Tetraploidy and Fe2O3 nanoparticles: dual strategy to reduce the Cd-induced toxicity in rice plants by ameliorating the oxidative stress and downregulation of metal transporters†
Fozia
Ghouri
 ab,
Munazzam Jawad
Shahid
c,
Shafaqat
Ali
cd,
Humera
Ashraf
ab,
Sarah Owdah
Alomrani
e,
Jingwen
Liu
ab,
Mohammed Ali
Alshehri
f,
Shah
Fahad
ab,
Munazzam Jawad
Shahid
c,
Shafaqat
Ali
cd,
Humera
Ashraf
ab,
Sarah Owdah
Alomrani
e,
Jingwen
Liu
ab,
Mohammed Ali
Alshehri
f,
Shah
Fahad
 *gh and
Muhammad Qasim
Shahid
*gh and
Muhammad Qasim
Shahid
 *ab
*ab
aState Key Laboratory for Conservation and Utilization of Subtropical Agro-Bioresources, Guangdong Laboratory for Lingnan Modern Agriculture, South China Agricultural University, Guangzhou, China. E-mail: foziaghouri@scau.edu.cn; humeraa49@gmail.com; jwliu@stu.scau.edu.cn; qasim@scau.edu.cn; Fax: +86 20 85280205; Tel: +86 20 85280205
bGuangdong Provincial Key Laboratory of Plant Molecular Breeding, South China Agricultural University, Guangzhou, China
cDepartment of Environmental Sciences, Government College University, Faisalabad 38000, Pakistan. E-mail: munazzam01@gamil.com; shafaqataligill@yahoo.com
dDepartment of Biological Sciences and Technology, China Medical University, Taichung 40402, Taiwan
eDepartment of Biology, College of Science and Arts, Najran University, Najran 66252, Saudi Arabia. E-mail: soalomrani@nu.edu.sa
fDepartment of Biology, Faculty of Science, University of Tabuk, Tabuk 71491, Saudi Arabia. E-mail: ma.alshehri@ut.edu.sa
gDepartment of Agronomy, Abdul Wali Khan University Mardan, Khyber Pakhtunkhwa 23200, Pakistan. E-mail: shah_fahad80@yahoo.com
hDepartment of Natural Sciences, Lebanese American University, Byblos, Lebanon
First published on 18th October 2024
Abstract
Whole-genome doubling or polyploidy increases plants' tolerance to biotic and abiotic stress. Cadmium (Cd) damages the plant's metabolic system, leading to decreased plant development. The role of tetraploidy and iron nanoparticles (Fe NPs) in minimizing Cd toxicity in rice was investigated in this work. Diploid (E285) and tetraploid (T485) rice lines were treated with Cd (100 μM) and different doses of Fe NPs (0, 10, 25, and 50 mg L−1). The Cd exposure substantially decreased agronomic traits (root and shoot length, shoot and root fresh weight), chlorophyll contents, and antioxidant enzyme activity and increased reactive oxygen species (ROS). The Cd toxicity effect was more pronounced in diploid rice than in tetraploid rice. The application of Fe NPs to Cd-contaminated rice plants reversed the detrimental consequences of Cd in tetraploid and diploid rice cultivars, verified by the substantial upturn in plant growth parameters, chlorophyll contents, decreased ROS, and increased levels of antioxidant enzymes. The Cd uptake was significantly reduced by tetraploidy and Fe NPs, which negatively controlled the expression patterns of Cd transporter genes (like OsNRAMP2 and OsHMA2). The strongest association was seen between diploid rice and cadmium levels in seedlings. Transmission electron microscopy revealed that Cd, especially in diploid rice, caused cell structure damage that Fe NPs and tetraploidy almost repaired. This study demonstrated that tetraploidy and Fe NPs could alleviate Cd toxicity by lowering Cd accumulation, ROS, and cell damage.
Environmental significanceCadmium is a non-biodegradable and persistent heavy metal that can cause acute and chronic health complications despite low quantities. Cd toxicity and uptake in plants can be reduced by applying vital nutrients such as iron, zinc, and nitrogen. Nanoparticles (NPs) are used extensively in agriculture due to their remarkable characteristics, such as their tiny size and massive surface area. The NPs can ease the toxicity of heavy metals by decreasing heavy metal availability and enhancing the plants' anti-metal defense systems. Given humanity's significant challenges in terms of food security and supply, understanding the beneficial role of nanoparticles and polyploidy in remediating cadmium-contaminated soil and improving plant tolerance to metal-induced stress may lead to improved breeding and crop-management strategies. This study aimed to determine whether Fe NPs could enhance the rice plant's resistance to high cadmium concentration and reduce the toxicity caused by cadmium in polyploid and diploid rice. This study assessed the relationship between polyploidy and resistance to metal-induced stress. Further, the combined effect of Fe NPs and polyploidy on reducing the toxicity caused by cadmium and their impact on various morpho-physiological mechanisms were examined. Our results revealed that combining Fe NPs and polyploid rice could be a valuable source for reducing Cd toxicity in rice plants. |
1. Introduction
Worldwide, agricultural lands are facing unprecedented challenges due to industrialization, uncontrolled population growth, and urbanization. Among the contaminants, soil contamination with heavy metals has seriously threatened food safety and humans due to detrimental effects on human health. Heavy metals do not degrade through microbial or chemical processes like organic pollutants, so they remain in soil for a long time. Cadmium is a non-biodegradable and persistent heavy metal that can cause acute and chronic health complications despite low quantities.1 Most of the crops grown on cadmium-contaminated soils frequently accumulate high concentrations of cadmium, which can have a deleterious impact on crop production and pose a risk to the health of consumers because Cd can accumulate in vital body organs and obstruct the normal function of organs.2 Cadmium is a potent inhibitor of the photosynthetic process as it disrupts the chloroplast, disturbs the photochemical and carboxylation reaction of photosynthesis, and inhibits the biosynthesis of chlorophyll.3 Rice is a staple cereal crop worldwide, but soil contamination with cadmium severely threatens the rice quality. Cadmium toxicity in paddy fields results in decreased seed germination, reduced plant growth, decreased mineral contents, reduced photosynthesis, and oxidative stress due to damage to the structure and metabolic process.4Cd toxicity and uptake in plants can be reduced by applying vital nutrition such as iron, zinc, and nitrogen.5 Nanoparticles are used extensively in agriculture due to their remarkable characteristics, such as their tiny size and huge surface area. The nanoparticles can enter the soil and plants, enhance the accessibility of mineral nutrients, and boost plant development and yield.6 The NPs can also ease the toxicity of heavy metals by decreasing heavy metal availability and enhancing the plants' anti-metal defense systems.7–9 However, NPs' responses to plants depend upon the method of NP application, soil type, plant species, and type of metals. For example, selenium and silicon NPs reduced Cd and Pb concentrations and ameliorated the development of rice plants.10 Nanoparticles may arrest the toxic metals in soil and readily change them into non-bioavailable metals through surface adsorption. Iron is essential for plant development and plays a key part in various biochemical and physiological processes. Fe-based NPs may enhance plant growth compared to Fe-based fertilizers and mitigate the toxic effect of metals because of their vast surface area.11
Polyploidy is the multiplication of the whole chromosomal set of a particular species and is widely distributed in the plant kingdom, such as flowering plants, gymnosperms, ferns, and diatoms. The diversification of plant species depends on polyploidy and contributes to genome evolution.12 The assumption is that polyploid plants retain higher adaptability to abiotic and biotic stress and survive in a harsh environment compared to their diploid species. Autotetraploid rice showed better resistance than its diploid counterpart to Cd and Pb toxicity.13,14 The new findings revealed that polyploidy enhanced stress tolerance, and plants such as autotetraploid Ziziphus jujuba showed better drought tolerance and enhanced regrowth after dehydration than their diploid counterparts.15 Polyploidy causes functional and structural changes in genomes, including gene loss, chromosomal rearrangement, and epigenetic modifications as well as the modification of gene expression. Among the many genetic modifications that happen during polyploidy, alteration in the action of transposable elements has a substantial impact on plant adaptation to several stresses through customizing stress-related genes.16
Given the significant challenges that humanity faces in terms of food security and supply, understanding the beneficial role of nanoparticles and polyploidy in remediating cadmium-contaminated soil and improving plant tolerance to metal-induced stress may lead to improved breeding and crop-management strategies. This study aimed to determine whether Fe NPs could enhance the rice plant's resistance to high cadmium concentration and reduce the toxicity caused by Cd in polyploid and diploid rice. In this study, the relationship between polyploidy and resistance to metal-induced stress was also assessed. Further, the combined effect of Fe NPs and polyploidy on reducing the toxicity caused by Cd and their impact on various morpho-physiological mechanisms were examined.
2. Materials and methods
2.1 Growing of rice plants
In this study, a cultivar of diploid rice (E285) and its counterpart tetraploid rice line (T485) were used to analyze the effect of Fe2O3 nanoparticles (Fe NPs) and cadmium on different growth parameters, photosynthetic activity, oxidative stress, antioxidant activity, and uptake of cadmium and Fe in rice plants. The rice line with genome doubling was developed by treating the diploid cultivar with colchicine. The dose of Fe NPs (mg L−1) and Cd (μM) stress level were selected through preliminary trials by providing different quantities of Fe NPs and Cd to rice seedlings and analyzing the growth response. The entire experiment was executed in pots at 20 to 25 °C and an average humidity of 70% in the greenhouse of South China Agricultural University (SCAU). The nanoparticles used in this study had a 99.9% purity and were obtained from Shanghai Chaowei Nanotechnology Co., Ltd. The NPs were characterized using XRD, and particle size and morphology were surveyed using scanning electron microscopy (SEM) with field emission (version 460, FEI). The experiment utilized a randomized complete block design with four replicates of each treatment.A few seeds of diploid and tetraploid rice cultivars were sterilized in a 0.5% NaClO solution for 30 min before washing with distilled water for this experiment. For germination, seeds were spread out on tissue paper. Seedlings with healthy root and shoot structures were chosen for the experiment's next stage after 15 days. A nutrient solution was applied to selected seedlings of diploid and tetraploid rice cultivars for 14 days to promote normal seedling growth. Plants with comparable root and shoot lengths were subjected to various concentrations of Fe NPs (0, 10, 25, and 50 mg L−1) and Cd (0 μM and 100 μM) for a period of 14 days.
2.2 Plant physiological parameters
The plants were cut down with scissors and a knife following a 14 day treatment period with tetraploid and diploid rice plants. After measuring the lengths of the roots and shoots with meter rods, the plants were divided into various parts for additional physicochemical analysis. 0.1 μM EDTA solution was used to wash and soak plant roots for 30 min. Absorbent paper was used to remove the water from the samples. Fresh root and shoot masses were quantified using a weight balance. The shoots and roots were then completely dried at 70 °C for 2 days, after which the mass of the dried roots and shoots was estimated.2.3 Chlorophyll and carotenoid contents
By examining the fully expanded leaves, the contents of chlorophyll and carotenoid were determined. 0.5 g of ground leaf tissue was subjected to centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min in 0.5 ml of acetone (3% v/v) to obtain the top aqueous solution. Chlorophyll and carotenoid levels of the supernatant were assessed using a spectrophotometer at wavelengths of 645 nm (chlorophyll b), 470 nm (carotenoids), and 663 nm (chlorophyll a).17
000 rpm for 10 min in 0.5 ml of acetone (3% v/v) to obtain the top aqueous solution. Chlorophyll and carotenoid levels of the supernatant were assessed using a spectrophotometer at wavelengths of 645 nm (chlorophyll b), 470 nm (carotenoids), and 663 nm (chlorophyll a).17
2.4 Activity of reactive oxygen species and antioxidant enzymes
Lipid peroxidation in the tissues of rice plants was analyzed by estimating the malondialdehyde (MDA) contents.18 About 0.5 g of leaves were blended in 0.1% C2HCl3O2 and centrifuged for 10 min at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The mixture was heated for half an hour at 95 °C, cooled and centrifuged at 1000g for 15 min at 4 °C to obtain the supernatant. The supernatant absorbance was analyzed by using a spectrophotometer at 532 nm.
000 rpm. The mixture was heated for half an hour at 95 °C, cooled and centrifuged at 1000g for 15 min at 4 °C to obtain the supernatant. The supernatant absorbance was analyzed by using a spectrophotometer at 532 nm.
The H2O2 concentration was assessed using the method reported by Patterson et al. (1984).19 Leaf tissue was centrifuged at 5000 rpm for 15 min after being homogenized in 10 mL of cold acetone. The top layer was once more extracted using acetone and 10 mL 2 N H2SO4, followed by centrifugation. The supernatant's absorbance was analyzed at 410 nm and compared with the standard curve to a known concentration of H2O2. The contents of electrolyte leakage (EL) were analyzed by putting root and shoot samples in glass tubes and heating at 32 °C for 2 h, and the mixture was designated as EC1. After cooling at ambient temperature, EC2 was measured after 20 min at 121 °C. The following equation measured the final electrolyte leakage.20
| EL (%) = (ECb − ECa)/ECc × 100 |
The antioxidant enzyme activity was determined by estimating the contents of glutathione (GSH), peroxidase (POD), catalase (CAT), and superoxide dismutase (SOD) using kits obtained from Jiancheng Bioengineering Institute, Nanjing, China.13 A 0.1 g root and shoot sample was crushed and placed in a 0.05 M phosphate solution (pH 7.0). The top layer was extracted by centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min (4 °C), and then absorbance was checked by a spectrophotometer to determine the POD and SOD contents.21 The catalase activity was assessed by analyzing the supernatant absorbance at 240 nm. High-performance liquid chromatography (HPLC) was used to estimate the GSH contents after modifying thiol compounds with iodoacetate and organofluorine (1-fluoro-2,4-dinitrobenzene), and to separate the various compounds.22
000 rpm for 10 min (4 °C), and then absorbance was checked by a spectrophotometer to determine the POD and SOD contents.21 The catalase activity was assessed by analyzing the supernatant absorbance at 240 nm. High-performance liquid chromatography (HPLC) was used to estimate the GSH contents after modifying thiol compounds with iodoacetate and organofluorine (1-fluoro-2,4-dinitrobenzene), and to separate the various compounds.22
2.5 Estimation of cadmium and iron content
By acid-digesting oven-dried samples of roots and shoots, rice plant roots and shoots' concentrations of Cd and iron were measured. The samples were broken down with analytical grade HNO3 and HClO4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in digestion tubes. The digested samples were examined for Cd and Fe accumulation by inductively coupled plasma mass spectrometry (ICP-MS) (Agilent Technologies, CA, USA).
