 Open Access Article
Open Access ArticleCarbon footprint of oil produced through enhanced oil recovery using carbon dioxide directly captured from air†
Antonio
Gasós
 a,
Ronny
Pini
a,
Ronny
Pini
 b,
Viola
Becattini
a and
Marco
Mazzotti
b,
Viola
Becattini
a and
Marco
Mazzotti
 *a
*a
aInstitute for Energy and Process Engineering, ETH Zurich, 8092 Zürich, Switzerland. E-mail: marcom@ethz.ch
bDepartment of Chemical Engineering, Imperial College London, SW7 2AZ London, UK
First published on 17th June 2025
Abstract
Some argue that using CO2 from direct air capture (DAC) in enhanced oil recovery (CO2-EOR) can produce carbon-neutral oil by permanently storing more CO2 than is emitted when using the extracted fossil fuels. However, existing analyses often provide case-specific insights based on short-term operations without considering the full life cycle of reservoir exploitation, including primary, secondary, and tertiary (EOR) recovery phases. Here, we present a general, top-down approach based on mass and volume conservation to assess the potential carbon footprint of oil production, applicable to different temporal perspectives of reservoir exploitation. Supported by field data from 16 EOR projects, our analysis shows that 30% of projects appear carbon-neutral when EOR is considered in isolation, but they all become significantly carbon-positive when the full reservoir lifetime is considered. The volume of emitted CO2 exceeds the pore space freed for storage by at least a factor of three, making carbon-neutral oil physically unattainable in conventional reservoirs. The favorable conditions for low-carbon oil production during CO2-EOR exist solely because of extensive prior oil extraction and water injection, and only residual oil zones may truly offer potential for carbon-neutral oil due to their low oil saturation and lack of legacy emissions. While omitting legacy emissions from previously depleted fields may be justifiable and may enable claims of carbon neutrality during the EOR phase, newly developed fields, i.e., developed now or in the future, should be held accountable for the full life-cycle emissions they generate. This necessitates clear and transparent accounting policy frameworks. Although CO2-EOR may reduce oil's carbon footprint, promoting it as a pathway to carbon-neutrality risks legitimizing continued fossil fuel production, ultimately undermining global climate targets.
Broader contextAs society struggles to find actionable pathways to reach net-zero CO2 emissions, carbon dioxide removal (CDR) technologies such as direct air capture (DAC) are attracting momentum. Some companies propose using DAC-derived CO2 for enhanced oil recovery (EOR), suggesting that the CO2 stored underground during this process could offset the emissions from burning the recovered oil—yielding so-called “carbon-neutral oil”. If valid, this approach could reshape how fossil fuels are positioned in the energy transition. However, these claims often overlook the emissions from earlier production stages. In this study, we present a simple, general framework based on mass and volume conservation—supported by field data from 16 CO2-EOR projects—to evaluate whether the emissions from oil production can be fully offset by geological CO2 storage. We show that, even under optimistic assumptions, conventional oil reservoirs lack the capacity to store enough CO2 to achieve true carbon neutrality. While some EOR projects may appear carbon-neutral during the EOR phase alone, this depends on omitting legacy emissions from previous production phases. Our findings highlight the need for transparent and consistent carbon accounting frameworks that address legacy emissions. Without them, misleading narratives could slow the transition away from fossil fuels and undermine climate goals. |
1 Introduction
To maximize oil extraction from a reservoir, oil production typically proceeds in three stages (Fig. 1). The first stage, primary recovery, relies on the natural reservoir pressure to produce oil. This is followed by secondary recovery, which involves injecting water, possibly seawater, to maintain the reservoir pressure and displace additional oil. Finally, tertiary recovery, or enhanced oil recovery (EOR), employs miscible fluids such as natural gas or carbon dioxide (CO2), or thermal methods such as steam injection, to mobilize trapped oil and enhance production.1 After oil extraction, depleted reservoirs can serve as sites for permanent CO2 storage.2,3 This work focuses specifically on CO2-EOR, where CO2 is used as the miscible fluid.Estimates suggest that approximately 180 Gt of oil could be recovered globally through CO2-EOR in known oil fields.4 For decades, this process has utilized CO2 sourced primarily from natural underground reservoirs, where CO2 has accumulated for millions of years,5 to maximize oil recovery per unit of CO2 injected, thus minimizing operational costs.6 As carbon capture and storage (CCS) technologies gained attention for their potential to reduce greenhouse gas emissions, CO2-EOR emerged as a possible method for permanently storing