 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Key considerations for cell selection in electric vertical take off and landing vehicles: a perspective†
Hamish T.
Reid
a,
Gaurav
Singh
 a,
Emma
Palin
a,
Emma
Palin
 c,
Yuhang
Dai
c,
Yuhang
Dai
 b,
Wei
Zong
b,
Wei
Zong
 b,
Limhi
Somerville
c,
Paul R.
Shearing
bd and
James B.
Robinson
b,
Limhi
Somerville
c,
Paul R.
Shearing
bd and
James B.
Robinson
 *ad
*ad
aAdvanced Propulsion Lab, Marshgate, UCL, London, E20 2AE, UK. E-mail: j.b.robinson@ucl.ac.uk
bThe ZERO Institute, Holywell House, Osney Mead, University of Oxford, Oxford OX2 0ES, UK
cVertical Aerospace, Unit 1 Camwal Court, Chapel Street, Bristol, BS2 0UW, UK
dThe Faraday Institution, Quad One, Becquerel Avenue, Harwell Science and Innovation Campus, Didcot OX11 0RA, UK
First published on 31st January 2025
Abstract
As battery performance has improved in recent years, all-electric aircraft have become a realistic prospect. Passenger electric vertical take-off and landing (eVTOL) vehicles have gained attention recently as a solution for intercity transport, reducing carbon emission, congestion and journey times. However, the performance demands of electrified flight are greater than that of ground-based vehicles, requiring high energy, power and safety characteristics. While electric vehicles typically use cylindrical, pouch and prismatic cells depending on the manufacturers’ needs, it is unclear which form factor is most suited to aerospace applications. This work appraises a range of commercial cells of different formats and their suitability for use in eVTOLs, considering their electrochemical, safety, cell-to-pack integration and future-proofing characteristics. The findings indicate that current prismatic cells lack the power density needed for take-off and landing. While pouch cells offer compelling energy density, there are concerns over their safety performance and ease of pack integration. While the geometry of cylindrical cells makes them difficult to pack and are unlikely to be used for emerging all solid-state chemistries, we believe they currently offer the best balance of safety and performance.
Broader contextSignificant innovation in Li-ion battery materials and cell and pack design has hastened the deployment of batteries in electric vehicles, with these applications now being relatively common. As the technology has matured the potential application of such devices to more demanding fields has increased. Of this aerospace is undoubtedly one of the most technically challenging. This field requires batteries to balance extremely high power and energy density whilst also delivering outstanding safety, lifetime and charging performance. Alongside these critical metrics the cells chosen must fulfil manufacturing and economic requirements. In recent years an emerging market of electric vertical take-off and landing vehicles (eVTOLs) has grown in prominence with multiple companies announcing their intention to develop vehicles in this area. This work assesses the requirements for Li-ion batteries to be deployed as a primary propulsion system in eVTOL applications. This assessment is conducted using currently available cell technology and provides a framework for cell selection for this application, considering all of the key metrics which are required for eVTOL systems. |
Introduction
As the world moves away from fossil fuels, electrification of all forms of transport is necessary to reduce carbon emissions and pollution. Typically, electric vehicles (EVs) are discussed in the context of ground transport electrification, where a battery provides propulsion rather than an internal combustion engine. However, increasingly, the wider electrification of transport, in particular aerospace is attracting attention, with the UK government putting electric vertical take-off and landing (eVTOL) aircraft at the centre of its ‘Future of Flight’ plan.1 Electrified aviation innovations offer opportunities for rapid, low operating cost, emission-free short haul urban and inter-city transport. In a similar manner to EVs, eVTOLs use electric propulsion as their primary power source instead of conventional fossil-fuel powered engines. The vertical take-off ability and comparatively small size of these aircraft is predicted to increase the utilisation of under-used airspace, opening new opportunities for passenger transport. eVTOLs are also flexible in their operational capability, being able to land on flat ground or small helipads, and recharge using infrastructure that is available or straightforward to implement in cities and urban environments.2The pace of eVTOL development has rapidly increased in recent years, with a range of companies announcing plans to develop urban air mobility systems. Predictions estimate that the eVTOL market is forecast to reach over US$ 22 billion by 2030.3 However, the technological and certification requirements for the aeronautical systems are significantly higher than those needed for EV power packs with various obstacles still to be overcome. Expanding charging infrastructure to allow for rapid charging times required for eVTOLs, the economic challenges of making electric flight a cost-worthy alternative to ground-based travel for consumers, and the regulatory issues of certifying electric aircraft are all key areas that will need addressing.4–7 Advances in battery technology and chemistry in the last few years are the critical factor that has made fully electric vertical flight a plausible reality. While several battery manufacturers, including Amprius, Molicel and Northvolt, have begun marketing cells for the eVTOL market, Joby, a major eVTOL technology company, have publicly disclosed the use of batteries manufactured for EV applications due to their reliable service record.8,9
As with all aerospace applications the mass and safety of an eVTOL aircraft are amongst the most significant considerations, with safety in particularly regulated to a greater extent than automotive or other, more conventional applications.4,10–12 The additional requirements for vertical take-off and landing also introduce challenges in delivering the required power for eVTOL systems during these mission phases, often requiring cells to deliver high power during both high and comparatively low states of charge. Perhaps most challengingly, the fast turnaround times between landing and take-off require active thermal management to keep the battery within it's safe operating range. Balancing both the pack infrastructure and lightweight mass requirements of the aircraft poses a major technical challenge. Each unit of mass limits the range of the aircraft and increases recharging time.13
Here we compare a range of commercially available cell geometries and chemistries to assess their viability in a primary propulsion system in an eVTOL application. Initially considering performance at cell level we also consider the challenges associated with pack integration and manufacturability to enable a comprehensive overview of the potential of the cell types. By focussing on these wide-ranging technical hurdles involved in the production of an energy storage system that can meet the significant energy density, safety, and power demands of aerospace applications we aim to support the nascent literature in this area and encourage the development of cell characterisation activity to accelerate the deployment of eVTOL applications in the field.
Summary of cell types considered
Cylindrical (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 650 vs. 21
650 vs. 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 700 vs. 4680)
700 vs. 4680)
Cylindrical cells were one of the first formats commercially adopted due to their high mechanical stability. Current collectors double-side coated with anode and cathode materials are wound together with a separator in between. The ‘jellyroll’ formed by the winding process is placed into a steel can, with positive interior tabs connected to the top cap and negative interior tabs connected to the bottom. Typically, the whole of the bare exterior of the cell can is electrically connected to the negative electrode. The top cap is crimped onto the can containing the jellyroll, with the cap insulated from the rest of the cell body by a plastic disk. Heat-shrink wrapping is then used to insulate the rest of cell from exterior shorting, although some pack manufacturers may choose to remove the sleeve to reduce mass.
Cylindrical cells come in several standardised sizes. The sizes of cylindrical cells are denoted by their diameter and height in millimetres. For example, the 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 cell is so-called because it is 18 mm in diameter and 65 mm in height. The most used sizes are 18
650 cell is so-called because it is 18 mm in diameter and 65 mm in height. The most used sizes are 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 and 21
650 and 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700; however, more recently Tesla and BMW have begun incorporating a 4680 design into their products. These cells have a relatively high cell wall thickness at 500 μm which reduces the overall energy density of the cells, but also adds to the mechanical stability of the cell during failure events.14
700; however, more recently Tesla and BMW have begun incorporating a 4680 design into their products. These cells have a relatively high cell wall thickness at 500 μm which reduces the overall energy density of the cells, but also adds to the mechanical stability of the cell during failure events.14
Cylindrical cells’ key advantages are their mechanical stability, where they can be more easily incorporated into packs and modules without the need for significant structural packaging. The steel can also give cells a degree of protection from impact or exterior damage. Most pressingly for aerospace applications the safety profile of cylindrical cells has been extensively studied with a range of failure modes identified.15
Pouch
Pouch cells involve either winding or stacking electrode and separator layers together before vacuum sealing in flexible aluminium laminate sheeting. Pouch cells offer flexibility in both physical size and capacity, ranging from credit card sized cell with capacities of circa. 3 Ah for mobile devices, to automotive cells such as Farasis P79B3 (295 × 115.5 × 13 mm) with a capacity of almost 80 Ah.Pouch cells are typically cuboid in shape, but their specific dimensions can depend on the application and space availability. This flexibility is one of the key advantages of the format. Another advantage is the low weight of the inactive packaging material relative to the rest of the cell. The lightweight packaging also allows for greater heat dissipation relative to cylindrical cells. As discussed later, pouch cells can achieve by far the highest energy densities at the cell level. However, unlike cylindrical and prismatic cells, pouch cells do not typically have integrated safety devices, such as positive temperature coefficient devices (PTCs) or current interruption devices (CIDs). Their lack of mechanical strength also means more consideration needs to be taken during the pack building stage, leading to a greater amount of inactive material at the application level. The flexible aluminium laminate packaging does little to protect cells from propagation during a thermal runaway event, raising concerns over safety when applied to eVTOL applications.
Prismatic
The prismatic cell format is gaining increasing prominence in the EV industry as manufacturers move towards a cell-to-pack type integrated propulsion unit and adopt the LiFePO4 (LFP) chemistry for their applications. This involves cells of significant capacity (>50 Ah) housed in a hard, metal casing. The electrodes in the cell are generally stacked rather than would, and as such the manufacturing costs of these cells are typically higher. Unlike their cylindrical equivalents prismatic cell manufacturers are yet to align upon a set of standardised sizes with cells as diverse as the EVE LFK22 (131.8 × 148.2 × 17.7 mm) to the BYD Blade (960 × 13.5 × 90 mm) seen in the market.The solid casing of the cell enables safety devices to be implemented, including directionally guided venting should a safety event occur.16 Despite the solid housing, external pressure is still typically required to maximise cell performance. Naturally this results in a reduced gravimetric cell to pack ratio hampering the potential deployment of this cell format in as weight sensitive an application as aviation. Prismatic cells are also typically produced for much higher capacities per cell than pouch or cylindrical, raising possible safety issues and difficulty in containing thermal runaway in the event of a single cell failure, which may in itself be catastrophic.
Candidate cells
In this work, we compare a range of cells from different manufacturers, form factors and chemistries. The cells chosen for this work are based on their high energy density, power density or, in the case of the BYD Blade battery, safety features, which provide, on at least one of these critical metrics, the potential for deployment in eVTOL applications.When considering the cylindrical format, Molicel have produced cells with some of the highest power densities available. The P28A and P45B models, built in 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 and 21
650 and 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 cylindrical formats respectively, are both capable of high discharge rates matched with a high gravimetric energy density when compared to alternative cells capable of delivering similar power. The Murata VTC6A has also been earmarked for aerospace applications, has been used in other eVTOL studies, and is capable of high discharge power.17,18 Finally, the new 4680 format, adopted by EV manufacturer Tesla is likely to be an attractive format for automotive applications once manufacturing capabilities mature. In particular, the ‘tabless’ design and innovative safety features, such as the directed vent, may offer compelling cases for eVTOL applications; however, the limited power density reported to date is likely to prevent the deployment of this cell format in eVTOLs. 4680s have a greater circumference than both 21
700 cylindrical formats respectively, are both capable of high discharge rates matched with a high gravimetric energy density when compared to alternative cells capable of delivering similar power. The Murata VTC6A has also been earmarked for aerospace applications, has been used in other eVTOL studies, and is capable of high discharge power.17,18 Finally, the new 4680 format, adopted by EV manufacturer Tesla is likely to be an attractive format for automotive applications once manufacturing capabilities mature. In particular, the ‘tabless’ design and innovative safety features, such as the directed vent, may offer compelling cases for eVTOL applications; however, the limited power density reported to date is likely to prevent the deployment of this cell format in eVTOLs. 4680s have a greater circumference than both 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700s and 18
700s and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650s, reducing their capacity to dissipate heat. The 4680 cells with data publicly available are only rated to a peak discharge of 0.84 kW kg−1 which is the lowest of the candidate cells reported here. As this format has only recently been put into widespread production, the discharge rating is expected to increase in the coming years.
650s, reducing their capacity to dissipate heat. The 4680 cells with data publicly available are only rated to a peak discharge of 0.84 kW kg−1 which is the lowest of the candidate cells reported here. As this format has only recently been put into widespread production, the discharge rating is expected to increase in the coming years.
For pouch cells, three different sizes and manufacturers have been chosen for this work. Both Northvolt and Ionblox have specifically introduced their cells as appropriate for passenger urban aircraft on account of their high power and energy densities. Lilium, a German aerospace company, have been using Ionblox cells to power their aircraft for several years. Amprius have also extensively marketed their cells for eVTOLs. However, data on Amprius cells is currently not publicly available, and Amprius did not respond to a request for information. The large LG E66A cell is used for high-performance EVs including the Porsche Taycan. The E66A is capable of high energy and power density, and the larger size means there is a lower ratio of packaging weight to active material. Farasis Energy have also expressed an interest in moving into the eVTOL space, and the P79B3 represents one of their best performing EV cells. Larger pouch cells typically display significant thermal gradients within the cell which, alongside their requirement for external pressure, will require a more complex pack design when deployed in high-power aerospace applications.
The BYD Blade prismatic format cell has been praised for its safety features and cell-to-pack integration. However, the LFP chemistry that gives the Blade cell it's promising safety performance also means it has a comparatively low energy density. Like the LG E66A pouch cell, the Samsung CS1200R prismatic is used in a high-performance EV, the BMW i3. Finally, Farasis have recently expressed an interest to enter the eVTOL space, with the P79B3 being the highest performing cell with information publicly available. Once again large aspect ratio prismatic cells are likely to require more complex thermal management systems and pack design to account for the thermal gradients which arise during high-rate operation.
A summary of the cells considered in this work are shown in Table 1 below, with these cells all featuring at least one outstanding characteristic for the cell format. Cells which have sufficient information publicly disclosed information have been assessed; however, it is likely that there are next generation prototype cells or pre-production cells which can offer improvements on the existing state-of-the-art. While this selection of cells represents a cross section of cell types that initially appeared to have the most appropriate performance, it is not a completely comprehensive list of all the cells and manufacturers available.
Cell requirements for eVTOL applications
As highlighted previously, eVTOL applications are amongst the most challenging environments for battery technologies. The aircraft requires a combination of high gravimetric energy and power density to be delivered simultaneously and must facilitate sufficiently rapid heat rejection to ensure the cell remains below the upper safe temperature limit even when delivering ultra-high rates during the take-off and landing phase of the flight. In addition to the technological requirements, to enable certification the cells must be extremely safe and resistant to failure propagation within the pack in the event of a thermal event. Beyond these three prerequisites for consideration, cells must also maintain these characteristics for an adequate cycle life to enable the economic deployment of the propulsion system in the aircraft. The economic considerations also likely require cells to be charged quickly to enable rapid turn-around of the vehicle, where cells may be charged at elevated temperatures to increase total utilisation of the aircraft. Furthermore, cells are also likely to experience variable external temperatures, with the expected temperature gradient of up to 6.5 °C km−1 while operating across a wide range of geographic regions.19 Finally, the wider cost of the cells, manufacturability of cells into packs and the potential reuse of cells in second-life applications should also be considered when identifying potential cells for eVTOL applications. This consideration should also be extended toward recycling due to impending legislative targets for recycled components within battery packs.20One of the key considerations for any aerospace grade lithium-ion cell is certification. The only cell specific means of compliance is ED-312 ‘Guidance on determining failure modes in Lithium-ion cells, this is a relatively new standard being released in 2023’. The most well-known and applied lithium-ion battery standard is the Radio Technical Commission for Aeronautics (RTCA) DO-311A. In most jurisdictions including the Federal Aviation Administration and the European Union Aviation Safety Agency, this standard must be followed prior to certification of lithium-ion battery systems for auxiliary power on an aircraft.21 Whilst this standard could not be applied directly to all battery systems because the largest category is energy systems >100 W h and is not used for critical applications, there is one part that is being applied in other jurisdictions, namely the means of dealing with a thermal runaway onboard the aircraft during flight. At present, under Appendix-C this standard requires two cells to be simultaneously ignited to initiate the thermal runaway condition.22 If we assume that approximately 2 L of gas is released per Ah of a cell during thermal runaway, then this requirement alone drives a significant impact on design considerations for the cell.23 If a large format automotive cell were used, then the gas release could be 136–272 L of gas simultaneously. Conversely a 5 Ah 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 cell used in automotive applications being deployed would reduce this gas volume to approximately 20 L. Given that containment and release only through a designated vent is required by this standard this is a significant factor in cell selection because it drives two different designs. Large format with CTP integration, such as those applied in automotive, are significantly hindered by this requirement as they would require containing very high pressures over large volumes resulting in increased packaging mass to provide sufficient housing strength. Even for relatively modest cells of 10–20 Ah this requirement can be difficult to meet. Current battery designs with such cells and complying with this requirement require smaller format packs containing a reduced number of lithium-ion cells allowing higher the containment of maximum pack whilst remaining contained. Other safety standards at pack level, such as those required by CS-27 require that a propulsion battery be able to sustain a drop from 50 feet.24 Whilst onerous, it is likely that pack housings will provide much of the required strength limiting, this standards impact on the cell choice.
700 cell used in automotive applications being deployed would reduce this gas volume to approximately 20 L. Given that containment and release only through a designated vent is required by this standard this is a significant factor in cell selection because it drives two different designs. Large format with CTP integration, such as those applied in automotive, are significantly hindered by this requirement as they would require containing very high pressures over large volumes resulting in increased packaging mass to provide sufficient housing strength. Even for relatively modest cells of 10–20 Ah this requirement can be difficult to meet. Current battery designs with such cells and complying with this requirement require smaller format packs containing a reduced number of lithium-ion cells allowing higher the containment of maximum pack whilst remaining contained. Other safety standards at pack level, such as those required by CS-27 require that a propulsion battery be able to sustain a drop from 50 feet.24 Whilst onerous, it is likely that pack housings will provide much of the required strength limiting, this standards impact on the cell choice.
Energy density
More so than land-based applications, aerospace applications place a high premium on gravimetric energy density as every kilogram of battery used to power the aircraft must also be lifted and transported by the powertrain. Unlike EVs, eVTOLs also have a requirement for redundant energy should the aircraft need to be diverted from the original flight path, known as baulking. This makes the ‘usable’ energy density is also important. In eVTOLs, the battery will be required to supply high currents from a low state-of-charge for emergency landings. In addition, eVTOL applications do not reduce in mass as fuel is consumed, this requires a modified approach to energy reserve management when compared to conventional take-off-and-landing vehicles. Fig. 1 shows the effect of loading on operation range for aircraft with varying performance.25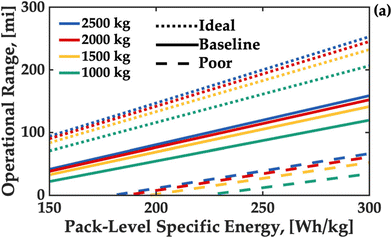 | ||
| Fig. 1 Cruise ranges for single passenger eVTOL based on varying tonnage and pack-level energy density. Adapted with permission from Fredericks et al.25 Copyright 2018 American Chemical Society. | ||
At the cell level, pouch cells generally have the highest energy density due to the lighter packaging materials. The steel can and vent cap of cylindrical cells adds inactive mass that reduces the overall energy density. However, when scaling up to the pack level, cylindrical cells require the least amount of additional pack casing mass as much of the mechanical strength is self-contained by the form factor. Of the three cylindrical cell formats discussed in this work, the 4680 and 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 design have the highest energy density. Surprisingly, the energy density of the 4680 is no better than the Molicel P45B 21
700 design have the highest energy density. Surprisingly, the energy density of the 4680 is no better than the Molicel P45B 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 cell. This is likely due to the extra safety features required in the larger 4680 cells. It is also important to note that data from the 4680 is confined to teardowns of early-stage production cells which have a comparatively immature design compared to 21
700 cell. This is likely due to the extra safety features required in the larger 4680 cells. It is also important to note that data from the 4680 is confined to teardowns of early-stage production cells which have a comparatively immature design compared to 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 cells. It is expected that the energy density and performance will improve in the coming years as Tesla scale-up and optimise production of this cell format.
700 cells. It is expected that the energy density and performance will improve in the coming years as Tesla scale-up and optimise production of this cell format.
The pouch cells analysed here have by a significant margin the highest energy density at the cell level, with Northvolt and Ionblox gearing their latest generation of cells towards eVTOL applications. However, although the energy densities advertised are attractive, pouch cells require the highest additional packing mass, including plates to apply pressure and supports for mechanical stability reducing the gravimetric cell to pack ratio. The two high-energy density pouch cells included in this work have relatively low capacity per cell, 5.1 and 12 Ah, when compared to the 65 Ah for the LG automotive cell. This reduced size of cells will further decrease the gravimetric cell-to-pack (GCTP) ratio due to the increased mass of cell packaging and pack componentry.
Prismatic cells provide a compromise, as the hard casing provides pressure to the electrode layers, but still requires significant extra packing material to provide mechanical stability. However, of the candidate cells discussed here, the prismatic cells have by far the lowest energy densities. For the EVE and BYD cells, this is due to the LFP chemistry which currently delivers a substantially lower energy density than NMC or NCA chemistries, but benefits from possible superior safety characteristics.26,27 The Samsung CS1200R does have a conventional Li-ion chemistry, and of the prismatic cells is closest to matching the other formats. However, at 205 W h kg−1 at the cell level, this is still too low for eVTOLs, especially when considering to the extra materials required for integrating into a working pack.
The >300 W h kg−1 energy density shown by the small pouch cells mark this format as a compelling form factor when simply considering energy density. However, despite the high packing density that can be achieved (Table 1) the loss of energy density due to pack integration and cooling will significantly impact the gravimetric cell to pack ratio of pouch cells reducing the benefits of this cell type when compared to cylindrical and prismatic formats.
Power density
Alongside energy density, power density is of major importance for eVTOLs. A typical eVTOL mission profile is broken down into the following phases: take-off, climb, cruise, descent, and landing. There may also be an additional baulking phase if the aircraft must undertake an emergency diversion, shown in (Fig. 2). The take-off and landing phases have the highest power requirements, with Yang et al. estimating a system-level range between 500–900 W kg−1.5 Several factors influence the ability of a cell to discharge energy quickly: chemistry, active material microstructure, electrode architecture, current collector, the number of tabs and their thicknesses.28–31 Of particular importance is thermal management, which is considered alongside safety later. Unfortunately, many features which improve power output are detrimental to energy density.28,30,32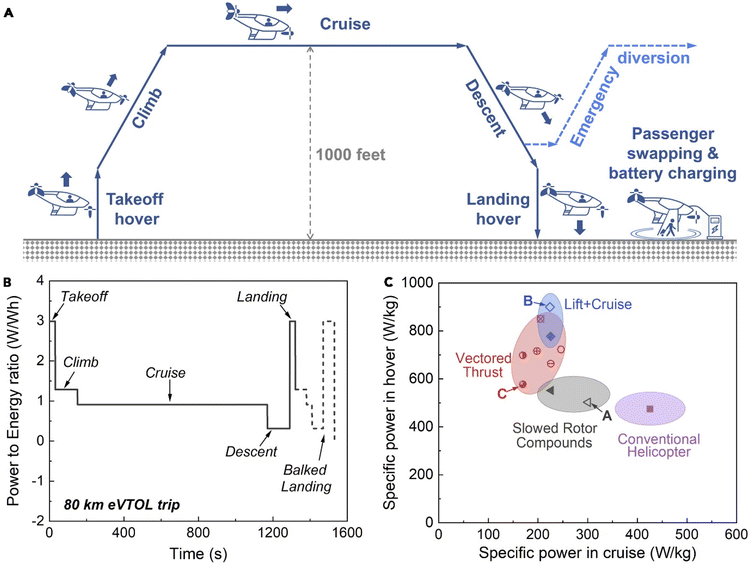 | ||
| Fig. 2 (A) Schematic illustration of a typical eVTOL trip.35 (B) Representative battery power profile during an eVTOL trip. (C) Required battery specific power in hover versus in cruise for the aircraft configurations being pursued by the industry. Adapted with permission from Yang et al.5 Copyright 2018 Elsevier. | ||
Of the cylindrical cells considered, the 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650s and 21
650s and 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700s offer the highest power capabilities, with calculated power densities of >2.2 kW kg−1. These cells are made with thick, multi-tab designs to reduce resistance and better distribute current and heat throughout the cell. How a manufacturer chooses to incorporate tabs into the cell is a key factor in the power density, as the conductivity and subsequent heat build-up at high currents can act as a limit on the rate of charge or discharge.30,33 The 4680 is pulse discharge power of 0.84 kW kg−1 and is insufficient for a typical eVTOL mission profile. The low current rating is likely due to the lower radial thermal diffusion and by extension heat rejection of this cell format, where the 4680s low volume specific surface area makes cooling challenging. However, key to the 4680s ability to function at all is its innovative tabless design. Rather than use the traditional tab strips spot welded onto specific points of uncoated current collector, the Tesla 4680 uses uncoated areas of current collector at the end of the electrode strips.14 This design gives improved heat and electrical conduction over the traditional welded tabs. At present, this still isn't enough to make the 4680 a feasible choice for eVTOLs. However, in the future, the tabless design could be incorporated into smaller cells or improved to make 4680s suitable for aerospace. Further it is likely that cooling strategies, alongside the direct conduction pathways through the cell provided by the current collectors will increase the potential power density of this cell.
700s offer the highest power capabilities, with calculated power densities of >2.2 kW kg−1. These cells are made with thick, multi-tab designs to reduce resistance and better distribute current and heat throughout the cell. How a manufacturer chooses to incorporate tabs into the cell is a key factor in the power density, as the conductivity and subsequent heat build-up at high currents can act as a limit on the rate of charge or discharge.30,33 The 4680 is pulse discharge power of 0.84 kW kg−1 and is insufficient for a typical eVTOL mission profile. The low current rating is likely due to the lower radial thermal diffusion and by extension heat rejection of this cell format, where the 4680s low volume specific surface area makes cooling challenging. However, key to the 4680s ability to function at all is its innovative tabless design. Rather than use the traditional tab strips spot welded onto specific points of uncoated current collector, the Tesla 4680 uses uncoated areas of current collector at the end of the electrode strips.14 This design gives improved heat and electrical conduction over the traditional welded tabs. At present, this still isn't enough to make the 4680 a feasible choice for eVTOLs. However, in the future, the tabless design could be incorporated into smaller cells or improved to make 4680s suitable for aerospace. Further it is likely that cooling strategies, alongside the direct conduction pathways through the cell provided by the current collectors will increase the potential power density of this cell.
The pouch cells here present maximum discharge rates of 1.7 kW kg−1 for the Northvolt-Cuberg and 1.58 kW kg−1 for the Ionblox cells. Despite their higher gravimetric energy density, this is lower than the maximum discharge rates available for the Molicel cylindrical cells. This reduced power performance is likely to result from the manufacturers’ desire to maximise energy density. As mentioned previously, there are number of design choices that manufacturers make to determine the trade-off between power and energy density in their cells. For example, thicker electrodes with a higher coating loading can increase the cell's energy density.34 However, this reduces Li diffusion and power capabilities. Thicker, heavier tabs and current collectors are also necessary for cells to be able to handle higher currents, which have a negative impact on gravimetric energy density. It is likely therefore that the cells examined in this work have been designed for optimised energy density at the cost of power. As with energy density, the values presented here are at the cell level. Pouch cells will require the largest amount of pack infrastructure before integration into the aircraft, meaning the pack-scale power density will be markedly lower.
On the prismatic side: at 0.33 kW kg−1 the BYD Blade cell has a power density too low for eVTOL operation. This is a product of the battery chemistry, LFP, and the low output voltage rather than the cell format. The Samsung CS1200R is much closer to the necessary power density at 1.09 kW kg−1. Similarly, the EVE LF105 cell has a greater power density than the BYD Blade, however, this is still less than half that of the best performing cylindrical cells, while still requiring a degree of reinforcement before integrating in the final pack for application use, further reducing performance. The prismatic cells here are designed for use in EVs, whereas some of the cylindrical and pouch cells are specifically geared towards aerospace. Their overall poor comparative performance is likely due to the requirements that they are designed for.
Overall, the cylindrical and pouch cells show the most promising electrochemical properties. Fig. 3 summarises the power and energy attributes of the cells discussed here.
 | ||
| Fig. 3 Ragone plot showing the specific energy density and specific power density of the cells examined in this work. | ||
Charging rate
As well as maximising energy and power density during discharge, the output of eVTOL cells, fast charging is paramount for practical as well as economic reasons. Mission profiles for eVTOLs require charge times of approximately 10 minutes.4 The same attributes that improve power density (thin electrodes, effective thermal management) also improve charging times. However, unlike discharging, charging too quickly can lead to Li plating on the graphite negative electrode.36–38 Alongside rapid degradation, Li plating and dendrite growth can lead to major safety concerns and internal shorting, as well as reducing the safe operating temperature of the cell.39 Several methods have been developed to detect and monitor Li plating during operation which could be incorporated into a pack. Common electrochemical methods include using electrochemical impedance spectroscopy (EIS), differential voltage (DV) and voltage plateau analysis to seek characteristic features in the charge and discharge profiles of the cells that indicate Li plating.40–47 Alternative approaches include those developed by Bommier et al. who have presented a direct monitoring approach for Li plating using acoustic measurements, while Huang et al. have used pressure measurements observe plating in cells. Both of these methods could provide additional monitoring signals for advanced battery management systems (BMS); however to date they have only been demonstrated on single pouch cells rather than modules/packs and would add further mass to the battery pack.48,49 To mitigate plating, the current available options are to increase the charging time or preheat the battery pack. While slowing the charge time presents economic issues by limiting the number of flights an aircraft could perform per day, the high discharge loads, and rapid turnaround times of eVTOLs subjected to fast charging, mean the battery is likely to already be at elevated temperatures negating the need for preheating. Indeed, keeping the battery cool during fast charging is likely to be a more significant challenge than Li plating. Furthermore, the ability of cells to charge quickly is predominantly a function of the battery chemistry, particularly the negative electrode and electrolyte, rather than the cell format. However, as with power density, heat generation is also a significant factor, which is affected by cell format.Again, the cylindrical cells show impressive charging rates, ranging from 2–3C, which translates to 20–30 minutes. Finding appropriate data for the Northvolt Cuberg cells was challenging, with data only available for a C/2 charging rate. Cells, which employ a lithium metal anode, suffer from a tendency to grow dendrites when operated at high areal current densities.50,51 This effect also poses integration challenges as maintaining pressure is even more vital to the operation of the cell. The charging rate may be also need limited to ensure a usable life span. Although finding data was also difficult, Ionblox claim they can charge from 10% to 80% capacity in 10 minutes, translating to roughly 6C charge. This is very fast and would be ideal for the quick turnaround times necessary for eVTOL applications. From a cell format perspective, this is a promising development and shows that with sufficiently advanced thermal management system pouch cells may be able to meet the charging requirements of electric-powered flight.
Cycle life
As discussed previously, for eVTOLs to be practical the cells that power them must be pushed to their operational limits. Both high charge and discharge rates will hamper the number of cycles that eVTOL batteries can perform before reaching the end of life. Fast charging will encourage lithium plating, compromising the safety characteristics and accelerating when aged batteries need to be decommissioned.36,37 Also of concern is the use of silicon negative electrode active material. Silicon has received significant attention in the literature and considerable effort is being made to increase the amount of silicon in batteries.52–54 Silicon in the negative electrode increases the capacity of cells, improving both the volumetric and gravimetric energy density, which are vital for several applications, and especially eVTOLs. However, irreversible changes to the microstructure of silicon particles limits their cycle life.55–57 Combined with extreme charge and discharge rates, eVTOL batteries are unlikely to last the minimum of 1000 duty cycles that are expected of their EV equivalents. Ionblox have championed the use of silicon anodes, having achieved over 700 cycles, albeit using a relatively slow 1C charge and discharge rate. This is likely to be significantly lower when undergoing eVTOL mission cycles, which will exacerbate the swelling and exfoliation issues commonly found in silicon anode materials.56 Likewise, for the Northvolt Cuberg cells, publicly available information gives approximately 670 cycles with a C/2 charge time. In comparison, the cylindrical cells show similar cycle life, with circa. 85% capacity remaining after 500 cycles.It is also important to note that eVTOL cells are unlikely to be used up to the 80% capacity loss limit set by the EV industry. The power requirements for aviation are much higher, and so packs are more likely to be retired once they reach approximately 90% of their original capacity to account for the more rapid power fade at high operating rates because of resistance growth during degradation. The short lifetime of eVTOL battery packs and necessity for second-life applications puts even greater emphasis on safety.
Safety
There have been several high-profile incidents in EVs where batteries have gone into thermal runaway.58–61 Battery safety remains a highly active area of research, as fire or explosion in even the most prosaic of applications is likely to have significant consequences for both the system and users or those adjacent to the event.15 In eVTOLs, the importance of safety is even greater due to an inability to evacuate a vehicle in an easy manner. Currently, the Radio Technical Commission for Aeronautics’ (RTCA's) DO-311A is used by regulatory bodies to outline the safety requirements of cells and battery packs before they are permissible for use on aircraft.22 Sripad et al. have published a comprehensive critical analysis of the DO-311A.6 The tests that packs are required to pass include drop testing, over-discharge, external short-circuit, rapid discharging, and operation in hot or cold temperatures. In large-scale battery packs used in all-electric eVTOLs, the key test that packs are required to pass is in Appendix C, where two cells in the pack are taken to thermal runaway either by overcharging or a thermal trigger (heating) as shown in Fig. 4.22 During this test, the pack must not “release any fragments outside of the battery system” or allow “escape of flames out of the battery system, except through designed venting provisions”. This effectively means that the pack must have suitable resistance to cell-to-cell thermal runaway propagation. The domino effect of one failing cell causing an adjacent cell to also go into thermal runaway is highly likely to fail the DO-311A regulations. Therefore, considering the safety requirements for cells it is also important to consider the resistance to propagation. In this case, pouch cells have the lowest resistance due to the nature of the housing used. Given the requirements of the DO-311A regulations it is unlikely that a pouch cell format would pass the safety testing for eVTOL applications. Given the high likelihood of propagation between cell formats in the event of a thermal failure on a safety basis alone pouch cells using Li-ion chemistries with conventional separators and safety components should be discounted for eVTOL and aerospace applications. With sufficient safety architecture installed to prevent cell-to-cell propagation, a pouch cell-based design may be able to pass the DO-311A tests, but the wider penalty on both gravimetric power and energy densities would likely make these cells unsuitable for eVTOL aircraft (Fig. 4).The steel casings of cylindrical cells make them the most resistant to cell propagation and are best placed to pass the necessary safety regulations. The inherent mechanical stability provided by the design also makes it best placed to pass drop testing as well. Previous work has shown that even when 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 cylindrical cells are placed flush together, the likelihood of propagation in a nail penetration test is approximately 50%.62 If the cylindrical cells used show a likelihood of this sidewall failure over typical ejection through the cap, there may be a need for extra safety measures. From a pouch perspective: the light, thin casing that gives high energy and power densities make them more vulnerable to propagation. Although the quick heat dissipation of pouch cell may counteract the lack of protection, safety still represents a real challenge without significant weight or space committed to stopping propagation. Furthermore, while silicon content in cylindrical cells is limited due to the mechanical stressing during manufacture, the cutting-edge pouch cells appraised in this work contain either silicon or lithium metal negative electrodes. Silicon as part of the negative electrode coating may have a detrimental effect on the safety performance, increasing the likelihood of thermal runaway and the severity if it does happen.63 Similarly, heat dissipating materials, such as aerogels, can be placed between cells in a pack to reduce the likelihood of propagation by increase heat dissipation efficiency. However, this increases the mass and volume of the system, decreasing the energy and power densities. Prismatic cells sit between the two, with the aluminium casing providing some protection from external damage and propagation, but not as much as in cylindrical cells. Many prismatic cells use an LFP chemistry, which can give improved safety characteristics, but this is a product of the choice of materials in manufacture rather than the form factor.
700 cylindrical cells are placed flush together, the likelihood of propagation in a nail penetration test is approximately 50%.62 If the cylindrical cells used show a likelihood of this sidewall failure over typical ejection through the cap, there may be a need for extra safety measures. From a pouch perspective: the light, thin casing that gives high energy and power densities make them more vulnerable to propagation. Although the quick heat dissipation of pouch cell may counteract the lack of protection, safety still represents a real challenge without significant weight or space committed to stopping propagation. Furthermore, while silicon content in cylindrical cells is limited due to the mechanical stressing during manufacture, the cutting-edge pouch cells appraised in this work contain either silicon or lithium metal negative electrodes. Silicon as part of the negative electrode coating may have a detrimental effect on the safety performance, increasing the likelihood of thermal runaway and the severity if it does happen.63 Similarly, heat dissipating materials, such as aerogels, can be placed between cells in a pack to reduce the likelihood of propagation by increase heat dissipation efficiency. However, this increases the mass and volume of the system, decreasing the energy and power densities. Prismatic cells sit between the two, with the aluminium casing providing some protection from external damage and propagation, but not as much as in cylindrical cells. Many prismatic cells use an LFP chemistry, which can give improved safety characteristics, but this is a product of the choice of materials in manufacture rather than the form factor.
Some of the safety implications of any of the cells considered here could be offset by charging the pack separately from the aircraft, where the pack is detached and removed to a protected location. This way, the temperature could be more closely checked, and diagnostic methods, such as acoustic monitoring, could be integrated to determine the state-of-health of the pack.48,64 However, this would present a considerable engineering challenge to make the packs easily accessible and detachable to keep turnaround times to a minimum. Furthermore, extra manpower would be required on landing to handle and remove heavy battery packs. Therefore, this method is not practical. Battery management system and thermocouple temperature monitoring and currently considered appropriate for determining the SOH of the packs, and when they need to be removed and replaced.
Cost and manufacturability
Currently, battery manufacturing costs are dominated by factors that are universal regardless of format. The price of cell casings, laminated aluminium or steel cans, are relatively cheap compared to other parts of the cell, so have a limited effect on the end price.65–71 After material costs, machine depreciation and labour are the major components contributing to cost. Costs can also be affected by the country of production, production volumes, and fluctuations in commodity material prices. At present, there are slightly higher startup costs for a pouch cell manufacturing line than for prismatic and cylindrical, with a Fraunhofer study by Neef et al. proposing a status quo of cell prices: cylindrical > prismatic > pouch.66,67 However, several studies, believe that in time pouch cells will become the cheapest format. The flexibility of sizes offered by pouch and prismatic cells also give manufacturers the option to increase cell volume for the cost benefit of reducing inactive material.66 On the other hand, analysis from Börner et al. shows cylindrical manufacturers can find cost and performance benefits in moving to a tabless design.72Comparing the relative costs of the candidate cells considered here is very difficult, the cylindrical cells are all available off-the-shelf and benefit from the economies of scale from mass manufacture. The LG E66A, Farasis P79B3 and the prismatic cells can be bought from overstock dealers or second-hand but are not free for purchase by the public in the same way. The Cuberg and Ionblox pouch cells are effectively bespoke, available only directly from the manufacturer or technology-transfer deals with current users. Furthermore, while cost sensitivity is important considering the short lifetimes of eVTOL packs, the cost of electric-powered aircraft will include expensive materials, specialty parts, maintenance, infrastructure development, and piloting. Furthermore, given the relatively early retirement criteria for eVTOL applications, with cells retaining no less than 90% of their initial capacity, alongside the need to ensure only high-quality cells are integrated into the propulsion battery pack it is likely that second life deployment will be leveraged for these systems. The sum of these extra costs makes the battery cost a tertiary concern against safety and electrochemical performance, as opposed to the EV industry where driving down costs is a key aim.
Pack integration
As discussed, when considering each cell format for transport applications, it is important to factor the pack level attributes as well as the cell level properties. Cylindrical cells are at an advantage here, in that the steel casing provides protection against thermal runaway, integrated safety features, compression on the electrode layers, resistance to expansion and delamination, and mechanical stability. However, suffers from low packing density and the need to weld each individual cell Prismatic cells share some of these features: integrated safety mechanisms and some pressure distribution on the electrode layers. Pouch cells require backing plates to provide compression to the layers, are prone to delamination, and uneven pressure distribution, and need protection from thermal runaway propagation. However, the larger surface area of pouch cells helps keep pouch cells cool through enhanced heat dissipation. This is key to reaching the high discharge currents required of eVTOL batteries. At the same time, slim cells with a large footprint demand larger plates to apply pressure, as well as reducing overall packing efficiency.73 The dense cylindrical cells have poorer heat dissipation characteristics and must be stopped from charging or discharging once they reach ca. 80 °C. As shown in Table 1, packing density is theoretically higher than pouch or prismatic cells when pack infrastructure is not considered.Chemistry and futureproofing
The cells considered in this work all make use of commercialised (Li-ion, LFP) and semi-commercialised (rechargeable Li-metal, ultrahigh silicon) chemistries and materials. While the cell manufacturers rarely disclose the specific of the chemical makeup of the different cell components, it is important to consider the impact of chemistry on the suitability of cell for eVTOL applications. High-nickel layered transition metal oxide compounds such as Li(NixMnyCoz)O2 (NMC) and Li(NixMnyAlz)O2 (NCA) are common positive electrode choices for high-energy cells, with a cell-level capacity of <200 mA h g−1 reported. NCA and NMC are also mature technologies that have been used for well-over a decade, with both produced at scale for automotive applications. By increasing the relative amount of Ni, Ni-rich positive electrodes have been able to offer both the power and energy density required for eVTOLs. However, the increase in Ni content has the knock-on effect of reducing cycle life and thermal stability,28,74 which results in a need to balance elevated power and energy densities with the need for more conservative cell and pack designs to maintain safety. Along with NMC and NCA, LFP is also commonly used in the automotive industry. Often deployed in prismatic formats, for instance the Eve LF105 and BYD Blade cells discussed in this work, LFP has the advantages of superior safety and lower cost when compared to layered transition metal oxides.75–77 Unfortunately, as demonstrated here, the moderate energy and power density (capacity of approximately 170 mA h g−1) of LFP cells makes them unlikely to be suitable.78 More recently, LMFP compounds have shown improved energy density performance and, with significant research effort, may eventually be used in eVTOLs.79 Li-rich compounds are typically defined as materials with more than 1 Li per molecule, for example Li1.2Ni0.2Mn0.6O2.80 While the values vary between materials, Li-rich electrodes offer improved energy density over traditional Li-ion electrodes, for example Li1.2Ni0.2Mn0.6O2 has a reversible capacity of 300 mA h g−1.81 However, Li-rich are unlikely to be used in eVTOLs soon due to their rapid energy fade and limited power density.81,82 At the time of writing Li-rich materials are still under development and have not been widely deployed in commercial cells. The early technology readiness level, and established long term behavioural understanding of these materials in the field is also an obstacle towards adoption in aerospace.Graphite is currently the main active material used in Li-ion negative electrodes. Silicon is often used in additive quantities to increase the energy and power density.54,83,84 However, the microstructure of silicon particle is unable to withstand the expansion and contraction of repeated cycling, so suffers from poor cycle life.52,56 The high reactivity of silicon has also been shown to result in comparatively poor safety performance which reduces it's viability in aerospace applications.15,85,86 As discussed previously, Li-metal negative electrodes offer high energy and power density, with a theoretical specific capacity of 3860 mA h g−1and are used by Cuberg in their cells specifically designed for eVTOL use.51 As commonly reported in the literature Li-metal electrodes suffer from dendrite formation during charging, limiting coulombic efficiency and safety performance.51,87
The electrolyte used by commercial cell manufacturers is particularly difficult to characterise via teardown and it's composition is rarely reported in datasheets. Most manufacturers are believed to use LiPF6 salt dissolved in a blend of organic solvents, but improving electrolyte formulations is an active area of research.88 During thermal runaway, reactions around liquid electrolyte, often comprised of organic solvents, are a key factor. Efforts have been made to improve the safety of Li-ion cells by replacing the organic solvents.89 For example, aqueous electrolytes, which use water instead of organic solvents offer improved safety performance.89,90 However, aqueous electrolytes also suffer from reduced electrochemical performances, in particular limited energy density.91 Aqueous electrolytes also require the use of more experimental electrode materials that are not yet use commercially.90 Improving the electronic conductivity of the electrolyte can also increase the rate that charge carrying ions intercalate in and out of the active material structure, increasing power density. Ionic liquids, which are molten salts that are liquid at room temperature, have been investigated as a method of increasing power density.92 Despite improvement in electrochemical performance, ionic liquids are significantly more expensive and difficult to produce compared to traditional electrolyte formulations, so would require improvements in scalability before adoption in any commercial application.93
There are a huge number of novel positive and negative electrode and electrolyte solutions that are currently being researched and are outside the scope of this work. Of these, the most likely to find widespread commercialisation in the next 5 years are solid-state electrolytes.
As the name suggests, solid-state electrolytes use a solid electrolyte instead of the traditional lithium salt in organic solvent. Solid-state electrolytes have gathered significant research attention, promising improved cyclability, energy density and better safety characteristics. At present, solid-state electrolytes still suffer from Li+ conductivity issues and high interfacial resistances, making them difficult to scale-up. The potential low power density and fast-charging ability of all solid-state batteries would likely prevent them from being used in eVTOL applications for several years. However, if the electrochemical performance could be brought to the appropriate level, the safety improvements would make them ideal for aerospace. Solid-state cells can only be made in pouch or prismatic cell format. The windings process required for cylindrical cells would break the electrolyte layer and make the device unusable. If high-performance solid-state cells become a reality, it would be easier for an eVTOL manufacturer who is already using pouch or prismatic cells to incorporate all solid-state cells into their current aircraft, as opposed to a manufacturer who has previous built their vehicles around cylindrical cells. Lithium sulfur cells are also considered to be comparatively close to commercialisation and are regularly discussed in the context of aviation.94 While this is the case for conventional take-off and landing vehicles and unmanned aircraft the power density demanded by eVTOL render this chemistry unlikely to be deployable in this application.
Conclusions
Electric flight has more demanding requirements for safety, energy and power than today's EV batteries. The aim of this work was to provide an assessment on the optimal choice of battery cell format for eVTOL applications, using a selection of candidate cells taken from the best performing available cells of each format. Based purely on electrochemical performance, pouch and cylindrical cells are the standout choices, with pouch cells giving the best performance due to their light inactive packaging. Prismatic cells typically use LFP chemistries and do not have the energy density required for eVTOLs, although Li-ion and Li-metal variants are emerging. The prismatic cells in this work are designed primarily for EVs, which have lower power requirements. However, when considering safety, cylindrical cells are the best choice as they are best placed to pass the rigorous safety standards required for electric flight, such as RTCA DO-311A. The cells used in eVTOLs must be resistant to cell-to-cell propagation during a failure event. The solid casing on the cylindrical cells makes them most resistant to external damage, whereas the thin pouch cells casings make them susceptible to external heating and thermal runaway. Overall, cylindrical cells are likely the best format for eVTOLs based on current technology. Although this may change in future with the maturity of next-generation chemistries.Author contributions
Hamish T. Reid: conceptualization, data curation, investigation, writing – original draft, writing – review & editing. Gaurav Singh: data curation, formal analysis, writing – original draft. emma palin: writing – review & editing. Yuhang Dai: writing – review & editing. Wei Zong: writing – review & editing. Limhi Somerville: conceptualization, funding acquisition, writing – review & editing. Paul R. Shearing: conceptualization, funding acquisition, project administration, supervision, writing – review and editing. James B. Robinson: conceptualization, funding acquisition, project administration, supervision, writing – review and editing.Data availability
The data supporting this article has been obtained using the methods provided in the ESI.† Any further request for information should be directed to the corresponding author.Conflicts of interest
Emma Palin and Limhi Somerville are employees of Vertical Aerospace, an eVTOL manufacturer.Acknowledgements
The authors would like to acknowledge Innovate UK and the Aerospace Technology Institute for funding through the CEBD programme (10050803). Shearing and Robinson would also like to acknowledge the Faraday Institution (Faraday.ac.uk; EP/S003053/1) for funding the energy storage work at the Advanced Propulsion Lab, UCL, and Department of Engineering Sciences, University of Oxford, (FIRG028, FIRG058, FIRG024, FIRG025, FIRG005). Shearing also acknowledges the Royal Academy of Engineering Chair in Emerging Technologies (CiET1718\59).References
- Department for Transport, UK Future of Flight Action Plan, 2024 Search PubMed.
- E. F. Dulia, M. S. Sabuj and S. A. M. Shihab, Benefits of Advanced Air Mobility for Society and Environment: A Case Study of Ohio, Appl. Sci., 2021, 12, 207, DOI:10.3390/app12010207.
- SNS Insider, EVTOL Aircraft Market Size, Share & Segmentation by Lift Technology (Vectored Thrust, Multirotor, Lift plus Cruise), by Propulsion Type (Fully Electric, Hybrid Electric, Hydrogen Electric), by System, by Range, by MTOW, by Mode of Operation, by Application, and by Region | Global Market Forecast to 2023–2030, 2023.
- V. Viswanathan, A. H. Epstein, Y.-M. Chiang, E. Takeuchi, M. Bradley, J. Langford and M. Winter, The challenges and opportunities of battery-powered flight, Nature, 2022, 601, 519–525, DOI:10.1038/s41586-022-04612-5.
- X.-G. Yang, T. Liu, S. Ge, E. Rountree and C.-Y. Wang, Challenges and key requirements of batteries for electric vertical takeoff and landing aircraft, Joule, 2021, 5, 1644–1659, DOI:10.1016/j.joule.2021.05.001.
- S. Sripad, A. Bills and V. Viswanathan, A review of safety considerations for batteries in aircraft with electric propulsion, MRS Bull., 2021, 46, 435–442, DOI:10.1557/s43577-021-00097-1.
- Federal Aviation Administration (FAA), Airworthiness Criteria: Special Class Airworthiness Criteria, Joby Aero, Inc. Model JAS4–1 Powered-Lift, USA, 2024 Search PubMed.
- Joby Aviation, Joby Investment Thesis, 2021.
- J. Aviation, Joby Completes Flight of More Than 150 Miles with Electric Vertical Take-Off Air Taxi, 2021 Search PubMed.
- M. Dixit, Balancing battery safety and performance for electric vertical takeoff and landing aircrafts, Device, 2023, 1, 100172, DOI:10.1016/j.device.2023.100172.
- A. Ayyaswamy, B. S. Vishnugopi and P. P. Mukherjee, Revealing hidden predicaments to lithium-ion battery dynamics for electric vertical take-off and landing aircraft, Joule, 2023, 7(9), 2016–2034, DOI:10.1016/j.joule.2023.07.014.
- EASA, Third Publication of Means of Compliance with the Special Condition VTOL, 2023 Search PubMed.
- A. Bills, S. Sripad, W. L. Fredericks, M. Singh and V. Viswanathan, Performance Metrics Required of Next-Generation Batteries to Electrify Commercial Aircraft, ACS Energy Lett., 2020, 5(2) DOI:10.1021/acsenergylett.9b02574.
- M. Ank, A. Sommer, K. Abo Gamra, J. Schöberl, M. Leeb, J. Schachtl, N. Streidel, S. Stock, M. Schreiber, P. Bilfinger, C. Allgäuer, P. Rosner, J. Hagemeister, M. Rößle, R. Daub and M. Lienkamp, Lithium-Ion Cells in Automotive Applications: Tesla 4680 Cylindrical Cell Teardown and Characterization, J. Electrochem. Soc., 2023, 170 DOI:10.1149/1945-7111/ad14d0.
- D. P. Finegan, J. Billman, J. Darst, P. Hughes, J. Trillo, M. Sharp, A. Benson, M. Pham, I. Kesuma, M. Buckwell, H. T. Reid, C. Kirchner-Burles, M. Fransson, D. Petrushenko, T. M. M. Heenan, R. Jervis, R. Owen, D. Patel, L. Broche, A. Rack, O. Magdysyuk, M. Keyser, W. Walker, P. Shearing and E. Darcy, The battery failure databank: Insights from an open-access database of thermal runaway behaviors of Li-ion cells and a resource for benchmarking risks, J. Power Sources, 2024, 597 DOI:10.1016/j.jpowsour.2024.234106.
- J. H. Kim, K. H. Lee, D. C. Ko, S. B. Lee and B. M. Kim, Design of integrated safety vent in prismatic lithium-ion battery, J. Mech. Eng. Sci., 2017, 31, 2505–2511, DOI:10.1007/s12206-017-0448-y.
- A. Bills, S. Sripad, L. Fredericks, M. Guttenberg, D. Charles, E. Frank and V. Viswanathan, A battery dataset for electric vertical takeoff and landing aircraft, Sci. Data, 2023, 10, 1–7, DOI:10.1038/s41597-023-02180-5.
- M. T. Phung, T. C. H. Nguyen, M. S. Akhtar and O. B. Yang, Machine learning approaches for assessing rechargeable battery state-of-charge in unmanned aircraft vehicle-eVTOL, J. Comput. Sci., 2024, 81 DOI:10.1016/j.jocs.2024.102380.
- S. Dunlop, A Dictionary of Weather, 2023 Search PubMed.
- The European Union , Regulation (EU) 2023/1542 of the European Parliament and of the Council of 12 July 2023 concerning batteries and waste batteries, amending Directive 2008/98/EC and Regulation (EU) 2019/1020 and repealing Directive 2006/66/EC.
- European Union Aviation Safety Agency (EASA), ETSO-C142b Non-Rechargeable Lithium Cells And Batteries, 2020.
- Radio Technical Commission for Aeronautics (RTCA), DO-311A – Minimum Operational Performance Standards for Rechargeable Lithium Batteries and Battery Systems, 2017 Search PubMed.
- A. W. Golubkov, D. Fuchs, J. Wagner, H. Wiltsche, C. Stangl, G. Fauler, G. Voitic, A. Thaler and V. Hacker, hermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes, RSC Adv., 2014, 4, 3633–3642, 10.1039/C3RA45748F.
- Easa, Easy Access Rules for Small Rotorcraft (CS-27), Initial Issue, 2023.
- W. L. Fredericks, S. Sripad, G. C. Bower and V. Viswanathan, Performance Metrics Required of Next-Generation Batteries to Electrify Vertical Takeoff and Landing (VTOL) Aircraft, ACS Energy Lett., 2018, 3, 12, DOI:10.1021/acsenergylett.8b02195.
- A. W. Golubkov, S. Scheikl, R. Planteu, G. Voitic, H. Wiltsche, C. Stangl, G. Fauler, A. Thaler and V. Hacker, Thermal runaway of commercial 18650 Li-ion batteries with LFP and NCA cathodes – impact of state of charge and overcharge, RSC Adv., 2015, 5, 57171–57186, 10.1039/C5RA05897J.
- M. Brand, S. Gläser, J. Geder, S. Menacher, S. Obpacher, A. Jossen and D. Quinger, Electrical safety of commercial Li-ion cells based on NMC and NCA technology compared to LFP technology, World Electr. Veh. J., 2013, 6, 572–580, DOI:10.3390/wevj6030572.
- F. Schipper, E. M. Erickson, C. Erk, J. Y. Shin, F. F. Chesneau and D. Aurbach, Review—Recent Advances and Remaining Challenges for Lithium Ion Battery Cathodes, J. Electrochem. Soc., 2017, 164, A6220–A6228, DOI:10.1149/2.0351701jes.
- S. T. Taleghani, B. Marcos, K. Zaghib and G. Lantagne, A Study on the Effect of Porosity and Particles Size Distribution on Li-Ion Battery Performance, J. Electrochem. Soc., 2017, 164, E3179–E3189, DOI:10.1149/2.0211711jes.
- P. Zhu, D. Gastol, J. Marshall, R. Sommerville, V. Goodship and E. Kendrick, A review of current collectors for lithium-ion batteries, J. Power Sources, 2021, 485, DOI:10.1016/j.jpowsour.2020.229321.
- I. Hwang, C. W. Lee, J. C. Kim and S. Yoon, Particle size effect of Ni-rich cathode materials on lithium ion battery performance, Mater. Res. Bull., 2012, 47, 73–78, DOI:10.1016/j.materresbull.2011.10.002.
- N. Nitta, F. Wu, J. T. Lee and G. Yushin, Li-ion battery materials: present and future, Mater. Today, 2015, 18, 252–264, DOI:10.1016/j.mattod.2014.10.040.
- A. Frank, J. Sturm, M. Steinhardt, A. Rheinfeld and A. Jossen, Impact of Current Collector Design and Cooling Topology on Fast Charging of Cylindrical Lithium-Ion Batteries, ECS Adv., 2022, 1(4), 040502, DOI:10.1149/2754-2734/ac97e0.
- Y. Kuang, C. Chen, D. Kirsch and L. Hu, Thick Electrode Batteries: Principles, Opportunities, and Challenges, Adv. Energy Mater., 2019 DOI:10.1002/aenm.201901457.
- Airbus, Urban Air Iconography, https://acubed.airbus.com/blog/airbus-utm/urban-air-mobility-iconography/, (accessed 20 November 2024).
- C. Mao, R. E. Ruther, J. Li, Z. Du and I. Belharouak, Identifying the limiting electrode in lithium ion batteries for extreme fast charging☆, Electrochem. Commun., 2018, 97, 37–41, DOI:10.1016/j.elecom.2018.10.007.
- P. P. Paul, V. Thampy, C. Cao, H. G. Steinrück, T. R. Tanim, A. R. Dunlop, E. J. Dufek, S. E. Trask, A. N. Jansen, M. F. Toney and J. Nelson Weker, Quantification of heterogeneous, irreversible lithium plating in extreme fast charging of lithium-ion batteries, Energy Environ. Sci., 2021, 14, 4979–4988, 10.1039/D1EE01216A.
- D. P. Finegan, A. Quinn, D. S. Wragg, A. M. Colclasure, X. Lu, C. Tan, T. M. M. Heenan, R. Jervis, D. J. L. Brett, S. Das, T. Gao, D. A. Cogswell, M. Z. Bazant, M. Di Michiel, S. Checchia, P. R. Shearing and K. Smith, Spatial dynamics of lithiation and lithium plating during high-rate operation of graphite electrodes, Energy Environ. Sci., 2020, 13, 2570–2584, 10.1039/D0EE01191F.
- T. Waldmann and M. Wohlfahrt-Mehrens, Effects of rest time after Li plating on safety behavior—ARC tests with commercial high-energy 18650 Li-ion cells, Electrochim. Acta, 2017, 230, 454–460, DOI:10.1016/j.electacta.2017.02.036.
- A. Straßer, A. Adam and J. Li, In operando detection of Lithium plating via electrochemical impedance spectroscopy for automotive batteries, J. Power Sources, 2023, 580 DOI:10.1016/j.jpowsour.2023.233366.
- Y. Pan, D. Ren, X. Han, L. Lu and M. Ouyang, Lithium Plating Detection Based on Electrochemical Impedance and Internal Resistance Analyses, Batteries, 2022, 8(11) DOI:10.3390/batteries8110206.
- M. Koseoglou, E. Tsioumas, D. Ferentinou, I. Panagiotidis, N. Jabbour, D. Papagiannis and C. Mademlis, Lithium plating detection using differential charging current analysis in lithium-ion batteries, J. Energy Storage, 2022, 54 DOI:10.1016/j.est.2022.105345.
- A. Adam, E. Knobbe, J. Wandt and A. Kwade, Application of the differential charging voltage analysis to determine the onset of lithium-plating during fast charging of lithium-ion cells, J. Power Sources, 2021, 495 DOI:10.1016/j.jpowsour.2021.229794.
- X. G. Yang, S. Ge, T. Liu, Y. Leng and C. Y. Wang, A look into the voltage plateau signal for detection and quantification of lithium plating in lithium-ion cells, J. Power Sources, 2018, 395, 251–261, DOI:10.1016/j.jpowsour.2018.05.073.
- N. Somasundaran, N. Fereshteh Saniee, T. Q. Dinh and J. Marco, Feasibility assessment of differential voltage analysis to detect lithium plating in battery modules for electric vehicles, 2022 25th International Conference on Mechatronics Technology (ICMT), 2022 DOI:10.3390/en16062537.
- I. D. Campbell, M. Marzook, M. Marinescu and G. J. Offer, How Observable Is Lithium Plating? Differential Voltage Analysis to Identify and Quantify Lithium Plating Following Fast Charging of Cold Lithium-Ion Batteries, J. Electrochem. Soc., 2019, 166, A725–A739, DOI:10.1149/2.0821904jes.
- T. Waldmann, B. I. Hogg and M., Wohlfahrt-Mehrens,Li plating as unwanted side reaction in commercial Li-ion cells – A review, J. Power Sources, 2018, 384 DOI:10.1016/j.jpowsour.2018.02.063.
- C. Bommier, W. Chang, Y. Lu, J. Yeung, G. Davies, R. Mohr, M. Williams and D. Steingart, In Operando Acoustic Detection of Lithium Metal Plating in Commercial LiCoO2/Graphite Pouch Cells, Cell Rep. Phys. Sci., 2020, 1(4), 100035, DOI:10.1016/j.xcrp.2020.100035.
- W. Huang, Y. Ye, H. Chen, R. A. Vilá, A. Xiang, H. Wang, F. Liu, Z. Yu, J. Xu, Z. Zhang, R. Xu, Y. Wu, L. Y. Chou, H. Wang, J. Xu, D. T. Boyle, Y. Li and Y. Cui, Onboard early detection and mitigation of lithium plating in fast-charging batteries, Nat. Commun., 2022, 13 DOI:10.1038/s41467-022-33486-4.
- Z. Li, J. Huang, B. Yann Liaw, V. Metzler and J. Zhang, A review of lithium deposition in lithium-ion and lithium metal secondary batteries, J. Power Sources, 2014, 254, 168–182, DOI:10.1016/j.jpowsour.2013.12.099.
- W. Xu, J. Wang, F. Ding, X. Chen, E. Nasybulin, Y. Zhang and J. G. Zhang, Lithium metal anodes for rechargeable batteries, Energy Environ. Sci., 2014, 7, 513–537, 10.1039/C3EE40795K.
- C. K. Chan, H. Peng, G. Liu, K. McIlwrath, X. F. Zhang, R. A. Huggins and Y. Cui, High-performance lithium battery anodes using silicon nanowires, Nat. Nanotechnol., 2008, 3, 31–35, DOI:10.1038/nnano.2007.411.
- P. Zheng, J. Sun, H. Liu, R. Wang, C. Liu, Y. Zhao, J. Li, Y. Zheng and X. Rui, Microstructure Engineered Silicon Alloy Anodes for Lithium-Ion Batteries: Advances and Challenges, Batter. Supercaps, 2023, 6(1) DOI:10.1002/batt.202200481.
- H. Zhao, F. Yang, C. Li, T. Li, S. Zhang, C. Wang, Z. Zhang and R. Wang, Progress and perspectives on two-dimensional silicon anodes for lithium-ion batteries, ChemPhysMater, 2023, 2, 1–19, DOI:10.1016/j.chphma.2022.03.005.
- X. Li, A. M. Colclasure, D. P. Finegan, D. Ren, Y. Shi, X. Feng, L. Cao, Y. Yang and K. Smith, Degradation mechanisms of high capacity 18650 cells containing Si-graphite anode and nickel-rich NMC cathode, Electrochim. Acta, 2019, 297, 1109–1120, DOI:10.1016/j.electacta.2018.11.194.
- N. Kirkaldy, M. A. Samieian, G. J. Offer, M. Marinescu and Y. Patel, Lithium-Ion Battery Degradation: Measuring Rapid Loss of ActiveSilicon in Silicon−Graphite Composite Electrodes, ACS Appl. Energy Mater., 2022, 5, 13367–13376, DOI:10.1021/acsaem.2c02047.
- K. Rhodes, N. Dudney, E. Lara-Curzio and C. Daniel, Understanding the Degradation of Silicon Electrodes forLithium-Ion Batteries Using Acoustic Emission, J. Electrochem. Soc., 2010, 157(12), A1354, DOI:10.1149/1.3489374.
- M. Loveridge, G. Remy, N. Kourra, R. Genieser, A. Barai, M. Lain, Y. Guo, M. Amor-Segan, M. Williams, T. Amietszajew, M. Ellis, R. Bhagat and D. Greenwood, Looking Deeper into the Galaxy (Note 7), Batteries, 2018, 4, 3, DOI:10.3390/batteries4010003.
- H. Jin and D. Shepardson, Reuters, 2021, https://www.reuters.com/business/autos-transportation/tesla-top-of-range-car-caught-fire-while-owner-was-driving-lawyer-says-2021-07-02/.
- T. P. Collinsworth, Jane/Jenna/John Doe vs. Apple Inc., Alphabet Inc., Microsoft Inc., Dell Technologies Inc., Tesla, Inc., - Class complaint for injunctive relief and damages Case 1:19–03737, Washington, D.C., 2019 Search PubMed.
- L. Bravo Diaz, X. He, Z. Hu, F. Restuccia, M. Marinescu, J. V. Barreras, Y. Patel, G. Offer and G. Rein, Review—Meta-Review of Fire Safety of Lithium-Ion Batteries: Industry Challenges and Research Contributions, J. Electrochem. Soc., 2020, 167, 090559, DOI:10.1149/1945-7111/aba8b9.
- M. Fransson, L. Broche, M. Buckwell, J. Pfaff, H. Reid, C. Kirchner-Burles, M. Pham, S. Moser, A. Rack, S. Nau, S. Schopferer, D. P. Finegan and P. Shearing, Sidewall breach during lithium-ion battery thermal runaway triggered by cell-to-cell propagation visualized using high-speed X-ray imaging, J. Energy Storage, 2023, 71 DOI:10.1016/j.est.2023.108088.
- X. Wu, K. Song, X. Zhang, N. Hu, L. Li, W. Li, L. Zhang and H. Zhang, Front. Energy Res., 2019, 7, 1–17 CrossRef.
- D. Williams, R. Copley, P. Bugryniec, R. Dwyer-Joyce and S. Brown, A review of ultrasonic monitoring: Assessing current approaches to Li-ion battery monitoring and their relevance to thermal runaway, J. Power Sources, 2024, 590 DOI:10.1016/j.jpowsour.2023.233777.
- D. L. Wood, J. Li and C. Daniel, Prospects for reducing the processing cost of lithium ion batteries, J. Power Sources, 2015, 275, 234–242, DOI:10.1016/j.jpowsour.2014.11.019.
- R. E. Ciez and J. F. Whitacre, Comparison between cylindrical and prismatic lithium-ion cell costs using a process based cost model, J. Power Sources, 2017, 340, 273–281, DOI:10.1016/j.jpowsour.2016.11.054.
- C. Neef, S. Link, T. Wicke, T. Hettesheimer, M. Diehl, O. Krätzig, F. Degen, F. Klein, P. Fanz, M. Burgard and R. Kleinert, Development perspectives for lithium-ion battery cell formats, Fraunhofer ISI, 2022, Available Online: https://www.isi.fraunhofer.de/en/blog/themen/batterie-update/lithium-ionen-batteriezellen-entwicklung-batteriezellformate.html.
- F. Duffner, L. Mauler, M. Wentker, J. Leker and M. Winter, Large-scale automotive battery cell manufacturing: Analyzing strategic and operational effects on manufacturing costs, Int. J. Prod. Econ., 2021, 232 DOI:10.1016/j.ijpe.2020.107982.
- R. Schmuch, R. Wagner, G. Hörpel, T. Placke and M. Winter, Performance and cost of materials for lithium-based rechargeable automotive batteries, Nat. Energy, 2018, 3, 267–278, DOI:10.1038/s41560-018-0107-2.
- L. Mauler, F. Duffner, W. G. Zeier and J. Leker, Battery cost forecasting: a review of methods and results with an outlook to 2050, Energy Environ. Sci., 2021, 14, 4712–4739, 10.1039/D1EE01530C.
- M. Wentker, M. Greenwood and J. Leker, A Bottom-Up Approach to Lithium-Ion Battery Cost Modeling with a Focus on Cathode Active Materials, Energies, 2019, 12, 1–18, DOI:10.3390/en12030504.
- M. F. Börner, A. M. Mohsseni, N. De, M. Faber, F. Krause, W. Li, S. Bihn, F. Ringbeck and D. U. Sauer, Manufacturing cost comparison of tabless vs. standard electrodes for cylindrical lithium-ion batteries, J. Energy Storage, 2024 DOI:10.1016/j.est.2023.109941.
- L. H. Saw, Y. Ye and A. A. O. Tay, Integration issues of lithium-ion battery into electric vehicles battery pack, J. Clean. Prod., 2016, 113 DOI:10.1016/j.jclepro.2015.11.011.
- H. J. Noh, S. Youn, C. S. Yoon and Y. K. Sun, Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries, J. Power Sources, 2013, 233, 121–130, DOI:10.1016/j.jpowsour.2013.01.063.
- P. J. Bugryniec, J. N. Davidson, D. J. Cumming and S. F. Brown, Pursuing safer batteries: Thermal abuse of LiFePO4 cells, J. Power Sources, 2019, 414, 557–568, DOI:10.1016/j.jpowsour.2019.01.013.
- S. Ohneseit, P. Finster, C. Floras, N. Lubenau, N. Uhlmann, H. J. Seifert and C. Ziebert, Thermal and Mechanical Safety Assessment of Type 21700 Lithium-Ion Batteries with NMC, NCA and LFP Cathodes–Investigation of Cell Abuse by Means of Accelerating Rate Calorimetry (ARC), Batteries, 2023, 9(5) DOI:10.3390/batteries9050237.
- S. Orangi, N. Manjong, D. P. Clos, L. Usai, O. S. Burheim and A. H. Strømman, Historical and prospective lithium-ion battery cost trajectories from a bottom-up production modeling perspective, J. Energy Storage, 2024, 76 DOI:10.1016/j.est.2023.109800.
- J. Hu, W. Huang, L. Yang and F. Pan, Structure and performance of the LiFePO4 cathode material: from the bulk to the surface, Nanoscale, 2020, 12, 10.1039/d0nr03776a.
- L. Yang, W. Deng, W. Xu, Y. Tian, A. Wang, B. Wang, G. Zou, H. Hou, W. Deng and X. Ji, Olivine LiMnxFe1−xPO4 cathode materials for lithium ion batteries: restricted factors of rate performances, J. Mater. Chem., 2021, 9 10.1039/d1ta01526e.
- J. Hong, D. H. Seo, S. W. Kim, H. Gwon, S. T. Oh and K. Kang, Structural evolution of layered Li1.2Ni0.2Mn0.6O2 upon electrochemical cycling in a Li rechargeable battery, J. Mater. Chem., 2010, 20, 10179–10186, 10.1039/C0JM01971B.
- K. Gu, Z. Shi, X. Li, B. Qiu and Z. Liu, Review on the synthesis of Li-rich layered oxide cathodes, J. Mater. Chem., 2024, 12 10.1039/d4ta03917c.
- Y. Xie, Y. Jin and L. Xiang, Li-rich layered oxides: Structure, capacity and voltage fading mechanisms and solving strategies, Particuology, 2022, 61, 1–10, DOI:10.1016/j.partic.2021.05.011.
- Y. F. Yang, J. L. Yang, F. Pan and Y. Cui, From Intercalation to Alloying Chemistry: Structural Design of Silicon Anodes for the Next Generation of Lithium-ion Batteries, Chin. J. Struct. Chem., 2020, 39(1), 16–19, DOI:10.14102/j.cnki.0254-5861.2011-2715.
- J. Asenbauer, T. Eisenmann, M. Kuenzel, A. Kazzazi, Z. Chen and D. Bresser, The success story of graphite as a lithium-ion anode material – fundamentals, remaining challenges, and recent developments including silicon (oxide) composites, Sustainable Energy Fuels, 2020, 4, 5387–5416, 10.1039/D0SE00175A.
- Q. Liu, T. Meng, L. Yu, S. Guo, Y. Hu, Z. Liu and X. Hu, Interface Engineering to Boost Thermal Safety of Microsized Silicon Anodes in Lithium-Ion Batteries, Small Methods, 2022, 6, 1–10, DOI:10.1002/smtd.202200380.
- Y. Wang, X. Feng, W. Huang, X. He, L. Wang and M. Ouyang, Challenges and Opportunities to Mitigate the Catastrophic Thermal Runaway of High-Energy Batteries, Adv. Energy Mater., 2023, 13, 15, DOI:10.1002/aenm.202203841.
- H. G. Lee, S. Y. Kim and J. S. Lee, Dynamic observation of dendrite growth on lithium metal anode during battery charging/discharging cycles, npj Comput. Mater., 2022, 8, 1–13, DOI:10.1038/s41524-022-00788-6.
- J. Xing, S. Bliznakov, L. Bonville, M. Oljaca and R. Maric, A Review of Nonaqueous Electrolytes, Binders, and Separators for Lithium-Ion Batteries, Electrochem. Ener. Reviews, 2022 DOI:10.1007/s41918-022-00131-z.
- S. Kainat, J. Anwer, A. Hamid, N. Gull and S. M., Khan,Electrolytes in Lithium-Ion Batteries: Advancements in the Era of Twenties (2020’s), Mater. Chem. Phys., 2024, 313 DOI:10.1016/j.matchemphys.2023.128796.
- X. Guo, H. He, S. Zhao, H. Dong, P. R. Shearing, R. Jervis and J. Lin, Unveiling aqueous lithium-ion batteries via advanced modelling and characterisation: A review, Energy Storage Mater., 2024, 70 DOI:10.1016/j.ensm.2024.103505.
- H. Ahn, D. Kim, M. Lee and K. W. Nam, Challenges and possibilities for aqueous battery systems, Communications Materials, 2023, 4 DOI:10.1038/s43246-023-00367-2.
- S. Rana, R. C. Thakur and H. S. Dosanjh, Ionic liquids as battery electrolytes for lithium ion batteries: Recent advances and future prospects, Solid State Ionics, 2023, 400 DOI:10.1016/j.ssi.2023.116340.
- J. Wang, L. Xu, G. Jia and J. Du, Challenges and Opportunities of Ionic Liquid Electrolytes for Rechargeable Batteries, Cryst. Growth Des., 2022, 22, 9, DOI:10.1021/acs.cgd.2c00706.
- S. Gifford, Powering the Skies: The Rise of Electric and Low-Carbon Aircraft, 2024, Available Online: https://www.faraday.ac.uk/wp-content/uploads/2024/04/Faraday_Insights_19_FINAL.pdf Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4eb00024b |
| This journal is © The Royal Society of Chemistry 2025 |


