Open Access Article
Open Access ArticleNon-TiO2-based photoanodes for photoelectrocatalytic wastewater treatment: electrode synthesis, evaluation, and characterization†
Jingyang
Liu
abc,
Huizhong
Wu
abc,
Jiangli
Sun
abc,
Shuaishuai
Li
abc,
Aydin
Hassani
 de and
Minghua
Zhou
de and
Minghua
Zhou
 *abc
*abc
aKey Laboratory of Pollution Process and Environmental Criteria, Ministry of Education, College of Environmental Science and Engineering, Nankai University, Tianjin 300350, China. E-mail: zhoumh@nankai.edu.cn
bTianjin Key Laboratory of Environmental Technology for Complex Trans-Media Pollution, College of Environmental Science and Engineering, Nankai University, Tianjin 300350, China
cTianjin Advanced Water Treatment Technology International Joint Research Center, College of Environmental Science and Engineering, Nankai University, Tianjin 300350, China
dDepartment of Materials Science and Nanotechnology Engineering, Faculty of Engineering, Near East University, 99138 Nicosia, TRNC, Mersin 10, Turkey
eResearch Center for Science, Technology and Engineering (BILTEM), Near East University, 99138 Nicosia, TRNC, Mersin 10, Turkey
First published on 29th May 2025
Abstract
To address the increasingly serious problem of water pollution, photoelectrocatalysis (PEC), one of the advanced oxidation processes (AOPs), has gained significant attention due to its ability to utilize sunlight and its low energy consumption. In PECs, TiO2 is the most widely used and established photoanode; however, non-TiO2-based photoanodes have increasingly become a focus for improving visible light utilization and meeting the requirements of specific reactions. The performance of these non-TiO2-based photoanodes in wastewater treatment varies based on different synthesis strategies and structures. Therefore, this paper critically reviews the synthesis, evaluation and characterization methods of non-TiO2-based photoanodes used in wastewater treatment. Specifically, it reveals the application potential of various non-TiO2-based photoanodes (such as WO3, ZnO, g-C3N4, and BiVO4), compares the costs and electrode stability of different synthesis methods from a practical application-oriented perspective, elucidates the synthesis–structure–mechanism–activity relationship, proposes an evaluation framework for PEC wastewater treatment based on multiple dimensions (including pollutant removal, electrode stability, light utilization efficiency, and environmental applicability), and introduces frontier theoretical simulations and characterization techniques of PEC wastewater treatment in depth according to the reaction process. Finally, an outlook on the preparation, evaluation and characterization of non-TiO2-based photoanodes is proposed, covering perspectives from the atomic level to large-scale applications. This work aims to provide a comprehensive understanding of these ‘rising stars’ and guide the synthesis of photoanodes with enhanced performance, as well as more accurate evaluation and characterization.
Broader contextPhotocatalysis is an innovative technique for addressing energy and environmental challenges, leveraging sunlight to generate green energy and facilitate the removal of pollutants. Nevertheless, conventional photocatalytic processes utilizing powdered semiconductors encounter significant limitations, such as the recombination of photogenerated charges and challenges in recycling, which impede their practical applications. In contrast, photoelectrocatalysis (PEC), which integrates photocatalysis with an externally applied electric field, presents a promising strategy to these challenges and has garnered considerable attention in the fields of energy production and pollutant remediation. Although TiO2 remains the most extensively studied photocatalyst, recent advancements have led to the development of non-TiO2-based photoanodes, which exhibit substantial potential for PEC wastewater treatment. This review summarizes progress in non-TiO2-based photoanodes, comparing their stability and cost across preparation strategies. It summarizes the evaluation frameworks for photoelectrodes in wastewater treatment and provides novel insights. Furthermore, it introduces characterization techniques for each PEC step and analyzes the synthesis–structure–mechanism–activity relationship. Finally, prospects, challenges, and opportunities from atomic to large-scale synthesis and characterization are discussed. By providing a comprehensive understanding of these materials, this review aims to guide the synthesis of photoelectrodes for environmental applications and enhance the mechanistic understanding of PEC processes. |
1. Introduction
Water pollution is closely related to human health and is one of the most concerning issues in the world. To tackle this issue, more and more wastewater treatment techniques have been used. However, when treating recalcitrant organics, biotechnology is limited and needs a large area to operate1,2 while physical methods can’t completely remove pollutants, causing secondary pollution.3 Advanced oxidation processes (AOPs) can efficiently remove pollutants and even mineralize them, showing promising application potential.4,5 AOPs include ultraviolet (UV)-based technologies,6 O3-based technologies,7 Fenton process,8 electrochemical advanced oxidation processes (EAOPs),9 photocatalysis (PC) process10,11etc., among which photocatalysis can work under sunlight, showing promise for solving environmental problems and alleviating energy stress at the same time. However, there are still many problems in PC, which limit its practical applications. For instance, photo-generated holes (h+) and electrons (e−) are easy to combine, thus decreasing the quantum yield of photocatalysts.12 What's more, photocatalysts are usually dispersed in solution, and they are difficult to separate from treated water.13To improve charge carriers’ separation efficiency and reusability, photoelectrocatalysis (PEC), the combination of PC and electrocatalysis (EC), is proposed. The photocatalysts are immobilized on conductive substrates; thus, the prepared photoelectrode can be easily cycled, and e−–h+ pairs are more likely to separate after applying an electric field.14 Compared with photocathodes, photoanodes are more popularly used in wastewater treatment because h+ accumulating at their surface can oxidize organic pollutants directly or indirectly.15Fig. 1 illustrates literature statistics on photoanodes since 2000. In Fig. 1a, it can be seen that TiO2-based photoanodes have been the most studied photoanodes since the beginning of this century. However, the research proportion of non-TiO2-based photoanodes has increased in recent years, which has increased from less than 20% before 2015 to more than half recently. On the one hand, non-TiO2-based photocatalysts have different band positions compared with TiO2, which can meet the requirements of different reactions. For example, BiVO4, WO3, and their composites are more suitable as photoanodes in the PEC–chlorine (PEC–Cl) system.16 On the other hand, many non-TiO2-based photoanodes offer unique advantages. For example, g-C3N4 is a metal-free polymer that exhibits visible-light-driven photocatalytic activity and high stability.17 It shows significant potential in future clean energy production and environmental compatibility. In the field of wastewater treatment, the eight most prominent non-TiO2-based photoanodes are shown in Fig. 1b, with WO3, ZnO, g-C3N4, and BiVO4 leading the way.
The preparation methods of these photoanodes are also different; some catalysts (WO3, BiVO4, etc.) can directly grow on substrates, while some photoanodes (g-C3N4, for example) have to be prepared by ex situ methods. Some synthesis methods are operated at room temperature and atmospheric pressure (e.g., successive ionic layer adsorption and reaction (SILAR)18), while some require special equipment, even high temperature and pressure (e.g., hydrothermal method19). Different synthesis strategies result in electrodes with different structures and hence different activities, so synthesis methods need to be summarized and compared. However, in previous reviews,20,21 synthesis methods of photoanodes are briefly introduced (e.g., their operations, advantages, and disadvantages), while the comparison of products (pollutant removal efficiency, stability, cost, etc.) is not conducted, and the synthesis–activity relationship is not revealed.
Moreover, to reveal the structure–activity relationship, light–matter interaction, and photocatalyst–pollutant interaction, sufficient evaluation and characterization should be carried out. The mechanism of PEC processes needs to be deeply understood. However, in previous reviews,21–24 when discussing characterization techniques, they are usually divided into characterization of morphology (scanning electron microscopy (SEM), transmission electron microscopy (TEM), etc.), chemical composition (X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD) spectra, etc.), and photoelectric properties (photoluminescence (PL) spectroscopy, UV/vis diffuse reflectance spectroscopy (UV-vis DRS), etc.). The relationship between the photoanodes and pollutant removal has not been thoroughly summarized. What's more, in recent years, in situ XPS, in situ infrared, density functional theory (DFT) calculation, computational fluid dynamics (CFD) simulation, etc. have gradually emerged to characterize the morphology, composition, and reaction mechanism of non-TiO2-based photoanodes. Therefore, an in-depth summary of existing and latest characterization techniques is needed.
Herein, this review compares and concludes the synthesis methods, evaluation systems, and characterization techniques of non-TiO2-based photoanodes for wastewater treatment. First, the fundamentals of PEC wastewater treatment are explained, and based on an analysis of the literature, the application trends of non-TiO2-based photoanodes are presented. Subsequently, the main synthesis methods for non-TiO2-based photoanodes are summarized and compared. Next, the evaluation of PEC wastewater treatment at the practical application level is presented, and the main techniques for characterizing non-TiO2-based photoanodes are described in depth from the PEC reaction process. Finally, an outlook on the preparation, evaluation, and characterization of non-TiO2-based photoanodes is proposed based on the perspective from the atomic level to large-scale applications. This review innovatively summarizes the impact of electrode synthesis on electrode structure and performance, systematically compares diverse synthesis strategies from the perspectives of product stability and cost-effectiveness, and highlights cutting-edge experimental and theoretical approaches for characterizing PEC wastewater treatment processes. We hope this review will inspire interested readers in the synthesis of superior photoanodes for wastewater treatment and in-depth characterization of them.
