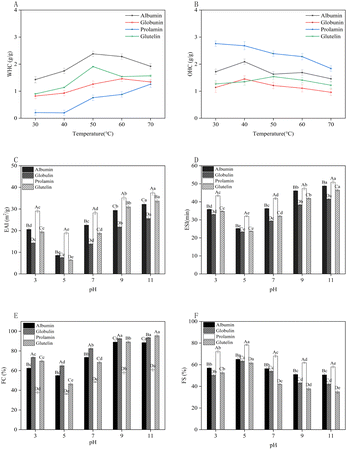
It was obvious that albumin solubility was the best and the worst for prolamin. Prolamin was hydrophobic due to its high hydrophobic AA content such as Leu, Ala, and Phe (Table 3), which made it difficult to dissolve in water. Glutelin is an alkali-soluble protein, and its solubility increased significantly in the alkaline environment, even exceeding that of albumin when the pH was beyond 9. As shown in the SDS-PAGE assay, the albumin fraction was mainly composed of low molecular weight peptides, which contributed greatly to its structural flexibility and solubility. The ionization of neutral and acidic AAs may also strongly affect solubility.47 The higher solubility fraction would facilitate the emulsifying and foaming capacity of proteins.52
The average particle size could reflect the average size of the protein particle population. The terms d4,3 and d3,2 represent the volume and surface mean diameter of particle diameter distributions, respectively, which could reflect the uniformity of particle sizes. The term D50 represents the particle size at which 50% of the sample's mass was composed of particles smaller than this size (Table 4). The d4,3 and d3,2 of albumin and globulin were similar, indicating that they had similar particle sizes. The D50 values of prolamin and glutelin were smaller than the other two proteins, reflecting that prolamin and glutelin had smaller particle sizes. It could be seen that the D50 values of prolamin and glutelin were more consistent with d4, 3, indicating that their particle sizes were relatively uniform. The small particle size indicated that protein molecules were difficult to aggregate, and the solution system was relatively stable. Although smaller protein aggregates were helpful to increase water solubility, more hydrophobic groups from hydrophobic AAs could be exposed on the molecular surface, thus reducing the solubility.54
| Index | Albumin | Globulin | Prolamin | Glutelin |
|---|---|---|---|---|
| a d 4,3, volume mean diameter of particle diameter distribution; d3,2, surface mean diameter of particle diameter distribution; D50, 50 percentile of particle diameter distribution. The averages within a row with different superscript letters are significantly different (p < 0.05). | ||||
| d 4,3 | 467.45 ± 19.26a | 446.30 ± 11.40a | 341.19 ± 14.42b | 263.64 ± 13.87c |
| d 3,2 | 337.67 ± 15.82a | 318.19 ± 15.79a | 241.44 ± 10.74b | 195.38 ± 7.51c |
| D50 | 396.06 ± 17.31b | 342.00 ± 10.79c | 295.31 ± 18.32a | 255.00 ± 12.99c |
Zeta potential generally represents the intensity of reciprocal repulsion or attraction between particles, expressed as the potential of electrostatic charge on the surface of a sample in a liquid.43 The absolute value of zeta potential could reflect the stability of the suspension. When the absolute value of zeta potential was more than 30 mV, it implied stability.15 It is shown in Fig. 4D that the zeta potentials of the four protein fractions from DMB were all negative because when the pH value was higher than their isoelectric point, the protein surface was negatively charged due to the existence of deprotonated carboxyl groups (COO–). The zeta potentials of albumin, globulin and prolamin were −13.45 mV, −14.52 mV and −7.28 mV, respectively, and they were all less than 30 mV, indicating that prolamin is more prone to precipitate due to its poor stability in a suspension, which echoed the above solubility results (Fig. 4A). However, the linear relationship between the zeta potential and solubility was not obvious.55 The absolute value of the zeta potential of glutelin was the highest (−34.97 mV) and more than 30 mV, indicating the high stability of glutelin suspension and long storage time.
