Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Functional and bioactive properties of chitosan produced from Acheta domesticus with fermentation, enzymatic and microwave-assisted extraction
Marios
Psarianos
 *a,
Nader
Marzban
a,
Shikha
Ojha
b,
Roland
Schneider
c and
Oliver K.
Schlüter
*ad
*a,
Nader
Marzban
a,
Shikha
Ojha
b,
Roland
Schneider
c and
Oliver K.
Schlüter
*ad
aDepartment Systems Process Engineering, Leibniz Institute for Agricultural Engineering and Bioeconomy (ATB), Max-Eyth-Allee 100, 14469 Potsdam, Germany. E-mail: mpsarianos@atb-potsdam.de; oschlueter@atb-potsdam.de
bDepartment of Land Sciences, School of Science and Computing, South East Technological University, Cork Road, X91 K0EK, Waterford, Ireland
cDepartment of of Microbiom Biotechnology, Leibniz Institute for Agricultural Engineering and Bioeconomy (ATB), Max-Eyth-Allee 100, 14469 Potsdam, Germany
dDepartment of Agricultural and Food Sciences, University of Bologna, Piazza Goidanich 60, 47521 Cesena, Italy
First published on 4th December 2024
Abstract
Edible insects are an important source of chitin and chitosan. Different methods, including the use of proteases, fermentation and microwave treatment, have been proposed to replace the conventional chitin isolation methods. House crickets are among the most commonly used insects for food applications. Chitosan was produced from house crickets from chitinous materials that were isolated via the conventional method, a biological process that combines fermentation with Lactococcus lactis and digestion with bromelain and a microwave-assisted chemical method. All chitosans were evaluated for their purity, functional properties and bioactive properties, namely their antioxidant and antimicrobial activity. All three methods generated chitosan with a purity higher than 85% and an exceptionally high oil binding capacity with a maximum of 1078.62 g oil per g chitosan for the chitosan produced with the conventional method. Furthermore, all cricket-derived chitosans showed a significant level of antioxidant activity with an effective concentration of 5 mg mL−1 or lower and antimicrobial activity against Escherichia coli, Staphylococcus aureus and Salmonella enterica ssp. Enterica Serovar Typhimurium. It was concluded that the biological chitin extraction method could lead to the generation of a chitosan material with high potential for application in different sectors.
Sustainability spotlightThe projected increase of global population will lead to a high protein demand. Edible insects constitute a variety of underexplored biomass that can address this demand with a lower environmental impact than conventional livestock. Insects have a rich nutritional profile with many valuable ingredients, including chitin and its derivative, chitosan. The present work aimed to valorize the house crickets, a commonly consumed species, as a source of chitosan. Therefore, green chitin extraction methods were applied, replacing alkaline and acidic treatments with fermentation and enzymatic treatments, followed by an evaluation of the produced chitosan. The use of edible insects and the reduction in chemical use contribute to SDG 2 (Zero Hunger) and partially 13 (climate action). |
1. Introduction
Edible insects have attracted considerable popularity in recent years due to their rich nutritional profiles and low environmental impact.1 They have lower feed1 and land requirements2 and produce lower gas emissions compared to conventional livestock.1 The house crickets (Acheta domesticus) are particularly interesting, because of their easy rearing process,3 high reproduction rate4 and rich nutritional profile.5 Furthermore, house crickets are being already consumed as food and feed in different parts of the world6 and they have been recently accepted as novel food under Regulation (EU) 2015/2283.7Apart from proteins and lipids, insects contain also chitin.8 Chitin is a polysaccharide that is abundant in fungal species and invertebrates9 and consists of a chain of β-(1-4)-N-acetyl-D-glucosamine.10 It can be found on the exoskeleton of insects9 and its content on insects varies, based on the insect species and life stage.8 The range of the chitin content of edible insects has been reported to be 43–108 mg per kg dry matter.11 The substitution of the N-acetyl group of chitin with an amine group is called deacetylation and describes the reaction of the production of chitosan from chitin.12
Chitosan offers potential for a variety of applications in the medicine, cosmetic, food and agricultural sectors.13 Chitosan has been characterized by antioxidant activity,14 antitumor activity15 and antimicrobial activity.16 A variety of edible insect species has been tested for chitin extraction and chitosan production.17 The insect-derived chitosan has exhibited some of the bioactive properties that are commonly reported for crustacean-derived chitosan, such as antioxidant activity18 and antimicrobial activity.19 Chitin is usually isolated from insects via processing pathways that include sequential delipidation, deproteinization, demineralization and decolorization, which often require the use of hazardous chemicals, such as HCl and NaOH at elevated temperatures, long durations17 and are not considered environmentally friendly and that generate pollution.20 Furthermore, the proteins and minerals that are dissolved in these reagents can be upcycled as food and feed easily because they are damaged and less biocompatible.21
A number of alternative and more environmentally friendly methods including fermentation, proteolysis and deep eutectic solvents have been proposed to replace the conventional treatments for chitin extraction.22,23 As mentioned before, the conventional method for extracting chitin through a sequential chemical process has several drawbacks, such as long durations that can last many hours or days, high energy requirements and the use of hazardous chemicals that can cause pollution and degrade the proteins and minerals in the by-products.24,25 Bioprocesses such as fermentation and enzymatic treatment can reduce the risk of using hazardous chemicals.26 Furthermore, microwave processing has been shown to facilitate the conventional method of chitin isolation by significantly reducing the treatment time.27
The treatment of a biomass with hydrolases is particularly interesting. Chitin and chitosan have been observed to undergo partial hydrolysis due to the treatment of proteases, lipases and glycosyl hydrolases.28 The hydrolysis of chitin and chitosan ca. lead to a chitosan with a lower molecular weight, which has been linked to improved bioactive properties.15 At the same time, the treatment of a chitin-rich biomass with proteases can be applied as a method for deproteinization.29 For instance, an enzymatic treatment with bromelain has been applied to house crickets as an alternative process to conventional chemical deproteinization in order to recover chitosan. It was found that the produced chitosan had a lower molecular weight when the enzymatic treatment was applied in comparison to chitosan that was recovered via the conventional process and to chitosan produced from standard chitin. Additionally, this chitosan exhibited higher antioxidant activity than the chitosan that was produced from standard chitin.30
These methods have also been underlined for the potential when applied to edible insects for chitin extraction.31,32 A chitinous material can be isolated from mealworms with fermentation,33 while proteolysis has been successfully applied to mealworms for chitin isolation.34 Moreover, a combination of fermentation with Lactococcus lactis and digestion with bromelain has been proposed to isolate a chitinous material from house crickets that can be used for chitosan production.30
The present study aims to compare the biological processing pathway that was applied by Psarianos et al.30 for the extraction of chitin from house crickets with the conventional method and a microwave-assisted method. After deacetylating this chitin into chitosan, the study aims to evaluate the properties of the produced chitosans in terms of functionality and bioactivity.
2. Materials and methods
2.1 Sample preparation
Living crickets (Acheta domestica) were purchased from Tropic Shop (Nordhorn, Germany) and were inactivated by freezing at −20 °C. They were thawed at 4 °C, separated from frass, washed with water and dried at 60 °C until constant weight. The dried insects were milled for 10 s with a laboratory Retsch mill (Haan, Germany) and were defatted using hexane (solid/liquid ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) for 1 h at room temperature. The hexane was recycled using a rotary evaporator (Büchi R-100, Flawil, Switzerland) and the defatted cricket biomass was stored for further use. Standard chitosan was used as a reference for all experiments. All chemicals were purchased from Carl Roth Gmbh & Co. KG (Karlsruhe, Germany), apart from 2,2-diphenyl-1-picrylhydrazyl (Alfa Aesar, Massachusetts, United States).
20) for 1 h at room temperature. The hexane was recycled using a rotary evaporator (Büchi R-100, Flawil, Switzerland) and the defatted cricket biomass was stored for further use. Standard chitosan was used as a reference for all experiments. All chemicals were purchased from Carl Roth Gmbh & Co. KG (Karlsruhe, Germany), apart from 2,2-diphenyl-1-picrylhydrazyl (Alfa Aesar, Massachusetts, United States).
2.2 Chitin extraction
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) for 24 h at 80 °C. Afterwards, they were demineralized with a 1 M HCl solution (solid/liquid ratio 1
50) for 24 h at 80 °C. Afterwards, they were demineralized with a 1 M HCl solution (solid/liquid ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) for 2 h at 98 °C. After both deproteinization and demineralization, the sample was washed with warm distilled water through a 0.063 mm filter, until the water reached a neutral pH. Afterwards, it was dried at 60 °C until constant weight.35,36
30) for 2 h at 98 °C. After both deproteinization and demineralization, the sample was washed with warm distilled water through a 0.063 mm filter, until the water reached a neutral pH. Afterwards, it was dried at 60 °C until constant weight.35,36
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), for 5 h at 60 °C, pH = 5.5 (as per supplier's specifications) and an enzyme/substrate ratio of 2% w/w. Between both steps, the samples were washed and afterwards dried, as described in Section 2.2.1.37,38
20), for 5 h at 60 °C, pH = 5.5 (as per supplier's specifications) and an enzyme/substrate ratio of 2% w/w. Between both steps, the samples were washed and afterwards dried, as described in Section 2.2.1.37,38
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) with a microwave treatment for 8 min at 500 W. Afterwards the samples were demineralized with a 1 M HCl solution (solid/liquid ratio 1
50) with a microwave treatment for 8 min at 500 W. Afterwards the samples were demineralized with a 1 M HCl solution (solid/liquid ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) with a microwave treatment for 8 min at 500 W. Between each steps the samples were washed and in the end, they were dried, as described in Section 2.2.1. The final temperature after the treatment was always 95–100 °C.
30) with a microwave treatment for 8 min at 500 W. Between each steps the samples were washed and in the end, they were dried, as described in Section 2.2.1. The final temperature after the treatment was always 95–100 °C.
2.3 Deacetylation
Deacetylation of all materials was performed with a 50% NaOH solution at 130 °C for 2 h. Afterwards, the samples were washed with water through a 0.063 mm filter until the neutrality of the water and dried at 60 °C until constant weight.392.4 Properties of chitosan
| Solubility (%) = (M1 − M2)/(M1 − M0) × 100 | (1) |
| OBC (g oil per g chitosan) = (mf − m0)/m0 | (2) |
2.5 Bioactivity
2.5.1.1 Free radical scavenging activity (2,2-diphenyl-1-picrylhydrazyl). The free radical scavenging activity was determined by mixing 1 mL of the chitosan solution at different concentrations with 3 mL of a DPPH solution of 6 × 10−5 M and incubating the mixture at room temperature in the dark for 30 min. Then the absorbance of the mixture was measured with a UV/Vis spectrometer at 515 nm. 1% acetic acid was used as a blank. Scavenging activity was expressed as:
| Scavenging activity (%) = (A0 − A1)/A0 × 100 | (3) |
The concentration of each solution that can result in 50% of scavenging activity (IC50) was determined from a linear regression of the concentration of the solution and the scavenging activity.44
2.5.1.2 Ferric iron reducing power (FRAP). The ferric iron reducing power was estimated as follows: 0.5 mL of each solution at different concentrations was mixed with 0.5 mL of sodium phosphate buffer (0.2 M, pH = 6.6) and 0.5 mL of potassium ferricyanide 1%. The mixture was incubated at 50 °C for 20 min and then was mixed with 0.5 mL of trichloroacetic acid 10%. Then 2 mL of water was added to the mixture and finally, 0.4 mL of ferric chloride 0.1%. The mixture was vortexed and the absorbance was measured with a UV/Vis spectrometer at 700 nm. A higher absorbance indicates a higher antioxidant activity. The effective concentration (EC50), which is the concentration that can lead to an absorbance of 0.5, was estimated with linear regression of the concentration of the solution and the absorbance.45
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 by mixing with 50 μL of the bacterial culture of all strains for 24 h at 37 °C and then adding 50 μL of iodonitrotetrazolium chloride (INT) solution and incubating for 1 h using a 96-well microplate. The MIC of each solution was determined as the lowest concentration at which the pink color did not occur. MHB was used as a blank and gentamycin sulfate 0.2 mg mL−1 was used as a positive control.46
2 by mixing with 50 μL of the bacterial culture of all strains for 24 h at 37 °C and then adding 50 μL of iodonitrotetrazolium chloride (INT) solution and incubating for 1 h using a 96-well microplate. The MIC of each solution was determined as the lowest concentration at which the pink color did not occur. MHB was used as a blank and gentamycin sulfate 0.2 mg mL−1 was used as a positive control.46
2.6 Statistical analysis
Each method used to produce chitosan was replicated three times. Every analytical test was performed for each replicate of production of each material at least twice. Data were analyzed with an analysis of variance (ANOVA) and Tukey's post hoc test to separate significantly different means (p ≤ 0.05). The Shapiro–Wilk test and the Levene test were performed prior to the analysis to verify if the data follow normal distribution and homogeneity of variance, respectively. The analysis was performed with IBM SPSS Statistics 23 (IBM Corp., Armonk, N.Y., USA).3. Results and discussion
3.1 Characterization of chitosan
Table 1 summarizes the properties of the chitosans that were produced from the crickets and of commercial chitosan. All chitosans produced from the insect biomass showed a high level of purity with a chitosan content higher than 87% for all samples, without significant differences (p > 0.05). A high chitosan content (%) was expected for all samples since the biological method has been successfully applied to house crickets to produce chitosan.30 Furthermore, the positive effect of microwave treatment has also been reported for shrimp chitosan.27 The process to extract chitin from insects involves the sequential removal of lipids, proteins, minerals and when necessary also pigments.17 The purity of chitosan is important, since it is one of the factors that determine its functionality and quality, together with the molecular weight, degree of deacetylation, crystallinity and viscocity.47| Commercial chitosan | Chitosan_C | Chitosan_B | Chitosan_M | |
|---|---|---|---|---|
| a Data are expressed as mean ± SD. Different superscript letters (a,b…) indicate significant differences (p < 0.05) among the means of the properties of samples that were generated with different methods. | ||||
| Chitosan content (%) | — | 89.18 ± 3.12a | 87.96 ± 5.33a | 87.11 ± 6.82a |
| Solubility (%) | 97.34 ± 0.48a | 70.73 ± 6.69b | 55.07 ± 4.40c | 42.56 ± 0.52d |
| OBC (g oil per g chitosan) | 411.70 ± 13.98a | 1078.62 ± 184.68b | 885.30 ± 163.03b | 860.92 ± 280.29b |
| pH | 3.32 ± 0.00a | 3.50 ± 0.12a | 3.78 ± 0.04b | 3.43 ± 0.15a |
| PDI | 0.43 ± 0.07a | 0.73 ± 0.21ab | 0.84 ± 0.22ab | 1.15 ± 0.28b |
| Zeta potential (mV) | 27.56 ± 2.20a | 38.86 ± 3.41ab | 50.58 ± 10.94b | 52.35 ± 5.64b |
The resulting chitosans from the crickets that have been produced with the methods described in the present study have exhibited insignificant ash residues, while their molecular analysis has revealed a notable presence of amino groups.30 It was therefore concluded that the remaining impurities are of protein origin. Chitin is mainly found in the procuticle of edible insects, where the cuticle is mainly composed of proteins.48 Additionally, insect chitin has been reported to form complexes with melanin, which is difficult to remove during the chitin extraction and chitosan production.49 The harsher processing conditions that led to the formation of Chitosan_C might have been more efficient in disrupting that complex in comparison to Chitosan_B and Chitosan_M, even if the level of purity is comparable.
Considering the low solubility of cricket proteins50 and the chitin–melanin complex in insects, it was hypothesized that the remaining impurities in the produced chitosan were mainly protein residues and melanin, while traces of minerals might also be present. This hypothesis would apply to all chitosans, since microwave heating has been reported to be applicable for the production of chitosan with similar structural properties in compare to conventional heating. Moreover, the biological processing pathway has been reported to lead to the production of chitosan with comparable structure to the one produced from chitin that is extracted with the conventional method.30,51
The solubility of the chitosans showed significant differences (p < 0.05). The commercial one had the highest solubility (97.34 ± 0.48) and the ones that were generated from the crickets showed a low solubility (70.73 ± 6.69 for Chitosan_C, 55.07 ± 4.40 for Chitosan_B and 42.56 ± 0.52 for Chitosan_M). Chitosan solubility is affected by several factors, including pH, temperature, solvent, ionic strength and degree of acetylation (DA%).52 The low solubility of the chitosans obtained from the crickets could be explained by reduced hydrophilicity due to a high DA% within the range of 20% and 50%.53 The chitosans produced from house crickets with the methods described in the present study have been reported to have a DA% of 30–40%.30
The pH is a significant factor that affects the solubility of chitosan, with chitosan being reported to be soluble at acidic pH values and precipitate at 6 < pH < 7.5.54 The aqueous acetic acid used in the present study has been considered among the solvents traditionally used for chitosan solubilization,55 while the low pH values would be expected to facilitate its solubility.56 Temperature affects chitosan solubility as well, with thermal treatments at temperatures >50 °C leading to the formation of hydrogels.55 In the present study, solubilization was performed at room temperature to avoid the formation of gels.
Therefore, the overall low solubility has been attributed mainly to the high degree of acetylation. However, the remains of the complex between insect chitosan with melanin and insoluble proteins is expected to lead to an overall reduced solubility and different physicochemical properties in compare to a chitosan produced by crustaceans.49
The extraction of cricket-derived chitin with the chemical method has been reported to be more efficient in removing the impurities and generate a purer chitin. On the contrary, for example the biological processing pathway was reported to generate a chitin-rich fraction, where chitin is the main component but the chitin content is <60% w/w.30 Due to their low solubility, the proteins from house crickets have been identified as the main impurity.50 During the deacetylation some of the protein impurities can be extracted to the alkaline medium, leading to a chitosan with a higher purity.57 This case was exhibited for the cricket-derived chitosan, which undergoes deacetylation and purification simultaneously during the treatment with the saturated NaOH.30
The insufficient deproteinization of chitin prior to deacetylation has been reported to have lower solubility and higher DA%.57 The difference in the chitin and protein content among the starting insect materials prior to deacetylation influenced the solubility of the resulting chitosan. Similarly, the DA% of the cricket derived-chitosans has been reported to be similar and high, with Chitosan_C being the least acetylated.30
One further parameter that can influence the solubility is particle size, with smaller particles exhibiting higher solubility.58 Chitosan is a molecule than can aggregate or form chitosan–protein aggregates generating larger complexes. The application of bromelain in the production process of Chitosan_B could generate oligopeptides that can form aggregates with chitosan at an acidic pH.59,60 In the case of the microwave treatment, it has been reported that microwave processing can form irreversible aggregates after degrading chitosan.61 Aggregation of chitosan is a further explanation of the low solubility, since the commercial chitosan shows a significantly lower particle size (Fig. 1) than the cricket-derived ones, which is consistent with its higher solubility, while Chitosan_B has the highest particle size and a low solubility.
The chitosans showed a significantly different OBC (p < 0.05), with the chitosan being produced from the crickets demonstrating over twice the OBC, compared to commercial chitosan. The OBC was high (>800 g oil per g chitosan) for all samples that were generated from the insects. The OBC of cricket-derived chitosans was not affected by the chitin isolation method (p > 0.05). A high value of OBC has also been reported for chitosan isolated from other insect species including cicadas, silkworms and mealworms (574–795%, 412–635%, 408–643% respectively).17,41 The differences in the OBC between commercial and insect-derived chitosans can be attributed to possible differences in crystallinity, salt-forming groups and protein residues in the samples.62
Cricket-derived chitosans exhibited a similar particle size distribution, according to Fig. 1, with a main fraction of higher particle size and a smaller one with a lower particle size, when isolated from chitin with chemical treatments. In particular, the larger fraction showed a particle size of 482.20 and 494.70 nm for Chitosan_C and Chitosan_M, respectively. The smaller fraction showed a particle size of 81.74 and 52.92 nm for Chitosan_C and Chitosan_M, respectively. On the contrary, Chitosan_B exhibited only one large fraction with 584.23 nm, similarly to the commercial chitosan that exhibited one fraction of 253.6 nm. Chitin can be partially hydrolyzed during the demineralization process due to the harsh acidic conditions, long treatment times and amount of solvent, resulting in some chitin losses.63 These losses could explain the larger particle size of Chitosan_B and the low particle size fraction of Chitosan_C and Chitosan_M. Additionally, the presence of residual impurities could influence the particle size distribution of the chitosans is expected to affect the particle size. It is also important to consider the values of the PDI since the chitosans that are produced from the insects show values higher than 0.5, which indicates a higher heterogeneity.64 Furthermore, Chitosan_B and Chitosan_C showed significantly higher (p < 0.05) zeta potential values than the commercial one (Table 1 and Fig. 1), which is an indication of higher colloidal stability and resistance to aggregation.65
The solubility of chitosan is related to its ionic strength, as well, due to the chitosan's ability to form pseudo colloidal dispersions when solubility is low. Fig. 2 presents the zeta potential of the commercial chitosan and the ones produced from the crickets. Chitosan has been reported to exhibit a large particle size >1000 nm and a high zeta potential (approx. 50 mV).55 Chitosans exhibit a positive charging at pH < 7 due to the presence of –NH3+. When the pH decreases and a solution becomes more acidic, the positive charge of chitosan increases and it becomes more soluble. Chitosan with higher molecular weight tend to aggregate and lose their positive charging as the pH increases due to the formation of hydrogen bonds. This aggregation causes chitosan to function as a micelle and was reported to lead to higher absolute values of the zeta potential.66 Chitosans with particularly high values of the zeta potential, as in the case of the cricket-derived chitosans, have been reported to have good rheological properties (because the viscosity of a chitosan-dispersion would decrease when the dispersion is stirred faster), as well as good colloidal stability.55 Additionally, a high positive charging of chitosan would indicate a stronger antimicrobial activity.55,66
The cricket-derived chitosans contains impurities and have a lower chitosan content in comparison to the commercial one that is of analytical grade. Additionally, the cricket-derived chitosans are more acetylated than the commercial one30 and therefore has a lower solubility. Considering that the residual impurities on the cricket-derived chitosan are expected to be insoluble proteins and melanin, which also contain amino groups and have colloidal properties, the cricket-based chitosans could show a higher zeta potential. Based on these properties, the cricket-derived chitosans show higher potential for more applications compared to the commercial one.
Based on the results presented in Table 1, the application of the treatment with L. lactis followed by the digestion with bromelain was considered appropriate alternative to the conventional method for chitin extraction. This alternative would address the disadvantages of the conventional method, such as the generation of large amounts of liquid waste and loss of nutrients,26 since the side streams can be more easily repurposed. The long treatment time of Chitosan_B is the main disadvantage of its production method. Therefore, it is important to optimize this process to reduce the duration. However, the application of microwaves is also applicable for the production of cricket-derived chitosan, since it can reduce the treatment time and the energy consumption of the process.
3.2 Bioactivity of chitosan
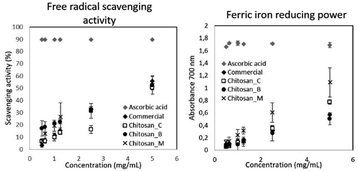
Both free radical scavenging activity and ferric iron reducing power were observed for all chitosan samples at a concentration range of 0.5–5 mg mL−1. This concentration range has been reported also for insect-derived chitosan from different species, including C. barbarous, O. decorus, Musca domestica and Chrysomya megacephala.18,44,67
Regarding the scavenging activity, Chitosan_B and commercial chitosan showed no significant differences (p > 0.05) of the IC50, while Chitosan_C was significantly higher (p < 0.05). On the contrary, regarding the EC50, the solution of Chitosan_C showed a significantly lower EC50 (p < 0.05). Commercial chitosan and Chitosan_B solutions showed no significant differences (p > 0.05), meaning that regarding both the scavenging activity and the reducing power, Chitosan_B could replace the commercial one.
Table 2 shows the IC50 and EC50 of all chitosans. The IC50 of chitosan refers to the chitosan concentration in a dispersion that is sufficient to achieve a 50% antioxidant activity in compare to a dispersion without chitosan. Consequently, a lower IC50 means that the amount of chitosan that is required to achieve a 50% antioxidant activity is lower and that this chitosan exhibits a stronger effect. The effective concentration of solutions of chitosan of crab14 or insect origin44,68 has been reported to be much higher than the one reported by the present study. The lower effective concentration indicates a stronger antioxidant activity and is attributed to the lower molecular weight that has been reported for chitosan produced from house crickets with the methods described in Section 2.2.30 It has been shown, that a chitosan with lower molecular weight exhibits stronger bioactive properties.15 The antioxidant activity of chitosan has been attributed to its amino group and its hydroxyl groups that react with free radicals.56 Chitosans with lower molecular weight exhibit a lower amount of intramolecular bonds and a higher molarity for the same respective mass and consequently a higher amount of amino and hydroxyl groups are available for reacting with free radicals.56,69,70
| IC50 (mg mL−1) | EC50 (mg mL−1) | |
|---|---|---|
| a Data are expressed as mean ± SD. Different superscript letters (a,b…) indicate significant differences (p < 0.05) among the means obtained for samples that were generated with different methods. | ||
| Commercial chitosan | 4.36 ± 0.28a | 4.26 ± 0.11ab |
| Chitosan_C | 5.31 ± 0.47b | 3.37 ± 0.25b |
| Chitosan_B | 4.62 ± 0.55a | 5.06 ± 1.13a |
| Chitosan_M | 4.46 ± 0.37a | 2.27 ± 0.54c |
Additional factors affecting the antioxidant activity of chitosan are the degree of acetylation, whereas more acetylated chitosans exhibit lower antioxidant activity,69 and solubility since chitosan needs to be solubilized to participate in oxidative reactions.71 Therefore, processing pathways and fractionation processes that generate chitosan with higher molecular weight and degree of acetylation will lead to chitosan with reduced antioxidant activity. The cricket-derived chitosans have been reported to have low molecular weight with Chitosan_B exhibiting particularly low molecular weight due to partial depolymerisation of chitosan from bromelain.30 Since, the processes that lead to depolymerisation of chitin and chitosan are known to generate chitosan with stronger bioactive properties,15 the cricket-based chitosans with the low molecular weight were expected to exhibit strong antioxidant activity.
The antioxidant activity of a biologically active molecule can be estimated with various methods that correspond to different reaction mechanisms. The DPPH method describes the free radical scavenging activity that is based on the transfer of electrons and H atoms, while the FRAP method describes the reduction of ferric ions due to the electron transfer reaction.72 The examination of several protocols for the in vitro antioxidant activity of a bioactive molecule can provide a more comprehensive argument of its activity and highlight the mechanism of the antioxidant activity of that molecule. A statistical correlation of these methods has been reported in the case of lignin.73
Insect-derived chitosan has been shown to require higher effective concentrations for reducing power than free radical scavenging.44 This was not the case for the chitosans produced from the house crickets, which showed a stronger free radical scavenging activity. Therefore, the antioxidant activity of chitosan from insects depends on both the production method and the species of origin. The lack of significant differences of the effective concentration of the cricket-derived chitosans and the commercial one highlights the importance of house crickets as a chitosan source alternative to the crustacean sources.
| Minimum inhibitory concentration for antimicrobial activity (mg mL−1) | |||
|---|---|---|---|
| Escherichia coli | Staphylococcus aureus | Salmonella enterica | |
| Commercial chitosan | 0.14 | 0.56 | 0.28 |
| Chitosan_C | 0.14 | 1.13 | 0.28 |
| Chitosan_B | 0.56 | 1.13 | 1.13 |
| Chitosan_M | 0.28 | 0.56 | 1.13 |
Similar to the antioxidant activity, the antimicrobial activity of chitosan has been mainly attributed to the presence of –NH3+.74 Regarding Gram-negative bacteria, chitosan interacts with anionic structures that are found on their surface, such as lipopolysaccharides and proteins.75 Regarding Gram-positive bacteria, chitosan interacts with their cell wall layer, consisting of negatively charges of peptidoglycans and teichoic acids.76 Chitosans with lower molecular weight and lower degree of acetylation would be expected to exhibit a higher amount of available reactive and positively charged amino groups.77 Additionally, chitosan with higher molecular weight was reported to form a barrier across Gram-positive bacteria blocking the transfer of nutrients, while chitosan with lower molecular weight can cross the cell of Gram-negative bacteria more easily,16 making chitosan with high molecular weight more effective against Gram-positive bacteria and vice versa.78
The antimicrobial properties of the chitosans produced from house crickets confirmed and were similar to the ones reported by Malm and Liceaga (2021), who suggested a minimum inhibitory concentration of higher than 0.5 mg mL−1 for chitosan produced from house crickets,19 while in the present study the respective concentration was 0.14–1.13 depending on the sample. The minimum inhibitory concentration of the cricket-derived chitosans against the examined bacteria is within the range that is reported for chitosan from marine sources (100–1000 ppm that would correspond to 0.1–1 mg mL−1).79 This finding highlights the potential of the cricket-derived chitosan as an antimicrobial agent comparable to crustacean-derived chitosan, which further underscores their potential in the agri-food chain, considering also their rich nutritional profile.48
4. Conclusions
A variety of processing pathways used for chitosan production from other resources can be applied to the house cricket biomass. Microwave processing, as well as, the combination of biological processes, such as fermentation and digestion, are efficient in replacing the conventional process for chitosan production based on its properties. Chitosans that were produced from the crickets were found to have significant bioactive properties. In specific, they showed high antioxidant activity and were quite efficient as antimicrobial agents against some commonly encountered pathogenic bacterial strains. The findings reported by the present study underline the applicability of alternative and more environmentally friendly methods for chitin isolation to house crickets, to produce a chitosan. The chitosan produced with these methods showed the highest zeta potential (52.35 mV for Chitosan_M), higher antioxidant activity with the lowest IC50 (4.46 mg mL−1 for Chitosan_M) and comparable EC50 (lowest 2.27 mg mL−1 for Chitosan_M), better antimicrobial activity (lowest minimum inhibitory concentration 0.28 mg mL−1 for Chitosan_M) and higher OBC (885.30 g oil per g chitosan for Chitosan_B). However, the isolating of high-value insect-derived chitin with environmentally friendly methods is still a challenging matter.Data availability
The data supporting the findings of this study are available within the article.Author contributions
MP: conceptualization, data curation, formal analysis, investigation, methodology, validation, writing, review – editing; NM: conceptualization, data curation, formal analysis, investigation, methodology, validation, writing, review – editing; SO: conceptualization, investigation, methodology, validation, writing, review – editing; RS: methodology, writing, review – editing; OS: fund acquisition, supervision, writing, review – editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was funded by the Federal Ministry of Education and Research (BMBF) based on a decision of the Parliament of the Federal Republic of Germany via the Project Management Jülich (PtJ) within the joint research project Agrarsysteme der Zukunft: F4F – Nahrung der Zukunft, Teilprojekt I (Grant No. 031B0730 I).References
- A. Van Huis and D. G. Oonincx, Agron. Sustainable Dev., 2017, 37, 1–14 CrossRef.
- G. Dennis and I. Oonincx, PLoS One, 2012, 7, e51145 CrossRef.
- R. Caparros Megido, E. Haubruge and F. Francis, Small-scale production of crickets and impact on rural livelihoods, in Insects as Food and Feed: From Production to Consumption, ed. A. van Huis and J. K. Tomberlin, Wageningen Academic Publishers, 2017 Search PubMed.
- C. Gillott, Entomology, Springer Science & Business Media, 2005 Search PubMed.
- B. A. Rumpold and O. K. Schlüter, Mol. Nutr. Food Res., 2013, 57, 802–823 CrossRef CAS.
- A. Van Huis, Annu. Rev. Entomol., 2013, 58, 563–583 CrossRef CAS.
- N. F. EFSA Panel on Nutrition, F. Allergens, D. Turck, T. Bohn, J. Castenmiller, S. De Henauw, K. I. Hirsch-Ernst, A. Maciuk, I. Mangelsdorf, H. J. McArdle and A. Naska, EFSA J., 2021, 19, e06779 Search PubMed.
- M. D. Finke, Zoo Biol., 2007, 26, 105–115 CrossRef CAS.
- W. J. Tang, J. G. Fernandez, J. J. Sohn and C. T. Amemiya, Curr. Biol., 2015, 25, 897–900 CrossRef CAS.
- E. Khor, Chitin: Fulfilling a Biomaterials Promise, Elsevier, 2014 Search PubMed.
- F. Kvasnička, L. Kouřimská, R. Bleha, P. Škvorová, M. Kulma and A. Rajchl, J. Chromatogr. A, 2023, 1695, 463952 CrossRef PubMed.
- M. N. R. Kumar, React. Funct. Polym., 2000, 46, 1–27 CrossRef CAS.
- N. Morin-Crini, E. Lichtfouse, G. Torri and G. Crini, in Sustainable Agriculture Reviews 35, Springer, 2019, pp. 49–123 Search PubMed.
- M.-T. Yen, J.-H. Yang and J.-L. Mau, Carbohydr. Polym., 2008, 74, 840–844 CrossRef CAS.
- I. Younes, S. Hajji, V. Frachet, M. Rinaudo, K. Jellouli and M. Nasri, Int. J. Biol. Macromol., 2014, 69, 489–498 CrossRef CAS PubMed.
- L.-Y. Zheng and J.-F. Zhu, Carbohydr. Polym., 2003, 54, 527–530 CrossRef CAS.
- K. Mohan, A. R. Ganesan, T. Muralisankar, R. Jayakumar, P. Sathishkumar, V. Uthayakumar, R. Chandirasekar and N. Revathi, Trends Food Sci. Technol., 2020, 105, 17–42 CrossRef CAS PubMed.
- H. Ai, F. Wang, Y. Xia, X. Chen and C. Lei, Food Chem., 2012, 132, 493–498 CrossRef CAS.
- M. Malm and A. M. Liceaga, Polysaccharides, 2021, 2, 339–353 CrossRef CAS.
- M. Healy, A. Green and A. Healy, Acta Biotechnol., 2003, 23, 151–160 CrossRef CAS.
- N. Islam, M. Hoque and S. F. Taharat, World J. Microbiol. Biotechnol., 2023, 39, 28 CrossRef CAS.
- S. Kaur and G. S. Dhillon, Crit. Rev. Biotechnol., 2015, 35, 44–61 CrossRef CAS.
- P. Zhou, J. Li, T. Yan, X. Wang, J. Huang, Z. Kuang, M. Ye and M. Pan, Carbohydr. Polym., 2019, 225, 115255 CrossRef PubMed.
- J. Luo, X. Wang, B. Xia and J. Wu, J. Macromol. Sci., Part A: Pure Appl. Chem., 2010, 47, 952–956 CrossRef CAS.
- K. Mohan, A. R. Ganesan, P. Ezhilarasi, K. K. Kondamareddy, D. K. Rajan, P. Sathishkumar, J. Rajarajeswaran and L. Conterno, Carbohydr. Polym., 2022, 287, 119349 CrossRef CAS PubMed.
- M. Psarianos, G. Baliota, C. I. Rumbos, C. G. Athanassiou, S. Ojha and O. K. Schlüter, in Insects as Food and Food Ingredients, Elsevier, 2024, pp. 129–143 Search PubMed.
- H. E. Knidri, J. Dahmani, A. Addaou, A. Laajeb and A. Lahsini, Int. J. Biol. Macromol., 2019, 139, 1092–1102 CrossRef PubMed.
- D. N. Poshina, S. V. Raik, A. N. Poshin and Y. A. Skorik, Polym. Degrad. Stab., 2018, 156, 269–278 CrossRef CAS.
- C. T. Doan, T. N. Tran, C.-L. Wang and S.-L. Wang, Polymers, 2020, 12, 2228 CrossRef CAS.
- M. Psarianos, S. Ojha, R. Schneider and O. K. Schlüter, Molecules, 2022, 27, 5005 CrossRef CAS PubMed.
- M. K. Lagat, S. Were, F. Ndwigah, V. J. Kemboi, C. Kipkoech and C. M. Tanga, Microorganisms, 2021, 9, 2417 CrossRef CAS PubMed.
- Y. N. Tan, Y. L. Chin and W. N. Chen, ACS Food Sci. Technol., 2021, 1, 698–706 CrossRef CAS.
- F. K. P. da Silva, D. W. Brück and W. M. Brück, FEMS Microbiol. Lett., 2017, 17, 364 Search PubMed.
- A. J. da Silva Lucas, E. Q. Oreste, H. L. G. Costa, H. M. López, C. D. M. Saad and C. Prentice, Food Chem., 2021, 343, 128550 CrossRef.
- M. Kaya and T. Baran, Int. J. Biol. Macromol., 2015, 75, 7–12 CrossRef CAS.
- A. Percot, C. Viton and A. Domard, Biomacromolecules, 2003, 4, 12–18 CrossRef CAS.
- W. D. P. Rengga, K. A. Salsabiil, S. E. Oktavia and M. Ansori, J. Phys.:Conf. Ser., 2019, 1367(1), 012080 CrossRef CAS.
- O. Aytekin and M. Elibol, Bioprocess Biosyst. Eng., 2010, 33, 393–399 CrossRef CAS.
- M. L. Tsaih and R. H. Chen, J. Appl. Polym. Sci., 2003, 88, 2917–2923 CrossRef CAS.
- A. Zamani, A. Jeihanipour, L. Edebo, C. Niklasson and M. J. Taherzadeh, J. Agric. Food Chem., 2008, 56, 8314–8318 CrossRef CAS PubMed.
- Q. Luo, Y. Wang, Q. Han, L. Ji, H. Zhang, Z. Fei and Y. Wang, Carbohydr. Polym., 2019, 209, 266–275 CrossRef CAS PubMed.
- M. Psarianos, G. Dimopoulos, S. Ojha, A. C. M. Cavini, S. Bußler, P. Taoukis and O. K. Schlüter, Innovative Food Sci. Emerging Technol., 2022, 76, 102908 CrossRef CAS.
- H. K. No, S. H. Kim, S. H. Lee, N. Y. Park and W. Prinyawiwatkul, Carbohydr. Polym., 2006, 65, 174–178 CrossRef CAS.
- M. Kaya, T. Baran, M. Asan-Ozusaglam, Y. S. Cakmak, K. O. Tozak, A. Mol, A. Mentes and G. Sezen, Biotechnol. Bioprocess Eng., 2015, 20, 168–179 CrossRef CAS.
- M. Oyaizu, Jpn. J. Nutr. Diet., 1986, 44, 307–315 CrossRef CAS.
- P. Stiefel, J. Schneider, C. Amberg, K. Maniura-Weber and Q. Ren, Sci. Rep., 2016, 6, 1–9 CrossRef PubMed.
- K. Piekarska, M. Sikora, M. Owczarek, J. Jóźwik-Pruska and M. Wiśniewska-Wrona, Polymers, 2023, 15, 793 CrossRef CAS PubMed.
- D. Oonincx and M. Finke, Food Sci. Technol. Res., 2021, 7, 639–659 Search PubMed.
- A. Khayrova, S. Lopatin and V. Varlamov, Int. J. Biol. Macromol., 2021, 167, 1319–1328 CrossRef CAS PubMed.
- N. Udomsil, S. Imsoonthornruksa, C. Gosalawit and M. Ketudat-Cairns, J. Insects Food Feed, 2019, 25, 597–605 CAS.
- H. El Knidri, R. El Khalfaouy, A. Laajeb, A. Addaou and A. Lahsini, Process Saf. Environ. Prot., 2016, 104, 395–405 CrossRef CAS.
- J. Lizardi-Mendoza, W. M. A. Monal and F. M. G. Valencia, in Chitosan in the Preservation of Agricultural Commodities, Elsevier, 2016, pp. 3–31 Search PubMed.
- C. Schatz, C. Viton, T. Delair, C. Pichot and A. Domard, Biomacromolecules, 2003, 4, 641–648 CrossRef CAS PubMed.
- K. M. Vårum, M. H. Ottøy and O. Smidsrød, Carbohydr. Polym., 1994, 25, 65–70 CrossRef.
- B. Li, L. Qiu, J. Zhang, S. Liu, M. Xu, J. Wang and H. Yang, Int. J. Biol. Macromol., 2024, 254, 127950 CrossRef CAS.
- I. Aranaz, A. R. Alcántara, M. C. Civera, C. Arias, B. Elorza, A. Heras Caballero and N. Acosta, Polymers, 2021, 13, 3256 CrossRef CAS PubMed.
- H. K. No, S. H. Lee, N. Y. Park and S. P. Meyers, J. Agric. Food Chem., 2003, 51, 7659–7663 CrossRef CAS.
- G. Buckton and A. E. Beezer, Int. J. Pharm., 1992, 82, R7–R10 CrossRef.
- K. F. Ahmed, A. Aschi and T. Nicolai, Colloids Surf., B, 2018, 169, 257–264 CrossRef CAS PubMed.
- A. D. Paulino-Gonzalez, H. Sakagami, K. Bandow, Y. Kanda, Y. Nagasawa, Y. Hibino, H. Nakajima, S. Yokose, O. Amano and G. Nakaya, In Vivo, 2020, 34, 1729–1738 CrossRef CAS PubMed.
- C. M. Journot, L. Nicolle, Y. Lavanchy and S. Gerber-Lemaire, Polymers, 2020, 12, 1274 CrossRef CAS PubMed.
- D. Knorr, J. Food Sci., 1982, 47, 593–595 CrossRef CAS.
- N. H. K. Al Shaqsi, H. A. S. Al Hoqani, M. A. Hossain and M. A. Al Sibani, Adv. Biomarker Sci. Technol., 2020, 2, 45–58 CrossRef CAS.
- L.-C. Chen, S.-K. Kung, H.-H. Chen and S.-B. Lin, Carbohydr. Polym., 2010, 82, 913–919 CrossRef CAS.
- D. Hanaor, M. Michelazzi, C. Leonelli and C. C. Sorrell, J. Eur. Ceram. Soc., 2012, 32, 235–244 CrossRef CAS.
- S.-H. Chang, H.-T. V. Lin, G.-J. Wu and G. J. Tsai, Carbohydr. Polym., 2015, 134, 74–81 CrossRef CAS PubMed.
- C. Song, H. Yu, M. Zhang, Y. Yang and G. Zhang, Int. J. Biol. Macromol., 2013, 60, 347–354 CrossRef CAS.
- C. Y. Soon, Y. B. Tee, C. H. Tan, A. T. Rosnita and A. Khalina, Int. J. Biol. Macromol., 2018, 108, 135–142 CrossRef CAS.
- S. Pu, J. Li, L. Sun, L. Zhong and Q. Ma, Carbohydr. Polym., 2019, 211, 161–172 CrossRef CAS.
- T. Sun, D. Zhou, J. Xie and F. Mao, Eur. Food Res. Technol., 2007, 225, 451–456 CrossRef CAS.
- D. G. Ivanova and Z. L. Yaneva, BioRes. Open Access, 2020, 9, 64–72 CrossRef CAS PubMed.
- F. Shahidi and Y. Zhong, J. Funct. Foods, 2015, 18, 757–781 CrossRef CAS.
- J. Rumpf, R. Burger and M. Schulze, Int. J. Biol. Macromol., 2023, 233, 123470 CrossRef CAS.
- D. Raafat and H. G. Sahl, Microb. Biotechnol., 2009, 2, 186–201 CrossRef CAS.
- H. Nikaido and M. Vaara, Microbiol. Rev., 1985, 49, 1–32 CrossRef CAS PubMed.
- M. Hosseinnejad and S. M. Jafari, Int. J. Biol. Macromol., 2016, 85, 467–475 CrossRef CAS PubMed.
- A. Guarnieri, M. Triunfo, C. Scieuzo, D. Ianniciello, E. Tafi, T. Hahn, S. Zibek, R. Salvia, A. De Bonis and P. Falabella, Sci. Rep., 2022, 12, 8084 CrossRef CAS PubMed.
- P. Eaton, J. C. Fernandes, E. Pereira, M. E. Pintado and F. X. Malcata, Ultramicroscopy, 2008, 108, 1128–1134 CrossRef CAS PubMed.
- R. C. Goy, D. D. Britto and O. B. Assis, Polímeros, 2009, 19, 241–247 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |