 Open Access Article
Open Access ArticleLavender essential oils as natural food protectants: myth or a viable substitute?
Dheeraj
Kumar
a,
Mahesh K.
Samota
 b,
Somnath
Roy
c,
Ana Sanches
Silva
b,
Somnath
Roy
c,
Ana Sanches
Silva
 d and
Abhay K.
Pandey
d and
Abhay K.
Pandey
 *a
*a
aDepartment of Mycology & Microbiology, Tea Research Association, North Bengal Regional R & D Center, Nagrakata, 735225, West Bengal, India. E-mail: abhaykumarpandey.ku@gmail.com
bHorticulture Crop Processing Division, ICAR – Central Institute of Post-Harvest Engineering & Technology, Abohar 152116, Punjab, India
cTea Research Association, Tocklai Tea Research Institute, Jorhat 785008, Assam, India
dUniversity of Coimbra, Faculty of Pharmacy, Polo III, Azinhaga de Sta Comba, 3000-548 Coimbra, Portugal
First published on 14th January 2025
Abstract
The widespread application of synthetic pesticides for food preservation and crop protection is a significant concern for both environmental sustainability and public health. Past and recent studies conducted worldwide revealed that botanical pesticides based on essential oils (EOs) have been developed against pests and pathogens deteriorating food commodities under both storage and field conditions. While EO-based botanical pesticides are less widely available, they offer considerable potential for managing pathogens and insects that affect food crops. The genus Lavandula also known as Lavender is one of the most important genera of the family Lamiaceae, comprising over 39 accepted species and many varieties distributed across the Iberian Peninsula, the Mediterranean coastline, parts of Southern & Eastern Africa, the Middle East, and South Asia. Lavandula species can potentially be used in the food and pharmaceutical industries as medicinal herbs. The genus is known for its abundance of EOs, which exhibit high variability in chemical constituents between species owing to various extrinsic (geographical origin) and intrinsic (genetic variation) factors. Despite broad scientific interest in the bioprospection of Lavandula species, there is a general lack of information regarding the use of Lavandula EOs (LEOs) in protection of food commodities/crops from harmful organisms. The objectives of this paper were to systematically review the scientific literature on the efficacy of LEOs against pathogens and pests deteriorating food commodities/crops under both storage and field conditions. Besides, studies on chemical analysis of LEOs originating from different countries and recommendations for their use as an alternative to synthetic pesticides in food protection are described. We also discussed the challenges in the use of LEOs and safety assessments so that they can be used as safe botanical pesticides in food systems.
Sustainability spotlightThe genus Lavandula also known as Lavender, is one of the most important genera of the family Lamiaceae, comprising over 39 species and many varieties distributed across the Iberian Peninsula, the Mediterranean coastline, parts of Southern & Eastern Africa, the Middle East, and South Asia. Lavandula species can potentially be used in the food and pharmaceutical industries as medicinal herbs. The present article summarizes that EOs and terpenoids derived from L. angustifolia and L. latifolia as well as other Lavandula species discussed in this review have broad antimicrobial and insecticidal properties against pathogens and pests deteriorating food commodities/hampering field food crops. There have been developments in the evaluation of LEO-based encapsulated products, such as thin films, biodegradable polymers, and nano-emulsion coatings against bacterial and fungal pathogens responsible for food spoilage, and investigators have found potential results. Therefore, botanical preservatives/pesticides derived from EOs of Lavandula species might be useful in combating microbial pathogens and insect pests in stored food commodities and field crops. A shift towards greener technologies directs an optimistic future towards safer deployment of LEOs in food preservation/crop protection. |
1. Introduction
Lavandula also known as Lavender, a genus of over 39 species and 79 intraspecific taxa and hybrids1 of higher plants in the mint family, Lamiaceae, has been recognized for its diverse therapeutic and aromatic properties since ancient times. The name Lavender is derived from the Latin “lavare” which means “to wash”.2 One of the most prevalent species, i.e., Lavandula angustifolia Mill. (commonly known as English lavender), is native to the Mediterranean region. It thrives in countries such as Morocco, Algeria, Tunisia, Spain, Greece, France, Italy, India, and Turkey while Lavandula latifolia Medik. (commonly known as spike lavender) shares a similar Mediterranean basin origin. Like its counterpart, Lavandula species have been widely studied for their traditional and pharmacological uses.3Lavandula is commonly used today in perfumes, candles, baths, soap, talc powers, scanted sachets, aromatherapy, and massage.4 In medieval and Renaissance Europe, Lavandula was commonly used in the preparation of 'nosegays' or small bouquets carried to ward off the plague, indicating its perceived antiseptic properties.5 Various folk traditions have used Lavandula for a variety of medical purposes such as Lavandula used in the traditional Chinese medicine, to treat infertility,6 infections, anxiety, and fever.6 It has also been used in Arabic medicine to treat stomach aches and kidney problems.7 It was considered an antispasmodic, antiemetic, antiflatulent, and antidepressant.8 The extract of the genus was used to enhance bile flow, cure varicose ulcers, and to reduce carpal tunnel syndrome.9Lavandula angustifolia and L. latifolia are highly valued for their essential oils (EOs), which are extensively used in aromatherapy and treating ailments like anxiety, depression, and sleep disorders. Several reviews have been published on Lavandula essential oils (LEOs).10,11 For instance, Lavandula aromatherapy has garnered attention for its potential cognitive enhancing effects, as reviewed by Aprotosoaie et al.10 In this review, they highlighted the chemical composition of LEOs, focusing on their main constituents such as linalool and linalyl acetate and discussed the variability in the chemical profiles due to different factors like geographical locations and harvesting times. Additionally, this review also emphasized the biological activities of LEOs, including their antimicrobial and antioxidant properties with reference to human pathogens, rather than against pathogens deteriorating food commodities.9
Another comprehensive review published by Malloggi et al.11 explored the cognitive effects of LEO inhalation, focusing on arousal, attention, and memory and concluded that LEOs, particularly their main components linalool and linalyl acetate have the potential to decrease arousal and enhance sustained attention. Their review highlighted the importance of EO's quality and administration methods, noting that different diffusion devices and EO compositions can influence outcomes. Besides, in 2021, Heral et al.1 described the taxonomy and morphology of Lavandula species along with a phytochemical analysis of their EOs. In particular, these reviews summarized the therapeutic and nutritional benefits as well as the phytochemical properties of LEOs rather than their application in food/crop protection. Therefore, a detailed review concerning the application of LEOs in food/crop protection is required to open opportunities for further research, especially in the areas of pest and pathogen control in food crops where the identification of potential biopesticides is still a limiting step. The objective of this paper was to provide a comprehensive review of the efficacy of LEOs, against insect pests and pathogens hampering/spoiling food commodities under both storage and field conditions. Besides, the chemical analysis of LEOs originating from different countries, challenges in using LEOs in food systems and safety assessments along with the scope of future research are also discussed.
The literature discussed in this review was explored and collected in 2023 and 2024. We surveyed the published research papers on the chemical composition and bio-efficacy of LEOs available through several online search engines, including Research Gate, Google Scholar, Sci Finder, Connected Papers, Web of Science, and Scopus using “Lavandula and essential oils” as the search keywords. Other keywords used for the survey of papers associated with LEOs were chemical composition, Gas Chromatography-Mass Spectrometry (GC-MS) analysis, medicinal, antibacterial, antifungal, insecticidal, nano-encapsulated LEOs, and antioxidant properties. The literature we have discussed here were mostly from Scopus and Web of Science indexed journals.
2. Phytochemical analysis of LEOs
Lavender EOs derived primarily from the genus Lavandula, are characterized by a complex composition of volatile compounds (Fig. 1), which confer their distinctive aromatic and therapeutic properties.12 The chemical composition of LEOs can be analysed and quantified using techniques such as gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS).13 This method allows for the accurate identification of individual components and their relative concentrations, providing insights into the quality and therapeutic potential of EOs.14 The majority of studies used GC and GC-MS analysis to identify the chemical constituents present in LEOs.14,15 In GC-MS analysis, the identification and quantification of compounds in EOs were performed based on their retention indices or retention times, and mass spectra. For instance, through GC-MS analysis, linalool, linalyl acetate, 1,8-cineole, camphor, and borneol were identified as the primary constituents of LEOs.16 It is found that each component contributes to the EO's unique chemical profile and biological activities.The chemical profile of EOs from L. angustifolia, L. latifolia and other Lavandula species, analysed by GC and GC-MS methods, originating from different countries, are summarized in Table 1 along with the extraction methods used to obtain the EOs. Investigators used various extraction methods for the isolation of EOs from Lavandula species. These include, hydro-distillation, headspace solvent microextraction, supercritical CO2 extraction, headspace solid-phase microextraction, microwave-assisted hydro distillation, steam distillation, solvent extraction, ultrasound-assisted distillation, turbo hydro-distillation, hydrodistillation–headspace solvent microextraction, supercritical water distillation, microwave-assisted extraction, and microdistillation (Table 1). Most researchers, however, used hydrodistillation methods because they are cost-effective and require less resources.
| Year | Lavandula spp. | Part used | Extraction methodsa | Major chemical compounds (%) | Origin | References |
|---|---|---|---|---|---|---|
| a Hydro-distillation (HD), headspace solvent microextraction (HSME), supercritical CO2 extraction (SCE), headspace solid-phase microextraction (HSPME), microwave-assisted hydro distillation (MAHD), steam distillation (SD), solvent extraction (SE), ultrasound-assisted distillation (UAD), turbo hydro-distillation (THD), and hydrodistillation-headspace solvent microextraction (HD-HSME), supercritical water distillation (SWD), microwave-assisted extraction (MAE), and microdistillation (MD). | ||||||
| 2005 | L. angustifolia | Aerial parts | HD-HSME | Linalool (32.8%), linalyl acetate (17.6%), and lavandulyl acetate (15.9%) | Iran | 17 |
| 2008 | L. angustifolia | Aerial parts | HD | Linalool (44.54%), geraniol (11.02%), and lavandulyl acetate (10.78%) | China | 18 |
| 2009 | L. angustifolia | Flowers | HD | Linalool (30.6%), linalyl acetate (14.2%), and geraniol (5.3%) | Poland | 19 |
| 2012 | L. angustifolia | Aerial parts | HD | 1,8-Cineole (29.0–38.0%), linalool (6.8–19.2%), and camphor (9.6–14.6%) | Iran | 20 |
| 2012 | L. dentata | Aerial parts | HD | Linalool (47.30%), linalyl acetate (28.65%), and camphor (2.32%) | Tunisia | 21 |
| 2012 | L. latifolia | Dried flowers | HD, SCE | Linalool (53%), linalyl acetate, camphor, and borneol | Australia | 22 |
| 2013 | L. angustifolia, L. x intermedia | Inflorescences | SD | Caryophyllene (24.1%), beta-phellandrene (16%), and eucalyptol (15.6%) | Romania | 23 |
| 2013 | L. angustifolia | Aerial parts | HS-SPME, MA–HS–SPME | 1,8-Cineole (41.37–54.84%), camphor (15.83–23.25%), and borneol (12.32–5.0%) | Iran | 24 |
| 2015 | L. angustifolia, L. latifolia | Aerial parts | HD | Linalool (35–51%), eucalyptol (26–32%), camphor (10–18%), α-pinene (1–2%), α-terpineol (1–2%), and α-bisabolene (1–2%) | Spain | 16 |
| 2015 | L. latifolia | Flowering stems | SD | Linalool, 1,8-cineole, camphor, borneol, α- and β-pinene | Europe | 25 |
| 2015 | L. latifolia | Aerial parts | SCE | Linalyl acetate (44.1–59.8%), and β-caryophyllene (6.3–7.3%) | India | 26 |
| 2016 | L. angustifolia | Aerial parts | HD, SCE | Linalyl acetate (44.1–59.8%), and β-caryophyllene (6.3–7.3%) | India | 27 |
| 2016 | L. angustifolia | Flowers | HD, SFE, SPME | Linalool (35.65%) and linalyl acetate (33.63%) | Jordan | 28 |
| 2016 | L. angustifolia | Flowers | HD | Linalool (24.63%), camphor (13.58%), and linalyl acetate (8.89%) | Iraq | 29 |
| 2016 | L. latifolia | Leaves, flower buds, flowers | SD | Linalool (23.9%), linalyl acetate (22.3%), γ-terpinene (3.3%), and terpinen-4-ol (5.0%) | Poland | 30 |
| 2016 | L. latifolia | Flowers | HD, SD, THD, UAD, SWD, MAE | Linalool, linalyl acetate, camphor, borneol, and 1.8-cineole | France | 31 |
| 2017 | L. angustifolia | Aerial parts | HD, MD | Linalool (22.1%), lavandulyl acetate (15.3%), and linalyl acetate (14.7%) | Turkey | 32 |
| 2017 | L. angustifolia | Flowers | HD, SFE, SPME | Linalool (51.8%), lavandulol, terpinen-4-ol, and α-terpineol | Bosnia and Herzegovina | 33 |
| 2018 | L. angustifolia | Aerial parts | HD, MAE | Linalool (34.70%), camphor (12.77%), and eucalyptol (11.50%) | Romania | 32 |
| 2018 | L. angustifolia | Flowers | HD | Linalool (34.70%), camphor (12.77%), and eucalyptol (11.50% | Syria | 34 |
| 2018 | L. stoechas | Flowers | HD | Fenchone (52.7%), camphor (25.94%), and 1,8-cineole (4.84%) | Algeria | 35 |
| 2018 | L. angustifolia | Fresh flowers, aerial parts and stems | HD | Linalool (26.5–34.7%), linalyl acetate (19.7–23.4%), terpinen-4-ol (2–4.9%), α-terpineol (2.8–5.1%), β-ocimene (2.9–10.7%), geranyl acetate (1.7–2.8%), and oct-1-en-3-yl acetate (0.9–3.6%) | Poland | 36 |