The OHC is defined as the protein's ability to bind the oil with its nonpolar (hydrophobic) side chains, which can preserve its flavor and make food more palatable.60 The OHC of foxtail millet protein concentrates (2.22–3.03 g g−1)58 was found to encompass the OHC range exhibited by the four DMBPs at 30 °C in our study. Fig. 5B shows that the OHC of the four protein fractions increased first and then decreased within 30–70 °C, which might be attributed to the low fluidity of oil at low temperatures, and it was easily intercepted by protein molecules and combined with small molecules, so the OHC increased.62 As the temperature increased, the protein exposed more polar groups and its capacity to bind oil decreased, thus the OHC reached saturation and decreased. Within the designed temperature range, prolamin exhibited the highest OHC of 2.76 g g−1 at 30 °C, which was equivalent to the OHC of soybean protein (2.61 g g−1),63 followed by albumin (2.09 g g−1), and glutelin had the lowest OHC (1.27 g g−1). The difference might be related to the structure and the ratio of surface hydrophilicity to hydrophobicity of the four protein fractions.61
Regarding each protein fraction, prolamin displayed the best EAI and ESI in tested pH, followed by albumin, glutelin, and globulin (Fig. 5C and D). This observation is consistent with the findings of Qi et al. regarding the EAI and ESI of millet flour proteins.64 Prolamin with high H0 might inhibit the interaction between protein and water,49 facilitate the adsorption of protein particles to the oil–water interface, and finally lead to higher EAI and ESI values in a strongly alkaline environment than the other three protein fractions. Additionally, a relatively small particle size might be helpful for the high EAI and ESI of prolamin. Zhu et al.65 found that proteins with high emulsion activity were tinier, which promoted the adsorption of protein particles onto the oil–water interface. Thus, prolamin could be used as an emulsifier in the food industry.
Regarding the FS, opposite results were observed, and the highest FS of the four protein fractions was found at the pI of proteins about 5.0 (Fig. 5F). This might be due to that the maximum electrostatic attraction at the pI of proteins and the rheological properties of the protein film, especially viscosity and rigidity, enhanced the FS. Denatured proteins could adsorb easily at the interface because of minimal repulsion of the molecules at the pI.68 Prolamin had a better FS than the other three fractions, and this might be due to its high H0 and the role of high content of SS at the interface, which could enhance protein–protein interactions, thus leading to thicker adsorbed films, improving the FS.68 The FC and FS of pearl millet protein were determined to be 18.35% and 51.33%, respectively,69 which were much lower than those of DMBPs we studied.
3.7. In vitro digestibility of protein fractions from DMB
IVPD indicates the amount of ingested protein absorbed in the body.27 Thus, IVPD affects the bioavailability of AAs and the nutritional quality of proteins.27 As shown in Fig. 6, the IVPD of albumin was the highest (46.67%), followed by globulin (41.68%), glutelin (38.55%), and prolamin (24.82%). Current research on the digestibility of DMBP remains limited. Moreover, variations in research methodologies and sample characteristics have led to inconsistent findings across studies. A study revealed that proteins in millet milk exhibited relatively low digestibility (35.2%), which was attributed to the presence of prolamins in millet.70 This is consistent with our experimental results. With an increase of protein's flexibility (α-helix) and solubility, the IVPD would increase, but it would decrease with increasing β-sheet content (structural stability).48 The albumin fraction had a high content of α-helix and high solubility, thus showing the highest IVPD; prolamin had a low α-helix content and low solubility, resulting in low IVPD. Additionally, a small protein molecule is easy to be digested and absorbed in the human body.36 As albumin and globulin fractions had relatively low molecular weights (Fig. 1), their IVPD was higher. It was reported that there was a strong negative correlation between IVPD and Cys.43 In addition, His, Val, Ser, Arg, total non-essential AAs, positive charge, hydrophilicity and hydroxyl AA positively correlated with IVPD.424. Conclusions
