Sustaining vacancy catalysis via conformal graphene overlays boosts practical Li–S batteries†
Jiaxi
Gu‡
a,
Zixiong
Shi‡
b,
Yongbiao
Mu‡
c,
Yuzhu
Wu‡
d,
Meng
Tian
 *e,
Ziang
Chen
a,
Kaihui
Chen
e,
Huicun
Gu
c,
Miaoyu
Lu
a,
Lin
Zeng
*e,
Ziang
Chen
a,
Kaihui
Chen
e,
Huicun
Gu
c,
Miaoyu
Lu
a,
Lin
Zeng
 *c,
Yuqing
Song
*d,
Qiang
Zhang
*c,
Yuqing
Song
*d,
Qiang
Zhang
 f and
Jingyu
Sun
f and
Jingyu
Sun
 *ad
*ad
aCollege of Energy, Soochow Institute for Energy and Materials Innovations, Key Laboratory for Advanced Carbon Materials and Wearable Energy Technologies of Jiangsu Province, Soochow University, Suzhou 215006, China. E-mail: sunjy86@suda.edu.cn
bMaterials Science and Engineering, Physical Science and Engineering Division, King Abdullah University of Science and Technology, Thuwal 23955-6900, Saudi Arabia
cShenzhen Key Laboratory of Advanced Energy Storage, Department of Mechanical and Energy Engineering, Southern University of Science and Technology, Shenzhen 518055, China. E-mail: zengl3@sustech.edu.cn
dBeijing Graphene Institute, Beijing 100095, China. E-mail: songyq@bgi-graphene.com
eSchool of New Energy, School of Intelligent Manufacturing, Nanjing University of Science and Technology, Jiangyin 214443, China. E-mail: tianmeng@njust.edu.cn
fDepartment of Chemical Engineering, Tsinghua University, Beijing 100084, China
First published on 8th May 2025
Abstract
Sluggish reaction kinetics and uncontrollable dendrite growth are deemed as the main bottlenecks for practical Li–S batteries. Notwithstanding fruitful advances in designing dual-functional mediators for both electrodes, cooperative efforts on protecting catalytically active sites and optimizing solid electrolyte interphase (SEI) by the employment of industrial catalysts are still lacking. Herein, an oxygen vacancy (VO)-sustained prototype mediator with layer-number controllable graphene modification (Al2O3@mG) is developed for concurrently accelerating redox kinetics at the S cathode and harvesting inorganic-rich SEI at the Li anode. Theoretical and experimental analyses reveal that VO enhances the electrocatalytic activity while the graphene overlay serving as a catalysis sustainer enables vacancy protection. Meanwhile, Al2O3@mG is conducive to homogenizing Li-ion flux and boosting preferential decomposition of anions, thereby stabilizing the Li metal anode. Benefiting from such dual-functional reformulation, Li–S batteries with Al2O3@mG modified separators achieve a low capacity decay of 0.032% per cycle over 1600 cycles at 1.0 C. The assembled pouch cell delivers high areal capacity and stable cycling operation. Such a vacancy-sustained graphene strategy showcases promising universality to be applied on various oxide candidates, offering meaningful guidance in mediator design toward pragmatic Li–S batteries.
Broader contextLithium–sulfur (Li–S) batteries stand out as one of the most promising energy storage systems owing to their energy density (2600 Wh kg−1), cost effectiveness, and environmentally friendly nature. The key issues impeding their development lie in severe polysulfide crossover and uncontrollable dendrite growth, which impose considerable limitations on the cycling lifetime of Li–S batteries. Dual-functional mediators have been widely investigated as a potential solution strategy. However, cooperative efforts on protecting catalytically active sites and optimizing solid electrolyte interphase (SEI) are still lacking. In this study, an oxygen vacancy (VO)-sustained Al2O3 prototype mediator with layer-number controllable graphene modification (Al2O3@mG) is developed for concurrently accelerating redox kinetics at the sulfur cathode and harvesting inorganic-rich SEI at the lithium anode. VO enhances the electrocatalytic activity of Al2O3 while the graphene overlay serving as a catalysis sustainer enables vacancy protection. Meanwhile, Al2O3@mG is conducive to homogenizing Li-ion flux and boosting the preferential decomposition of anions, thereby stabilizing the Li metal anode. This work delineates a vacancy-sustained graphene strategy, which showcases promising universality for application in various oxide candidates and further offering meaningful guidance to design dual-functional mediators toward pragmatic Li–S batteries. |
Introduction
Lithium–sulfur (Li–S) batteries have received extensive research attention in response to the growing demand for next-generation energy storage systems.1–5 The key issues impeding their development lie in severe polysulfide crossover and uncontrollable dendrite growth, which impose considerable limitations on the cycling lifetime, especially under harsh conditions such as elevated sulfur loading and large current density.6–10 Recent years have witnessed the appearance of a “one bird two stone” strategy to devise effective mediators with dual sulfiphilicity and lithiophilicity, simultaneously enabling facile sulfur redox kinetics and homogeneous lithium metal deposition.11–15To achieve boosted sulfur redox reaction and suppressed “shuttling effect”, a myriad of mediators with strong electrocatalytic effect have been developed, where vacancy engineering is regarded as a crucial solution to augmenting active sites.16–20 Despite enhanced catalytic activity for sulfur cathodes, insulating discharge products (i.e., Li2S2/Li2S) are prone to deposit over the limited catalyst surfaces without sufficient decomposition, hence the active sites are continuously deteriorated.21,22 In this case, it is anticipated that vacancies are vulnerable to various electronegative sulfur species, which would exacerbate the detrimental coverage on active sites along with severe accumulation of “dead” sulfides. Although such a catalyst failure has been identified as a universal phenomenon in the aprotic environment of Li–S batteries, strategies for sustaining the active sites are still lacking. Our recent endeavours unravelled that grown graphene coatings effectively armor the electrocatalysts, whereby both the catalytic activity and durability were elevated.23–25 Nevertheless, the strategic feasibility in protecting vacancy-rich electrocatalysts has not been explored. In this respect, it is imperative to decipher the function mechanism and actual sustainability of vacancies under the shielding of graphene chainmail.
In parallel, whether vacancy engineering would exert positive effects on stabilizing the Li metal anode is still questionable, wherein the fundamental understanding of the vacancy-mediated electrolyte decomposition pathway and chemical component distribution in as-formed solid electrolyte interphase (SEI) would be a key. Meanwhile, exploring the synergistic effect between vacancies and graphene on rendering a stable Li metal anode is also highly desirable. Inspired by these considerations, prevailing industrial catalyst Al2O3 is employed as a prototype dual-functional mediator, where oxygen vacancies (VO) are constructed during the chemical vapor deposition (CVD)-derived graphene growth process. Throughout the delicate thickness adjustment of graphene coatings, few-layer, multi-layer or thick-layer graphene is customized onto powdery Al2O3 (denoted as Al2O3@fG, Al2O3@mG and Al2O3@tG) with the aim to concurrently accelerate sulfur reaction kinetics and afford inorganic-rich SEI for long-life Li–S batteries (Fig. 1a). In one case, electrocatalytic activity of Al2O3 could be boosted with VO introduction. The multi-layer graphene armor can serve as a vacancy catalysis sustainer to mitigate the detrimental coverage of discharge products and restrain the “poisoning” of active sites. In another case, VO is beneficial for inducing homogenous Li deposition and promoting the preferential decomposition of anions, thereby generating and maintaining inorganic-rich SEI to suppress the dendritic growth at the anode side. Benefiting from such a dual-functional mediator effect, Li–S batteries equipped with Al2O3@mG modified separators harvest an initial capacity of 1307.5 mA h g−1 at 0.2 C, accompanied by a negligible fading rate of 0.032% for 1600 cycles at 1.0 C. The assembled pouch cell delivers an impressive areal capacity of 5.3 mA h cm−2, accompanied by a reasonable lifespan over 70 cycles. This strategy shows an appealing universality, which could be extended to other oxide catalysts (MgO, TiO2, and MoO3). Our work reveals the vital merits of synergistic vacancy engineering and graphene armoring for the optimization of interfacial electrochemistry, which affords practical insight into the rational design of advanced mediators for Li–S batteries.
Results and discussion
Vacancy-sustained graphene design
The Al2O3@G with varied layer numbers of graphene coating was achieved via a well-designed direct CVD synthesis. First, an Ar flow (100 sccm) was introduced into the furnace at room temperature while it was gradually increased to 1100 °C, followed by a preliminary treatment for 10 min with a H2 flow (50 sccm) to remove surface contaminants of Al2O3. Subsequently, CH4 (10 sccm) serves as the carbon precursor to enable controllable graphene growth under a mixed gas flow of H2 (50 sccm) and Ar (100 sccm), thus deriving Al2O3@G with a high production yield (Fig. 1b). It is worth noting that the layer number of the graphene shell can be tuned by altering the reaction duration. Likewise, TiO2@G, MgO@G and MoO3@G could be synthesized by changing the growth supports.X-ray diffraction (XRD) was utilized to probe the crystal phases of as-synthesized Al2O3@G. As illustrated in Fig. S1 (ESI†), the predominant signals of all samples match well with the (012) and (113) planes of α-Al2O3.26 Raman spectra were recorded to investigate the structural features of graphene. As shown in Fig. 1c, the typical peaks of the D band (1335 cm−1), G band (1570 cm−1) and 2D band (2668 cm−1) can be detected, corroborating graphene formation.27,28 Meanwhile, electron paramagnetic resonance (EPR) profiles were collected to gain insight into the defect characteristics. In this respect, distinct EPR signals that can be observed at a g-value of 2.003 for Al2O3@G are missing for pure Al2O3 (Fig. 1d and Fig. S2, ESI†), which is attributed to the partial removal of O atoms to generate unpaired electrons.29 This verifies the successful introduction of VO under thermal treatment with a reducing atmosphere. Notably, the concentration of VO is boosted with the extension of the CVD growth time, which might play a role in modulating the electrocatalytic activity of Al2O3.
The morphologies of Al2O3@G were inspected by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Upon graphene coating, there is no discernible difference in powdery morphology between bare Al2O3 and Al2O3@G (Fig. S3, ESI†). As shown in high-resolution TEM (HRTEM) images of Al2O3@mG, Al2O3@fG and Al2O3@tG (Fig. 1e and Fig. S4, ESI†), a lattice spacing of 1.81 Å for the inner core area could be clearly identified (Fig. 1f), corresponding to the (012) plane of α-Al2O3via the fast Fourier transform (FFT) pattern.30 Statistical analysis of the HRTEM images shows that the graphene shells grown on the Al2O3 core can be dictated at 4–6 layers (Fig. 1g and Fig. S5, ESI†), which is anticipated to achieve a balance between activity and stability for polysulfide electrocatalysis. Furthermore, the graphene shells of Al2O3@fG and Al2O3@tG are respectively adjusted to ca. 2 and 12 layers (Fig. 1h), again confirming the precise regulation of the layer numbers via our direct CVD route. The good tunability of the graphene overlay would help elucidate the evolution of vacancy-rich catalysts during the polysulfide conversion reaction. Energy-dispersive X-ray analysis of Al2O3@mG reveals the homogeneous element distribution (Fig. S6, ESI†), indicative of uniform graphene encapsulation over the inner Al2O3 core.
