 Open Access Article
Open Access ArticleSalting out and nitrogen effects on cloud-nucleating ability of amino acid aerosol mixtures †
Nahin
Ferdousi-Rokib
 a,
Kotiba A.
Malek
a,
Kotiba A.
Malek
 a,
Kanishk
Gohil‡
a,
Kanishk
Gohil‡
 a,
Kiran R.
Pitta
a,
Kiran R.
Pitta
 b,
Dabrina D
Dutcher
b,
Dabrina D
Dutcher
 cd,
Timothy M.
Raymond
cd,
Timothy M.
Raymond
 c,
Miriam Arak
Freedman
c,
Miriam Arak
Freedman
 be and
Akua A.
Asa-Awuku
be and
Akua A.
Asa-Awuku
 *a
*a
aDepartment of Chemical and Biomolecular Engineering, University of Maryland, College Park, MD 20742, USA. E-mail: asaawuku@umd.edu
bDepartment of Chemistry, Pennsylvania State University, University Park, PA 16802, USA
cDepartment of Chemical Engineering, Bucknell University, Lewisburg, PA 17837, USA
dDepartment of Chemistry, Bucknell University, Lewisburg, PA 17837, USA
eDepartment of Meteorology and Atmospheric Science, Pennsylvania State University, University Park, PA 16802, USA
First published on 28th January 2025
Abstract
Atmospheric aerosols exist as complex mixtures containing three or more compounds. Ternary aerosol mixtures composed of organic/organic/inorganic can undergo liquid–liquid phase separation (LLPS) under supersaturated conditions, affecting phase morphology and water uptake propensity. Phase separation and water uptake in ternary systems has previously been parameterized by oxygen to carbon (O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C) ratio; however, nitrogen containing organics, such as amino acid aerosols, also exist within complex mixtures. Yet, amino acid mixture CCN activity is poorly understood. In this study, we study the supersaturated hygroscopicity of three systems of internal mixtures containing ammonium sulfate (AS), 2-methylglutaric acid (2-MGA), and an amino acid. The three systems are AS/2-MGA/proline (Pro), AS/2-MGA/valine (Val), and AS/2-MGA/leucine (Leu). The amino acids are similar in O
C) ratio; however, nitrogen containing organics, such as amino acid aerosols, also exist within complex mixtures. Yet, amino acid mixture CCN activity is poorly understood. In this study, we study the supersaturated hygroscopicity of three systems of internal mixtures containing ammonium sulfate (AS), 2-methylglutaric acid (2-MGA), and an amino acid. The three systems are AS/2-MGA/proline (Pro), AS/2-MGA/valine (Val), and AS/2-MGA/leucine (Leu). The amino acids are similar in O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios but vary in solubility. Water-uptake, across a range of aerosol compositions in the ternary space, is measured using a cloud condensation nuclei counter (CCNC) from 0.4 to 1.7% supersaturation (SS). The single hygroscopicity parameter, κ, was calculated from CCNC measurements. All three systems exhibit two regions; one of these regions is phase separated mixtures when the composition is dominated by AS and 2-MGA; 2-MGA partitions to the droplet surface due to its surface-active nature and has a negligible contribution to water uptake. The second region is a homogeneous aerosol mixture, where all three compounds contribute to hygroscopicity. However, well mixed aerosol hygroscopicity is dependent on the solubility of the amino acid. Mixed Pro aerosols are the most hygroscopic while Leu aerosols are the least hygroscopic. Theoretical κ values were calculated using established models, including traditional κ-Köhler, O
C ratios but vary in solubility. Water-uptake, across a range of aerosol compositions in the ternary space, is measured using a cloud condensation nuclei counter (CCNC) from 0.4 to 1.7% supersaturation (SS). The single hygroscopicity parameter, κ, was calculated from CCNC measurements. All three systems exhibit two regions; one of these regions is phase separated mixtures when the composition is dominated by AS and 2-MGA; 2-MGA partitions to the droplet surface due to its surface-active nature and has a negligible contribution to water uptake. The second region is a homogeneous aerosol mixture, where all three compounds contribute to hygroscopicity. However, well mixed aerosol hygroscopicity is dependent on the solubility of the amino acid. Mixed Pro aerosols are the most hygroscopic while Leu aerosols are the least hygroscopic. Theoretical κ values were calculated using established models, including traditional κ-Köhler, O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility and O
C solubility and O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS models. To account for the possible influence of polar N–C bonds on solubility and water uptake, the X
C-LLPS models. To account for the possible influence of polar N–C bonds on solubility and water uptake, the X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C parameterization is introduced through the X
C parameterization is introduced through the X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility and X
C solubility and X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS models; X
C-LLPS models; X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C is obtained from the ratio of oxygen and nitrogen to carbon. The study demonstrates competing organic–inorganic interactions driven by salting out effects in the presence of AS. Traditional methods cannot further encapsulate the non-ideal thermodynamic interactions within nitrogen-containing organic aerosol mixtures thus predictions of LLPS and hygroscopicity in nitrogen containing ternary systems should incorporate surface activity, O–C, N–C bonds, and salting out effects.
C is obtained from the ratio of oxygen and nitrogen to carbon. The study demonstrates competing organic–inorganic interactions driven by salting out effects in the presence of AS. Traditional methods cannot further encapsulate the non-ideal thermodynamic interactions within nitrogen-containing organic aerosol mixtures thus predictions of LLPS and hygroscopicity in nitrogen containing ternary systems should incorporate surface activity, O–C, N–C bonds, and salting out effects.
Environmental significanceA significant population of aerosols within our atmosphere exist as complex mixtures containing both organic and inorganic mixtures. Organic/inorganic aerosol mixtures may also include the presence of nitrogen containing aerosols, such as amino acids. As a result, they may present varied mixing states that can affect current projections of aerosol-cloud interactions. Thus, it is critical to understand complex amino acid aerosol mixtures' ability to become cloud condensation nuclei in order to better predict their effect on our global radiative forcing projections. In this study, the water uptake of three amino acids, L-leucine, L-valine, and L-proline, as well as their mixtures with 2-methylglutaric acid and ammonium sulfate were investigated under supersaturated conditions. Our study shows that hygroscopicity of amino acids and their mixtures are dependent on amino acid structure and competing salting out effects due to the presence of AS. Our results demonstrate that several factors, including the presence of nitrogen and surface-active compounds, can influence droplet phase separation and aerosol water uptake ability. We further show that these effects can be parameterized to better predict hygroscopicity. Thus, physicochemical properties such as amino acid structure, the presence of nitrogen, surface activity, morphology and salting out effects must be accounted for when predicting amino acid mixture water uptake. By considering these parameters, we can better predict the effect or aerosol mixtures on our global climate. |
1. Introduction
Atmospheric aerosols are solid or liquid particles suspended in the air and can modify cloud properties. For example, an increase in aerosol particle concentration can increase cloud lifetime and reflectivity.1–3 Aerosol ability to form clouds, referred to as aerosol-cloud interactions, results in an overall cooling effect on our climate. Cloud formation and lifetime are driven by an aerosol's ability to uptake water, or hygroscopicity, under supersaturated (RH > 100%) conditions.4 In particular, aerosols exposed to supersaturated water vapor in the atmosphere presents a surface for water to condense onto; the composition of the aerosols present can influence cloud formation.4,5 However, cooling effects and subsequent radiative forcing projections from aerosol–cloud interactions present a large degree of uncertainty.1–3 Uncertainty in aerosol–cloud radiative forcing models is attributed to the complexity of aerosol particle size and chemical composition affecting water uptake ability.6–8Traditionally, droplet activation under supersaturated conditions and CCN activity of aerosols are predicted using Köhler theory.5 In particular, several previous studies have estimated supersaturated hygroscopicity assuming all aerosol solute components are dissolved within a well-mixed, aqueous phase.9–14 Thus, water uptake of aerosols and its mixtures can be parameterized by κ-Köhler theory.15 Traditional κ-Köhler theory predicts water uptake by assuming all compounds instantaneously dissolve into the droplet bulk and contribute to hygroscopicity.15 For aerosol mixtures, κ is based on the equal volume fraction contribution of individual solutes and is calculated by the Zdanovskii–Stokes–Robinson (ZSR) mixing rule.15 The assumption of full dissolution in supersaturated aerosol droplets is challenged by the presence of partially water-soluble and insoluble compounds. Previous studies have described the effect of a solubility distribution in the bulk; in particular, studies have shown that in the presence of partially water soluble to insoluble compounds, κ-hygroscopicity is overpredicted.15–26 Compounds with a solubility of 0.1–100 g L−1 can affect water uptake and CCN activity.16,18κ-Köhler theory can be modified by accounting for compound solubility and fraction of solute dissolved in the bulk.16,27 If the compound is known, water solubility values can be directly applied to hygroscopicity calculations.16,28 However, atmospheric organic aerosol composition is often unknown; readily available data from field studies provides elemental composition of organic aerosols including number of oxygen (O), carbon (C), and hydrogen (H) atoms.29–31 Water solubility is driven by polarity and the presence of O–C bonds can contribute to compound solubility. To extend hygroscopicity models to unknown organic aerosols and field measurements, solubility can be parameterized using oxygen to carbon (O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C) ratio; limited solubility range of 0.1–100 g L−1 corresponds to an O
C) ratio; limited solubility range of 0.1–100 g L−1 corresponds to an O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C range of 0.2–0.7.17,25,27,32
C range of 0.2–0.7.17,25,27,32
Parameterizations of solubility have been dependent on the presence of O–C bonds in compounds due to their polarity. However, organic aerosols may be composed of compounds containing elements other than O, C, and H; organics may also contain nitrogen (N) and N–C bonds. N–C bonds are also considered highly polar due to nitrogen's strong electronegativity. Nitrogen containing compounds are also present within our atmosphere; in this study, we focus on amino acids. Amino acids are composed of a carboxylic acid group and amino group; additionally, they are known as the building block of proteins and a source of nitrogen for organisms such as phytoplankton and bacteria.33,34 Previous field studies have detected the presence of amino acids; furthermore, amino acids can act as ice nucleating particles (INPs) and CCN.35–37 A study performed in Central Europe found amino acids composed up to 5% of atmospheric particulate matter within the region.38 Similarly, a study in Beijing, China measured a total concentration of 1.86 ± 1.29 μg m−3 during the 2014 Asia-Pacific Economic Cooperation (APEC) summit.35 However, amino acids are primarily found in sea spray aerosols (SSA).39,40 Ocean bubble bursting emits SSA into the atmosphere; as a result, 11–18% of dissolved organics within submicron SSA being composed of free amino acids.39 Previous studies have examined the water-uptake ability of amino acids as well as amino acid/AS mixtures under subsaturated (<100% RH) conditions; limited studies have evaluated amino acid hygroscopicity under supersaturated (>100% RH) conditions.37,41–44 In subsaturated conditions, the deliquescence and morphology of amino acid/AS aerosol mixtures was dependent on the solubility of the amino acid; amino acid aerosols were found to affect the phase state of ammonium sulfate and create a liquid state when the amino acid was sparingly soluble.44 Similarly, a study by Kristensson et al., 2010 also found that solubility effects prevailed in water uptake behavior of pure amino acids. Thus, amino acids are efficient CCN and its intrinsic solubility can present complexity in aerosol water uptake. A few studies have focused on select amino acids (e.g., aspartic acid, serine, glutamine), however supersaturated hygroscopicity data is not readily available for many other amino acids present in aerosols.35,37,39,40,44 For example, Leucine, Valine and Proline are three amino acids found within SSA and continental aerosol samples, yet CCN measurements are not readily available.35,39,45 The study of amino acids and nitrogen containing organic aerosol presents an opportunity to expand our scientific understanding of complex nitrogen containing aerosol species and their subsequent water-uptake. Recent studies have highlighted the growing significance of studying organic nitrogen (ON) containing aerosols due to its abundance in the atmosphere and making up a significant portion of nitrogen present in the atmosphere.45–52 For example, a study by Yu et al., 2024 found that 17–31% of nitrogen containing aerosols studied within several urban and rural sites in China were composed of organic nitrogen.53 Amino acids are encapsulated in these measurements; a study by Spitzy 1990 reported amino acid aerosol concentrations of 0.47–1.13 nM m−3 over the Bay of Bengal.46 Additionally, a study by Gorzelska and Galloway, 1990 identified free amino acid aerosols in the range of 0.003 to 1.63 nM m−3 over the North Atlantic Ocean.47 Thus, the characterization of amino acid hygroscopicity is important in further improving CCN activity predictions and projections of aerosol-cloud interaction radiative forcing.
Furthermore, the presence of amino acids may influence the morphology of aerosol particles. Both the chemical and physical (internal morphology) composition within inorganic/organic droplets can affect hygroscopicity predictions54–57 Uncertainty in aerosol mixture hygroscopicity predictions is attributed to organic aerosols (OA). Primary organic aerosols (POA) have the ability to oxidize, react, age, and interact with other organic/inorganic compounds.32,58–60 Additionally, volatile organic compounds (VOCs) present in the atmosphere can oxidize and condense onto the surface of existing aerosols present; through these reaction mechanisms, secondary organic aerosols (SOA) can be formed.58,61 OA are represented by a range of compound classes (e.g., carboxylic acids, alcohols) and have varied properties based on their composition; in particular, a distribution of solubilities are present in OA.18,29,62,63 Furthermore, organics can result in complex phase morphology within organic/inorganic aerosol mixtures.26,64–68 In particular, aerosol mixtures can present as an outer organic layer and inorganic core, also known as liquid–liquid phase separated (LLPS) morphology.26,64,66–68 Chemical composition, solubility and phase morphology of organic aerosols present complexity in predictions of droplet formation and CCN activity.26
Amino acid mixtures with inorganic compounds may further complicate solubility effects. Inorganic compounds such as AS are able to reduce the solubility of organic compounds, known as a “salting out” effect and is attributed to the presence of LLPS salting out strength is dependent on the ions present within the salt.65,69 The presence of salts (e.g., NaCl, AS) may further enhance intrinsic properties of organics. For example, surface-active compounds (e.g., 2-MGA, malonic acid) mixtures result in lowered surface tension depression; the salting out effect pushes organic out of the aqueous bulk and forms an organic monolayer on the droplet surface.70,71 Many protein studies have also described the use of AS to precipitate out proteins containing amino acids; the presence of AS can further reduce the partial water solubility of amino acids and as a result promote phase separation.72–75 Therefore, in mixtures of higher order (three or more compounds), there may be competing organic–inorganic interactions that affect CCN activity. To our knowledge, there are no studies that examine the CCN activity of higher order amino acid mixtures under supersaturated conditions.
Recent studies have used O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C to parameterize both limited solubility and the presence of LLPS. Studies have described the presence of partially soluble compounds promoting the presence of LLPS; in particular, compounds with fewer oxygen atoms (lower O
C to parameterize both limited solubility and the presence of LLPS. Studies have described the presence of partially soluble compounds promoting the presence of LLPS; in particular, compounds with fewer oxygen atoms (lower O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio) are more likely to phase separate in a mixture.13,65 LLPS within mixtures occurs when the Gibb's free energy is lower for a phase separated state as opposed to remaining in a homogeneous state.12,26,65,76 Previous studies have predicted the presence or lack of LLPS by mixture O
C ratio) are more likely to phase separate in a mixture.13,65 LLPS within mixtures occurs when the Gibb's free energy is lower for a phase separated state as opposed to remaining in a homogeneous state.12,26,65,76 Previous studies have predicted the presence or lack of LLPS by mixture O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio.26,67,68 For example, in mixtures containing ammonium sulfate (AS), LLPS is predicted when the mixture O
C ratio.26,67,68 For example, in mixtures containing ammonium sulfate (AS), LLPS is predicted when the mixture O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio <0.56; depending on the composition mixture and properties, LLPS may exist for AS mixtures of O
C ratio <0.56; depending on the composition mixture and properties, LLPS may exist for AS mixtures of O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 0.56–0.8.65,68 In studies by Ott et al., 2020 and Malek et al., 2023, LLPS in mixtures of 2-methylglutaric acid (2-MGA), AS, and sucrose was estimated to occur at O
C 0.56–0.8.65,68 In studies by Ott et al., 2020 and Malek et al., 2023, LLPS in mixtures of 2-methylglutaric acid (2-MGA), AS, and sucrose was estimated to occur at O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios <0.72 and 0.75, respectively. As more sucrose (a higher O
C ratios <0.72 and 0.75, respectively. As more sucrose (a higher O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C compound) is added to the system and O
C compound) is added to the system and O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C becomes greater than the threshold, the phase morphology shifts from phase separated to well mixed.26,67 Previous studies have assumed that salting out induced LLPS does not affect CCN activity due to mixed aerosols undergoing full deliquescence occurring above 100% RH.77 Malek et al., 2023 showed that phase separation does influence supersaturated hygroscopicity and incorporating O
C becomes greater than the threshold, the phase morphology shifts from phase separated to well mixed.26,67 Previous studies have assumed that salting out induced LLPS does not affect CCN activity due to mixed aerosols undergoing full deliquescence occurring above 100% RH.77 Malek et al., 2023 showed that phase separation does influence supersaturated hygroscopicity and incorporating O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C LLPS threshold in a hygroscopicity model best predicts κ.26 Instead, the less soluble organic partitions to the surface; however, the studies mentioned above do not account for phase separation within nitrogen containing mixtures, such as amino acid mixtures, and the possible influence of nitrogen on LLPS estimations.
C LLPS threshold in a hygroscopicity model best predicts κ.26 Instead, the less soluble organic partitions to the surface; however, the studies mentioned above do not account for phase separation within nitrogen containing mixtures, such as amino acid mixtures, and the possible influence of nitrogen on LLPS estimations.
In this study, we characterize the hygroscopicity of L-leucine (Leu), L-valine (Val), and L-proline (Pro) within ternary systems containing 2-MGA and AS. Though all three amino acids have a similar O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio (Leu = 0.33, Val and Pro = 0.4), solubility is varied (Leu < Val < Pro). κ-Hygroscopicity is first experimentally determined under supersaturated conditions for pure amino acids and their mixtures. Experimental hygroscopicity values are then compared against predicted hygroscopicity calculated from traditional κ-Köhler theory. In order to assess if O
C ratio (Leu = 0.33, Val and Pro = 0.4), solubility is varied (Leu < Val < Pro). κ-Hygroscopicity is first experimentally determined under supersaturated conditions for pure amino acids and their mixtures. Experimental hygroscopicity values are then compared against predicted hygroscopicity calculated from traditional κ-Köhler theory. In order to assess if O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C is an effective parameterization of solubility and LLPS for nitrogen containing organics, such as amino acids, the O
C is an effective parameterization of solubility and LLPS for nitrogen containing organics, such as amino acids, the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-solubility model and O
C-solubility model and O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model from Malek et al. 2023,26 are used to predict κ. To date, there have been no solubility and LLPS parameterizations that incorporate other polar bonds, such as N–C, that can have an effect on water uptake. We introduce a new parameterization, X
C-LLPS model from Malek et al. 2023,26 are used to predict κ. To date, there have been no solubility and LLPS parameterizations that incorporate other polar bonds, such as N–C, that can have an effect on water uptake. We introduce a new parameterization, X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C, to incorporate the polarity of both O–C and N–C within the amino acid in order to estimate solubility, LLPS and κ via the X
C, to incorporate the polarity of both O–C and N–C within the amino acid in order to estimate solubility, LLPS and κ via the X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-solubility, X
C-solubility, X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS and weighted average models. Ferdousi-Rokib et al., 2024 (in review)78 also studied 2-MGA/AS mixtures and found a surface tension depression effect due to the salting out of 2-MGA; 2-MGA is an organic compound that has the ability to partition the surface and reduce droplet surface tension, also referred to as being a surface-active organic. To account for surface activity, the Modified Monolayer Surface Coverage model is also incorporated into the O
C-LLPS and weighted average models. Ferdousi-Rokib et al., 2024 (in review)78 also studied 2-MGA/AS mixtures and found a surface tension depression effect due to the salting out of 2-MGA; 2-MGA is an organic compound that has the ability to partition the surface and reduce droplet surface tension, also referred to as being a surface-active organic. To account for surface activity, the Modified Monolayer Surface Coverage model is also incorporated into the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS, X
C-LLPS, X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS and weighted average models. We discuss each model's efficacy in predicting water uptake within the three amino acid ternary systems as well as the organic–inorganic interactions driving phase separation and CCN activity.
C-LLPS and weighted average models. We discuss each model's efficacy in predicting water uptake within the three amino acid ternary systems as well as the organic–inorganic interactions driving phase separation and CCN activity.
2. Experimental methods
2.1 Chemicals
For this study, all chemicals were purchased and used without further modifications: ammonium sulfate (AS, (NH4)2SO4; Thermo Fisher Scientific, >99.0%), 2-methylglutaric acid (2-MGA, C6H10O4; Sigma-Aldrich®, 98%), L-proline (Pro, C5H9NO2; Sigma-Aldrich®, ≥99%), L-valine (Val, C5H11NO2; Sigma-Aldrich®, ≥98%), and L-leucine (Leu, C6H13NO2; Sigma-Aldrich®, ≥98%). Compound chemical properties are listed in Table S1.†2.2 Solution preparation
Three ternary systems, the Pro system (AS, 2-MGA, and Pro), the Val system (AS, 2-MGA, and Val), and the Leu system (AS, 2-MGA, and Leu) were prepared at different weight percent compositions (Fig. S1–S3 and Tables S2–4†). For each ternary system, the three chemical compounds were weighed and dissolved in ultra purified Millipore water (18 MΩ cm). Mixtures range in O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C values (Tables S2–S4†) to include experiments where LLPS is present and where LLPS is not present. The phase transition from LLPS to well mixed (LLPS threshold) is estimated based on the O
C values (Tables S2–S4†) to include experiments where LLPS is present and where LLPS is not present. The phase transition from LLPS to well mixed (LLPS threshold) is estimated based on the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C LLPS model from Malek et al.26 The model predicts the O
C LLPS model from Malek et al.26 The model predicts the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C threshold to be 0.50, 0.48 and 0.43 for the Pro, Val and Leu systems, respectively. To determine the effect of LLPS on cloud condensation nuclei (CCN) activity of amino acid mixtures, supersaturated hygroscopicity measurements were performed. All CCN experiment solutions are provided in Tables S5–S7.†
C threshold to be 0.50, 0.48 and 0.43 for the Pro, Val and Leu systems, respectively. To determine the effect of LLPS on cloud condensation nuclei (CCN) activity of amino acid mixtures, supersaturated hygroscopicity measurements were performed. All CCN experiment solutions are provided in Tables S5–S7.†
2.3 Cloud condensation nuclei measurements
Hygroscopicity of pure amino acids and their respective ternary systems under supersaturated (SS) conditions (>100% RH) was estimated using a Cloud Condensation Nuclei Counter (CCNC, Droplet Measurement Technologies). The theory of the CCNC has been described in previous literature.56,79,80 Solutions were passed through a constant output Collision Nebulizer (Atomizer, TSI 3076) to generate polydisperse aerosols. Wet aerosols were dried (<5% RH) using two silica gel dryers in series.Dried polydisperse aerosols are passed through an electrostatic classifier (TSI 3080) in scanning mode from 8 nm to 352 nm for 135 seconds. The aerosol sample flow rate is 0.8 L min−1 and the aerosol to sheath flow rate is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The monodisperse size-selected particles were then sampled by a condensation particle counter (CPC, TSI 3776) and CCNC in parallel. The CPC and CCNC operated at 0.3 L min−1 and 0.5 L min−1, respectively. The CPC counted the number concentration of dry particles (condensation nuclei, CN) at a selected size. The particles are then exposed to 0.42 to 1.72% SS within the CCNC and the concentration of particles activated (CCN) were measured. The experimental set up was calibrated using ammonium sulfate.56 Calibration data and the experimental set up are provided in Table S8 and Fig. S4.†
10. The monodisperse size-selected particles were then sampled by a condensation particle counter (CPC, TSI 3776) and CCNC in parallel. The CPC and CCNC operated at 0.3 L min−1 and 0.5 L min−1, respectively. The CPC counted the number concentration of dry particles (condensation nuclei, CN) at a selected size. The particles are then exposed to 0.42 to 1.72% SS within the CCNC and the concentration of particles activated (CCN) were measured. The experimental set up was calibrated using ammonium sulfate.56 Calibration data and the experimental set up are provided in Table S8 and Fig. S4.†
Python-based CCN Analysis Toolkit (PyCAT 1.0) analyzed all CCNC ternary and calibration data. The open-source code is available on GitHub for public use.81 Briefly described here, PyCAT uses the scanning mobility CCN analysis (SMCA) of Moore et al., 2010 in Python;82 the activation ratio of CCN to CN was calculated for each dry particle size using PyCAT.83 A sigmoid was fit through the data to find the critical diameter (Dp,50) where ∼50% of the dry particles form cloud droplets. A charge correction is applied in PyCAT using the multi-charge correction algorithm described in Wiedensohler 1988.84,85 For each supersaturation, the critical diameters were found and used to calculate supersaturated single-hygroscopicity parameter, κCCN.
3. Theory
Traditionally, the water uptake of aerosol mixtures can be calculated using Köhler theory. However, Köhler theory assumes full solute dissolution and equal volume-weighted solute contributions to hygroscopicity. Previous studies have shown that hygroscopicity can be estimated for known mixtures containing partially to insoluble organic compounds.16,18,28,86 For known and unknown compounds, limited solubility has been parameterized by the oxygen to carbon (O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C) ratio and applied to hygroscopicity estimates.16,17,25,26 For this study, traditional Köhler theory will be compared against the four models that account for both solubility and internal particle morphology, O
C) ratio and applied to hygroscopicity estimates.16,17,25,26 For this study, traditional Köhler theory will be compared against the four models that account for both solubility and internal particle morphology, O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility model, X
C solubility model, X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility model, O
C solubility model, O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model and X
C-LLPS model and X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model. The theory and assumptions of each model are described in the following sections.
C-LLPS model. The theory and assumptions of each model are described in the following sections.
