Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Revisiting simultaneous sulfate reduction and ammonium oxidation in wastewater treatment – from inexplicable experimental observations to extended mechanistic hypotheses†
Bohan
Yu
 ab,
Di
Wu
bc,
Jianyong
Liu
d and
Eveline I. P.
Volcke
ab,
Di
Wu
bc,
Jianyong
Liu
d and
Eveline I. P.
Volcke
 *ab
*ab
aBioCo Research Group, Department of Green Chemistry and Technology, Ghent University, Ghent, Belgium. E-mail: Eveline.Volcke@ugent.be
bCentre for Advanced Process Technology for Urban REsource recovery (CAPTURE), Frieda Saeysstraat 1, 9052 Gent, Belgium
cCentre for Green Chemistry and Environmental Biotechnology, Ghent University Global Campus, Incheon, Republic of Korea
dSchool of Environmental and Chemical Engineering, Shanghai University, 333 Nanchen Road, Shanghai 200444, China
First published on 11th June 2025
Abstract
Over the last two decades, reference has been made to the ‘sulfammox’ conversion, comprising the anaerobic oxidation of ammonium with sulfate, with nitrogen gas (N2) and elemental sulfur (S0) as the main end products. However, this phenomenon has been associated with inexplicable experiment results in terms of variable end products and unclear reaction stoichiometry, besides the fact that it has been reported to occur under both heterotrophic and autotrophic conditions. This contribution sheds light on the ‘sulfammox’ phenomenon through a comprehensive revisit of experimental observations. The hypothesis for sulfammox-related reaction mechanisms was systematically extended, considering other end products than N2 and S0, and as well as potential syntrophic bioprocesses. This resulted in additional reactions which were more general than the specific sulfammox one and which were denoted by the term – simultaneous sulfate reduction and ammonium oxidation (SRAO). Multiple thermodynamically feasible reaction pathways of SRAO under heterotrophic and autotrophic conditions were identified in a systematic and intelligible way, and compared against previously reported experimental results regarding reactor performance and microbial community analysis.
Water impactThis work involves an emerging microbial process, namely simultaneous sulfate reduction ammonium oxidation, which has brought challenge to the current knowledge on the natural nitrogen and sulfur cycles. Unravelling this new phenomenon can help us better understand nitrogen and sulfur evolution in natural environment, and enable practical application in biological wastewater treatment as the real-world impacts. |
1. Introduction
Anaerobic ammonium oxidation (anammox), which utilises ammonium as electron donor and nitrite as electron acceptor, has been well investigated and become an established autotrophic nitrogen removal process in wastewater treatment.1 Over the last two decades, it has become clear that nitrite was not the only possible electron acceptor for microorganisms to oxidise ammonium under anaerobic condition.2 Anaerobic simultaneous sulfate reduction and ammonium oxidation (SRAO) was for the first time observed by Fdz-Polanco3 when running an anaerobic digestion reactor with high sulfate and ammonium concentration, where nearly 50% of ammonium “disappeared” in the experiment, leading to the postulation of a new anaerobic ammonium oxidation phenomenon (eqn (1)). The Gibbs free energy for this reaction was calculated as ΔG = −47.8 kJ mol−1,4 indicating its thermodynamic feasibility.| SO42− + 2NH4+ → S0 + N2 + 4H2O | (1) |
At present, the mechanism of SRAO conversion in wastewater treatment remains uncertain. Fdz-Polanco described the overall reaction (eqn (1)) as a combination of sulfate reduction to sulfide and ammonium oxidation to nitrite (eqn (2)), sulfur-based denitrification (eqn (3)), and anammox (eqn (4)).
| 3SO42− + 4NH4+ → 3S2− + 4NO2− + 4H2O + 8H+ | (2) |
| 3S2− + 2NO2− + 8H+ → 3S0 + N2 + 4H2O | (3) |
| 2NO2− + 2NH4+ → 2N2 + 4H2O | (4) |
| 10H+ + 2NH4+ + 2SO42− + 10Org. e− → 2HS− + N2 + 8H2O | (5) |
| NH4+ + SO42− → HS− + NO3− + H2O + H+ | (6) |
| 20Org. e− + 4NO3− + 24H+ → 2N2 + 12H2O | (7) |
Previously published review papers regarding SRAO phenomenon mainly summarised publication and citation records, potential environmental affecting factors11 and operational parameters affecting practical reactor operation.12 Other review studies, Liu et al.13 and Wu et al.14 recognised that the SRAO reactions could take place under both heterotrophic and autotrophic conditions, however they did not address potential differences in the corresponding reactions and underlying mechanisms. It is hereby clear that more fundamental insight needs to be gained to fully understand the SRAO phenomenon.
This contribution comprises a comprehensive revisit of experimental observations of the SRAO phenomenon under heterotrophic and autotrophic conditions, in order to identify and explain the (seemingly) inexplicable observations. Subsequently, a systematic extension of the hypothesis for the underlying reaction mechanism was made in order to reveal the SRAO phenomenon, considering all possible SRAO reactions, including also NO3−, NO2−, and S2− as possible end products, besides N2 and S0 which are considered in the sulfammox reaction. Multiple thermodynamically feasible pathways were identified. Furthermore, SRAO-related microbial communities reported in the literature have been reviewed and compared against the possibility of SRAO being an elementary or complex (combined) reaction. Lastly, the reaction kinetics were assessed based on batch tests in the literature.
2. Revisiting the experimental observations of the SRAO phenomenon
The experimental observations of studies on SRAO phenomenon were revisited. In order to investigate the end products and reaction stoichiometry, the steady state results obtained for continuous-flow reactors with or without organic carbon are summarised separately and subsequently compared.2.1. Steady-state results under heterotrophic conditions
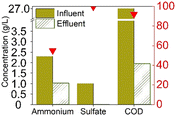
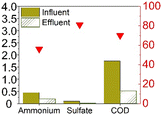
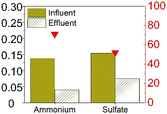
The earliest study to observe sulfammox was by Fdz-Polanco,3 involving an anaerobic digestion reactor fed with diluted vinasse from an ethanol distillery plant. Since then, a number of studies aimed to reproduce the observations under heterotrophic conditions, all of them were using synthetic wastewater organic carbon (chemical oxygen demand – COD) addition. Regarding the reactor type, most of them used a continuous flow reactor with or without biofilm carrier, except for Zhu et al.,15 who used a sequencing batch reactor (Table S5†). Table 1a shows the steady state experimental results under heterotrophic conditions reported in the literature. The influent ammonium concentration ranged from 38 mg N/L to 2300 mg N/L, the influent sulfate concentration ranged from 48 mg S/L to 1200 mg S/L, and the influent COD ranged from 400 mg L−1 to 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1. The removal efficiency of ammonium ranged from 55% to 80%, without a clear relation to influent concentration (Fig. S1†), nor to the ammonium/sulfate ratio (Table S1†). Regarding sulfate, the removal efficiency was mostly higher, between 85% to 100%, except for Zhu et al., where the sulfate removal efficiency was only 50%. In the case of Zhu et al., COD was completely consumed, while in the former studies, sulfate was largely used up. In sum, there was always 20% to 45% of the influent ammonium remained unconverted, whereas sulfate had a high removal efficiency (>85%) as long as COD was available.
000 mg L−1. The removal efficiency of ammonium ranged from 55% to 80%, without a clear relation to influent concentration (Fig. S1†), nor to the ammonium/sulfate ratio (Table S1†). Regarding sulfate, the removal efficiency was mostly higher, between 85% to 100%, except for Zhu et al., where the sulfate removal efficiency was only 50%. In the case of Zhu et al., COD was completely consumed, while in the former studies, sulfate was largely used up. In sum, there was always 20% to 45% of the influent ammonium remained unconverted, whereas sulfate had a high removal efficiency (>85%) as long as COD was available.
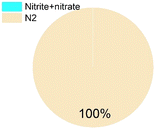
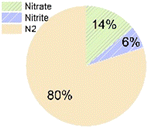
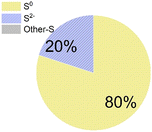
As for the end products, N2 was always the main end product (73% to 100% of the converted ammonium), while NO2− and NO3− were not always observed (Table 1), accounting for about 0–10%, and 0–15% of the converted ammonium, respectively. As for the sulfur compounds, elemental S was the main end product, corresponding to 29–88% of the converted sulfur, while some sulfide was produced as well (10–20% of the converted sulfur). In the case of Wang et al.,16 the reported total effluent sulfur species concentrations (Table S1†) corresponded to only 39% of the influent sulfur, while 61% of the converted sulfur seemed to be unidentified.
2.2. Steady-state results under autotrophic conditions
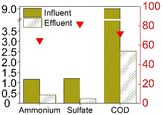
Table 1 provides a summary of the steady-state experimental results under autotrophic conditions (see Table S1† for corresponding numerical values). The influent ammonium concentration ranged from 50 mg N/L to 225 mg N/L, the influent sulfate concentration ranged from 73 mg S/L to 256 mg S/L. All studies were using synthetic wastewater and intended to examine the sulfammox reaction. Three studies used an up-flow reactor with or without biofilm carrier, while other studies applied a sequencing batch reactor and rotating contactor (Table S5†). The ammonium conversion efficiency decreased with decreasing influent ammonium concentration, from 80% down to 40% (Table S1 and Fig. S1†). The sulfate removal efficiency ranged between 10 and 78%, the highest sulfate removal efficiencies were obtained for the highest influent sulfate and the highest influent ammonium concentrations (Table S1 and Fig. S1†).In terms of the end products, N2 was the main end product (65% to 100% of the converted ammonium), while NO2− and NO3− were observed as well, accounting for 7–10%, and 13–25% of the converted nitrogen. As for the end products from sulfate conversion, the reported concentrations by Qin et al.17 and Yang et al.9 corresponded to one third of the converted sulfate ending up as sulfide and elemental sulfur, respectively, while two thirds remained unidentified in both studies (Table S1†).
2.3. Seemingly inexplicable experimental observations
Comparing the results under heterotrophic and autotrophic conditions, the ammonium removal efficiency was positively correlated to the influent ammonium concentration (Fig. S1c†) under autotrophic conditions, while no clear relation was found under heterotrophic conditions (Fig. S1a†). The removal efficiency of sulfate was found very high (>85%) under heterotrophic conditions (Fig. S1b†), except in the case where organic carbon was depleted (Table 1). Under autotrophic conditions, the sulfate removal efficiency was lower than under heterotrophic condition, probably due to absence of heterotrophic sulfate reduction. In both cases, a significant part of the ammonium remained unconverted. This may be due to the depletion of another component which is limiting the reaction, or it could be attributed to a slow reaction rate, possibly preventing the system from reaching steady state.According to the sulfammox reaction mechanism and stoichiometry postulated by Fdz-Polanco (eqn (1)), the converted ammonium: sulfate molar ratio is expected to be about 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. However, in the experimental results (Table 1 and S1†), the stoichiometric ammonium: sulfate molar ratio ranges between 0.6–7.2 in the presence of organic carbon and between 0.6–8.9 in the presence of bicarbonate, which significantly deviates from the expected value 2.0. This suggests that eqn (1) is not the (only) reaction occurring; the relatively wide range of consumption ratios observed also points towards the interference of one or more other reactions with the conversion of ammonium and/or sulfate. Some researchers hypothesised that the observed wide ammonium: sulfate ratio could be due to other bioprocesses, such as sulfate reduction and sulfur-based denitrification, interfering with the sulfammox phenomenon.13 Indeed, sulfate reduction, when taking place, increases sulfate consumption, while sulfur-based denitrification re-generates sulfate3 and thus reduces the net sulfate consumption. Overall, sulfate reduction could explain a higher sulfate consumption under heterotrophic conditions, and sulfur-based denitrification could explain a lower sulfate consumption under autotrophic conditions. However, the converted ammonium: sulfate molar ratio was found higher (>2
1. However, in the experimental results (Table 1 and S1†), the stoichiometric ammonium: sulfate molar ratio ranges between 0.6–7.2 in the presence of organic carbon and between 0.6–8.9 in the presence of bicarbonate, which significantly deviates from the expected value 2.0. This suggests that eqn (1) is not the (only) reaction occurring; the relatively wide range of consumption ratios observed also points towards the interference of one or more other reactions with the conversion of ammonium and/or sulfate. Some researchers hypothesised that the observed wide ammonium: sulfate ratio could be due to other bioprocesses, such as sulfate reduction and sulfur-based denitrification, interfering with the sulfammox phenomenon.13 Indeed, sulfate reduction, when taking place, increases sulfate consumption, while sulfur-based denitrification re-generates sulfate3 and thus reduces the net sulfate consumption. Overall, sulfate reduction could explain a higher sulfate consumption under heterotrophic conditions, and sulfur-based denitrification could explain a lower sulfate consumption under autotrophic conditions. However, the converted ammonium: sulfate molar ratio was found higher (>2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) under heterotrophic condition, whereas it was found lower (<2
1) under heterotrophic condition, whereas it was found lower (<2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) under autotrophic condition (Table S1†), which is contradictory to the explanation above.
1) under autotrophic condition (Table S1†), which is contradictory to the explanation above.
Nitrogen gas and elemental sulfur are typically considered as the end products of the sulfammox reaction (eqn (1)). However, nitrite and nitrate were also formed, under both heterotrophic and autotrophic conditions, albeit not in fixed proportions (Table 1), which suggests they could be either intermediate products or byproducts. Besides, the heterotrophic condition tended to have elemental sulfur and sulfide as main products, while the sulfur products were mostly unidentified under autotrophic conditions (Table 1).
The wide range in conversion efficiencies, converted substrate ratios, as well as the variety in the type and share of the observed end products, point out the value of a systematic and thorough investigation of all possible reactions which may either constitute an integral part of the SRAO reactions or take place in parallel.
3. Systematic extension of the hypothesis for the SRAO reaction mechanism
So far, two mechanisms have been put forward in the literature to explain the simultaneous sulfate reduction and ammonium oxidation, as denoted by eqn (2)–(4)3 and eqn (6)–(7),4 respectively. However, these may not be the only possible pathways. The objective of this section is to systematically identify all thermodynamically feasible pathways which result in simultaneous conversion of ammonium and sulfate. In this study, the term SRAO was adopted to denote all biochemical reactions in which sulfate reduction and ammonium oxidation take place simultaneously, considering more possible end products. Additional reaction pathways were identified in a systematic way, thus extending previous hypotheses.Elementary SRAO reactions, i.e. consisting of a single reaction step, are considered first and their thermodynamic feasibility will be evaluated. Next, an inventory is made of complex SRAO reactions, which are thermodynamically feasible combinations of elementary SRAO reactions with other, syntrophic reactions. A graphical overview of elementary and complex SRAO reactions is given in Fig. 1; their stoichiometry and thermodynamic feasibility is summarized in Table 2 (elementary SRAO) and Tables 3 and 4 (complex SRAO). This will be elaborated on in the following sections.
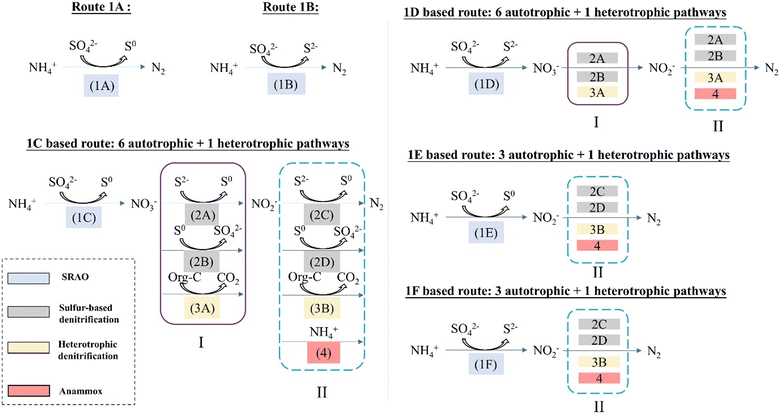 | ||
| Fig. 1 Extended hypothesis: overview of elementary SRAO reactions (1A, 1B, 1C, 1D, 1E, 1F) and of complex SRAO reactions with nitrogen gas as end product (18 combinations for autotrophic conditions and 4 combinations for heterotrophic conditions). Box I and box II group bioprocesses which are repeated. The reaction numbers and corresponding colours are consistent with Table 2; a full description is given in section 3.2. | ||
 have been corrected to match physiological conditions (pH = 7, T = 25 °C)
have been corrected to match physiological conditions (pH = 7, T = 25 °C)
3.1. Elementary SRAO reactions
In this study, the term SRAO is defined to denote all biochemical reactions in which sulfate reduction and ammonium oxidation take place simultaneously. By differentiating between nitrogen gas, nitrate and nitrite as three possible end products for the ammonium conversion, and between elemental sulfur and sulfide as products for the sulfate reduction, six elementary SRAO reactions are obtained (Table 2).For each of these six reactions, the standard Gibbs free energy change ΔG0 has been calculated (adapted to physiological conditions, i.e., pH = 7.0, atmospheric pressure, 25 celsius degree; the ionic strength was assumed to be ideal (I = 0); the detailed methodology is described in ESI† S4), showing that only two reactions (1A:N2–S0 and 1B:N2–S2−, Table 2) are thermodynamically feasible, both of which have nitrogen gas as the end product of ammonium conversion. The remaining four SRAO reactions, with nitrite or nitrate as the end product, are thermodynamically infeasible (1C:NO3−–S0, 1D:NO3−–S2−, 1E:NO2−–S0, and 1F:NO2−–S2−, Table 2).
The observation of nitrite and nitrate present in the experiments (Table 1) seems contradictory to the fact that the SRAO reactions of which they are the end product, are thermodynamically infeasible (1C, 1D, 1E, and 1F, Table 2).
It is important to recognise that the SRAO reactions listed in this paper are only the catabolic reactions. In practice, metabolic reactions combine both catabolism (energy supply) and anabolism (microorganism growth).18 It could be that the nitrate production during SRAO results from the anabolic reaction coupled to the catabolic reactions 1A and 1B, analogous as for the anammox stoichiometry.19,20
Assuming a typical microorganism composition of C5H7O2N, as suggested by Haug and McCarty,21 the biomass growth reaction can be written as:
| 5HCO3− + NH4+ + 20e− + 24H+ → C5H7O2N + 13H2O | (8) |
| NH4+ + 3H2O → NO3− + 10H+ + 8e− | (9) |
 | (10) |
| Met = λCat·Cat + A | (11) |
A second, alternative or even complementary explanation for the occasional presence of nitrite/nitrate is that the thermodynamically infeasible SRAO reactions are combined with syntrophic bioprocesses, resulting in complex SRAO reactions that are thermodynamically feasible.
Possible syntrophic bioprocesses that may occur under such conditions are sulfur-based denitrification (2A to 2D in Table 2), heterotrophic denitrification (3A to 3B), and anammox (4 in Table 2). In what follows, it will be investigated which combinations of thermodynamically infeasible SRAO reactions (1C to 1F) with syntrophic reactions result in thermodynamically feasible overall reactions. The focus hereby lies on the identification of thermodynamically feasible reactions which result in the conversion of ammonium to nitrogen gas, as aimed for during wastewater treatment.
3.2. Complex SRAO reactions
The SRAO reactions in which ammonium is converted to nitrogen gas with sulfate reduced to either elemental sulfur (1A in Table 2) or sulfide (1B in Table 2) constitute two obvious reaction mechanisms, in the form of elementary reactions.
The third SRAO reaction (1C) combines the oxidation of ammonium to nitrate with the reduction of sulfate to elemental sulfur. The produced nitrate can then be reduced to nitrite via sulfur-based denitrification (2A or 2B), or heterotrophic denitrification (3A). Subsequently, nitrite will be further reduced to nitrogen gas via sulfur-based denitrification (2C or 2D), or heterotrophic denitrification (3B), or anammox (4). The mechanisms involving heterotrophic denitrification require the presence of organic carbon, while the others take place under autotrophic conditions.
The fourth SRAO reaction (1D) connects ammonium oxidation to nitrate, and sulfate reduction to sulfide. The subsequent processes, i.e., nitrate reduction to nitrite, and nitrite reduction to nitrogen gas, are the same as described in the 1C based route, briefly denoted by (dotted) boxes I (nitrate to nitrite) and II (nitrite to nitrogen gas) in Fig. 1.
In the fifth (1E) and sixth (1F) SRAO reactions, ammonium is oxidised to nitrite, and sulfate is reduced to elemental sulfur or sulfide, respectively. Then nitrite is reduced to nitrogen gas in the same way denoted by box II (nitrite to nitrogen gas).
Overall, there are totally 24 possible pathways described in Fig. 1, in which 20 of them are not involving organic carbon, while 4 of them are. Therefore, 20 pathways under autotrophic conditions and 4 pathways under heterotrophic conditions are obtained.
Interestingly, some of the pathways were found to result in the same overall reactions. More specifically, seven complex pathways resulted in the same thermodynamically feasible overall reaction (‘complex autotrophic SRAO reaction 1’ in Table 3) as elementary reaction 1A, whereas two pathways led to another thermodynamically feasible overall reaction (‘complex autotrophic SRAO reaction 2’ in Table 3) as elementary reaction 1B. This indicates that reactions 1A and 1B may be either elementary reactions or complex (combined) reactions.
Additional thermodynamically feasible pathways were identified (Table 3). Since their substrates are not only ammonium and sulfate, they do not strictly match the definition of SRAO. Still, they may take place under the same conditions.
From the above analysis, it is clear that multiple reactions, with different stoichiometries, may take place simultaneously. This may explain the wide range of ammonium to sulfate conversion ratios and of end products reported in the sulfammox literature. Besides, the substrate consumption ratios and intermediate product accumulation will also be influenced by reaction kinetics, which is likely to differ as well between the different SRAO reactions.
| 8H+ + 2SO42− + 2NH4+ + 10Org. e− → 2S2− + N2 + 8H2O | (12) |
| 8H+ + 3SO42− + 4NH4+ + 12Org. e− → 3S2− + 2N2 + 12H2O | (13) |
| 40H+ + 8SO42− + 6NH4+ + 30Org. e− → 8S0 + 3N2 + 32H2O | (14) |
| 8H+ + 2SO42− + 2NH4+ + 6Org. e− → 2S0 + N2 + 8H2O | (15) |
It is clear that the combined occurrence of multiple feasible heterotrophic SRAO reactions may lead to a wide range of ammonium![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sulfate conversion ratios and different end products (S2− and/or S0), as observed in the experimental results.
sulfate conversion ratios and different end products (S2− and/or S0), as observed in the experimental results.
4. Functional microorganisms for SRAO
Since the first reports on the sulfammox, the functional microorganism(s) behind have remained unidentified. In this section, the SRAO-related microbial community analysis results from the literature are reviewed and analysed in light of the extended reaction mechanism hypothesis.4.1. Dedicated strain versus consortium
The most challenging puzzle comes from the functional microorganisms of the elementary SRAO reactions, for which various results have been reported in the literature.A commonly encountered hypothesis is that SRAO is carried out by anammox, through a dedicated metabolic pathway.22 Some latest studies were also in favour of this hypothesis,7,23 for instance, Liu et al.8 and Zhang et al.24 found Candidatus Brocadia sapporoensis prevailed in their SRAO reactors, which was potentially dedicated SRAO strain. However, this postulation seems only applicable for SRAO under autotrophic conditions, since it is contradictory to the fact that SRAO was discovered in anaerobic digestion reactor with high COD concentration, where anammox bacteria could not survive. Alternatively, as a similar hypothesis, SRAO could be carried out by another dedicated strain, other than anammox. Two strains were isolated from the biomass of SRAO reactors and claimed to be dedicated SRAO strains, namely Bacillus benzoevorans25 and Bacillus cereus.26 However, so far none of these strains nor consortium have been publicly verified and acknowledged for their function of conducting SRAO.
The other commonly encountered hypothesis is that SRAO is performed by consortium, for instance, Derwis et al. suggested a connection related to Thauera and Chloroflexi.27 Wimalaweera et al. indicated Desulfovibrio and Sulfurospirillum were the key species of SRAO.28
4.2. Comparison against experimental observations
A summary of microbial communities in SRAO reactors and their putative function is given in Table 5. These studies used high-throughput sequencing as analysis method. The hypothesised functional microorganisms were categorised into the dedicated strain or consortium, as proposed in the corresponding study. In general, strains with diverse functions were found in these studies. Under autotrophic conditions, Planctomycetes were the most suspected organisms for carrying out SRAO as a dedicated strain, while in the other studies, different combinations of consortium were suggested. As for heterotrophic conditions, there was limited literatures available – a consortium between denitrifiers and sulfate reduction bacteria was the most likely combination.For the syntrophic bioprocesses, autotrophic sulfur-based denitrification and (or) anammox were found in each study under autotrophic conditions, whereas heterotrophic denitrification was present as a major component in studies under heterotrophic conditions. This matches well what was postulated in the extended hypothesis, in light of SRAO under autotrophic and heterotrophic conditions.
Furthermore, the presence of nitrifiers, i.e., Nitrosomonas was found abnormally accumulated under such an anaerobic condition. The abundance of Nitrosomonas could somehow reach 15% of the microbial community.27 There are two possibilities for this: either all studies failed in keeping the environment anaerobic strictly29 and keeping the system from photosynthesis effect, or the nitrifiers may be related to the SRAO process, even though there is no known pathways of nitrifiers capable of oxidising ammonium in the absence of oxygen.30 A simple mass balance can be made to check whether SRAO is caused by oxygen intrusion: assuming the dissolved oxygen concentration in these experiments was 8–10 mg L−1, and all of this could be used for aerobic nitrification of ammonium to nitrate, this would correspond to 1.8–2.2 mg N/L nitrate produced (stoichiometry coefficient: 4.57 mg O2/mg NO3−). However, according to Table 1, the total nitrogen removal efficiency was typically over 40%, corresponded with a net nitrogen loss more than 100 mg N/L. Thus it can be seen that the unoptimized experimental conditions (e.g. oxygen intrusion) are most likely not (the only) cause of SRAO, albeit equipment failures and other major experimental design errors could not be completely excluded.
In addition, the heterotrophic denitrifiers were observed to be present together with the anaerobic fermentation bacteria even under autotrophic conditions, which indicated biomass decay within the sludge. It could be that the heterotrophic microorganisms were being fed by organic carbon from the decay of autotrophic biomass. This suggests a mixotrophic conditions, where the autotrophic SRAO and heterotrophic SRAO could take place simultaneously.
Furthermore, in the latest studies, the functional genes were detected (Table 6). For sulfur metabolism, sat (sulfate adenylyltransferase), apr (adenylylsulfate reductase), asr (anaerobic sulfite reductase), and sqr (sulfide quinone oxidoreductase) were often found, indicating sulfate reduction took place. As for nitrogen metabolism, hao (hydroxylamine oxidoreductase), nar (nitrate reductase), nap (periplasmic nitrate reductase), nirS (nitrite reductase (NO-forming)), nirK (copper-contating nitrite reductase), and amt (ammonium transporter) were detected, indicating co-occurrence of ammonium oxidation, nitrite oxidation and denitrification.
| Reference | Main functional genes related to sulfur metabolism | Main functional genes related to nitrogen metabolism |
|---|---|---|
| *The functional genes for sulfur metabolism are aprA (adenosine-5′-phosphosulfate reductase alpha subunit), aprB (adenosine-5′-phosphosulfate reductase beta subunit), aprAB (adenosine-5′-phosphosulfate reductase alpha and beta subunit), asrBC (assimilatory sulfite reductase), cysK (cysteine synthase), dsrA (dissimilatory sulfite reductase alpha subunit), dsrB(dissimilatory sulfite reductase beta subunit), sat (sulfate adenylyltransferase), soxA (sulfur oxidation protein A), soxX (sulfur oxidation protein X), soxZ (sulfur oxidation protein Z), soxB (sulfur oxidation protein B), sqr (sulfide:quinone oxidoreductase). **The functional genes for nitrogen metabolism are amt (ammonium transporter), amtB (ammonium transporter B), hao (hydroxylamine oxidoreductase), hdh (hydrazine dehydrogenase), hzs (hydrazine synthase), napA (periplasmic nitrate reductase A),napB (periplasmic nitrate reductase B), napAB (periplasmic nitrate reductase A and B), nar (nitrate reductase), narG (membrane-bound nitrate reductase G),narGHI (membrane-bound nitrate reductase complex), narH (membrane-bound nitrate reductase H), narI (membrane-bound nitrate reductase I), nirK (copper-containing nitrite reductase), nirS (cytochrome cd1 nitrite reductase), nifDKH (nitrogenase complex),norB (nitric oxide reductase B), norC (nitric oxide reductase C), nosZ (nitrous oxide reductase), nxrA (nitrite oxidoreductase A), nxrB (nitrite oxidoreductase B). | ||
| Liu et al., 202423 | asrBC, sat, aprAB, sqr | hao, nar, amt, nirS, nosZ |
| Wimalaweera et al., 2025 (ref. 28) | cysK, sqr, aprAB | nifDKH, narGHI, napAB, nosZ |
| Yin et al.,2025 (ref. 7) | dsrA,dsrB,sat, aprB, soxA,soxX,soxZ,soxB,sqr | hzs,hdh,nirK,narI,napA,napB,norC |
| Zhang et al., 2025 (ref. 24) | aprA,sat | amtB,hao,napA,narI,narH,narG,nirK,nirS,norC,norB,nosZ,nxrB,nxrA |
Overall, the experimental results in the literature match the postulation in the extended hypothesis well. The mass balance convinced that oxygen intrusion might not be (the only) reason for the cause of SRAO. Multiple syntrophic bioprocesses are observed to be involved and have played a significant role in the complex reaction of SRAO. Biomass decay was found to occur, which suggests a mixotrophic conditions, where autotrophic and heterotrophic SRAO could take place simultaneously.
5. SRAO kinetics
Fig. 2 shows the batch test results of SRAO under autotrophic conditions. Notably, ammonium and sulfate depletion occurred only in Liu et al. (2008),8 with the experiment lasting for 220 hours, whereas the other studies lasted less than 24 hours, with the removal efficiency all less than 50%. It suggested that the reaction of SRAO was slow and might prevent the reactors in the literature from reaching steady state.When the substrate concentration is fixed, higher biomass concentration is expected to result in higher substrate removal efficiency. In Fig. 2, a comparison between Zhu et al. (2022)15 and Prachakittikul et al. (2016),36 which had similar initial ammonium and sulfate concentrations, shows that the removal efficiencies of ammonium and sulfate were 10% and 8% (0.607 g VSS L−1), and 12% and 8%(4.9 g VSS L−1), respectively. Hence it seemed that higher volatile suspended solid concentration did not correspond to higher removal efficiency. The possible reason for this could be the functional microorganism only constitutes a small portion of the total biomass concentration.
To further quantify the reaction rate, the reaction rate of SRAO has been calculated based on batch test results from the literatures (Table 7) The first four hours were chosen for the calculation to ensure a fair comparison of the reaction rates (see ESI† S6).
| Batch volume (L) | Initial NH4+ (mg N) | NH4+ consumption (mg N) | Initial SO42− (mg S) | SO42− consumption (mg S) | Biomass (g L−1) | Ammonium reaction rate (mg N per g VSS L−1 h−1) | Sulfate reaction rate (mg S per g VSS L−1 h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Autotrophic condition | ||||||||
| 0.1 | 14.7 | 0.27 | 17.6 | 1.28 | 0.0544 | 124.1 | 588.2 | 8 |
| 0.25 | 75 | 12.5 | 125 | 28 | 2.654 | 15.08 | 33.76 | 32 |
| 0.3 | 15 | 1.05 | 16 | 1.92 | 4.900 | 0.60 | 1.1 | 36 |
| 0.7 | 49 | 3.5 | 280 | 7 | 0.509 | 2.81 | 5.61 | 6 |
| 1.5 | 112.5 | 9 | 270 | 25.5 | 0.607 | 1.32 | 3.73 | 15 |
| 1.5 | 195 | 30 | 225 | 48 | 3.730 | 0.89 | 1.15 | 31 |
| Heterotrophic condition | ||||||||
| 0.5 | 64.75 | 45.33 | 600 | 432 | 4.215 | 10.76 | 88.84 | 16 |
| 0.5 | 129.5 | 54.39 | 600 | 414 | 4.215 | 12.9 | 91.12 | 16 |
| 0.5 | 194.5 | 38.95 | 600 | 414 | 4.215 | 9.24 | 88.84 | 16 |
| 0.5 | 388.75 | 89.41 | 600 | 438 | 4.215 | 21.22 | 91.12 | 16 |
Under autotrophic conditions, the ammonium removal rate amounted to 0.6–124.1 mg N per g VSS L−1 h−1 (Table 7), showing a two order-of-magnitude variation. The highest reaction rate, 124.1 mg N per g VSS L−1 h−1, was observed in Liu et al. (2008),8 where SRAO was indicated to be induced by inoculating anammox sludge into the reactor. Also Lin et al. (2022)32 reported a relatively high ammonium removal rate, namely 15.08 mg N per g VSS L−1 h−1. All other studies had a much lower reaction rate, from 0.60 to 2.81 mg N per g VSS L−1 h−1. N isotope analysis also indicated the reaction rate to be very slow, with a rate of 2.4 mg N per g VSS L−1 h−1.6
For heterotrophic condition, the available literature was limited thus only Wang et al. (2017)16 could be used for the calculation. According to this study, the reaction rate was 9.24–21.22 mg N per g VSS L−1 h−1 for ammonium removal, and around 90 mg S per g VSS h−1 for sulfate removal, which were averagely 2 times faster than the reaction rate under autotrophic condition.
Overall, the SRAO reaction rates were very low, which may explain the low removal efficiency in the literature. The rate-limiting step of SRAO, i.e. whether it is sulfate reduction or ammonium oxidation, is hard to unravel, as they are not independent from each other. In the future study, a longer hydraulic retention time could be adopted in the experiments to deplete the substrates. Also, more favourable environmental conditions, e.g., thermophilic conditions and high substrate concentrations could be examined to facilitate SRAO conversions.
6. Application potential of SRAO
Despite the mechanism behind SRAO is still under investigation (as it is inherently being the result of multiple bioprocesses), its practical application in wastewater treatment process is definitely viable, which could be a supplement to lower substrate removal cost and energy consumption.27 Due to its slow kinetics, several researchers indicated that it is better to combine SRAO with other techniques when making the process design,38 instead of implementing SRAO alone. One example is the one from Zhang et al.,31 who combined anammox and SRAO in mature landfill leachate treatment containing high concentrations of sulfate. In their study, SRAO acted as a good supplementary process to remove nitrogen (27.5% contribution rate). Another scenario is the combination of SRAO with sulfur-based autotrophic denitrification32 and potentially also with anammox.7 Sulfur-based denitrification produces sulfate as the end product from sulfide oxidation, which can be in turn used by SRAO to oxidise ammonium, where SRAO could also become a good supplement in the system.In addition, concerns may also arise on the emission of nitrous oxide (N2O), a potent greenhouse gas. SRAO is most likely the result of multiple bioprocesses, some of which are known and some not. Even for the known ones, the N2O emissions are uncertain, for instance, heterotrophic denitrification may be both a sink or source of N2O; the studies on N2O emissions from sulfur-based denitrification showed contradictory results – some indicated it produces more N2O,33 while the rest indicated it reduces N2O.34,35 Besides, the mechanistic of ammonium oxidation of SRAO remains unknown, which warrants further investigation. The overall situation of N2O emissions will be thus very complicated, taking into account all the elements and the interactions between them.
7. Conclusions
Simultaneous sulfate reduction and ammonium oxidation (SRAO) has emerged as an intriguing biological conversion in wastewater treatment, on which this study sheds the following light:• Experimental results reported in the literature showed inexplicable results, such as a wide range of reaction stoichiometry, and variation in the types and proportions of observed end products.
• A systematic extended hypothesis for the underlying reaction mechanism was developed, considering multiple possible end products and the interaction with syntrophic bioprocesses, leading to complex SRAO reaction pathways under autotrophic and heterotrophic conditions.
• The thermodynamic feasibility of the complex SRAO reactions was calculated and discussed.
• Analysis of the functional microorganisms reported in the literature strengthened the hypothesis that SRAO is likely the result of multiple bioprocesses, with different functional organisms, and different possible combinations between heterotrophic conditions and autotrophic conditions.
• The reaction rate of SRAO was calculated as being very low.
Data availability
The data used in this study was selected from the literature, and could be found in Table S1 in ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
Bohan Yu acknowledges the support from China Scholarship Council (No. 202206890031).References
- M. Zheng, H. Li, H. Duan, T. Liu, Z. Wang, J. Zhao, Z. Hu, S. Watts, J. Meng, P. Liu, M. Rattier, E. Larsen, J. Guo, J. Dwyer, B. Van Den Akker, J. Lloyd, S. Hu and Z. Yuan, One-year stable pilot-scale operation demonstrates high fexibility of mainstream anammox application, Water Res.:X, 2023, 19, 100166 CAS.
- X. Kong, D. Castarède, E. S. Thomson, A. Boucly, L. Artiglia, M. Ammann, I. Gladich and J. B. C. Pettersson, A surface-promoted redox reaction occurs spontaneously on solvating inorganic aerosol surfaces, Science, 2021, 374, 747–752 CrossRef CAS PubMed.
- F. Fdz-Polanco, New process for simultaneous removal of nitrogen and sulphur under anaerobic conditions, Water Res., 2001, 35, 1111–1114 CrossRef CAS PubMed.
- H. N. Schrum, A. J. Spivack, M. Kastner and S. D'Hondt, Sulfate-reducing ammonium oxidation: A thermodynamically feasible metabolic pathway in subseafloor sediment, Geology, 2009, 37, 939–942 CrossRef CAS.
- E. E. Rios-Del Toro and F. J. Cervantes, Anaerobic ammonium oxidation in marine environments: contribution to biogeochemical cycles and biotechnological developments for wastewater treatment, Rev. Environ. Sci. Biotechnol., 2019, 18, 11–27 CrossRef CAS.
- M. Zhan, W. Zeng, H. Liu, J. Li, Q. Meng and Y. Peng, Simultaneous nitrogen and sulfur removal through synergy of sulfammox, anammox and sulfur-driven autotrophic denitrification in a modified bioreactor enhanced by activated carbon, Environ. Res., 2023, 232, 116341 CrossRef CAS PubMed.
- S. Yin, Y. Wang, C. Hou, J. Wang, J. Xu, X. Jiang, D. Chen, Y. Mu and J. Shen, Deciphering the key role of biofilm and mechanisms in high-strength nitrogen removal within the anammox coupled partial S0-driven autotrophic denitrification system, Bioresour. Technol., 2025, 419, 132020 CrossRef CAS PubMed.
- S. Liu, F. Yang, Z. Gong, F. Meng, H. Chen, Y. Xue and K. Furukawa, Application of anaerobic ammonium-oxidizing consortium to achieve completely autotrophic ammonium and sulfate removal, Bioresour. Technol., 2008, 99, 6817–6825 CrossRef CAS PubMed.
- Z. Yang, S. Zhou and Y. Sun, Start-up of simultaneous removal of ammonium and sulfate from an anaerobic ammonium oxidation (anammox) process in an anaerobic up-flow bioreactor, J. Hazard. Mater., 2009, 169, 113–118 CrossRef CAS PubMed.
- E. Rikmann, I. Zekker, M. Tomingas, T. Tenno, L. Loorits, P. Vabamäe, A. Mandel, M. Raudkivi, L. Daija, K. Kroon and T. Tenno, Sulfate-reducing anammox for sulfate and nitrogen containing wastewaters, Desalin. Water Treat., 2016, 57, 3132–3141 CrossRef CAS.
- D. Grubba, J. Majtacz and J. Mąkinia, Sulfate reducing ammonium oxidation (SULFAMMOX) process under anaerobic conditions, Environ. Technol. Innovation, 2021, 22, 101416 CrossRef.
- R. Madani, J. Liang, L. Cui, D. Zhang, T. Otitoju, R. Elsalahi and X. Song, Novel simultaneous anaerobic ammonium and sulfate removal process: A review, Environ. Technol. Innovation, 2021, 23, 101661 CrossRef CAS.
- L.-Y. Liu, G.-J. Xie, D.-F. Xing, B.-F. Liu, J. Ding, G.-L. Cao and N.-Q. Ren, Sulfate dependent ammonium oxidation: A microbial process linked nitrogen with sulfur cycle and potential application, Environ. Res., 2021, 192, 110282 CrossRef CAS PubMed.
- T. Wu, J. Ding, L. Zhong, H.-J. Sun, J.-W. Pang, L. Zhao, S.-W. Bai, N.-Q. Ren and S.-S. Yang, Sulfate-reducing ammonium oxidation: A promising novel process for nitrogen and sulfur removal, Sci. Total Environ., 2023, 893, 164997 CrossRef CAS PubMed.
- Y. Zhu, S. Yang, W. Wang, L. Meng and J. Guo, Applications of Sponge Iron and Effects of Organic Carbon Source on Sulfate-Reducing Ammonium Oxidation Process, Int. J. Environ. Res. Public Health, 2022, 19, 2283 CrossRef CAS PubMed.
- D. Wang, B. Liu, X. Ding, X. Sun, Z. Liang, S. Sheng and L. Du, Performance evaluation and microbial community analysis of the function and fate of ammonia in a sulfate-reducing EGSB reactor, Appl. Microbiol. Biotechnol., 2017, 101, 7729–7739 CrossRef CAS PubMed.
- Y. Qin, Q. Wei, Y. Zhang, H. Li, Y. Jiang and J. Zheng, Nitrogen removal from ammonium- and sulfate-rich wastewater in an upflow anaerobic sludge bed reactor: performance and microbial community structure, Ecotoxicology, 2021, 30, 1719–1730 CrossRef CAS PubMed.
- R. Kleerebezem and M. C. M. van Loosdrecht, A generalized method for thermodynamic state analysis of environmental systems, Crit. Rev. Environ. Sci. Technol., 2010, 40, 1–54 CrossRef.
- M. Strous, E. Pelletier, S. Mangenot, T. Rattei, A. Lehner, M. W. Taylor, M. Horn, H. Daims, D. Bartol-Mavel, P. Wincker, V. Barbe, N. Fonknechten, D. Vallenet, B. Segurens, C. Schenowitz-Truong, C. Médigue, A. Collingro, B. Snel, B. E. Dutilh, H. J. M. Op Den Camp, C. Van Der Drift, I. Cirpus, K. T. Van De Pas-Schoonen, H. R. Harhangi, L. Van Niftrik, M. Schmid, J. Keltjens, J. Van De Vossenberg, B. Kartal, H. Meier, D. Frishman, M. A. Huynen, H.-W. Mewes, J. Weissenbach, M. S. M. Jetten, M. Wagner and D. Le Paslier, Deciphering the evolution and metabolism of an anammox bacterium from a community genome, Nature, 2006, 440, 790–794 CrossRef PubMed.
- M. Jia, C. Castro-Barros, M. Winkler and E. I. P. Volcke, Effect of organic matter on the performance and N2O emission of a granular sludge anammox reactor, Environ. Sci.: Water Res. Technol., 2018, 4, 1035–1046 RSC.
- R. Haug and P. McCarty, Nitrification with Submerged Filters, J. - Water Pollut. Control Fed., 1972, 44, 2086–2102 CAS.
- D. R. Shaw, M. Ali, K. P. Katuri, J. A. Gralnick, J. Reimann, R. Mesman, L. Van Niftrik, M. S. M. Jetten and P. E. Saikaly, Extracellular electron transfer-dependent anaerobic oxidation of ammonium by anammox bacteria, Nat. Commun., 2020, 11, 2058 CrossRef CAS PubMed.
- L.-Y. Liu, X. Wang, C. Dang, Z. Zhao, D. Xing, B. Liu, N.-Q. Ren and G. Xie, Anaerobic ammonium oxidation coupled with sulfate reduction links nitrogen with sulfur cycle, Bioresour. Technol., 2024, 403, 130903 CrossRef CAS PubMed.
- Z. Zhang, C. Zhang, Y. Yang, Z. Zhang, K. Guo, X. Zhang, Z. Qin, J. Huang and Y. Li, Roles of nitrite in facilitating nitrogen and sulfur conversion in the hybrid bioreactor of sulfate-reduced ammonium oxidation and anaerobic ammonium oxidation, Bioresour. Technol., 2025, 419, 132085 CrossRef CAS PubMed.
- J. Cai, J. Jiang and P. Zheng, Isolation and identification of bacteria responsible for simultaneous anaerobic ammonium and sulfate removal, Sci. China:Chem., 2010, 53, 645–650 CrossRef CAS.
- R. Madani, J. Liang, L. Cui, R. H. Elsalahi, T. Otitoju, D. Zhang, X. Song, Y. Ma and S. Liu, Novel Simultaneous Removal of Ammonium and Sulfate by Isolated Bacillus cereus Strain from Sewage Treatment Plant, Water, Air, Soil Pollut., 2022, 233, 185 CrossRef.
- D. Derwis, J. Majtacz, P. Kowal, H. E. Al-Hazmi, J. Zhai, S. Ciesielski, G. Piechota and J. Mąkinia, Integration of the sulfate reduction and anammox processes for enhancing sustainable nitrogen removal in granular sludge reactors, Bioresour. Technol., 2023, 383, 129264 CrossRef CAS PubMed.
- I. Wimalaweera, F. Zuo, Q. Tang, Q. Sui, S. Jinadasa, S. Weragoda, T. Ritigala, R. Weerasooriya, Y. Wang, H. Zhong, M. Makehelwala and Y. Wei, Synchronised removal of nitrogen and sulphate from rubber industrial wastewater by coupling of Sulfammox and sulphide-driven autotrophic denitrification in anaerobic membrane bioreactor, Bioresour. Technol., 2025, 416, 131785 CrossRef CAS PubMed.
- Z. Bi, D. Wanyan, X. Li and Y. Huang, Biological conversion pathways of sulfate reduction ammonium oxidation in anammox consortia, Front. Environ. Sci. Eng., 2020, 14, 38 CrossRef CAS.
- B. Ni and Z. Yuan, Recent advances in mathematical modeling of nitrous oxides emissions from wastewater treatment processes, Water Res., 2015, 87, 336–346 CrossRef CAS PubMed.
- F. Zhang, S. Ren, H. Liang, Z. Wang, Y. Yan, J. Wang and Y. Peng, Efficient nitrogen removal and partial elemental sulfur recovery by combined nitrite-Anammox and sulfate-Anammox: A novel strategy for treating mature landfill leachate, J. Cleaner Prod., 2023, 399, 136553 CrossRef CAS.
- W. Lin, J. Feng, K. Hu, B. Qu, S. Song, K. He, C. Liu, Y. Chen and Y. Hu, Sulfidation forwarding high-strength Anammox process using nitrate as electron acceptor via thiosulfate-driven nitrate denitratation, Bioresour. Technol., 2022, 344, 126335 CrossRef CAS PubMed.
- Y. Liu, L. Peng, H. H. Ngo, W. Guo, D. Wang, Y. Pan, J. Sun and B. Ni, Evaluation of nitrous oxide emission from sulfide- and sulfur-based autotrophic denitrification processes, Environ. Sci. Technol., 2016, 50, 9407–9415 CrossRef CAS PubMed.
- W. Yang, Q. Zhao, H. Lu, Z. Ding, L. Meng and G.-H. Chen, Sulfide-driven autotrophic denitrification significantly reduces N2O emissions, Water Res., 2016, 90, 176–184 CrossRef CAS PubMed.
- Y.-F. Deng, D. Wu, H. Huang, Y.-X. Cui, M. C. M. van Loosdrecht and G.-H. Chen, Exploration and verification of the feasibility of sulfide-driven partial denitrification coupled with anammox for wastewater treatment, Water Res., 2021, 193, 116905 CrossRef CAS PubMed.
- P. Prachakittikul, C. Wantawin, P. Lek Noophan and N. Boonapatcharoen, ANAMMOX-like performances for nitrogen removal from ammonium-sulfate-rich wastewater in an anaerobic sequencing batch reactor, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2016, 51, 220–228 CrossRef CAS PubMed.
- P. C. Sabumon, Development of a novel process for anoxic ammonia removal with sulphidogenesis, Process Biochem., 2008, 43, 984–991 CrossRef CAS.
- L. Wu, Z. Yan, J. Li, S. Huang, Z. Li, M. Shen and Y. Peng, Low temperature advanced nitrogen and sulfate removal from landfill leachate by nitrite-anammox and sulfate-anammox, Environ. Pollut., 2020, 259, 113763 CrossRef CAS PubMed.
- D. Zhang, L. Cui, H. Wang and J. Liang, Study of sulfate-reducing ammonium oxidation process and its microbial community composition, Water Sci. Technol., 2019, 79, 137–144 CrossRef CAS PubMed.
- L. Zhang, P. Zheng, Y. He and R. Jin, Performance of sulfate-dependent anaerobic ammonium oxidation, Sci. China, Ser. B:Chem., 2009, 52, 86–92 CrossRef CAS.
- Q.-L. Zhao, W. Li and S.-J. You, Simultaneous removal of ammonium-nitrogen and sulphate from wastewaters with an anaerobic attached-growth bioreactor, Water Sci. Technol., 2006, 54, 27–35 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ew00283d |
| This journal is © The Royal Society of Chemistry 2025 |