 Open Access Article
Open Access ArticleImpact of chickpea aquafaba-based emulsion on the physicochemical, nutritional, rheological and structural characteristics of little millet (Panicum sumatrense Roth.) flour cake
Bijjam
Madhavi
a,
Niveditha
Asaithambi
a,
Alok
Kumar
b,
Pralay
Maiti
 b,
Dinesh
Chandra Rai
b,
Dinesh
Chandra Rai
 ac and
Raj Kumar
Duary
ac and
Raj Kumar
Duary
 *a
*a
aDepartment of Dairy Science and Food Technology, Institute of Agricultural Sciences, Banaras Hindu University (BHU), Varanasi, U.P. 221005, India. E-mail: bijjam0210@gmail.com; nivethaa94@gmail.com; dcrai@bhu.ac.in; rkduary@bhu.ac.in
bSchool of Materials Science and Technology, Indian Institute of Technology (BHU), Varanasi, 221005, India. E-mail: alokkumar.rs.mst23@iitbhu.ac.in; pmaiti.mst@itbhu.ac.in
cBabasaheb Bhimrao Ambedkar Bihar University, Muzaffarpur, Bihar 842001, India
First published on 2nd December 2024
Abstract
Recent patterns in food consumption indicate that consumers have a strong desire for vegan, gluten-free, and healthy diets. This study investigates the utility of chickpea aquafaba and little millet flour (LMF) as alternatives to egg and refined flour, respectively, for developing egg-free and gluten-free cake recipes. The cake and batter were analyzed for their physicochemical, structural, sensorial, rheological and textural properties, with a shelf-life study conducted over a period of 12 days (30 ± 2 °C). The batter's density (1.13 ± 0.01 g cm−3) and viscosity (15796.7 ± 0.09 cp) increased with the addition of LMF, and the batter predominantly exhibited an elastic behavior with G′ > G′′ for all cake formulations. These characteristic changes in cake batter caused the cake's weight to increase (by 10%) while causing its height, baking loss, volume, specific volume and symmetry index to decrease. The textural characteristics showed that adding LMF to cakes enhanced the hardness (145.00 ± 9.45 N) and firmness (19.36 ± 2.12 N) and decreased their cohesion, chewiness, gumminess, springiness and resilience. The image analysis showed more uniform bubble distribution in wheat flour (WF) cake than in LMF cakes, and an increase in LMF content resulted in a drop in cell circularity. The microbial degradation of cakes was observed from the 6th day of storage. LMF cake samples exhibited good texture and physical and sensory attributes comparable to those of the WF cake sample. Therefore, the study demonstrated the potential use of alternative ingredients, such as LMF and aquafaba emulsion, in the production of egg-free and gluten-free cakes. These ingredients could facilitate the scaling up of sustainable production practices for baked goods in the future.
Sustainability spotlightThis study explores sustainable food technology through ingredients like chickpea aquafaba and little millet flour (LMF) as alternative ingredients for developing egg-free and gluten-free cakes. With a rising consumer demand for vegan and healthy diets, the utilization of such ingredients not only reduces dependence on traditional animal-based products but also promotes the use of underutilized grains like LMF, which require fewer resources and support agricultural biodiversity. By transforming a waste product (aquafaba) into a functional baking component, this research exemplifies the principles of a circular economy, minimizing food waste and enhancing sustainability. Additionally, evaluating other sugar alternatives, such as unprocessed foods like raw honey, brown rice syrup, and jaggery, could significantly improve the health and sustainability of cakes produced in the industrial sector. The findings demonstrate that LMF cakes can achieve sensory and textural qualities comparable to those of conventional wheat flour cakes, making them a viable option for scaling up the production of sustainable baked goods that cater to diverse dietary needs. This work highlights the potential for innovative, environmentally friendly practices in the baking industry, contributing to a more sustainable food system. |
1. Introduction
Aquafaba, which translates to “bean water” in Latin, is a novel plant-based protein that has been increasingly used as an emulsifier. It is the residual water left from cooked beans (especially chickpeas), which is rich in water soluble proteins and complex carbohydrates.1 Its unique proportion of soluble carbohydrates and proteins comes through the soaking and cooking process. As an outcome, a more adaptable material, i.e. aquafaba, is obtained that can withstand a wide range of pH values and temperatures with multifunctional properties, exhibiting gelling, emulsifying, foaming, and water- and oil-holding capacities.People with phenylketonuria, a condition that affects protein metabolism, can now enjoy foods that are typically egg-free using aquafaba as an alternative. Additionally, those with egg allergy also follow a typical plant-based diet. Plant-based diets using pulses as an alternative to animal proteins have offered the most practical features as an essential protein source for use in culinary applications.2
Plant-based meals and nutrients are now widely available worldwide, offering a viable alternative to animal-based foods like dairy products, eggs, meat from cattle and poultry, and seafood.3 This shift acknowledges their benefits in terms of ethical considerations, environmental sustainability, and human health.4 Currently, one of the main focuses is on the development of egg alternatives. Aquafaba contains about one fourth of the protein relative to egg white by dry weight.5 Stantiall et al.6 reported that aquafaba can be utilized as an ingredient in place of egg white owing to its good gelling and foaming properties.
Health challenges such as phenylketonuria, egg allergy, excessive cholesterol, changes in dietary choices and religious convictions, along with monetary incentives, can all contribute to the replacement of eggs.7 Eggs have unique functional properties that make them highly versatile for various food applications, especially in the preparation of cakes in the bakery industry. In addition to egg less cakes, gluten free cakes are also in demand due to allergic reactions and other health related concerns like celiac disease, diabetes and obesity, which are linked to the active ingredients in cakes, like wheat flour (WF).8 To overcome this issue, wheat alternatives like millets have been recently researched for obtaining nutritional benefits and comparable textural properties.9,10
Little millet provides minerals, essential amino acids, and vitamin B complex—all of which are frequently deficient in our main meal diets.11 A phytochemical study has demonstrated that this millet has a lower glycemic index and greater antioxidant levels when compared to other dietary crops.12 Eating cereals made from these whole grains lowers the risk of cardiovascular disease and diabetes.13 Aquafaba has been recently explored by few authors as an egg-replacer in baked goods especially cakes.14–17 However, research on gluten free millet cakes using aquafaba has not yet been explored.
Thus, the current research aims to explore the feasibility of producing gluten free egg less cakes using little millet flour (LMF) and chickpea aquafaba with the goal of improving the nutritional and functional attributes similar to those of conventional cakes.
2. Materials and methods
2.1 Materials
The ingredients including chickpea, refined WF (maida), little millet (Panicum sumatrense Roth.), refined sugar, milk powder (Nestle Everyday Tea's Perfect Partner; ∼76% Solid Non Fat (SNF) and 15% fat), baking powder (Weikfield baking powder), vinegar (Dr Vaidya's apple cider vinegar) and vanilla essence (Flavor Mate) were purchased from a local supplier in Varanasi, Uttar Pradesh, India (82.9739° E, 25.3176° N). Little millet and sugar were powdered using a conventional mixer (Butterfly Smart mixer, 750 W), and screened with a 60-mesh size sieve to separate the particles with a smaller size (<200 μm). Analytical reagent (AR) grade chemicals were obtained from standard companies (Hi-Media, India, and Sigma Aldrich, India) for all preparation and analysis, and the reagents were made fresh using standard procedures.2.2 Aquafaba preparation
After cleaning, 100 g of chickpea was soaked at 4 °C for 16 h. The aquafaba was prepared based on the method followed by He et al.18 Soaked chickpeas were mixed with filtered water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), placed inside a glass jar, and autoclaved for 30 min at 115 °C. After that, it was left to cool for 24 h at room temperature, which was later filtered and placed in a refrigerator (2 to 4 °C) until further analysis.
4), placed inside a glass jar, and autoclaved for 30 min at 115 °C. After that, it was left to cool for 24 h at room temperature, which was later filtered and placed in a refrigerator (2 to 4 °C) until further analysis.