3.1 Traditional Köhler theory
Köhler theory describes the process of water vapor condensation on particles and droplet growth by considering aerosol physicochemical properties.5,15 To describe droplet growth, traditional Köhler theory accounts for the Kelvin effect and Raoult (solute) effect. The Kelvin effect accounts for the increase of water vapor saturation due to the curvature of the droplet. The solute effect accounts for the decrease in vapor pressure due to the presence of a soluble substance in the solvent; the solute effects contribute to the water activity term, aw.4,87 For compounds fully dissolved in water, aw can be parameterized using the single hygroscopicity parameter κ as:15,88 | (1) |
Combining both effects, the saturation ratio, S, over the droplet and the vapor pressure can described as:
 | (2) |
The κ parameter be calculated from the intrinsic properties of chemically known water-soluble aerosols as follows:88
 | (3) |
 | (4) |
κ can also be derived directly from experimental hygroscopicity measurements, such as from a CCNC. For supersaturated conditions, κCCN can be described as follows:15
 | (5) |
In traditional κ-Köhler theory, the volume fraction of all compounds fully contributes to overall hygroscopicity (eqn (4)). However, previous studies suggests that organic solubility distribution influences both phase morphology and aerosol hygroscopicity.16–18 Subsequent models modify traditional κ-Köhler theory to account for both organic solubility and phase morphology.
3.2 Parameterized solubility models
In the presence of partially water-soluble (0.1–100 g L−1) and effectively insoluble organic compounds, thermodynamically ideal water-interactions are complicated and not captured in traditional κ-Köhler theory.15,18–25 For known compositions, the partial water solubility can be directly applied to κ-hygroscopicity predictions.16,28 However, solubility limitations can be parameterized using oxygen to carbon (O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C) ratio to extend theory to unknown organic compounds.17,27 Volume based solubility, ξ, can be parameterized by O
C) ratio to extend theory to unknown organic compounds.17,27 Volume based solubility, ξ, can be parameterized by O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio viaeqn (6):17
C ratio viaeqn (6):17 | (6) |
Limited solubility is incorporated into κ-hygroscopicity by calculated the dissolved fraction of organic solute in the droplet, xi,j, through the following definitions:27
 | (7) |
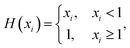 | (8) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility hygroscopicity, κO:C, can be defined as:
C solubility hygroscopicity, κO:C, can be defined as: | (9) |
Previous studies have used O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio to parameterize solubility due to the polarity of O–C bonds and its influence on water solubility.17 However, this parameterization is mainly based on organic compounds composed of O, C and H; this does not account for organic compounds containing nitrogen (N), such as amino acids. For example, amino acids contain polar amino groups that can also influence solubility. The O
C ratio to parameterize solubility due to the polarity of O–C bonds and its influence on water solubility.17 However, this parameterization is mainly based on organic compounds composed of O, C and H; this does not account for organic compounds containing nitrogen (N), such as amino acids. For example, amino acids contain polar amino groups that can also influence solubility. The O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility parameterization presented in eqn (6), based on the work of Kuwata et al., correlated several known organic compound O
C solubility parameterization presented in eqn (6), based on the work of Kuwata et al., correlated several known organic compound O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C to ξ to find the best fit parameterization.17 To incorporate possible nitrogen effects on solubility, a new parameterization X
C to ξ to find the best fit parameterization.17 To incorporate possible nitrogen effects on solubility, a new parameterization X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C is introduced where X is defined as the number of nucleophilic atoms; here, explicitly they are oxygen and nitrogen. The X
C is introduced where X is defined as the number of nucleophilic atoms; here, explicitly they are oxygen and nitrogen. The X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C of a mixture is defined as:
C of a mixture is defined as:
 | (10) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios (Table S9†);39,45ξ can be parameterized by X
C ratios (Table S9†);39,45ξ can be parameterized by X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio using eqn (10):
C ratio using eqn (10): | (11) |
The newly introduced X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility parameterization can then be used in eqn (7)–(9) to obtain κX:C.
C solubility parameterization can then be used in eqn (7)–(9) to obtain κX:C.
3.3 Parameterized solubility – LLPS models
Previous aerosol studies have demonstrated the influence of solubility on both water uptake and droplet phase morphology.26,67 In particular, Ott et al., 2020 found that transitions from phase separated to well mixed aerosols can be attributed by average mixture O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio; the O
C ratio; the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio where phase separation ceases to exist is considered the O
C ratio where phase separation ceases to exist is considered the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C threshold.67 For a ternary system containing 2-MGA, AS and sucrose the experimental O
C threshold.67 For a ternary system containing 2-MGA, AS and sucrose the experimental O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C threshold was 0.72.67 In Malek et al., 2023,26 liquid–liquid phase separation (LLPS) was parameterized by O
C threshold was 0.72.67 In Malek et al., 2023,26 liquid–liquid phase separation (LLPS) was parameterized by O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio for the 2-MGA/AS/sucrose ternary system and applied to κ-hygroscopicity predictions using the O
C ratio for the 2-MGA/AS/sucrose ternary system and applied to κ-hygroscopicity predictions using the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model.
C-LLPS model.
In the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model, it is assumed the organic compound with the lowest O
C-LLPS model, it is assumed the organic compound with the lowest O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C (i.e., the lowest solubility) will partition to the surface of the aqueous phase when phase separation is present; it is assumed that the most soluble organic compound is well-mixed with AS in the bulk.26 For this study, the three amino acids have lower O
C (i.e., the lowest solubility) will partition to the surface of the aqueous phase when phase separation is present; it is assumed that the most soluble organic compound is well-mixed with AS in the bulk.26 For this study, the three amino acids have lower O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C values than 2-MGA. However, 2-MGA has surface-active properties, especially when mixed with AS.26 In this study, 2-MGA is assumed surface-active and when LLPS is present, 2-MGA likely moves to the surface and the contribution to bulk hygroscopicity of AS and the amino acid is negligible (ε2-MGA = 0).
C values than 2-MGA. However, 2-MGA has surface-active properties, especially when mixed with AS.26 In this study, 2-MGA is assumed surface-active and when LLPS is present, 2-MGA likely moves to the surface and the contribution to bulk hygroscopicity of AS and the amino acid is negligible (ε2-MGA = 0).
The O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model uses bootstrap sampling method to estimate the O
C-LLPS model uses bootstrap sampling method to estimate the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C threshold of a ternary system. Briefly described here, the model simulates 100
C threshold of a ternary system. Briefly described here, the model simulates 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 possible O
000 possible O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C thresholds within a range to find the most probable occurrence of phase separation. The range is determined by the minimum and maximum O
C thresholds within a range to find the most probable occurrence of phase separation. The range is determined by the minimum and maximum O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C values; for the Leu system the O
C values; for the Leu system the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio range is 0.33–0.67 while for the Val and Pro systems the O
C ratio range is 0.33–0.67 while for the Val and Pro systems the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio range is 0.4–0.67. At activation, the solubility of the solute depends on Dw. Wet droplet diameter is estimated by using instrument SS, Dp,50 = Dd and κCCN from eqn (5) and (2); solubility is parameterized using eqn (6). For each possible O
C ratio range is 0.4–0.67. At activation, the solubility of the solute depends on Dw. Wet droplet diameter is estimated by using instrument SS, Dp,50 = Dd and κCCN from eqn (5) and (2); solubility is parameterized using eqn (6). For each possible O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C threshold, κO:C (eqn (9)) is calculated based on two conditions:
C threshold, κO:C (eqn (9)) is calculated based on two conditions:
(1) O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cmix < O
Cmix < O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cthresh: only Leu/Pro/Val and AS contribute to hygroscopicity within respective systems (ε2-MGA = 0)
Cthresh: only Leu/Pro/Val and AS contribute to hygroscopicity within respective systems (ε2-MGA = 0)
(2) O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cmix ≥ O
Cmix ≥ O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cthresh: all compounds contribute to hygroscopicity
Cthresh: all compounds contribute to hygroscopicity
O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C thresholds are tested at random across the range. For each threshold, a set of theoretical κO:C values are calculated; for each set the geometric mean is calculated and is referred to as κthresh; κthresh represents the potential average κ of the ternary system based on the presence of LLPS at the tested O
C thresholds are tested at random across the range. For each threshold, a set of theoretical κO:C values are calculated; for each set the geometric mean is calculated and is referred to as κthresh; κthresh represents the potential average κ of the ternary system based on the presence of LLPS at the tested O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cthresh. To effectively determine the true LLPS threshold, the mean κthresh is determined as this represents the most likely phase separated ternary system and O
Cthresh. To effectively determine the true LLPS threshold, the mean κthresh is determined as this represents the most likely phase separated ternary system and O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cthresh. The frequency of κthresh values are plotted as a Poisson distribution, where the mean κthresh bin is determined; the Poisson distribution represents the discrete distribution of an event's frequency count when the event randomly occurs. In this study, the Poisson distribution is used to represent the frequency of a mean κthresh to occur when the O
Cthresh. The frequency of κthresh values are plotted as a Poisson distribution, where the mean κthresh bin is determined; the Poisson distribution represents the discrete distribution of an event's frequency count when the event randomly occurs. In this study, the Poisson distribution is used to represent the frequency of a mean κthresh to occur when the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cthresh is tested at random. In other words, κthresh is the most probably hygroscopicity, driven by the composition and morphology that is most favorable for interactions with water. For example, the mean κthresh of the Pro system based on the Poisson distribution was found to be ∼0.39 (red line, Fig. 1A). κthresh is then plotted against their O
Cthresh is tested at random. In other words, κthresh is the most probably hygroscopicity, driven by the composition and morphology that is most favorable for interactions with water. For example, the mean κthresh of the Pro system based on the Poisson distribution was found to be ∼0.39 (red line, Fig. 1A). κthresh is then plotted against their O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C threshold (O
C threshold (O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cthresh) value and a curve fit is generated. An example of the κthreshvs. O
Cthresh) value and a curve fit is generated. An example of the κthreshvs. O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cthresh is shown for the Pro system in Fig. 1B. The curve fit and mean κthresh are used to determine its corresponding O
Cthresh is shown for the Pro system in Fig. 1B. The curve fit and mean κthresh are used to determine its corresponding O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cthresh (blue lines, Fig. 1B). The corresponding O
Cthresh (blue lines, Fig. 1B). The corresponding O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C threshold to the most frequent κthresh is considered the most probable O
C threshold to the most frequent κthresh is considered the most probable O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C threshold. The mean of the Poisson distribution indicates the most favored aerosol state where the thermodynamic limit is lowest. Using the mean O
C threshold. The mean of the Poisson distribution indicates the most favored aerosol state where the thermodynamic limit is lowest. Using the mean O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cthresh, κO:C is calculated using the previous two conditions; the hygroscopicity values are referred to as κO:C-LLPS.The Poisson distribution and corresponding κthreshvs. O
Cthresh, κO:C is calculated using the previous two conditions; the hygroscopicity values are referred to as κO:C-LLPS.The Poisson distribution and corresponding κthreshvs. O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cthresh results for the Val and Leu systems are shown in Fig. S7 and 8.†
Cthresh results for the Val and Leu systems are shown in Fig. S7 and 8.†
LLPS in all three amino acid ternary systems can also be parameterized with the newly introduced X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio. The X
C ratio. The X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model follows the methodology described above for the O
C-LLPS model follows the methodology described above for the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model with the exception of solubility parameterization; eqn (11) is used to parameterize volume-based solubility. The X
C-LLPS model with the exception of solubility parameterization; eqn (11) is used to parameterize volume-based solubility. The X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C bootstrap range is 0.5–0.67 for the Leu system and 0.6–0.67 for both Val and Pro systems. X
C bootstrap range is 0.5–0.67 for the Leu system and 0.6–0.67 for both Val and Pro systems. X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cthresh is calculated for each system using mean of κthresh distributions and corresponding X
Cthresh is calculated for each system using mean of κthresh distributions and corresponding X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cthresh value, shown in Fig. S9–11.† The calculated hygroscopicity values of the X
Cthresh value, shown in Fig. S9–11.† The calculated hygroscopicity values of the X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model are κX:C-LLPS.
C-LLPS model are κX:C-LLPS.