The millet bran protein fractions extracted from Huangjingu and separated by the Osborne method were found to be composed of albumin, globulin, prolamin, and glutelin. All protein fractions met the requirements of the FAO/WHO for most AAs in adults. FTIR had confirmed that β-turns and β-sheets were the main structures of all protein fractions. The fractionated proteins of millet bran could be developed as different functional ingredients according to their structural and functional properties. For instance, albumin with good solubility and WHC could serve as a suitable ingredient in food formulations; globulin with better FC could be used in ice cream; prolamin, which had poor solubility but excellent OHC and EAI, could be used as a good emulsifier. The primary motivation behind this work was to investigate the potential applications of each protein fraction of millet bran in the food industry, enhance the high-value utilization of millet bran proteins and promote the sustainable development of millet bran. Further studies on the optimization of the extraction conditions of millet bran protein and its fractions will be conducted.Data availability
Data will be made available on request.Author contributions
Wenjie Zhao: methodology, investigation, formal analysis, data curation, writing – original draft, visualization, validation. Juan Shen: writing – review & editing. Xia Fan: resources. Fanqiang Meng: writing – review & editing. Fengxia Lv: methodology, resources. Zhaoxin Lu: methodology, supervision, resources. Haizhen Zhao: writing – review & editing, supervision, resources, project administration, methodology, investigation, funding acquisition, conceptualization.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.References
- Á. J. Pastrana-Pastrana, R. Rodríguez-Herrera, J. F. Solanilla-Duque and A. C. Flores-Gallegos, Plant proteins, insects, edible mushrooms and algae: more sustainable alternatives to conventional animal protein, J. Future Foods, 2024, 5(3), 248–256 CrossRef.
- K. J. D. Sartagoda, R. N. Tiozon Jr and N. Sreenivasulu, Cereal-based proteins: Bridging health, sustainability, and future innovations for food industries, J. Food Compos. Anal., 2024, 136, 106720 CrossRef CAS.
- X. Gong, Q. An, L. Le, F. Geng, L. Jiang and J. Yan, et al., Prospects of cereal protein-derived bioactive peptides: Sources, bioactivities diversity, and production, Crit. Rev. Food Sci. Nutr., 2022, 62(11), 2855–2871 CrossRef CAS PubMed.
- N. Sachdev, S. Goomer, L. R. Singh, V. M. Pathak, D. Aggarwal and R. K. Chowhan, Current status of millet seed proteins and its applications: A comprehensive review, Appl. Food Res., 2023, 3(1), 100288 CrossRef CAS.
- J. Zhao, A. Yu, Y. Du, G. Wang, Y. Li and G. Zhao, et al., Foxtail millet (Setaria italica (L.) P. Beauv) CIPKs are responsive to ABA and abiotic stresses, PLoS ONE, 2019, 14(11), e0225091 CrossRef CAS PubMed.
- C. Yang, H. Y. Li, X. Y. Deng, Z. F. Tian and H. P. Wang, The amino acid composition of protein in millet and quality evaluation, Acad. Period. Farm Prod., 2008, 12(12), 8–12 Search PubMed.
- G. Jayaprakash, A. Bains, P. Chawla, M. Fogarasi and S. Fogarasi, A narrative review on rice proteins: Current scenario and food industrial application, Polymers, 2022, 14(15), 3003 CrossRef CAS PubMed.
- A. K. Anal, R. Singh, D. Rice, K. Pongtong, U. Hazarika and D. Trivedi, et al., Millets as supergrains: a holistic approach for sustainable and healthy food product development, Sustainable Food Technol., 2024, 2(4), 908–925 RSC.
- G. Kaur, S. Ahmadzadeh-Hashemi, S. Amir, Z. S. Khan, Z. Gulsunoglu-Konuskan and A. Karimidastjerd, et al., Exploring sustainable novel millet protein: A look at the future foods through innovative processing, Future Foods, 2024, 9, 100367 CrossRef CAS.
