Open Access Article
Open Access ArticleVisible-light photocatalytic CO2 hydrogenation using surface-alloyed plasmonic AgPt nanoprisms†
Garv
Bhardwaj
 a,
Fergus
McLaren
b,
Kishan S.
Menghrajani
c,
Sanje
Mahasivam
b,
Stefan A.
Maier
a,
Fergus
McLaren
b,
Kishan S.
Menghrajani
c,
Sanje
Mahasivam
b,
Stefan A.
Maier
 cd,
Murali
Sastry
ab and
Akshat
Tanksale
cd,
Murali
Sastry
ab and
Akshat
Tanksale
 *a
*a
aDepartment of Chemical and Biological Engineering, Monash University, VIC 3800, Australia. E-mail: Akshat.Tanksale@monash.edu
bDepartment of Material Science and Engineering, Monash University, VIC 3800, Australia
cSchool of Physics and Astronomy, Monash University, VIC 3800, Australia
dDepartment of Physics, Imperial College London, London SW7 2AZ, UK
First published on 22nd April 2025
Abstract
Development of suitable catalysts for light-driven CO2 hydrogenation is an alluring goal in catalysis. In this study, plasmonic Ag nanoprisms were combined with Pt to make surface-alloyed nanoparticles for aqueous-phase CO2 hydrogenation. The Pt loading favoured the product selectivity towards multi-electron C1 products and promoted acetic acid production via C–C coupling. Increasing the reaction pressure further improved acetic acid production where the highest yield of 0.491 mmol gcat−1 was achieved at 20 bar. Within the visible-light region, the in-plane dipole resonance peak of Ag91Pt9 at 600 nm contributed the highest apparent quantum yield of 26.7%. These investigations demonstrated the significance of designer plasmonic catalysts and highlighted their photocatalytic enhancement towards CO2 conversion.
Broader contextPhotocatalytic CO2 conversion to valuable C1–C2 chemicals offers a low carbon intensity pathway to combat climate change and replace fossil fuels as feedstock. This study focuses on enhancing photocatalytic activity by developing plasmonic surface-alloyed AgPt nanoprisms, to convert CO2 in an aqueous environment at room temperature. The addition of Pt enhances productivity towards a variety of chemicals, particularly acetic acid. The investigations on using visible-light illumination and achieving high apparent quantum yield underscore the ability of plasmon-mediated photocatalysis to enhance CO2 conversion. These findings highlight the potential of using plasmonic materials as photocatalysts. |
Introduction
Carbon capture, utilisation and storage (CCUS) technologies have expanded over the last decade, supporting the emissions reduction target towards creating a circular economy.1 Bulk chemical production from CO2 offers an opportunity to displace conventionally used fossil-derived chemicals. Carbon-neutral chemicals can be produced by hydrogenating the captured CO2 using green hydrogen.2 Photocatalytic CO2 hydrogenation is an emerging area of interest due to its potential to utilise sunlight.3Semiconductor photocatalysts have been studied extensively for CO2 hydrogenation to produce C1 chemicals such as carbon monoxide (CO),4,5 methane (CH4),4–7 formic acid (HCOOH),8–10 formaldehyde (HCHO)11,12 and methanol (CH3OH)13–15 and C2 chemicals including acetic acid (CH3COOH),8 acetaldehyde (CH3CHO),16 ethane (C2H6)5,12 and ethanol (C2H5OH).16,17 The major challenges of photocatalytic CO2 reduction and hydrogenation can be attributed to (1) poor multi-component catalyst design hindering light-to-energy transfer, (2) limited light absorption range, and (3) limited control of product selectivity because of a lack of understanding of reaction pathway. Plasmonic nanomaterials have garnered interest for various applications,18–20 including their potential as photocatalysts.21–24 Noble metal nanoparticles (NPs) made of silver (Ag), gold (Au) and copper (Cu) can capture the incident light through the collective excitation of conduction electrons. The spatial confinement of this excitation because of a NP's exterior boundary results in a resonant oscillation, known as the localised surface plasmon resonance (LSPR).25,26 LSPR produces a local electric field amplification and decay by creating hot charge carriers and phonon vibrations.27,28
Ag provides several advantages over other plasmonic metals (e.g. Au, Cu) including high plasmon quality factor, tunable optical properties and distinguishable interband and intraband transitions.29 Various factors affect LSPR such as particle size, morphology, composition and surrounding environment.30 Among these, silver nanoprism-like morphology has multiple tunable LSPR modes in the visible region and higher photocatalytic performance.31,32 Ag-based catalysts have shown CO2 activation. However, co-catalysts are required to enhance the photocatalytic properties. Combining Ag with a catalytically active metal such as platinum (Pt) can overcome the challenges with photocatalysts in hydrogenating CO2.33–37 Researchers have also found the plasmon energy transfer within Ag–Pt nanocubes useful for photochemical reactions.38,39
Here, we show plasmon-mediated photocatalytic capability of surface-alloyed AgPt nanoprisms (NPrs) in and aqueous-phase CO2 hydrogenation reaction to produce HCOOH, HCHO and CH3COOH. The AgPt NPrs were synthesised through galvanic replacement reaction (GRR) of Pt with Ag NPrs. The deposition of Pt on the Ag NPrs was surface-limited, which formed Ag–Pt alloys. The catalyst performance improved significantly with increasing Pt loading and reaction pressure. Control experiments confirmed the visible-light-driven photocatalysis consuming CO2 and H2. The in-plane dipole resonance peak was majorly responsible for the catalytic performance. We also investigated catalyst deactivation through colloidal instability. The reaction pathway was interpreted based on the catalyst selectivity and hydrogenation of selected intermediates.
Results
Materials characterisation
Ag nanoprisms (Ag NPrs) were synthesised according to the method developed by Aherne and co-authors with modifications to increase sample concentration.40 The colloidal synthesis produced a high proportion of platelet nanoprisms (NPr) (77%), which were categorised into triangles (29%) with an edge length of 63 ± 10 nm, and discs (48%) with a diameter of 42 ± 6 nm (Fig. S.1, S.2 and Table S.1, ESI†). Pt was incorporated in the Ag NPr by a galvanic replacement reaction (GRR) using K2PtCl4. The Pt loadings were calculated from the total moles of Ag and Pt used in synthesis, and samples were labelled Ag100−xPtx, where x corresponded with loadings from 1.5–9 mol% Pt, assuming complete GRR. The UV-vis spectra of Ag NPrs and AgPt NPrs showed both red-shift and dampening of the in-plane dipole resonance peak of Ag NPr at 600 nm, proportional to the Pt loadings (Fig. 1 and Table S.2, ESI†). This was attributed to the galvanic etching of Ag NPr and Ag–Pt alloy formation on the NPr surface.41–45 In comparison, the Pt NP spectra did not show any appreciable plasmon resonance peaks in the wavelength region (Fig. 1).In comparison with as-synthesised Ag NPrs (Fig. 2A), edge deformation was observed in bright field scanning transmission electron microscopy (BF-STEM) micrographs for all AgPt NPrs with varying Pt loadings (Fig. 2C, E, G and I). Between samples, the dimensions of the NPrs (Fig. S.2C, ESI†) and the degree of edge deformation did not vary significantly, but Kirkendall voids were present in at higher Pt loadings (Fig. 2I and J) which may contribute to the non-linear relationship between Pt concentration and in-plane dipole resonance wavelength (Table S.2, ESI†).
STEM energy dispersive spectroscopy (STEM-EDS) mapping of clusters of NPrs was used to quantify Ag/Pt composition of the samples (Table 1) and confirmed that free Pt NPs were not present. Mapping of individual NPrs at all loadings revealed Pt was distributed across the entire surface but was concentrated at the edges of the triangular face (Fig. 2C–J). As STEM-EDS is a depth-insensitive technique, EDS line scans of Ag91Pt9 at different orientations were collected (Fig. S.3, ESI†). Pt was most abundant at the edges of the NPrs in both orientations, and a pronounced minimum of Pt occurred at the centre of the NPr in the edge-on case. Given these results and previous literature showing the surface-limited nature of alloy formation through galvanic processes, the Pt deposition was determined to be surface-limited.42,46
| Sample | STEM-EDS | ICP-MS | XPS | ||||
|---|---|---|---|---|---|---|---|
| Ag (mol%) | Pt (mol%) | Ag (mol%) | Pt (mol%) | Ag0 (mol%) | Pt0 (mol%) | Pt2+ (mol%) | |
| Ag NPr | 100 | — | 100 | — | 100 | — | — |
| Ag98.5Pt1.5 | 98.9 ± 5.0 | 1.1 ± 0.1 | 98.5 ± 0.7 | 1.46 ± 0.07 | 97.6 | 2.25 | 0.10 |
| Ag98Pt2 | 98.8 ± 5.1 | 1.2 ± 0.2 | 97.9 ± 0.3 | 2.07 ± 0.30 | 95.5 | 3.85 | 0.67 |
| Ag96.5Pt3.5 | 97.4 ± 4.9 | 2.6 ± 0.2 | 96.4 ± 1.3 | 3.59 ± 0.12 | 94.2 | 4.89 | 0.93 |
| Ag91Pt9 | 92.6 ± 4.2 | 7.4 ± 0.4 | 92.2 ± 0.5 | 7.81 ± 0.46 | 87.2 | 7.04 | 5.73 |
The crystal structure of the NPrs was studied using selected area electron diffraction (SAED) (Fig. 2M). In agreement with Aherne and co-authors, peaks for HCP {1010}, FCC {111} and {220} planes were identified in the Ag NPr sample.40 Based on Vegard's Law and the lattice parameters of Ag (4.08 Å) and Pt (3.92 Å), a linear expansion of the real space inter-planar distances with increasing Pt concentration was expected. As Pt concentration increased up to Ag96.5Pt3.5, the HCP {1010} and FCC {111} peak positions remained unchanged, while the FCC {220} peak underwent a small reciprocal space expansion consistent with Ag–Pt alloying under Vegard's Law. HCP {1010} and FCC {111} planes occurred on the flat triangular faces and through the interior of the NPr, while FCC {220} planes were predominantly present at the edges and tips. The change to only the {220} peak position suggests that, at concentrations at or below 3.5%, Pt was mainly deposited along the NPr edges.
However, at Ag91Pt9, the crystallography of the sample changed considerably. The HCP {1010} peak was still present, though shifted from 4.06 nm−1 in Ag to 3.99 nm−1 in Ag91Pt9. Similarly, the FCC {220} peak shifted from 7.03 nm−1 in Ag to 6.87 nm−1 in Ag91Pt9 in a reversal of trend observed at lower Pt concentrations (where the peak gradually shifted from 7.03 nm−1 to 7.05 nm−1). The large peak at 4.18 nm−1 did not align with any of the expected reflections. Based on the lattice parameter of Ag, the FCC {111} reflection can be calculated to be 4.23 nm−1, but in the Ag NPr sample, FCC {111} was observed at 4.30 nm−1. It was unlikely that the 4.18 nm−1 peak is FCC {111}, as this would require a significant shift from the corresponding Ag NPr peak in the opposite direction to what was expected from AgPt alloying. However, given similar shifts occurred for the HCP {1010} and FCC {220} peaks in this sample, the FCC {111} assignment cannot be ruled out. The other explanation for the 4.18 nm−1 peak is an Ag–Pt alloy phase of HCP {1010}. Pt has been observed to adopt an HCP lattice structure when deposited on another HCP surface and may be responsible for the anomalous peak.47,48 High resolution TEM (HR-TEM) for single particle measurement do not give us conclusive indication of the existing changes as the lattice parameters of Ag and Pt are within the range of ∼5% error (Fig. S.4, ESI†). The atom-to-atom distance varied from 0.22 to 0.32 nm. These variations resulted in an average of 0.27 nm which are significantly higher than a 5% error (Fig. S.5, ESI†).
