Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cold plasma technology for sustainable food production: meeting the United Nations sustainable development goals
Fabiano A. N.
Fernandes
 *a and
Sueli
Rodrigues
*a and
Sueli
Rodrigues
 b
b
aUniversidade Federal do Ceará, Departamento de Engenharia Química, Campus do Pici, Bloco 709, 60440-900 Fortaleza, CE, Brazil. E-mail: fabiano@ufc.br
bUniversidade Federal do Ceará, Departamento de Engenharia de Alimentos, Campus do Pici, Bloco 858, 60440-900 Fortaleza, CE, Brazil. E-mail: sueli@ufc.br
First published on 8th October 2024
Abstract
This review explores the multifaceted contributions of cold plasma technologies to the United Nations Sustainable Development Goals (SDGs). Throughout this examination, we established linkages between various aspects of cold plasma technologies and the SDGs. Furthermore, we elucidated the primary technologies utilized in cold plasma, including dielectric barrier discharge, vacuum, jet, and gliding arc plasma. Additionally, we evaluated cold plasma's contributions, advantages, disadvantages, and limitations. While cold plasma food processing directly addresses Zero Hunger, its impact extends beyond food preservation. This technology holds the potential to promote well-being by facilitating the production of healthy foods and inspiring optimism about the future of sustainable food production. Our exploration of this technology encompassed its role in addressing from Zero Hunger to No Poverty.
Sustainability spotlightThis review explores the multifaceted contributions of cold plasma technologies to the United Nations Sustainable Development Goals (SDGs). Our exploration of this technology will encompass its role in addressing ten SDGs: “Zero Hunger”, “Good health and well-being”, “Affordable and clean energy”, “Decent work and economic growth”, “Industry, innovation, and infrastructure”, “Responsible consumption and production”, “Life below water”, “Life on land”, “Partnerships for the goals”, concluding with “No Poverty”. |
1. Introduction
The 2030 Agenda for Sustainable Development is a comprehensive framework for achieving peace and prosperity for people and the planet. It is a landmark agreement adopted by all member states of the United Nations Organization (UNO) that encompasses seventeen Sustainable Development Goals (SDGs). These goals outline many urgent calls to action for all countries, stressing the breadth of the challenges we face. They acknowledge the interconnected nature of global challenges and emphasize that ending poverty requires improvements in health, education, and work conditions, as well as the pursuit of sustainable economic growth, action on climate change, and the preservation of air, land, and oceans.Despite focusing on transforming the energy system to address climate change, it is important to recognize that achieving all Sustainable Development Goals (SDGs) requires numerous other transformations. In particular, significant changes are needed in the world's food system. Organizations such as the United Nations, the World Food Programme, the Columbia Center on Sustainable Investment, the International Monetary Fund, the World Bank, and others have all highlighted that the global food system is currently in crisis. The world faces significant challenges related to unhealthy diets, unsustainable food production, food waste, poverty in rural communities, and the food system's overall vulnerability to climate change and other crises.1–4
The global food system is highly intricate, involving millions of farmers, farm workers, food processing companies, logistics firms, vendors, employees, and consumers. In addition to this, there is an extremely diverse food production system due to varied food cultures and traditions. This complex system needs to transform into a sustainable food system. New methods of production and processing will be necessary, incorporating more technology. Cleaner, low-energy consuming, and sustainable technologies such as cold plasma, ultrasound, microwaves, UV light, high-pressure processing (HPP), pulsed electric field (PEF), and ozone are suitable for replacing older and less sustainable technologies.5–12
The sustainability of the food industry is an increasingly urgent concern as the demand for food grows. Food manufacturers and retailers are facing complex challenges, including the responsible use of land, improving production processes to reduce waste and conserve resources, finding eco-friendly packaging solutions, and minimizing emissions during transportation and distribution. Thoughtful and deliberate progress toward achieving greater sustainability is not only necessary but vital for the future of the food industry and the well-being of the planet.
Many conscious persons are adopting sustainable food practices, such as purchasing food from local farmers and producers, consuming fruits and vegetables when they are in their season, properly storing food, reducing the intake of meat, opting for products in eco-friendly packaging, and using energy-efficient cooking. These small steps individuals can adopt are a start for a sustainable lifestyle, but major steps need to be taken by the agro and food industry.
As previously cited, many technologies are available to bridge the gap between petro-based conventional production and more eco-friendly production. One such technology is cold plasma. Plasma is often referred to as the fourth state of matter. It is an ionized gas comprising several excited atomic, molecular, ionic, and radical species. These coexist with electrons, gas atoms, positive and negative ions, free radicals, molecules in the ground or excited state, and quanta of electromagnetic radiation such as UV photons and visible light. Its complexity makes it an interesting technology for sanitization, nutritional improvement, sensory quality improvement, and pesticide and allergenic removal. Above all, plasma runs exclusively on electrical energy that can come from renewable and sustainable sources.
This review explores this technology's role in addressing how cold plasma can contribute to the United Nations Organization (UNO) Sustainable Development Goals (SDGs) from Zero Hunger to No Poverty.
2. Cold plasma technology applied to food processing
Several articles and reviews have focused on the principles and cold plasma equipment used in food processing.13–22 Plasmas can be categorized as thermal or non-thermal. Thermal plasmas are characterized by ionization and chemical processes that are predominantly influenced by extremely high temperatures, which can exceed 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 K. These thermal plasma systems are employed for applications that demand intense heat, such as in coating technology, welding, cutting, and the treatment of hazardous wastes.23
000 K. These thermal plasma systems are employed for applications that demand intense heat, such as in coating technology, welding, cutting, and the treatment of hazardous wastes.23
In non-thermal plasmas, many plasma species are generated around room temperature. Non-thermal plasma uses energy more efficiently to achieve better chemical selectivity. In non-thermal plasmas, the electron temperature governs ionization and chemical processes.23 Plasma is in a metastable state with a roughly zero net electrical charge.24,25
Electrical discharges, such as corona, radiofrequency (RF), glow, pulsed corona, dielectric barrier (DBD), microwave, and plasma jet, can generate cold or non-thermal plasma. Among these plasma technologies, DBD plasma is especially interesting for food applications because it can operate at atmospheric pressure, use air as a gas source, and enable the continuous processing of materials.26 Glow discharge plasma has been used in food and materials processing; however, its application requires a vacuum, which makes it more complicated to use with some volatile materials. Plasma jet is more attractive for some biomedical applications than food processing due to the small surface areas that can be treated.27 Gliding arc and corona discharge plasma systems rely on forming voltaic arcs, which can degrade the quality of several food products. Both operate in the transition between non-thermal and thermal processing.
2.1. Dielectric barrier discharge plasma
Dielectric barrier discharges consist of two electrodes at different potentials separated by a dielectric material. The dielectric barrier restricts the current flow, preventing the formation of an electric arc and allowing the gas to ionize in the space between the electrodes (Fig. 1). The system is called dielectric barrier discharge (DBD) or surface dielectric barrier discharge (SDBD) depending on the electrodes and barrier configurations. When a high voltage is applied to one of the electrodes while the other is grounded, the gas in the gap experiences an increase in voltage, and the gas ionizes.28DBD plasma is suitable for in-package treatments, where plasma is generated directly inside sealed packages. These treatments eliminate the risk of postprocess contamination. Expensive gases, such as argon and helium or inexpensive nitrogen, can be employed, serving also as modified atmospheres in the package.29
2.2. Glow discharge plasma
In a glow-discharge plasma, gas is ionized inside a chamber maintained under a vacuum. The gas is fed into the vacuum chamber and ionizes due to the application of high voltage between two electrodes (Fig. 2). A vacuum pump maintains the vacuum inside the chamber and continually purges the ionized gas, ensuring a steady gas flow through the equipment. The power source in glow discharge plasma usually works at high frequency, using radio and microwave frequencies to ionize the gas.2.3. Jet plasma
Jet plasma is usually generated by a dielectric barrier discharge produced inside a cylindrical system at atmospheric pressure with a gas flow passing through (Fig. 3). The ground electrode, a rod, is placed in the middle of the tube, which drives the plasma to the environmental air. The high-voltage electrode, a ring, is placed over the tube.30Jet plasma systems can be found in various configurations. For example, the internal rod-shaped electrode (ground electrode) can be replaced by a second ring-shaped electrode positioned before the positive electrode. In this configuration, the electrodes are separated and insulated by a dielectric material to prevent arc formation outside the tube.31
Although numerous studies have examined jet plasma in food applications, its use in industrial-scale applications is not feasible because it can only treat very small surface areas on the order of mm2. However, the concept of jet plasma has been revitalized for use in bubbling columns to treat liquid food or produce plasma-activated water. In this new concept, the plasma is generated inside a tube and then introduced into a vessel or reactor containing a liquid.
2.4. Gliding arc plasma
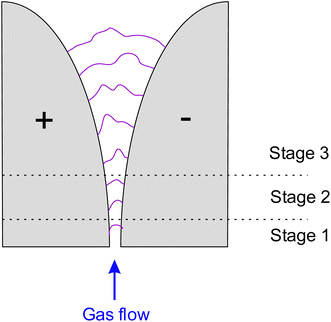
The gliding arc plasma is created between two angled electrodes (Fig. 4). Gas flows between the electrodes, generating the arc. The electrodes are typically knife-edged and insulated with glass or polymeric walls. When a sufficient electric field is applied across the gap, the arc is formed at the shortest distance between the electrodes, reaching a thermal equilibrium state (Stage 1). As the gas flows downstream, the arc elongates, and the voltage increases while the gas temperature remains constant, creating a transitional phase known as the quasi-equilibrium state (Stage 2). Elongation of the arc also increases heat loss to the surroundings. When the heat loss from the arc to the surroundings exceeds the power compensated to the arc from the power supply, the arc shifts from equilibrium to a non-equilibrium state involving rapid arc quenching (Stage 3). The arc extinguishes when the power supply cannot sustain an arc anymore, and the arc is reignited at the shortest gap between the electrodes (Stage 1).23Gliding arc discharge plasma is classified as warm plasma because of its intermediate energy density and gas temperature between cold and thermal plasmas. This technology may be interesting for drying applications, but few studies have been conducted on this equipment.
2.5. Corona discharge plasma
Corona discharge plasma is found at the interface between non-thermal and thermal arc processing. Corona discharges are relatively low-power electrical discharges at or near atmospheric pressure.32 They involve electric arcs flowing between positive and ground electrodes, typically needle-shaped. Some variations use an array of needles instead of the traditional two-needle system (Fig. 5).Corona discharges generate an environment consisting of photons, electric fields, charged species, glow discharge, streamer discharge, radicals, and other species. Due to this harsh environment, food processing is usually carried out downstream from the high-voltage gap, not between the gap. Some systems add a ground screen between the electrodes and the processed food sample. The ground screen intercepts all charged species and most electric flux densities, allowing only the neutral species to pass through the ground screen electrode.33
2.6. Plasma reactive species
The chemical reactions in cold plasma are complex, involving several species with lifetimes ranging from nanoseconds to hours. The reactive species and their concentration in cold plasma depend on several factors: the gas, the configuration of the plasma source, the power input to the gas, the duration of treatment, and the humidity levels.34The chemical reaction occurring during plasma application include electronic impact processes, such as attachment, dissociation, excitation, ionization, and vibration, ion–ion neutralization, ion–molecule reactions, penning ionization, quenching, three-body neutral recombination, and photo processes, such as photo-absorption, photo-emission, and photo-ionization.
Several gases can be used in plasma applications, including air, helium, argon, nitrogen, and oxygen. Noble gases, such as helium and argon, were used in the early development stages of plasma technology, but recent developments apply atmospheric air, synthetic air, or nitrogen.
Helium and argon have low ionization energy and are more easily ionized in an electric field. These inert gases do not produce active radicals involved in chemical reactions. Thus, they are used only where electrons and ion collisions are important, such as in sanitization processes.
Most reactions between plasma species occur in the gas phase. These gas-phase reactions can occur with electrons or between neutral and ionic species. Plasma produced in oxygen and nitrogen generates a large number of free radicals. Free radicals are unstable molecules that react rapidly with other molecules by electron donation reducing radicals, electron acceptance oxidizing radicals, hydrogen abstraction, self-annihilation, addition, and disproportionation.
Air plasma is a potent source of reactive oxygen species (ROS) and reactive nitrogen species (RNS). Air plasma free radicals include hydroxyl (HO˙), superoxide (O2˙−), alkoxyl (RO˙), peroxyl (ROO˙), and nitric oxide (NO˙). Air plasma non-radical species include ozone (O3), hydrogen peroxide (H2O2), and singlet oxygen (1O2).
Among the ROS, hydroxyl radical is the most powerful oxidant, followed by ozone and alkoxyl radical.35 These three species quickly react with nearby molecules upon formation, resulting in unavoidable damage that needs to be addressed through repair processes.36 Thus, forming high concentrations of hydroxyl radicals, ozone, and alkoxyl radicals can lead to fast and significant changes to the food product. Free radicals, such as superoxide, nitric oxide, and lipid hydroperoxides, are less reactive and may allow better control of the chemical changes induced by plasma application.
