Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Efficient freeze-drying of foamed strawberry puree: a study on drying kinetics and physicochemical properties†
Zhihao
Zhou
 ,
Carlos
Parra-Escudero
,
Hengjun
Du
,
Xiaojing
Guo
,
Qi
Wang
,
Hang
Xiao
* and
Jiakai
Lu
*
,
Carlos
Parra-Escudero
,
Hengjun
Du
,
Xiaojing
Guo
,
Qi
Wang
,
Hang
Xiao
* and
Jiakai
Lu
*
Department of Food Science, University of Massachusetts, Amherst, MA 01003, USA. E-mail: jiakailu@umass.edu
First published on 6th November 2024
Abstract
The production of high-quality strawberry powder is of significant interest in the food industry. This study examines the impact of shelf temperature (10, 20, and 40 °C) on the foaming properties and freeze-drying behavior of strawberry puree with added egg white (EW), with a particular emphasis on drying kinetics and the physicochemical properties of the dried powder, especially the retention of bioactive compounds. Evaluating various concentrations of EW addition revealed that incorporating 0.75% EW results in an optimal foam with maximum overrun (97.22%). Freeze-drying this optimized foamed strawberry puree significantly reduces the total drying time compared to its non-foamed counterpart, with reductions of 43.90%, 44.55%, and 36.00% at the respective shelf temperatures. The drying kinetics of the strawberry foam is effectively described by the modified Page model. Notably, foam mat dried strawberry powder produced at 40 °C exhibits the lowest moisture content (2.69 ± 0.09%) and water activity (0.189 ± 0.003, aw). The foaming treatment does not significantly impact the retention of total phenolic content, but higher shelf temperatures result in decreased retention. Furthermore, foaming pretreatment does not significantly influence the retention of total anthocyanin content in strawberry powders at 10 and 20 °C, but it enhanced the retention at the highest shelf temperature (40 °C). Our findings suggest that foam mat drying could be a more efficient and cost-effective method for producing high-quality strawberry powder.
Sustainability spotlightStrawberry foam-mat freeze drying is a cutting-edge technique that highlights sustainability in food preservation. From an environmental perspective, foam-mat freeze drying is particularly advantageous. The initial foaming treatment allows for a more rapid and uniform drying process, reducing energy consumption compared to traditional drying methods. Additionally, the lightweight dried product requires less packaging and has a smaller carbon footprint during transportation. This innovative technique not only extends the shelf life of strawberries and reduces food waste, but also ensures that consumers have access to healthy, nutritious snacks year-round. By utilizing foam-mat freeze drying, we have developed an approach towards more sustainable and efficient food preservation practices for strawberries. |
1. Introduction
Fruit powders, due to their wide range of applications in enhancing flavor, appearance, and functionality in various foods and beverages, have garnered significant commercial interest globally.1 Among these, strawberry (Fragaria × ananassa) powder is particularly popular due to the fruit's health benefits, attractive appearance, and desirable flavor. While fresh consumption of strawberries is ideal for obtaining all their sensory, nutritional and functional properties, their seasonal production and short shelf life, associated with high water content, limit their availability. Dehydration is a traditional method used to extend the shelf life of foods, allowing strawberries to be processed into dried fruit and consumed as snacks or food flavorings and colorants in various products.Strawberries are rich in anthocyanins, flavonoids, phenolic acids and ellagitannins, which contribute to their antioxidant, anti-inflammatory, immunomodulatory, antimicrobial, and hypoglycemic properties.2,3 Anthocyanins, in particular, are responsible for the color and health-promoting properties of strawberries but are highly sensitive to heat. Previous studies have shown that certain drying methods, such as spray drying, hot air drying, and microwave drying, can adversely affect the antioxidant, aromatic, and color properties of fruit powders.4–7 Freeze drying is considered superior to spray and hot air conductive drying as it can better preserve the flavor, nutrition, and function of strawberries.8 However, freeze drying is not economically friendly due to its low efficiency. Foam mat drying, a procedure that transforms a liquid food into a stable foam followed by drying, has shown promising results in improving the drying efficiency of liquid foods with low glass transition temperature.9–11 This method is especially suitable for heat-sensitive, high-sugar content, and viscous foods, such as strawberries.
Although hybrid freeze-drying techniques, such as foam-mat freeze drying, can highly retain the flavors, nutrition, and functions of dehydrated fruits compared with other drying methods because they use lower temperature and less drying time, their combined effects with shelf temperature are not well known.12 The temperature of loading shelves is a primary process parameter and critical system function that has a direct impact on the freeze-drying rate and the quality of final products. For instance, a study involving orange puree revealed that the optimum freeze-drying conditions for preserving nutrients were low pressures (5 Pa) and high shelf temperature (50 °C).13 A recent study indicated heating shelves to 40 °C could considerably shorten the freeze-drying time of kiwiberry pulp, and enhance the microbiological and functional stability during storage.14 Interestingly, another study showed that a specific shelf temperature setting of freeze-drying (50 °C–6 h, 40 °C–9 h, 30 °C–6 h) resulted in the highest retention of anthocyanin in freeze-dried strawberries compared to other drying shelf temperature profiles.15 Therefore, it is crucial to optimize the setting of the shelf temperature during foam-mat freeze drying to avoid exceeding critical thresholds that might deteriorate the quality of dehydrated strawberries.
This study aims to explore the use of foam-mat freeze-drying in the production of dehydrated strawberry powder. It examines the impact of shelf temperature on process efficiency and powder quality in foam-mat freeze-drying, while also comparing these outcomes to a conventional freeze-drying method (non-foamed) and evaluating the overall quality of strawberries. We established the drying kinetics and evaluated the drying efficiency at various shelf temperatures (10, 20, and 40 °C) for strawberry puree foam. Additionally, we measured and compared the quality of these dehydrated strawberry powders in terms of water content and activity, total phenolic content, anthocyanins, and color.
2. Materials and methods
2.1. Materials
Fresh strawberries were purchased from Driscoll's (California, U.S.). Egg white powder was purchased from JUDEE's (Plain City, OH, U.S.). Gallic acid, sodium carbonate (Na2CO3), and Folin–Ciocalteau reagent were purchased from Sigma-Aldrich Chemical Co. Methanol, acetonitrile, and formic acid of chromatographic grade were purchased from Fisher Scientific Co. Anthocyanin standards, including pelargonidin-3-glucoside, cyanidin-3-glucoside, delphinidin-3-galactoside chloride, pelargonidin-3-rutinoside chloride, and delphinidin-3,5-diglucoside chloride, were also purchased from Sigma-Aldrich Chemical Co.2.2. Sample preparation and foam production
Fresh strawberries were washed, and the green hull was manually removed. The fruit was blended using a kitchen blender until a puree formed. The strawberry puree was packed into ziplock bags and stored at −80 °C until use. An appropriate amount of strawberry puree was thawed at 4 °C and passed through a stainless-steel sieve (mesh size: 3 mm). Then, different concentrations of egg white (EW, 0–1.25%, wt/wt) as a foaming agent were added to strawberry puree. The foam production was achieved by whipping at a speed of 200 rpm for 270 seconds at room temperature.2.3. Determination of foam properties
The density of strawberry puree, both before and after foaming, was calculated using the ratio of mass to volume. Specifically, 100 mL of non-foamed or foamed strawberry puree was filled into a 100 mL graduated cylinder, and the weight was recorded. The foam density was calculated as follows:| Foam density (g cm−3) = m/v | (1) |
The foam expansion, which represents the amount of air incorporated into the strawberry puree by mechanical whipping, was calculated using the following equation:16
 | (2) |
The stability of the strawberry puree foam with different EW additions was measured using the method described in a previous study.16 Specifically, 100 mL of foamed strawberry puree of known weight was filled into a 100 mL graduated cylinder. The liquid that had drained from the foam was pipetted out and weighed after 30 min at room temperature. The stability of the foam was calculated according to the following equation:
 | (3) |
2.4. Experimental set-up
A pilot-scale freeze dryer (SP VirTis Genesis Pilot Lyophilizer) was used to conduct the freeze-drying process. The samples were dried at a chamber pressure of 200 mTorr, an ice condenser temperature of −80 °C and a set shelf temperature. Initially, the chamber pressure dropped to 200 ± 20 mTorr after 10 min for each run of freeze drying. Subsequently, the shelf temperature started to rise from −20 °C to 10, 20, and 40 °C at approximately 35, 45, and 65 min, respectively, and remained constant during the rest of the freeze-drying process. Strawberry puree and strawberry puree foamed by 0.75% EW addition were placed into glass Petri-dishes (6 mm × 100 mm) and immediately frozen at −20 °C for 24 h before freeze drying at set conditions.2.5. Drying kinetics
The modified Page model, a well-established mathematical model in the field of drying kinetics, is particularly useful in describing the drying kinetics of foods like fruits, vegetables, or porous materials, where the drying rate changes over time due to the evolving properties of the material during drying such as moisture content. In addition, this model is suitable due to its simplicity and flexibility in fitting experimental data over a wide range of conditions for freeze drying.17,18 This model, represented by eqn (4), where MR is the dimensionless moisture ratio, t is the drying time, and k and n are parameters associated with the drying rate and mechanism, respectively, is particularly useful for optimizing drying processes to enhance efficiency and quality.18| MR = e−(kt)n | (4) |
In this study, the moisture ratio was calculated using eqn (5), where M, Me and M0 are the current, equilibrium and initial moisture contents (dry basis), respectively.
 | (5) |
The drying process was carried out at three different temperatures: 10 °C, 20 °C, and 40 °C. The drying time td was calculated for every tested temperature by solving the fitted model for a cut-off of MR = 0.05. This cut-off was chosen as it represents a practically dry state for many food products, beyond which further drying might not significantly improve product quality but could lead to unnecessary energy consumption.18 The fitting in this section was performed by minimizing the least-squares within a 95% confidence interval using Wolfram Mathematica 14 (Champagne IL).