3.4 Predictions
Once the phase separation threshold is determined, one can predict the hygroscopicity of aerosols in ternary space. However, the influence of surface-active compounds such as 2-MGA, must be accounted for. Previous studies by Malek et al., 2023![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 and Ferdousi-Rokib et al., 2024 (in review)78 found that 2-MGA favors partitioning to the surface in mixtures with AS. Thus, the Monolayer Surface Coverage (MSC) model from Ferdousi-Rokib et al., 2024 (in review)78 was incorporated to only model 2-MGA/AS binary mixtures. Several studies have observed the presence of a monolayer influencing both droplet surface tension and water uptake.89–94 However, a previous study by Bain et al., 2023 describes how surface-active organic concentration of <100 mM can create a monolayer but droplet surface tension was reflective of more dilute organic concentrations; additionally, surface tension values can be as much as >40 mN m−1 higher than measured surface tension values from macroscopic solutions of the same concentration.94 Thus, the MSC model accounts for the probability of organic at the surface under dilute conditions and estimates the average droplet surface tension,
26 and Ferdousi-Rokib et al., 2024 (in review)78 found that 2-MGA favors partitioning to the surface in mixtures with AS. Thus, the Monolayer Surface Coverage (MSC) model from Ferdousi-Rokib et al., 2024 (in review)78 was incorporated to only model 2-MGA/AS binary mixtures. Several studies have observed the presence of a monolayer influencing both droplet surface tension and water uptake.89–94 However, a previous study by Bain et al., 2023 describes how surface-active organic concentration of <100 mM can create a monolayer but droplet surface tension was reflective of more dilute organic concentrations; additionally, surface tension values can be as much as >40 mN m−1 higher than measured surface tension values from macroscopic solutions of the same concentration.94 Thus, the MSC model accounts for the probability of organic at the surface under dilute conditions and estimates the average droplet surface tension,  , as:
, as: | (12) |
 and
and  are average experimental surface tension values of water and 2-MGA, respectively. Hygroscopicity is calculated by categorizing mixtures into three categories: (1) full dissolution (2) probability of surface coverage formation and (3) excess dissolution of surface-active organic into the bulk. For all systems, it is assumed that total AS mass dissolves into the bulk. In category 1 (φ ∼ 0), 2-MGA mass enters the bulk and hygroscopicity can be calculated using eqn (9). As 0 < φ < 1, 2-MGA no longer contributes to the bulk and the remaining 2-MGA mass partitions to the surface and presents a negligible contribution to hygroscopicity. Therefore, the 2-MGA bulk mass is limited to bulk mass where φ was formally ∼0. Finally, the droplet surface becomes saturated (φ ∼ 1) and the remaining organic mass enters the bulk. The modified monolayer formation probability φ is obtained from the dilute surface tension measurements of 2-MGA and 2-MGA/AS mixtures. For further details on the MSC model, see Ferdousi-Rokib et al., 2024 (in review).78 Hygroscopicity for 2-MGA/AS mixtures are calculated as:
are average experimental surface tension values of water and 2-MGA, respectively. Hygroscopicity is calculated by categorizing mixtures into three categories: (1) full dissolution (2) probability of surface coverage formation and (3) excess dissolution of surface-active organic into the bulk. For all systems, it is assumed that total AS mass dissolves into the bulk. In category 1 (φ ∼ 0), 2-MGA mass enters the bulk and hygroscopicity can be calculated using eqn (9). As 0 < φ < 1, 2-MGA no longer contributes to the bulk and the remaining 2-MGA mass partitions to the surface and presents a negligible contribution to hygroscopicity. Therefore, the 2-MGA bulk mass is limited to bulk mass where φ was formally ∼0. Finally, the droplet surface becomes saturated (φ ∼ 1) and the remaining organic mass enters the bulk. The modified monolayer formation probability φ is obtained from the dilute surface tension measurements of 2-MGA and 2-MGA/AS mixtures. For further details on the MSC model, see Ferdousi-Rokib et al., 2024 (in review).78 Hygroscopicity for 2-MGA/AS mixtures are calculated as: | (13) |
To determine if the MSC model must be applied for amino acid/AS systems, surface tension measurements were taken for Pro, Val, and Leu. The three amino acids were found to be less surface-active than 2-MGA; for dilute concentrations, all three amino acids have a surface tension value ∼71 N m−1 (water), while 2-MGA still presents depressed surface tension (Tables S10–12 and Fig. S12–S13†). Therefore, it is assumed 2-MGA is the only organic partitioning to the surface within LLPS aerosols due to its surface activity. Surface tension measurements predictions of the ternary aerosol hygroscopicity account for varying O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C and N
C and N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C bonds that influence solubility, hygroscopicity, and phase separation.
C bonds that influence solubility, hygroscopicity, and phase separation.
4. Results
4.1 Experimental results of ternary systems
The water uptake of the Pro, Val and Leu ternary systems were measured under supersaturated conditions (0.42, 0.61, 0.78, 0.99, 1.21, 1.57 and 1.72% SS) using a CCNC. Each system contained mixtures of varied compositions and O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios (Tables S2–S4†). Average experimental supersaturated hygroscopicity values were calculated for all mixtures across the supersaturated conditions and are reported as κCCN; the κCCN and standard deviation for all mixtures are reported in Tables S13–S15.† AS and 2-MGA κCCN were obtained from Ferdousi-Rokib et al., 2024 (in review).78κCCN of pure Pro, Val and Leu were 0.43 ± 0.09, 0.06 ± 0.02 and 0.01 ± 0.00, respectively. κCCN values of each compound are consistent with amino acid solubility, with Pro being most soluble/hygroscopic and Leu being least soluble/hygroscopic. Val is observed to have similar water uptake as Leu although Val has the same O
C ratios (Tables S2–S4†). Average experimental supersaturated hygroscopicity values were calculated for all mixtures across the supersaturated conditions and are reported as κCCN; the κCCN and standard deviation for all mixtures are reported in Tables S13–S15.† AS and 2-MGA κCCN were obtained from Ferdousi-Rokib et al., 2024 (in review).78κCCN of pure Pro, Val and Leu were 0.43 ± 0.09, 0.06 ± 0.02 and 0.01 ± 0.00, respectively. κCCN values of each compound are consistent with amino acid solubility, with Pro being most soluble/hygroscopic and Leu being least soluble/hygroscopic. Val is observed to have similar water uptake as Leu although Val has the same O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio as Pro; this is consistent with Val and Leu being partially soluble (88.5 and 22.4 g L−1, respectively). Previous studies have emphasized the influence of solubility distribution on κ-hygroscopicity. For moderately soluble compounds (>100 g L−1) such as Pro, the solute instantaneously dissolves and κCCN is driven by molar volume; for partially soluble compounds (0.1–100 g L−1), such as Val and Leu, hygroscopicity is limited by the dissolved fraction of organic solute in the droplet, xi,j, and lowers overall hygroscopicity.16,17,27,43,81
C ratio as Pro; this is consistent with Val and Leu being partially soluble (88.5 and 22.4 g L−1, respectively). Previous studies have emphasized the influence of solubility distribution on κ-hygroscopicity. For moderately soluble compounds (>100 g L−1) such as Pro, the solute instantaneously dissolves and κCCN is driven by molar volume; for partially soluble compounds (0.1–100 g L−1), such as Val and Leu, hygroscopicity is limited by the dissolved fraction of organic solute in the droplet, xi,j, and lowers overall hygroscopicity.16,17,27,43,81
Limited studies have investigated amino acid hygroscopicity; for example, Raymond and Pandis investigated Leu supersaturated hygroscopicity and observed a κCCN of ∼0.02, similar to experimental results of this study.15,95 However, to our knowledge, no prior studies have investigated hygroscopicity of Pro and Val as well as ternary mixtures containing amino acids under supersaturated conditions. κCCN values were also calculated for all ternary systems and evaluated against six hygroscopicity models – traditional Köhler model, O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility model, X
C solubility model, X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility model, O
C solubility model, O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model, X
C-LLPS model, X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model and a weighted average model.
C-LLPS model and a weighted average model.
Mixtures of the three systems, Pro/2-MGA/AS, Val/2-MGA/AS and Leu/2-MGA/AS were investigated. κCCN was calculated for each ternary system viaeqn (5) and is shown in Fig. 2 (data provided in Tables S13–S15†). All κCCN values are then presented on a contour ternary plot, where pure AS is represented on the bottom left vertex, 2-MGA on the bottom right vertex and amino acid on the top vertex. Hygroscopicity is presented as a color scale, ranging from dark purple (most hygroscopic, AS) to light yellow (least hygroscopic organic). Experimental κCCN values were used to extrapolate hygroscopicity values across the ternary contour plots (Fig. 2 and Tables S13–S15†).
For all ternary plots, κCCN range from 0.01 (pure Leucine) to 0.61 (pure AS). The overall Pro system is moderately hygroscopic, with κCCN values ranging between 0.14 (pure 2-MGA)–0.61 as seen in Fig. 2A. The ternary contour plot presents a darker purple (hygroscopic) region in the predominantly AS/2-MGA region, behaving closer to pure AS; a similar highly hygroscopic region is observed in Malek et al. for AS/2-MGA/sucrose ternary mixtures.26 The AS-dominated hygroscopic region is highlighted with a dash-white line (Fig. 2A–C). The hygroscopic region is attributed to surface partitioning of 2-MGA due to surface activity as well as salting out in the presence of AS.26 (Ferdousi-Rokib et al., 2024, in review).78
Therefore, 2-MGA is depleted from the droplet bulk and AS drives hygroscopicity within this region; this region is considered the LLPS region. Above this region, hygroscopicity presents a direct, almost linear, correlation with organic composition, reflecting full hygroscopic contribution of all compounds; as organic mass is increasing within the mixture, κCCN is decreasing. κCCN in the region above the dashed white line are moderately hygroscopic and range from ∼0.1 to 0.5. This trend has previously been observed in LLPS aerosol hygroscopicity and reflects the presence of both LLPS and well mixed regions.12,15,26
The Val ternary system presented similar water uptake behavior to the Pro ternary system. κCCN values range from 0.06 (pure Val) to 0.61 (pure AS); pure Val is presented as yellow in the ternary contour plot, shown in Fig. 2B. The Val ternary system is also the most hygroscopic in the predominantly 2-MGA/AS (LLPS) region. The most hygroscopic region suggests that 2-MGA continues to partition to the surface and drive LLPS in both ternary systems. However, κCCN in the well-mixed region (above the dashed white-line) is lower for Val than the Pro system although Pro and Val have the same O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio. This phenomenon is likely due to their solubilities, with Pro being more soluble than Val. As a result, the Val ternary system is less CCN active than the Pro system and the LLPS region is more apparent.
C ratio. This phenomenon is likely due to their solubilities, with Pro being more soluble than Val. As a result, the Val ternary system is less CCN active than the Pro system and the LLPS region is more apparent.
CCN activity of the ternary Leu system is shown in Fig. 2C. Pure Leu κCCN is represented as light yellow and experimental κCCN values ranged from 0.01 (pure Leu) to 0.61 (pure AS). The Leu ternary system presented the same LLPS and hygroscopicity trends as the Pro and Val systems. However, the well-mixed region (outside of LLPS, Fig. 2C) is the least hygroscopic of all three ternary systems. The CCN activity of all systems correlate with amino acid solubility; Leu is the least water soluble and as a result, mixed Leu aerosols are less likely to uptake water than Pro and Val aerosol mixtures. All three ternary systems present similar water uptake trends but the overall κ-hygroscopicity range is dependent on pure amino acid solubility.
4.2 Modeling results and comparisons
Fig. 3 shows that for all three ternary systems, the traditional κ-Köhler model (Fig. 3D–F) predicts a linear relationship between organic composition and hygroscopicity as mixtures become predominantly 2-MGA. The linear relationship does not include a phase separated hygroscopic region, as observed in experimental data (Fig. 3A–C). Previous studies have noted that traditional κ-Köhler theory is inadequate for explaining CCN activity of phase-separated or partitioned aerosols. This limitation may be due to solubility or surface activity.26,71,89,90,92,96–99 Therefore, traditional κ-Köhler is unable to predict the surface partitioning of 2-MGA due to its assumption that all compounds fully dissolve and contribute to the bulk droplet. As a result, the traditional κ-Köhler is ineffective in predicting κCCN of all three ternary systems.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) C and X
C and X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) C parameterized solubility predictions.
The deviation from traditional hygroscopicity predictions and experimental data has been attributed to the limited solubility distribution of organic compounds. To determine if organic solubility can effectively predict hygroscopicity of the ternary systems, we evaluate solubility for known compounds and as parameterized by O
C parameterized solubility predictions.
The deviation from traditional hygroscopicity predictions and experimental data has been attributed to the limited solubility distribution of organic compounds. To determine if organic solubility can effectively predict hygroscopicity of the ternary systems, we evaluate solubility for known compounds and as parameterized by O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility (eqn (6)). The solubility parameterizations were then used to calculate theoretical κO:C using eqn (7)–(9); κO:C was then plotted on ternary contour plots and are shown in Fig. 3G–I and Tables S16–18,† respectively. O
C solubility (eqn (6)). The solubility parameterizations were then used to calculate theoretical κO:C using eqn (7)–(9); κO:C was then plotted on ternary contour plots and are shown in Fig. 3G–I and Tables S16–18,† respectively. O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility predicts lower κ values for all mixtures. O
C solubility predicts lower κ values for all mixtures. O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility predicts pure Pro and pure Val κ ∼0.02; pure Leu κ is predicted to be ∼0.004. Pure Leu and pure Val are better predicted by O
C solubility predicts pure Pro and pure Val κ ∼0.02; pure Leu κ is predicted to be ∼0.004. Pure Leu and pure Val are better predicted by O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility than pure Pro. For mixtures, O
C solubility than pure Pro. For mixtures, O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility predicts similar theoretical κ for Pro and Val mixtures; for Leu mixtures, Leu dominant mixtures (>80 wt% Leu) are predicted to be less hygroscopic. All organics and their aerosol mixtures have O
C solubility predicts similar theoretical κ for Pro and Val mixtures; for Leu mixtures, Leu dominant mixtures (>80 wt% Leu) are predicted to be less hygroscopic. All organics and their aerosol mixtures have O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C values within 0.2–0.7. Previous studies show that the O
C values within 0.2–0.7. Previous studies show that the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility parameterization can be applied to organic aerosol with an O
C solubility parameterization can be applied to organic aerosol with an O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C range of 0.2–0.7.17,25,27 However, pure Pro is known to be fully water-soluble (365 g L−1, above the solubility limitation range of 0.1–100 g L−1)16 and the O
C range of 0.2–0.7.17,25,27 However, pure Pro is known to be fully water-soluble (365 g L−1, above the solubility limitation range of 0.1–100 g L−1)16 and the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility parametrization is inadequate for Pro and incorrectly predicts hygroscopicity. At the same time, the O
C solubility parametrization is inadequate for Pro and incorrectly predicts hygroscopicity. At the same time, the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility model still predicts a linear relationship between hygroscopicity and organic volume similar to traditional κ-Köhler model; the model is unable to predict the 2-MGA/AS dominated LLPS region. 2-MGA is predicted to be fully water-soluble by O
C solubility model still predicts a linear relationship between hygroscopicity and organic volume similar to traditional κ-Köhler model; the model is unable to predict the 2-MGA/AS dominated LLPS region. 2-MGA is predicted to be fully water-soluble by O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility (κ ∼ 0.16) but additionally partitions to the droplet surface. The LLPS region is likely dominated by surface-active 2-MGA partitioning; however, phase separation is not accounted for in the O
C solubility (κ ∼ 0.16) but additionally partitions to the droplet surface. The LLPS region is likely dominated by surface-active 2-MGA partitioning; however, phase separation is not accounted for in the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility model.
C solubility model.
Previous studies use O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C to parameterize solubility due to the polarity of O–C bonds driving water solubility and uptake. However, N–C bonds also present polarity that may affect solubility and water uptake properties. The X
C to parameterize solubility due to the polarity of O–C bonds driving water solubility and uptake. However, N–C bonds also present polarity that may affect solubility and water uptake properties. The X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility model uses a newly introduced solubility parameterization, X
C solubility model uses a newly introduced solubility parameterization, X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C, based on both O–C and N–C bonds (eqn (10) and (11)). κX:C is then calculated using eqn (7)–(9) (Tables S16–18†). The model predicts overall κX:C values greater than overall κO:C for all three ternary systems respectively (Fig. 3J–L). This is attributed to the X
C, based on both O–C and N–C bonds (eqn (10) and (11)). κX:C is then calculated using eqn (7)–(9) (Tables S16–18†). The model predicts overall κX:C values greater than overall κO:C for all three ternary systems respectively (Fig. 3J–L). This is attributed to the X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C parameterization predicting a higher solubility than the O
C parameterization predicting a higher solubility than the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C parameterization due to the presence of N–C bonds. As a result, full dissolution is predicted (H(xi,j) = 1) and the X
C parameterization due to the presence of N–C bonds. As a result, full dissolution is predicted (H(xi,j) = 1) and the X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility model presents a linear trend for all systems. Both solubility models do not estimate regions of LLPS driven hygroscopicity due to the assumption that all compounds still contribute to κ across the ternary mixture space.
C solubility model presents a linear trend for all systems. Both solubility models do not estimate regions of LLPS driven hygroscopicity due to the assumption that all compounds still contribute to κ across the ternary mixture space.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) C and X
C and X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) C parameterized solubility & LLPS model predictions.
Previous studies have observed complex morphologies in aerosol inorganic–organic and inorganic–organic–organic mixtures; these morphologies can influence water uptake.26,64,66–68 Studies have parameterized LLPS by using O
C parameterized solubility & LLPS model predictions.
Previous studies have observed complex morphologies in aerosol inorganic–organic and inorganic–organic–organic mixtures; these morphologies can influence water uptake.26,64,66–68 Studies have parameterized LLPS by using O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio.26,65,67,68,100,101 To model κCCN and account for LLPS, the O
C ratio.26,65,67,68,100,101 To model κCCN and account for LLPS, the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model, previously introduced in Malek et al., 2023
C-LLPS model, previously introduced in Malek et al., 2023![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 was used. The model predicts an O
26 was used. The model predicts an O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C threshold, or O
C threshold, or O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C mixture where LLPS reaches a thermodynamic limit. Above the threshold, aerosols are considered well mixed and κO:C-LLPS is calculated using eqn (9). Below the threshold, aerosols are considered phase separated and κ2-MGA is set to 0. The predicted O
C mixture where LLPS reaches a thermodynamic limit. Above the threshold, aerosols are considered well mixed and κO:C-LLPS is calculated using eqn (9). Below the threshold, aerosols are considered phase separated and κ2-MGA is set to 0. The predicted O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C thresholds are 0.49, 0.51, and 0.47 for the Pro, Val, and Leu systems, respectively (Table 1). For 2-MGA/AS mixtures, the MSC model is used to calculate theoretical κ (eqn (12) and (13)). κO:C-LLPS ternary contour plots are shown in Fig. 4D–F and Tables S16–18.†
C thresholds are 0.49, 0.51, and 0.47 for the Pro, Val, and Leu systems, respectively (Table 1). For 2-MGA/AS mixtures, the MSC model is used to calculate theoretical κ (eqn (12) and (13)). κO:C-LLPS ternary contour plots are shown in Fig. 4D–F and Tables S16–18.†
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS and X
C-LLPS and X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS models
C-LLPS models
| Ternary system | O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C threshold C threshold |
X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C threshold C threshold |
|---|---|---|
| Pro/AS/2-MGA | 0.49 | 0.65 |
| Val/AS/2-MGA | 0.51 | 0.64 |
| Leu/AS/2-MGA | 0.47 | 0.63 |
Compared to traditional Köhler, O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility, and X
C solubility, and X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility models, the O
C solubility models, the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model successfully estimates a hygroscopic LLPS region for all three systems. The model accounts for 2-MGA partitioning due to salting out and surface activity; 2-MGA partitioning is the main driver of LLPS in the amino acid ternary systems. Therefore, calculating water uptake based on a threshold value effectively predicts phase separation in all three systems and aligns with previous studies.26,65,67,68,100,101 However, the model is limited in predicting κ-hygroscopicity of well-mixed aerosols (above the white-dash line regions). For mixtures ≥O
C-LLPS model successfully estimates a hygroscopic LLPS region for all three systems. The model accounts for 2-MGA partitioning due to salting out and surface activity; 2-MGA partitioning is the main driver of LLPS in the amino acid ternary systems. Therefore, calculating water uptake based on a threshold value effectively predicts phase separation in all three systems and aligns with previous studies.26,65,67,68,100,101 However, the model is limited in predicting κ-hygroscopicity of well-mixed aerosols (above the white-dash line regions). For mixtures ≥O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cthresh, κO:C-LLPS = κO:C. As a result, κO:C-LLPS underpredicts water uptake for the Pro ternary system; the model continues to underpredict Pro κ as the O
Cthresh, κO:C-LLPS = κO:C. As a result, κO:C-LLPS underpredicts water uptake for the Pro ternary system; the model continues to underpredict Pro κ as the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C parameterization is unable to reflect Pro solubility. The O
C parameterization is unable to reflect Pro solubility. The O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model also underpredicts κ for a portion of the Val ternary system, shown on the Val/2-MGA axis (right side). For Val/2-MGA mixtures with >50 wt% Val, the O
C-LLPS model also underpredicts κ for a portion of the Val ternary system, shown on the Val/2-MGA axis (right side). For Val/2-MGA mixtures with >50 wt% Val, the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model predicts κO:C-LLPS of 0.02. However, κCCN reflects moderate water uptake properties and ranges from 0.06–0.16. The model best predicts κ for the Leu ternary system, especially for the Leu/2-MGA mixtures; this is represented by the light-yellow region in both Fig. 4C and F. O
C-LLPS model predicts κO:C-LLPS of 0.02. However, κCCN reflects moderate water uptake properties and ranges from 0.06–0.16. The model best predicts κ for the Leu ternary system, especially for the Leu/2-MGA mixtures; this is represented by the light-yellow region in both Fig. 4C and F. O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C parameterization is best able to reflect the limited solubility of Leu and as a result, the O
C parameterization is best able to reflect the limited solubility of Leu and as a result, the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model is able to predict CCN activity for both the LLPS and non-LLPS mixtures.
C-LLPS model is able to predict CCN activity for both the LLPS and non-LLPS mixtures.
In addition to the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model, the X
C-LLPS model, the X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model was also used to predict theoretical κ to determine if both O–C and N–C bonds can effectively incorporate solubility, LLPS, and water uptake (Fig. 4G–I). The model follows a similar approach to the previous parameterized-LLPS model; now solubility and phase separation are parameterized using X
C-LLPS model was also used to predict theoretical κ to determine if both O–C and N–C bonds can effectively incorporate solubility, LLPS, and water uptake (Fig. 4G–I). The model follows a similar approach to the previous parameterized-LLPS model; now solubility and phase separation are parameterized using X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C (eqn (10) and (11)). X
C (eqn (10) and (11)). X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C thresholds were calculated for each ternary system and are 0.65, 0.64 and 0.63 for Pro, Val and Leu, respectively (Tables S16–18†). The model is once again able to predict a hygroscopic phase separated region for predominantly 2-MGA/AS mixtures; the use of both a LLPS threshold and MSC model are effective in calculating hygroscopicity in amino acid ternary systems. For non-LLPS mixtures, κX:C-LLPS = κX:C. The X
C thresholds were calculated for each ternary system and are 0.65, 0.64 and 0.63 for Pro, Val and Leu, respectively (Tables S16–18†). The model is once again able to predict a hygroscopic phase separated region for predominantly 2-MGA/AS mixtures; the use of both a LLPS threshold and MSC model are effective in calculating hygroscopicity in amino acid ternary systems. For non-LLPS mixtures, κX:C-LLPS = κX:C. The X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C parameterization predicts higher water uptake than O
C parameterization predicts higher water uptake than O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C parameterization due to both O–C and N–C bonds contributing to polarity. The X
C parameterization due to both O–C and N–C bonds contributing to polarity. The X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model is effective in predicting water uptake for the Pro system; X
C-LLPS model is effective in predicting water uptake for the Pro system; X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility parameterization is able to predict pure Pro's higher solubility. However, the model overpredicts κ for both well mixed Val and Leu aerosol mixtures; the X
C solubility parameterization is able to predict pure Pro's higher solubility. However, the model overpredicts κ for both well mixed Val and Leu aerosol mixtures; the X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C parameterization overestimates solubility compared to O
C parameterization overestimates solubility compared to O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C. The effectiveness of the O
C. The effectiveness of the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS and X
C-LLPS and X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS models for the different amino acid systems may be attributed to the varied influence of N–C on amino acid solubility, including in the presence of AS.73,74,102
C-LLPS models for the different amino acid systems may be attributed to the varied influence of N–C on amino acid solubility, including in the presence of AS.73,74,102
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C LLPS and X
C LLPS and X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C LLPS models. To assess each model's predictive ability and characterize N–C influence on water uptake, a χ2 analysis was performed to determine the best model for each system; a smaller χ2 value corresponds to a better fit model representative of the experimental data. χ2 values for all ternary system models are reported in Table S19.† In addition to the previously mentioned models, an optimized weighted average of O
C LLPS models. To assess each model's predictive ability and characterize N–C influence on water uptake, a χ2 analysis was performed to determine the best model for each system; a smaller χ2 value corresponds to a better fit model representative of the experimental data. χ2 values for all ternary system models are reported in Table S19.† In addition to the previously mentioned models, an optimized weighted average of O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS and X
C-LLPS and X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model predictions assessed O
C-LLPS model predictions assessed O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C and N
C and N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C contribution and determined the best fit model for all three systems. The weighted average of the two models was calculated as:
C contribution and determined the best fit model for all three systems. The weighted average of the two models was calculated as:| κWA = a × κO:C-LLPS + b × κX:C-LLPS | (14) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C) and b (X
C) and b (X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C) range from 0 (no contribution) to 1 (full contribution). Coefficients are calculated by simulating different values for a and b to find the least χ2 fit for κCCN of each system; the best fit models and corresponding χ2 values are listed in Table 2 and κWA values are listed in Tables S16–S18.†
C) range from 0 (no contribution) to 1 (full contribution). Coefficients are calculated by simulating different values for a and b to find the least χ2 fit for κCCN of each system; the best fit models and corresponding χ2 values are listed in Table 2 and κWA values are listed in Tables S16–S18.†
| Ternary system | Best fit model | a | b | χ 2 |
|---|---|---|---|---|
| Pro/AS/2-MGA |
X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS C-LLPS |
0 | 1 | 5.23 |
| Val/AS/2-MGA | Weighted avg. | 0.30 | 0.70 | 2.85 |
| Leu/AS/2-MGA | Weighted avg. | 0.89 | 0.11 | 2.30 |
For the Pro ternary system, the X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model was the best fit (a = 0 and b = 1). However, Val and Leu ternary systems experimental κ are best predicted by a weighted average model. The Val system is modeled by a weighted average of 0.3 κO:C-LLPS and 0.7 κX:C-LLPS; the optimal model for the Leu system is a 0.89 κO:C-LLPS and 0.11 κX:C-LLPS weighted model. Amino acid best fit models are shown in Fig. 4J–L. Though all three amino acids have similar O
C-LLPS model was the best fit (a = 0 and b = 1). However, Val and Leu ternary systems experimental κ are best predicted by a weighted average model. The Val system is modeled by a weighted average of 0.3 κO:C-LLPS and 0.7 κX:C-LLPS; the optimal model for the Leu system is a 0.89 κO:C-LLPS and 0.11 κX:C-LLPS weighted model. Amino acid best fit models are shown in Fig. 4J–L. Though all three amino acids have similar O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios, O
C ratios, O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C and N
C and N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C contribution vary between all three systems. Pro presents equal contribution of O–C and N–C bonds; Val and Leu have greater O–C influence than N–C, with Leu being most dependent on O–C. Contributions of O–C and N–C to solubility and hygroscopicity in amino acid ternary systems is correlated with the structure of each amino acid.
C contribution vary between all three systems. Pro presents equal contribution of O–C and N–C bonds; Val and Leu have greater O–C influence than N–C, with Leu being most dependent on O–C. Contributions of O–C and N–C to solubility and hygroscopicity in amino acid ternary systems is correlated with the structure of each amino acid.
All three amino acids are categorized as nonpolar aliphatic; nonpolar aliphatic amino acids are defined by a carboxylic acid functional group and nonpolar, hydrophobic containing amino chain.72,74,103 Amino acid structures are listed in Table 3. Val and Leu are open chained whereas Pro is closed chained. Previous studies investigated nonpolar aliphatic amino acid solubility in the presence of salts, like AS.72–74 Salts such as AS and NaCl can reduce solubility (“salting out”) of organics due to its ionic behavior; salt ions disrupt organic molecule hydration because of its stronger affinity to interact with water molecules.72,74 Furthermore, salting out has been readily observed to be most effective in solutions containing salt and proteins composed of amino acids.75 AS's anion, SO42−, is considered an effective salting out agent.69
Specifically, amino acid side chain structure dictates salting out effect in the presence of salt ions. Amino acids such as Val and Leu contain a nonpolar, open side chain that is considered hydrophobic.72,74,103 Hydrophobicity of the side chain increases with additional CH2 groups; as a result, Leu has a more hydrophobic side chain than Val.69,72,74 When present in an aqueous solution, salt (AS) ions disrupt the hydration of the amino acid chain containing N–C bond, reducing amino acid solubility.72,74,103 Salting out effects are greater for Leu than Val due to the additional –CH2.72,74,102 Amino acids also contain a polar, carboxylic acid functional group containing only O–C and O–H bonds. The disruption of amino side chain (N–C) interaction with water enhances the effect of O–C in Leu and Val solubility in mixtures.74 However, N–C still presents influence on solubility and water uptake. Val and Leu are best represented by a weighted average model with O–C given more weight, as shown by its least χ2 fit; the contribution of N–C for the Leu system is less than the Val system (bLeu < bVal) and may be due to its longer chain enhancing salting out effects. However, Pro and its mixtures do not present salting out effects. Pro is also a nonpolar aliphatic amino acid but its side chain is cyclical (Table 3). Cyclic structures are more rigid and smaller; therefore, the compound efficiently dissolves in water and results in Pro having a higher solubility.104 Its small structure also limits salting out effects in the presence of AS and N–C contribution is not reduced.72 As a result, Pro is best modeled by the X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model alone; the X
C-LLPS model alone; the X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model predicts equal contribution of O–C and N–C on solubility and water uptake.
C-LLPS model predicts equal contribution of O–C and N–C on solubility and water uptake.
Modeling each amino acid ternary system based on LLPS, solubility and salting out effects best reflects hygroscopicity. The presence of nitrogen influences both solubility and water uptake behavior; to predict water uptake, contributions of O–C and N–C must be included. Contributions of the polar bonds are dependent on the structure of the amino acid; open chain amino acids (Leu, Val) have reduced N–C effects due to their propensity to salt out in the presence of salts. Amino acids with closed side chains, such as Pro, do not salt out and can therefore be modeled by an equal contribution of O–C and N–C bonds. Therefore, chemical structure can dictate CCN activity and should be considered in predicting water uptake of complex aerosol mixtures.105,106
2-MGA also salts out in the presence of AS and depresses surface tension. As a result, organic surface activity influences water activity of ternary mixtures by enhancing LLPS. Malek et al.26 correlated 2-MGA partitioning to its O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility; however, in this study, 2-MGA (the more soluble organic) partitioning is due to its surface activity. Accounting for both LLPS and surface activity by using parameterized LLPS and MSC model improves water uptake prediction of amino acid ternary systems. Thus, solubility, surface activity, phase morphology and CCN activity in amino acid/2-MGA/AS systems are driven by the salting-out effects of AS. Previous studies have investigated salting out, solubility, surface activity, and phase morphology influence on CCN activity, separately.15,16,19–25,28,89,90,93,107–109 However, studies have not accounted for all factors having a collective effect on CCN activity and subsequent κ-hygroscopicity. This study accounts for all factors having a collective effect on hygroscopicity through the incorporation of solubility parameterization and surface tension possibly driven by salting out effects in κ-hygroscopicity models.
C solubility; however, in this study, 2-MGA (the more soluble organic) partitioning is due to its surface activity. Accounting for both LLPS and surface activity by using parameterized LLPS and MSC model improves water uptake prediction of amino acid ternary systems. Thus, solubility, surface activity, phase morphology and CCN activity in amino acid/2-MGA/AS systems are driven by the salting-out effects of AS. Previous studies have investigated salting out, solubility, surface activity, and phase morphology influence on CCN activity, separately.15,16,19–25,28,89,90,93,107–109 However, studies have not accounted for all factors having a collective effect on CCN activity and subsequent κ-hygroscopicity. This study accounts for all factors having a collective effect on hygroscopicity through the incorporation of solubility parameterization and surface tension possibly driven by salting out effects in κ-hygroscopicity models.
Further work must investigate amino acids of different structures (e.g., longer/short side chain) and salts of lower order on the Hofmeister series (e.g., NaCl) to assess salting out and water uptake effects in nitrogen containing organic aerosol mixtures. Additionally, amino acids containing elements other than oxygen, nitrogen, carbon, and hydrogen exist. For example, methionine, cysteine and taurine each contain sulfur.110 Future work should study how these additional nucleophilic compounds may affect LLPS, salting out and hygroscopicity in mixtures. Organics of stronger surfactant strength can further complicate predictions of partitioning and LLPS by enhancing solubility and salting out effects.71,90 Therefore, studies must be performed to evaluate influence of surface activity in amino acid mixtures. In order to effectively predict κ-hygroscopicity for amino acid ternary mixtures, models must account for multiple factors (nitrogen effects on solubility, chemical structure, LLPS, surface activity), as shown by the X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS and weighted average models.
C-LLPS and weighted average models.
5. Summary and implications
Three amino acid ternary aerosol mixtures were investigated for their water uptake properties. Mixtures were composed of ammonium sulfate, an inorganic salt, 2-methylglutaric acid, a surface-active organic, and an amino acid; amino acids chosen for this study were proline (Pro), valine (Val), and leucine (Leu). The amino acids are similar in O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios (Leu O
C ratios (Leu O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C = 0.33 and Pro/Val O
C = 0.33 and Pro/Val O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C = 0.4), but vary in solubility, with Pro > Val > Leu. Hygroscopicity was measured under supersaturated conditions using a CCNC. The CCNC determined the activation ratio of particles from 0.4 to 1.7% SS and an experimental κCCN was calculated. Results for all three systems showed the presence of both LLPS and well mixed aerosol across the range of mixtures in the ternary space. Mixtures with phase separated morphology are dominated by AS/2-MGA and is likely due to 2-MGA bulk-surface partitioning. For well mixed aerosol mixtures, κCCN presents a quasi-linear trend between organic composition and hygroscopicity. However, water uptake in the well mixed region parallels the solubility of the amino acid; well mixed Pro (most soluble) mixtures are the most hygroscopic while Leu (least soluble) mixtures are the least hygroscopic.
C = 0.4), but vary in solubility, with Pro > Val > Leu. Hygroscopicity was measured under supersaturated conditions using a CCNC. The CCNC determined the activation ratio of particles from 0.4 to 1.7% SS and an experimental κCCN was calculated. Results for all three systems showed the presence of both LLPS and well mixed aerosol across the range of mixtures in the ternary space. Mixtures with phase separated morphology are dominated by AS/2-MGA and is likely due to 2-MGA bulk-surface partitioning. For well mixed aerosol mixtures, κCCN presents a quasi-linear trend between organic composition and hygroscopicity. However, water uptake in the well mixed region parallels the solubility of the amino acid; well mixed Pro (most soluble) mixtures are the most hygroscopic while Leu (least soluble) mixtures are the least hygroscopic.
Mixtures were compared to already existing hygroscopicity models, such as traditional κ-Köhler, O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility and the O
C solubility and the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS models. To account for the influence of nitrogen on amino acid solubility, a new parameter X
C-LLPS models. To account for the influence of nitrogen on amino acid solubility, a new parameter X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C was introduced through the X
C was introduced through the X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility and X
C solubility and X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS models. The traditional κ-Köhler, O
C-LLPS models. The traditional κ-Köhler, O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility and X
C solubility and X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C solubility models were unable to predict LLPS and hygroscopicity of the three amino acid ternary systems. The models predict a linear relationship between organic mass and hygroscopicity between all mixtures; this is due to the three models predicting full dissolution of 2-MGA. To account for LLPS and surface activity, the O
C solubility models were unable to predict LLPS and hygroscopicity of the three amino acid ternary systems. The models predict a linear relationship between organic mass and hygroscopicity between all mixtures; this is due to the three models predicting full dissolution of 2-MGA. To account for LLPS and surface activity, the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS (κO:C-LLPS), X
C-LLPS (κO:C-LLPS), X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS (κX:C-LLPS) models, and weighted average models were used to predict theoretical κ-values. The X
C-LLPS (κX:C-LLPS) models, and weighted average models were used to predict theoretical κ-values. The X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model was identified as the best fit for the Pro ternary system. Val and Leu ternary systems were best modeled by weighted average models; the Val system had a weighted average of 0.3 κO:C-LLPS and 0.7 κX:C-LLPS while the Leu system was 0.89 κO:C-LLPS and 0.11 κX:C-LLPS. The respective contributions of each model are attributed to the difference in amino acid side chain and subsequent salting out effects. Closed chain amino acids, such as Pro, are rigid and small and as a result, both O–C and N–C bonds contribute to solubility and the X
C-LLPS model was identified as the best fit for the Pro ternary system. Val and Leu ternary systems were best modeled by weighted average models; the Val system had a weighted average of 0.3 κO:C-LLPS and 0.7 κX:C-LLPS while the Leu system was 0.89 κO:C-LLPS and 0.11 κX:C-LLPS. The respective contributions of each model are attributed to the difference in amino acid side chain and subsequent salting out effects. Closed chain amino acids, such as Pro, are rigid and small and as a result, both O–C and N–C bonds contribute to solubility and the X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-LLPS model performs the best for Pro. Val and Leu are open chained and salt out in the presence of AS, with salting out effects being more prominent with increased chain length (e.g.; Leu chain > Val chain). Therefore, O–C bonds have more influence in Val and Leu ternary systems and are best reflected by a weighted average model dependent on their side chain length.
C-LLPS model performs the best for Pro. Val and Leu are open chained and salt out in the presence of AS, with salting out effects being more prominent with increased chain length (e.g.; Leu chain > Val chain). Therefore, O–C bonds have more influence in Val and Leu ternary systems and are best reflected by a weighted average model dependent on their side chain length.
AS/amino acid salting out are driven by amino acid chemical structure, which subsequently influences solubility and hygroscopicity, and indeed all thermodynamically driven processes. Previous studies utilize O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C parameterization for solubility, LLPS threshold, and κ-hygroscopicity predictions. However, O
C parameterization for solubility, LLPS threshold, and κ-hygroscopicity predictions. However, O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C alone cannot fully encapsulate the non-ideal interactions within amino acid containing mixtures, and possibly complex nitrogen containing mixtures. The newly introduced X
C alone cannot fully encapsulate the non-ideal interactions within amino acid containing mixtures, and possibly complex nitrogen containing mixtures. The newly introduced X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C parameter is a better parameterization or can be used in addition with O
C parameter is a better parameterization or can be used in addition with O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C these mixtures; this emphasizes that regardless of the amino acid structure, nitrogen still presents a degree of influence on solubility, LLPS, and hygroscopicity. Ultimately, functional group location and properties impact droplet activation as well and must be considered. Indeed, this study aligns with previous studies, such as Suda et al., 2014,111 that have also observed the influence of functional groups on aerosol properties, including hygroscopicity. Therefore, future work including cloud parcel-based modeling studies must put more emphasis on the presence of certain functional groups within aerosol mixtures, such as carbonyl and amino groups. By accounting for functional group and nucleophiles, this study shows that we can better encapsulate nonideal behavior (e.g., salting out, salting in) that can drive water uptake as well as chemical aging, aerosol surface-activity, and other possible aerosol mechanisms. However, relevant organic aerosol compounds may contain not only oxygen and nitrogen, but also sulfur. For example, methionine, an amino acid containing sulfur, has previously been attributed to lead to ultrafine particle formation within the Arctic.112,113 Future work must therefore further investigate how other additional elements such as sulfur may present an influence on cloud microphysics.
C these mixtures; this emphasizes that regardless of the amino acid structure, nitrogen still presents a degree of influence on solubility, LLPS, and hygroscopicity. Ultimately, functional group location and properties impact droplet activation as well and must be considered. Indeed, this study aligns with previous studies, such as Suda et al., 2014,111 that have also observed the influence of functional groups on aerosol properties, including hygroscopicity. Therefore, future work including cloud parcel-based modeling studies must put more emphasis on the presence of certain functional groups within aerosol mixtures, such as carbonyl and amino groups. By accounting for functional group and nucleophiles, this study shows that we can better encapsulate nonideal behavior (e.g., salting out, salting in) that can drive water uptake as well as chemical aging, aerosol surface-activity, and other possible aerosol mechanisms. However, relevant organic aerosol compounds may contain not only oxygen and nitrogen, but also sulfur. For example, methionine, an amino acid containing sulfur, has previously been attributed to lead to ultrafine particle formation within the Arctic.112,113 Future work must therefore further investigate how other additional elements such as sulfur may present an influence on cloud microphysics.
Additionally, the ability of ammonium sulfate to drive organic partitioning demonstrates how salting out effects may further emphasize organic compound characteristics that may be traditionally overlooked when projecting aerosol-cloud interaction radiative forcing. The presence of AS enhanced solubility limitations within AS/amino dominant mixtures and enhanced surface-activity within AS/2-MGA, challenging the traditional assumption of well mixed aerosols with droplet surface tension equivalent to water. The results of this work may improve understanding of mixed nitrogen containing aerosol water uptake properties, subsequent predictions of CCN activity in larger scale models, and projections of aerosol-cloud interaction radiative forcing.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
NF designed, collected, analyzed CCN experimental data, produced, and analyzed theoretical models. KM contributed to design and collection of CCN experimental data. KG contributed to the development and analysis of LLPS theoretical models. All authors contributed to the writing and preparation of the manuscript.Conflicts of interest
The authors have no competing interests to declareReferences
- Intergovernmental Panel on Climate, C., Climate Change 2021 – the Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, 2023 Search PubMed.
- S. Twomey, The nuclei of natural cloud formation part II: The supersaturation in natural clouds and the variation of cloud droplet concentration, Geofis. Pura Appl., 1959, 43(1), 243–249 CrossRef.
- S. Twomey, Pollution and the planetary albedo, Atmos. Environ., 1974, 8(12), 1251–1256 CrossRef.
- J. Seinfeld and S. Pandis, Atmospheric Chemistry and Physics: from Air Pollution to Climate Change, 1998, p.1326 Search PubMed.
- H. Köhler, The nucleus in and the growth of hygroscopic droplets, Trans. Faraday Soc., 1936, 32, 1152–1161 RSC.
- P. Saxena, et al, Organics alter hygroscopic behavior of atmospheric particles, J. Geophys. Res. Atmos., 1995, 100(D9), 18755–18770 CrossRef.
- D. M. Murphy, D. S. Thomson and M. J. Mahoney, In Situ Measurements of Organics, Meteoritic Material, Mercury, and Other Elements in Aerosols at 5 to 19 Kilometers, Science, 1998, 282(5394), 1664–1669 CrossRef CAS PubMed.
- K. A. Pratt and K. A. Prather, Aircraft measurements of vertical profiles of aerosol mixing states, J. Geophys. Res. Atmos., 2010, 115(D11), D11305 CrossRef.
- A. T. Lambe, et al., Laboratory studies of the chemical composition and cloud condensation nuclei (CCN) activity of secondary organic aerosol (SOA) and oxidized primary organic aerosol (OPOA), Atmos. Chem. Phys., 2011, 11(17), 8913–8928 CrossRef CAS.
- X. Tang, D. R. Cocker III and A. Asa-Awuku, Are sesquiterpenes a good source of secondary organic cloud condensation nuclei (CCN)? Revisiting β-caryophyllene CCN, Atmos. Chem. Phys., 2012, 12(18), 8377–8388 CrossRef CAS.
- L. Renbaum-Wolff, et al., Observations and implications of liquid–liquid phase separation at high relative humidities in secondary organic material produced by α-pinene ozonolysis without inorganic salts, Atmos. Chem. Phys., 2016, 16(12), 7969–7979 CrossRef CAS.
- M. B. Altaf, et al., Effect of Particle Morphology on Cloud Condensation Nuclei Activity, ACS Earth Space Chem., 2018, 2(6), 634–639 CrossRef CAS.
- Y. You, et al., Liquid–liquid phase separation in atmospherically relevant particles consisting of organic species and inorganic salts, Int. Rev. Phys. Chem., 2014, 33(1), 43–77 Search PubMed.
- T. Berkemeier, et al., Competition between water uptake and ice nucleation by glassy organic aerosol particles, Atmos. Chem. Phys., 2014, 14(22), 12513–12531 CrossRef.
- M. D. Petters and S. M. Kreidenweis, A single parameter representation of hygroscopic growth and cloud condensation nucleus activity, Atmos. Chem. Phys., 2007, 7(8), 1961–1971 CrossRef CAS.
- M. D. Petters and S. M. Kreidenweis, A single parameter representation of hygroscopic growth and cloud condensation nucleus activity – Part 2: Including solubility, Atmos. Chem. Phys., 2008, 8(20), 6273–6279 CrossRef CAS.
- M. Kuwata, et al., Classifying organic materials by oxygen-to-carbon elemental ratio to predict the activation regime of Cloud Condensation Nuclei (CCN), Atmos. Chem. Phys., 2013, 13(10), 5309–5324 CrossRef.
- I. Riipinen, N. Rastak and S. N. Pandis, Connecting the solubility and CCN activation of complex organic aerosols: a theoretical study using solubility distributions, Atmos. Chem. Phys., 2015, 15(11), 6305–6322 CrossRef CAS.
- C. N. Cruz and S. N. Pandis, A study of the ability of pure secondary organic aerosol to act as cloud condensation nuclei, Atmos. Environ., 1997, 31(15), 2205–2214 CrossRef CAS.
- C. E. Corrigan and T. Novakov, Cloud condensation nucleus activity of organic compounds: a laboratory study, Atmos. Environ., 1999, 33(17), 2661–2668 CrossRef CAS.
- A. J. Prenni, et al., The Effects of Low Molecular Weight Dicarboxylic Acids on Cloud Formation, J. Phys. Chem. A, 2001, 105(50), 11240–11248 CrossRef CAS.
- D. A. Hegg, et al., Laboratory studies of the efficiency of selected organic aerosols as CCN, Atmos. Res., 2001, 58(3), 155–166 CrossRef CAS.
- T. M. Raymond and S. N. Pandis, Cloud activation of single-component organic aerosol particles, J. Geophys. Res. Atmos., 2002, 107(D24), 1–8 CrossRef.
- D. Vu, et al., External and internal cloud condensation nuclei (CCN) mixtures: controlled laboratory studies of varying mixing states, Atmos. Meas. Tech., 2019, 12(8), 4277–4289 CrossRef CAS.
- P. N. Razafindrambinina, et al., Hygroscopicity of internally mixed ammonium sulfate and secondary organic aerosol particles formed at low and high relative humidity, Environ. Sci.: Atmos., 2022, 2(2), 202–214 CAS.
- K. Malek, et al., Liquid–Liquid Phase Separation Can Drive Aerosol Droplet Growth in Supersaturated Regimes, ACS Environ. Au, 2023, 3(6), 348–360 CrossRef CAS PubMed.
- S. Nakao, Why would apparent κ linearly change with O/C? Assessing the role of volatility, solubility, and surface activity of organic aerosols, Aerosol Sci. Technol., 2017, 51(12), 1377–1388 CrossRef CAS.
- K. Gohil and A. A. Asa-Awuku, Cloud condensation nuclei (CCN) activity analysis of low-hygroscopicity aerosols using the aerodynamic aerosol classifier (AAC), Atmos. Meas. Tech., 2022, 15(4), 1007–1019 CrossRef.
- J. De Gouw and J. L. Jimenez, Organic Aerosols in the Earth's Atmosphere, Environ. Sci. Technol., 2009, 43(20), 7614–7618 CrossRef CAS PubMed.
- I. Kourtchev, et al., Molecular composition of biogenic secondary organic aerosols using ultrahigh-resolution mass spectrometry: comparing laboratory and field studies, Atmos. Chem. Phys., 2014, 14(4), 2155–2167 CrossRef.
- L. M. Russell, R. Bahadur and P. J. Ziemann, Identifying organic aerosol sources by comparing functional group composition in chamber and atmospheric particles, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(9), 3516–3521 CrossRef CAS PubMed.
- J. L. Jimenez, et al., Evolution of Organic Aerosols in the Atmosphere, Science, 2009, 326(5959), 1525–1529 CrossRef CAS PubMed.
- N. J. Antia, P. J. Harrison and L. Oliveira, The role of dissolved organic nitrogen in phytoplankton nutrition, cell biology and ecology, Phycologia, 1991, 30(1), 1–89 CrossRef.
- K. G. McGregor and C. Anastasio, Chemistry of fog waters in California's Central Valley: 2. Photochemical transformations of amino acids and alkyl amines, Atmos. Environ., 2001, 35(6), 1091–1104 CrossRef CAS.
- S. Wang, et al., Occurrence of Aerosol Proteinaceous Matter in Urban Beijing: An Investigation on Composition, Sources, and Atmospheric Processes During the “APEC Blue” Period, Environ. Sci. Technol., 2019, 53(13), 7380–7390 CrossRef CAS PubMed.
- L. F. Evans, Ice Nucleation by Amino Acids, J. Atmos. Sci., 1966, 23(6), 751–752 CrossRef CAS.
- A. Kristensson, T. Rosenørn and M. Bilde, Cloud Droplet Activation of Amino Acid Aerosol Particles, J. Phys. Chem. A, 2010, 114(1), 379–386 CrossRef CAS PubMed.
- U. Pöschl, Atmospheric Aerosols: Composition, Transformation, Climate and Health Effects, Angew. Chem., Int. Ed., 2005, 44(46), 7520–7540 CrossRef PubMed.
- N. Triesch, et al., Sea Spray Aerosol Chamber Study on Selective Transfer and Enrichment of Free and Combined Amino Acids, ACS Earth Space Chem., 2021, 5(6), 1564–1574 CrossRef CAS.
- E. Rastelli, et al., Transfer of labile organic matter and microbes from the ocean surface to the marine aerosol: an experimental approach, Sci. Rep., 2017, 7(1), 11475 CrossRef PubMed.
- A. Marsh, et al., Influence of organic compound functionality on aerosol hygroscopicity: dicarboxylic acids, alkyl-substituents, sugars and amino acids, Atmos. Chem. Phys., 2017, 17(9), 5583–5599 CrossRef CAS.
- M. N. Chan, et al., Hygroscopicity of Water-Soluble Organic Compounds in Atmospheric Aerosols: Amino Acids and Biomass Burning Derived Organic Species, Environ. Sci. Technol., 2005, 39(6), 1555–1562 CrossRef CAS PubMed.
- S. Han, et al., Hygroscopicity of organic compounds as a function of organic functionality, water solubility, molecular weight, and oxidation level, Atmos. Chem. Phys., 2022, 22(6), 3985–4004 CrossRef CAS.
- Q. Luo, et al., Hygroscopicity of amino acids and their effect on the water uptake of ammonium sulfate in the mixed aerosol particles, Sci. Total Environ., 2020, 734, 139318 CrossRef CAS PubMed.
- P. J. Milne and R. G. Zika, Amino acid nitrogen in atmospheric aerosols: Occurrence, sources and photochemical modification, J. Atmos. Chem., 1993, 16(4), 361–398 CrossRef CAS.
- A. Spitzy, Amino acids in marine aerosol and rain.Facets of Modern Biogeochemistry, 1990, pp. 313–317 Search PubMed.
- K. Gorzelska and J. N. Galloway, Amine nitrogen in the atmospheric environment over the North Atlantic Ocean, Global Biogeochem. Cycles, 1990, 4(3), 309–333 CrossRef CAS.
- A. Laskin, J. Laskin and S. A. Nizkorodov, Chemistry of Atmospheric Brown Carbon, Chem. Rev., 2015, 115(10), 4335–4382 CrossRef CAS PubMed.
- M. Wedyan, Bioavailability of Atmospheric Dissolved Organic Nitrogen in The Marine Aerosol over the Gulf of Aqaba, Aust. J. Basic Appl. Sci., 2007, 1(3), 208–212 CAS.
- S. Cornell, et al., Organic nitrogen in Hawaiian rain and aerosol, J. Geophys. Res. Atmos., 2001, 106(D8), 7973–7983 CrossRef CAS.
- Q. Zhang, C. Anastasio and M. Jimenez-Cruz, Water-soluble organic nitrogen in atmospheric fine particles (PM2.5) from northern California, J. Geophys. Res. Atmos., 2002, 107(D11), 1–9 Search PubMed.
- K. A. Malek, et al., Hygroscopicity of nitrogen-containing organic carbon compounds: o-aminophenol and p-aminophenol, Environ. Sci.: Processes Impacts, 2023, 25(2), 229–240 RSC.
- X. Yu, et al., New measurements reveal a large contribution of nitrogenous molecules to ambient organic aerosol, npj Clim. Atmos. Sci., 2024, 7(1), 72 CrossRef CAS.
- D. J. Cziczo, et al., Infrared spectroscopy of model tropospheric aerosols as a function of relative humidity: Observation of deliquescence and crystallization, J. Geophys. Res. Atmos., 1997, 102(D15), 18843–18850 CrossRef CAS.
- J. H. Seinfeld, Tropospheric Chemistry And Composition | Aerosols/Particles, in Encyclopedia of Atmospheric Sciences, ed. J. R. Holton, Academic Press, Oxford, 2003. pp. 2349–2354 Search PubMed.
- D. Rose, et al., Calibration and measurement uncertainties of a continuous-flow cloud condensation nuclei counter (DMT-CCNC): CCN activation of ammonium sulfate and sodium chloride aerosol particles in theory and experiment, Atmos. Chem. Phys., 2008, 8(5), 1153–1179 CrossRef CAS.
- O. Laskina, et al., Size Matters in the Water Uptake and Hygroscopic Growth of Atmospherically Relevant Multicomponent Aerosol Particles, J. Phys. Chem. A, 2015, 119(19), 4489–4497 CrossRef CAS PubMed.
- M. Kanakidou, et al., Organic aerosol and global climate modelling: a review, Atmos. Chem. Phys., 2005, 5(4), 1053–1123 CrossRef CAS.
- D. M. Murphy, et al., Single-particle mass spectrometry of tropospheric aerosol particles, J. Geophys. Res. Atmos., 2006, 111(D23), D23S32 Search PubMed.
- Q. Zhang, et al., Ubiquity and dominance of oxygenated species in organic aerosols in anthropogenically-influenced Northern Hemisphere midlatitudes, Geophys. Res. Lett., 2007, 34(13), L13801 CrossRef.
- J. H. Kroll and J. H. Seinfeld, Chemistry of secondary organic aerosol: Formation and evolution of low-volatility organics in the atmosphere, Atmos. Environ., 2008, 42(16), 3593–3624 CrossRef CAS.
- M. Hallquist, et al., The formation, properties and impact of secondary organic aerosol: current and emerging issues, Atmos. Chem. Phys., 2009, 9(14), 5155–5236 CrossRef CAS.
- J. H. Kroll, et al., Carbon oxidation state as a metric for describing the chemistry of atmospheric organic aerosol, Nat. Chem., 2011, 3(2), 133–139 CrossRef CAS PubMed.
- A. K. Bertram, et al., Predicting the relative humidities of liquid-liquid phase separation, efflorescence, and deliquescence of mixed particles of ammonium sulfate, organic material, and water using the organic-to-sulfate mass ratio of the particle and the oxygen-to-carbon elemental ratio of the organic component, Atmos. Chem. Phys., 2011, 11(21), 10995–11006 CrossRef CAS.
- M. A. Freedman, Phase separation in organic aerosol, Chem. Soc. Rev., 2017, 46(24), 7694–7705 Search PubMed.
- N. Riemer, et al., Aerosol Mixing State: Measurements, Modeling, and Impacts, Rev. Geophys., 2019, 57(2), 187–249 Search PubMed.
- E.-J. E. Ott, E. C. Tackman and M. A. Freedman, Effects of Sucrose on Phase Transitions of Organic/Inorganic Aerosols, ACS Earth Space Chem., 2020, 4(4), 591–601 Search PubMed.
- M. Song, et al., Morphologies of mixed organic/inorganic/aqueous aerosol droplets, Faraday Discuss., 2013, 165, 289–316 RSC.
- B. Kang, et al., Hofmeister Series: Insights of Ion Specificity from Amphiphilic Assembly and Interface Property, ACS Omega, 2020, 5(12), 6229–6239 CrossRef CAS PubMed.
- D. Topping, An analytical solution to calculate bulk mole fractions for any number of components in aerosol droplets after considering partitioning to a surface layer, Geosci. Model Dev., 2010, 3(2), 635–642 CrossRef.
- N. L. Prisle, et al., Surfactant partitioning in cloud droplet activation: a study of C8, C10, C12 and C14 normal fatty acid sodium salts, Tellus B, 2008, 60(3), 416–431 Search PubMed.
- M. Aliyeva, et al., Electrolyte Effects on the Amino Acid Solubility in Water: Solubilities of Glycine, l-Leucine, l-Phenylalanine, and l-Aspartic Acid in Salt Solutions of (Na+, K+, NH4+)/(Cl–, NO3–), Ind. Eng. Chem. Res., 2022, 61(16), 5620–5631 CrossRef CAS.
- L. A. Ferreira, E. A. Macedo and S. P. Pinho, Effect of KCl and Na2SO4 on the Solubility of Glycine and dl-Alanine in Water at 298.15 K, Ind. Eng. Chem. Res., 2005, 44(23), 8892–8898 CrossRef CAS.
- P. Ramasami, Solubilities of Amino Acids in Water and Aqueous Sodium Sulfate and Related Apparent Transfer Properties, J. Chem. Eng. Data, 2002, 47(5), 1164–1166 Search PubMed.
- P. Novák and V. Havlíček, 4 – Protein Extraction and Precipitation, in Proteomic Profiling and Analytical Chemistry, ed. P. Ciborowski and J. Silberring, Elsevier, Boston, 2nd edn, 2016. pp. 51–62 Search PubMed.
- M. Song, et al., Liquid-liquid phase separation and morphology of internally mixed dicarboxylic acids/ammonium sulfate/water particles, Atmos. Chem. Phys., 2012, 12(5), 2691–2712 CrossRef CAS.
- C. N. Cruz and S. N. Pandis, Deliquescence and Hygroscopic Growth of Mixed Inorganic−Organic Atmospheric Aerosol, Environ. Sci. Technol., 2000, 34(20), 4313–4319 CrossRef CAS.
- N. Ferdousi-Rokib, K. A. Malek, I. Mitchell, L. M. Fierce and A. A. Asa-Awuku, Aerosol Hygroscopicity and SurfaceActive Coverage for the Droplet Growth of Aerosol Mixtures, Environ. Sci. Technol. Air, 2024 Search PubMed , in review.
- G. C. Roberts and A. Nenes, A Continuous-Flow Streamwise Thermal-Gradient CCN Chamber for Atmospheric Measurements, Aerosol Sci. Technol., 2005, 39(3), 206–221 Search PubMed.
- S. Lance, et al., Mapping the Operation of the DMT Continuous Flow CCN Counter, Aerosol Sci. Technol., 2006, 40(4), 242–254 Search PubMed.
- K. Gohil, et al., Hybrid water adsorption and solubility partitioning for aerosol hygroscopicity and droplet growth, Atmos. Chem. Phys., 2022, 22(19), 12769–12787 CrossRef CAS.
- R. H. Moore, A. Nenes and J. Medina, Scanning Mobility CCN Analysis—A Method for Fast Measurements of Size-Resolved CCN Distributions and Activation Kinetics, Aerosol Sci. Technol., 2010, 44(10), 861–871 Search PubMed.
- K. Gohil, kgohil27/PyCAT: v1.0 (v1.0), 2022 Search PubMed.
- N. A. Fuchs, On the stationary charge distribution on aerosol particles in a bipolar ionic atmosphere, Geofis. Pura Appl., 1963, 56(1), 185–193 Search PubMed.
- A. Wiedensohler, An approximation of the bipolar charge distribution for particles in the submicron size range, J. Aerosol Sci., 1988, 19, 387–389 Search PubMed.
- K. Gohil, et al., Solubility Considerations for Cloud Condensation Nuclei (CCN) Activity Analysis of Pure and Mixed Black Carbon Species, J. Phys. Chem. A, 2023, 127(17), 3873–3882 Search PubMed.
- H. Wex, et al., The Kelvin versus the Raoult Term in the Köhler Equation, J. Atmos. Sci., 2008, 65(12), 4004–4016 CrossRef.
- R. C. Sullivan, et al., Effect of chemical mixing state on the hygroscopicity and cloud nucleation properties of calcium mineral dust particles, Atmos. Chem. Phys., 2009, 9(10), 3303–3316 Search PubMed.
- C. R. Ruehl, J. F. Davies and K. R. Wilson, An interfacial mechanism for cloud droplet formation on organic aerosols, Science, 2016, 351(6280), 1447–1450 Search PubMed.
- J. Ovadnevaite, et al., Surface tension prevails over solute effect in organic-influenced cloud droplet activation, Nature, 2017, 546(7660), 637–641 CrossRef CAS PubMed.
- S. D. Forestieri, et al., Establishing the impact of model surfactants on cloud condensation nuclei activity of sea spray aerosol mimics, Atmos. Chem. Phys., 2018, 18(15), 10985–11005 CrossRef CAS.
- S. Vepsäläinen, et al., Comparison of six approaches to predicting droplet activation of surface active aerosol – Part 1: moderately surface active organics, Atmos. Chem. Phys., 2022, 22(4), 2669–2687 Search PubMed.
- J. Malila and N. L. Prisle, A Monolayer Partitioning Scheme for Droplets of Surfactant Solutions, J. Adv. Model. Earth Syst., 2018, 10(12), 3233–3251 Search PubMed.
- A. Bain, et al., Surface-Area-to-Volume Ratio Determines Surface Tensions in Microscopic, Surfactant-Containing Droplets, ACS Cent. Sci., 2023, 9(11), 2076–2083 CrossRef CAS PubMed.
- T. M. Raymond and S. N. Pandis, Formation of cloud droplets by multicomponent organic particles, J. Geophys. Res. Atmos., 2003, 108(D15), 4469 CrossRef.
- N. L. Prisle, et al., Surfactants in cloud droplet activation: mixed organic-inorganic particles, Atmos. Chem. Phys., 2010, 10(12), 5663–5683 CrossRef CAS.
- T. B. Kristensen, N. L. Prisle and M. Bilde, Cloud droplet activation of mixed model HULIS and NaCl particles: Experimental results and κ-Köhler theory, Atmos. Res., 2014, 137, 167–175 Search PubMed.
- N. L. Prisle, A predictive thermodynamic framework of cloud droplet activation for chemically unresolved aerosol mixtures, including surface tension, non-ideality, and bulk–surface partitioning, Atmos. Chem. Phys., 2021, 21(21), 16387–16411 CrossRef CAS.
- C. R. Ruehl and K. R. Wilson, Surface Organic Monolayers Control the Hygroscopic Growth of Submicrometer Particles at High Relative Humidity, J. Phys. Chem. A, 2014, 118(22), 3952–3966 CrossRef CAS PubMed.
- Y. C. Song, et al., Liquid–liquid phase separation and morphologies in organic particles consisting of α-pinene and β-caryophyllene ozonolysis products and mixtures with commercially available organic compounds, Atmos. Chem. Phys., 2020, 20(19), 11263–11273 CrossRef CAS.
- S. Ham, et al., Liquid–liquid phase separation in secondary organic aerosol particles produced from α-pinene ozonolysis and α-pinene photooxidation with/without ammonia, Atmos. Chem. Phys., 2019, 19(14), 9321–9331 CrossRef CAS.
- L. A. Ferreira, E. A. Macedo and S. P. Pinho, Solubility of amino acids and diglycine in aqueous–alkanol solutions, Chem. Eng. Sci., 2004, 59(15), 3117–3124 CrossRef CAS.
- C. Selvaraj, et al., Chapter 3 – Macromolecular chemistry: An introduction, in In Silico Approaches to Macromolecular Chemistry, ed. M. E. Thomas, et al., Elsevier, 2023. pp. 71–128 Search PubMed.
- D. G. Peiffer, The molecular factors affecting the solubility parameter, J. Appl. Polym. Sci., 1980, 25(3), 369–380 Search PubMed.
- S. R. Suda, et al., Influence of Functional Groups on Organic Aerosol Cloud Condensation Nucleus Activity, Environ. Sci. Technol., 2014, 48(17), 10182–10190 CrossRef CAS PubMed.
- S. S. Petters, et al., Hygroscopicity of Organic Compounds as a Function of Carbon Chain Length and Carboxyl, Hydroperoxy, and Carbonyl Functional Groups, J. Phys. Chem. A, 2017, 121(27), 5164–5174 Search PubMed.
- N. Good, et al., Consistency between parameterisations of aerosol hygroscopicity and CCN activity during the RHaMBLe discovery cruise, Atmos. Chem. Phys., 2010, 10(7), 3189–3203 CrossRef CAS.
- R. Sorjamaa, et al., The role of surfactants in Köhler theory reconsidered, Atmos. Chem. Phys., 2004, 4(8), 2107–2117 CrossRef CAS.
- R. Sorjamaa and A. Laaksonen, The influence of surfactant properties on critical supersaturations of cloud condensation nuclei, J. Aerosol Sci., 2006, 37(12), 1730–1736 CrossRef CAS.
- J. T. Brosnan and M. E. Brosnan, The Sulfur-Containing Amino Acids: An Overview12, J. Nutr., 2006, 136(6), 1636S–1640S CrossRef CAS PubMed.
- S. R. Suda, M. D. Petters, G. K. Yeh, C. Strollo, A. Matsunaga, A. Faulhaber, P. J. Ziemann, A. J. Prenni, C. M. Carrico, R. C. Sullivan and S. M. Kreidenweis, Influence of Functional Groups on Organic Aerosol Cloud Condensation Nucleus Activity, Environ. Sci. Technol., 2014, 48(17), 10182–10190 CrossRef CAS PubMed.
- C. Leck and E. K. Bigg, Aerosol production over remote marine areas-A new route, Geophys. Res. Lett., 1999, 26(23), 3577–3580 CrossRef CAS.
- E. Scalabrin, et al., Amino acids in Arctic aerosols, Atmos. Chem. Phys., 2012, 12(21), 10453–10463 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ea00128a |
| ‡ Present address: Center for Atmospheric Particle Studies, and the Department of Chemical Engineering, Carnegie Mellon University, Pittsburgh, PA. |
| This journal is © The Royal Society of Chemistry 2025 |