- Z. Peng, F. Wang, L. Yu, B. Jiang, J. Cao and Z. Sun, et al., Effect of ultrasound on the characterization and peptidomics of foxtail millet bran protein hydrolysates, Ultrason. Sonochem., 2024, 110, 107044 CrossRef CAS PubMed.
- S. Shan, Z. Li, I. P. Newton, C. Zhao, Z. Li and M. Guo, A novel protein extracted from foxtail millet bran displays anti-carcinogenic effects in human colon cancer cells, Toxicol. Lett., 2014, 227(2), 129–138 CrossRef CAS PubMed.
- Y. Zheng, J. Ma, Y. Guo, Y. Zhuang, Z. Yang and Z. Zhu, et al., In silico screening and characterization, inhibition mechanism on ACE, and stability of antihypertensive peptides with Zn-chelating capacity identified from millet bran albumin hydrolysates, Food Biosci., 2023, 56, 103419 CrossRef CAS.
- B. Xu, X. Wang, Y. Zheng, Y. Li, M. Guo and Z. Yan, Novel antioxidant peptides identified in millet bran glutelin-2 hydrolysates: Purification, in silico characterization and security prediction, and stability profiles under different food processing conditions, LWT--Food Sci. Technol., 2022, 164, 113634 CrossRef CAS.
- X. F. Ma, Characteristics and high-yield cultivation techniques of high-quality millet varieties in rural areas, Mod. Agric. Sci. Technol., 2011, 23, 127 CrossRef.
- J. Yang, I. Faber, C. C. Berton-Carabin, C. V. Nikiforidis, E. van der Linden and L. M. C. Sagis, Foams and air-water interfaces stabilised by mildly purified rapeseed proteins after defatting, Food Hydrocolloids, 2021, 112, 106270 CrossRef CAS.
- GB/T 5009.3-2016, Determination of Moisture in Foods, Ministry of Health of the People's Republic of China, Beijing, 2016 Search PubMed.
- GB/T 5009.4-2016, Determination of Ash in Foods, Ministry of Health of the People's Republic of China, Beijing, 2016 Search PubMed.
- GB/T 5009.6-2016, Determination of Fat in Foods, Ministry of Health of the People's Republic of China, Beijing, 2016 Search PubMed.
- GB/T 5009.88-2023, Determination of Crude Fiber in Foods, Ministry of Health of the People's Republic of China, Beijing, 2023 Search PubMed.
- GB/T 5009.5-2016, Determination of Protein in Foods, Ministry of Health of the People's Republic of China, Beijing, 2016 Search PubMed.
- J. Van de Vondel, M. A. Lambrecht and J. A. Delcour, Osborne extractability and chromatographic separation of protein from quinoa (Chenopodium quinoa Willd.) wholemeal, LWT--Food Sci. Technol., 2020, 126, 109321 CrossRef CAS.
- M. A. Redmile-Gordon, E. Armenise, R. P. White, P. R. Hirsch and K. W. T. Goulding, A comparison of two colorimetric assays, based upon Lowry and Bradford techniques, to estimate total protein in soil extracts, Soil Biol. Biochem., 2013, 67, 166–173 CrossRef CAS PubMed.
- J. Yang, Y. Duan, H. Zhang, F. Huang, C. Wan and C. Cheng, et al., Ultrasound coupled with weak alkali cycling-induced exchange of free sulfhydryl-disulfide bond for remodeling interfacial flexibility of flaxseed protein isolates, Food Hydrocolloids, 2023, 140, 108597 CrossRef CAS.
- S. Cui, D. J. McClements, X. Xu, B. Jiao, L. Zhou and H. Zhou, et al., Peanut proteins: Extraction, modifications, and applications: A comprehensive review, Grain & Oil Sci. Technol., 2023, 6(3), 135–147 Search PubMed.
- Q. Zhao, X. Hong, L. Fan, Y. Liu and J. Li, Solubility and emulsifying properties of perilla protein isolate: Improvement by phosphorylation in the presence of sodium tripolyphosphate and sodium trimetaphosphate, Food Chem., 2022, 382, 132252 CrossRef CAS PubMed.
- N. A. Mir, C. S. Riar and S. Singh, Effect of pH and holding time on the characteristics of protein isolates from Chenopodium seeds and study of their amino acid profile and scoring, Food Chem., 2019, 272, 165–173 CrossRef CAS PubMed.
- Q. Yang, Y. Wang, M. Yang, X. Liu, S. Lyu and B. Liu, et al., Effect of glycation degree on the structure and digestion properties of ovalbumin: A study of amino acids and peptides release after in vitro gastrointestinal simulated digestion, Food Chem., 2022, 373, 131331 CrossRef CAS PubMed.
- K. K. Meena, S. Meena, M. Joshi and A. V. Dhotre, Nutritional and functional exploration of pearl millet and its processing and utilization: An overview, Food and Humanity, 2024, 3, 100334 CrossRef.
- A. G. Wouters and J. A. Delcour, Cereal protein-based nanoparticles as agents stabilizing air–water and oil–water interfaces in food systems, Curr. Opin. Food Sci., 2019, 25, 19–27 CrossRef.
- X. L. He, T. W. Lei, Q. Z. Xu, Z. T. Wang, X. C. Mo and H. M. Li, Study on antioxidant activity in vitro of Tartary buckwheat proteins extracted by different methods, J. Anhui Agri. Sci., 2017, 45(25), 134–135 CAS.
- Y. Sun, D. Cai, Q. Xiang, W. W. Yan and Z. S. Zhong, Extraction and characterization of four proteins from wheat bran by Osborne, Food Sci. Technol. Ind., 2015, 36(9), 136–139 CAS.
- Y. Chen, W. Wang, Z. Shen, & D. Yu, The influences of extraction factors of rice bran soluble proteins, MFST, 2006, vol. 4, pp. 64–66 Search PubMed.
- F. Akharume, D. Santra and A. Adedeji, Physicochemical and functional properties of proso millet storage protein fractions, Food Hydrocolloids, 2020, 108, 105497 CrossRef CAS.
- F. N. Abd Rahim, W. Z. Wan Ibadullah, N. Saari, F. H. Brishti, N. A. Mustapha and N. Ahmad, et al., The effect of alkaline extraction and drying techniques on the physicochemical, structural properties and functionality of rice bran protein concentrates, Int. J. Biol. Macromol., 2023, 242, 124908 CrossRef CAS PubMed.
- S. R. Garcia, J. C. Orellana-Palacios, D. J. McClements, A. Moreno and M. Hadidi, Sustainable proteins from wine industrial by-product: Ultrasound-assisted extraction, fractionation, and characterization, Food Chem., 2024, 455, 139743 CrossRef CAS PubMed.
- S. Deb, Y. Kumar and D. C. Saxena, Functional, thermal and structural properties of fractionated protein from waste banana peel, Food Chem.:X, 2022, 13, 100205 CAS.
- J. Xue, M. Wang, Z. Yan, Y. Wang and Z. Chen, Extraction of grape seed protein components and subunit analysis, Food Ind., 2019, 40(3), 162–165 Search PubMed.
- K. K. Kumar and K. P. Parameswaran, Characterisation of storage protein from selected varieties of foxtail millet, J. Sci. Food Agric., 1998, 77, 535–542 CrossRef CAS.
- A. Singh, Arabinoxylan from pearl millet bran: Optimized extraction, structural characterization, and its bioactivities, Int. J. Biol. Macromol., 2024, 279, 135247 CrossRef CAS PubMed.
- L. M. Sang, C. Wang, Q. Y. Zhao, X. M. Diao, X. R. Wang and Q. Shen, Effect of different processing degree and processes on foxtail millet nutritional quality, J. Chin. Cereal. Oil., 2025, 41(2), 001071 Search PubMed.
- X. Y. Yu, Y. Zou, Q. W. Zheng, F. X. Lu, D. H. Li and L. Q. Guo, et al., Physicochemical, functional and structural properties of the major protein fractions extracted from Cordyceps militaris fruit body, Food Res. Int., 2021, 142, 110211 CrossRef CAS PubMed.