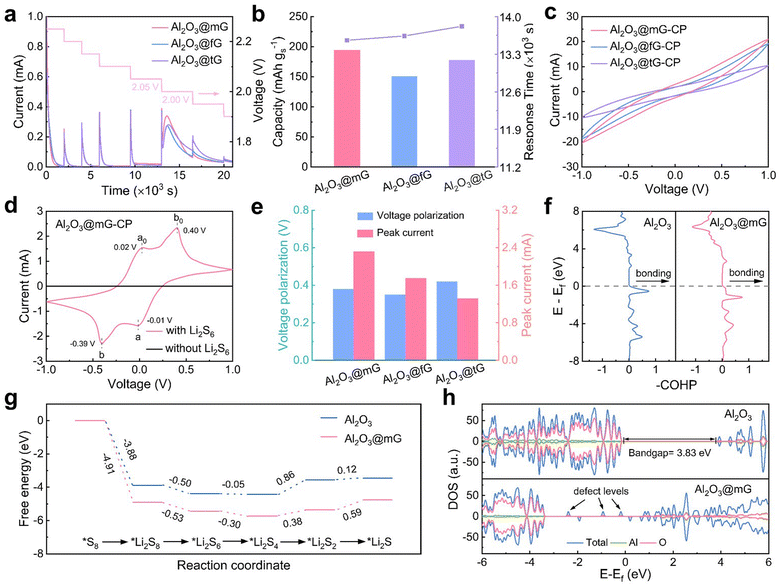
Polysulfide adsorption and catalytic conversion
To unravel the merits of VO-rich Al2O3 with tunable graphene chainmail in promoting bidirectional sulfur chemistry, a series of electro-kinetic analysis was performed.31 It is well accepted that both Li2S nucleation and dissociation afford a considerable energy barrier, which were investigated by potentiostatic intermittent titration technique (PITT) measurement.32 As for Li2S nucleation, the assembled battery was potentiostatically discharged from 2.25 to 1.90 V with an interval of 50 mV (Fig. 2a). The current peaks at 2.0 V are ascribed to Li2S precipitation from soluble polysulfide intermediates, wherein Al2O3@mG shows the highest peak intensity and the earliest time to reach the peak as compared to its counterparts (Fig. S7, ESI†). As manifested in Fig. 2b, the Al2O3@mG exhibits the largest capacity (194.3 mA h gs−1). In parallel, Li2S dissociation was investigated by potentiostatically charging the batteries from 2.20 to 2.50 V, with each interval of 100 mV, in which Al2O3@mG also displays the highest current response and fastest reaction kinetics (Fig. S8, ESI†). These results suggest that Al2O3@mG harnesses electrocatalytic robustness for expediting dual-directional sulfur redox reaction. In situ Raman probing of the Li–S cell with Al2O3@mG/PP was performed during a complete discharge/charge cycle, where the dynamic changes of LiPS speciation and concentration could be tracked. As indicated in Fig. S9 (ESI†), a gradual weakening of polysulfide signals including S82−, S62− and S32− can be observed during the discharging process, followed by a continuous signal enhancement during the subsequent charging, indicative of kinetically favorable LiPS conversion. Cyclic voltammetry (CV) profiles of the symmetric cells were collected in a Li2S6-containing electrolyte at a scan rate of 50 mV s−1. Apparently, Al2O3@mG enables the highest redox current response with improved reversibility (Fig. 2c and Fig. S10, ESI†). Meanwhile, it also harvests sharper and stronger redox peaks as compared to its counterparts at a scan rate of 0.5 mV s−1, suggestive of boosted electrocatalytic activity for polysulfide conversion (Fig. 2d and e and Fig. S11, ESI†). In this respect, other metal oxide catalysts also display strong electrocatalytic capability (Fig. S12, ESI†), manifesting that our graphene overlayer as a vacancy sustainer is widely applicable for electrocatalyst modification.Since optimized adsorption capability plays a vital role in mitigating the LiPS shuttle, the visualized Li2S6 adsorption test was carried out (Fig. S13, ESI†). Upon the addition of Al2O3@mG, the Li2S6 solution was rapidly decoloured, suggesting the efficient restriction of LiPS dissolution/diffusion. With respect to the UV-Vis spectroscopic study, the Li2S6 solution mixed with the Al2O3@mG exhibits the weakest absorption peak, further implying its strong polysulfide adsorption ability on account of VO sites. To further explore the synergistic effect of vacancy engineering and graphene armoring on propelling sulfur redox kinetics, density functional theory (DFT) calculation was performed. The crystal orbital Hamilton population (COHP) was employed to describe the orbital–pair interactions. As depicted in Fig. S14 (ESI†), three catalyst configurations are included: Al2O3, Al2O3 with single oxygen vacancy (Al2O3−x) and Al2O3 with double oxygen vacancies (Al2O3–2x). The results show that the adsorption energy of Li2S2 increased with the introduction of VO, in accordance with the integrated crystal orbital Hamilton population (–ICOHP) analysis. In the meantime, a similar trend can be observed with respect to the Al–S bond between Al2O3@G and Li2S4, demonstrative of enhanced polysulfide immobilization (Fig. S15, ESI†).33 Additionally, COHP was employed to resolve the states of the metal–sulfur bond, wherein the positive and negative COHP represent the bonding and anti-bonding contribution, respectively.34 Since fewer occupied electrons in the anti-bonding orbital represent stronger metal–sulfur interaction, Al2O3@G possesses a stronger Al–S bond for enhancing Li2S4 adsorption (Fig. 2f). Gibbs free energy change (ΔG) for multi-step sulfur reduction reactions was further calculated (Fig. 2g and Fig. S16 and S17, ESI†). Notably, Al2O3@G shows an apparently lower ΔG value than that of pristine Al2O3 for each step, indicative of promoted reaction kinetics. To investigate the underlying reasons, the electronic structure modification of the metal centre with the introduction of VO was analysed based on the density of states (DOS). As shown in Fig. 2h and Fig. S18 (ESI†), VO could introduce the defect levels, thereby narrowing the width of the band-gap. Consequently, it facilitates electron transfer from Al to S atoms and strengthens the Al–S hybridization bonding. Li2S dissociation pathways on Al2O3 with/without vacancy were modelled (Fig. S19 and S20, ESI†). As such, the lower energy barrier for Al2O3@G implies higher reaction activity for the promotion of Li2S decomposition.
