Open Access Article
Open Access ArticlePVA–nano cellulose active packaging films with clay nano particles and fennel seed essential oil for enhanced thermal, barrier, antimicrobial, antioxidant and biodegradation properties to improve the shelf life of tofu †
Ramesh
Shruthy
and
Radhakrishnan
Preetha
 *
*
Department of Food Process Engineering, School of Bioengineering, The College of Engineering and Technology, SRM Institute of Science and Technology, Kattankulathur-603203, Kancheepuram District, TamilNadu, India. E-mail: preethar@srmist.edu.in
First published on 24th December 2024
Abstract
In this study, Box Badam Pod Nano Cellulose Particles (BBNCPs) were prepared using a combination of physiochemical treatments. After each treatment, the removal of organic constituents from the raw material, other than cellulose, was confirmed by FTIR analysis. XRD analysis of BBNCPs showed a crystallinity of 88%. The morphological study of BBNCPs was conducted using SEM, TEM, and AFM. Their size was confirmed to be in the range of 4–8 nm. Then, a BBNCP-reinforced novel active packaging film was fabricated for the packaging of tofu. The casting solution of the optimized film contained 8% polyvinyl alcohol (PVA), 2.25 ml fennel seed essential oil, 1.75% BBNCP, and 6% montmorillonite nano clay. The active packaging material exhibited superior tensile strength (7 ± 0.01 N mm−2), thermal properties (complete degradation at 800 °C), oxygen transfer rate (2.6 cm3 per m2 per day), antioxidant activity (58.12 ± 0.08%), antimicrobial properties (against E. coli and Shigella flexneri) and biodegradation (complete degradation within 50 days) compared to neat PVA. After packing, the analysis of physiochemical properties, as well as microbial and sensory analysis of tofu was performed at regular intervals during storage (4 °C). A statistically significant difference (P < 0.05) was found for the quality parameters of tofu when packed in a nanocomposite film compared to when packed in a neat PVA film. Hence, BBNCP-reinforced active packaging is suggested to extend the shelf life of tofu.
Sustainability spotlightPreparation and characterization of Box Badam pod nano cellulose (4–8 nm) particles (BBNCPs) were carried out, followed by the fabrication of a film using PVA, BBNCPs, MCNP, and FSEO with improved antimicrobial/antioxidant properties. The film had enhanced mechanical, optical, barrier, thermal, and biodegradation properties. The sustainable active food packaging film developed will reduce the piling up of synthetic plastic, hence reducing its environmental impact. The active packaging improved the biochemical and microbial properties of tofu during storage. The active packaging extends the shelf life of tofu up to 20 days at 4 °C. |
1 Introduction
The prevalent use of non-biodegradable plastics in conventional packaging, although convenient for food handling, poses a considerable risk to the environment.1 Consequently, there is a growing interest in utilizing natural and renewable resource-based nanoparticles for food packaging development.2 Commonly used raw materials for fabricating biodegradable food packaging include hemp, bark, leaves, agro-waste, and bacterial cellulose. The utilization of agro-waste and food industry by-products as raw materials for biodegradable packaging can effectively mitigate environmental pollution.3 Notably, the study by Sodeinde et al.4 emphasized the limitations of using softwood due to deforestation concerns, leading to the exploration of alternative sources for Nano Cellulose Particle (NCP) preparation. Additionally, it was observed that pods and seeds have a higher cellulose content compared to straw and softwood.5 This led us to the selection of BoxBadam pods as the raw material for synthesizing cellulose nanoparticles. The BoxBadam tree is commonly found in East Africa, North Australia, Myanmar, Sri Lanka, and India.6 Furthermore, Das et al.7 outlined variations in the cross-linking properties of cellulose nanoparticles depending on their sources. Consequently, in the present study, a novel raw material was employed for the preparation of nano cellulose.Numerous ongoing research endeavours are currently directed towards enhancing the safety and quality of food packaging.8 Active packaging technologies have shown promise in prolonging the shelf life of food products while meeting heightened safety and quality standards.9 Lotfi et al.8 highlighted the antimicrobial properties of metallic nanoparticles, suggesting their potential application in the development of packaging films to extend the freshness of perishable food items such as chicken and fish. Additionally, Venezia et al.10 demonstrated the efficacy of an active film layer composed of electrospun hybrid TiO2 and PHBV in enhancing antimicrobial, thermal, optical, barrier, and mechanical properties. Furthermore, a study incorporating gelatin and humic substances from bio-waste resulted in the development of a hybrid hydrogel with favourable functional, rheological, and physiochemical properties.11 Jancy et al.12 reported that the reinforcement of nanocellulose derived from jackfruit waste with PVA in active packaging enhances the mechanical, thermal, and optical properties of the packaging film.
In this study, a polyvinyl alcohol (PVA)-active packaging film was fabricated with Box Badam Pod Nano Cellulose Particles (BBNCPs), fennel seed essential oil, and montmorillonite clay nanoparticles (MCNPs). The antimicrobial properties of montmorillonite clay nanoparticles (MCNPs) and fennel seed essential oil against food-borne pathogens were reported earlier,13,14 and this information prompted us to include montmorillonite clay nanoparticles and fennel seed essential oil in biocomposites.15,16 PVA is a biodegradable polymer, and the combination of PVA with other active additives makes the packaging material more efficient.17 In a previous report, prawns were packed in potato peel nanoparticles with PVA-based active packaging, achieving a shelf life of up to sixty-three days under frozen (−20 °C) conditions.18 Along with clay nanoparticles and nanocellulose particles, polyvinyl alcohol (PVA) was used as a key ingredient and binding agent due to its biodegradability and cost-effectiveness. However, the hydrophilic nature of PVA presents a major drawback, making it unsuitable for packaging food that contains moisture. MCNPs and fennel seed essential oil (FSEO) can mitigate the hydrophilic nature of PVA. Many studies have documented the antioxidant and antimicrobial properties of fennel essential oil, making its use in active packaging film an added advantage.12,19 Furthermore, numerous studies have reported the efficient mechanical properties of PVA–cellulose nanoparticle biocomposites.20 Van and Lee's study conducted in 2022 reveals that while the neat PVA film lacks mechanical properties compared to synthetic-conventional polymers,21 the incorporation of cellulose nanocrystals into PVA-based packaging not only enhances its mechanical properties but also imparts biodegradability. The study of a coconut shell cellulose nanofiber–linseed–lemon oil-based film showed a tensile strength of 6.72 ± 0.72 N mm−2, which is superior to that of a neat PVA film (2.56 ± 1.18 N mm−2).21 According to previous reports, the addition of cellulose nanoparticles can enhance the mechanical, thermal, barrier, and biodegradation properties of PVA.22,23 However, there remains significant potential for further enhancement of these properties, as they depend on the source of nanocellulose.23 This information prompts us to use a novel natural source for cellulose nanoparticle preparation.
The current study involves the preparation and characterization of BBNCPs in detail, followed by the fabrication and characterization of a PVA-based active packaging film incorporating BBNCPs, FSEO, and MCNPs. It is a unique packaging material for food. Tofu was selected to study the role of the above active packaging film in enhancing shelf life during storage. Tofu is a by-product of coagulated soy milk and is rich in calcium, protein, iron, and magnesium. Tofu is a vegetarian substitute for fish and meat; people allergic to lactose can consume it. It serves as the primary source of protein, accounting for 65–85%, and provides an abundance of nutrients.24 Hence, it was selected for the packaging study. During the packaging study, tofu was packed in the BBNCP-based nanocomposite film and stored at 4 °C. At regular intervals, the physiochemical and microbial quality of tofu was evaluated and compared with Tofu packed in a neat PVA film.
