Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Protection of Shewanella oneidensis MR-1 by manganese ferrite nanoparticles during chromate bio-reduction†
Diana S.
Raie
 ab,
Ioannis
Tsonas
c,
Stefanos
Mourdikoudis
ab,
Ioannis
Tsonas
c,
Stefanos
Mourdikoudis
 ab,
Evangelia
Delli
ab,
Evangelia
Delli
 d,
Antonios
Makridis
d,
Antonios
Makridis
 d,
Lena
Ciric
e and
Nguyen Thi Kim
Thanh
d,
Lena
Ciric
e and
Nguyen Thi Kim
Thanh
 *ab
*ab
aBiophysics Group, Department of Physics and Astronomy, University College London (UCL), London, WC1E 6BT, UK. E-mail: ntk.thanh@ucl.ac.uk; Web: https://www.ntk-thanh.co.uk
bUCL Healthcare Biomagnetics and Nanomaterials Laboratories, 21 Albemarle Street, London, W1S 4BS, UK
cUCL Electronic and Electrical Engineering, UCL, Gower Street, London, WC1E 7JE, UK
dLaboratory of Advanced Materials and Devices, Department of Physics, Aristotle University of Thessaloniki, 54124 Thessaloniki, Greece
eHealthy Infrastructure Research Group, Department of Civil, Environmental & Geomatic Engineering, UCL, Gower Street, WC1E 6BT London, UK
First published on 21st May 2025
Abstract
Shewanella oneidensis (S. oneidensis) MR-1 is a metal-reducing bacterium that can bio-reduce the carcinogenic hexavalent chromium (Cr6+) to a less toxic trivalent form (Cr3+). The bacteriocidal effect of Cr6+ challenges the above bio-reduction process. This work aims to illustrate the protective role of manganese ferrite nanoparticles (Mn0.2Fe2.8O4 NPs) to S. oneidensis MR-1 bacteria during the bio-reduction of Cr6+. Nanostructures were characterised by transmission electron microscopy (TEM) and X-ray diffraction (XRD). The interaction between S. oneidensis MR-1, Cr6+ and Mn0.2Fe2.8O4 NPs was monitored by X-ray photoelectron spectroscopy (XPS), which helped to unravel the oxidation states of Cr. The XPS analysis provided key insights into the oxidation states of Mn and Fe, confirming the redox interactions facilitating Cr6+ reduction. Mn0.2Fe2.8O4 NPs boosted the detoxification of the removed Cr6+ by 2.1 and 1.4 times compared to using S. oneidensis MR-1 alone and NPs alone, respectively. Scanning electron microscopy (SEM) imaging evaluated the changes in the morphology of bacterial cells. After exposure to Cr6+, S. oneidensis MR-1 cells revealed their inability to produce nanofibers, which are electrically conductive bacterial appendages. Yet, Mn0.2Fe2.8O4 NPs provoked the formation of bacterial nanofibers. These findings highlight the potential of Mn0.2Fe2.8O4 NPs for enhancing the bioremediation of Cr6+ contaminated environments.
Environmental significanceCarcinogenic hexavalent chromium leaks from industrial sites due to improper wastewater treatment into surface and groundwater, exposing flora and fauna to danger. The metal-reducing bacterium, Shewanella oneidensis MR-1, can reduce Cr6+ into less toxic Cr3+; bacteria lose their viability during treatment due to the toxicity of Cr6+. The novelty of this work is the discovery of a protective role of Mn-ferrite nanoparticles to S. oneidensis MR-1 bacteria during Cr6+ bio-reduction. We show that Mn0.2Fe2.8O4 NPs induced bacterial cell elongation and promoted nanofiber formation. Such morphological changes improve bacterial cell viability in response to the sub-lethal dose of Cr6+ and enhance their detoxification capability. Our findings provide a promising application of using nano-Mn0.2Fe2.8O4 in the bioremediation of Cr6+-contaminated environments. |
1. Introduction
Contamination of air, soil and water with heavy metals is hazardous to human health and the environment due to their toxicity, even at low concentrations1 as they are non-biodegradable materials.2 The Agency for Toxic Substances and Disease Registry (ATSDR) ranked chromium (Cr) the 17th on the substance priority list among many heavy metals.3Cr mainly occurs in two valence states: hexavalent (Cr6+) and trivalent (Cr3+). Human exposure to Cr6+ can cause liver damage, pulmonary congestion, oedema, skin irritation, ulcer formation,4 neurotoxicity,5 and carcinogenesis.6 U.S. Environmental Protection Agency (EPA) and WHO guidelines reported a permissible limit of Cr6+ in drinking water of 50 ppb.7 According to the EU drinking water directive, the regulation limit for the total Cr will be 25 μg L−1 by 12 January 2036.8 Since Cr3+ has low mobility, limited bio-absorptivity, and lower toxicity than Cr6+,9 Cr6+ should be reduced to Cr3+ for its safe removal.10
Bio-reduction of Cr6+ is a cost-effective and environmentally friendly method, attracting widespread interest.11 Some bacteria can reduce metals, acting as terminal electron acceptors under anaerobic conditions.12 So, metal-reducing bacteria can be used for the biotic reduction of heavy metals for detoxification purposes. Such a natural process is applicable for the biological reduction of the carcinogenic Cr6+ into less toxic Cr3+ form.13
Shewanella oneidensis MR-1 is a model metal-reducing bacteria for detoxifying Cr6+.14–18S. oneidensis MR-1 can employ Cr6+ as a terminal electron acceptor under anaerobic conditions.14,15,19 The biosafety of S. oneidensis MR-1 is an essential criterion for selecting bioremediation biological agents. In contrast, Pseudomonas aeruginosa bacteria can be used for Cr6+ removal but are not preferred for bioremediation because they cause diseases in humans and animals.20,21 Yet, the lethal effect of Cr6+ on the microbes during their respiration limited the bioremediation of Cr6+.22
Mn0.2Fe2.8O4 NPs showed a higher adsorption capacity for Cr6+ than Fe2O3 NPs and other tested MnxFe3−xO4 NPs.23 This chemical structure improved the bacterial viability and microbial detoxification of Cr6+.23 The adsorption of Cr6+ can limit the availability of the toxic cations to cells, which could improve their viability and bio-reduction efficiency.
Herein, to the best of our knowledge, we showed for the first time the protective role of the Mn0.2Fe2.8O4 NPs to S. oneidensis MR-1 during the bio-reduction of Cr6+. Raie et al.23 primarily investigated the adsorption and bio-removal of Cr6+ using Mn0.2Fe2.8O4 NPs and S. oneidensis MR-1, respectively.
This article builds upon findings by Raie et al.,23 and elucidates the reduction process of Cr6+ using XPS. In addition, this work presents bacterial imaging to visualise morphological changes in response to Cr6+ and NPs, providing deeper insights into the mechanism of Cr6+ reduction.
XPS revealed the possible reduction of Cr6+ to Cr3+ due to its interaction with Mn0.2Fe2.8O4 NPs. This allowed us to confirm the redox-based interaction among Cr6+ and Mn0.2Fe2.8O4 NPs. In addition, SEM showed the morphological change response of S. oneidensis MR-1 as a coping strategy in response to the toxic Cr6+ in the presence of Mn0.2Fe2.8O4 NPs. This article will advance the treatment of Cr6+ by demonstrating its removal, unravelling its reduction mechanism and the biological implications, thereby contributing novel insights and practical advancements to nanobiotechnology and environmental applications.
2. Materials and methods
2.1 NPs preparation and characterisation
Mn0.2Fe2.8O4 NPs were prepared by an adapted polyol solvothermal synthetic process24,25 at 250 °C as described in our recent work.23 In 20 mL of tetraethylene glycol (TEG), 0.3 M of iron(III) acetylacetonate (2.1 g) and 0.1 M manganese(II) acetylacetonate (0.5 g) were added. The mixture was added into a 45 mL Teflon-lined stainless-steel autoclave after being homogenised by vortex and sonication to be placed in an oven (Memmert, model UFP400) and heated within 30 min up to 250 °C for a 6 h hold at that temperature. In polyol synthesis, metal precursors are reduced by TEG, which acts as a high-temperature capping agent, solvent, and reductant. The formed metal nuclei grow and controllably coalesce together to produce the desired particles.26,27 The produced black dispersion underwent characterisation and functionalisation by tri-sodium citrate via ligand exchange.23 A JEOL JEM 1200-EX microscope operating at an acceleration voltage of 120 kV was employed to investigate the shape and size of the produced particles. The polydispersity index (PDI) is the ratio between the standard deviation and the mean nanoparticle diameter. To determine the crystal phase and the average crystallite size, we used XRD (PANalytical XPERT PRO MPD) coupled with Co Kα radiation source (λ = 1.789 Å) and an X'Celerator detector operated at 40 kV and 40 mA. An Optima 3100 XL Perkin Elmer Inductively Coupled Plasma Atomic Emission (ICP-AES) spectrometer was employed to determine the chemical composition of MnxFe3−xO4 particles. To quantify the iron content of the functionalised NPs dispersed in water, a colorimetric phenanthroline method was applied for the acid-digested NPs using a spectrophotometer (SpectraMax M2e, Molecular Devices, UK).2.2 Sources for bacteria of interest
A freeze-dried culture of S. oneidensis MR-1 (LMG 19005) was purchased from BCCM/LMG bacteria collection.2.3 Viability of S. oneidensis MR-1 to Mn0.2Fe2.8O4 NPs
The impact of Mn0.2Fe2.8O4 NPs on the viability of the S. oneidensis MR-1 was assessed using Guava easyCyte® flow cytometer (Merck, UK) following a protocol previously utilised by Raie et al.,23 under anaerobic conditions overnight. A homogeneous bacterial cell suspension (10 μL with OD measured at λ = 600 nm equal to 0.1) was added to 80 μL of M9 minimal salts (×2) medium, containing 20 mM sodium lactate as a sole electron source, 5 mL L−1 each of vitamins and minerals and pH was adjusted to 7.2 by 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer.23,28 Sodium fumarate (20 mM) was used as a terminal electron acceptor.23,28 Mn0.2Fe2.8O4 NPs (10 μL) were added to the mixture. The tested concentrations of NPs ranged from 1–60 mg mL−1 with an approximate total Fe content from 0.7 mg mL−1 to 40.6 mg mL−1.2.4 The exposure of S. oneidensis MR-1 to Cr6+ and Mn0.2Fe2.8O4 NPs
S. oneidensis MR-1 was exposed to Cr6+ and Mn0.2Fe2.8O4 NPs individually and also in a combined way overnight, in conditions similar to that mentioned in Section 2.3. Cr6+ (as a terminal electron acceptor) and Mn0.2Fe2.8O4 NPs were added to this medium with concentrations of 50 mg L−1 (sub-lethal dose, as reported by Raie et al.)23 and 1 mg mL−1, respectively.232.5 Analysis of oxidation state of Cr6+, Mnx+, and Fey+
The oxidation states of Mn and Fe in Mn0.2Fe2.8O4 NPs and Cr were investigated after being incubated together or separately with S. oneidensis MR-1 by XPS; a Kratos Analytical AXIS UltraDLD system with aluminium X-ray source (λKa= 1486.6 eV) was used, operated under ultra-high vacuum conditions (10−9 torr). The experimental curves were best fitted by combining Gaussian (70%) and Lorentzian (30%) distributions, while background subtraction was performed using the Shirley equation. A normalised peak area of each element is calculated by dividing its area by the sensitivity factor.29 To determine the redox interaction between Cr6+ and Mn0.2Fe2.8O4 NPs, we compared the normalised peak areas of Mn2+ to Mn3+, Fe2+ to Fe3+ and Cr3+ to Cr6+ in the high-resolution Mn 2p, Fe 2p and Cr 2p spectra, respectively, while only the ratios between that peak areas of Cr3+ to Cr6+ were analysed in the case of applying bacterial cells. The relative fold increase in Cr6+ bio-reduction was calculated by its equivalent atomic fraction to the reference values.2.6 Imaging bacteria by SEM
To acquire SEM images, 50 μL from the untreated or Mn0.2Fe2.8O4 NPs treated S. oneidensis MR-1 bacteria cell suspension were deposited on a microscope cover glass (Fisher, UK). The samples were imaged using Philips XL30 FEG SEM (FEI, Eindhoven, Netherlands), which operates at an accelerating voltage of 5 keV. Cell fixation was performed using glutaraldehyde (2.5% v/v in 0.01 M PBS) for 30 min at room temperature. Samples were washed three times in phosphate-buffered saline (PBS, 0.01 M) and dehydrated for 5 min in ethanol aqueous solutions. The concentrations of ethanol aqueous solutions were 10% v/v, 30% v/v, 50% v/v, 70% v/v, 90% v/v, 100% v/v, sequentially. A double-sided carbon tape (Agar Scientific, UK) was used to attach the glass slide with the SEM specimens onto aluminium stubs. Samples were then sputter-coated with gold–palladium at 20 mA and 1.25 kV for 90 s (Palaron E5000 sputter coater).3. Results and discussion
3.1 Characterisation of NPs
3.2 Interaction of Cr+6 with Mn0.2Fe2.8O4 NPs
Mn0.2Fe2.8O4 NPs adsorbed 16.8 ± 1.6 mg g−1 (around 61%) of Cr6+.23 The possible reduction of the adsorbed Cr6+ by Mn0.2Fe2.8O4 NPs was explored here by studying the oxidation state of Mn, Fe, and Cr of the adsorbent and adsorbate by XPS, as shown in Fig. 2A. | ||
| Fig. 2 Mn0.2Fe2.8O4 NPs treated by Cr6+: (A) wide scan XPS spectrum, and high-resolution XPS spectra of (B) Mn 2p, (C) Fe 2p, and (D) Cr 2p. | ||
In Mn0.2Fe2.8O4 NPs,23 Mn 2p peak, in Fig. 2B, was fitted by 5 contributions at 640.3 eV, 642.2 eV, 644.61 eV, 652.2 eV and 654.8 eV. Mn 2p3/2 was deconvoluted into 640.35 eV and 642.25 eV peaks, representing Mn2+ and Mn3+, respectively, as shown in Fig. 2B. The peak of Mn 2p1/2 was fitted into two contributions of Mn2+ and Mn3+ at 652.15 eV and 654.6 eV, respectively.36–39 The fifth small satellite peak at 645.2 eV was assigned to Mn2+ of MnO.38 Since stoichiometric MnFe2O4 can be expressed as MnO-Fe2O3, this pointed to the formation of MnxFe3−xO4 NPs.
3.3 Mn0.2Fe2.8O4 NP-assisted bacterial respiration of Cr6+
S. oneidensis MR-1 can respire Cr6+ under anaerobic conditions.48–50 The adsorption of Cr6+ (9 ± 1.5 mg g−1, i.e. 30 ± 0.5% of removal) by Mn0.2Fe2.8O4 NPs supported microbial survival in media supplemented by the tested S. oneidensis MR-1.23 The mechanism of bio-removal of Cr6+ can be attributed to the respiration of Cr6+ into Cr3+ (ref. 48–50) or bio-sorption51,52 by bacterial cells. Examining the oxidation state of Cr element via XPS analysis determines the interaction between Cr6+, Mn0.2Fe2.8O4 NPs and S. oneidensis MR-1, as illustrated in Fig. 3, which positively related to the enhanced Cr6+ bio-reduction by 2.7–3.6 fold.23 The reported significant drop in the XPS revealed the presence of peaks related to both Cr6+ and Cr3+ after exposing S. oneidensis MR-1 to Cr6+. | ||
| Fig. 3 Wide scan and high-resolution XPS spectra of (A) Cr 2p treated by S. oneidensis MR-1 alone, (B) Cr 2p after incubation of S. oneidensis MR-1 and Mn0.2Fe2.8O4 NPs. | ||
Peaks of Cr 2p XPS were observed at BE 576.7 eV and 585.9 eV, which were related to Cr3+, while peaks at 579.2 eV and 588.6 eV were assigned to Cr6+, as presented in Fig. 3A. S. oneidensis MR-1 can reduce Cr6+ into Cr3+, as confirmed by our XPS results in Fig. 3A and supported by the literature.48,53
Our findings reveal an extracellular interaction between Cr6+ and S. oneidensis MR-1 bacteria. A portion of Cr6+ was reduced to Cr3+, resulting in a [Cr3+]/[Cr6+] ratio of 1.7, while the remaining 41% of Cr6+ is adsorbed on the bacterial cell surface. The extracellular reduction of Cr6+ can occur via direct contact of Cr6+ with the metal-reducing protein complex on the cell surface and nanofiber. Also, S. oneidensis MR-1 can produce electron shuttles to promote mediated electron transfer between the cell and Cr6+. S. oneidensis MR-1 can uptake Cr6+ to be reduced inside the cell to Cr3+, but our results could not confirm the intracellular reduction of Cr6+ due to the depth limitation of XPS (7–10 nm).
Our XPS results revealed peaks related to both Cr6+ and Cr3+ after being incubated with S. oneidensis MR-1 in the presence of Mn0.2Fe2.8O4 NPs. Peaks of Cr 2p XPS observed at BE 576.7 eV and 585.9 eV denote the presence of Cr3+. Cr6+ is represented by one peak at 579.2 eV,38 as illustrated in Fig. 3B. Similar results were reported due to using Cr6+ as a terminal electron acceptor during the respiration process of S. oneidensis MR-1.48,53 The ratio between extracellular Cr3+ and Cr6+ was equal to 3.5. Bacteria can reduce Fe3+ to Fe2+, and biogenic Fe2+ can detoxify Cr6+ to Cr3+.54,55 The affinity of MnFe2O4 NPs to proteins on the bacterial outer membrane can improve the contact area between a single bacterium and Cr6+ as an external electron acceptor.56–59
In this work, the presence of both S. oneidensis MR-1 and Mn0.2Fe2.8O4 NPs removed Cr6+ 1.37 times more than using the NPs alone. Some possible scenarios could explain how NPs enhanced the bio-reduction of S. oneidensis MR-1 from Cr6+ to Cr3+. By adsorption, NPs can bridge the bacterial cell and Cr6+ to promote electron transfer. Cr6+ is adsorbed onto the MnFe2O4 NPs via partial chemisorption60,61 and partial physisorption.37 The Mn in MnFe2O4 can interact via ionic bonding with the O atoms of HCrO4−/CrO42−, facilitating Cr6+ adsorption.60,61 Mn2+ can reduce Cr6+ to Cr3+ and be oxidised to Mn3+. The disproportionation of oxidised Mn3+ produced Mn2+, causing Mn2+ to continue participating in the Cr6+ reduction. Cr3+ is deposited on the MnFe2O4 surface as Cr(OH)3 colloids.60,61
The limited availability of adsorbed Cr6+ improved the efficiency of microbial respiration,48,54 as was indicated by our results. Since MnFe2O4 NPs have electrochemical properties,59,62 metal oxides can link S. oneidensis MR-1 with Cr6+ to promote direct electron transfer and act as an electron mediator from the cell to Cr6+, a terminal electron acceptor.63
In addition, NPs can act as physical shields for bacterial surfaces from Cr6+, which could reduce the direct damage to bacteria caused by heavy metals. Encapsulating S. loihica by biochar reported that it could avoid the lethal effect of Cr6+.63 In addition, Mn0.2Fe2.8O4 NPs can sustain bacterial viability, as shown in Fig. S1† and supported by the literature.64 The Mn content in the chemical structure of Mn0.2Fe2.8O4 NPs improved the anti-oxidant activity of NPs and, in turn, cell viability.65 Substituting Fe2+ by Mn2+ in Mn0.2Fe2.8O4 NPs decreased the lethal effect of Fe2+ on bacterial viability. This explains how Mn0.2Fe2.8O4 NPs improved the viability of S. oneidensis MR-1 under the sub-lethal concentration of Cr6+ by 3.3 times.23
3.4 Boosting the bacterial tolerance to Cr6+ by Mn0.2Fe2.8O4 NPs
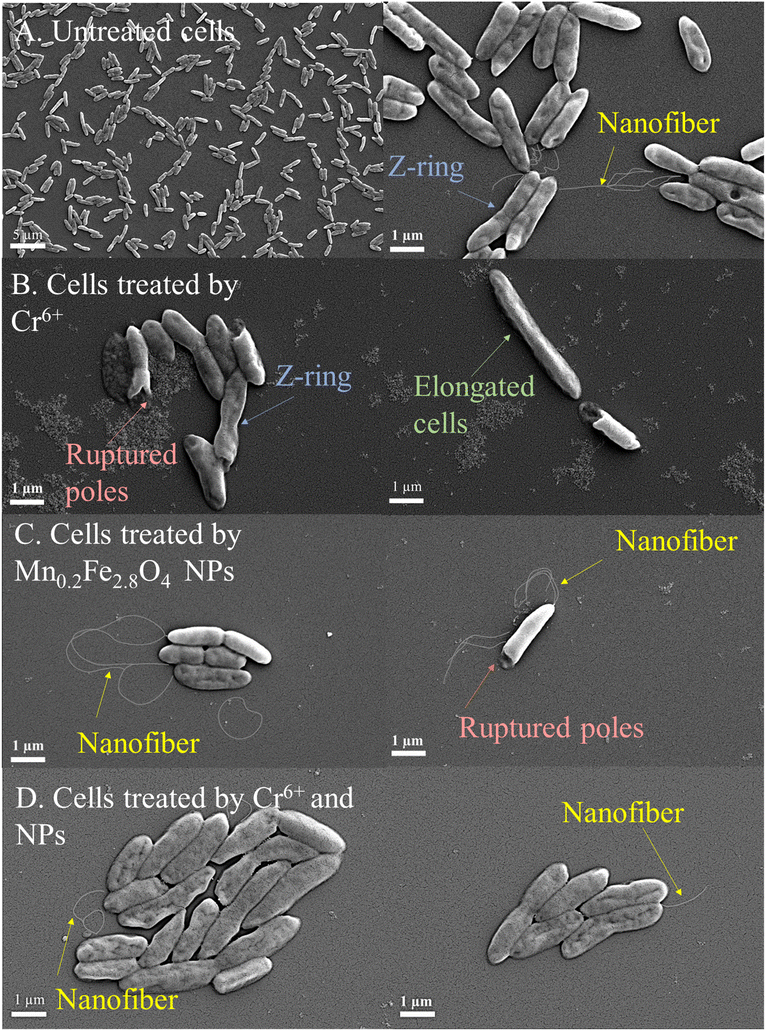
SEM imaging monitored the alterations in the morphology of bacterial cells following the bio-reduction.The capability of Shewanella to produce nanofibers in the presence of Mn0.2Fe2.8O4 NPs is shown in Fig. 4C. The poles of Shewanella cells were reported to be attractive to the metal oxide/hydroxides under both aerobic and anaerobic conditions,74,75 which explains the polar rupture of some cells in Fig. 4C.
Mn0.2Fe2.8O4 NPs provoked the formation of bacterial nanofiber in the presence of Cr6+, as depicted in Fig. 4D. Nanofibers are extensions of the outer membrane and periplasm, which are the extracellular electron transport components.66 Nanofibers are important for long-range extracellular electron transfer.53,66 The ability of NPs to regenerate bacterial nanofiber production agrees with the findings reported by Yu et al.53 Such observation in response to the interaction between MnxFe3−xO4 NPs and cells was confirmed in the present work by electron microscopy.
So, Fig. 5 summarises the protective role of Mn0.2Fe2.8O4 NPs to S. oneidensis MR-1 bacterial cells during Cr6+. The use of Mn0.2Fe2.8O4 NPs improved the viability of S. oneidensis MR-1 under a sub-lethal concentration of Cr6+ by 3.3 times, as shown in our previous report.23 Employing Mn0.2Fe2.8O4 NPs as the adsorbent can limit the availability of Cr6+ to S. oneidensis MR-1, boosting the tolerance to Cr6+.18,69 The positive adsorptive effect of NPs on Cr6+ concerning the viability of bacteria has been reported in the presence of goethite, humic acid,34 and ferric oxyhydroxide mediators.18,69 As reported in our recent investigation,23 Cr6+ was adsorbed on Mn0.2Fe2.8O4 NPs following the Langmuir adsorption isotherm model. Based on this model, the adsorption and desorption rates should be equal. Adsorption is the separation of molecules from the aqueous solution by being attached to the surface of the adsorbent. The desorption is inversely related to adsorption processes, where adsorbates are transferred from the adsorbed state to bulk solution.78 This possible continuous adsorption–desorption rate of Cr6+ can sustain a release of Cr6+ from the surface of NPs, which makes the exposure of cells to Cr6+ occur at a gradual rate.
 | ||
| Fig. 5 Illustration of the protective role of Mn0.2Fe2.8O4 NPs to S. oneidensis MR-1 during [CrO4]2− bio-reduction. | ||
Furthermore, Mn0.2Fe2.8O4 NPs reduce Cr6+ into Cr3+, as shown in Fig. 3B. Cr3+ is less toxic than Cr6+ towards S. oneidensis MR-1.22 Bacterial cells exposed to Cr3+ experienced viability loss but maintained some enzymatic activity and cellular integrity,22 which explains the morphological response of S. oneidensis MR-1 to Cr6+ in the presence of Mn0.2Fe2.8O4 NPs, as shown in Fig. 4B.
4. Conclusion
This study describes a possible protecting role of manganese ferrite nanoparticles (Mn0.2Fe2.8O4 NPs) to Shewanella oneidensis (S. oneidensis) MR-1 during hexavalent chromium (Cr6+) bio-reduction. Mn0.2Fe2.8O4 NPs can reduce the highly toxic Cr6+ to less toxic Cr3+. Under anaerobic conditions, we found that Mn0.2Fe2.8O4 NPs induced the elongation of the bacterial cells and promoted the formation of nanofibers. Such morphological change could improve the viability of S. oneidensis MR-1 cells in response to the sub-lethal dose of Cr6+ and, in turn, enhance their detoxification ability. Integrating both S. oneidensis MR-1 and Mn0.2Fe2.8O4 NPs enhanced Cr6+ detoxification by 2.1-fold compared to S. oneidensis MR-1 alone and 1.4-fold compared to NPs alone. Therefore, the present article provides evidence of Cr6+ bio-reduction and the bacterial response to Cr6+ and Mn0.2Fe2.8O4 NPs. This study will open a venue for applying nanotechnology in the bio-remediation of highly contaminated sites by heavy metals.Data availability
The data within this study is included in either the main article or ESI† figures.Author contributions
N. T. K. T. and L. C. devised and coordinated the project and provided resources. D. S. R. designed and did most of the experiments and wrote the manuscript. I. T. assisted in particle synthesis and data analysis. N. T. K. T. and S. M. provided expertise, revised the manuscript and helped to acquire funding. E. D. carried out XPS characterisation, processed data and corrected the manuscript. A. M. did a part of the characterisation and edited the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
D. S. R. was funded by Newton Mosharafa scholarship as a member in Egyptian Petroleum Research Institute. We extend our gratitude to UCL Grand Challenges and UCL Small Grant for doctoral school. We sincerely thank Adam Strange for participating in writing the UCL Small Grant application, and Professor Laurent Bozec for assisting in securing the mentioned funding. We appreciate Thithawat Trakoolwilaiwan for conducting ICP-AES analysis. Dr Linh Nguyen and Dr Nicola Mordan at UCL Eastman Dental Institute are appreciated for providing a Scanning Electron Microscopy facility. The authors thank EPSRC (EP/M015157/1), UK, for financial support. We acknowledge Konstantinos Simeonidis for the fruitful discussion.References
- A. Monga, A. B. Fulke and D. Dasgupta, Recent developments in essentiality of trivalent chromium and toxicity of hexavalent chromium: Implications on human health and remediation strategies, J. Hazard. Mater. Adv., 2022, 7, 100113 CAS , Available from: https://www.sciencedirect.com/science/article/pii/S2772416622000699.
- B. Pushkar, P. Sevak, S. Parab and N. Nilkanth, Chromium pollution and its bioremediation mechanisms in bacteria: A review, J. Environ. Manage., 2021, 287(February), 112279, DOI:10.1016/j.jenvman.2021.112279.
- E. Vaiopoulou and P. Gikas, Regulations for chromium emissions to the aquatic environment in Europe and elsewhere, Chemosphere, 2020, 254, 126876, DOI:10.1016/j.chemosphere.2020.126876.
- T. L. Desmarias and M. Costa, Toxicology Mechanisms of chromium-induced toxicity, Curr. Opin. Toxicol., 2019, 14, 1–7, DOI:10.1016/j.cotox.2019.05.003.
- J. P. Wise, J. L. Young, J. Cai and L. Cai, Current understanding of hexavalent chromium [Cr(VI)] neurotoxicity and new perspectives, Environ. Int., 2022, 158, 106877, DOI:10.1016/j.envint.2021.106877.
- International Agency for Research on Cancer, List of Classifications–IARC Monographs on the Identification of Carcinogenic Hazards to Humans, Agents classified by the IARC Monographs, 2025, vol. 1–138, Available from: https://monographs.iarc.who.int/agents-classified-by-the-iarc/.
- I. V. Y. Moffat, N. Martinova, C. Seidel and C. M. Thompson, Hexavalent Chromium in Drinking Water, J. - Am. Water Works Assoc., 2018, 110(5), E22–E35, DOI:10.1002/awwa.1044.
- European Parliament and the Council of the European Union, Directive (EU) 2020/2184 of the council of 16 December 2020 on the quality of water intended for human consumption (recast), Official Journal of the European Union, 2020, 435(December), 1–62 Search PubMed , Available from: https://eur-lex.europa.eu/eli/dir/2020/2184/oj/eng.
- A. Rai, N. Kumar Sharma, V. Kumar Singh, A. Rai, V. Kumar and A. Kumar, et al., Use of biowaste to ameliorate chromium-contaminated soils to improve crop productivity, Waste Manag. Bull., 2024, 2(1), 276–288, DOI:10.1016/j.wmb.2024.02.004.
- L. Li, Q. Liao, B. Hou, C. He, J. Liu and B. Li, et al., Synchronous reduction and removal of hexavalent chromium from wastewater by modified magnetic chitosan beads, Sep. Purif. Technol., 2023, 304, 122363 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/abs/pii/S1383586622019189.
- J. Ding, Y. Guo, M. Tang and S. Zhou, Effects of exogenous riboflavin or cytochrome addition on the cathodic reduction of Cr (VI) in microbial fuel cell with Shewanella putrefaciens, Environ. Sci. Pollut. Res., 2024, 31(20), 29185–29198 CrossRef CAS PubMed , Available from: https://link.springer.com/article/10.1007/s11356-024-33118-y.
- L. J. Liermann, E. M. Hausrath, A. D. Anbar and S. L. Brantley, Assimilatory and dissimilatory processes of microorganisms affecting metals in the environment, J. Anal. At. Spectrom., 2007, 22(8), 867–877 RSC , Available from: https://pubs.rsc.org/en/content/articlelanding/2007/ja/b705383e/unauth.
- J. Chen and Y. Tian, Hexavalent chromium reducing bacteria: mechanism of reduction and characteristics, Environ. Sci. Pollut. Res., 2021, 28(17), 20981–20997, DOI:10.1007/s11356-021-13325-7.
- M. Naveenkumar and K. Senthilkumar, Biomass and Bioenergy Microbial fuel cell for harvesting bio-energy from tannery effluent using metal mixed biochar electrodes, Biomass Bioenergy, 2021, 149, 106082, DOI:10.1016/j.biombioe.2021.106082.
- H. Gang, C. Xiao, Y. Xiao, W. Yan, R. Bai and R. Ding, et al., Proteomic analysis of the reduction and resistance mechanisms of Shewanella oneidensis MR-1 under long-term hexavalent chromium stress, Environ. Int., 2019, 127, 94–102, DOI:10.1016/j.envint.2019.03.016.
- R. Han, F. Li, T. Liu, X. Li, Y. Wu and Y. Wang, et al. Effects of incubation conditions on Cr(VI) reduction by c-type cytochromes in intact Shewanella oneidensis MR-1 cells, Front. microbiol., 2016, 7(MAY), 746, DOI:10.3389/fmicb.2016.00746/full.
- Y. Yin, C. Liu, G. Zhao and Y. Chen, Versatile mechanisms and enhanced strategies of pollutants removal mediated by Shewanella oneidensis: A review, J. Hazard. Mater., 2022, 440, 165187–165211, DOI:10.1016/j.jhazmat.2022.129703.
- X. Liu, G. Chu, Y. Du, J. Li and Y. Si, The role of electron shuttle enhances Fe(III)-mediated reduction of Cr(VI) by Shewanella oneidensis MR-1, World J. Microbiol. Biotechnol., 2019, 35(4), 64, DOI:10.1007/s11274-019-2634-9.
- A. Elahi and A. Rehman, Multiple metal resistance and Cr6+ reduction by bacterium, Staphylococcus sciuri A-HS1, isolated from untreated tannery effluent, J. King Saud Univ., Sci., 2019, 31(4), 1005–1013, DOI:10.1016/j.jksus.2018.07.016.
- S. Qin, W. Xiao, C. Zhou, Q. Pu, X. Deng and L. Lan, et al. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics, Signal Transduction Targeted Ther., 2022, 7(1), 199 CrossRef CAS , Available from: https://www.nature.com/articles/s41392-022-01056-1.
- F. Mat Arisah, A. F. Amir, N. Ramli, H. Ariffin, T. Maeda and M. A. Hassan, et al. Bacterial resistance against heavy metals in Pseudomonas aeruginosa RW9 involving hexavalent chromium removal, Sustainability, 2021, 13(17), 9797 CrossRef CAS , Available from: https://www.mdpi.com/2071-1050/13/17/9797.
- D. L. Parker, P. Borer and R. Bernier-Latmani, The response of Shewanella oneidensis MR-1 to Cr(III) toxicity differs from that to Cr(VI), Front. microbiol., 2011, 2(NOV), 223, DOI:10.3389/fmicb.2011.00223/full.
- D. S. Raie, I. Tsonas, M. Canales, S. Mourdikoudis, K. Simeonidis and A. Makridis, et al., Enhanced detoxification of Cr6+ by Shewanella oneidensis via adsorption on spherical and flower-like manganese ferrite nanostructures, Nanoscale Adv., 2023, 5(11), 2897–2910 RSC , Available from: https://pubs.rsc.org/en/content/articlelanding/2023/na/d2na00691j.
- X. Lasheras, M. Insausti, J. M. De La Fuente, I. Gil De Muro, I. Castellanos-Rubio and L. Marcano, et al. Mn-Doping level dependence on the magnetic response of MnxFe3−xO4 ferrite nanoparticles, Dalton Trans., 2019, 48(30), 11480–11491 RSC , Available from: https://pubs.rsc.org/en/content/articlelanding/2019/dt/c9dt01620a.
- K. Vamvakidis, M. Katsikini, G. Vourlias, M. Angelakeris, E. C. Paloura and C. Dendrinou-Samara, Composition and hydrophilicity control of Mn-doped ferrite (MnxFe3−xO4) nanoparticles induced by polyol differentiation, Dalton Trans., 2015, 44(12), 5396–5406 RSC , Available from: https://pubs.rsc.org/en/content/articlelanding/2015/dt/c5dt00212e.
- O. Antonoglou and C. Dendrinou-Samara, Polyols as a Toolbox for the Preparation of Inorganic-based Nanostructures. in Reducing Agents in Colloidal Nanoparticle Synthesis, 2021, pp. 51–72, Available from: https://books.rsc.org/books/edited-volume/912/chapter-abstract/708590/Polyols-as-a-Toolbox-for-the-Preparation-of?redirectedFrom=fulltext Search PubMed.
- N. T. K. Thanh, N. Maclean and S. Mahiddine, Mechanisms of nucleation and growth of nanoparticles in solution, Chem. Rev., 2014, 114(15), 7610–7630, DOI:10.1021/cr400544s.
- H. H. Hau, A. Gilbert, D. Coursolle and J. A. Gralnick, Mechanism and consequences of anaerobic respiration of cobalt by Shewanella oneidensis strain MR-1, Appl. Environ. Microbiol., 2008, 74(22), 6880–6886, DOI:10.1128/aem.00840-08.
- A. G. Shard, Practical guides for x-ray photoelectron spectroscopy: Quantitative XPS, J. Vac. Sci. Technol., A, 2020, 38(4), 041201 CrossRef CAS , Available from: https://pubs.aip.org/avs/jva/article/38/4/041201/246897/Practical-guides-for-x-ray-photoelectron.
- D. García-Soriano, R. Amaro, N. Lafuente-Gómez, P. Milán-Rois, Á. Somoza and C. Navío, et al., The influence of cation incorporation and leaching in the properties of Mn-doped nanoparticles for biomedical applications, J. Colloid Interface Sci., 2020, 578, 510–521 CrossRef PubMed , Available from: https://www.sciencedirect.com/science/article/abs/pii/S002197972030758X.
- J. M. Hughes, P. M. Budd, A. Grieve, P. Dutta, K. Tiede and J. Lewis, Highly monodisperse, lanthanide-containing polystyrene nanoparticles as potential standard reference materials for environmental “nano” fate analysis, J. Appl. Polym. Sci., 2015, 132(24), 42061, DOI:10.1002/app.42061.
- H. Etemadi and P. G. Plieger, Synthesis and characterisation of MnxFe3−xO4 (M = Fe, Mn, Zn) spinel nanoferrites through a solvothermal route, J. Mater. Sci., 2021, 56(31), 17568–17583, DOI:10.1007/s10853-021-06450-8.
- S. D. Oberdick, A. Abdelgawad, C. Moya, S. Mesbahi-Vasey, D. Kepaptsoglou and V. K. Lazarov, et al. Spin canting across core/shell Fe3O4/MnxFe3−xO4 nanoparticles, Sci. Rep., 2018, 8(1), 3425 CrossRef PubMed , Available from: https://www.nature.com/articles/s41598-018-21626-0.
- I. H. Fadhil Jasim, Thermal analysis and catalytic study of transition metals acetylacetonates, Thermochim. Acta, 1985, 93, 65–68 CrossRef , Available from: https://www.sciencedirect.com/science/article/abs/pii/0040603185850176.
- W. Shen, Z. Yao, Z. Liu, M. Xiao, J. Zhang and X. Zhang, et al., The effect of dissolved oxygen and ultrasonic pretreatment on the Cr(VI) removal efficiency with manganese ferrite, Desalin. Water Treat., 2022, 268, 48–56 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S1944398624111319.
- I. Desai, M. N. Nadagouda, M. Elovitz, M. Mills and B. Boulanger, Synthesis and characterization of magnetic manganese ferrites, Mater. Sci. Energy Technol., 2019, 2(2), 150–160, DOI:10.1016/j.mset.2019.01.009.
- J. Hu, I. M. C. Lo and G. Chen, Fast removal and recovery of Cr(VI) using surface-modified jacobsite (MnFe2O4) nanoparticles, Langmuir, 2005, 21(24), 11173–11179, DOI:10.1021/la051076h.
- M. C. Biesinger, B. P. Payne, A. P. Grosvenor, L. W. M. Lau, A. R. Gerson and R. S. C. Smart, Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni, Appl. Surf. Sci., 2011, 257(7), 2717–2730, DOI:10.1016/j.apsusc.2010.10.051.
- Z. Zhang, Y. Wang, Q. Tan, Z. Zhong and F. Su, Facile solvothermal synthesis of mesoporous manganese ferrite (MnFe2O4) microspheres as anode materials for lithium-ion batteries, J. Colloid Interface Sci., 2013, 398, 185–192, DOI:10.1016/j.jcis.2013.01.067.
- T. Yamashita and P. Hayes, Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials, Appl. Surf. Sci., 2008, 254(8), 2441–2449 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/abs/pii/S0169433207013748.
- T. Radu, C. Iacovita, D. Benea and R. Turcu, X-Ray Photoelectron Spectroscopic Characterization of Iron Oxide Nanoparticles, Appl. Surf. Sci., 2017, 405, 337–343, DOI:10.1016/j.apsusc.2017.02.002.
- B. P. Payne, M. C. Biesinger and N. S. McIntyre, X-ray photoelectron spectroscopy studies of reactions on chromium metal and chromium oxide surfaces, J. Electron Spectrosc. Relat. Phenom., 2011, 184(1–2), 29–37 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/abs/pii/S0368204810002586.
- K. Jagannathan, A. Srinivasan and C. N. R. Rao, An XPS study of the surface oxidation states of metals in some oxide catalysts, J. Catal., 1981, 69(2), 418–427 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/abs/pii/0021951781901779.
- R. Aymerich-Armengol, P. Cignoni, P. Ebbinghaus, J. Linnemann, M. Rabe and K. Tschulik, et al., Mechanism of coupled phase/morphology transformation of 2D manganese oxides through Fe galvanic exchange reaction, J. Mater. Chem. A, 2022, 10(45), 24190–24198 RSC , Available from: https://pubs.rsc.org/en/content/articlelanding/2022/ta/d2ta06552e.
- J. Chwastowski, P. Staroń, H. Kołoczek and M. Banach, Adsorption of hexavalent chromium from aqueous solutions using Canadian peat and coconut fiber, J. Mol. Liq., 2017, 248, 981–989 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/abs/pii/S0167732217341120.
- X. Liu, H. Dong, X. Yang, L. Kovarik, Y. Chen and Q. Zeng, Effects of citrate on hexavalent chromium reduction by structural Fe(II) in nontronite, J. Hazard. Mater., 2018, 343, 245–254 CrossRef CAS PubMed , Available from: https://www.sciencedirect.com/science/article/abs/pii/S0304389417307288.
- B. Sarkar, R. Naidu, G. S. R. Krishnamurti and M. Megharaj, Manganese(II)-catalyzed and clay-minerals-mediated reduction of chromium(VI) by citrate, Environ. Sci. Technol., 2013, 47(23), 13629–13636, DOI:10.1021/es401568k.
- A. Mohamed, B. Sun, C. Yu, X. Gu, N. Ashry and Y. Riahi, et al. Size effect of hematite particles on the Cr(VI) reduction by Shewanella oneidensis MR-1, J. Environ. Chem. Eng., 2021, 9(2), 105096, DOI:10.1016/j.jece.2021.105096.
- C. Ri, J. Tang, F. Liu, H. Lyu and F. Li, Enhanced microbial reduction of aqueous hexavalent chromium by Shewanella oneidensis MR-1 with biochar as electron shuttle, J. Environ. Chem. Eng., 2022, 113, 12–25, DOI:10.1016/j.jes.2021.05.023.
- R. Elmeihy, X. C. Shi, P. Ll Tremblay and T. Zhang, Fast removal of toxic hexavalent chromium from an aqueous solution by high-density Geobacter sulfurreducens, Chemosphere, 2021, 263, 128281, DOI:10.1016/j.chemosphere.2020.128281.
- J. Cheng, J. Gao, J. Zhang, W. Yuan, S. Yan and J. Zhou, et al., Optimization of Hexavalent Chromium Biosorption by Shewanella putrefaciens Using the Box-Behnken Design, Water, Air, Soil Pollut., 2021, 232(3), 92, DOI:10.1007/s11270-020-04947-7.
- Y. Xiao, C. Xiao and F. Zhao, Long-term adaptive evolution of Shewanella oneidensis MR-1 for establishment of high concentration Cr(VI) tolerance, Front. Environ. Sci. Eng., 2020, 14(1), 3, DOI:10.1007/s11783-019-1182-8.
- C. Yu, L. Yu, A. Mohamed, J. Fang, Y. Wu and K. Dai, et al. Size-dependent visible-light-enhanced Cr(VI) bioreduction by hematite nanoparticles, Chemosphere, 2022, 295(October 2021), 133633 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/abs/pii/S0045653522001266.
- H. Cheng, Z. Jing, L. Yang, A. Lu, G. Ren and J. Liu, Sunlight-triggered synergy of hematite and Shewanella oneidensis MR-1 in Cr(VI) removal, Geochim. Cosmochim. Acta, 2021, 305, 19–32, DOI:10.1016/j.gca.2021.04.034.
- G. Wang, B. Zhang, S. Li, M. Yang and C. Yin, Simultaneous microbial reduction of vanadium (V) and chromium (VI) by Shewanella loihica PV-4, Bioresour. Technol., 2017, 227, 353–358, DOI:10.1016/j.biortech.2016.12.070.
- L. Ma, Y. Du, S. Chen, F. Zhang, W. Zhan and D. Du, et al., Nanoscale zero-valent iron coupling with Shewanella oneidensis MR-1 for enhanced reduction/removal of aqueous Cr(VI), Sep. Purif. Technol., 2021, 277(August), 119488, DOI:10.1016/j.seppur.2021.119488.
- L. Liu, J. Zhao, W. Yin, S. Lv, M. Su and P. Li, et al., Enhanced immobilization of Cr(VI) by a Fe0–microorganisms composite system: Benchmark and pot experiments, J. Environ. Qua, 2021, 50(5), 1123–1134, DOI:10.1002/jeq2.20261.
- L. Ma, Y. Du, S. Chen, D. Du, H. Ye and T. C. Zhang, Highly efficient removal of Cr(VI) from aqueous solution by pinecone biochar supported nanoscale zero-valent iron coupling with Shewanella oneidensis MR-1, Chemosphere, 2022, 287(P2), 132184, DOI:10.1016/j.chemosphere.2021.132184.
- Y. Ma, X. Wu, Z. Shi, X. Li, S. Qian and X. Sun, et al. Photoactive Manganese Ferrite-Modified Bacterial Anode to Simultaneously Boost Both Mediated and Direct Electron Transfer Processes in Microbial Fuel Cells, ACS Sustainable Chem. Eng., 2022, 10(10), 3355–3362, DOI:10.1021/acssuschemeng.1c08683.
- J. Ifthikar, I. I. Shahib, A. Jawad, E. A. Gendy, S. Wang and B. Wu, et al., The excursion covered for the elimination of chromate by exploring the coordination mechanisms between chromium species and various functional groups, Coord. Chem. Rev., 2021, 437, 213868 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/abs/pii/S0010854521001028.
- M. Lu, Z. Su, Y. Zhang, H. Zhang, J. Wang and Q. Li, et al. Mn-Doped Spinel for Removing Cr(VI) from Aqueous Solutions: Adsorption Characteristics and Mechanisms, Materials, 2023, 16(4), 1553 CrossRef CAS , Available from: https://www.mdpi.com/1996-1944/16/4/1553.
- S. Khilari, S. Pandit, J. L. Varanasi, D. Das and D. Pradhan, Bifunctional Manganese Ferrite/Polyaniline Hybrid as Electrode Material for Enhanced Energy Recovery in Microbial Fuel Cell, ACS Appl. Mater. Interfaces, 2015, 7(37), 20657–20666, DOI:10.1021/acsami.5b05273.
- D. Zou, J. Tong, C. Feng, Y. Wang, X. Li and X. Zheng, et al., Synthesis of biochar@α-Fe2O3@Shewanella loihica complex for remediation of soil contaminated by hexavalent chromium: optimization of conditions and mechanism, Chemosphere, 2022, 303, 134858 CrossRef CAS PubMed , Available from: https://www.sciencedirect.com/science/article/abs/pii/S0045653522013510.
- C. R. Myers and K. H. Nealson, Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor, Dhaka Univ. Stud., Part B, 1988, 240(4857), 1319–1321, DOI:10.1126/science.240.4857.1319.
- I. L. Gunsolus, M. N. Hang, N. V. Hudson-Smith, J. T. Buchman, J. W. Bennett and D. Conroy, et al., Influence of nickel manganese cobalt oxide nanoparticle composition on toxicity toward Shewanella oneidensis MR-1: redesigning for reduced biological impact, Environ. Sci.:Nano, 2017, 4(3), 636–646 RSC , Available from: https://pubs.rsc.org/en/content/articlelanding/2017/en/c6en00453a.
- S. Pirbadian, S. E. Barchinger, K. M. Leung, H. S. Byun, Y. Jangir and R. A. Bouhenni, et al., Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components, Proc. Natl. Acad. Sci. U. S. A., 2014, 111(35), 12883–12888, DOI:10.1073/pnas.1410551111.
- N. S. Malvankar and D. R. Lovley, Microbial nanowires for bioenergy applications, Curr. Opin. Biotechnol., 2014, 27, 88–95, DOI:10.1016/j.copbio.2013.12.003.
- P. Subramanian, S. Pirbadian, M. Y. El-Naggar and G. J. Jensen, Ultrastructure of Shewanella oneidensis MR-1 nanowires revealed by electron cryotomography, Proc. Natl. Acad. Sci. U. S. A., 2018, 115(14), E3246–E3255, DOI:10.1073/pnas.1718810115.
- A. Mohamed, L. Yu, Y. Fang, N. Ashry, Y. Riahi and I. Uddin, et al. Iron mineral-humic acid complex enhanced Cr(VI) reduction by Shewanella oneidensis MR-1, Chemosphere, 2020, 247, 125902 CrossRef CAS PubMed , Available from: https://www.sciencedirect.com/science/article/abs/pii/S0045653520300941.
- H. B. Desai, S. Ghosh, R. Pandit and A. R. Tanna, Synergistic bacteriostatic effect of streptomycin-coated nanomagnetic functional oxides, BioNanoScience, 2021, 12, 62–73, DOI:10.1007/s12668-021-00923-5.
- J. A. Roberts, D. A. Fowle, B. T. Hughes and E. Kulczycki, Attachment behavior of Shewanella putrefaciens onto magnetite under aerobic and anaerobic conditions, Geomicrobiol. J., 2006, 23(8), 631–640, DOI:10.1080/01490450600964441.
- B. D. Bennett and J. A. Gralnick, Mechanisms of toxicity by and resistance to ferrous iron in anaerobic systems, Free Radical Biol. Med., 2019, 140(June), 167–171, DOI:10.1016/j.freeradbiomed.2019.06.027.
- B. D. Bennett, K. E. Redford and J. A. Gralnick, Survival of Anaerobic Fe2+ Stress Requires the ClpXP Protease, J. Bacteriol., 2018, 200(8), e00671-17, DOI:10.1128/jb.00671-17.
- S. Glasauer, S. Langley and T. J. Beveridge, Sorption of Fe (Hydr)Oxides to the Surface of Shewanella putrefaciens: Cell-Bound Fine-Grained Minerals Are Not Always Formed de Novo, Appl. Environ. Microbiol., 2001, 67(12), 5544–5550, DOI:10.1128/aem.67.12.5544-5550.2001.
- M. C. Grantham, P. M. Dove and T. J. DiChristina, Microbially catalyzed dissolution of iron and aluminum oxyhydroxide mineral surface coatings, Geochim. Cosmochim. Acta, 1997, 61(21), 4467–4477 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/abs/pii/S0016703797002652.
- S. S. Justice, D. A. Hunstad, L. Cegelski and S. J. Hultgren, Morphological plasticity as a bacterial survival strategy, Nat. Rev. Microbiol., 2008, 6(2), 162–168 CrossRef CAS PubMed , Available from: https://www.nature.com/articles/nrmicro1820.
- F. Li, H. Yu, B. Zhang, C. Hu, F. Lan and Y. Wang, et al. Engineered Cell Elongation Promotes Extracellular Electron Transfer of Shewanella Oneidensis, Adv. Sci., 2024, 11(41), 2403067, DOI:10.1002/advs.202403067.
- S. Azizian, S. Eris and L. D. Wilson, Re-evaluation of the century-old Langmuir isotherm for modeling adsorption phenomena in solution, Chem. Phys., 2018, 513, 99–104 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/abs/pii/S0301010418305317.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5en00204d |
| This journal is © The Royal Society of Chemistry 2025 |