- Y. Deng, L. Huang, C. Zhang, P. Xie, J. Cheng and X. Wang, et al., Physicochemical and functional properties of Chinese quince seed protein isolate, Food Chem., 2019, 283, 539–548 CrossRef CAS PubMed.
- P. W. Xu, X. F. Yuan and B. Zhao, A study on pH-mediated variations and differences in the structure and function of hemp seed albumin, globulin, and protein isolate, Food Biosci., 2023, 56, 103452 CrossRef CAS.
- P. V. Monteiro, T. K. Virupaksha and D. R. Rao, Proteins of Italian millet: Amino acid composition, solubility fractionation and electrophoresis of protein fractions, J. Sci. Food Agric., 1982, 33, 1072–1079 CrossRef CAS PubMed.
- M. J. Liu, F. F. Shi and Q. A. Zhang, Evaluation of the ultrasonically accelerated debitterizing with citric acid solutions of different pH: On the basis of amino acids changes in apricot kernels during debitterizing, J. Food Process. Preserv., 2021, 45(5), e15403 CAS.
- L. Li, L. N. Jin, P. P. Guo, D. H. Liu and J. Fu, Physicochemical properties, functional characteristics, and structure of selenium enriched millet protein, Food Ferment. Ind., 2024, 50(3), 259–267 Search PubMed.
- S. Momen, F. Alavi and M. Aider, Alkali-mediated treatments for extraction and functional modification of proteins: Critical and application review, Trends Food Sci. Technol., 2021, 110, 778–797 CrossRef CAS.
- R. Afkhami, M. J. Varidi, M. Varidi and F. Hadizadeh, Boosting emulsion properties: The role of β-sheet content and fibril length in soy protein isolate emulsions, Food Hydrocolloids, 2024, 149, 109513 CrossRef CAS.
- F. F. Liu, Y. Q. Li, C. Y. Wang, X. Z. Zhao, Y. Liang and J. X. He, et al., Impact of pH on the physicochemical and rheological properties of mung bean (Vigna radiata L.) protein, Process Biochem., 2021, 111, 274–284 CrossRef CAS.
- S. Benelhadj, S. Douiri, A. Ghouilli, R. B. Hassen, S. M. A. S. Keshk and A. El-kott, et al., Extraction of Arthrospira platensis (Spirulina) proteins via Osborne sequential procedure: Structural and functional characterizations, J. Food Compos. Anal., 2023, 115, 104984 CrossRef CAS.
- L. S. Sciarini, F. Maldonado, P. D. Ribotta, G. T. Perez and A. E. Leon, Chemical composition and functional properties of Gleditsia triacanthos gum, Food Hydrocolloids, 2013, 32(1), 211 CrossRef CAS.
- C. Li, J. Yang, L. Yao, F. Qin, G. Hou and B. Chen, et al., Characterisation, physicochemical and functional properties of protein isolates from Amygdalus pedunculata Pall seeds, Food Chem., 2020, 311, 125888 CrossRef CAS PubMed.
- C. Wang, L. Jiang, D. Wei, Y. Li, X. Sui and Z. Wang, et al., Effect of Secondary Structure determined by FTIR Spectra on Surface Hydrophobicity of Soybean Protein Isolate, Procedia Eng., 2011, 15, 4819–4827 CrossRef CAS.
- F. Alavi, L. Chen, Z. Wang and Z. Emam-Djomeh, Consequences of heating under alkaline pH alone or in the presence of maltodextrin on solubility, emulsifying and foaming properties of faba bean protein, Food Hydrocolloids, 2021, 112, 106335 CrossRef CAS.
- J. S. Yang, A sequential fractionation approach to understanding the physicochemical and functional properties of aqueous and enzyme-assisted aqueous extracted black bean proteins, Food Hydrocolloids, 2024, 146, 109250 CrossRef CAS.
- A. A. Scilingo and M. C. Anon, Calorimetric study of soybean protein isolates: Effect of calcium and thermal treatments, J. Agric. Food Chem., 1996, 44(12), 3751–3756 CrossRef CAS.
- L. D. Guo, L. Xu, C. Z. Ou, Y. Y. Ding, G. P. Zhang and C. L. Ni, et al., Molecular compositions and structural properties of proteins in millet, Food Sci., 2019, 40(24), 201–206 Search PubMed.
- K. Sanjaya, P. Wai and A. Anil Kumar, Microwave-assisted protein extraction from foxtail millet: Optimization, structural characterization, techno-functional properties, and bioactivity of peptides, Int. J. Biol. Macromol., 2025, 293, 139312 CrossRef PubMed.
- F. Tounkara, T. Amza, C. Lagnika, G. Le and Y. Shi, Extraction, characterization, nutritional and functional properties of Roselle (Hibiscus sabdariffa Linn) seed proteins, Songklanakarin J. Sci. Technol., 2013, 35(2), 159–166 CAS.
- M. H. Kamani, C. Neji, S. M. Fitzsimons, M. A. Fenelon and E. G. Murphy, Unlocking the nutritional and functional potential of legume waste to produce protein ingredients, Crit. Rev. Food Sci. Nutr., 2024, 64(21), 7311–7329 CrossRef CAS PubMed.
- A. Kheto, R. Sehrawat, K. Gul and L. Kumar, Effect of extraction pH on amino acids, nutritional, in-vitro protein digestibility, intermolecular interactions, and functional properties of guar germ proteins, Food Chem., 2024, 444, 138628 CrossRef CAS PubMed.
- Y. Zhang, W. Zheng, P. Zou and W. Chen, Study on the structure and physicochemical properties of olive kernel protein extracted by Osborne method, Cereals Oilseeds, 2022, 35(5), 129–133 Search PubMed.
- Y. Shen, X. Tang and Y. Li, Drying methods affect physicochemical and functional properties of quinoa protein isolate, Food Chem., 2021, 339, 127823 CrossRef CAS PubMed.
- W. Qi, J. W. Qiao, Y. Q. Li, S. J. Chen, P. Yang and L. Z. Zhang, Effect of extrusion treatment on the structural and functional properties of enzymatic hydrolysis of foxtail millet flour protein, Sci. Technol. Food Ind., 2023, 44(17), 59–67 Search PubMed.
- Z. Zhu, W. Zhu, J. Yi, N. Liu, Y. Cao and J. Lu, et al., Effects of sonication on the physicochemical and functional properties of walnut protein isolate, Food Res. Int., 2018, 106, 853–861 CrossRef CAS PubMed.
- I. Othmeni, R. Karoui and C. Blecker, Impact of pH on the structure, interfacial and foaming properties of pea protein isolate: Investigation of the structure–function relationship, Int. J. Biol. Macromol., 2024, 278, 134818 CrossRef CAS PubMed.
- L. Cui, N. Bandillo, Y. Wang, J. B. Ohm, B. Chen and J. Rao, Functionality and structure of yellow pea protein isolate as affected by cultivars and extraction pH, Food Hydrocolloids, 2020, 108, 106008 CrossRef CAS.
- J. F. Zayas, Functionality of Proteins in Food [Internet], Springer Berlin Heidelberg, Berlin, Heidelberg, 1997, Available from: http://link.springer.com/10.1007/978-3-642-59116-7 Search PubMed.
- T. Kavita, S. Rajan, K. K. Sunil, B. Hanuman, S. Baljit and S. Savita, Isolation and ultrasonication of pearl millet protein: Effect on techno-functional, structural, molecular interaction, and rheological properties, Food Res. Int., 2025, 201, 115583 CrossRef PubMed.
- J. T. Xu, S. S. Liu, H. Liu and S. T. Guo, Nutrition and digestion study of millet protein, J. Food Sci. Technol., 2014, 32(4), 15–20 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5fb00017c |
| This journal is © The Royal Society of Chemistry 2025 |