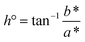Open Access Article
Open Access ArticleSustainable valorization of tropical fruit peels for sustainable production of natural antioxidants and functional food ingredients†
Panorjit
Nitisuk
a,
Pitchaporn
Wanyo
 *a,
Tossaporn
Chamsai
b and
Kiatipong
Charoenjit
a
*a,
Tossaporn
Chamsai
b and
Kiatipong
Charoenjit
a
aDepartment of Food Technology, Faculty of Agricultural Technology, Kalasin University, Kalasin 46000, Thailand. E-mail: pitchaporn.wa@ksu.ac.th
bDepartment of Mechanical Engineering, Faculty of Agriculture and Technology, Rajamangala University of Technology Isan, Surin Campus, Surin 32000, Thailand
First published on 13th June 2025
Abstract
Tropical fruit peels, frequently discarded during agricultural and food processing, represent an underutilized source of natural antioxidants and bioactive compounds. This study aimed to valorize tropical fruit peels, namely mango, banana, dragon fruit, pineapple, and papaya, by transforming them into functional food ingredients. Ethanol-based ultrasound-assisted extraction was applied to enhance the bioactive compounds and their antioxidant potentials. The variation in physicochemical characteristics, bioactive components, and antioxidant capacity of various fruit peel powders was investigated. 2,2-Diphenyl-1-picrylhydrazyl (DPPH), ferric-reducing antioxidant power (FRAP), and total antioxidant capacity assays were used to evaluate the antioxidant activities. The qualitative method was used for the phytochemical screening. Additionally, reverse-phase high-performance liquid chromatography (RP-HPLC) analysis reveals their phenolic compositions. Then, the selected high-potential components were incorporated into biscuits. The digestion method was conducted following the INFOGEST protocol. Comparing all tested powders, the mango peel powder (MPP) had the highest total phenolic content (TPC) with 69.81 mg GAE per g and the highest antioxidant activity, which corresponded with the presence of gallic acid (13.95 mg g−1), chlorogenic acid (9.62 mg g−1), and quercetin (11.59 mg g−1). The incorporation of MPP (5–15%) into biscuits improved nutritional composition and antioxidant potential while maintaining acceptable sensory characteristics at lower inclusion levels. Simulated in vitro digestion confirmed the retention and bioaccessibility of phenolics and flavonoids, indicating their functional relevance. This study addresses a gap in bioaccessibility and sensory acceptability of tropical fruit peel-fortified functional foods. This valorization framework also adopts circular economy principles, using residual biomass for composting. The findings demonstrate a green, scalable approach to transform fruit peel waste into health-promoting, functional ingredients aligned with sustainable food production.
Sustainability spotlightThe work described in this manuscript highlights the sustainable valorization of tropical fruit peels, and use of agricultural by-products as functional food ingredients using green processes. This approach is facilitating the circular economy by converting waste materials to high-value products and thus to reducing food waste and environmental impact. Furthermore, the present and potential utilization of natural antioxidants and bioactive compounds from fruit peels definitely belong to UN Sustainable Development Goals (SDGs) 12 (Responsible Consumption and Production) and 3 (Good Health and Well-being). In this contribution to the development of environmentally benign replacements for food additives, this work supports the development of sustainable food and environmental systems, and supports development of novel alleviating measures to global health and environmental challenges. |
1 Introduction
Global agricultural industries generate a significant amount of organic waste, a considerable portion of which originates from fruit processing. Tropical fruit peels—including those from mango, banana, papaya, pineapple, and dragon fruit—are frequently disposed of as by-products, despite their rich composition of polyphenols, flavonoids, carotenoids, and dietary fiber. These bioactive constituents exhibit potent antioxidant, antimicrobial, and anti-inflammatory properties,1 positioning them as valuable ingredients for functional foods and nutraceutical applications. Recent research has focused on the potential to repurpose fruit peels into value-added products. However, much of this work has been fragmented, often targeting either extraction of specific compounds or limited food applications, without offering a comprehensive sustainability-focused approach. Marquez Molina et al.2 evaluated the physicochemical properties of tropical fruit peel powders for their potential as functional ingredients. Similarly, Campos et al.3 explored green valorization of pineapple by-products to develop added-value ingredients, while Marcillo-Parra et al.4 and Suleria et al.5 reported high concentrations of phenolic compounds and antioxidant activity in mango and various fruit peels, respectively. Despite these advances, few studies bridge the gap between extraction and practical implementation, such as food product development and bioavailability analysis. Additionally, Imeneo et al.6 and Benmeziane-Derradji et al.7 demonstrated that incorporating fruit by-products (e.g., lemon pomace or apricot puree) into biscuits enhances both nutritional and sensory attributes, further supporting the application potential of fruit peel-derived ingredients.As pointed out by Villacís-Chiriboga et al. and Pathak et al., sustainable valorization requires low-energy, non-toxic extraction methods and downstream applications that promote waste minimization and value recovery.8,9 Applications of fruit-peel derived materials to food products offer the potential not only to enhance the nutritional quality of some foods but also to enhance sustainability more broadly.10,11 Mango peel, in particular, has emerged as a promising candidate due to its high phenolic content—especially gallic acid and quercetin—which contributes to strong antioxidant activity.2,12 Nevertheless, limited research has evaluated mango peel in food matrices with simulated gastrointestinal digestion, especially in functional prototypes such as baked products.
Furthermore, food processing is the pillar of our food system and food components can retain some of their properties of being changed or altered, leading to consequential influences in terms of health and nutrients.13,14 In addition, the bioaccessibility of all components of the dietary matrix is not guaranteed.15–17 Then, functional properties of some antioxidants can be altered by gastrointestinal tract digestion. Therefore, a comprehensive approach evaluating both bioaccessibility and stability via in vitro digestion models is essential to determine their efficacy in human health applications. This study addresses these gaps by: (1) applying ultrasound-assisted ethanol extraction as a green approach to recover bioactives from five tropical fruit peels; (2) comparing the antioxidant capacity and phytochemical profile across samples; (3) integrating the most potent peel (mango) into biscuits and evaluating sensory characteristics; and (4) assessing bioactive retention and antioxidant activity before and after in vitro digestion. The goal is to develop a sustainable, scalable model for valorizing tropical fruit peel waste into functional food ingredients, with added emphasis on antioxidant stability and consumer relevance.