CO2 from industrial sources, which is captured and transported to the injection site.5,7 Thus, the goal of CO2-EOR became that of producing oil while maximizing the volume of CO2 stored, as the utilization of CO2 changed from a cost to a potential climate service.8–10 However, using and thus burning the oil produced through EOR results in CO2 emissions that reduce or nullify the climate benefits of CO2 storage itself. An additional concern is the long-term permanence of CO2 stored via EOR, which operates under less stringent permitting and monitoring requirements (class II injection wells under US EPA) compared to dedicated geologic sequestration (class VI injection wells). CO2-EOR is now considered a form of CO2 utilization, whose attractiveness stems from being a profitable business rather than a means of counteracting climate change.11
In recent years, direct air capture (DAC) has gained significant attention as a carbon dioxide removal (CDR) technology, which enables the direct removal of CO2 from the atmosphere by technical means.12–14 At least one corporation engaged in hydrocarbon exploration has invested in DAC, viewing DAC as a way to offset the CO2 emissions generated by its products.15 Proponents of using CO2 derived from DAC in EOR argue that it can help finance the development and early deployment of DAC technologies, and that the oil produced in this manner could be carbon neutral.16,17 This latter argument hinges on the claim that the amount of CO2 ultimately stored in the reservoir exceeds that emitted during the refining and use (i.e., burning) of the extracted oil. If the CO2 has been captured from the atmosphere, using it for EOR could close the carbon cycle for the oil produced in this manner.
Robust, bottom-up approaches have assessed the climate impact of oil produced through EOR using life cycle analysis (LCA).18–21 These methods use operational field data or reservoir fluid dynamics models to estimate the amounts of both CO2 stored and hydrocarbons produced. The system boundaries are then extended to include factors such as emissions from oil utilization, EOR operation, and the CO2 source. While LCA studies provide detailed insights, they also rely heavily on case-specific data and assumptions, which can limit their ability to support broad conclusions about the feasibility of carbon-neutral oil.
The time frame considered is a critical factor in these assessments. CO2-EOR operations initially have a carbon-negative balance, meaning that more CO2 is stored than emitted, as significant volumes of CO2 are injected to pressurize the reservoir and displace fluids.22 However, over time—typically after about 10 years—the operation transitions to a net carbon-positive impact as hydrocarbon production increases while less new CO2 is injected, with a portion of the injected CO2 being produced at the extraction well and re-injected. Given that EOR operations usually last around 20 years, analyses focusing on shorter periods, such as under ten years,20 may provide a misleading assessment of EOR's net climate impacts.
Moreover, traditional LCA studies typically consider only the EOR phase, which represents a much shorter period than the entire life cycle of reservoir exploitation. Since EOR follows primary and secondary recovery phases (Fig. 1)—and its conditions are largely shaped by these earlier phases—we argue that assessments must cover the full life cycle of the reservoir to account properly for the overall climate impact.
This study proposes a novel top-down framework to evaluate the net climate impact of DAC-based CO2-EOR. While less detailed than bottom-up models, our analysis remains accurate and is broadly applicable. It enables the consistent assessment of carbon balance under varying temporal perspectives, whether isolating the EOR phase or considering the full life cycle of reservoir exploitation, revealing how temporal boundary choices shape carbon neutrality claims.
2 The conceptual framework
The feasibility of carbon neutral oil through CO2-EOR could be dismissed based on two figures: (1) burning one ton of oil generates at least three tons of CO223 and (2) under reservoir conditions, the density of oil is higher than that of CO2, with an oil-to-CO2 density ratio between 1.0 and 1.5.24 This means that all the CO2 generated by burning the recovered oil would occupy between 300% and 450% of the volume made available by extracting oil; thus, attaining carbon neutrality would be physically impossible given the reservoir's volume constraints.However, this perspective is incomplete, as it overlooks that injecting CO2 displaces not only oil but also other fluids present in the reservoir, namely a gas phase and an aqueous phase. In other words, there is an additional fraction of the pore space, previously occupied by less carbon-intensive fluids, that could be occupied by CO2. Here, we analyze the CO2-EOR system using a novel top-down approach, based on mass and volume conservation principles, accounting for all reservoir fluids. A schematic of the reservoir before and during exploitation is shown in Fig. 2.
2.1 Description of the reservoir
The analysis considers the reservoir as a fixed control volume, namely as a porous rock body with constant pore volume, Vp.Initially, the pore volume contains fluids at initial temperature and pressure, Ti and Pi. Based on the black-oil model, these fluids are grouped in three phases (see Fig. 2): an aqueous phase (w), a gaseous phase (g), and an oleic phase (o). For the sake of simplicity but without loss of generality, we assume that each phase consists of one pseudo-component only, namely water, methane, and oil. The initial state is described as:
| Vp = Vio + Vig + Viw | (1) |
After CO2-EOR, the pore volume is occupied by the residual fluids, not recovered, and by a dense phase, assumed to consist of pure CO2 only, under the final reservoir conditions, Tf and Pf. The final state is described as:
 | (2) |
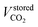 is the volume of CO2 stored and Vrj is the residual volume of phase j remaining in the reservoir.
is the volume of CO2 stored and Vrj is the residual volume of phase j remaining in the reservoir.
Eqn (2) assumes that all the stored CO2 exist at its dense phase density, even though it is partially evaporated or dissolved in the liquids. This assumption could be refined by using a lower effective CO2 density that accounts for these phases.
2.2 Exploitation of the reservoir
The extracted in-place fluids generate CO2 emissions upon their utilization, , which is calculated using emission factors, fj. These factors represent the volume of CO2 emitted per unit volume of phase j used, from gate to grave:25
, which is calculated using emission factors, fj. These factors represent the volume of CO2 emitted per unit volume of phase j used, from gate to grave:25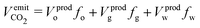 | (3) |
Here, Vprodj = (Vij − Vrjβj) is the volume of phase j produced, where βj is the phase density ratio after and before exploitation.
DAC and EOR operations have a site-specific carbon footprint, accounted for through an overall CO2 removal efficiency, ηCO2. Thus, the target volume of CO2 to be stored to fully compensate emissions is given by:
 | (4) |
2.3 Emission factors
The emission factors are calculated using: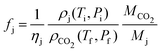 | (5) |
Here, ρj and ρCO2 are the densities of phase j and of CO2 at relevant temperature and pressure levels, respectively, while Mj and MCO2 are their molar masses, in mass per mole of carbon. We use Mo = 14 g mol−1 (for CH2, the building block of oil), Mg = 16 g mol−1 (methane), and Mw = 0 g mol−1 (water, being carbon-free).
The densities and molar masses estimate direct emissions from fuel combustion, while the variable ηj denotes the carbon efficiency in the utilization of phase j, accounting for indirect emissions. This efficiency depends on conditions and events beyond the scope of this analysis. Thus, we use a conservative value of 1, acknowledging that a LCA could provide a more precise estimate.
2.4 Carbon balance of the reservoir
The production of reservoir fluids may not provide enough pore volume to store the entire quantity of CO2 emitted. The ratio of the emitted CO2 volume to the reservoir's available storage volume from displaced fluids, ξ, is expressed as: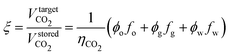 | (6) |
 | (7) |
Note that ϕj = Sij if there are no residual fluids remaining (Srj = 0).
If ξ = 1, the volume of displaced fluids is exactly sufficient to store the amount of CO2 emitted, enabling carbon-neutral oil production; if ξ < 1, there is excess storage capacity, allowing for negative emissions; and if ξ > 1, the storage capacity is insufficient, and EOR using DAC-derived CO2 ultimately emits more CO2 than it can store.
3 The climate impact of reservoir exploitation
3.1 Case study assumptions
The efficiency of DAC with storage typically ranges from 0.80 to 0.95 depending on the technology, energy source and geographical location,26,27 and lower efficiencies are possible but would likely not be deployed. Additionally, CO2 produced at the extraction well requires separation and re-injection to ensure effective storage, further reducing the overall ηCO2.21 Given these factors, a CO2 removal efficiency of ηCO2 = 0.85 is used in eqn (4).Emission factors are reported in Table 1 for a typical reservoir under identical initial and final conditions of P = 190 bar and T = 70 °C. The assumed densities for the calculations are 690 kg m−3 for the oleic phase28 (including dissolved gas), 640 kg m−3 for the CO2 dense phase,29 and 100 kg m−3 for the gas phase (ideal gas law). The oil emission factor is significantly larger than one, primarily due to stoichiometry rather than assumptions: even in an optimistic scenario with same CO2 and oil densities, and unitary efficiency, eqn (5) yields fo = MCO2/Mo = 3.14.
| Oil (fo) | Methane (fg) | Water (fw) |
|---|---|---|
| 3.4 | 0.44 | 0.0 |
3.2 Geometrical representation
The exploitation state of any reservoir can be represented as a point on the ternary diagram shown in Fig. 3, where the horizontal and the vertical coordinates are the volume fractions of gas and oil produced. The water fraction is the complement to one. The vertices of the triangle represent reservoirs filled with only one fluid phase, while the edges represent two-phase mixtures, with the excluded phase opposite the edge.Eqn (6) constrains the combination of produced phases, ϕj, compatible with a given value of ξ. By varying ξ values, one obtains straight isolines in the ternary diagram, representing loci of points where the volume occupied by the target CO2 to be stored is ξ times the pore volume freed in the reservoir upon extraction of the in-place fluids. Reservoir operations corresponding to points above the ξ = 1 isoline (red region) ultimately emit more CO2 than the reservoir can store, while those mapping in points below it (green region) may store more CO2 than they emit.
The ternary diagram may be used to effectively illustrate the specific scenarios of interest:
(1) Saline aquifer (ϕo = ϕg = 0): only water is displaced, providing CO2 storage capacity without extracting fossil fuels, resulting in ξ = 0.
(2) Gas reservoir (ϕo = 0): only gas and water are produced; since ηCO2 > fg > fw, more CO2 is stored than emitted, resulting in ξ < 1.
(3) Oil reservoir (ϕg = 0): only oil and water are produced; since fo > ηCO2 > fw, achieving carbon neutrality requires producing more than 70% water.
The impact assessment using the ternary diagram is based solely on production volume ratios and can be used regardless of whether legacy emissions from prior oil exploitation are included.
3.3 Sensitivity to assumptions
Fig. 4 illustrates the sensitivity of the ξ-isolines to variations in reservoir conditions, namely temperature and pressure with ranges based on reservoir data from Holm and Josendal24 (panel A), and in CO2 removal efficiency (panel B). The blue and red dash-dotted lines represent the best and worst climate impact scenarios within the considered sensitivity range. The effects of pressure and temperature were accounted for by modifying the densities of the dense CO2 phase (from 600 to 670 kg m−3)29 and of the gaseous phase (from 87 to 115 kg m−3, according to the ideal gas law), while the oil density remained unchanged.It is readily observed that the sensitivity of the position of the ξ-isolines, particularly of the ξ = 1 isoline, to reasonable changes of the above parameters is qualitatively and quantitatively rather small. This allows arguing that the conclusions drawn based on the specific scenario considered in Fig. 3 are indeed general.
3.4 Existing CO2-EOR projects
Fig. 5 illustrates the carbon balance as a function of the incremental oil recovered for 16 CO2-EOR projects reported by Azzolina et al.,30 supplemented with additional data detailed in Table S1 (ESI†).31,32 The carbon balance is also presented for these projects when accounting for emissions from oil produced before CO2-EOR, assuming a recovery of 35% of the original oil in place (OOIP) during primary and secondary production; this is considered to be a representative median value for reservoirs globally.1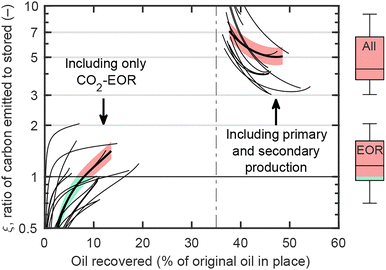 | ||
| Fig. 5 Ratio of carbon emitted to stored, ξ, as a function of oil recovery for 16 CO2-EOR projects.30 The bottom-left lines represent ξ considering only CO2-EOR, for each project, while the top-right lines include the entire reservoir lifetime, assuming 35% oil recovery before EOR. Colored areas illustrate the sensitivity to CO2 removal efficiency between 0.75 and 0.95 for one specific project. Box plots show the distribution of final ξ values, with the colored boxes indicating the interquartile range. | ||
Considering only EOR, all projects start carbon negative, as injected CO2 pressurizes the reservoir and displaces fluids before significant incremental oil is produced. The carbon balance then rises steeply due to increased oil production (effective mobilization) and reduced CO2 retention in the reservoir, which drops between 40 and 60% as CO2 breaks through at the production well,30 requiring separation and re-injection, thereby reducing the net-CO2 injected. The curve eventually flattens as the remaining oil becomes increasingly difficult to mobilize, thus requiring more injected CO2 per unit of oil produced. Most projects (11 out of 16) surpassed the ξ = 1 threshold within the temporal bounds of the CO2-EOR operation, typically after only 5–10% incremental recovery of OOIP, indicating that the oil produced during EOR ultimately emitted more CO2 than what was stored. The variability in carbon impact across projects is likely attributable to differences in: (1) CO2-EOR techniques, operating conditions, and exploitation duration, (2) reservoir physical properties, and (3) reservoir depletion before EOR commenced.
When emissions from primary and secondary production phases are also accounted for, the overall reservoir exploitation becomes significantly carbon positive. All projects start with an infinite value of ξ at 35% OOIP, as CO2 was emitted before EOR without any associated storage. Then, the value of ξ decreases as more CO2 is stored, indicating that EOR progressively reduces the average climate impact of the oil produced. However, the reduction is insufficient to achieve carbon neutrality. Notably, half of the reservoirs emitted between 370 and 660% of the stored CO2 over their lifetime, as shown with box plots in Fig. 5.
4 The temporal dimension of carbon-neutral oil
Some key observations are worth making based on the results presented in Fig. 3 and 5.First, the exploitation of oil reservoirs during their entire lifetime falls within the region where ξ > 1, indicating insufficient storage capacity to offset the CO2 emitted. Notably, the maximum allowable volume fraction of oil produced, or oil saturation if all reservoir fluids are recovered, that could enable carbon-neutral oil is only between 25 and 30% (Fig. 4 with ηCO2 = 1). Such saturation levels occur naturally only in residual oil zones, which are deep saline aquifers containing oil at residual saturation levels and are currently unexploited.33,34 These findings demonstrate that conventional oil reservoirs lack the capacity to store all the CO2 generated from the refining and combustion of the extracted fossil fuels, confirming that truly carbon-neutral oil production is physically unattainable within reservoir boundaries.
Second, as oil production advances through its various phases, the composition of reservoir fluids shifts. The volume freed by extracted oil is replaced by gas—previously dissolved in the oleic phase at higher reservoir pressures—and by water injected during secondary recovery. As a result, the oil fraction in the fluids produced decreases, shifting the operating point downward in the ternary diagram (Fig. 3). By the time CO2-EOR begins, the reservoir composition may fall below the ξ = 1 threshold depending on factors such as reservoir conditions, CO2 removal efficiency, and the extent of EOR exploitation—aligning with previous LCA findings.20,22 Although only 30% of EOR projects ultimately stored more CO2 than they emitted (Fig. 5), these cases illustrate how carbon-neutral oil may appear feasible when EOR is assessed in isolation.
From a physical perspective, EOR cannot be decoupled from earlier production stages, as its apparent favorable conditions for producing low-carbon oil exist solely due to prior exploitation. While DAC-based CO2-EOR may reduce the overall carbon footprint of oil by 10–32% (Fig. S2, ESI†), oil remains a carbon-intensive resource. To achieve net-zero emissions, the additional CO2 that cannot be stored in the reservoir could be sequestered elsewhere—such as in saline aquifers commonly located beneath oil reservoirs or in other suitable geological formations. However, this storage operation is independent of oil production itself, redirecting attention to the broader question of whether fossil fuel emissions should be offset through carbon removals.
From a policy perspective, there may be arguments for omitting certain legacy emissions from CO2 accounting—particularly for depleted fields where the environmental impact has already occurred. The long time frames of oil production, often spanning multiple operators and extraction phases, complicate accountability. However, the situation is fundamentally different for new oil and gas developments, i.e., initiated now or in the future. These projects are developed with full awareness of the emissions involved, and omitting earlier-phase emissions while claiming climate neutrality during EOR is wrong. Establishing transparent and consistent carbon accounting frameworks is essential to prevent misleading narratives about the climate impact of oil production and to ensure accountability in meeting climate targets.
5 Conclusion
We developed a general top-down approach that enables a consistent and efficient assessment of the carbon balance of oil and gas reservoirs, including and focusing on the EOR phase. This approach is based on simple material and volume balances and allows mapping the produced fluids onto a ternary diagram, thus yielding an insightful geometrical representation. Our assumptions provide a lower-bound estimate of the overall carbon footprint, as it excludes indirect emissions from hydrocarbon use. Project-specific insights from life cycle analyses can further refine the model parameters within the proposed framework.Our analysis demonstrates that CO2-EOR does not enable the production of carbon-neutral oil when the entire reservoir lifetime is considered, as oil reservoirs lack the capacity to store all the CO2 generated from extracted hydrocarbons. While carbon neutrality may appear achievable within limited time frames—such as during part or all of the CO2-EOR phase—this overlooks emissions from earlier production stages. These findings underscore the need for clear frameworks to address legacy emissions.
CO2-EOR has the potential to replace part of the conventional oil production, reducing the overall carbon footprint of oil while facilitating the development of subsurface CO2 injection technology.11,35 However, the prospect of significant oil recovery and CO2 storage could be misused as a pretext to continue promoting or funding fossil fuel production, which must be phased out to meet critical climate targets.36,37
Author contributions
A. G.: conceptualization, methodology, formal analysis, visualization, and writing – original draft. R. P.: methodology, formal analysis, and writing – review and editing. V. B.: writing – review and editing. M. M.: conceptualization, methodology, and writing – original draft.Conflicts of interest
There are no conflicts of interest to declare.Data availability
The primary data supporting the findings of this study are provided in the ESI.† A spreadsheet tool enables to construct ternary diagrams based on the selected reservoir conditions and operating parameters. Additional data sets and the MATLAB code used for the analysis are available upon request.Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.References
- L. Lake, R. Johns, B. Rossen and G. Pope, Fundamentals of Enhanced Oil Recovery, Society of Petroleum Engineers, 2014 Search PubMed.
- S. Bachu, Energy Convers. Manage., 2000, 41, 953–970 CrossRef CAS.
- M. D. Aminu, S. A. Nabavi, C. A. Rochelle and V. Manovic, Appl. Energy, 2017, 208, 1389–1419 CrossRef CAS.
- M. L. Godec, V. A. Kuuskraa and P. Dipietro, Energy Fuels, 2013, 27, 4183–4189 CrossRef CAS.
- B. Metz, O. Davidson, H. De Coninck, M. Loos and L. Meyer, IPCC special report on carbon dioxide capture and storage, Cambridge University Press, Cambridge, 2005 Search PubMed.
- M. Blunt, F. J. Fayers and F. M. Orr, Energy Convers. Manage., 1993, 34, 1197–1204 CrossRef CAS.
- F. Gozalpour, S. R. Ren and B. Tohidi, Oil Gas Sci. Technol., 2005, 60, 537–546 CrossRef CAS.
- K. Jessen, A. R. Kovscek and F. M. Orr, Energy Convers. Manage., 2005, 46, 293–311 CrossRef CAS.
- A. R. Kovscek and M. D. Cakici, Energy Convers. Manage., 2005, 46, 1941–1956 CrossRef CAS.
- A. Ettehadtavakkol, L. W. Lake and S. L. Bryant, Int. J. Greenhouse Gas Control, 2014, 25, 79–92 CrossRef CAS.
- N. Mac Dowell, P. S. Fennell, N. Shah and G. C. Maitland, Nat. Clim. Change, 2017, 7, 243–249 CrossRef CAS.
- R. Socolow, M. Desmond, R. Aines, J. Blackstock, O. Bolland, T. Kaarsberg, N. Lewis, M. Mazzotti, A. Pfeffer, K. Sawyer, J. Siirola, B. Smit and J. Wilcox, Direct Air Capture of CO2with Chemicals: A Technology Assessment for the APS Panel on Public Affairs, American physical society technical report, 2011.
- D. W. Keith, G. Holmes, D. S. Angelo and K. Heidel, Joule, 2018, 2, 1573–1594 CrossRef CAS.
- S. Fuss, W. F. Lamb, M. W. Callaghan, J. Hilaire, F. Creutzig, T. Amann, T. Beringer, W. de Oliveira Garcia, J. Hartmann and T. Khanna, et al. , Environ. Res. Lett., 2018, 13, 063002 CrossRef.
- Occidental Petroleum, Occidental Enters into Agreement to Acquire Direct Air Capture Technology Innovator Carbon Engineering, 2023, https://www.oxy.com/news/news-releases/occidental-enters-into-agreement-to-acquire-direct-air-capture-technology-innovator-carbon-engineering/, Accessed: 2024-09-17.
- Occidental Petroleum, Can we use CO2 in a beneficial way? EOR has the answer, 2024, https://www.oxy.com/operations/performance-production/eor/, Accessed: 2024-09-17.
- IEA, Can CO2-EOR really provide carbon-negative oil?, IEA, Paris, 2019, https://www.iea.org/commentaries/can-co2-eor-really-provide-carbon-negative-oil, Accessed: 2024-09-17.
- P. Jaramillo, W. M. Griffin and S. T. McCoy, Environ. Sci. Technol., 2009, 43, 8027–8032 CrossRef CAS PubMed.
- N. A. Azzolina, W. D. Peck, J. A. Hamling, C. D. Gorecki, S. C. Ayash, T. E. Doll, D. V. Nakles and L. S. Melzer, Int. J. Greenhouse Gas Control, 2016, 51, 369–379 CrossRef CAS.
- J. R. Sminchak, S. Mawalkar and N. Gupta, Energy Fuels, 2020, 34, 3566–3577 CrossRef CAS.
- J. Singh, U. Singh, G. R. Garcia, V. Vishal and R. Anex, Int. J. Greenhouse Gas Control, 2024, 139, 104281 CrossRef CAS.
- V. Núñez-López, R. Gil-Egui and S. A. Hosseini, Energies, 2019, 12, 448 CrossRef.
- EPA, Greenhouse Gas Equivalencies Calculator - Calculations and References, US Environmental Protection Agency, 2024, https://www.epa.gov/energy/greenhouse-gas-equivalencies-calculator-calculations-and-references, Accessed: 2024-12-06.
- L. W. Holm and V. A. Josendal, Soc. Pet. Eng. J., 1982, 22, 87–98 CrossRef CAS.
- M. Z. Hauschild, R. K. Rosenbaum and S. I. Olsen, Life cycle assessment, Springer, 2018 Search PubMed.
- S. Deutz and A. Bardow, Nat. Energy, 2021, 6, 203–213 CrossRef CAS.
- T. Terlouw, K. Treyer, C. Bauer and M. Mazzotti, Environ. Sci. Technol., 2021, 55, 11397–11411 CrossRef CAS PubMed.
- R. Labedi, J. Pet. Sci. Eng., 1990, 4, 375–390 CrossRef.
- S. Anwar and J. J. Carroll, Carbon Dioxide Thermodynamic Properties Handbook: Covering Temperatures from −20 to 250 °C and Pressures Up to 1000 Bar, John Wiley & Sons, 2016 Search PubMed.
- N. A. Azzolina, D. V. Nakles, C. D. Gorecki, W. D. Peck, S. C. Ayash, L. S. Melzer and S. Chatterjee, Int. J. Greenhouse Gas Control, 2015, 37, 384–397 CrossRef CAS.
- R. E. Hadlow, Soc. Pet. Eng. J., 1992, 24928 Search PubMed.
- W. R. Brock and L. A. Bryan, Soc. Pet. Eng. J., 1989, 18977 Search PubMed.
- V. Kuuskraa, R. Petrusak and M. Wallace, Energy Procedia, 2017, 114, 5438–5450 CrossRef CAS.
- R. J. Stewart, G. Johnson, N. Heinemann, M. Wilkinson and R. S. Haszeldine, Int. J. Greenhouse Gas Control, 2018, 75, 235–242 CrossRef CAS.
- V. Núñez-López and E. Moskal, Front. Clim., 2019, 1, 5 CrossRef.
- IEA, Net Zero Roadmap: A Global Pathway to Keep the 1.5 °C Goal in Reach, IEA, Paris, 2023, https://www.iea.org/reports/net-zero-roadmap-a-global-pathway-to-keep-the-15-0c-goal-in-reach, Accessed: 2024-10-03.
- IPCC, Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, IPCC, Geneva, Switzerland, 2023.
Footnote |
| † Electronic supplementary information (ESI) available: Data from existing CO2-EOR projects and additional analyses. See DOI: https://doi.org/10.1039/d5ee01752a |
| This journal is © The Royal Society of Chemistry 2025 |