Mechanism of active site failure and protection
To gain in-depth insight into the failure mechanism of Al2O3@G, ex situ EPR profiles were recorded. As shown in Fig. S21 (ESI†), the EPR intensities of both Al2O3@fG and Al2O3@tG exhibit an obvious decrease upon the discharge process, which might be attributed to the heavy deposition of discharge products without sufficient decomposition. In contrast, the EPR intensity of Al2O3@mG displays a negligible change after electrochemical cycling (Fig. 3a and b). In this sense, the vacancy retention rate of Al2O3@mG after 60 cycles at 1.0 C was derived to be 94.3%, which is higher than those of Al2O3@fG (75.4%) and Al2O3@tG (86.1%) (Fig. 3c). It is hence conjectured that the active sites of VO can be well maintained in the presence of graphene armor with an optimized thickness. To further verify the protective effect of graphene on catalytic activity, CV profiles were continuously collected at a scan rate of 50 mV s−1. As depicted in Fig. S22a (ESI†), Al2O3@mG can still exhibit a high redox current response with favourable reversibility after 30 cycles, suggestive of a sustainable electrocatalytic activity. Meanwhile, Li–S batteries equipped with Al2O3-modified separators display an apparent capacity decrease after resting for 20 h (Fig. S22b, ESI†). In stark contrast, Al2O3@mG/PP exhibits a negligible capacity loss (30.4 mA h g−1), indicating a durable electrocatalytic effect to mitigate an irreversible loss of active species. The conspicuous advantages of multi-layer graphene overlay are demonstrated in Fig. S22c (ESI†). Molecular dynamics simulation based on the large-scale atomic/molecular massively parallel simulator was also carried out to probe the structural features of VO.35 With respect to Al2O3−x substrates with the aid of a graphene sustainer, VO exhibits more stable states than that of bare Al2O3−x (Fig. S23, ESI†). Moreover, higher vacancy durability can be obtained when increasing the layer amount of graphene from one to three, suggesting that graphene chainmail can protect vacancy sites from “poisoning”. Collectively, one can conclude that Al2O3@fG with a thin graphene coating suffers from an insufficient shielding effect toward VO, whereas Al2O3@tG with thick layers hinders the complete release of the inner active sites. Both would lead to a rapid failure of active sites upon electrochemical reaction (Fig. 3d). The direct-CVD-enabled graphene with moderate thickness serves as a sustainer to protect vacancy sites, which is conducive to mitigating the detrimental coverage of discharge product while facilitating dual-directional sulfur redox (Fig. 3e).SEI compositional analysis and mechanistic investigation
To evaluate the impact of Al2O3@G on the lithium metal anode, Li||Li symmetric cell tests employing a PP separator were first carried out.36 Rate performances of Al2O3@mG/PP, Al2O3@fG/PP, Al2O3@tG/PP, Al2O3/PP, and PP were recorded under different current densities from 0.5 to 5.0 mA cm−2 with a fixed capacity of 1.0 mA h cm−2 (Fig. 4a and Fig. S24, ESI†). The cell with Al2O3@mG/PP delivers the lowest overpotential as well as the smallest voltage fluctuation. With respect to the Li plating/stripping test at 1.0 mA cm−2/1.0 mA h cm−2, Al2O3@mG/PP displays a superior cycling stability over 1800 h with a low voltage polarization while bare PP suffers from premature failure (Fig. 4b and Fig. S25 and S26, ESI†). Impressively, the cycling performance of Al2O3@mG/PP compares favourably with other advanced separator designs in Li–S batteries (Table S1, ESI†).12,13,17,36–40 Furthermore, deposited Li metal with VO-Al2O3@mG/PP exhibits a smoother morphology than its other counterparts after cycling, demonstrative of its key role in stabilizing the Li metal anode (Fig. S27, ESI†). As shown in Fig. S28 (ESI†), the voltage curve of the Li||Cu cell with the Al2O3@mG/Cu electrode also exhibits a smaller nucleation overpotential of 30.1 mV in comparison with that of Al2O3@fG/Cu (39.8 mV), Al2O3@tG/Cu (44.9 mV), Al2O3/Cu (48.3 mV), and bare Cu (75.9 mV). In addition, a high average Coulombic efficiency (CE) over 98.5% can be obtained on the Al2O3@mG/Cu electrode, indicative of its favourable lithiophilicity for homogeneous Li metal deposition.It is well established that the chemical components of SEI help govern the ion transport and interfacial stability at the anode/electrolyte interface.38,41,42 To investigate the roles of vacancy engineering in modulating SEI's composition, XPS depth profiling of the cycled Li metal anode was carried out. As for the Li 1s spectrum, three peaks appearing at 56.1, 55.2 and 54.0 eV respectively correspond to Li–CO32−, Li–N, and ROCO2–Li/Li–O (Fig. 4c and e).43 Two deconvoluted signals at 399.6 and 398.8 eV in the N 1s spectrum are ascribed to C–N and Li–N bonds, respectively (Fig. 4d and f).44 In the F 1s profile, C–F (688.8 eV) and Li–F (685.2 eV) bonds could be identified (Fig. S29, ESI†).45 Notably, the existence of ROCO2–Li/Li–O and C–F bonds indicates the formation of organic species from solvent decomposition, while the inorganic components (e.g., Li2CO3, Li3N and LiF) are usually derived from TFSI anions.46 With the increase of etching time, the proportion of ROCO2–Li/Li–O species formed via bare Al2O3 is gradually augmented. Even after Ar+ etching for 180 s, organic components still exhibit an overwhelming advantage, which is detrimental to Li-ion transportation and interfacial stability. Encouragingly, massive inorganic components exist in the SEI formed by Al2O3@mG, wherein the percentages of Li3N and LiF remain continuously high at different etching states. Upon Ar+ etching treatment, Li3N and LiF signals in the SEI layer are continuously increased for the Al2O3@mG electrode, which are stabilized at 60 s and become the dominant inorganic components. At 120 s of etching time, the ratio of N and F elements is almost twice as high as that formed by bare Al2O3 (Fig. S30, ESI†), again verifying the formation of an inorganic-rich SEI architecture under the regulation of VO and graphene armor. Fig. 4g displays the elemental ratio distribution at different etching stages, where a high ratio of inorganic to organic species can be observed for the SEI generated by Al2O3@mG (Fig. S31, ESI†). The spatial distribution of N and F elements on the Li metal anode was further analysed by time-of-flight secondary ion mass spectrometry (ToF-SIMS). By investigating the secondary ion F− and N−, the 3D spatial distribution of LiF and Li3N components can be unveiled.47 The F− intensity of Al2O3@mG is higher either on the surface or in the bulk than that for bare Al2O3 (Fig. 4h and i). In the meantime, a similar trend can be observed with respect to the distribution of N− (Fig. S32, ESI†). It is therefore concluded that the presence of VO promotes the formation of inorganic SEI constituents, thereby achieving a higher proportion of LiF and Li3N. Meanwhile, graphene armor plays a key role in sustaining vacancy sites without rapid deterioration (Fig. S33, ESI†).
Cryo-transmission electron microscopy (Cryo-TEM) was employed to further inspect the microstructure of SEI.48 As illustrated in Fig. 5a, a myriad of crystal domains could be witnessed in the SEI formed by Al2O3@mG, implying the richness of inorganic components. In response, the crystalline region mainly consists of Li3N, Li2O, Li2S, Li2CO3 and LiF, as evidenced by the corresponding FFT patterns (Fig. 5b–f).49 In particular, the marked areas 1, 4 and 5 can be indexed to the (111) planes of Li3N, LiF and Li2S, respectively.50 It is hence concluded that the SEI formed via Al2O3@mG is composed of many inorganic components derived from the decomposition of TFSI anions, validating the favorable SEI formation mediated by such a vacancy sustainer (Fig. 5g). In stark contrast, the SEI formed by bare Al2O3 is quite amorphous, in which Li2CO3 becomes the main component instead of LiF and Li3N (Fig. 5h–j). The proportion of organic components is also increased owing to dominant solvent decomposition, leading to an inhomogeneous thick SEI layer (Fig. 5k).
To clarify the mechanism of VO-mediated SEI formation, the adsorption energies between anions and Al2O3@mG were subject to DFT calculations (Fig. 5l).51 Although adsorption configurations are similar, the introduction of VO into Al2O3 enhances the capture of TFSI− with the adsorption energy augmenting from 0.69 eV to 4.10 eV (Fig. 5m). Likewise, the adsorption between NO3− and Al2O3@mG is also stronger than that for bare Al2O3, which promotes the breaking of N–O bonds (Fig. S34, ESI†). In terms of lithiophilicity, the adsorption configurations of Li+ on the Al-top site, O-top site and near O vacancy-top site were taken into consideration (Fig. S35 and S36, ESI†).52 Among them, Li+ near the O vacancy-top site affords a lower adsorption energy (−1.58 eV) than those of other configurations, confirming that Al2O3 with oxygen vacancies would exhibit stronger affinity with Li+. Moreover, the diffusion pathways of Li+ on Al2O3@mG and Al2O3 were investigated (Fig. S37, ESI†). In this respect, the apparently lower energy barrier for Al2O3@mG manifests that VO and graphene chainmail boost ion transport and facilitates interfacial kinetics (Fig. S38, ESI†). Finite element method simulations of the spatial Li+ distributions were performed to reveal the ion flux status over the Li electrode. In general, the reverse migration and accumulation of anions in the electrolyte deteriorate Li+ transport under a continuously applied electric field.53 The imbalance between Li+ consumption and transport would result in a depletion of Li+ near the anode surface. Encouragingly, Al2O3@mG helps boost the concentration of Li+ near the surface of Li anode, thereby enhancing interfacial charge transfer and homogenizing lithium deposition (Fig. 5n and Fig. S39, S40, ESI†).
Electrochemical performance
To comprehensively probe the electrochemical performances, coin-type Li–S batteries were assembled with Al2O3@mG or Al2O3 modified separators. Fig. 6a presents the CV profiles of full batteries within a voltage window of 1.7–2.8 V at a scan rate of 0.05 mV s−1. Two featured reduction peaks (peak i and peak ii) appear at 2.3–2.4 V and 1.9–2.1 V during the discharge process, corresponding to the formation of soluble polysulfides and insoluble Li2S, respectively. Meanwhile, the oxidation peaks are contributed by the Li2S decomposition during the charge process. Note that the Al2O3@mG/PP exhibits a higher current response and smaller voltage polarization among the tested systems, indicative of robust electrocatalytic activity toward the sulfur redox reactions. The as-derived Tafel slopes of both reduction and oxidation peaks were used to quantify the electrocatalytic activity.54 With respect to peak ii, the slope values for Al2O3@mG/PP, Al2O3/PP, and PP reach 25.0, 28.1, and 39.6 mV dec−1, respectively (Fig. S41, ESI†). As for the peak iii ascribed to Li2S dissociation, Al2O3@mG/PP also exhibits a smaller Tafel slope, suggesting a kinetically favorable sulfur reaction. Furthermore, CV curves at scan rates ranging from 0.1 to 0.5 mV s−1 were collected to investigate the Li-ion diffusion based on the Randles–Sevcik equation (Fig. S42, ESI†). The fitted curve for Al2O3@mG/PP is much sharper, signifying optimized Li-ion migration and improved reaction kinetics under the regulation of VO and graphene chainmail (Fig. S43, ESI†). As demonstrated by electrochemical impedance spectroscopy (EIS) analysis, the Al2O3@mG/PP shows smaller values for both R1 (equivalent series resistance) and Rct (charge-transfer resistance),55 which can be attributed to the high electrical conductivity and fast interfacial reaction (Fig. S44, ESI†).Rate performances of Al2O3@mG/PP, Al2O3/PP and PP were evaluated under different current densities (Fig. 6b). Al2O3@mG/PP demonstrates an obvious advantage, delivering capacities of 1307.5, 998.4, 772.9, 611.2, and 515.6 mA h g−1 at 0.2, 0.5, 1.0, 2.0 and 3.0 C, respectively. When the current density is switched back to 0.5 C, a capacity of 948.6 mA h g−1 could be recovered. Even at a high rate of 3.0 C, Al2O3@mG/PP enables two obvious discharge plateaus (Fig. S45, ESI†), indicative of efficient reaction kinetics. Alongside, galvanostatic charge/discharge (GCD) profiles at 0.2 C were collected (Fig. 6c). Notably, Al2O3@mG/PP not only delivers a higher capacity but also exhibits a lower voltage gap in comparison with its other counterparts, again confirming the kinetically favorable polysulfide conversion as well as reduced reaction polarization. The corresponding discharge capacities at high-voltage and low-voltage plateaus are shown in Fig. S46 (ESI†), where the Al2O3@mG/PP presents the larger values of 334 and 922 mA h g−1, respectively. As shown in Fig. 6d, the Al2O3@mG/PP harvests a high initial capacity with a favorable capacity retention after 100 cycles at 0.2 C, which is also superior to that of Al2O3/PP and PP.
When the current density is increased to 1.0 C, it is still able to achieve a reversible capacity of 374.2 mA h g−1 with a low decay rate of 0.032% per cycle after 1600 cycles, verifying the versatile functions of Al2O3@mG in restraining polysulfide shuttling and improving sulfur utilization (Fig. 6e). As presented in Fig. S47 (ESI†), Li–S batteries with an elevated sulfur loading and low electrolyte usage were also assembled. With a sulfur loading of 3.1 and 4.2 mg cm−2, they are able to operate stably for 40 cycles at 0.1 C, respectively yielding a maximum areal capacity of 3.9 and 4.1 mA h cm−2. More encouragingly, the Al2O3@mG/PP delivers an areal capacity of 4.7 mA h cm−2 at a sulfur loading of 5.1 mg cm−2, exceeding that of commercial Li-ion batteries (Fig. 6f and Table S2, ESI†).56–61 To further explore its practical potential, pouch cells with a thin Li anode (50 μm) and an E/S ratio of 4.8 μL mg−1 were assembled (Fig. 6g). With the aid of the Al2O3@mG modified separator, it realizes an initial areal capacity of 5.4 mA h cm−2, accompanied by a capacity retention of 73.1% at 0.2 C over 70 cycles (Fig. 6h). As shown in galvanostatic charge/discharge curves of a Li–S pouch cell, two typical discharge plateaus can be clearly observed (Fig. S48, ESI†), demonstrating a kinetically favorable sulfur reaction. Benefiting from such a dual-functional mediator and vacancy catalysis sustainer, the performances of Li–S batteries equipped with Al2O3@mG compare favorably with their related counterparts in both coin-type and pouch-cell configurations (Fig. 6i and j and Table S3, ESI†).13,38,58,60,62–72
Conclusions
We have designed a dual-functional VO-rich Al2O3 mediator for Li–S batteries, which was encapsulated by graphene chainmail with controlled layer number via the CVD technique. It is revealed that the electrocatalytically active sites of Al2O3 with a moderate vacancy concentration can be maintained with the aid of a tailored multi-layer graphene armor, which functions as a catalysis sustainer to mitigate the detrimental coverage of insulating discharge product and promote catalytic durability. Meanwhile, VO-rich Al2O3@mG is conducive to inducing homogeneous Li deposition and promoting the preferential decomposition of anions, thereby rendering a stable inorganic-rich SEI layer and inhibiting Li dendrite growth. Consequently, Li–S batteries equipped with Al2O3@mG modified separators exhibit impressive cycling stability with a low capacity decay rate at 0.032% per cycle after 1600 cycles at 1.0 C. The assembled pouch cells with a thin Li anode and a low E/S ratio acquire a high areal capacity with stable operation over 70 cycles. Our work explores the maintenance mechanism of vacancies and their role in mediating electrolyte decomposition as well as SEI generation, which opens an avenue for the rational modulation of dual-functional mediators toward Li–S commercialization.Author contributions
J. Sun conceived the idea. J. Gu, Y. Wu and Y. Song performed the synthesis of a dual-functional mediator. J. Gu, Y. Wu, Z. Chen and M. Lu carried out the material characterization and electrochemical measurements. Y. Mu, H. Gu and L. Zeng carried out the cryo-TEM measurements. M. Tian and K. Chen conducted the computational simulations. The manuscript was written by J. Gu, Z. Shi, Q. Zhang and J. Sun with input from all authors. All authors contributed to the analysis and discussion of the results leading to the manuscript. All authors have given approval to the final version of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no competing interests.Acknowledgements
This work was supported by the National Natural Science Foundation of China (T2188101, 22179089, and 22475026). The authors acknowledge the support from Suzhou Key Laboratory for Advanced Carbon Materials and Wearable Energy Technologies.References
- S. Chung and A. Manthiram, Adv. Mater., 2019, 31, 1901125 CrossRef PubMed.
- P. Bruce, S. Freunberger, L. Hardwick and J. Tarascon, Nat. Mater., 2011, 11, 19 CrossRef PubMed.
- Z. Shi, M. Li, J. Sun and Z. Chen, Adv. Energy Mater., 2021, 11, 2100332 CrossRef CAS.
- D. Liu, C. Zhang, G. Zhou, W. Lv, G. Ling, L. Zhi and Q. Yang, Adv. Sci., 2017, 5, 1700270 CrossRef PubMed.
- Z. Shi, Y. Ding, Q. Zhang and J. Sun, Adv. Energy Mater., 2022, 11, 2100332 CrossRef.
- C. Ye, Y. Jiao, H. Jin, A. Slattery, K. Davey, H. Wang and S. Qiao, Angew. Chem., Int. Ed., 2018, 57, 16703 CrossRef CAS PubMed.
- M. Jana, R. Xu, X. Cheng, J. S. Yeon, J. M. Park, J. Huang, Q. Zhang and H. S. Park, Energy Environ. Sci., 2020, 13, 1049 RSC.
- A. Hu, M. Zhou, T. Lei, Y. Hu, X. Du, C. Gong, C. Shu, J. Long, J. Zhu, W. Chen, X. Wang and J. Xiong, Adv. Energy Mater., 2020, 10, 2002180 CrossRef CAS.
- Y. Ding, Q. Cheng, J. Wu, T. Yan, Z. Shi, M. Wang, D. Yang, P. Wang, L. Zhang and J. Sun, Adv. Mater., 2022, 34, 2202256 CrossRef CAS PubMed.
- Z. Shi, Z. Tian, D. Guo, Y. Wang, Z. Bayhan, A. Alzahrani and H. Alshareef, ACS Energy Lett., 2023, 8, 3054 Search PubMed.
- Q. Ran, J. Liu, L. Li, Q. Hu, H. Zhao, S. Komarneni and X. Liu, Adv. Funct. Mater., 2024, 34, 2402872 CrossRef CAS.
- S. Zhai, Z. Ye, R. Liu, H. Xu, C. Li, W. Liu, X. Wang and T. Mei, Adv. Funct. Mater., 2023, 34, 2314379 Search PubMed.
- L. Chen, Y. Sun, X. Wei, L. Song, G. Tao, X. Cao, D. Wang, G. Zhou and Y. Song, Adv. Mater., 2023, 35, 2300771 CrossRef CAS PubMed.
- R. Razaq, M. M. U. Din, D. R. Småbråten, V. Eyupoglu, S. Janakiram, T. O. Sunde, N. Allahgoli, D. Rettenwander and L. Deng, Adv. Energy Mater., 2023, 14, 2302897 CrossRef.
- Y. Zhang, Y. Qiu, L. Fan, X. Sun, B. Jiang, M. Wang, X. Wu, D. Tian, X. Song, X. Yin, Y. Shuai and N. Zhang, Energy Storage Mater., 2023, 63, 103026 CrossRef.
- M. Wang, Z. Sun, H. Ci, Z. Shi, L. Shen, C. Wei, Y. Ding, X. Yang and J. Sun, Angew. Chem., Int. Ed., 2021, 60, 24558–24565 CrossRef CAS PubMed.
- H. Li, R. Gao, B. Chen, C. Zhou, F. Shao, H. Wei, Z. Han, N. Hu and G. Zhou, Nano Lett., 2022, 22, 4999 CrossRef CAS PubMed.
- Y. Shi, D. Zhang, H. Miao, X. Wu, Z. Wang, T. Zhan, J. Lai and L. Wang, Sci. China Chem., 2022, 65, 1829 CrossRef CAS.
- Z. Shi, Z. Sun, J. Cai, X. Yang, C. Wei, M. Wang, Y. Ding and J. Sun, Adv. Mater., 2021, 33, 2103050 CrossRef CAS PubMed.
- C. Zhao, B. Jiang, Y. Huang, X. Sun, M. Wang, Y. Zhang and N. Zhang, Energy Environ. Sci., 2023, 16, 5490 RSC.
- R. Wang, C. Luo, T. Wang, G. Zhou, Y. Deng, Y. He, Q. Zhang, F. Kang, W. Lv and Q. H. Yang, Adv. Mater., 2020, 32, 2000315 CrossRef CAS PubMed.
- J. He, A. Bhargav and A. Manthiram, ACS Nano, 2021, 15, 8583 CrossRef CAS PubMed.
- J. Gu, Z. Shi, T. Yan, M. Tian, Z. Chen, S. Chen, Y. Ding, M. Lu, Y. Zou, J. Zhang, L. Zhang and J. Sun, Small, 2024, 21, 2407196 CrossRef PubMed.
- J. Cai, Z. Sun, W. Cai, N. Wei, Y. Fan, Z. Liu, Q. Zhang and J. Sun, Adv. Funct. Mater., 2021, 31, 2100586 CrossRef CAS.
- H. Ci, J. Cai, H. Ma, Z. Shi, G. Cui, M. Wang, J. Jin, N. Wei, C. Lu, W. Zhao, J. Sun and Z. Liu, ACS Nano, 2020, 14, 11929 CrossRef CAS PubMed.
- P. Liu, H. Hao, A. Singla, B. Vishnugopi, J. Watt, P. Mukherjee and D. Mitlin, Angew. Chem., Int. Ed., 2024, 63, 202402214 CrossRef PubMed.
- M. Wang, Y. Song, N. Wei, Y. Shao, G. Sheng and J. Sun, Chem. Eng. J., 2021, 418, 129407 CrossRef CAS.
- M. Wang, Y. Zhu, Y. Sun, Y. Zhao, X. Yang, X. Wang, Y. Song, H. Ci and J. Sun, Adv. Funct. Mater., 2022, 33, 2211978 CrossRef.
- Y. Tian, G. Li, Y. Zhang, D. Luo, X. Wang, Y. Zhao, H. Liu, P. Ji, X. Du, J. Li and Z. Chen, Adv. Mater., 2020, 32, e1904876 CrossRef PubMed.
- P. Wang, J. Bao, K. Lv, N. Zhang, Z. Chang, P. He and H. Zhou, ACS Appl. Mater. Interfaces, 2019, 11, 35788–35795 CrossRef CAS PubMed.
- N. Wang, B. Chen, K. Qin, E. Liu, C. Shi, C. He and N. Zhao, Nano Energy, 2019, 60, 332 CrossRef CAS.
- B. Q. Li, L. Kong, C. X. Zhao, Q. Jin, X. Chen, H. J. Peng, J. L. Qin, J. X. Chen, H. Yuan, Q. Zhang and J. Q. Huang, InfoMat, 2019, 1, 533 CrossRef CAS.
- C. Zhao, Y. Huang, B. Jiang, Z. Chen, X. Yu, X. Sun, H. Zhou, Y. Zhang and N. Zhang, Adv. Energy Mater., 2023, 14, 2302586 CrossRef.
- M. Lu, T. Yan, Y. Ding, S. Chen, Z. Chen, J. Gu, X. Chen, L. Zhang, M. Tian and J. Sun, Energy Storage Mater., 2024, 70, 103458 CrossRef.
- A. Thompson, H. Aktulga, R. Berger, D. Bolintineanu, W. Brown, P. Crozier, P. In’t Veld, A. Kohlmeyer, S. Moore, T. Nguyen, R. Shan, M. Stevens, J. Tranchida, C. Trott and S. Plimpton, Comput. Phys. Commun., 2022, 271, 108171 CrossRef CAS.
- P. Xiong, F. Zhang, X. Zhang, Y. Liu, Y. Wu, S. Wang, J. Safaei, B. Sun, R. Ma, Z. Liu, Y. Bando, T. Sasaki, X. Wang, J. Zhu and G. Wang, Nat. Commun., 2021, 12, 4184 CrossRef CAS PubMed.
- J. Cai, J. Jin, Z. Fan, C. Li, Z. Shi, J. Sun and Z. Liu, Adv. Mater., 2020, 32, 2005967 CrossRef CAS PubMed.
- Y. Kong, L. Wang, M. Mamoor, B. Wang, G. Qu, Z. Jing, Y. Pang, F. Wang, X. Yang, D. Wang and L. Xu, Adv. Mater., 2024, 36, 2310143 CrossRef CAS PubMed.
- Y. Li, Z. Chen, X. Y. Zhong, T. Mei, Z. Li, L. Yue, J. L. Yang, H. J. Fan and M. Xu, Adv. Funct. Mater., 2024, 35, 2412279 CrossRef.
- J. Wang, S. Yi, J. Liu, S. Sun, Y. Liu, D. Yang, K. Xi, G. Gao, A. Abdelkader, W. Yan, S. Ding and R. V. Kumar, ACS Nano, 2020, 14, 9819 CrossRef CAS PubMed.
- W. Yao, J. Xu, L. Ma, X. Lu, D. Luo, J. Qian, L. Zhan, I. Manke, C. Yang, P. Adelhelm and R. Chen, Adv. Mater., 2023, 35, 2212116 CrossRef CAS PubMed.
- Q. Zhao, S. Stalin and L. A. Archer, Joule, 2021, 5, 1119 CrossRef CAS.
- P. Zhai, Q. He, H. Jiang, B. Gao, B. Zhang, Q. Chen, Z. Yang, T. Wang and Y. Gong, Adv. Energy Mater., 2023, 14, 2302730 CrossRef.
- X. Lian, Z. Ju, L. Li, Y. Yi, J. Zhou, Z. Chen, Y. Zhao, Z. Tian, Y. Su, Z. Xue, X. Chen, Y. Ding, X. Tao and J. Sun, Adv. Mater., 2024, 36, 2306992 CrossRef CAS PubMed.
- X. Wang, Z. Chen, X. Xue, J. Wang, Y. Wang, D. Bresser, X. Liu, M. Chen and S. Passerini, Nano Energy, 2025, 133, 110439 CrossRef CAS.
- S. Ni, M. Zhang, C. Li, R. Gao, J. Sheng, X. Wu and G. Zhou, Adv. Mater., 2023, 35, 2209028 CrossRef CAS PubMed.
- C. Bi, N. Yao, X. Li, Q. Zhang, X. Chen, X. Zhang, B. Q. Li and J. Huang, Adv. Mater., 2024, 36, 2411197 CrossRef CAS PubMed.
- Y. Mu, S. Yu, Y. Chen, Y. Chu, B. Wu, Q. Zhang, B. Guo, L. Zou, R. Zhang, F. Yu, M. Han, M. Lin, J. Yang, J. Bai and L. Zeng, Nano-Micro Lett., 2024, 16, 86 CrossRef CAS PubMed.
- X. Cheng, S. Yang, Z. Liu, J. X. Guo, F. Jiang, F. Jiang, X. Xiong, W. Tang, H. Yuan, J. Huang, Y. Wu and Q. Zhang, Adv. Mater., 2024, 36, e2307370 CrossRef PubMed.
- Y. Mu, Y. Chu, Y. Shi, C. Huang, L. Yang, Q. Zhang, C. Li, Y. Feng, Y. Zhou, M. Han, T. Zhao and L. Zeng, Adv. Energy Mater., 2024, 14, 2400725 CrossRef CAS.
- B. Xu, L. Ma, W. Wang, H. Zhu, Y. Zhang, C. Liang, L. Zhou, L. Wang, Y. Zhang, L. Chen, C. Zhang and W. Wei, Adv. Mater., 2024, 36, 2311938 CrossRef CAS PubMed.
- J. Qin, F. Pei, R. Wang, L. Wu, Y. Han, P. Xiao, Y. Shen, L. Yuan, Y. Huang and D. Wang, Adv. Mater., 2024, 36, e2312773 CrossRef PubMed.
- R. Xu, Y. Xiao, R. Zhang, X. Cheng, C. Z. Zhao, X. Zhang, C. Yan, Q. Zhang and J. Huang, Adv. Mater., 2019, 31, 1808392 CrossRef PubMed.
- B. Jiang, C. Zhao, X. Yin, Y. Zhang, X. Sun, S. Gu and N. Zhang, Energy Storage Mater., 2024, 66, 103237 CrossRef.
- J. Sun, Y. Sun, M. Pasta, G. Zhou, Y. Li, W. Liu, F. Xiong and Y. Cui, Adv. Mater., 2016, 28, 9797 CrossRef CAS PubMed.
- Z. Li, J. Zhang and X. W. Lou, Angew. Chem., Int. Ed., 2015, 54, 12886 CrossRef CAS PubMed.
- A. Joshi, T. S. Anand, J. Kala, M. Singh, A. Gupta, R. K. Srivastava and B. Nandan, ACS Appl. Energ. Mater., 2023, 7, 312 CrossRef.
- T. Sun, X. Zhao, B. Li, H. Shu, L. Luo, W. Xia, M. Chen, P. Zeng, X. Yang, P. Gao, Y. Pei and X. Wang, Adv. Funct. Mater., 2021, 31, 2101285 CrossRef CAS.
- P. Guo, P. Jiang, W. Chen, G. Qian, D. He and X. Lu, Electrochim. Acta, 2022, 428, 140955 CrossRef CAS.
- J. Xia, R. Gao, Y. Yang, Z. Tao, Z. Han, S. Zhang, Y. Xing, P. Yang, X. Lu and G. Zhou, ACS Nano, 2022, 16, 19133 CrossRef CAS PubMed.
- H. Park, S. Lee, H. Kim, H. Park, H. Kim, J. Kim, M. Agostini, Y. K. Sun and J. Y. Hwang, Carbon Energy, 2024, 6, 472 CrossRef.
- D. Cai, J. Yang, T. Liu, S. Zhao and G. Cao, Nano Energy, 2021, 89, 106452 CrossRef CAS.
- Z. Fang, J. Tan, L. Ma, P. Yi, W. Lu, Y. Xu, M. Ye and J. Shen, Nanoscale, 2024, 16, 17934 RSC.
- P. Geng, Y. Lin, M. Du, C. Wu, T. Luo, Y. Peng, L. Wang, X. Jiang, S. Wang, X. Zhang, L. Ni, S. Chen, M. Shakouri and H. Pang, Adv. Sci., 2023, 10, 2302215 CrossRef CAS PubMed.
- Y. Wang, H. Wang, Y. Jiang, G. Li, S. Liu and X. Gao, ACS Appl. Energy Mater., 2023, 6, 8377 CrossRef CAS.
- H. Cheng, S. Zhang, S. Li, C. Gao, S. Zhao, Y. Lu and M. Wang, Small, 2022, 18, 2202557 CrossRef CAS PubMed.
- M. Kim, M. Kim, V. Do, Y. Xia, W. Kim and W. I. Cho, J. Power Sources, 2019, 422, 104 CrossRef CAS.
- L. Luo, S. H. Chung, H. Yaghoobnejad Asl and A. Manthiram, Adv. Mater., 2018, 30, 1804149 CrossRef PubMed.
- L. Luo, J. Li, H. Yaghoobnejad Asl and A. Manthiram, ACS Energy Lett., 2020, 5, 1177 CrossRef CAS.
- R. Yang, F. Wang, W. Cui, W. Chen, T. Lei, D. Chen, D. CHen, X. Li, Z. Chi, C. Kai, D. Run, Y. Yi, N. Xiao and H. Yin, Energy Storage Mater., 2025, 75, 104030 CrossRef.
- W. Yao, J. Xu, Y. Cao, Y. Meng, Z. Wu, L. Zhan, Y. Wang, Y. Zhang, I. Manke, N. Chen, C. Yang and R. Chen, ACS Nano, 2022, 16, 10783 CrossRef CAS PubMed.
- X. Zhang, Q. Jin, Y. Nan, L. Hou, B. Q. Li, X. Chen, Z. H. Jin, X. Zhang, J. Huang and Q. Zhang, Angew. Chem., Int. Ed., 2021, 60, 15503 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ee01134e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |