Open Access Article
Open Access ArticleUnraveling nanoscale interfacial kinetics in battery cathodes through operando tip-enhanced Raman spectroscopy†
Beatrice
Laurenti‡
 a,
Juan Carlos
Gonzalez-Rosillo‡
a,
Juan Carlos
Gonzalez-Rosillo‡
 *a,
Francesco
Chiabrera
*a,
Francesco
Chiabrera
 a,
Alice
Fiocco
b,
Fernanda
Monteiro Freitas
a,
Alice
Fiocco
b,
Fernanda
Monteiro Freitas
 a,
Marc
Chaigneau
a,
Marc
Chaigneau
 b,
Alex
Morata
b,
Alex
Morata
 *a and
Albert
Tarancón
*a and
Albert
Tarancón
 *ac
*ac
aNanoionics and Fuel Cells, Catalonia Institute for Energy Research (IREC), Jardins de les Dones de Negre 1, 08930, Sant Adrià del Besòs, Barcelona, Spain. E-mail: jcgonzalez@irec.cat; amorata@irec.cat; atarancon@irec.cat
bHORIBA France SAS, 14 bd Thomas Gobert, 91120 Palaiseau, France
cCatalan Institution for Research and Advanced Studies (ICREA), Passeig Lluís Companys 23, 08010, Barcelona, Spain
First published on 1st July 2025
Abstract
Understanding interfacial phenomena at the micro- and nanoscale is essential for improving the performance of energy storage technologies such as lithium-ion batteries. However, probing the chemical and structural evolution of buried interfaces during operation remains a major experimental challenge. Here, we present the first implementation of operando Tip-Enhanced Raman Spectroscopy (TERS) to track nanoscale lithium-ion dynamics in working battery electrodes. Using LiMn2O4 and LiFePO4 thin films as model systems, we demonstrate that TERS can determine grain- and grain-boundary-specific behavior with spatial and temporal resolution. In LiMn2O4, we observe a delayed appearance of the λ-MnO2 phase at grain boundaries during delithiation, which is consistent with faster Li+ diffusion in these regions and is supported in this work by 2D finite-element simulations. In contrast, LiFePO4 exhibits reduced spectral visibility under operando conditions, yet systematic background modulation enables tracking of surface-level redox processes. These results establish operando TERS as a powerful technique for probing local ionic transport and interfacial chemistry in complex energy materials, with broad implications for the design and optimization of next-generation battery systems.
Broader contextThe global transition towards renewable energy sources and electrified transportation heavily relies on high-performance energy storage solutions, especially batteries. However, many promising battery technologies face fundamental limitations associated with interfacial phenomena occurring at extremely small length scales, which significantly affect overall battery efficiency, lifetime, and safety. These interfaces, such as grain boundaries and electrode–electrolyte interfaces, can either facilitate or hinder ion transport, making their detailed understanding crucial for optimizing battery performance. Despite advancements, conventional techniques used to study these phenomena generally provide averaged information and lack sufficient spatial resolution, limiting our ability to design better-performing, longer-lasting, and safer batteries. The development of operando Tip-Enhanced Raman Spectroscopy (TERS), as demonstrated in this study, addresses this critical gap by enabling direct visualization of chemical and structural changes at nanoscale interfaces under realistic operating conditions. |
Introduction
A battery consists of numerous interfaces, each playing a distinct and often dominant role in its overall performance, efficiency, and longevity. These interfacial interactions govern essential processes, such as ion transport, charge transfer, and chemical stability. Many studies have emphasized that interface design, particularly grain boundaries, is a crucial factor controlling both ionic and electronic transport.1–4 Since these nanometric-length scale interfaces undergo dynamic chemical and structural changes during operation, it is imperative to develop advanced characterization techniques with sufficient resolution and sensitivity capable of capturing the real-time chemical evolution of critical interfaces.One of the most widely used techniques for understanding the complex interplay of interfaces involved in electrochemical responses is Electrochemical Impedance Spectroscopy (EIS). EIS is widely used for decoupling phenomena at different time scales, which is of major importance for understanding ionic conduction, charge transfer mechanisms or diffusion-limited processes.5,6 However, EIS lacks the spatial resolution needed to directly visualize local heterogeneities, such as grain boundaries, offering instead an averaged response. To achieve higher spatial resolution, techniques such as Scanning Ion Conductance Microscopy (SICM) and Scanning Electrochemical Cell Microscopy (SECCM) have been developed. SICM measures localized ionic currents rather than electrochemical redox processes, while SECCM provides localized redox information but typically at lower spatial resolution (∼100 nm) compared to SICM.4,7–9 Both techniques, however, remain inherently confined to the nanoscale region beneath the nanopipette tip acting as the active electrode, limiting their effectiveness in probing very narrow interfaces. Electrochemical Strain Microscopy (ESM), with its superior spatial resolution (∼10 nm, limited mainly by tip geometry), uses a biased AFM tip to induce localized ionic transport and detects the resulting mechanical deformation due to ion migration.10–12 Despite its high resolution, ESM only indirectly probes electrochemical phenomena, and its signals are strongly influenced by mechanical coupling between the tip and sample. Furthermore, SICM, SECCM, and ESM can introduce high local electric fields and potential cross-talk between topographical and electrochemical signals, complicating the interpretation of electrochemical behavior, particularly in operando studies of structurally complex regions such as grain boundaries.
Over the past decades, significant advances have been made in the 2D and 3D analysis of grain boundaries. Advanced techniques such Atom Probe Tomography (APT) and High-Resolution Transmission Electron Microscopy (HRTEM) enable nanoscale characterization of grain boundaries.13 HRTEM provides high-resolution 2D images that reveal the atomic structure of materials, allowing direct visualization of grain boundary structures, defect distributions, and grain orientation with atomic-level precision.14 When coupled with Electron Energy Loss Spectroscopy (EELS) or Energy Dispersive X-ray Spectroscopy (EDS), HRTEM can provide additional compositional information.15 APT offers 3D images containing information of individual detected atomic species with sub-nanometer spatial resolution and extremely high analytical sensitivity.16,17 Very recently, our group demonstrated how APT can provide a 3D-resolved reconstruction with isotopic sensitivity,18 directly observing oxygen diffusion pathways at grain boundaries.19 However, these techniques require specific sample geometries, and the preparation process can be notably challenging and time-consuming and costly. These challenges highlight the need for more practical approaches, especially for operando studies.
A powerful tool for nanoscale chemical characterization that has emerged over the past two decades, is Scanning Probe Microscopy – Tip-Enhanced Raman Spectroscopy (SPM-TERS).20–23 This technique combines a Scanning Probe Microscope (SPM) and Raman Spectroscopy, taking advantage of Scanning Tunneling Microscopes (STM) or Atomic Force Microscopes (AFM). These TERS approaches offer nanometric resolution and high-sensitivity spectroscopy and imaging, enabling the acquisition of local chemical information. By focusing a laser onto a sharp metal (or metal-coated) SPM tip, it is possible to amplify the electromagnetic field – and thus the Raman signal – through a combination of localized surface plasmon resonance (LSP)24 and the lighting rod effect.25,26 This enhancement is confined to the vicinity of the SPM tip, enabling nanoscale resolution, limited by the probe itself. Our group recently studied LiMn2O4 thin film using AFM-based TERS, demonstrating the technique's sensitivity to surface defects and grain boundary chemistry.27 TERS offers significant benefits in terms of time efficiency and reduced complexity compared to the aforementioned techniques. In addition, TERS does not require any complex sample preparation, can be used in gas and temperature-controlled conditions and does not require long data acquisition times, enabling its use to monitor a variety of electrochemical processes, also in operando. Unlike ESM, SICM, or SECCM, operando TERS measurements offer the critical advantage that the entire sample can be electrochemically cycled rather than just the localized area directly beneath the tip, enabling more representative and accurate operando studies.
Despite the extensive scientific literature on batteries, relatively few studies have explored the potential of TERS in this context. This is likely due to challenges associated with performing “non-gap mode”28 measurements on rough or thick materials and achieving a strong TERS signal from bulk samples or thick films grown on non-transparent or metallic substrates. In gap mode, strong field enhancement arises from the confined electromagnetic field generated between a metallic tip and a metallic substrate, as opposed to non-gap mode configurations, which operate on non-metallic or irregular surfaces and rely solely on the tip for enhancement.29 The surface sensitivity of TERS makes it uniquely suited for detecting cathode–electrolyte interphase (CEI) or solid–electrolyte interphase (SEI) layers, providing spatially resolved insights into interfacial degradation processes. For that, among the limited studies available, the pioneering work of Nanda et al.30,31 on SEI composition in silicon thin film anodes and Dinda et al.32 study on both SEI and CEI in sodium batteries stand out. In the same direction, we recently reported the first ex situ studies of LiMn2O4 thin films cycled in aqueous electrolyte, revealing the presence of Mn3O4 and sulfate adsorbates at grain boundaries that evolve with cycling.28 However, to the best of our knowledge, no studies have reported the implementation of operando TERS in batteries.
Herein, we present the implementation of operando TERS, integrated into an atomic force microscope and coupled to a custom-designed electrochemical cell, to probe lithium-ion dynamics at the grain and grain boundary level in working battery materials. We focus primarily on LiMn2O4 thin films, a model system where grain boundaries are believed to promote fast Li+ transport.33–35 By positioning the TERS tip with nanometric precision on top of individual grains or at grain boundaries during cyclic voltammetry, we capture time-resolved vibrational signatures that reflect local lithiation and delithiation processes. Our experiments reveal a clear delay in the emergence of the λ-MnO2 phase at grain boundaries, consistent with faster Li+ diffusion compared to the grain interior. These findings are supported by two-dimensional finite-element simulations, confirming the ability of TERS to resolve interface-specific transport kinetics operando. To test the broader applicability of this method, we also investigate LiFePO4 thin films under similar conditions. Although operando spectral resolution is limited in this case, systematic background modulation remains detectable and correlated with the electrochemical state, demonstrating the sensitivity of TERS even under spectroscopically challenging environments. Together, these results establish operando TERS as a powerful platform for probing nanoscale ion transport and interfacial reactivity in complex electrochemical systems.
Results and discussion
Operando TERS in LiMn2O4
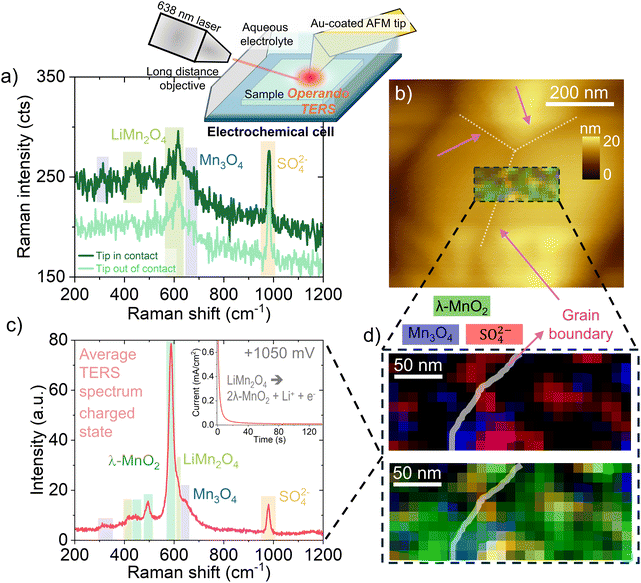
Building on our previous success in employing TERS to characterize LiMn2O4 thin films under ex situ conditions,28 we implemented a custom electrochemical cell to enable operando TERS measurements. This setup was integrated into a commercial AFM system from Horiba, allowing real-time monitoring of structural and compositional changes in LiMn2O4 as the entire film undergoes electrochemical cycling with the tip acting as a local nano-Raman probe. Further details on the cell design and experimental configuration are provided in the Methods section and the ESI (section I).†To establish the viability of TERS under liquid electrochemical conditions, we first evaluated signal enhancement in a LiMn2O4 thin film immersed in aqueous electrolyte. As shown in Fig. 1a, Raman spectra acquired with the tip in contact (TERS mode) exhibit an overall signal intensity amplification of approximately 25% compared to spectra collected with the tip out of contact (oscillating at ∼20 nm amplitude). Several Raman modes are observed and assigned based on previous literature:28,36,37 the dominant peaks (bands at ∼420 and 620 cm−1) correspond to LiMn2O4, while additional features are attributed to trace Mn3O4 (bands at ∼310 and 660 cm−1) impurities and a band near ∼980 cm−1 originating from sulfate ions in the aqueous electrolyte. This spectral fingerprint confirms that TERS enhancement persists in liquid environments and improves spectral resolution for weaker vibrational modes, in line with our previous ex situ studies on LiMn2O4 thin films. In particular, vibrational bands in the 200–400 cm−1 region become more prominent under TERS conditions, indicating enhanced sensitivity to lattice modes of the LiMn2O4 and Mn3O4 phases.
We next acquired an AFM topography image over a 600 × 600 nm2 region containing several grains (Fig. 1b), from which a 250 × 100 nm2 area was selected between two grains for spatially resolved TERS mapping. This quasi-operando experiment was conducted in the charged state after applying a constant potential of +1050 mV, as shown in the inset of Fig. 1c, ensuring surface transformation prior to spectral acquisition,38 according to the expected electrochemical reaction:
| LiMn2O4 → 2λ − MnO2 + Li+ + e− | (1) |
Averaging the collected spectra across the whole region yields the spectrum in Fig. 1c, where bands at ∼498 and ∼588 cm−1 are consistent with the formation of λ-MnO2, the fully delithiated phase. Notably, the Raman signal from λ-MnO2 is strongly enhanced compared to LiMn2O4, a well-known effect also observed at the macro-scale due to the resonance of the λ-MnO2 phase with the excitation laser.39 Residual features from LiMn2O4 and Mn3O4 are also present, along with the sulfate band from the electrolyte, in agreement with our previous ex situ studies.28 Following the methodology established in our earlier work, we constructed TERS intensity maps by tracking the spatial variation of selected vibrational modes (Fig. 1d). A white transparent line marks the approximate position of the grain boundary as inferred from the AFM topography. The maps reveal that Mn3O4 and sulfate signals are locally enhanced at the grain boundary, while the λ-MnO2 phase dominates the overall spectral response. These results are in line with our previous ex situ observations and confirm that TERS enables nanometric spatial resolution of electrochemical phase evolution. Having demonstrated the chemical sensitivity and spatial resolution of TERS under quasi-operando conditions, we then performed fully operando measurements during electrochemical cycling. We selected a well-defined grain from the same AFM topography map (Fig. 2a) and positioned the tip on top of a grain, far from the grain boundary. Cyclic voltammetry (CV) was performed while simultaneously acquiring time-resolved TERS spectra at a fixed point. The acquisition parameters (1.7 seconds per spectrum) were optimized to capture spectral changes with sufficient temporal resolution (see Methods for details). CV was used to drive lithium extraction (delithiation) and insertion (lithiation) in the LiMn2O4 film. During charge, the voltammogram displays two characteristic redox peaks: the first corresponds to the formation of a partially delithiated Li0.5Mn2O4 intermediate, while the second is associated with further delithiation toward the λ-MnO2 phase. These structural transitions are reversible upon discharge.
 | ||
| Fig. 2 TERS time map overlaid with the current density for grain (a) and grain boundary (b) with the inset showing the AFM topography with the tip position indicated with white crosses. (c) Raman spectra extracted at t = 10 s (green) and t = 50 s (red) from the TERS time maps of the grain. The two spectra are plotted using separate vertical axes for clarity: the red spectrum (left axis) spans from −1.1 to 0.8, while the green spectrum (right axis) spans from −0.05 to 0.4. No multiplication or artificial scaling was applied; both spectra belong to the same normalized data set. (d) and (e) Averaged TERS intensity (580–596 cm−1) with overlaid current density of the cyclic voltammetry for grain (d) and grain boundary (e). Note that these graphs correspond to the second cycle of a set of 3 consecutive scans, with time normalized for ease of visualization, see section II in the ESI.† | ||
Fig. 2a displays the changes of the Raman intensity over time, overlaid with the CV current profile using a common time axis. Notably, the Raman intensity around 590 cm−1, associated with the λ-MnO2 phase, increases in parallel with the positive current peak in the CV. This clearly demonstrates the ability of operando TERS to track phase transitions with nanoscale resolution, even at relatively fast scan rates of 5 mV s−1. To further illustrate the phase change, we compare two spectra from the TERS time series: one acquired at t = 10 s (before full delithiation), and another at t = 50 s (after delithiation). As shown in Fig. 2c, the λ-MnO2 spectral signature appears only after oxidation, confirming the formation of the delithiated phase following lithium extraction from LiMn2O4. Meanwhile, the prominent peak at ∼980 cm−1, assigned to the sulfate ion (SO42−) from the dissociation of the Li2SO4 electrolyte,27 remains unchanged throughout the CV. Note that the apparent difference in sulfate peak intensity between the two spectra in Fig. 2c is due to the different vertical scales used for plotting.
We next repeated this procedure, positioning the tip and laser on a grain boundary (white cross in the topography image, inset of Fig. 2b). As shown in Fig. 2b, the intensity of the λ-MnO2 peak again increases at the second delithiation peak in the CV. A closer examination of the high-intensity region in the time map (red zone) reveals a consistent shift in the onset of the phase transition, suggesting a delayed response at the grain boundary compared to the grain interior. To quantify this difference, we extracted the average Raman intensity in the 580–596 cm−1 range (corresponding to λ-MnO2 phase) for both the grain and grain boundary measurements and plotted them alongside the corresponding CV scans (Fig. 3d and e, respectively). Analysis of the λ-MnO2 peak intensity reveals a measurable delay of approximately 6 seconds in the emergence of the delithiated phase at the grain boundary. This observed delay suggests that lithium is extracted more efficiently from the grain boundary, resulting in slower local accumulation of delithiated products, which is a signature of higher Li-ion diffusivity.
In light of these observations, we propose that the delayed appearance of the λ-MnO2 phase at grain boundaries reflects a higher Li-ion diffusivity in these regions, which translates into faster deintercalation kinetics. Although this is the first time such behavior is observed via operando TERS, multiple studies have reported up to four orders of magnitude faster lithium diffusion along grain boundaries,33–35 often attributed to the presence of negatively charged lithium vacancies around the grain boundary core.35 Indeed, in our previous works we identified vacancy clusters of negatively charged vacancies accumulating at grain boundaries via Positron Annihilation Lifetime Spectroscopy.27,40 These defects typically surround the positively charged grain boundary core,34,41,42 balancing the electrostatic interactions and forming a space charge layer.34 Although space-charge layers are typically considered detrimental to Li-ion transport in most Li-based materials,43,44 LiMn2O4 appears to be an exception: here, ionic transport is significantly enhanced at grain boundaries. Remarkably, this effect is sufficiently pronounced and spatially extended to be detected by operando TERS, even with our spatial resolution limited by a ∼20 nm commercial tip radius.41
It is worth noting that the slightly longer persistence of the λ-MnO2 signal at the grain boundary may be influenced by subtle differences in the current level at the turning point of the voltage sweep (1.05 V) that arise from cycle to cycle. This current level is also different between the experimental and the simulated, as seen in Fig. 3a. However, this factor alone cannot account for the delayed onset of the phase transition, as measurements at both grain and grain boundary locations were independently repeated three times, consistently showing the same behavior. Post-mortem ex situ TERS analysis reveals early sulfate adsorption and preferential presence of Mn3O4 at grain boundaries, with stronger contrast than observed in conventional Raman (see ESI, section II†).
To support our experimental findings, we performed two-dimensional Finite Element Method (FEM) simulations that replicate an equivalent electrochemical experiment. The model describes electrochemical Li intercalation and diffusion in a layer with grain boundary diffusivity four order of magnitudes higher than the bulk one (see Methods for details). As a first step, we simulated a CV curve to verify that the model accurately reproduces the overall electrochemical response of the film (Fig. 3a), obtaining close agreement between the simulated and experimental CVs. The extracted values of bulk chemical diffusivity (10−11 cm2 s−1) and charge transfer resistance (0.6 Ω cm2) are comparable with literature values, endorsing the model.45–47 Discrepancies between model and experiments are mainly due to differences in the equilibrium voltage profiles, which were directly assumed from literature,46 and are not expected to influence the main conclusions of the simulations. We then used the model to track the Li+ concentration as a function of time (Fig. 3c) and spatial position across the film, extracting concentration maps representing the grain bulk and at the grain boundary, following the schematic shown in Fig. 3b. The concentration maps (Fig. 3d, snapshots at t = 35 s, 50 s, and 65 s) reveal the time evolution of the Li+ distribution. At the onset of lithium deintercalation, Li+ concentration remains higher at the grain boundaries, particularly near the subsurface, compared to the grain interior, consistent with the delayed phase transition observed via operando TERS. Conversely, during reintercalation (t ≈ 65 s), the Li+ front advances inward from the subsurface, while the grain boundaries exhibit relatively lower concentrations. To directly compare our time-resolved TERS measurements with the simulations, we set Li ≤0.4 in LixMn2O4 as the threshold for the phase transition into λ-MnO2,41 focusing on a 20 nm subsurface region to approximate the TERS sensitivity at both the grain center and the grain boundary. The resulting simulated profiles (Fig. 3f) reproduce the experimental delay observed between the grain and grain boundary regions (Fig. 3e) for both deintercalation and reintercalation processes. This agreement is achieved by incorporating a single assumption, well supported by literature, that lithium diffusion is faster at grain boundaries. Additional simulations investigating alternative explanations, such as local shifts in insertion potential or variations in charge transfer resistance, revealed negligible contributions to the observed delays (see ESI, section II†). To assist in visualizing this mechanism, a supplementary video (Video S1†) is provided, showing the time evolution of Li-ion concentration and simulated Raman response at both grain and grain boundary locations.
While our FEM model successfully captures the kinetic delay of lithium deintercalation between grain and grain boundary regions, a notable deviation remains: all simulated profiles peak around the maximum applied potential, whereas the Raman signal from operando TERS reaches its maximum earlier, during the rising portion of the voltage sweep (see ESI, section II†). This consistent discrepancy, observed both at grains and grain boundaries, cannot be explained by bulk transport dynamics alone. We propose that this behavior originates from surface-specific processes not captured in our model, revealing one of the most exciting strengths of operando TERS: its ability to selectively probe interfacial charge dynamics with nanometric resolution in realistic electrochemical environments.
Recent works have highlighted that surface-limited or interfacial processes can significantly influence the overall battery response. Halldin Stenlid et al.48 presented a state-of-the-art CIET (Coupled Ion-Electron Transfer) framework that combines constrained and constant potential DFT to predict charge transfer kinetics at the interface, explicitly incorporating solvent coordination and ion adsorption structures. Their results demonstrate that interfacial ion and electron transfer can dominate the local reaction landscape. While our model focuses solely on bulk diffusion, the TERS signal can still capture this interfacial complexity. Moreover, we add an additional layer of spatial resolution by specifically interrogating grain boundaries, which are interfaces that, despite their known influence on ionic transport, remain largely uncharacterized under operando conditions. The potential impact is particularly pronounced for small-grained materials, where the density of grain boundaries can directly shape device performance.
From a different angle, the work of Xiao et al.49 illustrates how space charge layers can give rise to interfacial lithium storage in mixed conductors, showing capacitive-like behavior localized at solid–solid interfaces. The early appearance of λ-MnO2 signatures in our TERS data may represent a related phenomenon, i.e., lithium depletion at the surface induced by space charge effects that could be studied as a function of the state-of-charge in operando conditions. Thickness-dependent studies will be performed for that, together with further defect-chemical modeling. Additionally, Huang et al.50 recently demonstrated that spontaneous H3O+ intercalation into the LiMn2O4 lattice during cycling in aqueous electrolytes can dramatically alter local redox behavior and structure by forming Mn4+-rich surface layers. Although our samples were not subjected to prolonged cycling to avoid such cumulative changes, these phenomena deserve focused investigation in future work.
Overall, despite the simplicity of our FEM model, which excludes surface and electrolyte-specific effects, its ability to reproduce key experimental trends highlights the robustness of the mechanistic picture proposed. Yet, the deviation in the timing of the phase transition peak is not a minor artifact, but rather a revealing clue: it points toward electrochemical complexity at the nanoscale that demands spatially and chemically resolved operando techniques. Operando TERS has proven capable of capturing these subtle but critical effects, often not visible by more conventional methods.
Extending operando TERS to LiFePO4
Having established the sensitivity and spatial selectivity of this technique in LiMn2O4, we now extend our operando TERS methodology to LiFePO4 thin films, a second model system with fundamentally different phase transformation mechanisms and transport properties. This comparison enables us to further test the universality and limits of nanoscale operando Raman as a probe of local redox dynamics under working conditions. Prior to operando measurements, we characterized LiFePO4 thin films via ex situ TERS and conventional Raman spectroscopy to establish a reference (see ESI, section III†). As shown in Fig. 4a, both techniques detect mainly the LiFePO4 modes (at ∼948 cm−1 the main mode, and minor modes at ∼430, 483, 570, 628, 990 and 1070 cm−1),12,16,22 but only TERS reveals an additional broad feature near 750 cm−1, attributed to a superficial Fe3O4 phase. This secondary signal, mapped across the film surface (Fig. 4b and c), highlights the enhanced surface sensitivity of TERS, revealing the presence of small regions in the map rich in Fe3O4. Control measurements confirmed the absence of laser-induced changes, indicating that these surface phases pre-exist in the as-deposited film.However, when measuring TERS under the same aqueous electrochemical conditions used for LiMn2O4, the LiFePO4 vibrational response effectively vanished. The in situ TERS spectra were dominated by a broad background (Fig. 4a, blue trace), likely due to fluorescence-like interference or surface reactions at the electrode–electrolyte interface.51–53 This signal loss is not observed in conventional Raman measurements under identical conditions, as shown in the ESI, section III,† nor in the LiMn2O4 case, and therefore likely reflects a near-surface effect specific to TERS. The origin of the strong fluorescence background observed under operando conditions may relate to hydrated or partially delithiated surface species51–53 and will be the focus of future investigations.
Despite the absence of clear vibrational features, operando TERS detected a consistent modulation in the spectral background during redox cycling. By positioning the tip on top of a grain and recording TERS spectra during a CV scan, we observed a reproducible baseline shift correlated with the state-of-charge. Furthermore, a relative intensity TERS map reflecting this background signal matched the topography precisely, clearly identifying the grains as regions of higher intensity (Fig. 4d and g). Although the characteristic LiFePO4 and FePO4 peaks could not be unambiguously identified, the background response reflects surface-level changes synchronized with lithium insertion and extraction. This suggests that TERS remains sensitive to interfacial phenomena even in chemically complex environments and highlights its potential as a diagnostic tool in such systems. Post-cycling ex situ TERS confirmed the coexistence of both LiFePO4 and FePO4 phases (ESI, section III†), indicating that redox activity indeed occurred, despite being masked during operando measurements. Further high-resolution TERS mapping would be needed to elucidate their spatial distribution and structural evolution in more detail.
By capturing distinct interfacial behaviors in two model cathode materials, this work demonstrates the versatility and diagnostic power of operando TERS. Even when full spectral resolution is limited, the technique yields valuable insight into local electrochemical dynamics. TERS provides unique perspectives to examine local chemical environments and reaction pathways with nanoscale resolution, which we believe is a critical requirement for advancing solid-state ionics and electrochemistry fields. Conventional Raman Spectroscopy cannot always capture subtle surface or interface phenomena, especially when secondary phases or defect-rich layers are confined to tens of nanometers or even below. In contrast, TERS probes these regions with spatial resolutions matching or even below the characteristic interface dimensions in many battery electrodes and solid electrolytes. This allows direct observation of local lithiation/delithiation, phase transformations, and defect-induced phenomena that would remain hidden when using other measurement techniques.
Besides its spatial resolution, TERS can be coupled with operando electrochemical setups, as demonstrated in this work. By correlating time-resolved current–voltage signals with chemical signature changes, TERS allows to visualize where and when ion transport bottlenecks or accelerations occur, whether in grains, grain boundaries, or near other functional interfaces (e.g., electrode–electrolyte surfaces). This time-resolved tracking of local phase evolution (including transient or mixed valence states) informs rational design of electrode architectures, doping strategies, and coatings that could enhance overall battery performance. Such in situ/operando insights could be equally key in understanding ion conduction in solid electrolytes or monitoring degradation in next-generation devices like composite electrodes in all-solid-state batteries. Equally exciting is the potential to extend TERS to systems beyond lithium-ion chemistries, such as sodium-ion, magnesium-ion, or solid oxide fuel cells, where complex reactions at electrode–electrolyte interfaces can limit device reliability. By revealing the interplay between local chemistry, morphological changes and electrochemical performance, TERS can become an essential technique that guides both fundamental materials research and practical energy-storage device engineering.
Conclusions
We have demonstrated the use of operando Tip-Enhanced Raman Spectroscopy (TERS) in two model cathode thin films, LiMn2O4 and LiFePO4, aiming to resolve grain- and grain-boundary-specific lithium-ion dynamics. In LiMn2O4, time-resolved TERS spectra captured phase transitions at the nanoscale, revealing a delayed appearance of the λ-MnO2 phase at grain boundaries indicative of enhanced Li+ transport in these regions, as supported by FEM simulations. The results directly link local kinetic asymmetries to structural features, establishing operando TERS as a powerful technique for probing interfaces in working battery materials.By contrast, operando TERS on LiFePO4 revealed no resolvable vibrational modes in situ, likely due to surface reactivity or amorphization in aqueous electrolyte. Nevertheless, we observed systematic background modulation correlated with the electrochemical cycle, enabling us to correlate Raman signal with electrochemical activity at the nanoscale. Post-cycling ex situ TERS confirmed the coexistence of LiFePO4 and FePO4 phases, further proving the sensitivity of the technique.
Our results highlight the nature of grain boundary transport phenomena and the dual advantage of TERS: its ability to spatially resolve functional interfaces and to extract chemically relevant signals even under challenging conditions. By integrating operando spectroscopy with electrochemical measurements and numerical modeling, this study opens the door to interrogating ion transport in complex architectures at the nanoscale. Operando TERS has the potential to become a useful technique for providing unprecedented insights by mapping dynamic processes at buried interfaces, including space charge layers, interfacial degradation, adsorbates, or proton intercalation, across a wide range of battery chemistries and energy-storage systems.
Methods section
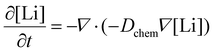 | (2) |
Conflicts of interest
The authors declare no conflict of interest.Data availability
Data for this article, including TERS maps and simulated profiles will be available at IREC's institutional repository, hosted in Zenodo at https://zenodo.org/communities/irec/.Acknowledgements
This project has received funding from the European Union's Horizon Europe research and innovation programme under grant agreement no. 101069743 (ADVAGEN) and from the European Union's Horizon 2020 research and innovation program under grant agreement no. 824072 (HARVESTORE). This project (PCI2022-132960) has partially been funded by MCIN/AEI/10.13039/501100011033 and the European Union “NextGenerationEU”/PRTR” (AfreeSSB project), the “Generalitat de Catalunya” (2021 SGR 00750, NANOEN, and 2021 SGR 01286) and the CERCA Programme/Generalitat de Catalunya. F. C. acknowledges financial support from Marie Skłodowska-Curie Actions Postdoctoral Fellowship grant (101107093). J.C.G.-R. acknowledges financial support from the Ramón y Cajal Fellowship from MICIU/AEI /10.13039/501100011033 and FSE+ (RYC2023-043274-I). The authors would like to thank the R&D team at HORIBA for their valuable contributions to the development and adaptation of the electrochemical solution integrated with the AFM-Raman system. Special thanks go to Vassily Gavrilyuk for the original design of the electrochemical cell, as well as to Alexey Belyaev and Alexander Yagovkin for their more recent involvement in the mechanical aspects and further modifications to enable optical coupling and EC-TERS applications. We also gratefully acknowledge Patrick Hsia for implementing the bipotentiostat into the electrical design of the AFM's conductive unit, with the support of Sergey Katsur. Finally, we thank Maxime Valay from Origalys for tuning the bipotentiostat to meet EC-TERS requirements and for providing dedicated support.References
- M. Lachal, R. Bouchet, A. Boulineau, S. Surblé, C. Rossignol, F. Alloin and S. Obbade, Solid State Ionics, 2017, 300, 187–194 CrossRef CAS.
- Q. Wang, C. Zhao, X. Hu, J. Wang, S. Ganapathy, S. Eustace, X. Bai, B. Li, H. Li, D. Aurbach and M. Wagemaker, J. Am. Chem. Soc., 2024, 146(6), 31778–31787 CrossRef CAS PubMed.
- T. Defferriere, D. Klotz, J. C. Gonzalez-Rosillo, J. L. M. Rupp and H. L. Tuller, Nat. Mater., 2022, 21, 438–444 CrossRef CAS PubMed.
- F. Chiabrera, I. Garbayo, L. López-Conesa, G. Martín, A. Ruiz-Caridad, M. Walls, L. Ruiz-González, A. Kordatos, M. Núñez, A. Morata, S. Estradé, A. Chroneos, F. Peiró and A. Tarancón, Adv. Mater., 2019, 31, 1805360 Search PubMed.
- A. C. Lazanas and M. I. Prodromidis, Am. Chem. Soc., 2023, 3(3), 162–193 Search PubMed.
- N. Goswami and R. Kant, J. Electroanal. Chem., 2019, 835, 227–238 CrossRef CAS.
- A. L. Lipson, R. S. Ginder and M. C. Hersam, Adv. Mater., 2011, 23, 5613–5617 Search PubMed.
- A. L. Lipson, K. Puntambekar, D. J. Comstock, X. Meng, M. L. Geier, J. W. Elam and M. C. Hersam, Chem. Mater., 2014, 26, 935–940 Search PubMed.
- K. L. Anderson and M. A. Edwards, ACS Meas. Sci. Au, 2025, 5(2), 160–177 CrossRef CAS PubMed.
- S. Jesse, A. Kumar, T. M. Arruda, Y. Kim, S. V. Kalinin and F. Ciucci, MRS Bull., 2012, 37, 651–658 CrossRef CAS.
- J. Yu, S. Duan, B. Huang, H. Jin, S. Xie and J. Li, Small Methods, 2020, 4(10), 2000308 Search PubMed.
- D. O. Alikin, A. V. Ievlev, S. Y. Luchkin, A. P. Turygin, V. Y. Shur, S. V. Kalinin and A. L. Kholkin, Appl. Phys. Lett., 2016, 108(11), 113106 CrossRef.
- J. F. Wu and X. Guo, Phys. Chem. Chem. Phys., 2017, 19, 5880–5887 RSC.
- A. K. Petford-Long and A. N. Chiaramonti, Annu. Rev. Mater. Res., 2008, 38, 559–584 CrossRef CAS.
- L. Yedra, A. Eljarrat, J. M. Rebled, L. López-Conesa, N. Dix, F. Sánchez, S. Estradé and F. Peiró, Nanoscale, 2014, 6, 6646–6650 RSC.
- T. F. Kelly and M. K. Miller, Rev. Sci. Instrum., 2007, 78(3), 031101 CrossRef PubMed.
- B. Gault, A. Chiaramonti, O. Cojocaru-Mirédin, P. Stender, R. Dubosq, C. Freysoldt, S. K. Makineni, T. Li, M. Moody and J. M. Cairney, Nat. Rev. Methods Primers, 2021, 1, 51 CrossRef CAS PubMed.
- F. Baiutti, F. Chiabrera, D. Diercks, A. Cavallaro, L. Yedra, L. López-Conesa, S. Estradé, F. Peiró, A. Morata, A. Aguadero and A. Tarancón, Adv. Mater., 2021, 33(48), 2105622 CrossRef CAS PubMed.
- F. Chiabrera, F. Baiutti, D. Diercks, A. Cavallaro, A. Aguadero, A. Morata and A. Tarancón, J. Mater. Chem. A, 2022, 10, 2228–2234 RSC.
- T. Deckert-Gaudig, A. Taguchi, S. Kawata and V. Deckert, Chem. Soc. Rev., 2017, 46, 4077–4110 RSC.
- D. Kurouski, M. Mattei and R. P. Van Duyne, Nano Lett., 2015, 15, 7956–7962 CrossRef CAS PubMed.
- X. Wang, S. C. Huang, T. X. Huang, H. S. Su, J. H. Zhong, Z. C. Zeng, M. H. Li and B. Ren, Chem. Soc. Rev., 2017, 46, 4020–4041 RSC.
- P. Verma, Chem. Rev., 2017, 117, 6447–6466 CrossRef CAS PubMed.
- X. Wang, S.-C. Huang, S. Hu, S. Yan and B. Ren, Nat. Rev. Phys., 2020, 2, 253–271 CrossRef.
- P. Alonso-González, P. Albella, M. Schnell, J. Chen, F. Huth, A. García-Etxarri, F. Casanova, F. Golmar, L. Arzubiaga, L. E. Hueso, J. Aizpurua and R. Hillenbrand, Nat. Commun., 2012, 3, 684 CrossRef PubMed.
- B. Pettinger, P. Schambach, C. J. Villagómez and N. Scott, Annu. Rev. Phys. Chem., 2012, 63, 379–399 CrossRef CAS PubMed.
- J. C. Gonzalez-Rosillo, M. Guc, M. O. Liedke, M. Butterling, A. G. Attallah, E. Hirschmann, A. Wagner, V. Izquierdo-Roca, F. Baiutti, A. Morata and A. Tarancón, Chem. Mater., 2024, 36, 6144–6153 CrossRef CAS PubMed.
- J. C. Gonzalez-Rosillo, M. Guc, M. O. Liedke, M. Butterling, A. G. Attallah, E. Hirschmann, A. Wagner, V. Izquierdo-Roca, F. Baiutti, A. Morata and A. Tarancón, Chem. Mater., 2024, 36, 6144–6153 CrossRef CAS PubMed.
- Z. Yang, J. Aizpurua and H. Xu, J. Raman Spectrosc., 2009, 40, 1343–1348 CrossRef CAS.
- J. Nanda, G. Yang, T. Hou, D. N. Voylov, X. Li, R. E. Ruther, M. Naguib, K. Persson, G. M. Veith and A. P. Sokolov, Joule, 2019, 3, 2001–2019 CrossRef CAS.
- G. Yang, X. Li, Y. Cheng, M. Wang, D. Ma, A. P. Sokolov, S. V. Kalinin, G. M. Veith and J. Nanda, Nat. Commun., 2021, 12, 578 CrossRef CAS PubMed.
- S. Dinda, S. Trivedi, A. Roy, F. D. Pammer and M. Fichtner, Adv. Energy Mater., 2023, 13(42), 2302176 CrossRef CAS.
- J. Mürter, S. Nowak, E. Hadjixenophontos, Y. Joshi and G. Schmitz, Nano Energy, 2018, 43, 340–350 CrossRef.
- C. Schwab, A. Höweling, A. Windmüller, J. Gonzalez-Julian, S. Möller, J. R. Binder, S. Uhlenbruck, O. Guillon and M. Martin, Phys. Chem. Chem. Phys., 2019, 21, 26066–26076 RSC.
- R. Wang, X. Chen, Z. Huang, J. Yang, F. Liu, M. Chu, T. Liu, C. Wang, W. Zhu, S. Li, S. Li, J. Zheng, J. Chen, L. He, L. Jin, F. Pan and Y. Xiao, Nat. Commun., 2021, 12, 3085 CrossRef CAS PubMed.
- C. M. Julien and M. Massot, Raman spectroscopic studies of lithium manganates with spinel structure, 2003, vol. 15 Search PubMed.
- C. M. Julien, Solid State Ionics, 2006, 177, 11–19 CrossRef CAS.
- M. Fehse, R. Trócoli, E. Ventosa, E. Hernández, A. Sepúlveda, A. Morata and A. Tarancón, ACS Appl. Mater. Interfaces, 2017, 9, 5295–5301 CrossRef CAS PubMed.
- K. Dokko, Q. Shi, I. C. Stefan and D. A. Scherson, J. Phys. Chem. B, 2003, 107, 12549–12554 CrossRef CAS.
- V. Siller, J. C. Gonzalez-Rosillo, M. N. Eroles, F. Baiutti, M. O. Liedke, M. Butterling, A. G. Attallah, E. Hirschmann, A. Wagner, A. Morata and A. Tarancón, ACS Appl. Mater. Interfaces, 2022, 14, 33438–33446 CrossRef CAS PubMed.
- N. Kuwata, Y. Matsuda, T. Okawa, G. Hasegawa, O. Kamishima and J. Kawamura, Solid State Ionics, 2022, 380, 115925 CrossRef CAS.
- J. S. Lee, U. Anselmi-Tamburini, Z. A. Munir and S. Kim, Electrochem. Solid-State Lett., 2006, 9, J34 CrossRef CAS.
- N. J. J. De Klerk and M. Wagemaker, ACS Appl. Energy Mater., 2018, 1, 5609–5618 CAS.
- R. Usiskin and J. Maier, Adv. Energy Mater., 2020, 11(2), 2001455 CrossRef.
- A. E. Bumberger, C. Boehme, J. Ring, S. Raznjevic, Z. Zhang, M. Kubicek and J. Fleig, Chem. Mater., 2023, 35, 5135–5149 CrossRef CAS PubMed.
- N. Kuwata, G. Hasegawa, D. Maeda, N. Ishigaki, T. Miyazaki and J. Kawamura, J. Phys. Chem. C, 2020, 124, 22981–22992 CrossRef CAS.
- C. Erinmwingbovo, V. Siller, M. Nuñez, R. Trócoli, D. Brogioli, A. Tarancón, A. Morata and F. La Mantia, ChemElectroChem, 2023, 202200759, 1–7 Search PubMed.
- J. Halldin Stenlid, P. Žguns, D. Vivona, A. Aggarwal, K. Gordiz, Y. Zhang, S. Pathak, M. Z. Bazant, Y. Shao-Horn, A. Baskin and J. W. Lawson, ACS Energy Lett., 2024, 9, 3608–3617 CrossRef CAS.
- C. Xiao, H. Wang, R. Usiskin, P. A. van Aken and J. Maier, Science, 2024, 386, 407–413 CrossRef CAS PubMed.
- J. Huang, L. Xue, Y. Huang, Y. Jiang, P. Wu, X. Fan and J. Zhu, Nat. Commun., 2024, 15, 6666 CrossRef CAS PubMed.
- P. He, X. Zhang, Y.-G. Wang, L. Cheng and Y.-Y. Xia, J. Electrochem. Soc., 2008, 155, A144 CrossRef CAS.
- I. Bezza, M. Kaus, R. Heinzmann, M. Yavuz, M. Knapp, S. Mangold, S. Doyle, C. P. Grey, H. Ehrenberg, S. Indris and I. Saadoune, J. Phys. Chem. C, 2015, 119, 9016–9024 CrossRef CAS.
- K. Zaghib, M. Dontigny, P. Charest, J. F. Labrecque, A. Guerfi, M. Kopec, A. Mauger, F. Gendron and C. M. Julien, J. Power Sources, 2008, 185, 698–710 CrossRef CAS.
- A. Morata, V. Siller, F. Chiabrera, M. Nuñez, R. Trocoli, M. Stchakovsky, A. Tarancón and A. Tarancón, J. Mater. Chem. A, 2020, 8, 11538–11544 RSC.
- F. Brosa Planella, W. Ai, A. M. Boyce, A. Ghosh, I. Korotkin, S. Sahu, V. Sulzer, R. Timms, T. G. Tranter, M. Zyskin, S. J. Cooper, J. S. Edge, J. M. Foster, M. Marinescu, B. Wu and G. Richardson, Prog. Energy, 2022, 4, 042003 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5eb00094g |
| ‡ Shared first co-authorship. |
| This journal is © The Royal Society of Chemistry 2025 |