| 2018 | L. latifolia | Fresh flowers, aerial parts and stems | HD | Linalool (26.5–34.7%), linalyl acetate (19.7–23.4%), terpinen-4-ol (2–4.9%), α-terpineol (2.8–5.1%), β-ocimene (2.9–10.7%), and geranyl acetate (1.7–2.8%) | Croatia | 37 |
| 2019 | Lavandula latifolia | Aerial parts | SD | Carvacrol (78.2%), 2-methoxy-4-vinylphenol (2.5%), and spathulenol (2.2%) | Morocco | 38 |
| 2020 | L. angustifolia | Flowers | HD | Linalool (23.51–27.39%), and linalyl acetate (26.60–40.66%) | Romania | 39 |
| 2020 | L. angustifolia | Flowers | HD | Linalyl acetate (28.89%), linalool (24.30%), caryophyllene (7.89%), and borneol (2.60%) | China | 40 |
| 2020 | Lavandula latifolia | Aerial parts | HD | Linalyl acetate (26.1%), linalool (19.7%), and lavandulol acetate (12.6%) | China | 41 |
| 2020 | Lavandula latifolia | Inflorescences | SD | Linalyl acetate (46.76%), lavandulyl acetate (14.21%), lavandulol (1.54%), and linalool (16.82%) | China | 42 |
| 2021 | L. angustifolia | Inflorescences | HD | 1,8-Cineol (eucalyptol) (2.0), β-caryophyllene (4.78%), (E)-β-farnesene (1.52%), and caryophyllene oxide (0.36%) | 43 | |
| 2021 | L. angustifolia | Aerial parts | HD | Linalool (32.19–46.83%), linalyl acetate (17.70–35.18%), and terpinen-4-ol (3.63–7.70%) | Romania | 44 |
| 2021 | L. latifolia | Aerial parts | HD | Gamma-terpinene (26.8%), camphor (13.8%), and 1,8-cineole (10.2%) | Saudi Arabia | 45 |
| 2022 | L. angustifolia, L. x intermedia | Aerial parts | HD | Linalool (26.14–57.07%) and linalyl acetate (9.08–24.45%) | Ukraine | 46 |
| 2022 | L. angustifolia, L. latifolia | Aerial parts | HD | Linalool (39.5%), linalyl acetate (26.7%), and eucalyptol (43.08%) | Egypt, France, Australia | 47 |
| 2022 | L. spica | Leaves | SE | Linalool (39.5%), eucalyptol (43.08%), and linalyl acetate (26.7%) | Egypt | 47 |
| 2022 | L. angustifolia | Flowers | HD | Linalool (29.95%) and linalyl acetate (18.86%) | Morocco | 48 |
| 2023 | L. angustifolia | Flowers | HD | Linalool (31.27%) and camphor (16.21%) | Algeria | 49 |
| 2023 | L. angustifolia | Aerial parts | HD | Linalool (20.0–45.0%) and linalyl acetate (20.79–39.91%) | Bulgaria | 50 |
| 2023 | L. latifolia | Aerial parts (leaves, stems, flowers) | SD | Linalool (14.93%), camphor (14.11%), linalyl acetate (11.17%), and eucalyptol (10.99%) | Morocco | 51 |
| 2024 | L. angustifolia | Flowers | MAHD | α-Terpinolene (24.25%) and (−)-borneol (19.55%) | Turkey | 52 |
| 2024 | L. angustifolia | Stems | HD | Linalool (33.27%) and linalyl acetate (21.01%) | Tajikistan | 53 |
Table 1 also shows that the chemical composition of LEOs can vary depending on the region and cultivation conditions. Using the GC-MS method, Jianu et al.54 analysed the EO of L. angustifolia grown in western Romania, and reported significant variations in the major chemical components. These differences can influence the biological activities of the EO, highlighting the importance of regional studies in understanding their efficacy. In addition, high-quality L. angustifolia EO was typically characterized by high levels of linalool and linalyl acetate through GC-MS analysis, whereas L. latifolia EO was distinguished by its higher camphor content.46 The table also provides evidence that linalool and linalyl acetate are the predominant compounds found in L. angustifolia EOs in the majority of the studies. In addition to the primary constituents, LEOs contain various secondary metabolites that contribute to their overall efficacy. These include terpenes such as lavandulol, geraniol, and α-terpineol, as well as sesquiterpenes like β-caryophyllene.16,36
In particular, L. angustifolia and L. latifolia are native to the Mediterranean region, with L. angustifolia being widely cultivated worldwide, including in China, Morocco, Italy and Algeria, while L. latifolia is commonly cultivated in Spain, France, and Italy (Table 1). Among these species, L. angustifolia from Romania has the highest concentration of linalool (73.0%) extracted via microwave-assisted hydro distillation.55 In contrast, L. latifolia, from Spain, shows a higher concentration of γ-terpinene (26.8%) and camphor (13.8%) extracted by hydro distillation.56 Besides, unique chemical constituents have also been identified in regional variants, such as L. angustifolia from Northeastern Algeria, which contains rare compounds like 2-furanmethanol (7.49%) and linalool oxide (11.98%), and in L. latifolia, known for its significant γ-terpinene content.49 Although, all these studies used GC-MS analysis for the chemical characterization of LEOs, these variations highlight the diverse chemical profiles of Lavandula species depending on their geological origin and extraction methods.39
3. Use of LEOs as antibacterial agents against foodborne pathogens
Bacterial phytopathogens are a significant cause of yield losses in legumes, cereals, vegetables, fruits, and other food commodities/foodstuffs, affecting crops/commodities during pre-harvest, transit, and storage. These pathogens can lead to annual yield reductions in food crops or foodstuffs ranging from 20% to 40%, posing a serious threat to global food security and agricultural sustainability.57 Their impact is particularly severe due to their ability to proliferate throughout the supply chain, from the field to the market, exacerbating economic losses and food waste.58 The major bacterial species involved in food spoilage or crop loss include Clavibacter michiganensis, Pseudomonas syringae, P. solanacearum, P. putida, Erwinia carotovora, E. amylovora, E. herbicola, Xanthomonas citri, X. axanopodis pv. malvacearum, X. campestris, Escherichia coli, Salmonella typhimurium, Listeria monocytogenes, Staphylococcus aureus, Bacillus cereus, and Pseudomonas aeruginosa, among others.57,59,60 Such bacterial pathogens cause substantial yield losses and quality degradation in many food crops of national and international significance.59 In the United States alone, foodborne illnesses caused by 14 major pathogens are estimated to cost $14 billion annually and result in a loss of 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 quality-adjusted life years (QALYs).61 Developing countries face amplified impacts due to inadequate food safety infrastructure, resulting in higher morbidity and mortality, particularly among children under five.62 For instance, in India, foodborne illnesses annually affect over 100 million people, contributing to significant economic losses from healthcare expenses and lost productivity.63 In Canada, the annual societal cost of bacterial foodborne illnesses ranges from $9.3 to $12.9 billion, driven by healthcare expenses and lost productivity.64 Such outbreaks also impact businesses through lawsuits, market losses, and consumer distrust, underlining the urgent need for improved food safety measures. Many EOs and plant extracts have been extensively evaluated by researchers from time to time for their antibacterial activity against these phytopathogenic and food spoilage bacteria under in vitro and in vivo conditions;65–68 although LEOs are known for their significant antibacterial properties, particularly against foodborne pathogens,69 there is limited information available about antibacterial properties of LEOs against phytopathogenic and food spoilage bacteria. The antibacterial activity of LEOs reported by researchers is summarized in Table 2.
000 quality-adjusted life years (QALYs).61 Developing countries face amplified impacts due to inadequate food safety infrastructure, resulting in higher morbidity and mortality, particularly among children under five.62 For instance, in India, foodborne illnesses annually affect over 100 million people, contributing to significant economic losses from healthcare expenses and lost productivity.63 In Canada, the annual societal cost of bacterial foodborne illnesses ranges from $9.3 to $12.9 billion, driven by healthcare expenses and lost productivity.64 Such outbreaks also impact businesses through lawsuits, market losses, and consumer distrust, underlining the urgent need for improved food safety measures. Many EOs and plant extracts have been extensively evaluated by researchers from time to time for their antibacterial activity against these phytopathogenic and food spoilage bacteria under in vitro and in vivo conditions;65–68 although LEOs are known for their significant antibacterial properties, particularly against foodborne pathogens,69 there is limited information available about antibacterial properties of LEOs against phytopathogenic and food spoilage bacteria. The antibacterial activity of LEOs reported by researchers is summarized in Table 2.
| Lavandula species | Bacterial pathogens | Sources | Effective doses | Country | References |
|---|---|---|---|---|---|
| L. angustifolia | Listeria innocua, Pseudomonas fluorescens, and Escherichia coli | Fish | Applied in biodegradable films | Spain | 70 |
| L. angustifolia | Staphylococcus aureus and E. coli | Ostrich meat | 2% EO in coating | Iran | 71 |
| L. angustifolia | Yersinia ruckeri, Aeromonas hydrophila, and Vibrio anguillarum | Fish | MIC: 62.5–500 μl mL−1 | Turkey | 72 |
| L. angustifolia | Bacillus subtilis, S. aureus, E. coli, and P. aeruginosa | Food pathogens | MIC: 0.4–4.5 mg mL−1 | Poland | 36 |
| L. officinalis | Aeromonas hydrophila, Lactococcus garvieae, and Vagococcus salmoninarum | Fish | MIC: 500–62.5 μl mL−1 | Turkey | 73 |
| L. stoechas | E. coli, L. monocytogenes, and Salmonella typhimurium | Common foodborne pathogens | MIC: 5–80 μl mL−1 | Turkey | 74 |
| L. angustifolia | Pseudomonas spp. and Enterobacteriaceae | Chicken | 0.2% EO in vacuum packaging | Slovakia | 75 |
| L. angustifolia | E. coli and L. monocytogenes | Chicken | 0.4 ml L−1 in drinking water | Poland | 76 |
| L. stoechas | Pseudomonas spp. and Enterobacteriaceae | Poultry meat | 100–200 ppm EO | Tunisia | 77 |
| L. angustifolia | L. monocytogenes and S. typhimurium | Common foodborne pathogens | MIC: 62.5 ml mL−1 | Turkey | 74 |
| L. angustifolia | E. coli | Pork sausages | (0.2%) | Seria | 78 |
| L. stoechas | E. carotovora | Causing potato soft rot | MIC: 5–10 μl mL−1 | Greece | 79 |
| L. angustifolia | Total mesophilic microorganisms | Cucumber | 100–200 ml L−1 of EO vapor | Greece | 80 |
| L. hybrida | E. coli, S. aureus, and B. cereus | Pathogenic food-borne bacteria | MIC: 0.25–0.5 mg mL−1 | Spain | 81 |
| L. angustifolia | S. aureus and E. coli | Food-borne bacteria | MIC: 0.16–20 mg mL−1 | Poland | 82 |
| L. angustifolia | S. aureus and E. coli | Cake | 600 ppm EO | Egypt | 83 |
| L. stoechas | E. coli | Milk | 59.4% | Tunisia | 84 |
| L. stoechas | S. aureus | Milk | 6.8% | Tunisia | 85 |
| L. angustifolia | Lactobacillus acidophilus, and Bifidobacterium bifidum | Fermented milk and Yogurt | 1–3% EO | Iran | 86 |
| L. angustifolia | Pseudomonas savastanoi | General food pathogens | 3000 mg mL−1 and 4000 mg mL−1 | Algeria | 49 |
| L. angustifolia | E. coli, S. aureus, S. abony, P. aeruginosa, and B. subtilis | Chocolate | MIC: 62.5–125 ml mL−1 | Italy | 87 |
| L. angustifolia | S. aureus and P. aeruginosa | General food pathogens | MIC: 0.25 ml mL−1 | South Africa | 88 |
| L. angustifolia | S. aureus, E. coli, and L. monocytogenes | Cherry tomatoes | 50–300 ml/10 mL in nano-emulsions | China | 89 |
| L. officinalis | Aerobic Mesophilic Bacteria, and Psychotropic bacteria | Lamb meat | 3% (W/W) | Turkey | 90 |
| L. angustifolia | Pseudomonas tolaasii | Button mushroom | 0.1–0.4% | Netherlands | 91 |
| L. angustifolia | S. aureus, E. coli, B. subtilis, and Staphylococcus epidermidis, P. aeruginosa, and S. enterica sub sp. enterica | — | MIC: 17–97 μg mL−1 | Bosnia | 33 |
| L. officinalis | E. coli and S. aureus | General food pathogens | MIC: 1000–1200 ppm | Argentina | 92 |
| L. x intermedia, L. angustifolia | S. enterica | Food pathogens | MIC≥ 10.0 μl mL−1 | Italy | 93 |
| L. angustifolia | E. coli and S. aureus | Beef | MIC 0.25 μl mL−1 | Algeria | 94 |
3.1. Antibacterial efficacy of free LEOs
Free LEOs have been extensively studied for their antibacterial activity. Researchers have demonstrated that LEOs exhibit broad-spectrum antibacterial properties, effectively inhibiting the growth of both Gram-positive and Gram-negative bacteria.95 These properties have been confirmed through various studies showing significant antibacterial effects against a range of pathogens. For instance, LEOs exhibited potent antibacterial activity against fish pathogenic bacteria isolated from olive flounder.73 The study demonstrated that LEOs were effective in inhibiting both Gram-negative (Aeromonas sobria, Aeromonas caviae, Aeromonas hydrophila, Vibrio anguillarum, Pseudomonas aeruginosa, Yersinia ruckeri, Edwardsiella tarda, Lactococcus garvieae, and Vagococcus salmoninarum) and Gram-positive (Staphylococcus warneri) bacteria, highlighting their potential as natural antibacterial agents in aquaculture. A similar study by Walasek-Janusz et al.82 highlighted the antimicrobial activity of L. angustifolia crude oil against bacteria and yeast and concluded that LEOs showed antibacterial properties with MICs ranging from 2.5–10 mg mL−1 against tested microorganisms. In addition to their antimicrobial properties, free LEOs also exhibit strong antioxidant activities,82 further enhancing their utility in food preservation. The presence of phenolic compounds such as rosmarinic acid and caffeic acid in free LEOs contributes to their ability to scavenge free radicals and reduce oxidative stress.In another study, L. angustifolia EO was shown to inhibit the growth of E. coli, S. aureus and P. aeruginosa, with average MICs of 3.33, 1.33 and 42.67 μl mL−1 respectively.96 Similarly, L. latifolia EO rich in camphor and 1,8-cineole exhibits significant antibacterial activity against Staphylococcus aureus and Listeria monocytogenes at 2.5 and 5 mg mL−1, respectively.97 The antibacterial efficacy of LEOs is primarily attributed to their major constituents, such as linalool, linalyl acetate, camphor, and 1,8-cineole, which possess strong antimicrobial properties.98 Few studies investigated the mode of action of LEO against bacterial cells. For instance, in the study by Benbrahim et al.,99L. dentata EO caused disruption of cell organelles of K. pneumoniae (Fig. 2). According to Benbrahim et al.,99 the antibacterial efficacy of LEOs is largely due to their ability to disrupt microbial cell membranes, which leads to leakage of cellular contents and eventual cell death.
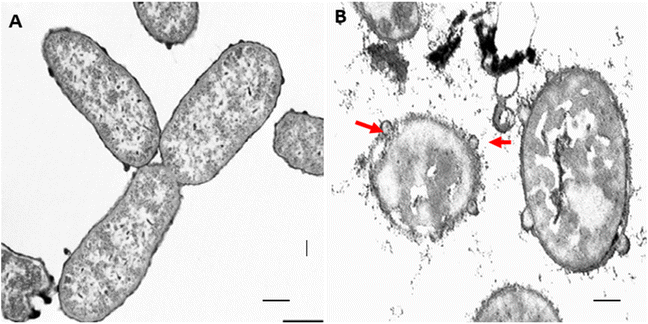 | ||
| Fig. 2 Images showing the impact of L. dentata EO on the cell membrane and cell organelles of Klebsiella pneumoniae, A-bacterial cells of the control and B-bacterial cells treated with L. dentata EO showing disruption of cell membrane cell organelles (indicated with red arrow). Adopted with permission from Benbrahim et al.99. | ||
In a study, Speranza et al.100 reported that LEOs showed strong antibacterial activity against L. monocytogenes and Salmonella enterica, two common pathogens in food products, with MICs as low as 0.3 μl mL−1. Another study carried out by Salavati Hamedani et al.98 demonstrated that LEOs effectively inhibit the growth of common foodborne pathogens such as E. coli, S. aureus, L. monocytogenes, B. cereus, and S. typhi. The oils caused significant leakage of intracellular components, leading to bacterial cell death even in the absence of linalool and linalyl acetate, compounds often thought to be key to Lavandula EOs' antibacterial properties. This ability to target multiple pathogens makes LEOs highly versatile as natural preservatives. In addition, Carrasco et al.16 found that the high linalool content in L. angustifolia EOs (37–54%) contributes to its broad-spectrum antibacterial activity by disrupting the integrity of microbial membranes, thus inhibiting the growth of bacterial pathogens involved in food spoilage.
Similarly, Benaiche et al.101 found that LEOs exhibited strong antibacterial effects against P. aeruginosa and S. aureus, pathogens known to contribute to foodborne illnesses. In this study, the EOs were especially effective in inhibiting P. aeruginosa growth, a notorious bacterium responsible for spoiling perishable foods. The study revealed that the MIC of L. angustifolia EO was as low as 0.3 μl mL−1, demonstrating significant potential as a food preservative. These findings underscore the potential of LEOs as natural antibacterial agents against foodborne pathogens, offering a safer alternative to synthetic antibiotics. In particular, Gram-positive bacteria are more susceptible towards EOs than Gram-negative bacteria. The cell membrane of Gram-negative bacteria contains hydrophilic lipopolysaccharides that acts as a barrier to macromolecules and hydrophobic compounds, thus providing enhanced tolerance to hydrophobic antimicrobial compounds such as those found in EOs.102 Therefore, it is difficult to predict the susceptibility of microorganisms to EOs due to broad genetic variations among species.
Although LEOs are well-known for their antimicrobial properties, some studies have shown that their efficacy can sometimes be lower than that of other EOs. For instance, in a study, Sienkiewicz et al.103 evaluated the antibacterial activities of LEOs and Thyme vulgaris EOs against 120 bacterial strains, and reported LEOs had lower efficacy than T. vulgaris EO screened against P. aeruginosa. Thyme oil exhibited significantly stronger antibacterial effects against Staphylococcus, Enterococcus, Escherichia, and Pseudomonas genera, making it a more potent option for food preservation and safety applications. In another similar study, Rota et al.104 specifically found L. angustifolia and L. latifolia EOs to be less effective than thyme (T. vulgaris L), and savory (Satureja montana L.) EOs in combating foodborne pathogens such as Salmonella enteritidis, S. typhimurium, Yersinia enterocolitica, E. coli, L. monocytogenes, Shigella flexneri, and S. aureus. They evaluated several EOs such as EOs from T. vulgaris from Spain and France, Salvia sclarea, S. officinalis, S. lavandulifolia, L. latifolia, L. angustifolia, three hybrids of L. latifolia × L. angustifolia (Lavandin ‘Super’, Lavandin ‘Abrialis’, and Lavandin ‘Grosso’), Rosmarinus officinalis, Hyssopus officinalis, and S. montana. In each experiment, thyme EO showed more antibacterial activity than LEOs against all the test pathogens at lower concentrations. The variation in efficacy of LEOs and thyme EO may be due to variations in their chemical constituents as well as their origin. Furthermore, in cases where LEOs possess poor efficacy, their synergistic application with other EOs can be recommended as antibacterial agents against foodborne pathogens.
3.2. Antibacterial efficacy of encapsulated LEOs
In recent years, encapsulation techniques have revolutionized the application of EOs as antimicrobial agents in food systems or crop protection by overcoming their inherent limitations, such as volatility, low solubility, and sensitivity to environmental factors.105 Nanoencapsulation, spray-drying, cyclodextrin encapsulation, and double-layer encapsulation techniques offer controlled release, improved stability, and targeted effects against harmful microorganisms in food systems.106 The different techniques used for the encapsulation of EOs are summarized in Fig. 3 and have been discussed in our previous reviews.107,108 Among these techniques, nanoencapsulation stands out for enhancing bioavailability and antimicrobial efficacy, as demonstrated by polymeric nanocapsules loaded with carvacrol and thymol, which exhibited superior bactericidal activity and stability over 20 days.109 Spray-drying methods, employing protective matrices such as maltodextrin, have shown to stabilize thyme EO, significantly extending its antimicrobial properties in meat products.110 Cyclodextrin inclusion complexes provide another approach, with hydroxypropyl β-cyclodextrin encapsulation ensuring controlled EO release and sustained antimicrobial effects.111 Double-layer encapsulation, combining proteins and polysaccharides, has been successful in protecting pink pepper EO and enhancing its bioactivity in dairy matrices.112 High-frequency ultrasound emulsification produces stable microemulsions, effectively delivering antimicrobial action against pathogens like Listeria monocytogenes and E. coli.113 These encapsulation strategies not only preserve the functional properties of EOs but also enhance their controlled release and applicability as natural food preservatives. LEOs have also been encapsulated using these techniques to determine their effectiveness against harmful organisms that degrade food commodities/crops.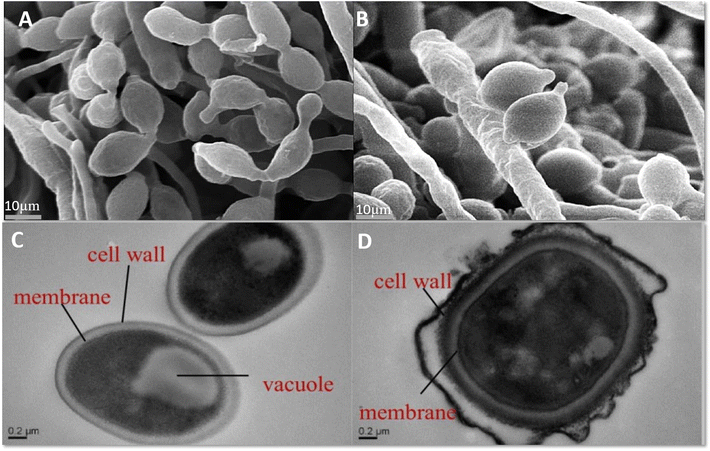 | ||
| Fig. 3 Images showing scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images of Monilinia fructicola, a fruit rot pathogen exposed to LEO (800 μL L−1). A and C are control sets, B and D are treated colonies showing abnormal cell structure in SEM and damage of cell membrane and cellular organelles in TEM analysis, respectively (Adopted with permission from Xiong et al.135). | ||
In many studies, encapsulated LEOs in various emulsion/particle systems have shown greater efficacy against foodborne bacteria than the LEOs used in crude form. For instance, encapsulated L. latifolia EOs exhibited enhanced antibacterial activity against L. monocytogenes compared to the crude oil.97 The study found that the encapsulated form was more effective in disrupting bacterial cell membranes and inhibiting cell growth, highlighting the potential of encapsulation to improve the bioavailability and efficacy of LEOs. In addition, Yuan et al.114 in their investigation found that encapsulated L. angustifolia EOs in alginate beads with 1.316 g hydroxypropyl-β-cyclodextrin which provided a controlled release of linalool and linalyl acetate, resulting in a sustained antibacterial effect against B. cereus and S. typhimurium.
A study by Türkoğlu et al.115 explored the antibacterial efficacy of encapsulated L. angustifolia EO with bead sizes of 2.2 μm and 5.2 μm and found that it was more potent than the crude EO against E. coli and S. aureus. The encapsulation in these forms was not only effective in inhibiting bacterial growth but also demonstrated potential for use as an antibacterial agent due to its sustained release properties. Another study carried out by Balasubramanian and Kodam116 encapsulated L. angustifolia EO in electrolyte-assisted polyacrylonitrile nanofibers. The encapsulated EO, with 88.44 nm bead size and concentrations ranging from 12.5 to 200 μg mL−1, exhibited potential growth inhibition (14–15 mm zone of inhibition) against S. aureus and Klebsiella pneumoniae. This method of encapsulation significantly enhanced the antibacterial properties of LEOs. Additionally, Silva et al.117 demonstrated the encapsulation of L. latifolia EO in gelatin nanoparticles with an average bead size of 100 nm and a concentration of 500 μg mL−1. The study showed significant antibacterial activity against S. aureus, highlighting the potential of this encapsulation method in enhancing the antimicrobial properties of EOs. Encapsulated LEOs also offer controlled release properties, allowing for a sustained and gradual release of active compounds over time. This controlled release mechanism ensures a prolonged antibacterial effect, which is particularly beneficial in food preservation. The controlled release system not only enhance the antibacterial efficacy but also minimize the sensory impact on food products, making it a promising approach for natural food preservation. Additionally, the literature on the efficacy of encapsulated LEOs against phytopathogenic bacteria hampering food crops is limited and needs further exploration in future research.
4. Use of LEOs as antifungal agents against food pathogens
Food commodities are also affected by several groups of fungi during preharvest and postharvest stages. The major fungal communities responsible for the biodeterioration of food commodities and field crops are described in our previous review.102 During infection, these pathogenic fungi also produce mycotoxins and render food unhealthy for consumption.118,119 In tropical climates fungal infection of food and foodstuffs is a major problem due to high humidity. Fungal infections deteriorate both quality and quantity of produce and losses have been estimated up to 60% worldwide.59 By affecting quality, fungal pathogens reduce the nutrient content of food commodities, e.g. proteins in pulses.120 During infection process, fungi also produce various types of mycotoxins depending upon the commodities, which can lead to famines in developing countries.121 In this regard, food contamination by Alternaria, Penicillium, Aspergillus, Rhizopus, and Fusarium spp. is of great significance because of the related health hazards and other environmental problems.122 Hence, prevention of fungal growth by EOs during storage and transit as well as at the field level could be a cost-effective approach to reduce economic losses of food commodities. Worldwide, the antifungal potential of LEOs and other EOs is increasingly considered as important.123–126 From time to time many investigations have focused on studying the antifungal activity of LEOs both in their crude and encapsulation forms against fungal pathogens infecting various food commodities/crops. Some of the potential findings are discussed below.4.1. Fungitoxic activity of free LEOs
The LEOs also exhibit notable antifungal activities, making them effective against various fungal pathogens that contaminate food products and infect field crops.58,107,108 The previously published research papers on LEOs revealed that they have potential fungitoxic activity against a wide range of fungal pathogens causing food contamination and mycotoxin development. Studies have demonstrated that LEOs can inhibit the growth and proliferation of fungi such as Aspergillus niger, Penicillium expansum, and Fusarium oxysporum infecting food commodities during storage.127 Another study found that the crude oil of L. stoechas exhibited strong antifungal activity against Candida albicans and Aspergillus niger.128 In one study, 60 μL of EO derived from L. stoechas L. ssp. stoechas was effective against Rhizoctonia solani and Fusarium oxysporum, while less effective against A. flavus.129 While in another study, L. stoechas subsp. luisieri EO showed strong fungitoxicity against all pathogens infecting strawberries fruits including A. carbonarius, Rhizopus stolonifer, Penicillium brevicompactum, Aureobasidium pullulans, and Saccothecium rubi, with MIC and MFC values ranging from 0.07–0.29 μl mL−1 and 0.58–9.33 μl mL−1, respectively.130Some important references on the antifungal activity of LEOs against fungal pathogens infecting food and food products/commodities are summarized in Table 3. As summarized in this table, the majority of studies showed that the potential antifungal activity of LEOs may be attributed due to presence of linalool, linalyl acetate, camphor, and 1,8-cineole.130 The study also revealed that the higher concentrations of 1,8-cineole and camphor present in the crude oil disrupt fungal cell wall synthesis and inhibit spore germination, thereby reducing fungal proliferation. For instance, Xiong et al.135 reported that LEO effectively inhibited the growth of Monilinia fructicola by damaging their cell walls and membranes. Later, Soylu et al.108 also revealed that exposure of Botrytis cinerea infecting tomatoes to L. stoechas EO at 25.6 μg mL−1 caused considerable morphological degeneration of the fungal hyphae such as vacuolations, cytoplasmic coagulation, hyphal shriveling and protoplast leakage and loss of conidiation (Fig. 4). In addition, recent reports showed that LEOs also interfere with protein synthesis and energy metabolism in fungal pathogens, e.g., Fu et al.162 found that treatment of Ustilaginoidea virens, an infectious agent responsible for rice false smut disease, with LEO resulted in the downregulation of genes related to cell wall synthesis, cell membrane synthesis, protein synthesis, and the energy metabolism pathway.
| Lavandula species | Target fungal species | Source | Effective doses | Origin | References |
|---|---|---|---|---|---|
| L. officinalis | Penicillium expansum and Botrytis cinerea | Apples | 1% EO | Italy | 131 |
| L. angustifolia | P. expansum and P. crustosum | Meat ball | 500 μl L−1 | Slovakia | 132 |
| L. angustifolia | Aspergillus niger and Penicillium spp. | Bread dough | 2.5% | Bulgaria | 133 |
| L. angustifolia | P. expansum, P. crustosum, and Aspergillus flavus | Bread | 125–500 μl L−1 | Slovakia | 134 |
| L. angustifolia | A. niger and P. expansum | Food pathogens | 0.4 to 4.5 μg mL−1 | Poland | 36 |
| L. angustifolia | Monilinia fructicola | Flat peaches | 800 μl L−1 | China | 135 |
| L. angustifolia | M. fructicola | Apricots | 1% | Pakistan | 136 |
| L. angustifolia | P. chrysogenum, Fusarium moniliforme, A. niger, and A. flavus | Chocolate | MIC: 62.5–125 lL mL−1 | Italy | 87 |
| L. stoechas | B. cinerea | Tomato | 1.6 μg mL−1 air | Turkey | 137 |
| L. stoechas | P. infestans | Late blight in tomato | 12.8–51.2 μg mL−1 | Turkey | 138 |
| L. angustifolia | A.nidulans, Leptosphaeria maculans and Sclerotinia sclerotiorum | Agricultural fungi | MIC: 0.5–2.0 μl mL−1 | Australia | 127 |
| L. angustifolia | Verticillium fungicola | Causing dry bubble in mushrooms | 0.5–1 mg cm−3 | Poland | 139 |
| L. angustifolia | Pseudomonas tolaasii | Button mushroom | 70% aqueous solution | Iran | 140 |
| L. stoechas subsp.Stoechas | P. infestans | Late blight disease in tomato | 0.4–2.0 μg mL−1 air | Turkey | 141 |
| Lavender | M. perniciosa | Button mushroom | 2000 μl L−1 | Iran | 142 |
| L. stoechas | Cladobotryum sp. | Causing cobweb disease in mushrooms | 1.6 μg mL−1 air concentration | Serbia | 143 |
| L. angustifolia | Phoma exigua var.foveata | Causing potato gangrene | 0.1–0.4% | Russia | 144 |
| L. angustifolia | B. cinerea, S. sclerotiorum, F. oxysporum, Phytophthora parasitica, Pythium aphanidermatum, Alternaria brassicae, Cladobotryum mycophilum, and Trichoderma aggressivum f.sp. europaeum | Vegetables and button mushroom | 5–32 μl mL−1 | Spain | 145 |
| L. angustifolia | Colletotrichum nymphaeae | Strawberry anthracnose | EC50: 12.97 ppm (mycelial inhibition) | Iran | 146 |
| L. viridis | Cryptococcus neoformans | Agricultural fungi | MIC 0.32–0.64 μl mL−1 | Portugal | 147,148 |
| L. angustifolia | A. niger and A. tubingensis | Grapes | 0.313 μL cm−3 | Slovakia | 149 |
| L. × hybrid | Botrytis cinerea | Grey mold in grapes | Vapors at 50 kPa reduced 65% | Italy | 150 |
| L. angustifolia | Epicoccum nigrum | Sugarcane, potatoes, and marine plant | MIC: 10.0 to 100.0 μl mL−1 | Serbia | 151 |
| L. stoechas | Fusarium oxysporum f.sp. radicis-cucumerinum | Cucumber | MIC: 0.125–1 μl mL−1 | Turkey | 152 |
| L. angustifolia | P. brevicompactum, P. citrinum, P. crustosum, P. expansum and P. griseofulvum | Stored fruits and vegetables | 2.5, 1.5, 3.5, 3.0, and 3.25 μl mL−1 | Slovakia | 153 |
| L. angustifolia | A. niger and A. flavus | Cereal grains, legumes, and tree nuts | 0.52 to 1.00 mg mL−1 | South Korea | 154 |
| L. officinalis | Monilinia laxa and B. cinerea | Stone fruits | 1% EO | Italy | 155 |
| L. angustifolia | Rhizopus stolonifer, B. cinerea, and Aspergillus niger | Pathogenic fungi | EC50 (311.24 ppm) | Iran | 156 |
| L. angustifolia | Fusarium roseum | Pathogenic fungi | MIC: 3000 μg mL−1 | Algeria | 49 |
| L. angustifolia | Eurotium amstelodami, E. herbariorum, E. repens, E. rubrum, A. flavus, A. niger, and Penicillium corylophilum | Bakery products | MIC: 500 μl L−1 | Slovakia | 157 |
| L. angustifolia | C. cladosporioides | Lesions on berries | 625 μl L−1 air | Slovakia | 158 |
| L. angusti folia | Verticillium fungicola and Trichoderma harzianum | Button mushroom | 0.1–0.4% | Netherlands | 91 |
| L. angustifolia | B. cinerea | Strawberry | 0.125–0.25 g per plate or sachet | Thailand | 159 |
| L. angustifolia | B. cinerea | Grey mold in grapes | 0.125–0.25 g in alginate beads | Thailand | 160 |
| L. angustifolia | Colletotrichum gloeosporioides | Avocado anthracnose | 0.05–0.2% EO | USA | 161 |
 | ||
| Fig. 4 Outline of the most common LEO delivery systems, materials used, fabrication techniques, and applications in food/crop protection. | ||
Aside from their antifungal mode of action, LEOs have also shown inhibition of mycotoxin production in food commodities. For example, L. angustifolia EO was found to be effective in reducing mycotoxin production by A. flavus.163 In their another study,164 LEO was found to be less effective than thyme, clove, oregano, cinnamon and lemongrass in inhibiting mycotoxin production in bread samples by four strains of Aspergillus (A. flavus, A. parasiticus, A. ochraceus and A. westerdijkiae). Similar results were reported by Hlebová et al.165 who found that L. angustifolia EO had a less toxic effect than cinnamon bark, lemongrass, and litsea EOs which were able to significantly inhibit the growth, sporulation, and mycotoxin production by toxigenic A. ochraceus and A. parasiticus. In the same line, LEO was less effective than star anise EO at 0.5 μl g−1 against A. flavus and A. parasiticus causing aflatoxin production in sorghum and peanut.166 Conversely, LEO completely inhibited the mycotoxin production and proliferation of Penicillium digitatum in lemon fruits at 350 μL per air and was found more effective than mint and basil EOs.167 The study attributed this effect to the high levels of linalool and linalyl acetate in the crude oil, which inhibited the enzyme activities involved in mycotoxin biosynthesis. The variation in efficacy might be due to variation in the chemical constituents of EOs or the origin of fungal strains. Furthermore, where LEOs show poor effectiveness, their synergistic application in food systems to prevent mycotoxin contamination/production should be adopted. These findings highlight the potential of LEOs as natural antifungal agents in food preservation, offering an alternative to synthetic fungicides that are often associated with health and environmental concerns.
4.2. Fungitoxic activity of encapsulated LEOs
As previously described, encapsulation enhances the antifungal activity of EOs by providing a controlled release mechanism and protecting the active compounds from degradation. Encapsulated LEOs offer several advantages over their crude form, particularly in terms of improving their antifungal efficacy and application in food preservation. Researchers encapsulated LEOs including L. angustifolia and L. latifolia EOs in various forms to enhance their antifungal properties against pathogens infecting food commodities/crops. One decade ago, Soylu et al.108 focused their study on L. angustifolia encapsulated oil with MIC values ranging from 0.32–0.64 μl mL−1, which exhibited significant antifungal activity against B. cinerea, infecting tomato. Previously, Inouye et al.168 also highlighted the encapsulated oil's inhibitory effects on A. fumigatus showcasing its broad-spectrum antifungal potential. The oils were composed of 3 groups. The first group included citron (Citrus medica L), LEO and tea tree oils (Camellia sinensis (L.) Kuntze). The 2nd group consisted of perilla (Perilla frutescens (L.) Britt.) and lemongrass oils (Cymbopogon citratus DC. Stapf.) and the third group consisted of cinnamon bark (Cinnamomum verum J.S. Presl) and thyme oil (Thymus vulgaris L).In 2020, Hammoudi et al.169 developed alginate–montmorillonite nanocomposite films incorporating LEOs. These films exhibited potent antifungal activity against common pathogenic fungi such as B. cinerea and Alternaria alternata infecting fruits and vegetables. The encapsulated LEOs provided sustained release and improved antifungal efficacy.169 Another study by Fagundes et al.170 tested the antifungal activity of food additives, including LEOs, in hydroxypropyl methylcellulose (HPMC)-lipid edible coatings. The coatings were effective in reducing fungal growth on cherry tomatoes, highlighting the potential of encapsulated LEOs in food preservation, because the antifungal properties of LEOs are preserved over a longer period, making them more effective in food preservation. The nanoemulsions provided a protective barrier around the oil droplets, preventing the loss of volatile compounds and maintaining their efficacy. The controlled release system not only enhanced the antifungal efficacy but also minimized the sensory impact on food products, making it a promising approach for natural food preservation.
5. Potential of LEOs in preservation of food commodities
A significant number of studies have been conducted on the potential applications of LEOs in food preservation, either in their free form or encapsulated, due to their potent antimicrobial and antioxidant properties. The active components of these oils, particularly linalool, linalyl acetate, and camphor, have been shown to combat foodborne pathogens and prevent oxidative degradation of food products. As consumers move toward more natural preservatives, these LEOs have emerged as effective alternatives to synthetic preservatives. LEOs exhibit both antibacterial and antifungal effects, helping to protect food products and perishables from microbial contamination while extending their shelf life.In 2018, Blažeković et al.37 demonstrated the potential of L. angustifolia EO against Candida albicans and food spoilage bacteria and reported that the EO showed a broad-spectrum of antibacterial (MICs 0.25–2.5 mg mL−1) and antifungal (MICs 0.1–2 mg mL−1) activities. The EO was able to inhibit spore germination and fungal growth, highlighting its potential use in controlling fungal spoilage in food products. Similarly, another study by Al-Ansari et al.45 explored the antifungal activity of L. latifolia EO against Trichophyton mentagrophytes, a major fruit-spoliating fungus in custard apple. The results revealed that L. latifolia EO, which is rich in camphor and 1,8-cineole, exhibited a strong fungicidal effect, with an MIC value of 0.125 μg mL−1. In another study, Sun et al.89 used LEO nano-emulsions in the range of 50–300 μl/10 mL incorporated into gelatine films and found strong antibacterial effects against food spoilage bacteria such as Staphylococcus aureus, Escherichia coli, and Listeria monocytogenes. Applied to cherry tomatoes, the films effectively extended shelf life for over 7 days at 25 °C by reducing weight loss, delaying acid and phenolic component degradation, and suppressing microbial growth, indicating improved preservation compared to untreated controls.
The antifungal food preservative properties of Lavandula EO were further supported by Kahramanoğlu et al.,171 who studied the effects of L. angustifolia EO on B. cinerea, the fungus responsible for gray mold in strawberries. In an in vivo vapor application, L. angustifolia EO significantly reduced the severity of fungal infections while maintaining the fruits' weight and sugar contents during storage. They also found that strawberries treated with L. angustifolia EO showed a 50% reduction in mold growth compared to untreated fruits, which contributed to a significant increase in their shelf life. Untreated strawberries typically developed mold within 4 to 5 days, while those treated with LEO remained mold-free for an additional 5 to 7 days, thereby extending the total shelf life to around 10–12 days. This finding suggests that LEOs are effective natural alternatives to synthetic fungicides, especially in the preservation of high-moisture fruits.
The use of LEOs in food preservation is further enhanced by their incorporation into active packaging materials. A study conducted in 2023 (ref. 172) explored the application of LEO as a treatment for packaging paper, showing its effectiveness in extending the shelf life of packaged food items by preventing microbial spoilage. In this study, LEO-treated paper exhibited a 60–90% reduction in microbial growth within the first two hours, maintaining its effectiveness for up to 120 hours. The EO was particularly effective against S. aureus, B. cereus, E. coli, P. aeruginosa, Salmonella abony, Saccharomyces cerevisiae, Aspergillus brasiliensis, and Fusarium moniliforme, making it a promising solution for food preservation in biodegradable and eco-friendly packaging materials. In another study, Tancinova et al.158 demonstrated that EOs from the Lamiaceae family, including L. angustifolia, significantly inhibited the growth of Cladosporium cladosporioides, a common pathogen responsible for post-harvest spoilage of berries. The study revealed that the application of L. angustifolia EO could extend the shelf life of fruits by inhibiting fungal growth by up to 100% during the 14-day cultivation period, compared to the control group, which showed complete fungal colonization and spoilage during this time. Such applications align with the growing consumer demand for natural preservatives and eco-friendly packaging solutions in the food industry.
Furthermore, Caprari et al.173 investigated the effect of L. angustifolia EO on the shelf life of stored apples. The results indicated that the application of LEOs delayed the onset of fungal spoilage by 30% to 50% compared to the control group. While untreated apples developed fungal infections within 14 days of storage, the lavender-treated apples remained mold-free for an additional 7–10 days, significantly prolonging their shelf life. Thus, these LEOs can be used for the fabrication of botanical fungicides for the safe storage of food commodities. However, before making recommendations, their large-scale evaluation at the warehouse/at field level is required.
6. Use of LEOs as insecticidal agents in food commodities/crops
Food commodities/crops are deteriorated by several insects during storage and pre-harvest, as noted by Gupta et al.174 Crops infested with insect pests have undergone significant changes since the dawn of the 21st century due to technological and ecological changes. While the numbers of many insect pests have declined, there has been an increase in the prevalence of other insect pests, including mealy bugs, particularly Phenacoccus solenopsis Tinsley, Spodoptera litura Fab., Callosobruchus species, diamond back moths, Plutella xylostella L., and Tribolium species, on various food crops or in stored grains. Globally, 40–80% crop losses have been estimated due to infestation by insect pests at both the field and storage levels.175 Plant-based insecticides have become more popular in food crops due to the health hazards associated with synthetic insecticides used extensively for crop protection and post-harvest grain storage. There is no doubt that plant-based botanical insecticides are among the most promising, eco-friendly, and sustainable approaches for controlling insect pests on food crops. Since insect pests have developed resistance to commercial insecticides, EO-based botanicals developed from many aromatic plants have been evaluated as potential sources of repellents, ovicides, antifeedants, and insecticides to control insect pests.176 In this section, we have summarized the efficacy of LEOs against insect pests under both storage and field conditions. The insecticidal activity of LEOs is well-supported by numerous studies compiled in Table 4.| Lavandula species | Target insect pests | Sources | Effective doses | Country | References |
|---|---|---|---|---|---|
| L. angustifolia | Sitophilus zeamais | Maize, cereals | 200 μl kg−1 | Cameroon | 177 |
| L. angustifolia | Tribolium castaneum, Rhyzopertha dominica, and Trogoderma granarium | Flour, grains, cereals | 2–6% | Pakistan | 178 |
| L. angustifolia | T. castaneum, Sitophilus oryzae, Stegobium paniceum, and Plodia interpunctella | Cereals | 100–300 μl g−1 | India | 179 |
| L. angustifolia | T. castaneum, Sitophilus granarius, and Oryzaephilus surinamensis | Stored cereals, dried fruits, and flour | 42.51–374.16 μl L−1 air | Iran | 180 |
| L.angustifolia | Sternechus pinguis and Rhyssomatus subtilis | Soybean plants | 0.40–1 μl cm−2 | Argentina | 181 |
| L. angustifolia | Plutella xylostella and Cotesia glomerata | Cruciferous vegetables, including cabbage and broccoli | 1–6 g L−1 | South Korea | 182 |
| L.angustifolia | Xanthogaleruca luteola | Elm tree leaves | 287.5 ppm | Iran | 183 |
| L. angustifolia | Sitophilus granaries | Wheat, barley, and rye | 0.449 mg per adult (contact toxicity) | Italy | 29 |
| L. angustifolia | S. oryzae | Stored rice, wheat, barley | 6 mg cm−2 (contact toxicity | Saudi Arabia | 184 |
| L. angustifolia | Ceratitis capitata | Citrus, stone fruits, and other soft fruits | 0.1 μL per fly (topical application) | Italy | 185 |
| L. angustifolia | C. capitata | Citrus, stone fruits, and other soft fruits | 0.1 μL per fly (topical application) | Italy | 185 |
| L. angustifolia | Tribolium confusum | Flour, stored grains, and cereal products | Various | Algeria | 186 |
| L. angustifolia | Plodia interpunctella | Dry food products, grains, and cereals | LD50 22.8 μg cm−2 (contact toxicity) | Argentina | 187 |
| L. latifolia | Drosophila suzukii | Soft fruits such as strawberries, cherries, and blueberries | EC50 3.79 μL oil per L air (fumigation) | Canada | 188 |
| L. angustifolia | Rhipicephalus annulatus | Cattle | 0.5–8% w/v (acaricidal) | Iran | 189 |
| L. angustifolia | Euphoria leucographa | Beetle larvae feed on crop roots | EC50 0.37% (fumigation | Saudi Arabia | 45 |
| L. angustifolia | Ephestia kuehniella | Stored flour and grains | 225 μl per L air (fumigation) | Turkey | 190 |
| L. angustifolia | Acanthoscelides obtectus | Bean seeds | 13.33–106.66 μl per L air (fumigation) | Algeria | 191 |
| L. angustifolia | Callosobruchus chinensis, and C. maculatus | Cowpeas, mung beans, and other legumes | 0.5–1% (contact toxicity) | Egypt | 192 |
| L. stoechas | Tetranychus cinnabarinus | Tomatoes, cucumbers, beans, and other greenhouse crops | LC50: 2.92 μg mL−1 | Turkey | 193 |
| L. angustifolia | S. granaries | Wheat, barley, oats | LC50: 1.5 mg L−1, LC90: 4.1 mg L−1 | Italy | 194 |
| L. spica | T. confusum | Flour, stored grains | LC50: 19.5 μL per L air | Algeria | 195 |
| L. angustifolia | S. oryzae | 100% mortality at 6 mg cm−2 | Saudi Arabia | 196 | |
| L. angustifolia | S. granarius | Wheat, barley, oats | LC50: 1.5 mg L−1 without wheat, 10.9 mg L−1 with wheat | Italy | 197 |
A study by Al-Ansar et al.45 evaluated the insecticidal activity of L. latifolia EO. The research revealed that gamma-terpinene, camphor, and 1,8-cineole were the predominant components, contributing to the oil's efficacy against Euphoria leucographa Gory and Percheron, a pest affecting custard apple fruit. The EO exhibited strong contact toxicity and fumigant activity, with an effective concentration (EC50) value of 0.37%, highlighting its potential as a natural insecticide. L. angustifolia EO has also demonstrated efficacy against the pea aphid, Acyrthosiphon pisum Harris.198 The investigators conducted fumigation tests that showed increased mortality rates of aphids with higher concentrations of the oil (Table 4). The major components, including linalool and linalyl acetate, played a crucial role in achieving an LC50 value of 11.2 μl L−1 of air. The study emphasized the importance of the complete mixture of constituents for maximum toxicity, suggesting the potential of L. angustifolia oil as a bioinsecticide.
Furthermore, the combined use of 1.14 and 1.7 μl L−1 air of L. angustifolia EO with 200 Gy gamma irradiation was explored for controlling the Mediterranean flour moth, Ephestia kuehniella Zeller. In this regard, Zallaghi and Ahmadi199 found that the combination treatment significantly increased mortality rates and reduced growth rates compared to treatments with either the oil or gamma radiation alone. The results suggest a synergistic effect, enhancing the insecticidal efficacy of L. angustifolia EOs.200 In addition to direct insecticidal effects, LEOs can enhance the efficacy of conventional insecticides when used together. In this regard, Faraone et al.200 investigated the synergistic effects of L. angustifolia and T. vulgaris EOs with imidacloprid and spirotetramat against the green peach aphid, Myzus persicae Sulzer. The effective dose for the EOs in the study was approximately 0.3% v/v for both LEO and thyme EOs. The study revealed that L. angustifolia EO significantly enhanced the toxicity of imidacloprid, indicating the potential for using EOs to reduce the required doses of synthetic insecticides.
Lavandula angustifolia EO has also been found to be effective against the lesser mulberry pyralid, Glyphodes pyloalis Walker.202 Yazdani et al.201 reported that the major constituents of LEO, such as borneol and linalool, significantly reduced the total protein, carbohydrate, and lipid contents in the larvae of G. pyloalis, impacting their growth and development. The study highlighted the potential of L. angustifolia EO as a natural insect growth regulator. Moreover, the insecticidal activity of L. angustifolia EO against the rice weevil, Sitophilus oryzae L., has also been investigated by Al-Harbi et al.196 who demonstrated that the EO caused 100% mortality at a concentration of 6 mg cm−2 within 24 hours of exposure. The study also noted a significant upregulation of detoxification and cytochrome P450 genes, indicating the impact of EOs on the metabolic pathways of the insect.
Besides, Germinara et al.203 evaluated the contact and fumigant toxicity of L. angustifolia EO against the granary weevil, Sitophilus granarius. The study found that the major components of the EO, including linalool and 1,8-cineole, provided significant protection against the pest, suggesting its use as a natural preservative for stored grains. Furthermore, LEOs have shown potential in integrated pest management programs. Modarres Najafabadi175 conducted a comparative study on the acaricidal activities of EOs from various plants, including L. angustifolia, against Tetranychus cinnabarinus Boisduval on cut roses. The study found that L. angustifolia EO significantly reduced the fecundity and fertility of the mites, supporting its inclusion in IPM strategies. Thus, these potential LEOs should be screened on a larger scale and after obtaining fruitful results they can be used for the development of botanical insecticides.
7. Challenges and safety assessment studies
While LEOs have received significant attention in food/crop research due to their natural origin, it is not easy to fix the dose for optimal in vitro effects in food preservation or crop protection. One of the major challenges in the application of LEOs is that their volatile natural compounds may interact with the proteins or carbohydrates present in food products/commodities, causing destabilization of LEOs and the development of new unwanted compounds, thereby decreasing their potential antimicrobial and insecticidal effects. This needs a higher amount of LEOs to ensure the effective preservation of food commodities. Nevertheless, the use of higher amounts of LEOs in food systems has been observed to modify the taste, quality and aroma of food commodities, making them less acceptable for consumption. But, the long-lasting aroma and flavour of LEOs allow for good effects at low doses instantaneously limiting negative organoleptic changes. The EOs derived from Lavandula species are considered safe for consumption due to their non-toxic nature and hypo allergenicity. The inappropriate application of LEOs and their derivatives in the preservation of food commodities or in pest protection may cause hostile side effects on human beings including respiratory issues, skin related problems, headache, and acute oral toxicity.204 With this perspective, anti-inflammatory and antinociceptive activities of LEO (L. angustifolia) has already been investigated by Silva et al.,117 which demonstrated its ability to decrease both acute and chronic forms of nociception. However, this aspect is not evaluated for EOs derived from other Lavandula species. It is important to be aware of their potential side effects before using these natural sources internally or as food additives or preservatives.Besides, the LEOs, if applied at higher doses, may cause harmful effects on the human organs such as the liver and stomach.205 Thus, LEOs and their derivatives must be tested for safety, appropriate doses, and toxicity. In this regard, many toxicological studies have been conducted on LEOs and reviewed by Cardia et al.206 For example, genotoxic and cytotoxic effects of LEOs are well studied.202 In another study, Arantes et al.207 studied the toxicological properties of L. stoechas subsp. luisieri EO in Alentejo (Portugal) and found that rats exhibited normal behaviour after administration of 200 mg per kg body weight, revealing low toxicity. Similar results were reported by Mekonnen et al.208 in Ethiopia; they found in their experiments that administering 2000 mg kg−1 of L. angustifolia EO to rabbits caused no significant changes (p > 0.05) in body weight, gross abnormalities, biochemical parameters, food and water intake. Furthermore, they did not find abnormality in kidneys and livers after histopathologic analysis. Besides, application of 10% ointment formulation did not cause any skin irritation which showed that the EO was nontoxic.208 Thus, LEOs can be promising candidates for use as food supplement applications.
The deployment of LEOs as food preservatives/crop protectants has many advantages due to their efficient activity against pathogens and insects hampering food crops both in storage and in the field with negligible harmful effects on beneficial organisms. To date, many LEOs including L. angustifolia EOs have demonstrated mosquitocidal activity against Culex pipiens larvae, a vector for West Nile virus,209 with an LC50 of 140 μg mL−1. At present, LEOs and some of their major constituents are used in aromatherapy as well as for the development of medicines for urogenital, respiratory, digestive, nervous, and vascular disorders.210 Additionally, past and recent studies have shown the potential of LEOs against pathogens and insect pests deteriorating food commodities197,211 and hampering field crops.212,213 In Asian, African, and European countries, as well as in the United States, the potential of LEOs has been recognized, but they have not yet been commercialized. Unfortunately, no bio-preservatives or biopesticides derived from LEOs are currently available on the market. Practical applications are rarer than published results at the field level, which is why published results are more common. Aside from the low production cost–benefit ratio, the low persistence of effects, and strict European Union regulations, LEOs have not yet been commercialized as botanical pesticides on a larger scale and remain confined to laboratory experiments.214 In addition, the direct application of LEOs as food preservatives against pathogens and insect pests deteriorating food commodities/hampering field crops has several limitations, including short shelf life, poor stability, and regulatory problems regarding their exposure to the environment.
Although, many LEOs have been investigated for their efficacy against food deteriorating pathogens and insect pests, the majority of investigations were conducted under laboratory conditions or on a smaller scale. During the application of LEOs in fields or storehouses, they may lose their efficacy. Therefore, to re-store their efficiency, stabilization methods including encapsulation technology can be considered. For instance, many research studies have shown that the efficacy and shelf life of LEOs are extended after their encapsulation.215,216 Thus, using LEOs to produce potential nano-preservatives could help to prevent their degradation. Thus, botanical preservatives fabricated from LEOs must be scientifically certified regarding their residual phytotoxicity on crops, deployment protocols, overcoming regulatory and toxicological barriers, and mitigating problems related to the environment for their long-term application for food preservation/crop protection. It is also challenging for LEOs to gain widespread approval for their compounds and legitimize them as biopesticides due to complex authorization procedures. Furthermore, their approval and registration procedures are very expensive due to their inherent toxicity costs and the need for a suitable evaluation environment.
8. Research gaps and future outlook
The LEOs and their derivatives have been evaluated for their functional properties in food system/crop protection including antibacterial, antifungal, and insecticidal activities; however, the majority of studies were focused on lab conditions. However, investigations regarding the field application of LEOs against phytopathogens and insect pests, as well as their impact on beneficial organisms including bees have not been conducted and need to be addressed in future research. Although, more than 39 species of Lavender exist worldwide, majority of pesticidal, food preservative, and toxicological studies have focused on EOs derived from L. angustifolia, L. latifolia, and L. stoechas. However, little attention has been given to L. lanata, L. viridis, L. dentata, among others, which could be evaluated against pests and pathogens of foods and food crops in future.A higher concentration of LEOs in food systems was observed to affect the taste, quality, and aroma of the food commodities, resulting in less consumer acceptance. The encapsulation technology can be one of the possible solutions to this problem. The encapsulation technology not only decreases the instability of LEOs (e.g., their reaction towards substrate protein), but also safeguards the pesticidal properties through controlled release. On this aspect, few investigators studied the antimicrobial and insecticidal properties of encapsulated LEOs, i.e., L. angustifolia and L. latifolia EOs; however the majority of other Lavandula species remain unexplored. To ensure food safety, safe dosage limits, and food preservation composition, this aspect needs to be concentrated in food research. Encapsulating LEOs improves their bio-efficacy, controlled release, shelf life and provides a relatively safer approach for the protection of food crops from pest/pathogen attacks. Preventive measures should be taken before commercialization to ensure that LEOs and their related derivatives are not harmful to beneficial organisms. Therefore, future studies are needed in order to achieve the (i) safe dosage limit, (ii) stability and bioactivity of LEOs and their related compounds, (iii) interaction of surface proteins on food commodities with LEOs' bioactive compounds, (iv) allergic reactions, (e) their optimal dosage limit to prevent deterioration/spoilage of taste, quality, and aroma of food commodities, and (f) improved encapsulation methods and controlled release to ensure increased shelf-life of food commodities and related products.
Lastly, the EOs of Lavandula species from various countries were characterized through GC-MS analysis and the results revealed that LEOs exhibit a broad range of variations in their constituents in different plant samples. However, there are knowledge gaps regarding the modes of action of a particular compound derived from EOs of Lavandula species against harmful organisms deteriorating food commodities/crops. However, based on prevailing toxicological and pharmaceutical investigations, LEOs have raised no concerns regarding their use in food preservation or crop protection and can be considered eco-friendly at the normally recommended doses reviewed here after their field trials. As a result of their multifaceted antimicrobial and insecticidal properties, LEOs may be used as botanical pesticides once the government of the concerned country determines the cost–benefit ratio and conducts other regulatory risk assessments. Last but not least, a robust and sustainable environment in the future requires a gradual and efficient approach for approving LEO-based botanical pesticides to mitigate pests and pathogens without interfering with marketable public interests.
9. Conclusion
The present article summarizes that EOs and terpenoids derived from L. angustifolia, L. latifolia, and L. stoechas have broad antimicrobial and insecticidal properties against pathogens and pests deteriorating food commodities/crops. In several investigations these EOs were found to be non cyto/geno toxic. There have been developments in the evaluation of LEO-based encapsulated products, such as thin films, biodegradable polymers, nano-emulsion coatings against bacterial and fungal pathogens responsible for food spoilage and investigators have found potential results. Based on the toxicological and pharmacological studies available, LEOs appear to be safer to the environment and consumers than synthetic pesticides. Therefore, LEO-based botanical pesticides might be useful in combating microbial pathogens and insect pests in stored food commodities and field crops. Unfortunately, LEOs that have been reported as efficient against pests and pathogens, are often the most phytotoxic. Therefore, sustainable protection of food commodities and crops from biodeterioration needs special attention. Like other alternatives, LEO based biopesticides are not a panacea for controlling harmful organisms hampering food commodities/field crops; however there will be prevailing market niches focused on worker and environmental safety, where LEO-based bio preservatives/biopesticides will find wide acceptance among farmers/retailers. Therefore, extensive research is required to address the challenges associated with LEOs, particularly regarding safe dosage limits and potential adverse effects. These challenges include unfavourable organoleptic properties, low stability, and a lack of standardization, all of which hinder their broader application as biopesticides. A shift towards greener technologies directs an optimistic future towards the safer deployment of LEOs in food preservation/crop protection.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare no conflict of interests.References
- B. Héral, É. Stierlin, X. Fernandez and T. Michel, Phytochem. Rev., 2021, 20, 751–771 CrossRef.
- N. Dobros, K. D. Zawada and K. Paradowska, Molecules, 2022, 28, 256 CrossRef PubMed.
- K. Yaghoobi, G. R. Kaka, D. Sh and H. Ashayeri, J. Norman Bethune Univ. Med. Sci., 2016, 17, 1–9 Search PubMed.
- M. Hancianu, O. Cioanca, M. Mihasan and L. Hritcu, Phytomedicine, 2013, 20, 446–452 CrossRef CAS PubMed.
- P. H. Koulivand, M. Khaleghi Ghadiri and A. Gorji, Evid. base Compl. Alternative Med., 2013, 2013, 1–10 CrossRef PubMed.
- M. Slighoua, I. Mahdi, N. Boucetta, F. Di Cristo, S. Boukhira, A. El youbi el Hamsas, M. I. Tattou, A. Grafov, A. Bari and D. Bousta, South Afr. J. Bot., 2022, 146, 354–364 CrossRef CAS.
- M. S. Ali-Shtayeh, S. Y. Abu-Zaitoun, N. Dudai and R. M. Jamous, Evid. base Compl. Alternative Med., 2020, 2020, 1–10 Search PubMed.
- T. S. Firoozeei, A. Feizi, H. Rezaeizadeh, A. Zargaran, H. R. Roohafza and M. Karimi, Compl. Ther. Med., 2021, 59, 102679 CrossRef PubMed.
- M. G. Evandri, L. Battinelli, C. Daniele, S. Mastrangelo, P. Bolle and G. Mazzanti, Food Chem. Toxicol., 2005, 43, 1381–1387 CrossRef CAS PubMed.
- A. C. Aprotosoaie, E. Gille, A. Trifan, V. S. Luca and A. Miron, Phytochem. Rev., 2017, 16, 761–799 CrossRef CAS.
- E. Malloggi, D. Menicucci, V. Cesari, S. Frumento, A. Gemignani and A. Bertoli, Appl. Psychol.: Health Well , 2022, 14, 663–690 Search PubMed.
- V. D. Rabotyagov, A. E. Palii and Y. S. Khokhlov, Agric. Biol., 2016, 53(3), 539–546 Search PubMed.
- M. Jalali-Heravi, R. S. Moazeni-Pourasil and H. Sereshti, J. Chromatogr. B, 2015, 983, 83–89 CrossRef PubMed.
- C. Virgiliou, C. Zisi, K. N. Kontogiannopoulos, A. Nakas, A. Iakovakis, V. Varsamis, H. G. Gika and A. N. Assimopoulou, J. Chromatogr. B, 2021, 1179, 122852 CrossRef CAS PubMed.
- R. Manor, E. Kumarnsit, N. Samerphob, T. Rujiralai, T. Puangpairote and D. Cheaha, J. Ethnopharmacol., 2021, 276, 114193 CrossRef CAS PubMed.
- A. Carrasco, R. Martinez-Gutierrez, V. Tomas and J. Tudela, Planta Med., 2015, 82, 163–170 CrossRef PubMed.
- A. R. Fakhari, P. Salehi, R. Heydari, S. N. Ebrahimi and P. R. Haddad, J. Chromatogr. A, 2005, 1098, 14–18 CrossRef CAS PubMed.
- Y. Cong, P. Abulizi, L. Zhi, X. Wang and Mirensha, Chem. Nat. Compd., 2008, 44, 810 CrossRef CAS.
- K. Smigielski, A. Raj, K. Krosowiak and R. Gruska, J. Essent. Oil Bear. Plants, 2009, 12, 338–347 CrossRef CAS.
- A. Tarakemeh, V. Rowshan and S. Najafian, Anal. Chem. Lett., 2012, 2, 244–249 CrossRef CAS.
- K. Msaada, N. Salem, S. Tammar, M. Hammami, M. Jamal Saharkhiz, N. Debiche, F. Limam and B. Marzouk, J. Essent. Oil Bear. Plants, 2012, 15, 1030–1039 CrossRef.
- L. T. Danh, N. D. A. Triet, J. Zhao, R. Mammucari and N. Foster, J. Supercrit. Fluids, 2012, 70, 27–34 CrossRef CAS.
- C. Jianu, G. Pop, A. T. Gruia and F. G. Horhat, Int. J. Agric. Biol., 2013, 15, 772–776 CAS.
- M. Torabbeigi and P. Aberoomand Azar, Acta Chromatogr., 2013, 25, 571–579 CrossRef CAS.
- L. Lesage-Meessen, M. Bou, J.-C. Sigoillot, C. B. Faulds and A. Lomascolo, Appl. Microbiol. Biotechnol., 2015, 99, 3375–3385 CrossRef CAS PubMed.
- H. Kamali, N. Aminimoghadamfarouj, E. Golmakani and A. Nematollahi, Acta Chromatogr., 2015, 7, 57 Search PubMed.
- G. D. K. Babu, V. Thakur and B. Singh, J. Herbs, Spices, Med. Plants, 2016, 22, 173–182 CrossRef.
- F. F. Afifia, Arab. J. Med. Aromat. Plants, 2016, 2, 71–85 Search PubMed.
- K. J. Hamad, S. J. A. Al-Shaheen, R. A. Kaskoos, J. Ahamad, M. Jameel and S. R. Mir, Int. Res. J. Pharm., 2013, 4, 117–120 CAS.
- M. Barazandeh, J. Essent. Oil Res., 2002, 14(2), 103–104 CrossRef CAS.
- A. Filly, A. S. Fabiano-Tixier, C. Louis, X. Fernandez and F. Chemat, C. R. Chim., 2016, 19, 707–717 CrossRef CAS.
- N. Kirimer, S. Mokhtarzadeh, B. Demirci, F. Goger, K. M. Khawar and F. Demirci, Ind. Crops Prod., 2017, 96, 120–125 CrossRef CAS.
- H. Niksic, E. Kovac-Besovic, E. Omeragic, S. Muratovic, J. Kusturica and K. Duric, J. Health Sci., 2017, 7, 35 Search PubMed.
- G. Al-Wassouf, Chem. Mater. Res., 2018, 10, 1–6 Search PubMed.
- L. Chekoual, A. Aissat, A. Ait-Kaciaourahoun and T. Benabdelkader, Ind. Crops Prod., 2018, 73, 16–27 Search PubMed.
- K. Smigielski, R. Prusinowska, A. Stobiecka, A. Kunicka-Styczyñska and R. Gruska, J. Essent. Oil Bear. Plants, 2018, 21, 1303–1314 CrossRef CAS.
- B. Blažeković, W. Yang, Y. Wang, C. Li, M. Kindl, S. Pepeljnjak and S. Vladimir-Knežević, Ind. Crop. Prod., 2018, 123, 173–182 CrossRef.
- B. Soulaimani, A. Nafis, A. Kasrati, A. Rochdi, N.-E. Mezrioui, A. Abbad and L. Hassani, South Afr. J. Bot., 2019, 125, 202–206 CrossRef CAS.
- M. Bogdan, S. Bungau, D. M. Tit, L. Copolovici, T. Behl, P. Otrisal, L. Aleya, G. Cioca, D. Berescu and D. Uivarosan, Res. Chin., 2020, 71, 307–315 CAS.
- X. Chen, L. Zhang, C. Qian, Z. Du, P. Xu and Z. Xiang, Microchem. J., 2020, 153, 104458 CrossRef CAS.
- G. Dong, X. Bai, A. Aimila, H. A. Aisa and M. Maiwulanjiang, Molecules, 2020, 25, 3166 CrossRef CAS PubMed.
- X. Guo and P. Wang, Molecules, 2020, 25, 5541 CrossRef CAS PubMed.
- A. Ciocarlan, L. Lupascu, A. Aricu, I. Dragalin, V. Popescu, E.-I. Geana, R. E. Ionete, N. Vornicu, O. G. Duliu and G. Hristozova, Plants, 2021, 10, 1829 CrossRef CAS PubMed.
- M. A. Bogdan, S. Bungau, D. M. Tit, D. C. Zaha, A. C. Nechifor, T. Behl, D. Chambre, A. I. Lupitu, L. Copolovici and D. M. Copolovici, Molecules, 2021, 26, 4381 CrossRef CAS PubMed.
- M. M. Al-Ansari, A. M. Andeejani, E. Alnahmi, R. H. AlMalki, A. Masood, P. Vijayaraghavan, A. A. Rahman and K. C. Choi, Ind. Crops Prod., 2021, 170, 113740 CrossRef CAS.
- K. Pokajewicz, M. Białoń, L. Svydenko, N. Hudz, R. Balwierz, D. Marciniak and P. P. Wieczorek, Molecules, 2022, 27, 2152 CrossRef CAS PubMed.
- H. I. Eldeghedy, A. E.-N. G. El-Gendy, A. A. Nassrallah, A. M. Aboul-Enein and E. A. Omer, J. Essent. Oil Bear. Plants, 2022, 25, 52–63 CrossRef CAS.
- C. Slimani, H. Sqalli, R. Chaimae, A. Farah, A. Lazraq, L. El Ghadraoui, S. Belmalha and G. Echchgadda, Not. Sci. Biol., 2022, 14, 11172 CrossRef CAS.
- H. H. Moussa, F. Benaliouche, I. Sbartai and H. Sbartai, Biodiversitas Journal of Biological Diversity, 2023, 24, 4535–4542 Search PubMed.
- V. Todorova, K. Ivanov, Y. Georgieva, D. Karcheva-Bahchevanska and S. Ivanova, Int. J. Environ. Anal. Chem., 2023, 2023, 1–6 CrossRef PubMed.
- K. Diass, M. Merzouki, K. Elfazazi, H. Azzouzi, A. Challioui, K. Azzaoui, B. Hammouti, R. Touzani, F. Depeint and A. Ayerdi Gotor, Plants, 2023, 12, 1571 CrossRef CAS PubMed.
- S. Kırkıncı, Y. C. Gercek, F. N. Baştürk, N. Yıldırım, B. Gıdık and N. E. Bayram, Sci. Rep., 2024, 14, 20922 CrossRef PubMed.
- S. R. Alieva, Z. U. Sherova, S. S. Mamadshoeva, S. R. Usmanova, Z. K. Muhidinov and S. Mou, Eur. J. Acad. Res., 2024, 4, 247–249 Search PubMed.
- C. Jianu, G. Pop, A. T. Gruia and F. G. Horhat, Int. J. Agric. Biol., 2013, 15, 772–776 CAS.
- I. Calinescu, A. Gavrila, M. Ivopol, G. Ivopol, M. Popescu and N. Mircioaga, Open Chem., 2014, 12, 829–836 CrossRef CAS.
- C. El-Kalamouni, P. R. Venskutonis, B. Zebib, O. Merah, C. Raynaud and T. Talou, Medicines, 2017, 4, 30 CrossRef PubMed.
- A. Di Matteo, M. Lavorgna, C. Russo, E. Orlo and M. Isidori, Appl. Food Res., 2024, 4, 100528 CrossRef CAS.
- A. K. Pandey, K. Dinesh, N. Sam Nirmala, A. Kumar, D. Chakraborti and A. Bhattacharyya, Curr. Res. Biotechnol., 2023, 6, 100144 CrossRef CAS.
- G. Agrios, Plant Pathology, Elsevier Academic Press, 5th edn, Amsterdam, 2005, vol. 26–27, pp. 398–401 Search PubMed.
- Y. El Abdali, G. Beniaich, A. M. Mahraz, A. El Moussaoui, Y. A. Bin Jardan, M. Akhazzane, M. Chebaibi, H.-A. Nafidi, N. Eloutassi, M. Bourhia and A. Bouia, Evid. base Compl. Alternative Med., 2023, 2023, 1–12 Search PubMed.
- M. Guenther, T. Driver and C. Saunders, The Overall Benefits of Food Safety – A Literature Review, Client Report prepared for New Zealand Food Safety Science and Research Centre, December, 2022, Lincoln University: Agribusiness and Economics Research Unit Search PubMed.
- E. T. Seyoum, T. Eguale, I. Habib, C. J. B. Oliveira, D. F. M. Monte, B. Yang, W. A. Gebreyes and W. Q. Alali, Animals, 2024, 14, 786 CrossRef PubMed.
- K. S. Almaary, Biosci., Biotechnol. Res. Asia, 2023, 20, 745–755 Search PubMed.
- A. Rana, S. Mishra, K. Soni, M. Samtiya, N. K. Taneja and T. Dhewa, Nutritional Science and Technology: Concept to Application, ed. T. Dhewa, A. K. Puniya and A. Panghal, Wiley, 2023, pp. 273–293 Search PubMed.
- S. I. Behiry, M. EL-Hefny and M. Z. M. Salem, Nat. Prod. Res., 2020, 34, 3394–3398 CrossRef CAS PubMed.
- T. Benali, A. Bouyahya, K. Habbadi, G. Zengin, A. Khabbach and K. Hammani, Biocatal. Agric. Biotechnol., 2020, 28, 101696 CrossRef.
- A. P. Martinazzo, R. de Oliveira Braga and C. E. de Souza Teodoro, Cienc. Nat., 2022, 44, e25 Search PubMed.
- E. Orlo, C. Russo, R. Nugnes, M. Lavorgna and M. Isidori, Foods, 2021, 10, 1807 CrossRef CAS PubMed.
- I. Dadalioǧlu and G. A. Evrendilek, J. Agric. Food Chem., 2004, 52, 8255–8260 CrossRef PubMed.
- J. Gómez-Estaca, A. L. De Lacey, M. E. López-Caballero, M. d C. Gómez-Guillén and P. Montero, Food Microbiol., 2010, 27, 889–896 CrossRef PubMed.
- S. Heydari, H. Jooyandeh, B. Alizadeh Behbahani and M. Noshad, Food Sci. Nutr., 2020, 8, 6497–6512 CrossRef CAS PubMed.
- B. Esin, Bol. do Inst. Pesca, 2020, 46(3), e565 Search PubMed.
- S. Metin, N. Kara, B. I. Didinen and A. Kubilay, Isr. J. Aquac., 2021, 73, 1305530 Search PubMed.
- I. Dadalioǧlu and G. A. Evrendilek, J. Agric. Food Chem., 2004, 52, 8255–8260 CrossRef PubMed.
- J. Petrová, M. Terentjeva, C. Puchalski, J. Hutková, A. Kántor, M. Mellen, J. Čuboň, P. Haščík, M. Kluz and R. Kordiaka, Slovak J. Food Sci., 2016, 10, 132–138 Search PubMed.
- M. Adaszyńska-Skwirzyńska and D. Szczerbińska, Anim. Physiol. Nutr., 2018, 102, 1020–1025 CrossRef PubMed.
- J. Sriti, M. Boulares, Y. Zarroug and R. Essid, J. Food Saf. Hyg., 2021, 7, 11–26 Search PubMed.
- I. V. Bulai, M. Georgescu, D. Tăpăloagă, O.-M. Ghimpeteanu and S.-M. Raita, Revista Lucrari stiintifice - Seria Agronomie, 2021, 64, 2 Search PubMed.
- D. Vokou, S. Vareltzidou and P. Katinakis, Agric. Ecosyst. Environ., 1993, 47, 223–235 CrossRef.
- P. Xylia, C. Goumenos, N. Tzortzakis and A. Chrysargyris, Agronomy, 2023, 13, 2493 CrossRef CAS.
- S. Varona, S. R. Rojo, Á. Martín, M. J. Cocero, A. T. Serra, T. Crespo and C. M. Duarte, Ind. Crops Prod., 2013, 42, 243–250 CrossRef CAS.
- M. Walasek-Janusz, A. Grzegorczyk, D. Zalewski, A. Malm, S. Gajcy and R. Gruszecki, Agronomy, 2022, 12, 2955 CrossRef CAS.
- Y. M. Riyad and E. A. Elkholany, J. Food Dairy Sci., 2020, 11, 113–120 CrossRef.
- H. Falleh, M. Ben Jemaa, K. Djebali, S. Abid, M. Saada and R. Ksouri, J. Food Process. Preserv., 2019, 43, e14257 CAS.
- H. Falleh, K. Djebali, M. B. Jemaa, M. Hammami, S. Khammasi and R. Ksouri, Food Measure, 2021, 15, 376–385 CrossRef.
- M. H. Marhamatizadeh, H. Mahmoudipour and E. Ehsandoost, BioTechnol.:Indian J., 2014, 9, 335–345 CAS.
- Z. Denkova, B. Goranov, D. Blazheva, T. Tomova, D. Teneva, R. Denkova-Kostova, A. Slavchev, R. Pagán, P. Degraeve and G. Kostov, Appl. Sci., 2023, 13, 11281 CrossRef CAS.
- S. De Rapper, A. Viljoen and S. Van Vuuren, Evid. base Compl. Alternative Med., 2016, 2016, 1–9 Search PubMed.
- X. Sun, J. Wang, H. Zhang, M. Dong, L. Li, P. Jia, T. Bu, X. Wang and L. Wang, Lwt, 2021, 142, 110987 CrossRef CAS.
- C. Doğan, N. Doğan, M. Gungor, A. K. Eticha and Y. Akgul, Food Packag. Shelf Life, 2022, 34, 100942 CrossRef.
- M. Soković and L. J. L. D. Van Griensven, Eur. J. Plant Pathol., 2006, 116, 211–224 CrossRef.
- J. F. Martucci, L. B. Gende, L. M. Neira and R. A. Ruseckaite, Ind. Crop. Prod., 2015, 71, 205–213 CrossRef CAS.
- R. Tardugno, A. Serio, F. Pellati, S. D'Amato, C. Chaves López, M. G. Bellardi, M. Di Vito, V. Savini, A. Paparella and S. Benvenuti, Nat. Prod. Res., 2019, 33, 3330–3335 CrossRef CAS PubMed.
- D. Djenane, M. Aïder, J. Yangüela, L. Idir, D. Gómez and P. Roncalés, Meat Sci., 2012, 92, 667–674 CrossRef CAS PubMed.
- M. H. Lodhia, K. R. Bhatt and V. S. Thaker, Indian J. Pharmaceut. Sci., 2009, 71, 134 CrossRef CAS PubMed.
- M. Moussi Imane, F. Houda, A. H. Said Amal, N. Kaotar, T. Mohammed, R. Imane and H. Farid, J. Essent. Oil Bear. Plants, 2017, 20, 1074–1082 CrossRef CAS.
- N. Karaca, G. Şener, B. Demirci and F. Demirci, Z. Naturforsch. C, 2021, 76, 169–173 CrossRef CAS PubMed.
- M. Salavati Hamedani, M. Rezaeigolestani and M. Mohsenzadeh, J. Nutr. Fasting Health, 2020, 8, 273–279 Search PubMed.
- C. Benbrahim, M. S. Barka, A. Basile, V. Maresca, G. Flamini, S. Sorbo, F. Carraturo, R. Notariale, M. Piscopo and A. Khadir, Appl. Sci., 2021, 11, 5688 CrossRef CAS.
- B. Speranza, A. Guerrieri, A. Racioppo, A. Bevilacqua, D. Campaniello and M. R. Corbo, Microbiol. Res., 2023, 14, 1089–1113 CAS.
- H. Benaiche, N. Bouredja, R. Terbeche and A. Alioua, Int. J. Environ. Stud., 2023, 80, 1853–1862 CrossRef CAS.
- P. S. X. Yap, B. C. Yiap, H. C. Ping and S. H. E. Lim, Open Microbiol., 2014, 7, 6–14 CrossRef PubMed.
- M. Sienkiewicz, M. Lysakowska, J. Ciecwierz, P. Denys and E. Kowalczyk, Med. Chem., 2011, 7, 674–689 CrossRef CAS PubMed.
- C. Rota, J. J. Carraminana, J. Burillo and A. Herrera, J. Food Protect., 2004, 67, 1252–1256 CrossRef CAS PubMed.
- A. K. Pandey, A. Sanches Silva, M. L. Chávez-González and P. Singh, Crit. Rev. Biotechnol., 2023, 43, 1257–1283 CrossRef CAS PubMed.
- A. K. Pandey, M. L. Chávez-González, A. S. Silva and P. Singh, Trends Food Sci. Technol., 2021, 111, 426–441 CrossRef CAS.
- S. Cassella, J. P. Cassella and I. Smith, Int. J. Aromather., 2002, 12, 2–15 CrossRef.
- E. M. Soylu, Ş. Kurt and S. Soylu, Int. J. Food Microbiol., 2010, 143, 183–189 CrossRef CAS PubMed.
- F. Hossain, P. Follett, S. Salmieri, K. D. Vu, C. Fraschini and M. Lacroix, Int. J. Food Microbiol., 2019, 295, 33–40 CrossRef CAS PubMed.
- L. D. Do Nascimento, K. S. Da Costa, M. M. Cascaes and E. H. De Aguiar Andrade, in Essential Oils, ed. M. Santana De Oliveira, Springer International Publishing, Cham, 2022, pp. 101–121 Search PubMed.
- G. Zhang, C. Yuan and Y. Sun, Molecules, 2018, 23, 1126 CrossRef PubMed.
- A. R. Locali-Pereira, N. A. Lopes, M. E. C. Menis-Henrique, N. S. Janzantti and V. R. Nicoletti, Int. J. Food Microbiol., 2020, 335, 108890 CrossRef CAS PubMed.
- M. Dávila-Rodríguez, A. López-Malo, E. Palou, N. Ramírez-Corona and M. T. Jiménez-Munguía, J. Food Sci. Technol., 2020, 57, 4133–4142 CrossRef PubMed.
- C. Yuan, Y. Wang, Y. Liu and B. Cui, Ind. Crops Prod., 2019, 130, 104–110 CrossRef CAS.
- G. C. Türkoğlu, G. Erkan, S. Y. Karavana, A. M. Sarıışık, A. Çetmeli Bakadur, B. Ütebay and A. Popescu, AATCC J. Res., 2023, 10, 332–345 CrossRef.
- K. Balasubramanian and K. M. Kodam, RSC Adv., 2016, 6, 75420–75421 RSC.
- L. S. Silva, J. M. Mar, S. G. Azevedo, M. S. Rabelo, J. A. Bezerra, P. H. Campelo, M. B. Machado, G. Trovati, A. L. Dos Santos, H. D. Da Fonseca Filho, T. P. De Souza and E. A. Sanches, J. Sci. Food Agric., 2019, 99, 685–695 CrossRef CAS PubMed.
- A. K. Pandey, P. Kumar, P. Singh, N. N. Tripathi and V. K. Bajpai, Front. Microbiol., 2017, 7, 2161 Search PubMed.
- P. A. Paranagama, K. H. T. Abeysekera, K. Abeywickrama and L. Nugaliyadde, Lett. Appl. Microbiol., 2003, 37, 86–90 CrossRef CAS PubMed.
- O. Dhingra, E. Mizubuti, I. Napoleao and G. Jham, Seed Sci. Technol., 2001, 29, 193–203 Search PubMed.
- J. M. Wagacha and J. W. Muthomi, Int. J. Food Microbiol., 2008, 124, 1–12 CrossRef CAS PubMed.
- A. K. Pandey and N. N. Tripathi, J. Indian Bot. Soc., 2011, 90, 326–331 Search PubMed.
- G. Arras and M. Usai, J. Food Prot., 2001, 64, 1025–1029 CrossRef CAS PubMed.
- P. Baruah, R. K. Sharma, R. S. Singh and A. C. Ghosh, J. Essent. Oil Res., 1996, 8, 411–412 CrossRef CAS.
- E. Bosquez-Molina, E. Ronquillo-de Jesús, S. Bautista-Baños, J. R. Verde-Calvo and J. Morales-López, Postharvest Biol. Technol., 2010, 57, 132–137 CrossRef CAS.
- V. Lalitha and K. A. Raveesha, Asian J. Microbiol. Biotechnol. Environ. Sci., 2006, 8, 483 Search PubMed.
- T. Moon, H. M. A. Cavanagh and J. M. Wilkinson, J. Essent. Oil Res., 2007, 19, 171–175 CrossRef CAS.
- M. Zuzarte, M. J. Gonçalves, C. Cavaleiro, M. T. Cruz, A. Benzarti, B. Marongiu, A. Maxia, A. Piras and L. Salgueiro, Ind. Crops Prod., 2013, 44, 97–103 CrossRef CAS.
- A. Angioni, A. Barra, V. Coroneo, S. Dessi and P. Cabras, J. Agric. Food Chem., 2006, 54, 4364–4370 CrossRef CAS PubMed.
- J. Domingues, F. Delgado, J. C. Gonçalves and C. S. Pintado, J. Pharm. Pharmacol., 2021, 9, 98–106 CrossRef CAS.
- J. G. Lopez-Reyes, D. Spadaro, M. L. Gullino and A. Garibaldi, Flavour Fragrance J., 2010, 25, 171–177 CrossRef CAS.
- A. H. Dincoglu and Z. Caliskan, Int. Food Res. J., 2022, 29, 991–1004 CAS.
- I. Vasileva, R. Denkova, R. Chochkov, D. Teneva, Z. Denkova, T. Dessev, P. Denev and A. Slavov, Food Chem., 2018, 253, 13–21 CrossRef CAS PubMed.
- V. Valková, H. Ďúranová, L. Galovičová, N. L. Vukovic, M. Vukic and M. Kačániová, Molecules, 2021, 26, 3859 CrossRef PubMed.
- X. Xiong, L. Zhang, X. Li, Q. Zeng, R. Deng, X. Ren and Q. Kong, Can. J. Microbiol., 2021, 67, 724–736 CrossRef CAS PubMed.
- Q. Ali Sultan and S. Wahab, Hortic., Environ. Biotechnol., 2023, 64, 643–654 CrossRef CAS.
- E. M. Soylu, Ş. Kurt and S. Soylu, Int. J. Food Microbiol., 2010, 143, 183–189 CrossRef CAS PubMed.
- E. M. Soylu, S. Soylu and S. Kurt, Mycopathologia, 2006, 161, 119–128 CrossRef CAS PubMed.
- R. Górski, H. Dorna, A. Rosińska, D. Szopińska and A. Kałużewicz, Ecol. Chem. Eng. S, 2021, 28, 411–427 Search PubMed.
- F. Farokhian, M. Jafarpour, M. Goli and O. Askari-Khorasgani, J. Food Process Eng., 2017, 40, e12432 CrossRef.
- E. M. Soylu, S. Soylu and S. Kurt, Mycopathologia, 2006, 161, 119–128 CrossRef CAS PubMed.
- M. Behnamian, Z. Najafi, M. Davari and S. Dezhsetan, Biol. Cult. Tests Control Plant Dis., 2017, 6, 111–119 Search PubMed.
- B. Tanović, I. Potočnik, G. Delibašić, M. Ristić, M. Kostić and M. Marković, Arch. Biol. Sci., 2009, 61, 231–237 CrossRef.
- M. S. Rabie and M. A. Vasilevich, Agric. News J., 2020, 11, 39–42 Search PubMed.
- F. Diánez, M. Santos, C. Parra, M. J. Navarro, R. Blanco and F. J. Gea, Lett. Appl. Microbiol., 2018, 67, 400–410 CrossRef PubMed.
- S. Hoseini, J. Amini, J. N. Rafei and J. Khorshidi, Eur. J. Plant Pathol., 2019, 155, 1287–1302 CrossRef CAS.
- M. Zuzarte, M. J. Gonçalves, C. Cavaleiro, J. Canhoto, L. Vale-Silva, M. J. Silva, E. Pinto and L. Salgueiro, J. Med. Microbiol., 2011, 60, 612–618 CrossRef CAS PubMed.
- M. Zuzarte, M. J. Gonçalves, M. T. Cruz, C. Cavaleiro, J. Canhoto, S. Vaz, E. Pinto and L. Salgueiro, Food Chem., 2012, 135, 1505–1510 CrossRef CAS PubMed.
- M. Císarová, D. Tančinová and J. Medo, Slovak J. Food Sci., 2016, 10(1), 83–88 Search PubMed.
- A. Servili, E. Feliziani and G. Romanazzi, Postharvest Biol. Technol., 2017, 133, 36–40 CrossRef CAS.
- M. Stupar, M. L. Grbić, A. Džamić, N. Unković, M. Ristić, A. Jelikić and J. Vukojević, South Afr. J. Bot., 2014, 93, 118–124 CrossRef CAS.
- E. M. Soylu and R. Incekara, J. Plant Pathol., 2017, 99, 437–444 Search PubMed.
- S. Felsociova, M. Kacaniova, E. Horská, N. Vukovic, L. Hleba, J. Petrová, K. Rovná, M. Stricik and Z. Hajduová, Ann. Agric. Environ. Med., 2015, 22, 38–42 CrossRef CAS PubMed.
- S. Shin, Arch Pharm. Res., 2003, 26, 389–393 CrossRef CAS PubMed.
- J. G. Lopez-Reyes, D. Spadaro, A. Prelle, A. Garibaldi and M. L. Gullino, J. Food Protect., 2013, 76, 631–639 CrossRef PubMed.
- S. Behnam, M. Farzaneh, M. Ahmadzadeh and A. S. Tehrani, Commun. Agric. Appl. Biol. Sci., 2006, 71, 1321–1326 CAS.
- M. E. Guynot, A. J. Ramos, L. Seto, P. Purroy, V. Sanchis and S. Marin, J. Appl. Microbiol., 2003, 94, 893–899 CrossRef CAS PubMed.
- D. Tancinová, Z. Barboráková, Z. Mašková, M. Mrvová, J. Medo, M. Golian, J. Štefániková and J. Árvay, J. Microbiol. Biotechnol. Food Sci., 2023, 13, 1–6 Search PubMed.
- J. Sangsuwan, T. Pongsapakworawat, P. Bangmo and S. Sutthasupa, LWT, 2016, 74, 14–20 CrossRef CAS.
- J. Sangsuwan and S. Sutthasupa, Packag. Technol. Sci., 2019, 32, 595–605 CrossRef CAS.
- A. Sarkhosh, A. I. Vargas, B. Schaffer, A. J. Palmateer, P. Lopez, A. Soleymani and M. Farzaneh, Food Packag. Shelf Life, 2017, 12, 16–22 CrossRef.
- R. Fu, L. Zhao, J. Wang, C. Chen, Y. Liu and D. Lu, LWT, 2024, 116315 CrossRef CAS.
- M. Císarová D. Tančinová, Scientific Papers Animal Science and Biotechnologies, 2015, 48, p. 55 Search PubMed.
- M. Císarová, L. Hleba, J. Medo, D. Tančinová, Z. Mašková, J. Čuboň, A. Kováčik, D. Foltinová, M. Božik and P. Klouček, Food Control, 2020, 110, 107007 CrossRef.
- M. Hlebová, L. Hleba, J. Medo, V. Uzsakova, P. Kloucek, M. Bozik, P. Haščík and J. Čuboň, Foods, 2021, 10, 2993 CrossRef PubMed.
- H. H. Abdel-Khalek, A. A. Hammad, R. M. A. El-Kader, K. A. Youssef and D. A. Abdou, Food Sci. Technol. Int., 2022, 28, 703–715 CrossRef CAS PubMed.
- R. M. Sumalan, R. Kuganov, D. Obistioiu, I. Popescu, I. Radulov, E. Alexa, M. Negrea, A. F. Salimzoda, R. L. Sumalan and I. Cocan, Molecules, 2020, 25, 1831 CrossRef CAS PubMed.
- S. Inouye, T. Tsuruoka, M. Watanabe, K. Takeo, M. Akao, Y. Nishiyama and H. Yamaguchi, Mycoses, 2000, 43, 17–23 CrossRef CAS PubMed.
- N. Hammoudi, H. Ziani Cherif, F. Borsali, K. Benmansour and A. Meghezzi, Mater. Technol., 2020, 35, 383–394 CrossRef CAS.
- C. Fagundes, M. B. Pérez-Gago, A. R. Monteiro and L. Palou, Int. J. Food Microbiol., 2013, 166, 391–398 CrossRef CAS PubMed.
- İ. Kahramanoglu, T. G. Kesimci, A. U. Bozhüyük, R. Gürbüz and H. Alptekin, Int. J. Agric. Environ. Food Sci., 2021, 5, 606–615 Search PubMed.
- D. Todorova, N. Yavorov, V. Lasheva, S. Damyanova and I. Kostova, Coatings, 2022, 13, 32 CrossRef.
- C. Caprari, F. Fantasma, P. Monaco, F. Divino, M. Iorizzi, G. Ranalli, F. Fasano and G. Saviano, Molecules, 2023, 28, 392 CrossRef CAS PubMed.
- S. Gupta, Glob. Pediatr. Health, 2019, 8, 81 Search PubMed.
- S. S. Modarres Najafabadi, J. Med. Plants By Prod., 2014, 3, 13–19 Search PubMed.
- A. K. Pandey, S. Tripathi and P. Singh, Arch. Phytopathol. Plant Protect., 2018, 51, 696–728 CrossRef CAS.
- L. D. Jacob, F. C. Ntungwen, S. Christopher, A. A. Wilson, T. T. Roli, N. E. Nchiwan, P. A. Iakovlev, A. J. Cheruvan, L. Ragesh and M. ul Hasan, 12th International Working Conference on Stored Product Protection (IWCSPP) in Berlin, Germany, October 7-11, 2018 Search PubMed.
- S. Akhtar, M. Sagheer and N. Javed, Pakistan J. Zool., 2015, 47(4), 1045 CAS.
- J. Brari and V. Kumar, Int. J. Pure Appl. Zool., 2019, 7, 41–45 Search PubMed.
- N. Bayramzadeh, F. Mehrkhou, A. A. Pourmirza and M. Mahmoudian, J. Agric. Sci. Technol., 2019, 21, 857–872 Search PubMed.
- M. P. Zunino, V. A. Areco and J. A. Zygadlo, Bol. Latinoam. Caribe Plantas Med. Aromat., 2012, 11, 269–277 CAS.
- M. P. Zunino, V. A. Areco and J. A. Zygadlo, Bol. Latinoam. Caribe Plantas Med. Aromat., 2012, 11, 269–277 CAS.
- B. Valizadeh, J. Jalali Sendi, M. Oftadeh, A. Ebadollahi and P. Krutmuang, Agronomy, 2021, 11, 2015 CrossRef CAS.
- N. A. Al-Harbi, N. M. Al Attar, D. M. Hikal, S. E. Mohamed, A. A. H. Abdel Latef, A. A. Ibrahim and M. A. Abdein, Plants, 2021, 10, 829 CrossRef CAS PubMed.
- G. Benelli, G. Flamini, A. Canale, P. L. Cioni and B. Conti, Crop Protect., 2012, 42, 223–229 CrossRef CAS.
- L. Kheloul, A. Kellouche, D. Bréard, M. Gay, C. Gadenne and S. Anton, Entomol. Exp. Appl., 2019, 167, 826–834 CrossRef CAS.
- E. N. Jesser, J. O. Werdin-González, A. P. Murray and A. A. Ferrero, J. Asia Pac. Entomol., 2017, 20, 1122–1129 CrossRef.
- L. A. Erland, M. R. Rheault and S. S. Mahmoud, Crop Protect., 2015, 78, 20–26 CrossRef CAS.
- K. Pirali-Kheirabadi and J. A. T. da Silva, Exp. Parasitol., 2010, 126, 184–186 CrossRef CAS PubMed.
- Y. N. Alpkent, Ö. Alaoğlu and H. Çetin, Plant Protect. Bull., 2013, 53, 115–125 Search PubMed.
- K. Khelfane-Goucem, N. Lardjane and F. Medjdoub-Bensaad, Afr. J. Agric. Res., 2016, 11, 1499–1503 CrossRef.
- M. M. Sabbour and S. E. El-Abd, Research on Crops, 2022, 23, 676–681 Search PubMed.
- E. Sertkaya, K. Kaya and S. Soylu, Ind. Crops Prod., 2010, 31, 107–112 CrossRef CAS.
- G. S. Germinara, M. G. Di Stefano, L. De Acutis, S. Pati, S. Delfine, A. De Cristofaro and G. Rotundo, Bull. Insectol., 2017, 70(1), 129–138 Search PubMed.
- L. Kheloul, S. Anton, C. Gadenne and A. Kellouche, J. Asia Pac. Entomol., 2020, 23, 320–326 CrossRef.
- N. A. Al-Harbi, N. M. Al Attar, D. M. Hikal, S. E. Mohamed, A. A. H. Abdel Latef, A. A. Ibrahim and M. A. Abdein, Plants, 2021, 10, 829 CrossRef CAS PubMed.
- G. S. Germinara, M. G. Di Stefano, L. De Acutis, S. Pati, S. Delfine, A. De Cristofaro and G. Rotundo, Bull. Insectol., 2017, 70, 121–128 Search PubMed.
- S. Attia, G. Lognay, S. Heuskin and T. Hance, J. Entomol. Zool. Stud., 2016, 4, 118–122 Search PubMed.
- N. Zallaghi and M. Ahmadi, Int. J. Pest Manag., 2021, 67, 203–215 CrossRef CAS.
- N. Faraone, N. K. Hillier and G. C. Cutler, PLoS One, 2015, 10, e0127774 CrossRef PubMed.
- E. Yazdani, J. J. Sendi, A. Aliakbar and S. Senthil-Nathan, Pestic. Biochem. Physiol., 2013, 107, 250–257 CrossRef CAS.
- A. Mesic, I. Mahmutović-Dizdarević, E. Tahirović, I. Durmišević, I. Eminovic, A. Jerković-Mujkić and R. Bešta-Gajević, Drug Chem. Toxicol., 2021, 44, 190–197 CrossRef CAS PubMed.
- G. Germinara, M. G. Stefano, L. De Acutis, S. Pati, S. Delfine, A. De Cristofaro and G. Rotundo, Bull. Insectol., 2017, 70, 129–138 Search PubMed.
- A. Jafari-Koulaee, F. Elyasi, Z. Taraghi, E. S. Ilali and M. Moosazadeh, Cent. Asian J. Global Health, 2020, 31(1), e442 Search PubMed.
- M. Antonelli and D. Donelli, Int. J. Ther. Massage Bodyw., 2020, 13, 32 Search PubMed.
- G. F. E. Cardia, F. M. de Souza Silva-Comar, E. M. T. da Rocha, S. E. Silva-Filho, M. Zagotto, N. S. Uchida, V. do Amaral, C. A. Bersani-Amado and R. K. N. Cuman, Res. Soc. Dev., 2021, 10, e23310514933 CrossRef.
- S. Arantes, F. Candeias, O. Lopes, M. Lima, M. Pereira, T. Tinoco, J. Cruz-Morais and M. Martins, Planta Med., 2016, 82, 1266–1273 CrossRef CAS PubMed.
- A. Mekonnen, S. Tesfaye, S. G. Christos, K. Dires, T. Zenebe, N. Zegeye, Y. Shiferaw and E. Lulekal, J. Toxicol., 2019, 2019, 1–8 CrossRef PubMed.
- F. El-Akhal, A. Ramzi, A. Farah, Y. Ez Zoubi, M. Benboubker, K. Taghzouti and A. El Ouali Lalami, Psyche, 2021, 2021, 1–7 CrossRef.
- H. M. A. Cavanagh and J. M. Wilkinson, Phytother Res., 2002, 16, 301–308 CrossRef CAS PubMed.
- M. Santamarina, M. Ibáñez, M. Marqués, J. Roselló, S. Giménez and M. Blázquez, Nat. Prod. Res., 2017, 31, 2675–2679 CrossRef CAS PubMed.
- Y. Akhtar, E. Pages, A. Stevens, R. Bradbury, C. A. G. Da Camara and M. B. Isman, Physiol. Entomol., 2012, 37, 81–91 CrossRef CAS.
- S. Dhaouadi, W. Rouissi, A. Mougou-Hamdane, I. Hannachi and B. Nasraoui, Tunis. J. Plant Prot., 2018, 13, 39–55 Search PubMed.
- A. F. Burlec, I. Macovei, A. Sacarescu, A. Corciova, C. Mircea, C. E. Iancu, O. Cioanca and M. Hancianu, Farmacia, 2020, 68, 992–998 CAS.
- P. Velmurugan, V. Ganeshan, N. F. Nishter and R. R. Jonnalagadda, Surface. Interfac., 2017, 9, 124–132 CrossRef CAS.
- R. Zhang, L. Huang, X. Xiong, M. C. Qian and H. Ji, Flavour Fragrance J., 2020, 35, 157–166 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |

