Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ecotoxicological assessment of MWCNT-reinforced MOC composites: impacts on model bacteria and eukaryotes with environmental relevance†
Simona
Lencova
 *ab,
Jana
Kofronova
bc,
Vaclav
Peroutka
*ab,
Jana
Kofronova
bc,
Vaclav
Peroutka
 a,
Anna-Marie
Lauermannova
a,
Anna-Marie
Lauermannova
 b,
Adela
Jirickova
b,
Michal
Lojka
b,
Ondrej
Jankovsky
b,
Adela
Jirickova
b,
Michal
Lojka
b,
Ondrej
Jankovsky
 b and
Radek
Vurm
b
b and
Radek
Vurm
b
aDepartment of Biochemistry and Microbiology, University of Chemistry and Technology, Prague, Czech Republic. E-mail: lencovas@vscht.cz
bDepartment of Inorganic Chemistry, University of Chemistry and Technology, Prague, Czech Republic
cDepartment of Environmental Chemistry, University of Chemistry and Technology, Prague, Czech Republic
First published on 21st May 2025
Abstract
Composite materials based on magnesium oxychloride cement (MOC), reinforced with multi-walled carbon nanotubes (MWCNT) and their oxidized form (MWCNT-ox), are promising eco-friendly building materials. However, little is known about their ecotoxicological impact. This study pioneers the evaluation of MWCNT-reinforced MOC effect on selected prokaryotic (Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa) and eukaryotic (Artemia salina, Sinapis alba, Desmodesmus subspicatus) organisms. Initially, the effects of MWCNT and MWCNT-ox at concentrations of 1 g L−1, 0.1 g L−1, and 0.01 g L−1 on the growth and proliferation of organisms were assessed. While MWCNT samples did not affect bacterial growth or eukaryotic viability, they significantly inhibited bacterial biofilm formation. The antibiofilm effect varied among the tested bacteria, with S. aureus and E. coli being significantly more inhibited than P. aeruginosa. No differences were observed between the effects of MWCNT and MWCNT-ox on bacteria, while MWCNT-ox exhibited higher toxicity toward the tested eukaryotic organisms. Subsequently, MOC reference (MOC-REF) and MWCNT-reinforced MOC samples (MOC-MWCNT, MOC-MWCNT-ox) were prepared and characterized using XRD and SEM-EDS. Ecotoxicological assays confirmed that the composites inhibited both bacterial growth and biofilm formation, a highly desirable outcome, as microbial degradation compromises the longevity of building materials. The incorporation of MWCNT enhanced the antibacterial effect of MOC. Further, the addition of MWCNT and MWCNT-ox to MOC did not affect A. salina mortality, S. alba seed germination, or D. subspitatus growth. However, inhibition of S. alba root growth was observed at the highest tested concentration (1 g L−1) for all MOC samples, regardless of MWCNT presence. Overall, the results indicate a low environmental impact of the prepared MOC-MWCNT and MOC-MWCNT-ox composites.
Environmental significanceComposite materials based on magnesium oxychloride cement (MOC) are promising eco-friendly building materials. Their physical and mechanical properties can be significantly enhanced by the incorporation of nanoadditives such as multi-walled carbon nanotubes (MWCNT) and their oxidized form (MWCNT-ox). However, their environmental impact remains largely unexplored. This study systematically investigates the effects of MWCNT, MWCNT-ox, and MOC reinforced by them on prokaryotic and eukaryotic organisms. The research provides fundamental insights into the interactions between these materials and biological systems, including their influence on bacterial viability and biofilm formation, and eukaryotic mortality, germination, and growth. The findings indicate that the tested materials have a low environmental impact, addressing emerging concerns regarding their potential ecotoxicity, and may accelerate their applications. |
1. Introduction
Composites based on magnesium oxychloride cement (MOC) are an eco-friendly alternative to Portland cement- (PC-) based construction composites.1 These composites are based on the MOC phase 5 matrix (Mg3(OH)5Cl·4H2O), which provides high strength, high abrasion resistance, low thermal conductivity, and fire resistance.2–4 The main advantage of MOC over PC is its increased environmental sustainability gained through the lower energy demands on the production of its raw materials and its increased ability to sequester carbon dioxide from the atmosphere.5–7 Current research on MOC-based composites mainly addresses their primary drawback, poor water resistance.8 Researchers generally take the approach of modification of the composites through the application of specific substances based on the additive-accommodation ability of MOC. In this regard, various types of inorganic and organic modifiers were tested, showing their efficiency as water-induced damage retardants.9–13 Among these MOC-based materials, carbon-based nanomaterials have shown significant promise. The used carbon-based materials can be divided into the layered (2D) and 1D groups. It was shown that the layered nanomaterials, such as graphene or graphene oxide, are beneficial mostly in the tuning of microstructural parameters, which directly influence the mechanical and hygric parameters.14 From the group of 1D carbon nanomaterials, carbon nanotubes (CNT) and their oxidized forms (CNT-ox) are among the most researched ones.The incorporation of CNT into construction composites is an experimental approach yielding positive results in terms of material properties. This is attributed to the outstanding mechanical, hygric, thermal, and electrical characteristics of CNTs.15 Furthermore, these carbon nanoadditives can help heal cracks by forming bridges, filling pores, and promoting hydration of the cementitious phases in the matrix.16 Utilization of CNT in MOC-based composites mainly focuses on the use of multi-walled carbon nanotubes (MWCNT) and oxidized multi-walled carbon nanotubes (MWCNT-ox). Studies have shown that MOC-MWCNT composites exhibit increased compressive strength (higher than 70 MPa), stiffness, and, importantly, improved water resistance (softening coefficient higher than 60, water absorption coefficient lower than 0.001 kg m−2 s−1/2), even with low MWCNT or MWCNT-ox concentrations (0.02–1.0 wt% relative to the weight of the matrix).17–19 While both MWCNT and MWCNT-ox improve the MOC material quite similarly, MWCNT-ox has a more pronounced effect on flexural strength due to the present oxygen functionalities, resulting in flexural strength values around 20 MPa.20
Given the promising industrial applications of MOC-MWCNT materials, their ecotoxicity has become a significant concern. Regarding MWCNT, studies on their toxicity in various organisms—microorganisms, plants, algae, crustaceans, and fish—have yielded inconsistent results.21 MWCNTs can either inhibit or promote microbial growth, depending on the microbial species, MWCNT type, concentration, structure (e.g., particle diameter, surface area), and environmental conditions.21–25 It is hypothesised that the primary mechanisms proposed for MWCNT microbial toxicity include: (i) production of reactive oxygen species (ROS),26 (ii) direct damage to bacterial cell membranes, and (iii) chemical–physical interactions between MWCNT and microbial cells.27 Reduced MWCNT toxicity may be attributed to CNT encapsulation by organic matter, limiting MWCNT mobility.28 In contrast, MWCNTs can promote microbial growth by inducing specific gene expression that accelerates cell division.29
For eukaryotic organisms, the effects of MWCNT are similarly varied, with both toxic and beneficial outcomes reported. Specific conclusions are difficult to draw as data on certain organisms remain limited.24 For example, a study by Yang, Deng30 on the plant Arabidopsis thaliana showed that MWCNT exposure can affect root growth and induce stress responses in plant cells, while another study reported enhanced photosynthesis, protein expression, and lateral root growth.24 In some cases, exposure to MWCNTs led to increased ROS accumulation and membrane disruption, inhibiting plant growth and physiological functions, as observed in lettuce Brassica rapa.31
In aquatic organisms, sublethal effects like altered swimming behavior and feeding rates were reported for the crustacean Daphnia magna following MWCNT exposure.32 Similarly, studies on fish (Danio rerio and Cyprinus carpio) demonstrated that MWCNTs can cause developmental abnormalities, increased embryo mortality (suggesting adverse effect on reproductive and developmental processes), oxidative stress, and tissue damage, specifically gill and liver.33,34
The diversity of MWCNT effects on organisms raises questions about their toxicity when incorporated into composite materials like MOC. While data is limited, as with MWCNT alone, the conclusions are inconsistent. Some studies have shown that MWCNTs combined with other materials, such as ZnO–Ag nanocomposites, enhance antibacterial activity against E. coli and S. aureus.23 Regarding eukaryotes, co-exposure of MWCNT with heavy metals35,36 or organic pollutants, such as phenanthrene,37 has been shown to increase toxicity and bioaccumulation in Daphnia magna. Negative effects of combining MWCNT with nonylphenol compared to the pure substance have also been demonstrated in the earthworm Eisenia fetida.38 In contrast, a reduction in toxicity was observed when MWCNTs were combined with bromodiphenyl ether in a study conducted on D. rerio.39 Additionally, exposure to MWCNT enhanced the growth of broccoli seeds subjected to salt stress.40
Despite the increasing research into MOC composites and the promising effects of their modification with MWCNTs, the ecotoxicological impact of MWCNT-reinforced MOC has not been systematically investigated yet. This study aims to fill that gap by evaluating the effects of two types of MWCNTs and MWCNT-modified MOC on the growth and proliferation of three prokaryotic (Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa) and three eukaryotic (Artemia salina, Sinapis alba, Desmodesmus subspicatus) organisms. Model organisms were selected to represent those commonly used in ecotoxicology and those relevant to microbial degradation and human health risks.
2. Materials and methods
2.1 Raw materials
The MgO powder (purity 98%) was sourced from penta, s.r.o., Prague, Czech Republic. MgCl2·6H2O (p.a. purity) was obtained from Lach-ner, s.r.o., Neratovice, Czech Republic. Three different size fractions of silica sand—PG1 (0.0–0.5 mm), PG2 (0.5–1.0 mm), and PG3 (1.0–2.0 mm)—were provided by Filtrační písky Ltd., Chlum u Doks, Czech Republic. MWCNT and MWCNT-ox, both with a purity >95%, were purchased from Chengdu Organic Chemicals Co. Ltd., Chinese Academy of Sciences (Chengdu, China). Purchased nano-dopants, MWCNT and MWCNT-ox, were studied using TEM and SEM (detailed information can be found in a previous publication41). MWCNT and MWCNT-ox were dissolved in sterile tryptone soy broth (TSB, Oxoid Ltd., United Kingdom) in concentrations of 1 g L−1, 0.1 g L−1, and 0.01 g L−1, and used for the ecotoxicological assay.2.2 MOC samples preparation and characterization
A reference material, MOC-REF, was prepared without MWCNT and MWCNT-ox for comparison purposes. Both composite samples, MOC-MWCNT and MOC-MWCNT-ox, contained their respective nano-dopants at a concentration of 1.0 wt% relative to the weight of the cement paste. Table 1 presents the composition of prepared MOC composites. To prepare the MOC samples, MgCl2·6H2O was dissolved and combined with MgO powder and silica sand using a mixer. For the MOC-based composites, the respective nano-dopant was homogenized in MgCl2·6H2O solution and mixed with MgO and silica sand. The wet mixtures were poured into prismatic molds. The samples were demolded after one day, and then left to cure in the air for 27 days. A more detailed preparation procedure is described in previous publications.18 The prepared composite samples were studied using XRD and SEM-EDS; the instrumentation information and settings can be found in previously published publications.41,42| Sample | MgO | MgCl2·6H2O | H2O | MWCNT | MWCNT-ox | PG1 | PG2 | PG3 |
|---|---|---|---|---|---|---|---|---|
| MOC-REF | 106.2 | 107.2 | 66.5 | — | — | 106.3 | 106.3 | 106.3 |
| MOC-MWCNT | 106.2 | 107.2 | 66.5 | 2.8 | — | 106.3 | 106.3 | 106.3 |
| MOC-MWCNT-ox | 106.2 | 107.2 | 66.5 | — | 2.8 | 106.3 | 106.3 | 106.3 |
2.3 Verification of MOC materials sterility
MOC composites both in solid and powder form were incubated in (i) sterile TSB for 48 h at 30 °C and (ii) plate count agar (PCA, Merck, Germany), respectively, for 72 h at 30 °C. After cultivation, the absorbance of the TSB was measured spectrophotometrically at 620 nm (Tecan, Switzerland) and compared to the values determined before cultivation, and colony-forming units (CFU) formed on PCA were counted.2.4 Bacterial strains, eukaryotic organisms, and culture conditions
Three bacterial strains (international standard reference strains for antibacterial disc susceptibility testing) obtained from the Czech Collection of Microorganisms (CCM, Czech Republic) were used as model microorganisms in this study: Gram-positive bacterium Staphylococcus aureus ATCC 25923 (eq. CCM 3953) and Gram-negative bacteria Escherichia coli ATCC 25922 (eq. CCM 3954) and Pseudomonas aeruginosa ATCC 27853 (eq. CCM 3955). Pure bacterial cultures, statically cultivated in sterile TSB for 24 h at 37 °C, were used for the ecotoxicological assays described below. Further, three eukaryotic organisms were included in testing: Artemia salina (aquatic crustacean; Easyfish, Czech Republic), Sinapis alba (mustard; Biom, Czech Republic), and Desmodesmus subspicatus (algae; Institute of Botany Třeboň, Czech Republic).2.5 Ecotoxicological assay – MWCNT and MWCNT-ox
Next, the impact of tested MWCNTs on bacterial biofilm formation was investigated. Biofilm was formed in a 96-well microtiter plate (Gama Group, Czech Republic) under the following conditions. Each well contained 160 μL of sterile TSB, 20 μL of single-species bacterial suspension with optical density 0.5 MacFarland (McF), and 20 μL of MWCNT (ev. MWCNT-ox) solution containing TSB to ensure the final concentration of MWCNT 1 g L−1, 0.1 g L−1, and 0.01 g L−1, respectively, in a well. Controls of microbial growth contained 180 μL of TSB and 20 μL of single-species bacterial suspension with optical density 0.5 McF; controls of MWCNT media contained 180 μL of TSB and 20 μL of MWCNT-containing TSB. Plates were incubated at 37 °C for 24 h. After cultivation, biofilms were washed five times with saline solution, dried for 45 min at laboratory temperature, and stained with 0.1% crystal violet (CV, Sigma-Aldrich, US) for 45 min at laboratory temperature. After washing three times with saline solution, the samples were either (i) microscope under optical microscopy at the 60× magnification or (ii) incubated with 200 μL of 96% ethanol for 15 min at laboratory temperature, from which 100 μL was transferred into a sterile 96-well microtiter plate and the A595nm of the suspensions was measured spectrophotometrically (Tecan, Switzerland). Further, we tested the effect of MWCNTs on the formation of ROS; similar conditions as in previous microbial tests were used. H2O2 (oxidative stress marker) production was quantified using the ROS-Glo™ H2O2 Assay (Promega, US) according to the manufacturers protocol, with final measurement of luminescence (BioTek Synergy H1, Agilent, US) and determination of percentage increase of ROS generation compared to the control (bacteria without MWCNT addition).
The growth of mustard Sinapis alba was tested according to a standard procedure with modifications. Prior to testing, the samples were prepared by dispersion of nanopowder in a medium and left for sonication for 30 min. Then, the series of dilutions 1 g L−1, 0.1 g L−1, and 0.01 g L−1 was prepared by dilution with standard control medium. A control was established using demineralized water enriched with salt solution. Additionally, a solution of potassium dichromate was used as a reference substance in the positive control. The experiments were carried out in 120 mm diameter Petri dishes comprising cellulose filter papers with holes, with a total volume of 5 mL of the prepared samples or control added to each dish. A total of fifteen seeds were placed into the holes, after which each dish was covered with a lid. Subsequently, the samples were incubated at 20 °C for a period of 72 h in the absence of light. Following the incubation period, root length was quantified utilizing ImageJ software (National Institutes of Health, United States). A seed was deemed germinated when a root of at least 1 mm was observable. The germination of seeds was enumerated, and root elongation inhibition was calculated by comparison with the control results.
The algal growth inhibition of Desmodesmus subspicatus was conducted in accordance with the standard EN ISO 8692, with the incorporation of modifications. The green algae were obtained as a sterile culture of D. subspicatus (R. Chodat), E. Hegewald et A. Schmidt, Brinkmann 1953/SAG 8681. Three days prior to the inhibition test, the algae were subjected to a pre-cultivation process in a 500 mL Erlenmeyer flask, which was filled with Bold's Basal growth medium (BBM) and sealed with a pulp plug. The incubation was on an orbital shaker (ELMI DOS-20L, ELMI USA) at 23 °C under continuous illumination provided by daylight lamps, with an intensity of 7000 lx. The samples were diluted with BBM medium and subjected to ultrasonication for a period of 30 min. The final concentration was 0.01 g L−1 and 0.1 g L−1, as a precaution against potential shading effects. Thereafter, the samples were transported under sterile conditions to Erlenmeyer flasks with a total volume of 15 mL, together with the algal inoculum. The volume of added algal inoculum was dependent on the initial density determined under microscopy (Primo Star, Zeiss, Germany) with 400× magnification and Bürker counting chamber. The flasks were cultivated under identical heat and light conditions to those employed during the pre-cultivation phase, which was conducted under orbital shaking (150 rpm). BBM served as a control medium. Following a period of 72 h, the cell concentration of D. subspicatus was quantified utilising a microscope. The resulting growth and inhibition rates were calculated according to the standard.
2.6 Ecotoxicological assay – MOC samples
Firstly, the impact of MOC samples on bacterial growth was studied. Pieces (1 × 1 × 1 cm) of MOC samples, respectively, were incubated in 3 mL of single-species bacterial suspensions of E. coli, S. aureus, and P. aeruginosa of optical density 0.5 McF at 37 °C for 48 h. Controls of bacterial growth contained only 3 mL of bacterial suspension; control of MOC sterility contained 3 mL of sterile TSB and an MOC piece. Both before and after the cultivation, A620 nm of the suspension was measured spectrophotometrically (Tecan, Switzerland). Further, the pH of suspensions both before and after cultivation was measured with a calibrated pH meter (Radiometer Analytical, France).In the next step, the impact of MOC samples on bacterial biofilm formation was determined. Pieces (1 × 1 × 1 cm) of MOC samples, respectively, were incubated in 3 mL of single-species bacterial suspensions of E. coli, S. aureus, and P. aeruginosa of optical density 0.5 McF at 37 °C for 48 h. Controls of bacterial growth contained only 3 mL of bacterial suspension; control of MOC sterility contained 3 mL of sterile TSB and an MOC piece. After the cultivation, MOC samples were washed in saline solution and sonicated in 3 mL of sterile saline solution for 3 min at room temperature in a sonication bath (Polsonic, Poland). Physiological solution with released biofilm-forming cells was decimally diluted in sterile saline solution up to the eighth dilution. Droplets (20 μl) of all dilutions were plotted on PCA (Merck, Germany) in triplicates and cultivated. After the cultivation (24 h at 37 °C), CFUs were counted, values were converted to 1 cm2, and compared to the control. Furthermore, SEM analysis of bacteria adhered to the MOC surface was performed, along with EDS for confirmation of organic matter presence according to the previously published publications.41–43 Further, H2O2 production in MOC presence was quantified using the ROS-Glo™ H2O2 Assay (Promega, US) similarly as described in the section 2.5.1.
Finally, determination of MOC-MWCNT toxicity on eukaryotic organisms was performed. A. salina mortality, S. alba growth, and D. subspicatus growth in the presence of MOC samples, respectively, was determined similarly as described in 2.5.
2.7 Statistical analysis
All the experiments were performed at least in biological and technical triplicates. Data analysis was provided in the R programming language. Outliers were discarded according to Dean-Dixon's Q-test. The Shapiro–Wilk test was applied to data sets to verify normal distribution; the data were considered normally distributed at p > 0.05. The final data were subjected to multiple comparisons by one-way analysis of variance (ANOVA) with a significance level α = 0.05, α = 0.01, and α = 0.001. In case of significant results, Tukey HSD (Tukey honest significant differences) and Bonferroni correlation were applied to perform multiple pairwise comparisons of groups' mean.Statistical analysis was performed for the evaluation of the initial hypotheses. We presumed that both tested forms of MWCNT, as well as the prepared MOC samples, influence bacterial and eukaryotic growth and viability, and the different effects of MWCNT and MWCNT-ox (eventually MOC-MWCNT and MOC-MWCNT-ox) were expected.
3. Results and discussion
For better clarity and understanding, a visualization of sample preparation and characterization can be seen in Scheme 1.3.1 MWCNT and MWCNT-ox characterization
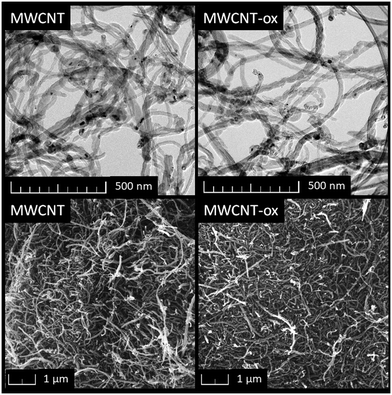
The purchased nano-dopants, MWCNT and MWCNT-ox, were characterized using TEM and SEM (Fig. 1). Both nano-dopants exhibited the typical morphology of carbon nanotubes, appearing as disorganized, long carbon tubes with an approximate diameter of 25 nm. No significant difference between MWCNT and MWCNT-ox was observed. SEM-EDS analysis confirmed that oxygen content in MWCNT-ox was significantly higher compared to MWCNT.3.2 Impact of MWCNT on prokaryotes and eukaryotes
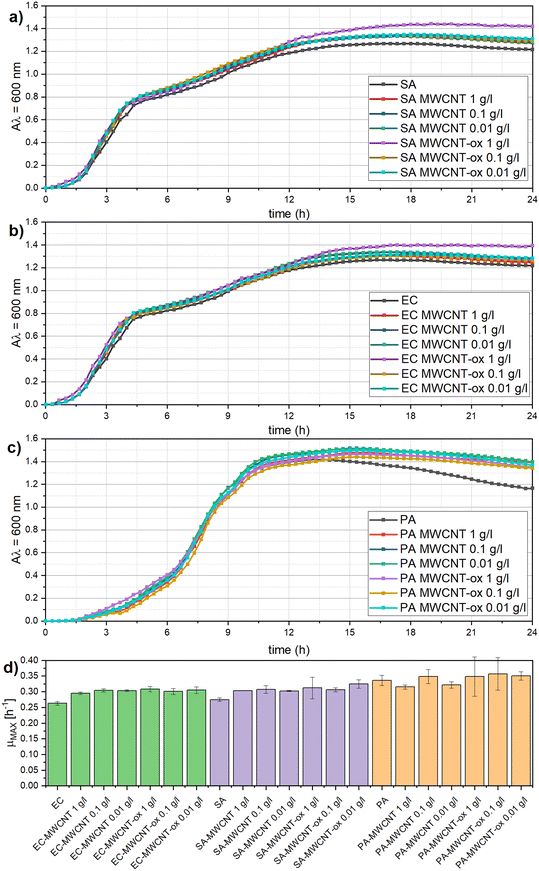
First, the impact of MWCNT and MWCNT-ox on bacterial growth was examined. Spectrophotometric measurement (Fig. 2) showed that the presence of MWCNT and MWCNT-ox at all tested concentrations (1 g L−1, 0.1 g L−1, 0.01 g L−1) did not significantly affect the growth of any of the tested bacteria (α = 0.05). This is probably caused by the aggregation and settling of MWCNT and MWCNT-ox particles. Growth curves and quantitative growth characteristics, such as the maximum specific growth rate, did not differ significantly across the tested bacteria.Our data are in correspondence with recent studies,21,44 also concluding that MWCNT do not necessarily influence bacterial growth. MWCNTs are generally considered less toxic than single-walled carbon nanotubes (SWCNTs), and their effect can vary depending on several factors, such as particle size, concentration, dispersion quality, functionalization, and the type of target bacteria.22,23 However, studies reporting that MWCNT inhibit bacterial growth prevail, particularly at higher concentrations of MWCNT. For example, Saleemi, Fouladi25 reported an antibacterial effect of MWCNT at concentrations between 60 and 100 μg mL−1, while lower concentrations had no significant impact. In the study of Liu, Wei,22 a high dose of 500 μg mL−1 significantly inhibited the growth of E. coli and S. aureus. Given the variability in MWCNTs' effects, it is critical to test each type of MWCNT before application due to the complexity and inconsistency of its inhibition potential.
In contrast, both MWCNT and MWCNT-ox exhibited strong antibiofilm activity at all tested concentrations (1 g L−1, 0.1 g L−1, 0.01 g L−1) against the three bacterial species (Fig. 3). Biofilm formation in the presence of both MWCNT and MWCNT-ox was significantly reduced compared to the control (p < 0.05). However, the inhibition effect varied between the bacteria, with S. aureus and E. coli being significantly more inhibited than P. aeruginosa (p < 0.001). No significant differences were observed between MWCNT and MWCNT-ox in their effect on S. aureus and E. coli across all concentrations. However, P. aeruginosa was more strongly inhibited at a concentration of 0.1 g L−1 for both MWCNT and MWCNT-ox. The differential effects observed on the bacterial species are likely due to differences in cell wall structure and shape between Gram-positive and Gram-negative bacteria, which are considered the main factors influencing bacterial resilience to external factors.45–47
Our results demonstrate that while MWCNT and MWCNT-ox do not inhibit bacterial growth, they exert strong antibiofilm effects. This could be due to the settling of MWCNT particles at the bottom of the culture plate, preventing bacterial adhesion and continuous biofilm formation. Additionally, MWCNTs are thought to induce oxidative stress and generate ROS, which hinder biofilm formation.26 In the literature, antibiofilm effects of MWCNT themselves have been studied rarely, as they are more often tested in composites that enhance this effect. For instance, Gomes, Gomes46 observed strong antibiofilm activity of MWCNT/poly(dimethylsiloxane) composites against E. coli and Enterococcus faecalis, with increasing MWCNT content correlating with stronger antibiofilm activity. Similarly, Al-Gaashani, Pasha23 reported antibiofilm activity inducing effect of MWCNT in ZnO-Ag-MWCNTs nanocomposites against E. coli and S. aureus.
Prokaryotes and eukaryotes exhibit fundamental physiological differences, which result in varied responses to environmental factors. In addition to the bacterial tests mentioned above, the mortality rate of A. salina, germination and root growth inhibition in S. alba, and growth inhibition of D. subspicatus were evaluated.
To investigate the effects of oxidized and non-oxidized MWCNT on crustaceans, A. salina nauplii were used. This early life stage is generally considered more sensitive than the adult form. No significant mortality or bioaccumulation was observed for MWCNT and MWCNT-ox at concentrations of 0.01 and 0.1 g L−1 (Table 2). A slight increase in mortality was observed at 0.1 g L−1 for MWCNT-ox, possibly due to its lower potential for aggregation and thus higher bioavailability.48 These findings are consistent with studies of Mesarič, Gambardella49 and Trompeta, Preiss,50 which also reported the absence of MWCNT-induced mortality at a concentration of 0.1 g L−1. However, they did observe bioaccumulation of black MWCNT aggregates in the intestinal tracts of A. salina, which were cleared within 24 hours, restoring normal functionality, indicating that there is no short-term impact on the organism's functionality.50 For a concentration of 1 g L−1, 100% mortality was observed, likely due to restricted movement in the higher-concentration environment51 or, physical interference caused by the elongated shape of MWCNTs. The toxicity at this level is likely mechanical rather than chemical.52 Nevertheless, such a high concentration is not environmentally relevant, and real-world exposure levels are expected to be far lower.
| Sample | c (g L−1) | A. salina | S. alba | D. subspicatus |
|---|---|---|---|---|
| Mortality (%) | I R (%) | I (%) | ||
| MWCNT | 0.01 | 0 | −4.7 ± 3.4b,c | 8.3 ± 3.4b |
| 0.1 | 0 | −16.2 ± 4.2c | 20.3 ± 3.2a,b | |
| 1 | 100 | 22.0 ± 4.7a | — | |
| MWCNT-ox | 0.01 | 0 | −14.4 ± 10.4c | 25.2 ± 7.4a |
| 0.1 | 13.3 ± 5.7 | −14.7 ± 1.1c | 24.8 ± 4.1a | |
| 1 | 100 | 10.2 ± 8.4a,b | — |
The exposure of S. alba to both MWCNT types resulted in less than 10% inhibition of germination across all concentrations when compared to the control. These findings are in line with the observations of Lin and Xing,53 who reported no germination inhibition for radish, rape, ryegrass, lettuce, corn, or cucumber at MWCNT concentrations of up to 2 g L−1. With regard to the impact on root growth, a stimulatory effect was observed at concentrations of 0.01 and 0.1 g L−1 for both materials (Table 2), with the oxidized form showing a stronger effect. However, at 1 g L−1, an inhibitory effect was observed, with MWCNT causing twice the inhibition compared to MWCNT-ox. These results are consistent with those of Mondal, Basu,54 who noted a beneficial effect on Brassica juncea (brown mustard) seeds at low MWCNT-ox concentrations compared to non-oxidized MWCNT. The stimulatory effect is likely due to MWCNT's ability to enhance water and nutrient absorption by plants.55 However, at concentrations exceeding 0.1 g L−1, these stimulatory effects diminish, leading to inhibition.56
Further, algal growth of D. subspicatus was inhibited following exposure to MWCNT and MWCNT-ox (Table 2). MWCNT-ox demonstrated lower inhibition rates in a concentration-dependent manner, with less than 10% inhibition at 0.01 g L−1 and 20% at 0.1 g L−1. In contrast, the oxidized form demonstrated an inhibitory effect of approximately 25% at both concentrations. These differences in toxicity of the two forms of MWCNTs may be attributed to structural factors, whereby MWCNT-ox can interact more readily with biological molecules due to their reactive functional groups.57
In summary, at a concentration of 0.1 g L−1, both MWCNT and MWCNT-ox exhibited similar toxicity levels, suggesting that general mechanisms like oxidative stress and interference with cellular functions—typical for MWCNTs—likely dominate.58–60 Nevertheless, at higher concentrations, insufficient illumination or diminished accessibility of nutrients complexed with nanoparticle agglomerates may also contribute to the observed inhibition.59 Identifying the exact source of inhibition can be challenging, as it often results from the complex interplay of multiple factors.
3.3 MOC samples preparation and characterization
As the tested MWCNT and MWCNT-ox were shown to be harmless to bacterial and eukaryotic growth but exhibited significant antibiofilm effect – an advantageous property for building materials – they were incorporated into MOC-based composites. Two types of MOC-based composites were prepared: a reference sample containing only MOC phase 5 and silica sand filler, and two modified samples doped with a small dosage of either MWCNT or MWCNT-ox.The phase composition of the prepared samples was analyzed using XRD, with diffraction patterns shown in Fig. 4. In both samples, MOC phase 5 and silicon oxide in the form of quartz were detected. The presence of quartz originated from the utilization of silica sand as a filler. Even though the samples MOC-MWCNT and MOC-MWCNT-ox contained MWCNT and MWCNT-ox, these cannot be seen in the diffraction pattern, as their content is very low. The MOC phase 5 is considered crucial for the superior microstructural and mechanical properties of MOC-based composites.
 | ||
| Fig. 4 Diffraction patterns of the samples MOC-REF (top), MOC-MWCNT (middle), and MOC-MWCNT-ox (bottom) obtained from XRD. | ||
SEM analysis of the fracture surfaces of the composites revealed a dense microstructure composed of interlocking, needle-shaped crystals typical of MOC phases (Fig. 5). These needle crystals are responsible for the excellent material performance of MOC-based materials. Silica sand filler grains are also visible in the micrographs. At higher magnifications, MWCNT and MWCNT-ox bundles were observed, formed due to their hydrophobic nature and propensity for agglomeration. In the future, it will be necessary to mitigate the agglomeration of the nanomaterials by using specific additives, such as surfactants,61,62 or techniques, such as ultrasonication.63
During SEM analysis, EDS was employed to determine the chemical composition of the studied fracture surface. The detected elements included Mg, O, Cl, Si, and C (Fig. 6). Along with the qualitative analysis, the quantity of the individual elements on the fracture surface was studied using EDS. However, these results are influenced by the small area of the studied surface, and thus the elemental quantities are not presented as they do not reflect the true chemical composition of the samples.
3.4 Impact of MOC-MWCNT on prokaryotes and eukaryotes
After verifying the influence of MWCNT and MWCNT-ox on selected organisms, MWCNT-reinforced MOC samples were prepared and tested in terms of their ecotoxicological impact. First, we evaluated MOC sterility by testing the capture of microorganisms that can be cultivated by conventional cultivation methods. No microbial growth was observed after culturing the materials (MOC-REF as well as MOC-MWCNT and MOC-MWCNT-ox), in both solid and powder forms, in nutrient media, indicating that these materials are resistant to microbial contamination from the environment (Fig. S1†). This suggests that their surface and structure do not support microbial colonization, and that MOC is inherently sterile, without being influenced by the addition of MWCNT/MWCNT-ox. These results are promising for the use of MOC materials in applications where microbial resistance is critical, as microbial degradation is a major factor affecting material durability.64Regarding bacterial interactions with the tested MOC materials, the results (Fig. 7) show that all three MOC samples inhibited bacterial growth in their surroundings. MOC-REF and MWCNT-reinforced MOC samples significantly reduced bacterial growth (p < 0.001). The addition of both types of MWCNTs to MOC enhanced the inhibition of S. aureus and E. coli growth (p < 0.05). In case of P. aeruginosa, only the addition of MWCNT significantly affected the growth, while the addition of MWCNT-ox to MOC had no observable effect.
Near-total inhibition of growth occurred for S. aureus and E. coli; P. aeruginosa showed lower growth inhibition compared to other tested bacteria, reaching a maximum of 30% growth compared to the control. This may be caused by a notable increase in medium pH caused by the MOC. The pH shifted from neutral to a basic environment (pH 9–10) during cultivation, consistent with other studies reporting MOC-induced alkalinity of pH 8–10.65 Bacteria typically prefer neutral pH and can slightly alter the medium's pH through acidic or alkaline metabolites production during growth.66 However, at pH levels of 9–10, bacterial growth may be affected due to disruptions in various cellular processes, including protein denaturation and membrane damage. That aligns with the observation that P. aeruginosa was the most resistant to the alkaline conditions.67 An additional anomaly was observed in the behavior of P. aeruginosa—at pH 9 or higher in the presence of MOC, the bacterium did not produce its typical phycocyanin pigment, which gives the bacterial suspension a blue-green color (Fig. S2†). Phycocyanin is produced within an optimal pH range of 6.4–7.4 and is not produced at pH levels of 9 or higher.68,69
Further, we studied bacterial biofilm formation on the surface of MOC samples (Fig. 8). Both MOC-REF and MWCNT-reinforced MOCs inhibited biofilm formation in all tested bacteria, including the most durable one, P. aeruginosa. The biofilm formation in control samples reached values of 7.7 ± 0.0.2–8.5 ± 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log (CFU cm−2), while notably lower values were observed for MOC surfaces.
log (CFU cm−2), while notably lower values were observed for MOC surfaces.
Biofilm formation on MOC-REF ranged between 1.8 ± 0.1 and 2.5 ± 0.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log (CFU cm−2), while MWCNT-/MWCNT-ox-reinforced MOC samples showed biofilm formation between 1.1 ± 0.2 and 2.3 ± 0.1
log (CFU cm−2), while MWCNT-/MWCNT-ox-reinforced MOC samples showed biofilm formation between 1.1 ± 0.2 and 2.3 ± 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log (CFU cm−2). The most significant effect was observed for MOC-MWCNT on E. coli (p < 0.05); no significant difference between the effect of MOC-REF and MOC-MWCNT-ox was confirmed (p > 0.05). SEM analysis revealed that only a minimal number of bacterial cells adhered to the surface of the tested samples (Fig. 9). The bacterial cells showed visible damage to their cell walls, which appeared wrinkled, and predominantly attached to areas with surface cracks or areas without nanotubes. This indicates that the surface of the material does not facilitate bacterial adhesion, preventing the formation of a continuous biofilm, a typical feature of the tested bacterial species.70 The presence of prokaryotic organisms on selected samples was further confirmed by EDS. In the elemental maps (Fig. 10), areas containing S. aureus embedded in the MOC-MWCNT-ox composite showed increased carbon content.
log (CFU cm−2). The most significant effect was observed for MOC-MWCNT on E. coli (p < 0.05); no significant difference between the effect of MOC-REF and MOC-MWCNT-ox was confirmed (p > 0.05). SEM analysis revealed that only a minimal number of bacterial cells adhered to the surface of the tested samples (Fig. 9). The bacterial cells showed visible damage to their cell walls, which appeared wrinkled, and predominantly attached to areas with surface cracks or areas without nanotubes. This indicates that the surface of the material does not facilitate bacterial adhesion, preventing the formation of a continuous biofilm, a typical feature of the tested bacterial species.70 The presence of prokaryotic organisms on selected samples was further confirmed by EDS. In the elemental maps (Fig. 10), areas containing S. aureus embedded in the MOC-MWCNT-ox composite showed increased carbon content.
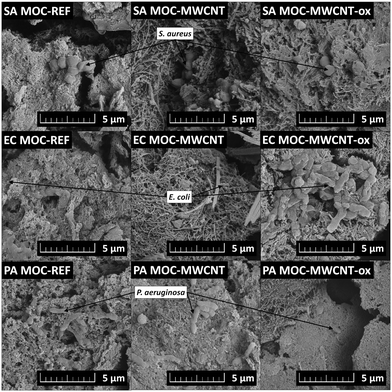 | ||
| Fig. 9 SEM micrographs of the prepared MOC-based composites after exposure to prokaryotes S. aureus (SA), E. coli (EC), and P. aeruginosa (PA). | ||
To further investigate the mechanism of action of the tested materials on bacterial cells, oxidative stress induced by MWCNTs and MOCs was assessed using the model bacterium S. aureus (Table 3). H2O2 levels were measured as a marker of material-induced oxidative stress in bacteria, as H2O2 has the longest half-life compared to other ROS and reflect general changes in ROS levels over time. As the applied assay is based on luminescence detection, measurements of highly concentrated MWCNT and MWCNT-ox solutions are subject to greater statistical error and should be interpreted with caution. This bias arises from the blackness and increased turbidity of the solution, which absorb emitted light and thereby distort luminometric readings. Conversely, at lower concentrations, where these interfering effects are less pronounced, the assay revealed a notable increase in ROS production—by several tens of percent—in the presence of MWCNT and MWCNT-ox. However, for MOC composites (both reference and MWCNT-incorporated ones), the observed increases were not statistically significant.
| Sample | Control | MWCNT 1 g l−1 | MWCNT-ox 1 g l−1 | MWCNT 0.1 g l−1 | MWCNT-ox 0.1 g l−1 | MOC-ref | MOC-MWCNT | MOC-MWCNT-ox |
|---|---|---|---|---|---|---|---|---|
| ROS (H2O2) luminiscence (RLU) | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 964.0 ± 160.7 964.0 ± 160.7 |
2057.3 ± 51.1 | 2125.3 ± 25.7 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 849.0 ± 161.6 849.0 ± 161.6 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 444.7 ± 122.0 444.7 ± 122.0 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 675.0 ± 160.1 675.0 ± 160.1 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 389.3 ± 39.8 389.3 ± 39.8 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 636.3 ± 21.2 636.3 ± 21.2 |
| Increase of ROS generation (average %) | 0.0 | −89.2 | −88.8 | 62.7 | 71.1 | −22.6 | −18.8 | 3.5 |
Following the bacterial tests, the effect of MOC-MWCNT on A. salina mortality rate, S. alba germination and root growth inhibition, and D. subspicatus growth inhibition were investigated. We revealed that the toxicity of bulk MOC to A. salina was higher than that of the MOC-nanotube combination at a concentration of 1 g L−1 (see Fig. 11). One potential explanation is the increased compactness and stability of MOC in aqueous media when combined with nanotubes. At concentrations of 0.1 g L−1 and higher, a slight haze formed in the pure MOC solution, but not in the MWCNT mixture. An alternative explanation could relate to the chemical nature of MOC itself or residual substances from the preparation process.71,72 At the lowest exposed concentration, all three materials exhibited identical toxicity (13%), indicating that the MOC is responsible for this effect.
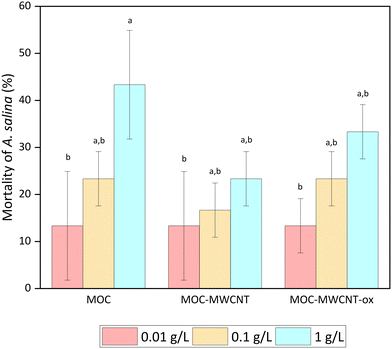 | ||
| Fig. 11 A. salina mortality; means that do not share a letter are statistically significant at p < 0.05 (ANOVA and Tukey's test with Bonferroni correction). | ||
To better understand the toxic effects on A. salina, it is important to consider not only mortality but also potential bioaccumulation, particularly in the intestinal tract. Despite the absence of bulk MWCNT in A. salina gut, our study revealed the presence of MOC-MWCNT and MOC-MWCNT-ox particles at the highest exposed concentration of 1 g L−1 (Fig. 12). The combination of MOC with nanotubes resulted in a shape modification that may have enhanced the particles' attractiveness to Artemia, thereby more closely mimicking natural food sources and increasing the probability of ingestion. Black particles were observed throughout the gut, indicating that they should be emptied over time. This is consistent with the findings of the study by Zhu, Luo73 following exposure to oxidized MWCNT, where most of it was excreted after 72 h. Some particles were also attached to the body surface, which could cause mortality.73
 | ||
| Fig. 12 Bioaccumulation of MWCNT by A. salina gut: (A) control, (B) accumulated MOC-MWCNT in 1 g L−1, (C) accumulated MOC-MWCNT-ox in 1 g L−1. Scale bars: 300 μm. | ||
Further, no significant inhibition of S. alba seed germination was observed, with inhibition levels not exceeding 10%. However, a notable inhibition of S. alba root growth was detected (Fig. 13), exceeding 30% at a concentration of 1 g L−1 for pure MOC and its combinations with MWCNT or MWCNT-ox. This effect could be attributed to the elevated magnesium ion levels in the solution. While magnesium is essential for plant growth and photosynthesis, excess magnesium can disrupt the mineral balance and adversely affect root development.74,75 Inhibitory effects, approximately 20%, were also observed in MOC combinations with MWCNT and MWCNT-ox at a notably lower concentration of 0.1 g L−1. At 0.01 g L−1, significant root growth inhibition was only noted for MWCNT-ox. These results suggest that the combination of MOC and MWCNTs enhances the toxic potential in S. alba seeds at certain concentrations.
 | ||
| Fig. 13 S. alba root growth inhibition; means that do not share a letter are statistically significant at p < 0.05 (ANOVA and Tukey's test with Bonferroni correction). | ||
Next, we evaluated the inhibition of D. subspicatus growth by MOC samples. The potential for growth inhibition was assessed by direct cell counting of D. subspicatus following exposure to MOC and its combinations with MWCNT. While a concentration of 0.01 g L−1 MOC stimulated growth, a 10% increase in inhibition was observed at a concentration of 0.1 g L−1 compared to the control (Fig. 14). The combination of MOC with nanotubes yielded unexpected antagonistic effects, with inhibition at both tested concentrations not exceeding 5%. The results were not found to be statistically significant. This suggests that the concentration of MWCNT/MWCNT-ox plays a key role in influencing algal growth inhibition.
 | ||
| Fig. 14 D. subspicatus growth inhibition. Means are not statistically significant at p < 0.05 (ANOVA and Tukey's test). | ||
At higher concentrations, the dispersion of pure MOC in BBM resulted in the formation of a slight haze, which may have created a shielding effect. This phenomenon is commonly observed when particles accumulate on the surface of organisms, leading to inhibition of photosynthetic activity.76 However, the dispersion of MOC combined with MWCNT did not show this effect. Based on these findings, it can be inferred that the release of small quantities of these materials into the environment, such as through degradation,77,78 is unlikely to significantly impact freshwater algae like D. subspicatus.
3.5 Summary of results
To summarize all the results, the following conclusions were made. MWCNT and MWCNT-ox, at concentrations of 1 g L−1, 0.1 g L−1, and 0.01 g L−1, did not significantly influence bacterial growth, although they provided strong antibiofilm effects. No significant difference was observed between the non-oxidized and oxidized forms of MWCNT in their effects on the tested bacterial strains. Regarding the tested eukaryotes, both MWCNT and MWCNT-ox slightly influenced growth and viability. For A. salina, no significant mortality or bioaccumulation was detected at 0.01 and 0.1 g L−1, but the highest concentration of 1 g L−1 caused total mortality. S. alba root growth inhibition varied by concentration, with a stimulatory effect observed at 0.01 and 0.1 g L−1 for both MWCNT and MWCNT-ox, while the 1 g L−1 concentration resulted in significant inhibition, with MWCNT showing twice the inhibitory effect compared to MWCNT-ox. Low inhibition levels were observed for D. subspicatus growth, with a concentration-dependent effect.Additionally, the prepared MOC samples inhibited bacterial growth in the surroundings and provided strong antibiofilm properties, primarily due to the MOC itself. No significant difference between MOC-MWCNT and MOC-MWCNT-ox effects was detected. For the tested eukaryotes, MOC samples affected growth and viability, with bulk MOC showing higher toxicity to A. salina than its combination with nanotubes. For S. alba, root growth inhibition exceeded 30% at a concentration of 1 g L−1, likely due to elevated magnesium levels. In the case of D. subspicatus, no significant toxic effects were observed for MOC or its combinations with nanotubes.
Based on these results, we consider the prepared MOC materials to be environmentally safe, with minimal ecological impact. As MWCNT characteristics may vary between organisms, we recommend testing the toxicity of both MWCNT additives and MOC-reinforced composites in specific ecotoxicological assessments before their application.
Conclusion
MOC/MWCNT composites present an eco-friendly alternative to PC-based composites. The proposed composite system is easily synthesizable, manifests high values of mechanical parameters, and, due to the utilization of the functional additive, MWCNTs, it provides sufficient water resistance, which is a crucial application potential-limiting factor in the case of MOC-based binders. Given the intended use in construction, it is critical to study the ecotoxicological impacts of these composites. To address this need, the effects of MOC-MWCNT and MOC-MWCNT-ox on six selected organisms were investigated. The acquired findings confirmed that the characteristics and concentrations of MWCNTs play a crucial role in their interactions with both prokaryotes and eukaryotes. Overall, the addition of MWCNTs to MOC did not dramatically increase inhibitory effects on the tested organisms, though specific differences were observed depending on the organism and MWCNT concentration. Notably, the tested MOC samples displayed strong antibiofilm properties, suggesting low risk of microbial degradation. These results highlight the potential of MWCNT-reinforced MOC as environmentally safe alternatives to traditional building materials. However, it is recommended to verify the toxicity of specific CNTs and their reinforced composites prior to application, as the effects may vary depending on the physicochemical properties of the CNTs, their concentrations, and the characteristics of the target organisms. After this, the upscaling of the MOC-MWCNT and MOC-MWCNT-ox production, and their use in construction applications such as flooring or the fabrication of construction elements, such as panels or specific prefabricated elements, will be possible. This solution will provide a competitive, eco-friendly material able to replace conventional construction composites with increased microbial resistance.Data availability
According to open science principles, data can be found at DOI: https://doi.org/10.5281/zenodo.13935318.Author contributions
Simona Lencova: conceptualization, methodology, investigation, data curation, formal analysis, writing – original draft, writing – review & editing. Jana Kofronova: methodology, investigation, visualization, data curation, formal analysis, writing – original draft. Vaclav Peroutka: methodology, investigation, formal analysis, visualization, writing – original draft, writing – review & editing. Anna-Marie Lauermannova: writing – original draft, investigation, data curation, formal analysis, visualization, writing – review & editing. Adela Jirickova: writing – original draft, investigation, data curation, formal analysis, writing – review & editing. Michal Lojka: investigation, data curation. Ondrej Jankovsky: project administration, writing – original draft, resources, supervision. Radek Vurm: investigation, data curation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Czech Science Foundation (project 23-05194M), while the infrastructure has been utilized in the frame of project No. CZ.02.01.01/00/22_008/0004631. Materials and technologies for sustainable development within the Jan Amos Komensky Operational Program financed by the European Union and from the state budget of the Czech Republic.References
- G. Kastiukas, S. Ruan, C. Unluer, S. Liang and X. Zhou, Environmental Assessment of Magnesium Oxychloride Cement Samples: A Case Study in Europe, Sustainability, 2019, 11(24), 6957 CrossRef CAS.
- S. Tang, Y. Hu, W. Ren, P. Yu, Q. Huang and X. Qi, et al., Modeling on the hydration and leaching of eco-friendly magnesium oxychloride cement paste at the micro-scale, Constr. Build. Mater., 2019, 204, 684–690 CrossRef CAS.
- A. Singh, R. Kumar and P. Goel, Factors influencing strength of magnesium oxychloride cement, Constr. Build. Mater., 2021, 303, 124571 CrossRef CAS.
- M. Davraz, M. Koru and A. E. Akdağ, The Effect of Mixing Ratios on Physical, Mechanical, and Thermal Properties in Lightweight Composite with Magnesium Oxychloride Cement, Int. J. Thermophys., 2022, 44(1), 3 CrossRef.
- S. Ruan and C. Unluer, Comparison of the Environmental Impacts of Reactive Magnesia and Calcined Dolomite and Their Performance under Different Curing Conditions, J. Mater. Civ. Eng., 2018, 30(11), 04018279 CrossRef.
- I. M. Power, G. M. Dipple and P. S. Francis, Assessing the carbon sequestration potential of magnesium oxychloride cement building materials, Cem. Concr. Compos., 2017, 78, 97–107 CrossRef CAS.
- O. Jankovský, M. Lojka, A.-M. Lauermannová, F. Antončík, M. Pavlíková, Z. Pavlík and D. Sedmidubský, Carbon Dioxide Uptake by MOC-Based Materials, Appl. Sci., 2020, 10(7), 2254 CrossRef.
- C. Chang, L. An, J. Dong, W. Zheng, J. Wen, F. Yan and X. Xiao, Study on Deterioration Process of Magnesium Oxychloride Cement under the Environment of Dry–Wet Cycles, Materials, 2023, 16(5), 1817 CrossRef CAS PubMed.
- M. Xu, X. Chen and L. Han, Effect of tartaric acid on the early hydration process and water resistance of magnesium oxychloride cement, J. Build. Eng., 2023, 66, 105838 CrossRef.
- J. Sun, Z. Song, Y. Zhang, Y. Zhang, S. Zhao, M.-Z. Guo and L. Jiang, Effect of red mud and phosphate on water resistance and hydration mechanism of magnesium oxychloride cement, Constr. Build. Mater., 2024, 413, 134844 CrossRef CAS.
- Y. Xie, H. Wang, Y. Guo, C. Wang, H. Cui and J. Xue, Mechanical performance and water resistance of biochar admixture lightweight magnesium oxychloride cement, Sci. Total Environ., 2024, 912, 168773 CrossRef CAS PubMed.
- S. Zhao, Z. Song, Y. Zhang, Y. Zhang, J. Sun and M.-z. Guo, et al., Utilization of phosphogypsum and phosphate in improving water resistance of magnesium oxychloride cement, Constr. Build. Mater., 2024, 444, 137777 CrossRef CAS.
- M. Xu, Y. Bu, J. Du, L. Zhao, A. Zhou, Y. Zhang and Z. Lu, Magnesium oxychloride cement with hydrophobic pore network for utilizing as oil well cement, Constr. Build. Mater., 2023, 409, 133745 CrossRef CAS.
- O. Jankovský, M. Lojka, A.-M. Lauermannová, F. Antončík, M. Pavlíková and M. Záleská, et al., Towards novel building materials: High-strength nanocomposites based on graphene, graphite oxide and magnesium oxychloride, Appl. Mater. Today, 2020, 20, 100766 CrossRef.
- Z. Ali, S. Yaqoob, J. Yu and A. D'Amore, Critical review on the characterization, preparation, and enhanced mechanical, thermal, and electrical properties of carbon nanotubes and their hybrid filler polymer composites for various applications, Compos., Part C: Open Access, 2024, 13, 100434 Search PubMed.
- P. Zhang, J. Su, J. Guo and S. Hu, Influence of carbon nanotube on properties of concrete: A review, Constr. Build. Mater., 2023, 369, 130388 CrossRef CAS.
- M. Lojka, A.-M. Lauermannová, D. Sedmidubský, M. Pavlíková, M. Záleská and Z. Pavlík, et al., Magnesium Oxychloride Cement Composites with MWCNT for the Construction Applications, Materials, 2021, 14(3), 484 CrossRef CAS PubMed.
- A. Jiříčková, A.-M. Lauermannová, O. Jankovský, M. Lojka, M. Záleská and A. Pivák, et al., Impact of nano-dopants on the mechanical and physical properties of magnesium oxychloride cement composites – Experimental assessment, J. Build. Eng., 2024, 87, 108981 CrossRef.
- Effect of carbon nanotubes (CNTs) on the physicomechanical properties of magnesium oxychloride cement pastes, Proceedings of the International Conference On Nano-Technology for Green and Sustainable Construction, ed. A. Kandeel, M. Etman, A. Sharara, W. Sufe and S. Shebl, Cairo, Egypt, 2010 Search PubMed.
- A.-M. Lauermannová, O. Jankovský, M. Lojka, I. Faltysová, J. Slámová and M. Pavlíková, et al., Co-Doped Magnesium Oxychloride Composites with Unique Flexural Strength for Construction Use, Materials, 2022, 15(2), 604 CrossRef PubMed.
- M. Chen, Y. Sun, J. Liang, G. Zeng, Z. Li and L. Tang, et al., Understanding the influence of carbon nanomaterials on microbial communities, Environ. Int., 2019, 126, 690–698 CrossRef CAS PubMed.
- S. Liu, L. Wei, L. Hao, N. Fang, M. W. Chang and R. Xu, et al., Sharper and Faster “Nano Darts” Kill More Bacteria: A Study of Antibacterial Activity of Individually Dispersed Pristine Single-Walled Carbon Nanotube, ACS Nano, 2009, 3(12), 3891–3902 CrossRef CAS PubMed.
- R. Al-Gaashani, M. Pasha, K. A. Jabbar, A. R. Shetty, H. Baqiah and S. Mansour, et al., Antimicrobial activity of ZnO-Ag-MWCNTs nanocomposites prepared by a simple impregnation–calcination method, Sci. Rep., 2023, 13(1), 21418 CrossRef CAS.
- X. Fan, J. Xu, M. Lavoie, W. J. G. M. Peijnenburg, Y. Zhu and T. Lu, et al., Multiwall carbon nanotubes modulate paraquat toxicity in Arabidopsis thaliana, Environ. Pollut., 2018, 233, 633–641 CrossRef CAS.
- M. A. Saleemi, M. H. Fouladi, P. V. Yong and E. H. Wong, Elucidation of Antimicrobial Activity of Non-Covalently Dispersed Carbon Nanotubes, Materials, 2020, 13(7), 1676 CrossRef CAS PubMed.
- S. Martel Martín, R. Barros, B. Domi, C. Rumbo, M. Poddighe and S. Aparicio, et al., Low Toxicological Impact of Commercial Pristine Multi-Walled Carbon Nanotubes on the Yeast Saccharomyces cerevisiae, Nanomaterials, 2021, 11(9), 2272 CrossRef.
- A. Nel, T. Xia, L. Mädler and N. Li, Toxic Potential of Materials at the Nanolevel, Science, 2006, 311(5761), 622–627 CrossRef CAS PubMed.
- S. Kang, M. Pinault, L. D. Pfefferle and M. Elimelech, Single-Walled Carbon Nanotubes Exhibit Strong Antimicrobial Activity, Langmuir, 2007, 23(17), 8670–8673 CrossRef CAS PubMed.
- F. Abbasian, R. Lockington, T. Palanisami, M. Megharaj and R. Naidu, Multiwall carbon nanotubes increase the microbial community in crude oil contaminated fresh water sediments, Sci. Total Environ., 2016, 539, 370–380 CrossRef CAS PubMed.
- Z. Yang, C. Deng, Y. Wu, Z. Dai, Q. Tang and C. Cheng, et al., Insights into the mechanism of multi-walled carbon nanotubes phytotoxicity in Arabidopsis through transcriptome and m6A methylome analysis, Sci. Total Environ., 2021, 787, 147510 CrossRef CAS PubMed.
- M. Hong, J.-L. Gong, W.-C. Cao, R. Fang, Z. Cai and J. Ye, et al., The combined toxicity and mechanism of multi-walled carbon nanotubes and nano zinc oxide toward the cabbage, Environ. Sci. Pollut. Res., 2022, 29(3), 3540–3554 CrossRef CAS PubMed.
- J. K. Stanley, J. G. Laird, A. J. Kennedy and J. A. Steevens, Sublethal effects of multiwalled carbon nanotube exposure in the invertebrate Daphnia magna, Environ. Toxicol. Chem., 2016, 35(1), 200–204 CrossRef PubMed.
- J. Cheng, C. M. Chan, L. M. Veca, W. L. Poon, P. K. Chan and L. Qu, et al., Acute and long-term effects after single loading of functionalized multi-walled carbon nanotubes into zebrafish (Danio rerio), Toxicol. Appl. Pharmacol., 2009, 235(2), 216–225 CrossRef CAS PubMed.
- K. Rezaei Tavabe, M. Yavar, S. Kabir, P. Akbary and Z. Aminikhoei, Toxicity effects of multi-walled carbon nanotubes (MWCNTs) nanomaterial on the common carp (Cyprinus carpio L. 1758) in laboratory conditions, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2020, 237, 108832 Search PubMed.
- L. Qin, Q. Huang, Z. Wei, L. Wang and Z. Wang, The influence of hydroxyl-functionalized multi-walled carbon nanotubes and pH levels on the toxicity of lead to Daphnia magna, Environ. Toxicol. Pharmacol., 2014, 38(1), 199–204 CrossRef CAS PubMed.
- X. Wang, R. Qu, J. Liu, Z. Wei, L. Wang and S. Yang, et al., Effect of different carbon nanotubes on cadmium toxicity to Daphnia magna: The role of catalyst impurities and adsorption capacity, Environ. Pollut., 2016, 208, 732–738 CrossRef CAS PubMed.
- F. Zindler, B. Glomstad, D. Altin, J. Liu, B. M. Jenssen and A. M. Booth, Phenanthrene Bioavailability and Toxicity to Daphnia magna in the Presence of Carbon Nanotubes with Different Physicochemical Properties, Environ. Sci. Technol., 2016, 50(22), 12446–12454 Search PubMed.
- C. Hu, Y. Cai, W. Wang, Y. Cui and M. Li, Toxicological effects of multi-walled carbon nanotubes adsorbed with nonylphenol on earthworm Eisenia fetida, Environ. Sci.: Processes Impacts, 2013, 15(11), 2125–2130 RSC.
- W. Wang, X. Zhao, X. Ren and X. Duan, Antagonistic effects of multi-walled carbon nanotubes and BDE-47 in zebrafish (Danio rerio): Oxidative stress, apoptosis and DNA damage, Aquat. Toxicol., 2020, 225, 105546 CrossRef CAS.
- M. C. Martínez-Ballesta, L. Zapata, N. Chalbi and M. Carvajal, Multiwalled carbon nanotubes enter broccoli cells enhancing growth and water uptake of plants exposed to salinity, J. Nanobiotechnol., 2016, 14(1), 42 CrossRef.
- A.-M. Lauermannová, M. Pavlíková, Z. Pavlík, A. Pivák, A. Jiříčková and J. Sklenka, et al., Magnesium oxychloride cement with phase change material: Novel environmentally-friendly composites for heat storage, J. Mater. Res. Technol., 2022, 21, 3327–3342 CrossRef.
- A. Jiříčková, A.-M. Lauermannová, O. Jankovský, J. Fathi, M. Záleská and A. Pivák, et al., Utilization of waste carbon spheres in magnesium oxychloride cement, Case Stud. Constr. Mater., 2023, 19, e02374 Search PubMed.
- S. Lencova, M. Stindlova, K. Havlickova, V. Jencova, V. Peroutka and K. Navratilova, et al., Influence of Fiber Diameter of Polycaprolactone Nanofibrous Materials on Biofilm Formation and Retention of Bacterial Cells, ACS Appl. Mater. Interfaces, 2024, 16(20), 25813–25824 CrossRef CAS PubMed.
- K. Weise, L. Winter, E. Fischer, D. Kneis, M. C. Barron and S. Kunze, et al., Multiwalled Carbon Nanotubes Promote Bacterial Conjugative Plasmid Transfer, Microbiol. Spectrum, 2022, 10(2), e00410–e00422 Search PubMed.
- B. Kramer, J. Thielmann, A. Hickisch, P. Muranyi, J. Wunderlich and C. Hauser, Antimicrobial activity of hop extracts against foodborne pathogens for meat applications, J. Appl. Microbiol., 2015, 118(3), 648–657 CrossRef CAS.
- M. Gomes, L. C. Gomes, R. Teixeira-Santos, M. F. R. Pereira, O. S. G. P. Soares and F. J. Mergulhão, Carbon nanotube-based surfaces: Effect on the inhibition of single- and dual-species biofilms of Escherichia coli and Enterococcus faecalis, Results Surf. Interfaces, 2022, 9, 100090 CrossRef.
- S. Lencova, H. Stiborova, M. Munzarova, K. Demnerova and K. Zdenkova, Potential of Polyamide Nanofibers With Natamycin, Rosemary Extract, and Green Tea Extract in Active Food Packaging Development: Interactions With Food Pathogens and Assessment of Microbial Risks Elimination, Front. Microbiol., 2022, 13, 857423 CrossRef.
- R. Dubey, D. Dutta, A. Sarkar and P. Chattopadhyay, Functionalized carbon nanotubes: synthesis, properties and applications in water purification, drug delivery, and material and biomedical sciences, Nanoscale Adv., 2021, 3(20), 5722–5744 RSC.
- T. Mesarič, C. Gambardella, T. Milivojević, M. Faimali, D. Drobne and C. Falugi, et al., High surface adsorption properties of carbon-based nanomaterials are responsible for mortality, swimming inhibition, and biochemical responses in Artemia salina larvae, Aquat. Toxicol., 2015, 163, 121–129 CrossRef.
- A.-F. A. Trompeta, I. Preiss, F. Ben-Ami, Y. Benayahu and C. A. Charitidis, Toxicity testing of MWCNTs to aquatic organisms, RSC Adv., 2019, 9(63), 36707–36716 RSC.
- P. Jackson, N. R. Jacobsen, A. Baun, R. Birkedal, D. Kühnel and K. A. Jensen, et al., Bioaccumulation and ecotoxicity of carbon nanotubes, Chem. Cent. J., 2013, 7(1), 154 CrossRef.
- S. Kang, J.-E. Kim, D. Kim, C. G. Woo, P. V. Pikhitsa, M.-H. Cho and M. Choi, Comparison of cellular toxicity between multi-walled carbon nanotubes and onion-like shell-shaped carbon nanoparticles, J. Nanopart. Res., 2015, 17(9), 378 CrossRef.
- D. Lin and B. Xing, Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth, Environ. Pollut., 2007, 150(2), 243–250 CrossRef CAS.
- A. Mondal, R. Basu, S. Das and P. Nandy, Beneficial role of carbon nanotubes on mustard plant growth: an agricultural prospect, J. Nanopart. Res., 2011, 13(10), 4519–4528 CrossRef CAS.
- S. Mathew, D. K. Tiwari and D. Tripathi, Interaction of carbon nanotubes with plant system: a review, Carbon Lett., 2021, 31(2), 167–176 CrossRef.
- O. Zaytseva, Z. Wang and G. Neumann, Phytotoxicity of carbon nanotubes in soybean as determined by interactions with micronutrients, J. Nanopart. Res., 2017, 19(2), 29 Search PubMed.
- A. Kumar, K. Kaladharan and F.-G. Tseng, Nanomaterials: Surface Functionalization, Modification, and Applications, in Nanomaterials and Their Biomedical Applications, ed. T. S. Santra and L. Mohan, Springer Singapore, Singapore, 2021, pp. 405–38 Search PubMed.
- D. Pantarotto, J.-P. Briand, M. Prato and A. Bianco, Translocation of bioactive peptides across cell membranes by carbon nanotubes, Chem. Commun., 2004, 16–17 RSC.
- M. M. Pereira, L. Mouton, C. Yéprémian, A. Couté, J. Lo and J. M. Marconcini, et al., Ecotoxicological effects of carbon nanotubes and cellulose nanofibers in Chlorella vulgaris, J. Nanobiotechnol., 2014, 12(1), 15 CrossRef PubMed.
- S. Orlanducci, G. Fulgenzi, A. Margonelli, G. Rea, T. K. Antal and M. D. Lambreva, Mapping Single Walled Carbon Nanotubes in Photosynthetic Algae by Single-Cell Confocal Raman Microscopy, Materials, 2020, 13(22), 5121 CrossRef CAS PubMed.
- O. A. Mendoza Reales, Y. P. Arias Jaramillo, J. C. Ochoa Botero, C. A. Delgado, J. H. Quintero and R. D. Toledo Filho, Influence of MWCNT/surfactant dispersions on the rheology of Portland cement pastes, Cem. Concr. Res., 2018, 107, 101–109 CrossRef CAS.
- J. Yu, N. Grossiord, C. E. Koning and J. Loos, Controlling the dispersion of multi-wall carbon nanotubes in aqueous surfactant solution, Carbon, 2007, 45(3), 618–623 CrossRef CAS.
- T. Manzur and N. Yazdani, Strength Enhancement of Cement Mortar with Carbon Nanotubes: Early Results and Potential, Transp. Res. Rec., 2010, 2142(1), 102–108 Search PubMed.
- C. Gaylarde, M. Ribas Silva and T. Warscheid, Microbial impact on building materials: an overview, Mater. Struct., 2003, 36(5), 342–352 CrossRef CAS.
- A. Maier and D. L. Manea, Perspective of Using Magnesium Oxychloride Cement (MOC) and Wood as a Composite Building Material: A Bibliometric Literature Review, Materials, 2022, 15(5), 1772 CrossRef CAS PubMed.
- Q. Jin and M. F. Kirk, pH as a Primary Control in Environmental Microbiology: 1. Thermodynamic Perspective, Front. Environ. Sci., 2018, 6, 21 CrossRef.
- F. Ahmed, Z. A. Mirani, P. N. Mirani, M. J. Imdad, F. Z. Khan and M. N. Khan, et al., Pseudomonas aeruginosa Response to Acidic Stress and Imipenem Resistance, Appl. Sci., 2022, 12(16), 8357 CrossRef CAS.
- T. Gonçalves and U. Vasconcelos, Colour Me Blue: The History and the Biotechnological Potential of Pyocyanin, Molecules, 2021, 26(4), 927 CrossRef.
- A. A. Abdelaziz, A. M. A. Kamer, K. B. Al-Monofy and L. A. Al-Madboly, Pseudomonas aeruginosa's greenish-blue pigment pyocyanin: its production and biological activities, Microb. Cell Fact., 2023, 22(1), 110 CrossRef CAS.
- S. Lencova, M. Stindlova, K. Havlickova, V. Jencova, V. Peroutka and K. Navratilova, et al., Influence of Fiber Diameter of Polycaprolactone Nanofibrous Materials on Biofilm Formation and Retention of Bacterial Cells, ACS Appl. Mater. Interfaces, 2024, 25813–25824 CrossRef CAS.
- J. Nobre, H. Ahmed, M. Bravo, L. Evangelista and J. de Brito, Magnesia (MgO) Production and Characterization, and Its Influence on the Performance of Cementitious Materials: A Review, Materials, 2020, 13(21), 4752 CrossRef PubMed.
- Y. Tan, C. Wu, H. Yu, Y. Li and J. Wen, Review of reactive magnesia-based cementitious materials: Current developments and potential applicability, J. Build. Eng., 2021, 40, 102342 Search PubMed.
- S. Zhu, F. Luo, X. Tu, W.-C. Chen, B. Zhu and G.-X. Wang, Developmental toxicity of oxidized multi-walled carbon nanotubes on Artemia salina cysts and larvae: Uptake, accumulation, excretion and toxic responses, Environ. Pollut., 2017, 229, 679–687 CrossRef CAS PubMed.
- M. Hauer-Jákli and M. Tränkner, Critical Leaf Magnesium Thresholds and the Impact of Magnesium on Plant Growth and Photo-Oxidative Defense: A Systematic Review and Meta-Analysis From 70 Years of Research, Front. Plant Sci., 2019, 10, 766 CrossRef PubMed.
- F. J. Romera, P. Lan, J. Rodríguez-Celma and R. Pérez-Vicente, Nutrient Interactions in Plants, Front. Plant Sci., 2021, 12, 782505 CrossRef.
- E. Navarro, A. Baun, R. Behra, N. B. Hartmann, J. Filser and A.-J. Miao, et al., Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi, Ecotoxicology, 2008, 17(5), 372–386 CrossRef CAS.
- F. Ahmad, S. Rawat and Y. Zhang, Magnesium Oxychloride Cement: Development, Opportunities and Challenges, Appl. Sci., 2024, 14(7), 3074 CrossRef CAS.
- A.-M. Lauermannová, M. Lojka, M. Záleská, M. Pavlíková, A. Pivák and Z. Pavlík, et al., Magnesium oxychloride cement-based composites for latent heat storage: The effect of the introduction of multi-walled carbon nanotubes, J. Build. Eng., 2023, 72, 106604 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Verification of MOC-REF, MOC-MWCNT, and MOC-MWCNT-ox sterility (Fig. S1); P. aeruginosa suspension after the incubation with MOC materials (Fig. S2). See DOI: https://doi.org/10.1039/d4en01088d |
| This journal is © The Royal Society of Chemistry 2025 |