XPS analysis was conducted to investigate the efficiency of Pt deposition by GRR and determine the surface composition of the Ag Pt NPs (Fig. 3). Ag 3d spectra of Ag NPr showed binding energy peaks at 367.7 eV (3d5/2 spin–orbit component), corresponding to Ag049 (Fig. 3A). The Ag 3d5/2 peaks in AgPt NPrs also showed similar peak profiles at ∼367.7 eV, while no signals were observed for Ag+. For AgPt, Pt 4f spectra showed both Pt0 and Pt2+ signals with binding energies at 70.1 and 72 eV of 4f7/2 spin orbital50,51 (Fig. 3B). The intensity of the Pt2+ signal was proportional to Pt loading, thus showing the limited extent of GRR. The XPS results were used to quantify the elemental composition of Ag0, Pt0 and Pt2+ in the different samples (Table 1). C 1s spectra for Ag NPr showed peaks at 285 eV (C–H), 286.5 eV (C–O) and 288 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), which likely results from the citrate used as capping agent.52 Similarly, O 1s spectra for Ag NPr showed peaks at ∼531 eV (C
O), which likely results from the citrate used as capping agent.52 Similarly, O 1s spectra for Ag NPr showed peaks at ∼531 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and 533 eV (C–O/C–OH) (Fig. S.6, ESI†).53 The peak signals corresponding to C
O) and 533 eV (C–O/C–OH) (Fig. S.6, ESI†).53 The peak signals corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O were significantly lower on Ag91Pt9, resulting from the loss of capping agent on the surface.49
O were significantly lower on Ag91Pt9, resulting from the loss of capping agent on the surface.49
Table 1 summarises the metal composition measured through various techniques. The measurements from STEM-EDS of particle maps and ICP-MS of bulk solution were in good agreement and were close to theoretical estimates. Results from XPS quantification corresponded to the surface composition, which showed higher Pt loading. This was expected as XPS is a surface-sensitive technique. This is in agreement with the surface-limited bimetallic structure seen in STEM line scans (Fig. S.3, ESI†). Of the total Pt signals, Pt2+ increased from ∼4.3% to ∼45% with increasing Pt loading. This indicated that the degree of galvanic reduction was inversely correlated with the Pt loading.
Photocatalytic CO2 hydrogenation
In situ temperature profile was measured during the reaction to investigate the effect of Pt loading on the plasmon decay pathway (Fig. 4B). The trend of photothermal response indicated that low Pt loading did not significantly affect the photothermal response, suggesting that electron relaxation to thermalisation was not affected. The highest in situ temperature of ~0.35 °C/mgcat was observed in Ag98Pt2 whereas Ag91Pt9 showed a relatively lower rate and steady-state temperature. The correlation between dampened extinction intensity of the plasmon resonance peaks and temperature increase was similarly reported for Au/Pd system.54 The plasmon decay for Ag–Pt system has been reported to under electron and energy transfer, therefore reducing heat generation.41,55
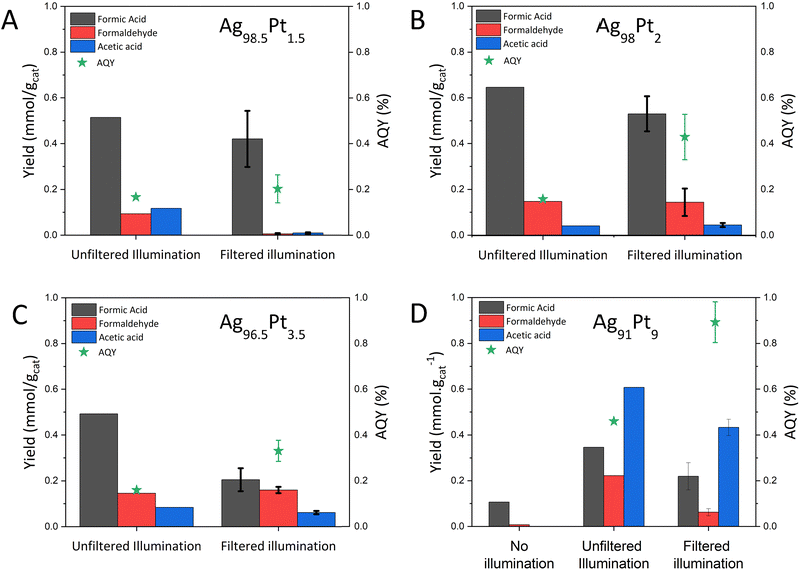
A series of control reactions verified the visible-light photocatalytic activity of AgPt NPrs. Catalysts were irradiated with a 300 W Xe Lamp in unfiltered and filtered modes corresponding to different spectral intensity profiles (Fig. S.7C, ESI†). As seen in Fig. 5, the product selectivity between the illumination modes varied at lower Pt loadings. This difference in the selectivity at lower Pt loading was due to electrons generated by the interband transitions in Ag that lied in the UV region (Fig. 1 and 5A, B). As the Pt loading increased, the product selectivity was relatively similar as the plasmon decay pathway dissipated towards surface AgPt sites, thus governing the catalytic pathway (Fig. 5C and D).38 A control reaction with no illumination (reaction in dark) was also conducted for Ag91Pt9, which produced 0.11 mmol gcat−1 HCOOH and <0.01 mmol gcat−1 HCHO. The aqueous production of HCOOH has a negative Gibbs free energy and is favourable at these conditions.56 The apparent quantum yield (AQY) was calculated for the cumulative production of HCOOH, HCHO and CH3COOH against the photons emitted in the two illumination modes. The increase in AQY in the filtered mode increased proportional to the Pt loading, thus confirming the visible-light driven nature of the Ag100−xPtx nanoprisms.
Further control reactions were conducted using Ag91Pt9 to validate the consumption of CO2 and H2 (Fig. S.13, ESI†). The reaction did not proceed without any catalyst. A 100% CO2 environment yielded 0.012 mmol gcat−1 HCOOH and 0.053 mmol gcat−1 HCHO. While Ag-based catalysts have shown HCOOH and HCHO production in similar applications using H2O as the electron-donating species, this reaction relied on the presence of H2 as the electron donor.57–59 To confirm the absence of any other carbon sources in the reaction, a 100% H2 environment was used. We observed the formation of HCOOH at 0.11 mmol gcat−1, HCHO at 0.026 mmol gcat−1 and CH3COOH at 0.01 mmol gcat−1. This contradicted our initial assumption. However, we detected ~280 μM bicarbonate (HCO3−) from HPLC analysis in the solution before the reaction commenced which decreased to 190 μM, indicating that CO2 from the air was absorbed and converted into aqueous species, such as HCO3− and carbonate (CO32−), in the feed solution.
To validate the source of carbon species responsible for forming the C1 and C2 products in this study, the feed solution was dosed with 0.5 mM HCO3− and H2. The results showed increased product yields, with the maximum yields of HCOOH, HCHO and CH3COOH at 0.19 mmol gcat−1, 0.072 mmol gcat−1, and 0.125 mmol gcat−1, respectively. This confirmed that HCO3− was a precursor to the hydrogenation reaction in a similar yield trend to that of H2 + CO2 environment. Photo-oxidation of adsorbed citrate capping on Ag NPr to CO2 has been observed.60 Hence, we dosed a reaction containing 0.5 mM sodium tricitrate and H2. No products were detected until 1 h, similar to the 100% H2 environment case, which showed negligible contribution from citrate.
The optical properties of the NPrs were analysed ex situ at different reaction times (Fig. S.8, ESI†). The plasmon resonance peaks observed in Ag NPr did not change. However, a blue shift was observed in the AgPt NPrs. This suggested changes in the adsorbed surface species on the NPrs, although further investigations were conducted to determine possible causes.58,61 XPS analysis of the NPrs post-reaction showed some BE shifts for Ag 3d and Pt 4f regions compared to as-synthesised NPrs (Fig. S.9, ESI†). The Ag 3d5/2 peak for Ag NPr shifted the most from 367.7 eV to 368 eV. The BE shift was reduced with increasing Pt loading. Similarly, the highest BE shift in Pt 4f7/2 was observed for Ag98.5Pt1.5 from 70.1 eV to 70.5 eV. We deduce these shifts in binding energy indicate diffusion of Ag to form an Ag–Pt surface alloy adlayer.62
Aggregation of NPrs was observed among all samples under reaction conditions (Fig. S.10A, ESI†), which was hypothesised to be because of either the loss of capping agent or structural changes. Upon the introduction of reaction gases, a decrease in pH occurred due to. the solvation of CO2, which created an acidic medium. The decrease in pH increases the protonation of trisodium citrate, which could cause NPrs aggregation.63 Analysis of the solutions pre- and post-reaction revealed an average pH reduction from 6.9 to 4.9 (Fig. S.10B, ESI†). The shift in pH would cause protonation of [Citrate]3− to H2[Citrate]− and affect the surface charge of the NPr. Therefore, we investigated the change in surface charge by measuring the zeta potential and hydrodynamic radius of the NPrs post-reaction (Fig. S.10C, ESI†). The zeta potential of as-synthesised AgPt NPrs averaged at −28 mV with a mean hydrodynamic radius of 48 nm. The zeta potential of the NPrs post-reaction increased compared to the initial measurement, where this increase was proportional to the Pt loading. Similarly, the hydrodynamic radius increased proportionally to the Pt loading with a mean hydrodynamic radius of ∼80 nm for Ag91Pt9. XPS analysis of post-reaction AgPt NPrs in C 1s region (Fig. S.9C, ESI†) also showed a significant decrease in the signal area for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288 eV), confirming the cause of aggregation from loss of citrate from the particle surface. This implied that the acidic environment formation of acidic products further caused citrate protonation and particle aggregation.64
O (288 eV), confirming the cause of aggregation from loss of citrate from the particle surface. This implied that the acidic environment formation of acidic products further caused citrate protonation and particle aggregation.64
On the nanoscale, examples of NPrs with structural deformations, such as holes on particle faces, were seen in pre- and post-reaction samples. The frequency of hole formation was higher in the post-reaction samples (Table S.4 and Fig. S.11, ESI†). Catalyst composition was also quantified post-reaction (Table S.5, ESI†). The composition changed compared to as-synthesised samples. However, there were discrepancies among the measurements. We considered these discrepancies as part of sample preparation techniques and analysed the data separately. In comparison, a relative increase in Pt loading of samples post-reaction was observed from ICP-MS and XPS. The increase in Pt loading implied a loss of Ag, which was not detected as Ag+ in XPS measurements. The total catalyst recovered was also calculated using ICP-MS which showed significant Ag loss of up to 81.7% while only 10.8% Pt loss occurred in the same sample (Table S.6, ESI†). Hence, we proposed this as an unrecoverable catalyst lost during the reaction by aggregation and colloidal instability. Measurements from STEM-EDS indicated no significant compositional change occurred to the imaged NPrs (Table S.5, ESI†).