2. Fundamentals of PEC wastewater treatment
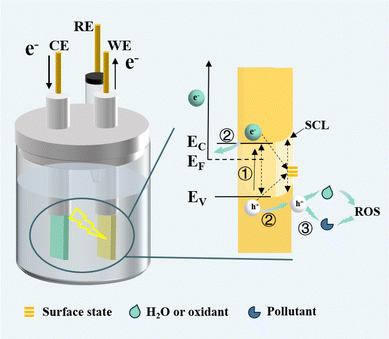
The PEC wastewater treatment process can be divided into three steps: light absorption and exciton excitation, separation and transport of carriers, and interface reaction.10 The relationship between the three steps is shown in Scheme 1. Due to the difference between the Fermi energy level of the photoanode and the redox potential of the solution, electron transfer occurs at the interface between the semiconductor and the solution, forming an energy band bending and space charge layer (SCL) on the surface.25 In step one, when the energy of absorbed light is higher than the bandgap energy (Eg), e− in the valence band (VB) transit to the conduction band (CB), causing h+ to be left in the VB.14 In this step, the e− and h+ generated in the bulk phase, if not effectively separated, will quickly recombine26 (as demonstrated by the dashed arrows in Scheme 1, including bulk-phase recombination, SCL recombination, and surface-state recombination), and can’t participate in the pollutant degradation reaction. In the second step, the simultaneous presence of light and anode bias leads to a more pronounced energy band bending, which favors the separation of photogenerated carriers.25 Within the SCL, h+ move towards the electrode surface, while e− migrate to the cathode via an external circuit. In the interfacial reaction in the third step, the accumulated h+ may oxidize the contaminant directly, or they may oxidize water or other oxidants to generate reactive species with strong oxidizing properties.16 The type of oxidation reaction that occurs depends on the catalyst's CB and VB potentials, the electrode bias, and the mass transfer of the contaminant. Throughout the process, the lifetimes of electrons and holes in the bulk phase are only picoseconds to nanoseconds, whereas the reactions to degrade pollutants are on the timescale of milliseconds to seconds, suggesting that the kinetics of carriers within the photoanode are very critical.27 The selection of photoanode materials and different synthesis methods may affect carrier migration and redox reaction kinetics, which in turn affect the pollutant removal efficiency. Therefore, a summary of photoanode synthesis methods and characterization of the PEC process is essential for understanding and breaking through the barriers to pollutant degradation kinetics.3. Application trend of non-TiO2 based photoanodes in PEC water treatment
The energy band structures of the eight most common non-TiO2-based photoanodes are shown in Fig. 2a, in which MoS2 and Cu2O are p-type semiconductors and the others are n-type semiconductors. Metrological analysis of the retrieved literature reveals the research hotspots and trends of non-TiO2-based photoanodes in the field of wastewater treatment. The results show that compared with TiO2-based photoanodes, the two most obvious application trends in non-TiO2 photoanodes are as follows: one is that the proportion of the literature with “fuel cell” and “electricity” as keywords is higher than that with TiO2, which is represented by ZnO and WO3; another is that the proportion of “visible-light” as the keyword is also significantly higher than that with TiO2 as the keyword, represented by WO3, g-C3N4, BiVO4, and α-Fe2O3.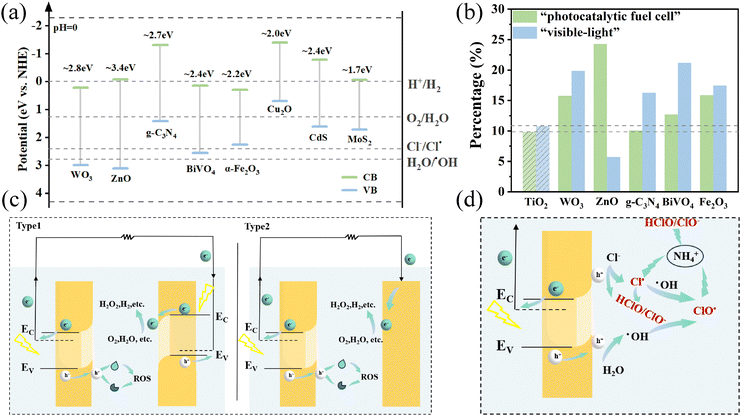 | ||
| Fig. 2 (a) The band structures of eight main non-TiO2-based photoanodes. Data from ref. 33–36. (b) Bibliometric analysis of TiO2 and the five most frequently used non-TiO2-based photoanodes. (Counting the keywords in the titles of the literature, filtering out words like ‘and’, ‘of’, ‘degradation’, and ‘photoanode’ that do not reflect the key information, and the most significant remaining keywords are “fuel cell”, “visible-light”, and the proportion of the literature containing these keywords to the total literature was calculated.) (c) The schematic of two types of PFCs with non-TiO2-based photoanodes. (d) A schematic diagram of the possible sources of RCS generation in the PEC–Cl system.34 | ||
A photocatalytic fuel cell (PFC) is a special PEC water treatment system. The non-TiO2-based photoanode can be combined with a photocathode (type 1) or a cathode without light response (type 2) to form a PFC (Fig. 2c). It relies on the photovoltage generated by the photoelectrode after illumination to drive the reaction without external bias.28 The theoretical maximum value of the photovoltage between the two electrodes depends on the Fermi energy level difference between the electrodes, so it is necessary to select the appropriate anode and cathode.29 In the photoanode–photocathode system, under light illumination, the photoanode undergoes energy band bending (upward) near the surface of the solution, and e− migrate towards the cathode, while h+ accumulate on the surface of the photoanode, and oxidation reactions take place.30 The photocathode undergoes downward energy band bending, which facilitates the aggregation of electrons towards the cathode for reduction reactions to occur at its surface. Contaminants can be oxidized at the anode, and electrons gathered at the cathode may undergo a variety of reduction reactions, depending on the electrode material and the redox potential. Wang et al.31 used oxygen vacancy-rich BiVO4 as a photoanode, Pt/C cocatalyst-coated Si as a photocathode, and the pollutant triethanolamine as “fuel” to construct a PFC system for the simultaneous degradation of pollutants and hydrogen production, which can achieve bias-free H2 production with a current density of 10.17 mA cm−2. Dong et al.32 constructed an artificial leaf with simultaneous H2O2 production at the cathode and anode using SnO2−x/BiVO4/WO3 as a photoanode and Mo-SACs/mrG as a cathode without the need for applied voltage, achieving an unassisted H2O2 production rate of 0.77 μmol (min−1 cm−2) under 1 sun AM 1.5 illumination. The most obvious advantage of a PFC is that it can generate electricity while degrading pollutants, making it a promising technology to address the environmental and energy crisis.
One of the key issues in PEC wastewater treatment is the use of light, with only 5% of sunlight being the most energetic UV light and 43% being visible light.10 Semiconductors possessing too high a bandgap energy can only utilize UV light, as can be seen in Fig. 2a, where all photoanode materials except ZnO have smaller bandgap energies compared with TiO2 (∼3.2 eV). This is why all photoanodes except for TiO2 and ZnO have been reported in such a high percentage of visible-light studies. However, most of the current studies on “visible light PECs” use simulated sunlight, and the use of actual sunlight for pollutant degradation needs to be taken seriously. Sun et al.37 studied a MgO/g-C3N4 S-scheme heterojunction photoanode, which showed superior visible light utilization prospects. This anode was combined with modified carbon felt to construct a new PEC system. In the actual PEC degradation experiment under sunlight, 98.12% of tetracycline was removed within 30 min. Xie et al.38 synthesized a BiVO4-decorated WO3 photoanode, which was combined with an electrodeposited polyaniline-decorated carbon fiber cathode to construct a solar-driven wastewater resuscitation system. The system was operated under natural sunlight and achieved 99.1% uranium reduction and 98.4% oxytetracycline hydrochloride removal, showing superior practical application potential.
It is noteworthy that for non-TiO2-based photoanodes, the keyword “ammonia” also appears more frequently in WO3 and BiVO4 due to the increasing application of PEC–Cl systems in the treatment of ammonia-containing wastewater. The active chlorine species (RCS) have obvious advantages over ˙OH in the treatment of NH3–N,39 so the core of PEC–Cl is the formation of RCS (Fig. 2d). The selection of photoanode materials is extremely critical in controlling the generation of RCS and inhibiting the generation of toxic chlorine-containing by-products. The valence band potential of some photoanodes (e.g., Fe2O3 and g-C3N4) is not sufficient to oxidize Cl−, and therefore, they are not suitable as photoanode materials (see Fig. 2a). Some photoanodes have too large a bandgap and require UV excitation (e.g., ZnO), which are also not suitable as photoanode materials. Therefore, BiVO4, WO3, and their composites are most often considered for PEC–Cl.34 It has also been shown by some researchers that too much oxidizing capacity of the valence band generates more ˙OH, which is detrimental to the generation of RCS. For example, Zhang et al.16 reported a self-driven PEC–Cl system with a BiVO4/WO3 heterojunction photoanode. In order to control toxic chlorate and nitrate caused by the excessive oxidation capacity of ˙OH, they realized the predominant production of Cl˙ by regulating the valence band edge of WO3 through modifying BiVO4. The results showed that 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mg
mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L−1 ammonia-N was completely removed in 120
L−1 ammonia-N was completely removed in 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min, and toxic byproducts chlorate and nitrate were inhibited by 79.3% and 31%, respectively, compared to the WO3 photoanode. The PEC–Cl system has also demonstrated potential in addressing combined organic–inorganic contamination. For instance, Zhang et al.40 fabricated a novel WO3/BiVO4-CoBi photoanode, which could remove 99% of carbamazepine (CBZ) within 40 min and 75.4% of NH4+ within 120 min.
min, and toxic byproducts chlorate and nitrate were inhibited by 79.3% and 31%, respectively, compared to the WO3 photoanode. The PEC–Cl system has also demonstrated potential in addressing combined organic–inorganic contamination. For instance, Zhang et al.40 fabricated a novel WO3/BiVO4-CoBi photoanode, which could remove 99% of carbamazepine (CBZ) within 40 min and 75.4% of NH4+ within 120 min.
4. Synthesis of non-TiO2-based photoanodes
The synthesis of photoanodes differs significantly from that of powder photocatalysts, necessitating careful consideration of substrate selection and growth methodology employed. Synthesis techniques encompass wet-chemical methods, electrochemical methods, other chemical methods, and physical methods. Based on whether photocatalysts are directly grown on substrates or whether pre-prepared powder photocatalysts are affixed to them, synthesis methods can be classified into in situ and ex situ categories. A summary of several in situ synthesis methods for non-TiO2-based photoanodes utilized in wastewater treatment is presented in Table 1 and Fig. 3.| Category | Synthesis method | Photoanode | Modification method | Synthesis conditiona | Main equipment | Pollutant (mg L−1) | Removal efficiency | Stability of photoanode | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| a Some photoanodes involve the combination of multiple catalysts and require more than one preparation method. The catalyst synthesis condition mentioned is only for a certain catalyst. When involving multiple catalysts, the words representing the target catalyst are thickened. In addition, the conditions here do not include post-processing conditions. b BPA: bisphenol A. c NiFe-LDH: NiFe-layered double hydroxide. d FTO: fluorine-doped tin oxide. e CA: clofibric acid. f 4-CP: 4-chlorophenol. g OTC: oxytetracycline. h TC: tetracycline hydrochloride. i CIP: ciprofloxacin. j MO: methyl orange. k RhB: rhodamine B. l DC: direct current. m PFOA: perfluorooctanoic acid. n ATZ: atrazine. o ALD: atomic layer deposition. p MB: methylene blue. | |||||||||
| Wet-chemical methods | Hydrothermal | hm-m-WO3/W mesh | Heterophase junction | 160 °C for 12 h | Teflon-lined stainless-steel autoclave; oven | BPAb (20) | 99.9% (150 min) | Still removed 99.8% of BPA, 5 cycles | 46 |
| 60.9% (TOC, 150 min) | |||||||||
| k = 0.054 min−1 | |||||||||
| NiFe-LDHc/Co3O4/Ni foam | Heterojunction | 90 °C for 8 h | BPA (10) | 100% (120 min) | Still removed more than 90% BPA and Cr(VI), 10 cycles | 47 | |||
| Cr(VI) (10) | ∼65 (TOC, 120 min) | ||||||||
| α-Fe 2 O 3 /g-C3N4/FTOd | Heterojunction | 120 °C for 4 h at pH 1.0 | CAe (30) | 99.7% (300 min) | Still removed 98% of CA, 5 cycles | 48 | |||
| Chemical bath deposition | WO3−x/FTO | Doping | 85 °C for 2 h | Beaker; constant temperature heating magnetic stirrer | 4-CPf (10) | 98.9% (180 min) | Insignificant decrease in activity, 8 cycles | 49 | |
| k = 0.016 min−1 | |||||||||
| MoO3/ZnO/Zn | Heterojunction | 90 °C for 4 h | Phenol (40) | 89.5% (COD, 240 min) | Insignificant decrease in activity, 4 cycles | 50 | |||
| Cu2+ (200) | 93.5% (Cu2+, 240 min) | ||||||||
| WO 3 /Ag/FTO | Modifying with noble metals | 90 °C for 3 h | Hg2+ (40.12) | 100% (12 min) | Kept photocurrent stable at pH 2, 200 min | 51 | |||
| Liquid phase deposition | Cu2O/α-Fe2O3/FTO | Heterojunction | 55 °C for 30 min | Beaker; constant temperature heating magnetic stirrer | OTCg (10) | 73.3% (60 min) | About 3% loss of PEC activity, 5 cycles | 42 | |
| k = 0.021 min−1 | |||||||||
| α-Fe 2 O 3 /Ti Ni-ZnO/FTO | Doping | 40 °C for 1.5 h | TCh (10) | 87.5% (180 min) | Not mentioned | 52 | |||
| Successive ionic layer adsorption and reaction | BiVO4/α-Fe2O3/FTO | Morphological modification and heterojunction | Room temperature, normal pressure | Beaker | Phenol (50) | 68.89% (COD, 120 min) | Photocurrent density decayed by ∼8%, 7000 s | 53 | |
| ZnS/Bi 2 S 3 /ZnONR array/FTO | Heterojunction | Room temperature, normal pressure | H2 production | 112.8 μmol cm−2 h−1 | ∼70% retention of initial photocurrent density, 2 h | 54 | |||
| Ag 2 S/BiVO4/FTO | Heterojunction | Room temperature, normal pressure | CIPi (10) | 80% (120 min) | Insignificant decrease in activity, 3 cycles | 55 | |||
| 69% (TOC, 120 min) | |||||||||
| Drop-coating | Sn/Ti:α-Fe2O3@CuxO/FTO | Doping and heterojunction | 60 °C for 5 min | Beaker; constant temperature heating magnetic stirrer | MOj | 99% (120 min) | Kept photocurrent steady, 10 h | 56 | |
| BiClO/Ti | No modification | Room temperature for 1 h | RhBk (10) | 89% (180 min) | Kept photocurrent steady, 3 h | 57 | |||
| Spin-coating | BiVO 4 /FTO | No modification | 2000 rpm for 15 s, 15 cycles | UO22+ (20); TC (20) | 100% (UO22+, 40 min) | Insignificant decrease in activity, 20 cycles | 58 | ||
| 99% (TC, 40 min) | |||||||||
| Electrospinning | BiFeO3/BiVO4/Al | Heterojunction | 12 kV, 0.01 mL min−1 | Electrostatic spinning machine | RhB (10) | 90.88% (180 min) | Insignificant decrease in activity, 15 h | 59 | |
| Pd-ZnO/CNF/FTO | Modifying with noble metals | 15 kV, 0.5 mL min−1, 300 rpm | Paracetamol (15.12) | 100% (150 min) | Less than 4% loss of PEC activity, 8 cycles | 60 | |||
| k = 0.009 min−1 | |||||||||
| 71.2% (TOC, 240 min) | |||||||||
| Electrochemical methods | Anodic oxidation | Fe2WO6/ZnO/Zn | Heterojunction | 1.1 V for 2 h | DCl power supply | TC (20), total nitrogen (5.1) | 100% (TC, 120 min) | ∼20% loss of PEC activity, 5 cycles | 61 |
| 88.4% (total nitrogen, 120 min) | |||||||||
| CN-WO3/W | Modifying with carbonaceous materials | 70 V | DC power supply | PFOAm (5) | 95% (120 min) | Kept current stable, 24 h | 62 | ||
| WO3 nanostructures | Morphological modification | 20 V, 50 °C for 4 h, hydrodynamic condition (375 rpm) | Rotating disk electrode | ATZn (20) | 100% (180 min) | Not mentioned | 63 | ||
| k = 0.023 min−1 | |||||||||
| Electro-chemical deposition | Ov-Fe2O3@BiVO4/FTO | Heterojunction | −0.1 V (vs. Ag/AgCl), 5 min | Electrochemical workstation | COD (320), NH3–N (59.2) | 89.38% (COD, 2 h) | Still removed 84.75% of COD, 4 cycles | 64 | |
| 100% (NH3–N, 2 h) | |||||||||
| Ar-Fe2O3/Ti3+-TiO2-NTs | Heterojunction | Switch between the cathode pulse (0.25 A, 10 ms) and the anode pulse (0.25 A, 3 ms) | Electrochemical workstation | TC (20) | 100% (90 min) | Still removed 99.5% of TC, 5 cycles | 65 | ||
| k = 0.0684 min−1 | |||||||||
| Ov:BiVO4/FTO | Oxygen vacancy | No details | DC power supply | Triethanolamine (0.5 M) | The faradaic efficiency of H2 production is 97.26% | Kept current stable, 6 h | 31 | ||
| Other chemical methods | Atomic layer deposition | Pd-ZnO/CNF/FTO | Modifying with noble metals | Pre-heated at 220 °C | Low-pressure thermal ALDo reactor | Paracetamol (15.12) | 100% (150 min) | Less than 4% loss of PEC activity, 8 cycles | 60 |
| k = 0.009 min−1 | |||||||||
| 71.2% (TOC, 240 min) | |||||||||
| Sn-Fe2O3/NiFeOx/FTO | Doping | 106.66 Pa, 125 °C–225 °C, N2 atmosphere | In-house built flow-type ALD reactor | TC (20) | 96% (120 min) | Kept photocurrent steady, 18 cycles | 66 | ||
| k = 0.024 min−1 | |||||||||
| Chemical vapor deposition | NTAs/g-C3N4 | Morphological modification and heterojunction | 550 °C for 4 h (5 °C min−1) | Ceramic crucible | Aniline (10) | 31% (60 min) | Almost no decreases in PEC performances, 5 cycles | 67 | |
| N-ZnO/Si | Doping | 700 °C for 45 min | CVD reactor | MBp (5) | 95% (90 min) | ∼10% loss of PEC activity, 5 cycles | 68 | ||
| Physical methods | Sputtering | BiVO4/FTO | Doping | 2.0 Pa, O2/Ar flow rate: 40/100 sccm, 300/250 W | Sputter gun | TC (20) | 79% (12 min) | Kept photocurrent steady, ∼8000 s | 69 |
4.1 Wet-chemical methods
Wet-chemical methods are the most popular and facile methods for the preparation of non-TiO2-based photoanodes. The sol–gel method is a very popular wet chemistry method for the preparation of TiO2-based photoanodes;21 however, this method is not commonly used for the preparation of non-TiO2-based photoanodes. Hydrothermal methods can grow catalysts on conductive substrates in a Teflon-lined stainless-steel autoclave at high temperature and pressure; it is always followed by annealing (Fig. 4a). Solvothermal methods use organic solvents as the reaction medium41 and are also conducted in autoclaves; however, this approach has limited applications. Similar to the hydrothermal method, chemical bath deposition (CBD) grows films by heating the precursor and substrate, but it doesn’t require an enclosed space and high pressure, and it is sometimes referred to as the dip-coating method. Liquid phase deposition (LPD), based on the ligand-exchange hydrolysis of the metal-fluoro complex and the F− consumption reaction of boric acid,42 is another wet-chemical method. CBD and LPD are “softer” in conditions because they don’t require high pressure and temperature (Fig. 4b). SILAR is conducted by alternately immersing the substrate in different anionic and cationic solutions for many cycles (Fig. 4c). Ratnayake et al.43 deposited a BiVO4 thin film on FTO using the SILAR method, and they studied the effect of deposition parameters, including precursor concentration, number of immersion cycles, and annealing temperature, on the properties and PEC efficiency of the BiVO4 photoanode. Instead of immersing the substrate in the solution directly, photoanodes can also be prepared using coating techniques. Drop-coating is conducted by dropping the precursor on the substrate (Fig. 4d), while spin-coating is operated using a spin-coater and can achieve a more uniform film (Fig. 4e). Electrospinning is a promising technique to synthesize nano-fiber structure non-TiO2-based photoanodes. In the presence of a high voltage electric field, the liquid supply device promotes the flow of the electrospinning precursor, and a large amount of solvent volatilizes to produce spray, forming micro-nanofibers on the substrates (Fig. 4f).Coating methods are popular in the ex situ preparation of photoanodes. For instance, Fan and coworkers44 prepared MoS2 nanosheets via liquid exfoliation, dispersed them in ethanol via sonication, and then drop-cast them onto TiO2 electrodes. Notably, g-C3N4-based photoanodes are often prepared using ex situ methods because g-C3N4 is usually prepared by heating melamine or urea. For instance, Sun et al.37 first obtained MgO/g-C3N4 powder by calcining mixed alkaline magnesium carbonate and melamine in a muffle furnace, which was subsequently mixed with ethanol and Nafion and then loaded onto FTO by the coating method. Perylene diimide (PDI), a promising organic semiconductor, was also loaded onto indium–tin-oxide (ITO) glass by the dip-coating method.45 Therefore, ex situ methods allow sought-after materials to be coated onto the substrates and to exert their strengths, significantly expanding the variety of non-TiO2-based photoanodes.
Exploiting the relatively mild operating conditions and low equipment costs (refer to Table 1 and Fig. 3b), wet-chemical methods predominate in the current synthesis of non-TiO2-based photoanodes (see Fig. 3a). Electrodes produced via this methodology are extensively utilized for the degradation of organic compounds, the reduction of heavy metals, and the generation of energy. Nevertheless, Fig. 3a indicates that the convenience of wet-chemical methods is often accompanied by low stability, particularly in the cases of SILAR and LPD, with an average electrode lifetime of less than 500 min. This lack of stability may be attributed to inadequate adhesion and the occurrence of oxygen evolution reaction (OER) alongside the oxidation of contaminants.70 In contrast, the photoanodes synthesized through hydrothermal methods and electrospinning techniques demonstrate relatively high stability. He et al.71 employed a combination of metal-assisted chemical etching and hydrothermal methods to fabricate a Si/ZnO photoanode for application in PFCs. The resulting system maintained the ability to degrade over 90% of RhB after 20 cycles (7200 min), showcasing remarkable stability.
4.2 Electrochemical methods
Electrochemical methods are less reliant on specialized equipment and harsh conditions, allowing for precise adjustments in film thickness by varying electrolysis conditions. Anodic oxidation operates by applying a positive voltage to clean metal foils, sheets, or rods, followed by annealing to obtain the corresponding metal oxide semiconductor (Fig. 4g). For instance, Fernández-Domene and coworkers63 prepared a WO3 photoanode with nanostructures by adding H2O2 to the electrolyte. The charge transfer resistance of this nanostructure was significantly lower than that of the WO3 compact layer, which was prepared without H2O2. Similarly, ZnO and Fe2O3 photoanodes can also be synthesized using this method. This approach reduces interfacial resistance between the film and the substrate72 by enabling the direct formation of nanostructures while ensuring good stability. Feng et al.73 demonstrated that the current of their photoanode remained stable for 40 days.In contrast to anodic oxidation, the electrochemical deposition method applies a negative voltage, facilitating the deposition of anions from the electrolyte onto conductive substrates (Fig. 4h). In the preparation of the BiVO4 photoanode, Zheng et al.74 initially deposited BiOI onto the FTO substrate. Subsequently, they coated the precursor with a solution of VO(acac)2 in dimethyl sulfoxide (DMSO) solution and immersed it in NaOH solution following the annealing process. This methodology allows for the deposition on various substrates, not limited to the corresponding metal, as FTO and other conductive electrodes can also be utilized. Furthermore, the deposition process is not confined to metal oxide semiconductors.
As shown in Fig. 3a, electrochemical deposition has a very wide application in the preparation of non-TiO2-based photoanodes (second only to the hydrothermal method), and the electrodes prepared by electrochemical methods are moderately stable. Moreover, electrochemical methods can be carried out using electrochemical workstations or even DC power supplies, and the cost is not high (see Fig. 3b).
4.3 Other chemical methods and physical methods
In addition to the above chemical methods, several photoanode preparation techniques are not commonly used in wastewater treatment. ALD is a method of forming thin films by alternating pulses of gas-phase precursors into a reaction chamber and gas–solid-phase chemisorption reactions on the surface of the substrate (Fig. 4i). The films prepared by this method have excellent 3D conformality, stability, and homogeneity. Through ALD, Kim's group66 formed a NiFeOx film on the surface of Fe2O3 to achieve surface reconstruction. At the same time, they attained an accurate stoichiometric ratio using the super-cycle method. The as-prepared photoanode was used for water splitting and degradation of TC; as a result, it showed great PEC performance and could maintain a stable photocurrent after 18 cycles. However, as shown in Table 1 and Fig. 3b, this preparation method requires a special ALD reactor, which limits its application due to its high cost.CVD is a technology employed for the precise fabrication of photoanodes. This method utilizes substances in gaseous or vaporous states, which react at a gas–solid interface to yield solid deposits (Fig. 4j). Compared with wet-chemical methods, films produced via CVD exhibit superior adherence to the substrate, and the control over film thickness is highly manageable. Mane et al.68 reported the development of an N–ZnO–Si photoanode fabricated through metal–organic chemical vapor deposition (MOCVD) employing a nitrogen-doping technique. This approach effectively addressed the challenges of wide bandgap and low resistance to photo-corrosion of ZnO nanowires. As illustrated in Fig. 3a, however, the high cost associated with this method does not yield a corresponding enhancement in electrode stability.
Sputtering is an uncommon physical method that involves bombarding the source material (metal or metal oxide) under vacuum conditions with energetic ions and depositing atoms onto the substrate75 (Fig. 4k). Benefiting from its large-scale coating ability, non-selectivity of the substrate, high controllability, and versatility, sputtering has been widely applied in the industry; hence, it is a promising technique for preparing photoanodes used for wastewater treatment in the future. For instance, Huang et al.69 prepared a BiVO4 photoanode with excellent performance by co-sputtering V and BiVO4 targets, demonstrating excellent removal ability for TC (79% within 12 min). In addition, to demonstrate scalability, they prepared a large-area BiVO4 (100 × 100 mm), offering additional insights for promoting the proposed photoanode toward the practical application of PEC degradation. However, the expensive and specialized equipment (Fig. 3b) and harsh operating conditions prevent it from being extensively studied at the laboratory scale.
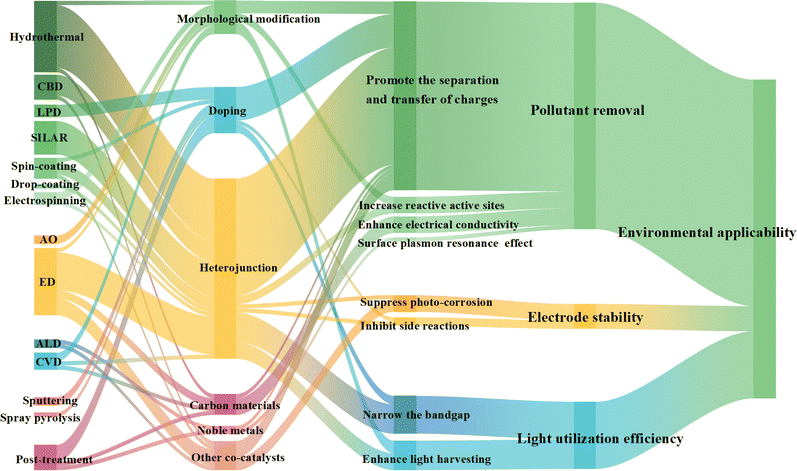
4.4 Modification of photoanodes
Pristine photoanodes may suffer from problems including large bandgap energy, low stability, or easy recombination of photo-induced carriers, so it is necessary to modify photoanodes. Typical modification methods for non-TiO2-based photoanodes include morphological modification, doping modification, heterojunction construction, and modification with carbonaceous materials, noble metals, and other co-catalysts. Modification can be achieved during the preparation of photoanodes, and the corresponding preparation methods are listed in Table 1 and Fig. 5.The morphological modification encompasses geometric shape control, nanometer-scale adjustments, and facet engineering, as illustrated in Fig. 4l. Variations in catalyst dimensionality result in distinct properties. For instance, Co3O4 predominantly exhibits a one-dimensional (1D) nanowire structure,76 whereas and MoS2 is characterized as a typical two-dimensional (2D) material.77 1D fibers or tubes possess a reduced charge carrier diffusion distance, which effectively inhibits the recombination of e− and h+; conversely, 2D sheets demonstrate high adhesion, facilitating reactions with organic contaminants.78 The crystal structure of a semiconductor significantly influences its properties, including stability, adsorption capacity, and photocatalytic reactivity.79–81 BiVO4 has garnered considerable attention in the realm of crystal facet engineering. For example, Yang et al.81 fabricated SnO2/010 facet-exposed BiVO4 nanocomposites utilizing the hydrothermal method. Their findings indicated that (010) facet-exposed BiVO4, in comparison to the (100) facets, exhibits higher surface energy and more exposed Bi atoms. Consequently, augmenting the exposure ratio of the (010) facet is advantageous for enhancing the adsorption of 2,4-dinitrophenol.
The doping modification includes metal doping and non-metal doping, and sometimes the construction of defects is also considered (Fig. 4m). Wu et al.82 devised a photoanode with simultaneous boron doping and oxygen vacancie (OV) production on the Bi2Sn2O7 photoanode. The synergistic effects of B-doping and OVs narrowed the bandgap of Bi2Sn2O7, allowed the surface of Bi2Sn2O7 to be more electron-rich and created intermediate levels inhibiting the recombination of e−–h+ pairs. As a result, it exhibited efficient and stable PEC degradation of SMT.
Constructing heterojunctions is one of the most popular strategies to modify photoanodes (Fig. 5) because it can effectively separate e−–h+ pairs by a built-in electric field (Fig. 4n). In addition to heterojunctions, some scholars have studied other forms of interface composites; for instance, Huang and Zhang's group83 synthesized a BiVO4 homojunction with staggered band alignment without incorporating any heteroatoms. Furthermore, Wang and coworkers84 fabricated a black/red phosphorus in situ junction, which was further utilized to prepare a novel multi-heterojunction TiO2–BiVO4–BP/RP film.
Combining photoanodes with co-catalysts is also a perspective strategy (Fig. 4o), for example, Wang et al.51 demonstrated that the deposition of Ag on WO3 facilitated the simultaneous reduction and detection of Hg within a concentration range of 0.296 nM to 12.5 μM when the electrode was operated in a solution containing Hg2+. This approach achieved a remarkable removal efficiency of 97 ± 2% for Hg2+ in industrial wastewater containing various pollutant ions. Furthermore, PEC performance can be significantly enhanced through the incorporation of carbonaceous materials, attributed to their superior electrical conductivity.85 Additionally, the introduction of co-catalysts has been shown to accelerate sluggish kinetics and electron transfer processes.86,87
Notably, certain modification approaches, though less commonly employed in PEC wastewater treatment systems, deserve attention for their potential to enhance photoanode performance. A prominent example is surface reconstruction. Seenivasan et al.66 demonstrated this strategy by applying an ultra-thin NiFeOx catalyst coating to hematite photoanodes via ALD. Benefiting from ALD's precise thickness control, the conformal NiFeOx coating not only passivated surface states but also facilitated rapid charge transfer to the electrolyte. This strategy effectively suppressed e−–h+ recombination within the photoanode.
5. Performance evaluation of non-TiO2-based photoanodes
Different preparation methods yield photoanodes with distinct structures, which in turn influence the electrode's performance in various ways. The evaluation of photoanode performance includes pollutant removal efficiency, electrode stability, light utilization efficiency, and environmental applicability. In order to further investigate how different structures affect electrode performance, the underlying mechanisms were characterized. A synthesis–structure–mechanism–activity diagram for non-TiO2-based photoanodes was developed after an extensive literature review (Fig. 5).5.1 Pollutant removal efficiency
Contaminant degradation experiments are the most commonly used method to test photoelectrodes. The pollutant removal efficiencies of prepared electrodes under different operational parameters are compared. Although higher light source power generally favors contaminant removal, our group has opted for lower-power LED lights from an energy consumption perspective, achieving satisfactory results.14,37,88 Anodic bias is another critical parameter. While higher bias voltages enhance the separation of photogenerated carriers by external electric fields and increase dark current density, excessively high bias may compromise anode stability and raise energy consumption.5 No clear patterns emerge regarding non-TiO2-based photoanode performance across varying pH levels. For instance, the WO3/TiO2 photoanode prepared by Li et al.19 exhibited optimal urea degradation at pH 3, whereas the Fe2WO6/ZnO photoanode prepared by Lam et al.61 achieved peak TC degradation at pH 7, with such variations attributable to differences in both pollutant and photoanode properties. Additionally, this discrepancy may also be influenced by the dominant active species. For example, in the TC treatment system reported by Sun et al.,37 where 1O2 acts as the primary reactive species, the photoanode exhibits continuously enhanced performance within the pH range of 7–9.These degradation experiments under varied conditions determine the optimal operational parameters for photoanodes. To demonstrate the superiority of the developed photoanodes, researchers further conduct comparative evaluations with other electrodes. However, variations in experimental conditions and lack of standardized testing methods make it difficult to compare and evaluate the published results. In order to assess the practical application potential of electrodes rationally, the attention must be paid to the selection of water matrix. Most photoanodes perform well in solutions containing deionized water, simple-component electrolytes, and target contaminants, while actual water has a complex composition that can reduce the efficiency. For instance, Rather et al.89 collected sewage from three different treatment locations in Hong Kong to use as the electrolyte in PEC experiments. The results showed that sewage with extremely high concentrations of Cl− and SO42− reduced charge (h+) transport, thereby decreasing degradation efficiency. It is also reasonable to consider how co-existing ions affect degradation efficiency or to simulate pollutants in real wastewater as comprehensively as possible. Wu et al.90 investigated the effect of co-existing anions on the degradation of SMT in their PEC system and found that the order of effect of the co-existing anions was PO43− > CO32− > Cl− (Fig. S1, ESI†). Zhang et al.91 constructed a 4-liter reactor and used ammonia, glucose, bovine serum albumin, and E. coli to represent inorganic matter, organic matter, macromolecules, and microbial pollutants contained in wastewater, respectively. The results showed that large protein molecules were much more difficult to destroy than E. coli. Alternatively, from another perspective, researchers can use some bulky indicators when expressing degradation efficiency, such as total organic carbon (TOC), chemical oxygen demand (COD), and biochemical oxygen demand (BOD), which are common in actual wastewater treatment. Therefore, the selection of actual wastewater as a contaminant, the consideration of the influence of co-existing ions, or the selection of bulky indicators can help to evaluate the performance of photoanodes more comprehensively.
5.2 Stability of photoanodes
Another fundamental purpose of studying photoanodes for actual wastewater treatment is to assess their stability after repeated use. The most common methods for characterizing the stability of photoanodes are illustrated in Fig. S2 (ESI†).In PEC wastewater treatment, studies identifying the causes of stability loss are limited. The Fourier-transform infrared (FTIR) spectra of the MoS2@BL-BiVO4 photoanode, after recycling tests in sewage, displayed new peaks compared with those observed after use in a NaCl solution. These peaks were attributed to the adsorption of natural organic matter (NOM) as noted by Zheng et al.92 The review of Zuo et al.93 clarified that the stability of electrodes is influenced by an electrochemical window and organic fouling or inorganic scaling. Understanding the key factors influencing the stability of photoanodes can help formulate strategies to maintain their performance. Liu et al.94 identified that the primary cause of photoanode deactivation is the reconstruction of the oxide surface structure, which occurs due to the coordination of the oxide with Cl− during seawater splitting. They further enhanced the stability of the β-Fe2O3 photoanode by improving the metal–oxygen interaction. By introducing Sn into the crystal lattice, the Sn/β-Fe2O3 photoanode demonstrated stability during seawater splitting for 3000 h. Li et al.95 modified the BiVO4/Cu2O heterojunction photoanode by using the co-catalyst cobalt–phosphate (Co–Pi). Co–Pi can effectively capture and release holes through the chemical state change of Co, which, in turn, inhibits photo-corrosion and improves electrode stability (Fig. S2h, ESI†). In summary, the stability of the photoanode can be improved by inhibiting the occurrence of both side reactions and photo-corrosion (Fig. 5).
Moreover, the cycling tests are mainly performed in the laboratory and use solutions containing certain target contaminants. Tests in real applications or using sewage are insufficient, and stability results may change under such conditions.
5.3 Light utilization efficiency
The enhancement of light harvesting through specialized geometrical structures and the reduction of the bandgap width of photocatalysts can significantly improve the sunlight utilization of photoanodes (Fig. 5). The most common indicators used to evaluate light utilization efficiency, along with corresponding examples, are summarized in eqn (S1)–(S4) and Fig. S3 (ESI†). Among these indicators, internal quantum efficiency (IQE) reflects the intrinsic efficiency of the material, while η accounts for the contribution of the external bias voltage. Altogether these two indicators effectively represent the light utilization efficiency of non-TiO2-based photoanodes. Furthermore, the evaluation methods primarily stem from PEC water splitting, and there is currently no established evaluation system for PEC wastewater treatment. Here, we refer to the commonly used equations (eqn (S3) and (S4) (ESI†)) for PEC water splitting and propose the photoelectrochemical mineralization efficiency (PME) by considering both pollutant degradation efficiency and externally input electrical energy, as shown in eqn (1): | (1) |
Herein, ΔG0 is the standard Gibbs free energy change (J mol−1) for the complete mineralization of pollutants, ΔTOC is the concentration of TOC removed (mg L−1), V is the volume of the reaction solution (L), nC is the number of carbon atoms in the pollutant, A is the effective reaction area of the electrode (m2), t is the reaction time (s), PE is the power consumed by the external bias (W m−2), and PL is the incident radiation power (W m−2).
5.4 Environmental applicability
The aforementioned metrics ultimately serve the environmental applicability of photoanodes, i.e., their application potential. Life cycle assessment (LCA) systematically evaluates the environmental impact of products throughout their life cycle by quantifying resource consumption, energy use, and environmental emissions. For example, Gao et al.96 prepared a NbClOx/BiVO4 photoanode using an electrochemical deposition method, which directly synthesized ClO− from seawater while simultaneously recovering high-value-added products. In the LCA of this work, the functional unit was defined as 1 kg of NaClO, and indicators such as fossil abiotic depletion potential, human toxicity potential, and global warming potential were used to quantify the environmental impact. Compared with dimensionally stabilized anodes, the NbClOx/BiVO4 photoanode reduced CO2 emissions by 75.31% and lowered electricity costs by 77.16% when producing the same amount of ClO− using conventional grid electricity. Zhang et al.97 developed a system that utilized electrons generated from PEC phenol degradation for cathodic ammonia synthesis. LCA results indicated that electricity consumption was the only critical factor affecting its overall economic and sustainability performance. Compared with standalone electrochemical ammonia synthesis, the integrated system reduced electricity consumption by 51.8% and exhibited lower greenhouse gas emissions.In studies on non-TiO2-based photoanodes, LCA applications remain limited, with most research focusing only on greenhouse gas emissions during pollutant degradation or techno-economic analyses. In the work conducted by Zheng et al.74 on reduced BiVO4 photoanodes for simultaneous organic pollutant degradation, ammonia nitrogen removal, bacterial inactivation, and hydrogen production, they categorized PEC process carbon emissions into direct and indirect emissions. Indirect emissions were linked to PEC electricity consumption and were compensated using the energy of the produced H2; the compensated electricity consumption was multiplied by the emission factor to calculate the indirect emissions. Techno-economic analyses typically calculate electricity consumption per unit volume of wastewater treated,14,98 per unit mass of pollutant removed46,99 or per order of pollutant concentration reduction.5 Notably, these analyses often focus solely on pollutant degradation, while electrode preparation also requires significant energy input, such as kilowatt-level oven usage for hydrothermal reactions, far exceeding the energy demands of electrochemical deposition. When the entire life cycle is considered, evaluation outcomes may differ. Additionally, most experiments are not conducted under direct sunlight, and simulated light sources consume significantly more energy than the electricity required to drive PEC processes,5 which is often overlooked during techno-economic analyses and deserves attention as well.
6. Mechanism characterization of PECs
To reveal how modified catalysts improve PEC performance, the study of the PEC mechanism is needed; thus, the characterization methods to reveal the mechanism are deep-level and vital work.6.1 Light absorption and exciton excitation
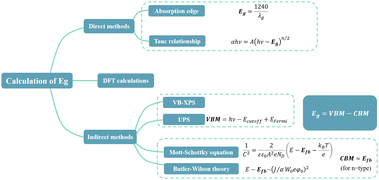
The crux of this process is to determine the light absorption efficiency of the semiconductor (see Section 5.3) and its bandgap energy (Eg).E g calculation methods can be divided into three categories: direct methods, indirect methods, and DFT calculations (Fig. 6). An introduction to these methods, along with corresponding examples, is summarized in eqn (S5)–(S12) and Fig. S4 (ESI†).
Table 2 presents the bandgap energy of some non-TiO2-based photoanodes. It is evident that the Eg values differ from the pristine Eg shown in Fig. 2a, which is attributed to the modification of catalysts. Additionally, the Tauc plot is the most widely used method for calculating Eg, yet few studies employ more than one method to calculate Eg. Since the Eg, CBM, and VBM positions are crucial for explaining the reaction mechanism, researchers must characterize this information in a mutually verifiable manner. Moreover, it is important to consider the suitability of characterization methods prior to conducting analysis. For instance, UV-vis DRS is not appropriate for semiconductors with intermediate energy states, which are often caused by defects.23 Additionally, the Kubelka–Munk function may introduce uncertainty in the analysis of doped semiconductors.100,101
| Photoanode | Calculation method | E g (eV) | Carrier lifetime | Calculation method | Ref. |
|---|---|---|---|---|---|
| hm-m-WO3/W mesh | Tauc plot | 2.87 | 6.21 ns | TRPL | 46 |
| WO3/BiVO4/FTO | Not mentioned | Not mentioned | 22.7 ms (transit time) | IMPS | 105 |
| BiVO4/ITO | Tauc plot | 2.45 | Not mentioned | Not mentioned | 12 |
| DFT | 2.23 | ||||
| BiVO4/FTO | Tauc plot; M–S plot, UPS | 2.54 | Not mentioned | Not mentioned | 106 |
| SnO2@BiVO4/FTO | Tauc plot; M–S plot, UPS | 2.46 | Not mentioned | Not mentioned | 86 |
| IrxZn1−xO/Ti | Tauc plot; DFT | 1.42–2.26 | 0.5 ms (charge relaxation time) | EIS | 107 |
| MgO/g-C3N4/FTO | Tauc plot | 3.34 | 5.67 ns | TRPL | 37 |
| α-Fe2O3/g-C3N4/FTO | Tauc plot | 2.08 | 4.2 ms | EIS | 48 |
| Sn-Fe2O3/NFO25/FTO | Tauc plot | 2.20 | Not mentioned | Not mentioned | 66 |
| ZnO/CdS/MoS2/FTO | Tauc plot | 2.25 | 10 ns | TPV | 108 |
| MoS2/Ti | Not mentioned | 1.80 | Not mentioned | Not mentioned | 77 |
| In2O3/In2S3/CdS/FTO | Tauc plot | ∼1.80 | 3.12 ns | TRPL | 41 |
| BiVO4/Cu2O/Co–P/FTO | Tauc plot | ∼2.40 | Not mentioned | OCP | 95 |
| Cu2O/Ag3PO4/FTO | Tauc plot | Not mentioned | 47.8 ms | EIS | 109 |
| BiVO4@TiO2/Ti | Tauc plot | 2.98 | 17.45 ns | TRPL | 110 |
6.2 Separation and transfer of carriers
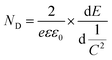 | (2) |
The meaning of each symbol has been explained in eqn (S10) and (S11) (ESI†). Some researchers used ND to approximate carriers’ density,48 and it is inversely related to the slope of the Mott–Schottky plot (Fig. 7a).
 | ||
| Fig. 7 Techniques used to characterize separation efficiency: schematic diagram of (a) the Mott–Schottky plot, (b) charge separation efficiency, (c) steady-state SPV results, and (d) steady-state PL spectra (where it is assumed that the separation efficiency of photoanode 1 is higher than that of photoanode 2); charge transfer kinetics: schematic diagram of (e) TRPL decay spectra, (f) transient photovoltage result, (g) Bode plot, and (h) normalized OCP decay curves (where it is assumed that photoanode 1 has a longer decay lifetime than photoanode 2); charge transfer pathway: (i) schematic XPS spectra of the composite photoanode under different conditions, (j) and (k) SEM images of the WO3 photoanode after photochemical deposition of Ag and Co3O4, reprinted from ref. 46, Copyright (2022), with permission from Elsevier. (l) and (m) DDA simulations for the electric field intensity at the WO3 nanoplate and Ag nanocrystal interface before and after Hg2+ pretreatment, reprinted with permission from ref. 51 Copyright (2023) Wiley-VCH. | ||
The photocurrent is an important indicator to imply carrier density and separation efficiency. Chronoamperometry is usually used to reveal how photocurrent changes over time; current (density)–time curves (Fig. S6a, ESI†) and transient photocurrent (density) (Fig. S6b, ESI†) are two main forms of it. LSV (Fig. S6c, ESI†) and chopped LSV (Fig. S6d, ESI†) are usually used to depict how photocurrent changes with applied potential. The measurement of photocurrent in different electrolytes can be used to calculate charge separation efficiency according to eqn (3):103
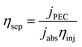 | (3) |
 | (4) |
 | (5) |
 | (6) |
Herein, λ0 is the absorption edge of the photoanode, Jflux is the current flux, Nph is the photon flux and can be obtained from the spectrum of the light; and hν is the energy of the photon. After obtaining the above information, the ηsep plot of the photoanode at different bias potentials can be plotted (Fig. 7b). For example, Wang et al.103 found that the charge separation efficiency of GaN:ZnO photoanodes increases with decreasing moisture exposure time; corresponding information is shown in Fig. S7 (ESI†).
In addition to the photocurrent, as shown in Scheme 1, the e−–h+ pairs generated by the light excitation of the photoanode create a surface photovoltage (SPV) after separation within the SCL. The steady-state SPV reflects the separation efficiency of the photogenerated charges at the surface/interface (Fig. 7c).111 PL spectra can also be used to analyze separation efficiency, as the recombination of h+ and e− results in photon emission; a smaller PL response indicates more efficient charge separation (Fig. 7d). Finally, DFT can provide insights into the separation and migration properties of charges by calculating the effective masses of holes and electrons since the efficiency of charge separation and migration is inversely proportional to the effective mass.112
 | (7) |
 | (8) |
Lastly, similar to the form of eqn (7), Zeng et al.114 carried out intensity-modulated photocurrent spectroscopy (IMPS) to measure the majority charge carrier transit time—the average time required for the photogenerated carriers to reach the back contact of the substrate—of their WO3/BiVO4 photoanode, as described in eqn (9):
 | (9) |
However, characterizing the lifetime of photoinduced carriers is complex due to various factors. First, do different techniques yield similar results for the same catalysts? As shown in Table 2, the carrier lifetimes calculated using TRPL and TPV are in the nanosecond range, while those obtained from EIS and IMPS are in the millisecond range. It is crucial to determine whether this significant discrepancy arises from the catalysts themselves or the characterization methods employed. Additionally, the literature presents conflicting explanations regarding the relationship between carrier lifetime and separation efficiency. Gao et al.10 argued that the shorter lifetime measured via TRPL indicated more effective photoexcited charge separation and transfer. Similarly, Song et al.15 suggested that the shorter lifetimes of photogenerated holes measured through transient absorption spectra (TAS) also reflects an efficient charge separation and transfer process. These interpretations contradict the aforementioned view that longer lifetimes signify more effective separation. When considering the time carriers spent in the external circuit or at the interface of the photoanode and solution, a shorter carrier lifetime suggests faster transfer and more efficient utilization. However, when examining transfer within the bulk of the photoanode, a longer lifetime implies reduced recombination. Therefore, it is essential to compare measurement techniques for carrier lifetimes to enhance the credibility of results, and the interpretation of these results should be as clear as possible.
Apart from these methods, Li and coworkers46 synthesized photoanodes featuring WO3/W heterophase junction structures, significantly enhancing the separation of photoinduced h+ and e−. They proposed a matched band structure for monoclinic WO3 and hexagonal WO3 based on the results of UV-vis DRS and Mott–Schottky curves, further verifying their assumption through photochemical deposition experiments. As shown in the SEM images in Fig. 7j and k, the deposition of Ag nanoparticles on monoclinic WO3 and the formation of Co3O4 nanoparticles on hexagonal WO3 indicated the accumulation of e− and h+, respectively, which aligned with the charge transfer pathway they proposed.
WO3/Ag Schottky heterojunction photoanodes were prepared by Wang et al.51 Under light exposure, hot electrons were generated at the interface of WO3 and Ag due to the localized surface plasmon resonance (LSPR) effect. However, this effect was quenched upon the combination of Ag and Hg2+. As a result, the corresponding photocurrent decreased, enabling both the reduction of Hg and the simultaneous detection of Hg concentration. Utilizing discrete dipole approximation (DDA) simulations, they explored the spatial distribution of the LSPR-induced electric field. In the absence of Hg2+, the WO3–Ag interface exhibited the highest electric field enhancement (EFE) (Fig. 7l). Conversely, in the presence of Hg2+, the formation of surface Ag2−xHgx resulted in diminished oscillation and polarization, leading to reduction in EFE (Fig. 7m).
Furthermore, internal electric fields (IEF) are commonly referenced in discussions of charge separation in PECs; hence, characterizing IEF is crucial for understanding the intrinsic mechanisms involved. Recently, Yuan's group115 concluded characterization techniques to identify IEF. These techniques include determining the work function of semiconductors using UPS, Kelvin probe force microscopy (KPFM), and DFT calculations, as well as measuring the surface potential through KPFM, piezo-response force microscopy (PFM), and SPV. Additionally, they indirectly demonstrated the formation of IEF through free radical quenching experiments and electron paramagnetic resonance (EPR) studies.
6.3 Interface reaction process
This process is influenced by two factors. The first factor is the electrode itself, which includes its redox capacity, the active species produced, and the interfacial charge transfer rate. The second factor is the contaminant, encompassing its mass transfer processes, adsorption characteristics, and reactive sites.When the applied bias is low and is used solely to accelerate the separation of e−–h+ pairs, the redox ability is primarily determined by the band position of the photocatalyst. The positions of the VB and CB can be determined using various methods described in Section 5.2.1. Under certain conditions, once the bandgap of semiconductors is determined, it can also facilitate the calculation of the conduction band minimum (CBM) and valence band maximum (VBM). For instance, Yang et al.116 calculated the VB potential of C3N4–MoS2 using eqn (10):
| VBM = X − Ee + 0.5Eg | (10) |
| CBM = −Φ + 0.5Eg | (11) |
| VBM = −Φ − 0.5Eg | (12) |
By comparing the band edge position with the potentials of various redox reactions, we can deduce possible reactions and reactive species. As the applied potential increases, it is important to consider electro-oxidation or electro-reduction.118
Like other AOPs, in PECs, some reactions are dominated by reactive species, while others may be influenced more by electron transfer following surface adsorption. The primary reactive species utilized in non-TiO2-based PECs include radicals such as hydroxyl radicals (˙OH) and superoxide radicals (O2˙−). Occasionally, chlorine radicals (Cl˙),40 chlorite radicals (ClO˙),119 sulfate radicals (SO4˙−),44 carbonate radicals (CO3˙−),120 and others are considered when PECs are combined with other AOPs. The nonradical pathway typically involves the participation of h+, e−, and sometimes singlet oxygen (1O2).121 To assess the contributions of these reactive species, researchers commonly employ quenching experiments, probe techniques, and EPR to characterize the roles of these species qualitatively and quantitatively. However, some researchers have proposed that adding high-concentration ROS quenchers may alter the catalytic mechanisms within their systems.122 Moreover, in the PEC process, quenching of holes or electrons can enhance contaminant removal efficiency by promoting the separation of holes and electrons.81 Recently, Yang's group123 supplemented the reaction rate constants of probes and quenchers with commonly used reactive species, and general recommendations were put forward for the selection of appropriate probes and quenchers.
Several techniques can be employed to characterize interfacial charge transfer efficiency. As noted in eqn (3) and (4), charge injection efficiency can be derived from eqn (13):
 | (13) |
 | ||
| Fig. 8 Techniques for characterizing interfacial reaction processes: (a) photocurrent–time curves of pristine BiVO4, SnO2@BiVO4, and SnO2@BiVO4/Co–Pi photoanodes, reprinted with permission from ref. 86, Copyright (2019) Royal Society of Chemistry. Schematic diagram of (b) Tafel curves and (c) Nyquist plots (where it is assumed that photoanode 1 has faster interfacial charge transfer kinetics than photoanode 2). (d) IMPS Nyquist plots of the Bi2S3/ZnO NRA and ZnS/Bi2S3/ZnO NRA, respectively, reprinted with permission from ref. 54, Copyright (2022) American Chemical Society. (e) CFD simulations of the flow velocity in the plate and network electrode in flowing water, reprinted from ref. 46, Copyright (2022), with permission from Elsevier. (f) Contact angle of EG and MoS2/Ag@WO3/EG photoanodes, reprinted from ref. 124, Copyright (2023), with permission from Elsevier. | ||
Mass transfer at the interface of the pollutant electrode can be calculated using computer simulations. To analyze the fluid behavior around the electrode, Ma et al.46 conducted computational fluid dynamics (CFD) simulations on both the plate electrode and the WO3/W mesh electrode. As shown in Fig. 8e, the grids in the mesh electrode enhanced fluid flow and facilitated contact between the contaminant and the catalyst compared to the plate electrode.
To investigate the adsorption of pollutants at the electrode, N2 adsorption–desorption isotherms can help to determine the specific surface area.46,81 FTIR provides insights into the adsorption mechanism,81 and contact angle measurements can evaluate hydrophobicity. Mafa et al.124 synthesized a visible light-responsive MoS2/Ag@WO3/EG photoanode. They compared bare EG and MoS2/Ag@WO3/EG photoanodes using contact angle measurements; the results showed that the contact angles of the two were 84.76° and 64.87°, respectively (Fig. 8f). A smaller contact angle indicates better hydrophilicity, facilitating full contact between the anode and pollutant molecules and enhancing the generation of ˙OH from water molecules on the electrode surface.
The electronic structure of pollutants can help to understand the degradation mechanisms. The highest occupied molecular orbital (HOMO), lowest unoccupied molecular orbital (LUMO), and Fukui index are commonly used to describe the charge distribution of organic contaminants.125 For instance, Zhang et al.48 employed DFT simulation to illustrate the HOMO and LUMO orbital distributions, charge distribution, and Fukui index of clofibric acid. Their simulated results facilitated predictions regarding the feasibility of specific sites in clofibric acid for free radical attacks. By integrating their computational findings with data from a high-resolution mass spectrometer, they proposed pathways for the catalytic degradation of clofibric acid.
7. Conclusion and perspectives
7.1 Conclusion
In recent years, non-TiO2-based photoanodes used for wastewater treatment have been a field of interest for researchers and have undergone rapid development. Given that the reasonable synthesis of photoanodes can help achieve the best performance of photocatalysts, comprehensive evaluation can help to reasonably analyze the application prospects, and sufficient characterization can help to intuitively understand the intrinsic mechanisms; this review introduces the synthesis, evaluation, and characterization methods of non-TiO2-based photoanodes used for wastewater treatment.Compared with TiO2-based photoanodes, different non-TiO2-based photoanodes have potential applications in various scenarios. For example, WO3, g-C3N4, BiVO4, and Fe2O3 have the potential for visible light response; WO3, ZnO, and Fe2O3 have numerous applications in PFC; and WO3, BiVO4, and their composites are suitable for the removal of ammonia nitrogen.
The techniques for electrode synthesis include wet-chemical methods, electrochemical methods, other chemical methods, and physical methods. When considering practical applications, it is important to evaluate the cost of the synthesis method, the stability of the electrode, and the potential for large-scale production. Currently, the wet-chemical and electrochemical methods are the most commonly used methods for synthesizing non-TiO2-based photoanodes. However, overly simple operating conditions may compromise electrode stability. In contrast, the less commonly used physical method is costly but offers large-scale coating capabilities.
When evaluating the effect of electrode degradation on pollutants, it is necessary to pay attention to the ability of the photoanode to treat actual sewage. When assessing stability, it is also essential to identify the key factors affecting it. When evaluating light utilization efficiency, it is necessary to develop more sophisticated equations to incorporate pollutant degradation and electrical energy input. Finally, the assessment of the environmental applicability of the photoanode is indispensable, as it determines the potential for its practical application.
To deeply reveal the mechanism of PEC wastewater treatment, the bandgap of the catalyst should be confirmed through various methods. Additionally, the separation efficiency, carrier kinetics, and transfer pathways of the carriers should be further investigated. It is also important to consider the redox ability of the electrode interface, as well as the physical and chemical conversion processes of pollutants during PEC wastewater treatment.
Despite the aforementioned recommendations for photoanode synthesis and a systematic summary of non-TiO2-based photoanode evaluation and characterization methods, several fundamental questions still remain in the following areas.
7.2 Perspectives: from the atomic level to large-scale applications
The synthesis, evaluation, and characterization of non-TiO2-based photoanodes should be closely interconnected. The synthesis method influences the evaluation and characterization results, while the characterization outcomes can, in turn, enhance the synthesis approach. This review paper proposes a comprehensive overview of synthesis, evaluation, and characterization methods, spanning from the micro-level to the electrode level and extending to large-scale applications (Scheme 2).(i) At the micro-level, synthesizing catalysts should prioritize the micro-morphology of photocatalysts, the regulation of exposed crystal facets, and the enhancement of synergy among different components in composite materials. It is also important to improve the dispersion and utilization efficiency of cocatalysts, such as single-atom catalysts (SACs). Furthermore, there are some catalysts with excellent performance in PEC water splitting that can be considered for PEC wastewater treatment. For instance, Ta3N5 exhibits potential in photocatalytic pollutant removal due to its superior visible-light responsiveness (Eg = 2.1 eV)126 and low raw material supply risk.127 Notably, its lower valence band position128 makes it more suitable for constructing heterojunctions with other photocatalysts.129,130
To better understand the catalyst structure at the atomic level, more advanced characterization methods should be introduced, for example, electron microscopy with higher resolution (e.g., high-angle angular dark field-scanning transmission electron microscopy (HAADF-STEM)), electron microscopy that changes the properties of the photoanode as little as possible during observation (e.g., cryogenic electron microscopy (cryo-EM)), and energy spectroscopy that can analyze finer coordination environments (e.g., extended X-ray absorption fine structure (EXAFS)). Furthermore, to infer the mechanism of photoanode action more intuitively, it is necessary to represent the changes in the photoanode during the PEC water treatment process; therefore, in situ characterization techniques are very important. In situ photoelectrochemical characterization should be performed while the PEC system degrades pollutants, and in situ characterization results can also be obtained by controlling the input light and voltage.
(ii) At the electrode level, maximizing catalyst efficiency requires careful consideration of the most suitable substrate, including factors such as conductivity and stability of the catalyst film. The in situ growth method should be chosen for preparing the photoanode. Additionally, the selection of reactor configuration, electrolyte, and light source will also impact the efficiency of non-TiO2-based photoanodes.
Correspondingly, attention should also be given to characterizing the physical properties of the photoanode, including hydrophobicity, specific surface area, and interface resistance, as well as the interfacial physical processes related to pollutants, such as adsorption and mass transfer.
(iii) At the large-scale application level, although researchers have made attempts to carry out degradation under sunlight and prepare large-area electrodes (see Sections 3 and 4.3), there are currently few large-scale applications for PEC wastewater treatment. From the perspective of electrode preparation, the challenges that limit scale-up mainly include equipment that does not support large-area preparation, time-consuming preparation methods, and the high costs of catalysts and substrates. To address these difficulties, researchers could employ scalable preparation methods such as sputtering, modularly produce small-area electrodes for assembly into large-area electrodes,131 and select metal foils, sheets, or rods rather than conductive glass as electrode substrates. What's more, it is also worth considering the supply risk of PEC materials, with Hillenbrand et al.127 showing that hematite is the material with the lowest current supply risk, while bismuth vanadate has the highest future supply risk.
At this level, attention to characterization and evaluation should not focus solely on pollutant removal efficiency. A photoanode with strong stability, low energy consumption, or high light utilization efficiency can also enhance its overall performance. Additionally, the degradation of a single target pollutant may produce more toxic byproducts, making high degradation efficiency appear misleadingly one-sided. Thus, it is essential to develop a more comprehensive evaluation system that encompasses multiple dimensions.
(iv) Notably, machine learning is instructive in both the synthesis and characterization of photoanodes, enhancing the interaction and feedback between synthesis and characterization methods. For instance, screening suitable photocatalysts or substrates quickly and accurately is a massive task, while machine learning can help solve this problem. It can not only guide the screening, preparation, and optimization of new catalysts under different environmental application scenarios by combining the data obtained from different routes but also improve the analysis of characterization results by integrating characterization techniques, such as electron microscope image recognition and extraction of information from wave spectra.
Once the aforementioned suggestions are addressed, it will greatly benefit the development of photoelectrocatalysis in the environmental field. This will advance the preparation of photoanodes for industrial applications and enhance deeper mechanistic characterization. We anticipate that this review will inspire more frontier research in the synthesis, evaluation, and characterization of non-TiO2-based photoanodes, thereby attracting significant attention in the field of PEC wastewater treatment.
Conflicts of interest
There are no conflicts of interest to declare.Data availability
No primary research results, software or code have been included and no new data have been generated or analyzed in this review.Acknowledgements
This work was supported by the National Key Research and Development Program International Cooperation Project (2023YFE0108100), Tianjin Research Innovation Project for Postgraduate Students (2022SKY005), and Fundamental Research Funds for the Central Universities, Nankai University.References
- W. Xiong, H. Guo, Y. Liu, Y. Meng, Y. Jiang, B. Li, R. Liu and C. Yang, Chem. Eng. J., 2024, 489, 151389 CrossRef CAS.
- G. Ren, M. Zhou, Q. Zhang, X. Xu, Y. Li and P. Su, Chem. Eng. J., 2020, 387, 124037 CrossRef CAS.
- Y. Xie, Q. Rong, F. Mao, S. Wang, Y. Wu, X. Liu, M. Hao, Z. Chen, H. Yang, G. I. N. Waterhouse, S. Ma and X. Wang, Nat. Commun., 2024, 15, 2671 CrossRef CAS PubMed.
- F. Li, K. Liu, Y. Bao, Y. Li, Z. Zhao, P. Wang and S. Zhan, Water Res., 2024, 254, 121373 CrossRef CAS PubMed.
- J. Liu, R. Liang, Z. Hu, X. Zhang and M. Zhou, Chem. Eng. J., 2024, 491, 152088 CrossRef CAS.
- B. Li, H. Pan and B. Chen, Water Res., 2023, 244, 120537 CrossRef CAS PubMed.
- S. Li, J. Xie, J. Gu and M. Zhou, Chin. Chem. Lett., 2023, 34, 108204 CrossRef CAS.
- F. Deng, H. Olvera-Vargas, M. Zhou, S. Qiu, I. Sirés and E. Brillas, Chem. Rev., 2023, 123, 4635–4662 CrossRef CAS PubMed.
- S. Li, W. Wang, H. Wu, X. Zhang, R. Liang, X. Zhang, G. Song, J. Jing, S. Li and M. Zhou, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e1890002175 Search PubMed.
- C. Gao, J. Low, R. Long, T. Kong, J. Zhu and Y. Xiong, Chem. Rev., 2020, 120, 12175–12216 CrossRef CAS PubMed.
- Q. Zhou, Y. Guo and Y. Zhu, Nat. Catal., 2023, 6, 574–584 CrossRef CAS.
- F. Li, W. Zhao and D. Y. C. Leung, Appl. Catal., B, 2019, 258, 117954 CrossRef CAS.
- V. Andrei, G. M. Ucoski, C. Pornrungroj, C. Uswachoke, Q. Wang, D. S. Achilleos, H. Kasap, K. P. Sokol, R. A. Jagt, H. Lu, T. Lawson, A. Wagner, S. D. Pike, D. S. Wright, R. L. Z. Hoye, J. L. MacManus-Driscoll, H. J. Joyce, R. H. Friend and E. Reisner, Nature, 2022, 608, 518–522 CrossRef CAS PubMed.
- R. Liang, H. Wu, Z. Hu, J. Sun, C. Fu, S. Li, X. Zhang and M. Zhou, Appl. Catal. B-Environ. Energy, 2024, 352, 124042 CrossRef CAS.
- R. Song, H. Chi, Q. Ma, D. Li, X. Wang, W. Gao, H. Wang, X. Wang, Z. Li and C. Li, J. Am. Chem. Soc., 2021, 143, 13664–13674 CrossRef CAS PubMed.
- Y. Zhang, Y. Ji, J. Li, J. Bai, S. Chen, L. Li, J. Wang, T. Zhou, P. Jiang, X. Guan and B. Zhou, J. Hazard. Mater., 2021, 402, 123725 CrossRef CAS PubMed.
- Y. Yu and H. Huang, Chem. Eng. J., 2023, 453, 139755 CrossRef CAS.
- R. Dhawle, D. Mantzavinos and P. Lianos, Appl. Catal., B, 2021, 299, 120706 CrossRef CAS.
- L. Li, J. Li, F. Fang, Y. Zhang, T. Zhou, C. Zhou, J. Bai and B. Zhou, Appl. Catal., B, 2023, 333, 122776 CrossRef CAS.
- M. B. Costa, M. A. de Araújo, M. V. D. L. Tinoco, J. F. D. Brito and L. H. Mascaro, J. Energy Chem., 2022, 73, 88–113 CrossRef CAS.
- S. Garcia-Segura and E. Brillas, J. Photochem. Photobiol., C, 2017, 31, 1–35 CrossRef CAS.
- L. Y. Ozer, C. Garlisi, H. Oladipo, M. Pagliaro, S. A. Sharief, A. Yusuf, S. Almheiri and G. Palmisano, J. Photochem. Photobiol., C, 2017, 33, 132–164 CrossRef CAS.
- F. Parrino, V. Loddo, V. Augugliaro, G. Camera-Roda, G. Palmisano, L. Palmisano and S. Yurdakal, Catal. Rev.: Sci. Eng., 2019, 61, 163–213 CrossRef CAS.
- X. Wang, H. Xu, Y. Nan, X. Sun, J. Duan, Y. Huang and B. Hou, J. Oceanol. Limnol., 2020, 38, 1018–1044 CrossRef CAS PubMed.
- Z. Zhang and J. T. Yates, Chem. Rev., 2012, 112, 5520–5551 CrossRef CAS PubMed.
- R. Das Adhikari, M. J. Patel, H. Baishya, D. Yadav, M. Kalita, M. Alam and P. K. Iyer, Chem. Soc. Rev., 2025, 54, 3962–4034 RSC.
- S. Corby, R. R. Rao, L. Steier and J. R. Durrant, Nat. Rev. Mater., 2021, 6, 1136–1155 CrossRef CAS.
- J. Zhu, D. Shao, W. Wen, Z. Tian, X. Zhang and S. Wang, Coord. Chem. Rev., 2024, 518, 216095 CrossRef CAS.
- M. Li, Y. Liu, L. Dong, C. Shen, F. Li, M. Huang, C. Ma, B. Yang, X. An and W. Sand, Sci. Total Environ., 2019, 668, 966–978 CrossRef CAS PubMed.
- Z. Hu, M. Zhou, H. A. Maitlo, R. Liang, Y. Zheng, H. Wu, X. Song and O. A. Arotiba, Appl. Catal., B, 2023, 331, 122676 CrossRef CAS.
- Z. Wang, X. Liu, Y. Mao, H. Zhang, P. Wang, Z. Zheng, Y. Liu, Y. Dai, H. Cheng, Z. Wang and B. Huang, Adv. Funct. Mater., 2024, 34, 2313950 CrossRef CAS.
- C. Dong, Y. Yang, X. Hu, Y. Cho, G. Jang, Y. Ao, L. Wang, J. Shen, J. H. Park and K. Zhang, Nat. Commun., 2022, 13, 4982 CrossRef CAS PubMed.
- T. Tong, M. Zhang, W. Chen, X. Huo, F. Xu, H. Yan, C. Lai, W. Wang, S. Hu, L. Qin and D. Huang, Coord. Chem. Rev., 2024, 500, 215498 CrossRef CAS.
- P. Jiang, T. Zhou, J. Bai, Y. Zhang, J. Li, C. Zhou and B. Zhou, Water Res., 2023, 235, 119914 CrossRef CAS PubMed.
- H. Ullah, Z. Haneef, A. Ahmad, I. S. Butler, R. Nasir Dara and Z. Rehman, Inorg. Chem. Commun., 2023, 153, 110775 CrossRef CAS.
- X. Pang, R. Liu, X. Lv, W. Lu, L. Sun, Q. Wang, Z. Li, Q. Kang, J. Xie, Y. Pang and F. Zhou, RSC Adv., 2024, 14, 32883–32892 RSC.
- J. Sun, H. Wu, C. Fu, C. Zhang, Z. Hu and M. Zhou, Appl. Catal. B-Environ. Energy, 2024, 351, 123976 CrossRef CAS.
- C. Xie, Q. Zhang, Q. Zeng, Q. Zhang, Y. Liu, G. Tang, J. Lv, T. Yu and Q. Zeng, Chem. Eng. J., 2024, 480, 148363 CrossRef CAS.
- Z. Yan, W. Kuang, Y. Lei, W. Zheng, H. Fu, H. Li, Z. Lei, X. Yang, S. Zhu and C. Feng, Environ. Sci. Technol., 2023, 57, 20915–20928 CrossRef CAS PubMed.
- Y. Zhang, J. Cui and Y. Pei, Chem. Eng. J., 2023, 464, 142652 CrossRef CAS.
- Y. Xiao, G. Tian, Y. Chen, X. Zhang, H. Fu and H. Fu, Appl. Catal., B, 2018, 225, 477–486 CrossRef CAS.
- L. Cheng, Y. Tian and J. Zhang, J. Colloid Interface Sci., 2018, 526, 470–479 CrossRef CAS PubMed.
- S. P. Ratnayake, J. Ren, J. van Embden, C. F. McConville and E. Della Gaspera, J. Mater. Chem. A, 2021, 9, 25641–25650 RSC.
- X. Fan, Y. Zhou, G. Zhang, T. Liu and W. Dong, Appl. Catal., B, 2019, 244, 396–406 CrossRef CAS.
- H. Miao, J. Yang, G. Peng, H. Li and Y. Zhu, Sci. Bull., 2019, 64, 896–903 CrossRef CAS PubMed.
- Q. Ma, R. Song, F. Ren, H. Wang, W. Gao, Z. Li and C. Li, Appl. Catal., B, 2022, 309, 121292 CrossRef CAS.
- W. Fei, J. Gao, N. Li, D. Chen, Q. Xu, H. Li, J. He and J. Lu, J. Hazard. Mater., 2021, 402, 123515 CrossRef CAS PubMed.
- L. Zhang, X. Zhang, C. Wei, F. Wang, H. Wang and Z. Bian, Chem. Eng. J., 2022, 435, 134873 CrossRef CAS.
- X. Liu, H. Zhou, S. Pei, S. Xie and S. You, Chem. Eng. J., 2020, 381, 122740 CrossRef CAS.
- S. Lam, J. Sin, H. Zeng, H. Lin, H. Li, Z. Qin, J. W. Lim and A. R. Mohamed, Sep. Purif. Technol., 2021, 265, 118495 CrossRef CAS.
- J. Wang, S. Liu, T. Liu, J. Wang, F. Liu, M. Jia, J. Li, Y. Lai, Y. Zhao, L. Jiang, Y. Li and T. Zhai, Adv. Funct. Mater., 2023, 33, 2302809 CrossRef CAS.
- J. Feng, Y. Tian, O. K. Okoth, L. Cheng and J. Zhang, J. Electrochem. Soc., 2019, 166, H685–H690 CrossRef CAS.
- L. Xia, J. Bai, J. Li, Q. Zeng, L. Li and B. Zhou, Appl. Catal., B, 2017, 204, 127–133 CrossRef CAS.
- Y. Lu, R. Popescu, D. Gerthsen, Y. Feng, W. Su, Y. Hsu and Y. Chen, ACS Appl. Mater. Interfaces, 2022, 14, 7756–7767 CrossRef CAS PubMed.
- B. O. Orimolade and O. A. Arotiba, Sci. Rep., 2020, 10, 5348 CrossRef CAS PubMed.
- L. R. Nagappagari, J. Lee, H. Lee, B. Jeong and K. Lee, Environ. Pollut., 2021, 271, 116318 CrossRef CAS PubMed.
- K. Li, Y. Xu, Y. He, C. Yang, Y. Wang and J. Jia, Environ. Sci. Technol., 2013, 47, 3490–3497 CrossRef CAS PubMed.
- Q. Zhang, Q. Deng, Y. Zhang, Y. Xiao, C. Zhang, H. Gong, Q. Zeng, Q. Zhang and Q. Zeng, Appl. Catal. B-Environ. Energy, 2024, 347, 123808 CrossRef CAS.
- P. Teng, J. Zhu, X. Wen, Z. Li, S. Gao, K. Li, N. Copner, Z. Liu, H. Jiang, Y. Zhang and F. Tian, Opt. Mater., 2023, 146, 114318 CrossRef CAS.
- A. A. Nada, B. O. Orimolade, H. H. El-Maghrabi, B. A. Koiki, M. Rivallin, M. F. Bekheet, R. Viter, D. Damberga, G. Lesage, I. Iatsunskyi, E. Coy, M. Cretin, O. A. Arotiba and M. Bechelany, Appl. Mater. Today, 2021, 24, 101129 CrossRef.
- S. Lam, J. Sin, H. Zeng, H. Li, H. Lin, L. Huang, J. Lim and K. Dong, Sep. Purif. Technol., 2024, 329, 125249 CrossRef CAS.
- D. Zhang, W. Zhang, J. Zhang, L. Dong, X. Chen, Y. Guan, Z. Wang and Y. Li, Chem. Eng. J., 2024, 480, 147910 CrossRef CAS.
- R. M. Fernández-Domene, R. Sánchez-Tovar, B. Lucas-granados, M. J. Muñoz-Portero and J. García-Antón, Chem. Eng. J., 2018, 350, 1114–1124 CrossRef.
- T. Dong, Z. Zheng, J. H. K. Man and I. M. C. Lo, Chem. Eng. J., 2024, 499, 155840 CrossRef CAS.
- M. Jia, Q. Liu, W. Xiong, Z. Yang, C. Zhang, D. Wang, Y. Xiang, H. Peng, J. Tong, J. Cao and H. Xu, Appl. Catal., B, 2022, 310, 121344 CrossRef CAS.
- S. Seenivasan, S. Adhikari and D. Kim, Chem. Eng. J., 2021, 422, 130137 CrossRef CAS.
- G. Li, Z. Lian, W. Wang, D. Zhang and H. Li, Nano Energy, 2016, 19, 446–454 CrossRef CAS.
- P. Mane, I. V. Bagal, H. Bae, M. A. Kulkarni, A. Abdullah, S. Ryu and J. Ha, J. Electroanal. Chem., 2022, 922, 116729 CrossRef CAS.
- J. Huang, T. Lin, L. Lin, G. Ma, Z. Zhang, S. Handschuh-Wang, A. Meng, P. Han and B. He, ACS Appl. Energy Mater., 2024, 7, 10710–10720 CrossRef CAS.
- J. Li, X. Li, S. Yu, S. Gao, Y. Zhang, Y. Li, C. Wang and Q. Wang, Chin. Chem. Lett., 2023, 34, 107417 CrossRef CAS.
- S. He, D. Xie, B. Wang, M. Zhu and S. Hu, J. Colloid Interface Sci., 2023, 650, 1993–2002 CrossRef CAS PubMed.
- X. Z. Li and H. S. Liu, Environ. Sci. Technol., 2005, 39, 4614–4620 CrossRef CAS PubMed.
- H. Feng, Y. Liang, K. Guo, N. Li, D. Shen, Y. Cong, Y. Zhou, Y. Wang, M. Wang and Y. Long, Water Res., 2016, 102, 428–435 CrossRef CAS PubMed.
- Z. Zheng, J. H. K. Man and I. M. C. Lo, Environ. Sci. Technol., 2022, 56, 16156–16166 CrossRef CAS PubMed.
- O. Samuel, M. H. D. Othman, R. Kamaludin, O. Sinsamphanh, H. Abdullah, M. H. Puteh and T. A. Kurniawan, Ceram. Int., 2022, 48, 5845–5875 CrossRef CAS.
- F. Zhao, W. Li, Y. Song, Y. Fu, X. Liu, C. Ma, G. Wang, X. Dong and H. Ma, Appl. Mater. Today, 2022, 26, 101390 CrossRef.
- Y. Zhou, X. Fan, G. Zhang and W. Dong, Chem. Eng. J., 2019, 356, 1003–1013 CrossRef CAS.
- X. Wang, G. Liu, J. Liu, G. Ren and P. Wang, Chem. Eng. J., 2024, 495, 153170 CrossRef CAS.
- Y. Peng and S. C. E. Tsang, Nano Today, 2018, 18, 15–34 CrossRef CAS.
- R. A. Rather, A. Mehta, Y. Lu, M. Valant, M. Fang and W. Liu, Int. J. Hydrogen Energy, 2021, 46, 21866–21888 CrossRef CAS.
- J. Yang, N. Sun, Z. Zhang, J. Bian, Y. Qu, Z. Li, M. Xie, W. Han and L. Jing, ACS Appl. Mater. Interfaces, 2020, 12, 28264–28272 CrossRef CAS PubMed.
- H. Wu, Z. Hu, R. Liang, X. Zhang, M. Zhou and O. A. Arotiba, J. Hazard. Mater., 2023, 456, 131696 CrossRef CAS PubMed.
- H. Wang, S. Wang, M. T. Oo, Y. Yang, J. Zhou, M. Huang and R. Zhang, J. Colloid Interface Sci., 2023, 646, 687–694 CrossRef CAS PubMed.
- Y. Wang, J. Wu, Y. Yan, L. Li, P. Lu, J. Guan, N. Lu and X. Yuan, Chem. Eng. J., 2021, 403, 126313 CrossRef CAS.
- F. Khodabandeloo, M. Sheydaei, P. Moharramkhani, M. Masteri-Farahani and A. Khataee, Chemosphere, 2023, 330, 138766 CrossRef CAS PubMed.
- J. Liu, J. Li, M. Shao and M. Wei, J. Mater. Chem. A, 2019, 7, 6327–6336 RSC.
- K. Guima, L. E. Gomes, J. Alves Fernandes, H. Wender and C. A. Martins, ACS Appl. Mater. Interfaces, 2020, 12, 54563–54572 CrossRef CAS PubMed.
- H. Wu, J. Jing, S. Li, S. Li, J. Liu, R. Liang, Y. Zhang, Z. Xia and M. Zhou, Appl. Catal. B-Environ. Energy, 2025, 360, 124517 CrossRef CAS.
- R. A. Rather and I. M. C. Lo, Water Res., 2020, 168, 115166 CrossRef CAS PubMed.
- H. Wu, Z. Hu, R. Liang, O. V. Nkwachukwu, O. A. Arotiba and M. Zhou, Appl. Catal., B, 2023, 321, 122053 CrossRef CAS.
- G. Zhang, Z. Zhang, D. Xia, Y. Qu and W. Wang, J. Hazard. Mater., 2020, 392, 122292 CrossRef CAS PubMed.
- Z. Zheng and I. M. C. Lo, Appl. Catal., B, 2021, 299, 120636 CrossRef CAS.
- K. Zuo, S. Garcia-Segura, G. A. Cerrón-Calle, F. Chen, X. Tian, X. Wang, X. Huang, H. Wang, P. J. J. Alvarez, J. Lou, M. Elimelech and Q. Li, Nat. Rev. Mater., 2023, 8, 472–490 CrossRef.
- C. Liu, N. Zhang, Y. Li, R. Fan, W. Wang, J. Feng, C. Liu, J. Wang, W. Hao, Z. Li and Z. Zou, Nat. Commun., 2023, 14, 4266 CrossRef CAS PubMed.
- X. Li, J. Wan, Y. Ma, Y. Wang and X. Li, Chem. Eng. J., 2021, 404, 127054 CrossRef CAS.
- R. Gao, Z. Gao, N. T. Nguyen, J. Chen, X. Liu, L. Wang and L. Wu, Nat. Sustainability, 2025, 1–10, DOI:10.1038/s41893-025-01530-y.
- J. Zhang, G. Zhang, H. Lan, H. Liu and J. Qu, Chem. Eng. J., 2022, 428, 130373 CrossRef CAS.
- M. S. Koo, X. Chen, K. Cho, T. An and W. Choi, Environ. Sci. Technol., 2019, 53, 9926–9936 CrossRef CAS PubMed.
- Y. Zhang, J. Li, J. Bai, L. Li, S. Chen, T. Zhou, J. Wang, L. Xia, Q. Xu and B. Zhou, Environ. Sci. Technol., 2019, 53, 6945–6953 CrossRef CAS PubMed.
- T. Le Bahers and K. Takanabe, J. Photochem. Photobiol., C, 2019, 40, 212–233 CrossRef CAS.
- A. B. Murphy, Sol. Energy Mater. Sol. Cells, 2007, 91, 1326–1337 CrossRef CAS.
- C. M. Tian, W. W. Li, Y. M. Lin, Z. Z. Yang, L. Wang, Y. G. Du, H. Y. Xiao, L. Qiao, J. Y. Zhang, L. Chen, D. Qi, J. L. MacManus-Driscoll and K. H. L. Zhang, J. Phys. Chem. C, 2020, 124, 12548–12558 CrossRef CAS.
- Z. Wang, X. Zong, Y. Gao, J. Han, Z. Xu, Z. Li, C. Ding, S. Wang and C. Li, ACS Appl. Mater. Interfaces, 2017, 9, 30696–30702 CrossRef CAS PubMed.
- B. Lei, D. Xu, B. Wei, T. Xie, C. Xiao, W. Jin and L. Xu, ACS Appl. Mater. Interfaces, 2021, 13, 4785–4795 CrossRef CAS PubMed.
- Q. Zeng, J. Li, L. Li, J. Bai, L. Xia and B. Zhou, Appl. Catal., B, 2017, 217, 21–29 CrossRef CAS.
- X. Li, M. Kan, T. Wang, Z. Qin, T. Zhang, X. Qian, Y. Kuwahara, K. Mori, H. Yamashita and Y. Zhao, Appl. Catal., B, 2021, 296, 120387 CrossRef CAS.
- K. Feng, Y. Lin, J. Guo, Z. Ye, Y. Zhang, Q. Ma, Y. Shao, K. Chen, J. Zhuang, D. Lin and T. Lin, J. Hazard. Mater., 2020, 393, 122488 CrossRef CAS PubMed.
- Y. Tang, Z. Zheng, X. Sun, X. Li and L. Li, Chem. Eng. J., 2019, 368, 448–458 CrossRef CAS.
- B. A. Koiki, B. O. Orimolade, B. N. Zwane, O. V. Nkwachukwu, C. Muzenda, D. Nkosi and O. A. Arotiba, Chemosphere, 2021, 266, 129231 CrossRef CAS PubMed.
- Y. Jin, P. Zhang, X. Wang, H. Ma and Y. Zhang, Chem. Eng. J., 2024, 491, 152168 CrossRef CAS.
- Y. Yang, H. Chen, X. Zou, X. Shi, W. Liu, L. Feng, G. Suo, X. Hou, X. Ye, L. Zhang, C. Sun, H. Li, C. Wang and Z. Chen, ACS Appl. Mater. Interfaces, 2020, 12, 24845–24854 CrossRef CAS PubMed.
- H. L. Tan, R. Amal and Y. H. Ng, J. Mater. Chem. A, 2017, 5, 16498–16521 RSC.
- H. Zhang, J. H. Park, W. J. Byun, M. H. Song and J. S. Lee, Chem. Sci., 2019, 10, 10436–10444 RSC.
- Q. Zeng, J. Li, L. Li, J. Bai, L. Xia and B. Zhou, Appl. Catal., B, 2017, 217, 21–29 CrossRef CAS.
- L. Chen, J. Ren and Z. Yuan, Adv. Energy Mater., 2023, 2203720 CrossRef CAS.
- W. Yang and Y. Wang, Appl. Catal., B, 2021, 282, 119574 CrossRef CAS.
- J. Liu, J. Li, Y. Li, J. Guo, S. Xu, R. Zhang and M. Shao, Appl. Catal., B, 2020, 278, 119268 CrossRef CAS.
- F. Liang and Y. Zhu, Appl. Catal., B, 2016, 180, 324–329 CrossRef CAS.
- T. Su, Y. Gong, X. Cui, X. Wang, Y. Zhang and H. Yu, Chem. Eng. J., 2024, 497, 155036 CrossRef CAS.
- L. Xia, F. Chen, J. Li, S. Chen, J. Bai, T. Zhou, L. Li, Q. Xu and B. Zhou, J. Hazard. Mater., 2020, 389, 122140 CrossRef CAS PubMed.
- Z. Feng, Q. Tian, Q. Yang, Y. Zhou, H. Zhao and G. Zhao, Appl. Catal., B, 2021, 286, 119908 CrossRef CAS.
- Y. Guo, J. Long, J. Huang, G. Yu and Y. Wang, Water Res., 2022, 215, 118275 CrossRef CAS PubMed.
- Y. Lei, Y. Yu, X. Lei, X. Liang, S. Cheng, G. Ouyang and X. Yang, Environ. Sci. Technol., 2023, 57, 5433–5444 CrossRef CAS PubMed.
- P. J. Mafa, M. E. Malefane, F. Opoku, B. B. Mamba and A. T. Kuvarega, Chem. Eng. J., 2023, 464, 142462 CrossRef CAS.
- P. Zhang, Y. Yang, X. Duan, Y. Liu and S. Wang, ACS Catal., 2021, 11, 11129–11159 CrossRef CAS.
- W. Yu, C. Feng, R. Li, B. Zhang and Y. Li, Chin. J. Catal., 2025, 68, 51–82 CrossRef.
- M. Hillenbrand, C. Helbig and R. Marschall, Energy Environ. Sci., 2024, 17, 2369–2380 RSC.
- J. Fu, Z. Fan, M. Nakabayashi, H. Ju, N. Pastukhova, Y. Xiao, C. Feng, N. Shibata, K. Domen and Y. Li, Nat. Commun., 2022, 13, 729 CrossRef CAS PubMed.
- X. Zhan, T. Lei, L. Wang, H. Zhang, D. Ou, M. Yang, M. Guo, Y. Luo, L. Tang, H. Yang, W. Yang and H. Hou, J. Photochem. Photobiol., A, 2024, 451, 115538 CrossRef CAS.
- S. Li, J. Chen, S. Hu, H. Wang, W. Jiang and X. Chen, Chem. Eng. J., 2020, 402, 126165 CrossRef CAS.
- Z. Hu, H. Wu, S. Li, C. Zhang, R. Liang and M. Zhou, Chem. Eng. J., 2024, 498, 155308 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ey00068h |
| This journal is © The Royal Society of Chemistry 2025 |