 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Improving microalgae harvesting efficiency: electrochemical techniques and parameter optimization
Elif
Yakamercan
ab,
Samriti
Guleria
cd,
Mahmoud
Karimi
e,
Ahmet
Aygun
b,
Aparajita
Bhasin
c and
Halis
Simsek
 *a
*a
aDepartment of Agricultural & Biological Engineering, Purdue University, West Lafayette, IN, USA. E-mail: simsek@purdue.edu
bDepartment of Environmental Engineering, Bursa Technical University, Bursa, Turkey
cDepartment of Food Technology and Nutrition, Lovely Professional University, Phagwara, India
dDepartment of Food Science and Technology, Khalsa College, Amritsar, India
eDepartment of Biosystems Engineering, Arak University, Arak, Iran
First published on 18th September 2025
Abstract
The harvesting microalgae is a challenging process that requires innovative and efficient technologies to make large-scale cultivation economically viable. This study investigated the effectiveness of electrochemical methods for harvesting microalgae Chlorella vulgaris. The operational parameters, such as electrolysis time, electrical current, and pH, were optimized using the response surface methodology based on the Box–Behnken design. The boron-doped diamond (BDD), aluminum (Al), and iron (Fe) electrodes were tested and compared. BDD–Al showed 99.3% of harvesting efficiency (time: 20 min, current: 100 mA, pH: 9), which is the highest value and a pH of 9. The physicochemical properties of the harvested algae, including lipids, proteins, carbohydrates, total suspended solids, and chlorophyll-a content, were examined. The content of harvested algae was found as 41.07–46.63% for protein, 5.5–16.9% for lipid, and 9.02–12.08% for carbohydrates (sugar). The chlorophyll-a concentrations varied from 6.7 to 8.36 μg mL−1. Optimized operating conditions for electrolysis time, pH, and current were determined, and harvesting efficiency was achieved at more than 99%. Energy consumptions for the highest harvesting efficiencies were found to be 0.2, 0.35, and 0.4 kWh kg−1 for BDD–Al, Al–Al, and Al–BDD electrode pairs, respectively. These values were lower than those of conventional algae harvesting methods. The results showed that the electrochemical harvesting techniques are promising alternatives with a high harvesting efficiency and low energy consumption.
Water impactThis study presents an energy-efficient and high-yield method for microalgae harvesting using electrochemical processes, with significant implications for sustainable water management. Conventional microalgae harvesting techniques—such as centrifugation and chemical flocculation—consume high energy or produce secondary pollutants, making them less viable for large-scale use. In contrast, the electrochemical harvesting method optimized in this work (using BDD–Al and Al-based electrodes) achieved up to 99.3% efficiency with energy consumption as low as 0.2 kWh kg−1. By employing electrocoagulation with minimal chemical input, the approach reduces reliance on synthetic coagulants and limits residual contaminants in the effluent. Moreover, the integration of egg-washing wastewater as a nutrient medium aligns with circular water use practices, turning a high-strength wastewater stream into a resource. The findings support the development of decentralized, low-cost, and low-impact algae harvesting technologies, contributing to water reuse, biomass recovery, and improved resource efficiency in wastewater treatment systems. |
1 Introduction
Microalgae, primitive plant-like organisms, play a vital role in commercial products, food supplies, and energy sources. They are rich in lipids and proteins, and are used in animal feed, medicinal products, and food additives.1,2 They are also a promising biofuel source, offering higher oil yields per acre and rapid growth compared to other sources.3 As a potential third-generation biofuel, microalgae are being increasingly researched for their sustainable energy potential. Their lipids can be converted into environmentally friendly biodiesel, thereby reducing CO, hydrocarbons, and greenhouse gas emissions. However, microalgae biomass harvesting remains a significant challenge and energy-intensive operation.4,5 An estimated 20–30% of the biomass production cost is attributed to the harvesting of microalgae.6 The harvesting method chosen depends on factors such as algal species, cost, time, water reuse options, and waste generation.7 Common harvesting techniques include sedimentation, centrifugation, flocculation, and filtering.8It has been pointed out that filtration and sedimentation processes are not suitable for algae harvesting because of the low cell density and small size of algae.9 Microsized (∼5 μm) algae can pass through a filter during filtration.10 Furthermore, it is also highlighted that algae can be harvested using the flocculation process with metal coagulants such as Al3+ and Fe2+ salts, but the main disadvantages of this process are the chemical requirements and the generation of secondary pollutants such as sludge.6,11 Centrifugation is one of the most common methods for harvesting algae, using mechanical and gravitational forces to separate algae from water. However, the main drawback of this process is its high operating costs.5 To overcome these problems, electrochemical methods such as electrocoagulation (EC) and electrooxidation (EO) are promising alternatives to harvest microalgae and can be used for a variety of algal species.7 Electrochemical processes enable cost-effective algae harvesting compared to other methods.12 In addition, compared to energy composition in centrifugation and the electrochemical process for harvesting Ankistrodesmus falcatus, it is reported that centrifugation consumes 65.34 kwh kg−1 of energy, which is much higher than 1.76 kWh kg−1 required by the electrochemical process.9 Electrochemical processes are generally used for water and wastewater treatment. In this method, coagulants are generated in situ using an electricity supply and an electrolysis reaction occurs. During electrolysis, these electrodes release hydrogen (cathode) and oxygen (anode) gases.13 In the electrochemical process, cations are released from the anode, and hydroxyl anions and hydrogen bubbles are generated at the cathode when a current is applied. These released cations destabilize the negatively charged algae, leading to aggregation. The release of cations and hydroxyl anions produces metal hydroxides that promote coagulation.14 The main advantages of this process are high efficiency, easy operation, and no chemical requirement.8 In addition, compared with other microalgae harvesting methods, electrochemical processes are cost-effective and appropriate for a variety of algal species.7 Electrochemical processes use both sacrificial materials such as aluminum (Al) and iron (Fe), and non-sacrificial materials such as boron-doped diamond (BDD), carbon, or Ti electrodes. BDD is preferred for its stability and efficiency in treating industrial and municipal wastewater, although its use in algae-related studies is limited and mostly focuses on removal rather than harvesting.15 Response surface methodology (RSM) is a statistical and mathematical tool that is commonly used in experimental design and analysis. It utilizes mathematical models to evaluate the effects and interactions of these variables and optimize the responses of the system.6,16 RSM is commonly used to determine operational parameters and optimize a variety of electrochemical wastewater treatment studies.17,18 In addition, Savvidou et al. (2021) used microwave-synthesized magnetite to optimize the magnetic harvesting of C. vulgaris.6
Few studies have optimized the operating parameters for electrochemical algal harvesting. Pandey et al., (2020) achieved complete harvesting of Scenedesmus sp. using an Al electrode at pH 5, with 15 minutes of electrolysis and 12 mA cm−2 current density,19 while Wong et al., (2017) used RSM to assess C. vulgaris harvesting, both with efficiencies under 25%, which is very low.20 These studies did not address energy consumption or operating parameter (pH, current, and time) optimization. The current study aims to fill these gaps by exploring electrochemical process for harvesting C. vulgaris, focusing on optimizing the pH, current, electrolysis time, and electrode configuration. Using RSM, the optimal parameters were identified, and various electrode pairs (BDD–Al, Al–BDD, Al–Al, and Fe–Fe) were compared to determine the best harvesting efficiency. Electrode configurations in electrochemical processes can significantly impact the electrochemical treatment performance.21 In literature, studies have predominantly employed a single electrode configuration, and the influence of various electrodes on algae harvesting has not been thoroughly examined.13,22 This study distinguishes itself by employing both inert (BDD) and sacrificial electrodes (Al or Fe) to compare their effects on algae harvesting, thereby contributing novel insights to the field. To the best of our knowledge, no previous study has investigated the various electrode pairs' effect on algae harvesting efficiency and harvesting cost. It is essential to observe the modifications in the physicochemical characteristics of algae post-electrochemical treatment, particularly for their utilization in advanced sectors like biodiesel production. However, there is a notable scarcity of research on this topic within the existing literature.3 In the scope of this study, the properties of the harvested algae, such as carbohydrate, lipid, protein, and chlorophyll contents, were determined. In electrochemical harvesting, the cost of the process is as important as the harvesting efficiency. Within the scope of this study, the optimization of operating parameters to achieve the highest harvesting efficiency at the lowest cost constitutes its innovative aspect.
2 Materials and methods
2.1 Microalgae cultivation
Pure culture microalgae, C. vulgaris strain, was obtained from the University of Texas at Austin Culture Collection of Algae (UTEX). Before harvesting, the algae were inoculated in BG-11 broth medium was prepared as previously described.23 The detailed composition of the BG-11 medium is provided in the SI (Table S1). Cell density was determined by measuring the optical density of the algal samples at 680 nm using a spectrophotometer.24 When the optical density of algae reached 0.5 at 680 nm, they were transferred to a 22.7 L photobioreactor tank. Egg-washing wastewater (Egg Innovations, Warsaw, Indiana, United States) was added to the photobioreactor as a growth medium for algae. The characteristics of the egg-washing wastewater have been described in a previous study.25 Air was supplied using an air pump and light was provided using LED lamps with a light/dark cycle of 12:12 h. After 21 days of cultivation, the algae were ready for harvesting, with a dry weight of 0.263 g L−1.2.2 Experimental setup
Electrochemical algae harvesting experiments were conducted using a 1.0 L reactor in batch mode (Fig. 1). A 700 mL C. vulgaris solution was added to the reactor, and a magnetic stirrer was used at a constant speed of 200 rpm to ensure the homogeneity of the solution. A DC power supply was used to continuously apply a voltage to the electrodes. NaCI was selected as the supporting electrolyte and was added at a constant concentration of 1.0 g L−1 in the experiments. It has been reported that NaCI decreases electrolysis time and increases algae harvesting efficiency.13 1.0 M HCI or NaOH were used for pH adjustment to the desired value (5, 7, and 9). This study tested three different currents, including 0.1, 0.3, and 0.5 A (current density values: 1.64, 4.92, and 8.20 mA cm−2). Three types of electrodes, including Al, Fe, and BDD were used, and the inter-electrode gap was kept constant at 1.0 cm throughout the study based on previous studies.26,27 In addition, short distances like 1 cm decrease electrode power consumption and electrode passivation while enhancing process efficiency.15 Four different electrode configurations, including BDD–Al, Al–Al, Fe–Fe, and Al–BDD, were used, and their effects on harvesting efficiencies were compared. The dimensions of the electrodes were 6.6 cm (width), 12 cm (length), and 2 mm (thickness), and the effective surface area was 61 cm2. The electrodes were cleaned after each operation using nitric acid and acetone solutions and rinsed with distilled water to remove residual pollutants. All the experiments were conducted in the Agricultural & Biological Engineering Department at Purdue University in 2023. | ||
| Fig. 1 (a) The schematical diagram of electrocoagulation of algae (1: power supply, 2: anode, 3: cathode, 4: magnetic stirrer); (b) a real application of electrochemical algae harvesting. | ||
2.3 Analytical methods
The initial, final, and harvested algae samples were collected, and before each analysis, the samples were kept at 4 °C to inhibit any biological activity. Harvesting efficiency, chlorophyll-a, volatile and total suspended solids, lipids, sugars, and protein contents were measured. | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 minutes, then 2 mL of sample was taken from the supernatant, 4.5 mL of reagent (0.0075 g bromophenol blue +15 mL 95% ethanol +2.5 mL glacier acetic acid) was added. The mixture was allowed to sit for 15 minutes, and the peaks were read on the spectrophotometer at 610 nm.
000 rpm for 5 minutes, then 2 mL of sample was taken from the supernatant, 4.5 mL of reagent (0.0075 g bromophenol blue +15 mL 95% ethanol +2.5 mL glacier acetic acid) was added. The mixture was allowed to sit for 15 minutes, and the peaks were read on the spectrophotometer at 610 nm.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 minutes, and the supernatant was discarded. The pellet was resuspended in 1.0 mL of methanol and heated at 65 °C for 15 minutes in a water bath. After cooling the tube in a freezer at −20 °C for 15 minutes, the sample was centrifuged again at 10
000 rpm for 5 minutes, and the supernatant was discarded. The pellet was resuspended in 1.0 mL of methanol and heated at 65 °C for 15 minutes in a water bath. After cooling the tube in a freezer at −20 °C for 15 minutes, the sample was centrifuged again at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 minutes. Subsequently, 1.0 mL of the extract was mixed with 2.0 mL of methanol in a cuvette, and the optical density (OD) was measured across 200–800 nm. Chlorophyll concentration was calculated using eqn (2).32
000 rpm for 10 minutes. Subsequently, 1.0 mL of the extract was mixed with 2.0 mL of methanol in a cuvette, and the optical density (OD) was measured across 200–800 nm. Chlorophyll concentration was calculated using eqn (2).32| Chla (mg L−1) = 12.7 × (Abs663) − 2.69 × (Abs645) | (2) |
 | (3) |
Energy consumption is the main part of the total cost. In this current study, energy consumption (kWh kg−1) was calculated based on previous study11 given in eqn (4). Where U, I, t, V, ηa, and ci are the voltage (V), current (I), electrolysis time (h), the volume of the solution (m3), algae harvesting efficiency, and initial algae biomass concentration (kg m−3), respectively. The cost for power consumption is calculated using energy consumption multiplied by the unit price of electricity in Indiana, US that is 0.1245$ per kWh.33
 | (4) |
2.4 RSM design and optimization
The effect of operating variables, including electrolysis time (X1), pH (X2), and current (X3) on the algae harvesting was evaluated using Box–Behnken design (BBD) under the application of response surface methodology (RSM). Preliminary experiments were carried out to determine the experimental design parameters. Design Expert software version 11.0.3 (Stat-Ease Inc., MN, USA) was used for experimental design, and three independent levels with 15 runs were conducted in two replications. The experimental design was created based on independent test ranges, and the codes are given in Table 1.| Factor | Variable | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| X 1 | Electrolysis time (min) | 15 | 20 | 25 |
| X 2 | pH | 5 | 7 | 9 |
| X 3 | Current (mA) | 100 | 300 | 500 |
The second-order polynomial model was used to predict optimal operating values. Response (Y) and independent (Xi and Xj) variables were described, respectively (eqn (5)). A number of independent values, random error, constant coefficient, coefficient of linear, interaction and quadratic terms were k, ε, β0, βi, βii, and βij, respectively.
 | (5) |
3 Result and discussion
Electrode selection is a critical factor in the electrochemical process, as electrodes significantly influence performance.26 In this study, BDD, Al, and Fe electrodes were used in four configurations: Al–Al, BDD–Al, Al–BDD, and Fe–Fe. The BDD electrode was chosen as an inert electrode, while Al and Fe were selected as sacrificial electrodes.3.1 Electrochemical harvesting of C. vulgaris and RSM
Box–Behnken design was used to create the model and optimize the electrochemical harvesting of C. vulgaris. Fifteen experiments were conducted with two replicates for each electrode pair to determine the effect of electrolysis time, current, and pH on algae harvesting efficiency. The maximum harvesting efficiency of 99.3% was obtained under an electrolysis time of 20 minutes, a current of 100 mA, and pH of 9 using the BDD–Al electrode pair. Similar to this electrode pair, a maximum efficiency of 99.10% for Al–Al under an electrolysis time of 25 minutes, a current of 300 mA, and pH of 5 was observed, and 99.03% for Al–BDD at an electrolysis time of 25 minutes, a current of 300 mA and, pH of 5.The p values in the ANOVA results were used to determine model significance. In this reseach, p values were found as <0.0001, 0.0001, 0.0008, and <0.0001 for Al–Al, Al–BDD, BDD–Al, and Fe–Fe, respectively. This situation indicated that the model is significant and accurate. To determine the correlation of dependent and independent variables, the R2 values were determined. R2 values were found as 0.995, 0.992, 0.965, and 0.985 for Al–Al, Al–BDD, BDD–Al, and Fe–Fe, respectively. This situation indicated that there is a high correlation between process variables and harvesting efficiency. Adequate precisions were found to be 45.46, 25.11, 16.43, and 27.64 for Al–Al, Al–BDD, BDD–Al, and Fe–Fe, respectively. The models can be used to navigate the design space due to higher adequate precision values (>4).
A high F value indicates a strong influence on harvesting efficiency. Among the independent variables, the maximum F values were 173.91 for the Al–Al electrode pair, 65.85 for Al–BDD, 20.78 for BDD–Al, and 49.52 for Fe–Fe. These results suggest that the model for the Al–Al electrode pair is the most significant, followed by Al–BDD, Fe–Fe, and BDD–Al. The highest F value was found as 668.42 and 261.02 for the current in Al–Al and Al–BDD electrode pairs, and this situation indicated that current was the most important factor and pH and electrolysis time followed the current for Al–Al; however, electrolysis time and pH followed the current for Al–BDD electrode pair. On the other hand, the F value for pH was found as 477.97 for BDD–Al electrode pair, and this situation indicated that pH was the most effective parameter and current and electrolysis time followed the pH.
Equations were created for each electrode pair and are given in Table 2. Where Y is the harvesting efficiency, X1, X2, and X3 are the electrolysis time, pH, and current, respectively. X1X2 and X2X3 model terms were significant model terms for each electrode pair. Among all variables, the X1X3 model terms were found to be insignificant (p > 0.05) for the Al–Al electrode pair.
| Electrode configuration | Equations |
|---|---|
| Al–Al | y = 88.02 + 3.21X1 − 8.03X2 + 12.60X3 + 1.58X1X2 + 1.42X2X3 + 3.16X12 − 1.04X22 − 13.68X32 |
| BDD–Al | y = 89.60 + 4.05X1 + 7.73X2 + 5.58X3 − 21.18X1X2 − 3.20X1X3 − 9.00X2X3 − 10.29X12 − 3.10X22 |
| Al–BDD | y = 98.29 + 2.17X1 − 0.355X2 + 5.10X3 − 0.643X1X2 − 1.53X1X3 − 2.88X2X3 + 1.05X12 − 2.6X22 − 6.45X32 |
| Fe–Fe | y = 74.91 + 0.98X1 + 2.62X2 − 2.89X3 + 3.04X1X2 + 2.03X1X3 + 7.66X2X3 + 8.03X12 + 2.43X22 |
The positive-signed model terms indicated a synergistic effect and increased the harvesting efficiency, while negative-signed model terms had an adverse effect and decreased the harvesting efficiency. In the present study, electrolysis time and current had a synergistic effect on the response for each electrode pair. pH had a synergistic effect on BDD–Al but had an adverse impact on the Al–Al and Al–BDD electrode pairs. All interactions had a synergistic effect on harvesting efficiency when the Fe–Fe electrode pair was used.
Fig. 2 presents the 3D response surface graphs generated by the predictive model for each electrode pair. These plots depict algae harvesting efficiency as a function of electrolysis time versus pH, pH versus current, and electrolysis time versus current. The graphs show that at a constant electrolysis time of 20 minutes, current has a greater impact on harvesting efficiency than pH. Similarly, in the electrolysis time versus current plot, current is more influential than electrolysis time at a constant pH of 7. For the electrolysis time versus pH plot, electrolysis time exerts a stronger influence than pH at a constant current of 300 mA for the Al–Al and Al–BDD electrode pairs. In contrast, for the BDD–Al and Fe–Fe electrode pairs, electrolysis time and pH have similar effects on harvesting efficiency.
It was observed that the algae harvesting efficiency varies from 51.75 to 99.10% for Al–Al, 41.07 to 99.31% for BDD–Al, 82.00 to 99.03% for Al–BDD, and 63.36 to 91.10% for Fe–Fe electrode pairs (Table 3). These results align with previous studies20,34 that demonstrated the effectiveness of electrochemical processes in enhancing algae harvesting efficiency. The findings also confirm that Al electrodes are more effective than Fe electrodes for algae harvesting, consistent with earlier research.11,26 This superiority is likely due to the higher effectiveness of Al(OH)3 as a coagulant compared to Fe(OH)2.11
| Run number | Operating conditions | Harvesting efficiency (%) | |||||
|---|---|---|---|---|---|---|---|
| Electrolysis time (min) | pH | Current (mA) | BDD–Al | Al–BDD | Al–Al | Fe–Fe | |
| 1 | 15 | 9 | 300 | 96.10 | 95.74 | 78.00 | 83.12 |
| 2 | 25 | 7 | 100 | 72.14 | 92.00 | 67.00 | 84.71 |
| 3 | 20 | 9 | 100 | 99.31 | 85.88 | 51.75 | 76.40 |
| 4 | 20 | 9 | 500 | 92.87 | 90.72 | 78.00 | 85.31 |
| 5 | 15 | 7 | 100 | 76.66 | 84.00 | 61.00 | 86.74 |
| 6 | 25 | 9 | 300 | 68.06 | 98.20 | 87.00 | 91.10 |
| 7 | 20 | 5 | 500 | 92.62 | 98.35 | 92.00 | 63.36 |
| 8 | 25 | 7 | 500 | 89.29 | 98.72 | 95.00 | 83.61 |
| 9 | 20 | 7 | 300 | 84.89 | 98.06 | 89.00 | 74.05 |
| 10 | 20 | 7 | 300 | 90.04 | 98.68 | 88.07 | 76.42 |
| 11 | 15 | 5 | 300 | 41.07 | 94.00 | 96.44 | 85.32 |
| 12 | 25 | 5 | 300 | 97.74 | 99.03 | 99.10 | 81.14 |
| 13 | 15 | 7 | 500 | 81.00 | 96.85 | 87.00 | 77.52 |
| 14 | 20 | 5 | 100 | 63.07 | 82.00 | 71.45 | 85.10 |
| 15 | 20 | 7 | 300 | 91.99 | 98.14 | 87.00 | 73.47 |
3.2 Influence of process variables and their optimization for electrochemical algae harvesting
The results proved that electrolysis time positively affected harvesting efficiency by using sacrificial electrodes (Fe or Al). In this current study, increasing electrolysis time from 15 to 25 minutes, the harvesting efficiency improved from 78 to 87% with Al–Al, and from 83.3 to 91.1% with Fe–Fe at constant pH (9) and current (0.3 A). Fayad et al., (2017) evaluated the effect of electrolysis time on C. vulgaris harvesting efficiency.35 They found that increasing electrolysis time from 15 to 25 minutes improved their harvesting efficiency from approximately 8 to 20% for Fe electrodes and from 18 to 38% for Al electrodes at a constant pH of 8 and a current density of 6.7 mA.35 The slight difference in harvesting efficiencies can be raised from variations in the operations of the process between the two studies.
The results demonstrated that the maximum algae harvesting efficiency was found to be 99% under a current of 0.3 A for Al–Al and Al–BDD. When the current was increased to 0.5 A at the same pH, the harvesting efficiencies were found to be 98.35 and 92% for Al–Al and Al–BDD, respectively. The reason for the marginal difference can be explained as the formation of Al(OH)3 layers on electrode surfaces that hinder the release of Al3+ ions and due to passivation.37 A similar study also reported that increasing the current beyond a certain point has no marginal effect on algae harvesting.34
Increasing the current significantly reduces the electrolysis time needed to achieve maximum harvesting efficiency. Acurrent of 0.5 A resulted in over 80% harvesting efficiency within 15 minutes, while a current of 0.1 A failed to reach 80% even after 25 minutes using BDD–Al electrodes. These findings are similar to previous study by Li et al., (2022), which used a non-sacrificial anode and stainless steel cathode and found that increasing current enhances algae harvesting efficiency.7 Additionally, higher currents improved algae harvesting efficiency. With BDD–Al electrodes, increasing the current from 0.1 to 0.5 A raised efficiency from 63 to 92% at a constant pH of 5 and an electrolysis time of 20 minutes. However, at pH 7, the efficiency increase was smaller, rising from 72 to 89% over 25 minutes. In pH 5, more Al is released from the electrode surface with increasing current, which enhances Al(OH)3 generation. On the other hand, under pH 7, hydroxyl ions increase, causing some Al3+ to remain in soluble form or transform into Al(OH)4−, which limits coagulation efficiency.38,39 Additionally, under high pH, undesired oxidation processes can occur at the anode, reducing the performance of the electrochemical process.40
The applied current facilitates the release of Al, enhancing the generation of Al coagulants. These positively charged coagulants destabilize the negatively charged algae, thereby increasing algae harvesting efficiency.41 In this current study, a maximum harvesting efficiency of 99% was achieved using an Al–Al electrode pair with a current of 0.3 A, an electrolysis time of 25 minutes, and a pH of 5. These results are comparable to or exceed those of previous studies. For instance, Javan et al. (2024) reported a maximum C. vulgaris harvesting efficiency of 68.5% at a current density of 3 mA cm−2 and an electrolysis time of 60 minutes using Al–Al electrodes.37 Similarly, Hawari et al. (2020) found that increasing the current boosts harvesting efficiency, achieving a maximum of 97.6% with Al–Al electrodes.42 Fayad et al. (2017) also reported a 99% harvesting efficiency in similar conditions for C. vulgaris harvesting.35
In the Al–BDD electrode configuration, increasing the current from 0.1 A to 0.5 A enhanced harvesting efficiency from 92 to 98% at a constant pH of 9 and an electrolysis time of 20 minutes. Similarly, at a constant pH of 7 and an electrolysis time of 25 minutes, efficiency increased from 94 to 98%. These results align with previous studies. For instance, an electrochemical harvesting study of Scenedesmus quadricauda showed that increasing the current density from 7.5 to 30 mA cm−2 (with an electrode area of 70 × 40 mm2) raised the harvesting efficiency from 91.8 to 100%.41 The slight difference may arise from the type of algae. Additionally, Li et al. (2022) studied C. vulgaris using a non-sacrificial anode and found that increasing the current from 1 A to 3 A boosted harvesting efficiency from 40 to 80% over 120 minutes of electrolysis.7
In the Fe–Fe electrode configuration, increasing the current from 0.1 to 0.5 A at a pH of 7 did not result in significant changes in algae harvesting efficiency. In pH 9, the medium is alkaline, more conductive, and Fe(OH)3 is the stable form. Therefore, the coagulation mechanism is enhanced with increasing current due to increased OH− generation. On the other hand, at a pH 7, there are not enough OH− in the medium, and the effect of current is limited.43,44 However, at a pH of 9, the efficiency increased from 76.4 to 85.3%. This finding aligns with previous research by Maleki et al. (2014), which reported a rise in Dunaliella salina harvesting efficiency from 33 to 75% when the current was increased from 0.3 to 1 A at a pH of 7.5.45 Unlike other electrode configurations, the Fe–Fe electrodes generally showed a decrease in harvesting efficiency with increased current, except under alkaline conditions. This decrease could be due to the change in the solution's color because of excess dissolved from the Fe metal, which interferes with spectrophotometric measurements, as Vandamme et al. (2011) noted.11 Additionally, side reactions and electrode corrosion may contribute to this outcome. Algae separation via flotation benefits from increased current, as it raises microbubble density and reduces bubble size, thus enhancing flotation efficiency.46 While higher currents generally improve harvesting efficiency, they also lead to increased energy consumption.7,47 Therefore, optimizing the current to balance low energy consumption with high harvesting efficiency is crucial.
The significant release of protective extracellular polymers in alkaline conditions above pH 9 is primarily responsible for changes in the surface charge of algal cells. In acidic conditions, variations in the dissociation of carboxyl and amine groups in the algal cell wall also influence surface charge.10 Generally, harvesting efficiency is lower in basic conditions compared to neutral or acidic conditions, which may be due to changes in water charge. In basic environments, aluminum dissolves as Al(OH)4, shifting the water's charge from positive to negative. Since algae naturally carry a negative charge, this reduces harvesting efficiency.14
The current study achieved high algae harvesting efficiencies with the BDD–Al electrode pair at pH 9, under low current (0.1 A), and longer electrolysis time (20 minutes). This improvement may be due to increased flotation from the generation of H2 and O2 gases. Conversely, at pH 5, harvesting efficiency dropped to 63%. This reduction was more pronounced at high currents and low pH, with efficiency falling to 41%. In acidic conditions, electrooxidation dominates for this electrode pair due to the high O2 overpotential of the BDD electrode.17 Additionally, generated OH radicals at low pH may contribute to algae biomass degradation, leading to reduced harvesting as biomass is degraded instead of collected.
High harvesting efficiency was generally observed under acidic conditions for the Al–Al electrode pair. However, the combined effects of pH-current and pH-electrolysis time on harvesting efficiency should be considered. Elevated harvesting efficiencies were achieved at high current settings, even at a high pH of 9. For instance, 71% efficiency was recorded at pH 5 with a 20 minute electrolysis time and 0.1 A current, while 87% efficiency was observed at pH 9 with a 25 minute electrolysis time and 0.3 A current. Similarly, Zhang et al. (2015) reported 91.9% efficiency at pH 9.47 Literature suggests that the formation of insoluble Al(OH)3 in alkaline conditions supports sweeping coagulation–flocculation, a key mechanism for algae harvesting.11
In the Fe–Fe electrode configuration, an increase in pH generally enhances harvesting efficiency compared to other electrode pairs. However, under low current conditions (0.1 A), reducing the pH from 9 to 5 increased algae harvesting efficiency from 76.40% to 85.10% in this study. A previous study by Beiramzadeh et al. (2022) found that higher pH levels improve precipitation in the EC process with iron electrodes.48 This is attributed to Fe(II) oxidizing to Fe(III) in basic conditions, which enhances EC performance. Additionally, iron hydroxide formation increases in alkaline conditions, further improving harvesting efficiency. The pH also influences the formation of iron coagulants: in acidic conditions, species such as Fe(OH)2+, Fe(OH)2+, and Fe(OH)3 dominate, while in alkaline conditions, Fe(OH)63− and Fe(OH)4− are the predominant species.5
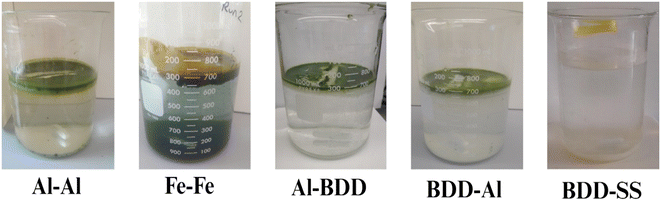 | ||
| Fig. 3 Electrochemical algae harvesting using different electrode pairs (Al: aluminum, Fe: iron, BDD: boron-doped diamond, SS: stainless still electrodes). | ||
Compared to the Al–BDD configuration, the BDD–Al setup exhibited lower harvesting efficiency, likely due to the higher degradation potential associated with the BDD anode. Preliminary experiments using a BDD anode and stainless-steel (SS) cathode showed complete algae removal, as hydroxyl radicals generated during the electrochemical process degrade algae. When the BDD anode is used, electrooxidation becomes the dominant mechanism, particularly in acidic conditions. Under acidic conditions (pH 5), with a current of 0.3 A and an electrolysis time of 15 minutes, the lowest algae harvesting efficiency (41%) was observed in the BDD–Al configuration.
During the electrochemical process, the algae suspension turned milky white, producing green foam that floated as the voltage was applied after a certain time. The subnatant became colorless as the foam rose to the surface. The type of electrode significantly influenced the characteristics of the flocs. In the BDD–SS electrode configuration, a layer of white flocs appeared on the surface, likely due to the electrooxidation-driven degradation of algae. The biomass was fully broken down, changing the floc color from green to white. In contrast, the Al–Al and BDD–Al configurations produced greenish algae flocs, which floated on the surface. These flocs likely contained algae cells and aluminum hydroxide.46 Previous studies have reported that algae cells primarily comprise carbon, nitrogen, and oxygen.46
In the Fe–Fe configuration, yellowish-green flocs formed at the surface after a certain time, attributed to the presence of Fe(II) and Fe(III), which caused the flocs and water to take on a yellow or brown hue. The subnatant remained transparent in the Al–Al, BDD–Al, and Al–BDD configurations. These observations are consistent with previous research.14
While electrode type influences algae harvesting efficiency, sacrificial electrodes like Al and Fe can introduce contaminants into the harvested algae, leading to complications. According to Faraday's law, increasing current and electrolysis time elevates the concentration of dissolved Al or Fe.47 Optimizing electrolysis time and current is essential to mitigate this issue, or using inert electrodes like BDD may be preferred to avoid contamination. The generation of H2 in cathode through electroflotation was the dominant mechanism and algae generally collected on the surface. In addition, at anode Fe and Al ions dissolved and the metal cations generated in solution caused aggregation of algae.
The mechanisms occurring at the anode and cathode for electrode configurations differ, and this situation also affects harvesting efficiency. The mechanisms in electrochemical processes are summarised in Table 4.
| Electrode pairs | Dominant mechanism at anode | Dominant mechanism at cathode | Harvesting efficiency |
|---|---|---|---|
| BDD–Al | OH* radical generation and electrooxidation | Electrocoagulation | Low–very high |
| Al–BDD | Electrocoagulation | H2 generation, electroflotation | Optimum condition, high–very high harvesting efficiency |
| Al–Al | Electrocoagulation | H2 generation, electroflotation | Moderate–very high |
| Fe–Fe | Electrocoagulation | H2 generation, electroflotation | Moderate–very high |
| BDD–SS | OH* radical generation and electrooxidation | Limited electrofloatation | Biomass degraded, no algae harvesting |
In this study, Al, Fe, and BDD electrodes generally ensured effective algae harvesting potential. Despite the high initial investment cost, BDD electrodes are long-lasting, durable, inert in structure, and do not produce secondary pollutants like sludge, enabling high algae harvests.15 On the other hand, the dissolution of sacrificial electrodes may require periodic replacement of Al and Fe electrodes, which could incur additional costs. Moreover, the accumulation of Al released from these electrodes within the system may be toxic to living organisms. To overcome this issue, lower current and shorter electrolysis time were recommended.26 It is desired that the Al content in the harvested algae be less than 1% or less than 2 mg L−1 in solution. To achieve this, it is suggested to operate at low current and short electrolysis times.26 In this current study, the aluminum accumulated in the harvested algae was not analyzed; however, when compared to the conditions in the literature, it is estimated that the aluminum content in the algae will be less than 1% because the current and electrolysis times used in this study are shorter than those in the current literature.11,26,49
3.3 Electrochemical harvesting of algae
In the present study, the TSS of algae before electrochemical harvesting was 0.26 g L−1, increasing to 3.27 g L−1 post-harvesting due to the higher sample concentration. The electrochemical harvesting process provides algae aggregation, which increases the algal TSS value. Similarly, Parmentier et al., (2020) reported that TSS values increased after electrocoagulation–flotation using Al and Fe electrodes to harvest C. vulgaris.14 In this study, the VSS/TSS ratio after electrochemical harvesting averaged 0.8, aligning with previous research that reported a ratio of 0.69–0.84.51
Similarly, Pandey et al. (2020) found that the electrocoagulation flotation process had no impact on the chlorophyll-a content of Scenedesmus sp., reporting a value of 8.3 μg mL−1.19 Landels et al. (2019) also evaluated the impact of electrochemical harvesting on pigment changes in various algal species, concluding that these methods did not significantly alter pigment profiles. However, in some cases, the electrocoagulation flotation process can cause inaccurate chlorophyll measurements due to spectrophotometric interference.52
3.4 Energy consumption for electrochemical harvesting and sustainability
By employing Al–BDD, Al–Al, Fe–Fe, and BDD–Al electrode configurations, the average energy consumption obtained from RSM experiment values were 1.74, 2.21, 2.23, and 2.6 kWh kg−1, respectively. These values were lower than those of conventional algae harvesting methods. For instance, the energy consumption for centrifugation varies from 16 to 65.34 kWh kg−1,13,56 4.62 kWh kg−1 for electrocoagulation,34 3.84 kWh kg−1 for electrocoagulation–flotation.3 0.7–19.71 kWh kg−1 for membrane filtration, and 9.33 kWh kg−1 for electrocoagulation–flotation and membrane technology.12 In this current study, the minimum energy consumptions were observed as 0.18, 0.19, 0.2, and 0.21 kWh kg−1 for Fe–Fe, Al–Al, Al–BDD, and BDD–Al, respectively, in conditions of current at 0.1 A, pH of 5, and electrolysis time of 20 minutes. The low energy consumptions in these results were produced because of the lowest current application. On the other hand, maximum energy consumptions were observed at another set of experiments with a current of 0.3 A, pH of 5, and electrolysis time of 15 minutes. The maximum energy consumptions were 5.16 kWh kg−1 for Al–BDD, 5.42 kWh kg−1 for Fe–Fe, 5.67 kWh kg−1 for Al–Al, and 11.81 kWh kg−1 for BDD–Al configuration. It is indicated that increasing current increases energy consumption. Similar results were also reported by Vandamme et al., (2011). They studied the electrocoagulation flotation process for C. vulgaris harvesting and found energy consumption of 1.3 kWh kg−1 at 0.3 A, which increased to 9.5 kWh kg−1 at 1.2 A.11In this study, the inert cathode consumed less energy than the sacrificial electrode. Similarly, Javan et al. (2024) compared the energy consumption of different cathode materials in algae harvesting. They found that Al cathodes required more energy than graphite.37 Wong, (2016) also reported that in the electrochemical harvesting of C. vulgaris, aluminum electrodes consumed the highest energy (3.7 kWh kg−1), followed by iron (3.6 kWh kg−1) and carbon (1.9 kWh kg−1) electrodes.57 This current study used 1 g L−1 NaCI as the electrolyte. Increasing salt concentration was found to reduce energy consumption, consistent with findings from Al-Yaqoobi and Al-Rikabey, (2023).8 Fayad et al. (2017) also reported a significant reduction in energy consumption from 5.7 to 1.6 kWh kg−1 when NaCI concentration increased from 0.5 to 1.5 g L−1.35
Operating parameters such as current, electrolysis time, and pH significantly influence energy consumption. Current was found to have the greatest impact, followed by electrolysis time, while pH had no statistically significant effect. In general, increasing current and electrolysis time leads to higher energy consumption. The highest harvesting efficiency (99.3%) was achieved with the BDD–Al electrode pair at an energy consumption of 0.2 kWh kg−1. In comparison, the Al–Al (99.01%) and Al–BDD (99.03%) electrode pairs required 0.35 kWh kg−1 and 0.4 kWh kg−1 as energy consumption, respectively. These results align with a previous study, which reported optimal energy consumption of around 0.38 kWh kg−1 using an Al–Al electrode with 1.0 g L−1 NaCI as the electrolyte.8
For the Fe–Fe electrode pair, energy consumption was 5.42 kWh kg−1 with a harvesting efficiency of 91%. This high energy demand resulted from the use of elevated current and extended electrolysis time to achieve harvesting efficiency. However, for a lower harvesting efficiency of 85%, the energy consumption decreased significantly to 0.29 kWh kg−1. It has been reported that Al electrodes consume less energy than Fe electrodes.14 This is due to the formation of a passivating corrosion layer on the Fe electrode surface, which requires higher current to maintain performance, leading to increased energy consumption.14,58
In this current study, the average energy cost from power consumption for Al–BDD, Al–Al, Fe–Fe, and BDD–Al electrode configurations was 0.216, 0.275, 0.277, and 0.32 $ per kg, respectively. These values are lower than the centrifugation process, which reported 1.5 $ per kg, but similar to the drum drying energy cost of 0.34 $ per kg.59 In addition, the electrochemically (using Al electrode) biomass production costs of C. vulgaris and Nannochloropsis were reported as 0.339 and 0.169 $ per kg, respectively.60 Studies indicated that energy costs are a substantial part of the overall harvesting cost. These costs generally range from 20–30% of the total operational costs.60 It is worth noting that chemical consumption and electrode renewal costs can be considered when calculating the total cost. Algae species and operational conditions can also impact the harvesting cost. The electrochemical algae harvesting method, an environmentally friendly approach, enables the fast and cost-effective harvesting of algae biomass without significantly changing the algae's structure. Compared to traditional algae harvesting methods, it is cheaper and does not need chemicals (only small amounts may be needed for pH and conductivity), ensuring economic sustainability.61
A study on the life cycle analysis of biocrude production from microalgae revealed that algae cultivation and harvesting contribute significantly to greenhouse gas emissions across the entire process, primarily due to energy consumption and resource utilization.62 On the other hand, life cycle study on algae harvesting indicates that electrochemical processes have lower greenhouse gas emissions compared to traditional methods.63 There is a limited study about the life cycle assessment (LCA) of electrochemical algae harvesting. Based on the harvesting of 1 kg of dry algae mass, greenhouse gas emissions calculated on a gate-to-gate basis were reported as 77 g CO2 eq J−1 kg−1 algae using the electrochemical harvesting method, while this value was found to be 94.3 g CO2 eq J−1 kg−1 for harvesting using flocculation with chitosan.63 On the other hand, an LCA study on the electrocoagulation of wastewater treatment indicated that global warming, terrestrial ecotoxicity, fossil resource scarcity, and human non-carcinogenic toxicity were the parameters with the highest environmental impact, while the impacts of other parameters were less than 2%. In the same study, it was reported that global warming was mainly attributed to electricity consumption; terrestrial ecotoxicity resulted from both electricity consumption and metal release during the EC process; fossil resource scarcity was linked to electricity consumption during the EC process, as well as the production of electrodes and chemicals; and human non-carcinogenic toxicity was associated with chemical release during electrode production.64 Additionally, the environmental impacts of electrochemical processes can be minimized by reducing greenhouse gas emissions when electricity is provided from renewable energy sources.65
4 Conclusion
This study thoroughly examined the influence of various process variables on algae harvesting efficiency, focusing on electrolysis time, current density, pH, and electrode configuration. The comparison of electrode configurations revealed that Al-based electrodes performed better than Fe-based ones, primarily due to superior coagulant properties. The BDD–Al configuration showed high harvested efficiency at a low current. The Al–Al configuration consistently achieved high harvesting efficiencies and was more energy-efficient than Fe–Fe and BDD–Al configurations. Increasing electrolysis time significantly enhanced harvesting efficiency across all electrode configurations. Current density also played a crucial role; higher currents generally improved harvesting efficiency by increasing coagulant production and bubble density, although excessive currents led to diminishing returns and higher energy consumption. The pH significantly impacted harvesting efficiency. Acidic conditions (pH 5–7) generally resulted in higher efficiencies due to enhanced charge neutralization and coagulation. In contrast, alkaline conditions (pH 9) often reduce efficiency. The harvested algae's TSS, VSS, and biochemical content (lipids, proteins, and carbohydrates) were consistent with previous studies, indicating that electrochemical-based methods are effective for algae harvesting with manageable energy consumption. This paper focuses solely on C. vulgaris due to its widespread use as an algae species; however, other algae species have different cell wall compositions that can be impacted in various ways. Further study will compare different algae species based on optimized electrochemical operation conditions. The accumulation of iron and aluminum in harvested algae will be examined in future studies.Author contributions
E. Y.: conceptualization, methodology, formal analysis, writing – original draft, writing – review & editing; S. G.: conceptualization, writing – review & editing, M. K.: methodology, creation of models,writing – review & editing, A. A.: validation, visualization, methodology, writing – review & editing, validation; A. B.: data curation, formal analysis, writing – review & editing, H. S.: investigation, resources, supervision, writing – review & editing, supervision, funding acquisition.Conflicts of interest
There are no conflicts to declare.Data availability
Supplementary information: recipe of BG-11 media and experimental design of the model. See DOI: https://doi.org/10.1039/D5EW00518C.The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request. All experimental data, including raw measurements, RSM design outputs, and energy consumption calculations, have been archived and can be provided to qualified researchers for verification or further analysis.
Acknowledgements
This research was funded by the USDA National Institute of Food and Agriculture, AFRI project number 2023-68016-39718. The opinions, findings, conclusions, and recommendations presented in this study are the sole responsibility of the authors and do not necessarily represent the perspectives of the funding agencies. The authors extend their sincere gratitude to the anonymous reviewers and the editor for their valuable and constructive feedback, which significantly contributed to the improvement of this paper.References
- K. Goiris, W. Van Colen, I. Wilches, F. León-Tamariz, L. De Cooman and K. Muylaert, Impact of Nutrient Stress on Antioxidant Production in Three Species of Microalgae, Algal Res., 2015, 7, 51–57, DOI:10.1016/j.algal.2014.12.002.
- M. Roy and K. Mohanty, A Comprehensive Review on Microalgal Harvesting Strategies: Current Status and Future Prospects, Algal Res., 2019, 44, 101683, DOI:10.1016/j.algal.2019.101683.
- R. Misra, A. Guldhe, P. Singh, I. Rawat, T. A. Stenström and F. Bux, Evaluation of Operating Conditions for Sustainable Harvesting of Microalgal Biomass Applying Electrochemical Method Using Non Sacrificial Electrodes, Bioresour. Technol., 2015, 176, 1–7, DOI:10.1016/j.biortech.2014.11.014.
- C. Nitsos, R. Filali, B. Taidi and J. Lemaire, Current and Novel Approaches to Downstream Processing of Microalgae: A Review, Biotechnol. Adv., 2020, 45, 107650, DOI:10.1016/j.biotechadv.2020.107650.
- S. Lucakova, I. Branyikova, S. Kovacikova, M. Pivokonsky, M. Filipenska, T. Branyik and M. C. Ruzicka, Electrocoagulation Reduces Harvesting Costs for Microalgae, Bioresour. Technol., 2021, 323, 124606, DOI:10.1016/j.biortech.2020.124606.
- M. G. Savvidou, M. M. Dardavila, I. Georgiopoulou, V. Louli, H. Stamatis, D. Kekos and E. Voutsas, Optimization of Microalga Chlorella Vulgaris Magnetic Harvesting, Nanomaterials, 2021, 11(6), 1614, DOI:10.3390/nano11061614.
- W. Li, Y. Zhang, Y. Hu, S. Luo, X. Wu, Y. Liu, A. Min and R. Ruan, Harvesting Chlorella Vulgaris by Electro-Flotation with Stainless Steel Cathode and Non-Sacrificial Anode, Bioresour. Technol., 2022, 363, 127961, DOI:10.1016/j.biortech.2022.127961.
- A. M. Al-Yaqoobi and M. N. Al-Rikabey, Electrochemical Harvesting of Chlorella Sp.: Electrolyte Concentration and Interelectrode Distance, Chem. Ind. Chem. Eng. Q., 2023, 29(1), 23–29, DOI:10.2298/CICEQ210815010A.
- A. Guldhe, R. Misra, P. Singh, I. Rawat and F. Bux, An Innovative Electrochemical Process to Alleviate the Challenges for Harvesting of Small Size Microalgae by Using Non-Sacrificial Carbon Electrodes, Algal Res., 2016, 19, 292–298, DOI:10.1016/j.algal.2015.08.014.
- I. A. Matter, V. K. Hoang Bui, M. Jung, J. Y. Seo, Y. E. Kim, Y. C. Lee and Y. K. Oh, Flocculation Harvesting Techniques for Microalgae: A Review, Appl. Sci., 2019, 9(15), 3069, DOI:10.3390/app9153069.
- D. Vandamme, S. C. V. Pontes, K. Goiris, I. Foubert, L. J. J. Pinoy and K. Muylaert, Evaluation of Electro-Coagulation-Flocculation for Harvesting Marine and Freshwater Microalgae, Biotechnol. Bioeng., 2011, 108(10), 2320–2329, DOI:10.1002/bit.23199.
- M. Taghavijeloudar, A. K. Ahangar, R. Keshmiri-Naqab, P. Yaqoubnejad and H. Shabanizadeh, Amalgamation of Electrocoagulation-Flotation and Membrane Technology: Rapid and Efficient Microalgal Biomass Recovery and Fouling Mitigation, Chem. Eng. J., 2025, 506, 160131, DOI:10.1016/j.cej.2025.160131.
- R. Misra, A. Guldhe, P. Singh, I. Rawat and F. Bux, Electrochemical Harvesting Process for Microalgae by Using Nonsacrificial Carbon Electrode: A Sustainable Approach for Biodiesel Production, Chem. Eng. J., 2014, 255, 327–333, DOI:10.1016/j.cej.2014.06.010.
- D. Parmentier, D. Manhaeghe, L. Baccini, R. Van Meirhaeghe, D. P. L. Rousseau and S. Van Hulle, A New Reactor Design for Harvesting Algae through Electrocoagulation-Flotation in a Continuous Mode, Algal Res., 2020, 47, 101828, DOI:10.1016/j.algal.2020.101828.
- E. Yakamercan, P. Bhatt, A. Aygun, A. W. Adesope and H. Simsek, Comprehensive Understanding of Electrochemical Treatment Systems Combined with Biological Processes for Wastewater Remediation, Environ. Pollut., 2023, 330, 121680, DOI:10.1016/j.envpol.2023.121680.
- S. Sharma, A. Aygun and H. Simsek, Electrochemical Treatment of Sunflower Oil Refinery Wastewater and Optimization of the Parameters Using Response Surface Methodology, Chemosphere, 2020, 249, 126511, DOI:10.1016/j.chemosphere.2020.126511.
- E. Yakamercan, A. Aygün and H. Simsek, Antibiotic Ciprofloxacin Removal from Aqueous Solutions by Electrochemically Activated Persulfate Process: Optimization, Degradation Pathways, and Toxicology Assessment, J. Environ. Sci., 2024, 143, 85–98, DOI:10.1016/j.jes.2023.08.013.
- S. Sharma and H. Simsek, Sugar Beet Industry Process Wastewater Treatment Using Electrochemical Methods and Optimization of Parameters Using Response Surface Methodology, Chemosphere, 2020, 238, 124669, DOI:10.1016/j.chemosphere.2019.124669.
- A. Pandey, R. Shah, P. Yadav, R. Verma and S. Srivastava, Harvesting of Freshwater Microalgae Scenedesmus Sp. by Electro–Coagulation–Flocculation for Biofuel Production: Effects on Spent Medium Recycling and Lipid Extraction, Environ. Sci. Pollut. Res., 2020, 27(3), 3497–3507, DOI:10.1007/s11356-019-06897-y.
- Y. K. Wong, Y. H. Ho, H. M. Leung, K. C. Ho, Y. H. Yau and K. K. L. Yung, Enhancement of Chlorella Vulgaris Harvesting via the Electro-Coagulation-Flotation (ECF) Method, Environ. Sci. Pollut. Res., 2017, 24(10), 9102–9110, DOI:10.1007/s11356-016-7856-x.
- K. Zuo, S. Garcia-Segura, G. A. Cerrón-Calle, F. Y. Chen, X. Tian, X. Wang, X. Huang, H. Wang, P. J. J. Alvarez, J. Lou, M. Elimelech and Q. Li, Electrified Water Treatment: Fundamentals and Roles of Electrode Materials, Nat. Rev. Mater., 2023, 8(7), 472–490, DOI:10.1038/s41578-023-00564-y.
- N. M. Nasir, A. J. Razif Harun, N. N. L. Nik Ibrahim and W. A. W. Abdul Karim Ghani, Optimization of Aquaculture Wastewater Bioremediation and Harvesting Utilizing Response Surface Methodology (RSM), Chem. Eng. Trans., 2021, 83, 151–156, DOI:10.3303/CET2183026.
- P. Bhatt, P. B. Brown, J. Y. Huang, A. S. Hussain, H. T. Liu and H. Simsek, Algae and Indigenous Bacteria Consortium in Treatment of Shrimp Wastewater: A Study for Resource Recovery in Sustainable Aquaculture System, Environ. Res., 2024, 250, 118447, DOI:10.1016/j.envres.2024.118447.
- J. M. Do, S. W. Jo, I. S. Kim, H. Na, J. H. Lee, H. S. Kim and H. S. Yoon, A Feasibility Study of Wastewater Treatment Using Domestic Microalgae and Analysis of Biomass for Potential Applications, Water, 2019, 11(11), 1–14, DOI:10.3390/w11112294.
- P. Bhatt, B. A. Engel, K. B. Shivaram, R. F. Turco, Z. Zhou and H. Simsek, Treatment and Optimization of High-Strength Egg-Wash Wastewater Effluent Using Electrocoagulation and Electrooxidation Methods, Chemosphere, 2024, 347, 140632, DOI:10.1016/j.chemosphere.2023.140632.
- N. Fayad, T. Yehya, F. Audonnet and C. Vial, Harvesting of Microalgae Chlorella Vulgaris Using Electro-Coagulation-Flocculation in the Batch Mode, Algal Res., 2017, 25, 1–11, DOI:10.1016/j.algal.2017.03.015.
- E. Yakamercan, R. F. Turco, B. Nas, A. S. Hussain, A. Aygun, L. Meador and H. Simsek, Optimizing Electrochemical Methods for Fish Wastewater Treatment in Recirculating Aquaculture Systems, J. Water Process Eng., 2024, 66, 105891, DOI:10.1016/j.jwpe.2024.105891.
- E. Bligh and W. J. Dyer, A Rapid Method of Total Lipid Extraction and Purification, Can. J. Biochem. Physiol., 1959, 37(8), 911–917 CrossRef CAS PubMed.
- APHA, Standard Methods for the Examination of Water and Wastewater, United Book Press, USA, 23rd edn, 2017 Search PubMed.
- R. J. Porra, W. A. Thompson and P. E. Kriedemann, Determination of Accurate Extinction Coefficients and Simultaneous Equations for Assaying Chlorophylls a and b Extracted with Four Different Solvents: Verification of the Concentration of Chlorophyll Standards by Atomic Absorption Spectroscopy, Biochim. Biophys. Acta, 1989, 975, 384–394 CrossRef CAS.
- S. Bhushan, U. Jayakrishnan, N. Johnson, S. K. Prajapati, K. A. S. Lakshan, K. Kaphle, S. Eshkabilov and H. Simsek, UV-C Pretreatment of Wastewater-Grown Algal Biomass for Recover of Biofuel Precursors, J. Environ. Chem. Eng., 2024, 12(2), 112087, DOI:10.1016/j.jece.2024.112087.
- A. R. Wellburn, The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution, J. Plant Physiol., 1994, 144(3), 307–313, DOI:10.1016/S0176-1617(11)81192-2.
- Electricity Rates, https://www.electricchoice.com/ (accessed 2025-05-25) Search PubMed.
- A. H. Hawari, A. M. Alkhatib, P. Das, M. Thaher and A. Benamor, Effect of the Induced Dielectrophoretic Force on Harvesting of Marine Microalgae (Tetraselmis Sp.) in Electrocoagulation, J. Environ. Manage., 2020, 260, 110106, DOI:10.1016/j.jenvman.2020.110106.
- N. Fayad, T. Yehya, F. Audonnet and C. Vial, Harvesting of Microalgae Chlorella Vulgaris Using Electro-Coagulation-Flocculation in the Batch Mode, Algal Res., 2017, 25, 1–11, DOI:10.1016/j.algal.2017.03.015.
- W. A. Khatib, A. Ayari, A. T. Yasir, M. Talhami, P. Das, M. A. Quadir and A. H. Hawari, Enhancing the Electrocoagulation Process for Harvesting Marine Microalgae (Tetraselmis Sp.) Using Interdigitated Electrodes, J. Environ. Manage., 2021, 292, 112761, DOI:10.1016/j.jenvman.2021.112761.
- R. H. Javan, M. Seyyedi and B. Ayati, Evaluation of Treatment and Energy Efficiencies of an Advanced Electrochemical System for Chlorella Removal Equipped with Aluminum , Graphite , and RGO Nanoparticles-Coated Cathodes, Water Sci. Eng., 2024, 143, 85–98, DOI:10.1016/j.wse.2023.12.004.
- A. Prasetyaningrum, B. Jos, Y. Dharmawan and I. R. Praptyana, The Effect of PH and Current Density on Electrocoagulation Process for Degradation of Chromium (VI) in Plating Industrial Wastewater, J. Phys.: Conf. Ser., 2019, 1295(1), 012064, DOI:10.1088/1742-6596/1295/1/012064.
- G. Mouedhen, M. Feki, M. D. P. Wery and H. F. Ayedi, Behavior of Aluminum Electrodes in Electrocoagulation Process, J. Hazard. Mater., 2008, 150(1), 124–135, DOI:10.1016/j.jhazmat.2007.04.090.
- E. Hoseinzadeh, A. Gholi-fam and M. Faramarzi, Treatment of Real Carwash Wastewater Using High-Efficiency and Energy-Saving Electrocoagulation Technique, Sustainable Chem. Pharm., 2024, 41, 101688, DOI:10.1016/j.scp.2024.101688.
- B. G. Ryu, J. Kim, J. I. Han, K. Kim, D. Kim, B. K. Seo, C. M. Kang and J. W. Yang, Evaluation of an Electro-Flotation-Oxidation Process for Harvesting Bio-Flocculated Algal Biomass and Simultaneous Treatment of Residual Pollutants in Coke Wastewater Following an Algal-Bacterial Process, Algal Res., 2018, 31, 497–505, DOI:10.1016/j.algal.2017.06.012.
- A. H. Hawari, A. M. Alkhatib, P. Das, M. Thaher and A. Benamor, Effect of the Induced Dielectrophoretic Force on Harvesting of Marine Microalgae (Tetraselmis Sp.) in Electrocoagulation, J. Environ. Manage., 2020, 260, 110106, DOI:10.1016/j.jenvman.2020.110106.
- Y. Gendel and O. Lahav, A New Approach to Increasing the Efficiency of Low-PH Fe-Electrocoagulation Applications, J. Hazard. Mater., 2010, 183(1–3), 596–601, DOI:10.1016/j.jhazmat.2010.07.066.
- D. Lakshmanan, D. A. Clifford and G. Samanta, Ferrous and Ferric Ion Generation during Iron Electrocoagulation, Environ. Sci. Technol., 2009, 43(10), 3853–3859, DOI:10.1021/es8036669.
- H. M. Maleki, M. Almassi, M. Amin and S. Minaei, Harvesting of Microalgae by Electro-Coagulation-Flocculation for Biodiesel Production: An Investigation of the Effect of Operational Parameters and Forecast Model Using Response Surface Methodology, Int. J. Biosci., 2014, 6655, 258–269, DOI:10.12692/ijb/4.7.258-269.
- S. Gao, J. Yang, J. Tian, F. Ma, G. Tu and M. Du, Electro-Coagulation-Flotation Process for Algae Removal, J. Hazard. Mater., 2010, 177(1–3), 336–343, DOI:10.1016/j.jhazmat.2009.12.037.
- D. Zhang, Y. Yu, C. Li, C. Chai, L. Liu, J. Liu and Y. Feng, Factors Affecting Microalgae Harvesting Efficiencies Using Electrocoagulation-Flotation for Lipid Extraction, RSC Adv., 2015, 5(8), 5795–5800, 10.1039/c4ra09983d.
- Z. Beiramzadeh, M. Baqersad and M. Aghababaei, Application of the Response Surface Methodology (RSM) in Heavy Metal Removal from Real Power Plant Wastewater Using Electrocoagulation, Eur. J. Environ. Civ. Eng., 2022, 26(1), 1–20, DOI:10.1080/19648189.2019.1640139.
- C. T. Matos, M. Santos, B. P. Nobre and L. Gouveia, Nannochloropsis Sp. Biomass Recovery by Electro-Coagulation for Biodiesel and Pigment Production, Bioresour. Technol., 2013, 134, 219–226, DOI:10.1016/j.biortech.2013.02.034.
- Y. Su, A. Mennerich and B. Urban, A Comparison of Feasible Methods for Microalgal Biomass Determinations during Tertiary Wastewater Treatment, Ecol. Eng., 2016, 94, 532–536, DOI:10.1016/j.ecoleng.2016.06.023.
- W. Cai, Z. Zhao, D. Li, Z. Lei, Z. Zhang and D. J. Lee, Algae Granulation for Nutrients Uptake and Algae Harvesting during Wastewater Treatment, Chemosphere, 2019, 214, 55–59, DOI:10.1016/j.chemosphere.2018.09.107.
- A. Landels, T. A. Beacham, C. T. Evans, G. Carnovale, S. Raikova, I. S. Cole, P. Goddard, C. Chuck and M. J. Allen, Improving Electrocoagulation Floatation for Harvesting Microalgae, Algal Res., 2019, 39, 101446, DOI:10.1016/j.algal.2019.101446.
- A. O. Ijaola, D. O. Akamo, T. T. George, A. Sengul, M. Y. Adediji and E. Asmatulu, Algae as a Potential Source of Protein: A Review on Cultivation, Harvesting, Extraction, and Applications, Algal Res., 2024, 77, 103329, DOI:10.1016/j.algal.2023.103329.
- H. Nezammahalleh, M. Nosrati, F. Ghanati and S. A. Shojaosadati, Exergy-Based Screening of Biocompatible Solvents for in Situ Lipid Extraction from Chlorella Vulgaris, J. Appl. Phycol., 2017, 29(1), 89–103, DOI:10.1007/s10811-016-0921-5.
- A. Rahmani, D. Zerrouki, L. Djafer and A. Ayral, Hydrogen Recovery from the Photovoltaic Electroflocculation-Flotation Process for Harvesting Chlorella Pyrenoidosa Microalgae, Int. J. Hydrogen Energy, 2017, 42(31), 19591–19596, DOI:10.1016/j.ijhydene.2017.06.123.
- A. Guldhe, F. A. Ansari, P. Singh and F. Bux, Heterotrophic Cultivation of Microalgae Using Aquaculture Wastewater: A Biorefinery Concept for Biomass Production and Nutrient Remediation, Ecol. Eng., 2017, 99, DOI:10.1016/j.ecoleng.2016.11.013.
- Y. K. Wong, Feasibility of Using Chlorella Vulgaris for the Production of Algal Lipids, for Advancement towards a Potential Application in the Manufacture of Commodity Chemicals and the Treatment of Wastewater, PhD thesis, Hong Kong Baptist University, 2016 Search PubMed.
- A. I. Barros, A. L. Gonçalves, M. Simões and J. C. M. Pires, Harvesting Techniques Applied to Microalgae: A Review, Renewable Sustainable Energy Rev., 2015, 41, 1489–1500, DOI:10.1016/j.rser.2014.09.037.
- B. Zhang, C. Peng, S. Zhang, M. Zhang, D. Li, X. Wang and B. Mao, Comprehensive Analysis of the Combined Flocculation and Filtration Process for Microalgae Harvesting at Various Operating Parameters, Sci. Total Environ., 2023, 857, 159658, DOI:10.1016/j.scitotenv.2022.159658.
- S. J. McGrath, C. A. Laamanen, G. N. A. Senhorinho and J. A. Scott, Microalgal Harvesting for Biofuels – Options and Associated Operational Costs, Algal Res., 2024, 77, 103343, DOI:10.1016/j.algal.2023.103343.
- T. Mathimani and N. Mallick, A Comprehensive Review on Harvesting of Microalgae for Biodiesel - Key Challenges and Future Directions, Renewable Sustainable Energy Rev., 2018, 1103–1120, DOI:10.1016/j.rser.2018.04.083.
- M. Karimi and H. Simsek, Life Cycle Assessment and Techno-Economic Optimization of Biocrude Oil Production from Microalgae via Hydrothermal Liquefaction Using Genetic Algorithms, J. Cleaner Prod., 2025, 520, 146104, DOI:10.1016/j.jclepro.2025.146104.
- R. Shi, R. M. Handler and D. R. Shonnard, Life Cycle Assessment of Novel Technologies for Algae Harvesting and Oil Extraction in the Renewable Diesel Pathway, Algal Res., 2019, 37, 248–259, DOI:10.1016/j.algal.2018.12.005.
- O. Sedaghat, N. Bahramifar, M. Nowrouzi and H. Younesi, Life Cycle Assessment of Industrial Wastewater Treatment: Evaluating the Environmental Impact of Electrocoagulation Technologies, J. Water Process Eng., 2025, 71, 107257, DOI:10.1016/j.jwpe.2025.107257.
- S. Nath, Electrochemical Wastewater Treatment Technologies through Life Cycle Assessment: A Review, ChemBioEng Rev., 2024, 11(4), 1–12, DOI:10.1002/cben.202400016.
| This journal is © The Royal Society of Chemistry 2025 |