2.3 Sponge cake preparation
The sponge cakes using little millet flour (LMF) were prepared according to Mustafa et al.14 with some modification. Using a hand blender (Braun MQ9047X, New Castle, USA), 110 mL of aquafaba and 10 mL of apple vinegar were combined, and the mixture was whipped for 7 min at an optimal speed until the majority of the aquafaba became frothy and little liquid persisted. When the frothy blend was whipped into a dense peak, 130 g of granulated sugar was added. Using a paddle, 130 g of WF, 7 g of baking powder, and 13 g of milk powder were carefully added and blended into the froth. Lastly, 5 mL of vanilla essence was added and mixed gently. The ratio of WF to LMF was varied to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 75
1, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 50
25, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 25
50, 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 and 1
75 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 on weight basis and the samples were labelled as C-100, T-25, T-50, T-75, and T-100, respectively (Table 1). The samples of cake batter were then transferred to 14.7 × 7.7 cm rectangular pans and baked in a conventional micro-oven (Panasonic NN-DS596, Japan) set at 150 °C for 10 min and 180 °C for 20 min. The pans were then taken out of the micro-oven after baking and allowed to cool for 30 min at room temperature.
100 on weight basis and the samples were labelled as C-100, T-25, T-50, T-75, and T-100, respectively (Table 1). The samples of cake batter were then transferred to 14.7 × 7.7 cm rectangular pans and baked in a conventional micro-oven (Panasonic NN-DS596, Japan) set at 150 °C for 10 min and 180 °C for 20 min. The pans were then taken out of the micro-oven after baking and allowed to cool for 30 min at room temperature.
| C-100 | T-25 | T-50 | T-75 | T-100 | |
|---|---|---|---|---|---|
| a C-100: 100% WF; T-25: 75% WF + 25% LMF; T-50: 50% WF + 50% LMF; T-75: 25% WF + 75% LMF; T-100: 100% LMF. | |||||
| Aquafaba (mL) | 110.0 | 110.0 | 110.0 | 110.0 | 110.0 |
| WF (g) | 130.0 | 97.5 | 65.0 | 32.5 | — |
| LMF (g) | — | 32.5 | 65.0 | 97.5 | 130.0 |
| Sugar powder (g) | 130.0 | 130.0 | 130.0 | 130.0 | 130.0 |
| Milk powder (g) | 13.0 | 13.0 | 13.0 | 13.0 | 13.0 |
| Baking powder (g) | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 |
| Vinegar (mL) | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Vanilla essence (mL) | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
2.4 Physical measurements of the cake and batter
All the analyses of batter were done in triplicate using the procedures outlined by He et al.18 The quantity of air included into a batter can be determined by the measurement of the batter density (ρ). The batter density (ρ) was measured using an Elcometer 1800 pycnometer (Manchester, UK). The viscosity of the batter was determined using a Brookfield DV-11 + Pro Viscometer (Middleborough, MA; spindle no. 4 (S64)) set at 5 rpm at room temperature (21.8 ± 2 °C). The pH of the cake batter was determined using a pH meter (Model 1761, Jenco Electronics Ltd, Taiwan) at a temperature of 25 °C.Proximate analysis of the cake was conducted to measure its moisture content, protein content, ash content, fat content, carbohydrate content and calorific value.19 The physical measurements including baking loss, cake weight and volume of each cake formulation were performed according to Gómez et al.20 For determining the baking loss, a vernier caliper was used to measure the cake's height, breadth and length in centimeters. The cake's weight (g) was measured in triplicate using an electronic weighing scale (Metler Toledo, JB1603-C, Switzerland). The difference between the total mass of the dough before baking (Wu) and the weight of the cake after baking (Wv) was used to quantify the baking loss of the cake samples. A laser sensor with a BVM-L370 (TexVol Instruments, Viken, Sweden) was used to measure the cake's volume. The ratio of the size of the cake's volume to its weight provided the cake's specific volume.
According to Grossi Bovi Karatay et al.,21 the symmetry index was calculated after 24 h of storage using eqn (1). This measurement is taken at specified positions to ensure a representative sample of the cake's overall structure. For each cake slice, the height measurements were taken from the center of the slice (C), height at one quarter (B) and height at three quarters (D) of the cake length.
| Symmetry index = 2 × C − B − D | (1) |
2.6 Significant rheological characteristics of the prepared doughs
The dynamic rheological measurements of storage modulus (G′), loss modulus (G′′) and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ (G′′/G′) were performed according to Li et al.22 using a rheometer (AR2000ex, TA Instruments, New Castle, USA) with a parallel plate geometry (40 mm in diameter) at 1 mm gap. The dough sample was obtained by manually mixing 2 g of prepared batter with 1.3 mL of distilled water and rested for 20 min to relax any residual stress. The frequency sweep test was carried out at a frequency of 0.1–10 Hz and a strain of 0.1%. The proportion of G′′ to G′ was used to determine tan
δ (G′′/G′) were performed according to Li et al.22 using a rheometer (AR2000ex, TA Instruments, New Castle, USA) with a parallel plate geometry (40 mm in diameter) at 1 mm gap. The dough sample was obtained by manually mixing 2 g of prepared batter with 1.3 mL of distilled water and rested for 20 min to relax any residual stress. The frequency sweep test was carried out at a frequency of 0.1–10 Hz and a strain of 0.1%. The proportion of G′′ to G′ was used to determine tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ.
δ.
2.7 Instrumental textural profile analysis
The interior crumb texture characteristics of the sponge cake and exterior crust were analyzed in accordance with He et al.18 The texture profile analysis was performed in a Texture analyser (Texture Pro CT V1.6 build, Brookfield) using Texture Lab Pro Software, Version 1, 13-002, and a TMS-Pro Texture Press (Food Technology Corp., Sterling, VA). Sponge cake pieces of 25 × 15 mm dimension were cut out to make the crumb, making sure to leave out the outermost layer and borders. At a cross-head speed of 100 mm min−1, each sample was vertically crushed to 50% of its initial height (7.5 mm). Hardness cycle 1 (N), hardness cycle 2 (N), firmness (N), gumminess (mm), chewiness (N), cohesiveness, resilience (mJ) and springiness (mm) were obtained from the software and an average of triplicate readings was calculated.2.8 Image analysis
Image analysis of the sponge cake was done according to Tsatsaragkou et al.23 Cakes were sliced vertically into 1.5 cm thick pieces. Each slice was photographed at 300 dpi for precision. The cake height was measured with Image J 2.9.0 software (National Institutes of Health, Palo Alto, CA, USA) and the scanned picture was analyzed for obtaining the crumb cell structure. To perform the aforementioned analysis, a 3.5 × 3.5 cm portion with a uniform thickness was clipped from the center of each slice. The image was divided into colour channels first, to which contrast was added, and at last the image was binarized following a grayscale threshold. The image was later analyzed for cell count, average size and number of bubbles and the measurements were taken in triplicate.2.9 Bright field microscopy
Sponge cake crumb structure was imaged using bright field microscopy (Olympus CX23, Olympus, Japan) as per the protocol given by Tsatsaragkou et al.23 with LED illumination and 30 μm magnification. The image was first divided into color channels using Image J 2.9.0 (National Institutes of Health, Palo, Alto, CA, USA), then the contrast effect was increased, and finally the image was binarized following the grayscale threshold. Cell circularity and average cell area (mm2) were computed by eqn (2). The values range from 0.0 to 1.0 for cell circularity. A circularity value of 1.0 denotes a circle that is perfectly circular, whereas values closer to 0.0 suggest an increasingly extended polygon. Triplicate measurements were taken from the center of each cake. | (2) |
2.10 Fourier transform infrared spectroscopy analysis of the sponge cake
The infrared spectra of the materials were obtained using a PerkinElmer Spectrum 100 FTIR spectrometer with the KBr pellet technique, at room temperature. Pellets were formed by gradually combining the samples with KBr powder that had been micronized. Each sample underwent twenty scans, with a spectral resolution of 4 cm−1, and spectra were collected between 4000 and 400 cm−1 wavenumber. Regular background spectrum collection was done to ensure data accuracy and dependability.2.11 Shelf-life studies
The shelf-life study of cakes was done for 12 days and measurements of moisture, protein, ash, total solids, fat, weight gain, total plate count, coliform, yeast and mold were performed. The samples were kept in a PET box at room temperature (30 ± 2 °C). 10 g of cake sample was ground in 90 mL of a sterile sodium chloride solution (0.9%) diluted for microbiological analysis in accordance with the protocol of Salfinger and Tortorello.242.12 Sensory evaluation
A sensory examination was done on cakes that were baked using LMF and served as 2.5 cm cubes. Thirty semi-trained panelists (15 each male and female), aged between 22 and 25 years, were selected and samples were served at 25 ± 2 °C. The samples were presented to panelists in a random order and they were requested to assess the samples' color, appearance, texture, flavor, aftertaste and overall acceptability based on the 9-point hedonic scale. In the rating system, 1 denotes strong dislike and 9 denotes high liking.2.13 Statistical analysis
Each experiment was conducted in triplicate, and the results were expressed in terms of mean ± SD. Using SPSS version 20.0 software (SPSS Inc., Chicago, IL, USA), ANOVA (one-way analysis of variance) and Turkey's test (p < 0.05) were performed.3. Results and discussion
3.1 Physicochemical properties of the cake and batter
| C-100 | T-25 | T-50 | T-75 | T-100 | ||
|---|---|---|---|---|---|---|
| a Dissimilar alphabets (a, b, c, d) in the similar row indicate significance variance (P < 0.05). C-100: 100% WF; T-25: 75% WF + 25% LMF; T-50: 50% WF + 50% LMF; T-75: 25% WF + 75% LMF; T-100: 100% LMF. | ||||||
| Physicochemical analysis | ||||||
| Moisture | Crumb (%) | 24.37 ± 1.54c | 23.64 ± 0.37c | 22.17 ± 0.56bc | 20.41 ± 1.65 ab | 18.69 ± 1.03a |
| Crust (%) | 16.04 ± 0.83a | 15.83 ± 0.33a | 15.46 ± 1.65a | 14.23 ± 0.03a | 13.84 ± 1.05a | |
| Ash (%) | 1.24 ± 0.04a | 1.29 ± 0.20a | 1.34 ± 0.05a | 1.4 ± 0.07a | 1.44 ± 0.03a | |
| Protein (%) | 13.19 ± 0.36c | 12.94 ± 0.28c | 12.34 ± 0.52bc | 11.58 ± 0.34 ab | 11.13 ± 0.56a | |
| Fat (%) | 16.26 ± 0.53a | 16.61 ± 0.24a | 16.97 ± 0.42a | 17.23 ± 0.5a | 18.35 ± 0.16a | |
| Carbohydrates (%) | 58.48 ± 0.42a | 59.19 ± 0.36 ab | 59.90 ± 0.47b | 60.37 ± 0.58b | 61.88 ± 0.42c | |
| Calorific value (kcal) | 344.00 ± 1.02a | 347.00 ± 1.34a | 356.00 ± 1.22b | 364.00 ± 1.11c | 378.00 ± 1.47d | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Cake characterization | ||||||
| Length (cm) | 14.60 ± 0.30a | 14.50 ± 0.20a | 14.40 ± 0.20a | 14.40 ± 0.10a | 14.40 ± 0.30a | |
| Breadth (cm) | 7.70 ± 0.20a | 7.70 ± 0.30a | 7.60 ± 0.40a | 7.60 ± 0.30a | 7.50 ± 0.20a | |
| Height (cm) | 4.20 ± 0.03b | 4.20 ± 0.01b | 4.10 ± 0.05 ab | 4.10 ± 0.05 ab | 4.00 ± 0.07a | |
| Weight (g) | 170.50 ± 1.23a | 175.25 ± 0.98b | 180.00 ± 0.15c | 184.47 ± 0.12d | 190.03 ± 1.67e | |
| Volume (cm3) | 462.84 ± 0.04e | 449.68 ± 0.3d | 448.29 ± 0.02c | 440.09 ± 0.02b | 437.52 ± 0.04a | |
| Baking loss (%) | 11.40 ± 1.02a | 11.09 ± 0.84a | 10.88 ± 0.65a | 10.35 ± 0.56a | 9.30 ± 0.98a | |
| Cake specific volume (cm3 g−1) | 2.71 ± 0.01d | 2.62 ± 0.05c | 2.60 ± 0.02bc | 2.53 ± 0.03 ab | 2.51 ± 0.01a | |
| Symmetry index | 2.60 ± 0.02a | 2.40 ± 0.03a | 2.50 ± 0.01a | 2.20 ± 0.30a | 2.30 ± 0.24a | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Batter characterization | ||||||
| Density (g cm−3) | 1.06 ± 0.01a | 1.09 ± 0.03 ab | 1.11 ± 0.01b | 1.11 ± 0.02b | 1.13 ± 0.01b | |
| Viscosity (cp) | 14450.00 ± 0.07a | 14492.13 ± 0.07b | 15423.44 ± 0.05c | 15678.91 ± 0.12d | 15796.7 ± 0.09e | |
| pH | 6.60 ± 0.01a | 6.70 ± 0.01b | 6.60 ± 0.01a | 6.80 ± 0.01c | 6.70 ± 0.01b | |
The dimensions such as length, breadth, and height (as shown in Fig. 1) and volume of the sponge cake are presented in Table 2. Not much variation in the dimensions of different cake formulations was observed; however, variations in volume, specific volume and weight were found. This could be attributed to the lower content of structure-forming proteins and reduced gluten in the LMF, which led to decreased carbon dioxide gas retention and resulted in a less dense texture. Consequently, the observed decrease in cake samples was significantly attributed to the dilution of gluten in the wheat mixes. Similar outcomes were obtained by Mudau et al.30 by the addition of millet flour. The cake weights ranged from 170.50 ± 1.23 g (C-100) to 190.03 ± 1.67 g (T-100), with all samples showing significant differences (p < 0.05). The heavier dough could be attributed to factors such as particle size, improved moisture absorption, and decreased air entrapment. Kayitesi et al.31 noted that the increased weight and larger particle size of fiber-rich flours result in greater bulk density and water absorption capacity.
 | ||
| Fig. 1 Physical structural dimensions of aquafaba and little millet flour incorporated cake formulations. (A) length; (B) height; (C) breadth. | ||
Additionally, not much significant (p ≥ 0.05) variation in cake's baking loss and specific volume was observed, with a decreasing trend in baking loss, being highest for C-100 (11.40% ± 1.02%) and lowest for T-100 (9.30% ± 0.98%). de la Hera et al.32 found that lower gluten concentration flours had less baking loss because of their poor water binding capacity. All symmetry index values in the current investigation were positive, indicating no evidence of cake collapse.33
3.2 Rheological characteristics of the prepared dough
Understanding the rheological properties of dough, specifically its viscoelasticity, is crucial for dough preparation. Measuring the batter's rheological properties immediately after preparation offers valuable insights that significantly predict the volume of the cakes together with their cohesiveness.37 The storage modulus (G′) of the batter ranged from 3135.3 Pa (C-100) to 2758.4 Pa (T-100) (at 0.1 rad s−1) (see Fig. 2). The storage modulus of a batter reflects the elasticity or solid nature of the dough, which was highly influenced by the addition of LMF. The addition of LMF enhanced the dough structure and stiffness by forming a more interconnected network. Moreover, the proteins and fibers in LMF contribute to a stronger, more cohesive gel network, which potentially reduced the storage modulus. Unlike WF, LMF lacks gluten, which aids in a stable protein network, resulting in higher G′. Consequently, the LMF dough exhibited less elasticity compared to the WF batter, resulting in a less elastic structure.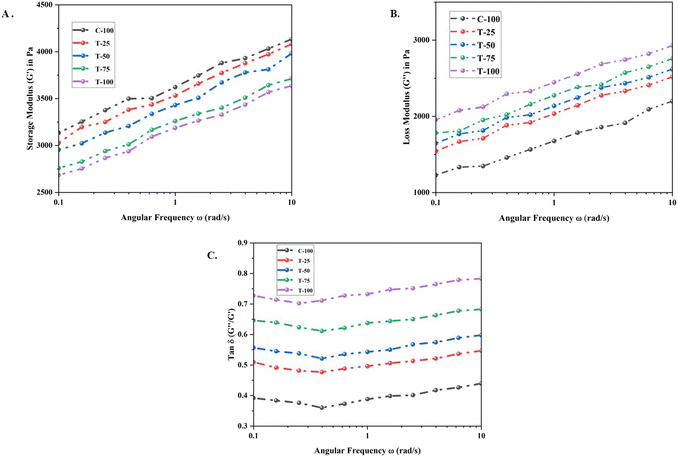 | ||
Fig. 2 Dynamic rheological properties of formulated cake batters. (A) Storage modulus (G′); (B) loss modulus (G′′); (C) tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ (G′′/G′). δ (G′′/G′). | ||
The loss modulus (G′′) of the batter ranged from 1228.6 Pa (C-100) to 1995.44 Pa (T-100) (as shown in Fig. 2). The loss modulus quantifies a material's viscous behavior by indicating the amount of energy dissipated as heat during deformation. An increase in LMF content typically raises the batter's viscosity due to the higher fiber content, which in turn increased the loss modulus.38 Enhanced hydration from LMF contributes to improved viscosity characteristics, as greater water absorption leads to more energy dissipation during deformation. LMF, with its higher starch content compared to WF, further elevates the batter's viscosity, resulting in a higher loss modulus. Consequently, the LMF batter exhibited a more viscous behavior than the WF batter. Supporting this, Yu et al.39 found that an increase in barley flour proportion led to a higher loss modulus and a lower storage modulus of the dough. The addition of millet flour may have created new interactions between starch and protein, resulting in higher viscosity in the formulated dough.
The tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ values ranged from 0.39 (C-100) to 0.72 (T-100) (as shown in Fig. 2) with a significant difference among the samples (p < 0.05). The tan
δ values ranged from 0.39 (C-100) to 0.72 (T-100) (as shown in Fig. 2) with a significant difference among the samples (p < 0.05). The tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ ratio represents the dough's viscosity-to-elasticity ratio, where a lower tan
δ ratio represents the dough's viscosity-to-elasticity ratio, where a lower tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ indicates a more elastic structure. The data show that the tan
δ indicates a more elastic structure. The data show that the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ value initially decreased and then increased with increasing frequency, suggesting that elasticity is not dominant at higher frequencies. Throughout the tested frequencies, G′ consistently exceeds G′′, indicating that elasticity is the predominant characteristic of the formulated sponge cakes. The addition of the low-moisture flour, i.e., the LMF, notably altered the dough's viscoelastic properties. Varying LMF levels resulted in distinct changes in G′ and G′′, likely due to differences in cross-linking and mechanical changes in the gluten network.40 Consequently, the dough with a more elastic structure (C-100) exhibited the lowest tan
δ value initially decreased and then increased with increasing frequency, suggesting that elasticity is not dominant at higher frequencies. Throughout the tested frequencies, G′ consistently exceeds G′′, indicating that elasticity is the predominant characteristic of the formulated sponge cakes. The addition of the low-moisture flour, i.e., the LMF, notably altered the dough's viscoelastic properties. Varying LMF levels resulted in distinct changes in G′ and G′′, likely due to differences in cross-linking and mechanical changes in the gluten network.40 Consequently, the dough with a more elastic structure (C-100) exhibited the lowest tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ value.
δ value.
3.3 Instrumental textural profile analysis
Table 3 presents the texture profile analysis for the crust and crumb of cakes. The data indicated that cakes with LMF exhibited higher hardness compared to WF cakes, but the data was insignificant (p ≥ 0.05). The hardness of the cake crust ranged from 140.00 ± 10.24 N (C-100) to 145.00 ± 9.45 N (T-100); however, not much significant difference between the values was observed (p ≥ 0.05). For the crumb the hardness values ranged from 15.00 ± 1.4 N (C-100) to 20.00 ± 1.69 N (T-100). The increased hardness observed in cakes with higher LMF content is likely due to a denser batter. Lee et al.41 also reported an increase in cake hardness with the addition of oat bran. With respect to firmness, the crust values ranged from 16.91 ± 2.34 N (C-100) to 19.36 ± 2.12 N (T-100), while crumb values ranged from 6.56 ± 1.86 N for C-100 to 6.96 ± 0.96 N for T-100 with no significant difference among cake formulations (p ≥ 0.05). Similar trends of crust and crumb firmness were reported by Martinez et al.42 Moreover, significant (p < 0.05) variation in chewiness was also observed, which represents the internal strength of the sponge cake, which could be related to the dilution of gluten content by the millet flour. Similar findings were reported by Vinay and Singh.38 in muffins prepared with pearl millet flour.| C-100 | T-25 | T-50 | T-75 | T-100 | |
|---|---|---|---|---|---|
a Dissimilar alphabets (a, b, c) in the similar row indicate significance variance (P < 0.05). C-100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100% WF; T-25 100% WF; T-25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75% WF + 25% LMF; T-50 75% WF + 25% LMF; T-50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50% WF + 50% LMF; T-75 50% WF + 50% LMF; T-75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25% WF + 75% LMF; T-100 25% WF + 75% LMF; T-100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100% LMF. 100% LMF.
|
|||||
| Crust | |||||
| Hardness cycle 1 (N) | 140.00 ± 10.24a | 135.00 ± 8.64a | 125.00 ± 14.86a | 130.00 ± 11.34a | 145.00 ± 9.45a |
| Hardness cycle 2 (N) | 95.00 ± 6.87b | 75.00 ± 6.41 ab | 65.00 ± 10.53a | 70.00 ± 9.34a | 80.00 ± 7.92 ab |
| Firmness (N) | 16.91 ± 2.34a | 19.76 ± 1.68a | 18.45 ± 2.36a | 18.62 ± 3.52a | 19.36 ± 2.12a |
| Cohesiveness | 0.39 ± 0.01b | 0.38 ± 0.02b | 0.34 ± 0.02 ab | 0.34 ± 0.02 ab | 0.32 ± 0.03a |
| Gumminess (mm) | 18.00 ± 2.21a | 17.00 ± 1.24a | 15.00 ± 3.21a | 14.00 ± 2.36a | 13.00 ± 1.69a |
| Chewiness (N) | 240.50 ± 3.98c | 210.40 ± 5.34b | 205.00 ± 5.47b | 199.50 ± 4.26 ab | 186.00 ± 7.23a |
| Resilience (mJ) | 0.25 ± 0.02b | 0.24 ± 0.02 ab | 0.22 ± 0.01 ab | 0.21 ± 0.01a | 0.21 ± 0.01a |
| Springiness (mm) | 8.65 ± 0.23c | 7.98 ± 0.78bc | 6.34 ± 0.46 ab | 5.45 ± 1.34a | 4.84 ± 0.54a |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Crumb | |||||
| Hardness cycle 1 (N) | 15.00 ± 1.4a | 25.00 ± 1.02c | 25.00 ± 1.68c | 20.00 ± 1.62b | 20.00 ± 1.69b |
| Hardness cycle 2 (N) | 9.50 ± 1.34a | 15.00 ± 1.9b | 15.00 ± 1.58b | 10.00 ± 0.72a | 10.00 ± 1.76a |
| Firmness (N) | 6.56 ± 1.86a | 6.84 ± 1.68a | 5.42 ± 0.86a | 6.64 ± 2.05a | 6.96 ± 0.96a |
| Cohesiveness | 0.50 ± 0.03b | 0.45 ± 0.01 ab | 0.44 ± 0.04 ab | 0.43 ± 0.01a | 0.42 ± 0.02a |
| Gumminess (mm) | 15.00 ± 3.67a | 15.00 ± 2.02a | 13.00 ± 1.55a | 11.00 ± 1.86a | 10.00 ± 2.68a |
| Chewiness (N) | 95.00 ± 4.78c | 80.00 ± 8.42b | 69.00 ± 3.24 ab | 63.00 ± 5.98a | 58.00 ± 3.86a |
| Resilience (mJ) | 0.24 ± 0.01a | 0.24 ± 0.02a | 0.23 ± 0.03a | 0.22 ± 0.01a | 0.22 ± 0.01a |
| Springiness (mm) | 6.48 ± 1.12a | 6.39 ± 0.92a | 6.23 ± 0.56a | 6.18 ± 0.86a | 5.56 ± 1.34a |
Not much variation in cohesiveness, springiness, gumminess and resilience of crust and crumb of cake formulations was observed. However, a decreasing trend with the addition of LMF was observed, which could also be attributed to the reduced gluten content of LMF. This can be correlated to the results of G′ of cake batter, where the elasticity decreased with decreased gluten content due to LMF addition, leading to reduced cohesiveness. Rajiv et al.43 observed a similar trend in muffins prepared with finger millet flour, where cohesiveness and springiness decreased with increased flour substitution.
3.4 Image analysis
Due to water evaporation and gas diffusion, the low viscosity of the batter enhanced the mobility of bubbles, which contributed to surface irregularities. A significant central hole (Fig. 3) was observed in the cake crumb, resulting from substantial gas formation. Turabi et al.44 reported similar findings in rice flour cakes, where low-viscosity batters allowed air bubbles to easily rise to the surface and escape. The samples with WF (C-100) exhibited a significant (p < 0.05) increase in expansion height compared to the other samples (Fig. 3), with average bubble sizes categorized as <0.5 mm, 0.5–1.0 mm, 1.0–1.5 mm, 1.5–2.0 mm, 2.0–3.0 mm, 3.0–4.0 mm, 4.0–5.0 mm, and >5.0 mm in a 3 × 3 cm area (Fig. 4). These results suggest improved gas retention and the effective action of leavening agents during baking. The results were consistent with those of Sanz et al.,45 who suggested that dough viscosity influenced bubble incorporation and mobility, both of which are crucial in determining final cake volume. These findings emphasize the importance of batter viscosity in obtaining the overall cake structure. Conversely, T-100, with a greater density and broader range of bubble sizes, resulted in a noticeably smaller cake height. This aligns with the results of Zahn et al.,46 who found that muffins made with added fiber i.e. 50% inulin instead of fat had reduced volume without showing significant changes in mass loss during baking. | ||
| Fig. 3 Image analysis of aquafaba and little millet flour incorporated cakes. (A) Batter; (B) cake; (C) crumb slice; (D) crumb structure and (↓) indicates the central hole. | ||
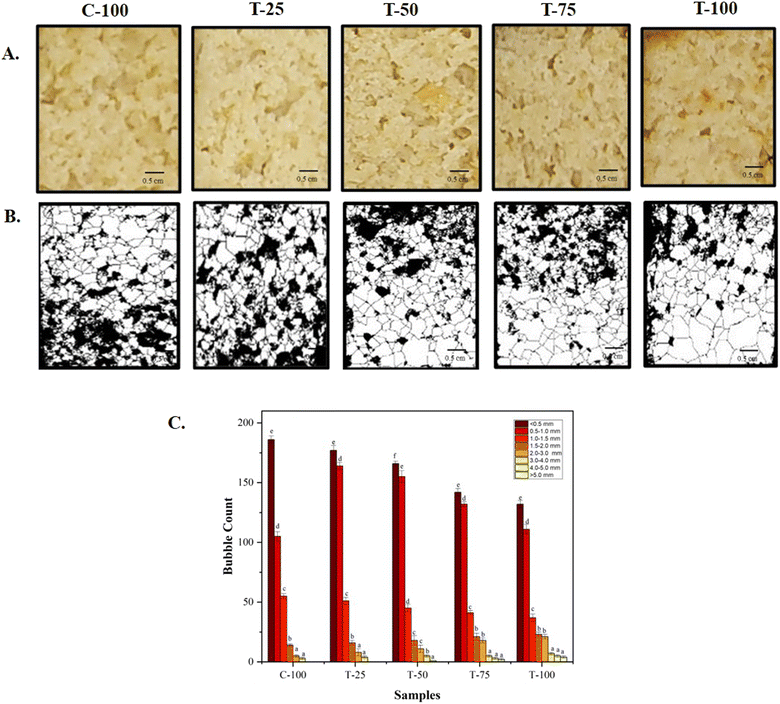 | ||
| Fig. 4 Bubble count of aquafaba and little millet flour incorporated cake formulations. (A) Crumb (3 × 3 cm); (B) crumb bubbles (3 × 3 cm); (C) bubble count (3 × 3 cm). | ||
3.5 Bright field microscopy images
The variation in physicochemical characteristics of each cake formulation can be due to the difference in cake morphology. From Fig. 3 and 4 it can be inferred that the crumb structures of all cake formulations differed notably from one another. Specifically, T-100, which did not include WF, restricted the expansion of cakes, resulting in decreased cake height. The cell circularity and average cell area varied among the crumbs, with T-100 showing larger, more mobile bubbles that were capable of greater growth. Consequently, T-100's crumb structure was characterized by large, interconnected cells and crack-like diffusion channels (Fig. 5). As noted by Kocer et al.,47 increased gas phase mobility facilitated the formation of such diffusion paths. The T-100 cake made solely with LMF had the least height of all the samples, indicating reduced cake expansion. In contrast, the control cake featured a uniform distribution of smaller cells as compared with other formulated cakes (see Fig. 3 and 4). T-100's crumb showed a more heterogeneous cell distribution, with larger cells and denser regions, reflected by a significant (p < 0.05) increase in mean cell size and decrease in circularity values. Specifically, the circularity values for C-100 and T-100 were 0.77 and 0.71, while cell area values were 0.61 μm2 and 0.78 μm2, respectively (see Fig. 5). This can be attributed to higher loss modulus (G′′) values, which typically indicates greater energy dissipation, which can result in a less stable structure. If the batter is too fluid due to a high G′′, it may not support the formation of well-defined air cells, leading to a more irregular shape, leading to reduced circularity. These findings aligned with those of Tsatsaragkou et al.,23 who observed lower cell circularity values in cakes and biscuits made with inulin as a sugar replacer.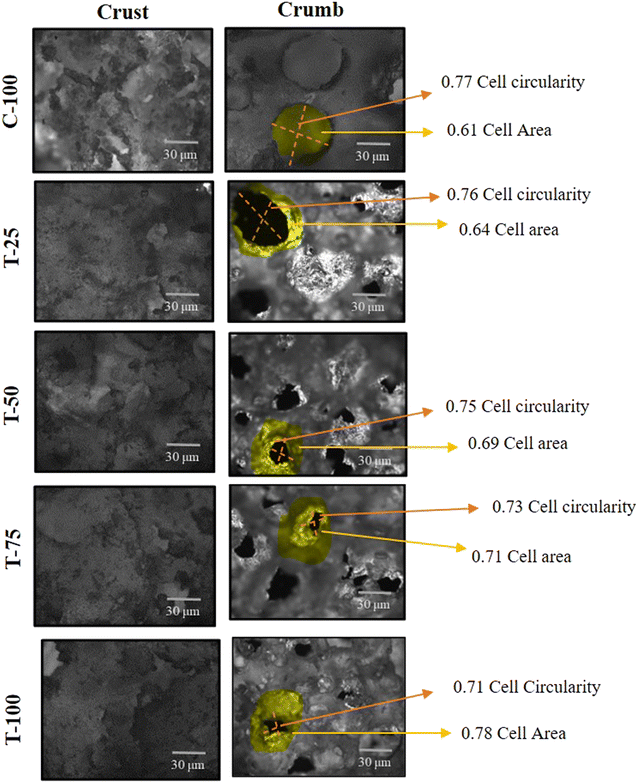 | ||
| Fig. 5 Bright field microscopic images of aquafaba and little millet flour incorporated cakes representing cell circularity. Sample were: C-100; T-25, T-50, T-75, and T-100 respectively. | ||
3.6 Fourier transform infrared spectroscopy analysis of sponge cakes
The examination of FTIR spectra (Fig. 6) revealed notable differences among the samples, particularly between C-100 and T-100, while other cake formulations showed minimal variations. Peaks in the 3800–2500 cm−1 range were associated with O–H bond stretching, which potentially indicated the water molecules, visibly present in all samples. Specifically, a peak at 3786.5 cm−1 was observed in both C-100 and T-100. Peaks at 2931.32 cm−1 and 2845.14 cm−1 appeared in all samples, which represent the C–H stretching of methoxyl groups related to the lignin component.48 The peak near 2845.14 cm−1 was clearly visible in all the samples batter (LMF) except for C-100, inferring the absence of fibre. The 3500–3100 cm−1 range, linked to the N–H stretching of amide A, was consistent across all samples, with a peak at 3447.6 cm−1.49,50 Additionally, peaks associated with amide groups were identified at 1660.73 cm−1 and 1454.71 cm−1, representing amide-I and amide-II, respectively. These peaks were significantly diminished with baking, reflecting typical denaturation of proteins while baking.Carbohydrates, identified by peaks in the 1200–900 cm−1 range, were present in all spectra, with notable peaks at 936 cm−1, 1024.62 cm−1, and 1158.41 cm−1. The presence of a peak at 1158.41 cm−1 in T-100 suggests distinct starch derivatives from LMF, influenced by heat treatment and biological origin. This peak corresponds to the crystalline area of starch,50 while the peak at 1022 cm−1 is the amorphous phase of starch granules and the range of 995–900 cm−1 marks the molecular order and crystalline areas.51
3.7 Shelf-life
The shelf-life analysis of the cake is summarized in Table 3, which examines the effects of ambient storage at 30 ± 1 °C for over 12 days on the cake's bacteriological quality, particularly when WF is replaced by LMF. During storage, the cake's moisture content, ash content, fat content, and weight decreased, while microbial populations increased. Total plate count showed no significant change during the first 3 days of storage at 30 °C, suggesting a lag phase for bacterial adaptation. Yeast and mold counts, as well as coliform bacteria, increased more slowly compared to total plate counts and remained low throughout the storage period. By the end of 12 days, the total plate count had risen to 17.2 × 103 ± 0.3 CFU g−1, which correlated with a decline in the cake's sensory properties. This increase in total plate count, particularly on the 9th and 12th days, can be attributed to the ideal growth conditions of 30 °C ± 1 °C. The temperature range of 4.4–60 °C is generally recognized as the danger zone where microorganisms proliferate most rapidly.52 Extended exposure to ambient temperatures can lead to higher microbial loads, potentially reaching unsafe levels, posing significant health risks to humans (Table 4).| 0th Day | 3rd Day | 6th Day | 9th Day | 12th Day | ||
|---|---|---|---|---|---|---|
| a Dissimilar alphabets (a, b, c, d) in the similar row indicate significance variance (P < 0.05). C-100: 100% WF; T-25: 75% WF + 25% LMF; T-50: 50% WF + 50% LMF; T-75: 25% WF + 75% LMF; T-100: 100% LMF. | ||||||
| Moisture (%) | C-100 | 24.37 ± 1.54c | 24.01 ± 0.83c | 22.83 ± 0.11d | 20.74 ± 1.01 cd | 20.36 ± 0.34c |
| T-25 | 23.64 ± 0.37c | 23.43 ± 0.73c | 22.28 ± 0.23 cd | 21.57 ± 0.85d | 21.38 ± 0.74c | |
| T-50 | 22.17 ± 0.56bc | 21.25 ± 1.02b | 20.91 ± 0.54bc | 19.38 ± 0.22bc | 18.28 ± 0.69b | |
| T-75 | 20.41 ± 1.65 ab | 20.14 ± 0.56 ab | 19.79 ± 0.85b | 18.48 ± 0.45 ab | 17.63 ± 0.63 ab | |
| T-100 | 18.69 ± 1.03a | 18.40 ± 0.34a | 18.11 ± 0.79a | 16.82 ± 0.37a | 16.40 ± 0.91a | |
| Ash (%) | C-100 | 1.24 ± 0.04a | 1.24 ± 0.02a | 1.25 ± 0.11a | 1.25 ± 0.12a | 1.26 ± 0.75a |
| T-25 | 1.29 ± 0.2a | 1.29 ± 0.01 ab | 1.30 ± 0.03a | 1.31 ± 0.23a | 1.32 ± 0.56a | |
| T-50 | 1.34 ± 0.05a | 1.34 ± 0.1 ab | 1.35 ± 0.04a | 1.36 ± 1.01a | 1.37 ± 0.68a | |
| T-75 | 1.40 ± 0.07a | 1.40 ± 0.07b | 1.50 ± 0.06a | 1.50 ± 0.68a | 1.60 ± 0.22a | |
| T-100 | 1.44 ± 0.03a | 1.44 ± 0.02b | 1.50 ± 0.3a | 1.56 ± 0.45a | 1.60 ± 0.23a | |
| Fat (%) | C-100 | 13.19 ± 0.53c | 12.99 ± 0.54b | 12.47 ± 0.43c | 11.98 ± 0.22c | 11.02 ± 0.11c |
| T-25 | 12.94 ± 0.24c | 12.81 ± 0.75 ab | 11.84 ± 0.57c | 11.39 ± 0.17c | 10.31 ± 0.24c | |
| T-50 | 12.34 ± 0.42bc | 11.91 ± 0.55 ab | 11.60 ± 0.15bc | 11.29 ± 0.76bc | 10.25 ± 0.87bc | |
| T-75 | 11.58 ± 0.5 ab | 11.19 ± 0.98a | 10.80 ± 0.22 ab | 9.85 ± 0.71 ab | 8.79 ± 0.67 ab | |
| T-100 | 11.13 ± 0.16a | 11.09 ± 0.32a | 10.50 ± 0.24a | 9.53 ± 0.62a | 8.22 ± 0.45a | |
| Weight (g) | C-100 | 170.00 ± 1.34a | 168.00 ± 0.67a | 167.00 ± 0.81a | 164.00 ± 0.79 ab | 161.00 ± 0.56bc |
| T-25 | 171.00 ± 1.56a | 170.00 ± 1.11 ab | 168.00 ± 0.64 ab | 166.00 ± 0.69b | 163.00 ± 0.66d | |
| T-50 | 172.00 ± 0.57a | 171.00 ± 1.33 ab | 168.00 ± 0.55 ab | 164.00 ± 0.67 ab | 160.00 ± 0.34 ab | |
| T-75 | 173.00 ± 2.1a | 171.00 ± 1.45 ab | 167.00 ± 0.43a | 163.00 ± 1.1a | 159.00 ± 0.11a | |
| T-100 | 174.00 ± 1.86a | 172.00 ± 0.95b | 169.00 ± 0.21b | 166.00 ± 1.3b | 162.00 ± 0.38 cd | |
| Total plate count (CFU g−1) | C-100 | 10.0 ± 2a | 2.0 ± 0.1 × 102b | 7.1 ± 0.2 × 102c | 10.3 ± 0.3 × 103b | 1.72 ± 0.03 × 104b |
| T-25 | 8.0 ± 2a | 1.5 ± 0.1 × 102a | 6.9 ± 0.3 × 102bc | 9.4 ± 0.3 × 103a | 1.69 ± 0.04 × 104 ab | |
| T-50 | 8.0 ± 1a | 1.3 ± 0.1 × 102a | 6.6 ± 0.3 × 102abc | 8.9 ± 0.4 × 103a | 1.64 ± 0.04 × 104 ab | |
| T-75 | 7.0 ± 2a | 1.2 ± 0.2 × 102a | 6.4 ± 0.2 × 102 ab | 8.8 ± 0.3 × 103a | 1.63 ± 0.05 × 104 ab | |
| T-100 | 6.0 ± 1a | 1.2 ± 0.2 × 102a | 6.2 ± 0.2 × 102a | 8.6 ± 0.3 × 103a | 1.59 ± 0.02 × 104a | |
| Yeast and Moulds (CFU g−1) | C-100 | 4.0 ± 1a | 1.5 ± 0.3 × 101b | 3.0 ± 0.4 × 102b | 5.0 ± 0.3 × 102c | 7.5 ± 0.3 × 103c |
| T-25 | 4.0 ± 1a | 1.5 ± 0.3 × 101b | 3.0 ± 0.3 × 102b | 4.3 ± 0.3 × 102bc | 5.0 ± 0.4 × 103a | |
| T-50 | 3.0 ± 1a | 1.0 ± 0.3 × 101a | 2.5 ± 0.2 × 102 ab | 4.0 ± 0.4 × 102 ab | 6.5 ± 0.3 × 103b | |
| T-75 | 3.0 ± 1a | 0.5 ± 0.2 × 101a | 2.5 ± 0.2 × 102 ab | 3.2 ± 0.4 × 102a | 5.5 ± 0.2 × 103a | |
| T-100 | 3.0 ± 1a | 0.5 ± 0.1 × 101a | 2.0 ± 0.1 × 102a | 3.7 ± 0.3 × 102 ab | 6.5 ± 0.3 × 103b | |
| Coliform bacteria (CFU g−1) | C-100 | 3.0 ± 2a | 1.0 ± 0.2 × 101a | 4.0 ± 0.2 × 101b | 9.0 ± 0.3 × 101d | 14.0 ± 0.3 × 101d |
| T-25 | 3.0 ± 1a | 1.0 ± 0.4 × 101a | 3.0 ± 0.2 × 101a | 7.0 ± 0.4 × 101b | 12.0 ± 0.2 × 101b | |
| T-50 | 2.0 ± 1a | 0.7 ± 0.3 × 101a | 4.0 ± 0.2 × 101b | 8.0 ± 0.3 × 101c | 13.0 ± 0.2 × 101c | |
| T-75 | 2.0 ± 2a | 1.0 ± 0.3 × 101a | 4.0 ± 0.3 × 101b | 7.0 ± 0.4 × 101b | 11.0 ± 0.2 × 101a | |
| T-100 | 2.0 ± 2a | 0.9 ± 0.2 × 101a | 3.0 ± 0.3 × 101a | 6.0 ± 0.4 × 101a | 12.0 ± 0.2 × 101b | |
3.8 Sensory evaluation
