Open Access Article
Open Access ArticlePasteurization of mandarin juice by ohmic heating and evaluation of its shelf-life under refrigerated and ambient conditions
Somnath
Basak
 ,
Piyush
Thakur
and
Snehasis
Chakraborty
,
Piyush
Thakur
and
Snehasis
Chakraborty
 *
*
Food Engineering and Technology Department, Institute of Chemical Technology, Matunga, Mumbai, 400019, India. E-mail: snehasisftbe@gmail.com; sc.chakraborty@ictmumbai.edu.in
First published on 11th November 2024
Abstract
Mandarin juice was treated inside a bath ohmic heater at 15 different combinations of voltages (120, 160, and 200 V) and treatment times (30, 60, 90, 120, and 150 s) targeting 85 °C. The come-up time to reach 85 °C for 120, 160, and 200 V were 240, 180, and 100 s, respectively. The system performance ranged between 88% and 98%. Ohmic heating rates increased with the applied voltage reaching 0.545 °C s−1 at 200 V. Ohmic heating at 200 V/30 s and 160 V/90 s produced a microbially safe mandarin juice (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction in natural microflora); whereas 200 V/30 s, 160 V/90 s, and 120 V/150 s treatments led to an enzymatically stable (>99% spoilage enzyme inactivation) mandarin juice. Under the pasteurized condition of 200 V/30 s, there was only a 10% and 8.4% loss in vitamin C and total phenolics in the juice. Elevated antioxidants were observed after ohmic heating. Lightness and total color change (ΔE*) of the juice increased with ohmic heating. The maximum browning of 66.74 was observed in the juice treated at 120 V/150 s. The juice treated at 200 V/30 s showed a shelf life of 42 and 15 days under refrigerated and ambient conditions, respectively.
log reduction in natural microflora); whereas 200 V/30 s, 160 V/90 s, and 120 V/150 s treatments led to an enzymatically stable (>99% spoilage enzyme inactivation) mandarin juice. Under the pasteurized condition of 200 V/30 s, there was only a 10% and 8.4% loss in vitamin C and total phenolics in the juice. Elevated antioxidants were observed after ohmic heating. Lightness and total color change (ΔE*) of the juice increased with ohmic heating. The maximum browning of 66.74 was observed in the juice treated at 120 V/150 s. The juice treated at 200 V/30 s showed a shelf life of 42 and 15 days under refrigerated and ambient conditions, respectively.
Sustainability spotlightTo achieve adequate microbial lethality and enzymatic stability in food products, the conventional pasteurization methods require long holding times at high temperatures. This detrimentally results in a loss of bioactive compounds and nutritional appeal of the product. Ohmic heating helps in attaining the desired temperature in a smaller duration, leading to lower process time. The advantages of uniform heating, high energy conversion efficiencies, no surface fouling, and less maintenance cost due to no moving parts advocate for ohmic heating as a sustainable technology. This also aligns well with the United Nation's Sustainable Development Goal 12 of “Responsible Consumption and Production”; wherein ohmic heating can sustainably help in the improvement of the shelf-life of fresh produce and prevent food wastage. |
1 Introduction
Citrus juices are an important source of bioactive compounds and micronutrients; and their consumption has been correlated with a reduced incidence of several chronic diseases.1 The citrus fruits can be classified into four major categories, namely mandarins, sweet oranges, grapefruit & pomelo group, and the common acid group members such as the acid lime and the sweet lime.2 The common mandarin fruit (Citrus reticulata Blanco) is rich in nutrients and bioactive compounds, possessing nutritional value.3 The popularity of mandarin fruit due to its “simple-to-peel” characteristic has propelled an increase in mandarin consumption in comparison to the “hard-to-peel” grapefruit and pomelo.4 Mandarin is reported to exhibit antioxidant, anti-diabetic, anti-inflammatory, and antihyperlipidemic activities.5Although the acidity of citrus juices acts as a barrier to the growth of foodborne pathogens, outbreaks of Salmonella, and Escherichia coli O157:H7 in fruit juices have been reported quite often.6 In such a scenario, the pasteurization of fruit juices is extremely necessary to prevent the emergence of fruit juice-associated outbreaks.7 Some of the pasteurization conditions employed for fruit juices include 65 °C|30 min, 77 °C|1 min, and 88 °C|15 s.8 The conventional thermal processing used for pasteurization has a very high come-up time (in the range of 15–30 min), drastically reducing the nutritional quality and sensory acceptability of juices.9 Therefore, novel thermal methods are being employed to preserve the nutritional quality and also achieve microbial and enzymatic lethality. Ohmic heating is being projected as a great alternative to conventional thermal pasteurization.10
Ohmic heating, or Joule effect heating, is a process in which heat is generated internally within a food material when an electric current is passed through it. The internal heat generation in the food material is due to the resistance offered by food materials to an electric current; i.e., food material acts as a resistor in the circuit. Ohmic heating does not depend on the heat transfer between solid–liquid interfaces or solid–solid interfaces; therefore, it is a form of internal energy generation technology, not thermal transfer technology.11 The advantages of ohmic heating include uniform heating, high energy conversion efficiencies, no surface fouling, target temperature being achieved very quickly, and less maintenance cost due to no moving parts,12 increasing demand in the food industry. Due to uniform or rapid heating, nutritional or structural properties of food materials are minimally affected by ohmic heating.13 Apart from the electric field, a concentric magnetic field is also created near the current carrying electrodes. A Lorentz force is experienced by the charged particles, wherein the particle attains a velocity which is perpendicular to the direction of the force and the magnetic field.14 A higher rate of temperature rise is expected as the charged particles in the juice will experience several forces from different directions at a particular point, resulting in greater agitation of the particles. Ohmic heating has several potential applications such as extraction,15 fermentation,16 pasteurization,17 drying,18 juice expression,19 and frying.20 Ohmic heating is best suited for the aseptic processing of viscous fluids or particulates.21 However, no study has been undertaken to recommend a pasteurization condition for mandarin juice. Therefore, this study aims to study the effect of various ohmic heating conditions on the various quality attributes of mandarin juice.
In this study, the ohmic heating treatment of mandarin juice was undertaken at different voltage and treatment durations. The physicochemical properties, along with the microbial and enzymatic quality of juice, were examined to understand the impact of process variables. Furthermore, a microbially safe and enzymatically stable sample after ohmic heating was selected. Finally, the properties of the juice were also studied during storage under refrigerated and ambient conditions to determine the shelf-life of the ohmic heating-pasteurized and equivalent thermally treated juice.
2 Materials and methods
2.1 Materials
Fresh, ripe and mature mandarins (Citrus reticulata) were purchased from the local market in Matunga, Mumbai, India. The fruits (∼10 cm diameter) were free from any physical damage and infestation. The fruits were washed with 200 ppm sodium hypochlorite solution, peeled, deseeded, and extracted using a juice extractor (JL02, Anjali Kitchenware, India). The average juice yield was around 55%, excluding the peel, seeds, and pulp. The juice was filtered using a press filter (Microfilt India, Mumbai, India) with a 100 μm mesh. All the other chemicals were of analytical grade and procured from HiMedia Pvt. Ltd, Mumbai, India. The pH, total soluble solids (TSS), and Brix to acid ratio of juice were 3.2 ± 0.2, 10.82 ± 0.37 °Bx, and 17.60 ± 1.3, respectively. The viscosity of strained juice was 496.64 ± 0.02 mPa s.2.2 Pasteurization in a batch ohmic heating unit
The heating section had a capacity of 6 L and consisted of a cuboidal shape cell (620 mm × 100 mm × 100 mm). Six stainless steel (SS-304) electrodes (Φ 2 mm) with a silver coating were placed 1.25 cm apart to facilitate temperature uniformity. A J type thermocouple (Pencil type, Thermonic India) was placed between the electrodes (Fig. 1). The thermocouple is attached to a temperature controller (SELEC TC523 PID, Mumbai, India). The voltage regulator (Variacco Electricals, Mumbai, India) is connected to regulate the voltage between electrodes. The variable power supply was obtained using a voltage regulator (0–250 V) from a domestic supply (220 V and 50 Hz). Electrodes were attached to a voltage regulator, and a thermocouple is connected to the temperature controller. The voltage was varied at three levels, i.e., 120, 160, and 200 V and the time was varied on five levels, i.e., 30, 60, 90, 120, and 150 s, making the cumulative number of treatments 3 × 5 = 15. The treatment temperature was maintained constant at 85 °C. The come-up time for 2 L of juice to reach 85 °C for 120, 160 and 200 V was 240, 180, and 100 s, respectively. The ohmic heating rate of the mandarin juice was calculated by plotting come-up time with respect to temperature.To understand the performance of the ohmic heating system, the system performance coefficient (SPC) was calculated as the ratio of the sensible heat absorbed by the juice (Heatabs) and the energy delivered to the juice system (Energydel), as given in eqn (1).9 The specific heat of the juice was assumed to be constant in the studied temperature range. The electrical energy given to a system and heat required to reach the final temperature (Tf) are calculated using the voltage (V), current (I), and initial temperature (Ti). The SPC during the ohmic heating was calculated from the mass of the juice (m) with a specific heat (Cp) that was processed in t seconds.
 | (1) |
For high-moisture foods, the Cp of the juice above the freezing point can be calculated using the Seibel empirical formula, wherein Xm refers to the moisture content of the juice.22
| Cp = 3.35Xm + 0.837 | (2) |
The current (I) during the treatment of mandarin juice at 120, 160, and 200 V was recorded between 35 and 60 °C to determine the electrical conductivity (σ).23
 | (3) |
The L/A represents the cell constant of a continuous ohmic heater where L is the length of the electrode through which current is generated in the sample and A is the cross-section area normal to the current flow. V represents the voltage and I represents the current. The cell constant of the ohmic heater was 1.59 cm−1 when filled with up to 2 L of juice. The methodology for calculating electrical conductivity for a multiple-electrode assembly has been adopted from Bhattacharjee and Chakraborty.9 The setup for determining electrical conductivity includes two multimeters functioning as voltage and current indicators. The multimeters were calibrated before each set of current and voltage readings to ensure accuracy. In this configuration, two adjacent electrodes were connected to one terminal of the voltage transformer, while the other two electrodes were connected to the opposite terminal. A known current was applied between the two outer electrodes, and the resulting voltage drop was measured across the inner electrode pair.
2.3 pH, total soluble solids and titratable acidity
The pH of the juice was estimated by using a bench-top pH meter (Labserv, Fisher Scientific). A handheld refractometer (Erma 0–32 ° Brix, 182 Japan) was used to determine the TSS. The titratable acidity (TA) was determined by titrating the juice against a standard NaOH solution. The TA was expressed in % (wt/v) citric acid (anhydrous).2.4 Color
CIE L* (lightness), a* (green to red) and b* (blue to yellow) values of the juice were recorded using a HunterLab colorimeter (LabScan XE, Hunter Associates Laboratory, USA) in reflection mode (D65/10°). The browning index (BI) and total color difference (ΔE*) were calculated using the following equations:24 | (4) |
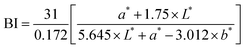 | (5) |
2.5 Ascorbic acid, total phenolics, and antioxidant activity
The ascorbic acid (AA), total phenolic content (TPC), and antioxidant capacity (AOX) of the juice were determined using the spectrophotometric methods described by Chakraborty et al.25 The ascorbic acid (AA) content was expressed as mg AA/100 mL of juice. TPC was estimated using Folin–Ciocalteu's phenol reagent method and expressed in mg gallic acid equivalent (GAE)/100 mL of juice. The AOX activity was reported in mg GAE/100 mL of the juice.2.6 Enzyme activity
The assays of peroxidase (POD), polyphenol oxidase (PPO), and pectin methyl esterase (PME) were conducted as per the protocol suggested by Chakraborty et al.26 and Sahoo & Chakraborty.27 The protein concentration in the crude extract was estimated using the Bradford method, which used bovine serum albumin as the reference protein. The rate of change in product formation per unit mass of the protein was considered to be enzyme activity. The residual activity was calculated, considering the untreated sample's activity to be 100%.2.7 Microbial enumeration
The indigenous microflora of the untreated and the enzymatically stable (complete inactivation, i.e., >99% inactivation) juices were enumerated using the serial dilution-pour plate method.28 While the aerobic mesophiles (AM) were incubated on the plate count agar (HIMEDIA® M091) for 24 h at 37 °C, the yeasts and molds (YM) were incubated on yeast and mold agar (HIMEDIA® M424) at 30 °C for 3–5 days. The limit of detection was 10 cfu mL−1.2.8 Shelf-life estimation
The best ohmic heating condition was selected as the minimum intensity required to achieve 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log cycle reduction in the natural microflora population along with >99% enzyme inactivation. The best treatment (200 V|30 s) was chosen for further shelf-life experiments. Soon after the ohmic heating treatment, the pasteurized juice was transferred to sterile coextruded ethylene vinyl alcohol (EVOH) copolymer pouches (thickness 90 microns) with a water vapor permeability of 0.047 g mm2 m−2 h−1 kPa−1. The AM and YM counts of the juice were tracked on every 7th day till 42 days of refrigerated (4 ± 1 °C) and ambient storage (27 ± 1 °C) at 85% R. H. The PME activity, color, and TPC were also monitored.
log cycle reduction in the natural microflora population along with >99% enzyme inactivation. The best treatment (200 V|30 s) was chosen for further shelf-life experiments. Soon after the ohmic heating treatment, the pasteurized juice was transferred to sterile coextruded ethylene vinyl alcohol (EVOH) copolymer pouches (thickness 90 microns) with a water vapor permeability of 0.047 g mm2 m−2 h−1 kPa−1. The AM and YM counts of the juice were tracked on every 7th day till 42 days of refrigerated (4 ± 1 °C) and ambient storage (27 ± 1 °C) at 85% R. H. The PME activity, color, and TPC were also monitored.
A control untreated sample and equivalent thermally treated sample (90 °C for 60 s of holding) were also checked for their shelf-lives for comparison. The thermal treatment condition was selected for its ability to achieve greater than 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 cfu mL−1 reduction in YMC and AMC, along with undetectable enzyme activity. While an open-tank treatment was performed for the batch ohmic heating, in-pouch thermal treatment was performed as the reference treatment. The in-pouch treatment was performed to prevent any possibility of post-processing contamination. The processing temperature was maintained at 90 °C in a thermostatic water bath (EIC23, Expo-Hi-Tech, Mumbai, India). 500 mL of the juice was packed in an EVOH pouch and placed in the center of the water bath to facilitate heating of the juice from 27 to 90 °C. A dummy pouch containing the juice and J-type pencil thermocouple (Thermonic India) was also placed in the water bath to estimate the come-up time. Throughout the heating process, it was ensured that the juice inside the pouch is completely dipped in the water. The come-up time for the thermal treatment (90 °C) was 12.1 ± 0.8 min. The thermally pasteurized and the ohmic heated samples have been referred to as ETT and OHT, respectively. The samples stored under refrigerated conditions have been referred to as ETTR and OHTR, while the pasteurized juices stored under ambient conditions have been referred to as ETTA and OHTA throughout the manuscript.
log10 cfu mL−1 reduction in YMC and AMC, along with undetectable enzyme activity. While an open-tank treatment was performed for the batch ohmic heating, in-pouch thermal treatment was performed as the reference treatment. The in-pouch treatment was performed to prevent any possibility of post-processing contamination. The processing temperature was maintained at 90 °C in a thermostatic water bath (EIC23, Expo-Hi-Tech, Mumbai, India). 500 mL of the juice was packed in an EVOH pouch and placed in the center of the water bath to facilitate heating of the juice from 27 to 90 °C. A dummy pouch containing the juice and J-type pencil thermocouple (Thermonic India) was also placed in the water bath to estimate the come-up time. Throughout the heating process, it was ensured that the juice inside the pouch is completely dipped in the water. The come-up time for the thermal treatment (90 °C) was 12.1 ± 0.8 min. The thermally pasteurized and the ohmic heated samples have been referred to as ETT and OHT, respectively. The samples stored under refrigerated conditions have been referred to as ETTR and OHTR, while the pasteurized juices stored under ambient conditions have been referred to as ETTA and OHTA throughout the manuscript.
2.9 Statistical analysis
All the tests and analyses were conducted in triplicate. One-way analysis of variance (ANOVA) at a significance level of 0.05 was performed using Tukey's HSD test using SPSS software (IBM SPSS Statistics, version 16.0, USA).3 Results and discussion
3.1 Heating rate, electrical conductivity of mandarin juice and SPC of ohmic heating
The potential difference across the electrodes significantly affects the heating rate of mandarin juice during ohmic treatment. Heating rates of the juice at three different voltages iare shown in Fig. 2. The ohmic heating rates were 0.234 °C s−1 0.333 °C s−1, 0.545 °C s−1, at 120, 160, and 200 V respectively (Fig. 2(A)). The time required to reach 60 °C at 200 V was 1.6 and 2.2 times lower than that for 160 and 120 V. The heating rate is affected by the electrical conductivity of juice and the electric field strength during the ohmic heating treatment. Ohm's law states that the current flowing through the two points between a conductor is directly proportional to the potential difference across those points. Thus, when higher voltage gradients were applied, the samples showed a linear trend of temperature rise from 30 to 60 °C. At higher voltages, the augmentation of the movement of water molecules and ions is greater, leading to higher heating rates. This significantly reduces the process time. However, bubble formation was observed above 95 °C, which reduces the heating rate significantly. Darvishi et al.29 reported a similar bubble formation in pomegranate juice during ohmic heating. The formation of a bubble is due to electrochemical reactions, and fruit juices are generally acidic, leading to hydrogen gas formation by electrochemical reactions.30The range of electrical conductivity of mandarin juice was between 1.2 and 2 S m−1. The electrical conductivity of mandarin juice showed a linear relationship with the temperature at three different voltages (Fig. 2(B)). The increasing movement of ions and the decreasing viscosity of the juice at higher temperatures and voltages result in greater electrical conductivities.31,32 Jha et al.33 and Yildiz et al.34 reported that electrical conductivity increases with temperature due to a reduction in drag force for the movement of ions. The electrical conductivity of juice also depends on the solid content of juice; at low solid content, the drag force for the movement of ions is less, which provides high electrical conductivity.30 The current intensity and electrical conductivity are expected to increase with temperature, probably due to a decrease in the viscosity of the juice and frictional drag force between the ions.35 Karakavuk et al.36 observed that the electrical conductivity of the grape juice ranged between 0.19 and 0.85 S m−1, when exposed to a voltage gradient of 13 to 17 V cm−1. Norouzi et al.35 also observed electrical conductivity in the range of 0.70 to 2.52 S m−1 when sour cherry juice was pasteurized by ohmic heating at voltage levels of 30 to 50 V.
The SPC of the system was 98% when treatment was conducted at 120 V, and as the voltage increased, the SPC value started to decrease. At 200 and 160 V, efficiency of a system falls to 88% and 90%, respectively. These values of SPC indicate that at a higher voltage gradient, the energy provided to the system is not thoroughly utilized for heating the juice. Heat loss (2%) was significantly less at 120 V, indicating efficient conversion of electrical energy to heat energy. This is in line with the findings of Icier et al.,23,37 wherein SPC increases from 47 to 92%, with a decrease in voltage gradient. The low energy losses at low voltage gradient can be attributed to the small heat transfer area of the cell and thereby lower convection losses. Higher voltages induce chemical, physical, and electrochemical changes in the juice, which result in greater energy losses.38
3.2 pH, total soluble solids and titratable acidity
Initially, the pH of untreated mandarin juice was 3.31, which largely remained unaffected after the ohmic heating treatment. Similarly, the total soluble solids of the untreated sample were 10.82 ± 0.37 °Brix, which remained constant after the ohmic heating treatments. In other words, ohmic heating did not induce any major hydrolysis of complex polysaccharides into monosaccharides and disaccharides. The titratable acidity of the juice decreased from 0.58% to 0.41 and 0.48% at a treatment voltage of 200 V and 160 V for 150 s, respectively (Table 1). There is some literature reporting a similar trend. For instance, Darvishi et al.29 observed a slight decrease in acidity after ohmic heating due to the hydrolysis of juice and corrosion of electrodes. The reason behind this is the conversion or degradation of organic acids such as citric and ascorbic acid. Besides, ascorbic acid can participate in non-enzymatic browning reactions, which gets aggravated at higher voltage and long treatment durations.39| Voltage (V) | Time (s) | pH | TSS (°Bx) | Titratable acidity (% (wt/v) citric acid) |
|---|---|---|---|---|
| a Different superscript letters within one column indicate significant (p ≤ 0.05) differences among means determined by Tukey's test. | ||||
| Untreated | 3.32 ± 0.02a | 10.82 ± 0.37a | 0.58 ± 0.04a | |
| 120 | 30 | 3.34 ± 0.01a | 10.76 ± 0.28a | 0.56 ± 0.03a |
| 60 | 3.33 ± 0.01a | 10.47 ± 0.38a | 0.56 ± 0.04a | |
| 90 | 3.33 ± 0.01a | 10.81 ± 0.49a | 0.58 ± 0.04a | |
| 120 | 3.33 ± 0.01a | 10.78 ± 0.39a | 0.58 ± 0.05a | |
| 150 | 3.34 ± 0.01a | 10.72 ± 0.29a | 0.52 ± 0.05a | |
| 160 | 30 | 3.36 ± 0.01a | 10.69 ± 0.34a | 0.56 ± 0.04a |
| 60 | 3.37 ± 0.01a | 10.72 ± 0.44a | 0.55 ± 0.05a | |
| 90 | 3.37 ± 0.01a | 10.66 ± 0.35a | 0.52 ± 0.04ab | |
| 120 | 3.37 ± 0.01a | 10.45 ± 0.43a | 0.51 ± 0.04ab | |
| 150 | 3.37 ± 0.01a | 10.56 ± 0.51a | 0.48 ± 0.03b | |
| 200 | 30 | 3.35 ± 0.01a | 10.45 ± 0.54a | 0.57 ± 0.04a |
| 60 | 3.35 ± 0.01a | 10.44 ± 0.22a | 0.56 ± 0.05ab | |
| 90 | 3.35 ± 0.01a | 10.63 ± 0.11a | 0.53 ± 0.04ab | |
| 120 | 3.35 ± 0.01a | 10.52 ± 0.23a | 0.49 ± 0.05bc | |
| 150 | 3.34 ± 0.01a | 10.45 ± 0.44a | 0.41 ± 0.04c | |
3.3 Ascorbic acid, total phenolics and antioxidant activity
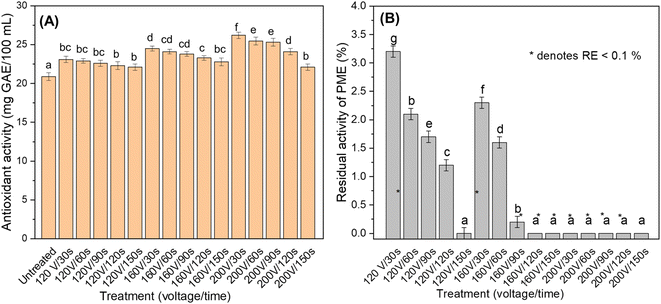
The ascorbic acid content of fresh mandarin juice was 48 mg/100 mL. The AA degradation was in the range of 10 to 53.8% for the tested voltage–time combinations. As the voltage increases, the come-up time decreases, leading to a shorter cumulative treatment time. The minimum ascorbic acid degradation was observed for a treatment duration of 30 s at 200 V. A reduced come-up time correlated well with greater ascorbic acid retention. The highest loss of 53.8% was observed at 120 V for a treatment duration of 150 s. This can be attributed to the highest come-up time at a lower voltage. The ascorbic acid (AA) retention of guava, sapota, and papaya was tested by Athmaselvi et al.40 at different voltage gradients (10 and 20 V cm−1), temperatures (70, 80, 90 and 100 °C) and electrode combinations (stainless steel and titanium electrodes). A higher voltage resulted in greater ascorbic acid degradation due to electrochemical reactions and electrode corrosion. This result is contrary to our results. This can be attributed to the lower come-up time at higher voltages and the type of electrode (silver coated SS 304) which reduces the corrosion and electrochemical reactions during treatment. However ascorbic acid degradation is more sensitive to the time of treatment compared to an electric field. Assiry et al.38 reported that ascorbic acid degradation of orange juice within a temperature range of 65–90 °C was unaffected by the electric field strength. Interestingly, Doan et al.41 observed an increase in AA content in pomelo juice by 5.9% for a treatment at 30 V cm−1. The ohmic heating treatment breaks down the bonds that are associated with AA and the pomelo juice matrix, thereby leading to an increase in AA content.A similar trend was also observed for the phenolics and antioxidant capacity (Fig. 3(B) and 4(A)). The total phenolic content of untreated juice was 53.3 mg GAE/100 mL. The least and greatest reduction in phenolics was 8.4 and 27.1%, under treatment conditions of 200 V for 30 s and 120 V for 150 s, respectively (Fig. 3). According to Brochier et al.,42 the TPC degradation of sugarcane juice was 11–18% between 50 and 80 °C for 6–12 min at a fixed voltage. Brochier et al.42 reported that the degradation of phenolics in sugarcane juice was 14% at 3.9 V cm−1 and 10% at 20.5 V cm−1. The antioxidant capacity (AOC) of untreated mandarin juice was 21.2 mg GAE/100 mL. Interestingly, the antioxidant activity of the treated juices was significantly greater than that of the untreated juice. It increased with the treatment voltage. The maximum antioxidant activity (26.3 mg GAE/100 mL) was observed at 200 V/30 s (85 °C) at the same point where the least AA and TPC degradation was observed. While a lower come-up time at higher voltages is expected to better preserve the AOC, the greater electro-permeabilization at higher voltages can facilitate greater extraction of antioxidants while maintaining the integrity of the product.43
3.4 Color
The visual and sensory acceptability of juices is highly dependent on the color of the juice. The L* value of untreated mandarin juice was 16.13 which increased with an increase in treatment voltage, implying an increase in the brightness of the juice. Similarly, the a* and b* values significantly increased after the ohmic heating treatment. Notably, the b* values for ohmic-treated samples were in the range of 3.89 to 4.88, which is substantially greater than the b* value of the untreated mandarin juice (2.69). This is indicative of the increase in the yellowness of juice with voltage. The lower degradation of carotenoids at higher voltage due to a lower come-up time can be attributed to this trend.17 It is worth noting that L* significantly increased after the ohmic heating treatment compared to the untreated sample. This increase was so significant that it was a major reason for a drastic increase in ΔE*. For instance, the maximum ΔE* was significantly higher for 200 V-treated juice; however, the BI values of the juice treated at 120 and 160 V were similar. The increased L* or lightness of the juice can probably be due to the better extraction of carotenoids and phenolics at higher voltages. The higher b* values also support the trend, indicating a greater yellowness in the juice. Therefore, the higher ΔE* should not be mistaken for greater browning of the juice.The browning index of ohmic-treated mandarin juice was in the range of 62.65 to 66.74 (Table 2). The maximum browning was observed in the juice treated at 120 V for 150 s, which can be attributed to the highest come-up time as compared to the other voltage treatments. Non-enzymatic browning results in the loss of nutrients, formation of brown pigments, off-flavour and loss of colour. Makroo et al.44 observed a similar phenomenon for ohmic heating of watermelon juice. Although darkening of the juice was observed after the treatment of the juice, the L* decreased to a greater extent for thermal processing than ohmic heating. The authors attributed this trend to the greater degree of PPO inactivation in ohmic heating. Ascorbic acid degradation is considered one of the main reasons for browning in citrus juices. Leizerson and Shimoni17 reported the same trend in ohmic-treated orange juice. The change in color (ΔE*) of juice increases as the treatment time increases. The maximum change was seen in the sample treated at 150 s and minimum in a sample treated at 30 s at any given voltage. The sample treated at 200 V shows less color change compared to the sample treated at 120 V (Table 2). For instance, the total color change of guava juice during ohmic heating decreases with an increase in voltage gradient.45 The reason is due to less exposure time at a higher voltage. The color change may occur due to the heat-induced browning of juice in the presence of metal ions, oxygen, and carbonyl groups that act as a precursor in enzymatic browning.17 Saberian et al.46 did not report any change in browning in aloe vera gel after the ohmic heating. Sabancı and Icier47 ascribed the color change and browning in sour cherry juice to the degradation of monomeric anthocyanins after the ohmic heating.
| Voltage (V) | Time (s) | L* | a* | b* | ΔE* | BI |
|---|---|---|---|---|---|---|
| a Different superscript letters within one column indicate significant (p ≤ 0.05) differences among means determined by Tukey's test. The ΔE* and BI refer to the total color difference and browning indices, respectively. | ||||||
| Untreated | 16.13 ± 0.12a | 0.93 ± 0.11a | 2.69 ± 0.12a | — | 62.65 ± 0.12a | |
| 120 | 30 | 17.98 ± 0.11d | 1.87 ± 0.21cd | 3.89 ± 0.05b | 2.40 ± 0.11b | 65.55 ± 0.10b |
| 60 | 17.89 ± 0.06d | 1.99 ± 0.13d | 3.78 ± 0.12b | 2.33 ± 0.12b | 65.52 ± 0.13b | |
| 90 | 17.81 ± 0.10d | 2.02 ± 0.06d | 3.99 ± 0.08b | 2.39 ± 0.09b | 66.07 ± 0.10c | |
| 120 | 17.53 ± 0.06c | 1.85 ± 0.13cd | 3.94 ± 0.09b | 2.10 ± 0.08a | 65.92 ± 0.09c | |
| 150 | 16.98 ± 0.12b | 2.11 ± 0.09d | 3.99 ± 0.10b | 1.95 ± 0.10a | 66.74 ± 0.09e | |
| 160 | 30 | 18.87 ± 0.11f | 1.64 ± 0.11c | 4.12 ± 0.12b | 3.17 ± 0.11d | 65.25 ± 0.10b |
| 60 | 18.33 ± 0.13e | 1.89 ± 0.12cd | 4.01 ± 0.11b | 2.74 ± 0.12c | 65.63 ± 0.10b | |
| 90 | 18.42 ± 0.12e | 1.78 ± 0.10cd | 4.21 ± 0.09b | 2.88 ± 0.10c | 65.86 ± 0.08b | |
| 120 | 18.21 ± 0.15de | 1.75 ± 0.09cd | 4.16 ± 0.10b | 2.68 ± 0.14c | 65.85 ± 0.13bc | |
| 150 | 17.98 ± 0.09d | 1.83 ± 0.11cd | 4.23 ± 0.13b | 2.58 ± 0.11c | 66.24 ± 0.12d | |
| 200 | 30 | 21.33 ± 0.11h | 1.24 ± 0.21b | 4.76 ± 0.21c | 5.61 ± 0.17f | 64.78 ± 0.16b |
| 60 | 20.76 ± 0.09g | 1.68 ± 0.18c | 4.98 ± 0.12c | 5.22 ± 0.13ef | 65.95 ± 0.14c | |
| 90 | 20.88 ± 0.10g | 1.59 ± 0.12c | 4.99 ± 0.13c | 5.32 ± 0.12f | 65.81 ± 0.13c | |
| 120 | 20.55 ± 0.12g | 1.62 ± 0.09c | 4.86 ± 0.09c | 4.98 ± 0.10e | 65.78 ± 0.11c | |
| 150 | 20.42 ± 0.22g | 1.51 ± 0.12c | 4.88 ± 0.11c | 4.85 ± 0.16e | 65.76 ± 0.15c | |
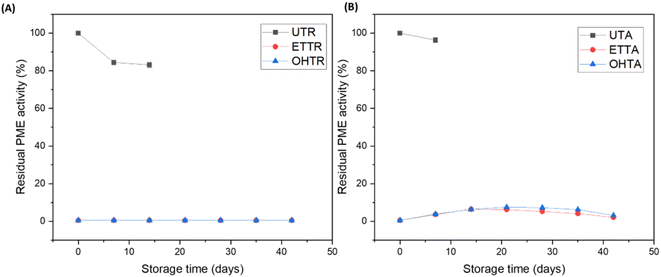
3.5 Enzymatic activity
PPO and POD result in enzymatic browning of juices, leading to a loss of nutritional content, sensory appeal and economic value of the product. Furthermore, enzymes like PME result in a loss of turbidity and viscosity, leading to phase separation in juices.48 The PPO and POD enzymes were more susceptible than PME in mandarin juice and were completely inactivated after ohmic heating treatments of 120 V for 60 s, 160 V for 30 s and 200 V for 30 s, respectively. PPO and POD were inactivated by 92.4 and 95.7%, respectively, after ohmic heating at 120 V for 30 s. PME was also completely inactivated after ohmic heating at 160 V for 90 s and 120 V for 150 s (Fig. 4(B)). For a specific voltage, the enzyme activity decreased with increasing duration of the treatment. Brochier et al.42 also established that PPO is more labile than POD in sugarcane juice during ohmic heating. Demirdöven & Baysal49 reported 96% reduction in PME activity in orange juice after a treatment of 42 V cm−1 at 69 °C. Similarly, Saxena et al.50 noticed a 97.8% reduction in PPO activity after a treatment of 32 V cm−1 at 90 °C for 5 min. The mechanism of enzyme inactivation by ohmic heating is a combination of thermal and nonthermal mechanisms. Thermal denaturation of enzymes occurs when heat disrupts the enzyme's secondary and tertiary structures by breaking hydrogen bonds, ionic bonds, and hydrophobic interactions. Apart from the heat-induced denaturation of enzymes, the presence of an electric field can modify the molecular spacing and promote inter-chain reactions in enzymes.44 The non-thermal effect of ohmic heating is due to the translation and rotation of the enzyme molecules in response to the applied external electric field. This can be majorly attributed to the charge and dipole moment in the enzyme molecule. A greater electric field gradient results in greater collision between the molecules, that can have a detrimental effect on enzyme activity.42 The electrostatic forces generated by the electrodes during ohmic heating result in conformational alterations in the tertiary structure.51 The induction of molecular movement due to the dissipation of energy by friction resulting from the oscillating electric field reduces the enzyme activity.43 The susceptibility of the enzymes to denaturation by ohmic heating is increased due to the metallic prosthetic groups interacting with electric fields generated during the treatment.523.6 Microbial quality
It has been reported that spoilage enzymes exhibit greater resistance to thermal treatment compared to microbes in fruit juices.48 Our goal is to identify a treatment condition that achieves both microbial safety (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction) and enzymatic stability (99% inactivation of the target enzyme). Therefore, only samples showing 99% enzyme inactivation after treatment were further analyzed for microbial enumeration. It is evident that the remaining treatment conditions, while potentially meeting microbial safety, do not satisfy both criteria of microbial safety and enzymatic stability. The enzymatically stable samples of mandarin juice (i.e., 200 V for 30 s, 160 V for 90 s, and 120 V for 150 s) were taken for microbial testing. The initial AM and YM counts in the untreated juice sample were 6.9 and 6.2
log reduction) and enzymatic stability (99% inactivation of the target enzyme). Therefore, only samples showing 99% enzyme inactivation after treatment were further analyzed for microbial enumeration. It is evident that the remaining treatment conditions, while potentially meeting microbial safety, do not satisfy both criteria of microbial safety and enzymatic stability. The enzymatically stable samples of mandarin juice (i.e., 200 V for 30 s, 160 V for 90 s, and 120 V for 150 s) were taken for microbial testing. The initial AM and YM counts in the untreated juice sample were 6.9 and 6.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 cfu mL−1, respectively. No aerobic mesophiles were detected in the juice samples treated at 200 V for 30 s and 160 V for 90 s. A 5.5
log10 cfu mL−1, respectively. No aerobic mesophiles were detected in the juice samples treated at 200 V for 30 s and 160 V for 90 s. A 5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction in AM population was observed in the 120 V|150 s treated juice. The YM count was undetected in all the enzymatically stable samples. The reduction of AM and YM population was greater than 5
log reduction in AM population was observed in the 120 V|150 s treated juice. The YM count was undetected in all the enzymatically stable samples. The reduction of AM and YM population was greater than 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 cycle, indicating adequate pasteurization. The application of ohmic heating treatment reduced all microbiological counts, with values which are below the quantification levels. The microbial inactivation induced by ohmic heating is a combination of thermal and nonthermal effects. While the thermal effects of ohmic heating result in protein denaturation, nonthermal effects such as electroporation result in cell lysis and death. Yoon et al.53 revealed that the exuded intracellular material was greater in ohmic heating than in the conventional heating treatment. This suggests that nonthermal effects of ohmic heating play a significant role in microbial inactivation. The external electric field induces a transmembrane potential in the phospholipidic membrane.54 The increase in permeability thereof would result in irreversible leakage of proteins, nucleic acids and coenzymes.