1) in digestion tubes. The digested samples were examined for Cd and Fe accumulation by inductively coupled plasma mass spectrometry (ICP-MS) (Agilent Technologies, CA, USA).
2.6 Transmission electron microscopy (TEM)
Rice seedling root tips were removed, cleaned, fixed, dehydrated, mounted, and finally coated with platinum for 60 seconds using a sputter coater to obtain TEM specimens. The samples were filtered and implanted in Spurr resin after dehydration in a succession of ethanol concentrations (50%, 60%, 70%, 80%, 90%, 95%, and 100%) and acetone. Transmission electron microscopy (TALOS L120C) was used to examine the ultrathin specimens.232.7 Analysis of the properties of iron nanoparticles
Fe NPs (99.9%, 2030 nm) were obtained from Pantian Nanomaterials Co., Ltd. (Shanghai, China) for Fe-based nanoparticles. The iron nanoparticles were analyzed by SEM and TEM. From SEM images, we can see that our nano-oxide iron materials are rod-like and distributed in tight clusters. The long axis length is about 50–150 nm, and the short axis is about 15–25 nm (Fig. S1†). Through EDS energy spectrum analysis using a transmission electron microscope, it is confirmed that the important elements in the material are oxygen and iron, and there are no other impurities.2.8 Metal transporter gene expression levels
Using a plant RNA kit and manufacturer's recommendations, the total RNA from rice roots was extracted, and DNase was removed from the genomic DNA samples. A NanoDrop 2000 spectrophotometer was used to measure the quantity of RNA, and Primer Premier 5.0 software was utilized for creating the qRT-PCR primers; their sequences are displayed in Table S1.† The internal reference gene was ACTIN, and the expression patterns were analyzed using the 2−ΔΔCt approach.24 Triplicate qRT-PCR assays were conducted for every sample.2.9 Statistical analysis
The data presented here are averages from the four replicates of each treatment. Using SPSS Statistics V26 software, the data were subjected to a one-way analysis of variance (ANOVA). To determine the significance level (p < 0.05) between the treatment and control groups, Tukey's post hoc analysis was performed. The letters on the graphs were used to distinguish between significant and insignificant differences in treatments.3. Results
3.1 Morphological traits
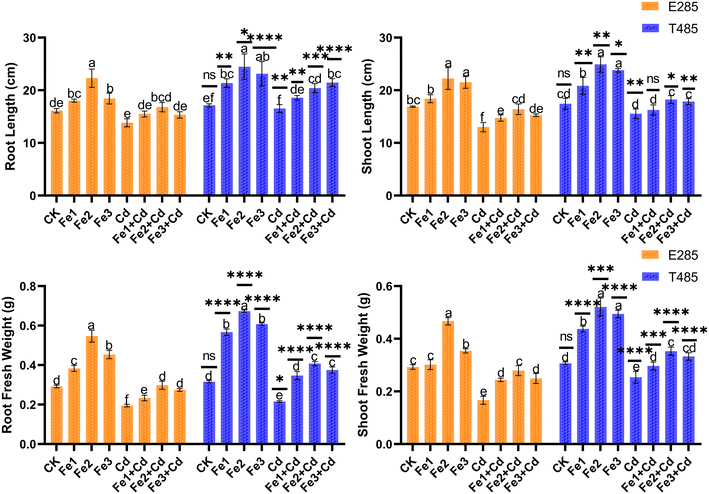
The results of this research revealed that Cd had a negative influence on all the morphological parameters of the rice plants (Fig. 1). The Fe NPs enhanced the shoot length, root fresh weight, root length, and shoot fresh weight in all treatments. Under 100 μM Cd, all the applied concentrations of Fe NPs increased the rice plant's growth parameter in diploid (E285) and tetraploid (T485) rice cultivars. The maximum improvement in growth parameters of both cultivars was observed at an Fe NP concentration of 25 mg L−1, which trended downward at 50 mg L−1 Fe. The tetraploid cultivar showed significantly better agronomic growth than the diploid cultivar under Cd and all concentrations of Fe NPs. The Fe NPs (25 mg L−1) at 100 μM Cd increased the fresh root and shoot weight in the tetraploid by 31.51% and 14.98% against the control.3.2 Photosynthetic activity
Cadmium significantly reduced the carotenoids, Chl a, Chl b, and total chlorophyll contents in diploid and tetraploid rice cultivars (Fig. S2†). Applying Fe NPs augmented the Chl a, Chl b, chlorophyll ab and carotenoid contents in the two rice genotypes, with the highest increase at 25 mg L−1 Fe NPs and a slight decrease at 50 mg L−1 Fe NPs. The influence of Fe NPs on the photosynthetic parameters with and without Cd was more prominent in tetraploid than in diploid rice. Applying Fe NPs (25 mg L−1) with Cd, the Chl a and Chl b contents increased to 52.82% and 52.64% in tetraploid rice and 21.55% and 23.65% in diploid versus control.3.3 Reactive oxygen species (EL, MDA, and H2O2)
Cadmium caused significant cellular damage in rice cultivars and increased the activity of ROS obviously from the high level of H2O2, electrolyte leakage (EL), and malondialdehyde (MDA) (Fig. 2). Polyploid plants had lower ROS levels than diploid rice. Under Cd toxicity, Fe NPs reduced ROS contents in E285 and T485 lines, with a maximum reduction at 25 mg L−1 Fe NPs. Even with the Cd toxicity, the levels of EL, MDA, and H2O2 were significantly lesser in autotetraploid than in diploid cultivars, and this was further reduced by using Fe NPs. However, applying 50 mg L−1 Fe NPs led to a small increase in EL, MDA, and H2O2 compared to 25 mg L−1 Fe NPs.3.4 Antioxidant enzyme activities
The stress induced by Cd affected the activity of antioxidant enzymes with a maximum decrease in rice cultivars in the treatment with only cadmium (100 μM) (Fig. S3†). Polyploid plants exhibited the highest antioxidant enzyme activities under Cd treatment compared to diploid rice. The applied Fe NPs enhanced the activity of SOD, POD, GSH, and CAT in diploid and tetraploid cultivars of rice with a maximum increase in tetraploid cultivars. Even when Fe NPs and Cd were combined, the tetraploid cultivar had significantly higher antioxidant enzyme activity than the diploid. In the case of antioxidant enzymes, increasing the Fe NP concentration increased antioxidant enzyme activity, with the highest activity at 50 mg L−1 Fe NPs in both diploid and tetraploid cultivars.3.5 Uptake of Fe and Cd
The Cd concentration increased in all groups relative to control, with the highest Cd level in rice plants treated with only cadmium (100 μM) (Fig. 3). The roots contained the most Cd levels than the shoots. Fe NPs significantly decreased the root and shoot Cd uptake, with a maximum reduction at 50 mg L−1 Fe NPs. Tetraploid rice cultivars depicted significantly less Cd uptake with and without Fe NPs, while Fe NPs further repressed Cd accumulation in the tetraploid rice line. Applying Fe NPs amplified the level of Fe in shoots and roots of both diploid and tetraploid cultivars of rice. However, just like Cd, the roots of diploid rice contained considerably more Fe than the shoots of tetraploid cultivar.3.6 Ultrastructure observations by transmission electron microscopy
Root ultrastructure was normal when treated with Fe NPs (25 mg L−1), and higher numbers of mitochondria and normal cell organelles were observed in both rice lines (Fig. 4A and B). When exogenous Fe NPs (25 mg L−1) were applied to the Cd-treated plants, meristematic root cells appeared to be exclusively produced and reduced the abnormalities caused by Cd in diploid and tetraploid rice seedlings (Fig. 4C and F). Transmission electron microscopy revealed a variety of abnormalities in rice seedlings under Cd stress, including vacuole enlargement or an increase in the number of vacuoles, U- or V-shaped cell structure, deformed mitochondria, and broken karyotheca (Fig. 4G and J). However, these anomalies were less prevalent in tetraploid rice.3.7 Heatmap, correlation and principal component analysis (PCA)
We discovered from the heatmap that T485 had higher carotenoid, chlorophyll, chlorophyll a, chlorophyll b, chlorophyll ab, root length, root fresh weight, aboveground length, aboveground fresh weight, and biomass contents than E285 under Cd exposure (Fig. 5A). Similarly, after Fe treatment, these physiological indicators were greater in T485 than in E285 and significantly higher than under Cd toxicity. Under Cd treatment, however, MDA, Cd root contents, Cd contents in the shoot, H2O2 content, and electrolyte content were higher in E285 than in T485. Principal component analysis revealed that the total variance of PCA's two components accounts for 87.33%, while the total variance of PC1 accounts for 72.80% and that of PC2 accounts for 14.53% (Fig. 5B). The correlation between root length and Cd concentration in T485 and E285 was positive, but the correlation was stronger in T485. The majority of variables, including POD, CAT, MDA, and Chl levels, were affected by PC1. E285 and T485 did not overlap during the Fe + Cd treatment, showing that the two materials reacted to this treatment differently (Fig. S4†). E285 and T485 responded more consistently when treated with Fe alone. T485 demonstrated a more significant association with Cd treatment than E285, which showed minimal differences between Cd and control treatments.The majority of the morphological and physiological changes in rice in E285 were strongly connected with the cadmium level in roots and shoots, and each morphological and physiological change also had a strong correlation, as shown by the correlation coefficient (Table S2†). There were strong correlations between the contents of Cd in roots and shoots, and the levels of EL, H2O2, and MDA. Cadmium concentrations were found to have a very substantial negative correlation with Chl ab, Chl a, root length, Chl b, shoot fresh weight, and shoot length. Similarly, a strong correlation was also detected in the T485 material (Table S3†). MDA was shown to be highly associated with other variables. Fe in roots and Chl ab, Chl a, and Chl b, as well as GSH and Chl ab, Chl a, and Chl b, were positively associated in T485 material, although the correlation was not statistically significant when compared to E285.
3.8 Metal transporter gene expression levels
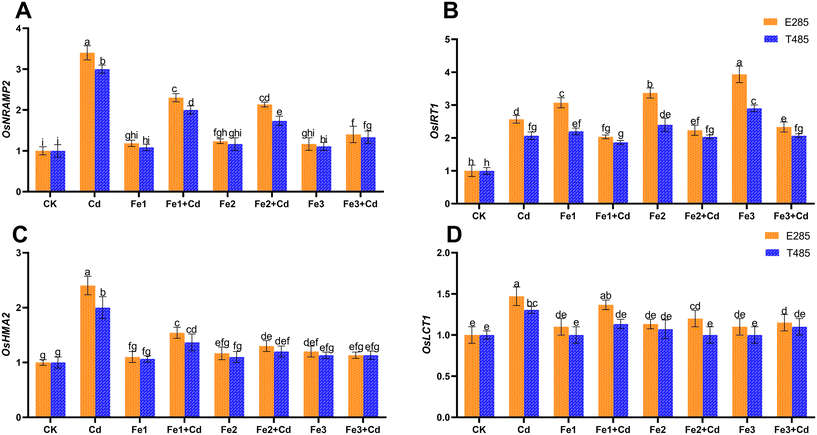
We used qRT-PCR to examine the expression patterns of Fe and Cd transporter genes (Fig. 6). Our findings showed that Fe nanoparticles inhibit Cd transporter gene expression patterns. For example, OsNRAMP2, OsHMA2, and OsLCT1 expression levels were noticeably lower in Fe- and Fe + Cd-treated plants than in Cd-treated plants. However, compared to diploid rice, their level of expression was lower in tetraploid rice. OsIRT2 transports Fe in plants, which was positively regulated by Fe nanoparticles in both rice lines. Overall, these results explained that polyploidy and Fe nanoparticles suppressed the expression patterns of Cd transporter genes, which reduced Cd toxicity and cell damage, and thus enhanced plant resistance against Cd stress.4. Discussion
4.1 Effects of polyploidy and Fe NPs on agronomic parameters
Gene duplication, especially whole-genome duplication, develops changes in the genome and creates genotypic and phenotypic changes. Polyploid species are more resilient to biotic and abiotic stress due to high genetic diversity and the protective influence of gene redundancy.25 In this study, autotetraploid rice outperformed its diploid counterpart regarding metal tolerance (Fig. 1). Even after the toxicity of Cd, the autotetraploid rice line significantly outperformed the diploid rice line in terms of shoot length, root length, fresh root weight, and fresh shoot weight. It is widely known that cadmium and plant nutrients compete for the same transporters, and a high concentration of cadmium in soil and/or hydroponic medium can result in mineral and nutrient deficiency in plants. High concentrations of cadmium can also disturb the uptake of vital nutrients to the plant, leading to a disturbance in plant metabolism.26 Plants with polyploidy are generally resilient to nutritional stress such as nitrogen assimilation, and shoot accumulation was increased under low nitrogen levels in allohexaploid wheat concerning its diploid and tetraploid lines.27 Similarly, in citrus plants, polyploidy was found effective in increasing the tolerance to nutrient deficiency.28The enlarged growth of agronomic parameters in polyploid compared to diploid rice line can be credited to the high tolerance of the tetraploid plants to cadmium stress and the ability to perform better even at low concentrations of critical nutrients. It is possible to lessen the toxicity of Cd in plants in a stressed environment by enhancing the Fe contents.29 Fe NPs further enhanced all agronomic parameters in diploid and tetraploid plants by diminishing Cd exposure. Nanoparticles can increase the bioavailability of essential nutrients in plants by increasing their absorption capability. It is well documented that the exogenous use of nanoparticles can lessen the uptake of trace elements by many plant species. Application of Fe NPs boosted wheat biomass and reduced condensed Cd toxicity in the plants.10 Applying Fe NPs on rice plants reduced chromium stress by decreasing the bioavailability of chromium ions and enhancing biomass and yield by increasing the availability of essential nutrients.11
The uptake of Fe NPs by rice is contingent upon the interaction between the nanoparticles and the plant's root system. Iron nanoparticles infiltrate the soil environment and frequently experience alteration, disintegration, or aggregation. Fe NPs often undergo partial dissolution in the rhizosphere, releasing Fe3+ ions that are subsequently absorbed by rice plants in their ionic state. Plants usually assimilate iron in the form of Fe2+ (ferrous) or Fe3+ (ferric) ions, with Fe2+ exhibiting greater bioavailability in anaerobic or reduced soil environments. Consequently, rice predominantly assimilates iron from iron nanoparticles in ionic forms (Fe2+/Fe3+) rather than as elemental nanoparticles. The absorption of these nanoparticles is contingent upon their size, concentration, and the species-specific reaction of the plant, which may vary from growth enhancement to potential toxicity at elevated concentrations.30,31 Iron nanoparticles can influence rice plants based on their concentration and exposure parameters. Some potential alterations encompass increased growth (at low to moderate concentrations) via functioning as a micronutrient supplement, particularly in iron-deficient environments. This may enhance root development, produce greener leaves, and promote healthier plant growth. Since iron is essential for chlorophyll synthesis, Fe NPs may enhance chlorophyll concentrations, which signifies improved photosynthetic activity. Elevated concentrations of Fe NPs may result in inhibited development or root deformities due to stress reactions. The shoots may exhibit discoloration or diminished leaf size if the iron supply becomes high or causes oxidative stress.7 Cadmium, a recognized toxic heavy metal, accumulates in rice plants and causes more detrimental effects than Fe NPs, as it disrupts nutrient absorption (including iron), potentially leading to chlorosis (yellowing of leaves) due to chlorophyll deficiency or compromised photosynthesis. Elevated Cd levels frequently result in inhibited growth of both roots and shoots.32 Rice plants may have diminished plant height and inadequate root systems, thus affecting the overall plant size.9
4.2 Effects of polyploidy and Fe NPs on Cd and iron uptake modification model
Polyploidy can modify the root anatomy, which can alter the ability of plants to uptake nutrients and prevent the uptake of unnecessary elements. Anatomical root alterations in tetraploid citrus plants due to modified gene expression reduced boron uptake in comparison to its diploid counterpart.33 The polyploid rice exhibited a significantly decreased absorption of Cd and Fe nanoparticles compared to its diploid counterpart across different treatments, potentially associated with the resistance capabilities of polyploid rice (Fig. 3). TEM revealed that tetraploid rice possesses thicker cell walls compared to diploid rice, potentially decreasing cadmium absorption and transportation in polyploid rice. Citrus grown on tetraploid rootstocks showed higher Cr sequestration to roots and less transport to leaves than citrus grown on diploid rootstocks.34 Fe NPs also regulated Cd accumulation in the rice lines. Fe availability can significantly mitigate Cd injury by reducing Cd accumulation and uptake in plants.32 The reduced uptake of Cd can be linked to the properties of NPs that adsorb heavy metals and reduce their bioavailability to plants; for example, Fe NPs reduce Cd mobility in plants.35 Similarly, ZnO nanoparticles and tetraploid rice significantly reduced Cd uptake in seedlings.13Fe NPs mitigate metal toxicity by synergistic actions.14 Some research suggests that Fe NPs may diminish Cd absorption in rice plants by immobilizing Cd in the soil, hence alleviating its toxic effects. In such instances, plants may exhibit diminished indicators of Cd-induced stress, demonstrating healthier development than anticipated solely from Cd exposure. Conversely, high exposure to Fe NPs and Cd may induce combined or synergistic stress beyond the plant's defense mechanisms.31 This may lead to a more pronounced effect on growth, root structure, and leaf morphology. Our findings indicated that low to moderate concentrations of Fe NPs promoted superior root and shoot development. Still, high concentrations resulted in stunted growth, root deformities, and potential indicators of oxidative stress. Cadmium exposure inhibited development, elevated oxidative stress, and reduced chlorophyll levels.13 Fe NPs combined with Cd may serve as a possible remedy for Cd toxicity at ideal nanoparticle concentrations;7 however, elevated amounts could intensify stress symptoms. Evaluating the physical characteristics in conjunction with physiological and molecular data elucidates the effects of Fe NPs and Cd treatments on rice plants, providing insights into their possible applications in agriculture or environmental contexts.
4.3 Effects of polyploidy and Fe NPs on chlorophyll contents and cell structure
In this study, despite the Cd toxicity, tetraploid rice had significantly higher Chl a, Chl b, chlorophyll, and carotenoid contents than diploid rice plants (Fig. S2†). Fe NPs also significantly enhanced the chlorophyll contents in tetraploid and diploid rice due to their ability to regulate the uptake of essential nutrients and decrease the bioavailability of Cd by adsorption with NPs and reduced mobility in soil.35 It is documented that iron oxide-based NPs can improve photosynthesis by increasing the bioavailability of essential nutrients such as N2 and P and by protecting the photosynthetic apparatus against metal toxicity.7 Fe3O4 NPs enhanced the photosynthetic activity in wheat plants by increasing the contents of Fe, P, and K in leaves.7 The significantly high chlorophyll and carotenoid contents in tetraploid rice can be attributed to the enhanced accumulation and remobilization of mineral nutrients and increased resistance to nutrient deprivation in polyploid plants.36 Plants with polyploidy can perform the photosynthetic activity more efficiently than diploid plants due to the large cells having more chloroplast and thus high contents of chlorophyll and RuBisCO in contrast to their diploid lines.37 Polyploidy has a pronounced effect on photosynthetic characteristics in rice plants. Similarly, Yang et al. reported high photosynthetic ability in tetraploid rice plants compared to diploid.38The application of Fe NPs resulted in normal cell shape in both rice lines, whereas Cd exposure resulted in several abnormalities in rice seedlings, particularly in diploid rice. For example, vacuole enlargement or an increase in the number of vacuoles, U- or V-shaped cell structure, deformed mitochondria, abnormal endoplasmic reticulum shape/size, and broken karyotheca (Fig. 4). These abnormalities, on the other hand, were significantly lower in tetraploid and Fe NP-treated plants under Cd stress, indicating that Fe NPs and polyploidy can alleviate Cd stress in rice. These findings agreed with earlier research that found Cd exposure to be harmful to plant cells.13,39 Similarly, nanoparticles such as Si and ZnO nanoparticles minimized Cd harmful effects in rice.39–41
4.4 Effects of polyploidy and Fe NPs on reactive oxygen species and antioxidant enzymes
Cadmium exposure strengthened oxidative stress in rice plants, as demonstrated by high levels of electrolyte leakage (EL), malonaldehyde (MDA), and hydrogen peroxide (H2O2) (Fig. 2). Numerous studies showed that harmful metals and metalloids cause an imbalance in peroxisomal oxidative metabolism, chloroplast, and mitochondrial electron transfer activities, and production of ROS initiates oxidative stress in plants.42 Due to Cd toxicity, rice plants produced excessive amounts of MDA and had higher levels of H2O2, which increased lipid peroxidation.43 Plants primarily deal with oxidative stress through endogenous defensive mechanisms composed of various enzymatic and non-enzymatic antioxidants. In this study, rice plants demonstrated changes in antioxidant activities in response to high levels of ROS caused by cadmium (Fig. S3†). The Fe NPs reduced the oxidative stress in both diploid and tetraploid rice lines due to the Cd toxicity and enhanced the activity of antioxidant enzymes. Nanoparticles can reduce oxidative stress by strengthening antioxidant scavenging defense in plants.44 The NPs' oxidant and antioxidant properties aid in their entry into cells and interaction with the cell organelles, and prevent cell injury as a result of oxidative stress.45 Here, cell abnormalities were also lower in Fe NP-treated plants in diploid and tetraploid rice, especially in polyploid rice. Incorporating NPs with antioxidant enzymes enhances the potential of antioxidant enzymes and serves as nano-antioxidants. The joint usage of zinc and iron NPs reduced ROS, such as EL, MDA, and H2O2, in wheat under Cd exposure.46 In another study, Fe NPs reduced oxidative injury by increasing enzymatic activities in rice plants induced by the high chromium concentrations.11The polyploid rice line showed significantly high antioxidant enzymatic and non-enzymatic activity by the increased levels of CAT, SOD, POD, and GSH than the diploid rice line. Under different abiotic stresses, such as salt stress, several polyploid plant species showed significantly higher production of essential antioxidants such as SOD, POD, and GSH than their diploid relatives, decreased the level of MDA, which is a crucial indicator of membrane damage, and lipid peroxidation.47 Plant polyploidy alters gene expression in reaction to a stressful environment. A recent comparative proteomic and genomic analysis of diploid and polyploid counterparts revealed that photosynthetic activity and ROS scavenging enzymes were more upregulated in polyploid genotypes.48 Triploid citrus genotypes showed less oxidative damage due to the more potent antioxidant enzymatic defense system than the diploid genotype under abiotic stress.49
4.5 Impact of polyploidy and Fe NPs on gene expression levels of metal transporters
In this study, polyploid plants showed better root and shoot growth even under Cd toxicity without the supply of Fe (Fig. 1). This may be linked with the downregulation of OsNRAMP2, responsible for increased shoot biomass even in Fe deficiency in polyploid plants. Our results reflected that Fe NPs suppressed the expression patterns of Cd transporter genes and enhanced the expression of Fe transporter genes. The OsNRAMP2 gene is responsible for transporting Fe and Cd from vacuole to cytosol and has a vital role in increasing biomass production.50 The Fe NPs amplified the expression of Fe transporter genes, enhanced iron availability to the rice plant, and reduced cadmium uptake (Fig. 6).In rice plants, a vital Cd transporter from the roots to the shoots is OsHMA2, and its expression is primarily apparent in the roots.51,52 Polyploidy and Fe NPs lessened the expression pattern of OsHMA2, which ultimately reduced Cd contents in rice plants. Another finding suggested that OsHMA2 accumulates Cd and Zn in the xylem and translocates these metals from root to shoot. The suppressed expression of OsHMA2 also lessened the Cd concentration in rice leaves and raised Zn accumulation in roots.52,53
OsIRT2 is an Fe transporter gene related to iron homeostasis in rice plants.54 This gene also plays a crucial function in regulating rice plants' Cd, Fe, and Mn uptake. The Fe deficit in the presence of a Cd-enriched environment may upregulate the expression of OsIRT2, resulting in enhanced Cd uptake in rice plants' roots. However, in Fe-enriched culture, OsIRT2 may limit the uptake of Cd.55 Here, Fe NPs upregulated the expression patterns of OsIRT2, which ultimately enhanced iron uptake and reduced cadmium uptake in both diploid and tetraploid rice. Similar findings were reported by another study in which Fe-lacking rice accumulated more Cd than Fe-enriched rice, proposing the role of OsIRT2 in Cd and Fe transport.56,57 The lower uptake of Fe and Cd in tetraploid rice may be related to the downregulation of the OsIRT2 gene than in diploid rice, as it is well documented that gene duplication and altered gene expression may enhance the resistance to abiotic and biotic stress.
OsLCT1 transports Cd to the grains and regulates Cd deposition into phloem and grains.58 Here, applying Fe NPs decreased the expression patterns of OsLCT1 in the roots of diploid and tetraploid rice, which was more prominent in polyploid rice. When plants were exposed to Cd, OsLCT1 expression levels increased, and the amount of Cd accumulated in the roots was boosted. This shows that polyploidy and Fe NPs could reduce the expression level of OsLCT1 and ultimately limit uptake of Cd through roots.
In the context of the findings, the framework of Fe NPs and polyploidy in reducing the toxicity of Cd was proposed. Genome doubling decreased the Fe and Cd transporter gene expression levels and Cd uptake in polyploid plants. Moreover, polyploid rice with thicker cell walls and fewer cell abnormalities displayed low toxicity or adsorption of Cd. The Fe NPs tend to accumulate around the cell wall and prevent Cd entrance into the cell. Further, the Fe NPs controlled the expression patterns of Fe and Cd transporter genes and decreased the root and shoot's uptake and accumulation of Cd (Fig. 7). From this, we can infer that (1) Fe NPs can bind the Cd ions and inhibit their diffusion into the cells, resulting in decreased accumulation of Cd into the cell. (2) Overall, polyploidy and Fe NPs suppressed the patterns of OsNRAMP2, OsHMA2, and OsLCT1, which transport Cd, resulting in decreased Cd uptake in tetraploid compared to diploid plants. (3) The Fe NPs and gene doubling enhanced the Fe uptake and reduced Cd as OsIRT2 tends to uptake more Fe and reduce Cd uptake under high concentrations of Fe. (4) Overall, genome doubling boosted plant growth and suppressed the Fe and Cd transporter genes, which reduced Cd uptake in plants. Here, high expression levels of OsIRT2 revealed enhanced uptake of Fe, and downregulation of OsHMA2, OsNRAMP2, and OsLCT1 genes depicted low Cd adsorption in plants. These results were consistent with Fe and Cd levels in shoots and roots of diploid and tetraploid rice.