The sensory evaluations revealed significant differences in appearance, color, flavor, body, texture, aftertaste, and overall acceptability among the cakes (Fig. 7). Sensory scores generally improved with increasing amounts of LMF in the cake composition. Notably, the crust color became darker with higher LMF content, reflecting a trend toward more intense coloration. The addition of LMF, known for its nutritional benefits, enriched the overall flavor profile of the cakes, imparting a better taste. The texture of the cake is influenced by its moist and tender mouthfeel. A higher storage modulus (G′) indicates a well-developed gluten network that provides a light and airy texture, enhancing the perception of softness. Conversely, a higher loss modulus (G′′) negatively affects the texture. These results align with rheological characteristics, showing a significantly (p < 0.05) lower textural acceptability for cakes incorporated with LMF. However, the flavor score improved with the addition of LMF, with the highest being observed for the sample incorporated with 75% LMF (T-75), influencing the overall acceptance of the cake incorporated with LMF for substituting WF. These findings aligned with the results of Vinay and Singh.38 who found the highest flavor score for eggless muffins incorporated with pearl millet flour compared to refined WF.4. Conclusion
This study demonstrated the potential utilization of aquafaba based emulsion, which is often discarded as waste. Substituting wheat four (WF) with little millet flour (LMF) showed a significant increase in calorific value. It was found that for all the cake formulations, there was an increase in both hardness and firmness values with LMF substitution. The differences in cake dimensions with the incorporation of LMF were clearly observed from image analysis, which showed that the C-100 sample had an even distribution of cells while the T-100 sample had a more uneven distribution of cells. The microbial shelf-life analysis revealed higher shelf life for the T-100 cake sample compared to C-100 with an average shelf life of 6 days. Sensory evaluation revealed higher acceptability for LMF cakes. Thus, it was concluded that the substitution of LMF for WF in aquafaba incorporated egg-less cake formulations was successful in improving the sensory properties, shelf life and calorific values without much significant changes in cake quality characteristics. Future research could explore the utilization of LMF and aquafaba in largescale production of eggless and gluten free formulations for other baked goods for broader application. Furthermore, a comparative analysis with other sugar alternatives, such as unprocessed sugars like raw honey, brown rice syrup, and jaggery powder, could further enhance the health and sustainability aspects of formulated cakes in the industrial sector.Abbreviations
| WF: | Wheat flour |
| LMF | Little millet flour |
| SNF | Solid non-fat |
| FTIR | Fourier transform infrared spectroscopy |
| mL | Milliliters |
| L | Liters |
| mg | Milligrams |
| G′ | Storage modulus |
| G′′ | Loss modulus |
| cP | Centipoise |
| Pa | Pascal |
| Hz | Hertz |
| N | Newton |
Data availability
The data supporting this article have been included in the main document.Author contributions
Bijjam Madhavi: investigation; methodology; data curation; formal analysis; visualization; validation; writing – original draft. Niveditha Asaithambi: investigation; methodology; data curation; formal analysis; visualization; validation; writing – original draft; Alok Kumar: investigation; methodology; data curation; formal analysis; Pralay Maiti: visualization; writing – reviewing and editing; Dinesh Chandra Rai: formal analysis; visualization; writing – reviewing and editing; Raj Kumar Duary: conceptualization; visualization; formal analysis; resources; supervision; writing – reviewing and editing.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We are grateful to Banaras Hindu University (Central University, India) for providing facilities to carry out the study. Also, extending sincere thanks to Institution of Eminence (IoE) Scheme, Banaras Hindu University, Varanasi (UP) India for support under Credit-Research Incentive to the Faculty Members (Dev. Scheme No. 6031(A); PFMS Scheme No. 3254) and Raja Jwala Prasad Post-Doctoral Fellowship Scheme (RJP-PDF; (Dev. Scheme No. 6031(A); PFMS Scheme No. 3254 to Dr Niveditha Asaithambi)). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.References
- R. Mustafa and M. J. T. Reaney, in Food Wastes and By-products, 2020, pp. 93–126, DOI:10.1002/9781119534167.ch4.
- F. B. Alsalman, M. Tulbek, M. Nickerson and H. S. Ramaswamy, Legume Sci., 2020, 2(2), 181–193, DOI:10.1002/leg3.30.
- Y. He, V. Meda, M. J. T. Reaney and R. Mustafa, Trends Food Sci. Technol., 2021, 111, 27–42, DOI:10.1016/j.tifs.2021.02.035.
- F. Boukid and M. Gagaoua, Foods, 2022, 11(2), 161, DOI:10.3390/foods11020161.
- T. F. Buhl, C. H. Christensen and M. Hammershøj, Food Hydrocolloids, 2019, 96, 354–364, DOI:10.1016/j.foodhyd.2019.05.041.
- S. E. Stantiall, K. J. Dale, F. S. Calizo and L. Serventi, Eur. Food Res. Technol., 2017, 244, 97–104, DOI:10.1007/s00217-017-2943-x.
- G. N. Yazici and M. S. Ozer, Trends Food Sci. Technol., 2021, 111, 346–359, DOI:10.1016/j.tifs.2021.02.071.
- S. A. Oyeyinka, A. T. Oyeyinka, D. O. Opaleke, O. R. Karim, F. L. Kolawole, G. O. Ogunlakin and O. H. Olayiwola, Ann.:Food Sci. Technol., 2014, 15(1), 20–28 Search PubMed.
- A. K. Anal, R. Singh, D. Rice, K. Pongtong, U. Hazarika, D. Trivedi and S. Karki, Sustainable Food Technol., 2024, 2, 908–925, 10.1039/d4fb00047a.
- E. V. de Aguiar, A. C. L. S. Centeno, F. Garcia dos Santos and V. D. Capriles, Appl. Food Res., 2024, 4, 100520, DOI:10.1016/j.afres.2024.100520.
- N. A. N. Gowda, K. Siliveru, P. V. V. Prasad, Y. Bhatt, B. P. Netravati and C. Gurikar, Foods, 2022, 11(4), 499, DOI:10.3390/foods11040499.
- P. Prasad and J. K. Sahu, Food Biosci., 2023, 56, 103105, DOI:10.1016/j.fbio.2023.103105.
- A. Ahamed V.P, A. Joshi, A. Mudey, S. Choudhari, J. Raut and S. Ahmed, Cureus, 2024 DOI:10.7759/cureus.59283.
- R. Mustafa, Y. He, Y. Y. Shim and M. J. T. Reaney, Int. J. Food Sci. Technol., 2018, 53, 2247–2255, DOI:10.1111/ijfs.13813.
- N. Ertaş and M. Aslan, Harran Tarım ve Gıda Bilimleri Dergisi, 2020, 24, 1–8, DOI:10.29050/harranziraat.569397.
- K. Crawford, W. Kerr and C. Tyl, Int. J. Food Sci. Technol., 2023, 59, 552–559, DOI:10.1111/ijfs.16492.
- G. N. Yazici, T. Taspinar, H. Binokay, C. Dagsuyu, A. Kokangul and M. S. Ozer, J. Food Meas. Charact., 2023, 17, 5759–5776, DOI:10.1007/s11694-023-02077-2.
- Y. He, Y. Y. Shim, R. Mustafa, V. Meda and M. J. T. Reaney, Foods, 2019, 8(12), 685, DOI:10.3390/foods8120685.
- A. O. A. C., Official Methods of Analysis of the Association of Official Analytical Chemists, Sec.985.29, The Association: Arlington, VA, 15th edn, 1990, vol. 2 Search PubMed.
- M. Gómez, A. Moraleja, B. Oliete, E. Ruiz and P. A. Caballero, LWT–Food Sci. Technol., 2010, 43, 33–38, DOI:10.1016/j.lwt.2009.06.026.
- G. Grossi Bovi Karatay, A. P. Rebellato, C. Joy Steel and M. Dupas Hubinger, Foods, 2022, 11(16), 2484, DOI:10.3390/foods11162484.
- S. Li, Y. Liu, J. Tong, L. Yu, M. Ding, Z. Zhang, A.-u. Rehman, M. Majzoobi, Z. Wang and X. Gao, Food Res. Int., 2020, 130, 108914, DOI:10.1016/j.foodres.2019.108914.
- K. Tsatsaragkou, L. Methven, A. Chatzifragkou and J. Rodriguez-Garcia, Foods, 2021, 10(5), 951, DOI:10.3390/foods10050951.
- Y. Salfinger and M. L. Tortorello, Compendium of Methods for the Microbiological Examination of Foods, 2015, DOI:10.2105/mbef.0222.
- N. Y. Njintang, C. M. F. Mbofung, F. Balaam, P. Kitissou and J. Scher, J. Sci. Food Agric., 2007, 88, 273–279, DOI:10.1002/jsfa.3085.
- A. Sharoba, Ann. Agric. Sci., 2021, 59, 445–454, DOI:10.21608/assjm.2021.195011.
- K. Pawar and S. Thorat, Bioinfolet, 2018, 15, 299–300 Search PubMed.
- C. Amandikwa, M. O. Iwe, A. Uzomah and A. I. Olawuni, Niger. Food J., 2015, 33, 12–17, DOI:10.1016/j.nifoj.2015.04.011.
- F. R. Adasi, R. M. K. Kubabom and M. McArthur-Floyd, NVEO, 2022, 888–897 CAS.
- M. Mudau, S. E. Ramashia, M. E. Mashau and H. Silungwe, Braz. J. Food Technol., 2021, 24, e2020123, DOI:10.1590/1981-6723.12320.
- L. Kayitesi, K. G. Vijayalakshmi, K. G. Nath and H. S. Surendra, Mysore J. Agric. Sci., 2012, 46(2), 265–273 Search PubMed.
- E. de la Hera, C. M. Rosell and M. Gomez, Food Chem., 2014, 151, 526–531, DOI:10.1016/j.foodchem.2013.11.115.
- B. Herranz, W. Canet, M. J. Jiménez, R. Fuentes and M. D. Alvarez, Int. J. Food Sci. Technol., 2016, 51, 1087–1098, DOI:10.1111/ijfs.13092.
- S.-C. Wu, Y.-S. Shyu, Y.-W. Tseng and W.-C. Sung, Processes, 2020, 8(3), 318, DOI:10.3390/pr8030318.
- M. Majzoobi, Z. V. Poor, J. Jamalian and A. Farahnaky, Int. J. Food Sci. Technol., 2016, 51, 1369–1377, DOI:10.1111/ijfs.13104.
- S. Bhaduri and A. K. Mukherjee, Int. J. Food Sci., Nutr. Diet., 2016, 325–329, DOI:10.19070/2326-3350-1600058.
- M. Christaki, P. Verboven, T. Van Dyck, B. Nicolaï, P. Goos and J. Claes, J. Cereal Sci., 2017, 77, 219–227, DOI:10.1016/j.jcs.2017.07.001.
- G. M. Vinay and A. K. Singh, J. Food Process. Preserv., 2024, 2024, 1–11, DOI:10.1155/2024/5519265.
- L. Yu, Y. Ma, Y. Zhao, Y. Pan, R. Tian, X. Yao, Y. Yao, X. Cao, L. Geng, Z. Wang, K. Wu and X. Gao, Front. Nutr., 2021, 8, 785847, DOI:10.3389/fnut.2021.785847.
- C. Chang and J. Lin, J. Food Process Eng., 2017, 40(3), e12480, DOI:10.1111/jfpe.12480.
- S. Lee, G. E. Inglett and C. J. Carriere, Cereal Chem., 2004, 81, 637–642, DOI:10.1094/cchem.2004.81.5.637.
- M. Martinez, A. K. Stone, A. G. Yovchev, R. Peter, A. Vandenberg and M. T. Nickerson, Eur. Food Res. Technol., 2016, 242, 1903–1911, DOI:10.1007/s00217-016-2690-4.
- J. Rajiv, C. Soumya, D. Indrani and G. Venkateswara Rao, J. Texture Stud., 2011, 42, 478–489, DOI:10.1111/j.1745-4603.2011.00309.x.
- E. Turabi, G. Sumnu and S. Sahin, Food Hydrocolloids, 2010, 24, 755–762, DOI:10.1016/j.foodhyd.2010.04.001.
- T. Sanz, A. Salvador, R. Baixauli and S. M. Fiszman, Eur. Food Res. Technol., 2009, 229, 197–204, DOI:10.1007/s00217-009-1040-1.
- S. Zahn, F. Pepke and H. Rohm, Int. J. Food Sci. Technol., 2010, 45, 2531–2537, DOI:10.1111/j.1365-2621.2010.02444.x.
- D. Kocer, Z. Hicsasmaz, A. Bayindirli and S. Katnas, J. Food Eng., 2007, 78, 953–964, DOI:10.1016/j.jfoodeng.2005.11.034.
- N. Y. Abu-Thabit, A. A. Judeh, A. S. Hakeem, A. Ul-Hamid, Y. Umar and A. Ahmad, Int. J. Biol. Macromol., 2020, 155, 730–739, DOI:10.1016/j.ijbiomac.2020.03.255.
- M. Carbonaro and A. Nucara, Amino Acids, 2009, 38, 679–690, DOI:10.1007/s00726-009-0274-3.
- N. F. Nagai, N. S. Argel and S. C. Andrés, Cereal Chem., 2022, 99, 850–859, DOI:10.1002/cche.10541.
- J. Xu, Z. Ma, N. Ren, X. Li, L. Liu and X. Hu, Food Chem., 2019, 289, 582–590, DOI:10.1016/j.foodchem.2019.03.093.
- S. N. Chaudhari, S. B. Palve, K. R. Choudhari, D. H. Pawar and S. S. Gaikwad, Int. J. Curr. Microbiol. Appl. Sci., 2017, 6, 3519–3525, DOI:10.20546/ijcmas.2017.612.409.
| This journal is © The Royal Society of Chemistry 2025 |