Living organisms are constantly exposed to reactive oxygen species produced as by-products of metabolism, respiration, stress, and oxidation. Antioxidant defenses regulate the amount of reactive oxygen species in living organisms. An imbalance between the reactive oxygen species and antioxidant defenses causes oxidative stress. Plasma treatment generates a large number of reactive oxygen species in a short period of time, causing extreme oxidative stress that the antioxidant defense system cannot easily control.
In processed food products, where there is no longer a living organism, the reactive oxygen species will cause similar effects but without any defense system to regulate the concentration of these species. Thus, the effect of plasma application in non-living cells may be more significant in terms of oxidation reactions. In minimally processed food products, where there is no living organism but enzymes may still be active, oxidative stress may encounter a limited defense response, with different outcomes regarding chemical changes.
This means that the chemical changes induced by plasma treatment in a strawberry may not have the same outcome as those in strawberry juice or a dried strawberry. The analysis of plasma-induced changes in some food products must consider its defense system.
In living organisms, the oxidative stress caused by reactive oxygen species deregulates cellular functions, leading to oxidative reactions, tissue injury, and accelerated cellular death.37,38 Plasma technology has been used as a non-thermal sanitization technology. However, besides its use in sanitization, food processing can benefit from the defense system's response to reactive oxygen species, such as sugar reduction, since sugar is the primary energy source needed to keep the defense system active.
The reaction of oxygen plasma species with carbon-based compounds generates alkoxy radical (RO˙) and peroxyl radical (ROO˙). Alkoxy radicals and peroxyl radicals can be generated by the oxidation of lipids, proteins, amino acids, and nucleic acid.39 These compounds are good oxidizing agents and tend to accept electrons.40
Water species may be produced in gaseous and liquid phases. A series of reactions occur between water and electrons, producing several radicals and ionic species. Most of the species produced in water are classified as reactive oxygen species.
Gaseous plasma species can react with solid surface molecules, changing the surface's physical and chemical properties. One of the main reactions is surface etching, where a diatomic plasma species reacts with the solid surface, removing atoms and molecules from the surface.
2.7. Half-life of plasma species
The stability of the plasma species can be correlated to their half-life, which is the time required for the number of plasma species to be reduced to half of its initial value.41Table 1 presents the half-life of several ROS and RNS.Most ROS and RNS exist for less than a second and cease to exist when the plasma application finishes. The exceptions concern ozone, nitrogen dioxide, and carboxyl radicals (not shown in the table).
Current legislation worldwide requires that approved food processing technology not produce harmful substances and that no harmful substances may come into contact with the food. Approval of plasma technology for food processing currently faces this barrier since it is almost impossible to assess the toxicology of species that exist for less than a second or detect all types of species generated during plasma treatment. Only a change in the legal understanding of the irradiation processes and how to assess food safety for these processes will allow the commercial use of plasma for food production.
3. Role of cold plasma technology on SDG #2: zero hunger
The second Sustainable Development Goal (SDG #2) demands the eradication of hunger, the attainment of food security, the improvement of nutrition, and the promotion of sustainable agriculture. In 2022, the United Nations emphasized that wars, conflicts, climate change, pandemics, and growing inequalities significantly impact global food security. Presently, 10% of the world's population suffers from hunger, and almost 30% lack regular access to healthy, nutritious food.42 War and regional conflicts have impacted important agriculture-based countries, leading to food shortages for the world's most impoverished individuals.43–45Addressing the issue of hunger and undernourishment requires a comprehensive and constructive approach. It involves tackling various interconnected factors such as food production, distribution logistics, government policies, and financial considerations.46 As highlighted earlier, a significant portion of produced food goes to waste, emphasizing the need for improved efficiency in the global food supply chain. While it may seem that the world produces enough food to end hunger, it's crucial to recognize that a more nuanced and constructive approach is necessary to address these complex challenges. This entails implementing proactive solutions that improve the entire food production and distribution system to ensure food reaches those in need.
The upcoming sections and subsections will discuss how cold plasma technologies align with and support the goals established by the United Nations.
3.1. Effect of cold plasma on the shelf life of the products
Cold plasma has the potential to alleviate hunger by ensuring the availability of safe food with extended shelf life and heightened nutritional benefits. Additionally, it helps make food more affordable while playing a role in environmental conservation through reduced energy usage.Cold plasma technology plays a crucial role in ensuring the safety and quality of food products. By using cold plasma, food products can be effectively sanitized, which helps to minimize the presence of harmful pathogens and extend the shelf life of the food.
The main mechanism for microbial inactivation may vary among plasma systems. In systems that produce UV radiation, this may be the primary inactivation mechanism because UV photons transmitted in plasma discharge correspond to the absorption band of nucleic acids.
When plasma discharge happens in humid air, NO˙ and OH˙ radicals are formed. High concentrations of NO˙ lower the pH of the environment due to the formation of HNO2 and HNO3. If acidification is rapid (pH decreasing from 7.0 to 4.0 in up to 20 min), bacteria and other microbes have no time to adapt and die.
The OH˙ and O˙ radicals can damage the cell wells. High concentrations of OH˙ radicals act as a strong oxidizer, inducing rapid degradation of organic matter, and can oxidize the unsaturated fatty acids in the cell walls' lipid bilayer, oxidize amino acid side changes, and cleave peptide bonds. O˙ radicals can oxidize proteins and be involved in the etching process. The OH˙ radicals combine with H2O2 in an aqueous medium, which is also an oxidizing agent with high sterilization activity. These reactive species induce alterations in enzymatic activities, peroxidation of lipids, protein degradation, and DNA alterations. Reactive nitrogen species cause oxidative stress, damaging several cellular structures, including lipids, membranes, proteins, and DNA.48
Charged particles can inactivate bacterial cells through the rupture of the cytoplasmic membrane. The electrostatic force caused by charge accumulation on the cell membrane's outer surface can overcome the membrane's tensile strength, rupturing it.49 With their irregular surfaces, Gram-negative bacteria are more prone to this kind of membrane rupture.
Microbial inactivation can persist during storage because microbial cells are exposed to the active species generated by the plasma for an extended time. This continuous inactivation of microorganisms may occur for hours or even days during storage. The progressive inactivation may be caused by the generation of active antimicrobial compounds, such as hydrogen peroxide (H2O2), hydroperoxy radicals (OOH˙), and peroxynitrite (ONOO−), which have extended lifetimes, compared to ˙OH and 1O2.14,50
Some intrinsic factors are important regarding the efficacy of plasma treatment. Surface properties, such as surface roughness, are highly related to the effectiveness of microbial inactivation. Studies on the inactivation efficacy of Enterobacter aerogenes in fruit systems showed a significantly lower inactivation (2.52 ± 0.46 log CFU per surface) in a rough surface (spiny gourd) than in a smooth surface (grape tomatoes; 5.31 ± 0.14 log CFU per surface).51 Furthermore, accelerated death of microbial cells occurs in food matrixes with low sugar content because they do not have sufficient energy sources to trigger their defense mechanism against plasma-induced oxidative stress effectively.50
Most information regarding sanitization using cold plasma concerns Salmonella, Campylobacter, Escherichia coli, and Listeria monocytogenes, which are responsible for most major food-borne outbreaks. Other microorganisms have been studied, and their inactivation follows similar pathways. Gram-negative bacteria are, in general, inactivated more easily than Gram-positive bacteria. Gram-positive bacteria have relatively more stable and thicker cell walls and present slower initial reduction in CFU and higher resistance to cold plasma treatment than gram-negative bacteria. However, it must be noted that due to environmental and processing factors, in some cases, Gram-positive bacteria may inactivate faster than Gram-negative.
In general, increasing the treatment time and voltage has a positive impact on reducing the bacterial population. Applying higher gas flow rates in gliding arc plasma increased bacterial inactivation.52
The effectiveness of plasma sanitization depends on many factors, such as the plasma system, operating conditions, food matrix, and packaging. Fig. 6 presents the maximal log CFU reductions observed for some bacteria, showing the potential of plasma systems on some microorganisms. Plasma was very effective in sanitizing some health-treating bacteria, such as Salmonella enterica, Listeria monocytogenes, and Escherichia coli.60–64
 | ||
| Fig. 6 Maximal log CFU reductions observed for 11 species of bacteria reported in the literature.49,53–59 | ||
Fungi are eukaryotic (nucleated), single-celled, or complex multicellular micro-organisms larger than bacteria, thus usually more difficult to inactivate. The application of plasma breaks the conidiophores and vesicles of fungi, resulting in cell leakage and loss of viability. Plasma also damages the cell walls and cell membrane structures, resulting in cytoplasm leakage. Inactivation of fungi is particularly efficient when ROS are produced during plasma application.65–67
Fungi tend to display progressive inactivation during storage due to the presence of extended-life plasma species such as hydrogen peroxide (H2O2), hydroperoxy radicals (OOH), and peroxynitrite (ONOO−), which remain active as antimicrobial ingredients for about five days.50
Although fungi tend to be more difficult to inactivate, some studies have demonstrated that yeast and mold may be more susceptible to DBD plasma than most bacteria. This observation is supported by the fact that reactive species and ultraviolet radiation generated by plasma can diffuse more easily in fungi than in bacteria and directly react with the organelles inside the fungal cell.68Fig. 7 shows the maximal log CFU reductions reported for some fungi.
 | ||
| Fig. 7 Maximal log CFU reductions observed for 4 species of fungi reported in the literature.65,69,70 | ||
Cold plasma provides satisfactory sanitization efficiency compared to other technologies. Many studies reported log reductions of 5 and above for several microorganisms, including the main microorganisms of concern, such as Listeria, Salmonella, and E. coli. Such log reductions are comparable to those achieved by pasteurization71–73 but with the advantage of not compromising food nutritional quality. Compared to other non-thermal technologies, cold plasma provides better sanitization than ultrasound and UV light.74–79 Direct comparison is not available with many technologies, but cold plasma performs similarly to high-pressure processing and ozone treatment on the sanitization of several microorganisms.9,80–85 An advantage of cold plasma concerns fungi's inactivation, often higher than other technologies.
Plasma treatment destroys cell walls, making them permeable and allowing the leakage of intracellular components. The treatment may also destroy the DNA of a fungal spore, which confirms a decaying CD spectrum signature and loss of band intensity after gel electrophoresis.48 When the plasma active species density is not sufficiently high to destroy spore structures, physiological changes occur due to apoptosis, causing an increase in the accumulation of lipid bodies.67 The inactivation of fungi spores usually requires the application of plasma for several minutes (∼5 to 20 min).
 | ||
| Fig. 8 Maximal log CFU reductions observed for microorganisms in powdered foods reported in the literature.86–88 | ||
Sanitizing dried and powdered foods is often challenging because most sanitization technologies work better with foods containing water or food products immersed in water. Cold plasma, ozonation, and high-pressure processing are usually more effective when sanitizing low-water-activity foods than other technologies. Most thermal technologies present lower efficacy due to problems related to uniform heat distribution in solid particles and irregularly shaped solids.
4. Role of cold plasma technology on SDG #3: good health and well-being
Goal #3 of the United Nations' Sustainable Development Goals (SDGs) strives to ensure that people of all ages enjoy healthy lives and well-being. This objective highlights the essential role of food in promoting and sustaining good health. Food is a direct source of a wide range of vital components, including important vitamins, bioactive compounds, sugars, fibers, and other nutrients, all crucial for overall well-being and health. By emphasizing providing access to nutritious and well-balanced food, societies can effectively enhance the health and wellness of their populations, thereby contributing to the achievement of Goal #3.4.1. Effect on nutrient composition
Free radicals, ions, photons, and other plasma species can also react with nearly all food constituents, affecting the physicochemical (for example, pH, carbohydrate, vitamins, anthocyanidin, and respiration rate) and organoleptic (for example, taste, color, and texture) attributes of food products. The effects will depend on the operating conditions and the processing time. Longer processing or contact times will induce more significant changes in food than very short exposure to plasma.Our group has already written an extensive review of the chemical changes induced by plasma; thus, we will focus on the observed main changes in this review. Details on the chemical mechanisms can be found in Fernandes & Rodrigues.89
Sugar increase is less common but has been reported.90–92 Such an increase is usually related to the depolymerization of starch and oligosaccharides. An increase in fructose and glucose concentration is related to the breaking of sucrose. In fruits, a slight increase in sugar content can also be due to the extraction of sugars from the suspended pulp.
The average chain length of oligosaccharides tends to decrease during plasma processing. Depolymerization of oligosaccharides can occur to varying extents, depending on the type of oligosaccharide and the operating conditions. Typically, larger molecules are more susceptible to depolymerization than smaller molecules. As a result, the concentration of oligosaccharides with a higher degree of polymerization tends to decrease, leading to an increase in the concentration of oligosaccharides with a lower degree of polymerization.93
As with oligosaccharides, starch's molecular structure may change. These changes include depolymerization, cross-linking, formation of functional groups, and grafting of functional groups.94 Cross-linking of starch molecules can occur and increase their molecular weight. A common feature is the increase in amylopectin content. Small starch fragments react with the linear amylose chain, forming a branched structure (amylopectin).94–97
The effects of plasma on other vitamins are still uncertain because very few studies report on vitamins A, B, D, E, and K. The limited reports available have not been able to establish a trend or propose a mechanism.
The concentration of anthocyanins can either increase or decrease. An increase in content is related to the extraction of anthocyanins from the vacuoles to the extracellular space, while a decrease in content is related to oxidative degradation. Although anthocyanins are susceptible to plasma species, the aglycone anthocyanins are less vulnerable to degradation than the aglycon molecule.101
Anthocyanins are located in the cell vacuoles in most plants. When plasma species react, they can break down cell and vacuole membranes, releasing these compounds into the extracellular space, where they can be more easily detected by analytical instruments. Many anthocyanins have strong colors due to their chemical structure containing chromophores, which are chemical groups that absorb light, giving rise to color. Ozone and hydroxyl radicals can cause chromophores' oxidative cleavage, resulting in anthocyanins' decay.102,103
The combined effects of various bioactive compounds determine the antioxidant capacity. Therefore, increasing the total phenolic content or other bioactive chemical groups does not necessarily lead to a higher antioxidant capacity. If the concentration of compounds with high antioxidant capacity decreases while the concentration of compounds with low antioxidant capacity increases, the overall antioxidant capacity may end up being higher due to a greater total concentration of bioactive compounds, but with a lower concentration of antioxidant capacity. Plasma treatment may initially increase a product's antioxidant capacity, but prolonged exposure to plasma species can eventually decrease this capacity.
The most susceptible amino acid residues to oxidation are residues containing sulfur because of the high reactivity of the thiol group105 Oxidation of thiol groups can occur in two different pathways. The first pathway involves the reaction of the side chains with free radicals, forming the radicals. The thiyl radical further reacts with other thiol groups or with oxygen to produce peroxy radicals (RSOO˙). The second pathway involves the reaction of the side chains with hydrogen peroxide or singlet oxygen, generating sulfur-containing acids.
In meat products, the unsaturated fatty acyl groups of phospholipids are the first to be oxidized by plasma species. Adverse plasma effects can be higher in pork than in ruminants because pork has higher amounts of unsaturated fatty acids.106 Lipid changes are caused by hydroperoxyl radicals, superoxide radicals, and singlet oxygen that reacts with unsaturated fatty acids. Contact with light and the absence of plant or animal defense mechanisms increase lipid peroxidation. The presence of myoglobin in meat products also enhances lipid peroxidation.
Although changes in food flavor and aroma are usually not desirable, plasma applications open new possibilities for improving the flavor and aroma of many products. Most researchers think of plasma as a sanitization tool. Still, plasma systems can be used with success to mitigate off-flavors, correct flavors and aromas, and provide new sensory experiences to customers.107–109
Most food processing technologies, especially those applied for sanitizing, drying, concentration, and cooking, change food properties and nutritional quality. Thermal processes lead to a series of chemical changes, from Maillard reactions to thermal degradation of compounds. Thus, they often impart the most changes in food. Non-thermal processes tend to induce fewer changes in food, so in practice, little change is sensed by the consumers. High-pressure processing, pulsed UV-light, and UV treatments are the technologies that usually lead to the slightest changes in food.9,10,90,110,111 Ozonation tends to induce the oxidation of several compounds. The changes caused by cold plasma depend on the extent and goal of its application. The generation of free radicals in food activates several enzymatic and non-enzymatic reactions that lead to changes in sensory quality. However, cold plasma offers the opportunity to control the changes that can be used to improve the product. When properly used, cold plasma is a tool for quality control and provides customers new sensory experiences.
5. Role of cold plasma technology on SDG #7: affordable and clean energy
Goal #7 ensures everyone can access affordable, reliable, sustainable, and modern energy. According to the United Nations, approximately 733 million people worldwide lack electricity access. If the current trend continues, over 670 million people will still be without access to electricity in 2030. While significant progress in electrification has occurred over the last three decades, this progress has slowed down in recent years.42Between 2010 and 2019, the annual energy-intensity improvement rate was 1.9%. However, to reach the Sustainable Development Goals (SDGs) target, a higher rate of 3.2% is required. Achieving this improvement rate depends on global progress in energy efficiency. Low energy efficiency in key economic sectors like agriculture, industry, and services is a barrier to everyone's access to energy. Due to energy restrictions and high prices, in 2020, 2.4 billion people still used inefficient and polluting cooking systems.42
Plasma technology, which is utilized in a variety of industrial and scientific applications, operates exclusively on electrical energy. This electrical energy can be sustainably sourced from renewable resources, including wind, hydroelectric, and solar energy. This reliance on renewable energy sources reduces environmental impact and contributes to a more sustainable approach to utilizing plasma technology.
The comparison between plasma and conventional technologies' energy consumption remains unclear. Theoretically, plasma systems are expected to consume less energy than traditional thermal processes. While some lab-scale plasma systems consume less than 500 W, lab-scale thermal processes may consume over 2000 W. However, a simple assessment of equipment energy is too basic because it fails to account for processing time and total energy requirements. Future research should focus on better assessing this aspect of plasma processing.
Some articles have assessed plasma processes as pretreatments for drying fruits,112–114 showing the higher production efficiency and lower energy consumption of the processes employing plasma as pretreatment. For example, Du et al.112 applied a DBD pretreatment to wolfberry, attaining a 50.6% reduction in drying time with a consequent 46% reduction in energy consumption. In a similar approach, Khudyakov et al.113 applied cold filamentary plasma pretreatment to apple slices, attaining between 20 and 30% reduction in the total energy consumption for drying apples. A similar trend was observed when using gliding arc plasma as pretreatment for saffron drying, as carried out by Tabibian et al.,114 where total energy consumption was reduced by up to 40%.
Elmizadeh et al.115 have studied plasma processes as pretreatments for extracting bioactive compounds and their impact on energy consumption. In their work, plasma pretreatments reduced energy consumption (kW h g−1) to extract tanshinone from Salvia sp. by 12 and 19% when associated with heat reflux and ultrasonic extraction.
The few works that investigated the energy consumption of plasma systems concluded that plasma is less energy-consuming than its counterpart processes. However, further investigation on this subject is required if pilot or industrial-scale plasma systems are to be planned.
The few articles and industrial reports on energy consumption of non-thermal systems prevent us from accurately comparing technologies. Based on lab- and small-scale equipment, it is possible to indicate that cold plasma, ozonation, UV-light, and pulsed-UV light are the less energy-consuming technologies. High-pressure processing, ultrasound, and pulsed electric fields usually require more energy than the latter.
6. Role of cold plasma technology on SDG #8: decent work and economic growth
Goal #8 focuses on promoting decent work and economic growth. Plasma processing supports two specific targets within Goal #8: Target 8.2, which aims to achieve higher levels of economic productivity through diversification, technological upgrading, and innovation, and Target 8.3, which aims to promote development-oriented policies that support productive activities, decent job creation, entrepreneurship, creativity, and innovation, and encourage the formalization and growth of micro-, small- and medium-sized enterprises, including through access to financial services.6.1. Labor issue
The production of food usually requires a lot of labor. Facilities of various sizes rely on workers to pick and cut food, operate machinery, transport products, ensure quality, handle packaging, and carry out other tasks. While larger facilities may have more automation, they still need many employees. Smaller facilities typically depend on manual labor and are very labor-intensive.Lower production costs directly impact food prices, as reduced costs translate to lower consumer prices. As a result, consumers can afford to purchase more products and subsequently demand increased production from the industry. This higher demand fuels economic growth, creating a favorable supply and demand cycle.
The food industry is highly responsive to energy prices because they affect several stages of food production, including transportation, processing, and distribution. Higher fuel and fertilizer prices directly impact food production costs. Plasma technology, as described in Section 5 (“Affordable and clean energy”), can help diminish the energy consumption for food production.
6.2. Food shelf-life and enzyme inactivation
A second aspect to consider relates to food shelf life. The shelf life of food products and their associated costs are interconnected. Foods with longer shelf lives are usually wasted less. This means these longer-lasting foods will have more chance of being produced, sold, and consumed. The cost of disposing of spoiled and unsold food is accounted for in the selling price. Thus, foods with longer shelf life will need to account less for disposal costs and will probably cost less. Plasma processing helps economic growth by reducing the cost of food production and food disposal.The time that food remains edible and nutritious depends on several factors, such as temperature, moisture, the growth rate of spoilage-causing organisms, and low enzymatic degradation. In Section 3 (“Zero Hunger”), we have addressed sanitization and reduction in the growth rate of spoilage-causing organisms. In this section, we will discuss how plasma processing can reduce enzymatic degradation, one of the main problems of extending shelf life.
Enzyme inactivation is a critical process to prevent unwanted changes in the composition and quality of food products during storage. However, enzymatic activity can also lead to the deterioration of food products, causing changes in color, flavor, aroma, and nutritional content.
Traditionally, heat treatments have been the go-to method for enzyme inactivation. While heat treatments are effective at deactivating enzymes, they can also significantly impact the sensory characteristics and nutritional properties of food products.
In recent years, plasma technology has emerged as a promising alternative for enzymatic inactivation in the food industry. This innovative technology has shown satisfactory results in deactivating enzymes while mitigating the adverse effects on color, flavor, aroma, and nutritional quality, thus offering a potential solution to the limitations associated with heat treatments.
The more accepted mechanism of plasma-induced enzyme inactivation is primarily related to the compromise of enzymatic activity and the unfolding of secondary structure. The reactive species produced during plasma application can cause the breakdown of specific bonds and chemical modifications of the side chains of the amino acids.
Reactive oxygen species, mainly atomic oxygen and OH radicals, cleave peptide bonds, oxidize amino acid side chains, and form protein–protein cross-linkages.116 The oxidation or breakdown of one or more amino acids in a protein may affect the enzyme function, reducing or inactivating enzyme activity. Plasma species such as hydroxyl (OH˙), superoxide anion (O2−), hydrogen peroxide (HOO˙), and nitric oxide (NO˙) radicals lead to chemical changes in amino acid residues such as cysteine, phenylalanine, tyrosine, and tryptophan, decreasing the enzymatic activity.117
Endogenous enzymatic browning is a factor that leads to the decay of food produced, causing undesirable or unacceptable organoleptic attributes for consumption. PPO and POD were partially inactivated due to chemical modification of their secondary structures, a reduction in the alpha-helix content, and an increase in the beta-sheet region. Reactive nitrogen species can act as quenchers of tryptophan fluorescence, reducing POD activity. The quenching effect increases the oxidation of tryptophan during plasma application, increasing the inactivation of POD.
The stability of the PPO and POD depends on the food matrix. Reports on different matrices show that sometimes PPO is more stable than POD, and sometimes POD is more stable than PPO.
The inactivation of PPO increases with increasing voltage, processing time, and oxygen concentration in the plasma gas. The inactivation of PPO may occur in two stages. A fast inactivation first stage followed by a slower inactivation stage.118 The two-stage inactivation behavior may be related to the penetration depth of plasma species. The first rapid stage occurs while the plasma species have good accessibility to the enzymes, while the second slow stage occurs when the accessibility to the enzymes worsen.119
The inactivation of POD also increases with increasing voltage, processing time, and oxygen concentration in the plasma gas. It may also occur in two stages, but the inactivation obtained in the first stage is usually lower than for PPO. The first stage is followed by a much slower second inactivation stage, which may not lead to total inactivation of enzyme activity.118
Most research on enzymatic inactivation refers to PPO and POD. At the same time, very few reports exist on the effects of plasma on other enzymes such as ALS, SOD, PAX, and several others. Further research is needed on this subject, but publishing articles on enzyme inhibition is not considered innovative by many journals, driving away much important information that has not been investigated.
There is still a lack of comprehensive understanding about how enzymes are activated and inactivated in relation to ultrasound, electromagnetic fields, pressure, and UV. Therefore, it is currently not possible to predict how several enzymes will behave when exposed to these factors. Thermal processes and cold plasma expose enzymes to heat and free radicals, respectively, and there is a much better understanding of enzyme behavior in these conditions. Depending on the treatment goal, cold plasma has an advantage over thermal processes. For instance, thermal treatment tends to break down enzymes related to the production of phenolics, while cold plasma tends to activate them, resulting in generally healthier products.
7. Role of cold plasma technology on SDG #9: industry, innovation, and infrastructure
Goal #9 pertains to building resilient infrastructure, promoting inclusive and sustainable industrialization, and fostering innovation. The United Nations states that higher-technology industries are more resilient in crises than their lower-tech counterparts. The food market is dominated by a few large industries and thousands of medium-sized and small local industries. The large industries mainly use conventional and high technology, producing a limited variety of products for the global market. On the other hand, the medium and small-sized industries offer a wider range of food products but typically do not employ high technology. Within SDG #9, these medium and small-sized industries must adopt new technologies to improve their products, lower prices, and increase productivity.It is undoubtful that cold plasma poses high innovation in food processing with several advantages and minimal disadvantages. However, its use in food processing has still not been approved. The major problem with the legislation is that plasma is considered an irradiation process. Most countries only allow irradiation processes when electron beams, X-rays, and gamma rays are employed. Other forms of irradiation are considered in a case-by-case scenario, and very few processes have been approved, such as using UV light to treat mushrooms.