4.6 The impact of elevated Fe NP concentrations on various physicochemical parameters
In this study, the increasing intensity of Fe NPs up to a specific limit enhanced the positive impact on chlorophyll and plant growth. However, the agronomic parameters and chlorophyll contents of plants decrease as the concentration of Fe NPs increases, which can be attributed to the lethal impact of metal based on plants. The high amount of nanoparticles can damage the plant's physiological and metabolic process by damaging cellular structures, DNA, and mitochondrial structure and enhancing oxidative stress.39,59 In a study, applying 50 μg mL−1 ZnO NPs caused a genotoxic and cytotoxic effect on root meristems, inhibiting root growth of Allium cepa.605. Conclusion
The toxicity of cadmium slowed plant growth due to damage to the cell structure, metabolic process, and disturbance to enzymatic/non-enzymatic activity in rice plants. Polyploidy or genome doubling showed great potential to reinforce the plant defense system against Cd-induced toxicity because Cd transporter genes (OsHMA2 and OsNRAMP2) were suppressed. Fe NPs reduced the toxicity of Cd due to their interaction with the different cellular processes and their influence on Cd and Fe transporter genes in rice. Applying Fe NPs accelerated plant development by lowering the contents of EL, H2O2, and MDA and enhanced the activity of antioxidant enzymes, including SOD, CAT, GSH, and POD, which reduced Cd adsorption by plants. We detected the strongest correlation between diploid rice and aboveground cadmium levels. The tetraploid rice line showed more capability to tolerate Cd stress with less impact on growth traits and cell structure due to gene doubling. The tetraploid rice line and Fe NPs diminished Cd uptake in rice plants and maintained cell shape, which may relate to the reduced expression patterns of Cd transporters. The use of tetraploid rice lines can enhance the production of rice crops, which have limited chances of transferring Cd to humans through the food chain due to their ability to sequestrate Cd in roots. Field research can further investigate the role of Fe NPs and polyploid rice lines in remediating the metals' harmful effects under natural field conditions.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Fozia Ghouri: data curation, investigation, writing – original draft, visualization, methodology. Munazzam Jawad Shahid: writing – original draft, review & editing, data analysis. Shafaqat Ali: writing – review & editing, conceptualization. Humera Ashraf: data analysis, investigation, visualization. Sarah Owdah Alomrani: formal analysis, writing – review & editing. Jingwen Liu: investigation, visualization. Mohammed Ali Alshehri: writing – review & editing. Shah Fahad: writing – review & editing, conceptualization. Muhammad Qasim Shahid: conceptualization, project administration, supervision, funding acquisition, methodology, resources, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the National Natural Science Foundation of China (NSFC) (32350610253) and the Laboratory of Lingnan Modern Agriculture Project (NT2021001). Ms Yu Shuhong, Ms Sun Lixia, Ms Lai Mingyu and the other lab employees' assistance with the tests and analyses is gratefully acknowledged by the authors.References
- K. A. Alaboudi, et al., Phytoremediation of Pb and Cd contaminated soils by using sunflower (Helianthus annuus) plant, Ann. Agric. Sci., 2018, 63, 123–127 CrossRef.
- M. Mourato, et al., in Cadmium Toxicity and Tolerance in Plants, ed. M. Hasanuzzaman, M. N. V. Prasad and M. Fujita, Academic Press, 2019, ch 13 – The Effect of Cd Stress in Mineral Nutrient Uptake in Plants, pp. 327–348 Search PubMed.
- H. Zhao, et al., Effects of cadmium stress on growth and physiological characteristics of sassafras seedlings, Sci. Rep., 2021, 11, 1–11 CrossRef PubMed.
- T. El Rasafi, et al., Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies, Crit. Rev. Environ. Sci. Technol., 2020, 52, 675–726 CrossRef.
- U. Zulfiqar, et al., Cadmium Toxicity in Plants: Recent Progress on Morpho-physiological Effects and Remediation Strategies, J. Soil Sci. Plant Nutr., 2022, 22, 212–269 CrossRef CAS.
- X. Wang, et al., Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root, Materials, 2023, 16, 3097 CrossRef CAS.
- Y. Feng, et al., Effects of Iron Oxide Nanoparticles (Fe3O4) on Growth, Photosynthesis, Antioxidant Activity and Distribution of Mineral Elements in Wheat (Triticum aestivum) Plants, Plants, 2022, 11, 1894 CrossRef PubMed.
- F. Ghouri, et al., The protective role of tetraploidy and nanoparticles in arsenic-stressed rice: Evidence from RNA sequencing, ultrastructural and physiological studies, J. Hazard. Mater., 2023, 458, 132019 CrossRef CAS.
- M. Lai, et al., Modulation of metal transporters, oxidative stress and cell abnormalities by synergistic application of silicon and titanium oxide nanoparticles: A strategy for cadmium tolerance in rice, Chemosphere, 2023, 345, 140439 CrossRef CAS PubMed.
- A. Hussain, et al., Combined use of different nanoparticles effectively decreased cadmium (Cd) concentration in grains of wheat grown in a field contaminated with Cd, Ecotoxicol. Environ. Saf., 2021, 215, 112139 CrossRef CAS.
- H. F. Alharby, et al., Combined Role of Fe Nanoparticles (Fe NPs) and Staphylococcus aureus L. in the Alleviation of Chromium Stress in Rice Plants, Life, 2022, 12, 338 CrossRef CAS PubMed.
- Y. Van de Peer, et al., Polyploidy: an evolutionary and ecological force in stressful times, The Plant Cell, 2021, 33, 11–26 CrossRef PubMed.
- F. Ghouri, et al., Polyploidy and zinc oxide nanoparticles alleviated Cd toxicity in rice by modulating oxidative stress and expression levels of sucrose and metal-transporter genes, J. Hazard. Mater., 2023, 448, 130991 CrossRef CAS PubMed.
- F. Ghouri, et al., Silicon and iron nanoparticles protect rice against lead (Pb) stress by improving oxidative tolerance and minimizing Pb uptake, Sci. Rep., 2024, 14, 5986 CrossRef CAS.
- M. Li, et al., Multiple responses contribute to the enhanced drought tolerance of the autotetraploid Ziziphus jujuba Mill. var. spinosa, Cell Biosci., 2021, 11, 1–20 CrossRef.
- L. Quadrana, et al., Transposition favors the generation of large effect mutations that may facilitate rapid adaption, Nat. Commun., 2019, 10, 1–10 CrossRef CAS.
- H. K. Lichtenthaler, [34] Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes, in Methods in Enzymology, Elsevier, 1987, vol. 148, pp. 350–382 Search PubMed.
- H. Siddiqui, et al., Comparative effect of 28-homobrassinolide and 24-epibrassinolide on the performance of different components influencing the photosynthetic machinery in Brassica juncea L, Plant Physiol. Biochem., 2018, 129, 198–212 CrossRef CAS PubMed.
- B. D. Patterson, et al., Estimation of hydrogen peroxide in plant extracts using titanium(IV), Anal. Biochem., 1984, 139, 487–492 CrossRef CAS.
- M. L. Dionisio-Sese, et al., Antioxidant responses of rice seedlings to salinity stress, Plant Sci., 1998, 135, 1–9 CrossRef CAS.
- X. Z. Zhang, The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system, 1992 Search PubMed.
- M. W. Fariss, et al., High-performance liquid chromatography of thiols and disulfides: Dinitrophenol derivatives, in Methods in Enzymology, Academic Press, 1987, vol. 143, pp. 101–109 Search PubMed.
- M. Riaz, et al., Nano-silicon mediated alleviation of Cd toxicity by cell wall adsorption and antioxidant defense system in rice seedlings, Plant Soil, 2022, 486, 1–15 Search PubMed.
- K. J. Livak, et al., Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method, Methods, 2001, 25, 402–408 CrossRef CAS PubMed.
- N. Arrigo, et al., Rarely successful polyploids and their legacy in plant genomes, Curr. Opin. Plant Biol., 2012, 15, 140–146 CrossRef CAS.
- F. U. Haider, et al., Cadmium toxicity in plants: Impacts and remediation strategies, Ecotoxicol. Environ. Saf., 2021, 211, 111887 CrossRef CAS.
- C. Yang, et al., A newly formed hexaploid wheat exhibits immediate higher tolerance to nitrogen-deficiency than its parental lines, BMC Plant Biol., 2018, 18, 1–12 CrossRef.
- J. Oustric, et al., Nutrient Deficiency Tolerance in Citrus Is Dependent on Genotype or Ploidy Level, Front. Plant Sci., 2019, 10, 127 CrossRef.
- R. Nazar, et al., Cadmium Toxicity in Plants and Role of Mineral Nutrients in Its Alleviation, Am. J. Plant Sci., 2012, 03, 1476–1489 CrossRef.
- H. Zhu, et al., Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants, J. Environ. Monit., 2008, 10, 713–717 RSC.
- L.-B. Wu, et al., Shoot tolerance mechanisms to iron toxicity in rice (Oryza sativa L.), Plant, Cell Environ., 2017, 40(4), 570–584 CrossRef CAS.
- X. Meng, et al., Ectopic expression of IMA small peptide genes confers tolerance to cadmium stress in Arabidopsis through activating the iron deficiency response, J. Hazard. Mater., 2022, 422, 126913 CrossRef CAS PubMed.
- M. Ruiz, et al., Tetraploidy Enhances Boron-Excess Tolerance in Carrizo Citrange (Citrus sinensis L. Osb. × Poncirus trifoliata L. Raf.), Front. Plant Sci., 2016, 7, 701 Search PubMed.
- R. M. Balal, et al., Kinnow mandarin plants grafted on tetraploid rootstocks are more tolerant to Cr-toxicity than those grafted on its diploids one, Environ. Exp. Bot., 2017, 140, 8–18 CrossRef CAS.
- M. F. Khalid, et al., Nanoparticles: The Plant Saviour under Abiotic Stresses, Nanomaterials, 2022, 12, 3915 CrossRef CAS.
- F. B. Ulum, et al., Polyploidy Improves Photosynthesis Regulation within the Ranunculus auricomus Complex (Ranunculaceae), Biology, 2021, 10, 811 CrossRef.
- Z. Münzbergová, et al., Effects of polyploidization on the contents of photosynthetic pigments are largely population-specific, Photosynth. Res., 2019, 140, 289–299 CrossRef.
- P. M. Yang, The mechanism of starch content increase in grain of autotetraploid rice (Oryza sativa L.), Photosynthetica, 2019, 57, 680–687 CrossRef CAS.
- J. Zhu, et al., Increased ZnO nanoparticle toxicity to wheat upon co-exposure to phenanthrene, Environ. Pollut., 2019, 247, 108–117 CrossRef CAS PubMed.
- M. Faizan, et al., Zinc oxide nanoparticles alleviates the adverse effects of cadmium stress on Oryza sativa via modulation of the photosynthesis and antioxidant defense system, Ecotoxicol. Environ. Saf., 2021, 220, 112401 CrossRef CAS.
- X. Ma, et al., Simultaneous reduction of arsenic (As) and cadmium (Cd) accumulation in rice by zinc oxide nanoparticles, Chem. Eng. J., 2020, 384, 123802 CrossRef CAS.
- A. Shoaib, et al., Oxidative Stress in Plants Exposed to Heavy Metals, in Organic Solutes, Oxidative Stress, and Antioxidant Enzymes Under Abiotic Stressors, CRC Press, 2021 Search PubMed.
- M. Rizwan, et al., Cadmium stress in rice: toxic effects, tolerance mechanisms, and management: a critical review, Environ. Sci. Pollut. Res., 2016, 23, 17859–17879 CrossRef CAS PubMed.
- S. Sharma, Effect of Nanoparticles on Oxidative Damage and Antioxidant Defense System in Plants, Mol. Plant Abiotic Stress, 2019, 315–333 CAS.
- A. Singh, et al., Role of nanoparticles in crop improvement and abiotic stress management, J. Biotechnol., 2021, 337, 57–70 CrossRef CAS PubMed.
- M. Rizwan, et al., Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat, Chemosphere, 2019, 214, 269–277 CrossRef CAS PubMed.
- V. E. Tossi, et al., Impact of polyploidy on plant tolerance to abiotic and biotic stresses, Front. Plant Sci., 2022, 13, 869423 CrossRef.
- T. Liao, et al., Adaptive photosynthetic and physiological responses to drought and rewatering in triploid Populus populations, Photosynthetica, 2018, 56, 578–590 CrossRef CAS.
- R. Lourkisti, et al., Enhanced Photosynthetic Capacity, Osmotic Adjustment and Antioxidant Defenses Contribute to Improve Tolerance to Moderate Water Deficit and Recovery of Triploid Citrus Genotypes, Antioxidants, 2022, 11, 562 CrossRef CAS PubMed.
- J.-D. Chang, et al., The vacuolar transporter OsNRAMP2 mediates Fe remobilization during germination and affects Cd distribution to rice grain, Plant Soil, 2022, 476, 79–95 CrossRef CAS.
- H. Huang, et al., Synergistic effect of silicon and selenium on the alleviation of cadmium toxicity in rice plants, J. Hazard. Mater., 2021, 401, 123393 CrossRef CAS.
- R. Takahashi, et al., The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice, Plant, Cell Environ., 2012, 35, 1948–1957 CrossRef CAS.
- M. F. Adil, et al., Cadmium-zinc cross-talk delineates toxicity tolerance in rice via differential genes expression and physiological/ultrastructural adjustments, Ecotoxicol. Environ. Saf., 2020, 190, 110076 CrossRef CAS PubMed.
- H. Li, et al., Inhibition of nitric oxide production under alkaline conditions regulates iron homeostasis in rice, Physiol. Plant., 2021, 172, 1465–1476 CrossRef CAS.
- A. Rasheed, et al., Role of genetic factors in regulating cadmium uptake, transport and accumulation mechanisms and quantitative trait loci mapping in rice. A REVIEW, Appl. Ecol. Environ. Res., 2020, 18, 4005–4023 CrossRef.
- H. Nakanishi, et al., Iron deficiency enhances cadmium uptake and translocation mediated by the Fe 2+ transporters OsIRT1 and OsIRT2 in rice, Soil Sci. Plant Nutr., 2006, 52, 464–469 CrossRef CAS.
- A. U. Rehman, et al., Toxicity of heavy metals in plants and animals and their uptake by magnetic iron oxide nanoparticles, J. Mol. Liq., 2021, 321, 114455 CrossRef.
- J. Cui, et al., Silica nanoparticles alleviate cadmium toxicity in rice cells: Mechanisms and size effects, Environ. Pollut., 2017, 228, 363–369 CrossRef CAS.
- S. U. Rahman, et al., A review of the influence of nanoparticles on the physiological and biochemical attributes of plants with a focus on the absorption and translocation of toxic trace elements, Environ. Pollut., 2022, 310, 119916 CrossRef CAS PubMed.
- Z. Sun, et al., Influences of zinc oxide nanoparticles on Allium cepa root cells and the primary cause of phytotoxicity, Ecotoxicology, 2019, 28, 175–188 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4en00470a |
| This journal is © The Royal Society of Chemistry 2025 |