2 Materials and methods
2.1 Chemicals and analysis instruments
Standard phenolic acids (e.g., gallic acid, vanillic acid, and ferulic acid) and flavonoids (e.g., rutin and quercetin) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Other reagents and enzymes, such as α-amylase, pepsin, pancreatin, and lipase, were also from Sigma-Aldrich. Other analytical chemicals were used, while HPLC-grade acetonitrile was used for HPLC analysis. Absorbance was measured using a Biochrom Libra S22 UV/Visible spectrophotometer. Reverse Phase High-Performance Liquid Chromatography (RP-HPLC) was conducted using Shimadzu LC-20AC pumps and SPD-M20A diode array detection on an Inertsil ODS-3 C18 column (4.6 mm × 250 mm, 5 μm) from Hichrom Limited, UK.2.2 Fruit materials and preparation of fruit peel powders
Five regionally-grown fruits including green (unripe) banana (Musa sapientum L.), red-fleshed dragon fruit (Selenicereus costaricensis), green (unripe) mango (Mangifera indica L.), pineapple (Ananas comosus), and green (unripe) papaya (Carica papaya L.) were purchased from a local produce market in Kalasin, Thailand. Peels were manually separated, washed, and cut into 1 × 1 cm pieces. Tray drying was performed at 50 °C for 6–18 h until the moisture content (wet basis) fell below 10%. The dried peels were milled and sieved (300 μm), and yield (%) was calculated as the weight of peel powder relative to the fresh peel. Powders were stored in airtight polyethylene bags at −20 °C until further analysis.2.3 Determination of physicochemical properties
The moisture content of the fruit peel powders was determined gravimetrically using AOAC Official Method 925.10.17 The drying rate (% h−1) was calculated from the moisture of materials at two consecutive times divided by the period.Colour properties (L*, a*, b*) of peel powders were assessed using a colorimeter (Ultra Scan Pro, Hunter Lab, Germany), against a white calibration standard. Mean values from 10 readings per sample were recorded. Chroma (C*) and hue angle (h°) were computed from a* and b* using:
2.4 Extraction of fruit peel powders
Fruit peel powders (1 g) were extracted using 90% ethanol (10 mL), following the method of Monteiro et al.18 The mixture was sonicated for 10 min in an ultrasonic bath (Powersonic 405, frequency: 40 kHz, power: 400 W) at ambient temperature, ensuring the temperature remained below 40 °C. This method was chosen for its efficiency and alignment with green chemistry principles. Ethanol, recognized for its low toxicity and biodegradability, is suitable for extracting a broad spectrum of phytochemicals. Importantly, this extraction technique is not limited to phenolic compounds but has also been validated for the effective recovery of carotenoids from plant materials, including fruit peels, due to ethanol's intermediate polarity.3 After sonication, the mixture was centrifuged at 5600×g and 25 °C for 10 min. The supernatant was transferred into a 30 mL vial. This procedure was performed in triplicate. These extracts were used for bioactive compounds and antioxidant activity assessments.2.5 Determination of bioactive compounds
Bioactive substances of fruit peel powders were evaluated in terms of total phenolic content (TPC), total flavonoid content (TFC) and total carotenoid content (TCC). The TPC and TFC were calculated using the procedure of Wanyo et al.19 For TPC absorbance was measured at 725 nm and reported in milligrams of gallic acid equivalents per gram (mg GAE per g) of dry weight. For TFC, the absorbance was measured at 510 nm and results were reported as milligrams of quercetin equivalents per gram (mg QE per g) dry weight. TCC was calculated on the basis of the method of Marcillo-Parra et al., absorbance at 450 nm was measured, and measurements were reported as micrograms of β-carotene equivalents per gram (μg BCE per g) of dry weight.4The mobile phase for the individual phenolic acids and flavonoids of fruit peel powders was made up of purified water with acetic acid (pH 2.74) (solvent A) and acetonitrile (solvent B) flowing at a rate of 0.8 mL min−1. Previous descriptions of the gradient elution settings were provided by Wanyo et al.19 Operating parameters included a 20 μL injection volume, a column temperature of 38 °C, and UV-diode array detection at 280 nm for hydroxybenzoic acids, 320 nm for hydroxycinnamic acids, and 370 nm for flavonoids. An external standard approach for detection was used to identify the phenolic compounds in the samples by comparing their retention periods and UV spectra to those of standard compounds. Chromatograms for phenolic acids and flavonoids are provided in ESI (Fig. S1).† Milligrams per gram (mg g−1) dry weight was the unit of measurement used to express the results.
2.6 Determination of antioxidant activity
Fruit peel powders were tested for antioxidant activity utilizing total antioxidant capacity (TAC), ferric-reducing antioxidant power (FRAP) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity assays. The DPPH and FRAP assays were described by Wanyo et al.19 In the context of DPPH analysis, absorbance readings were taken at 517 nm and the percent inhibition activity was computed using the formula [(Ao − AS)/Ao] × 100 (where Ao represents absorbance without the sample and AS denotes absorbance with the sample). Meanwhile, the FRAP assay required absorbance measurement at 593 nm, and the resultant FRAP value was expressed in μM of ferrous sulfate per gram (μM FeSO4 per g) of dry weight. Furthermore, the TAC assay, which quantifies the reduction of Mo(VI) to Mo(V), entails the interaction of the extract with a reagent solution; this process ultimately yields a green-colored phosphate/Mo(V) complex under acidic conditions.20 Milligrams of ascorbic acid equivalents per gram (mg AAE per g) of dry weight was the unit of measurement for the absorbance in this instance, which was measured at 695 nm.2.7 Application of mango peel powder in biscuits
Among the evaluated tropical fruit peels, mango peel powder (MPP) exhibited the highest levels of TPC, TFC, and antioxidant activities (DPPH, FRAP, and TAC), making it a suitable candidate for food application. Moreover, mango peel has favorable sensory characteristics and a mild flavor profile, which support its incorporation into bakery products without compromising consumer acceptability. These properties, alongside its previously demonstrated bioaccessibility in digestion studies, justify its selection for functional biscuit formulation in this study.In accordance with the established procedure of the American Association of Cereal Chemists,21 biscuit samples were prepared from doughs that contained 5.0, 7.5, 10.0, and 15.0% of MPP as replacing levels for wheat flour. 100 g of wheat flour, 38 g of powdered sugar, 20 g of butter, 7 g of skim milk powder, 34 g of egg, 0.5 g of salt, and 0.5 g of baking powder were the ingredients utilized. A smooth paste was created by creaming butter, egg, and powdered sugar. Next, wheat flour or mixed flour, milk powder, baking powder, and salt were added. After fully mixing, the dough was given a brief rest. After being sheeted to a thickness of 0.5 mm using a stainless-steel roller, the dough was cut out using a 50 mm diameter dough cutter. 20 min of baking at 170 °C, followed by cooling, packing, and room temperature storage.
Sensory tests were carried out in a sensory laboratory, Department of Food Technology, Kalasin University. Fifty untrained panelists—25 men and 25 women—who were knowledgeable about baked products' attributes evaluated the biscuit samples' sensory characteristics. They scored the various samples' sensory quality attributes. Each sample was blind-coded with random three-digit numbers and presented in a randomized order under controlled lighting and temperature. A glass of plain water was served to participants for mouth cleaning before and between samples evaluation. A 9-point hedonic scale was used to score the biscuit samples' appearance, color, odor, texture, taste and overall acceptability (1–9 being the lowest and highest acceptance, respectively). The obtained results were presented as means with standard deviations. According to national research ethics guidelines, no formal ethics approval was required as no sensitive data were collected, and participation was voluntary. Sensory evaluation in this study involved all participants were fully informed on the nature and purpose of the study. Based on their consent, the participants agreed to have their data used for research only, and they were free to withdraw their participation from the study at any time.
Proximate analysis was used to assess the nutritional value of the chosen biscuit samples.17 Based on the energy-producing components (fats, proteins, and carbohydrates); the energy quantity was calculated.
Biscuit samples were extracted according to Monteiro et al.,18 followed by TPC, TFC, TCC and antioxidant activity were determined as described above.
