Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Characterizing amino compounds in indoor poultry farms: air quality and its impact on workers and chickens in Canadian egg farms†
Xinyang
Guo
 a,
Rowshon
Afroz
a,
Rowshon
Afroz
 a,
Shuang
Wu
a,
Shuang
Wu
 ab,
Kimberly
Wong
ab,
Kimberly
Wong
 a,
Valerie
Carney
c,
M. J.
Zuidhof
a,
Valerie
Carney
c,
M. J.
Zuidhof
 c,
Joey
Saharchuk
d,
Hans
Osthoff
c,
Joey
Saharchuk
d,
Hans
Osthoff
 d and
Ran
Zhao
d and
Ran
Zhao
 *a
*a
aDepartment of Chemistry, University of Alberta, Edmonton, Alberta T6G 2G2, Canada. E-mail: rz@ualberta.ca
bAlberta Energy Regulator, Edmonton, Alberta T6B 2X3, Canada
cDepartment of Agriculture, Food and Nutrition Science, University of Alberta, Edmonton, Alberta T6G 2G2, Canada
dDepartment of Chemistry, University of Calgary, Calgary, Alberta T2N 1N4, Canada
First published on 26th February 2025
Abstract
Indoor air pollution is a common problem in poultry and many livestock facilities. Small airborne amino chemicals (AACs), such as ammonia and short-chain amines, are common air pollutants in poultry farms. An elevated concentration of AACs can reduce the indoor air quality (IAQ) of the farm, affecting the production of chicken eggs, the welfare of the animals, and the occupational health of producers. Recent studies have identified ammonia and small volatile organic pollutants in poultry farms. However, the characterization of large AACs, such as uric acid (UA) and large amines, has rarely been reported, although many of them have been proposed as the main form of biological nitrogen waste. Our goal is to provide information on organic amino pollutants in poultry farms. This project includes an online aerosol sample using a particle-into-liquid sampler (PILS) and an offline chemical analysis using liquid chromatography mass spectrometry (LC-MS). With a selective characterization of AACs in a poultry farm, we found that UA and suspended particles are correlated with onsite management practices, such as barn lights. Among the three major indoor phases (gas, particles, and litter) in the facility, we report the phase partition of UA, NH3, NH4+, and large amines. The observation of these indoor pollutants has implications on the formation of dust particles and ammonia, and the results can benefit the poultry industry in solving persistent IAQ problems.
Environmental significanceAirborne amino chemicals (AACs) are common pollutants in the poultry industry, which are of great concern for the welfare of animals and the occupational health of producers. It is important to characterize these chemicals in poultry farms, as many of these AACs are precursors to ammonia which can significantly degrade air quality. Our research has discovered many AACs on the farm and highlighted their partitioning between air, particles, and litter phases. In addition, we report on the diurnal trend of uric acid, one of the main AACs in the air. Our work has explored the origin of ammonia pollution on a poultry farm, which can be applied to other livestock facilities. At the same time, we have emphasized the implications of indoor air pollution on the welfare of animals and the occupational health of producers. |
Introduction
Indoor air quality (IAQ) has become increasingly recognized for its impact on public health and well-being in past years.1 Recent studies have shown that the air quality in residential homes is influenced by human emissions,2,3 animal or biological activities, and chemical processes.4,5 The IAQ of the workplaces is just as crucial as that of residential settings, given that many contemporary occupations occur indoors.6 While government agencies have established general IAQ protocols,7 workers may be exposed to air pollutants unique to their specific occupations, indicating a need of tailored standards. For industries that are the main emitters of air pollutants, workers can experience prolonged exposure to concentrations that exceed safety thresholds, threatening both their productivity and occupational health.8,9The US Department of Labor has identified common biological, chemical, and particulate pollutants in commercial and institutional indoor buildings, but has also provided only general administrative and control guidance.10 Managing IAQ in workplaces with diverse indoor environments remains challenging, as general benchmarks are insufficient to ensure clean air. Research has shown that air pollution in office environments not only causes discomfort, but also contributes to cardiovascular or respiratory diseases.11–14 For industries that predominantly involve indoor activities, such as exhibitions,15,16 entertainment,17,18 and beauty industry,19,20 exposure to volatile organic compounds (VOCs) is a significant concern. Similarly, the poultry industry faces severe air quality challenges. With elevated levels of air pollutants such as carbon dioxide (CO2),21 ammonia (NH3), particulate matter (PM10 and PM2.5),22 and VOCs.23 These pollutants are often associated with reduced chicken productivity and welfare,24–26 yet systematic studies remain limited. Despite the widespread of air pollution problems in commercial poultry farms,27,28 the cost of implementing additional air quality control measures often discourages producers from taking action.29
The primary source of air pollutants in poultry facilities is manure, which can be easily resuspended by birds' activities.30 Airborne amino chemicals (AACs) are common air pollutants in these environments, often characterized by their odorous and toxic nature.31 Small and highly volatile aliphatic amines and NH3 are the most frequently detected and monitored compounds, knowing their strong odors and high concentrations.28,32,33 NH3 serves as a key indicator of IAQ in poultry facilities, and should be kept below 25 ppm, according to Canadian Council on Animal Care (CCAC).34 This level also represents the threshold at which hens exhibit aversion.35 High concentration of NH3 can be negatively impact chickens, leading to reduced body weight gain, poorer calorie conversion, and weakened immune system functions.36 However, chickens are typically not the direct source of NH3; instead, various organic amino wastes from animals act as its precursors. For example, enzyme-assisted microbial decomposition of uric acid (UA) is a major contributor to indoor NH3.37,38 Therefore, it is necessary to comprehensively understand these compounds within poultry farms. By implementing targeted measures, producers can indirectly improve their management of NH3 pollution, thus mitigate the risks associated with both farmer health and animal welfare.
UA is a common biogenic amino chemical found in both animal and plant bodies,39,40 and is abundant in agricultural facilities that contain animal and plant waste. Although UA is the main source of nitrogen in such settings,41–43 its presence in aerosols and the indoor atmosphere is rarely documented. Airborne UA can not only contribute to an elevated concentration of total AAC, but can also enter the respiratory system.44,45 It remains unclear whether airborne UA can facilitate the accumulation of suspended dust or if any adverse health effects are associated with chronic exposure. Furthermore, the microbial decomposition of UA produces CO2 and NH3, both of which are critical indicators of IAQ.46,47
In addition to UA, numerous organic AACs are known to serve as precursors of small inhalable amino species.48 Characterizing these compounds can be challenging23,49,50 largely due to limited analytical techniques.51 Amines, such as cadaverine (CAD), putrescine (PUT), urea and guanine (GUA), are often found in chicken products and are decomposition products of large bio-molecules.52,53 These AACs can also been emitted into the atmosphere and contribute to total VOCs.54,55 Amines are often involved in acid–base chemical reactions due to the basic amino group. Consequently, changes in surrounding environmental conditions, such as temperature, ions, or pH, can influence their emission into the farm air.56,57
The objective of this study is to provide detailed information on nitrogen emissions in indoor poultry farms. First, we will demonstrate a time-resolved collection and quantification of AACs. Second, we will evaluate the distributions of the AACs in different indoor phases, including air, particles, and litter. Third, using UA as an indicator, we will explore the correlation between AACs and IAQ parameters by monitoring aerosol time profiles. The findings of this work would expand our understanding of air quality in the animal husbandry industry, thereby supporting future efforts to improve animal productivity and the well-being of producers.
Material and methods
Chemicals and materials
The deionized water used in this study was made using a Thermo-Fisher Scientific Barnstead™ E-Pure™ Ultrapure Water Purification System. HPLC grade acetonitrile, boric acid (>99.5%), formic acid (98–100%), ammonium hydroxide (NH4OH) solution (28% NH3 in water), uric acid (>99%), cadaverine (95%), putrescine (99%), guanine (98%), allantoin (>98%), urea (99.0–100.5%), p-toluenesulfonyl chloride (TsCl) (>99%) were purchased from Sigma-Aldrich. Sodium hydroxide pellets were purchased from Fisher Chemical.Two buffers were prepared for sample collection and extraction. A 0.25 M sodium borate buffer was prepared by dissolving boric acid solids in deionized water, with its pH then adjusted to 9.0 by NaOH. A 0.1% formic acid scrubbing solution (pH = 2.7) was prepared by dissolving formic acid in deionized water. These two solutions are herein referred to as the basic buffer and the acidic scrubber to be used in subsequent steps.
Sample collection and treatment
Aerosol samples were collected using a particle-into-liquid sampler (PILS) (Model 4001) and an auto collector manufactured by Brechtel Inc. The PILS is equipped with a gas denuder, a particle impacter, and a sample inlet tube. The gas denuder contains active charcoal strips that can remove gaseous compounds from the sample air. The particle impactor allows particles smaller than 2.5 μm in diameter to pass through. The inlet tube is a 30 cm long stainless steel tube, whose diameter is 0.5 inch. The number, mass and size of aerosols are monitored by an optical particle counter (OPC) (Model 11-C) manufactured by Grimm Inc. The density of all types of aerosols was assumed to be 2 g cm−3 during its operation. However, we acknowledge that the atmosphere of poultry farms is distinct from ambient atmosphere, hence this assumption may not be accurate in farm-alike environments, hence would bring errors when calculating the particle mass concentration.Fig. 1 is a schematic of the approaches taken to measure AACs in indoor poultry facilities. Preliminary functionality tests of all but intercomparison instruments involved in Fig. 1 were performed at the Poultry Research Center (PRC) of the University of Alberta. The PRC farm had floor-pen housings for a small flock of 70–75 birds, and the entire barn area had about 1200 birds. Commercial farm samples involved in this study were collected on a farm located near Camrose, Alberta, Canada (Fig. S1†). The farm was a completely indoor, organic, and free-range table egg facility. The barn we sampled was the home of 8000 birds at approximately 60 to 70 weeks of age. On the commercial farm, four trials of instrument testing and sampling were carried out between November 2022 and March 2023. During these preliminary activities, we identified the most suitable location for the instruments in the barn, such that our collection would receive minimized impact from farmers' activities and farm machines. We also determined the intake rate of PILS and OPC, which will be used for quantification in the latter sections.
Gas samples were collected using a homemade impinger driven by a diaphragm pump, and the gas flow rate was controlled by an Alicat mass flow controller at 0.7 L min−1 for 30 min. Upstream of the pump, a 0.2 μm Watman filter was installed to remove the incoming particles. This filter was extracted by stirring in the basic buffer for 1 hour, and the resulting extract was submitted to the Natural Resource Analytical Laboratory (NRSL) at the University of Alberta for anion analysis. The acidic scrubber described above was used to maximize the collection of the gaseous NH3. We also evaluated the scrubbing capacity of our acidic solution. Under the maximum regulated indoor concentration NH3 (25 ppm), the molarity of formic acid in the solution is still approximately 40 times higher than NH3 collected throughout the gas sampling period. The acidic solution thus will not lose the scrubbing efficiency during sampling. However, the collection efficiency of an impinger can be affected by its design, for example, residence time, surface area of contact, or flow rate. These parameters were not optimized in our study and there is no other NH3 analyzer on site for reference. While we assumed that the impinger achieved a 100% collection efficiency, our reported NH3 concentration may underestimate the actual value.
The temporal profile of particles was collected by the OPC, and time-resolved aerosol samples were collected by PILS and its autocollector in the basic buffer for subsequent chemical analysis. Here, the collection was carried out in the basic buffer, as it showed a stronger response to most AACs, including UA, than the acidic scrubber solution (Section S2†). We note that the basic buffer can compromise the collection of NH4+ as the pH of the buffer is close to the pKa of NH4+. We expect that a portion of NH4+ may evaporate after being collected, which means that our reported value can underestimate the actual value. The solvent was driven by a peristaltic pump at a rate of 0.3 mL min−1, the resulting solution was then injected directly into a 1.8 mL autosampler vial every 2 min. Due to limited slots in the autosampler, sometimes, these samples were also collected in a 12 mL vial every 20 minutes for prolonged collections.
Chicken litter samples were collected by hand picking five random locations within the farm. The five samples were pooled by shaking them in a 20 mL glass vial after collection. In the laboratory, a portion of the litter was weighed and extracted using an orbital shaker in 20 mL of basic buffer at room temperature for an hour. We noted that the litter sample was a mixture of chicken manure and bedding materials made primarily of wood pellets (Fig. S3 in the ESI†). Therefore, the concentration of AACs will vary depending on the ratio between chicken manure and wood pallets. For example, if one portion of the litter contains more chicken manure materials, it would likely have more AACs.
In this paper, we report a set of results obtained from one sampling campaign on the commercial farm. This campaign was conducted on April 13, 2023, and involved all types of samples mentioned above. On this day, we collected a set of samples containing 50 PILS samples, a time series of OPC samples, one vial of gas impinger sample, and approximately 10 grams of homogenized litter sample. April is in the winter season of Alberta, Canada. During this season, the ventilation of the air in the barn was usually minimized to reduce the loss of heat to the outdoor environment, hence the farm IAQ is expected to be the worst of the year. The lighting in the barn is governed by incandescent light bulbs that are covered with red plastic covers. Farmers advised us that red light could reduce chicken anxiety. After the sampling campaign, all field samples were analyzed on the same day in the laboratory. A sketch of the barn can be found in Fig. S2† in the ESI.†
Derivatization and chemical analysis of AACs
The primary chemical analysis instrument was the Thermo-Fisher Accela Liquid Chromatography (LC) system and Thermo-Fisher LTQ-XL mass spectrometer operated in positive electrospray ionization mode (ESI+). The ESI is a soft ionization source that retains molecular ions at the source, and hence it is suited for observing unknown species. The LTQ-XL offers a rapid scanning rate, while also having adequate resolution for the preliminary speciation and tracking of selected TsCl derivatives. The column for LC separation was a Phenomex Luna Omega polar C-18 column, dimension 150 mm × 2.1 mm × 3 μm. An Orbitrap high-resolution mass spectrometer (Thermo-Fisher Exactive Orbitrap) was also used for identification. This Orbitrap MS offers an MS scanning resolution greater than 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, thus it is very suitable to obtain the elemental composition of unknown compounds.
000, thus it is very suitable to obtain the elemental composition of unknown compounds.
The derivatization was performed directly inside the autosampler vial. The derivatization method was developed and modified from Nalazek-Rudnicka et al.58 All samples were mixed with 0.052 M TsCl solution in acetonitrile and prepared in the basic buffer. The derivatization takes at least two hours in a 50 °C water bath. All derivatized samples were analyzed using the positive mode of LC-ESI-MS, in which molecular ions are detected as [M + H]+. Details and settings for this instrument are listed in Section S3 of the ESI.†
TsCl is known to be selective towards amino and alcohol groups, forming sulfonamides and sulfonates by nucleophilic tosylation.59,60 The resulting sulfonates and sulfonamides have higher molecular weight and lower polarity, thus improving the separation of amino compounds in the reverse phase C18 column. In addition, since alcohol-derivatized sulfonates are only stable under highly basic conditions,61 the mildly basic condition used here only retains stable amine-derivatized sulfonamides. All TsCl derivatives were first identified according to their molecular weights in high-resolution Orbitrap MS with empirical formulae. Identities of potential amines are further confirmed by referring to commercial standards.
We selectively quantified UA and NH4+ in our sample with external calibration methods. UA calibration curve was performed in the basic buffer and had five data points ranging from 0 to 400 μM, with the R2 value greater than 0.9990. NH4+ calibration curve had six data points ranging from 0 to 20 mM, with the R2 value greater than 0.9990.
Identification of TsCl derivatives
TsCl derivatives were identified by signature isotopic profiles (Fig. S8†), as the sulfur in the TsCl derivative can result in separated peaks at the mass of [M + 2]+ position. Due to the mass of [34S–32S] being smaller than 2× [13C–12C], the lighter peak at this position is [M(34S)]+, and the heavier peak is the combination of [M(13C2)]+ and [M(14C)]+. Furthermore, since the natural abundance of 34S is higher than 14C or 13C, the lighter peak will be more intense.62 Suppose that the only source of sulfur in our samples was TsCl, any compounds that contain this isotopic peak pattern are its derivatives. Details about this identification method can be found in Section S5.†Modeling and assumptions
The phase partition of selected AACs between the gas phase and suspended particles was estimated using the Extended Aerosol Inorganic Model (E-AIM, http://www.aim.env.uea.ac.uk/aim/aim.php). Concentrations of neutral and ionic forms of these AACs in both the aqueous phase and the gas phase were estimated using Model 2. Input parameters of the model are based on our observation and are listed in Section S3.† The primary reason for using E-AIM is that the sample of particles and litter collected on the poultry farm is rich in anions. In addition, the partition of many primary and secondary amines is strongly influenced by inorganic ion concentrations and aqueous dissociation.63,64 Hence, using E-AIM is appropriate as it considers thermodynamic equilibrium between the neutral and protonated form of amines.A few assumptions are made to execute the model simulation. (1) The partition of AACs is assumed to take place only between the particle and gas phase. (2) Modeled water-soluble ions are only H+, NH4+, SO42−, and NO3− per the model design. (3) The extraction efficiency of anions from the filter sample is assumed to be 100%, as the NH4+ form of sulfate and nitrate are very water-soluble. (4) Modeled organic compounds are only dimethylamine (DMA), PUT, and CAD, due to the limited availability of thermodynamic properties in the E-AIM library.56,57 (5) Since we did not observe CAD in particles, the modeled CAD concentration in the particle is assumed to be approximately 100 times lower than PUT, according to their ratio in litter samples (shown in the later section).
Quality control and instrument validation
Thermo 17i NH3 analyzer and a scanning mobility particle sizer (SMPS, TSI Inc.) were used to evaluate the efficiency of PILS. The SMPS includes a diffusion mobility analyzer (Model 3080) and a condensation particle counter (Model 3775).Although the PILS is designed to collect particles, a trace amount of gaseous chemicals may penetrate the gas denuder. To determine this potential bias, we performed an inter-comparison experiment between PILS and Thermo 17i ammonia analyzer. During this experiment, both PILS and Thermo 17i had collected laboratory-generated ammonia and ammonium bisulfate particles from the same chamber. In addition to the efficiency of the gas denuder, we have also obtained the standard error of the PILS collection (6.7%), which will serve as error bars in the following quantitative analysis in this study. Detailed information on this experiment can be found in Section S4 in the ESI.†
Due to technical constraints, our lab-generated NH3 could only reach approximately 100 ppb in the gas phase (Table S4†). This is about 1–2% of the concentration we observed in the farm (presented in following sections). Hence, we were unable to fully reproduce the farm environment in our inter-comparison experiment, and some assumptions were necessary. First, the PILS operation parameters are the same between the laboratory and the farm, such as the peristaltic pump rate, the air pump rate, the denuder efficiency, and the particle impacter efficiency. Second, our reference instrument (Thermo 17i) reflects the actual NH3/NH4+ concentration in the chamber, although it has been shown that its accuracy varies between 3.7% to 10.5% on average according to the US Environment Protection Agency (EPA).65
Without the gas denuder, PILS collected 59.1% of the gas phase NH3− related to the NH3 analyzer. When the denuder was mounted, the penetrated concentration of NH3 was below the detection limit of LC-MS (LOD, 20 ppb, converted to gas phase), which can over-estimate the denuder efficiency. As a result, we performed another test using DMA, which has a better LOD (0.25 ppb), to help determine the denuder efficiency (96.3%). We acknowledge that the farm contains a much higher concentration of gases than our chamber, and the denuder penetration was expected to be higher on the farm. As a result, the actual denuder efficiency was expected to be lower than our reported value. This limitation implies that the characterization of PILS denuder efficiency requires a more dedicated experiment setup in future studies.
PILS was designed to collect fine particles (30 nm and above). Although the literature has shown that the collection efficiency between 30 nm and 10 μm is greater than 97%,66 a portion of the salt particles generated in this experiment was less than 30 nm, which was outside the optimal range of PILS. We noted that the NH3 analyzer is also capable of measuring aerosols containing NH4+. Its oven (approximately 800 °C) evaporates the NH4+ particles into NH3 which is then oxidized by catalysts. NH3 is converted to NO, which is then oxidized by O3, eventually being detected as photons emitted from excited NO2. This process is known as chemiluminescence. In this specific inter-comparison, PILS-LCMS has quantified 71.8% of NH4+ particles relative to Thermo 17i. Higher efficiency can be achieved when the particle size is larger according to the PILS working fundamentals.66,67 According to the size distribution collected by the OPC (shown in Fig. 5 in the latter section), many particles in the poultry farm are large particles between 2–20 μm. Therefore, biases due to ultrafine particles would be rather negligible.
A test for UA extraction and derivatization efficiency was performed by injecting a standard solution with a known concentration into litter bedding samples during extraction. Two sets of samples containing five pairs of spike–non-spike samples were prepared. The recovery value obtained was 72.7% ± 11.5%. Furthermore, we also performed a stability test of the derivatized sample to account for the sequence queueing time in the autosampler. This was done by repetitively analyzing the same derivatized UA. Through this experiment, the decay rate of the calibrated UA chromatography peak is found to be approximately 0.04% per minute, with the R2 value equals to 0.82. We determined that this signal decay is due to a gradual conversion of single-derived UA to its double-derived form, as samples are waiting to be analyzed. Additional details can be found in Sections S4 and S5,† and the corresponding correction to sample degradation has been applied to our time-resolved data series.
Results and discussion
Identification of AACs in different phases
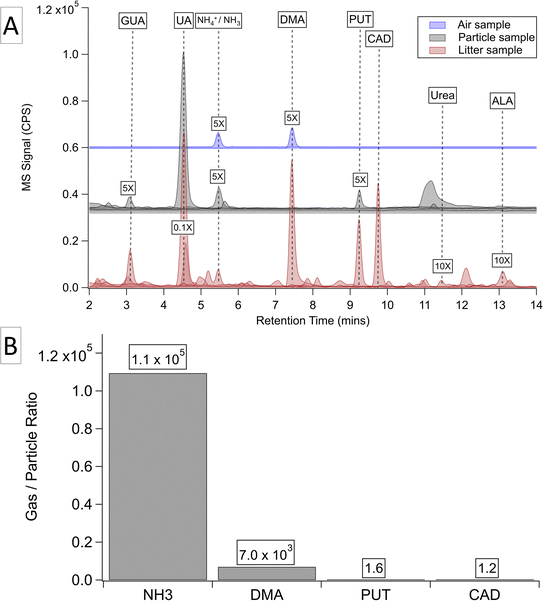
Using high-resolution mass spectrometry, our initial analysis detected 15 potential AACs 297 and proposed 10 species through library matching, as summarized in Section S5.† Using commercial standards, we further identified NH3, DMA, GUA, UA, PUT, and CAD, urea, and allantoin (ALA). It should be noted that trimethylamine is often abundant and is frequently reported in livestock facilities.31,32,68,69 However, it is inert to derivatization because TsCl cannot react with any tertiary amine due to the absence of active amino groups.The phase distribution of AACs among gas, particles, and litter was evaluated using a targeted analysis (Fig. 2A). We observed a general variation of AACs in different phases, indicating that phase partition of AACs plays a key role in the indoor environment. Volatile organic AACs tend to remain in the gas phase rather than in the particle phase, as the dust contains a limited moisture content (with a relative humidity of roughly 0.3 according to farm monitors) to dissolve volatile compounds. Larger AACs, such as UA, are restricted by their low volatility and are therefore absent in the gas phase. In contrast, the litter has a higher moisture content than the dust, allowing it to retain volatile species. To support our observations, we simulated the phase partition of NH3, DMA, CAD, and PUT using the E-AIM model in Fig. 2B.56,57,70 The model predicts the concentration of AACs in each phase, with the gas–particle ratio calculated based on these predicted concentrations. Input parameters, including anion concentrations and organic AAC concentrations, are derived from results obtained from NRSL and our LC-MS analysis (Table S3†).
Fig. 2A shows that the gas phase contains only NH3 and DMA. According to the E-AIM model prediction, these two compounds have high predicted gas–particle ratios (1.1 × 105 and 7.0 × 103, respectively), indicating their strong preferences for partitioning into the gas phase. In contrast, the gas–particle ratios of CAD and PUT are as low as 1.6 and 1.2, indicating that a relatively small fraction of compounds are volatile, compared to NH3 and DMA. The low concentrations of gaseous CAD and PUT could fall below our LOD and hence be absent in our gas sample.
UA, GUA, NH4+, and PUT are detected in the particle phase, suggesting that they are the dominant amino component in suspended dust particles. Therefore, these AACs are more likely to be inhaled by chickens and farm workers. Depending on the size of the particles to which they have been attached, these chemicals can be deposited in different sections of the respiratory system. The absence of DMA in the particle phase agrees with the prediction from the E-AIM model. Because the gas–particle ratio is 7 × 103, most DMA would be volatile. In addition, unlike NH3, which can form inorganic salts, there are very few ionic compounds that DMA can produce. Hence, it is unlikely for DMA to remain in the particle phase.
Compared to gas and dust samples, chicken litter contains the widest range of AACs. The peak of UA in this phase is much higher than that of other chemicals, exemplifying its potential dominance in the litter. The presence of volatile DMA in the litter could be due to its attachment to the moisture content of the litter, or its entrapment within the porous structure of litter particles. Urea and ALA are detected exclusively in the litter, suggesting their inability to be retained in particles or gas. Additionally, the detection of these two compounds indicates that the litter is the reaction site of UA decomposition, as they are known intermediates in this process.46,47 This observation implies that the litter serves as a continuous source and reservoir for NH3 within the barn.
Distribution of amino species in each phase
In the previous section, AACs exhibited a diverse distribution pattern across three indoor phases. This section focuses on determining the concentration of individual AACs in these three phases, as shown in Fig. 3, with anion molarity ratios shown as insets. Gas phase concentrations were calculated on the basis of the total volume of air sampled using the impinger. Particle phase concentrations were derived from LC-MS calibration results and then compared to the total particle mass (TPM) obtained by the OPC. Litter phase concentrations were calculated related to the dry mass of fresh litter. To determine the dry mass, the litter sample was baked in a 60 °C oven overnight, with the loss of mass approximated as the water content. For extraction, we decided to extract fresh litter instead of dry litter because the loss of volatile AACs would be inevitable during the drying procedure. The anion molarity ratios were determined using the colorimetric method of the US EPA,71 performed by NRSL. We did not conduct a cation analysis due to the scope of the study and limited instrument availability. As a result, our anion measurement reflects only the distribution of ammonium salts in the sample.For the anion molarity ratios, only phosphate, chloride, sulfate, nitrite, and nitrate were determined. These five anions are among the most abundant anion species in our sample. Other anions are likely present, such as conjugated bases of organic acids, which could also contribute to total ammonium. However, we were unable to provide a comprehensive overview as a result of the limited instrument and method capacity. We acknowledge that our reported percentages may be overestimated and serve as a preliminary quantification.
According to the pie chart inset in Fig. 3A, a moderate correlation is observed between the litter and the particle phase. In the particle phase, phosphate accounts for approximately half of the total ion content, followed by chloride, sulfate, and nitrate. In contrast, the litter (pie chart in Fig. 3B) contains a dominating fraction of phosphate compared to other anions, followed by nitrate and sulfate. Unlike the particle phase, chloride has only occupied a minimal ratio in the litter.
The high phosphate content in chicken litter is consistent with the literature, indicating that it originates primarily from direct excretion through manure.72 This suggests that the significant presence of phosphates in airborne particles may be due to the suspension of litter caused by air circulation and animal movement. Chloride is the second most abundant anion in particles; however, its relatively low proportion in the litter implies the existence of other sources of airborne chloride in addition to chicken manure. According to the producer, chicken feed contains various chloride compounds, such as choline chloride, suggesting that airborne chloride may partly originate from the feed. Overall, the presence of anions in both particles and litter has indicated a significant inhalable exposure to phosphate salts for both animals and workers, raising concerns about potential phosphate toxicity.73
The gas phase contains 5.40 ppm of NH3 and 0.047 ppm of DMA, shown by the box inserted in Fig. 3A. In the particle phase, the mass fractions of each AACs are calculated relative to an hourly averaged TPM concentration of 19 mg m−3, according to the OPC. NH4+ account for over 18% of the TPM, while this proportion is approximately 14 times lower in the litter (1.29%, Fig. 3B). This substantial difference suggests the presence of unidentified sources of NH4+ in airborne particles. The litter had a higher mass concentration of UA (2.64%) than that of particles (1.43% ± 0.28%), indicating that the litter is a major source of airborne UA. CAD occupies 0.32% of the litter mass, ranking as the third most dominant amino chemical. DMA has the lowest mass ratio among all AACs (0.011%), likely due to its high volatility and low molecular mass. It should be noted that we are unable to determine the source of these amines, but the existing literature suggested that the microbial metabolism of amino acids could be a contributing pathway.74
The commercial poultry farm presents a highly dynamic indoor environment, making it challenging to define typical concentrations for most of the indoor pollutants. Airborne chemicals are often influenced by factors such as chicken activity, ventilation, and farm infrastructure. To provide context, we compared our observation with existing studies in Table 1. In the gas phase, our measured NH3 concentration falls within the range reported in the literature and remains below the CCAC limit of 25 ppm.34 In the particle phase, our measured NH4+ concentration is of the same order of magnitude as the reported value but is three times higher, likely due to varying farm conditions. Our high time-resolution measurements captured periods of intense chicken activity, leading to an elevated average NH4+ and greater variability. Consequently, poultry farms are expected to exhibit rapid temporal fluctuations in suspended chemicals, closely related to chicken activities. Beyond NH or NH +, many other AACs remain unreported in the existing literature, making our results a potential reference for future research.
| Chemicals | Gas | Particle (mass) | Litter (mass) | |||
|---|---|---|---|---|---|---|
| Literature | This work | Literature | This work | Literature | This work | |
| a All percentage (%) and ppm units represent the mass concentration of the compound relative to the mass of the corresponding matrix (gas, particle, or litter). | ||||||
| NH3/NH4+ | 2.8–24.2 ppm (ref. 21, 43, 75 and 76) | 5.40 ppm | 5.45% ± 1.53% (ref. 77) | 18.41% ± 7.76% | 0.36–0.78% (ref. 78 and 79) | 1.29% ± 0.15% |
| DMA | <0.57 mg m−3 (ref. 32 and 80) | 0.058 mg m−3 | N/A | Below LOD | N/A | 0.011% |
| UA | N/A | Below LOD | N/A | 1.43% ± 0.28% | 0.78–3.0% (ref. 81 and 82) | 2.64% ± 0.17% |
| Total particle | N/A | N/A | 0.05–9.61 mg m−3 (ref. 28, 77 and 83) | 7.2–36.8 mg m−3 | N/A | N/A |
Dust and chemical correlation
To explore the correlations of AACs with other indoor conditions, such as farm lighting and common IAQ parameters, we obtained PILS samples near the evening of the sampling day. This particular day was chosen for several reasons: first, the outdoor temperature was moderate, representing a typical winter day in the local Alberta. Second, the producers planned to remove the birds from the farm in the evening, providing a unique opportunity to observe the direct impact of human-induced chicken activities on airborne compounds. Third, this timing allowed us to study the diurnal cycle of IAQ on the farm, as it covers the complete sleep–wake cycle of chickens within a short evening.Fig. 4A shows the time profile of UA and TPM measured by PILS-LCMS and OPC, 412 and the shading of the background indicates the change in the lighting conditions in the barn. UA and TPM concentrations were plotted against each other to elucidate their correlations (Fig. 4B). We differentiated our sampling period into three zones: day-time, sunset, and night-time, each of them stands for different light intensities. The farm light had the maximum output during the day-time (white zone) and gradually dimmed during the sunset (light gray zone). During the night-time, there were no lights inside the barn (dark gray zone).
During the day-time, the TPM fluctuated around 3 × 104 μg m−3 while the UA concentration can be as high as 500 μg m−3. The UA concentration constitutes approximately 1.5% of the TPM, which is consistent with the results presented in Fig. 3. To highlight the potential occupational health risks due to polluted farm air, we conducted a brief estimation of human exposure to airborne chemicals at these TPM and UA levels. Assuming TPM and UA concentrations of 20 mg m−3 and 250 μg m−3, respectively, and using a typical adult breath rate of 6 L min−1,84 farm workers are estimated to inhale 1.5 μg of UA per minute. Considering the previously mentioned NH4+ ratio of 18%, farm workers would also inhale 21.6 μg of NH4+ per minute during their shift.
The elevated day-time airborne dust and chemical are primarily attributed to chicken activities. Based on our on site visual observations, most of the birds gather on the ground during this period, and were in direct contact with the chicken litter. Bird movement can stir up dust from the litter bedding, leading to increased concentration of both UA and TPM. Fluctuations in the TPM levels are likely due to local activities of chickens, which creates plumes of dust reaching our instrument. For instance, a notable spike in both TPM and UA concentrations was observed at 17:05 during an intense activity event when chickens became agitated.
During the sunset period, the chickens started moving to upper layers, which is a steel rack and served as sleeping places for birds. As the steel rack was free of litter, the movement of chickens could not suspend litter particles, leading to a gradual reduction of TPM in the air. When night arrived, the chickens fell asleep quickly, so on regular days the concentration of UA and TPM should have been maintained at a low level until the next morning. However, as the producers were in the process of removing the flock from the facility, sleeping birds awakened as the worker turned on the light. An increase in UA and TPM was observed after 19:00. The time profile of the TPM exhibited multiple sharp peaks that were not observed during the day-time. It is likely caused by farmer-induced localized and sporadic bird activities. The UA profile has shown rather a single broad peak than multipeaks, primarily because of the reduced PILS sampling frequency according to our sequence design.
The correlation between PILS and OPC results (R2 > 0.8) is shown in Fig. 4B. These two instruments were co-located during measurement. The regression analysis indicates that (1) particles are the main carrier of airborne UA, which is consistent with the discussions in previous sections, and (2) the fluctuating concentration of airborne UA reflects changing chicken activities on the farm. This consistency also indicates that UA shares a relatively stable ratio in airborne particles, which also reflects that airborne UA has a consistent source, such as the manure suspension.
During this case study, we also assessed the temporal size distribution of airborne particles, considering PILS has a minimum particle size threshold of 30 nm for optimal collection efficiency. A 2-D contour plot was generated to illustrate the concentrations of particles in different OPC size bins, ranging from 0.25 μm to 32 μm (Fig. 5). It is important to note that this size range is determined by the instrumental cut-off, as the OPC operates based on the Mie scattering of particles. According to Fig. 5, most of the particles were found to exceed the minimum size requirement of PILS, indicating that the collection would fall within the effective working range of PILS, therefore maximizing collection efficiency (97%).66
 | ||
| Fig. 5 2-D plot of the mass concentration distribution of all particle sizes throughout the experimental period. Temporal profile of PM2.5 concentration is illustrated against the right axis. | ||
A higher particle count was observed in all size bins during the active period of the chickens. The corresponding PM2.5 concentration was well above 1000 μg m−3 until 16:30. Combining with the observed 1.5% ratio, the estimated UA in PM2.5 was more than 15 μg m−3 during this period. Although there is no current indoor PM2.5 exposure limit set by the Canadian government, our observed concentration is a few orders of magnitude higher than the Canada-wide 24-hour standard (27 μg m−3).85 Therefore, farmers should be aware that their working environment is much worse than the federal standard. In addition, chicken pulmonary systems have been found to be highly susceptible to pathogens.86 Exposure to elevated particulate matter can induce cardiotoxicity in chicken embryos87 and reduce growth performance in hatched birds.88
Conclusions
Our project has demonstrated the most comprehensive exploration of airborne amino chemicals (AACs) within a commercial poultry farm. This study has identified and quantified a range of organic and inorganic AACs, many of which have never been previously evaluated. Amino species share a large proportion of chemicals in commercial poultry farms. Elevated concentrations of these chemicals can directly degrade indoor air quality, which can pose risks to the occupational health of workers. More importantly, a high level of AACs can negatively impact the welfare of birds, reduce productivity, and undermine the cost-effectiveness of investments in the farm ventilation system.Although existing research focuses mainly on small volatile compounds,21,23 our results revealed the presence of a wide range of organic AACs and NH4+ salts in the air of a commercial poultry farm. Large organic AACs can act as potential precursors to NH3. Elevated concentrations of organic AACs in the indoor environment could influence the production and removal of NH3, ultimately affecting the nitrogen cycle in these settings. AACs exhibit a variable distribution across three indoor phases. In the gas phase, NH3 and DMA were quantified, with concentrations comparable to those reported in the literature.21,32 In the particle phase, NH4+ concentration was notably higher than that of the litter, suggesting that there may be unrecognized sources of NH4+. Large organic AACs, including UA, GUA, and many other amines, were also detected in airborne particles. These compounds could be inhaled directly or serve as precursors to NH3. The litter bedding serves as the primary reservoir for all AACs present in other phases, especially for the formation of NH3, as it offers a potential reaction site for microbial decomposition.
Our time-resolved measurements have revealed clear and novel relationships between animal activity, total suspended particles, and individual inhalable chemicals. These observations suggest that (1) there are significant differences between day and night concentrations of TPM and AACs, (2) spikes in both TPM and AACs levels are associated to events that trigger intense animal activity, and (3) total AACs occupy a notable proportion in the TPM. Prolonged exposure to airborne AACs and dust particles by chickens can not only decrease the quality of life of chickens, but can also undermine the effectiveness of investments in breeding. In addition, events that cause sudden increases in airborne AACs can pose health risks to farmers, especially when proper personal protective equipment is not used.
Our study has provided new insights into air pollutants that contribute to the formation of gaseous NH3. Based on our findings, addressing indoor air pollution in poultry housing is not a simple task. Controlling NH3 formation requires the management of its precursors, which includes a wide range of organic AACs. Therefore, the removal of AAC precursors in the environment would be beneficial, and future studies should focus on developing technologies that facilitate this process. Furthermore, our study highlights the importance of chemical partitioning of AACs within farm environments. Pollutants are unevenly distributed across three indoor phases. More studies are needed to develop new waste management and ventilation strategies.
Ethical statements
Ethics approval and patient consent statements do not apply to the study. This study does not contain any reproduction material from other sources.Data availability
The data that support the findings of this study are available from the corresponding author on a reasonable request.Author contributions
Xinyang Guo: led the project, identified all carbonyl compounds in the sample, built experimental procedures, processed all data, and wrote the manuscript. Rowshon Afroz: helped to dispatch instruments on-site. Shuang Wu: provided critical inputs in the E-AIM simulation. Kimberly Wong: helped to construct the calibration curves. M. J. Zuidhof: advised the research team with respect to dust collection and measurement in poultry facilities and proofread the manuscript. Valerie Carney: advised the research team on poultry management, connected the team with the commercial poultry farm, and proofread the manuscript. Joey Saharchuk: involved in the inter-comparison of instrument. Hans Osthoff: involved in the inter-comparison of instrument, and proofread the manuscript. Ran Zhao: the PI, oversaw the entire project with advice and proofread the manuscript.Conflicts of interest
No conflict of interest was declared.Acknowledgements
The authors acknowledge the Poultry Research Center at the University of Alberta for preliminary field experiments; farmers at the commercial poultry farm for providing sites and helping to set up instruments; Dr Randy Whittal from the University of Alberta Mass Spectrometry Facility for technical support; Kimberly Wong thanks the Undergraduate Research Initiative at the University of Alberta for funding. This research project was supported by the Canada Foundation for Innovation (Project Number 38334), the NSERC Discovery Grant (RGPIN2018-03814), Egg Farmers of Canada, Egg Farmers of Alberta, 548 Result Driven Agriculture Research, Canadian Hatching Egg Producers, and the NSERC Alliance Grant (ALLRP 586332-23).References
- J. M. Samet and J. D. Spengler, Indoor Environments and Health: Moving Into the 21st Century, Am. J. Public Health, 2003, 93, 1489–1493 Search PubMed.
- N. Notman, How human biology and behavior affect indoor air quality, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 22619–22622 CrossRef CAS PubMed.
- E. C. Pagel, N. C. Reis Júnior, C. E. d. Alvarez and J. M. Santos, Impact of human activities on the concentration of indoor air particles in an antarctic research station, Ambient. Constr., 2018, 18, 463–477 Search PubMed.
- L. I. Robins, S. Napier, C. M. Seek, X. Gao, C. Flegler and C. D. Mackenzie, Control of felinine-derived malodor in cat litter, J. Feline Med. Surg., 2021, 24, 123–130 Search PubMed.
- K. Kim, S. Lee, Y. Choi and D. Kim, Emissions of Fungal Volatile Organic Compounds in Residential Environments and Temporal Emission Patterns: Implications for Sampling Methods, Int. J. Environ. Res. Public Health, 2022, 19, 12601 Search PubMed.
- J. M. Cox-Ganser and P. K. Henneberger, Occupations by Proximity and Indoor/Outdoor Work: Relevance to COVID-19 in All Workers and Black/Hispanic Workers, Am. J. Prev. Med., 2021, 60, 621–628 CrossRef PubMed.
- T. Nathanson, Canada. Health Canada; Federal-Provincial Advisory Committee on Environmental and Occupational Health (Canada) Indoor air quality in office buildings: A technical guide : A report of the federal-provincial advisory committee on environmental and occupational health, Health, 1995, 10–17 Search PubMed.
- K. Toren, I. A. Bergdahl, T. Nilsson and B. Jarvholm, Occupational exposure to particulate air pollution and mortality due to ischaemic heart disease and cerebrovasculardisease, Occup. Environ. Med., 2007, 64, 515–519 Search PubMed.
- P. Carrer and P. Wolkoff, Assessment of Indoor Air Quality Problems in Office-Like Environments: Role of Occupational Health Services, Int. J. Environ. Res. Public Health, 2018, 15, 741 Search PubMed.
- OSHA Indoor Air Quality in Commercial and Institutional Buildings, https://www.osha.gov/sites/default/files/publications/3430indoor-air-quality-sm.pdf, assessed 2024-01-29.
- P. Wolkoff, Indoor air pollutants in office environments: Assessment of comfort, health, and performance, Int. J. Hyg. Environ. Health, 2013, 216, 371–394 CrossRef CAS PubMed.
- P. Wolkoff and G. D. Nielsen, Effects by inhalation of abundant fragrances in indoor air – An overview, Environ. Int., 2017, 101, 96–107 CrossRef CAS PubMed.
- S. T. Glass, E. Lingg and E. Heuberger, Do ambient urban odors evoke basic emotions?, Front. Psychol., 2014, 5, 00340 Search PubMed.
- H. Salonen, A.-L. Pasanen, S. Lappalainen, H. Riuttala, T. Tuomi, P. Pasanen, B. Bäck and K. Reijula, Volatile Organic Compounds and Formaldehyde as Explaining Factors for Sensory Irritation in Office Environments, J. Occup. Environ. Hyg., 2009, 6, 239–247 CrossRef CAS PubMed.
- G. Dabanlis, G. Loupa, G. A. Tsalidis, E. Kostenidou and S. Rapsomanikis, The In-terplay between Air Quality and Energy Efficiency in Museums, a Review, Appl. Sci., 2023, 13, 5535 Search PubMed.
- O. Chiantore and T. Poli, Indoor Air Quality in Museum Display Cases: Volatile Emissions, Materials Contributions, Impacts, Atmosphere, 2021, 12, 364 CrossRef CAS.
- S. Varughese, K. Teschke, M. Brauer, Y. Chow, C. van Netten and S. M. Kennedy, Effects of theatrical smokes and fogs on respiratory health in the entertainment industry, Am. J. Ind. Med., 2005, 47, 411–418 Search PubMed.
- X. Guo, T. Ehindero, C. Lau and R. Zhao, Impact of glycol-based solvents on indoor air quality—Artificial fog and exposure pathways of formaldehyde and various carbonyls, Indoor Air, 2022, 32, e13100 CAS.
- M. Tagesse, M. Deti, D. Dadi, B. Nigussie, T. T. Eshetu and G. T. Tucho, Non-Combustible Source Indoor Air Pollutants Concentration in Beauty Salons and Associated Self-Reported Health Problems Among the Beauty Salon Workers, Risk Manag. Healthc. Policy, 2021, 14, 1363–1372 Search PubMed.
- M. Evtyugina, E. D. Vicente, A. M. Vicente, T. Nunes, F. Lucarelli, G. Calzo-lai, S. Nava, C. Blanco-Alegre, A. I. Calvo, A. Castro, R. Fraile, F. Oduber, M. Cerqueira and C. A. Alves, Air quality and particulate matter speciation in a beauty salon and surrounding outdoor environment: Exploratory study, Atmos. Pollut. Res., 2021, 12, 101174 Search PubMed.
- I. Kilic and E. Yaslioglu, Ammonia and Carbon Dioxide Concentrations in a Layer House, Asian-Australas. J. Anim. Sci., 2014, 27, 1211–1218 CrossRef CAS PubMed.
- Z. Wang, T. Gao, Z. Jiang, Y. Min, J. Mo and Y. Gao, Effect of ventilation on distriutions, concentrations, and emissions of air pollutants in a manure-belt layer house, J. Appl. Poult. Res., 2014, 23, 763–772 CrossRef CAS.
- S. Trabue, K. Scoggin, H. Li, R. Burns, H. Xin and J. Hatfield, Speciation of volatile organic compounds from poultry production, Atmos. Environ., 2010, 44, 3538–3546 CrossRef CAS.
- G. Gržinić, A. Piotrowicz-Cieślak, A. Klimkowicz-Pawlas, R. L. Górny, A. Ławniczek Wałczyk, L. Piechowicz, E. Olkowska, M. Potrykus, M. Tankiewicz, M. Krupka, G. Siebielec and L. Wolska, Intensive poultry farming: A review of the impact on the environment and human health, Sci. Total Environ., 2023, 858, 160014 CrossRef PubMed.
- B. Kocaman, N. Esenbuga, A. Yildiz, E. Lacin and M. Macit, Effect of environmental conditions in poultry houses on the performance of laying hens, Int. J. Poult. Sci., 2006, 5, 26–30 Search PubMed.
- A. Laca, A. Laca and M. Diaz, Environmental Impact of Agro-Food Industry and Food Consumption, Elsevier, 2021, pp. 81–100 Search PubMed.
- R. Mitroi, O. Stoian, C. I. Covaliu and D. Manea, Pollutants resulting from intensive poultry farming activities and their impact on the environment, E3S Web Conf., 2021, 286, 03018 CrossRef CAS.
- L. Guo, B. Zhao, Y. Jia, F. He and W. Chen, Mitigation Strategies of Air Pollutants for Mechanical Ventilated Livestock and Poultry Housing—A Review, Atmosphere, 2022, 13, 452 CrossRef CAS.
- I. Saleeva, A. Sklyar, T. Marinchenko, M. Postnova and A. Ivanov, Efficiency of poultry house heating and ventilation upgrading, IOP Conf. Ser.: Earth Environ. Sci., 2020, 433, 012041 CrossRef.
- T. Kabelitz, O. Biniasch, C. Ammon, U. Nübel, N. Thiel, D. Janke, S. Swaminathan, R. Funk, S. Münch, U. Rösler, P. Siller, B. Amon, A. J. Aarnink and T. Amon, Particulate matter emissions during field application of poultry manure - The influence of moisture content and treatment, Sci. Total Environ., 2021, 780, 146652 Search PubMed.
- A. Nowak, T. Bakula, K. Matusiak, R. Ga-lecki, S. Borowski and B. Gutarowska, Odor-ous Compounds from Poultry Manure Induce DNA Damage, Nuclear Changes, and Decrease Cell Membrane Integrity in Chicken Liver Hepatocellular Carcinoma Cells, Int. J. Environ. Res. Public Health, 2017, 14, 933 Search PubMed.
- J. Skóra, K. Matusiak, P. Wojewódzki, A. Nowak, M. Sulyok, A. Ligocka, M. Okrasa, J. Hermann and B. Gutarowska, Evaluation of Microbiological and Chemical Contaminants in Poultry Farms, Int. J. Environ. Res. Public Health, 2016, 13, 192 CrossRef PubMed.
- E.-C. Hong, H.-K. Kang, J.-J. Jeon, A.-S. You, H.-S. Kim, J.-S. Son, H.-J. Kim, Y.-S. Yun, B.-S. Kang and J.-H. Kim, Studies on the concentrations of particulate matter and ammonia gas from three laying hen rearing systems during the summer season, J. Environ. Sci. Health., Part B, 2021, 56, 753–760 Search PubMed.
- T. Tennessen, L. Connor, A. Marie de Passillé, I. Duncan, J. Feddes, M. Keaney, H. Kochhar, S. MacDonald, J. Rushen, F. Silversides, K. Stanford, M. von Keyerserlingk and G. Griffin, Laurie Connor CCAC Guidelines on: the Care and Use of Farm Animals in Research, Teaching and Testing, Canadian Council on Animal Care, 2009 Search PubMed.
- H. H. Kristensen, L. R. Burgess, T. G. Demmers and C. M. Wathes, The preferences of laying hens for different concentrations of atmospheric ammonia, Appl. Anim. Behav. Sci., 2000, 68, 307–318 CrossRef PubMed.
- A. A. Swelum, M. T. El-Saadony, M. E. Abd El-Hack, M. M. Abo Ghanima, M. Shukry, R. A. Alhotan, E. O. Hussein, G. M. Suliman, H. Ba-Awadh, A. A. Ammari, A. E. Taha and K. A. El-Tarabily, Ammonia emissions in poultry houses and microbial nitrification as a promising reduction strategy, Sci. Total Environ., 2021, 781, 146978 Search PubMed.
- C. Ritz, B. Fairchild and M. Lacy, Implications of Ammonia Production and Emissions from Commercial Poultry Facilities: A Review, J. Appl. Poult. Res., 2004, 13, 684–692 Search PubMed.
- H. E. Schefferle, The Decomposition of Uric Acid in Built Up Poultry Litter, J. Appl. Bacteriol., 1965, 28, 412–420 Search PubMed.
- M. Kacprzak, K. Malińska, A. Grosser, J. Sobik-Szo-ltysek, K. Wystalska, D. Dróżdż, A. Jasińska and E. Meers, Cycles of carbon, nitrogen and phosphorus in poultry manure management technologies – environmental aspects, Crit. Rev. Environ. Sci. Technol., 2022, 53, 914–938 CrossRef.
- R. M. Hafez, T. M. Abdel-Rahman and R. M. Naguib, Uric acid in plants and microorganisms: Biological applications and genetics - A review, J. Adv. Res., 2017, 8, 475–486 Search PubMed.
- J. A. Shannon, The excretion of uric acid by the chicken, J. Cell. Comp. Physiol., 1938, 11, 135–148 Search PubMed.
- D. Dróżdż, K. Wystalska, K. Malińska, A. Grosser, A. Grobelak and M. Kacprzak, Management of poultry manure in Poland – Current state and future perspectives, J. Environ. Manage., 2020, 264, 110327 Search PubMed.
- R. B. Bist, S. Subedi, L. Chai and X. Yang, Ammonia emissions, impacts, and mitigation strategies for poultry production: A critical review, J. Environ. Manage., 2023, 328, 116919 CrossRef CAS PubMed.
- J. J. R. Feddes, M. J. Zuidhof and H. Cook, Characterization of Airborne Dust Particles in turkey Housing, 1992 Search PubMed.
- H. Valenzuela, Ecological Management of the Nitrogen Cycle in Organic Farms, Nitrogen, 2023, 4, 58–84 CrossRef CAS.
- S. Naseem and A. J. King, Ammonia production in poultry houses can affect health of humans, birds, and the environment—techniques for its reduction during poultry production, Environ. Sci. Pollut. Res., 2018, 25, 15269–15293 Search PubMed.
- H. Li, C. Lin, S. Collier, W. Brown and S. White-Hansen, Assessment of frequent litter amendment application on ammonia emission from broilers operations, J. Air Waste Manage. Assoc., 2013, 63, 442–452 Search PubMed.
- J. Y. Balta, G. Blom, A. Davidson, K. Perrault, J. F. Cryan, S. M. O'Mahony and J. P. Cassella, Developing a quantitative method to assess the decomposition of embalmed human cadavers, Forensic Chem., 2020, 18, 100235 CrossRef CAS.
- W. E. Burnett, Air pollution from animal wastes. Determination of malodors by gas chromatographic and organoleptic techniques, J. Environ. Sci. Technol., 1969, 3, 744–749 CrossRef CAS.
- M. S. Smith, A. J. Francis and J. M. Duxbury, Collection and analysis of organic gases from natural ecosystems: application to poultry manure, J. Environ. Sci. Technol., 1977, 11, 51–55 CrossRef CAS.
- H. H. T. Dinh, Analysis of Ammonia and Volatile Organic Amine Emissions in a Confined Poultry Facility, 2010, vol. 707 Search PubMed.
- W. Wojnowski, J. P-lotka-Wasylka, K. Kalinowska, T. Majchrzak, T. Dymerski, P. Szweda and J. Namieśnik, Direct determination of cadaverine in the volatile fraction of aerobically stored chicken breast samples, Monatsh. Chem., 2018, 149, 1521–1525 CrossRef CAS PubMed.
- M. Rokka, S. Eerola, M. Smolander, H.-L. Alakomi and R. Ahvenainen, Monitoring of the quality of modified atmosphere packaged broiler chicken cuts stored in different temperature conditions, Food Control, 2004, 15, 601–607 CrossRef CAS.
- J.-Q. Ni, W. P. Robarge, C. Xiao and A. J. Heber, Volatile organic compounds at swine facilities: A critical review, Chemosphere, 2012, 89, 769–788 CrossRef CAS PubMed.
- E. Matthews, et al., Airborne observations over the North Atlantic Ocean reveal the importance of gas-phase urea in the atmosphere, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2218127120 CrossRef CAS PubMed.
- X. Ge, A. S. Wexler and S. L. Clegg, Atmospheric amines – Part I. A review, Atmos. Environ., 2011, 45, 524–546 CrossRef CAS.
- X. Ge, A. S. Wexler and S. L. Clegg, Atmospheric amines – Part II. Thermodynamic properties and gas/particle partitioning, Atmos. Environ., 2011, 45, 561–577 CrossRef CAS.
- K. Nalazek-Rudnicka and A. Wasik, Development and validation of an LC–MS/MS method for the determination of biogenic amines in wines and beers, Monatsh. Chem., 2017, 148, 1685–1696 CrossRef CAS PubMed.
- T. Devadoss, Synthetic applications of p-toluenesulfonyl chloride: A recent update, J. Mol. Struct., 2023, 1289, 135850 Search PubMed.
- M. A. Pasha, R.-u.-R. Khan and N. Shrivatsa, N-Sulfonylation of amines, imides, amides and anilides using p-TsCl in presence of atomized sodium in EtOH–THF under sonic condition, Ultrason. Sonochem., 2015, 26, 15–21 CrossRef CAS PubMed.
- G. Huang, G. Deng, H. Qiao and X. Zhou, Determination of Trace C1C4 Aliphatic Alcohols in Aqueous Samples by 9-Fluorenylmethyl Chloroformate Derivatization and Reversed-Phase High-Performance Liquid Chromatography, Anal. Chem., 1999, 71, 4245–4249 CrossRef CAS PubMed.
- S. D.-H. Shi, C. L. Hendrickson and A. G. Marshall, Counting individual sulfur atoms in a protein by ultra high resolution Fourier transform ion cyclotron resonance mass spectrometry: Experimental resolution of isotopic fine structure in proteins, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 11532–11537 Search PubMed.
- J. Jiang, D. S. Stevenson, A. Uwizeye, G. Tempio and M. A. Sutton, A climate-dependent global model of ammonia emissions from chicken farming, Biogeosciences, 2021, 18, 135–158 CrossRef CAS.
- E. Nemitz, M. A. Sutton, J. K. Schjoerring, S. Husted and G. Paul Wyers, Resistance modelling of ammonia exchange over oilseed rape, Agric. For. Meteorol., 2000, 105, 405–425 CrossRef.
- EPA Environmental Technology Verification Report – Thermo Electron Corporation Model 17C Ammonia Analyzer, https://archive.epa.gov/nrmrl/archive-etv/web/pdf/01_vr_thermo.pdf, assessed 2024-02-21 Search PubMed.
- D. A. Orsini, Y. Ma, A. Sullivan, B. Sierau, K. Baumann and R. J. Weber, Refinements to the particle-into-liquid sampler (PILS) for ground and airborne measurements of water soluble aerosol composition, Atmos. Environ., 2003, 37, 1243–1259 Search PubMed.
- R. J. Weber, D. Orsini, Y. Daun, Y.-N. Lee, P. J. Klotz and F. Brechtel, A Particle- into-Liquid Collector for Rapid Measurement of Aerosol Bulk Chemical Composition, Aerosol Sci. Technol., 2001, 35, 718–727 CrossRef CAS.
- A. Nowak, K. Matusiak, S. Borowski, T. Bakula, S. Opaliński, R. Ko-lacz and B. Gutarowska, Cytotoxicity of Odorous Compounds from oultry Manure, Int. J. Environ. Res. Public Health, 2016, 13, 1046 Search PubMed.
- J. Sintermann, S. Schallhart, M. Kajos, M. Jocher, A. Bracher, A. Münger, D. Johnson, A. Neftel and T. Ruuskanen, Trimethylamine emissions in animal husbandry, Biogeosciences, 2014, 11, 5073–5085 CrossRef.
- S. L. Clegg, P. Brimblecombe and A. S. Wexler, Thermodynamic Model of the System H+NH4+SO42-NO3-H2O at Tropospheric Temperatures, J. Phys. Chem. A, 1998, 102, 2137–2154 Search PubMed.
- EPA National Environmental Methods Index, https://www.nemi.gov/home, assessed2024-01-29.
- J. Choi, R. Miles and R. Harms, The Phosphorus Excretion Pattern and Balance during One Egg Cycle of the Laying Hen Fed a Phosphorus Deficient Diet with or without a Single Dose of Phosphoric Acid, Poult. Sci., 1979, 58, 1535–1540 CrossRef CAS PubMed.
- M. S. Razzaque, Phosphate toxicity: new insights into an old problem, Clin. Sci., 2010, 120, 91–97 CrossRef PubMed.
- H. Berthoud, D. Wechsler and S. Irmler, Production of Putrescine and Cadaverine by Paucilactobacillus wasatchensis, Front. Microbiol., 2022, 13, 842403 Search PubMed.
- Y. Liang, H. Xin, E. F. Wheeler, R. S. Gates, H. Li, J. S. Zajaczkowski, P. A. Topper, K. D. Casey, B. R. Behrends, D. J. Burnham and F. J. Zajaczkowski, Ammonia Emissions from U.S. Laying Hen Houses in Iowa and Pennsylvania, Trans. ASAE, 2005, 48, 1927–1941 CAS.
- C. M. Wathes, M. R. Holden, R. W. Sneath, R. P. White and V. R. Phillips, Concentrations and emission rates of aerial ammonia, nitrous oxide, methane, carbon dioxide, dust and endotoxin in UK broiler and layer houses, Br. Poult. Sci., 1997, 38, 14–28 Search PubMed.
- D. Shen, S. Wu, P. Dai, Y. Li and C. Li, Distribution of particulate matter and ammonia and physicochemical properties of fine particulate matter in a layer house, Poult. Sci., 2018, 97, 4137–4149 Search PubMed.
- S. Mahimairaja, N. S. Bolan, M. J. Hedley and A. N. Macgregor, Evaluation of methods of measurement of nitrogen in poultry and animal manures, Fert. Res., 1990, 24, 141–148 Search PubMed.
- D. Miles, D. Rowe and P. Owens, Winter broiler litter gases and nitrogen compounds: Temporal and spatial trends, Atmos. Environ., 2008, 42, 3351–3363 CrossRef CAS.
- B. Gutarowska, K. Matusiak, S. Borowski, A. Rajkowska and B. Brycki, Removal of odorous compounds from poultry manure by microorganisms on perlite – bentonite carrier, J. Environ. Manage., 2014, 141, 70–76 CrossRef CAS PubMed.
- K. Murakami, M. Hara, T. Kondo and Y. Hashimoto, Increased total nitrogen content of poultry manure by decreasing water content through composting processes, Soil Sci. Plant Nutr., 2011, 57, 705–709 Search PubMed.
- J. Mowrer, D. E. Kissel, M. Cabrera and S. M. Hassan, Nondegradative Extraction and Measurement of Uric Acid from Poultry Litter, Soil Sci. Soc. Am. J., 2013, 77, 1413–1417 Search PubMed.
- A. Peña Fernández, T. G. Demmers, Q. Tong, A. Youssef, T. Norton, E. Vranken and D. Berckmans, Real-time modelling of indoor particulate matter concentration in poultry houses using broiler activity and ventilation rate, Biosyst. Eng., 2019, 187, 214–225 CrossRef.
- J. D. Pleil, M. Ariel Geer Wallace, M. D. Davis and C. M. Matty, The physics of human breathing: flow, timing, volume, and pressure parameters for normal, on-demand, and ventilator respiration, J. Breath Res., 2021, 15, 042002 CrossRef CAS PubMed.
- CAREX Outdoor Air Pollution Profile, https://www.carexcanada.ca/profile/outdoor_air_pollution, assessed 2024-09-19.
- J. N. Maina, A critical assessment of the cellular defences of the avian respiratory system: are birds in general and poultry in particular relatively more susceptible to pulmonary infections/afflictions?, Biol. Rev., 2023, 98, 2152–2187 Search PubMed.
- Q. Jiang, C. Zhang, S. Chen, L. Shi, D. C. Li, N. Lv, L. Cui, Y. Chen and Y. Zheng, Particulate Matter 2.5 Induced Developmental Cardiotoxicity in Chicken Embryo and Hatchling, Front. Pharmacol, 2020, 11, 00841 CrossRef CAS PubMed.
- Z. Chen, Y. Bai, C. Lou and B. Wu, Serum metabolome responses induced by long-term inoculation of suspended PM2.5 in chicken, Poult. Sci., 2024, 103, 103283 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4em00254g |
| This journal is © The Royal Society of Chemistry 2025 |