Given the broad spectrum of potential outcomes arising from the interaction of the plasma system, the product, the working gas, and the treatment process, it is imperative to conduct thorough experimental validation for each specific manufacturing process. This rigorous approach is crucial for obtaining regulatory approval in most countries. To gain approval, it is necessary to demonstrate that cold plasma treatment of food is effective, safe, and consistent. If the process results in a noticeable change in nutrition, performance, or appearance, setting it apart from untreated food, further testing and data collection are required to ensure safety. The data collection for regulatory review and approval must be conducted using industrial equipment, not at the laboratory scale. Hence, a substantial capital investment is needed to construct the equipment and gather regulatory data. This means that it is unlikely that small and medium-sized industries will endeavor with this technology before large economic groups, at least with the current legislation.
There has been some confusion in Europe regarding implementing cold plasma technologies. The Directive 1999/2/EC of the European Community does not provide a clear definition of “ionizing radiation,” which has created uncertainty about the scope of the legislation concerning new decontamination technologies such as cold plasma and ultraviolet treatment. This uncertainty has led to discrepancies among member states. Some believe these new technologies are not listed in the directive because they are forbidden. In contrast, others believe that they are authorized but not listed due to not falling under the definition of ionizing radiation and, therefore, not within the directive's scope.
Current legislation may jeopardize the use of cold plasma in the food industry, but advances in its approval are happening. Cold plasma has been approved for the treatment of food packaging, seed treatment for agriculture, and in several medical applications, such as skin treatment and cancer tumor treatments. These new approvals may change future political views on cold plasma for food processing.
8. Role of cold plasma technology on SDG #12: responsible consumption and production
Goal #12 emphasizes the need for sustainable consumption and production patterns to prevent climate change, biodiversity loss, and pollution, as stated in the 2030 Agenda. In every country, a significant amount of food is lost or wasted each day. According to the United Nations, 13% of the world's food is lost after harvesting and before it reaches retail markets. These losses occur during harvest, transport, storage, and processing. Another 17% of food is wasted at the consumer level, including in our homes, grocery stores, and restaurants. This means that a total of 30% of all food produced is never eaten.428.1. Sensory quality
Responsible consumption is often linked to food's sensory quality. Food that looks, smells, and tastes better is less likely to be wasted. Plasma technology can improve food's sensory characteristics, making it more appealing for purchase and consumption.Significant color changes may occur in food due to pigment oxidation. Products subjected to plasma in environments with high ozone levels may be brighter due to a superficial bleaching effect. A significant decrease in chlorophyll (>15%) may occur after plasma application. Chlorophyll degradation occurs due to a reaction with oxygen radicals produced during plasma application. Oxygen radicals induce membrane breakdown, intensifying in minimally processed fruits and vegetables and chlorophyll oxidation.120
Chlorophyll presents multiple breakdown pathways. Some pathways maintain the porphyrin ring intact, leading to colored compounds. Further oxidation of these intermediates produces discolored products due to cleavage of the porphyrin ring.121 Chemical modification of the periphery of the porphyrin ring leads to green-colored compounds. The most common degradation of this kind occurs through the removal of phytol.122 The removal of chelated magnesium from the center of the porphyrin ring leads to the formation of olive-brown and olive-yellow-colored products named pheophytins. In this pathway, the chelated magnesium is substituted by a hydrogen ion in the porphyrin ring; thus, plasma systems that tend to hydrogenate molecules may lead to faster chlorophyll degradation.
Acid conditions promote this degradation pathway, and it is the main degradation pathway of chlorophylls.122 Plasma treatment tends to decrease the pH. Thus, application of pH control may be required to avoid excess degradation of chlorophylls. Alkalizing agents, such as sodium bicarbonate, disodium glutamate, hexametaphosphate, sodium hydroxide, and magnesium hydroxide, are commonly used to raise pH during green vegetable processing.123 Despite the significant decrease in chlorophyll, studies in kiwis showed that a reduction of this pigment was insufficient to change the fruit's color.124
Slight color changes may occur with the changes induced in phenolic compounds. Non-flavonoid phenolic compounds are colorless or slightly yellow. They do not directly contribute to the color, but their presence stabilizes and enhances the color of flavonoid compounds due to co-pigmentation. Flavonoid compounds include anthocyanins, flavonols, and flavan-3-ols, which display intense colors. Among this group, changes in anthocyanin content have the highest effect on color.125 Plasma treatment can alter the phenolic compounds in food, either increasing or decreasing specific compounds depending on the technology and operating conditions used. This treatment may lead to changes in color, particularly noticeable in fruits with higher anthocyanin content.
Anthocyanins are natural pigments in fruits and vegetables that display various colors, from orange to blue. Many anthocyanins have strong colors due to their chemical structure, which contains chromophores. Chromophores are chemical groups that absorb light, giving rise to color. Ozone and hydroxyl radicals can cause chromophores' oxidative cleavage, resulting in anthocyanins' decay and color.102,103
Plasma treatment partially converts anthocyanins into their aglycone form (anthocyanidins) by cleaving the glycoside. This chemical transformation may slightly alter the color of the product. However, the color stability of anthocyanins depends on the molecular structure of the anthocyanin, concentration, pH, temperature, and the presence of complexing agents; thus, other factors can play a more significant role in color change than the application of plasma.126
Color changes can also occur in anthocyanin-rich solutions due to changes in pH. At different pH levels, anthocyanin can take on different conformations, potentially leading to changes in color. At pH lower than 2, it exists mainly in the form of the red-orange colored flavylium cation. At pH higher than 2, water addition on the pyrylium nucleus converts the anthocyanin into hemiketal and chalcone, which are colorless. Water addition increases when the anthocyanin is linked to a diglycoside. In this case, more colorless products are formed when pH increases.127
However, at a pH higher than 2, fast deprotonation of the most acidic hydroxyl groups forms quinonoids, which are purple-colored. A thermodynamic equilibrium occurs between the colored anthocyanin and quinonoid forms and the colorless hemiketal and chalcone forms. The relative amounts of these forms depend on the anthocyanin structure, glycoside linked to the structure, and the pH.127
Plasma systems that emit more visible and UV radiation can accelerate the breakdown of anthocyanins, leading to the creation of colorless chalcones through photodegradation. However, anthocyanins and their color are less likely to degrade when combined with phenolic acids and flavonols. These compounds efficiently absorb harmful UV radiation and protect the anthocyanins from degradation.127
Heme proteins, such as myoglobin, hemoglobin, and cytochrome c, are responsible for the color of meat products. In meats, myoglobin accounts for more than 70% of the total heme protein.128 Cytochrome c is present in low amounts in meat products and has minimal impact on color. The primary issue with plasma involves systems that produce higher ozone levels, which reacts with the hemoglobin's prosthetic group, disrupting heme into oxidized compounds.129 In such cases, meat color may slightly change.
Myoglobin can be bonded to oxygen, forming the bright red-colored oxymyoglobin (MbFe(II)O2), or not bonded to oxygen, forming the purple-red colored deoxymyoglobin (deoxyMbFe(II)). Oxidation of Fe(II) to Fe(III) leads to the formation of the colorless metmyoglobin (MbFe(III)). Furthermore, peroxynitrite anion (ONO2−) may oxidize the ferrous ion in heme proteins, leading to a color change from red to brown.130
Fruits that undergo plasma treatment may experience either an increase or loss of firmness, which are linked to changes in the fruit surface caused by plasma species. Loss of firmness in some fruits (strawberries, blueberries, and tomatoes) may be related to high levels of ozone in the plasma environment. The loss is seldom caused by the softening of the fruit surface and the damage of the outermost cells as a consequence of internal structure degradation.131–133 Loss of crunchiness was related to the destruction of surface cells by plasma oxidant radicals.134 Minimal changes in texture have been reported for meat products and vegetables (lettuce and cabbage).
Most research indicates that cold plasma does not significantly change the visual aspect of food or produce off-flavors that would prevent someone from buying cold plasma-treated products. On the contrary, research indicates that cold plasma improves the sensory aspects of food. The only exception is in the case of fish treated with cold plasma, where lipid oxidation and the release of undesired odors can occur under certain treatment conditions.
Enhancing food properties through cold plasma technology depends on a thorough understanding of the chemical changes induced by plasma. Over the years, numerous studies have reported the chemical reactions and mechanisms associated with sugars.,91,135 furans, pyrazines, and pyridines,107,136 oligosaccharides,90,92 esters and thioesters,137 amino acids,138,139 terpenes and sesquiterpenes,108,140 styrene141 when subjected to plasma. However, due to the complexity of functional groups in foods, much work must be done to address this technology's capabilities fully.
Cold plasma treatment of fruit juices has effectively altered their aroma and reduced off-flavors and undesirable smells. Research on orange juice demonstrated a significant decrease in the undesired off-flavor caused by 4-terpineol.108,109 Moreover, 4-terpineol was transformed into limonene, the key flavor compound in orange juice. Research on pineapple juice demonstrated the ability to chemically alter ester compounds, reducing the presence of methyl hexanoate, which has a strong sweet flavor, thus enhancing the juice's appeal.137 The plasma treatment conducted using camu-camu juice, an exotic Amazonian fruit, demonstrated the ability to modify the juice's aroma. This was achieved by adjusting the operating conditions of the treatment, resulting in an increase or decrease in the odor activity value of several descriptors.140,142
The processing of ground coffee and ready-to-drink coffee using cold plasma caused various chemical reactions in the compounds contributing to its aroma. Aldehydes and furans underwent significant chemical changes, while pyrroles and pyrazines showed minimal modifications. The excitation frequency and processing time significantly influenced the reactions, particularly regarding C–C bond scission and oxidation processes.107,109,140 The main benefit of plasma processing was the enhancement of desirable nutty notes and the ability to impart green and fruity notes without relying on artificial aromas. The chemical alterations caused by plasma could be useful in addressing aroma defects in coffees.
The plasma-induced changes did not produce toxic or undesired compounds that could compromise its use in flavor modulation and off-flavor mitigation. Cold plasma is an interesting process for engineering natural fruit juices and coffee aromas. The technology may help in quality control to maintain similar aromas between batches and create new sensory experiences for consumers. The primary drawback of plasma processing in some juices and coffee was a general decrease in volatile compounds with high odor value activity, which can result in a less prominent aroma.
It is not possible to modulate and correct flavor with any other technology. Quality flavor control usually relies on blending and adding extracts or artificial flavorings. Thus, cold plasma has a significant advantage over most technologies in modulating flavor without requiring additives.
9. Role of cold plasma technology on SDG #14 and #15: life below water and life on land
Upon entering a processing facility, fruit, vegetables, grains, and other materials are washed and sanitized to remove dirt, pesticides, herbicides, and microorganisms. Typically, washing and sanitization are carried out using chlorine or other sanitizers. However, chlorine and other sanitizers have been linked to negative health effects. Furthermore, the water used for washing containing chlorine and other substances is often not properly treated. It ends up in rivers, lakes, and the ocean, contributing to pollution and impacting aquatic and terrestrial life. While some countries have prohibited the use of chlorine, they still allow the use of other environmentally harmful sanitizers. It is crucial to minimize the use of chlorine and other sanitizers as this is in line with the Sustainable Development Goals (SDGs). Plasma application can be a valuable method in reducing the use of chlorine and other sanitizers.Along with dirt, fruits come to the industry with many endemic pathogens on their surface. Microorganisms such as aerobic bacteria, molds, yeasts, lactic acid bacteria, coliforms, Pseudomonas, Salmonella, Escherichia, and Listeria have been reported on the surface of fruits and vegetables.143–147 Plasma-activated water (PAW) can be used to reduce the amount of microorganisms during washing, preventing the use of chlorine and other chemical sanitizers. Studies with model solutions containing microorganisms proved PAW could be used as a sanitizing agent.148–152 Furthermore, several studies showed that PAW was effective against pathogens and other microorganisms when treating apples,153 eggs,154 and fish.155 Recent studies have evaluated the synergy between PAW and other green processes, such as ultrasound.156,157
Microorganisms are not the only health hazard; herbicides and pesticides used in crops also pose a health risk. Consuming fruits and vegetables without washing or in minimally processed form can lead to the ingestion of pesticides, which is a major concern. Removing pesticides from food products is an effective way to prevent the harmful effects of pesticides on the human body.
Reactive plasma species from cold plasma can degrade pesticide residues (herbicides, insecticides, fungicides) into less toxic compounds. The efficacy of plasma treatment in degrading pesticides is correlated with the concentration of reactive plasma species and the average energy of electrons.158
The degradation mechanism mostly relies on the oxidation of pesticides and herbicides by the reactive plasma species.159 UV irradiation also contributes to the degradation of the organic pesticide molecules.160 Degradation usually increases with increasing processing time and voltage. The type of gas used in the treatment may also enhance degradation.
Fig. 9 presents the efficacy of plasma treatment in reducing pesticides and herbicides in food products, mainly fruits, vegetables, and grains. Until now, only parathion and paraxon showed 100% degradation after plasma treatment.170 High degradation percentages were observed for omethoate, dichlorvos, malathion, and imidacloprid.161,162
 | ||
| Fig. 9 Maximal percentual reductions observed on several pesticides and herbicides after plasma treatment of foods reported in the literature.28,158,161–169 | ||
Plasma performs well against pesticides and herbicides. However, as most of the works refer to the static application of plasma to food products, further research on the application of plasma in continuous systems with better fluidodynamics may improve these numbers.
Compared to other non-thermal technologies, plasma performs satisfactorily in degrading herbicides and pesticides, performing similarly to hydrodynamic cavitation and better than UV light alone. However, other advanced oxidation technologies based on catalysis and electrochemistry, alone or combined with other non-thermal technologies, usually perform better than cold plasma.158,171–175 An advantage of cold plasma is related to the degradation of pesticides and herbicides in food products, which is one of the most effective technologies for this goal.166,167,176
10. Role of cold plasma technology on SDG #17: partnerships for the goals
Goal #17 focuses on “strengthening the means of implementation and revitalizing the global partnership for sustainable development.” Targets 17.7 and 17.8 specifically address science and technology. Target 17.7 aims to promote the development, transfer, dissemination, and diffusion of sustainable technologies. Meanwhile, Target 17.8 calls for increased funding to support science, technology, and innovation.One of the main points that still need to be addressed regarding plasma technology concerns its security. Few works report on plasma-treated food security, which is of utmost importance if plasma technology becomes a reality in the industry. In this aspect, part of the problem is not exactly a lack of research but a lack of interest in journals to publish such research and of reviewers to understand its importance. Many studies are being rejected on the grounds of poor innovation. Food security studies are not novel and, most of the time, are a repetition of an established protocol, which is why they are labeled as non-innovative. However, without this kind of research, the regulatory agencies will not approve plasma technology.
The lack of approval drastically reduces the number of studies on scaling up the technology for food applications. While pilot and large-scale plasma systems for ammonia production, seed, and water treatment are being built and put into commercial practice, plasma processing systems for food processing are not a reality, with few prototypes available.
Nowadays, thousands of articles have evaluated the effects of plasma on all sorts of foods. Many trends have been established, but the chemistry involved is still not completely understood. Research on food-related plasma chemistry should become deeper, trying to correlate the main plasma species to its direct effect on food constituents. This kind of research can help us understand what to expect when treating food and the possible advantages and disadvantages.
11. Conclusions
Cold plasma technology contributes to several Sustainable Development Goals (SDGs). One of plasma technologies' main benefits is the extended shelf life of products and improved microbiological safety, which significantly contributes to achieving “Zero Hunger” (Goal #2). Additionally, plasma processes may enable the production of long-shelf-life healthy foods, promoting well-being (Goal #3). Improved food quality with reduced losses directly impacts responsible production and consumption (Goal #12).Plasma-activated water can be used in the early stages of food processing for washing and sanitizing, which reduces the requirement for sanitizing chemicals. This reduces toxic wastewater and contributes to land and water life (Goals #14 and #15). Since plasma is an electrical system, it can be powered by renewable energy sources and replace some thermal processes, reducing energy consumption (Goal #7).
The innovation brought by cold plasma technologies is directly related to Goal #9. It involves advanced technology, higher productivity, and lower costs in the processing industry, which calls for better-paid workers, more decent workplaces, and, consequently, more economic growth (Goal #8). To support industrial innovation, more research is needed. Therefore, more effective interaction between industry, universities, governments, and research centers will flourish (Goal #17).
Plasma processing can significantly impact various sustainable development goals. It can help reduce hunger, improve food quality, contribute to a better environment, lower industrial costs, save energy, and create more job opportunities. Ultimately, plasma technology may contribute to achieving one of the most important goals: eradicating poverty (Goal #1).
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
F. A. N. Fernandes: conceptualization; data curation; writing – original draft; writing – review & editing. S. Rodrigues: writing – original draft; writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) through the “INCT Frutos Tropicais” grant, and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Finance Code 001).References
- N. Madirossian, G. Espinosa, D. M. Rincón-Rico, E. O'Dwyer, A. Marrero, R. Plekenpol, et al., Handbook for SDG-Aligned Food Companies, Columbia Center on Sustainable Investment, Columbia, 1st edn, 2021, p. 244 Search PubMed.
- G. Laganda, Responding to loss and damage in food systems, Nat. Food., 2023, 4(2), 133–134 CrossRef PubMed.
- L. Hunter, S. Gerritsen and V. Egli, Changes in eating behaviours due to crises, disasters and pandemics: a scoping review, Nutr. Food Sci., 2023, 53(2), 358–390 CrossRef.
- J. Ammann, A. Arbenz, G. Mack, T. Nemecek and N. El Benni, A review on policy instruments for sustainable food consumption, Sustain. Prod. Consum., 2023, 36, 338–353 Search PubMed.
- L. Paniwnyk, Applications of ultrasound in processing of liquid foods: A review, Ultrason. Sonochem., 2017, 38, 794–806 CrossRef CAS PubMed.
- E. Ozen and R. K. Singh, Atmospheric cold plasma treatment of fruit juices: A review, Trends Food Sci. Technol., 2020, 103, 144–151 CrossRef CAS.
- P. J. Asl, V. Rajulapati, M. Gavahian, I. Kapusta, P. Putnik and A. Mousavi Khaneghah, et al., Non-thermal plasma technique for preservation of fresh foods: A review, Food Control, 2022, 134, 108560 CrossRef CAS.
- V. K. Sharma and N. J. D. Graham, Oxidation of amino acids, peptides, proteins by ozone: a review, Ozone Sci. Eng., 2010, 32, 81–90 CrossRef CAS.
- U. Roobab, M. A. Shabbir, A. W. Khan, R. N. Arshad, A. E. D. Bekhit and X. A. Zeng, et al., High-pressure treatments for better quality clean-label juices and beverages: Overview and advances, LWT, 2021, 149, 111828 CrossRef CAS , https://linkinghub.elsevier.com/retrieve/pii/S0023643821009816.
- B. Kruszewski, K. Zawada and P. Karpiński, Impact of High-Pressure Homogenization Parameters on Physicochemical Characteristics, Bioactive Compounds Content, and Antioxidant Capacity of Blackcurrant Juice, Molecules, 2021, 26(6), 1802 CrossRef CAS PubMed.
- R. N. Arshad, Z. Abdul-Malek, U. Roobab, M. A. Munir, A. Naderipour and M. I. Qureshi, et al., Pulsed electric field: A potential alternative towards a sustainable food processing, Trends Food Sci. Technol., 2021, 111, 43–54 CrossRef CAS , https://linkinghub.elsevier.com/retrieve/pii/S0924224421001485.
- F. J. Barba, C. M. Galanakis, M. J. Esteve, A. Frigola and E. Vorobiev, Potential use of pulsed electric technologies and ultrasounds to improve the recovery of high-added value compounds from blackberries, J. Food Eng., 2015, 167, 38–44 CrossRef CAS.
- R. Bahrami, R. Zibaei, Z. Hashami, S. Hasanvand, F. Garavand and M. Rouhi, et al., Modification and improvement of biodegradable packaging films by cold plasma; a critical review, Crit. Rev. Food Sci. Nutr., 2022, 62(7), 1936–1950, DOI:10.1080/10408398.2020.1848790.
- M. Laroussi, I. Alexeff and W. L. Kang, Biological decontamination by nonthermal plasmas, IEEE Trans. Plasma Sci., 2000, 28(1), 184–188 CrossRef , http://ieeexplore.ieee.org/document/842899/.
- C. Chen, D. Liu, A. Yang, H. L. Chen and M. G. Kong, Aqueous Reactive Oxygen Species Induced by He + O2 Plasmas: Chemistry Pathways and Dosage Control Approaches, Plasma Chem. Plasma Process., 2018, 38(1), 89–105 CrossRef CAS.
- R. Thirumdas, C. Sarangapani and U. S. Annapure, Cold Plasma: A novel Non-Thermal Technology for Food Processing, Food Biophys., 2014, 10(1), 1–11 CrossRef.
- M. Turner, Physics of Cold Plasma, in Cold Plasma in Food and Agriculture, ed. Misra N. N., Schluter O. and Cullen P. J., Elsevier, 2016, pp. 17–51, available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128013656000020 Search PubMed.
- K. Zhang, C. A. Perussello, V. Milosavljević, P. J. Cullen, D. W. Sun and B. K. Tiwari, Diagnostics of plasma reactive species and induced chemistry of plasma treated foods, Crit. Rev. Food Sci. Nutr., 2019, 59(5), 812–825, DOI:10.1080/10408398.2018.1564731.
- X. Lv, C. Ren, T. Ma, Y. Feng and D. Wang, An atmospheric large-scale cold plasma jet, Plasma Sci. Technol., 2012, 14(9), 799–801 CrossRef CAS.
- P. J. Cullen, N. N. Misra, L. Han, P. Bourke, K. Keener and C. O'Donnell, et al., Inducing a dielectric barrier discharge plasma within a package, IEEE Trans. Plasma Sci., 2014, 42(10), 2368–2369 Search PubMed.
- L. Bárdos and H. Baránková, Plasma processes at atmospheric and low pressures, Vacuum, 2008, 83(3), 522–527 CrossRef.
- D. Mehta and S. K. Yadav, Recent Advances in Cold Plasma Technology for Food Processing, Food Eng. Rev., 2022, 14, 555–578 CrossRef CAS.
- J. Ananthanarasimhan, R. Lakshminarayana, M. S. Anand and S. Dasappa, Influence of gas dynamics on arc dynamics and the discharge power of a rotating gliding arc, Plasma Sources Sci. Technol., 2019, 28(8), 085012 CrossRef CAS.
- N. N. Misra, B. K. Tiwari, K. S. M. S. Raghavarao and P. J. Cullen, Nonthermal Plasma Inactivation of Food-Borne Pathogens, Food Eng. Rev., 2011, 3(3–4), 159–170 CrossRef.
- N. N. Misra, A. Martynenko, F. Chemat, L. Paniwnyk, F. J. Barba and A. R. Jambrak, Thermodynamics, transport phenomena, and electrochemistry of external field-assisted nonthermal food technologies, Crit. Rev. Food Sci. Nutr., 2018, 58(11), 1832–1863 CrossRef CAS.
- M. Turner, Physics of Cold Plasma, in Cold Plasma in Food and Agriculture, Elsevier, 2016. pp. 17–51 Search PubMed.
- T. M. C. Nishime, A. C. Borges, C. Y. Koga-Ito, M. Machida, L. R. O. Hein and K. G. Kostov, Non-thermal atmospheric pressure plasma jet applied to inactivation of different microorganisms, Surf. Coat. Technol., 2017, 312, 19–24 CrossRef CAS.
- S. K. Pankaj, C. Bueno-Ferrer, N. N. Misra, V. Milosavljevic, C. P. O'Donnell and P. Bourke, et al., Applications of cold plasma technology in food packaging, Trends Food Sci. Technol., 2014, 35, 5–17 CrossRef CAS.
- N. N. Misra, S. Patil, T. Moiseev, P. Bourke, J. P. Mosnier and K. M. Keener, et al., In-package atmospheric pressure cold plasma treatment of strawberries, J. Food Eng., 2014, 125(1), 131–138 CrossRef CAS.
- S. Förster, C. Mohr and W. Viöl, Investigations of an atmospheric pressure plasma jet by optical emission spectroscopy, Surf. Coat. Technol., 2005, 200, 827–830 CrossRef.
- B. L. Sands, B. N. Ganguly and K. Tachibana, A streamer-like atmospheric pressure plasma jet, Appl. Phys. Lett., 2008, 92, 151503 CrossRef.
- J. S. Chang, P. A. Lawless and T. Yamamoto, Corona discharge processes, IEEE Trans. Plasma Sci., 1991, 19(6), 1152–1166 CrossRef CAS.
- S. Xie and P. D. Pedrow, A Novel Active Needle Probe in an Atmospheric Pressure Corona-Based Cold Plasma Reactor with Admixtures of Helium and Dry Air, IEEE Trans. Plasma Sci., 2020, 48, 2418–2430 CAS.
- N. N. Misra and C. Jo, Applications of cold plasma technology for microbiological safety in meat industry, Trends Food Sci. Technol., 2017, 64, 74–86 CrossRef CAS.
- A. Segat, N. N. Misra, A. Fabbro, F. Buchini, G. Lippe and P. J. Cullen, et al., Effects of ozone processing on chemical, structural and functional properties of whey protein isolate, Food Res. Int., 2014, 66, 365–372 CrossRef CAS.
- İ. Gulcin, Antioxidants and antioxidant methods: an updated overview, Arch. Toxicol., 2020, 94(3), 651–715 CrossRef.
- V. Sindhi, V. Gupta, K. Sharma, S. Bhatnagar, R. Kumari and N. Dhaka, Potential applications of antioxidants – A review, J. Pharm. Res., 2013, 7(9), 828–835 CAS.
- R. Apak, M. Özyürek, K. Güçlü and E. Çapanoğlu, Antioxidant Activity/Capacity Measurement. 3. Reactive Oxygen and Nitrogen Species (ROS/RNS) Scavenging Assays, Oxidative Stress Biomarkers, and Chromatographic/Chemometric Assays, J. Agric. Food Chem., 2016, 64(5), 1046–1070 CrossRef CAS.
- O. Augusto, S. Miyamoto, Oxygen radicals and related species, in Principles of Free Radical Biomedicine, ed. Pantopoulos K. and Schipeer H. M., Nova Science Publishers, New York, 2011, pp. 19–42 Search PubMed.
- M. Gutowski and S. Kowalkzyk, A study of free radical chemistry: their role and pathophysiological significance, Acta Biochim. Pol., 2013, 60, 1–16 CrossRef CAS.
- C. Papuc, G. V. Goran, C. N. Predescu and V. Nicorescu, Mechanisms of Oxidative Processes in Meat and Toxicity Induced by Postprandial Degradation Products: A Review, Compr. Rev. Food Sci. Food Saf., 2017, 16(1), 96–123 CrossRef CAS PubMed.
- United Nations SESASD, The 17 Goals, 2023, https://sdgs.un.org/goals.
- H. T. Ben and H. El Bilali, Impacts of the Russia-Ukraine War on Global Food Security: Towards More Sustainable and Resilient Food Systems?, Foods, 2022, 11(15), 2301 CrossRef PubMed.
- M. Alsabri, A. Alhadheri, L. M. Alsakkaf and J. Cole, Conflict and COVID-19 in Yemen: beyond the humanitarian crisis, Glob. Health, 2021, 17(1), 83 CrossRef.
- J. Sowers and E. Weinthal, Health and Environmental Tolls of Protracted Conflicts in the Middle East and North Africa, Curr. Hist., 2021, 120(830), 339–345 CrossRef.
- L. Amoroso, Post-2015 Agenda and Sustainable Development Goals: Where Are We Now? Global Opportunities to Address Malnutrition in all Its Forms, Including Hidden Hunger, in Hidden Hunger: Strategies to Improve Nutrition Quality, ed. Biesalski H. K. and Birner R., Karger, Basel, 2018, pp. 45–56 Search PubMed.
- R. Mandal, A. Singh and A. Pratap Singh, Recent developments in cold plasma decontamination technology in the food industry, Trends Food Sci. Technol., 2018, 80(July), 93–103 CrossRef CAS.
- H. Lee, J. E. Kim, M. S. Chung and S. C. Min, Cold plasma treatment for the microbiological safety of cabbage, lettuce, and dried figs, Food Microbiol., 2015, 51, 74–80 CrossRef CAS.
- B. Surowsky, A. Fröhling, N. Gottschalk, O. Schlüter and D. Knorr, Impact of cold plasma on Citrobacter freundii in apple juice: Inactivation kinetics and mechanisms, Int. J. Food Microbiol., 2014, 174, 63–71, DOI:10.1016/j.ijfoodmicro.2013.12.031.
- A. Starek, J. Pawłat, B. Chudzik, M. Kwiatkowski, P. Terebun and A. Sagan, et al., Evaluation of selected microbial and physicochemical parameters of fresh tomato juice after cold atmospheric pressure plasma treatment during refrigerated storage, Sci. Rep., 2019, 9(1), 1–11 CrossRef CAS PubMed.
- I. Joshi, D. Salvi, D. W. Schaffner and M. V. Karwe, Characterization of Microbial Inactivation Using Plasma-Activated Water and Plasma-Activated Acidified Buffer, J. Food Prot., 2018, 81(9), 1472–1480 CrossRef CAS PubMed , https://meridian.allenpress.com/jfp/article/81/9/1472/175126/Characterization-of-Microbial-Inactivation-Using.
- A. Mai-Prochnow, M. Clauson, J. Hong and A. B. Murphy, Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma, Sci. Rep., 2016, 6(1), 38610 CrossRef CAS.
- M. Moreau, M. G. J. Feuilloley, N. Orange and J. L. Brisset, Lethal effect of the gliding arc discharges on Erwinia spp, J. Appl. Microbiol., 2005, 98(5), 1039–1046 CrossRef CAS PubMed.
- D. Ziuzina, L. Han, P. J. Cullen and P. Bourke, Cold plasma inactivation of internalised bacteria and biofilms for Salmonella enterica serovar Typhimurium, Listeria monocytogenes and Escherichia coli, Int. J. Food Microbiol., 2015, 210, 53–61 CrossRef PubMed.
- D. Ziuzina, L. Han, P. J. Cullen and P. Bourke, Cold plasma inactivation of internalised bacteria and biofilms for Salmonella enterica serovar Typhimurium, Listeria monocytogenes and Escherichia coli, Int. J. Food Microbiol., 2015, 210, 53–61, DOI:10.1016/j.ijfoodmicro.2015.05.019.
- A. S. Chiper, W. Chen, O. Mejholm, P. Dalgaard and E. Stamate, Atmospheric pressure plasma produced inside a closed package by a dielectric barrier discharge in Ar/CO2 for bacterial inactivation of biological samples, Plasma Sources Sci. Technol., 2011, 20(2), 025008 CrossRef.
- I. Albertos, A. B. Martín-Diana, P. J. Cullen, B. K. Tiwari, S. K. Ojha and P. Bourke, et al., Effects of dielectric barrier discharge (DBD) generated plasma on microbial reduction and quality parameters of fresh mackerel ( Scomber scombrus ) fillets, Innovat. Food Sci. Emerg. Technol., 2017, 44, 117–122 CrossRef CAS , https://linkinghub.elsevier.com/retrieve/pii/S1466856416302399.
- H. I. Yong, H. Lee, S. Park, J. Park, W. Choe and S. Jung, et al., Flexible thin-layer plasma inactivation of bacteria and mold survival in beef jerky packaging and its effects on the meat's physicochemical properties, Meat Sci., 2017, 123, 151–156 CrossRef CAS PubMed , https://linkinghub.elsevier.com/retrieve/pii/S0309174016303242.
- A. Patange, D. Boehm, C. Bueno-Ferrer, P. J. Cullen and P. Bourke, Controlling Brochothrix thermosphacta as a spoilage risk using in-package atmospheric cold plasma, Food Microbiol., 2017, 66, 48–54 CrossRef CAS , https://linkinghub.elsevier.com/retrieve/pii/S0740002016307948.
- S. Khatami, G. B. Pour, S. F. Aval and M. Amini, Cold Atmospheric Plasma Brush Effect on Population Reduction of Different Bacterial Spectrums, Plasma Chem. Plasma Process., 2023, 43(5), 1131–1147 CrossRef CAS.
- Y. J. Park, S. Y. Kim and W. J. Song, Inactivation of Salmonella Typhimurium and Listeria monocytogenes on buckwheat seeds through combination treatment with plasma, vacuum packaging, and hot water, J. Appl. Microbiol., 2023, 134(11), lxad272 CrossRef.
- B. Abdoli, M. H. Khoshtaghaza, H. Ghomi, M. A. K. Torshizi, S. A. Mehdizadeh and G. Pishkar, et al., Cold atmospheric pressure air plasma jet disinfection of table eggs: Inactivation of Salmonella enterica, cuticle integrity and egg quality, Int. J. Food Microbiol., 2024, 410, 110474 CrossRef CAS PubMed.
- A. Pandiscia, P. Lorusso, A. Manfredi, G. Sánchez, V. Terio and W. Randazzo, Leveraging Plasma-Activated Seawater for the Control of Human Norovirus and Bacterial Pathogens in Shellfish Depuration, Foods, 2024, 13(6), 850 CrossRef CAS.
- J. Y. Han, S. C. Min, H. S. Lee, S. Eom, S. Ryu and S. Cho, et al., Pilot-scale plasma activated water treatment for decontaminating romaine lettuce and steamed rice cakes, LWT, 2024, 200, 116176 CrossRef CAS.
- B. G. Dasan, M. Mutlu and I. H. Boyaci, Decontamination of Aspergillus flavus and Aspergillus parasiticus spores on hazelnuts via atmospheric pressure fluidized bed plasma reactor, Int. J. Food Microbiol., 2016, 216, 50–59 CrossRef CAS PubMed , https://linkinghub.elsevier.com/retrieve/pii/S0168160515301197.
- Q. Lu, D. Liu, Y. Song, R. Zhou and J. Niu, Inactivation of the Tomato Pathogen Cladosporium fulvum by an Atmospheric-Pressure Cold Plasma Jet, Plasma Processes Polym., 2014, 11(11), 1028–1036 CrossRef CAS.
- K. Panngom, S. H. Lee, D. H. Park, G. B. Sim, Y. H. Kim and H. S. Uhm, et al., Non-Thermal Plasma Treatment Diminishes Fungal Viability and Up-Regulates Resistance Genes in a Plant Host, PLoS One, 2014, 9(6), e99300 CrossRef PubMed.
- B. J. Park, D. H. Lee, J. C. Park, I. S. Lee, K. Y. Lee and S. O. Hyun, et al., Sterilization using a microwave-induced argon plasma system at atmospheric pressure, Phys. Plasmas, 2003, 10(11), 4539–4544 CrossRef CAS.
- C. Pignata, D. D'Angelo, D. Basso, M. C. Cavallero, S. Beneventi and D. Tartaro, et al., Low-temperature, low-pressure gas plasma application on Aspergillus brasiliensis, Escherichia coli and pistachios, J. Appl. Microbiol., 2014, 116(5), 1137–1148, DOI:10.1111/jam.12448.
- U. Schnabel, R. Niquet, O. Schlüter, H. Gniffke and J. Ehlbeck, Decontamination and Sensory Properties of Microbiologically Contaminated Fresh Fruits and Vegetables by Microwave Plasma Processed Air (PPA), J. Food Process. Preserv., 2015, 39(6), 653–662, DOI:10.1111/jfpp.12273.
- R. K. Kesavan, S. Gogoi and P. K. Nayak, Influence of thermosonication and pasteurization on the quality attributes of kutkura (Meyna spinosa) juice, Appl. Food Res., 2023, 3(1), 100268 CrossRef CAS.
- V. Santhirasegaram, Z. Razali, D. S. George and C. Somasundram, Comparison of UV-C treatment and thermal pasteurization on quality of chokanan mango (Mangifera indica L.) juice, Food Bioprocess Technol., 2015, 94, 313–321 CAS.
- T. F. F. Silveira, M. Cristianini, G. G. Kuhnle, A. B. Ribeiro, J. Teixeira-Filho and H. T. Godoy, Anthocyanins, non-anthocyanin phenolics, tocopherols and antioxidant capacity of açaí juice (Euterpe oleracea) as affected by high pressure processing and thermal pasteurization, Innovative Food Sci. Emerging Technol., 2019, 55, 88–96 CrossRef.
- M. C. de Moraes Motta Machado, B. M. Lepaus, P. C. Bernardes and J. F. B. de São José, Ultrasound, Acetic Acid, and Peracetic Acid as Alternatives Sanitizers to Chlorine Compounds for Fresh-Cut Kale Decontamination, Molecules, 2022, 27(20), 7019 CrossRef CAS PubMed.
- J. F. B. d. São José and M. C. D. Vanetti, Application of ultrasound and chemical sanitizers to watercress, parsley and strawberry: Microbiological and physicochemical quality, LWT–Food Sci. Technol., 2015, 63, 946–952 CrossRef.
- A. T. Mustapha, C. Zhou, R. Amanor-Atiemoh, T. A. A. Ali, H. Wahia and H. Ma, et al., Efficacy of dual-frequency ultrasound and sanitizers washing treatments on quality retention of cherry tomato, Innovat. Food Sci. Emerg. Technol., 2020, 62, 102348 CrossRef CAS.
- J. F. Brilhante São José and M. C. Dantas Vanetti, Effect of ultrasound and commercial sanitizers in removing natural contaminants and Salmonella enterica Typhimurium on cherry tomatoes, Food Control, 2012, 24(1–2), 95–99 CrossRef.
- T. Koutchma, UV light for processing foods, Ozone-Sci Eng., 2008, 30, 93–98 CrossRef CAS.
- K. Krishnamurthy, J. C. Tewari, J. Irudayaraj and A. Demirci, Microscopic and spectroscopic evaluation of inactivation of Staphylococcus aureus by pulsed UV light and infrared heating, Food Bioprocess Technol., 2010, 3, 93–104 CrossRef.
- P. A. Klockow and K. M. Keener, Safety and quality assessment of packaged spinach treated with a novel ozone-generation system, LWT–Food Sci. Technol., 2009, 42(6), 1047–1053 CrossRef CAS , https://linkinghub.elsevier.com/retrieve/pii/S0023643809000589.
- A. T. Mustapha, C. Zhou, H. Wahia, R. Amanor-Atiemoh, P. Otu and A. Qudus, et al., Sonozonation: enhancing the antimicrobial efficiency of aqueous ozone washing techniques on cherry tomato, Ultrason. Sonochem., 2020, 64, 105059 CrossRef PubMed.
- T. V. Fonteles, M. K. d. A. Barroso, E. d. G. Alves Filho, F. A. N. Fernandes and S. Rodrigues, Ultrasound and Ozone Processing of Cashew Apple Juice: Effects of Single and Combined Processing on the Juice Quality and Microbial Stability, Processes, 2021, 9(12), 2243 CrossRef CAS.
- F. Liu, X. Zhang, L. Zhao, Y. Wang and X. Liao, Potential of high-pressure processing and high-temperature/short-time thermal processing on microbial, physicochemical and sensory assurance of clear cucumber juice, Innovat. Food Sci. Emerg. Technol., 2016, 34, 51–58 CrossRef CAS.
- S. F. V. M. Evelyn, High pressure processing pretreatment enhanced the thermosonication inactivation of Alicyclobacillus acidoterrestris spores in orange juice, Food Control, 2016, 62, 365–372 CrossRef.
- R. M. Uckoo, G. K. Jayaprakasha, J. A. Somerville, V. M. Balasubramaniam, M. Pinarte and B. S. Patil, High pressure processing controls microbial growth and minimally alters the levels of health promoting compounds in grapefruit (Citrus paradisi Macfad) juice, Innovat. Food Sci. Emerg. Technol., 2013, 18, 7–14 CrossRef CAS.
- J. E. Kim, D. U. Lee and S. C. Min, Microbial decontamination of red pepper powder by cold plasma, Food Microbiol., 2014, 38, 128–136, DOI:10.1016/j.fm.2013.08.019.
- J. E. Kim, Y. J. Oh, M. Y. Won, K. S. Lee and S. C. Min, Microbial decontamination of onion powder using microwave-powered cold plasma treatments, Food Microbiol., 2017, 62, 112–123 CrossRef CAS PubMed , https://linkinghub.elsevier.com/retrieve/pii/S0740002015301908.
- C. Hertwig, K. Reineke, J. Ehlbeck, D. Knorr and O. Schlüter, Decontamination of whole black pepper using different cold atmospheric pressure plasma applications, Food Control, 2015, 55, 221–229 CrossRef CAS , https://linkinghub.elsevier.com/retrieve/pii/S0956713515001462.
- F. A. N. Fernandes and S. Rodrigues, Cold Plasma Processing on Fruits and Fruit Juices: A Review on the Effects of Plasma on Nutritional Quality, Processes, 2021, 9, 2098 CrossRef CAS.
- E. G. Alves Filho, P. J. Cullen, J. M. Frias, P. Bourke, B. K. Tiwari and E. S. Brito, et al., Evaluation of plasma, high-pressure and ultrasound processing on the stability of fructooligosaccharides, Int. J. Food Sci. Technol., 2016, 51, 2034–2040 CrossRef CAS.
- T. R. B. Farias, E. G. Alves Filho, L. M. A. Silva, E. S. De Brito, S. Rodrigues and F. A. N. Fernandes, NMR evaluation of apple cubes and apple juice composition subjected to two cold plasma technologies, LWT–Food Sci. Technol., 2021, 150, 112062 CrossRef CAS , https://linkinghub.elsevier.com/retrieve/pii/S0023643821012159.
- F. D. L. Almeida, W. F. Gomes, R. S. Cavalcante, B. K. Tiwari, P. J. Cullen and J. M. Frias, et al., Fructooligosaccharides integrity after atmospheric cold plasma and high-pressure processing of a functional orange juice, Food Res. Int., 2017, 102, 282–290 CrossRef CAS PubMed.
- F. D. L. Almeida, R. S. Cavalcante, P. J. Cullen, J. M. Frias, P. Bourke and F. A. N. Fernandes, et al., Effects of atmospheric cold plasma and ozone on prebiotic orange juice, Innovative Food Sci. Emerging Technol., 2015, 32, 127–135 CrossRef CAS.
- F. Zhu, Plasma modification of starch, Food Chem., 2017, 232, 1–7, DOI:10.1016/j.foodchem.2017.04.024.
- P. Bie, H. Pu, B. Zhang, J. Su, L. Chen and X. Li, Structural characteristics and rheological properties of plasma-treated starch, Innovative Food Sci. Emerging Technol., 2016, 34, 196–204, DOI:10.1016/j.ifset.2015.11.019.
- G. A. Marenco-Orozco, M. F. Rosa and F. A. N. Fernandes, Effects of multiple-step cold plasma processing on banana (Musa sapientum) starch-based films, Packag. Technol. Sci., 2022, 35, 589–601 CrossRef CAS.
- C. Y. Lii, C. D. Liao, L. Stobinski and P. Tomasik, Behaviour of granular starches in low-pressure glow plasma, Carbohydr. Polym., 2002, 49, 499–507 CrossRef CAS.
- D. A. Jacobo-Velázquez, G. B. Martinéz-Hernández, S. C. Rodriguez, C. M. Cao and L. Cisneros-Zevallos, Plants as biofactories: Physiological role of reactive oxygen species on the accumulation of phenolics antioxidants in carrot tissue underwounding and hyperoxia stress, J. Agric. Food Chem., 2011, 59, 6583–6593 CrossRef PubMed.
- Z. Herceg, D. B. Kovačević, J. G. Kljusurić, A. R. Jambrak, Z. Zorić and V. Dragović-Uzelac, et al., Gas phase plasma impact on phenolic compounds in pomegranate juice, Food Chem., 2016, 190, 665–672 CrossRef CAS PubMed.
- K. Robards, P. D. Prenzler, G. Tucker, P. Swatsitang and W. Glover, Phenolic compounds and their role in oxidative processes in fruits, Food Chem., 1999, 66, 401–436 CrossRef CAS.
- F. Grzegorzewski, S. Rohn, L. W. Kroh, M. Geyer and O. Schluter, Surface morphology and chemical composition of lamb's lettuce (Valerianella locusta) after exposure to a low-pressure oxygen plasma, Food Chem., 2010, 122, 1145–1152 CrossRef CAS.
- C. Sarangapani, G. O'Toole, P. J. Cullen and P. Bourke, Atmospheric cold plasma dissipation efficiency of agrochemicals on blueberries, Innovative Food Sci. Emerging Technol., 2017, 44, 235–241 CrossRef CAS.
- B. K. Tiwari, K. Muthukumarappan, C. P. O'Donnell and P. J. Cullen, Kinetics of freshly squeezed orange juice quality changes during ozone processing, J. Agric. Food Chem., 2008, 56, 6416–6422 CrossRef CAS PubMed.
- M. N. Lund, M. Heinonen, C. P. Baron and M. Estévez, Protein oxidation in muscle foods: a review, Mol. Nutr. Food Res., 2011, 55, 83–95 CrossRef CAS.
- W. Zhang, S. Xiao and D. U. Ahn, Protein oxidation: basic principles and implication for meat quality, Crit. Rev. Food Sci. Nutr., 2013, 53, 1191–1201 CrossRef CAS PubMed.
- M. Enser, K. Hallett, B. Hewitt, G. A. J. Fursey and J. D. Wood, Fatty acid composition of English beef, lamb and pork at retail, Meat Sci., 1996, 42, 443–456 CrossRef CAS.
- S. Rodrigues and F. A. N. Fernandes, Changing Ready-to-Drink Coffee Aroma Using Dielectric Barrier Discharge Plasma, Processes, 2022, 10(10), 2056 CrossRef CAS.
- S. Rodrigues and F. A. N. Fernandes, Glow Discharge Plasma Processing for the Improvement of Pasteurized Orange Juice's Aroma and Off-Flavor, Processes, 2022, 10, 1812 CrossRef CAS.
- S. Rodrigues and F. A. N. Fernandes, Effect of Dielectric Barrier Discharge Plasma Treatment in Pasteurized Orange Juice: Changes in Volatile Composition, Aroma, and Mitigation of Off-flavors, Food Bioprocess Technol., 2023, 16(4), 930–939, DOI:10.1007/s11947-022-02976-0.
- F. González-Cebrino, R. Durán, J. Delgado-Adámez, R. Contador and R. Ramírez, Changes after high-pressure processing on physicochemical parameters, bioactive compounds, and polyphenol oxidase activity of red flesh and peel plum purée, Innovat. Food Sci. Emerg. Technol., 2013, 20, 34–41 CrossRef.
- F. V. M. Silva and A. Sulaiman, Control of Enzymatic Browning in Strawberry, Apple, and Pear by Physical Food Preservation Methods: Comparing Ultrasound and High-Pressure Inactivation of Polyphenoloxidase, Foods, 2022, 11, 1942 CrossRef CAS PubMed.
- Y. Du, H. Wang, S. Yuan, H. Yu, Y. Xie and Y. Guo, et al., Dielectric barrier discharge plasma pretreatment: A cleaner new way to improve energy efficiency and quality of wolfberry drying, J. Cleaner Prod., 2024, 450, 141951 CrossRef.
- D. Khudyakov, M. Sosnin, I. Shorstkii and C. O. R. Okpala, Cold filamentary microplasma pretreatment combined with infrared dryer: Effects on drying efficiency and quality attributes of apple slices, J. Food Eng., 2022, 329, 111049 CrossRef CAS.
- S. A. Tabibian, M. Labbafi, G. H. Askari, A. R. Rezaeinezhad and H. Ghomi, Effect of gliding arc discharge plasma pretreatment on drying kinetic, energy consumption and physico-chemical properties of saffron (Crocus sativus L.), J. Food Eng., 2020, 270, 109766 CrossRef CAS.
- A. Elmizadeh, S. A. H. Goli and M. Rahimmalek, Application of cold plasma pretreatment to improve the extraction efficiency of tanshinone compounds from Salvia subg. Perovskia root, Ind. Crops Prod., 2023, 204, 117337 CrossRef CAS.
- B. S. Berlett and E. R. Stadtman, Protein oxidation in aging, disease, and oxidative stress, J. Biol. Chem., 1997, 272, 20313–20316 CrossRef CAS PubMed.
- E. Takai, K. Kitano, J. Kuwabara and K. Shiraki, Protein inactivation by low temperature atmospheric pressure plasma in aqueous solution, Plasma Processes Polym., 2012, 9, 77–82 CrossRef CAS.
- B. Surowsky, A. Fischer, O. Schüeter and D. Knorr, Cold plasma effects on enzyme activity in a model food system, Innovat. Food Sci. Emerg. Technol., 2013, 19, 146–152 CrossRef CAS.
- Z. Xiong, T. Du, X. Lu, Y. Cao and Y. Pan, How deep can plasma penetrate into a biofilm?, Appl. Phys. Lett., 2011, 98, 221503 CrossRef.
- Z. L. Queiroz, E. Jacob-Lopes and M. Roca, Catabolism and bioactive properties of chlorophylls, Curr. Opin. Food Sci., 2019, 26, 94–100 CrossRef.
- J. L. Lafeuille, S. Lefèvre and J. Lebuhotel, Quantitation of Chlorophylls and 22 of Their Colored Degradation Products in Culinary Aromatic Herbs by HPLC-DAD-MS and Correlation with Color Changes During the Dehydration Process, J. Agric. Food Chem., 2014, 62(8), 1926–1935 CrossRef CAS PubMed.
- J. L. Lafeuille, S. Lefèvre and J. Lebuhotel, Quantitation of Chlorophylls and 22 of Their Colored Degradation Products in Culinary Aromatic Herbs by HPLC-DAD-MS and Correlation with Color Changes During the Dehydration Process, J. Agric. Food Chem., 2014, 62(8), 1926–1935 CrossRef CAS.
- N. Koca, F. Karadeniz and H. S. Burdurlu, Effect of pH on chlorophyll degradation and colour loss in blanched green peas, Food Chem., 2007, 100(2), 609–615 CrossRef CAS.
- I. Ramazzina, A. Berardinelli, F. Rizzi, S. Tappi, L. Ragni and G. Sacchetti, et al., Effect of cold plasma treatment on physico-chemical parameters and antioxidant activity of minimally processed kiwifruit, Postharvest Biol. Technol., 2015, 107, 55–65 CrossRef CAS.
- H. Kelebek, A. Canbas, M. Jourdes and P. L. Teissedre, Characterization of colored and colorless phenolic compounds in Öküzgözü wines from Denizli and Elazig regions using HPLC-DAD–MS, Ind. Crops Prod., 2010, 31(3), 499–508 CrossRef CAS.
- P. Markakis, Stability of anthocyanins in foods, in Anthocyanins as Food Colors, ed. Markakis P., Academic Press, New York, 1982, pp. 163–180 Search PubMed.
- C. Malien-Aubert, O. Dangles and M. J. Amiot, Color Stability of Commercial Anthocyanin-Based Extracts in Relation to the Phenolic Composition. Protective Effects by Intra- and Intermolecular Copigmentation, J. Agric. Food Chem., 2001, 49(1), 170–176 CrossRef CAS PubMed.
- K. C. Nam and D. U. Ahn, Carbon monoxide-heme pigment is responsible for the pink color in irradiated raw turkey breast meat, Meat Sci., 2002, 60, 25–33 CrossRef CAS PubMed.
- F. Cataldo, Ozone degradation of biological macromolecules: proteins, hemoglobin, RNA, and DNA, Songklanakarin J. Sci. Technol., 2006, 28, 317–328 CAS.
- A. Vasilescu, A. Vezeanu, Y. Liu, I. S. Hosu, R. M. Worden and S. F. Peteu, Meat freshness: peroxynitrites oxydative role, its natural scavengers, and new measuring tools, in Instrumental Methods for the Analysis and Identification of Bioactive Molecule, ed. Society A. C., ACS Sympos, 2014, pp. 302–333 Search PubMed.
- C. Sarangapani, G. O'Toole, P. J. Cullen and P. Bourke, Atmospheric cold plasma dissipation efficiency of agrochemicals on blueberries, Innovative Food Sci. Emerging Technol., 2017, 44, 235–241 CrossRef CAS.
- S. Tappi, G. Gozzi, L. Vannini, A. Berardinelli, S. Romani and L. Ragni, Cold plasma treatment for fresh-cut melon stabilization, Innovat. Food Sci. Emerg. Technol., 2016, 33, 225–233 CrossRef CAS.
- A. Lacombe, B. A. Niemira, J. B. Gurtler, X. Fan, J. Sites and G. Boyd, et al., Atmospheric cold plasma inactivation of aerobic microorganisms on blueberries and effects on quality attributes, Food Microbiol., 2015, 46, 479–484 CrossRef CAS PubMed.
- S. Tappi, A. Berardirielli, L. Ragni, M. D. Rosa, A. Guarnieri and P. Rocculi, Atmospheric gas plasma treatment of fresh-cut apples, Innovat. Food Sci. Emerg. Technol., 2014, 21, 114–122 CrossRef CAS.
- T. R. B. Farias, S. Rodrigues and F. A. N. Fernandes, Effect of dielectric barrier discharge plasma excitation frequency on the enzymatic activity, antioxidant capacity and phenolic content of apple cubes and apple juice, Food Res. Int., 2020, 136, 109617 CrossRef CAS , https://linkinghub.elsevier.com/retrieve/pii/S0963996920306426.
- S. Rodrigues and F. A. N. Fernandes, Green Chemistry Applied to Ground Coffee Volatile Compounds Modification Aiming Coffee Aroma Improvement, J. Food Process. Preserv., 2023, 2023, 4921802 Search PubMed.
- E. C. M. Porto, E. S. de Brito, S. Rodrigues and F. A. N. Fernandes, Effect of Atmospheric Cold Plasma on the Aroma of Pineapple Juice: Improving Fresh and Fruity Notes and Reducing Undesired Pungent and Sulfurous Aromas, Processes, 2023, 11, 2303 CrossRef CAS.
- E. Takai, T. Kitamura, J. Kuwabara, S. Ikawa, S. Yoshizawa, K. Shiraki, H. Kawasaki, R. Arakawa and K. Kitano, et al., Chemical modification of amino acids by atmospheric-pressure cold plasma in aqueous solution, J. Phys. D Appl. Phys., 2014, 47(28), 285403 CrossRef.
- P. Pal, P. Kaur, N. Singh, A. P. Kaur, N. N. Misra and B. K. Tiwari, et al., Effect of nonthermal plasma on physico-chemical, amino acid composition, pasting and protein characteristics of short and long grain rice flour, Food Res. Int., 2016, 81, 50–57, DOI:10.1016/j.foodres.2015.12.019.
- P. H. Campelo, E. G. Alves Filho, L. M. A. Silva, E. S. de Brito, S. Rodrigues and F. A. N. Fernandes, Modulation of aroma and flavor using glow discharge plasma technology, Innovat. Food Sci. Emerg. Technol., 2020, 62, 102363 CrossRef CAS.
- D. L. H. Maia, S. Rodrigues and F. A. N. Fernandes, Influence of Glow Discharge Plasma Treatment on Cashew Apple Juice's Aroma Profile and Volatile Compounds, J. Food Process. Preserv., 2023, 2023, 1–13 CrossRef.
- P. H. Campelo, E. G. Alves Filho, L. M. A. Silva, E. S. de Brito, S. Rodrigues and F. A. N. Fernandes, Modulation of aroma and flavor using dielectric barrier discharge plasma technology in a juice rich in terpenes and sesquiterpenes, LWT, 2020, 130, 109644 CrossRef CAS , https://linkinghub.elsevier.com/retrieve/pii/S0023643820306332.
- A. Gani, W. N. Baba, M. Ahmad, U. Shah, A. A. Khan and L. A. Wani, et al., Effect of ultrasound treatment on physico-chemical, nutraceutical and microbial quality of strawberry, LWT–Food Sci. Technol., 2016, 66, 496–502 CrossRef CAS.
- S. Cao, Z. Hu, B. Pang, H. Wang, H. Xie and F. Wu, Effect of ultrasound treatment on fruit decay and quality maintenance in strawberry after harvest, Food Control, 2010, 21, 529–532 CrossRef CAS.
- P. Elizaquivel, G. Sánchez, M. v. Selma and R. Aznar, Application of propidium monoazide-qPCR to evaluate the ultrasonic inactivation of Escherichia coli O157:H7 in fresh-cut vegetable wash water, Food Microbiol., 2012, 30, 316–320 CrossRef CAS PubMed.
- H. Zhang, S. Tsai and R. v Tikekar, Inactivation of Listeria innocua on blueberries by novel ultrasound washing processes and their impact on quality during storage, Food Control, 2021, 121, 107580 CrossRef CAS.
- E. A. Alenyorege, H. Ma, J. H. Aheto, I. Ayim, F. Chikari and R. Osae, et al., Response surface methodology centred optimization of mono-frequency ultrasound reduction of bacteria in fresh-cut Chinese cabbage and its effect on quality, LWT–Food Sci. Technol., 2020, 122, 108991 CrossRef CAS.
- Q. Xiang, C. Kang, L. Niu, D. Zhao, K. Li and Y. Bai, Antibacterial activity and a membrane damage mechanism of plasma-activated water against Pseudomonas deceptionensis CM2, LWT, 2018, 96, 395–401 CrossRef CAS.
- C. Smet, M. Govaert, A. Kyrylenko, M. Easdani, J. L. Walsh and J. F. Van Impe, Inactivation of Single Strains of Listeria monocytogenes and Salmonella Typhimurium Planktonic Cells Biofilms With Plasma Activated Liquids, Front. Microbiol., 2019, 10 DOI:10.3389/fmicb.2019.01539.
- J. Guo, K. Huang, X. Wang, C. Lyu, N. Yang and Y. Li, et al., Inactivation of Yeast on Grapes by Plasma-Activated Water and Its Effects on Quality Attributes, J. Food Prot., 2017, 80(2), 225–230 CrossRef CAS PubMed.
- A. Mai-Prochnow, R. Zhou, T. Zhang, K. Ostrikov, S. Mugunthan and S. A. Rice, et al., Interactions of plasma-activated water with biofilms: inactivation, dispersal effects and mechanisms of action, npj Biofilms Microbiomes, 2021, 7(1), 11 CrossRef CAS.
- B. L. Fina, B. Santamaría, M. G. Ferreyra, L. P. Schierloh, J. C. Chamorro and E. Cejas, et al., Innovative application of plasma-activated water in the inactivation of Escherichia coli: Temperature-dependent chemical processes leading to the synergistic microbicidal effect, Food Control, 2024, 163, 110530 CrossRef CAS.
- C. Liu, C. Chen, A. Jiang, X. Sun, Q. Guan and W. Hu, Effects of plasma-activated water on microbial growth and storage quality of fresh-cut apple, Innovat. Food Sci. Emerg. Technol., 2020, 59, 102256 CrossRef CAS.
- S. L. Narasimhan, D. Salvi, D. W. Schaffner, M. V. Karwe and J. Tan, Efficacy of cold plasma-activated water as an environmentally friendly sanitizer in egg washing, Poult. Sci., 2023, 102(10), 102893 CrossRef CAS.
- Z. Ke, X. Peng, S. Jia, S. Liu, X. Zhou and Y. Ding, Mechanisms underlying the potent antimicrobial effects of plasma-activated seawater (PASW) on fish spoilage bacterium Shewanella putrefaciens, Food Chem., 2024, 455, 140147 CrossRef CAS.
- R. Sun, W. Xu, L. Xiong, N. Jiang, J. Xia and Y. Zhu, et al., The combined effects of ultrasound and plasma-activated water on microbial inactivation and quality attributes of crayfish during refrigerated storage, Ultrason. Sonochem., 2023, 98, 106517 CrossRef CAS PubMed.
- W. Xu, R. Sun, N. Jiang, Q. Wang, C. Wang and Q. Liu, et al., Synergistic effects of ultrasound and plasma-activated water against Listeria innocua in crayfish disinfection by metabolomics analysis, Food Biosci., 2024, 61, 104597 CrossRef CAS.
- Y. Bai, J. Chen, Y. Yang, L. Guo and C. Zhang, Degradation of organophosphorus pesticide induced by oxygen plasma: Effects of operating parameters and reaction mechanisms, Chemosphere, 2010, 81(3), 408–414 CrossRef CAS , https://linkinghub.elsevier.com/retrieve/pii/S0045653510007459.
- M. Gavahian and A. M. Khaneghah, Cold plasma as a tool for the elimination of food contaminants: Recent advances and future trends, Crit. Rev. Food Sci. Nutr., 2020, 60(9), 1581–1592 CrossRef CAS.
- R. Tsao and M. Eto, Effect of some natural photosensitizers on photolysis of some pesticides, in Aquatic and Surface Photochemistry, CRC Press, Boca Raton, USA, 1994, pp. 163–171 Search PubMed.
- Y. Bai, J. Chen, H. Mu, C. Zhang and B. Li, Reduction of dichlorvos and omethoate residues by O2 plasma treatment, J. Agric. Food Chem., 2009, 57, 6238–6245 CrossRef CAS PubMed.
- W. C. Zhu, B. R. Wang, H. L. Xi and Y. K. Pu, Decontamination of VX Surrogate Malathion by Atmospheric Pressure Radio-frequency Plasma Jet, Plasma Chem. Plasma Process., 2010, 30(3), 381–389, DOI:10.1007/s11090-010-9221-z.
- N. N. Misra, The contribution of non-thermal and advanced oxidation technologies towards dissipation of pesticide residues, Trends Food Sci. Technol., 2015, 45(2), 229–244 CrossRef CAS.
- C. Sarangapani, G. O'Toole, P. J. Cullen and P. Bourke, Atmospheric cold plasma dissipation efficiency of agrochemicals on blueberries, Innovative Food Sci. Emerging Technol., 2017, 44, 235–241, DOI:10.1016/j.ifset.2017.02.012.
- C. Sarangapani, N. N. Misra, V. Milosavljevic, P. Bourke, F. O'Regan and P. J. Cullen, Pesticide degradation in water using atmospheric air cold plasma, J. Water Proc. Eng., 2016, 9, 225–232 CrossRef.
- K. T. K. Phan, H. T. Phan, D. Boonyawan, P. Intipunya, C. S. Brennan and J. M. Regenstein, et al., Non-thermal plasma for elimination of pesticide residues in mango, Innovat. Food Sci. Emerg. Technol., 2018, 48, 164–171 CrossRef CAS , https://linkinghub.elsevier.com/retrieve/pii/S1466856417314339.
- R. Zhou, R. Zhou, F. Yu, D. Xi, P. Wang and J. Li, et al., Removal of organophosphorus pesticide residues from Lycium barbarum by gas phase surface discharge plasma, Chem. Eng. J., 2018, 342, 401–409 CrossRef CAS , https://linkinghub.elsevier.com/retrieve/pii/S1385894718303206.
- N. S. Heo, M. K. Lee, G. W. Kim, S. J. Lee, J. Y. Park and T. J. Park, Microbial inactivation and pesticide removal by remote exposure of atmospheric air plasma in confined environments, J. Biosci. Bioeng., 2014, 117, 81–85 CrossRef CAS PubMed.
- S. H. Kim, J. H. Kim and B. K. Kang, Decomposition Reaction of Organophosphorus Nerve Agents on Solid Surfaces with Atmospheric Radio Frequency Plasma Generated Gaseous Species, Langmuir, 2007, 23(15), 8074–8078, DOI:10.1021/la700692t.
- C. Fang, S. Wang, C. Shao, C. Liu, Y. Wu and Q. Huang, Study of detoxification of methyl parathion by dielectric barrier discharge (DBD) non-thermal plasma at gas-liquid interface:mechanism and bio-toxicity evaluation, Chemosphere, 2022, 307, 135620 CrossRef CAS.
- K. Yogesh Kumar, M. K. Prashanth, H. Shanavaz, L. Parashuram, F. Alharethy and B. H. Jeon, et al., Ultrasound assisted fabrication of InVO4/In2S3 heterostructure for enhanced sonophotocatalytic degradation of pesticides, Ultrason. Sonochem., 2023, 100, 106615 CrossRef CAS PubMed.
- H. Azarpira, T. Rasolevandi, A. H. Mahvi and M. Karimy, Diazinon pesticide photocatalytic degradation in aqueous matrices based on reductive agent release in iodide exciting under UV Irradiation, Environ. Sci. Pollut. Res., 2022, 29(38), 58078–58087 CrossRef CAS PubMed.
- B. Li, S. Li, L. Yi, H. Sun, J. Qin and J. Wang, et al., Degradation of organophosphorus pesticide diazinon by hydrodynamic cavitation: Parameters optimization and mechanism investigation, Process Saf. Environ. Prot., 2021, 153, 257–267 CrossRef CAS.
- T. Ahmadifard, R. Heydari, M. J. Tarrahi and G. S. Khorramabadi, Photocatalytic Degradation of Diazinon in Aqueous Solutions Using Immobilized MgO Nanoparticles on Concrete, Int. J. Chem. React. Eng., 2019, 17(9), 1–13 Search PubMed.
- T. J. Al-Musawi, N. Mengelizadeh, W. M. S. Kassim, M. Sillanpää, S. H. Siddiqui and S. Shahbaksh, et al., Sonophotocatalytic degradation and operational parameters optimization of diazinon using magnetic cobalt–graphene nanocomposite as a catalyst, J. Water Proc. Eng., 2022, 46, 102548 CrossRef.
- S. M. Mousavi, S. Imani, D. Dorranian, K. Larijani and M. Shojaee, Effect of cold plasma on degradation of organophosphorus pesticides used on some agricultural products, J. Plant Prot. Res., 2017, 57, 25–35 CAS.
| This journal is © The Royal Society of Chemistry 2025 |