2.8 Simulated in vitro digestions
The in vitro digestion method used was based on INFOGEST, described by Brodkorb et al. with minor modifications.22 It was composed of a three-stage procedure that imitated oral, gastric, and intestinal digestion. Previous descriptions of simulated saliva fluid (SSF), simulated gastric fluid (SGF), and simulation intestinal fluid (SIF) preparation were provided by Wanyo et al.23 The biscuit samples were digested in three steps. The oral phase: biscuits (1 g) were chewed and mixed with 10 mL of SSF (with α-amylase) and the mixtures were subjected to the oral phase for 2 min in a 37 °C shaking water bath at 120 rpm. The gastric phase: the samples subjected to the oral phase were further diluted to pH 2.0 with 5 M HCL and 10 mL of SGF (mixtures were continuously shaken for 2 h in a 37 °C shaking water bath). The intestine phase: the pH of digested samples from the gastric phase was brought to 7.0 with 1 M NaOH and 9 mL of SIF containing pancreatin and bile salt was added. Then 1.5 mL of NaCl solution and 1.5 mL of KCl solution were added, and all the mixtures were transferred to a shaking water bath set at 37 °C for 2 h. The enzymatic reactions were stopped using cold distilled water which had been tempered at a low temperature using ice. Aliquots of the digested products were collected and then centrifuged for 10 min at 2500 rpm. The supernatants were then spun down and the liquid was stored at −20 °C till further use. These simulated digestions were carried out in triplicate. These fractions, after intestinal phase digestion, were used for TPC, TFC, TCC, DPPH, FRAP, and TAC analysis.2.9 Statistical analyses
All measurements and calculations were done at least in triplicate, and the data obtained were presented as the mean standard deviation. The statistical software, IBM SPSS Version 19, was used for all calculations. Statistical significance was determined using ANOVA in a Completely Randomized Design, and the means were compared using Duncan's Multiple Range Test. The following are the results of the T-tests used in performing pairwise comparisons. The variations in the sensory scores of the biscuits with MPP were evaluated using Principal Component Analysis (PCA). All statistical parameters were calculated at p < 0.05 significance level.3 Results and discussion
3.1 Characterization of dried tropical fruit peel powders
The physicochemical properties (moisture content, drying rate, yield, and color values) of dried tropical fruit peel powder from banana, dragon fruit, mango, pineapple and papaya are shown in Table 1. Moisture content across all samples ranged from 6.70% to 7.29%, indicating no significant variation. These moisture content values are consistent with standard drying protocols for fruit peel powders that are intended to uphold product stability.24 Drying rate, calculated as percent moisture loss per hour, varied significantly (p < 0.05) among samples. Mango peel powder (MPP) showed the highest drying rate (11.05% h−1), while banana peel powder (BPP) recorded the lowest (4.46% h−1). The drying rate reflects water diffusivity and process efficiency—a critical factor in energy use and thermal stability of phytochemicals. This trend is consistent with structural compositions of mango peels, which are fibrous and contain low lipid content, facilitating rapid dehydration.25,26 Yield, defined as the percentage of dry powder obtained from fresh peel, was highest for MPP (34.15%), followed by pineapple peel powder (PAPP, 25.32%). Dragon fruit peel powder (DFPP) and papaya peel powder (PPP) exhibited lower yields. Similar findings were reported for pitaya peels, where high water content negatively affected powder yield.27| Parameters | BPP | DFPP | MPP | PAPP | PPP |
|---|---|---|---|---|---|
| a BPP: banana peel powder; DFPP: dragon fruit peel powder; MPP: mango peel powder; PAPP: pineapple peel powder; PPP: papaya peel powder; L*: lightness (white/black); a*: redness (red/green); b*: yellowness (yellow/blue); C*: chroma; h°: hue angle. Values are expressed as means ± standard deviation. Measurements were performed in triplicate (N = 3) for physicochemical properties, and ten replicates (N = 10) for color measurements. Means with different letters in the same columns were significantly different at p < 0.05. NS indicates non-significant. | |||||
| Moisture content (%)NS | 6.70 ± 0.19 | 7.28 ± 0.30 | 6.72 ± 0.24 | 7.29 ± 0.40 | 6.85 ± 0.47 |
| Drying rate (%/h) | 4.46 ± 0.01d | 4.55 ± 0.02c | 11.05 ± 0.04a | 6.27 ± 0.03b | 4.53 ± 0.02c |
| Yield (%) | 20.14 ± 0.10c | 18.54 ± 0.18e | 34.15 ± 0.16a | 25.32 ± 0.11b | 18.82 ± 0.22d |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Color values | |||||
| L* | 69.57 ± 1.34c | 59.94 ± 1.22d | 78.11 ± 1.72a | 55.41 ± 1.02e | 72.68 ± 1.07b |
| a* | −2.58 ± 0.35d | 24.52 ± 0.85a | −4.75 ± 0.67e | 8.34 ± 0.19b | 2.33 ± 0.18c |
| b* | 28.63 ± 0.84b | 4.33 ± 0.40d | 37.16 ± 0.73a | 14.44 ± 0.12c | 37.41 ± 1.65a |
| C* | 28.74 ± 0.81b | 24.90 ± 0.91c | 37.47 ± 0.79a | 16.67 ± 0.17d | 37.48 ± 1.66a |
| h° | 95.17 ± 0.83b | 10.00 ± 0.55e | 97.28 ± 0.88a | 59.99 ± 0.44d | 86.44 ± 0.11c |
Regarding CIELAB color parameters, MPP displayed the highest lightness (L* = 78.11) and lowest a* value (−4.75), indicating a bright, greenish-yellow hue with minimal browning. In contrast, DFPP had the highest a* value (24.52), consistent with its betacyanin pigmentation. The chroma (C*) and hue angle (h°) were also calculated to better describe color saturation and tone. Notably, high L* and C values in MPP suggest minimal thermal degradation, reinforcing its potential as a visually appealing functional powder. Previous reports have shown that high lightness values and low a* are desirable indicators of controlled drying.25 Additionally, the bright coloration of DFPP is consistent with betalain retention in pitaya, which enhances its color attributes.28
Collectively, the low moisture, high yield, and stable color parameters observed in MPP support its suitability as a candidate for incorporation into functional food matrices, particularly where color and stability are essential attributes. These findings contribute to reducing food waste and promoting circular economy practices in the food industry.
3.2 Phytochemical contents and antioxidant activity
The phytochemical content and antioxidant activity of tropical fruit peel powders are summarized in Table 2 and Fig. 1. Among all samples, MPP exhibited the highest total phenolic content (TPC: 69.81 mg GAE per g) and total flavonoid content (TFC: 29.61 mg QE per g), significantly exceeding those of other peels (p < 0.05). BPP and DFPP showed moderate levels, while PPP and pineapple peel powder (PAPP) had comparatively lower TPC and TFC values. The elevated TPC and TFC in MPP align with previous research that indicates mango peels are abundant in phenolic compounds and flavonoids—this is particularly true for gallic acid and quercetin—because of their structural and compositional characteristics.5 The phenolic profile of mango peel underscores its potential for antioxidant and health-promoting activities. In terms of total carotenoid content (TCC), DFPP recorded the highest value (84.91 μg BCE per g), attributed to its betalains and carotenoid-rich red pigmentation. The elevated TCC found in DFPP corresponds with its well-documented pigmentation, which is primarily due to the presence of betalains and carotenoids.25 Pineapple and papaya peels, however, exhibit lower levels of carotenoids, a finding that is consistent with previous research indicating that their peels are less pigmented.25,29| Compounds | BPP | DFPP | MPP | PAPP | PPP |
|---|---|---|---|---|---|
| a BPP: banana peel powder, DFPP: dragon fruit peel powder, MPP: mango peel powder, PAPP: pineapple peel powder, PPP: papaya peel powder, TPC: total phenolic content, TFC: total flavonoid content, TCC: total carotenoid content, GA: gallic acid, PCCA: protocatechuic acid, ρ-OH: ρ-hydroxybenzoic acid, VA: vanillic acid, SyA: syringic acid, ChA: chlorogenic acid, CFA: caffeic acid, ρ-CA: ρ-coumaric acid, FA: ferulic acid, SNA: sinapic acid, and nd: not detected. Values are expressed as means ± standard deviation (N = 3). Means with different letters in the same rows were significantly different at p < 0.05. | |||||
| TPC (mg GAE per g) | 20.95 ± 0.12b | 19.79 ± 0.25b | 69.81 ± 1.93a | 15.57 ± 0.63c | 13.85 ± 0.39d |
| TFC (mg QE per g) | 15.86 ± 0.21c | 16.95 ± 0.17b | 29.61 ± 0.92a | 12.82 ± 0.27d | 8.92 ± 0.19e |
| TCC (μg BCE per g) | 19.52 ± 0.63c | 84.91 ± 3.80a | 28.84 ± 1.07b | 4.48 ± 0.14d | 2.74 ± 0.12d |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Phenolic acids (mg g− 1 ) | |||||
| GA | 1.41 ± 0.04 cd | 1.08 ± 0.07d | 13.95 ± 0.42a | 1.51 ± 0.04c | 2.29 ± 0.06b |
| PCCA | 0.44 ± 0.03c | nd | 2.48 ± 0.04a | 2.18 ± 0.03b | 0.43 ± 0.02c |
| ρ-OH | 4.93 ± 0.08b | 1.81 ± 0.05d | 8.13 ± 0.16a | 1.23 ± 0.04e | 2.94 ± 0.09c |
| VA | 0.19 ± 0.02b | nd | 0.50 ± 0.02a | nd | nd |
| SyA | 3.30 ± 0.04c | 3.38 ± 0.06b | 7.96 ± 0.05a | 1.00 ± 0.03e | 2.50 ± 0.04d |
| ChA | 4.51 ± 0.04b | 3.99 ± 0.06c | 9.62 ± 0.03a | 0.68 ± 0.03d | 0.03 ± 0.01e |
| CFA | 0.18 ± 0.02c | nd | 3.39 ± 0.05a | 1.01 ± 0.03b | nd |
| ρ-CA | nd | 2.86 ± 0.05c | 4.12 ± 0.04a | 2.97 ± 0.06b | 0.45 ± 0.03d |
| FA | 1.51 ± 0.04c | 2.92 ± 0.09b | 4.78 ± 0.05a | 0.18 ± 0.02c | 1.52 ± 0.03d |
| SNA | nd | nd | 2.95 ± 0.08a | 2.00 ± 0.03b | 0.41 ± 0.03c |
| Total phenolic acids | 16.47 ± 0.19b | 16.04 ± 0.17c | 57.89 ± 0.44a | 12.76 ± 0.08d | 10.56 ± 0.09e |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Flavonoids (mg g− 1 ) | |||||
| Catechin | 1.95 ± 0.07c | 7.75 ± 0.09a | 6.97 ± 0.06b | 0.62 ± 0.04d | 0.04 ± 0.01e |
| Rutin | 8.45 ± 0.08a | nd | 0.28 ± 0.01b | nd | 0.04 ± 0.00c |
| Myricetin | 0.11 ± 0.03b | nd | nd | 0.03 ± 0.01c | 0.36 ± 0.05a |
| Quercetin | 4.84 ± 0.06b | 4.97 ± 0.14b | 11.59 ± 0.07a | 4.00 ± 0.11c | 4.91 ± 0.10b |
| Kaempferol | nd | 3.46 ± 0.05c | 9.42 ± 0.08a | 7.02 ± 0.10b | 3.29 ± 0.06d |
| Total flavonoids | 15.36 ± 0.09c | 16.19 ± 0.20b | 28.26 ± 0.05a | 11.66 ± 0.09d | 8.63 ± 0.12e |
Ten phenolic acids and five flavonoids were quantified using RP-HPLC (Table 2). Phenolic acids, five were classified as hydroxybenzoic acids (namely, gallic, protocatechuic, ρ-hydroxybenzoic, vanillic and syringic) and the other five were hydroxycinnamic acids (including chlorogenic, caffeic, ρ-coumaric, ferulic and sinapic). The findings revealed that both the content and composition of phenolic acids varied significantly across the analyzed samples. Notably, MPP exhibited the most varied phenolic acid profile, showcasing high concentrations of gallic acid (13.95 mg g−1) and chlorogenic acid (9.62 mg g−1). On the other hand, DFPP contained remarkable levels of ferulic acid (2.92 mg g−1), whereas BPP was characterized by elevated amounts of syringic acid (3.30 mg g−1) and chlorogenic acid (4.51 mg g−1). Additionally, the high concentrations of gallic acid, chlorogenic acid and ferulic acid found in mango peels further support studies that identify mango peel as a valuable source of these bioactive compounds.30
Five flavonoids (catechin, rutin, myricetin, quercetin and kaempferol) were significantly (p < 0.05) among samples. MPP exhibited significant levels of quercetin (11.59 mg g−1) and kaempferol (9.42 mg g−1). DFPP was notable for its catechin content (7.75 mg g−1), although BPP contained moderate amounts of rutin (8.45 mg g−1). The elevated levels of quercetin and kaempferol found in mango peels are supported by findings indicating that flavonoids in fruit peels play a crucial role in antioxidant defense mechanisms.31
DFPP's catechin content underscores its potential as a functional ingredient, because of its antioxidant properties. However, the specific contributions of each flavonoid require further investigation to fully understand their health benefits.
HPLC chromatograms for major phenolic acids and flavonoids are presented in Fig. S1 (ESI),† confirming compound identities based on retention time and UV spectra compared with standards. These results indicate distinct polyphenolic profiles among fruit peel types, with MPP possessing a diversified phenolic matrix beneficial for antioxidant function.
Antioxidant capacity was assessed using DPPH radical scavenging activity, FRAP (ferric-reducing antioxidant power), and TAC (total antioxidant capacity) assays (Fig. 1). Overall, the results for antioxidant activities assessed across five tropical fruit peel powders indicated that MPP had the highest values. DPPH inhibition ranged from 54.70% (PPP) to 93.30% (MPP), reflecting scavenging efficiency. FRAP values ranged from 119.67 to 591.63 μM FeSO4 per g, highest in MPP due to strong electron-donating polyphenols. TAC, expressed as mg AAE per g, followed the same trend: MPP > BPP > DFPP > PAPP ≈ PPP.
The high antioxidant activities of MPP were closely associated with its phenolic composition—particularly gallic acid, chlorogenic acid and quercetin. These compounds are known to stabilize free radicals and reduce oxidative stress.12,32,33 In contrast, the lower antioxidant activity in PPP and PAPP may result from limited phenolic and flavonoid diversity. Notably, DFPP showed strong DPPH and FRAP responses despite moderate TPC, likely due to contributions from betalains and carotenoids.10,34 Literature review shows that phenolics have a strong relationship with TAC especially in the tropical fruit peels.5 Interestingly, similar rankings of antioxidant activity have been reported for various fruit peels, with mango peel consistently surpassing others regarding DPPH and FRAP values.35 In contrast, studies focusing on pineapple and papaya peels reveal diminished antioxidant activity, which correlates with their comparatively low phenolic levels.2 Overall, the results confirm strong correlations between polyphenolic composition and antioxidant capacity, highlighting MPP's superior potential as a functional food antioxidant source.
3.3 Application of mango peel powder in biscuits
Mango peel powder (MPP) was used to substitute wheat flour in biscuit products to increase the bioactivity and add value to tropical fruit by-products. The sensory properties of biscuits fortified with MPP at different inclusion levels (0%, 5%, 7.5%, 10%, and 15%) were evaluated using a 9-point hedonic scale. The radar plot (Fig. 2) was created to provide a comprehensive comparison of all sensory parameters. There was a noticeable decline in sensory attribute scores as the level of MPP increased. The plot illustrates that the 5% MPP biscuit exhibited a sensory profile closely matching the control, especially in terms of color, taste, and overall acceptability. The 7.5% MPP biscuit showed moderate reductions in scores but maintained acceptable sensory integrity. Beyond 10% MPP, all attributes declined markedly, confirming that high fortification levels compromise consumer preference.Table 3 provides quantitative evidence that corroborates the visual findings; significant differences (p < 0.05) were observed across all sensory attributes—appearance, color, odor, texture, taste, and overall acceptability—as the proportion of MPP increased. Biscuits with 0% (control) and 5% MPP showed the highest appearance (7.90 and 7.82, respectively) and color scores (8.02 and 7.93), with no significant difference between them. However, higher substitution levels (10–15%) significantly decreased these scores, likely due to the darker hue and particulate structure introduced by MPP. There were no significant differences in odor and texture between the control and 5% MPP biscuits, but a notable decline was observed at 10% and 15% levels. This may be due to the fibrous content and phenolic compounds in MPP contributing to an earthier odor and denser texture. The taste score was highest in the control (8.08) and 5% MPP (8.02), with statistically similar values. A progressive decline in taste was observed as MPP increased, reaching 6.68 in the 15% MPP group. The lower taste acceptability at higher levels could be attributed to the bitterness and astringency of phenolic compounds such as gallic acid and quercetin. Control and 5% MPP biscuits received the highest overall acceptability scores (7.82 and 7.75, respectively), showing no significant difference. Acceptability decreased with increasing MPP content, with 15% MPP scoring significantly lower (6.57). These findings align with prior research emphasizing the trade-off between functional ingredient incorporation and sensory quality.6,36,37
| Sensory attributes | Score | ||||
|---|---|---|---|---|---|
| Control | 5% MPP | 7.5% MPP | 10% MPP | 15% MPP | |
| a MPP: mango peel powder. Values are expressed as means ± standard deviation (N = 50). Means with different letters in the same rows were significantly different at p < 0.05. | |||||
| Appearance | 7.90 ± 0.28a | 7.82 ± 0.21 ab | 7.41 ± 0.15bc | 7.10 ± 0.23 cd | 6.80 ± 0.29d |
| Color | 8.02 ± 0.21a | 7.93 ± 0.15a | 7.48 ± 0.27b | 6.80 ± 0.23c | 6.35 ± 0.18d |
| Odor | 7.86 ± 0.23a | 7.73 ± 0.28a | 7.27 ± 0.25 ab | 7.07 ± 0.19bc | 6.78 ± 0.26c |
| Texture | 7.58 ± 0.22a | 7.60 ± 0.25a | 7.08 ± 0.23b | 6.85 ± 0.30bc | 6.51 ± 0.20c |
| Taste | 8.08 ± 0.19a | 8.02 ± 0.20a | 7.62 ± 0.23b | 6.95 ± 0.29c | 6.68 ± 0.16c |
| Overall acceptability | 7.82 ± 0.30a | 7.75 ± 0.26a | 7.22 ± 0.20b | 6.85 ± 0.26bc | 6.57 ± 0.20c |
Principal Component Analysis (PCA) was performed to visualize the correlation and variation among sensory attributes across samples. The first two principal components (PCs) captured the majority of variation in sensory data, which is consistent with findings in similar studies on fruit- and vegetable-fortified biscuits.38 In our analysis, the PCA biplot (Fig. 3) identified taste and color as the primary drivers of sensory differentiation (PC1 and PC2), collectively explaining the majority of variance. The tight clustering of control and 5% MPP samples (p < 0.05) confirmed their sensory equivalence, while ≥10% MPP formulations exhibited pronounced divergence due to lower texture, taste, and color scores. This aligns with studies on other fortified baked products, where ≤5% fruit and vegetable byproducts (e.g., apricot kernel flour, dragon fruit powder, citrus lemon pomace) maintained acceptability.7,39 The negative correlation of higher MPP levels (7.5–15%) with key attributes reflects polyphenol-induced bitterness and darkening—a trend also observed in biscuits fortified with date seed or peanut shell fibers.40 PCA statistically validates 5% MPP as the optimal level, beyond which sensory quality declines significantly—mirroring findings in chickpea or soy protein isolate fortified biscuits.41 Declining acceptability at higher concentrations is linked to bioactive compounds (e.g., polyphenols) in mango peel, informing strategies to mitigate bitterness or color changes in future formulations. Nutritional composition, antioxidant properties as well as in vitro digestion of biscuits incorporated with 5% MPP and control biscuits (0% MPP) were then compared.