2 Materials and methods
BoxBadam (Sterculia foetida) pods were obtained from different localities in Kattankulathur, Chennai, and Tamil Nadu, India. After that, retting processes such as beating and soaking of the raw BoxBadam pods were conducted to get rid of organic compounds, including pectin, hemicellulose, and other volatile fatty acids bound with the fiber constituent.13 Following the retting process, the pods were washed with water, sun-dried, and blended using a milling machine (Rajalakshmi flour-making electric machine, India) and stored in an airtight box for the study. The chemicals used for synthesizing BBNCPs include sodium hydroxide, 30% hydrogen peroxide, montmorillonite nano clay particles (MNCP) and polyvinyl alcohol (PVA). These were purchased from Lobha Chemie Pvt Ltd, Mumbai, India. Additionally, sulphuric acid and hydrochloric acid were provided by Rankem TM, Avantor Performance Material India Limited, Haryana, India. Fennel Seed Essential Oil (FSEO) was supplied by Devine's, India.3 Preparation of BoxBadam nano cellulose particles
BoxBadam pod fibers were used for the preparation of nano cellulose particles. The preparation was carried out using a combination of physiochemical treatment processes, which have already been reported in the literature.12 The chemical treatment started with an alkaline treatment to remove the non-cellulosic constituents present in the BoxBadam pod, followed by bleaching to remove the color.15,25 Then, the fiber obtained was washed using water, followed by acid hydrolysis treatment for three hours at 40 °C using 68% sulphuric acid. Finally, the acid hydrolysis process with ice-cold water effectively terminated the treatment. The obtained nano cellulose particle sample was again washed using H2O to achieve pH 7. Then, the physical treatment was performed for BBNCPs by ultra-homogenization (1000 rpm) and ultra-sonication (60 kHz) for 30 minutes each, as described in our earlier study with slight modifications.15,263.1 Characterization studies of BBNCPs
This technique was carried out to confirm the reduction of hemicellulose and pectin in BBNCPs. The Fourier Transform Infrared (FTIR) analysis was conducted within the frequency range of 4000–1000 cm−1, utilizing a spectral resolution of 4 cm−1.22
XRD (Aeris-High Resolution Bench Top XRD, Malvern analytics, United Kingdom) analysis of raw BoxBadam pod fiber, chemically treated fiber, and physiochemically treated BBNCPs was performed to determine the crystallinity index; the analysis utilized copper K-α radiation with 154 nm wavelength operating at 30 mA and 40 kV and the crystallinity index was determined using the Scherrer formula and the Segal method.13
The TEM (transmission electron microscope) technique was used to obtain the size distribution and perform a morphological study of BBNCPs. TEM analysis was performed by transferring BBNCP suspension on carbon-coated copper grids to enhance contrast and image resolution.27 HR-TEM analyzed BBNCPs over a carbon-coated copper grid at 400 kV, and a CCD camera captured the images.22
Atomic force microscopy (AFM), equipped with a high-resolution camera (5Mp), was used for the quick determination of the BBNCP sample (AFM, D13100, NY). The, raw data were processed using Nanoscope Analysis Software to obtain real images of BBNCPs with high resolution.20
3.2 Invention of the BBNCP-based nanocomposite and optimization of ingredients
The nanocomposite films were prepared with different concentrations of ingredients such as PVA (1, 2, 3, 4, 5, 6, 7, 8, 9 and 10%) along with BBNCPs (0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, and 5%), FSEO (1.75, 2.25, 2.75, 3.25, 3.75, 4.25, 4.75, 5.25, 5.75 and 6.25 ml) and MCNPs (1, 2, 3, 4, 5, 6, 7, 8, 9 and 10%). The ranges for ingredient concentration were fixed based on the literature.22,28 The nanocomposite was fabricated (using the solvent casting method) by dissolving PVA in 0.1 L of H2O at 60 °C for 45 minutes using a magnetic stirrer and a hot plate. Afterward, BBNCPs, FSEO, and MCNPs were combined with warm PVA. The resulting film-forming solution mixture was then poured over the glass plate and dried at room temperature, and the nanocomposite film was peeled off. The film with maximum mechanical, antimicrobial, and antioxidant properties was selected for further studies.Thermal property characterization of the nanocomposite was performed using a ASTM E-1131-20 using SDT Q 600 (simulated DTA-TGA, TA Instruments, USA). The reference material used for the study was calcined alumina, and platinum cups were used as the sample and reference containers. The test was conducted in nitrogen with 99.99% purity, moisture at one ppm, and oxygen at one ppm with a purge rate of 100 ± 5 ml min−1. The temperature range used for the study was from 22 °C to 800 °C, with a heating rate of 10 °C min−1. The transition temperatures, enthalpies of fusion, and crystallization of the nanocomposite were determined using Differential Scanning Calorimetry (DSC-Q600, TA Instrument Inc., USA), and the test method used for the study was ASTM D 3418-15. The barrier property study of the BBNCP-MCNP- FSEO-PVA-based nanocomposite was conducted by determining the OTR (oxygen transfer rate) (ISO. 15105). A film with a thickness of 84 μm was used for analysis at 32 °C and a relative humidity of 35%. The sample cells were analyzed at a pressure of 0.1 MPa for 24 hours. The slope of pressure versus time was determined using linear fitting to calculate the oxygen transmission rate.
The Desiccant Gravimetric WVTR (Water Vapor Transmission Rate) equipment from Labthink Instruments Co LTD, China, was utilized for this study. The WVTR of the BBNCP–MCNP–FSEO–PVA-based nanocomposite was evaluated in accordance with the ASTM E 96/E96M14 standard method. The samples were hermetically sealed in a test dish containing discolored silica gel (12 g) and placed in a desiccator with a saturated sodium chloride solution under controlled conditions of 38 °C and 90% relative humidity for 24 hours. Subsequently, the WVTR was determined by periodically weighing the samples. The water vapor transmission rate was calculated using eqn (1) as reported by Morris et al.,28 where, ‘G’ is the change in weight, ‘T’ is the time, and ‘A’ is the exposed area of the film
 | (1) |
A solubility study of the BBNCP-based nanocomposite and control tofu packed in neat PVA was performed by measuring their weight before and after immersion in water at ambient temperature. Both the BBNCP-based nanocomposite film and the control neat PVA film (5 g each) were immersed in 100 ml of distilled water in glass beakers. After that, the samples were kept on a magnetic stirrer at 600 rpm. The degree of solubility was determined using formula (2):
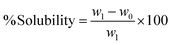 | (2) |
A biodegradation study of the BBNCP-based nanocomposite and neat PVA was conducted using the soil burial method at ambient temperature, and the % weight loss was analyzed as described in ASTM.29
3.3 Food packaging application study using BBNCP based nanocomposite active packaging
Tofu was used for the packaging application study. To prepare tofu, soybeans were procured from a local grocery shop at Kattankulathur, Chennai, and then washed and soaked. 500 g of soybeans were soaked in 1 L of water for 24 hours. After that, the soybeans were ground with 2000 ml of water using a grinder (Sujatha Mixer grinder, Powermatic plus SUJATA, Mitta electronics, Delhi, India). Then, the obtained soy mixture was cooked at 70 °C for 10 minutes indirectly to get rid of beany flavor and anti-nutritional factors. Then, it was strained, and the soy milk and cake (okara) were separated using cheesecloth. The coagulation of the soy milk was achieved by adding the coagulant CaSO4·2H2O at a temperature of 20 °C. After that, the coagulated tofu was broken, and the curd was pressed in perforated molds for 30 minutes to obtain a perfectly shaped tofu. Then, the prepared tofu was kept in chilled water to reduce the inside-out temperature.24 For the shelf life study, tofu was cut into small pieces (3 g each) and then packed using BBNCP-based nanocomposite films, and stored at 4 °C for further analysis. Before the quality check, the thawing process of tofu was done by placing it in 20 °C warm water, as described in the study of Fuchigami et al.31The textural properties of the Tofu were studied using a digital texture analyzer (TA.XT Plus stable Microsystem, UK). Parameters such as hardness, springiness, and chewiness were recorded using a textural analyzer.35 For the textural analysis, 3 g of thawed tofu were positioned beneath the probe on a 75 mm diameter platen. A pre-test speed of 2 mm s−1 and a post-test speed of 10 mm s−1 were employed, with 80% compression. The two bite test was followed for the textural analysis based on the already-reported study of Hansen et al.34 Textural analysis was performed for the tofu packed in BBNCP-based nanocomposite and neat PVA films. The study was performed continuously for 20 days with triplicate measurements.
The pH of the tofu packed in control neat PVA and the BBNCP-based nanocomposite films was observed using a digital pH meter, as described by Gopinath et al.35
Water activity (aw) of the tofu packed in control neat PVA and the BBNCP-based nanocomposite films was measured using a water activity meter (Lab Touch, Novasina, Model: 2600775, Switzerland). Water activity is the measurement of water availability for the growth and metabolic activity of microbes. For the analysis, 1 g of tofu sample was placed inside the sample holder at the water activity meter, and the sensor inside the instrument analyzes the water activity.36
The tofu packed in control neat PVA and the BBNCP-based nanocomposite films was precisely analyzed using a Color Quest XE Hunter color meter (A60-1011-610, Hunter Association Laboratory, Virginia, USA). These measurements were conducted three times. The L*, a*, and b* values, represents lightness, redness-greenness, and yellowness-blueness, respectively. Furthermore, the whiteness index, a crucial parameter in color analysis, was calculated from the obtained data using the precise equation as reported by Anbarasu and Vijayalekshmi.36
Yeast and mold counts were determined by the spread plate method; 0.1 ml samples of diluents (tofu samples) were plated on Rose Bengal Chloramphenicol agar (Himedia) and incubated at 30 °C for 120 hours.38 Triplicate readings were observed and recorded for the study.
3.4 Statistical analysis
In order to identify any significant changes between treatments, the shelf life research data were statistically evaluated using one-way ANOVA in Microsoft Excel (MS Office 2010). The findings were presented as means with standard deviation, and the statistical approach revealed significant differences (P < 0.05). In the textural property study, the experimental values were analyzed by using ANOVA in the SPSS statistical package, (SPSS statistics for Windows, Version 29) and the values in the table are represented as mean ± standard deviation, and Tukey's posthoc test (SPSS v.22) was used to determine significant differences (P < 0.05), which are indicated by distinct superscripts.4 Results and discussion
4.1 Preparation of nano cellulose particles from BoxBadam
The successful extraction of BBNCPs was done, as shown in Fig. 1, and the yield of BBNCPs obtained after extraction was 48 ± 0.8%. The yield of BBNCPs was higher compared with the already reported nanoparticle extraction yields from sources such as areca nut (35%), banana rachis (28.6%), coir (23.5%), kapok (33.7%), sisal fiber (9%) and so on.13,39,404.2 Characterization studies of BBNCPs
Fig. 2(a) shows the strong spectral absorption peak at 1145 cm−1, which results from stretching vibrations of the C–O–C bond which is an important bond between the glucose units in the cellulose.40 The stretching vibration peak at 1255 cm−1 confirms the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the raw sample, which corresponds to the aromatic ring of lignin. The acid-hydrolyzed sample shows the absence of C
O in the raw sample, which corresponds to the aromatic ring of lignin. The acid-hydrolyzed sample shows the absence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, confirming the elimination of the lignin in the BBNCPs; a similar result was reported in the study of Arun et al.21 The alkaline treated and bleached samples showed significant reduction in lignin content. The acid hydrolyzed sample confirmed the elimination of lignin, which indicates the proper chemical treatment during BBNCP synthesis.41 Overall, the FTIR analysis study confirms the purity of the BBNCPs synthesized by substantial reduction/elimination of lignin and hemicellulose. The percentage crystallinity index of the BBNCPs was determined using an X-ray diffractometer. The analysis was done before the treatment (raw BoxBadam fiber) and after the treatments (alkaline, bleaching, and acid hydrolysis). The diffractogram obtained after the analysis is shown in Fig. 2(b).
O, confirming the elimination of the lignin in the BBNCPs; a similar result was reported in the study of Arun et al.21 The alkaline treated and bleached samples showed significant reduction in lignin content. The acid hydrolyzed sample confirmed the elimination of lignin, which indicates the proper chemical treatment during BBNCP synthesis.41 Overall, the FTIR analysis study confirms the purity of the BBNCPs synthesized by substantial reduction/elimination of lignin and hemicellulose. The percentage crystallinity index of the BBNCPs was determined using an X-ray diffractometer. The analysis was done before the treatment (raw BoxBadam fiber) and after the treatments (alkaline, bleaching, and acid hydrolysis). The diffractogram obtained after the analysis is shown in Fig. 2(b).
The major diffraction peaks observed at 16.6° and 22.45° correspond to the structure of typical cellulose; similar patterns were observed in the study of coconut waste nanofiber and areca nut husk fiber.22 The crystallinity index of the raw BoxBadam pod fiber was determined to be 68.66%. Following alkaline treatment, the fiber exhibited a crystallinity index of 74%, while the bleached fiber demonstrated 80% crystallinity. Subsequently, the acid-hydrolyzed and physically treated BBNCPs revealed a notably higher crystallinity index of 88%. The particle size for BBNCPs was determined to be 5.9 nm, obtained using the Scherrer formula. Studies have already reported that the crystallinity index is 80.9% for banana fiber, coir (84.5%), and kapok (86.5%). However, the BBNCP exhibits an improved crystallinity index of 88%, which is superior to the values reported in similar studies on nanofibers in the literature. An increased crystallinity index indicates increased hardness and density of the nanoparticles.13 Nanocellulose has a high crystallinity index, indicating reduction of the amorphous region of cellulose due to hydrolysis.22 However, at high concentrations, strong acids not only eliminate the amorphous region but also degrade the crystalline region of cellulose. The degree of crystallinity significantly influences thermal degradation.13 Nanocelluloses with a more amorphous structure exhibit lower resistance to temperature compared to those with a crystalline structure.13
Raw BoxBadam fibers are clumsy rod-like structures, as shown in Fig. 3(a). However, alkaline treated (b) and bleached (c) fibers were observed to have chopped needle-like structures. At the same time, acid-hydrolyzed physically treated BoxBadam fiber shows the defibrillated clumsy aggregates of chopped BBNCPs. The agglomeration results from the hydrogen bonding interaction between various cellulose fiber molecules.22 The absence of skepticism was resolved through the TEM and AFM analysis of BBNCPs. The morphology and size distribution were analyzed by Transmission Electron Microscopy, as shown in Fig. 3(e). The BBNCPs were observed on a scale of 50 nm and analyzed in the size range of 10 nm–25 nm, consisting of aggregated and chopped nano cellulose particles. Furthermore, the dimensional study of BBNCPs was performed using AFM.
The AFM study provides insights into the morphology and surface topography of the BBNCPs, as shown in Fig. 3(f). The size of the nano cellulose particles was studied in the scale range of 3 μm × 3 μm. The 3D topographical analysis confirms the needle-like morphology of BBNCPs, with a size range of 4–8 nm. In the research conducted by Ramesh and Radhakrishnan,13 it was documented that areca nut fiber nanocrystals typically fall within the range of 130–230 nm, while potato cellulose nanoparticles exhibit a size range of 50–100 nm.41 In the present study, further size reduction was observed for BBNCPs. Hence, the current study shows an efficient size range. Arun et al.21 made similar observations for coconut shell cellulose nanocrystals, which were in the size range of 12–16 nm. Hence, the physiochemical treatments used to produce BBNCPs were found to enhance the nano cellulose particle size.
4.3 Invention of the BBNCP-based nanocomposite with optimum composition
The nanocomposite film was prepared using a film casting solution with a composition of 1.75% BBNCP, 2.25 ml FSEO, 6% MCNP and 8% PVA. The nanocomposite film with the above composition was selected for further studies since it exhibited maximum mechanical, microbial, and antioxidant properties. The picture of the active packaging film is shown in Fig. 4. The cost analysis study of the BBNCP-based nanocomposite is given in the ESI data†Table 1. | ||
| Fig. 4 Tofu packed in BBNCP–PVA–FSEO–nano clay based nanocomposite films for the shelf life study: (a) nanocomposite film, (b) Tofu packed on day 1 and (c) aged Tofu on the 20th day. | ||
| Characteristics | Neat PVA | PVA-BBNCP | PVA-FSEO | PVA-MCNP | BBNCP-FSEO-MCNP-PVA nanocomposite |
|---|---|---|---|---|---|
| a PVA-polyvinyl alcohol, BBNCP-BoxBadam nano cellulose particles, FSEO-fennel seed essential oil, and MNCP-montmorillonite nano clay particles. Data are expressed as mean ± standard deviation (n = 3). In each row, each value followed by a different letter is significantly different (P ≤ 0.05) as determined by Tukey's posthoc test (SPSS v.22). | |||||
| Mechanical property | |||||
| Tensile strength (TS) (N mm−2) | 3.2 ± 0.05d | 5.01 ± 0.03b | 3.04 ± 0.03e | 4 ± 0.09c | 7.03 ± 0.01a |
| Elongation at break (%) | 178 ± 0.02b | 86 ± 0.01c | 215 ± 0.04a | 52 ± 0.03e | 74 ± 0.02d |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Antioxidant property (%) | |||||
| 7.06 ± 0.02d | 13 ± 0.03c | 23.2 ± 0.02b | 2.08 ± 0.06e | 58.12 ± 0.08a | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Antimicrobial property: zone of inhibition (cm) | |||||
| Escherichia coli | 0.15 ± 0.001e | 0.18 ± 0.003d | 1.46 ± 0.006c | 1.73 ± 0.004b | 2.54 ± 0.007a |
| Staphylococcus aureus | 0.12 ± 0.002d | 0.09 ± 0.001e | 1.21 ± 0.001c | 1.50 ± 0.003b | 2.00 ± 0.001a |
| Salmonella typhi | 0.011 ± 0.001e | 1.015 ± 0.006d | 1.062 ± 0.002c | 2.00 ± 0.001b | 2.42 ± 0.003a |
| Shigella flexneri | 0.09 ± 0.005e | 0.28 ± 0.002d | 2.26 ± 0.003b | 2.08 ± 0.006c | 2.90 ± 0.006a |
Thermal properties of the BBNCP–FSEO–MCNP–PVA nanocomposite film, including TGA-DSC, were analyzed using a SDT Q600. The thermal stability of the film depends on the interaction between the molecules present in it.22 As shown in Fig. 5(a), the nanocomposite film underwent three significant thermal degradation steps spanning a temperature range of 100–800 °C. The nanocomposite film displayed initial degradation at 121.74 °C, signifying the loss of water. The second degradation step ranges from 236.33–425.85 °C, which is attributed the degradation of PVA and cellulose components to carbon.13 The final degradation step was observed at 800 °C with a 1.05% weight loss, which indicates that incorporating BBNCPs leads to thermal stability efficiency. In a similar study by Ramesh and Radhakrishnan,15 it was reported that the potato peel CNP-PVA film showed ultimate degradation at 794.51 °C with a weight loss of 2.68%, and the reason for improved thermal stability was the presence of potato peel CNPs. Hence, the current study demonstrated superior thermal stability by comparing it with the potato peel CNP-PVA film. The DSC thermograph of the BBNCP–FSEO–MCNP–PVA film exhibits a glass transition temperature at 100 °C (infers absorbed moisture), a melting temperature at 200 °C (degradation of the cellulose), and oxidative degradation at 650 °C.45 In a similar study, the initial endothermic peak at 100 °C was identified and linked to the evaporation of water molecules.15 By comparing with the already reported studies, the current study shows an increased enthalpy, which is the main reason for the increased melting temperature of the BBNCP–FSEO–MNCP–PVA-based film.12,46 An increase in enthalpy serves as evidence of the effectiveness of cellulose nanofibers as a nucleating agent, concurrently bolstering the rate of crystallization.12 Moreover, most of the studies achieved a maximum thermal property of up to 600 °C, but the current study obtained a maximum thermal stability of 800 °C. Overall, the TGA-DSC results confirmed that the newly fabricated nanocomposite film has superior thermal properties compared to similar nanocomposites reported to date and can be used as a high-quality active packaging material for food products.
A spectrophotometer was utilized to assess the light transmittance of the nanocomposite film and the control PVA. Light transmittance denotes the degree of light penetration through the material's surface. In the present study, the control PVA exhibited a light transmittance of 0.083 ± 0.02 A mm−1, whereas the BBNCP–FSEO–MCNP–PVA nanocomposite film demonstrated a light transmittance of 1.369 ± 0.33 A mm−1. The diminished transparency of the nanocomposite film is attributed to its heightened opacity.13
The barrier property study provides an oxygen transmission rate (OTR) of 2.6 cm3 per m2 per day, which is less than the OTR value reported for potato cellulose nano particle–polyvinyl alcohol–fennel seed essential oil-based film (3 cm3 per m2 per day) reported in the literature. Similarly, the PVA–piperic acid-based composite film studied for its potential in food packaging exhibited an OTR of 4.97 cm3 per m2 per day.46 The OTR is an essential factor in maintaining the storage stability and quality of pasteurized food products, thereby improving the keeping quality of the food product.47 In the current study, BBNCPs increased molecular interactions and thereby decreased the OTR of the bio nanocomposite. According to the study by Mousavi et al.,48 the decreased oxygen permeability coefficient of the polymer reduces the oxidation rate of food products, hence extending the shelf life of food commodities. The current study achieved a lower OTR compared to previous studies on similar packaging materials. This suggests its suitability for maintaining quality and extending the shelf life of perishable processed food products.
The water vapor transmission rate (WVTR) is a critical consideration for evaluating food packaging materials, particularly for packaging high-moisture food products. In the current study, the BBNCP-based nanocomposite displayed a WVTR of 4.42 ± 0.07 g per m2 per day, while neat PVA demonstrated an increased WVTR of 10.02 ± 0.01 g per m2 per day. Water vapor permeability is a material property that measures how quickly water vapor passes through it. A lower WVTR indicates the packaging material's effectiveness in reducing the transfer of water vapor molecules.40 Morris et al.28 reported that the decreased WVTR can be attributed to the incorporation of cellulose nanofibers with PVA. The cellulose nanofibers create a tortuous path, altering the direct diffusion lanes of water molecules within the composite, thereby impeding the passage of water vapor through the film. Consequently, this study establishes the efficacy of the newly developed packaging material for preserving perishable food products.
The water solubility of packaging materials is a critical parameter. Percentage solubility of the neat PVA (control) and BBNCP based nanocomposite films are given in the ESI data,†Table 2. The PVA control film exhibited complete solubility, reaching 100% dissolution within 60 minutes. The study of the PVA–pomegranate peel nanoparticle-based film reported 29% solubility rate within 60 minutes.49 At the same time, BBNCP-based nanocomposite film had a solubility of 25 ± 0.02% within 60 minutes. This disparity indicates a reduced solubility rate in the newly developed packaging film, attributed to the presence of BBNCPs and their cross-linking properties with PVA. In this scenario, the newly developed packaging film is suggested for food packaging.
| Parameters | Number of days | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 4 | 7 | 10 | 13 | 16 | 20 | |
| a Data are expressed as mean ± standard deviation (n = 3). In each row, each value followed by a different letter is significantly different (P ≤ 0.05) as determined by Tukey's posthoc test (SPSS v.22). | |||||||
| Chewiness (N mm) | |||||||
| a | 13.27 ± 0.002a | 12.4 ± 0.001b | 11.54 ± 0.003c | 10.01 ± 0.002d | 9.36 ± 0.003e | 5.43 ± 0.002f | 0.11 ± 0.003g |
| b | 13.27 ± 0.002a | 12.9 ± 0.002b | 12.47 ± 0.003c | 11.84 ± 0.045d | 10.8 ± 0.003e | 9.33 ± 0.002f | 4.32 ± 0.0025g |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Springiness (mm) | |||||||
| a | 3.25 ± 0.002a | 3 ± 0.001b | 2.99 ± 0.002c | 2.39 ± 0.0015d | 2.07 ± 0.003e | 1.70 ± 0.002f | 1.20 ± 0.003g |
| b | 3.25 ± 0.002a | 3.10 ± 0.002b | 2.96 ± 0.001c | 2.81 ± 0.0020d | 2.66 ± 0.001e | 2.10 ± 0.0021f | 1.72 ± 0.002g |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Hardness (N) | |||||||
| a | 12.40 ± 0.005a | 11.8 ± 0.002b | 11.15 ± 0.001c | 10.22 ± 0.001d | 8.14 ± 0.002e | 5.40 ± 0.015f | 2.55 ± 0.015g |
| b | 12.40 ± 0.005a | 12.02 ± 0.02b | 11.24 ± 0.026c | 10.72 ± 0.015d | 10.1 ± 0.057e | 9.30 ± 0.002f | 7.22 ± 0.025g |
The biodegradation of the BBNCP-based nanocomposite and neat PVA was observed for 50 days at ambient temperature, as shown in Fig. 5(b). On day 1, the BBNCP-based nanocomposite and neat PVA weighed 10 ± 0.08%. Later, after 28 days, the neat PVA showed a weight loss % of 8.03 ± 0.01, and the BBNCP-based nanocomposite showed a weight loss % of 45.88 ± 0.03. After that, on day 42, the neat PVA showed a weight loss % of 23.51 ± 0.022, and the BBNCP-based nanocomposite showed a weight loss % of 59.04 ± 0.04. Finally, at day 50, the BBNCP-based nanocomposite was degraded completely in the soil, while 55.33 ± 0.01% weight loss was observed for neat PVA. A similar study of coconut shell nano crystal–PVA–linseed and lemon essential oil-based packaging material also showed a degradation period of 45 days.22
Overall, the newly fabricated BBNCP-based nanocomposite showed improved mechanical, barrier, and biodegradation properties and is recommended for food packaging, especially for perishable food items.
Antimicrobial properties of neat PVA and BBNCP–FSEO–MCNP–PVA active packaging were evaluated using the zone of inhibition method, and the corresponding results are provided in Table 1. The analysis demonstrates that the nanocomposite exhibits an enhanced zone of inhibition compared to the neat PVA film. Importantly, a statistically significant difference of P < 0.05 is observed between tofu packed in BBNCP-based biocomposites and that packed in neat PVA.
Ciprofloxacin antibiotic disks showed a zone of inhibition of 3 ± 0.001 cm, which emphasizes that the prepared nanocomposite possesses antimicrobial properties similar to those of the antibiotic disk. In a study conducted by Hong et al.,50 it was observed that natural clay exhibits inhibitory effects against the food-borne pathogen Staphylococcus aureus. Several studies have corroborated the antimicrobial properties of fennel essential oil against foodborne pathogens.12,20 Furthermore, research by Ramesh and Radhakrishan13 indicates that montmorillonite clay contains antimicrobial agents, including Al2O3, CaCO3, SiO2, Fe, and Ti, contributing to its ability to inhibit microbes. The mechanism behind the inhibition of microbes is that the antimicrobial agents affect the cell membrane and produce reactive oxygen species in large amounts that can inactivate the inner cellular constituents and lead to the disruption of the microbes.49 In the case of FESO, the mechanism behind its inhibitory effect is that FSEO is composed of antimicrobial agents such as aldehyde, terpenes, ketones, and phenols, which can disrupt the microbial cell membrane (cytoplasmic membrane), leading to impairment of the microbial respiratory function. In addition, FSEO has a hydrophobic nature; it promotes antibacterial properties by depositing over cell membranes and blocking the passage of microbes.20
4.4 Application study using BBNCP based nanocomposite films for tofu packaging
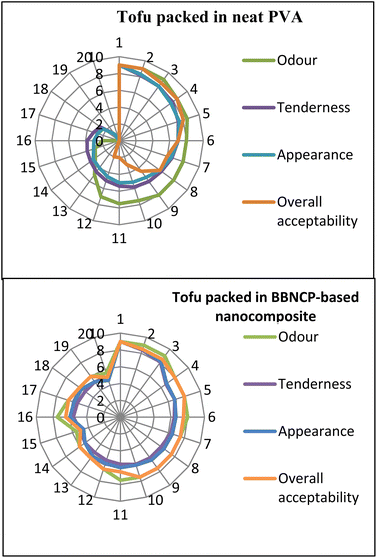
Tofu was packed in BBNCP-based nanocomposite films and stored at 4 °C for the application study, as shown in Fig. 4 (tofu packed on day 1; aged tofu on the 20th day). Tofu packed in neat PVA was used as the control. The shelf life of the tofu packed in the BBNCP-based nanocomposite and control tofu packed in neat PVA was studied for three weeks. The quality analyses, including physiochemical, microbial, and sensory analyses, were performed in triplicates at regular intervals for three weeks.Tofu packaged in BBNCP-based nanocomposites and control tofu packaged in neat PVA were studied for water activity (aw) during the storage period. On day 1, neat PVA and BBNCP-based nanocomposite showed a water activity of 0.956 ± 0.005. After that, on day 7 there was a slight increase to 0.968 ± 0.002 for neat PVA; later, on day 20, the water activity of neat PVA showed a drastic change to 0.098 ± 0.003. In the case of the nanocomposite-based film, the water activity was 0.960 ± 0.001 on day 7 and 0.965 ± 0.002 on day 20. The results indicate a drastic difference in aw between tofu packed in neat PVA and nanocomposite films. In brief, a statistically significant difference P < 0.05 is evident between tofu packaged in neat PVA and the BBNCP-based nanocomposite. According to ref. 20, the inhibition of microbial growth can be reduced by decreasing the moisture content. In the current study, the BBNCP-based nanocomposite packaging material is superior in terms of antimicrobial and antioxidant properties.
Moreover, the crosslinking bond between the BBNCP and PVA in the nanocomposite and the presence of FSEO blocks the passage of the moisture content inside and out of the package. The increase in water activity denotes the hydrolysis of lipids to fatty acids/oxidation of fatty acids.39 Overall, the current study proves that the tofu packed in BBNCP-based nanocomposites enhanced the water activity compared with tofu packed in neat PVA, hence proving and guaranteeing the extension of the shelf life of tofu.
Protein degradation refers to microbial spoilage in food products. The change in protein content of tofu packed in BBNCP-based nanocomposites and control tofu packed in neat PVA during the storage study is shown in Fig. 6. Initially, the protein content of tofu packed in BBNCP-based nanocomposites and control tofu packed in neat PVA was 47.678 ± 0.03%. Later, on day 10, the protein % of tofu packed in BBNCP films was 41.683 ± 0.02%, whereas the control tofu packed in neat PVA showed a decreased protein % of 40.541 ± 0.06%, indicating the initiation of spoilage of tofu packed in neat PVA. On day 20, it was observed that the protein % of the tofu packed in neat PVA films was the lowest at 8.326 ± 1.11%, indicating the microbial deterioration of Tofu. Tofu packed in BBNCP-based nanocomposites showed an acceptable protein percentage (28.207 ± 0.06%) and it was significantly higher than that of tofu packed in neat PVA (P < 0.05). Based on FSSAI standard regulation, 2011,53 the acceptable minimum range of protein % is 8.0. A similar study of soy protein storage at 4 °C reported the degradation of soy protein due to microbial growth.38 The reason for protein degradation in tofu is the excessive growth of microbes, which leads to rehydration and the production of proteolytic enzymes. Then the produced enzymes will break down the bonds between the cross-linked protein present in tofu, leading to the development of free amino acids by hydroxylation.38 Hence, the current study proved that the newly developed nanocomposite exhibited superior barrier properties towards spoilage by microbes.
 | ||
| Fig. 6 Protein % and fat % in tofu packed in BBNCP-based nanocomposites and control tofu packed in neat PVA during the storage study. | ||
Tofu is rich in polyunsaturated fatty acids (PUFA); the fat % during the storage study of tofu packed in BBNCP-based nanocomposites and control tofu packed in neat PVA is shown in Fig. 6. Day 1 shows a fat % of 8.6 ± 0.02 for both the tofu packed in BBNCP-based nanocomposite and control tofu packed in neat PVA. Later, at day 10, the fat % decreased due to lipid peroxidation in tofu, but compared with the control tofu package, the BBNCP-based nanocomposite package showed an improved fat % of 7.33 ± 0.07; which in turn indicated reduced rancidity due to the decreased peroxidation of fat content of tofu packed in BBNCP nanocomposites. Fat with a high polyunsaturated fatty acid content is prone to chemical reactions with oxygen, resulting in oxidative rancidity leading to the development of undesirable off-flavors and odors. The main reason for the improved fat % in the BBNCP-based nanocomposite Tofu package is the efficient antioxidant properties of the active packaging film. FSEO is the main ingredient in the nanocomposite preparation, and FSEO has been reported for its efficiency in improving antioxidant and antimicrobial properties.12 Afterwards, on day 20, tofu packed in BBNCP-based nanocomposite films showed a fat % of 4.00 ± 0.01, which is an improved fat % compared with the control Tofu packed in neat PVA (0.529 ± 0.02%). Overall, there is a significant difference (P < 0.05) in quality parameters of tofu packed in BBNCP-based biocomposites and neat PVA. It means that the tofu packed in neat PVA is much more susceptible to fat peroxidation. The FSSAI regulation,53 reports that the acceptable limit of fat content in tofu is in the range of 2.0–5.0%. In the current study, the results show that the tofu packed in neat PVA exceeds its acceptable limit whereas the tofu packed in nanocomposite falls within the acceptable limit. Hence, the study proved and guaranteed that the prepared nanocomposite efficiently extends the shelf life of tofu.
According to the study of Anbarasu and Vijyalekshmi,36 the tulsi extract-treated tofu showed a decrease in fat peroxidation, and the reason behind the decreased lipid peroxidation is the metal chelating properties of tulsi, which also improves the antimicrobial properties of tofu. Accordingly, the BBNCP-based nanocomposite incorporated with FSEO has efficient antioxidant and antimicrobial properties in the present study. Hence, it can be inferred that the tofu packed in BBNCP–PVA–FSEO–MCNP can synergistically reduce the peroxidation of fat, thereby improving the shelf life of tofu.
In the plot, the different letter is significantly different (P ≤ 0.05) as determined by Tukey's posthoc test (SPSS v.22).
Table 3 shows the results of the textural property study of tofu packed in BBNCP-based nanocomposites and control tofu packed in neat PVA during the storage study. According to the hardness study, on day 1, both the samples had a hardness of 12.403 ± 0.005 N, and after 10 days, the control tofu packed in neat PVA showed a decreased hardness of 10.220 ± 0.001 N. The BBNCP-based tofu package showed a hardness of 10.72 ± 0.015 N, which is five times less compared to the control. The reason behind the improved hardness of the BBNCP-based tofu package is the effective amount of BBNCPs, FSEO, and MCNPs. The BBNCPs have crosslinking properties and hence they reduce the gas exchange through the packaging material; also, their antioxidant and antimicrobial properties can reduce the spoilage of tofu by inhibiting bacterial growth. So, it is evident from the current study that the control tofu package shows a drastic decrease (2.556 ± 0.0152 N) in hardness, indicating complete spoilage, while the tofu packed in the BBNCP-based package shows an improved hardness of 7.226 ± 0.025 N.
| CIELAB color space | Day 1 | Day 5 | Day 10 | Day 15 | Day 20 | |
|---|---|---|---|---|---|---|
| a Data are expressed as mean ± standard deviation (n = 3). In each row, each value followed by a different letter is significantly different (P ≤ 0.05) as determined by Tukey's posthoc test (SPSS v.22). | ||||||
| L* | Control tofu packed in neat PVA | 64.80 ± 0.57a | 57.33 ± 0.09b | 49.42 ± 0.66c | 43 ± 0.25d | 37.31 ± 0.17e |
| BBNCP-based nanocomposite packaged tofu | 64.80 ± 0.57a | 59.15 ± 0.16b | 54.13 ± 0.86c | 50.95 ± 0.06d | 45.03 ± 0.23e | |
| a* | Control tofu packed in neat PVA | 0.11 ± 0.04e | 0.26 ± 0.05d | 0.40 ± 0.04c | 0.55 ± 0.06b | 0.67 ± 0.02a |
| BBNCP-based nanocomposite packaged tofu | 0.11 ± 0.04e | 0.13 ± 0.01d | 0.26 ± 0.02c | 0.38 ± 0.01b | 0.41 ± 0.03a | |
| b* | Control tofu packed in neat PVA | 11.73 ± 0.56e | 21.22 ± 0.44d | 28.33 ± 0.42c | 34.20 ± 0.35b | 43.49 ± 0.46a |
| BBNCP-based nanocomposite packaged tofu | 11.73 ± 0.56e | 13.26 ± 0.30d | 15.00 ± 0.39c | 22.99 ± 0.11b | 25.05 ± 0.24a | |
| Whiteness index | Control tofu packed in neat PVA | 63.01 ± 0.13a | 50.23 ± 0.23b | 43.45 ± 0.41c | 40 ± 0.31d | 35.11 ± 0.25e |
| BBNCP-based nanocomposite packaged tofu | 63.01 ± 0.13a | 58.50 ± 0.06b | 51.04 ± 0.58c | 46.12 ± 0.68d | 44.37 ± 0.03e | |
In the case of the springiness of tofu, both the tofu packed in the BBNCP-based nanocomposite and control tofu packed in neat PVA show a value of 3.251 ± 0.0015 mm on day 1. Whereas on day 10, the control tofu packed in a neat PVA film shows a decreased springiness of 2.386 ± 0.0015 mm.
Compared with the control, the tofu packed in the BBNCP-based nanocomposite shows a more elastic nature, indicating improved freshness at day 10. Later, on the 20th day, there is a steep decrease in the elastic nature of the Tofu packed in neat PVA, which implies spoilage. Tofu packed in a BBNCP-based package shows an effective/efficient keeping quality.
For tofu, chewiness is an essential factor, especially in Indian cooking. The usage/consumption of tofu is expected to be economically favorable. According to the study by Anbarasu and Vijyalekshmi,36 tofu should be more rigid and stiffer during consumption. In this context, the present study of tofu packed in BBNCP-based nanocomposites and control Tofu packed in neat PVA shows a chewiness of 13.265 ± 0.0020 N mm. However, later, day by day, the chewiness of the tofu packed in neat PVA (control) decreased, as shown in Table 2, hence proving the spoilage of Tofu on the 20th day. However, the tofu packed in a BBNCP-based nanocomposite package showed a chewiness of 4.322 ± 0.0025 N mm, which is an acceptable quality range according to the study of Anbarasu and Vijayalekshmi.36 Overall, the BBNCP-based nanocomposite tofu package showed an acceptable texture quality until the 20th day. Hence, the newly prepared packaging material can be used to improve the shelf life of tofu. The reason behind the improved textural properties of tofu is the efficient composition of the packaging material, with BBNCP, FSEO, and MCNP having the ability to improve the antioxidant and antimicrobial properties.35
The color of a food product plays a significant role in influencing consumer acceptability. The changes in color values during storage (at 4 °C) for tofu packed in BBNCP-based nanocomposite films and neat PVA films (control) are outlined in Table 3. Throughout the twenty-day storage period, color monitoring was conducted for tofu packed in BBNCP-based nanocomposite films and neat PVA films. However, tofu packed in a BBNCP-based nanocomposite packaging exhibited no discernible color variation over the 20-day storage, as indicated in Table 3. In contrast, a color change was evident within ten days for the control tofu packed in neat PVA, possibly attributed to a comparably higher spoilage rate, as documented in the literature for paneer.54
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU g−1 on day 20 for tofu packed in BBNCP based nanocomposites. However, the tofu packed in control films showed a drastic increase in the total bacterial count than that packed in BBNCP based nanocomposite films (P < 0.05). The total bacterial count in the control was not within the acceptable limit as per the literature.38 Many studies have reported that the standard quality level for microbial analysis of tofu is not available. However, a comparison between the control tofu package and the BBNCP-based tofu package from the present study shows an improved quality for the tofu packaged in BBNCP-based nanocomposite films . The yeast and mold count for the BBNCP-based nanocomposite tofu package showed an effective/increased keeping quality compared with the control tofu package. On day 1, the yeast and mold count for both BBNCP and control packages was 0 CFU g−1; on the 5th day, it increased to 0.69 ± 0.004 CFU g−1 for the control tofu package. However, for the BBNCP-based package, it was only 0.3 ± 0.002 CFU g−1 on the 5th day, indicating a decreased growth level compared with the control tofu package. The BBNCP based tofu package, on day 20, showed a yeast and mold count of 2.22 ± 0.004 CFU g−1 which is lower compared with that observed in the control tofu package (4.65 ± 0.001 CFU g−1), which indicates the efficiency of the newly developed BBNCP package. In the study by Anbarasu and Vijayalekshmi, 2017,36 the shelf life study of tofu treated with Ocimum sanctum exhibited a yeast and mold count of 2.33 × 105 CFU g−1 on the 7th day itself; in comparison the current study shows the efficiency of the BBNCP packaging in maintaining the quality of tofu.
CFU g−1 on day 20 for tofu packed in BBNCP based nanocomposites. However, the tofu packed in control films showed a drastic increase in the total bacterial count than that packed in BBNCP based nanocomposite films (P < 0.05). The total bacterial count in the control was not within the acceptable limit as per the literature.38 Many studies have reported that the standard quality level for microbial analysis of tofu is not available. However, a comparison between the control tofu package and the BBNCP-based tofu package from the present study shows an improved quality for the tofu packaged in BBNCP-based nanocomposite films . The yeast and mold count for the BBNCP-based nanocomposite tofu package showed an effective/increased keeping quality compared with the control tofu package. On day 1, the yeast and mold count for both BBNCP and control packages was 0 CFU g−1; on the 5th day, it increased to 0.69 ± 0.004 CFU g−1 for the control tofu package. However, for the BBNCP-based package, it was only 0.3 ± 0.002 CFU g−1 on the 5th day, indicating a decreased growth level compared with the control tofu package. The BBNCP based tofu package, on day 20, showed a yeast and mold count of 2.22 ± 0.004 CFU g−1 which is lower compared with that observed in the control tofu package (4.65 ± 0.001 CFU g−1), which indicates the efficiency of the newly developed BBNCP package. In the study by Anbarasu and Vijayalekshmi, 2017,36 the shelf life study of tofu treated with Ocimum sanctum exhibited a yeast and mold count of 2.33 × 105 CFU g−1 on the 7th day itself; in comparison the current study shows the efficiency of the BBNCP packaging in maintaining the quality of tofu.
| Parameters (CFU g−1) | Day 1 | Day 5 | Day 10 | Day 15 | Day 20 |
|---|---|---|---|---|---|
| a Data are expressed as mean ± standard deviation (n = 3). In each row, each value followed by a different letter is significantly different (P ≤ 0.05) as determined by Tukey's posthoc test (SPSS v.22). | |||||
| Control | 2.14 ± 0.007e | 2.18 ± 0.003d | 3.± 0.005c | 3.85 ± 0.002b | 5.96 ± 0.003a |
| Total bacterial count | 2.14 ± 0.009e | 2.50 ± 0.001d | 3.03 ± 0.004c | 3.20 ± 0.007b | 3.84 ± 0.001a |
| Control | 0 | 0.69 ± 0.004d | 2 ± 0.002c | 3.56 ± 0.003b | 4.65 ± 0.001a |
| Yeast and mold count | 0 | 0.3 ± 0.002d | 0.82 ± 0.007c | 1.67 ± 0.005b | 2.22 ± 0.004a |
5 Conclusion
In the present study, nanocellulose particles were prepared from BoxBadam pods to produce a novel nanocomposite for active packaging applications. The characterization studies of the active packaging material showed excellent antioxidant, antimicrobial, mechanical, optical, thermal, barrier, and degradation properties compared to the those reported in previous studies. The storage studies showed that the tofu packed in PVA–BBNCP–FSEO–MCNP-based packaging films had a shelf life of up to 20 days at 4 °C. At the same time, the tofu packaged in neat PVA films exceeded the microbial and biochemical parameter limits on the eighth day itself. Hence, the study suggests the BBNCP–PVA–FSEO–MCNP nanocomposite as an effective packaging material to enhance shelf life of tofu and perishable processed food products for improved quality during storage. Furthermore, the research proposes that the synthesized nanocomposite could be explored for uses in the pharmaceutical and medicinal sectors in addition to the food sector.Ethical approval
The authors declared that the sensory analysis work described has been carried out and approved in accordance with FSSAI (Reg. No. 44/S/FSSAI/2017).Data availability
No data were used for the research described in the article.Author contributions
The manuscript was written through contributions of all authors/all the authors contributed equally.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We sincerely acknowledge CLIF, University of Kerala; SCTMIST, Trivandrum, Kerala; the Research Facility, School of Bioengineering, Departments of Physics and Nanotechnology and the Nanotechnology Research Centre (NRC) at the SRM Institute of Science and Technology (SRMIST). We thank the Department of Biotechnology, Govt. of India, for the financial support (BT/PR48100/BCE/8/1776/2023). We would also like to thank Prof. C. Muthamizchelvan, VC, SRMIST, and Dr M. Vairamani, Chairperson, School of Bioengineering, SRMIST, for their cordial support.References
- A. Dghoughi, F.-E. Nazih, A. Halloub, M. Raji, H. Essabir, M. O. Bensalah, R. Bouhfid and A. E. K. Qaiss, Int. J. Biol. Macromol., 2023, 242, 125077 CrossRef CAS PubMed.
- D. S. Nascimento, A. M. Da Cruz Rodrigues and L. H. M. Da Silva, Food Sci. Technol., 2020, 40, 245–249 CrossRef.
- X. Song, L. Cheng and L. Tan, Food Sci. Technol., 2019, 39, 971–979 CrossRef.
- K. O. Sodeinde, A. M. Ojo, S. O. Olusanya, O. S. Ayanda, A. O. Adeoye, T. M. Dada and O. S. Lawal, Ind. Crops Prod., 2021, 160, 113138 CrossRef CAS.
- N. Neitzel, R. Hosseinpourpia, T. Walther and S. Adamopoulos, Materials, 2022, 15, 4542 CrossRef CAS PubMed.
- A. M. Orwa, R. Kindt, R. Jamnadass and S. Anthony, Agroforestree Database 3.0, a Tree Species Reference and Selection Guide: Tree Seed Suppliers Directory, Botanic Nomenclature for Agroforestry Species Database, 2005 Search PubMed.
- P. P. Das, P. Kalyani, R. Kumar and M. Khandelwal, Sustainable Food Technol., 2023, 1, 528–544 RSC.
- S. Lotfi, H. Ahari and R. Sahraeyan, J. Food Saf., 2019, 39(3), 12625 CrossRef.
- A. D. Santos, A. P. Ingle and M. Rai, Appl. Microbiol. Biotechnol., 2020, 104, 2373–2383 CrossRef PubMed.
- V. Venezia, C. Prieto, Z. Evtoski, C. Marcoaldi, B. Silvestri, G. Vitiello, G. Luciani and J. M. Lagaron, J. Ind. Eng. Chem., 2023, 124, 510–522 CrossRef CAS.
- V. Venezia, M. Verrillo, P. R. Avallone, B. Silvestri, S. Cangemi, R. Pasquino, N. Grizzuti, R. Spaccini and G. Luciani, Biomacromolecules, 2023, 24, 2691–2705 CrossRef CAS PubMed.
- S. Jancy, R. Shruthy and R. Preetha, Int. J. Biol. Macromol., 2020, 142, 63–72 CrossRef CAS PubMed.
- S. Ramesh and P. Radhakrishnan, Appl. Nanosci., 2020, 12, 295–307 CrossRef.
- P. Chawla, K. Sridhar, A. Kumar, P. K. Sarangi, A. Bains and M. Sharma, Int. J. Biol. Macromol., 2023, 242, 124805 CrossRef CAS PubMed.
- S. Ramesh and P. Radhakrishnan, Appl. Surf. Sci., 2019, 484, 1274–1281 CrossRef CAS.
- N. Rüegg, B. Röcker and S. Yildirim, Food Packag. Shelf Life, 2022, 31, 100771 CrossRef.
- W. Wei, Y.-T. Zhang, Q.-S. Huang and B.-J. Ni, Water Res., 2019, 163, 114881 CrossRef CAS PubMed.
- R. Shruthy, S. Jancy and R. Preetha, Int. J. Food Sci. Technol., 2020, 56, 3991–3999 CrossRef.
- C. Xie, F. Wang, Z. He, H. Tang, H. Li, J. Hou, Y. Liu and L. Jiang, Int. J. Biol. Macromol., 2023, 242, 125045 CrossRef CAS PubMed.
- S. Van Nguyen and B.-K. Lee, Int. J. Biol. Macromol., 2022, 207, 31–39 CrossRef CAS PubMed.
- R. Arun, R. Shruthy, R. Preetha and V. Sreejit, Chemosphere, 2022, 291, 132786 CrossRef CAS PubMed.
- S. P. Bangar, W. S. Whiteside, P. Kajla and M. Tavassoli, Int. J. Biol. Macromol., 2023, 243, 125320 CrossRef CAS PubMed.
- E. Kavakebi, A. A. Anvar, H. Ahari and A. A. Motalebi, Food Sci. Technol., 2021, 41, 267–278 CrossRef.
- T. N. Baite, M. K. Purkait and B. Mandal, Int. J. Biol. Macromol., 2023, 235, 123880 CrossRef CAS PubMed.
- S. Roy, R. Priyadarshi, Ł. Łopusiewicz, D. Biswas, V. Chandel and J.-W. Rhim, Int. J. Biol. Macromol., 2023, 239, 124248 CrossRef CAS PubMed.
- M. M. Rahman, S. Afrin, P. Haque, Md. M. Islam, M. S. Islam and Md. A. Gafur, Int. J. Chem. Eng., 2014, 2014, 1–7 CrossRef.
- Y. Sul, P. Ezati and J.-W. Rhim, Int. J. Biol. Macromol., 2023, 246, 125600 CrossRef CAS PubMed.
- B. A. Morris, in Elsevier eBooks, 2016, pp. 259–308 Search PubMed.
- J. C. Alvarez-Zeferino, M. Beltran-Villavicencio and A. Vazquez-Morillas, Open J. Polym. Chem., 2015, 5, 55 CrossRef CAS.
- R. Preetha, N. Jayaprakash and I. B. Singh, Dis. Aquat. Org., 2007, 74, 243–247 CrossRef CAS PubMed.
- M. Fuchigami, A. Teramoto and N. Ogawa, J. Food Sci., 1998, 63, 1054–1057 CrossRef CAS.
- AOAC, Official Methods of Analysis of Association of Official Analytical Chemists, Washington, DC, 18th edn, 2010 Search PubMed.
- AOAC, Official Methods of Analysis of the Association of Official's Analytical Chemists, Association of Official Analytical Chemists (AOAC), Arlington, Virginia, 17th edn, 2003 Search PubMed.
- L. T. Hansen, T. Gill and H. H. Hussa, Food Res. Int., 1995, 28, 123–130 CrossRef CAS.
- G. Himanath, R. Shruthy, R. Preetha and V. Sreejit, ACS Food Sci. Technol., 2021, 1, 1538–1549 CrossRef CAS.
- K. Anbarasu and G. Vijayalakshmi, J. Food Sci., 2007, 72, M300–M305 CrossRef CAS PubMed.
- P. Downes, K. Ito, K. Itō and A. P. H. Association, Compendium of Methods for the Microbiological Examination of Foods, Ignatius Press, 2001 Search PubMed.
- H. S. Yang, M. Chaliha and A. James, Nutr. Food Sci., 2015, 4, 207–215 CrossRef PubMed.
- Z.-S. Liu and S. K. C. Chang, J. Food Process. Preserv., 2008, 32, 39–59 CrossRef CAS.
- S. Sheela Devi, J. Dhanalakshmi and S. Selvi, Int. J. Curr. Biotechnol., 2013, 1, 10–14 Search PubMed.
- M.-C. Li, Q. Wu, K. Song, C. F. De Hoop, S. Lee, Y. Qing and Y. Wu, Ind. Eng. Chem. Res., 2015, 55, 133–143 CrossRef.
- E. B. M. Viana, N. L. Oliveira, J. S. Ribeiro, M. F. Almeida, C. C. E. Souza, J. V. Resende, L. S. Santos and C. M. Veloso, Food Packag. Shelf Life, 2022, 31, 100776 CrossRef CAS.
- P. P. Das, P. Kalyani, R. Kumar and M. Khandelwal, Sustainable Food Technol., 2023, 1, 528–544 RSC.
- F. A. M. Gowsia and J. A. Banday, Prog. Biomater., 2022, 11, 281–295 CrossRef PubMed.
- C. R. Sonar, J. Tang and S. S. Sablani, in Elsevier eBooks, 2022, pp. 307–322 Search PubMed.
- A. Bhatia, R. K. Gupta, S. N. Bhattacharya and H. J. Choi, J. Nanomater., 2012, 1–11 CAS.
- L. B. Williams, D. W. Metge, D. D. Eberl, R. W. Harvey, A. G. Turner, P. Prapaipong and A. T. Poret-Peterson, Environ. Sci. Technol., 2011, 45, 3768–3773 CrossRef CAS PubMed.
- S. M. Mousavi, S. A. Hashemi, S. Salahi, M. Hosseini, A. M. Amani and A. Babapoor, in InTech eBooks, 2018 Search PubMed.
- W. Yoong, W. Norfazilah and W. Ismail, Biointerface Res. Appl. Chem., 2020, 10, 5484–5489 CAS.
- S.-I. Hong, L.-F. Wang and J.-W. Rhim, Food Packag. Shelf Life, 2022, 31, 100784 CrossRef CAS.
- A. S. Sadadekar, R. Shruthy, R. Preetha, N. Kumar, K. R. Pande and G. Nagamaniammai, J. Food Sci. Technol., 2022, 60, 938–946 CrossRef PubMed.
- B. Deepa, E. Abraham, N. Cordeiro, M. Mozetic, A. P. Mathew, K. Oksman, M. Faria, S. Thomas and L. A. Pothan, Cellulose, 2015, 22, 1075–1090 CrossRef CAS.
- FSSAI, https://www.fssai.gov.in/cms/food-safety-and-standards-regulations.php.
- M. Kim, I. Son and J. Han, J. Food Qual., 2004, 27, 27–40 CrossRef CAS.
- M. J. Mukkadan, R. Preetha and V. Sreejit, Int. J. Food Sci. Technol., 2024, 59, 2033–2041 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fb00238e |
| This journal is © The Royal Society of Chemistry 2025 |