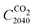Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Transition pathways to electrified chemical production within sector-coupled national energy systems†
Patricia
Mayer
 a,
Florian Joseph
Baader
a,
David Yang
Shu
a,
Ludger
Leenders
a,
Christian
Zibunas
a,
Florian Joseph
Baader
a,
David Yang
Shu
a,
Ludger
Leenders
a,
Christian
Zibunas
 b,
Stefano
Moret
a and
André
Bardow
b,
Stefano
Moret
a and
André
Bardow
 *a
*a
aEnergy & Process Systems Engineering, ETH Zürich, Switzerland. E-mail: abardow@ethz.ch
bInstitute of Technical Thermodynamics, RWTH Aachen University, Germany
First published on 3rd July 2025
Abstract
The chemical industry's transition to net-zero greenhouse gas (GHG) emissions is particularly challenging due to the carbon inherently contained in chemical products, eventually released to the environment. Fossil feedstock-based production can be replaced by electrified chemical production, combining carbon capture and utilization (CCU) with electrolysis-based hydrogen. However, electrified chemical production requires vast amounts of clean electricity, leading to competition in our sector-coupled energy systems. In this work, we investigate the pathway of the chemical industry towards electrified production within the context of a sector-coupled national energy system's transition to net-zero emissions. Our results show that the sectors for electricity, low-temperature heat, and mobility transition before the chemical industry due to the required build-up of renewables, and to the higher emissions abatement of heat pumps and battery electric vehicles. The chemical industry transitions last together with high-temperature heat, beginning with methanol, then ammonia, the olefins, and finally the aromatics. To achieve the net-zero target, the energy system relies on clean energy imports to cover 41% of its electricity needs, largely driven by the high energy requirements of a fully electrified chemical industry. Nonetheless, a partially electrified industry combined with dispatchable production alternatives provides flexibility to the energy system by enabling electrified production when renewable electricity is available. Hence, a partially electrified, diversified chemical industry can support the integration of intermittent renewables, serving as a valuable component in net-zero energy systems.
Broader contextThe chemical industry contributes substantially to global greenhouse gas emissions. These emissions are considered hard to abate due to the carbon needed in chemical products, currently supplied by fossil-based feedstocks. Electrified chemical production could replace fossil-based feedstocks with captured CO2 and green H2. However, electrified chemical production requires vast amounts of intermittent renewable electricity, leading to competition with other energy sectors and to potential operational challenges. Given these dependencies on other energy sectors, the transition towards electrified chemical production must be resolved alongside the energy system's transition to net-zero emissions. In this work, we present for the first time this interconnected transition, focusing on the timing of the chemical industry's transition and on an electrified industry's interactions with a renewables-dominated energy system. We show that although priority should first be placed on transitioning other sectors, an electrified chemical industry can provide valuable flexibility to the energy system, revealing the potential contributions of an electrified chemical industry beyond reducing its own hard-to-abate emissions. |
1 Introduction
The chemical industry consumes 14% of oil and 8% of natural gas supply globally.1 Fossil-based resources used in the chemical industry account for 7% of global greenhouse gas (GHG) emissions.2 Thus, defossilizing the chemical industry is crucial for meeting net-zero greenhouse gas (GHG) emissions targets. However, defossilization of the chemical industry is challenging due to the inherent need for carbon in chemical products, traditionally supplied by fossil-based hydrocarbons. The need for a material input accounts for 58% of the chemical industry's fossil demand.3 The carbon in the feedstock is usually released to the environment either as direct emissions during chemicals production, as is the case for conventional ammonia,4 or during the chemicals use and end-of-life phases, as is the case for waste incinerating carbon-containing chemicals such as plastics.One way to reduce the chemical industrys reliance on fossil-based feedstocks is through electrified production, where the feedstocks are obtained through carbon capture and utilization (CCU) and electrolytic hydrogen (H2).5,6 With CCU, CO2 is captured from industrial point sources or directly from the air and is used as a carbon-based feedstock for chemicals, thus enabling a circular chemical industry. Electrolytic H2 is produced through water-splitting into H2 and oxygen (O2) by applying electricity, thus avoiding the CO2 emissions from conventional fossil-based H2 production. Using renewable electricity for both CCU and electrolytic H2 can result in a low-emission chemical industry, with a global emissions reduction potential of 3.5 Gt CO2-eq if abundant clean electricity is available.7
CCU-based methanol has been identified as a promising precursor for all high value chemicals8 and was shown to safely operate within the Earth's carbon emissions planetary boundaries.9 A future highly electrified chemical industry has been predicted to have lower annualized costs than a biomass-based industry, indicating that an electrified chemical industry may be the most economical solution for a future green chemical industry.6
Thus, electrification provides a pathway to a sustainable chemical industry, but requires an abundance of electricity that is low in GHG-intensity. Reducing global GHG emissions by 3.5 Gt CO2-eq would require 18.1 PWh of low carbon electricity,7 corresponding to 103% of the global renewable electricity production targets in 2030 even under optimistic assumptions on technology development10 (Stated Policies Scenario). Obtaining a mostly electrified chemical industry by 2050, considering future demand growth, would require nearly 40 PWh of electricity, comprising 150% of today's global electricity generation.6 This high demand for clean electricity is the main barrier in the transition to an electrified chemical industry.11–17
Renewables can potentially be expanded massively to supply an electrified chemical industry with sufficient clean electricity by 2050.10 However, during the energy transition, clean electricity will be limited and used more efficiently in other sectors. Particularly, single-technology comparisons indicate that heat pumps and battery electric vehicles might reduce emissions more than utilizing the electricity in an electrified chemical industry, which has a low energy return on investment.11,18 Thus, the transition towards electrified chemical production must be considered in the context of a sector-coupled energy system.
Considering the combined transition also enables identification of the effects of intermittent renewable electricity supply on electrified chemical production,19 as well as the potential flexibility provision from electrified chemical processes to the energy system.20 For instance, Almajed et al.21 show that the profitability of electrified syngas production depends on renewable electricity availability and price. With respect to flexibility to the energy system, electrified chemical processes can help manage grid congestion in a renewables-dominated power grid through flexible operation.22 Chemicals can also provide flexibility over varying timescales, from hours to seasons,15 and have been deemed essential, together with other CCU options, as flexibility providers to renewables-dominated energy systems.23 However, as highlighted by Guerra et al.,24 the value of this flexibility to the energy system is not well understood, and requires further investigation.
Studies have investigated transition pathways of sector-coupled energy systems including industry, showing the importance of resolving the fully coupled system for the costs and technological transitions of each sector.25–32 For instance, Bogdanov et al.25 find that including more electrified industry increases flexibility while reducing the system's levelized cost of energy. While insightful regarding important interactions between industry and the energy system, these studies either neglect the chemical industry altogether,26,27 or simplify its representation. Simplifications include aggregating individual chemicals into a single chemical product (i.e. aggregated aromatics or high-value chemicals),28–31 or focusing on the electrified feedstock transition rather than chemical products and thus limiting the production options for chemicals further downstream.25,32 As their focus is not on the chemical industry, these analyses disregard the timing and evolution of the chemical industry's transition, as well as its interactions with the energy system.
Other works have focused on the interactions between a sustainable chemical industry and other energy sectors.33–38 However, these studies either neglect the transition pathway and only consider a future chemical industry,33,34 or focus on alternative production routes such as biomass and recycling.35–37 While biomass and recycling are promising solutions for GHG mitigation of the chemical industry,39–41 they both have limitations such as biomass availability and competition36 and recycling ramp-up challenges.42 Hence, electrified chemical production is an important component in the portfolio of potential solutions.35
In this work, we investigate transition pathways towards an electrified chemical industry while considering the interactions with a transitioning sector-coupled national energy system. We consider the German energy system for our case study due to the importance of its chemical industry, being the third largest industry in Germany43 and the third largest chemical exporter worldwide.44 We consider the electricity, residential heat, industrial heat, and private mobility sectors within the energy system.45 For the chemical industry, we consider the seven base and high value chemicals: ammonia, methanol, ethylene, propylene, benzene, xylene, and toluene. More than 90% of the oil and gas entering the chemical industry as feedstock, by mass, is used for the production these chemicals.1 Moreover, the energy requirements for these seven chemicals account for two thirds of the chemical sector's energy consumption,46 making these chemicals a good subset for representing the industry. We gather process data for the electrified chemical production processes from a comprehensive literature review, combining published data with private databases.
We determine the optimal timing of the chemical industrys transition relative to the transition of the other energy sectors, finding that the chemical industry transitions after the build-up of renewable electricity and the transition to heat pumps and battery electric vehicles. We also take a deep dive into the chemical industry's transition, determining the optimal transition order of the individual chemicals. We introduce the Cost-Avoided, a metric quantifying the cost reduction from utilizing 1 MWh of renewable electricity in a chemical's electrified process versus producing the same amount via its fossil-based process. We identify this metric as a key indicator of the chemicals' order of transition, with methanol transitioning first and the aromatics last. Finally, we evaluate the interplay between the chemical industry and the overall energy system, uncovering the flexibility provision to the energy system that incentivizes an earlier transition of the chemical industry.
In Section 2, we introduce the model setup used to represent the sector-coupled energy system together with the chemical industry. In Section 3, we present the results and discuss the findings. In Section 4, we summarize our findings and highlight the key takeaways.
2 Modeling the chemical industry transition pathway within a sector-coupled energy system
In this section, we introduce the modeling of the integrated sector-coupled energy system with the chemical industry to calculate the optimal transition pathway towards net-zero GHG emissions. Section 2.1 describes the representation of the chemical industry and its implementation within the sector-coupled energy system. Section 2.2 describes the representation of the German sector-coupled energy system used in our case study. Section 2.3 introduces SecMOD, the modeling framework used to calculate the transition pathways and describes the optimization setup details. Section 2.4 introduces the Cost-Avoided metric which guides the prioritization for the operation of electrified technologies across the energy system.2.1 The chemical industry
Our representation of the chemical industry consists of the base and high value chemicals: ammonia, methanol, ethylene and propylene (olefins), and benzene, xylene, and toluene (aromatics). We introduce exogenous demands for the production of each chemical corresponding to historic German production volumes,47 and a constant hourly demand for every hour of the year. The yearly demands (Table 1) are maintained constant throughout our transition pathway, as studies project constant or even declining chemical production volumes as countries transition towards carbon neutrality.48,49 For each chemical, we include fossil-based and electrified production options (Table 1), considering all process energy and material requirements. For olefins and aromatics electrified processes, we consider the methanol-to-olefins (MTO) and methanol-to-aromatics (MTA) processes. We do not consider the direct conversion of CO2 to olefins and aromatics due to their low technology readiness levels.6 Besides the main fossil-based and electrified processes for each chemical, we consider upstream processes for the production of chemical intermediates such as synthesis gas and pyrolysis gas (Fig. 1). In total, our chemical industry models over 30 processes gathered from a literature review (Section S1, ESI†).| Chemical | Fossil-based process | Electrified process | Demand [Mtonne per year] |
|---|---|---|---|
| a Mixed xylenes. | |||
| Ammonia | SMR + HB | e-H2 + HB | 2.56 |
| Methanol | From synthesis gas | e-H2 + CCU | 1.40 |
| Ethylene | Steam cracking | MTO | 4.52 |
| Propylene | of naphtha | 3.44 | |
| Benzene | Solvent extraction | MTA | 1.51 |
| Toluene | from pyrolysis gasoline | 0.55 | |
| Xylenea | 0.40 | ||
 | ||
| Fig. 1 Schematic of processes included in the chemical industry model for producing base and high-value chemicals (blue boxes on the right). Olefins comprise ethylene and propylene, BTX comprise the aromatics benzene, toluene, xylene. Processes are in boxes, whereas products are in circles. Processes are grouped by color based on the main product. SMR: steam methane reforming, HB: Haber Bosch, DAC: direct air capture, syngas: synthesis gas, pyr. gas: pyrolysis gas, NG: natural gas, MTO: methanol to olefins, MTA: methanol to aromatics, NH3: ammonia, H2: hydrogen, CO2: carbon dioxide, MeOH: methanol, CCU: carbon capture and utilization. * Three SMR processes are considered: one for H2 production, and two for syngas production. For syngas, SMR with H2 skimming and SMR with CO2 import are considered.50 | ||
The two key molecules needed for an electrified chemical industry are CO2 and H2. For the sourcing of CO2, we include direct air capture (DAC)51 and industrial point source capture from the modelled chemical processes that separate a concentrated CO2 stream (i.e. CO2 from steam methane reforming). For the sourcing of H2, we include domestic production through steam methane reforming or electrolysis, and green H2 imports. To isolate the effect of inter-sectoral competition for limited renewable electricity, we place a high price penalty on imported green H2 such that the system prioritizes domestic energy resources.
Our chemical industry emissions are calculated as CO2-eq following the IPCC GWP-100 methodology52 for life cycle assessment. We consider the direct process emissions, life cycle emissions from the process energy requirements, life cycle emissions of imported products, and emissions from the chemicals use-phase and end-of-life. For the direct process emissions, we follow the methodology employed by Meys et al.,40 closing the atom balances around the chemical processes. Emissions from the process energy requirements are accounted for in the respective production technologies modelled in the energy system (Section 2.2). Emissions of imported products are taken from the ecoinvent database.53 For the chemicals use phase and end-of-life emissions, we follow the methodology employed by Zibunas et al.,54 assuming complete combustion of the produced chemicals such that the carbon contained in the chemicals is converted to CO2 in the year that the chemicals are produced. We do not consider emissions associated with the construction of the chemical facilities. However, because we only constrain the system's operational emissions, in line with current carbon accounting practice,55 neglecting the emissions associated with facility construction does not affect the results.
To account for existing fossil-based production capacities, we follow the methodology employed by Zibunas et al.,54 assuming an existing capacity equal to each chemical's hourly demand, and a uniform age distribution for the facilities such that they retire uniformly throughout the transition pathway. This implementation results in an equal share of facilities retiring in each investment period, introducing the decision to either reinvest in fossil-based production capacity, or replace it with electrified production capacity.
To achieve net-zero operational GHG emissions, the chemical industry in our model needs to fully electrify by 2045. Through this setup, we can address our research questions regarding the timing of the chemical industry's transition relative to the other energy sectors, as well as the interactions between a transitioning chemical industry and the energy system.
2.2 The sector-coupled energy system
For our case study, we adopt the German energy system model developed by Baumgärtner et al.45 with the extensions implemented by Shu et al.51 The energy system considers the sectors: electricity, private mobility, residential heating, and industrial heating at three temperature levels (low temperature below 100 °C, medium temperature heat between 100–400 °C, and high temperature heat above 400 °C). Sectoral energy demands are exogenously provided with an hourly resolution. Yearly emissions targets are also exogenously provided considering the historical emissions of the aforementioned sectors. The underlying assumptions can be found in the supplementary information of Baumgärtner et al.45To include the chemical industry in the sector-coupled energy system model, we subtract the chemical industry electricity and heat demands from the original exogenous demands, as these demands are accounted for in the chemical processes (Tables S4 and S5, ESI†). We add the direct process emissions associated with the chemical industry56 and the use phase and end-of life emissions of the considered chemical products (Section 2.1) to the GHG emissions limit. We also update the exogenous emissions targets to reflect the most recent reduction targets of 65% of 1990 levels by 2030 and net-zero by 204557 (Table S6, ESI†). We explicitly model CO2 as a product to account for CO2 production and consumption across various processes. Finally, we add resistance heaters and H2 boilers to introduce electrified high-temperature heat options. Due to the low technology readiness levels of high-temperature resistance heaters58–60 and hydrogen boilers61,62 for large-scale industrial applications, we assume high capital costs for these technologies (Table S3, ESI†). We include a scenario with optimistic capital cost assumptions in Section S5 (ESI†).
Because we consider life cycle emissions calculated as CO2-eq,52 and we do not include CO2 sequestration in our system set-up, residual emissions are unavoidable. For instance, the life cycle emissions associated with the sorbent for direct air capture, cannot be offset when CO2 is captured and used as a feedstock. Aditionally, even electrified technologies that run on fully renewable electricity are subject to maintenance and degradation that would lead to life cycle emissions. To address this limitation and still reach our net-zero emissions target, a high price penalty is placed on remaining CO2-eq representing a high-cost CO2 removal option for achieving net-zero emissions.
2.3 The SecMOD framework and optimization setup
We employ the open-source, linear optimization framework SecMOD, used for sector-coupled energy system modeling, optimization, and life cycle assessment (LCA).63 The framework minimizes a user-defined objective function, such as cost minimization, subject to user-defined constraints, such as GHG emissions limits. SecMOD considers both spatial and temporal resolution, with the temporal resolution occurring at two levels: the time steps considered within the optimization of a single investment period, and the number of investment periods considered for a transition pathway optimization. For a single investment period, the full hourly time series is aggregated into user-defined typical periods using the TSAM package.64 For the full transition pathway, the number of investment periods is defined by the user. Each investment period is optimized individually, with the user specifying the foresight regarding future periods. The investment periods can either be solved independently with no foresight of future periods, all together with perfect foresight of all investment periods, or with limited foresight by employing a rolling-horizon strategy.45 Further details of the modeling framework, including the mathematical formulation, can be found in Reinert et al.63 and in the open source repository.‡In this study, SecMOD is used to calculate the cost-optimal transition pathway of the coupled German energy system (Section 2.2) plus chemical industry (Section 2.1), subject to annual emissions constraints. We consider both investment and operating costs in our objective function, while only considering operating emissions in our constraint, in line with current accounting practice.55 We solve the transition pathway for the years 2020 to 2045 in 5-year increments, instantiating the model for the base year 2016.45,51 We use a rolling horizon-strategy for the optimization of each investment period with a foresight of 4 periods, or 20 years. We represent the sector-coupled energy system with one node, and aggregate the hourly time series into 6 typical periods of 6 hours each. The temporal resolution was taken from Shu et al.51 By excluding spatial resolution in our system set-up, we potentially underestimate the energy systems flexibility needs65 and disregard the spatial distribution of a transitioning chemical industry. However, as we are interested in the timing of the chemical industry's transition relative to other sectors, and we do consider temporal resolution, we believe that our setup is sufficient for our research objectives and leave the spatial component for future work.
2.4 Cost-Avoided and the merit order curve: guiding electrified production across the energy system
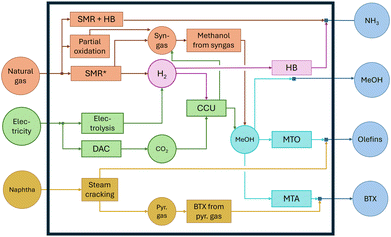
The Cost-Avoided by electrification, or ΔCeleci,t [k€/MWh], quantifies the system's cost reduction per MWh of renewable electricity used in the electrified production of product i compared to producing an equivalent amount via its fossil-based alternative (eqn (1)). This Cost-Avoided can be interpreted as an economic Power-to-X efficiency following the methodology introduced by Sternberg and Bardow.18 The Cost-Avoided depends on time, t, based on the time-dependent operation of the sector-coupled energy system. A positive Cost-Avoided indicates a decrease in the system costs. | (1) |
The Cost-Avoided is comprised of three parts:
1. ΔCopi,t [k€/MWh]: the difference in operating costs between 1 MWh-worth of product i produced via its electrified process versus producing the same amount via its fossil-based process at time t (eqn (2)):
| ΔCopi,t = Mi,t·(Cop,fossili,t − Cop,eleci,t) | (2) |
2. 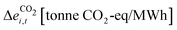 : the difference in CO2-eq emissions between 1 MWh-worth of product i produced via its electrified process versus producing the same amount via its fossil-based process at time t (eqn (3)). This calculation is based on Sternberg and Bardow18 with an added time component.
: the difference in CO2-eq emissions between 1 MWh-worth of product i produced via its electrified process versus producing the same amount via its fossil-based process at time t (eqn (3)). This calculation is based on Sternberg and Bardow18 with an added time component.
 | (3) |
 and
and  are the emissions of the electrified and fossil-based processes per amount of product i at time t, respectively.
are the emissions of the electrified and fossil-based processes per amount of product i at time t, respectively.
3.  the energy system's CO2 price, represented by the endogenous shadow price of the optimization model's CO2-eq emissions constraint. We obtain one CO2 price per investment period, y, from our total annual emissions constraint.
the energy system's CO2 price, represented by the endogenous shadow price of the optimization model's CO2-eq emissions constraint. We obtain one CO2 price per investment period, y, from our total annual emissions constraint.
To calculate Mi,t, we take the inverse of the electricity demand per unit of a product's electrified production at time t, Ei,t [MWh/uniti] (eqn (4)):
| Mi,t = (Ei,t)−1 | (4) |
The time component is introduced into the electricity demands (Ei,t), operating costs (Cop,eleci,t, Cop,fossili,t) and emissions  because these terms consider the direct production processes and the underlying supply chains of the process material and energy inputs (Fig. S1, ESI†). The underlying supply chains depend on the temporal results of our optimization model. For example, let's consider electrified methanol production (using H2 from electrolysis and captured CO2) which requires medium-temperature heat. This heat can be produced via fossil fuel combustion depending on the endogenously optimized heat supply mix of the energy system at a given time-step. The costs and emissions for the underlying heat production are thus included in the costs, Cop,eleci,t, and emissions,
because these terms consider the direct production processes and the underlying supply chains of the process material and energy inputs (Fig. S1, ESI†). The underlying supply chains depend on the temporal results of our optimization model. For example, let's consider electrified methanol production (using H2 from electrolysis and captured CO2) which requires medium-temperature heat. This heat can be produced via fossil fuel combustion depending on the endogenously optimized heat supply mix of the energy system at a given time-step. The costs and emissions for the underlying heat production are thus included in the costs, Cop,eleci,t, and emissions,  of the electrified methanol process for the given time-step (Section S3, ESI†).
of the electrified methanol process for the given time-step (Section S3, ESI†).
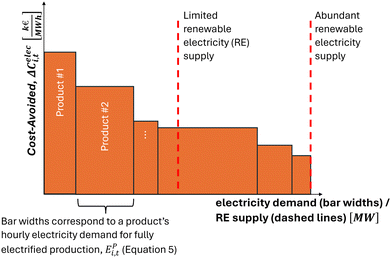
The Cost-Avoided creates a merit order of electrified products, ranking products by their cost savings from electrified production. We combine this merit order with the products' hourly electricity demands, EPi,t [MWh/hour] (eqn (5)), to create a time-dependent merit order curve. Based on its intersection with the energy system's availability of renewable electricity at a given time-step, this curve determines which products are produced electrically and to what extent (Fig. 2). This merit order curve complements the electricity-market merit order and is induced through the CO2 emissions constraint. The Cost-Avoided and the resulting merit order curve therefore guide the hourly production of electrified products in the energy system.
The products' hourly electricity demands for fully electrified production, EPi,t [MWh/hour], are used as the bar widths for the merit order curve (eqn (5)). These electricity demands are calculated from the minimum of a product's electrified installed capacity in investment year, y, Pi,y [tonne/hour] and a product's exogenously provided hourly demand, Di,t [tonne/hour]. We take the minimum since some products, such as methanol, serve as intermediates. Taking the minimum ensures that the electricity demand of the additional installed capacity for intermediary production is allocated to the final product rather than to the intermediate.
| EPi,t = Ei,t·min(Pi,y, Di,t) | (5) |
The Cost-Avoided and the corresponding merit order curve provide a tool for evaluating the hourly deployment of electrified products across the energy system given renewables intermittency. This tool is particularly useful for determining the prioritization of products for electrification and for calculating and comparing utilization rates across the various electrified products. These rates can be calculated by aggregating the hourly deployment provided by the merit order curve. Furthermore, products positioned further down in the merit order can become valuable flexibility providers to the energy system by adapting their production between electrified and fossil-based depending on the availability of renewable electricity (Fig. 2). Thus, the merit order curve is also a valuable tool for evaluating the flexibility provision from individual products or energy sectors.
3 Results and discussion
Here, we present the results and discuss the findings of the coupled energy system and chemical industry's transition to net-zero emissions. We first focus on the overall system's transition pathway in Section 3.1. We then take a deep dive into the chemical industry's transition in Section 3.2. In Section 3.3, we focus on the interactions between a transitioning chemical industry and energy system with particular emphasis on flexibility provision.3.1 Transition pathway of the coupled energy system and chemical industry
In its combined transition pathway with the energy system, the chemical industry starts implementing electrified production in 2040, after the transitions of the electricity, residential and low-temperature heat, and mobility sectors (Fig. 3). The chemical industry starts transitioning together with medium and high-temperature heat. Hence, in energy systems with limited renewable electricity, priority is first placed on building up renewable electricity, transitioning to heat pumps and to battery electric vehicles before electrifying chemical production. Electrification of chemical production takes place along with the electrification of other hard-to-abate, electricity-intensive sectors.To enable the transition to net-zero emissions, the electricity sector first transitions away from lignite and coal, building up wind and photovoltaic capacities (Fig. S2, ESI†). Electricity from natural gas combined cycle is used for dispatchable electricity between 2025 and 2040, causing most emissions from the electricity sector in that time period. The residential and low temperature heat sectors also transition from natural gas boilers to heat pumps together with the electricity sector (Fig. S3 and S4, ESI†), taking advantage of the increasing renewable electricity availability. Next, the mobility sector transitions from a mix of gasoline, diesel, and natural gas cars to battery electric vehicles in 2035 (Fig. S5, ESI†). The transition of the mobility sector occurs in one investment period due to the existing vehicle fleet reaching the end of its lifetime, and the foresight to the net-zero emissions target in 2045 influencing the decision to invest in electric vehicles rather than reinvest in fossil fuel vehicles. Finally, the chemical industry starts transitioning to electrified production in 2040 together with medium and high temperature heat. In 2040, 77% of medium temperature heat is produced via electrode boilers rather than natural gas boilers (Fig. S6, ESI†), whereas only 0.5% of high temperature heat is produced via resistance heaters (Fig. S7, ESI†). The bulk of the high temperature heat transition occurs in 2045, when 90% of high temperature heat is produced via resistance heaters and H2 boilers and the remainder is produced as a by-product of an electrified chemical industry.
The late transition of the high temperature heat sector is driven in part by the high capital costs assumed for resistance heaters and H2 boilers (Section 2.2). In a scenario with more optimistic cost assumptions (Section S5, ESI†), the high-temperature heat sector still transitions late, but the bulk occurs in 2040 rather than 2045. In this scenario, a portion of the chemical industry transition is delayed to 2045. This finding highlights the delayed transition of the chemical industry compared to other energy sectors, and underscores how closely its timeline is tied to the transition of other hard-to-abate, electricity-intensive sectors.
Despite the net-zero emissions target in 2045, 4.2 Mtonne of residual CO2-eq emissions remain. Although small, equivalent to only 0.7% of Germany's emissions in 2020,66 the residual emissions indicate that a fully net-zero energy system is not possible without carbon dioxide removal. 75% of these residual emissions come from the operation of battery electric vehicles (BEVs). These operational emissions come from our allocation of road and vehicle degradation to the operational life-cycle of BEVs, which we adopt from Baumgärtner et al.45 20% of the residual emissions come from the chemical industry, mainly from the supply chain of the direct air capture units used to procure CO2 for electrified chemical production. The remaining residual emissions come from the operation of renewable electricity production technologies and from the H2 import supply chain.
To reach the nearly net-zero energy system in 2045 with a fully electrified chemical industry, the energy system imports 483 TWh per year of clean energy in the form of green H2, equivalent to 41% of the energy system's electricity demand. These imports are needed due to insufficient domestic renewable electricity availability during some hours of the year. 43% of these imports are required for a fully electrified chemical industry either as direct H2 feedstock or for process energy. Although these imports are lower than present-day fossil-based energy imports to Germany, which imported 968 TWh of natural gas alone in 2023,67 they require an eight-fold increase in present-day global low-carbon hydrogen production68 by 2045, which is a matter with much uncertainty.69 Hence, fully electrifying the chemical industry in countries with limited renewable electricity availability requires green energy imports of magnitudes which may not be available.
An interesting intermediate configuration, however, can be seen in 2040, when the chemical industry is partially electrified together with fossil-based production. The energy system imports 400 TWh of fossil-based energy, 33% of which is used in the chemical industry as naphtha feedstock. No clean energy is imported due to the high cost assumption. The reduced reliance on clean energy imports from a mixed chemical industry suggests that combining electrified production with other production options can yield an energy system more resilient to global green energy availability. It must be noted, however, that this mixed chemical industry configuration still requires nearly 21 GW of domestic electrolyzer capacity. Although in line with European targets of 40 GW by 2030,70 this requirement is not trivial considering the present-day global capacity of 1.3 GW.71 Hence, accelerating electrolyzer deployment and integrating them on-site in chemical facilities is an important factor in transitioning towards an electrified chemical industry.
In summary, our study shows that the chemical industry transitions towards the end of the pathway, following the build-up of renewable electricity, the transition to heat pumps, and to battery electric vehicles. We find that a fully net-zero energy system is not possible, requiring carbon dioxide removal to offset residual emissions. Finally, a nearly net-zero system with a fully electrified chemical industry requires substantial clean energy imports, largely due to the chemical industry's high energy requirements. However, although the required energy imports of a fully electrified chemical industry are much lower than present-day fossil imports, our intermediate 2040 results show that electrified chemical production can potentially be combined with other production options to reduce import dependencies.
3.2 Transition towards an electrified chemical industry
Electrified chemical production begins in the year 2040, when methanol, ammonia, and the olefins (ethylene and propylene) are partly produced via their electrified processes. Their yearly production mix is split between fossil-based and electrified production (Fig. 4). Before this transition year, all chemicals are produced via their fossil-based processes except for 20% of methanol, which is produced via CCU by combining by-product H2 from synthesis gas production with CO2 from chemical industry point sources. The aromatics (benzene, toluene, and xylene) transition at the end of the pathway in 2045 when all chemicals are fully produced electrically to meet the net-zero emissions target. | ||
| Fig. 4 Transition of chemical production from 2035 to 2045. Years prior to 2035 have the same production mix as 2035 and are therefore excluded from the figure. Methanol becomes an important intermediate for the production of electrified olefins and aromatics, leading to a 25-fold increase in methanol production between 2035 and 2045. The specific electrified, fossil, and methanol-based processes are found in Table 1. Elec (via methanol) refers to the methanol-to-olefins and methanol-to-aromatics processes. elec (H2 by-product) refers to methanol produced via CCU using by-product H2 from synthesis gas production and CO2 from chemical industry point sources. SMR + HB (CH4 by-product) refers to ammonia produced via steam methane reforming + Haber–Bosch using by-product CH4 from other electrified processes. | ||
The need for methanol as an intermediate for electrified production of olefins and aromatics increases methanol production drastically by 2045. In a fully electrified chemical industry, methanol production increases 25-fold compared to levels before 2040. This increase indicates the need for a massive scale-up of methanol production to transition to a fully electrified chemical industry when relying on high TRL methanol-to-olefins and methanol-to-aromatics processes.
The chemicals' order of transition can be understood by the merit order of chemicals created by the Cost-Avoided (Section 2.4). In the transition year, 2040, the merit order stays the same for every time-step despite the time dependency of the Cost-Avoided, with methanol consistently first, followed by ammonia, the olefins, and finally the aromatics (Table 2). The resulting order is attributed to two main components: the electricity requirements for the chemicals' electrified production (Ei,t), and the emissions-intensity of their respective fossil-based processes  . Methanol has the highest Cost-Avoided, despite the higher electricity requirement per tonne of production than for ammonia (Table 2). Methanol's lead is due to the high emissions associated with its fossil-based production, resulting in a high
. Methanol has the highest Cost-Avoided, despite the higher electricity requirement per tonne of production than for ammonia (Table 2). Methanol's lead is due to the high emissions associated with its fossil-based production, resulting in a high 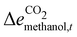 (eqn (3)). The emissions abated via methanol's electrified production are high enough that a smaller mass of electrified methanol production abates more emissions than a larger mass of ammonia replacing its fossil-based process. The placement of the aromatics as last in the merit order is due to their high electricity requirement, particularly for production of methanol as a feedstock.
(eqn (3)). The emissions abated via methanol's electrified production are high enough that a smaller mass of electrified methanol production abates more emissions than a larger mass of ammonia replacing its fossil-based process. The placement of the aromatics as last in the merit order is due to their high electricity requirement, particularly for production of methanol as a feedstock.
 , ΔCopi,2040, and
, ΔCopi,2040, and  , using the aggregated production mix for the year 2040 as the underlying supply chain. Olefins represent both ethylene and propylene which have the same values. Aromatics represent benzene, toluene, xylene which have the same values. Due to the low ΔCeleci,2040 of the aromatics, no installed electrified capacities exist in 2040
, using the aggregated production mix for the year 2040 as the underlying supply chain. Olefins represent both ethylene and propylene which have the same values. Aromatics represent benzene, toluene, xylene which have the same values. Due to the low ΔCeleci,2040 of the aromatics, no installed electrified capacities exist in 2040
The Cost-Avoided does not consider capital costs, which are a key component of the cost optimization that drives the chemical industry's transition. Nonetheless, the Cost-Avoided reveals itself as a good metric for explaining the order of transition of the various chemicals. This adequacy arises because the same drivers behind a chemicals Cost-Avoided often also dominate its capital costs: for example, the low Cost-Avoided of the aromatics is driven by the high electricity requirement of the methanol feedstock. This methanol feedstock also increases the capital costs associated with aromatics production. Therefore, despite not capturing the full cost structure, the Cost-Avoided serves also as a good indicator of the chemicals order of transition.
Overall, our results indicate an order of transition for the individual chemicals, with methanol first, followed by ammonia, the olefins, and finally the aromatics. A fully electrified chemical industry would require a massive scale-up of methanol production, requiring 25 times more production than today. The order of transition follows the merit order of the Cost-Avoided, with a chemicals position in the merit order dictated by both its electricity demand for electrified production, and the emissions-intensity of its fossil-based alternative processes. Hence, prioritization of chemicals for electrified production should consider both their electricity requirements, and the emissions intensity of their fossil-based alternatives.
While the order of transition follows the merit order of Cost-Avoided, an interplay between the chemical industry and the energy system orchestrates the partial electrification in the transition year. We explore this interplay in the following section (Section 3.3).
3.3 Flexibility provision from a transitioning chemical industry
The partial electrification of multiple chemicals observed in 2040 (Section 3.2) contrasts previous studies that indicate full sequential transitions of individual chemicals.7,33,54 However, these studies do not consider the interactions with the energy system. By considering these interactions, we find that the partial electrification is beneficial due to flexibility that the chemical industry provides to the energy system once investments in both fossil-based and electrified production capacities are made.In the transition year, 2040, which still has a positive emissions budget, the system re-invests in the phased-out fossil-based production capacities to meet the full hourly demand (Section 2.1), and, at the same time, invests in electrified production capacities to meet between 50% (propylene) to 100% (methanol, ammonia, ethylene) of the hourly demand. These investments lead to an oversizing of the chemicals' installed capacities and to diversification in production options. Therefore, in a given hour, chemicals can either be produced from fossil-based or electrified processes depending on the renewable electricity supply and on the renewable electricity demand from the other sectors. The ability to choose between chemical production options thus introduces a flexibility lever to the energy system.
The mechanism for flexibility provision is depicted in Fig. 5: renewable electricity supply is prioritized in the electricity, residential and low-temperature heat, and mobility sectors due to their higher Cost-Avoided (Section 2.4). These sectors are always 100% electrified. The remaining renewable electricity we thus consider as excess renewables that can then be used by the medium to high-temperature heat and the chemical sectors (Fig. 5, top). The merit order curve, (Section 2.4), guides the electrified portion of these additional sectors in a given hour based on the curve's intersection with the renewable electricity supply (Fig. 5, bottom). In hours with abundant excess renewables, all of the medium to high-temperature heat and chemicals are produced electrically up to the electrified installed capacities (Fig. 5, dashed green line). However, in hours with limited excess renewables, only a portion of these sectors is fulfilled with electrified production, with the remainder produced via fossil-based processes. This switching between fossil-based and electrified production in the individual hours of the year leads to the partial electrification of chemicals over the course of the year 2040.
 | ||
| Fig. 5 Top: Electricity supply and demand for each hour in the year 2040. The hours are ordered from highest to lowest excess renewables after full electrification of the electricity, residential and low-temperature (LT) heat, and mobility sectors. Due to the time series aggregation, the hours repeat themselves, causing the steps in the figure. Electricity storage is excluded from the figure. Middle: Load-duration curve for the year 2040, with the hourly breakdown of electrified vs. fossil-based production for medium and high-temperature (MT + HT) heat and for each chemical product. The hours are in the same order as in the electricity balance plot (top figure). In hours with high excess renewables (dashed green line), all chemicals and heat are produced via their electrified process up to the installed capacities. In hours with low excess renewables (dashed red line), only a subset of chemicals are produced via their electrified processes. This behavior is explained by the merit order curves created by the Cost-Avoided and the electricity demand of each product (bottom figures). Bottom: Merit order curves of electrified products in the sector-coupled energy system. Each curve corresponds to a separate hour, identified by the red and green dashed lines crossing the top and middle figures. The red and green dashed lines show the renewable electricity supplied for the electrified energy sectors. Everything to the left of the intersection between the renewable electricity supply and the merit order curve is produced electrically for that hour. Mobility, residential heat, and LT heat are fully electrified in every hour (dashed gray lines). The excess renewables (red and green brackets) are then used for electrification of chemicals and MT + HT heat. There is a tranche of electrified ammonia that is prioritized over electrified MT + HT heat in the middle figure while having a lower Cost-Avoided. Although not shown in this figure, the Cost-Avoided of ammonia can be split into two parts depending on whether the point-source CO2 emissions from fossil-based ammonia can be used downstream to produce CCU-based methanol (Section S3, ESI†) Thus, the prioritized tranche of electrified ammonia corresponds to the portion with a higher Cost-Avoided. *Heat production is shown in tonne natural gas equivalents using a heating value of 15.4 MWh per tonne. | ||
This ability to switch between production processes introduces an added benefit to flexibility provision: decreased carbon intensity of the produced chemicals. Electrified technologies are only utilized when renewable electricity is available and not with fossil-based electricity, which would increase the chemicals' carbon intensity. As the electricity sector transitions towards more renewables penetration, electrified production technologies can be increasingly utilized, further decreasing the chemicals' carbon intensity.
A closer look at the hourly chemical production mix for 2040 (Fig. 5, middle) shows that electrified methanol achieves an overall yearly utilization rate of 86%, reflecting its favorable position in the Cost-Avoided merit order following low-temperature heat. Electrified ammonia has an overall yearly utilization of 67%, while electrified olefins, positioned at the back of the merit order, have a utilization of 47%. The large variation in the Cost-Avoided across the different chemicals together with the chemical industry's large electricity demand make the chemical sector span a large portion of the merit order curve, introducing a large flexibility lever to the energy system. This flexibility lever makes earlier investments in electrified chemical production capacities cost-optimal despite yearly utilization rates as low as 47%. Despite the flexibility benefits of an oversized and diversified chemical industry, however, it is important to highlight that this underutilization phenomenon is limited to the transition period, as this flexibility enhances renewables penetration. Once the net-zero energy system is established, the chemical industry switches to 100% utilization of its electrified technologies.
Furthermore, while the operational flexibility offered by an oversized and diversified chemical industry can accelerate its transition to electrified production, the timing of investments remains strongly influenced by capital costs. We already show that the capital costs of high-temperature resistance heaters and H2 boilers can influence the transition timing of the chemical industry (Section 3.1 and Section S5, ESI†). To better understand the effect of capital costs on the chemical industry transition, we perform two sensitivity analyses: one regarding facility lifetimes and another regarding costs (Section S6, ESI†). Both analyses reveal that while capital cost increases do not significantly affect the transitions of methanol and ammonia, cost increases can affect the olefins transition. Particularly, cost increases for direct air capture and electrolyzers can delay the olefins transition to 2045 (Fig. S12 and S13, ESI†). Nonetheless, electrified capacities for methanol and ammonia are always built and underutilized in 2040 regardless of cost increases. This finding reinforces the value of flexibility provision from an oversized and diversified chemical industry to the energy system.
In conclusion, our results reveal that oversizing and diversifying the chemical industry with electrified production capacities provides valuable flexibility to a renewables-dominated energy system. This flexibility provision makes earlier investments in electrified capacities advantageous despite lower utilization rates. Sensitivity analyses on facility lifetimes and capital costs confirm that electrified methanol and ammonia capacities are consistently built by 2040 despite underutilization. This consistent deployment, supported by their favorable Cost-Avoided and resulting flexibility provision, underscores the robustness of their early adoption. The system-wide benefits of an oversized and diversified chemical industry thus present a strategic opportunity for the industry to accelerate its transition to electrified production.
3.4 Real-world plausibility of an oversized and diversified chemical industry
In this study, we adopt the viewpoint of a central planner, a modeling approach that is well-established in the energy system modeling literature.72–74 From this perspective, we optimize costs for the entire energy system assuming perfect cooperation across the individual actors. Under this paradigm, increased costs for an individual actor, such as the chemical industry, can result in a larger overall cost reduction for the entire system.However, real-world decisions are rarely made from a central planners perspective, but rather by the individual actors. This difference in perspective becomes particularly relevant when considering the early deployment of an oversized and diversified chemical industry. Accessing the flexibility from such a configuration would require companies to invest in multiple chemical production capacities in parallel - a challenging investment strategy given the decreased utilization and prolonged payback periods that do not capture the economic benefits realized by the energy system. To illustrate the imbalance between benefits to the energy system and costs incurred by industry, we evaluate a scenario where the chemical industry continues operating its fossil-based facilities at full utilization rather than investing in underutilized electrified capacities in 2040. We find that the chemical industry capital costs decrease by 2.1 B€ per year, confirming the economic unattractiveness of the investments from the industry perspective. However, energy system costs increase by 5.3 B€ per year, more than double the chemical industry savings. To overcome this imbalance, government-led support, such as subsidy programs, could help redistribute the economic benefits to incentivize industry into making such investments. Initiatives such as the Inflation Reduction Act75 and the European Green Deal76 already demonstrate the feasibility of such support.
Furthermore, companies can realize operational benefits from these investments through market dynamics, such as fluctuating electricity and feedstock prices, and through risk mitigation, such as disruptions in supply chains. Studies already show that making electrified chemical production flexible to market dynamics, either via process diversification77 or through overcapacities24 can yield economic gains despite lower utilization rates. Guerra et al.24 demonstrate this phenomenon for electrolyzer operation, showing how lower utilization rates translate to lower electricity prices for the electrolyzer.
Another challenge of accessing flexibility from a diversified chemical industry lies in the ramping limitations of production facilities. Our results demonstrate the benefits from flexible dynamic operation of chemical production facilities in response to renewables availability. However, dynamically switching between production routes is incompatible with the real-world ramping limitations of chemical facilities, which often require weeks or even months to ramp up or down. To evaluate the effect of this real-world ramping limitation on the industrys transition, we evaluate a scenario where we constrain facility ramping to occur over longer time scales (Section S7, ESI†). We find that even with this requirement, the energy system invests in underutilized electrified capacities which provide flexibility on a monthly and seasonal basis (Fig. S15, ESI†). In this new scenario, the energy system costs increase by only 0.1%. Hence, flexibility from a diversified and underutilized chemical industry retains its value even considering more realistic ramping rates, making this underutilized configuration viable for real-world implementation.
In summary, while an underutilized and dynamically operated chemical industry presents challenges related to investment incentives and operational limitations, our findings demonstrate that the benefits of such a chemical industry configuration could be obtained under more realistic conditions. The economic benefits to the energy system outweigh the capital cost burden borne by industry, suggesting a clear rationale for public support mechanisms such as subsidies to bridge this imbalance. Furthermore, adapting production over monthly or seasonal time scales can also provide flexibility to the energy system due to varying renewables availability over these same time horizons. Therefore, we find that flexibility from an oversized and diversified chemical industry is not only theoretically beneficial, but seems also practically achievable with the right policy structures in place.
4 Conclusions
In this work, we investigate the chemical industry's pathway to electrified production within the context of a sector-coupled national energy system's transition to net-zero emissions. We determine the timing of the chemical industry's transition to electrified production relative to other sectors, and resolve the interactions between a transitioning chemical industry and the energy system.Our results show the prioritization of the build-up of renewable electricity, the transition to heat pumps for low-temperature heat and to battery electric vehicles for mobility over electrified chemical production. We find that a fully net-zero energy system requires 473 TWh year−1 of green energy imports, equivalent to 41% of its annual electricity demand, with 43% of these imports used by a fully electrified chemical industry. Although much lower than present day fossil-based energy imports, these imports require a massive scale-up in global green energy production. Furthermore, full chemical electrification requires a 25-fold increase in annual methanol production compared to today to serve as a platform chemical for the chemical industry.
Green energy imports and methanol production can be reduced via partial electrification of the chemical industry. We find that methanol, ammonia, and the olefins start transitioning to electrified production together with the medium and high-temperature heat sectors with lower import dependencies. These chemicals are distinguished by their high Cost-Avoided, a metric quantifying the system cost reduction per MWh of renewable electricity used in their electrified processes. Of our considered chemicals, methanol has the highest Cost-Avoided due to the high emissions of its fossil-based process, which its electrified process abates. Aromatics have the lowest Cost-Avoided due to their high electricity requirement, particularly for production of methanol as a feedstock. Thus, prime targets for electrification are chemicals with lower electricity requirements or high-emission fossil-based alternatives, such as methanol and ammonia.
Our results expose an additional benefit of partial electrification of the chemical industry: flexibility provision to the energy system. Methanol, ammonia, and the olefins provide flexibility when both fossil-based and electrified production capacities are available. Production of these chemicals adapts to the hourly availability of renewable electricity by adjusting the production mix accordingly. This flexibility provision makes earlier investments in electrified capacities worthwhile despite yearly utilization rates as low as 47%. This accelerated deployment of underutilized electrified chemical production capacities occurs even under extreme cost scenarios, emphasizing the value of flexibility provision from an oversized and diversified chemical industry to the energy system. The energy system benefits of a partially electrified chemical industry should be shared with individual companies in the form of subsidies or tax credits to incentivize an earlier roll-out of electrified chemical production capacities.
To more holistically determine the role of electrified chemical production, it should be evaluated together with other sustainable chemical industry alternatives like biomass, recycling, and CCS. A dedicated consideration of green hydrogen imports would also be beneficial, as these imports can influence an electrified chemical industry's demand for domestic renewable electricity as well as the energy system's flexibility requirements. Nonetheless, our study shows that electrified chemical production could be a valuable part of the transition to net-zero emissions if prioritized after other energy sectors. Building up electrified production capacities while diversifying with other dispatchable production options provides both an avenue to defossilization of chemical production and flexibility to the energy system, thus serving as a valuable component in net-zero energy systems.
Author contributions
Patricia Mayer: conceptualization, formal analysis, methodology, investigation, writing – original draft, writing – review & editing, visualization. Florian Joseph Baader: conceptualization, methodology, supervision, writing – review & editing. Stefano Moret: supervision, visualization, writing – review & editing. David Yang Shu: conceptualization, investigation, writing – review & editing. Ludger Leenders: conceptualization, supervision. Christian Zibunas: resources. André Bardow: conceptualization, methodology, supervision, writing – review & editing, funding acquisition.Conflicts of interest
A. B. served on review committees for research and development at ExxonMobil and TotalEnergies, oil and gas companies that are also active in carbon capture, transport, utilization and storage. A. B. has ownership interests in firms that render services to industry, some of which may work on carbon dioxide capture, transport, utilization and storage.Data availability
The data supporting this article have been included as part of the ESI.†Acknowledgements
This work was funded by the Swiss Federal Office of Energys SWEET program as part of the project PATHFNDR.References
- International Energy Agency, The Future of Petrochemicals: Towards More Sustainable Plastics and Fertilisers, 2018 Search PubMed.
- M. Van der Hoeven, Y. Kobayashi and R. Diercks, Int. Energy Agency, Paris, 2013, 56 Search PubMed.
- International Energy Agency, Tracking Clean Energy Progress 2017, 2017 Search PubMed.
- P. Mayer, A. Ramirez, G. Pezzella, B. Winter, S. M. Sarathy, J. Gascon and A. Bardow, iScience, 2023, 26(8), 107389 CrossRef CAS PubMed.
- Z. J. Schiffer and K. Manthiram, Joule, 2017, 1, 10–14 CrossRef.
- G. Lopez, D. Keiner, M. Fasihi, T. Koiranen and C. Breyer, Energy Environ. Sci., 2023, 16(7), 2879–2909 RSC.
- A. Kätelhön, R. Meys, S. Deutz, S. Suh and A. Bardow, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 11187–11194 CrossRef PubMed.
- I. Yarulina, A. D. Chowdhury, F. Meirer, B. M. Weckhuysen and J. Gascon, Nat. Catal., 2018, 1, 398–411 CrossRef CAS.
- A. González-Garay, M. S. Frei, A. Al-Qahtani, C. Mondelli, G. Guillén-Gosálbez and J. Pérez-Ramírez, Energy Environ. Sci., 2019, 12, 3425–3436 RSC.
- International Energy Agency, World Energy Outlook 2024, 2024 Search PubMed.
- N. Mac Dowell, P. S. Fennell, N. Shah and G. C. Maitland, Nat. Clim. Change, 2017, 7, 243–249 CrossRef CAS.
- International Energy Agency, Exploring clean energy pathways, OECD, 2019 Search PubMed.
- A. Bazzanella and F. Ausfelder, Low carbon energy and feedstock for the European chemical industry: Technology Study, DECHEMA, Gesellschaft für Chemische Technik und Biotechnologie eV, 2017 Search PubMed.
- P. Gabrielli, M. Gazzani and M. Mazzotti, Ind. Eng. Chem. Res., 2020, 59, 7033–7045 CrossRef CAS.
- D. S. Mallapragada, Y. Dvorkin, M. A. Modestino, D. V. Esposito, W. A. Smith, B.-M. Hodge, M. P. Harold, V. M. Donnelly, A. Nuz and C. Bloomquist, et al. , Joule, 2023, 7, 23–41 CrossRef CAS.
- S. Brethauer and M. H.-P. Studer, Chimia, 2021, 75, 788 CrossRef CAS PubMed.
- E. Palm, L. J. Nilsson and M. Åhman, J. Cleaner Prod., 2016, 129, 548–555 CrossRef.
- A. Sternberg and A. Bardow, Energy Environ. Sci., 2015, 8, 389–400 RSC.
- C. Ganzer and N. Mac Dowell, Sustainable Energy Fuels, 2020, 4, 3888–3903 RSC.
- P. Mayer, M. Heer, D. Y. Shu, N. Zielonka, L. Leenders, F. J. Baader and A. Bardow, Front. Energy Res., 2024, 12, 1443506 CrossRef.
- H. M. Almajed, O. J. Guerra, A. Somoza-Tornos, W. A. Smith and B.-M. Hodge, LAPSE, 2024, 1588, 641–651 Search PubMed.
- S. S. Foslie, B. R. Knudsen, S. Bjarghov and M. Korpås, Energy Environ. Sci., 2024, 17, 8838–8854 RSC.
- H. Mikulčić, I. R. Skov, D. F. Dominković, S. R. W. Alwi, Z. A. Manan, R. Tan, N. Duić, S. N. H. Mohamad and X. Wang, Renewable Sustainable Energy Rev., 2019, 114, 109338 CrossRef.
- O. J. Guerra, H. M. Almajed, W. A. Smith, A. Somoza-Tornos and B.-M. S. Hodge, Joule, 2023, 7, 1111–1133 CrossRef CAS.
- D. Bogdanov, A. Gulagi, M. Fasihi and C. Breyer, Appl. Energy, 2021, 283, 116273 CrossRef.
- T. Aboumahboub, R. J. Brecha, H. B. Shrestha, U. Fuentes, A. Geiges, W. Hare, M. Schaeffer, L. Welder and M. J. Gidden, Energies, 2020, 13, 3805 CrossRef CAS.
- T. Burandt, B. Xiong, K. Löffler and P.-Y. Oei, Appl. Energy, 2019, 255, 113820 CrossRef CAS.
- E. Kawai, A. Ozawa and B. D. Leibowicz, Appl. Energy, 2022, 328, 120183 CrossRef CAS.
- S. D. Manuel, T. Floris, W. Kira, S. Jos and F. André, Adv. Appl. Energy, 2022, 7, 100105 CrossRef CAS.
- R. Martnez-Gordón, M. Sánchez-Diéguez, A. Fattahi, G. Morales-España, J. Sijm and A. Faaij, Adv. Appl. Energy, 2022, 5, 100080 CrossRef.
- B. Pickering, F. Lombardi and S. Pfenninger, Joule, 2022, 6, 1253–1276 CrossRef CAS PubMed.
- M. Ram, D. Bogdanov, R. Satymov, G. Lopez, T. Mensah, K. Sadovskaia and C. Breyer, Lappeenranta, Brussels, 2022 Search PubMed.
- X. Rixhon, D. Tonelli, M. Colla, K. Verleysen, G. Limpens, H. Jeanmart and F. Contino, Front. Energy Res., 2022, 10, 904777 CrossRef.
- I. Ioannou, Á. Galán-Martín, J. Pérez-Ramírez and G. Guillén-Gosálbez, Energy Environ. Sci., 2023, 16, 113–124 RSC.
- P. Stegmann, V. Daioglou, M. Londo, D. P. van Vuuren and M. Junginger, Nature, 2022, 612, 272–276 CrossRef CAS PubMed.
- V. Daioglou, B. Wicke, A. P. Faaij and D. P. Van Vuuren, GCB Bioenergy, 2015, 7, 1321–1334 CrossRef CAS.
- I. Tsiropoulos, R. Hoefnagels, S. de Jong, M. van den Broek, M. Patel and A. Faaij, Biofuels, Bioprod. Biorefin., 2018, 12, 665–693 CrossRef CAS.
- F. Hofmann, C. Tries, F. Neumann, E. Zeyen and T. Brown, Nature Energy, 2025, 1–10 Search PubMed.
- J. Zheng and S. Suh, Nat. Clim. Change, 2019, 9, 374–378 CrossRef.
- R. Meys, A. Kätelhön, M. Bachmann, B. Winter, C. Zibunas, S. Suh and A. Bardow, Science, 2021, 374, 71–76 CrossRef CAS PubMed.
- M. Bachmann, C. Zibunas, J. Hartmann, V. Tulus, S. Suh, G. Guillén-Gosálbez and A. Bardow, Nat. Sustainability, 2023, 6, 599–610 CrossRef.
- Ellen MacArthur Foundation, The New Plastics Economy: Catalysing action, 2017.
- Cefic, Germany Chemical and pharmaceutical industry snapshot, https://cefic.org/a-pillar-of-the-european-economy/landscape-of-the-european-chemical-industry/germany/, Accessed: 2024-29-30.
- Statista, Leading chemical exporting countries worldwide in 2021 based on value, https://www.statista.com/statistics/272369/export-volume-of-the-chemical-industry-by-country/, Accessed: 2024-22-08.
- N. Baumgärtner, S. Deutz, C. Reinert, N. Nolzen, L. E. Kuepper, M. Hennen, D. E. Hollermann and A. Bardow, Front. Energy Res., 2021, 9, 621502 CrossRef.
- IEA, Primary chemical production in the Sustainable Development Scenario, 2000–2030, https://www.iea.org/data-and-statistics/charts/primary-chemical-production-in-the-sustainable-development-scenario-2000-2030, Licence: CC BY 4.0.
- VCI, The German Chemical Industry in Figures Online.
- Y. Kloo, A. Scholz and S. Theisen, Towards a net-zero chemical industry: a meta-analysis of recent scenario studies and roadmaps. Results from the ongoing research project GreenFeed. Wuppertal Institute, 2023, p. 48.
- CEFIC, Molecule Managers – A journey into the Future of Europe with the European Chemical Industry, 2019.
- M. Bachmann, S. Volker, J. Kleinekorte and A. Bardow, ACS Sustainable Chem. Eng., 2023, 11, 5356–5366 CrossRef CAS.
- D. Y. Shu, S. Deutz, B. A. Winter, N. Baumgärtner, L. Leenders and A. Bardow, Renewable Sustainable Energy Rev., 2023, 178, 113246 CrossRef CAS.
- O. Edenhofer, Climate change 2014: mitigation of climate change, Cambridge University Press, 2015, vol. 3 Search PubMed.
- Ecoinvent, Ecoinvent Data V. 3.6. Swiss Centre for Life Cycle Inventories, 2020 Search PubMed.
- C. Zibunas, R. Meys, A. Kätelhön and A. Bardow, Comput. Chem. Eng., 2022, 162, 107798 CrossRef CAS.
- K. Rypdal, N. Paciornik, S. Eggleston, J. Goodwin, W. Irving, J. Penman and M. Woodfield, IPCC guidelines for national greenhouse gas inventories: Chapter 1, Institute for Global Environmental Strategies, Hayama, Kanagawa, Japan, 2006.
- Federal Ministry for the Environment, Nature Conservation and Nuclear Safety and, https://www.bmu.de/en.
- D. Bundestag, Bundesgesetzblatt I, 2021, 59, 3905–3907 Search PubMed.
- S. T. Wismann, J. S. Engbæk, S. B. Vendelbo, F. B. Bendixen, W. L. Eriksen, K. Aasberg-Petersen, C. Frandsen, I. Chorkendorff and P. M. Mortensen, Science, 2019, 364, 756–759 CrossRef CAS PubMed.
- U. Y. Qazi, Energies, 2022, 15, 4741 CrossRef CAS.
- M. Rieks, R. Bellinghausen, N. Kockmann and L. Mleczko, Int. J. Hydrogen Energy, 2015, 40, 15940–15951 CrossRef CAS.
- J. Leicher, A. Giese and C. Wieland, J, 2024, 7, 439–456 CAS.
- R. Lowes, 2023, https://www.raponline.org/wp-content/uploads/2023/09/RAP-Lowes-Regret-Ready-Hydrogen-Ready-Boilers-2023-1-Mar-FINAL-properties.pdf.
- C. Reinert, L. Schellhas, J. Mannhardt, D. Y. Shu, A. Kämper, N. Baumgärtner, S. Deutz and A. Bardow, Front. Energy Res., 2022, 10, 884525 CrossRef.
- M. Hoffmann, L. Kotzur, D. Stolten and M. Robinius, Energies, 2020, 13, 641 CrossRef.
- S. Pfenninger, A. Hawkes and J. Keirstead, Renewable Sustainable Energy Rev., 2014, 33, 74–86 CrossRef.
- International Energy Agency, Germany 2020, 2020.
- Bundesnetzagentur, Bundesnetzagentur publishes gas supply figures for 2023.
- D. Tonelli, L. Rosa, P. Gabrielli, K. Caldeira, A. Parente and F. Contino, Nat. Commun., 2023, 14, 5532 CrossRef CAS PubMed.
- A. Odenweller, F. Ueckerdt, G. F. Nemet, M. Jensterle and G. Luderer, Nat. Energy, 2022, 7, 854–865 CrossRef.
- European Commission, A hydrogen strategy for a climate-neutral Europe, 2020.
- International Energy Agency (IEA), Global Hydrogen Review 2024, 2024.
- J. M. Weinand, F. Scheller and R. McKenna, Energy, 2020, 203, 117817 CrossRef.
- U. J. Frey, S. Sasanpour, T. Breuer, J. Buschmann and K.-K. Cao, Front. Environ. Economics, 2024, 3, 1398358 CrossRef.
- M. G. Prina, G. Manzolini, D. Moser, B. Nastasi and W. Sparber, Renewable Sustainable Energy Rev., 2020, 129, 109917 CrossRef.
- J. Bistline, G. Blanford, M. Brown, D. Burtraw, M. Domeshek, J. Farbes, A. Fawcett, A. Hamilton, J. Jenkins and R. Jones, et al. , Science, 2023, 380, 1324–1327 CrossRef CAS PubMed.
- C. Fetting, ESDN Rep., 2020 Search PubMed , https://www.esdn.eu/fileadmin/ESDN_Reports/ESDN_Report_2_2020.pdf.
- D. J. Laky, N. P. Cortes, J. C. Eslick, A. A. Noring, N. Susarla, C. Okoli, M. A. Zamarripa, D. A. Allan, J. H. Brewer and A. K. Iyengar, et al. , Energy Environ. Sci., 2024, 9509–9525 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ee01118c |
| ‡ https://git-ce.rwth-aachen.de/ltt/secmod. |
| This journal is © The Royal Society of Chemistry 2025 |