2.6. Moisture content and water activity
Moisture content and water activity of the foam-mat freeze-dried strawberry powder were determined by using a moisture analyzer (A&D Company, Limited, N92, Japan) and water activity analyzer (AQUALAB, Pre, USA), respectively. Triplicates were performed for each sample.2.7. Color determination
The color (L*, a* and b*) of foam-mat dried strawberry powder was analysed using a colorimeter (Hunterlab, ColorFlex EZ, USA). Triplicates were performed for each sample.2.8. Determination of total phenolic content (TPC)
100 mg of SP was extracted using 5 mL of 80% methanol containing 0.1% HCl (v/v) and 5 μM internal standard with the assistance of ultrasound at room temperature for 15 min twice. During extraction, the samples were covered with aluminium foil to avoid light exposure. The sample was then centrifuged at 5000 rpm for 10 min, and the supernatants from two extractions were combined and diluted to 50 mL by using 80% methanol. Similarly, a 10 g sample of fresh strawberry was extracted by using 50 mL of 80% methanol twice, and then a 5-fold diluted solution was prepared. The prepared extracts were stored at −80 °C until further analysis.Estimation of TPC was performed based on the Folin–Ciocalteu procedure described in a previous study with slight modifications.19 Specifically, 50 μL of the prepared extract was mixed with 50 μL of Folin–Ciocalteau reagent and 1.0 mL of distilled water, followed by the addition of 1.0 mL of 7.5% Na2CO3. The mixture was vortexed for 5 seconds. After incubation at room temperature for 60 min and centrifugation at 5000 rpm for 10 min, the absorbance was determined at 760 nm using a BioTek Microplate Spectrophotometer. Standard gallic acid (GA) was used for the determination of a calibration curve.
2.9. Determination of anthocyanin
UPLC-MS/MS studies were performed using a Xevo TQD triple-quadrupole mass spectrometer from Waters (Milford, MA, USA) equipped with an electrospray ionization (ESI) source operating in positive ionization modes. The UPLC was equipped with a 150 × 2.1 mm (i.d.), 1.7 μM particle size, Waters ACQUITY UPLC@BEH C18 column. The anthocyanins in all samples were identified in positive mode, with the composition of the mobile phase varying as follows: 0–4 min, 20–80% B; 4–4.01 min, 80–20% B; 6–8 min, isocratic condition, 20% B. All solvents and solutions were filtered through a 0.22 μm polyamide filter from CELLTREAT® (Massachusetts, U.S.). The injection volume was 5 μL. The temperature of the column was 35 °C and the desolvation temperature in the ionization source was 450 °C. The gas flows for desolvation and the cone were 1000 and 60 L h−1, respectively. The capillary voltage was 3 kV. Detection was performed in the dynamic-multiple reaction monitoring (dynamic-MRM) mode, and the dynamic-MRM peak areas were integrated for quantification. The most abundant product ion was used for quantitation, and the others for qualification. The specific time window for each compound (Δ retention time) was set at 2 min. The selected ion transitions and the mass spectrometer parameters for the analyzed compounds are reported in the ESI.†2.10. Statistical analysis
For each sample and all the tests, triplicates were prepared. In total, a three-fold determination was conducted and was reported as means and standard deviations, calculated using Excel (Microsoft, Redmond, USA). The comparison of two or more groups was analyzed by one-way ANOVA followed by Tukey's multiple comparison test using GraphPad Prism 9.5.1. The two-way analysis of variance (ANOVA) was performed on the collected data, using GraphPad Prism 9.5.1, to evaluate the significance of interaction between the freeze-drying parameters of foamed strawberry puree.3. Results and discussion
3.1. Foam properties
During mechanical whipping, air is incorporated into a liquid containing a foaming agent to form a dispersion of gas. As shown in Table 1, the density of whole strawberry puree without foaming was 0.96 g cm−3. The density of foamed strawberry puree with different EW additions showed a significant decrease compared to the non-foamed one, ranging from 0.49 to 0.84 g cm−3. Based on previous studies, the recommended foam density for the foam-mat drying process was 0.3–0.6 g cm−3.20 A low density foam with high porosity can facilitate the water removal during the drying process as it extensively increases the surface area of mass transfer.21 In this study, the density of foamed strawberry puree containing 0.75% and 1.25% EW was 0.49 and 0.51 g cm−3, respectively, which were within the recommended foam density range. Similarly, the density of beetroot slurry foamed with 5% and 10% fresh egg white (wt/wt) was 0.72 and 0.60 g cm−3, respectively.22 Sour cherry foam with various densities was produced by different egg white and methylcellulose additions, which ranged from 0.43 to 0.65 g cm−3.23| EW concentration (%) | Foam density (g cm−3) | Foam expansion (%) |
|---|---|---|
| a Values are mean ± standard deviation, n = 3, mean values in the column with different letters are significantly different at p < 0.05. | ||
| 0 | 0.96 ± 0.01a | |
| 0.25 | 0.84 ± 0.01b | 14.60 ± 1.66c |
| 0.75 | 0.49 ± 0.01c | 97.22 ± 4.42a |
| 1.25 | 0.51 ± 0.01c | 90.07 ± 2.23b |
The foam expansion was calculated based on the density of strawberry puree foam using eqn (2). As shown in Table 1, the expansion of foamed strawberry puree was found to be in the range of 31.11–97.22%. The EW concentration significantly influenced the foam expansion of strawberry puree. As the concentration of EW increased, the foam expansion increased significantly and reached the highest value at 0.75% EW concentration. However, the expansion slightly dropped to 90.07% by 1.25% EW addition. This could be because the addition of EW above 0.75% increased the liquid viscosity and thus limited the air incorporation during the foaming process. This was in accordance with the report by Suet Li et al. (2021).11 In addition, Rin-ut & Rattanapitigorn (2020) reported that the foam expansion of an aqueous extract of pandan leaves with 10% egg white protein addition had around 900% overrun, which was far more than that of foamed strawberry puree.24 The insoluble solids in strawberry puree may inhibit the foam expansion as hydrophobic particles could destabilize the surface of foam.25
The foam stability is critical throughout the foam-mat drying process. Many stabilizers, such as pectin, maltodextrin and methylcellulose, etc., have been widely utilized in foam preparation to generate stable foam.10,22,23,26 In this study, we found that the foamed strawberry puree exhibited high stability at each EW concentration even without a foaming stabilizer. This could be explained as fresh strawberry contains high sugar and pectin, which might contribute to maintaining the foam stability. In this paper, we focus on the impact of shelf temperature and will use only the 0.75% EW addition for subsequent studies, as it yields the largest foam capacity (Table 1) and higher drying efficiency.27,28 Using strawberry puree foamed with 0.75% EW, we determined the drying kinetics at various freeze-drying temperatures and evaluated the physicochemical properties of the resulting SP in the following sections.
3.2. Drying kinetics
The drying curves of non-foamed and foamed (0.75% EW) strawberry puree were used to establish the drying kinetics using the modified Page model (Table 2) at various shelf temperatures, as shown in Fig. 1. The results showed that higher temperatures led to a decrease in the time needed to reach a zero-moisture ratio, in line with the general understanding that elevated temperatures accelerate evaporation rates, resulting in faster drying times.29 The inclusion of 0.75% EW consistently led to an earlier achievement of MR = 0, indicating a positive impact on drying rates. This enhancement was attributed to the improved foam structure of the strawberry puree due to the addition of EW, which likely increased the surface area and porosity, facilitating greater evaporation and faster drying.29| Sample (°C) | Non-foamed | Foamed | ||||
|---|---|---|---|---|---|---|
| k | n | R 2 | k | n | R 2 | |
| 10 | 0.00267 | 1.488 | 0.997 | 0.00440 | 1.240 | 0.990 |
| 20 | 0.00404 | 1.634 | 0.998 | 0.00769 | 1.460 | 0.993 |
| 40 | 0.00667 | 2.521 | 0.998 | 0.01127 | 2.009 | 0.996 |
Table 2 presents the coefficients and fittings of the modified Page model for the different drying conditions. The parameters (k) and (n) were determined through non-linear regression analysis and are indicative of the drying rate and mechanism, respectively. The values of (k) increased with higher temperatures, reflecting the accelerated drying rates at elevated temperatures. Similarly, the parameter (n) showed variations that correspond to changes in the drying mechanism due to temperature and the presence of EW.
The higher (k) values for the foamed samples compared to the non-foamed samples indicate that the addition of 0.75% EW significantly enhances the drying rate. This can be attributed to the improved foam structure, which increases the surface area and porosity, facilitating more efficient moisture removal. The consistency of these findings across different temperatures underscores the reliability of the modified Page model in capturing the drying kinetics of strawberry puree under varying conditions.
Fig. 1 visually supports these findings by showing the drying kinetics of non-foamed and foamed strawberry puree at three different temperatures: 10 °C, 20 °C, and 40 °C. The graphs illustrate that the foamed samples generally have a higher moisture ratio than the non-foamed samples at any given time, indicating faster drying rates for the foamed samples.
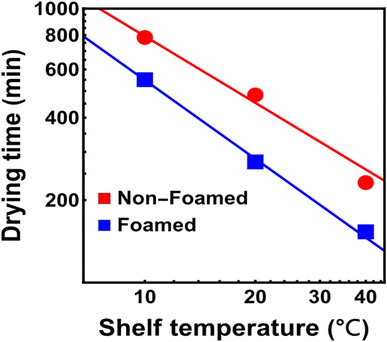
Drying times were calculated using the modified Page model for a cut-off MR of 0.05, and a power law was applied due to its scale invariance property which can be particularly useful for predicting drying times across a wide range of temperatures as shown in Fig. 2.29 The relationship indicated that drying time decreases as a power function of temperature, a common observation in drying kinetics. Notably, for both non-foamed and foamed samples, the slopes of the lines on the log–log plot were similar, suggesting a comparable temperature dependence of drying time, irrespective of the addition of EW.29 However, the line for the foamed sample consistently appeared below the control line, indicating a shorter drying time when EW was added, further emphasizing the positive effect of EW on enhancing the drying efficiency of strawberry puree.29
Research on the freeze drying of grapefruit puree demonstrated that increasing temperature to 40 °C resulted in a 57.5%-time reduction.30 This finding aligns with the observations in the drying kinetics of strawberry puree, where increased temperatures expedited the drying process.29,31 In addition, a study on the preparation and sensory evaluation of functional drinks based on papaya pulp revealed that the drying time of foamed pulp was significantly lower than that of non-foamed pulp, consistent with the results seen in the strawberry puree study where the addition of EW reduced drying times.32 This consistency in findings across different fruit pulps underscores the positive impact of foaming agents like egg white on drying kinetics.29,32
3.3. Moisture content and water activity
The moisture content of strawberry powder (SP) is presented in Table 3. The non-foamed SP had the highest moisture content, which was in the range of 6.01–7.47%. It was evident that the foaming pre-treatment could significantly decrease the moisture content in the freeze-dried SP. The lowest moisture content was found in the foam-mat freeze-dried SP produced at the highest shelf temperature (40 °C). This result aligns with previous reports,11,33 where their experiments revealed that foam-mat dried cantaloupe and date powder both had lower moisture content than non-foamed ones. In addition, an increased shelf temperature of freeze drying showed a significant reduction in moisture content. Similar trends were observed in freeze-dried orange puree and hot air dried yacon powder.13,34 By two-way ANOVA analysis, it was found that there was a significant interaction between the foaming pretreatment and shelf temperature of freeze drying (P < 0.0001) for moisture reduction. It indicated that foaming pretreatment could promote the impact of shelf temperature on the reduction of moisture content. For instance, the moisture content in foam-mat freeze-dried SP produced at 20 °C showed a 59.87% reduction compared to the non-foamed one, while this reduction was only 35.33% at 10 °C. However, there was no significant difference between the moisture contents of the foam-mat freeze-dried powders produced at 20 °C and 40 °C.| Samples | Moisture content (%) | Water activity (aw) | ||||
|---|---|---|---|---|---|---|
| 10 °C | 20 °C | 40 °C | 10 °C | 20 °C | 40 °C | |
| a a w = ERH/100, where ERH is the equilibrium relative humidity (%). Values are mean ± standard deviation, n = 3, mean values in the column with different letters are significantly different at p < 0.05. | ||||||
| Non-foamed | 7.47 ± 0.25a | 6.93 ± 0.06b | 6.01 ± 0.04c | 0.265 ± 0.006a | 0.250 ± 0.004a | 0.209 ± 0.005c |
| Foamed | 4.84 ± 0.10d | 2.78 ± 0.07e | 2.69 ± 0.09e | 0.239 ± 0.003a | 0.226 ± 0.004b | 0.189 ± 0.003d |
Water activity showed a similar decreasing trend in SP with foaming pre-treatment and increase in shelf temperature of freeze drying. As shown in our results, shelf temperature (P < 0.0001) and foaming pretreatment (P < 0.0001) both exhibited a significant effect on reducing the water activity of SP, but the synergistic effect of these two factors was not significant (P = 0.3321). It has been reported that as the concentration of the foaming agent (gum arabic) increased, the water activity decreased in foam-mat freeze-dried date powder.33 A similar trend was also revealed in foam-mat freeze-dried tapai powder.35 Water activity is one of the critical parameters, which is responsible for the shelf life of powder foods. Normally, the water activity below 0.6 aw will inhibit microbial activity and chemical degradation, thus extending the shelf life. In our study, the water activity of SP ranged from 0.189 to 0.265 aw (Table 3), which could be generally considered as microbiologically and chemically stable. Similar water activity was also found in foam-mat freeze-dried blueberry powder (less than 0.4 aw) and pandan powders (0.23–0.34 aw).24,36
3.4. Color determination
Color is a crucial parameter representing the quality of SP as fruit powders are commonly used as natural colorants in many foods, such as yogurt, baked treats, and frosting.37 The effect of foaming pre-treatment and shelf temperature on the color parameters of freeze-dried SP is shown in Fig. 3. The L* value (lightness) of SP increased with foaming pre-treatment and shelf temperature, reaching up to 52.17 for foam-mat freeze-dried SP produced at the highest shelf temperature (40 °C), indicating that the powder was whiter. In this case, EW has a white color, and its addition could lighten the color of SP by diluting strawberry pigments. Additionally, the higher shelf temperature may accelerate the degradation of anthocyanin, which might also be responsible for this result. In contrast, the non-foamed freeze-dried SP exhibited elevated values for both a* (indicating redness) and b* (indicating yellowness), and this was also observed at lower shelf temperature. Particularly for the redness, it experienced a sharp reduction. This was consistent with the report by Shishehgarha, Makhlouf, & Ratti (2002), who found that freeze-drying caused a pronounced increase in the red color of strawberries and the degree of redness reduction was positively correlated with temperature increase within the range of 30 to 50 °C.38 Moreover, compared to the a* value of SP produced at 10 °C, the redness was decreased by 19.45% and 11.08% for non-foamed and foam-mat freeze-dried SP produced at 20 °C, respectively. The less redness reduction in foam-mat freeze-dried SP was probably because the completion of the drying process took less time. However, the protective effect of foaming pre-treatment on redness reduction of SP produced at 40 °C was not as obvious as that at 20 °C. This might be due to the fact that raising temperature to 40 °C significantly reduced the drying time, but the reaction rate of anthocyanin degradation via enzymatic and non-enzymatic approaches may have increased as well. | ||
| Fig. 3 Hunter color parameters of non-foamed and foamed freeze-dried strawberry powders produced at different freeze-drying shelf temperatures. Strawberry foam was made by 0.75% EW addition (wt/wt). | ||
3.5. Effect of drying on the retention of TPC and TAC
The antioxidant activity of strawberry powder is highly correlated with its anthocyanin and total phenolic contents.39,40 In our study, non-foamed and foamed strawberry puree were freeze-dried under various shelf temperatures. As shown in Fig. 4, the total phenolic content (TPC) in strawberry powder produced by foaming and non-foaming methods at various shelf temperatures ranged from 11.92 to 15.56 mg GAE per g dry matter. By t-tests, it was found that foaming treatment did not show significant impact on the retention of TPC, but increasing the shelf temperature by 20 °C significantly reduced the retention of TPC. Specifically, the SPs produced at 10 °C showed the highest retention of TPC, 94.88 ± 1.40% and 95.47 ± 4.35% for non-foamed and foamed strawberry puree, respectively. This aligns with previous reports that the TPC in strawberry powder was highly influenced by drying methods, with freeze-drying being the most efficient way to retain the bioactive polyphenols in strawberry powder, compared with convective drying and spray drying.8 However, there were no significant changes in the retention of TPC between SP produced at 20 °C and 40 °C. This could be explained as the degradation of phenolic compounds can be attributed either to the extent of drying time or the drying temperature.41 Moreover, it should be noted that foaming pretreatment did not exhibit an impact on the retention of TPC among SPs produced at 10, 20 and 40 °C, respectively. Foaming pretreatment significantly shortened the drying time of strawberry puree, but the air containing oxygen that was incorporated in the bubbles during the foaming process might accelerate the degradation of phenolic compounds.42The three major anthocyanins (pelargonidin-3-glucoside, pelargonidin-3-rutinoside and cyanidin-3-glucoside) were identified and quantified in fresh strawberry puree and freeze-dried SP. Pelargonidin-3-glucoside (74.35 ± 0.86 mg/100 g FW) was the predominant anthocyanin in the fresh strawberry puree, followed by pelargonidin-3-rutinoside (8.11 ± 0.26 mg/100 g FW) and cyanidin-3-glucoside (1.35 ± 0.10 mg/100 g FW). This was in agreement with a previous report.43 After freeze-drying, anthocyanins showed a significant reduction in SP production at various shelf temperatures. The results indicated that the lowest shelf temperature (10 °C) had the highest retention of TAC. Foaming pretreatment did not exhibit significant impact on the retention of TAC in the SPs produced at 10 and 20 °C, but foaming pretreatment helped retain more TAC in the SP produced at the highest shelf temperature (40 °C). Anthocyanin stability is also affected by several other processing conditions, the structure of the anthocyanins, and structural damage during sample preparation and drying, which promote degradative reactions.41 Furthermore, the decrease in water content seems to promote oxidative mechanisms and alter the structure of anthocyanin pigments.44 Anthocyanins are degraded enzymatically in the presence of PPO and these enzymes could play a role in the degradation of anthocyanins in strawberries. Denaturation of PPO by the effect of the drying temperature may explain the retention of anthocyanins when lower temperatures are used (Fig. 5).
4. Conclusion
In summary, our study demonstrated that whipping strawberry puree with 0.75% egg white addition at a speed of 200 rpm for 270 seconds at room temperature resulted in foams with the least density and maximum foam expansion. The application of the modified Page model proved highly effective in predicting the drying process, yielding a remarkable coefficient of determination (R2) of 0.99. Both foaming treatment and elevating shelf temperature during freeze drying exerted notable positive influences on reducing the moisture content and water activity of the final strawberry powder. These factors synergistically impacted the moisture content but not water activity. While EW addition contributed to lightening the color of the strawberry powder, it showed less effectiveness in preserving its redness compared to non-foamed freeze-dried powder, particularly at 20 °C. Foaming treatment did not significantly affect the retention of total phenolic content (TPC), although higher shelf temperatures led to decreased retention. Additionally, foaming pretreatment did not significantly impact the retention of total anthocyanin content (TAC) in strawberry powders produced at 10 and 20 °C, but it enhanced the TAC retention at the highest shelf temperature (40 °C). Nonetheless, further investigations are warranted to elucidate the effects of different foaming agents and their varying concentrations on the drying kinetics and overall quality attributes of strawberry powder.Data availability
The authors declare that all data will be available upon reasonable request.Conflicts of interest
There are no conflicts of interest declared by the authors.Acknowledgements
This research was partially funded by the USDA MAS00612, NIFA 2022-67021-37014 and NIFA 2023-67017-40248.References
- M. G. Aziz, Y. Yusof, C. Blanchard, M. Saifullah, A. Farahnaky and G. Scheiling, Material properties and tableting of fruit powders, Food Eng. Rev., 2018, 10, 66–80 CrossRef CAS.
- M. da Silva Pinto, F. M. Lajolo and M. I. Genovese, Bioactive compounds and quantification of total ellagic acid in strawberries (Fragaria × ananassa Duch.), Food Chem., 2008, 107(4), 1629–1635 CrossRef CAS.
- F. Van De Velde, A. M. Tarola, D. Güemes and M. E. Pirovani, Bioactive compounds and antioxidant capacity of Camarosa and Selva strawberries (Fragaria × ananassa Duch.), Foods, 2013, 2(2), 120–131 CrossRef.
- D. Abouelenein, A. M. Mustafa, S. Angeloni, G. Borsetta, S. Vittori, F. Maggi, G. Sagratini and G. Caprioli, Influence of freezing and different drying methods on volatile profiles of strawberry and analysis of volatile compounds of strawberry commercial jams, Molecules, 2021, 26(14), 4153 CrossRef CAS.
- M. C. Bustos, D. Rocha-Parra, I. Sampedro, S. de Pascual-Teresa and A. E. León, The influence of different air-drying conditions on bioactive compounds and antioxidant activity of berries, J. Agric. Food Chem., 2018, 66(11), 2714–2723 CrossRef CAS PubMed.
- B. Han, S. Tian, R. Fan, R. Chen, Y. Wang, H. Gong and M. Bian, Selection of drying technology based on dynamic effects on physicochemical properties and flavours of mulberry, Czech J. Food Sci., 2023, 41(4), 295–303 CrossRef CAS.
- S. Kittibunchakul, P. Temviriyanukul, P. Chaikham and V. Kemsawasd, Effects of freeze drying and convective hot-air drying on predominant bioactive compounds, antioxidant potential and safe consumption of maoberry fruits, LWT, 2023, 184, 114992 CrossRef CAS.
- A. Sadowska, F. Świderski and E. Hallmann, Bioactive, physicochemical and sensory properties as well as microstructure of organic strawberry powders obtained by various drying methods, Appl. Sci., 2020, 10(14), 4706 CrossRef CAS.
- S. Darniadi, P. Ho and B. S. Murray, Comparison of blueberry powder produced via foam-mat freeze-drying versus spray-drying: evaluation of foam and powder properties, J. Sci. Food Agric., 2018, 98(5), 2002–2010 CrossRef CAS PubMed.
- M. Ozcelik, A. Heigl, U. Kulozik and S. Ambros, Effect of hydrocolloid addition and microwave-assisted freeze drying on the characteristics of foamed raspberry puree, Innovative Food Sci. Emerging Technol., 2019, 56, 102183 CrossRef CAS.
- T. Suet Li, R. Sulaiman, Y. Rukayadi and S. Ramli, Effect of gum Arabic concentrations on foam properties, drying kinetics and physicochemical properties of foam mat drying of cantaloupe, Food Hydrocolloids, 2021, 116, 106492 CrossRef CAS.
- C. S. Nwankwo, E. O. Okpomor, N. Dibagar, M. Wodecki, W. Zwierz and A. Figiel, Recent developments in the hybridization of the freeze-drying technique in food dehydration: a review on chemical and sensory qualities, Foods, 2023, 12(18), 3437 CrossRef CAS.
- M. A. Silva-Espinoza, C. Ayed, T. Foster, M. d. M. Camacho and N. Martínez-Navarrete, The impact of freeze-drying conditions on the physico-chemical properties and bioactive compounds of a freeze-dried orange puree, Foods, 2019, 9(1), 32 CrossRef PubMed.
- R. Bogusz, M. Nowacka, K. Rybak, D. Witrowa-Rajchert and E. Gondek, Foam-Mat freeze drying of kiwiberry (Actinidia arguta) pulp: drying kinetics, main properties and microstructure, Appl. Sci., 2024, 14(13), 5629 CrossRef CAS.
- Ö. Kılıç, B. Azak and S. Ersus, The effect of different drying temperature profiles on the shrinkage and physical quality of the freeze-dried strawberries, J. Food Process Eng., 2024, 47(1), e14523 CrossRef.
- M. Ozcelik, S. Ambros, A. Heigl, E. Dachmann and U. Kulozik, Impact of hydrocolloid addition and microwave processing condition on drying behavior of foamed raspberry puree, J. Food Eng., 2019, 240, 83–91 CrossRef CAS.
- D. A. Delfiya, K. Prashob, S. Murali, P. Alfiya, M. P. Samuel and R. Pandiselvam, Drying kinetics of food materials in infrared radiation drying: a review, J. Food Process Eng., 2022, 45(6), e13810 CrossRef.
- D. I. Onwude, N. Hashim, R. B. Janius, N. M. Nawi and K. Abdan, Modeling the thin-layer drying of fruits and vegetables: a review, Compr. Rev. Food Sci. Food Saf., 2016, 15(3), 599–618 CrossRef PubMed.
- S. Yan, H. Shao, Z. Zhou, Q. Wang, L. Zhao and X. Yang, Non-extractable polyphenols of green tea and their antioxidant, anti-α-glucosidase capacity, and release during in vitro digestion, J. Funct. Foods, 2018, 42, 129–136 CrossRef CAS.
- C. Ratti and T. Kudra, Drying of foamed biological materials: opportunities and challenges, Drying Technol., 2006, 24(9), 1101–1108 CrossRef.
- O. S. Qadri, A. K. Srivastava and B. Yousuf, Trends in foam mat drying of foods: special emphasis on hybrid foam mat drying technology, Crit. Rev. Food Sci. Nutr., 2020, 60(10), 1667–1676 CrossRef PubMed.
- M. L. Ng and R. Sulaiman, Development of beetroot (Beta vulgaris) powder using foam mat drying, LWT, 2018, 88, 80–86 CrossRef CAS.
- E. Abbasi and M. Azizpour, Evaluation of physicochemical properties of foam mat dried sour cherry powder, LWT--Food Sci. Technol., 2016, 68, 105–110 CrossRef CAS.
- S. Rin-ut and P. Rattanapitigorn, Effect of foaming agents on process conditions, characteristics, and stability of foam-mat freeze-dried pandan (Pandanus amaryllifolius) powder, J. Food Process. Preserv., 2020, 44(9), e14690 CAS.
- P. R. Garrett, Defoaming: antifoams and mechanical methods, Curr. Opin. Colloid Interface Sci., 2015, 20(2), 81–91 CrossRef CAS.
- S. Rin-ut and P. Rattanapitigorn, Effect of foaming agents on process conditions, characteristics, and stability of foam-mat freeze-dried pandan (Pandanus amaryllifolius) powder, J. Food Process. Preserv., 2020, 44(9), e14690 CAS.
- Z. Hardy and V. Jideani, Foam-mat drying technology: a review, Crit. Rev. Food Sci. Nutr., 2017, 57(12), 2560–2572 CrossRef CAS PubMed.
- O. S. Qadri, A. K. Srivastava and B. Yousuf, Trends in foam mat drying of foods: special emphasis on hybrid foam mat drying technology, Crit. Rev. Food Sci. Nutr., 2020, 60(10), 1667–1676 CrossRef PubMed.
- Z. Wang, J. Sun, X. Liao, F. Chen, G. Zhao, J. Wu and X. Hu, Mathematical modeling on hot air drying of thin layer apple pomace, Food Res. Int., 2007, 40(1), 39–46 CrossRef.
- L. A. Egas-Astudillo, N. Martínez-Navarrete and M. Camacho, Impact of biopolymers added to a grapefruit puree and freeze-drying shelf temperature on process time reduction and product quality, Food Bioprod. Process., 2020, 120, 143–150 CrossRef CAS.
- K. O. Falade and O. J. Solademi, Modelling of air drying of fresh and blanched sweet potato slices, Int. J. Food Sci. Technol., 2010, 45(2), 278–288 CrossRef CAS.
- N. Dev, M. S. Hossain and A. Iqbal, Preparation and sensory evaluation of functional drink based on papaya (Carica papaya L.) pulp: Processing of functional drink from papaya, J. Bangladesh Agril. Univ., 2019, 17(3), 388–395 CrossRef.
- T. Seerangurayar, A. Manickavasagan, A. M. Al-Ismaili and Y. A. Al-Mulla, Effect of carrier agents on flowability and microstructural properties of foam-mat freeze dried date powder, J. Food Eng., 2017, 215, 33–43 CrossRef CAS.
- T. S. Franco, C. A. Perussello, L. d. S. N. Ellendersen and M. L. Masson, Foam mat drying of yacon juice: experimental analysis and computer simulation, J. Food Eng., 2015, 158, 48–57 CrossRef CAS.
- N. Fauziyah, I. Ifie, O. Syarief and S. Darniadi, Impact of hydrocolloid and foaming agent on the physicochemical, microstructural and bioactive characteristics of foam-mat freeze-dried tapai (fermented black glutinous rice) powder, Food Sci. Nutr., 2022, 11(1), 578–589 CrossRef PubMed.
- S. Darniadi, P. Ho and B. S. Murray, Comparison of blueberry powder produced via foam-mat freeze-drying versus spray-drying: evaluation of foam and powder properties, J. Sci. Food Agric., 2018, 98(5), 2002–2010 CrossRef CAS PubMed.
- R. Różyło, Recent trends in methods used to obtain natural food colorants by freeze-drying, Trends Food Sci. Technol., 2020, 102, 39–50 CrossRef.
- F. Shishehgarha, J. Makhlouf and C. Ratti, Freeze-drying characteristics of strawberries, Drying Technol., 2002, 20(1), 131–145 CrossRef.
- H. Li, Z. Deng, H. Zhu, C. Hu, R. Liu, J. C. Young and R. Tsao, Highly pigmented vegetables: anthocyanin compositions and their role in antioxidant activities, Food Res. Int., 2012, 46(1), 250–259 CrossRef CAS.
- S. Skrovankova, D. Sumczynski, J. Mlcek, T. Jurikova and J. Sochor, Bioactive compounds and antioxidant activity in different types of berries, Int. J. Mol. Sci., 2015, 16(10), 24673–24706 CrossRef CAS PubMed.
- L. Méndez-Lagunas, J. Rodríguez-Ramírez, M. Cruz-Gracida, S. Sandoval-Torres and G. Barriada-Bernal, Convective drying kinetics of strawberry (Fragaria ananassa): effects on antioxidant activity, anthocyanins and total phenolic content, Food Chem., 2017, 230, 174–181 CrossRef.
- F. A. Lobo, M. A. Nascimento, J. R. Domingues, D. Q. Falcão, D. Hernanz, F. J. Heredia and K. G. de Lima Araujo, Foam mat drying of Tommy Atkins mango: effects of air temperature and concentrations of soy lecithin and carboxymethylcellulose on phenolic composition, mangiferin, and antioxidant capacity, Food Chem., 2017, 221, 258–266 CrossRef CAS PubMed.
- T. Dzhanfezova, G. Barba-Espín, R. Müller, B. Joernsgaard, J. N. Hegelund, B. Madsen, D. H. Larsen, M. M. Vega and T. B. Toldam-Andersen, Anthocyanin profile, antioxidant activity and total phenolic content of a strawberry (Fragaria × ananassa Duch) genetic resource collection, Food Biosci., 2020, 36, 100620 CrossRef CAS.
- M. C. N. Nunes, J. K. Brecht, A. M. Morais and S. A. Sargent, Possible influences of water loss and polyphenol oxidase activity on anthocyanin content and discoloration in fresh ripe strawberry (cv. Oso Grande) during storage at 1 C, J. Food Sci., 2005, 70(1), S79–S84 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fb00227j |
| This journal is © The Royal Society of Chemistry 2025 |