 | ||
| Fig. 3 Principal component analysis (PCA) biplot showing the variation of sensory attribute scores among the biscuits with mango peel powder (MPP). | ||
Nutritional composition (Table 4) showed that biscuits with MPP had significantly higher fiber (2.19 g per 100 g) and ash content (1.45 g per 100 g) compared to control biscuits (1.42 and 1.16 g per 100 g, respectively; p < 0.05). Moisture also increased slightly due to the hydrophilic nature of dietary fiber. Protein and fat contents were not significantly different (p > 0.05). The total energy value decreased in MPP biscuits (390.16 kcal per 100 g) compared to control (397.18 kcal per 100 g), attributable to lower carbohydrate density and fiber substitution.
| Parameters | Control biscuit | Biscuit with MPP | p |
|---|---|---|---|
| a MPP: mango peel powder. Values are expressed as means ± standard deviation. Values are expressed as means ± standard deviation (N = 3). The data were compared with an independent t-test (p < 0.05). | |||
| Moisture (g per 100 g) | 3.96 ± 0.06 | 4.48 ± 0.09 | 0.001 |
| Protein (g per 100 g) | 14.23 ± 0.08 | 13.99 ± 0.14 | 0.060 |
| Fat (g per 100 g) | 4.66 ± 0.15 | 4.52 ± 0.18 | 0.359 |
| Carbohydrate (g per 100 g) | 74.57 ± 0.11 | 73.37 ± 0.07 | 0.000 |
| Fiber (g per 100 g) | 1.42 ± 0.07 | 2.19 ± 0.08 | 0.000 |
| Ash (g per 100 g) | 1.16 ± 0.04 | 1.45 ± 0.06 | 0.002 |
| Total energy (kcal) | 397.18 ± 1.38 | 390.16 ± 1.12 | 0.002 |
This observation is consistent with the findings of Ajila et al.11 There were no significant differences in the protein and fat contents of the control and MPP biscuits. Similar results were reported in other research where fruit peel powders contained almost no amount of protein or fat.36 Overall, the observed great increase in the fiber and ash contents in MPP biscuits suggest that they can play a major role in increasing fiber consumption, which is critical in the promotion of gut health and the prevention of chronic diseases. This aligns with previous findings that MPP incorporation increases dietary fiber and enhances mineral content.12 Addition of MPP decreased the total energy value as has been observed previously in other studies by dietary fiber decreasing carbohydrate content.37 It has been demonstrated that biscuits containing MPP show enhanced values for dietary fiber and antioxidant activity of this product. Studies on similar applications with other fruit peels, such as pomegranate and citrus, show comparable effects on nutritional and antioxidant properties.42
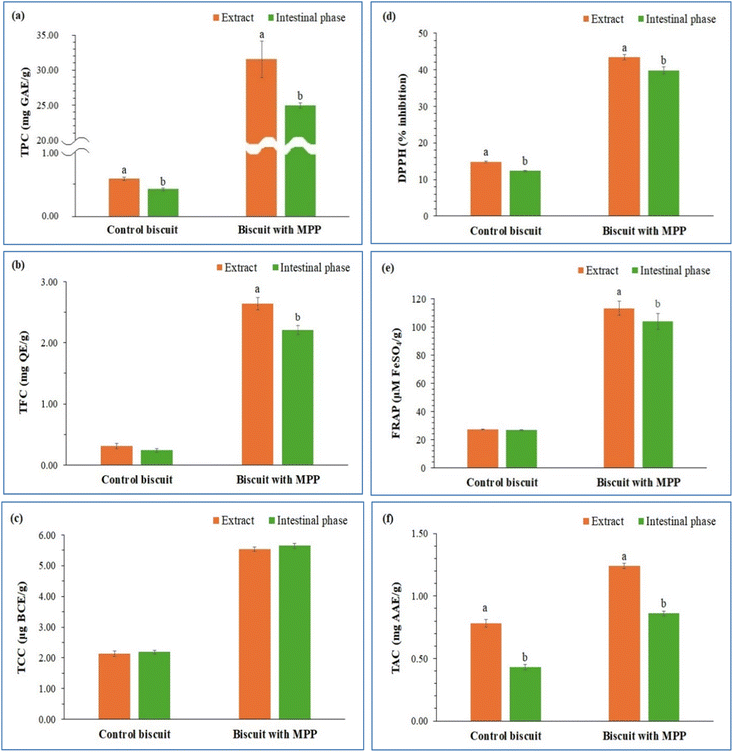
Fig. 4 shows the comparative analysis of TPC, TFC, TCC, DPPH, FRAP, and TAC values of the control biscuit and the biscuit with MPP. Biscuits containing MPP demonstrated significantly greater TPC (31.54 mg GAE per g) in comparison to control biscuits (0.58 mg GAE per g). The addition of MPP increased the phenolic content, owing to the polyphenol profile of MPP. TFC was also significantly greater in MPP biscuits (2.64 mg QE per g) than it was in control biscuits (0.31 mg QE per g). Mango peel's natural flavonoid composition contributed to this enhancement. MPP biscuits show a significant increase in TCC (5.54 μg BCE per g) in comparison to control biscuits (2.14 μg BCE per g) due to addition of carotenoid-rich mango peel. This is consistent with the reported relative high phenol content of mango peel powder.43 The significant increase in TPC, TFC, and TCC in MPP biscuits is consistent with previous research on mango peel's special polyphenolic and carotenoids. Ajila et al., mentioned that the incorporation of MPP increased the phenolic and carotenoid contents of the biscuits by 4–5 folds.11
This study highlighted the viability of MPP in enhancing functional food properties through its antioxidant activity. MPP biscuits also showed significantly increased antioxidant activity where DPPH inhibition, FRAP, and TAC have increased many folds as compared to control biscuits. The results of DPPH radical scavenging activity revealed that MPP biscuits had higher inhibition (43.42%) while control biscuits possessed (14.85%) showing increased free radical scavenging capacity. According to FRAP, MPP biscuits showed better reducing power (113.25 μM FeSO4 per g) than the control biscuits (27.11 μM FeSO4 per g). The TAC of MPP biscuit (1.24 mg AAE per g) was significantly higher than that of control biscuit (0.78 mg AAE per g) and its antioxidant activity was also confirmed (Fig. 4). These antioxidant improvements were directly attributed to the bioactive compounds in MPP, particularly gallic acid, chlorogenic acid, and quercetin. These compounds are particularly known for their antioxidant activity by neutralizing free radicals.43 These results echo similar trends seen in biscuits fortified with citrus or pomegranate peel.6,37
Incorporation of MPP in biscuits significantly improved their nutraceutical properties without compromising sensory acceptability at moderate inclusion levels, as demonstrated by earlier research.35 This makes MPP a promising ingredient for functional food development. Similar trends were observed in studies using other fruit peels. For instance, biscuits with addition of citrus and pomegranate peel had enhanced antioxidant potential because of polyphenols.5 However, again mango peel emerged supreme over other fruit peels as far as increasing antioxidant capacity was concerned because of higher phenolic and carotenoid contents.
Incorporation of MPP in biscuits enhances nutritional value and antioxidant properties, with optimal acceptability achieved at 5–7.5% substitution levels. While higher levels improve bioactivity, they compromise sensory attributes. These findings demonstrate the potential of mango peel as a functional ingredient for food applications. Therefore, a massive improvement in antioxidant activity of MPP biscuits make them candidate for combating oxidative stress-related diseases. These functional foods may help provide dietary antioxidant nutrients which are healthy alternatives to synthetic sources of antioxidants. Hence, addition of mango peel powder improves the biscuits' bioactive compound and antioxidant level notably, making mango peel powder a viable functional food ingredient.
3.4 Effect of in vitro digestion on bioactive components in biscuits
The fact that not all components in the food matrix are definitely bioaccessible is well established.15,16 Digestion in the gastrointestinal tract can alter the functional properties of antioxidants. Therefore, the stability of antioxidant compounds present in in vitro digestion is an essential parameter that can provide information about their potential positive impacts on human health.22,44 The amount of TPC, TFC and TCC and the antioxidant activity using the DPPH, FRAP and TAC assay of the undigested extract biscuits and the fractions of the biscuits after the intestinal phase digestion were measured. Changes in bioactive compounds and antioxidant activity after the simulated in vitro gastrointestinal digestion of biscuits are presented in Fig. 5. Impact of in vitro digestion, biscuits with MPP showed significantly higher TPC both before and after digestion compared to control biscuits. The TPC decreased after digestion, with a 21% reduction in MPP biscuits, which still retained a considerably higher TPC than the control (Fig. 5a). MPP biscuits retained high flavonoid levels after digestion, indicating good bioaccessibility, though levels decreased by 16% (Fig. 5b). The observed degradation of TPC and TFC is consistent with previous findings that phenolics are sensitive to pH variations, enzymatic hydrolysis, and micellar interactions in digestive fluids.6,37 However, specific compounds such as quercetin and gallic acid, prevalent in MPP, have been reported to be structurally resistant to digestive degradation.45 In this case, digestion also leads to a decrease in TPC and TFC in other fruit peels, including citrus and pomegranate bioaccessibility depending on the phenolic structure.5 Similarly for TCC, there were insignificant reductions in content after the digestion processes in both control and MPP biscuits (Fig. 5c). The stable TCC values confirmed that carotenoids of MPP have good bioaccessibility during digestion processes. Previous research has also confirmed that mango peel carotenoids remain stable to intestinal degradation and thus their functionality.44The DPPH scavenging activity reduced after digestion in both control and MPP biscuits as seen in Fig. 5d, though the MPP biscuits had relatively higher values (39.81% inhibition) than the control biscuits (12.34% inhibition). As seen in Fig. 5e, FRAP values reduced after digestion while were still significantly higher in MPP biscuits (103.78 μM FeSO4 per g) compared to control biscuits (27.00 μM FeSO4 per g). Similarly with TAC, it was higher with MPP biscuits after digestion (0.86 mg AAE per g) compared to the control (0.43 mg AAE per g), as seen in Fig. 5f. Despite these reductions, post-digestion values in MPP biscuits remained significantly higher than controls (p < 0.05), confirming their functional resilience during digestion.
Antioxidant activities (DPPH, FRAP, and TAC) were in agreement with other studies on fruit peel-enriched products. The high antioxidant activity of the MPP biscuits after digestion is consistent with the reports on mango peel extracts which demonstrate high radical scavenging and reducing power owing to polyphenols and carotenoids.12 The inhibition of antioxidant activity after digestion has also been reported for passion fruit and citrus peels due to phenolic degradation, while mango peel bioactive compounds seem to be more stable.16 The stability of bioactives post-digestion is attributed to both compound structure and matrix interaction—fiber–polyphenol complexes can modulate release and protect against enzymatic degradation.44
These findings are comparable to studies involving citrus, passion fruit, and pomegranate peels, which also showed partial loss but notable retention of polyphenols after digestion.15–17,31,46 Therefore, MPP-enriched biscuits can provide bioaccessible antioxidants, potentially contributing to dietary management of oxidative stress and inflammation-linked chronic diseases.10,12 The incorporation of MPP into baked products thus represents a sustainable, functional food approach for improving nutritional quality and health relevance.
3.5 Sustainability aspects and potential impact
The schematic diagram shown in Fig. 6 indicates a sustainable valorization process of tropical fruit peels into functional food ingredients. This process encompasses several key stages: include raw material collection, preliminary processing, drying, powder production, phytochemical extraction, functional testing, incorporation of the powder into functional food products, bioavailability assessment, waste management, and evaluation of sustainability impact. | ||
| Fig. 6 Schematic of sustainable valorization process for tropical fruit peels into functional food ingredients. | ||
The valorization process starts by the collection of tropic fruit peels, such as mango, banana, dragon fruit, pineapple, and papaya from agricultural and industrial waste. This strategy fosters circular economy principles by repurposing by-products into valuable resources, aligning with the UN Sustainable Development Goal (SDG) 12: Responsible Consumption and Production. Cleansing and cutting enhance drying as well as preserve bioactive compounds, as acknowledged by Marquez Molina et al. on the subject of sustaining materials' quality.2
The peels then undergo hot air drying monitored at temperatures such as 50 °C to attain microbial inactivity and preserve bioactive compounds. Through milling and sieving produces fine and standardized powder. This stage highlights the energy-efficient methods essential for maintaining product quality while minimizing environmental impact, contributing to SDG 13: Climate Action. Pathak et al. have emphasized the steps in developing functional ingredients as well as the saving on energy levels.9
To extract bioactive compounds including phenolic acids and flavonoids, green extraction methods were applied. The use of ethanol—though an organic solvent—was justified as food-safe, biodegradable, and compatible with current green chemistry guidelines.18 Moreover, ultrasound-assisted extraction improves mass transfer and reduces solvent use, representing a viable transition toward sustainable phytochemical recovery. Compared to conventional solvent extraction, this method significantly reduces processing time and thermal degradation.18,40
As the data show, these compounds contain considerable antioxidant activity (DPPH, FRAP assays), which confirms their perspectives for the usage in functional products with health beneficial effects. The alignment with SDG 3: there is strong linkage with Good Health and Well Being which can be evidenced by the fact that the process will allow creation of natural antioxidant in place of synthetic ones. Similar extraction methods of phytochemicals were discussed by Rather et al. with more emphasis on their profitability and environmental conservation.10
The powders are added into biscuits, improving their nutritional and functional properties. Sensory assessment enables acceptance of the ingredients at moderate inclusion levels among consumers. In vitro digestion models give valuable information regarding the stability and bioavailability of the bioactive compounds after digestion and thereby supporting the possible health benefits. This aligns with SDG 9: industry, innovation, and infrastructure, as such as food products which can provide solutions to existing health and sustainability issues can be developed.
From a techno-economic perspective, mango peel, which comprises up to 20–25% of fruit mass, is abundantly available in tropical countries and currently underutilized. As demonstrated in this study, even 5% peel powder inclusion in bakery products can deliver nutritional and functional enhancements while remaining sensorially acceptable. Furthermore, the remaining post-extraction residues can be revalorized as compost or animal feed, closing the loop and eliminating landfill disposal. This show commitment with SDG12 since it is considered as zero waste that reduces environmental impacts and improved resource utilization. The enhancement of waste management solutions within the valorization process improves a more general correspondence of Biorefinery concepts.9
The sustainable valorization process as presented in Fig. 6 is not only circumvents the addition of values to agricultural by-products but also resolves the global food waste issues. This process makes it possible to create a scalable model of rational production and application of natural bioactive compounds that contribute to the innovation, more sustainable types of food products. The results herein concur with SDG 12 and SDG 3 goals and objectives focusing on efficient use of resources and enhanced nutrition and health benefits from functional foods.
4 Conclusion
This study utilized tropical fruit peels, particularly mango peel powder (MPP), as a potent natural source of antioxidants to demonstrate a sustainable approach to functional food valorization. Among the five tested peels, MPP exhibited the highest phenolic, flavonoid, and antioxidant activity profiles. The addition of 5% MPP into biscuits enhanced their fiber content, antioxidant potential, and phytochemical concentration while maintaining their sensory acceptability. Simulated in vitro digestion revealed that essential bioactive compounds in MPP remained partially bioaccessible and functionally active post-digestion, supporting their nutritional relevance. The proposed ultrasound-assisted extraction and low-temperature drying methods serve as green processing strategies, while the reuse of residual peel waste for compost aligns with zero-waste, circular economy objectives. The proposed methodologies of ultrasound-assisted extraction and low-temperature drying signify environmentally sustainable processing strategies, whereas reutilization of residual peel waste for composting aligns with the principles of a zero-waste, circular economy. These findings contribute directly to sustainable food engineering by providing a scalable and economically viable approach for transforming fruit waste into high-value components for functional foods. Future research should explore industrial-scale application, storage stability, and clinical assessments of bioactive bioavailability and health benefits. Additionally, the examination of additional underutilized tropical by-products utilizing similar frameworks has the potential to expand the impact of sustainable valorization systems.Data availability
All data underlying the results are available as part of the article and no additional source data are required.Author contributions
Panorjit Nitisuk: validation, formal analysis, investigation, resources; Pitchaporn Wanyo: conceptualization, methodology, formal analysis, investigation, writing – original draft, writing – review & editing; Tossaporn Chamsai: conceptualization, methodology, validation, formal analysis, writing – review & editing; Kiatipong Charoenjit: resources, visualization.Conflicts of interest
There are no conflicts to declare.References
- C. Can-Cauich, E. Sauri-Duch, D. Betancur-Ancona, L. Chel-Guerrero, G. González-Aguilar, L. Cuevas-Glory, E. Pérez-Pacheco and V. Moo-Huchin, Tropical fruit peel powders as functional ingredients: Evaluation of their bioactive compounds and antioxidant activity, J. Funct. Foods, 2017, 37, 501–506, DOI:10.1016/J.JFF.2017.08.028.
- O. Marquez Molina, J. A. Domínguez-Avila, S. Pareek, T. J. Madera Santana, G. A. González Aguilar and L. X. Lopez-Martínez, Valorization of tropical fruit peel powders: Physicochemical composition, techno-functional properties, and in vitro antioxidant and antidiabetic activities, Emir. J. Food Agric., 2023, 35(6), 577–587, DOI:10.9755/ejfa.2023.v35.i6.3105.
- D. A. Campos, T. B. Ribeiro, J. A. Teixeira, L. Pastrana and M. M. Pintado, Integral valorization of pineapple (Ananas comosus L.) by-products through a green chemistry approach towards added value ingredients, Foods, 2020, 9(1), 60, DOI:10.3390/foods9010060.
- V. Marcillo-Parra, M. Anaguano, M. Molina, D. S. Tupuna-Yerovi and J. Ruales, Characterization and quantification of bioactive compounds and antioxidant activity in three different varieties of mango (Mangifera indica L.) peel from the Ecuadorian region using HPLC-UV/VIS and UPLC-PDA, NFS J., 2021, 23, 1–7, DOI:10.1016/j.nfs.2021.02.001.
- H. Suleria, C. Barrow and F. Dunshea, Screening and characterization of phenolic compounds and their antioxidant capacity in different fruit peels, Foods, 2020, 9(9), 1026, DOI:10.3390/foods9091206.
- V. Imeneo, R. Romeo, A. Gattuso, A. De Bruno and A. Piscopo, Functionalized biscuits with bioactive ingredients obtained by citrus lemon pomace, Foods, 2021, 10(10), 2460, DOI:10.3390/foods10102460.
- F. Benmeziane-Derradji, A. Taguida, L. Arkoub, R. Raigar and S. Bellagoune, Physiochemical and sensorial attributes of apricot fortified wheat biscuits, Carpathian J. Food Sci. Technol., 2022, 14, 59–71, DOI:10.34302/crpjfst/2022.14.1.5.
- J. Villacís-Chiriboga, K. Elst, J. V. Camp, E. Vera and J. Ruales, Valorization of byproducts from tropical fruits: Extraction methodologies, applications, environmental, and economic assessment, Compr. Rev. Food Sci. Food Saf., 2020, 19(2), 405–447, DOI:10.1111/1541-4337.12542.
- P. D. Pathak, A. R. Jadhav, S. K. Deokar, S. Jogalekar and V. Gedam, Sustainable fruit peel waste biorefinery: Challenges and future perspectives, in Biorefinery: A sustainable approach for the production of biomaterials, biochemicals and biofuels, ed. P. D. Pathak and S. A. Mandavgane, Springer, Singapore, 2023, pp. 377–389, DOI:10.1007/978-981-19-7481-6_14.
- J. A. Rather, N. Akhter, Q. Ayaz, S. A. Mir, A. Singh, G. Goksen, D. Majid, H. A. Makroo and B. N. Dar, Fruit peel valorization, phytochemical profile, biological activity, and applications in food and packaging industries: Comprehensive review, Curr. Food Sci. Technol. Rep., 2023, 1, 63–79, DOI:10.1007/s43555-023-00007-3.
- C. Ajila, K. Leelavathi and U. Rao, Improvement of dietary fiber content and antioxidant properties in soft dough biscuits with the incorporation of mango peel powder, J. Cereal Sci., 2008, 48, 319–326, DOI:10.1016/J.JCS.2007.10.001.
- J. F. Cui, C. Y. Zhao, L. P. Feng, Y. H. Han, H. J. Du, H. Xiao and J. K. Zheng, Pectins from fruits: Relationships between extraction methods, structural characteristics, and functional properties, Trends Food Sci. Technol., 2021, 110, 39–54, DOI:10.1016/j.tifs.2021.01.077.
- H. M. C. Azeredo, R. V. Tonon and D. J. Mcclements, Designing healthier foods: reducing the content or digestibility of key nutrients, Trends Food Sci. Technol., 2021, 118, 459–470, DOI:10.1016/j.tifs.2021.10.023.
- X. Luo, M. Tian, Y. Cheng, C. Ji, S. Hu, H. Liu, J. Lu and J. Ren, Effects of simulated in vitro gastrointestinal digestion on antioxidant activities and potential bioaccessibility of phenolic compounds from K. coccinea fruits, Front. Nutr., 2022, 9, 1024651, DOI:10.3389/fnut.2022.1024651.
- Q. Cao, J. Teng, B. Wei, L. Huang and N. Xia, Phenolic compounds and bioaccessibility of passion fruit peel ethanol extracts, Food Chem., 2021, 356, 129682, DOI:10.1016/j.foodchem.2021.129682.
- B. Gullon, M. E. Pintado, J. Fernández-López, J. A. Pérez-Álvarez and M. Viuda-Martos, In vitro gastrointestinal digestion of pomegranate peel (Punica granatum) flour obtained from co-products: Changes in the antioxidant potential and bioactive compounds stability, J. Funct. Foods, 2015, 19, 617–628, DOI:10.1016/j.jff.2015.09.056.
- The Association of Official Analytical Chemists (AOAC), Official Methods of Analysis, AOAC, Washington DC, 17th edn, 2000 Search PubMed.
- S. F. Monteiro, E. L. N. Costa, R. S. B. Ferreira and R. C. Chisté, Simultaneous extraction of carotenoids and phenolic compounds from pulps of orange and yellow peach palm fruits (Bactris gasipaes) by ultrasound-assisted extraction, Food Sci. Technol., 2022, 42, e34021, DOI:10.1590/fst.34021.
- P. Wanyo, N. Kaewseejan, N. Meeso and S. Siriamornpun, Bioactive compounds and antioxidant properties of different solvent extracts derived from Thai rice by-products, Appl. Biol. Chem., 2016, 59, 373–384, DOI:10.1007/s13765-016-0173-8.
- N. Dasgupta and B. De, Antioxidant activity of Piper betle L. leaf extract in vitro, Food Chem., 2004, 88(2), 219–224, DOI:10.1016/j.foodchem.2004.01.036.
- American Association of Cereal Chemists (AACC), Baking Quality of Cookie Flour, Approved Methods of the American Association of Cereal Chemists, AACC, St. Paul, USA, 10th edn, 2000 Search PubMed.
- A. Brodkorb, L. Egger, M. Alminger, P. Alvito, R. Assunção and S. Ballance, et al., INFOGEST static in vitro simulation of gastrointestinal food digestion, Nat. Protoc., 2019, 14(4), 991–1014, DOI:10.1038/s41596-018-0119-1.
- P. Wanyo, T. Chamsai, N. Toontom, L. K. Nghiep and K. Tudpor, Differential effects of in vitro simulated digestion on antioxidant activity and bioaccessibility of phenolic compounds in purple rice bran extracts, Molecules, 2024, 29, 2994, DOI:10.3390/molecules29132994.
- C. Bezerra, L. Silva, D. Corrêa and A. Rodrigues, A modeling study for moisture diffusivities and moisture transfer coefficients in drying of passion fruit peel, Int. J. Heat Mass Transf., 2015, 85, 750–755, DOI:10.1016/J.IJHEATMASSTRANSFER.2015.02.027.
- P. Dias, J. Sajiwanie and R. Rathnayaka, Chemical composition, physicochemical and technological properties of selected fruit peels as a potential food source, Int. J. Fruit Sci., 2020, 20, S240–S251, DOI:10.1080/15538362.2020.1717402.
- T. Kongpichitchoke, E. Gnoumou, A. Noomhorm and C. Ho-Hsien, Study on factors affecting physicochemical properties of spray dried mango powder using taguchi experimental design approach, Int. J. Eco-Innov. Sci. Eng., 2021, 2(1), 7–13, DOI:10.33005/IJEISE.V2I01.34.
- F. Santos, R. Figueirêdo, A. Queiroz and D. Santos, Drying kinetics and physical and chemical characterization of white-fleshed ‘pitaya’ peels, Rev. Bras. Eng. Agric. Ambient., 2017, 21, 872–877, DOI:10.1590/1807-1929/AGRIAMBI.V21N12P872-877.
- P. Pichayajittipong and S. Thaiudom, Optimum condition of beta-cyanin colorant production from red dragon fruit (Hylocercus polyrhizus) peels using response surface methodology, Chiang Mai Univ. J. Nat. Sci., 2014, 13(1), 483–496, DOI:10.12982/CMUJNS.2014.0051.
- D. Morais, E. Rotta, S. Sargi, E. Schmidt, E. G. Bonafe, M. Eberlin, A. Sawaya and J. Visentainer, Antioxidant activity, phenolics, and UPLC–ESI(–)–MS of extracts from different tropical fruits parts and processed peels, Food Res. Int., 2015, 77(3), 392–399, DOI:10.1016/j.foodres.2015.08.036.
- C. Ajila and U. Rao, Mango peel dietary fibre: Composition and associated bound phenolics, J. Funct. Foods, 2013, 5, 444–450, DOI:10.1016/J.JFF.2012.11.017.
- B. Singh, J. Singh, A. Kaur and N. Singh, Phenolic composition, antioxidant potential and health benefits of citrus peel, Food Res. Int., 2020, 132, 109114, DOI:10.1016/j.foodres.2020.109114.
- C. Ajila, K. Naidu, S. Bhat and U. Rao, Bioactive compounds and antioxidant potential of mango peel extract, Food Chem., 2007, 105, 982–988, DOI:10.1016/J.FOODCHEM.2007.04.052.
- D. Peng, H. Zahid, S. Ajlouni, F. Dunshea and H. Suleria, LC-ESI-QTOF/MS profiling of australian mango peel by-product polyphenols and their potential antioxidant activities, Processes, 2019, 7(10), 764, DOI:10.3390/pr7100764.
- H. Chung, Comparative study of antioxidant activity of imported tropical and subtropical fruits, Korean J. Food Preserv., 2015, 22(4), 577–584, DOI:10.11002/KJFP.2015.22.4.577.
- Z. Marina and A. Noriham, Quantification of total phenolic compound and in vitro antioxidant potential of fruit peel extracts, Int. Food Res. J., 2014, 21(5), 1925–1929 CAS.
- I. Ashoush and M. Gadallah, Utilization of mango peels and seed kernels powders as sources of phytochemicals in biscuit, World J. Dairy Food Sci., 2011, 6(1), 35–42 Search PubMed.
- H. Aslam, M. Ianam, U. Raheem, R. Ramzan, A. Shakeel, M. Shoaib and H. Sakandar, Utilization of mango waste material (peel, kernel) to enhance dietary fiber content and antioxidant properties of biscuit, J. Glob. Innov. Agric. Sci., 2014, 2, 76–81, DOI:10.17957/JGIASS/2.2.533.
- M. Kour, J. Mitra and A. Datta, Principal component analysis of hedonic and fuzzy approach sensory evaluation for compositional optimization of Refractance-Window dried jackfruit leather, J. Food Meas. Charact., 2023, 17, 4318–4331, DOI:10.1007/s11694-023-01933-5.
- B. Bheemisetti, K. Kumar, A. Bhagwan, J. Shankaraswamy and G. Sathish, Sensory evaluation of biscuits enriched with composite flour of barnyard millet and dragon fruit powder, Int. J. Adv. Biochem. Res., 2024, 8(10), 485–487, DOI:10.33545/26174693.2024.v8.i10g.2511.
- M. Ranasinghe, M. Alghaithi, C. Stathopoulos, B. Sundarakani and S. Maqsood, Valorizing date seeds through ultrasonication to enhance quality attributes of dough and biscuit: Part 2 – Study on bioactive properties, sensory acceptance, in vitro gastrointestinal digestion and shelf life of biscuits, Ultrason. Sonochem., 2025, 112, 107160, DOI:10.1016/j.ultsonch.2024.107160.
- T. Rababah, M. Al-Mahasneh and K. Ereifej, Effect of chickpea, broad bean, or isolated soy protein additions on the physicochemical and sensory properties of biscuits, J. Food Sci., 2006, 71, S438–S442, DOI:10.1111/j.1750-3841.2006.00077.x.
- B. R. Salem, K. M. El-Sahy, A. M. Sulieman and M. R. Gouda, Use of tomato pomace, mango seeds kernel and pomegranate peels powders for the production of functional biscuits, Zagazig J. Agric. Res., 2020, 47(4), 1011–1023, DOI:10.21608/zjar.2020.110329.
- S. Marçal and M. Pintado, Mango peels as food ingredient/additive: nutritional value, processing, safety and applications, Trends Food Sci. Technol., 2021, 114, 472–489, DOI:10.1016/j.tifs.2021.06.012.
- F. Blancas-Benitez, G. Mercado-Mercado, A. Quirós-Sauceda, E. Montalvo-González, G. González-Aguilar and S. Sáyago-Ayerdi, Bioaccessibility of polyphenols associated with dietary fiber and in vitro kinetics release of polyphenols in Mexican 'Ataulfo' mango (Mangifera indica L.) by-products, Food Funct., 2015, 6(3), 859–868, 10.1039/c4fo00982g.
- G. Velderrain-Rodríguez, L. Salvia-Trujillo, A. Wall-Medrano, G. González-Aguilar and O. Martín-Belloso, In vitro digestibility and release of a mango peel extract encapsulated within water-in-oil-in-water (W1/O/W2) emulsions containing sodium carboxymethyl cellulose, Food Funct., 2019, 10(9), 6110–6120, 10.1039/C9FO01266D.
- Y. Cai, W. Qin, S. Ketnawa and Y. Ogawa, Impact of particle size of pulverized citrus peel tissue on antioxidant properties during digestion, Food Sci. Hum. Wellness, 2020, 9(1), 58–63, DOI:10.1016/j.fshw.2019.12.008.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fb00371c |
| This journal is © The Royal Society of Chemistry 2025 |