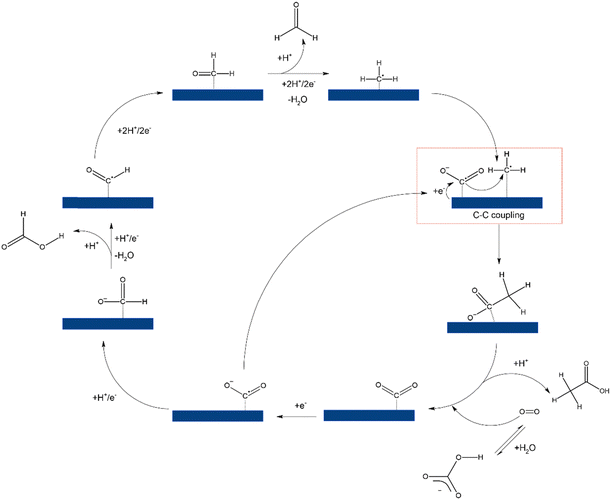
Based on the liquid products observed, the reaction pathway was elucidated based on similar findings from previous studies.66,68 As shown in Scheme 1, the reaction initiates with the adsorption of CO2 resulting from dissolved CO2 and HCO3−. The formation of HCOOH was followed by a two-step electron reduction involving COO− and *HCOO−. HCHO was produced by further hydrogenating *HCOO− to *CHO and *CH2O. *CH3 was produced from *CH2O, undergoing a nucleophilic reaction from COO− to produce CH3COO−/CH3COOH.
Discussion
The CO2 hydrogenation performance of Ag91Pt9 NPrs was comparable with other metal-based photocatalysts reported in the literature (Table S.8, ESI†). While HCOOH and HCHO were produced in similar yields, CH3COOH was produced at a much higher yield. This could be attributed to the favourability of C–C coupling on AgPt NPrs and optimal reaction conditions. Although most reaction conditions involved H2O as the electron donor to reduce CO2 to CO, using H2 in our experiments significantly improved the product yields. H2 is used in CO2-to-CH3OH, which favours *CH3 formation. Using a liquid-phase reaction also favoured certain products, and the reaction pressure improved the mass transfer of reactants in the solution. This study conducted the reactions at room temperature and confirmed the catalyst was primarily active in the visible light region. Therefore, the reaction was considered to be plasmon-mediated and driven by visible-light illumination. We observed correlations between the LSPR peaks and in situ temperature; however, this did not confirm the exact mechanism of plasmon decay. With the addition of Pt, the plasmon decay mechanism shifted towards energy transfer and hot-electron generation. Further investigation would provide insight into this mechanism. Increasing Pt loading and reaction pressure affected the colloidal stability. The aggregation presented a limitation on the parameters that could be investigated. An alternative capping agent such as polyvinylpyrrolidone (PVP) and cetyltrimethylammonium bromide (CTAB) could improve the colloidal stability. In order to create a scalable synthesis, catalyst immobilisation on a support or encapsulation in microparticle frameworks could help overcome this limitation. Further improvement in achieving high AQY could be approached by designing a modular reactor system which ideal wavelength illumination in a continuous mode.Conclusions
We have developed a bimetallic plasmon-active catalyst consisting of a combination of the Ag–Pt system for photocatalysis. The incorporation of Pt into Ag NPrs as surface-limited alloys significantly improved the product yield and selectivity by altering the plasmon decay pathway towards energy transfer from Ag to Pt. The maximum yields of HCOOH (0.52 mmol gcat−1 with Ag98Pt2), HCHO (0.16 mmol gcat−1 with Ag96.5Pt3.5 and CH3COOH (0.43 mmol gcat−1 with Ag91Pt9) suggested multi-electron hydrogenation and C–C coupling of *CH3 and COO− species. Notably, a peak AQY of 26.7% was observed at the in-dipole resonance (600 nm) for Ag91Pt9, highlighting the wavelength dependence in driving the reaction. Catalyst deactivation due to colloidal instability and long-term performance validation remains a challenge. Therefore, future work on improving catalyst stability and investigating surface species will provide deeper insight on the viability for scale-up. These findings highlight the role of designer plasmonic catalysts in enhancing photocatalytic CO2 conversion. The results demonstrate that careful tuning of catalyst composition and morphology significantly improve the light-to-energy transfer and product selectivity.Experimental
Chemicals
Silver nitrate (AgNO3), trisodium citrate dihydrate, sodium borohydride (NaBH4), poly(4-styrenesulfonic acid)sodium salt (PSSS, 1000 kDa), L-ascorbic acid, potassium tetrachloroplatinate(II) (K2PtCl4), formic acid (reagent grade), acetic acid (reagent grade) and methanol (ACS reagent) were purchased from Sigma Aldrich and used as received. CO2 (ultra-high purity) and H2 (ultra-high purity) was purchased from BOC. MilliQ water (18.2 mΩ) was used for all the experiments.Synthesis of Ag NPr and Ag100−xPtx nanoparticles
Characterisations
UV-vis extinction spectra of the samples were obtained using a PerkinElmer Lambda 365 UV-vis spectrophotomer. Samples to be imaged in STEM were centrifuged three times at 10k RPM for 30 minutes. The supernatant was removed and replaced with Milli-Q H2O. 1.4 μL of sample solution was drop cast on plasma-cleaned carbon-film-coated Cu grids. STEM imaging was captured on both the FEI Tecnai G2 T-20 TWIN TEM and F20 S-TWIN TEM at Monash Centre for Electron Microscopy (MCEM). STEM-EDS was conducted on the F20 using a Bruker 30 mm2 123 eV windowless SDD and Quantax analysis system. 107Ag and 195Pt isotope concentrations were analysed in the bulk catalyst samples using Inductively Coupled Plasma – Mass Spectroscopy (Thermo iCAP TQe Triple Quadrupole). 2 mL of catalyst samples were diluted in 70% v/v HNO3 followed by serial dilutions in 2% v/v HNO3. Sample zeta potentials and hydrodynamic radius were measured and recorded by Malvern Nano Zetasizer using disposable folded capillary cells (DTS1070). XPS analysis of as-synthesised nanoparticles was conducted on Thermo Scientific K-alpha XPS (monochromated Al Kα, Ephoton = 1487.6 eV, 1 × 10−9 torr). Scans were obtained using a pass energy of 50 eV with 0.1 eV resolution. Post-reaction samples were analysed on the Thermo Nesa Surface Analysis System (monochromated Al Kα, Ephoton = 1487.6 eV). The spectra were charge corrected to adventitious carbon at 285 eV in the C 1s region using Avantage software. Ag 3d and P 4f regions were background corrected with Shirley algorithm. Ag0 and Pt0 peaks were fitted with a Doniac-Sunjic asymmetry model. The oxidation states (Pt2+), C 1s and O 1s regions were equipped with Gaussian–Lorentzian function in Origin software (2022b).Photocatalytic CO2 hydrogenation
The reaction was carried out in batch mode in a PARR (A239M) photoreactor with a K-type thermocouple inserted at the base. 5–10 mg of as-synthesised catalysts were concentrated, re-dispersed in 50 mL Milli-Q H2O and added to the reaction vessel. The reactor was sealed, and H2 was introduced for 15 min to flush the reactor. This was followed by venting the reactor and introducing CO2 and H2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to the specified pressure. A 300 W Xe lamp (Zolix 300P) irradiated the sample through the photoreactor window. For visible light (380–780 nm) irradiation, a UV + IR cutoff filter was used. Liquid products were analysed using high-performance liquid chromatography (Agilent 1220 Infinity). An ion exclusion column (Rezex RHM-Monosaccharide H+ 8%) with refractive index detector was used to detect HCOOH and CH3COOH). A C18 column (Xterra MS C18) with diode array detector was used to detect HCHO by derivatisation with Nash reagent. Gaseous products (CO, CO2, CH4 and H2) were analysed using gas chromatography (Agilent MicroGC 990) equipped with a TCD detector. Wavelength-dependent studies were performed using the same photoreactor setup as NKT Supercontinuum source (SuperK Fianium FIU-15) with a LTTF Contrast VIS giving a bandwidth <2.5 nm.
1) to the specified pressure. A 300 W Xe lamp (Zolix 300P) irradiated the sample through the photoreactor window. For visible light (380–780 nm) irradiation, a UV + IR cutoff filter was used. Liquid products were analysed using high-performance liquid chromatography (Agilent 1220 Infinity). An ion exclusion column (Rezex RHM-Monosaccharide H+ 8%) with refractive index detector was used to detect HCOOH and CH3COOH). A C18 column (Xterra MS C18) with diode array detector was used to detect HCHO by derivatisation with Nash reagent. Gaseous products (CO, CO2, CH4 and H2) were analysed using gas chromatography (Agilent MicroGC 990) equipped with a TCD detector. Wavelength-dependent studies were performed using the same photoreactor setup as NKT Supercontinuum source (SuperK Fianium FIU-15) with a LTTF Contrast VIS giving a bandwidth <2.5 nm.
Equations
where,
N
formic![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acid = 2
acid = 2
N formaldehyde = 4
N
acetic![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acid = 8
acid = 8
Data availability
All data has been included in the manuscript and ESI.† Raw data may be provided upon request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is supported by the Australian Research Council (ARC) Research Hub for Carbon Utilisation & Recycling (RECARB Hub) (IH220100012), Monash University, BASF Australia Ltd and Woodside Energy Technologies Pty Ltd. The authors would like to thank Woodside Monash Energy Partnership for financial support. The authors acknowledge the use of the instruments and scientific and technical assistance at the Monash Centre for Electron Microscopy, a node of Microscopy Australia. This research used equipment funded by the Australian Research Council grant LE110100223. The authors acknowledge the use of facilities within the Monash X-ray Platform. The authors acknowledge the facilities, and the scientific and technical assistance of the RMIT University's Microscopy & Microanalysis Facility, a linked laboratory of Microscopy Australia, enabled by NCRIS The authors acknowledge the assistance of Prof. Terence Turney and thank him for his advice and feedback. Prof. Stefan A. Maier acknowledges the Lee-Lucas Chair in Physics.Notes and references
- G. Garcia-Garcia, M. C. Fernandez, K. Armstrong, S. Woolass and P. Styring, ChemSusChem, 2021, 14, 995–1015 CrossRef CAS PubMed.
- W. Ahmad, G. Bhardwaj, R. Lakshman, P. Koley and A. Tanksale, Energy Fuels, 2023, 37, 19377–19399 CrossRef CAS.
- Y. Wang, E. Chen and J. Tang, ACS Catal., 2022, 12, 7300–7316 CrossRef CAS PubMed.
- M. Tahir and N. A. S. Amin, Int. J. Hydrogen Energy, 2017, 42, 15507–15522 CrossRef CAS.
- J. Zhao, B. Liu, L. Meng, S. He, R. Yuan, Y. Hou, Z. Ding, H. Lin, Z. Zhang, X. Wang and J. Long, Appl. Catal., B, 2019, 256, 117823 CrossRef CAS.
- G. Chen, R. Gao, Y. Zhao, Z. Li, G. I. N. Waterhouse, R. Shi, J. Zhao, M. Zhang, L. Shang, G. Sheng, X. Zhang, X. Wen, L.-Z. Wu, C.-H. Tung and T. Zhang, Adv. Mater., 2018, 30, 1704663 CrossRef PubMed.
- S. Xue, C. Wei, M. Shen, X. Liang, J. Wang, C. Yang, W. Xing, S. Wang, W. Lin, Z. Yu, Y. Hou, J. C. Yu and X. Wang, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2319751121 CrossRef CAS PubMed.
- D. Zeng, H. Wang, X. Zhu, H. Cao, W. Wang, Y. Zhang, J. Wang, L. Zhang and W. Wang, Appl. Catal., B, 2023, 323, 122177 CrossRef CAS.
- K. Tamaki, P. Verma, T. Yoshii, T. Shimojitosho, Y. Kuwahara, K. Mori and H. Yamashita, Catal. Today, 2023, 411–412, 113795 CrossRef CAS.
- R. Kuriki, K. Sekizawa, O. Ishitani and K. Maeda, Angew. Chem., Int. Ed., 2015, 54, 2406–2409 CrossRef CAS.
- L. F. Garay-Rodríguez and L. M. Torres-Martínez, J. Mater. Sci.: Mater. Electron., 2020, 31, 19248–19265 CrossRef.
- W. Hou, W. H. Hung, P. Pavaskar, A. Goeppert, M. Aykol and S. B. Cronin, ACS Catal., 2011, 1, 929–936 CrossRef CAS.
- Z.-j Wang, H. Song, H. Pang, Y. Ning, T. D. Dao, Z. Wang, H. Chen, Y. Weng, Q. Fu, T. Nagao, Y. Fang and J. Ye, Appl. Catal., B, 2019, 250, 10–16 CrossRef CAS.
- D. Wu, K. Deng, B. Hu, Q. Lu, G. Liu and X. Hong, ChemCatChem, 2019, 11, 1598–1601 CrossRef CAS.
- M. Izadpanah Ostad, M. Niknam Shahrak and F. Galli, J. CO2 Util., 2021, 43, 101373 CrossRef CAS.
- N. Li, X. Liu, J. Zhou, W. Chen and M. Liu, Chem. Eng. J., 2020, 399, 125782 CrossRef CAS.
- Y. Meng, L. Zhang, H. Jiu, Q. Zhang, H. Zhang, W. Ren, Y. Sun and D. Li, Mater. Sci. Semicond. Process., 2019, 95, 35–41 CrossRef CAS.
- S. Unser, I. Bruzas, J. He and L. Sagle, Sensors, 2015, 15, 15684–15716 CrossRef.
- Z. Zhang, W. Shen, J. Xue, Y. Liu, Y. Liu, P. Yan, J. Liu and J. Tang, Nanoscale Res. Lett., 2018, 13, 54 CrossRef.
- C. Clavero, Nat. Photonics, 2014, 8, 95–103 CrossRef CAS.
- C. Hu, X. Chen, J. Jin, Y. Han, S. Chen, H. Ju, J. Cai, Y. Qiu, C. Gao, C. Wang, Z. Qi, R. Long, L. Song, Z. Liu and Y. Xiong, J. Am. Chem. Soc., 2019, 141, 7807–7814 CrossRef CAS.
- H. Yin, Z. Sun, K. Liu, A. A. Wibowo, J. Langley, C. Zhang, S. E. Saji, F. Kremer, D. Golberg, H. T. Nguyen, N. Cox and Z. Yin, Nanoscale Horiz., 2023, 8, 1695–1699 RSC.
- P. Christopher, H. Xin and S. Linic, Nat. Chem., 2011, 3, 467–472 CrossRef CAS PubMed.
- I. García-García, E. C. Lovell, R. J. Wong, V. L. Barrio, J. Scott, J. F. Cambra and R. Amal, ACS Sustainable Chem. Eng., 2020, 8, 1879–1887 CrossRef.
- U. Kreibig and M. Vollmer, Optical Properties of Metal Clusters, Springer Berlin/Heidelberg, Berlin, Heidelberg, Berlin, Heidelberg, 1995 Search PubMed.
- S. A. Maier, Plasmonics: Fundamentals and Applications, Imprint, Springer, New York, NY: Springer US, 1st edn, 2007 Search PubMed.
- A. Manjavacas, J. G. Liu, V. Kulkarni and P. Nordlander, ACS Nano, 2014, 8, 7630–7638 CrossRef CAS.
- K. Kolwas, Plasmonics, 2019, 14, 1629–1637 CrossRef.
- A. G. M. da Silva, T. S. Rodrigues, J. Wang and P. H. C. Camargo, Chem. Commun., 2022, 58, 2055–2074 RSC.
- W. Xie, K. Zhang, R. Grzeschik and S. Schlücker, Plasmonic Catalysis, 2021, pp. 71–108 DOI:10.1002/9783527826971.ch3.
- V. V. Pham, T. Q. Nguyen, H. V. Le and T. M. Cao, Nanoscale Adv., 2024, 6, 2380–2389 RSC.
- A. G. M. da Silva, T. S. Rodrigues, J. Wang, L. K. Yamada, T. V. Alves, F. R. Ornellas, R. A. Ando and P. H. C. Camargo, Langmuir, 2015, 31, 10272–10278 CrossRef CAS PubMed.
- S. Kattel, B. Yan, J. G. Chen and P. Liu, J. Catal., 2016, 343, 115–126 CrossRef CAS.
- E. S. Gutterød, A. Lazzarini, T. Fjermestad, G. Kaur, M. Manzoli, S. Bordiga, S. Svelle, K. P. Lillerud, E. Skúlason, S. Øien-Ødegaard, A. Nova and U. Olsbye, J. Am. Chem. Soc., 2020, 142, 999–1009 CrossRef PubMed.
- F. L. Chan, G. Altinkaya, N. Fung and A. Tanksale, Catal. Today, 2018, 309, 242–247 CrossRef CAS.
- S. Bai, Q. Shao, Y. Feng, L. Bu and X. Huang, Small, 2017, 13, 1604311 CrossRef PubMed.
- Z. He, Q. Qian, J. Ma, Q. Meng, H. Zhou, J. Song, Z. Liu and B. Han, Angew. Chem., Int. Ed., 2016, 55, 737–741 CrossRef CAS PubMed.
- U. Aslam, S. Chavez and S. Linic, Nat. Nanotechnol., 2017, 12, 1000–1005 CrossRef CAS PubMed.
- V. G. Rao, U. Aslam and S. Linic, J. Am. Chem. Soc., 2019, 141, 643–647 CrossRef CAS PubMed.
- D. Aherne, D. M. Ledwith, M. Gara and J. M. Kelly, Adv. Funct. Mater., 2008, 18, 2005–2016 CrossRef CAS.
- S.-C. Lin, C.-S. Hsu, S.-Y. Chiu, T.-Y. Liao and H. M. Chen, J. Am. Chem. Soc., 2017, 139, 2224–2233 CrossRef CAS.
- W. He, X. Wu, J. Liu, K. Zhang, W. Chu, L. Feng, X. Hu, W. Zhou and S. Xie, Langmuir, 2010, 26, 4443–4448 CrossRef CAS.
- J. Chen, B. Wiley, J. McLellan, Y. Xiong, Z.-Y. Li and Y. Xia, Nano Lett., 2005, 5, 2058–2062 CrossRef CAS PubMed.
- L. Ma, S.-J. Ding and D.-J. Yang, Dalton Trans., 2018, 47, 16969–16976 RSC.
- C. Tiburski and C. Langhammer, ACS Photonics, 2023, 10, 253–264 CrossRef CAS.
- F. Merkoçi, J. Patarroyo, L. Russo, J. Piella, A. Genç, J. Arbiol, N. G. Bastús and V. Puntes, Mater. Today Adv., 2020, 5, 100037 CrossRef.
- Y. Chen, Z. Lai, X. Zhang, Z. Fan, Q. He, C. Tan and H. Zhang, Nat. Rev. Chem., 2020, 4, 243–256 CrossRef CAS.
- Z. Cao, Q. Chen, J. Zhang, H. Li, Y. Jiang, S. Shen, G. Fu, B.-A. Lu, Z. Xie and L. Zheng, Nat. Commun., 2017, 8, 15131 CrossRef.
- K. Qi, Y. Zhang, J. Li, C. Charmette, M. Ramonda, X. Cui, Y. Wang, Y. Zhang, H. Wu, W. Wang, X. Zhang and D. Voiry, ACS Nano, 2021, 15, 7682–7693 CrossRef CAS PubMed.
- E. Fidiani, G. Thirunavukkarasu, Y. Li, Y.-L. Chiu and S. Du, J. Mater. Chem. A, 2020, 8, 11874–11883 RSC.
- S.-S. Chen, X.-X. Lin, A.-J. Wang, H. Huang and J.-J. Feng, Sens. Actuators, B, 2017, 248, 214–222 CrossRef CAS.
- T. Shao, D. Bai, M. Qiu, Y. Li, Q. Zhang, Z. Xue, S. He, D. Zhang and X. Zhou, J. Ind. Eng. Chem., 2022, 108, 456–465 CrossRef CAS.
- M. H. Ullah, K. Il and C.-S. Ha, Mater. Lett., 2006, 60, 1496–1501 CrossRef CAS.
- J. Gargiulo, M. Herran, I. L. Violi, A. Sousa-Castillo, L. P. Martinez, S. Ezendam, M. Barella, H. Giesler, R. Grzeschik, S. Schlücker, S. A. Maier, F. D. Stefani and E. Cortés, Nat. Commun., 2023, 14, 3813 CrossRef CAS PubMed.
- J. Bi, H. Cai, B. Wang, C. Kong and S. Yang, Chem. Commun., 2019, 55, 3943–3946 RSC.
- S. Moret, P. J. Dyson and G. Laurenczy, Nat. Commun., 2014, 5, 4017 CrossRef CAS.
- Q. Jianping, T. Juntao, S. Jie, W. Cuiwei, Q. Mengqian, H. Zhiqiao, C. Jianmeng and S. Song, Electrochim. Acta, 2016, 203, 99–108 CrossRef.
- G. Kumari, R. Kamarudheen, E. Zoethout and A. Baldi, ACS Catal., 2021, 11, 3478–3486 CrossRef CAS PubMed.
- D. Devasia, A. J. Wilson, J. Heo, V. Mohan and P. K. Jain, Nat. Commun., 2021, 12, 2612 CrossRef CAS PubMed.
- P. L. Redmond, X. Wu and L. Brus, J. Phys. Chem. C, 2007, 111, 8942–8947 CrossRef CAS.
- G. Kumari, X. Zhang, D. Devasia, J. Heo and P. K. Jain, ACS Nano, 2018, 12, 8330–8340 CrossRef CAS PubMed.
- M. T. Schaal, M. P. Hyman, M. Rangan, S. Ma, C. T. Williams, J. R. Monnier and J. W. Medlin, Surf. Sci., 2009, 603, 690–696 CrossRef CAS.
- M. Barbalinardo, G. Ori, L. Lungaro, G. Caio, A. Migliori and D. Gentili, J. Phys. Chem. C, 2024, 128, 16220–16226 CrossRef CAS.
- T. C. Prathna, N. Chandrasekaran and A. Mukherjee, Colloids Surf., A, 2011, 390, 216–224 CrossRef CAS.
- P. G. Jessop, F. Joó and C.-C. Tai, Coord. Chem. Rev., 2004, 248, 2425–2442 CrossRef CAS.
- C. Genovese, C. Ampelli, S. Perathoner and G. Centi, Green Chem., 2017, 19, 2406–2415 RSC.
- C.-T. Lin, M.-H. Shiao, M.-N. Chang, N. Chu, Y.-W. Chen, Y.-H. Peng, B.-H. Liao, H. J. Huang, C.-N. Hsiao and F.-G. Tseng, Nanoscale Res. Lett., 2015, 10, 74 CrossRef PubMed.
- J. Dankar, V. Rouchon, M. Rivallan, C. Pagis and M. El-Roz, ACS Appl. Mater. Interfaces, 2024, 16, 42210–42220 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ey00046g |
| This journal is © The Royal Society of Chemistry 2025 |