log10 cycle, indicating adequate pasteurization. The application of ohmic heating treatment reduced all microbiological counts, with values which are below the quantification levels. The microbial inactivation induced by ohmic heating is a combination of thermal and nonthermal effects. While the thermal effects of ohmic heating result in protein denaturation, nonthermal effects such as electroporation result in cell lysis and death. Yoon et al.53 revealed that the exuded intracellular material was greater in ohmic heating than in the conventional heating treatment. This suggests that nonthermal effects of ohmic heating play a significant role in microbial inactivation. The external electric field induces a transmembrane potential in the phospholipidic membrane.54 The increase in permeability thereof would result in irreversible leakage of proteins, nucleic acids and coenzymes.
3.7 Shelf-life analysis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log cycle reduction in the natural microflora population along with >99% enzyme inactivation. Furthermore, the greatest retention in ascorbic acid (90.2%) and phenolics (91.6%) was also observed under the treatment condition of 200 V|30 s.
log cycle reduction in the natural microflora population along with >99% enzyme inactivation. Furthermore, the greatest retention in ascorbic acid (90.2%) and phenolics (91.6%) was also observed under the treatment condition of 200 V|30 s.
The AM and YM are the main microbes responsible for the spoilage of fruit juices under refrigerated conditions.55 The AM and YM counts of untreated juice stored at 4 °C crossed the value of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 cfu mL−1 on the 7th day of storage. The AM and YM counts of the untreated juice sample stored at 27 °C exhibited the same trend wherein the counts increased to 7.6
log10 cfu mL−1 on the 7th day of storage. The AM and YM counts of the untreated juice sample stored at 27 °C exhibited the same trend wherein the counts increased to 7.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 cfu mL−1 and 7.4
log10 cfu mL−1 and 7.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 cfu mL−1; on the 7th day of ambient storage. The ETTR and OHTR exhibited undetectable AM and YM counts throughout the storage period of 42 days. The microbial counts in the sample stored at 27 °C started increasing rapidly from the 14th day of the storage period. On the 27th day of ambient storage, the respective AM and YM counts were 6.7 and 6.3
log10 cfu mL−1; on the 7th day of ambient storage. The ETTR and OHTR exhibited undetectable AM and YM counts throughout the storage period of 42 days. The microbial counts in the sample stored at 27 °C started increasing rapidly from the 14th day of the storage period. On the 27th day of ambient storage, the respective AM and YM counts were 6.7 and 6.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 cfu mL−1 for the ETT sample, while the respective AM and YM counts in the juice pasteurized by ohmic heating were 6.1
log10 cfu mL−1 for the ETT sample, while the respective AM and YM counts in the juice pasteurized by ohmic heating were 6.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 cfu mL−1 and 6.4
log10 cfu mL−1 and 6.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 cfu mL−1 on the 27th day (Fig. 5). The sample stored at 4 °C exhibited no growth in microbial counts compared to the sample stored at 27 °C, in which microbial count rapidly increased from the 27th day of storage. As per the microbiological standards for fruit products set by the Food Safety and Standards Authority of India (FSSAI), the permissible AM and YM counts in pasteurized juices are 4 and 3
log10 cfu mL−1 on the 27th day (Fig. 5). The sample stored at 4 °C exhibited no growth in microbial counts compared to the sample stored at 27 °C, in which microbial count rapidly increased from the 27th day of storage. As per the microbiological standards for fruit products set by the Food Safety and Standards Authority of India (FSSAI), the permissible AM and YM counts in pasteurized juices are 4 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 cfu mL−1, respectively.56 While the standard set for unpasteurized juices mandates a maximum permissible limit of 7
log10 cfu mL−1, respectively.56 While the standard set for unpasteurized juices mandates a maximum permissible limit of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 cfu mL−1 of natural microflora. For the ETTR and OHTR samples, the microbial counts were well below the prescribed limit for 42 days. For the juices stored at 27 °C. the AM and YM counts crossed the value of 4 and 3
log10 cfu mL−1 of natural microflora. For the ETTR and OHTR samples, the microbial counts were well below the prescribed limit for 42 days. For the juices stored at 27 °C. the AM and YM counts crossed the value of 4 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 cfu mL−1 after 15 days. Therefore, it can be summarized that ohmic heating treatment increased the shelf life of juice stored under refrigerated and ambient conditions to 42 and 15 days, respectively. Alcántara-Zavala et al.57 reported that ohmic heating at 65 °C for 5 min preserved pulque, a probiotic beverage until 22 days with greater sensory acceptability. The sudden spurt in growth under ambient conditions indicates a sublethal effect on the microorganisms, which regenerate after cell damage. Saxena et al.50 observed that the untreated sugarcane juice converted to a viscous gel on the 6th day of refrigerated and ambient storage. Similar to our study, the authors also did not observe any significant increase in AM and YM at the end of 10 days of refrigerated storage. The juice at room temperature had a rapid growth of YM, thereby limiting the shelf-life to less than 10 days of storage. Debbarma et al.58 also achieved a shelf-life of 10 days of refrigerated storage of carrot juice treated by ohmic heating treatment at an electric field intensity of 17 V cm−1 for a processing time of 40 s.
log10 cfu mL−1 after 15 days. Therefore, it can be summarized that ohmic heating treatment increased the shelf life of juice stored under refrigerated and ambient conditions to 42 and 15 days, respectively. Alcántara-Zavala et al.57 reported that ohmic heating at 65 °C for 5 min preserved pulque, a probiotic beverage until 22 days with greater sensory acceptability. The sudden spurt in growth under ambient conditions indicates a sublethal effect on the microorganisms, which regenerate after cell damage. Saxena et al.50 observed that the untreated sugarcane juice converted to a viscous gel on the 6th day of refrigerated and ambient storage. Similar to our study, the authors also did not observe any significant increase in AM and YM at the end of 10 days of refrigerated storage. The juice at room temperature had a rapid growth of YM, thereby limiting the shelf-life to less than 10 days of storage. Debbarma et al.58 also achieved a shelf-life of 10 days of refrigerated storage of carrot juice treated by ohmic heating treatment at an electric field intensity of 17 V cm−1 for a processing time of 40 s.
