Reversible metal–organic polymer template for enhanced platinum nanoparticle self-assemblies and accelerated POD-like catalysis for the rapid and ultrasensitive detection of multiple forms of mercury†
Received
26th August 2024
, Accepted 19th September 2024
First published on 24th September 2024
Abstract
Monitoring the distribution characteristics of the various forms of mercury is crucial for understanding its biogeochemical cycling and assessing its health risks. The accurate detection of inorganic and organic mercury still relies on techniques requiring large instruments such as CVAAS, ICP-MS, and LC-ICP-MS. Although these traditional methods can accurately analyze and differentiate the different forms of mercury, their complexity, high cost, and inability to facilitate immediate on-site analysis at environmental sample locations represent significant limitations. Therefore, based on a reversible metal–organic polymer template for platinum nanoenzymes, we developed a colorimetric detection method that does not require sample pretreatment to rapidly and sensitively detect mercury in multiple forms. This method detected Hg2+ within a range of 0.001–5000 nM in 3 min, with a detection limit of 0.0006 nM (equivalent to 0.12 ng L−1). Additionally, the total mercury content in samples could be quantified by this colorimetric method, enabling the precise analysis of organic mercury with an accuracy on par with traditional ICP-MS. Notable enhancements in sensitivity and analysis speed were demonstrated, which could be attributed to the distinct molecular structure of the PNAs(HCl). Studies on the reaction mechanisms revealed that the dense assembly of platinum nanoparticles within PNAs(HCl) and their porous external structure not only effectively protect the catalytic active sites and offer ample reaction space, thereby efficiently mimicking the POD-like enzymatic activity, but also facilitate effective and specific binding to Hg2+. To further affirm the reliability and practicality of this analysis method, a variety of real environmental water samples were evaluated, including from rivers, lakes, polluted waters, and garden soil extracts. The analytical data were consistent with those obtained from traditional ICP-MS analysis. Our results demonstrated that the PNAs(HCl)-based colorimetric method offers a rapid and precise technique for the analysis of Hg2+ and organic mercury, making it ideally suited for immediate on-site analysis following environmental sample collection.
Environmental significance
Both organic and inorganic mercury possess persistence, mobility, bioaccumulation, and significant neurotoxicity, making mercury a critical indicator for assessing environmental quality. The ultrasensitive detection of mercury and its chemical states enables the precise identification of the biogeochemical cycling characteristics of mercury in the environment, offering a promising analytical strategy for mercury pollution control and its health risk assessment. Considering the significant environmental impacts and health risks, there is an urgent requirement for innovative technologies that can facilitate the convenient and rapid detection and identification of both inorganic and organic mercury in environmental contexts. Traditionally, qualitative and quantitative mercury detection methods include cold vapor atomic absorption spectroscopy (CVAAS), inductively coupled plasma mass spectrometry (ICP-MS), liquid chromatography-ICP-MS, gas chromatography-mass spectrometry (GC-MS), and liquid chromatography-atomic fluorescence spectrometry (LC-AFS). While these methods offer high precision, they are frequently hindered by complex pre-treatment procedures, costly equipment, the necessity for skilled operators, and considerable mercury memory effects. Therefore, it is essential to develop a simple and convenient method for the rapid detection of mercury in various forms.
|
Introduction
Mercury (Hg), a metal detrimental to humans, animals, plant life, and the broader natural environment, can have pronounced toxic effects on major physiological systems and vital organs.1,2 Mercury exists in the natural environment in various forms, including inorganic mercury (Hg2+, Hg(0)) and organic mercury (MeHg, EtHg, (Me)2Hg+, (Et)2Hg+, PhHg, etc.).3 Hg2+ is the predominant form in water bodies, soils, and sediments, while organic Hg and Hg(0) are the main forms found in biota and the atmosphere, respectively. Hg2+ in the environment can be readily methylated by microbial action into MeHg, which is more lipophilic and toxic.4 Owing to its persistence, mobility, bioaccumulation, and pronounced neurotoxicity, along with its enduring presence in the food chain and direct participation in trans-regional cycles, both organic and inorganic mercury serve as essential indicators for assessing environmental quality.5,6 The mobility, bioavailability, and toxicity of mercury are contingent upon its chemical form. In complex environmental systems, results obtained for total mercury provide limited chemical information about the mercury. Therefore, the development of analytical methods to differentiate mercury species is crucial for understanding their biogeochemical cycles and assessing their toxicity and health risks.
Currently, a variety of methods are available for total mercury detection, including cold vapor atomic absorption spectroscopy (CVAAS), cold vapor atomic fluorescence spectroscopy (CVAFS), inductively coupled plasma mass spectrometry (ICP-MS), and X-ray absorption spectroscopy (XAS).7–10 Additionally, mercury speciation analysis frequently employs chromatographic coupling techniques, such as liquid chromatography-atomic fluorescence spectroscopy (LC-AFS), liquid chromatography-inductively coupled plasma mass spectrometry (LC-ICP-MS), and gas chromatography–mass spectrometry (GC-MS).11,12 Although these analytical techniques enable the precise and sensitive detection of mercury and its species, they necessitate substantial equipment and experienced operators, resulting in elevated testing costs and rendering them impractical for the rapid on-site analysis of environmental samples. Furthermore, these instruments are particularly prone to mercury memory effects, necessitating the specialized pretreatment of samples to ensure analytical accuracy. In contrast, electrochemical analysis and colorimetric methods can serve as alternative analytical approaches for the on-site detection of mercury.13,14 While these techniques offer simple and rapid testing, they are typically limited to detecting Hg2+ and often suffer from lower sensitivity and poor reproducibility.
Nanoenzymes, based on inorganic nanoparticles, are artificially engineered substances that demonstrate stable and efficient natural enzyme-like activities and are widely employed in colorimetric detection.15–17 However, these reported colorimetric methods for Hg detection are limited to Hg2+, and their detection limits have yet to match those of existing conventional analytical techniques. For example, in the standard analysis methods for mercury within the Chinese Surface Water Environmental Quality Standards (GB3838-2002), the prescribed detection techniques are CVAAS or CVAFS, with a detection limit of 0.00005 mg L−1 (equal to 0.25 nM). Therefore, the development of an accurate, trace-level mercury detection method based on colorimetric analysis is critically important for facilitating the practical implementation of convenient, rapid, and precise environmental sample testing.
The sensitivity and detection time of colorimetric methods employing peroxidase-like activity are fundamentally dependent on the catalytic properties of those nanoenzymes.18–20 Hence, this study developed a synthesis approach based on a reversible MOF template, employing the structural changes in MOFs driven by dynamic covalent bonds to produce dense Pt nanoparticles assemblies (PNAs), thereby enhancing their activity as peroxidase-mimicking enzymes. These PNAs form mesoporous nanospheres, whose porous structure not only protects the catalytic active centers but also facilitates substrate binding, improving the colorimetric response sensitivity. Moreover, the high concentration of Pt NPs per unit area ameliorates mercury amalgamation (Pt–Hg) interactions, thereby accelerating the color change process of the chromogenic substrate. In addition to specifically detecting trace Hg2+, this platinum nanoparticle-based colorimetric method, as described in Scheme 1 and following appropriate sample pretreatment, enables the quantification of the total mercury content in samples, thereby achieving accuracy comparable to traditional ICP-MS. Given that the PNAs-based colorimetric method can accurately measure both Hg2+ and total mercury, we conducted Hg2+ and total mercury assessments at six different sample locations across a river basin, analyzing the environmental distribution characteristics of Hg2+ and organic mercury in this segment of the river. Overall, this detection approach using PNAs is an ultrasensitive technique well-suited for the rapid testing of Hg2+ and total mercury, providing an innovative strategy for different mercury speciation analysis in environmental samples.
 |
| | Scheme 1 Schematic illustration of detection process for mercury ions and total mercury in samples. | |
Materials and method
Materials
2,4,6-Tris(4-aminophenyl)-1,3,5-triazine, 4-pyridinecarboxaldehyde, trifluoroacetic acid, N,N-dimethylformamide-d7, and tetramethylbenzidine were purchased from Aldrich (USA). Tetrahydrofuran, potassium tetrachloroplatinate, sodium carbonate, diethyl ether, terephthalic acid, hydrogen peroxide, nitric acid, hydrochloric acid, sulfuric acid, zinc sulfate heptahydrate, potassium chloride, magnesium chloride, ferrous sulfate, manganese sulfate, dihydrate calcium chloride, silver sulfate, sodium chloride, copper chloride dihydrate, cadmium chloride, nickel chloride, lead perchlorate trihydrate, lithium chloride, hexahydrate chromium chloride, and cobalt chloride hexahydrate were purchased from Innochem (Beijing, China).
Preparation and characterization of PNAs(HCl)
Imine-traizine-benzylpyridine derivative was synthesized using 2,4,6-tris(4-aminophenyl)-1,3,5-triazine, 4-pyridylaldehyde (1.32 mL, 14.1 mmol) and hydrochloric acid (HCl) as described previously.21 The imine-triazine-benzylpyridine derivative (0.01 mmol) and hydrochloric acid (4.125 μL) were dissolved in 5 mL of DMF. Moreover, potassium tetrachloroplatinate (0.03 mmol) was dissolved in 5 mL of deionized water. These two solutions were combined in a stainless-steel reactor and incubated at 100 °C for 12 h. The precipitates were isolated by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, washed three times with DMF and water, and subsequently dried under vacuum to yield the final product.
000 rpm, washed three times with DMF and water, and subsequently dried under vacuum to yield the final product.
The features of the platinum nanoparticles assemblies (PNAs(HCl)) were extensively studied. Transmission electron microscopy (TEM) analysis was performed using a JEOL JEM-2100F microscope operated at 200 kV to determine the morphology. The surface morphology and elemental composition were assessed using a scanning electron microscopy system equipped with an energy-dispersive X-ray spectroscopy unit (SEM-EDS, Hitachi SU8010). Dynamic light scattering (DLS) (Zetasizer Nano ZS90, Malvern Instruments, UK) was employed to measure the hydrodynamic diameters and zeta potential of the PNAs(HCl). Additionally, the chemical states and elemental compositions were evaluated by X-ray photoelectron spectroscopy (XPS, Axis HSi, Kratos Ltd., UK) and inductively coupled plasma atomic emission spectrometry (ICP-AES). The other chemical properties of PNAs(HCl) were determined by UV-vis-NIR spectrophotometry (Lambda 950, PerkinElmer, UK) and micro infrared spectrophotometry. Nitrogen sorption isotherms and the pore-size distribution were measured at 77 K with a surface area analyzer (TriStar II 3020, Micromeritcs, USA).
POD-like activity of PNAs(HCl)
The peroxidase (POD)-like activity of the platinum nanoparticles assemblies PNAs(HCl) was evaluated using H2O2 or TMB (3,3′,5,5′-tetramethylbenzidine) as substrates. Using H2O2 as a substrate, the reaction involved PNAs (10 μg mL−1) and TMB (0.2 mg mL−1) in HAc-NAc buffer (pH 5.5), with H2O2 concentrations varied from 0.001–0.04 mM (equal to 0.034–1.36 μg mL−1) at 25 °C. In the case of TMB as the substrate, the assay was conducted with H2O2 (0.136 mg mL−1) and various concentrations of TMB (0.04–0.8 mg mL−1). The dynamic changes in absorbance at 652 nm were monitored in a time-scanning mode to quantitatively determine the POD-like activity (n = 3).
Method for calculating the initial reaction rate
The initial reaction rate was determined following the method reported. According to the Lambert–Beer law (eqn (1)), the derivation formulas were as follows (eqn (2) and (3)):| | | (d[c])/(d[t]) = (d[A])/(d[t])1/(εL) | (3) |
where d[c]/d[t] is the initial velocity (v) and d[A]/d[t] was obtained by calculating the slope (k) of the initial absorbance changes with time. Therefore, eqn (3) can be replaced by eqn (4).where ε is the molar absorption coefficient for TMB-derived oxidation products (39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) and L is 1 cm.
000 M−1 cm−1) and L is 1 cm.
The reaction times of the POD-like activity of PNAs(HCl) were determined first with H2O2 or TMB as the substrate. The absorbance determination was performed in 900 s or 600 s.
Method for calculating the apparent kinetic parameters
Based on the data of the initial velocity and the concentrations of the substrate, the apparent kinetic parameters of PNAs(HCl) were obtained from the Michaelis–Menten equation.
Colorimetric and UV-vis analysis of Hg2+
First, 100 μL Hg2+ solution with various concentrations (0.001, 0.005, 0.01, 0.02, 0.04, 0.08, 0.1, 0.5, 5, 50, 500 nM) and 100 μL of PNAs(HCl) solution (50 μg mL−1) were prepared. TMB (0.12 mg mL−1) and H2O2 (2.04 mg mL−1) were dissolved in a pH 4.0 PBS solution. The mixture was reacted at room temperature for 3 min and then centrifugated at 12000 rpm. The supernatant was then collected for analysis. The absorbance at 652 nm was measured by a UV-vis spectrophotometer. The sensitivity value was calculated according to the 3σ/k formula,20 where σ is the standard deviation of 10 blank samples (Fig. S7†) and k is the slope of the standard curve.
Interference study
The interference study was conducted using a UV-vis spectrophotometer to evaluate the specificity of the assay in the presence of various potentially interfering ions, including Zn2+, K+, Mg2+, Fe2+, Mn2+, Ca2+, Ag+, Na+, Cu2+, Cd2+, Ni2+, Co2+, Cr3+, Li+, and Pb2+. The peak of TMB at 652 nm was recorded for a 10 μM Hg2+ solution in the presence of 100 μM of each interfering ion and mixtures of them.
Detection of Hg2+ in actual samples
To further determine the capability of PNAs(HCl) in the Hg2+ detection of complex samples with the PNAs-based detection method, various actual samples, including water from the Beijiang, Dongjiang, and Xijiang tributaries, and estuary water of the river, an artificial lake water, and soil were selected for analysis. In the standard protocol, 100 μL of the actual sample was filtered with a 0.22 μm needle filter, and 100 μL each of PNAs(HCl) solution (50 μg mL−1), 0.5 mM TMB, and 60 mM H2O2 were mixed in the PBS buffer (pH 4.0), and then incubated in the dark for 3 min. The mixture was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 1 min. The absorbance at 652 nm was measured by a UV-vis spectrophotometer.
000 rpm for 1 min. The absorbance at 652 nm was measured by a UV-vis spectrophotometer.
Detection of total Hg
First, 3 mL of the sample and 0.5 mL H2SO4 (98%) were mixed and heated at 100 °C for 24 h and then neutralized to pH 7.0 by adding 5 M NaOH solution to obtain the treated sample. Next, 100 μL of the treated sample and 100 μL each of PNAs(HCl) solution (50 μg mL−1), TMB (0.12 mg mL−1), and H2O2 (2.04 mg mL−1) were dissolved in a pH 4.0 PBS solution. The mixture was reacted at room temperature for 3 min and centrifugated at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The supernatant was collected and analyzed by a UV-vis spectrophotometer.
000 rpm. The supernatant was collected and analyzed by a UV-vis spectrophotometer.
Results and discussion
Synthesis and mechanism formation of PNAs(HCl)
PNAs(HCl) were synthesized through a hydrothermal method with acid catalysis, utilizing an imine-triazinebenzylpyridine ligand and K2PtCl4.21 The synthesis of the imine-triazinebenzylpyridine ligand involved the condensation of 4-pyridinecarbaldehyde with 2,4,6-tris(4-aminophenyl)-1,3,5-triazine in the presence of hydrochloric acid. A 2D reversible metal–organic polymer was obtained via the reaction with the triazine-imine-type molecular ligand and platinum ions, which exhibited a planar sheet structure in the TEM images (Fig. 1a and S1†). In the presence of HCl, the dynamic covalent imine bonds within the structure of the 2D reversible metal–organic polymer progressively dissociated, leading to the formation of iminium intermediates.22,23 Simultaneously, the coordination bonds between the ligand and the platinum ions progressively diminished in strength. The aldehyde groups released during this process reduced the dispersed Pt(II) into Pt (0) nanoparticles. This transformation was distinctly observed using TEM in a reaction system consisting solely of 4-pyridinecarboxaldehyde and K2PtCl4, thereby confirming the Pt (0) nanoparticles formation (Fig. S2†). Additionally, the 2D reversible metal–organic polymer prompted the planar sheets to curl inward from the edges, providing a template for the assembly of reduced platinum nanoparticles. The Pt (0) nanoparticles, formed in situ, encountered diffusion constraints and were tightly encapsulated by the curling structures of the 2D reversible metal–organic polymer, ultimately resulting in densely packed Pt nanospheres (Fig. 1a).
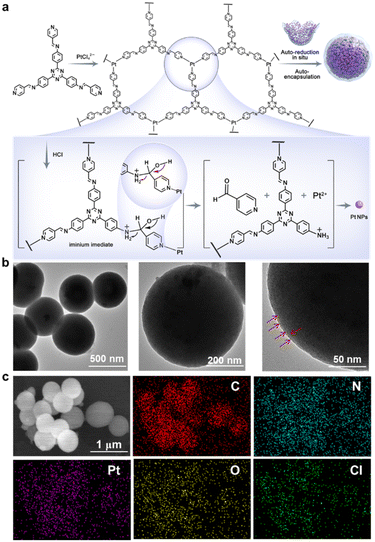 |
| | Fig. 1 Synthesis and characterization of PNAs(HCl). a) Schematic illustration of the process and mechanism of PNAs(HCl) formation. b) Representative TEM images of the as-prepared PNAs(HCl). c) Representative SEM and elemental mapping images of the as-prepared PNAs(HCl). | |
Characterization of the ligand and PNAs(HCl)
The imine-type ligand was characterized by 1H-NMR (Fig. S3†). The transmission electron microscopy (TEM) results demonstrated the formation of well-defined, uniformly sized spherical PNAs(HCl). The high-magnification TEM images showed the platinum nanoparticles (Pt NPs), ranging from 2 to 10 nm, encapsulated within the supporting structure, offering detailed insights into the architecture of the PNAs(HCl) (Fig. 1b). Scanning electron microscopy coupled with energy-dispersive spectroscopy (SEM-EDS) confirmed the morphology and elemental composition, revealing a uniform distribution of Pt, C, N, Cl, and O elements on the surfaces of the PNAs(HCl) (Fig. 1c). Dynamic light scattering (DLS) measurements indicated that the PNAs(HCl) possessed an average particle diameter of 554 ± 13 nm with a zeta potential of −36.1 mV, corresponding with the TEM and SEM findings (Fig. 2a and S4†). X-Ray photoelectron spectroscopy (XPS) provided further chemical characterization details, displaying significant peaks corresponding to O, N, C, Pt, and Cl (Fig. 2b). The Pt content of PNAs(HCl) was quantitatively assessed at 49.54 wt% through inductively coupled plasma atomic emission spectroscopy (ICP-AES). The absorbance of PNAs(HCl) were also obviously observed in the region of UV and visible light (300–700 nm) (Fig. S5†).
 |
| | Fig. 2 Characterization of PNAs(HCl). a) DLS pattern of PNAs(HCl). b) XPS spectra of PNAs(HCl). c) Initial absorbance changes with time at different concentrations of H2O2 in the presence of PNAs (10 μg mL−1), TMB (0.2 mg mL−1) in HAc–NaAc buffer at pH 5.5 and 25 °C. d) Michaelis–Menten curve of the POD-like activity of PNAs(HCl) using H2O2 as a substrate. e) Initial absorbance changes with time at different concentrations of TMB in the presence of PNAs (10 μg mL−1), H2O2 (0.34 mg mL−1) in HAc–NaAc buffer at pH 5.5 and 25 °C. f) Michaelis–Menten curve of the POD-like activity of PNAs(HCl) using TMB as a substrate. g) N2 adsorption–desorption isotherms of PNAs(HCl) and the corresponding surface area and pore size. h) N2 adsorption–desorption isotherms of PNAs(TPA) with terephthalic acid as a catalyst and the corresponding surface area and pore size. | |
POD enzyme-mimicking activity of PNAs(HCl)
To assess the ability of PNAs(HCl) to emulate the activity of peroxidase (POD), we utilized substrates commonly engaged by these enzymes under physiological conditions. Peroxidase (POD), a biological enzyme, plays a crucial role in detoxifying H2O2 into H2O. Typically, 3,3′,5,5′-tetramethylbenzidine (TMB) has been used as a chromogenic substrate for assessing POD enzyme activity. In the enzymatic reaction, POD catalyzes the conversion of H2O2 into H2O, simultaneously oxidizing TMB from a colorless state to a blue oxidized form (TMBox), which exhibits an absorption band at 652 nm. We compared the POD-like activity of PNAs(HCl) with H2O2 as the substrate and TMB as the chromogenic indicator. We found that the reaction rates of PNAs(HCl) increased proportionally with the substrate concentration. Subsequently, leveraging the Michaelis–Menten kinetics, we analyzed the catalytic activity of PNAs(HCl) using H2O2 and TMB as substrates (Fig. 2c and d). The kinetic analysis for both substrates yielded Michaelis constants (Km) of 2.66 mM for H2O2 and 12.1 mM for TMB, with maximum reaction velocities (Vmax) of 6.91 × 10−8 M S−1 and 8.48 × 10−8 M S−1, respectively (Fig. 2e and f). We next evaluated the catalytic performance of PNAs(HCl) relative to previously published PNAs(TPA) that employed terephthalic acid as a synthetic catalyst. Enzymatic kinetic analysis demonstrated that PNAs(HCl) displayed an obviously higher substrate affinity (2.66 nM) compared to PNAs(TPA) (3.53 mM) when using hydrogen peroxide as a substrate, along with an enhanced reaction velocity (Vmax/PNAs (HCl): 6.91 × 10−8 M S−1; Vmax/PNAs (TPA): 2.58 × 10−8 M S−1). To elucidate this phenomenon, Brunauer–Emmett–Teller (BET) N2 adsorption–desorption measurements were performed to analyze the molecular structure of both types of PNAs. Fig. 2g illustrates that the surface area of the PNAs(HCl) was found to be 35.06 m2 g−1, with a pore size of 22.93 nm. In contrast, PNAs(TPA) displayed a surface area of only 27.95 m2 g−1, and a pore size of 13.76 nm (Fig. 2h). These data indicated that PNAs(HCl) had a greater surface area and larger pore sizes. A larger specific surface area reveals an increase in reactive sites per unit area, while larger pore sizes enable the molecular structure to accommodate more substrate, thereby increasing the substrate affinity and catalytic reaction velocity. The enhanced peroxidase-mimetic catalytic performance of PNAs(HCl) thus has the potential to significantly improve both the sensitivity and the rapidity of the colorimetric Hg2+ detection method.
Sensitivity of Hg2+ detection with PNAs(HCl)
The sensitivity of PNAs(HCl) as a Hg2+ detection probe was evaluated under optimized conditions. With varied concentrations of 0–5000 nM Hg2+, an inverse relationship was observed between the Hg2+concentration and the absorption peak of TMB at 652 nm, which decreased as the Hg2+ concentration increased (Fig. 3a). A robust linear correlation, evidenced by the linear regression equation y = −1.01x + 0.5341 with the correlation coefficient of 0.99, was established between the Hg2+concentration (0.001–0.1 nM) and the absorption maxima at 652 nm (Fig. 3b). According to the 3σ/k, the detection limit was calculated to be 0.0006 nM (equal to 0.12 ng L−1), obviously lower than the maximum permissible concentration of Hg2+ in drinking water set by the United States Environmental Protection Agency (US EPA) at 10 nM. Furthermore, the process from TMB color development to Hg2+ introduction for detection necessitated no further sample treatment and was completed within 3 min, enabling rapid instrumental analysis and immediate data acquisition with optimal detection stability (RSD ≤ 3%, Fig. S6†). To elucidate the distinct characteristics of the Hg2+ detection method utilizing PNAs(HCl), an analytical comparison was performed with other Hg2+ detection methodologies reported with precious metals (Table 1).13,18–20,24–29 This investigation highlights that our approach provides notable advantages in terms of the sensitivity and detection time, and it was validated across a range of environmental samples. Overall, the reasonable detection range, sensitive detection limit, and rapid assay duration make the PNAs(HCl) method exceptionally well-suited for the rapid and sensitive analysis of Hg2+ in environmental samples.
 |
| | Fig. 3 Sensitivity and specificity of Hg2+ detection with PNAs(HCl). a) UV-visible spectra of H2O2, TMB, PNAs(HCl) with various concentrations of Hg2+ (0.001–5000 nM). b) Plot of the absorption at 652 nm with different concentrations of Hg2+; inset shows the linear response curve for Hg2+. c) Photograph depicting the color progression with various metal ions in the TMB-H2O2 reaction system (pH 4.0) catalyzed by PNAs(HCl) at 25 °C. d) A652 values in the TMB-H2O2 reaction system (pH 4.0) catalyzed by PNAs(HCl) in the presence of Hg2+ or other interfering ions (Zn2+, K+, Mg2+, Fe2+, Mn2+, Ca2+, Ag+, Na+, Cu2+, Cd2+, Ni2+, Co2+, Cr3+, Li+, Pb2+), where A0 and A represent the A652nm values generated by PNAs(HCl) alone and PNAs(HCl) with the respective metal ions, respectively. | |
Table 1 Comparison of some previously reported methods with the present method for Hg2+ detection (n = 3)
| Method |
Matrix |
Selectivity |
LOD (nM) |
Recovery (%) |
Time (min) |
Ref. |
| PNAs(HCl) (nanozyme) colorimetric |
River |
Zn2+, K+, Mg2+, Fe2+, Mn2+, Ca2+, Ag+, Na+, Cu2+, Cd2+, Ni2+, Co2+, Cr3+, Li+, Pb2+ |
0.0006 |
94.03–102.63 |
3 |
This work
|
| Lake water |
| Soil |
| PtNPs@UiO 66-NH2 (nanozyme) colorimetric |
Tap water |
N.A |
0.35 |
97.2–110.6 |
15 |
18
|
| Pt NPs (nanozyme) colorimetric |
Tap water |
K+, Ca2+, Ba2+, Cr3+, Ca2+, Mg2+, Mn2+, Co2+, Ni2+, Cu2+, Zn2+, Fe3+, Fe2+, Pb2+ |
0.0085 |
96.57–101.88 |
10 |
19
|
| OEG-AuNPs (nanozyme) colorimetric |
Bottled and tap water |
K+, Zn2+, Na+, Mn2+, Ag+, Al3+, Co2+, Ca2+, Fe2+, Mg2+, Bi3+, Li+, Sn4+ |
50 |
76–124 |
45 |
20
|
| Seawater |
| Cu2O/Pt cube (nanozyme) colorimetric |
Waste water |
K+, Na+, Ag+, Ni2+, Co2+, Mg2+, Mn2+, Pb2+, Zn2+, Cd2+, Ba2+, Cu2+, Cr3+, Fe3+, Al3+ |
0.56 |
99.30–104.33 |
20 |
24
|
| NiSe2 (nanozyme) colorimetric |
Drinking water |
K+, Na+, Li+, Ba2+, Ca2+, Cd2+, Co2+, Cu2+, Fe2+, Mg2+, Ni2+, Pb2+, Zn2+, Al3+, Cr3+, CH3Hg, C2H5Hg |
5.18 |
92.06–112.28 |
15 |
25
|
| Tap water |
| TMB@CMs: (nanozyme) colorimetric |
N.A |
Na+, Ca2+, Ba2+, Cr3+, Al3+, Co2+, Pb2+, Mg2+, Zn2+, Cu2+ |
104 |
NA |
60 |
26
|
| Pt N-rGO |
Waste water |
Cr3+, Co2+, Fe3+, Zn2+, Mn2+, Fe2+, Cd2+, Ag+, Cu2+, Ni2+, Pb2+, Sn2+ |
0.75 |
95.1–99.03 |
N.A |
27
|
| Electro-chemical |
| AuNSt NPs |
Seawater CRM |
Bi3+, Ag+, Sn4+, Co2+, Zn2+, Al3+, Mg2+, Na+, Li+, Ca2+, K+, Mn2+, Fe2+ |
1 |
95.2–104.5 |
20 |
28
|
| Colorimetric/SERS Raman |
| Ag NPs |
Reservoir water |
Zn2+, Al3+, Fe2+, Cu2+, Pb2+,Co2+, Ba2+, Cr3+, Ca2+ |
0.014 |
94.03–102.94 |
N.A |
29
|
| SERS Raman |
Lake water |
| River water |
| (ppy/CpPc-btz)Pt |
River water |
Cd2+, Pb2+ |
3110 |
97.4 |
N.A |
13
|
| Electrode |
| DPASV |
Specificity test
To evaluate the specificity of the PNAs(HCl)-based nanoenzyme toward Hg2+, 15 common interference ions (Zn2+, K+, Mg2+, Fe2+, Mn2+, Ca2+, Ag+, Na+, Cu2+, Cd2+, Ni2+, Co2+, Cr3+, Li+, Pb2+) were selected for their effect on the detection. Despite these ions being present at ten times concentrations higher than that of Hg2+, no significant inhibition of the POD-like activity was observed in the case of PNAs(HCl) for any of the tested interference ions, as depicted in Fig. 3c. The color responses image indicated a minimal change for all the tested interference ions compared to the blank sample. In contrast, the presence of Hg2+ significantly altered the colorimetric reaction of TMB, resulting in the fading of the blue oxidized state of TMB. Furthermore, we conducted a specificity test by mixing Hg2+ with a series of common interference ions in a composite sample. As shown in Fig. 3d, the mixture of common interference ions demonstrated negligible influence on the Hg2+ sensing. Due to the unique ability of Hg to engage in amalgamation reactions with noble metals, such as Pt, a distinctive Pt–Hg interaction was established on the surface of Pt NPs, which was not exhibited by the other metal ions.
Mechanism of Hg detection with PNAs(HCl) POD-like activity
This rapid and highly sensitive detection method benefits from the PNAs(HCl) being porous assemblies of Pt NPs, which exhibit significant peroxidase-like activity. As shown in Fig. 4e, the substrate H2O2 dissociated and adsorbed on the surface of the Pt atoms, forming a dihydroxy adsorbed intermediate state. The desorption of HO*/HO* promoted electron transfer to the colorimetric reagent TMB, catalyzing the TMB oxidation from the reduced state (TMBred) to the oxidized state (TMBox), thereby transforming the reaction system from colorless to blue. Owing to its expansive specific surface area and porous architecture, PNAs(HCl) can rapidly adsorb Hg2+ from mercury-containing solutions onto its surface, where Hg2+ undergoes electron transfer on the surface of Pt atoms, further generating Hg(0) and Pt2+. The valence state transitions of Hg and Pt were validated through XPS analysis both pre- and post-testing (Fig. 4a–d). Finally, the Hg(0) transferred electrons back to TMBox, reducing back to the reduced state TMBred, thereby changing the system from blue back to colorless, completing the colorimetric detection process for Hg2+.
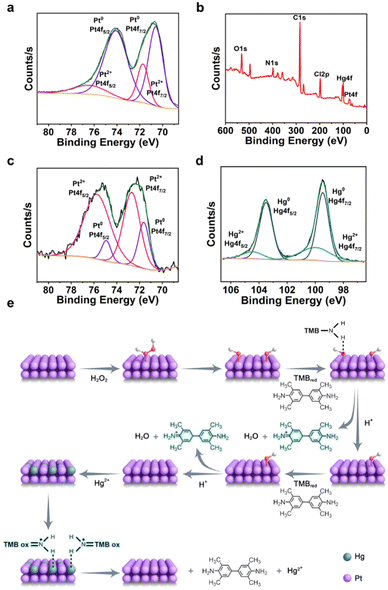 |
| | Fig. 4 Mechanism of Hg detection with PNAs(HCl) POD-like activity. a) XPS spectra of PNAs(HCl) for Pt2+: 4f7/2 and 4f5/2 pink; Pt (0): 4f7/2 and 4f5/2 blue. b) XPS spectra of PNAs(HCl) after being treated with Hg2+. c) Pt(4f) XPS spectra of PNAs(HCl) after being treated with Hg2+. d) Hg(4f) XPS spectra of PNAs(HCl) after being treated with Hg2+. e) Proposed mechanism of POD-like activity of PNAs(HCl), and the TMB decolorization upon the addition of Hg2+. | |
Hg2+ determination in actual samples
The efficiency of Hg2+ detection in actual environmental samples via PNAs(HCl) was verified with various actual samples, including from natural water bodies from the eastern, western, and northern regions, as well as from the estuary of the river, artificial lake water, industrial wastewater, and soil extract. After filtering impurities from the actual samples, Hg2+ detection was performed following the standard procedure based on PNAs(HCl). Subsequently, known quantities of Hg2+ were added to the corresponding actual samples to determine the final concentrations, with each experimental group repeated 3 times. As indicated in Table 2, the spiking of these samples achieved recovery rates ranging from 94% to 104%, with a relative standard deviation (RSD) of ≤10%. These results confirm that the PNAs(HCl)-based colorimetric method satisfies analytical requirements for actual sample testing, demonstrating its ability to detect nearly 100% of Hg2+ with high sensitivity, and also complied with the State Environmental Protection Standards for “quality control methods in water and wastewater monitoring and analysis” (GB/T5750.5-2006), which stipulate spike-recovery rates between 80% and 120%. Consequently, this PNAs(HCl)-based colorimetric method provides a convenient, rapid, and highly sensitive method for trace levels of Hg2+ measurement in actual environmental analysis. Furthermore, we collected surface water samples from a river basin and performed Hg2+ analyses to evaluate the environmental analytical efficacy of the PNAs(HCl)-based colorimetric method. Hg2+ concentrations at the S1 location on the north river, S2 and S3 on the west river, S4 and S5 on the east river, and S6 as estuaries ranged from 0.03168–0.6142 nM. The distribution of Hg2+concentrations across these six sample locations indicated that contamination was predominantly concentrated in river channels associated with urban and industrial areas (west river and east river), while the upstream site on the north river at S1 exhibited relatively lower Hg2+ concentrations (Fig. S8†).
Table 2 Hg2+ concentration and sensitivity of the PNAs(HCl)-based colorimetric method in different sample locations of the river (S1–S6), artificial lake water, pollution water, and garden soil extract. Validation was conducted to assess the relative standard deviation (RSD) (%) and% recovery (n = 3)
| Sample |
Hg2+ by PNAs(HCl)-based colorimetric method (nM) |
Added (nM) |
Spiked (nM) |
Recovery (%) |
RSD (%) |
| S1 |
0.03168 |
0.05 |
0.08168 |
102.63 |
9.91 |
| S2 |
0.6142 |
1.00 |
1.6142 |
101.0 |
2.57 |
| S3 |
0.1726 |
0.20 |
0.3726 |
103.72 |
6.29 |
| S4 |
0.1290 |
0.20 |
0.3290 |
97.79 |
4.31 |
| S5 |
0.1488 |
0.20 |
0.3488 |
101.99 |
4.98 |
| S6 |
0.1152 |
0.20 |
0.3152 |
94.03 |
4.12 |
| Artificial lake |
0.2234 |
0.50 |
0.72234 |
99.54 |
2.50 |
| Pollution water |
0.3264 |
0.50 |
0.8208 |
99.32 |
5.49 |
| Garden soil extract |
0.02310 |
0.05 |
0.07310 |
99.77 |
6.42 |
Mercury speciation analysis
In natural aquatic environments, Hg2+ is readily transformed into organic Hg through microbial activity. Therefore, only measuring the Hg2+ content in samples may fail to provide an accurate representation of the environmental distribution of mercury species. Here, the samples were mixed with concentrated sulfuric acid and heated to convert all the organic Hg in the samples to mercuric sulfate via sulfuric acid's oxidizing properties. Subsequently, total Hg was quantified using the PNAs(HCl)-based colorimetric method and compared with data analyzed by ICP-MS. These data confirmed that the sensitivity and accuracy of the PNAs(HCl)-based colorimetric method were on par with the ICP-MS results, with a relative standard deviation (RSD by the colorimetric method) of ≤5% and a relative standard deviation (RSD by ICP-MS) of ≤17%. Furthermore, we processed and analyzed varied environmental samples, including river water, artificial lake, pollution water, and garden soil extract using the two methods (Table 3).
Table 3 Comparison of the accuracy of total mercury measurement between ICP-MS and PNAs(HCl)-based colorimetric methods. Validation was conducted to assess the relative standard deviation (RSD) (%) and % recovery (n = 3)
| Sample |
Total Hg by ICP-MS (nM) |
Total Hg by PNAs(HCl)-based colorimetric method (nM) |
Accuracya (%) |
RSD by colorimetric method (%) |
RSD by ICP-MS (%) |
Organic Hgb (nM) |
|
Accuracy (%) calculated by total Hg by PNAs(HCl)-based colorimetric method (nM)/total Hg by ICP-MS (nM) × 100%.
Organic Hg (nM) calculated by total Hg by PNAs(HCl)-based colorimetric method (nM) –Hg2+ by PNAs(HCl)-based colorimetric method (nM).
|
| S1 |
1.470 |
1.435 |
97.57 |
5.167 |
9.044 |
1.403 |
| S2 |
2.142 |
2.099 |
97.98 |
2.772 |
1.528 |
1.485 |
| S3 |
1.896 |
1.899 |
100.17 |
4.246 |
5.237 |
1.727 |
| S4 |
2.850 |
2.679 |
93.98 |
2.366 |
11.63 |
2.550 |
| S5 |
1.289 |
1.428 |
110.79 |
4.131 |
8.264 |
1.279 |
| S6 |
1.015 |
0.9928 |
97.80 |
4.675 |
17.25 |
0.8776 |
| Artificial lake |
3.975 |
4.288 |
107.88 |
4.898 |
9.821 |
4.065 |
| Pollution water |
3.411 |
3.214 |
94.21 |
4.02 |
1.050 |
3.693 |
| Garden soil extract |
3.403 |
3.281 |
96.41 |
3.98 |
18.85 |
3.258 |
Due to the discharge of industrial and domestic wastewater, the river samples at S1–S6 are at risk of mercury pollution in the aquatic systems.30 Analysis of the water from the six sample locations within the region revealed total mercury concentrations ranging from 0.9928–2.679 nM (equal to 0.0002–0.0005 mg L−1) based on the PNAs(HCl)-based colorimetric method, thereby classifying these as category IV surface waters in accordance with the Surface Water Environmental Quality Standards (GB3838-2002). The distribution of total mercury indicated a higher total mercury in the main river channels compared to inn the tributaries and estuarial locations (Fig. 5a). Among the six sample locations, S2 and S4 exhibited the highest total mercury content. Notably, S2 had the highest concentration of Hg2+, closely associated with the area's developed marine engineering, construction, and marine chemical industries.31 S4 contained significantly higher levels of organic mercury than the other sample locations, likely due to the environmental conditions more conducive to the biomethylation of mercury ions32 (Fig. 5b, S8 and S9†). Sample location S6, located at the estuary, experiences water exchange where pollutants are diluted by seawater, resulting in lower concentrations of heavy metal ions along the coast compared to nearby areas33 (Fig. 5a and b).
 |
| | Fig. 5 Mean concentrations of total Hg in the six investigated sample locations of the river. a) Total Hg distribution in the six investigated sample locations. b) Relationship between Hg2+, organic Hg, and total Hg concentrations in the six investigated sample locations (n = 3). | |
Conclusions
Based on PNAs(HCl), a rapid and ultrasensitive colorimetric analysis method for Hg was developed, capitalizing on the robust POD-like activity and specific affinity for Hg2+. This method was validated for both the rapid and precise detection of Hg2+ and the total mercury in environmental samples and for the accurate analysis of organic mercury, achieving an accuracy comparable to that of ICP-MS. Consequently, this economical and accurate colorimetric method holds great potential to replace the traditional, expensive, and complex instrumentation currently used in the analysis of mercury speciation in environmental samples, making it more suitable for high-throughput analysis with actual samples. In our study, the focus of the environmental samples analysis was predominantly on the PNAs(HCl)-based colorimetric method validation, without conducting comprehensive monitoring of the environmental samples. Future efforts may extend this method to a more systematic analysis of environmental samples, facilitating a more objective evaluation of mercury speciation distribution within the sampled regions. In conclusion, this PNAs-based colorimetric method will offer a novel analytical tool for investigations of the biogeochemical cycling of mercury and its toxicity and associated health risks evaluation.
Data availability
The data supporting this article have been included in the experimental section of the manuscript and the ESI.† No database or code were utilized in this work.
Author contributions
F. Z.: research design, experiments execution, data analyze, and writing. T. G. and L. F. experiments execution, data analyze. T. G. creates Scheme 1 with https://BioRender.com, and scheme1 has been paid the copyright fee by authors. Y. L. and D. W.: reviewing and discussing. T. L.: research design, writing, reviewing, and editing. All the authors have read and approved the final version of the manuscript.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
This study was financially supported by National Natural Science Foundation of China (No. 22127810); Key Research and Development Program of Guangdong Province, China (2020B1111350003); Guangdong Provincial Laboratory of Chemistry and Fine Chemical Engineering Jieyang foundation (Guangdong Provincial Rongjiang Laboratory, RJDT240008).
References
- M. Yeganeh, M. Afyuni, A. H. Khoshgoftarmanesh, L. Khodakarami, M. Amini, A. R. Soffyanian and R. Schulin, Mapping of human health risks arising from soil nickel and mercury contamination, J. Hazard. Mater., 2013, 244-245, 225–239 CrossRef CAS.
- W. Zhu, Z. Li, P. Li, B. Yu, C. J. Lin, J. Sommar and X. Feng, Re-emission of legacy mercury from soil adjacent to closed point sources of Hg emission, Environ. Pollut., 2018, 242, 718–727 CrossRef CAS.
- L. H. Reyes, J. L. G. Mar, A. Hernández-Ramírez, J. M. Peralta-Hernández, J. M. A. Barbosa and H. M. S. Kingston, Microwave assisted extraction for mercury speciation analysis, Microchim. Acta, 2010, 172, 3–14 CrossRef.
- T. Wang and D. Obrist, Inorganic and methylated mercury dynamics in estuarine water of a salt marsh in Massachusetts, USA, Environ. Pollut., 2022, 294, 118657 CrossRef CAS PubMed.
- B. Gworek, W. Dmuchowski and A. H. Baczewska-Dąbrowska, Mercury in the terrestrial environment: a review, Environ. Sci. Eur., 2020, 32, 128 CrossRef CAS.
- K. R. Mahbub, K. Krishnan, R. Naidu, S. Andrews and M. Megharaj, Mercury toxicity to terrestrial biota, Ecol. Indic., 2017, 74, 451–462 CrossRef CAS.
- S. Kulomaki, S. Peramaki and A. Vaisanen, Addition of thiourea and hydrochloric acid: Accurate nanogram level analysis of mercury in humic-rich natural waters by inductively coupled plasma mass spectrometry, Talanta, 2020, 218, 121125 CrossRef CAS.
- N. Manousi, E. Rosenberg, E. A. Deliyanni and G. A. Zachariadis, Sample Preparation Using Graphene-Oxide-Derived Nanomaterials for the Extraction of Metals, Molecules, 2020, 25, 2411 CrossRef CAS.
- F. Mercader-Trejo, R. Herrera-Basurto, E. R. de San Miguel and J. de Gyves, Mercury determination in sediments by CVAAS after on line preconcentration by solid phase extraction with a sol-gel sorbent containing CYANEX 471X®, Int. J. Environ. Anal. Chem., 2011, 91, 1062–1076 CrossRef CAS.
- A. A. Elezz, H. Mustafa Hassan, H. Abdulla Alsaadi, A. Easa, S. Al-Meer, K. Elsaid, Z. K. Ghouri and A. Abdala, Validation of Total Mercury in Marine Sediment and Biological Samples, Using Cold Vapour Atomic Absorption Spectrometry, Methods Protoc., 2018, 1, 31 CrossRef CAS.
- F. A. Da Silva Cunha, M. J. de Oliveira, P. P. Florez-Rodriguez and J. C. C. Santos, Mercury speciation in estuarine water using dithiol-based magnetic solid-phase extraction and cold vapor atomic fluorescence spectrometry, Spectrochim. Acta, Part B, 2022, 192, 106412 CrossRef CAS.
- H. Shirkhanloo, M. Habibnia, A. Rashidi, A. Faghihi Zarandi and M. Dehghani Mobarake, Simultaneously speciation of mercury in water, human blood and food samples based on pyrrolic and pyridinic nitrogen doped porous graphene nanostructure, Food Chem., 2023, 403, 134394 CrossRef CAS.
- S. Shoba, A. Mambanda and I. N. Booysen, Electrocatalytic detection of Hg(II) using a platinum electrode modified with composite film of cobalt(II) phthalocyanine tetra-substituted with 1-(methoxymethyl)-benzotriazole groups and co-electropolymerized polypyrrole, Int. J. Electrochem. Sci., 2024, 19, 100541 CrossRef CAS.
- G. Aragay, J. Pons and A. Merkoci, Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection, Chem. Rev., 2011, 111, 3433–3458 CrossRef CAS.
- P. Mishra, J. Lee, D. Kumar, R. O. Louro, N. Costa, D. Pathania, S. Kumar, J. Lee and L. Singh, Engineered Nanoenzymes with Multifunctional Properties for Next-Generation Biological and Environmental Applications, Adv. Funct. Mater., 2022, 32, 2108650 CrossRef CAS.
- A. J. Kora and L. Rastogi, Peroxidase activity of biogenic platinum nanoparticles: A colorimetric probe towards selective detection of mercuric ions in water samples, Sens. Actuators, B, 2018, 254, 690–700 CrossRef CAS.
- J. Tao, S. Chen, E. K. Fodjo, W. Deng and D. Li, Tailoring dual-functional gold nanoplasmonic rods for colorimetric and SERS detection of mercury species in complex matrices, Chem. Eng. J., 2023, 452, 139026 CrossRef CAS.
- H. Li, H. Liu, J. Zhang, Y. Cheng, C. Zhang, X. Fei and Y. Xian, Platinum Nanoparticle Encapsulated Metal-Organic Frameworks for Colorimetric Measurement and Facile Removal of Mercury(II), ACS Appl. Mater. Interfaces, 2017, 9, 40716–40725 CrossRef CAS PubMed.
- G. W. Wu, S. B. He, H. P. Peng, H. H. Deng, A. L. Liu, X. H. Lin, X. H. Xia and W. Chen, Citrate-capped platinum nanoparticle as a smart probe for ultrasensitive mercury sensing, Anal. Chem., 2014, 86, 10955–10960 CrossRef CAS.
- N. Logan, C. McVey, C. Elliott and C. Cao, Amalgamated gold-nanoalloys with enhanced catalytic activity for the detection of mercury ions (Hg2+) in seawater samples, Nano Res., 2020, 13, 989–998 CrossRef CAS.
- F. Zhang, L. Feng, C. Jia, Y. Wu, J. Liu, X. Shuai and Z. Cao, Mixed-valence Pt(0)/Pt2+ nanoassemblies as high-Z radiosensitizers and metallo-immune regulators for potent radiotherapy of breast cancer, Nano Today, 2023, 48, 101708 CrossRef CAS.
- X. Huang, X. Li, Q. Luan, K. Zhang, Z. Wu, B. Li, Z. Xi, W. Dong and G. Wang, Highly dispersed Pt clusters encapsulated in MIL-125-NH2 via in situ auto-reduction method for photocatalytic H2 production under visible light, Nano Res., 2021, 14, 4250–4257 CrossRef CAS.
- M. E. Belowich and J. F. Stoddart, Dynamic imine chemistry, Chem. Soc. Rev., 2012, 41, 2003–2024 RSC.
- G. Sun, Y. Zhang, D. Qian, Q. Xu, J. Li and H. Li, Morphology-controlled peroxidase-like cuprous oxide-platinum cubes for dual-mode sensing of mercury ions, Sens. Actuators, B, 2024, 410, 135709 CrossRef CAS.
- N. Nataraj, P. Dash, R. Sakthivel, Y.-C. Lin, H.-W. Fang and R. J. Chung, Simultaneous electrochemical and colorimetric detection of tri-heavy metal ions in environmental water samples employing 3D-MOF/nickel selenide as a synergistic catalyst, Chem. Eng. J., 2024, 485, 149965 CrossRef CAS.
- H. Zhang, Y. Xu, Y. Xu, J. Lu, X. Song and X. Luo, An ingenious cellulose membrane sensor design strategy for colorimetric detection of Ag(+)/Hg(2+) based on redox reaction, Talanta, 2023, 255, 124209 CrossRef CAS PubMed.
- M. R. Mahmoudian, W. J. Basirun, P. M. Woi and Y. Alias, Synthesis of polyaniline microtubes/Pt reduced N-graphene oxide in the presence of L-glutamine for the detection of Hg2+, J. Appl. Electrochem., 2020, 50, 1269–1280 CrossRef CAS.
- N. Logan, J. Lou-Franco, C. Elliott and C. Cao, Catalytic gold nanostars for SERS-based detection of mercury ions (Hg2+) with inverse sensitivity, Environ. Sci.: Nano, 2021, 8, 2718–2730 RSC.
- B. Hao, X. Bu, J. Wu, Y. Ding, L. Zhang, B. Zhao and Y. Tian, Determination of Hg2+ in water based on acriflavine functionalized AgNPs by SERS, Microchem. J., 2020, 155, 104736 CrossRef CAS.
- Y. Yu, L. Liu, X. Chen, M. Xiang, Z. Li, Y. Liu, Y. Zeng, Y. Han and Z. Yu, Brominated flame retardants and heavy metals in common aquatic products from the pearl river delta, south china: Bioaccessibility assessment and human health implications, J. Hazard. Mater., 2021, 403, 124036 CrossRef CAS PubMed.
-
J. H. Xiao and S. H. Guo, Empirical Analysis and Countermeasure Study on the Development Capability of Marine Industry in Jiangmen City, Advances in Economics, Business and Management Research, 2020, vol. 159, pp. 98–101 Search PubMed.
- K. A. Merritt and A. Amirbahman, Mercury methylation dynamics in estuarine and coastal marine environments — A critical review, Earth-Sci. Rev., 2009, 96, 54–66 CrossRef CAS.
- W. Hou, X. Chen, J. Wu, C. Zhang, J. Yu, J. Bai and T. Chen, Sources and spatiotemporal variations of nitrogen and phosphorus in Liaodong Bay, China, Mar. Pollut. Bull., 2022, 185, 114191 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  abe,
Tianyu
Guo
c,
Liwen
Feng
g,
Yaobin
Lu
bc,
Jiewei
Deng
abe and
Tiangang
Luan
abe,
Tianyu
Guo
c,
Liwen
Feng
g,
Yaobin
Lu
bc,
Jiewei
Deng
abe and
Tiangang
Luan
 *abcdf
*abcdf
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, washed three times with DMF and water, and subsequently dried under vacuum to yield the final product.
000 rpm, washed three times with DMF and water, and subsequently dried under vacuum to yield the final product.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) and L is 1 cm.
000 M−1 cm−1) and L is 1 cm.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 1 min. The absorbance at 652 nm was measured by a UV-vis spectrophotometer.
000 rpm for 1 min. The absorbance at 652 nm was measured by a UV-vis spectrophotometer.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The supernatant was collected and analyzed by a UV-vis spectrophotometer.
000 rpm. The supernatant was collected and analyzed by a UV-vis spectrophotometer.