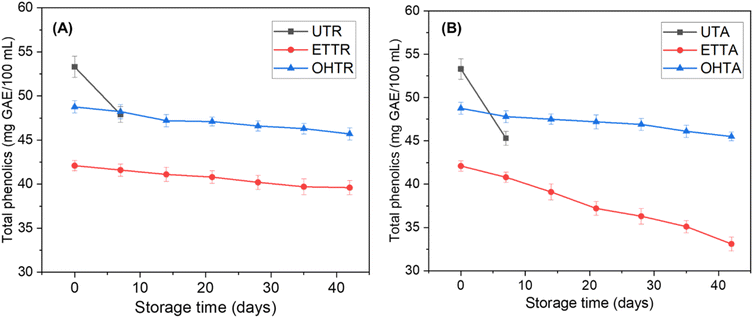
Enzyme activity is a crucial factor for determination of cloud stability and sensory acceptability of juice. The residual PME activity in untreated juice stored at 4 and 27 °C exhibited a decrease in activity. The ETTR and OHTR maintained low enzyme activities (<0.5%) throughout the refrigerated storage period of 42 days (Fig. 6). However, under ambient conditions, the ETTA and OHTA exhibited an increase in enzyme activities up to 10%. This indicates that the ohmic heating treatment successfully reduced the enzyme activity soon after the treatment and achieved a low enzyme activity throughout the storage period. Saxena et al.50 reported a similar trend in PPO activity in ohmic heated sugarcane juice during refrigerated storage. This was also true in the case of PPO and POD in carrot juice after ohmic heating followed by storage.43 A slight increase in PME activity after 2-week storage of a pasteurized orange-carrot juice blend was reported by Rivas et al.59 In this study, the slight increase in PME activity might be due to the activation of the reversible latent form at ambient temperature.
| L* | a* | b* | ΔE* | BI | ||
|---|---|---|---|---|---|---|
| a Different superscript letters within one column indicate significant (p ≤ 0.05) differences among means determined by Tukey's test. The ΔE* and BI refer to the total color difference and browning indices, respectively. UTR: untreated juice stored under refrigerated conditions; OHTR: ohmic heating-pasteurized juice stored under refrigerated conditions; ETTR: thermally pasteurized juice stored under refrigerated conditions. | ||||||
| UTR | 0 | 16.13 ± 0.12b | 0.93 ± 0.05a | 2.69 ± 0.10b | 62.65 ± 0.09a | |
| 7 | 14.13 ± 0.11a | 1.05 ± 0.11b | 2.28 ± 0.12a | 2.05 ± 0.11c | 62.83 ± 0.09a | |
| OHTR | 0 | 21.33 ± 0.12i | 1.24 ± 0.09b | 4.76 ± 0.22f | 5.60 ± 0.15h | 64.78 ± 0.13b |
| 7 | 20.81 ± 0.11h | 1.26 ± 0.08b | 4.21 ± 0.14e | 4.93 ± 0.16g | 64.03 ± 0.11b | |
| 14 | 19.12 ± 0.13g | 1.31 ± 0.11bc | 4.19 ± 0.18de | 3.37 ± 0.18f | 64.86 ± 0.15b | |
| 21 | 19.01 ± 0.21g | 1.43 ± 0.14c | 4.07 ± 0.17de | 3.23 ± 0.21f | 64.82 ± 0.18b | |
| 28 | 18.71 ± 0.19f | 1.56 ± 0.11c | 3.91 ± 0.21de | 2.92 ± 0.15e | 64.80 ± 0.16b | |
| 35 | 18.22 ± 0.17e | 1.61 ± 0.13cd | 3.86 ± 0.13de | 2.49 ± 0.15d | 65.03 ± 0.09b | |
| 42 | 17.75 ± 0.15d | 1.67 ± 0.11cd | 3.67 ± 0.15de | 2.03 ± 0.12c | 64.96 ± 0.11b | |
| ETTR | 0 | 19.25 ± 0.13g | 1.28 ± 0.09b | 4.13 ± 0.11de | 3.45 ± 0.10f | 64.63 ± 0.09b |
| 7 | 18.91 ± 0.22f | 1.35 ± 0.21bc | 4.07 ± 0.09de | 3.13 ± 0.16f | 64.77 ± 0.13b | |
| 14 | 18.65 ± 0.23f | 1.47 ± 0.12c | 4.01 ± 0.13de | 2.89 ± 0.16e | 64.93 ± 0.14b | |
| 21 | 17.73 ± 0.19d | 1.67 ± 0.23cd | 3.91 ± 0.16de | 2.14 ± 0.15c | 65.49 ± 0.13b | |
| 28 | 17.11 ± 0.15c | 1.72 ± 0.11d | 3.73 ± 0.15d | 1.63 ± 0.12b | 65.54 ± 0.11b | |
| 35 | 16.65 ± 0.32b | 1.85 ± 0.22d | 3.61 ± 0.22cd | 1.40 ± 0.23a | 65.73 ± 0.16b | |
| 42 | 16.19 ± 0.29b | 1.87 ± 0.17d | 3.49 ± 0.18c | 1.24 + 0.08a | 65.78 ± 0.12b | |
| L* | a* | b* | ΔE* | BI | ||
|---|---|---|---|---|---|---|
| a Different superscript letters within one column indicate significant (p ≤ 0.05) differences among means determined by Tukey's test. The ΔE* and BI refer to the total color difference and browning indices, respectively. UTA: untreated juice stored under ambient conditions; OHTR: ohmic heating-pasteurized juice stored under ambient conditions; ETTR: thermally pasteurized juice stored under ambient conditions. | ||||||
| UTA | 0 | 16.13 ± 0.11c | 0.93 ± 0.08a | 2.69 ± 0.03b | 62.65 ± 0.10a | |
| 7 | 13.12 ± 0.15a | 1.07 ± 0.09b | 2.01 ± 0.15a | 3.09 ± 0.13d | 62.70 ± 0.12a | |
| OHTA | 0 | 21.33 ± 0.23h | 1.24 ± 0.03c | 4.76 ± 0.07e | 5.61 ± 0.12g | 64.78 ± 0.20c |
| 7 | 20.65 ± 0.22g | 1.29 ± 0.08c | 4.17 ± 0.13d | 4.77 ± 0.16f | 64.06 ± 0.19b | |
| 14 | 19.42 ± 0.23f | 1.35 ± 0.03c | 3.94 ± 0.22d | 3.54 ± 0.23e | 64.26 ± 0.18b | |
| 21 | 18.46 ± 0.24e | 1.46 ± 0.02d | 3.81 ± 0.05d | 2.64 ± 0.18c | 64.61 ± 0.18b | |
| 28 | 17.31 ± 0.26d | 1.57 ± 0.10d | 3.73 ± 0.19d | 1.70 ± 0.17b | 65.22 ± 0.17c | |
| 35 | 15.12 ± 0.11b | 1.69 ± 0.05de | 3.42 ± 0.12c | 1.46 ± 0.11ab | 66.11 ± 0.11d | |
| 42 | 14.89 ± 0.13b | 1.77 ± 0.04e | 3.31 ± 0.19c | 1.62 ± 0.16b | 66.12 ± 0.10d | |
| ETTA | 0 | 19.25 ± 0.21f | 1.28 ± 0.09c | 4.13 ± 0.17d | 3.45 ± 0.19e | 64.63 ± 0.16c |
| 7 | 18.85 ± 0.20e | 1.37 ± 0.12cd | 4.01 ± 0.13d | 3.06 ± 0.08d | 64.71 ± 0.15c | |
| 14 | 18.32 ± 0.20e | 1.51 ± 0.13d | 3.84 ± 0.04d | 2.54 ± 0.17c | 64.81 ± 0.14c | |
| 21 | 17.65 ± 0.19d | 1.69 ± 0.09de | 3.62 ± 0.22c | 1.94 ± 0.16b | 64.94 ± 0.19c | |
| 28 | 17.01 ± 0.18d | 1.75 ± 0.04e | 3.41 ± 0.13c | 1.40 ± 0.16ab | 64.92 ± 0.13c | |
| 35 | 16.52 ± 0.13c | 1.87 ± 0.03f | 3.28 ± 0.15c | 1.18 ± 0.12a | 65.08 ± 0.13c | |
| 42 | 15.65 ± 0.21bc | 1.91 ± 0.09f | 3.19 ± 0.18c | 1.20 ± 0.20a | 65.48 ± 0.19c | |
Furthermore, a lower enzyme activity at lower temperatures can also be one of the possible reasons for lower degradation at 4 °C than at 27 °C. To summarize, the ohmic heating-treated sample had 12.5% greater phenolics than thermally treated juice at the end of their respective shelf-lives at 4 °C.
4 Conclusion
The ohmic heating rates increased with applied voltage reaching 0.545 °C s−1 at 200 V. The system performance decreased with applied voltage; however, 88% efficiency was obtained in every case. The ohmic heating at 200 V/30 s produced a microbially safe (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction in natural microflora) and enzymatically stable (>99% inactivation of PPO, POD, and PME) mandarin juice. Under the pasteurized condition of 200 V/30 s, 90% vitamin C and 91% phenolics were retained in the juice. The juice treated at 200 V/30 s showed a shelf life of 42 and 15 days under refrigerated and ambient conditions, respectively. Further study should explore a continuous ohmic heating system for the juice and its industrial scale-up.
log reduction in natural microflora) and enzymatically stable (>99% inactivation of PPO, POD, and PME) mandarin juice. Under the pasteurized condition of 200 V/30 s, 90% vitamin C and 91% phenolics were retained in the juice. The juice treated at 200 V/30 s showed a shelf life of 42 and 15 days under refrigerated and ambient conditions, respectively. Further study should explore a continuous ohmic heating system for the juice and its industrial scale-up.
Data availability
Data will be made available on request.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the All India Council for Technical Education (AICTE), Government of India.References
- G. C. Rampersaud and M. F. Valim, 100% citrus juice: Nutritional contribution, dietary benefits, and association with anthropometric measures, Crit. Rev. Food Sci. Nutr., 2017, 57, 129–140, DOI:10.1080/10408398.2013.862611.
- J. Singh, V. Sharma, K. Pandey, S. Ahmed, M. Kaur and G. S. Sidhu, Horticultural classification of citrus cultivars, in Citrus Research, Development and Biotechnology, ed. M. S. Khan and I. A. Khan, IntechOpen, 2021, DOI:10.5772/intechopen.96243.
- M. Shorbagi, N. M. Fayek, P. Shao and M. A. Farag, Citrus reticulata Blanco (the common mandarin) fruit: An updated review of its bioactive, extraction types, food quality, therapeutic merits, and bio-waste valorization practices to maximize its economic value, Food Biosci., 2022, 47, 101699, DOI:10.1016/j.fbio.2022.101699.
- Z. Tietel, A. Plotto, E. Fallik, E. Lewinsohn and R. Porat, Taste and aroma of fresh and stored mandarins, J. Sci. Food Agric., 2011, 91, 14–23, DOI:10.1002/jsfa.4146.
- S. Wang, C. Yang, H. Tu, J. Zhou, X. Liu, Y. Cheng and J. Xu, et al., Characterization and metabolic diversity of flavonoids in citrus species, Sci. Rep., 2017, 7, 10549, DOI:10.1038/s41598-017-10970-2.
- M. Krug, T. Chapin, M. Danyluk, R. Goodrich-Schneider, K. Schneider, L. Harris and R. Worobo, Outbreaks of Foodborne Disease Associated with Fruit and Vegetable Juices, Edis, 2020, 2020(5), 1–7 Search PubMed.
- A. O. Oladunjoye and E. U. Awani-Aguma, Effect of thermosonication on physicochemical, bioactive, sensory and microbiological qualities of African mango fruit (Irvingia gabonensis) juice, Meas.: Food, 2023, 11, 100103, DOI:10.1016/j.meafoo.2023.100103.
- H. S. Ramaswamy and C. Abbatemarco, Thermal processing of fruits, Process. Fruits: Sci. Technol., 1996, 1, 25–65 Search PubMed.
- A. Bhattacharjee and S. Chakraborty, Design of a batch Ohmic heater and evaluating the influence of different treatment conditions on quality attributes of kinnow (Citrus nobilis× Citrus deliciosa) juice, Innovative Food Sci. Emerging Technol., 2022, 82, 103186, DOI:10.1016/j.ifset.2022.103186.
- R. Silva, R. S. Rocha, G. L. P. Ramos, D. Xavier-Santos, T. C. Pimentel, J. M. Lorenzo and A. G. Cruz, et al., What are the challenges for ohmic heating in the food industry? Insights of a bibliometric analysis, Food Res. Int., 2022, 157, 111272, DOI:10.1016/j.foodres.2022.111272.
- M. C. Knirsch, C. A. Dos Santos, A. A. M. de Oliveira Soares and T. C. V. Penna, Ohmic heating–a review, Trends Food Sci. Technol., 2010, 21, 436–441, DOI:10.1016/j.tifs.2010.06.003.
- A. I. Muhammad, A. Shitu and M. A. Tadda, Ohmic Heating as Alternative Preservation Technique-A Review, Arid Zone Journal of Engineering, Technology and Environment, 2019, 15, 268–277 Search PubMed.
- J. R. Sarkis, G. D. Mercali, I. C. Tessaro and L. D. F. Marczak, Evaluation of key parameters during construction and operation of an Ohmic heating apparatus, Innovative Food Sci. Emerging Technol., 2013, 18, 145–154, DOI:10.1016/j.ifset.2013.02.001.
- J. Walker, D. Halliday and R. Resnick, in Halliday & Resnick Fundamentals of Physics, Wiley, 10th edn, 2022, pp. 803–856 Search PubMed.
- M. Sánchez, P. Ferreira-Santos, J. S. Gomes-Dias, C. Botelho, A. Laca and C. M. Rocha, Ohmic heating-based extraction of biocompounds from cocoa bean shell, Food Biosci., 2023, 54, 102886, DOI:10.1016/j.fbio.2023.102886.
- H. Y. Cho, A. E. Yousef and S. K. Sastry, Growth kinetics of Lactobacillus acidophilus under Ohmic heating, Biotechnol. Bioeng., 1996, 49, 334–340, DOI:10.1002/(sici)1097-0290(19960205)49:3%3C334::aid-bit12%3E3.0.co;2-e.
- S. Leizerson and E. Shimoni, Effect of ultrahigh-temperature continuous Ohmic heating treatment on fresh orange juice, J. Agric. Food Chem., 2005, 53, 3519–3524, DOI:10.1021/jf0481204.
- M. Lima and S. K. Sastry, The effects of Ohmic heating frequency on hot-air drying rate and juice yield, J. Food Eng., 1999, 41, 115–119 CrossRef.
- W. C. Wang and S. K. Sastry, Effects of moderate electrothermal treatments on juice yield from cellular tissue, Innovative Food Sci. Emerging Technol., 2002,(3), 371–377, DOI:10.1016/S1466-8564(02)00054-1.
- S. Salengke and S. K. Sastry, Effects of Ohmic pretreatment on oil uptake of potato slices during frying and subsequent cooling, J. Food Process Eng., 2007, 30, 1–12, DOI:10.1111/j.1745-4530.2007.00096.x.
- S. Ghnimi, N. Flach-Malaspina, M. Dresch, G. Delaplace and J. F. Maingonnat, Design and performance evaluation of an Ohmic heating unit for thermal processing of highly viscous liquids, Chem. Eng. Res. Des., 2008, 86, 626–632, DOI:10.1016/j.cherd.2008.02.005.
- H. S. Ramaswamy and M. Marcotte, Food Processing: Principles and Applications. CRC Press, 2005 Search PubMed.
- F. Icier and C. Ilicali, Temperature dependent electrical conductivities of fruit purees during Ohmic heating, Food Res. Int., 2005, 38, 1135–1142, DOI:10.1016/j.foodres.2005.04.003.
- S. Basak, T. Jha and S. Chakraborty, Pasteurization of tender coconut water by pulsed light treatment: Microbial safety, enzymatic inactivation, and impact on physicochemical properties, Innovative Food Sci. Emerging Technol., 2023, 84, 103302, DOI:10.1016/j.ifset.2023.103302.
- S. Chakraborty, S. Mahale, R. Dhar and S. Basak, Development of a mixed fruit beverage and pulsed light treatment thereof to obtain a microbially safe and enzymatically stable product, Food Biosci., 2022, 45, 101508, DOI:10.1016/j.fbio.2021.101508.
- S. Chakraborty, P. S. Rao and H. N. Mishra, Kinetic modeling of polyphenoloxidase and peroxidase inactivation in pineapple (Ananas comosus L.) puree during high-pressure and thermal treatments, Innovative Food Sci. Emerging Technol., 2015, 27, 57–68, DOI:10.1016/j.ifset.2014.11.003.
- P. Sahoo and S. Chakraborty, Influence of pulsed light, ultrasound, and series treatments on quality attributes, pectin methyl esterase, and native flora inactivation in sweet orange juice (Citrus sinensis L. Osbeck), Food Bioprocess Technol., 2023, 16, 2095–2112, DOI:10.1007/s11947-023-03042-z.
- S. Basak, S. Mahale and S. Chakraborty, Changes in quality attributes of pulsed light and thermally treated mixed fruit beverages during refrigerated storage (4 °C) condition, Innovative Food Sci. Emerging Technol., 2022, 78, 103025, DOI:10.1016/j.ifset.2022.103025.
- H. Darvishi, M. H. Khostaghaza and G. Najafi, Ohmic heating of pomegranate juice: Electrical conductivity and pH change, J. Saudi Soc. Agric. Sci., 2013, 12, 101–108, DOI:10.1016/j.jssas.2012.08.003.
- S. Palaniappan and S. K. Sastry, Electrical conductivity of selected juices: influences of temperature, solids content, applied voltage, and particle size, J. Food Process Eng., 1991, 14, 247–260, DOI:10.1111/j.1745-4530.1991.tb00135.x.
- A. Kumar, M. Kumar, M. R. Mahboob and B. Srivastava, Influence of° Brix/Acid, and flow rate of pineapple juice and electric field strength on the performance of continuous ohmic heating system, J. Food Sci. Technol., 2024, 61, 1–13, DOI:10.1007/s13197-024-05961-x.
- M. Cevik, Electrical conductivity and performance evaluation of verjuice concentration process using ohmic heating method, J. Food Process Eng., 2021, 44(5), e13672, DOI:10.1111/jfpe.13672.
- S. N. Jha, K. Narsaiah, A. L. Basediya, R. Sharma, P. Jaiswal, R. Kumar and R. Bhardwaj, Measurement techniques and application of electrical properties for nondestructive quality evaluation of foods—a review, J. Food Sci. Technol., 2011, 48, 387–411, DOI:10.1007/s13197-011-0263-x.
- H. Yildiz, H. Bozkurt and F. Icier, Ohmic and conventional heating of pomegranate juice: effects on rheology, color, and total phenolics, Food Sci. Technol. Int., 2009, 15, 503–512, DOI:10.1177/1082013209350352.
- S. Norouzi, A. Fadavi and H. Darvishi, The Ohmic and conventional heating methods in concentration of sour cherry juice: Quality and engineering factors, J. Food Eng., 2021, 291, 110242, DOI:10.1016/j.jfoodeng.2020.110242.
- E. Karakavuk, A. Goksu and S. Sabanci, Investigation of electrical conductivity and bioactive quality during ohmic evaporation process of Apple juice, J. Food Process. Preserv., 2022, 46, e17036, DOI:10.1111/jfpp.17036.
- F. Icier and C. Ilicali, Electrical conductivity of apple and sourcherry juice concentrates during Ohmic heating, J. Food Process Eng., 2004, 27, 159–180, DOI:10.1111/j.1745-4530.2004.tb00628.x.
- A. Assiry, S. K. Sastry and C. Samaranayake, Degradation kinetics of ascorbic acid during Ohmic heating with stainless steel electrodes, J. Appl. Electrochem., 2003, 33, 187–196, DOI:10.1023/A:1024076721332.
- S. Basak, L. Shaik and S. Chakraborty, Effect of ultraviolet and pulsed light treatments on ascorbic acid content in fruit juices-A review of the degradation mechanism, Innovative Food Sci. Emerging Technol., 2023, 2, 100333, DOI:10.1016/j.focha.2023.100333.
- K. A. Athmaselvi, C. Kumar and P. Poojitha, Influence of temperature, voltage gradient and electrode on ascorbic acid degradation kinetics during Ohmic heating of tropical fruit pulp, J. Food Meas. Char., 2017, 11, 144–155, DOI:10.1007/s11694-016-9381-5.
- N. K. Doan, Q. D. Lai, T. K. P. Le and N. T. Le, Influences of AC frequency and electric field strength on changes in bioactive compounds in ohmic heating of pomelo juice, Innovative Food Sci. Emerging Technol., 2021, 72, 102754, DOI:10.1016/j.ifset.2021.102754.
- B. Brochier, G. D. Mercali and L. D. F. Marczak, Influence of moderate electric field on inactivation kinetics of peroxidase and polyphenol oxidase and on phenolic compounds of sugarcane juice treated by Ohmic heating, LWT, 2016, 74, 396–403, DOI:10.1016/j.lwt.2016.08.001.
- L. M. N. Rodríguez, R. Arias, T. Soteras, A. Sancho, N. Pesquero, L. Rossetti and N. Szerman, et al., Comparison of the quality attributes of carrot juice pasteurized by Ohmic heating and conventional heat treatment, LWT, 2021, 145, 111255, DOI:10.1016/j.lwt.2021.111255.
- H. A. Makroo, J. Saxena, N. K. Rastogi and B. Srivastava, Ohmic heating assisted polyphenol oxidase inactivation of watermelon juice: Effects of the treatment on pH, lycopene, total phenolic content, and color of the juice, J. Food Process. Preserv., 2017, 41, e13271, DOI:10.1111/jfpp.13271.
- I. Chakraborty and K. A. Athmaselvi, Changes in physicochemical properties of guava juice during Ohmic heating, J. Ready Eat Food, 2014, 1, 152–157 Search PubMed.
- H. Saberian, Z. Hamidi Esfahani and S. Abbasi, Effect of conventional and Ohmic pasteurization on some bioactive components of aloe Vera gel juice, Iran. J. Chem. Chem. Eng., 2015, 34, 99–108, DOI:10.30492/ijcce.2015.14756.
- S. Sabancı and F. Icier, Enhancement of the performance of sour cherry juice concentration process in vacuum evaporator by assisting Ohmic heating source, Food Bioprod. Process., 2020, 122, 269–279, DOI:10.1016/j.fbp.2020.05.004.
- S. Basak and S. Chakraborty, The potential of nonthermal techniques to achieve enzyme inactivation in fruit products, Trends Food Sci. Technol., 2022, 123, 114–129, DOI:10.1016/j.tifs.2022.03.008.
- A. Demirdöven and T. Baysal, Optimization of Ohmic heating applications for pectin methylesterase inactivation in orange juice, J. Food Sci. Technol., 2014, 51, 1817–1826, DOI:10.1007/s13197-012-0700-5.
- J. Saxena, H. Ahmad Makroo and B. Srivastava, Effect of Ohmic heating on Polyphenol Oxidase (PPO) inactivation and color change in sugarcane juice, J. Food Process Eng., 2017, 40, e12485, DOI:10.1111/jfpe.12485.
- C. P. Samaranayake and S. K. Sastry, In-situ activity of α-amylase in the presence of controlled-frequency moderate electric fields, LWT, 2018, 90, 448–454, DOI:10.1016/j.lwt.2017.12.053.
- F. Schottroff, D. Biebl, M. Gruber, N. Burghardt, J. Schelling, M. Gratz and H. Jaeger, et al., Inactivation of vegetative microorganisms by Ohmic heating in the kilohertz range–Evaluation of experimental setups and non-thermal effects, Innovative Food Sci. Emerging Technol., 2020, 63, 102372, DOI:10.1016/j.ifset.2020.102372.
- S. W. Yoon, C. Y. J. Lee, K. M. Kim and C. H. Lee, Leakage of cellular materials from Saccharomyces cerevisiae by Ohmic heating, J. Microbiol. Biotechnol., 2002, 12, 183–188 Search PubMed.
- W. A. Müller, L. D. F. Marczak and J. R. Sarkis, Microbial inactivation by Ohmic heating: Literature review and influence of different process variables, Trends Food Sci. Technol., 2020, 99, 650–659, DOI:10.1016/j.tifs.2020.03.021.
- S. Yildiz, P. R. Pokhrel, S. Unluturk and G. V. Barbosa-Cánovas, Shelf life extension of strawberry juice by equivalent ultrasound, high pressure, and pulsed electric fields processes, Food Res. Int., 2021, 140, 110040, DOI:10.1016/j.foodres.2020.110040.
- Food Safety and Standards Authority of India, Revised Microbiological Standards for Fruits and Vegetables and their products, 2018, https://fssai.gov.in/upload/uploadfiles/files/Gazette_Notification_Fruits_Vegetables_04_04_2018.pdf, accessed on 9th June 2024.
- A. E. Alcántara-Zavala, J. de Dios Figueroa-Cárdenas, E. Morales-Sánchez, J. A. Aldrete-Tapia, S. M. Arvizu-Medrano and H. E. Martínez-Flores, Application of Ohmic heating to extend shelf life and retain the physicochemical, microbiological, and sensory properties of pulque, Food Bioprod. Process., 2019, 118, 139–148, DOI:10.1016/j.fbp.2019.09.007.
- T. Debbarma, S. Thangalakshmi, M. Tadakod, R. Singh and A. Singh, Comparative analysis of Ohmic and conventional heat-treated carrot juice, J. Food Process. Preserv., 2021, 45, e15687, DOI:10.1111/jfpp.15687.
- A. Rivas, D. Rodrigo, A. Martínez, G. V. Barbosa-Cánovas and M. Rodrigo, Effect of PEF and heat pasteurization on the physical–chemical characteristics of blended orange and carrot juice, LWT, 2006, 39, 1163–1170, DOI:10.1016/j.lwt.2005.07.002.
- S. Bhat, C. S. Saini and H. K. Sharma, Changes in total phenolic content and color of bottle gourd (Lagenaria siceraria) juice upon conventional and ohmic blanching, Food Sci. Biotechnol., 2017, 26, 29–36, DOI:10.1007/s10068-017-0004-7.
- S. Salari and S. M. Jafari, The influence of ohmic heating on degradation of food bioactive ingredients, Food Eng. Rev., 2020, 12, 191–208, DOI:10.1007/s12393-020-09217-0.
- L. Shaik and S. Chakraborty, Effect of different storage conditions on the quality attributes of sweet lime juice subjected to pulsed light and thermal pasteurization, Sustainable Food Technol., 2023, 1, 722–737, 10.1039/D3FB00023K.
| This journal is © The Royal Society of Chemistry 2025 |