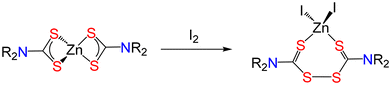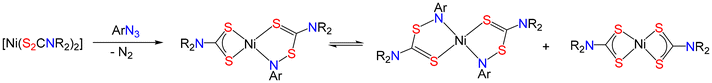Open Access Article
Open Access ArticleAddressing misconceptions in dithiocarbamate chemistry
David
Pugh
 and
Graeme
Hogarth
and
Graeme
Hogarth
 *
*
Department of Chemistry, King's College London, Britannia House, 7 Trinity Street, London SE1 1DB, UK. E-mail: graeme.hogarth@kcl.ac.uk
First published on 20th June 2025
Abstract
Dithiocarbamates are monoanionic chelating ligands, easily prepared from CS2 and secondary or primary amines, that find widespread use in agriculture, medicine, materials science and coordination chemistry. This is (in part) due to their ability to stabilise metals in a wide range of oxidation states, a result of soft (dithiocarbamate) and hard (thioureide) resonance forms. However, in the many thousands of publications on dithiocarbamate chemistry, some common misconceptions have arisen, often being accepted as truth. In this perspective we address some of these in the hope that, moving forward, the wider community will better grasp the nuances of the chemistry of this important ligand type.
1. Introduction
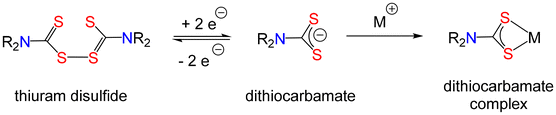
Dithiocarbamates, their complexes, and oxidised counterparts the thiuram disulfides (Fig. 1) find widespread uses in a diverse range of areas including: agriculture,1,2 analytical chemistry,3–7 coordination chemistry,8–18 environmental remediation,19–23 medicine,24–29 enzyme inhibition,30–36 medical imaging,37–39 living polymerisation,40–43 materials science44–48 and as precursors to metal-sulfide nanomaterials.49–52 They are a subset of the widely studied 1,1′-dithiolate ligands and close relatives of thiocarbamates and carbamates.8,9 Despite being known for at least 150 years they remain an area of significant research activity. For example, with the recent identification of a new cell death mechanism termed cuprotosis,53 the anti-cancer activity of [Cu(S2CNEt2)2], which has been known for over 40 years,54–59 has once again come under the spotlight.60–64 Thus, tetraethyl thiuram disulfide (Et4TDS), better known as Disulfiram (Antabuse), is a drug that finds widespread use in the treatment of alcoholism.65 It is rapidly metabolised in the gut (it is normally given orally) or in blood66 to give diethyldithiocarbamate, which in turn can bind to Cu(II) to afford [Cu(S2CNEt2)2] in situ.Dithiocarbamates are easily prepared, generally in high yields, upon reaction of CS2 with secondary or primary amines generally in the presence of an added base.8 Water is often used as the solvent, although reactions also proceed in MeOH and some other organic solvents. Reactions can be carried out in air, making dithiocarbamates easily accessible in less sophisticated lab environments. Consequently, this has led to an extremely large volume of research output: a search for “dithiocarbamate” in SciFinder© giving almost 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hits and for “dithiocarbamato” another ca. 1500. Many of these publications are of excellent quality, some being cited thousands of times. However others contain errors and misconceptions, some of which are repeated so frequently as to be erroneously accepted as truth.
000 hits and for “dithiocarbamato” another ca. 1500. Many of these publications are of excellent quality, some being cited thousands of times. However others contain errors and misconceptions, some of which are repeated so frequently as to be erroneously accepted as truth.
As researchers who are active in the area67–72 and have written significant reviews,8,24,49 we have read and closely scrutinised a considerable number of papers on dithiocarbamate chemistry. In doing so we have seen some errors-misconceptions being regularly repeated. In this contribution we address these, with the hope that they may appear less frequently in the future. In general, our intention is not to highlight individual contributions where errors have been made or perpetuated, however at times this is unavoidable. We accept that the majority are made in good faith and hope to avoid demonising individuals.
2. Synthesis of dithiocarbamates
(i) Amines are deprotonated by base, and it is the amide that undergoes nucleophilic attack at CS2 to afford the dithiocarbamate
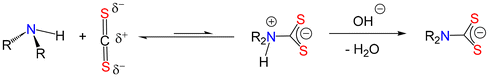
Primary and secondary amines are extremely basic, having pKbs of ca. 4. Consequently, they are not acidic and have pKas of ca. 40. Thus, upon dissolution in water (or an organic solvent), even following the addition of a strong base such as NaOH, they are not deprotonated. They are, however, nucleophilic and like many N-based nucleophiles can react directly with the electrophilic carbon in CS2.73 This generates a zwitterion which cannot be isolated or spectroscopically identified, presumably since the equilibrium lies to the left-hand side. It can, however, be deprotonated by the added base, which if it is a hydroxide then simply affords water and the desired dithiocarbamate (Fig. 2).Unfortunately, there are many examples in the literature that show the secondary (or primary) amine being deprotonated by the base and then suggesting that it is the generated amide [NR2]− or [NHR]− that reacts with CS2. As stated above, most bases used in the formation of dithiocarbamates are not basic enough to deprotonate the amine, and even if they were, the generated amides are very susceptible to hydrolysis and would immediately regenerate the amine. We note that alkali metal amides such as LiNMe2 are highly pyrophoric and should be handled in a rigorously anhydrous environment (also see section 2-iii).
(ii) In the absence of an added base, CS2 reacts with a primary or secondary amine to afford the dithiocarbamic acid
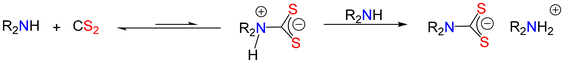
Many secondary and primary amines react directly with CS2 and do not require added base. Thus, it is tempting to suggest that the reaction furnishes the free dithiocarbamic acid. This is, however, erroneous and rather this reaction generates the corresponding ammonium salt of the dithiocarbamate (Fig. 3).Thus, it can be viewed in the same way as that above (Fig. 2), with the zwitterion being deprotonated by the amine which acts as both nucleophile and base. Thus, the overall stoichiometry is not 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 but rather 2
1 but rather 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
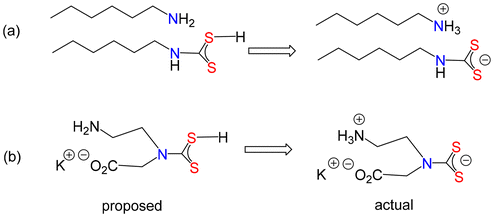
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, amine to CS2.74–76 Indeed, three purported crystal structures of dithiocarbamic acids have been reported77–79 (one later revised).80 The crystal structure of an adduct of hexylamine (HexNH2) and the dithiocarbamic acid, HexNHCS2H
1, amine to CS2.74–76 Indeed, three purported crystal structures of dithiocarbamic acids have been reported77–79 (one later revised).80 The crystal structure of an adduct of hexylamine (HexNH2) and the dithiocarbamic acid, HexNHCS2H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78 is really the ammonium salt (Fig. 4a), but the proton has been incorrectly located on sulfur rather than hexylamine. Likewise, in the proposed amino acid derivative (Fig. 4b) the proton is actually located on the basic nitrogen.79
78 is really the ammonium salt (Fig. 4a), but the proton has been incorrectly located on sulfur rather than hexylamine. Likewise, in the proposed amino acid derivative (Fig. 4b) the proton is actually located on the basic nitrogen.79
Sometimes, even when a second base has been added, the ammonium dithiocarbamate is (in part) generated in the reaction especially when the amine is a strong base, as the two compete to deprotonate the zwitterion. This is the case in the reaction of iBu2NH and CS2 in water with added NaOH, a reaction we have carried out numerous times in our lab. Thus, the off-white ammonium salt has poor water solubility and can precipitate, often being observed as a “scum” in the reaction vessel. Simply stirring in a warm (ca. 50–60 °C) water bath for 20–30 min leads to complete dissolution and formation of a clear pale-yellow solution of NaS2CNBui2.
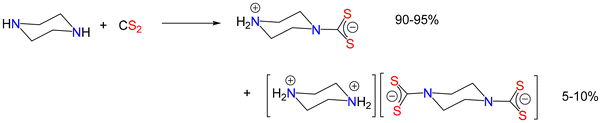
Nice illustrations of an amine acting as both a base and a nucleophile are in the reaction of diamines with CS2. For example, reaction of piperazine with CS2 affords as the major product a zwitterionic product (Fig. 5) together with a small amount (ca. 5–10%) of the (so-called) double salt, thus providing an elegant method of desymmetrising a cheap diamine which can then be used to build up novel multimetallic arrays.81–86
(iii) Reactions of diarylamines with CS2 in the presence of MOH afford diaryldithiocarbamates
Anilines are nucleophilic enough to react directly with CS2 and thus dithiocarbamates, [ArNHCS2]− are readily available.87 In contrast, diphenylamine and other diarylamines are not nucleophilic enough and will not react directly with CS2 in the presence of standard bases. Thus, to prepare [Ar2NCS2]−, the corresponding amides [NAr2]− need to be accessed; these are nucleophilic enough to react with CS2. Reagents that are basic enough to deprotonate Ar2NH include nBuLi,88,89 NaBH4,90 sodium amide91,92 and KOBut.67 All require water-free conditions otherwise the neutral amines will simply be regenerated. Thus, we have recently developed a preparation88 that uses nBuLi in thf to afford LiNAr2 at low temperatures, which in turn react with CS2 to form LiS2CNAr2 in (essentially) quantitative yields67 (Fig. 6). Once formed these dithiocarbamate salts are very stable and can be stored indefinitely as solids in air. We have also had success with using KOBut in thf but found this not to be reproducible in regular lab grade solvents. As stated above, other authors have used NaBH4 but in our hands this was not successful.(iv) Amides such as phthalimide and succinimide react with CS2 to generate dithiocarbamates
As discussed above, amines need to be relatively nucleophilic if a dithiocarbamate is to be made directly, and amides are not nucleophilic enough to react directly with CS2. Despite this, several publications claim the synthesis of amide-derived dithiocarbamates and their complexes,93–97 some of which we have failed to reproduce.93,94 As highlighted for diarylamines, a second approach is to initially deprotonate to yield the more nucleophilic anion. In this way, we have tried reacting commercially available sodium phthalimide with CS2 under a variety of conditions but in all instances, we found no evidence for the formation of the corresponding dithiocarbamate (Fig. 7).In contrast, 2-pyrrolidone does react with CS2 in the presence of bases such as KOH, to afford a dithiocarbamate which can be quenched with electrophiles to give the corresponding ester.98–100 Formamide also reacts with CS2 in the presence of base as confirmed by the crystal structure of KS2CNH(CHO).101 There is a short paper on metal complexes of 2-pyrrolidone dithiocarbamate,102 which suggests this ligand type could be further developed, along with that of iso-indolinone, for which the dithiocarbamate has not been reported but is likely accessible.
There are several authenticated examples of amide-functionalised dithiocarbamate complexes, being accessible via dithiocarbimate complexes.103 For example, deprotonation of the corresponding primary amine-derived nickel complexes, [Ni(S2CNHR)2], followed by quenching with electrophiles such as acetic anhydride and benzoyl chloride (Fig. 8).104–107 These amide-dithiocarbamate complexes are very stable and show interesting physical properties, something we have recently been reinvestigating in our own research.107
 | ||
| Fig. 8 Synthesis and molecular structure (R = iBu) of amide-functionalised nickel bis(dithiocarbamate) complex. | ||
3. Stability of dithiocarbamates and thiuram disulfides
(i) Dithiocarbamic acids are stable and isolable entities
A major misconception in dithiocarbamate chemistry, being especially prevalent in the biological domain, is that dithiocarbamic acids are stable entities. Thus, as has been well studied, disulfiram is rapidly reduced in vivo to give two equivalents of diethyldithiocarbamate, which is often erroneously written as the free acid.108 Chemists are also not immune to this mistake and often (as noted above) the direct reaction of an amine with CS2 is purported to give the dithiocarbamic acid rather than the ammonium salt of the dithiocarbamate.77–79,109–112 Dithiocarbamates are basic, especially those with two alkyl substituents, and at pH 7 or below (indeed even above this in some cases) they are protonated to give the dithiocarbamic acids. However, these are unstable and, in most cases, decompose rapidly to give CS2 and the corresponding ammonium salt (Fig. 9). Many publications have addressed the decomposition process, and mechanistic aspects have been elucidated.113–124 Here is not the place to go into detail but the main decomposition route involves a hydrogen-bonded intermediate, rather than zwitterion formation resulting from proton transfer from sulfur to nitrogen. Decomposition is accelerated upon lowering the pH and this is why dithiocarbamates are generated under basic conditions. Recently, the generation and decomposition of dithiocarbamic acids has been repurposed as a route for the release of CS2.125 These studies show that their lifetimes are highly dependent upon the nature of the substituents, those with two aryl groups being stable for up to 24 h at pH 7.4.125 Dithiocarbamic acids of primary amines are especially unstable119 but still appear in the literature.126 Dithiocarbamates of primary amines are widely used as precursors for the generation of organic isothiocyanates127,128 and other sulfur-containing organics.129,130(ii) Dithiocarbamate halides are accessible
A relatively uncommon misconception, but one that is increasingly appearing in the literature, is the idea that halides of dithiocarbamates, especially the iodide, are accessible.131 It is well known that iodine acts as an oxidising agent, converting dithiocarbamates into thiuram disulfides, rather than forming the corresponding iodide (Fig. 10). With primary amine dithiocarbamates this (likely) generates the unstable thiuram disulfide (see below), and not the iodide.132,133It is worth adding that, while not a misconception, the redox chemistry of the dithiocarbamate ligand is often overlooked, which can lead to an over-simplification of discussion of redox events when coordinated to metal centres. Examples are reactions of [M(S2CNR2)2] (M = Zn, Cd) with I2.134,135 Thus, reduction of iodine is rapid at the non-redox active Zn(II) centre as it is the dithiocarbamate that is oxidised, on metal, to form the corresponding thiuram disulfide complexes (Fig. 11).
(iii) Thiuram disulfides of primary amines are stable
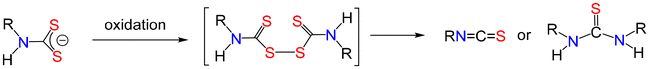
Thiuram disulfides generated from secondary amines are generally very stable and can be easily isolated, purified and stored. Indeed, they are often easier to store than the corresponding dithiocarbamates and can then be reduced in situ to provide a source of dithiocarbamate. In contrast, oxidation of primary amine dithiocarbamates affords thiuram disulfides with poor stability, decomposing to afford isothiocyanates and/or thioureas, depending upon the reaction conditions used127,136–140 (Fig. 12).4. Accessibility and air-stability of [M(S2CNR2)2]
One of the beauties of doing transition metal dithiocarbamate chemistry is their ease of synthesis, isolation, and purification, much of which can be carried out in air. Thus, complexes normally have moderate to good solubility in polar organic solvents, and many can be crystallised in air, either by slow evaporation or the (careful) addition of an anti-solvent such as hexane or petroleum ether. In this way, the crystal structures of thousands of dithiocarbamate complexes have found their way into the Cambridge Crystallographic Data Centre (CCDC).141 However, not all dithiocarbamate complexes are air stable. For example, while bis(dithiocarbamate) complexes, [M(S2CNR2)2] (M = Ni, Cu, Zn) are air and moisture stable, in contrast those of the other first row transition elements can only be prepared (if at all) under rigorously oxygen-free conditions.142–144 Thus, generally, if they can be prepared, [M(S2CNR2)2] complexes are readily oxidised in air, with M(III) complexes being favoured. Below we discuss each metal type individually as their chemistry differs. We also describe some related chemistry (where appropriate) to provide context.(i) M = Ti, V, Cr
The chemistry of titanium is dominated by the +4 and +3 oxidation states, and dithiocarbamates are no exception. A Ti(II) complex has briefly been mentioned in the literature,145 reaction of [Ti(NEt2)3] and CS2 being reported to give a mixture of [Ti(S2CNEt2)2] and [Ti(S2CNEt2)4]. However, no characterising data or reaction details were given. The existence and stability of red Ti(IV) complexes [Ti(S2CNR2)4] are without doubt, being prepared upon insertion of CS2 into [Ti(NR2)4],146 or upon addition of LiS2CNR2 to TiBr4.147 Both [Ti(S2CNEt2)4]148 and [Ti(S2CNPri2)4]147 have been crystallographically characterised. One report suggests that reaction of NaS2CNR2 with TiCl3 in EtOH affords [Ti(S2CNR2)3] as brown- or yellow-green solids,149 but there are no later mentions of this type of complex in the literature. Thus, the stability of [Ti(S2CNR2)3], their potential disproportionation to [Ti(S2CNR2)2] and [Ti(S2CNR2)4], and structure and stability of the former remain issues that require clarification. Interestingly, it has recently been established that dithiocarbamates can coordinate to Ti(0), thus, oxidation of [Ti(CO)6]2− by thiuram disulfides affords [Ti(CO)4(S2CNR2)]− which adopt an unexpected trigonal prismatic geometry.150 There may yet be new things to discover in titanium dithiocarbamate chemistry.The only V(II) dithiocarbamate complex reported is light brown [V(S2CNEt2)2], formed under rigorously oxygen-free conditions upon addition of two equivalents of NaS2CNEt2 to VCl2 in MeCN in a dry box.143 Unfortunately, no characterising data was given, but it was reported to be soluble in warm 1,2-dichlorobenzene, so may be worthy of reinvestigation. In air, addition of [R2NH2][S2CNR2] to VBr2·6H2O affords [V(S2CNR2)3].151 The diethyl derivative has been crystallographically characterised152 and magnetic measurements show a S = 1 ground state with two unpaired electrons.153 Possibly, [V(S2CNR2)2] are fleetingly formed but readily oxidised, and putative [V(S2CNR2)2]+ reacts with further dithiocarbamate. The reduction chemistry of [V(S2CNEt2)3] has been investigated and reveals a reversible one-electron process.154 Eight-coordinate V(IV) complexes, [V(S2CNR2)4], are accessible from the insertion of CS2 into [V(NR2)4],155 but upon heating they undergo intramolecular electron transfer resulting in elimination of thiuram disulfide and formation of [V(S2CNR2)3]. Formal oxidation products of [V(S2CNR)2], namely vanadyl complexes [VO(S2CNR2)2], are easily prepared156 and have been extensively investigated as potential insulin mimetics157 and molecular qubits.158 Thus, akin to the titanium chemistry, even the simple dithiocarbamate chemistry of vanadium requires further investigation.
Chromium bis(dithiocarbamate) complexes are indisputably accessible, but extremely air sensitive, so unless rigorous experimental methods are utilised it is the analogous d3 Cr(III) complexes, [Cr(S2CNR2)3] that are isolated.159–161 The first synthesis of [Cr(S2CNEt2)2] was reported by Fackler and Holah in 1966,162 being described as a pyrophoric yellow-green solid. It has subsequently been prepared from thermolysis of [Cr(CO)6] and [Hg(S2CNEt2)2].163 In solution [Cr(S2CNEt2)2] is extremely sensitive to air oxidation, converting rapidly to blue [Cr(S2CNEt2)3]. Very few derivatives have been prepared and there are no crystal structure determinations, thus molecular structure(s) of [Cr(S2CNR2)2] remain unknown, although most likely they are polymeric in the solid state, and tetrahedral in the gas phase or solution. Electrochemical studies of [Cr(S2CNR2)3] show that they undergo a one-electron reduction, with the generated [Cr(S2CNR2)3]− rapidly losing a dithiocarbamate to give [Cr(S2CNR2)2]164 (Fig. 13). However, all attempts to isolate or characterise [Cr(S2CNR2)2] generated in this way have led only to rapid back oxidation and regeneration of [Cr(S2CNR2)3], again highlighting their extreme air-sensitivity.
Non-homoleptic Cr(II) dithiocarbamate complexes can be prepared. For example, room temperature addition of [CpCr(CO)3]2 and tetraethyl thiuram disulfide affords [CpCr(CO)2(S2CNEt2)] in good yields.165
(ii) M = Mo, W
We briefly consider the heavier group 6 homologues, [Mo(S2CNR2)2] and [W(S2CNR2)2]. The synthesis and X-ray crystal structure of [Mo(S2CNEt2)2] has been claimed166 but is erroneous: the structure presented is that of [Ni(S2CNEt2)2] with a misassigned metal atom.167 Complexes of the stoichiometry Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dithiocarbamate 1
dithiocarbamate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 are known. Thus, reactions of [Mo2(μ-OAc)4] with four equivalents of dithiocarbamate afford [Mo2(μ-S2CNR2)4] as green solids.168 However, the latter are thermally unstable and upon heating undergo C–S bond scission to yield Mo(V) thiocarboxamide complexes, [Mo(μ-S)(S2CNR2)(SCNR2)]2 as shown crystallographically (R = iPr).169 Related W(II) complexes, [W(S2CNR2)2], remain unreported, but both cis-[W(CO)2(S2CNR2)2] and [W(CO)3(S2CNR2)2] are known170 as are the corresponding Mo(II) carbonyl complexes.171
2 are known. Thus, reactions of [Mo2(μ-OAc)4] with four equivalents of dithiocarbamate afford [Mo2(μ-S2CNR2)4] as green solids.168 However, the latter are thermally unstable and upon heating undergo C–S bond scission to yield Mo(V) thiocarboxamide complexes, [Mo(μ-S)(S2CNR2)(SCNR2)]2 as shown crystallographically (R = iPr).169 Related W(II) complexes, [W(S2CNR2)2], remain unreported, but both cis-[W(CO)2(S2CNR2)2] and [W(CO)3(S2CNR2)2] are known170 as are the corresponding Mo(II) carbonyl complexes.171
(iii) [Mn(S2CNR2)2]
Bright yellow [Mn(S2CNR2)2] complexes can easily be prepared, but both the reaction and work-up must be carried out under rigorously inert conditions.172,173 The molecular structure of only one example, namely [Mn(S2CNEt2)2], has been reported (Fig. 14).174 It is a coordination polymer containing octahedral Mn(II) centres resulting from the dithiocarbamates binding in a bridging manner. | ||
| Fig. 14 Part of the polymeric structure of [Mn(S2CNEt2)2].174 | ||
Many papers detail reactions of two equivalents of dithiocarbamates with Mn(II) salts that have been carried out in water and air, and invariably the generated products are dark (often violet-purple) paramagnetic solids formulated as [Mn(S2CNR2)2]. Authors often cite elemental analysis data as proof of this formulation. Indeed, fits with calculated values are often good, albeit in many cases a small amount of bound water (between 0.5 and 2 molecules) are suggested. Recently, this issue has been addressed, and it is now unequivocally established that the dark product described is a Mn(III) oxygen adduct.175 The precise nature of this adduct is less clear, with the authors suggesting it is monomeric, but a recent crystal structure (Fig. 15) has shown that (at least in part) it is a dimeric oxygen-bridged complex.176
 | ||
| Fig. 15 Oxidation of [Mn(S2CNR2)2] with proposed Mn(III) adducts and the molecular structure of [Mn2(S2CNEt2)4(μ-O2)].175,176 | ||
The colour of the oxygen adducts is very similar to that for Mn(III) complexes, [Mn(S2CNR2)3], generated upon addition of three equivalents of dithiocarbamate to Mn(II) salts in air, as is the spectroscopic data. Thus, it is not easy to differentiate between the two. If two equivalents of dithiocarbamate are added to Mn(II) salts in water under argon then heavy yellow precipitates result, which are unequivocally [Mn(S2CNR2)2]. Further, later exposure to oxygen (bubbling through solution works best to ensure full oxidation) reliably affords the pure oxygen adduct.175 Addition of more dithiocarbamate salt to the latter gives [Mn(S2CNR2)3], although it is hard to know exactly when the reaction is complete and to ensure that it is, a slight excess of dithiocarbamate is recommended.175
Manganese dithiocarbamate complexes are becoming widely used as single source precursors (SSPs) to manganese sulfide nanomaterials.177–180 In this respect, the accidental use of the oxygen adduct may be advantageous. Thus, oxygen adducts have a higher manganese content than [Mn(S2CNR2)3] and, as shown by thermogravimetric analysis (TGA) for the diethyl derivatives (Fig. 16), decompose at lower temperatures. Indeed, TGA is a good way of differentiating between these two Mn(III) dithiocarbamate complexes.176 Finally, we note that [Mn(S2CNR2)3] decomposes in a two-step process, the former being associated with loss of a dithiocarbamate and in situ formation of [Mn(S2CNR2)2], thus negating the need to isolate air sensitive Mn(II) complexes.
 | ||
| Fig. 16 TGA data for [Mn(S2CNEt2)3] and [Mn2(S2CNEt2)4(μ-O2)].176 | ||
An early application of dithiocarbamates was as fungicides, with manganese containing Maneb© being widely used. It is formed from the reaction of Mn(II) salts with Nabam©, the bis(dithiocarbamate) generated from ethylene diamine (Fig. 17). More recently it has been linked to the development of Parkinson's disease181,182 and since 2009 has been banned in the European Union, a fate also experienced by the zinc complex, Zineb©, which was also previously used as a pesticide.
Maneb© and Zineb© were used in agriculture for many years and are proposed to have polymeric structures, as has the double hydration product of Maneb© namely [Mn(S2CNHC2H4NHCS2)·2H2O]n. Recently a good quality molecular structure of the dimethylformamide (dmf) adduct [Mn(S2CNHC2H4NHCS2)·2dmf]n was reported183 (Fig. 18) and confirms this. Manganese is in the +2 oxidation state but, importantly, has an octahedral coordination geometry with coordination of two cis dmf molecules (there are another two non-coordinated dmfs not shown). Likely hydrated Maneb© has a very similar structure except with bound water. Another interesting observation is that while pure samples of this adduct and Maneb© are yellow, the latter ages over time, becoming dark.
 | ||
| Fig. 18 Part of the polymeric structure of [Mn(S2CNHC2H4NCS2)·2dmf]n.183 | ||
(iv) [Fe(S2CNR2)2]
Like their manganese counterparts, [Fe(S2CNR2)2] can be prepared and isolated but are extremely air sensitive and should be handled in a dry box or a high quality Schlenk line environment. When prepared under the correct conditions they are described as chocolate brown,162 pink142 or red184 solids. Crystal structures of two polymorphs of [Fe(S2CNEt2)2]185,186 show that it adopts a dimeric structure in the solid state (Fig. 19). Each iron centre is 5-coordinate, being best described as a distorted trigonal bipyramid. No other molecular structures have been determined, but there is some early evidence from Mössbauer studies that some derivatives may be coordination polymers.187,188 | ||
| Fig. 19 The molecular structure of [Fe(S2CNEt2)2]2.185 | ||
As with the manganese chemistry described above, many authors describe the purported synthesis of Fe(II) bis(dithiocarbamate) complexes in air, and thus their assignment is clearly wrong. Unlike the manganese chemistry, however, it is less clear exactly what they have made if only two equivalents of dithiocarbamate are used. Thus, oxygen adducts of [Fe(S2CNR2)2] have not been reported. These complexes do undergo rapid addition, for example addition of bis(perfluoromethyl)-1,2-dithietene affords Fe(III) complexes (Fig. 20) showing how prone they are to oxidation.189 It is also known that [Fe(S2CNR2)2] react with excess dithiocarbamate to afford yellow [Fe(S2CNR2)3]−, which are also extremely air sensitive, being rapidly oxidised to brown Fe(III), [Fe(S2CNR2)3].190 Thus, it is most likely that reaction of two equivalents of dithiocarbamate with Fe(II) salts in air simply gives reduced yields of [Fe(S2CNR2)3] along with some unreacted Fe(II) salt.
 | ||
| Fig. 20 Reactions of [Fe(S2CNR2)2] with a dithietane resulting in oxidation, and addition of further dithiocarbamate followed by oxidation. | ||
Bis(dithiocarbamate) complexes [Fe(S2CNR2)2] also bind neutral donor ligands, as shown in crystal structures of a dmf adduct of the morpholine-dithiocarbamate complex (Fig. 21a)191 and a DABCO adduct of [Fe(S2CNEt2)2] which co-crystallises with C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 144 (Fig. 21b). Thus, from aqueous solutions, it may be that a species [Fe(S2CNR2)2·H2O] precipitates out. Interestingly, an NMR spectrum of [Fe(S2CNC4H8O)2·dmf] run in CDCl3 was found to be identical to that of paramagnetic [Fe(S2CNC4H8O)3], suggesting oxidation and ligand arrangement upon dissolution in this weakly acidic solvent.191 Thus, it might be that Fe(II) is reducing enough to convert H+ into H2 with concomitant formation of Fe(III).
144 (Fig. 21b). Thus, from aqueous solutions, it may be that a species [Fe(S2CNR2)2·H2O] precipitates out. Interestingly, an NMR spectrum of [Fe(S2CNC4H8O)2·dmf] run in CDCl3 was found to be identical to that of paramagnetic [Fe(S2CNC4H8O)3], suggesting oxidation and ligand arrangement upon dissolution in this weakly acidic solvent.191 Thus, it might be that Fe(II) is reducing enough to convert H+ into H2 with concomitant formation of Fe(III).
 | ||
| Fig. 21 Molecular structure of (a) [Fe(S2CNC4H8O)2·dmf]191 and (b) [{Fe(S2CNEt2)2}2DABCO] as part of a large structure with C60·2DABCO.144 | ||
The Fe(II) oxidation state can be also stabilised by binding of carbonyls in cis-[Fe(CO)2(S2CNR2)2]192,193 and NO in [Fe(NO)(S2CNR2)2].194,195 Indeed, the binding of NO to [Fe(S2CNEt2)2], as determined by the distinctive EPR spectrum of [Fe(NO)(S2CNEt2)2], is an accepted way of determining the presence of this important signalling gas.196–198 It is noteworthy, however, that reaction of NO with [Fe(S2CNEt2)3] also affords [Fe(NO)(S2CNEt2)2] (together with thiuram disulfide as a byproduct).198 Photolysis of [Fe(S2CNEt2)3] also results in elimination of thiuram disulfide and formation of [Fe(S2CNEt2)2], which can be trapped with bis(diphenylphosphino)ethane (dppe) to afford [Fe(κ2-dppe)(S2CNEt2)2].199 This suggests that there is a fine redox balance between the reducible Fe(III) centre and the oxidisable dithiocarbamate. Indeed, we have found using in situ EXAFS, that upon warming [Fe(S2CNiBu2)3] in oleylamine at 60 °C, reductive elimination of thiuram disulfide results with concomitant formation of the corresponding Fe(II) complex.193 Further, TGA measurements show that cis-[Fe(CO)2(S2CNR2)2] lose both carbonyls at relatively low temperatures to afford [Fe(S2CNR2)2]. Thus, [Fe(S2CNR2)2], [Fe(S2CNR2)3] and cis-[Fe(CO)2(S2CNR2)2] are all effectively equivalents when used as SSPs193 thus negating the need to isolate air sensitive [Fe(S2CNR2)2].
Neither [M(S2CNR2)2] (M = Ru, Os) appear in the reliable literature, with both metals favouring the M(III) state [M(S2CNR2)3], but like iron, carbonyl-stabilised M(II) complexes cis-[Ru(CO)2(S2CNR2)2]200 and cis-[Os(CO)2(S2CNR2)2]201–203 are accessible.
(v) [Co(S2CNR2)2]
The theme continues into the chemistry of cobalt. Like iron and manganese, cobalt forms stable M(III) tris(dithiocarbamate) complexes [Co(S2CNR2)3], the low spin d6 electronic configuration allowing NMR characterisation.204–206 In contrast, while the corresponding M(II) species [Co(S2CNR2)2] are accessible, they are extremely air-sensitive and paramagnetic, with [Co(S2CNEt2)2] being described as a dark green-brown solid.142 It is telling that there are no crystal structures of [Co(S2CNR2)2] and examples of authentic syntheses and characterisation are rare. Most authors report the formation of green precipitates from reactions of dithiocarbamate salts with CoCl2·6H2O in air, i.e. they actually generate [Co(S2CNR2)3]. Recently, Khitrich and co-workers provided some evidence for the formation of [Co(S2CNR2)2],207 suggesting that, provided pH is kept between 6–7, then even in air aqueous solutions of [CoCl2] react with two equivalents of NaS2CNR2 to afford [Co(S2CNR2)2] and note that in the solid state they are air stable. Good elemental analysis data are presented, and powder X-ray diffraction studies reveal (for those that are not amorphous) the absence of [Co(S2CNR2)3]. Further, magnetic moments of 2.19–2.45 BM suggest that they have one unpaired electron, consistent with a square planar geometry. Interestingly, when dissolved in organic solvents they oxidise rapidly with formation of [Co(S2CNR2)3] and [Co(OH)2] (Fig. 22). Clearly more work in this area is warranted. | ||
| Fig. 22 Proposed decomposition of [Co(S2CNR2)2] in organic solvents.207 | ||
Electrochemical reduction of [Co(S2CNR2)3] leads to dithiocarbamate loss to give [Co(S2CNR2)2], but back oxidation is facile.208 Chemical reduction of [Co(S2CNR2)3] by excess Zn/Hg in CH2Cl2 results in a slow (ca. 3 h) darkening of the green solution and when canulated under nitrogen onto PhI = NTs, the Co(II) complexes react via insertion into the Co–S bonds to afford crystallographically characterised [Co{TsNSC(NR2)SNTs}2]209 (Fig. 23). Thus, it is chemically possible to prepare [Co(S2CNR2)2] in organic solvents but oxygen must be rigorously excluded.
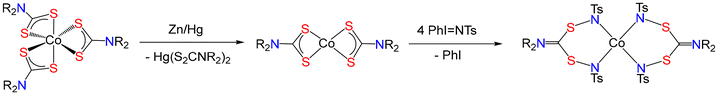 | ||
| Fig. 23 Reductive generation of [Co(S2CNR2)2] and trapping via multiple NTs insertion in the Co–S bonds. | ||
We note that there are no authentic examples of [Rh(S2CNR2)2] or [Ir(S2CNR2)2] complexes,210,211 all purported syntheses being likely that of the analogous [M(S2CNR2)3] complexes.212,213 Electrochemical reduction of [Rh(S2CNR2)3] is irreversible and shown to be a two-electron process with formation of [Rh(S2CNR2)2]− and dithiocarbamate being suggested.214
5. Structure and stability of dithiocarbamate complexes
(i) [M(S2CNR2)2] (M = Mn, Fe, Co) are all square planar
In solution or the gas phase, the molecular structures of group 10 bis(dithiocarbamate) complexes and [Cu(S2CNR2)2] are square planar. In the solid state, the 4-coordinate square planar arrangement is maintained for [M(S2CNR2)2] (M = Ni, Pd, Pt), but the precise metal coordination environment for copper varies with substituents due to secondary Cu⋯S interactions.8,13 The solution and gas phase structures of [M(S2CNR2)2] (M = Mn, Fe, Co) remain unknown, although some authors suggest that all are square planar. This seems highly unlikely for [Mn(S2CNR2)2] as for a d5 electronic conformation one would not expect the difference in crystal field stabilisation energies (CFSEs) between tetrahedral and square planar geometries to override steric effects, even when the preferred bite angle for dithiocarbamates is less than 90°. An early communication suggested that, in ethanol, [Mn(S2CNEt2)2] adopts a spin quartet state, i.e. has 3 unpaired electrons,215 although it was later shown that this result was erroneous, as measurements had been made on a partially oxidised sample. Upon rigorous exclusion of air, magnetic measurements on [Mn(S2CNEt2)2] and other derivatives show that they adopt a ground state with 5 unpaired electrons.172 Thus, it seems likely that [Mn(S2CNR2)2] have a distorted tetrahedral coordination geometry, although a recent DFT study suggests a square-planar arrangement, which may be a local minimum.216 In a similar vein, iron complexes, [Fe(S2CNR2)2], with a d6 electronic configuration have been shown to have 4 unpaired electrons,186 being inconsistent with a square-planar array. Nevertheless, recent DFT calculations propose a local FeS4 geometry with D2h symmetry, consistent with a square planar geometry.217 Clearly, accurate calculations are required to corroborate the actual coordination geometry. The absence of any crystallographic data makes it harder to predict the molecular geometry of d7 [Co(S2CNR2)2], but they are likely square-planar, as supported by the magnetic susceptibility measurements and DFT calculations.218(ii) Ru(III) complexes [Ru(S2CNR2)3] and [Ru(S2CNHR)3] are diamagnetic
Iron tris(dialkylDTC) complexes, [Fe(S2CNR2)3], have been widely studied as spin crossover complexes as they can adopt either high spin (HS) 6A1 or low spin (LS) 2T2 electronic configurations, the position of the equilibrium being affected by the nature of the alkyl-substituents and external factors such as temperature and pressure.219–222 Receiving less attention are analogous Ru(III) complexes, [Ru(S2CNR2)3], although a number of early papers established their fluxional distorted octahedral structures223 and paramagnetic nature,224,225 the latter being as expected for a d5 electronic configuration. Nevertheless, several publications claim to have prepared diamagnetic Ru(III) tris(dithiocarbamate) complexes of both secondary and primary amines, characterised by (almost perfect) elemental analysis data and 1H NMR peaks in the standard 0–10 ppm range.226–228 Interestingly, 1H NMR spectroscopy can be used to part-characterise [Ru(S2CNR2)3], although peaks are observed across a ca. 40 ppm range, as expected for a weak (low spin) paramagnetic system.229–231 Diamagnetic complexes can be formed from the reaction of RuCl3 and dithiocarbamates, indeed many studies have reported mixtures of paramagnetic [Ru(S2CNR2)3] and diamagnetic [Ru2(S2CNR2)5]+ (which can exist in two isomeric forms) from these reactions.229–232 Thus, it may be that such diamagnetic cations are the real products of the reactions described above.226–228 Akin to their ruthenium analogues, osmium complexes, [Os(S2CNR2)3], are also low spin d5 systems that show resonances across a wide chemical shift range in their 1H NMR spectra and are easily oxidised.233(iii) Stable dithiocarbamate complexes of high-valent metal oxides are always accessible
One of the major benefits of the dithiocarbamate ligand is its ability to stabilise metals in a wide range of oxidation states, being (in simple terms) attributed to the accessibility of dithiocarbamate and thioureide resonance forms. Thus, the former is a relatively soft (strong field) and the latter a hard (weak field) donor. While not unique to dithiocarbamates, some ligand sets, for example the related xanthates (ROCS2−), cannot adopt the latter form as the oxygen is too electronegative to act as an electron-pair donor.234 Adoption of the thioureide form also accounts for the partial double bond character of the backbone C–N vector which leads to hindered rotation about it.235 Thus, rotamers of complexes [M(S2CNR1R2)2] (M = Ni, Pd) can be distinguished, and their rate of interconversions measured.236 The thioureide form can also account for the stability of high-valent species such as of Mo(VI) and W(VI) complexes.8,9 However, one should not forget that the dithiocarbamate is redox active, being relatively easily oxidised, and thus when bound to a highly oxidised metal centre, the system is on a cliff edge, as beautifully illustrated by a series of publications from Stiefel and co-workers.237–241While a wide range of metal centres and oxidation states can be tolerated by dithiocarbamates,8 this does not extend to the most oxidising. For example, reaction of dithiocarbamate salts with potassium dichromate, K2[Cr2O7], affords a mixture of two Cr(III) products: ring-expanded [Cr(S2CNR2)2(OS2CNR2)] as the major component together with [Cr(S2CNR2)3] (R = Me, Et)242 (Fig. 24). Thus, (at least formally) three equivalents of dithiocarbamate act as one-electron reducing agents and the other three as ligands. The precise mode of formation of the ring-expanded complex is not clear. In related work, Farmer and co-workers showed that addition of H2O2 to [Ru(κ2-2,2′-bipy)2(S2CNMe2)]+ afforded both ring-expanded sulfur-oxidised isomers but they did not interconvert, suggesting that they are formed via two separate pathways,243 although in other work they showed that [Zn(S2CNEt2)(OS2CNEt2)] was a product of the oxygenation of [Zn(S2CNEt2)2], a transformation that can be reversed upon addition of a phosphine.244 Interestingly, reaction of chromate with excess dithiocarbamate has been repurposed as an analytical method of measuring relative amounts of Cr(III) and Cr(IV).245–247 Thus, Cr(III) reacts to form [Cr(S2CNR2)3] and Cr(VI) a mixture of this and the ring-expanded products. Importantly the two products have different chromatographic retention times and thus by understanding the precise ratio of products formed from the Cr(VI) reaction it is possible to determine relative amounts of Cr(III) and Cr(VI), although not all publications seem to do it this way, with some suggesting that Cr(III) is unreactive towards dithiocarbamates.
Likely dithiocarbamates cannot stabilise high oxidation states such as Os(VIII) and Re(VII), since these metal centres are powerful oxidising agents. There are reports of Os(VI) dithiocarbamate complexes, trans-[OsO2(S2CNR2)2],248,249 which show distinctive vibrations at 839 and 888 cm−1 in the IR spectrum, being assigned to asymmetric and symmetric trans-OsO2 vibrations respectively.249 Further investigations are needed to unequivocally establish the validity of these claims, but they seem quite likely to be correct given that the trans-[Os(VI)O2] moiety is particularly stable, being found for example in complexes such as trans-[OsO2(CO)4]2+ and trans-[OsO2(OH)4]2−.
(iv) Au(III) complexes, [Au(S2CNR2)2]+ and [AuCl2(S2CNR2)], are easily prepared in their pure forms
Gold(III) dithiocarbamate complexes of the type [AuX2(S2CNR2)] (where X = Cl, Br, I) have been widely studied as potential anti-cancer metallo-pharmaceuticals. Structurally they are comparable to cis-platin and the mechanism of action is suspected to be similar.250 These compounds were first synthesised in 1964 by oxidation of the Au(I) complex [Au(S2CNR2)]n with elemental X2.251 This supposedly afforded the Au(III) dithiocarbamate [AuX2(S2CNR2)] as a single compound in solution. Alternatively, the addition of one equivalent of dithiocarbamate to Au(III) halides is claimed to result in the sole formation of [AuX2(S2CNR2)]. In a similar fashion, addition of two equivalents of dithiocarbamate to Au(III) halides supposedly resulted in the sole formation of the cationic complex [Au(S2CNR2)2]+.9 Often in this system X− is invoked as the counterion, but a haloaurate species is more likely to be present.We recently investigated the [AuX2(S2CNR2)] system thoroughly and discovered that the established reactivity is not correct.252 The series of compounds [AuX2(S2CNR2)] where X = halide cannot be isolated as a single species in solution; instead, they exist in an equilibrium with [Au(S2CNR2)2][AuX4] (Fig. 25). This equilibrium is present irrespective of the method of synthesis, or the ratio of dithiocarbamate to Au(III). A careful look at the older literature reveals that this equilibrium has always been present even though it had not previously been acknowledged. For example, the 1H and 13C{1H} NMR spectra of dibenzyl-dithiocarbamate- and methylsarcosine-dithiocarbamate-derived Au(III) complexes both clearly show the presence of two dithiocarbamate environments, one for the neutral species and one for the ion pair.253,254 Mass spectrometry of [AuX2(S2CNR2)] revealed that the cation [Au(S2CNR2)2]+ was also present in the sample, although it was explained as decomposition in solution.255 A single crystal containing [AuCl2(S2CNiPr2)], two [Au(S2CNiPr2)2]+ cations and two [ClO4]− anions was obtained, showing that both components of the equilibrium were present in a single reaction mixture.256 Recently, it was shown that an equilibrium mixture where [Au(S2CNiPr2)2][AuCl4] was the majority product could be converted to a mixture where [AuCl2(S2CNiPr2)] was the majority product by refluxing in acetone.257
We note here that it is possible to obtain [Au(S2CNR2)2]+ as a single species in solution, but the counterion must be chosen with care and haloaurate anions should be avoided. For example, use of a weakly coordinating anion such as [BF4]− led to the isolation of [Au(S2CNR2)2][BF4] which showed no sign of existing as an equilibrium, even upon standing in solution for several weeks. However, as soon as the anion was metathesized to [AuCl4]−, the equilibrium restarted.252 In a similar vein, it is possible to isolate [AuX2(S2CNR2)] as a single pure product in solution by avoiding the use of halide ions: stable systems are known to exist for X = Me, C6F5, mesityl and thiolate, for example.258–261
The above notwithstanding, it is possible to fractionally crystallise [AuX2(S2CNR2)] as a single compound. The neutral species forms orange crystals (in contrast to the ion pair, which is yellow); these are indefinitely stable in the solid phase, but as soon as the crystals are dissolved in an organic solvent then the equilibration process resumes. Researchers are urged to be cautious here by not mistaking this for a single pure product in solution.261 It is also noteworthy that elemental analysis is not a useful characterization technique since the two components of the equilibrium, namely [AuX2(S2CNR2)] and [Au(S2CNR2)2][AuX4], have identical empirical formulae and cannot be used to distinguish the two isomers.262
(v) Primary amine-derived dithiocarbamate complexes are always stable
The majority of dithiocarbamate chemistry focuses on those derived from secondary amines. There are several reasons for this, one being their greater stability vs. those derived from primary amines. Thus, while all dithiocarbamates are unstable in acidic media, the relatively acidic nature of the unique proton in RNHCS2− also makes them prone to instability in basic media. Nevertheless, there are some relatively simple and highly reproducible syntheses of primary amine dithiocarbamate salts. Examples of these are group 10263–265 and group 12266–269 [M(S2CNHR)2] complexes, but also [Co(S2CNHR)3]270,271 and 99mTc complexes with radiopharmaceutical applications.272–274 The authenticity of homoleptic primary amine dithiocarbamate complexes of other metals is far less certain and likely the majority are too unstable to be isolated.Upon metal binding the acidity of the backbone proton is retained, its removal generating the dianionic dithiocarbimate (or imidomethanedithiolate) ligand, S2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR2−. Such ligands form complexes with a wide range of metals,103 being especially prevalent for Group 10 elements and when the substituent is strongly electron-withdrawing, for example aryl or cyanide. For Group 10 elements, as discussed earlier (Fig. 8), double deprotonation upon addition of a strong base of [M(S2CNHAr)2] and [M(S2CNHR)2] affords the corresponding dithiocarbimate dianions that, provided they are kept under anhydrous and oxygen-free conditions, have significant lifetimes and can be used to prepare functionalised dithiocarbamate derivatives. Stability of the dithiocarbimate complexes (presumably) results from delocalisation of the excess electron density into the vacant d-orbital. However, this is not the case for most other metals. For example, addition of base to Group 12 complexes [M(S2CNHR)2] and [M(S2CNHAr)2] (M = Zn, Cd) results in rapid decomposition to give ZnS or CdS nanomaterials.266–268 Indeed, this is advantageous, as is the case for [Ni(S2CNHR)2]275 as they provide low temperature routes to these nanomaterials (Fig. 26).
NR2−. Such ligands form complexes with a wide range of metals,103 being especially prevalent for Group 10 elements and when the substituent is strongly electron-withdrawing, for example aryl or cyanide. For Group 10 elements, as discussed earlier (Fig. 8), double deprotonation upon addition of a strong base of [M(S2CNHAr)2] and [M(S2CNHR)2] affords the corresponding dithiocarbimate dianions that, provided they are kept under anhydrous and oxygen-free conditions, have significant lifetimes and can be used to prepare functionalised dithiocarbamate derivatives. Stability of the dithiocarbimate complexes (presumably) results from delocalisation of the excess electron density into the vacant d-orbital. However, this is not the case for most other metals. For example, addition of base to Group 12 complexes [M(S2CNHR)2] and [M(S2CNHAr)2] (M = Zn, Cd) results in rapid decomposition to give ZnS or CdS nanomaterials.266–268 Indeed, this is advantageous, as is the case for [Ni(S2CNHR)2]275 as they provide low temperature routes to these nanomaterials (Fig. 26).
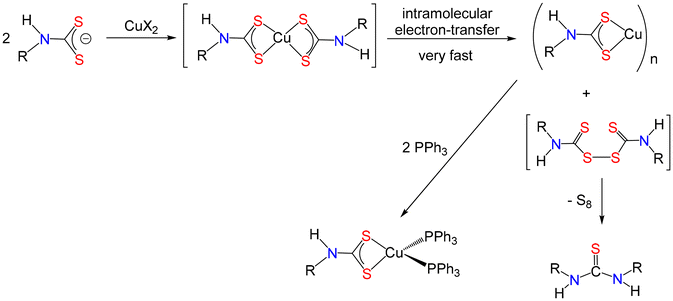
For organic chemists reading this it may ring bells as the base-induced decomposition of primary amine dithiocarbamates to afford organic isothiocyanates is a well-developed preparative method.276–278 Indeed, often a metal ion is added to facilitate the process. Thus, such complexes are not stable, certainly under basic conditions, rapidly decomposing to afford the isothiocyanate. Nevertheless, there are (purported) examples of [Fe(S2CNHR)3]279,280 and [Cu(S2CNHR)2]281–285 in the literature, some of the latter being touted as enzyme inhibitors.285 We have recently looked more closely at both systems in our laboratory and will publish our results in due course. The iron system is complex and is not appropriate to discuss here, but [Fe(S2CNHR)3] have at best a fleeting stability. Reactions of copper(II) salts with a range of primary amine derived dithiocarbamates in water leads to isolation of high yields of bright yellow polymeric Cu(I) complexes, [Cu(S2CNHR)]n (R = Bu, Cy) along with the corresponding thiourea.286 Their poor solubility hinders full characterisation, but simple addition of a slight excess of PPh3 affords the soluble and crystallographically characterised complexes, [Cu(S2CNHR)(PPh3)2]286 (Fig. 27). In this regard we note that [Cu(S2CNHPh)(PPh3)2] has previously been prepared from reaction of [Cu(BH4)(PPh3)2] with PhNCS.287 Thus, primary amine dithiocarbamates are stable at a Cu(I) centre but not Cu(II).
We briefly note here that [NH4][S2CNH2] is easily prepared288,289 and has been used to generate a range of stable homoleptic complexes, including crystallographically characterised: [Ni(S2CNH2)2],290 [Zn(S2CNH2)2],291 [Cr(S2CNH2)3],292 [Co(S2CNH2)3],288,293 [Rh(S2CNH2)3],288 [Ag(S2CNH2)],294 [Au(S2CNH2)],295 [Au(S2CNH2)2][SCN],296 [Cu(S2CNH2)]297 and [Cu(S2CNH2)2][NH4].297 Considering the discussion above, the finding that this ligand is only stabilised at the Cu(I) centre is interesting. Further several non-homoleptic Ru(II) derivatives have been prepared and crystallographically characterised288 suggesting that there is a relatively rich coordination chemistry of this ligand still to be explored.
(v) Complexes with two different dithiocarbamate ligands and dithiocarbamate-containing mixed-ligand complexes are (easily) accessible in a pure state
Many homoleptic dithiocarbamate complexes are known, being easy to prepare even in the most basic of laboratory settings.8,9 In developing this chemistry further, it is tempting to consider preparing related complexes in which two (or more) different dithiocarbamates bind to a single metal centre, thus providing easy tuning of chemical and physiochemical properties. Consequently, over the past decade, there has been an increasing number of publications claiming the formation of pure complexes containing two different dithiocarbamate ligands of Ni(II),298 Cu(II),299–301 zinc and mercury302–306 and other transition metal307–309 and main group310–315 elements. In all these reports, the simple addition of equimolar equivalents of two different dithiocarbamates to a metal salt is reported to (cleanly) afford the mixed-ligand complex, without any explanation as to why a mixed-ligand complex might be thermodynamically preferable to either the two homoleptic complexes or a statistical mixture of homo- and heteroleptic complexes. Very few of these reports are supported with single crystal X-ray data, or compelling characterising data. Crystal structures of [Hg(S2CNMePh)(S2CNEtPh)]304 and [Zn(S2CNMePh)(S2CNEtPh)(2,2′-bipy)]305 are in the literature but in both there is a significant disorder of the Me/Et groups. The only good quality (non-disordered) example of a molecular structure of a heteroleptic dithiocarbamate complex is that of the anion [Cd(S2CNPr2)2(S2CNMeBu)]−, formed (in a similar manner to other examples) upon addition of pyrrolidinium salts of [S2CNMeBu]− to [Cd(S2CNPr2)2].316 However, while the crystal structure shows a well-ordered mixed-dithiocarbamate complex, both 1H and 13C{1H} NMR spectra show that in solution multiple products are present, resulting from ligand-exchange. Thus, while complexes with two different dithiocarbamates are accessible as part of a mixture and can (at least potentially) be selectively crystallised out, they are not accessible in pure form in solution.For diamagnetic species, NMR spectroscopy should be a simple way of probing such equilibria, however, the (relatively) slow timescale coupled with low intensity of the quaternary backbone carbon signal in the 13C{1H} NMR spectrum and its insensitivity to substituent changes317 renders it unsuitable. In contrast, HPLC has been shown to be an excellent analytical tool for investigating these systems, being used effectively, for example, by Moriyasu and Hashimoto,318–320 Liska and co-workers321,322 and others.323 Thus, Moriyasu and Hashimoto mixed homoleptic bis(dithiocarbamate) complexes of nickel and copper (which can't easily be studied by NMR spectroscopy) and in all cases found that they were labile and in equilibrium with the mixed-ligand counterpart (Fig. 28). Rate constants depend on both metal and substituents, for Ni(II) being of the order of 101–102 M−1 s−1 and for Cu(II) slower at ca. 103 M−1 s−1.317–320 These studies were later extended to other metals and in all cases, the dithiocarbamates were found to be labile with equilibrium constants being ca. 4, i.e. a statistical mixture of the three complexes.324 Similar equilibria have also been noted between homoleptic nickel complexes of primary and secondary amines, affording [Ni(S2CNR12)(S2CNHR2)],318–320 and also between [Ni(S2CNR2)2] and xanthate complexes, [Ni(S2COR)2].325 Most studies support a bimolecular exchange process likely proceeding through a dimeric intermediate, the form of which is common in crystallographic studies of [Zn(S2CNR2)2] and [Cu(S2CNR2)2].8 Rapid dithiocarbamate exchange has also been noted at Fe(III)326–328 and Hg(II)329,330 centres.
For low spin d6 [Co(S2CNR2)3] complexes, the CFSE is so high that exchange rates are slow331 and isolation of mixed-ligand complexes is possible. Thus, addition of a dithiocarbamate salt, NaS2CNR12 to [Co2(S2CNR22)5]+ affords a mixture of [Co(S2CNR12)(S2CNR22)2] and [Co2(S2CNR22)3]332 (Fig. 29). Further, adding equimolar amounts of two different dithiocarbamates to Co(III) salts in water affords a statistical mixture of products,333 and a similar mixture of products can be obtained upon heating equimolar amounts of [Co(S2CNEt2)3] and [Co(S2CNiPr2)3] either in the solid-state or at 155 °C in chloronaphthalene for 4–5 h.333 Importantly, these mixtures can be fully separated by column chromatography allowing their individual characterisation. Interestingly, while it is hard to differentiate them by 1H or 13C{1H} NMR spectroscopy, they can be distinguished by 59Co NMR spectroscopy and mass spectrometry and have tuneable oxidation potentials.333
The ease of accessibility of mixed-ligand complexes containing dithiocarbamates also needs some careful consideration. There are many such examples8,9 but for solution stability in their pure form they need to feature either a relatively non-labile metal centre with either bulky non-dithiocarbamate ligands that preclude formation of a bimetallic intermediate or secondary inter-ligand interactions (e.g. hydrogen-bonding). Following on from the discussion above, Co(III) is a good example of a non-labile metal centre. Thus, [Co2(S2CNR2)5]+ reacts with a range of nucleophiles to afford [Co(S2CNR2)3] and a mixed-ligand complex, a recent case being the formation of Co(III) bis(dithiocarbamate)dithiolane complexes from addition of dithiones (Fig. 29).334 Other examples of a high CFSE leading to stable mixed-ligand complexes relate to the iron dithietane complexes189 (Fig. 20) which exist in temperature-dependent spin-equilibrium between the singlet (S = 0) ground state and a low-lying triplet (S = 1) excited state. Thus, not only are these mixed-ligand complexes stable, but their NMR spectra are also accessible.
However, simultaneously adding a dithiocarbamate and a different ligand to a metal salt will not automatically afford the mixed-ligand species as has been suggested in several publications.335–338 Just as for complexes with two different dithiocarbamates, it is hard by simple spectroscopic methods to tell the difference between a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of two homoleptic complexes and a mixed-ligand species, and in many cases the three likely co-exist. A recent publication highlights this nicely (Fig. 30).339 Thus, while insertion of an imide group into a Ni–S bond of [Ni(S2CNR2)2] affords mixed-ligand complexes, examples of which can be crystallographically characterised, upon dissolution they are shown to be in equilibrium with the two homoleptic complexes.
1 mixture of two homoleptic complexes and a mixed-ligand species, and in many cases the three likely co-exist. A recent publication highlights this nicely (Fig. 30).339 Thus, while insertion of an imide group into a Ni–S bond of [Ni(S2CNR2)2] affords mixed-ligand complexes, examples of which can be crystallographically characterised, upon dissolution they are shown to be in equilibrium with the two homoleptic complexes.
(vi) Reacting two different dithiocarbamate complexes gives a pure mixed metal product
Given the extremely large number of homonuclear transition metal dithiocarbamate complexes that are known, it is perhaps surprising that dithiocarbamate-bridged heteronuclear complexes are rare. Thus, as shown by Robinson,340 complexes obtained by successive addition of metal salts MX2 (M = Zn, Cd or Hg) and M′Y2 (M′ = VO, Mn, Fe, Co, Ni, Cu or Zn) to a solution of a sodium dithiocarbamate are not heterobimetallic [{M′M(S2CNR2)4}n] but rather contain a mixture of homonuclear complexes. Likewise, reactions of Pd(II) salts [PdCl2L2] (L = PhCN, L2 = dppe) with a series of homoleptic dithiocarbamate complexes affords palladium-dithiocarbamate cations [Pd(S2CNR2)L2]+ with the second metal being incorporated into the counterion.341–343 More recently, Torimoto and co-workers added Na(S2CNEt2) to mixtures of metal salts and used the ensuing “complex” as a precursor to a range of nanoscale metal sulfides with quantum dot properties.344–347 While the method is effective and efficient, the suggestion that a mixed-metal complex is formed is erroneous. Rather the precursor “complex” is an intimate mixture of several different complexes. There are some well-authenticated examples of dithiocarbamate-bridged heterobimetallic complexes, being especially prevalent amongst the coinage metals348–351 and there are also a small number of other dithiocarbamate-containing heteronuclear complexes that have been crystallographically characterised.352,353 Nevertheless, the vast majority of dithiocarbamate complexes contain a single metal type.6. Dithiocarbamate complexes as single source precursors
Dithiocarbamate complexes find widespread use as single source precursors for a range of nanoscale and thin film metal sulfides.49–52 Thus, simply heating, either in the solid-state or solution, the complex or mixture of complexes, results in S–C and other bond scission(s) affording the thermodynamically stable metal-sulfide(s) and various organic species, the latter (normally) being easily removed either by washing or evaporation. The utility of this simple approach is that air-stable dithiocarbamate complexes from across the periodic table can be (relatively easily) prepared and stored, and by judicious control of reaction conditions and stoichiometry a wide range of binary, ternary and quaternary metal sulfides can be accessed. A nice example of this is their use towards the synthesis of Cu2ZnSnS4 (CZTS) a quaternary semiconductor with a power conversion efficiency of ca. 10% that has been suggested as a viable material for thin film solar cells. Thus, decomposing mixtures of copper, zinc and tin dithiocarbamates leads to the formation of CZTS thin films or quantum dots.354,355However, such transformations are not as simple as they might appear. Firstly, in the solid state, thermogravimetric analysis studies have shown that temperatures of between 200–400 °C are required to cleave the S–C (and other) bonds within dithiocarbamate complexes.356–359 Decomposition temperatures can be tuned (normally within a small range) upon changing substituents, and there is also a correlation between the ease of thermal decomposition and ionic radius of the metal ion; the smaller the metallic ionic radius, the greater the thermal stability.360 In solution, decomposition temperatures can be significantly lower than in the solid-state.275,361,362 For example, the water-soluble SSP [Cu{S2CN(CH2CO2H)2}2] decomposes at 180 °C in the solid state but at ca. 80 °C in water.362 Another issue to address when preparing ternary, quaternary or multinary sulfides, is matching of decomposition temperatures, such that all SSPs decompose within a relatively small range, thus ensuring that the different molecular building blocks are all available at the nucleation stage. This is nicely exemplified by O'Brien's synthesis of CZTS.363,364 Thus, as the decomposition temperature of [Sn(S2CNEt2)2] (174 °C) is significantly lower than those of [Cu(S2CNEt2)2] (220 °C) and [Zn(S2CNEt2)2] (240 °C), heating this mixture will lead to the premature formation of tin sulfides and hence the tin precursors was replaced by the Sn(IV) SSPs, [Sn(S2CNBu2)4]363 and [Bu2Sn(S2CNBu2)2]364 which decompose at higher temperatures. A study by Torimoto and co-workers nicely highlights the need to carefully control decomposition conditions.365 Thus, for the synthesis of Ag(InxGa1−x)S2 quantum dots, simply heating a mixture of silver, indium and gallium precursors initially results in formation of polydisperse Ag2S as the silver dithiocarbamate decomposes at lower temperatures than others, and upon further heating as indium and gallium SSPs decompose they give a shell of these sulfides around the Ag2S (Fig. 31a).366 However, when a silver source is injected into the decomposition reaction then co-nucleation of all components occurs, initially to give a core–shell structure, while further heating affords the desired ternary phase sulfide as quantum dots.365
 | ||
| Fig. 31 Schematic illustrations of the synthesis of Ag(InxGa1−x)S2 quantum dots form (a) heating of the precursor mixture and (b) injecting a silver source into the decomposition mixture.365 | ||
Given these complications and constraints, it is surprising that there are an increasing number of reports seemingly suggesting that simply preparing mixtures of dithiocarbamates at room temperature results in the formation of metal sulfides i.e. there is no mention of a heating or other decomposition process367–371 although decomposition appears to be supported by powder X-ray diffraction data. Further, many of these “decompositions” are proposed to give composite mixtures of individual metal sulfides, some of which such as BaS3, are normally prepared at 400 °C.372 Clearly such reports require some scrutiny and perhaps further explanation(s) from the authors.
7. Summary and conclusions
In this perspective we have tried to address common misconceptions and errors that we have found in the literature on dithiocarbamates and their complexes. Amongst the challenges faced by those working in this area are the vast number of publications and patents, and the widespread reach of this simple ligand type across many different fields of research. Thus, it is hard for anyone to fully keep up to date and understand all aspects of their chemistry. Likely many misconceptions-errors that have crept into the literature result are further exacerbated by the recent (seemingly unstoppable) proliferation of scientific journals. It is a testament to the wide applicability of dithiocarbamate chemistry that this perspective cites over 200 different journal titles: some of us long for the days when our library reading covered 10–15 journals, each with a clear remit. Thus, we need to understand that newcomers to this mature field of research have a lot of reading and understanding to do to get up to speed with what has gone before. Dithiocarbamate chemistry has been an active area of research for over 150 years and unlike some fields of scientific research, some early papers have relevance today. For example, while recently developing a primary-amine derived dithiocarbamate as a H2S release-vector we came across a paper from 1891373 which had effectively already studied this and provided us with invaluable insight into our work.Having said this, there is also some evidence of tardy work in the dithiocarbamate literature, with researchers either cutting corners, or not being totally honest in reporting their findings-data. Sadly, this appears to be an issue that has proliferated through all aspects of scientific research and one that seriously undermines the efforts of those who play fair. It is hard to understand why anyone would deliberately mis-represent their work, but undoubtably the high speed of modern living and (sometimes) excessive pressures put on academics to publish may be part of the problem. Linking publication to financial gain or making it a pre-requisite for promotion will tempt some to cut corners.
Conflicts of interest
There are no conflicts to declare.Data availability
There is no data associated with this submission.Acknowledgements
We thank Dr Jagodish C. Sarker for reading through the manuscript and providing valuable comments, and Dr Shishir Ghosh for attempting to reproduce a reaction discussed herein.References
- C. Campanale, M. Triozzi, A. Ragonese, D. Losacco and C. Massarelli, Dithiocarbamates: Properties, Methodological Approaches and Challenges to Their Control, Toxics, 2023, 11, 851 CrossRef CAS PubMed.
- J. M. Veiga-del-Bano, S. Martinez-Lopez, G. Perez-Lucas, J. J. Cuenca-Martinez and P. Andreo-Martinez, Trends in dithiocarbamates food research: A bibliometric vision, Chemosphere, 2023, 313, 137342 CrossRef CAS PubMed.
- R. J. Magee and J. O. Hill, The analytical chemistry of metal dithiocarbamate complexes, Rev. Anal. Chem., 1985, 8, 5–72 CAS.
- L. Ye, A. T. Dinkova-Kostova, K. L. Wade, Y. Zhang, T. A. Shapiro and P. Talalay, Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans, Clin. Chim. Acta, 2002, 316, 43–53 CrossRef CAS PubMed.
- K. W. Bruland, R. P. Franks, G. A. Knauer and J. H. Martin, Sampling and analytical methods for the determination of copper, cadmium, zinc, and nickel at the nanogram per liter level in sea water, Anal. Chim. Acta, 1979, 105, 233–245 CrossRef CAS.
- C. Yuan, B. Liu, F. Liu, M.-Y. Han and Z. Zhang, Fluorescence “Turn On” Detection of Mercuric Ion Based on Bis(dithiocarbamato)copper(II) Complex Functionalized Carbon Nanodots, Anal. Chem., 2014, 86, 1123–1130 CrossRef CAS PubMed.
- O. H. J. Szolar, Environmental and pharmaceutical analysis of dithiocarbamates, Anal. Chim. Acta, 2007, 582, 191–200 CrossRef CAS PubMed.
- G. Hogarth, Transition metal dithiocarbamates: 1978–2003, Prog. Inorg. Chem., 2005, 53, 71–561 CAS.
- D. Coucouvanis, The chemistry of the dithioacid and 1,1-dithiolate complexes, 1968–1977, Prog. Inorg. Chem., 1979, 26, 301–469 CAS.
- P. J. Heard, Main group dithiocarbamate complexes, Prog. Inorg. Chem., 2005, 53, 1–69 CAS.
- F. Bonati and R. Ugo, Organotin(IV) N,N-disubstituted dithiocarbamates, J. Organomet. Chem., 1967, 10, 257–268 CrossRef CAS.
- E. R. T. Tiekink, Tin dithiocarbamates: applications and structures, Appl. Organomet. Chem., 2008, 22, 533–550 CrossRef CAS.
- G. Hogarth and D. C. Onwudiwe, Copper Dithiocarbamates: Coordination Chemistry and Applications in Materials Science, Biosciences and Beyond, Inorganics, 2021, 9, 70 CrossRef CAS.
- A. M. Bond and R. L. Martin, Electrochemistry and redox behavior of transition metal dithiocarbamates, Coord. Chem. Rev., 1984, 54, 23–98 CrossRef CAS.
- Y. S. Tan, C. I. Yeo, E. R. T. Tiekink and P. J. Heard, Dithiocarbamate Complexes of Platinum Group Metals: Structural Aspects and Applications, Inorganics, 2021, 9, 60 CrossRef CAS.
- J. Cookson and P. D. Beer, Exploiting the dithiocarbamate ligand in metal-directed self-assembly, Dalton Trans., 2007, 1459–1472 RSC.
- A. Torres-Huerta, B. Rodriguez-Molina, H. Hopfl and M. A. Garcia-Garibay, Synthesis and Solid-State Characterization of Self-Assembled Macrocyclic Molecular Rotors of Bis(dithiocarbamate) Ligands with Diorganotin(IV), Organometallics, 2014, 33, 354–362 CrossRef CAS.
- E. R. T. Tiekink, On the coordination role of pyridyl-nitrogen in the structural chemistry of pyridyl-substituted dithiocarbamate ligands, Crystals, 2021, 11, 286 CrossRef CAS.
- L. Monser and N. Adhoum, Modified activated carbon for the removal of copper, zinc, chromium and cyanide from wastewater, Sep. Purif. Technol., 2002, 26, 137–146 CrossRef CAS.
- M. Tuzen, K. O. Saygi and M. Soylak, Solid phase extraction of heavy metal ions in environmental samples on multiwalled carbon nanotubes, J. Hazard. Mater., 2008, 152, 632–639 CrossRef CAS PubMed.
- P. I. Girginova, A. L. Daniel-da-Silva, C. B. Lopes, P. Figueira, M. Otero, V. S. Amaral, E. Pereira and T. Trindade, Silica coated magnetite particles for magnetic removal of Hg2+ from water, J. Colloid Interface Sci., 2010, 345, 234–240 CrossRef CAS PubMed.
- S. Kanchi, P. Singh and K. Bisetty, Dithiocarbamates as hazardous remediation agent: A critical review on progress in environmental chemistry for inorganic species studies of 20th century, Arabian J. Chem., 2014, 7, 11–25 CrossRef CAS.
- K. Nakakubo, M. Endo, Y. Sakai, F. B. Biswas, K. H. Wong, A. S. Mashio, T. Taniguchi, T. Nishimura, M. Katsuhiro and H. Hasegawa, Crosslinked dithiocarbamate-modified cellulose with enhanced thermal stability and dispersibility as a sorbent for arsenite removal, Chemosphere, 2022, 307, 135671 CrossRef CAS PubMed.
- G. Hogarth, Metal-dithiocarbamate complexes: chemistry and biological activity, Mini-Rev. Med. Chem., 2012, 12, 1202–1215 CrossRef CAS PubMed.
- C. Nardon, G. Boscutti and D. Fregona, Beyond platinums: gold complexes as anticancer agents, Anticancer Res., 2014, 34, 487–492 CAS.
- L. Ronconi, L. Giovagnini, C. Marzano, F. Bettio, R. Graziani, G. Pilloni and D. Fregona, Gold Dithiocarbamate Derivatives as Potential Antineoplastic Agents: Design, Spectroscopic Properties, and in Vitro Antitumor Activity, Inorg. Chem., 2005, 44, 1867–1881 CrossRef CAS.
- L. Ronconi, C. Marzano, P. Zanello, M. Corsini, G. Miolo, C. Macca, A. Trevisan and D. Fregona, Gold(III) Dithiocarbamate Derivatives for the Treatment of Cancer: Solution Chemistry, DNA Binding, and Hemolytic Properties, J. Med. Chem., 2006, 49, 1648–1657 CrossRef CAS PubMed.
- F. K. Keter, I. A. Guzei, M. Nell, W. E. van Zyl and J. Darkwa, Phosphinogold(I) Dithiocarbamate Complexes: Effect of the Nature of Phosphine Ligand on Anticancer Properties, Inorg. Chem., 2014, 53, 2058–2067 CrossRef CAS PubMed.
- J. O. Adeyemi and D. C. Onwudiwe, Organotin(IV) dithiocarbamate complexes: chemistry and biological activity, Molecules, 2018, 23, 1–27 CrossRef CAS PubMed.
- R. Schreck, B. Meier, D. N. Maennel, W. Droege and P. A. Baeuerle, Dithiocarbamates as potent inhibitors of nuclear factor κB activation in intact cells, J. Exp. Med., 1992, 175, 1181–1194 CrossRef CAS PubMed.
- Q. W. Xie, Y. Kashiwabara and C. Nathan, Role of transcription factor NF-κB/Rel in induction of nitric oxide synthase, J. Biol. Chem., 1994, 269, 4705–4708 CrossRef CAS.
- M. Meyer, R. Schreck and P. A. Baeuerle, Hydrogen peroxide and antioxidants have opposite effects on activation of NF-κB and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor, EMBO J., 1993, 12, 2005–2015 CrossRef CAS PubMed.
- N. Marui, M. K. Offermann, R. Swerlick, C. Kunsch, C. A. Rosene, M. Ahmad, R. W. Alexander and R. M. Medford, Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells, J. Clin. Inhib. Med. Chem., 1993, 92, 1866–1874 CAS.
- C. T. Supuran, Structure-based drug discovery of carbonic anhydrase inhibitors, J. Enzyme Inhib. Med. Chem., 2012, 27, 759–772 CrossRef CAS PubMed.
- F. Carta, M. Aggarwal, A. Maresca, A. Scozzafava, R. McKenna and C. T. Supuran, Dithiocarbamates: a new class of carbonic anhydrase inhibitors. Crystallographic and kinetic investigations, Chem. Commun., 2012, 48, 1868–1870 RSC.
- F. Carta, M. Aggarwal, A. Maresca, A. Scozzafava, R. McKenna, E. Masini and C. T. Supuran, Dithiocarbamates strongly inhibit carbonic anhydrases and show antiglaucoma action in vivo, J. Med. Chem., 2012, 55, 1721–1730 CrossRef CAS PubMed.
- D. J. Berry, R. T. Martin de Rosales, P. Charoenphun and P. J. Blower, Dithiocarbamate complexes as radiopharmaceuticals for medical imaging, Mini-Rev. Med. Chem., 2012, 12, 1174–1183 CrossRef CAS PubMed.
- R. Pasqualini, A. Duatti, E. Bellande, V. Comazzi, V. Brucato, D. Hoffschir, D. Fagret and M. Comet, Bis(dithiocarbamato) nitrido technetium-99 m radiopharmaceuticals: a class of neutral myocardial imaging agents, J. Nucl. Med., 1994, 35, 334–341 CAS.
- R. T. Martin de Rosales, R. Tavare, R. L. Paul, M. Jauregui-Osoro, A. Protti, A. Glaria, G. Varma, I. Szanda and P. J. Blower, Synthesis of 64CuII-Bis(dithiocarbamatebisphosphonate) and Its Conjugation with Superparamagnetic Iron Oxide Nanoparticles: In Vivo Evaluation as Dual-Modality PET-MRI Agent, Angew. Chem., Int. Ed., 2011, 50, 5509–5513 CrossRef CAS PubMed.
- R. T. A. Mayadunne, E. Rizzardo, J. Chiefari, Y. K. Chong, G. Moad and S. H. Thang, Living Radical Polymerization with Reversible Addition-Fragmentation Chain Transfer (RAFT Polymerization) Using Dithiocarbamates as Chain Transfer Agents, Macromolecules, 1999, 32, 6977–6980 CrossRef CAS.
- G. Moad, E. Rizzardo and S. H. Thang, Radical addition-fragmentation chemistry in polymer synthesis, Polymer, 2008, 49, 1079–1131 CrossRef CAS.
- M. Destarac, D. Charmot, X. Franck and S. Z. Zard, Dithiocarbamates as universal reversible addition-fragmentation chain transfer agents, Macromol. Rapid Commun., 2000, 21, 1035–1039 CrossRef CAS.
- G. Moad, A Critical Survey of Dithiocarbamate Reversible Addition-Fragmentation Chain Transfer (RAFT) Agents in Radical Polymerization, J. Polym. Sci., Part A: Polym. Chem., 2019, 57, 216–227 CrossRef CAS.
- Y. Zhao, W. Perez-Segarra, Q. Shi and A. Wei, Dithiocarbamate Assembly on Gold, J. Am. Chem. Soc., 2005, 127, 7328–7329 CrossRef CAS PubMed.
- C. M. Wolff, P. D. Frischmann, M. Schulze, B. J. Bohn, R. Wein, P. Livadas, M. T. Carlson, F. Jaeckel, J. Feldmann and F. Wuerthner, et al., All-in-one visible-light-driven water splitting by combining nanoparticulate and molecular co-catalysts on CdS nanorods, Nat. Energy, 2018, 3, 862–869 CrossRef CAS.
- F. Dubois, B. Mahler, B. Dubertret, E. Doris and C. Mioskowski, A Versatile Strategy for Quantum Dot Ligand Exchange, J. Am. Chem. Soc., 2007, 129, 482–483 CrossRef CAS PubMed.
- E. R. Knight, A. R. Cowley, G. Hogarth and J. D. E. T. Wilton-Ely, Bifunctional dithiocarbamates: a bridge between coordination chemistry and nanoscale materials, Dalton Trans., 2009, 607–609 RSC.
- J. He, J. Liu, Y. Hou, Y. Wang, S. Yang and H. G. Yang, Surface chelation of caesium halide perovskite by dithiocarbamate for efficient and stable solar cells, Nat. Commun., 2020, 11, 4237 CrossRef CAS PubMed.
- J. C. Sarker and G. Hogarth, Dithiocarbamate Complexes as Single Source Precursors to Nanoscale Binary, Ternary and Quaternary Metal Sulfides, Chem. Rev., 2021, 121, 6057–6123 CrossRef CAS PubMed.
- D. Pan, L. An, Z. Sun, W. Hou, Y. Yang, Z. Yang and Y. Lu, Synthesis of Cu-In-S Ternary Nanocrystals with Tuneable Structure and Composition, J. Am. Chem. Soc., 2008, 130, 5620–5621 CrossRef CAS PubMed.
- M. A. Malik, N. Revaprasadu and P. O'Brien, Air-Stable Single-Source Precursors for the Synthesis of Chalcogenide Semiconductor Nanoparticles, Chem. Mater., 2001, 13, 913–920 CrossRef CAS.
- T. Trindade, P. O'Brien, X.-M. Zhang and M. Motevalli, Synthesis of PbS nanocrystallites using a novel single molecule precursors approach: X-ray single-crystal structure of Pb(S2CNEt-iso-Pr)2, J. Mater. Chem., 1997, 7, 1011–1016 RSC.
- P. Tsvetkov, S. Coy, B. Petrova, M. Dreishpoon, A. Verma, M. Abdusamad, J. Rossen, L. Joesch-Cohen, R. Humeidi, R. D. Spangler, J. K. Eaton, E. Frenkel, M. Kocak, S. M. Corsello, S. Lutsenko, N. Kanarek, S. Santagata and T. R. Golub, Copper induces cell death by targeting lipoylated TCA cycle proteins, Science, 2022, 375, 1254–1261 CrossRef CAS PubMed.
- D. Chen, Q. C. Cui, H. Yang and Q. P. Dou, Disulfiram, a Clinically Used Anti-Alcoholism Drug and Copper-Binding Agent, Induces Apoptotic Cell Death in Breast Cancer Cultures and Xenografts via Inhibition of the Proteasome Activity, Cancer Res., 2006, 66, 10425–10433 CrossRef CAS PubMed.
- D. Cen, D. Brayton, B. Shahandeh, F. L. Meyskens Jr. and P. J. Farmer, Disulfiram Facilitates Intracellular Cu Uptake and Induces Apoptosis in Human Melanoma Cells, J. Med. Chem., 2004, 47, 6914–6920 CrossRef CAS PubMed.
- D. J. Lewis, P. Deshmukh, A. A. Tedstone, F. Tuna and P. O'Brien, On the interaction of copper(II) with disulfiram, Chem. Commun., 2014, 50, 13334–13337 RSC.
- B. Cvek, V. Milacic, J. Taraba and Q. P. Dou, Ni(II), Cu(II), and Zn(II) Diethyldithiocarbamate Complexes Show Various Activities Against the Proteasome in Breast Cancer Cells, J. Med. Chem., 2008, 51, 6256–6258 CrossRef CAS PubMed.
- Z. Skrott, M. Mistrik, K. K. Andersen, S. Friis, D. Majera, J. Gursky, T. Ozdian, J. Bartkova, Z. Turi, P. Moudry, I. Vrobel, P. Pouckova, J. Sedlacek, A. Miklovicova, A. Kutt, J. Li, J. Mattova, C. Driessen, Q. P. Dou, J. Olsen, M. Hajduch, B. Cvek, R. J. Deshaies and J. Bartek, Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4, Nature, 2017, 552, 194–199 CrossRef CAS PubMed.
- Z. Skrott and B. Cvek, Diethyldithiocarbamate complex with copper: the mechanism of action in cancer cells, Mini-Rev. Med. Chem., 2012, 12, 1184–1192 CrossRef CAS PubMed.
- E. J. Ge, A. I. Bush, A. Casini, P. A. Cobine, J. R. Cross, G. M. DeNicola, Q. P. Dou, K. J. Franz, V. M. Gohil, S. Gupta, S. G. Kaler, S. Lutsenko, V. Mittal, M. J. Petris, R. Polishchuk, M. Ralle, M. L. Schilsky, N. K. Tonks, L. T. Vahdat, L. Van Aelst, D. Xi, P. Yuan, D. C. Brady and C. J. Chang, Connecting copper and cancer: from transition metal signalling to metalloplasia, Nat. Cancer Rev., 2022, 22, 102–113 CrossRef CAS.
- E. Ekinci, S. Rohondia, R. Khan and Q. P. Dou, Repurposing Disulfiram as An Anti-Cancer Agent: Updated Review on Literature and Patents, Recent Pat. Anti-Cancer Drug Discovery, 2019, 14, 113–132 CrossRef CAS.
- Q. Yang, Y. Yao, K. Li, L. Jiao, J. Zhu, C. Ni, M. Li, Q. P. Dou and H. Yang, An Updated Review of Disulfiram: Molecular Targets and Strategies for Cancer Treatment, Curr. Pharm. Des., 2019, 25, 3248–3256 CrossRef CAS PubMed.
- X. Kang, S. Jadhav, M. Annaji, C.-H. Huang, R. Amin, J. Shen, C. R. Ashby Jr., A. K. Tiwari, R. J. Babu and P. Chen, Advancing Cancer Therapy with Copper/Disulfiram Nanomedicines and Drug Delivery Systems, Pharmaceutics, 2023, 15, 1567 CrossRef CAS PubMed.
- Z. Skrott, D. Majera, J. Gursky, T. Buchtova, M. Hajduch, M. Mistrik and J. Bartek, Disulfiram's anti-cancer activity reflects targeting NPL4, not inhibition of aldehyde dehydrogenase, Oncogene, 2019, 38, 6711–6722 CrossRef CAS PubMed.
- C. Wright and R. D. Moore, Disulfiram treatment of alcoholism, Am. J. Med., 1990, 88, 647–655 CrossRef CAS PubMed.
- J. Cobby, M. Mayersohn and S. Selliah, The rapid reduction of disulfiram in blood and plasma, J. Pharmacol. Exp. Ther., 1977, 202, 724–731 CrossRef CAS.
- J. C. Sarker, R. Nash, S. Boonrungsiman, D. Pugh and G. Hogarth, Diaryldithiocarbamates: Synthesis, oxidation to thiuram disulfides, Co(III) complexes [Co(S2CNAr2)3] and use as single source precursors to CoS2, Dalton Trans., 2022, 51, 13061–13070 RSC.
- J. C. Sarker, F. Alam, P. McNaughter, D. Pugh, J. K. Cockcroft, D. J. Lewis and G. Hogarth, Synthesis of diaryl dithiocarbamate complexes of zinc and their uses as single source precursors for nanoscale ZnS, Inorg. Chim. Acta, 2023, 556, 121663 CrossRef CAS.
- J. C. Sarker, X. Xu, F. Alam, R. Nash, S. Boonrungsiman, D. Pugh, J. K. Cockcroft, D. J. Lewis and G. Hogarth, Copper diaryl-dithiocarbamate complexes and their application as single source precursors (SSPs) for copper sulfide nanomaterials, New J. Chem., 2023, 47, 12718–12727 RSC.
- M. Kashif, A. G. Hogarth, Z. Khan, M. Imran and Z. Rehman, Platinum(II) dithiocarbamate complexes [Pt(S2CNR2)Cl(PAr3)] as anticancer and DNA-damaging agents, Inorg. Chim. Acta, 2020, 512, 119853 CrossRef.
- M. Imran, Z. Rehman, G. Hogarth, D. A. Tocher, G. Chaudhry, I. S. Butler, F. Belanger-Gariepy and T. Kondratyuk, Two new monofunctional platinum(II) dithiocarbamate complexes: phenanthriplatin-type axial protection, equatorial-axial conformational isomerism, and anticancer and DNA binding studies, Dalton Trans., 2020, 49, 15385–15396 RSC.
- H.-U. Islam, A. Roffey, N. Hollingsworth, W. Bras, G. Sankar, N. H. De Leeuw and G. Hogarth, Understanding the role of zinc dithiocarbamate complexes as single source precursors to ZnS nanomaterials, Nanoscale Adv., 2020, 2, 798–807 RSC.
- W.-D. Rudorf, Reactions of carbon disulfide with N-nucleophiles, J. Sulfur Chem., 2007, 28, 295–339 CrossRef CAS.
- A. A. Aly, A. B. Brown, T. M. I. Bedair and E. A. Ishak, Dithiocarbamate salts: biological activity, preparation, and utility in organic synthesis, J. Sulfur Chem., 2012, 33, 605–617 CrossRef CAS.
- Z.-Y. Wang, J. Li, N. Wang, H. Liu, W. Ding and K.-K. Wang, A Decade of Advances of CS2/Amines in Three-Component Reactions, Synthesis, 2023, 55, 1159–1171 CrossRef CAS.
- A. Z. Halimehjani, R. Mohtasham, A. Shockravi and J. Martens, Multicomponent synthesis of dithiocarbamates starting from vinyl sulfones/sulfoxides and their use in polymerization reactions, RSC Adv., 2016, 6, 75223–75226 RSC.
- R. Biswas, P. Thakur, G. Kaur, S. Som, M. Saha, V. Jhajhria, H. Singh, I. Ahmed, B. Banerjee and D. Chopra, et al. Interfacial Engineering of CuCo2S4/g-C3N4 Hybrid Nanorods for Efficient Oxygen Evolution Reaction, Inorg. Chem., 2021, 60, 12355–12366 CrossRef CAS.
- A. A. Pradhan, M. C. Uible, S. Agarwal, J. W. Turnley, S. Khandelwal, J. M. Peterson, D. D. Blach, R. N. Swope, L. Huang and S. C. Bart, et al. Synthesis of BaZrS3 and BaHfS3 Chalcogenide Perovskite Films Using Single-Phase Molecular Precursors at Moderate Temperatures, Angew. Chem., Int. Ed., 2023, 62, e202301049 CrossRef CAS PubMed.
- B. Prelesnik, K. Andjelkovic, Z. Markovic, T. Sabo and S. Trifunovic, Potassium 3-dithiocarboxy-3-aza-5-aminopentanoate dihydrate, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1997, 53, 719–720 CrossRef.
- R. Biswas, P. Thakur, G. Kaur, S. Som, M. Saha, V. Jhajhria, H. Singh, I. Ahmed, B. Banerjee and D. Chopra, et al. Correction to Interfacial Engineering of CuCo2S4/g-C3N4 Hybrid Nanorods for Efficient Oxygen Evolution Reaction, Inorg. Chem., 2022, 61, 13226–13227 CrossRef CAS PubMed.
- J. D. E. T. Wilton-Ely, D. Solanki and G. Hogarth, Multifunctional dithiocarbamates as ligands towards the rational synthesis of polymetallic arrays: An example based on a piperazine-derived dithiocarbamate ligand, Eur. J. Inorg. Chem., 2005, 4027–4030 CrossRef CAS.
- J. D. E. T. Wilton-Ely, D. Solanki, E. R. Knight, K. B. Holt, A. L. Thompson and G. Hogarth, Multimetallic assemblies using piperazine-based dithiocarbamate building blocks, Inorg. Chem., 2008, 47, 9642–9653 CrossRef CAS.
- E. R. Knight, N. H. Leung, A. L. Thompson, G. Hogarth and J. D. E. T. Wilton-Ely, Multimetallic Arrays: Bi-, Tri-, Tetra-, and Hexametallic Complexes Based on Gold(I) and Gold(III) and the Surface Functionalization of Gold Nanoparticles with Transition Metals, Inorg. Chem., 2009, 48, 3866–3874 CrossRef CAS.
- E. R. Knight, N. H. Leung, Y. H. Lin, A. R. Cowley, D. J. Watkin, A. L. Thompson, G. Hogarth and J. D. E. T. Wilton-Ely, Multimetallic arrays: symmetrical bi-, tri- and tetrametallic complexes based on the group 10 metals and the functionalization of gold nanoparticles with nickel-phosphine surface units, Dalton Trans., 2009, 3688–3697 RSC.
- K. Oliver, A. J. P. White, G. Hogarth and J. D. E. T. Wilton-Ely, Multimetallic complexes of group 10 and 11 metals based on polydentate dithiocarbamate ligands, Dalton Trans., 2011, 40, 5852–5864 RSC.
- M. J. Macgregor, G. Hogarth, A. L. Thompson and J. D. E. T. Wilton-Ely, Multimetallic Arrays: Symmetrical and Unsymmetrical Bi-, Tri-, and Tetrametallic Organometallic Complexes of Ruthenium(II) and Osmium(II), Organometallics, 2009, 28, 197–208 CrossRef CAS.
- E. Humeres, N. A. Debacher, J. D. Franco, B. S. Lee and A. Martendal, Mechanisms of Acid Decomposition of Dithiocarbamates. 3. Aryldithiocarbamates and the Torsional Effect, J. Org. Chem., 2002, 67, 3662–3667 CrossRef CAS PubMed.
- S. C. Ball, I. Cragg-Hine, M. G. Davidson, R. P. Davies, A. J. Edwards, I. Lopez-Solera, P. R. Raithby and R. Snaith, Lithium intermediates during the α-lithiation and subsequent α-substitution of heterocyclic amines in the presence of CO2, Angew. Chem., Int. Ed. Engl., 1995, 34, 921–923 CrossRef CAS.
- P. Padungros and A. Wei, Practical Synthesis of Aromatic Dithiocarbamates, Synth. Commun., 2014, 44, 2336–2343 CrossRef CAS PubMed.
- M. Uchiyama, K. Satoh and M. Kamigaito, Cationic RAFT Polymerization Using ppm Concentrations of Organic Acid, Angew. Chem., Int. Ed., 2015, 54, 1924–1928 CrossRef CAS PubMed.
- H. Ma, G. Wang, G. T. Yee, J. L. Petersen and M. P. Jensen, Scorpionate-supported models of nickel-dependent superoxide dismutase, Inorg. Chim. Acta, 2009, 362, 4563–4569 CrossRef CAS.
- B. Ballard, D. Wycoff, E. R. Birnbaum, K. D. John, J. W. Lenz, S. S. Jurisson, C. S. Cutler, F. M. Nortier, W. A. Taylor and M. E. Fassbender, Selenium-72 formation via natBr(p,x) induced by 100 MeV Protons: Steps towards a novel 72Se/72As generator system, Appl. Radiat. Isot., 2012, 70, 595–601 CrossRef CAS PubMed.
- K. S. Siddiqi and N. Nishat, Synthesis and characterization of succinimide and phthalimide dithiocarbamates and their complexes with some transition metal ions, Synth. React. Inorg. Met.-Org. Chem., 2000, 30, 1505–1518 CrossRef CAS.
- I. F. Alshdoukhi, A. S. M. O. Al-Barwari, N. M. Aziz, T. Khalil, A. S. Faihan, S. A. Al-Jibori and A. S. Al-Janabi, Pd(II), Pt(II), Zn(II), Cd(II) and Hg(II) complexes of the newly prepared 1,2-benzoisothiazol-3(2H)-dithiocarbamate (BIT-DTC) ligand, Bull. Chem. Soc. Ethiop., 2024, 38, 889–899 CrossRef CAS.
- M. Y. Mohammed, Z. M. Mustafa and F. N. Aziz, Synthesis, Characterization, and Antibacterial Activity of Co(II), Ni(II), and Pd(II) Complexes with Bisdithiocarbamate and Cyclic Amines Ligands, Macromol. Symp., 2022, 401, 2100341 CrossRef CAS.
- M. Y. Mohammed, M. G. Abdel Karim and H. M. Abdul Karim, Synthesis, Characterization, and Bacterial Activity of Co(II), Ni(II), and Pd(II) Complexes with Ligands of Thymine, Uracil Thiuram Disulfur, and Cyclic Amines, Macromol. Symp., 2022, 401, 2100310 CrossRef CAS.
- H. W. Omar, M. Y. Mohammed and A. I. Yaseen, Synthesis and Characterization of Silver(I) Macromolecules Containing Macrocyclic Dithiocarbamate Ligand and Phosphines, Macromol. Symp., 2025, 414, 2400217 CrossRef CAS.
- T. Takeshima, M. Ikeda, M. Yokoyama, N. Fukada and M. Muraoka, Reaction of carbon disulfide with cyclic amides and related compounds. Free N-acyl- and N-carbamoyl-dithiocarbamic acids, J. Chem. Soc., Perkin Trans. 1, 1979, 692–695 RSC.
- J. Chiefari, R. T. A. Mayadunne, C. L. Moad, G. Moad, E. Rizzardo, A. Postma, M. A. Skidmore and S. H. Thang, Thiocarbonylthio Compounds (S:C(Z)S-R) in Free Radical Polymerization with Reversible Addition-Fragmentation Chain Transfer (RAFT Polymerization). Effect of the Activating Group Z, Macromolecules, 2003, 36, 2273–2283 CrossRef CAS.
- H. Aoshima, M. Uchiyama, K. Satoh and M. Kamigaito, Interconvertible Living Radical and Cationic Polymerization through Reversible Activation of Dormant Species with Dual Activity, Angew. Chem., Int. Ed., 2014, 53, 10932–10936 CrossRef CAS PubMed.
- R. Gerner, G. Kiel and G. Gattow, Chalcogenolates. 148. Reaction of formamide with carbon disulfide. 2. Crystal structure of potassium N-formyldithiocarbamate, Z. Anorg. Allg. Chem., 1985, 523, 76–88 CrossRef CAS.
- C. C. Hadjikostas and G. A. Katsoulos, Synthesis and characterization of 2-pyrrolidone-N-carbodithioato complexes of several transition metals, Int. J. Chem., 1994, 5, 49–57 CAS.
- P. J. Heard, Y. S. Tan, C. I. Yeo and E. R. T. Tiekink, The Coordination Chemistry of Imidomethanedithiolate Di-anions: A Structural Comparison with Their Dithiocarbamate Analogs, Inorganics, 2021, 9, 71 CrossRef CAS.
- C. C. Hadjikostas, G. A. Katsoulos, M. P. Sigalas and C. A. Tsipis, Carbodithioato derivatives of weak nitrogenous nucleophiles. II. Nondirect syntheses and structural studies of nickel(II) N-carbodithioates with substituted ureas, carbamic esters and sulfonamides, Inorg. Chim. Acta, 1989, 163, 173–176 CrossRef CAS.
- C. C. Hadjikostas, G. A. Katsoulos, M. P. Sigalas and C. A. Tsipis, Carbodithioato derivatives of weak nitrogenous nucleophiles. I. Electronic structure and ground state properties of nickel(II) amide N-carbodithioates, Can. J. Chem., 1989, 67, 902–909 CrossRef CAS.
- C. C. Hadjikostas, G. A. Katsoulos, M. P. Sigalas, C. A. Tsipis and N. V. Duffy, Convenient, straightforward routes to carbodithioato derivatives of weak nitrogenous nucleophiles, Polyhedron, 1989, 8, 2637–2639 CrossRef CAS.
- J. Salah, H. P. Bridges, D. Pugh and G. Hogarth, unpublished results.
- B. Cvek, The Promiscuity of Disulfiram in Medicinal Research, ACS Med. Chem. Lett., 2023, 14, 1610–1614 CrossRef CAS PubMed.
- A. Bera, B. Busupalli and B. L. V. Prasad, Solvent-Less Solid State Synthesis of Dispersible Metal and Semiconducting Metal Sulfide Nanocrystals, ACS Sustainable Chem. Eng., 2018, 6, 12006–12016 CrossRef CAS.
- A. Bera, D. Mandal, P. N. Goswami, A. K. Rath and B. L. V. Prasad, Generic and Scalable Method for the Preparation of Monodispersed Metal Sulfide Nanocrystals with Tuneable Optical Properties, Langmuir, 2018, 34, 13459–13460 CrossRef CAS PubMed.
- A. Bera, B. Busupalli and B. L. V. Prasad, Solvent-Less Solid State Synthesis of Dispersible Metal and Semiconducting Metal Sulfide Nanocrystals, ACS Sustainable Chem. Eng., 2018, 6, 12006–12016 CrossRef CAS.
- C. Jin, Y. Li, J. Lin, X. Guo, Y. Shi, H. Zhu, Y. Wang and X. Zhang, Efficient Transition Metal Sulfide Electrocatalysts Prepared by Laser-Induced Precursor Decomposition, Appl. Energy Mater., 2024, 7, 8546–8533 CrossRef CAS.
- S. J. Joris, K. I. Aspila and C. L. Chakrabarti, Monobasic or dibasic character of dithiocarbamic acids, Anal. Chem., 1969, 41, 1441–1445 CrossRef CAS.
- S. J. Joris, K. I. Aspila and C. L. Chakrabarti, On the Mechanism of Decomposition of Dithiocarbamates, J. Phys. Chem., 1970, 74, 860–865 CrossRef CAS.
- R. R. Vandebeek, S. J. Joris, K. I. Aspila and C. L. Chakrabarti, Decomposition of some cyclic dithiocarbamates, Can. J. Chem., 1970, 48, 2204–2209 CrossRef CAS.
- K. I. Aspila, S. J. Joris and C. L. Chakrabarti, Solvent Isotope Effects on Decomposition of N,N′-Dialkyldithiocarbamic Acids, Anal. Chem., 1971, 43, 1529–1530 CrossRef.
- D. De Filippo, P. Deplano, F. Devillanova, E. F. Trogu and G. Verani, Inductive effect in dithiocarbamate decomposition mechanism, J. Org. Chem., 1973, 38, 560–563 CrossRef CAS.
- F. Takami, K. Tokuyama, S. Wakahara and T. Maeda, Decomposition of dithiocarbamates. VI. Decomposition of N-monosubstituted dithiocarbamic acids in acidic solutions, Chem. Pharm. Bull., 1973, 21, 594–599 CrossRef CAS.
- E. Humeres, N. A. Debacher, M. de Marta, J. D. Franco and A. Schutz, Mechanisms of Acid Decomposition of Dithiocarbamates. 1. Alkyl Dithiocarbamates, J. Org. Chem., 1998, 63, 1598–1603 CrossRef CAS.
- E. Humeres, N. A. Debacher and M. M. D. S. Sierra, Mechanisms of Acid Decomposition of Dithiocarbamates. 2. Efficiency of the Intramolecular General Acid Catalysis, J. Org. Chem., 1999, 64, 1807–1813 CrossRef CAS.
- E. Humeres, N. A. Debacher, J. D. Franco, B. S. Lee and A. Martendal, Mechanisms of Acid Decomposition of Dithiocarbamates. 3. Aryldithiocarbamates and the Torsional Effect, J. Org. Chem., 2002, 67, 3662–3667 CrossRef CAS PubMed.
- E. Humeres, B. S. Lee and N. A. Debacher, Mechanisms of Acid Decomposition of Dithiocarbamates. 5. Piperidyl Dithiocarbamate and Analogues, J. Org. Chem., 2008, 73, 7189–7196 CrossRef CAS PubMed.
- M. L. Riekkola and H. Siren, The rate of decomposition of sodium diisobutyldithiocarbamate in aqueous solution and extractability of the corresponding acid with methylene chloride, Finn. Chem. Lett., 1983, 68–73 CAS.
- V. Amarnath, K. Amarnath and W. M. Valentine, Mechanism of decomposition of N,N-dialkyl dithiocarbamates, Curr. Top. Toxicol., 2007, 4, 39–44 CAS.
- A. W. DeMartino, M. L. Souza and P. C. Ford, Uncaging carbon disulfide. Delivery platforms for potential pharmacological applications: a mechanistic approach, Chem. Sci., 2017, 8, 7186–7196 RSC.
- Y. Gao and B. Xia, Microdroplet accelerated reaction for high-efficiency carbon disulfide conversion, Chem. Commun., 2023, 59, 10773–10776 RSC.
- B. Maeda and K. Murakami, Recent advancement in the synthesis of isothiocyanates, Chem. Commun., 2024, 60, 2839–2864 RSC.
- R. Wong and S. J. Dolman, Isothiocyanates from Tosyl Chloride Mediated Decomposition of in Situ Generated Dithiocarbamic Acid Salts, J. Org. Chem., 2007, 72, 3969–3971 CrossRef CAS PubMed.
- S. Murru, H. Ghosh, S. K. Sahoo and B. K. Patel, Intra- and Intermolecular C-S Bond Formation Using a Single Catalytic System: First Direct Access to Arylthiobenzothiazoles, Org. Lett., 2009, 11, 4254–4257 CrossRef CAS PubMed.
- B. Luo, Q. Cui, H. Luo, Y. Hu, P. Huang and S. Wen, N-Benzyldithiocarbamate Salts as Sulfur Sources to Access Tricyclic Thioheterocycles Mediated by Copper Species, Adv. Synth. Catal., 2016, 358, 2733–2738 CrossRef CAS.
- A. Z. Halimehjani, S. Shokrgozar and P. Beier, Transition-Metal-Free Coupling Reaction of Dithiocarbamates with Indoles: C-S Bond Formation, J. Org. Chem., 2018, 83, 5778–5783 CrossRef CAS PubMed.
- J. Nath, H. Ghosh, R. Yella and B. K. Patel, Molecular Iodine Mediated Preparation of Isothiocyanates from Dithiocarbamic Acid Salts, Eur. J. Org. Chem., 2009, 1849–1851 CrossRef CAS.
- J. Nath, P. Jamir and B. K. Patel, Improved procedure for the preparation of isothiocyanates via iodine-mediated desulfurization of dithiocarbamic acid salts, Green Chem. Lett. Rev., 2011, 4, 1–34 CrossRef CAS.
- H. C. Brinkhoff, J. A. Cras, J. J. Steggerda and J. Willemse, Oxidation of dithiocarbamate complexes of nickel, copper, and zinc, Recl. Trav. Chim. Pays-Bas, 1969, 88, 633–640 CrossRef CAS.
- S. Thirumaran, K. Ramalingam, G. Bocelli and A. Cantoni, Spectral and single crystal X-ray structural studies on disulfide complexes: reaction of bis(dialkyldithiocarbamato)M(II) with iodine and crystal structure determination of diiodo(bipiperidinethiuramdisulfide)M(II) (M = zinc, cadmium), Polyhedron, 2000, 19, 1279–1282 CrossRef CAS.
- F. Liang, J. Tan, C. Piao and Q. Liu, Carbon tetrabromide promoted reaction of amines with carbon disulfide: facile and efficient synthesis of thioureas and thiuram disulfides, Synthesis, 2008, 3579–3584 CrossRef CAS.
- S. Techapanalai, R. M. Annuur, M. Sukwattanasinitt and S. Wach, arasindhu, One-Pot Synthesis of Isothiocyanates from Amines Mediated by Carbon Tetrabromide, ChemistrySelect, 2023, 8, e202302045 CrossRef CAS.
- Z. Fu, W. Yuan, N. Chen, Z. Yang and J. Xu, Na2S2O8-mediated efficient synthesis of isothiocyanates from primary amines in water, Green Chem., 2018, 20, 4484–4491 RSC.
- D. Li, Y. Shu, P. Li, W. Zhang, H. Ni and Y. Cao, Synthesis and structure-activity relationships of aliphatic isothiocyanate analogs as antibiotic agents, Med. Chem. Res., 2013, 22, 3119–3125 CrossRef CAS.
- J. Ma, F. Li, C. Wang, Z. Wang, C. Du and L. Wang, Synthesis of Isothiocyanates from Primary Amines via Visible-Light Photocatalysis, Org. Lett., 2023, 25, 5692–5696 CrossRef CAS PubMed.
- There were 3749 hits in the CSD version 5.45 (November 2023) for metal dithiocarbamate complexes.
- D. V. Konarev, A. Y. Kovalevsky, S. S. Khasanov, G. Saito, D. V. Lopatin, A. V. Umrikhin, A. Otsuka and R. N. Lyubovskaya, Synthesis, crystal structures, magnetic properties and photoconductivity of C60 and C70 complexes with metal dialkyldithiocarbamates M(R2dtc)x, where M = CuII, CuI, AgI, ZnII, CdII, HgII, MnII, NiII, and PtII; R = Me, Et, and nPr, Eur. J. Inorg. Chem., 2006, 1881–1895 CrossRef CAS.
- D. V. Konarev, S. S. Khasanov, D. V. Lopatin, V. V. Rodaev and R. N. Lyubovskaya, Fullerene complexes with divalent metal dithiocarbamates: structures, magnetic properties, and photoconductivity, Russ. Chem. Bull., 2007, 56, 2145–2161 CrossRef CAS.
- D. V. Konarev, S. S. Khasanov, A. Y. Kovalevsky, D. V. Lopatin, V. V. Rodaev, G. Saito, B. Nara, L. Forro and R. N. Lyubovskaya, Supramolecular approach to the synthesis of [60]fullerene-metal dithiocarbamate complexes, {(MII(R2dtc)2)x·L}·C60 (M = Zn, Cd, Hg, Fe, and Mn; x = 1 and 2). The study of magnetic properties and photoconductivity, Cryst. Growth Des., 2008, 8, 1161–1172 CrossRef CAS.
- E. C. Alyea, D. C. Bradley, M. F. Lappert and A. R. Sanger, Lower valent dialkylamides of titanium and vanadium, J. Chem. Soc., Chem. Commun., 1969, 1064–1065 RSC.
- D. C. Bradley and M. H. Gitlitz, Preparation and Properties of N,N-Dialkyldithiocarbamates of Early Transition Elements, J. Chem. Soc. A, 1969, 1152–1156 RSC.
- A. C. Behrle, A. J. Myers, A. Kerridge and J. R. Walensky, Coordination Chemistry and QTAIM Analysis of Homoleptic Dithiocarbamate Complexes, M(S2CNiPr2)4 (M = Ti, Zr, Hf, Th, U, Np), Inorg. Chem., 2018, 57, 10518–10524 CrossRef CAS PubMed.
- M. Colapietro, A. Vaciago, D. C. Bradley, M. B. Hursthouse and I. F. Rendall, Structural studies of metal dithiocarbamates. VI. Crystal and molecular structure of tetrakis(N,N-diethyldithiocarbamato)titanium(IV), J. Chem. Soc., Dalton Trans., 1972, 1052–1057 RSC.
- G. L. Campbell, G. D. Ellis and M. R. Chakrabarty, The synthesis and characterization of some titanium(III) tris-N,N-dialkyldithiocarbamates, J. Inorg. Nucl. Chem., 1981, 43, 2265–2268 CrossRef CAS.
- R. E. Jilek, G. Tripepi, E. Urnezius, W. W. Brennessel, V. G. Young Jr. and J. E. Ellis, Zerovalent titanium-sulfur complexes. Novel dithiocarbamato derivatives of Ti(CO)6: [Ti(CO)4(S2CNR2)]−, Chem. Commun., 2007, 2639–2641 RSC.
- L. F. Larkworthy and M. W. O'Donoghue, Synthesis and properties of vanadium(III) dithiocarbamates, Inorg. Chim. Acta, 1983, 74, 155–158 CrossRef CAS.
- H. P. Zhu, Y.-H. Deng, X.-Y. Huang, C.-N. Chen and Q.-T. Liu, Tris(N,N-diethyldithiocarbamato-S,S’)vanadium(III), Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1997, 53, 692–693 CrossRef.
- S. Sproules, T. Weyhermueller, S. De Beer and K. Wieghardt, Six-Membered Electron Transfer Series [V(dithiolene)3]z (z = 1+, 0, 1-, 2-, 3-, 4-). An X-ray Absorption Spectroscopic and Density Functional Theoretical Study, Inorg. Chem., 2010, 49, 5241–5261 CrossRef CAS PubMed.
- T. L. Riechel, L. J. De Hayes and D. T. Sawyer, Electrochemical studies of vanadium(III), -(IV), and -(V) complexes of diethyldithiocarbamate in acetonitrile, Inorg. Chem., 1976, 15, 1900–1904 CrossRef CAS.
- D. C. Bradley, I. F. Rendall and K. D. Sales, Covalent compounds of quadrivalent transition metals. VI. Spectroscopic studies on titanium, vanadium, and zirconium diethyldithiocarbamates, J. Chem. Soc., Dalton Trans., 1973, 2228–2233 RSC.
- B. J. McCormick, Structure and spectra of dithiocarbamate complexes of oxovanadium(IV), Inorg. Chem., 1968, 7, 1965–1970 CrossRef CAS.
- H. Sakurai, H. Watanabe, H. Tamura, H. Yasui, R. Matsushita and J. Takada, Insulin-mimetic vanadyl-dithiocarbamate complexes, Inorg. Chim. Acta, 1998, 283, 175–183 CrossRef CAS.
- M. Atzori, L. Tesi, S. Benci, A. Lunghi, R. Righini, A. Taschin, R. Torre, L. Sorace and R. Sessoli, Spin Dynamics and Low Energy Vibrations: Insights from Vanadyl-Based Potential Molecular Qubits, J. Am. Chem. Soc., 2017, 139, 4338–4341 CrossRef CAS.
- R. M. Golding, P. C. Healy, P. Colombera and A. H. White, NMR studies of some chromium(III) dithicarbamate complexes, Aust. J. Chem., 1974, 27, 2089–2097 CrossRef CAS.
- C. L. Raston and A. H. White, Crystal structure of tris(N,N-diethyldithiocarbamato) chromium(III), Aust. J. Chem., 1977, 30, 2091–2094 CrossRef CAS.
- D. J. Lewis, A. A. Tedstone, X. L. Zhong, E. A. Lewis, A. Rooney, N. Savjani, J. R. Brent, S. J. Haigh, M. G. Burke and C. A. Muryn, et al., Thin Films of Molybdenum Disulfide Doped with Chromium by Aerosol-Assisted Chemical Vapor Deposition (AACVD), Chem. Mater., 2015, 27, 1367–1374 CrossRef CAS.
- J. P. Fackler Jr. and D. G. Holah, Sulfur chelates. II. Five-coordinate transition metal complexes, Inorg. Nucl. Chem. Lett., 1966, 2, 251–255 CrossRef.
- R. Lancashire and T. D. Smith, Syntheses of diethyldithiocarbamate chelates of chromium(III) and molybdenum(V) by oxidative decarbonylation, J. Chem. Soc., Dalton Trans., 1982, 845–846 RSC.
- A. M. Bond and G. G. Wallace, Influence of oxygen insertion on the electrochemistry of chromium(III) dithiocarbamate complexes, Inorg. Chem., 1984, 23, 1858–1865 CrossRef CAS.
- L. Y. Goh, Z. Weng, W. K. Leong and P. H. Leung, C-S bond cleavage and C-C coupling in cyclopentadienylchromium complexes to give the first dithiooxamide-bridged and doubly dithiocarbamate-bridged double cubanes: [Cp6Cr8S8{(C(S)NEt2)2}] and [Cp6Cr8S8(S2CNEt2)2], Angew. Chem., Int. Ed., 2001, 40, 3236–3239 CrossRef CAS.
- G.-Y. He, F.-L. Bei, H.-Q. Chen and X.-Q. Sun, Solid-phase synthesis, crystal structure, and quantum chemical calculation of a molybdenum(II) complex with bis(diethyldithiocarbamate), J. Chem. Crystallogr., 2006, 36, 481–486 CrossRef CAS.
- R. Selvaraju, K. Panchanatheswaren, A. Thiruvalluvar and V. Parthasarathi, Redetermination of Bis(N,N-diethyldithiocarbamato)nickel(II), Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1995, 51, 606–608 CrossRef.
- P. Vella and J. Zubieta, Preparation, characterization and electrochemical investigation of dimeric molybdenum thioxanthate complexes, Mo2(S2CSR)4, and their relationship to analogous binuclear dithioacid complexes, J. Inorg. Nucl. Chem., 1978, 40, 477–487 CrossRef CAS.
- L. Ricard, J. Estienne and R. Weiss, Formation of a thiocarboxamidomolybdenum complex by oxidative bond cleavage. Crystal and molecular structure of bis[μ-sulfido-thiocarboxamido(dipropyldithiocarbamato)molybdenum], Inorg. Chem., 1973, 12, 2182–2186 CrossRef CAS.
- J. A. Broomhead and C. G. Young, Tungsten carbonyl complexes with dithiocarbamate ligands, Aust. J. Chem., 1982, 35, 277–285 CrossRef CAS.
- S. J. N. Burgmayer and J. L. Templeton, Synthesis and structure of a seven-coordinate molybdenum carbonyl fluoride derivative: [Et4N][Mo(CO)2(S2CNEt2)2F], Inorg. Chem., 1985, 24, 2224–2230 CrossRef CAS.
- D. M. Hill, L. F. Larkworthy and M. W. O'Donoghue, Manganese(II) compounds of N,N-disubstituted dithiocarbamates, J. Chem. Soc., Dalton Trans., 1975, 1726–1728 RSC.
- G. Reale, F. Calderoni, T. Ghirardi, F. Porto, F. Illuminati, L. Marvelli, P. Martini, L. Uccelli, E. Tonini, L. Del Bianco, F. Spizzo, M. Capozza, E. Cazzola, A. Carnevale, M. Giganti, A. Turra, J. Esposito and A. Boschi, Development and Evaluation of the Magnetic Properties of a New Manganese(II) Complex: A Potential MRI Contrast Agent, Int. J. Mol. Sci., 2023, 24, 3461 CrossRef CAS PubMed.
- M. Ciampolini, C. Mengozzi and P. Orioli, Structure and magnetic properties of bis(diethyldithiocarbamato)manganese(II), J. Chem. Soc., Dalton Trans., 1975, 2051–2054 RSC.
- P. Martini, A. Boschi, L. Marvelli, L. Uccelli, S. Carli, G. Cruciani, E. Marzola, A. Fantinati, J. Esposito and A. Duatti, Synthesis and Characterization of Manganese Dithiocarbamate Complexes: New Evidence of Dioxygen Activation, Molecules, 2021, 26, 5954 CrossRef CAS PubMed.
- J. Qu, A. Elgendy, R. Cai, M. A. Buckingham, A. A. Papaderakis, H. de Latour, K. Hazeldine, G. F. S. Whitehead, F. Alam, C. T. Smith, D. J. Binks, A. Walton, J. M. Skelton, R. A. W. Dryfe, S. J. Haigh and D. J. Lewis, A Low-Temperature Synthetic Route Toward a High-Entropy 2D Hexernary Transition Metal Dichalcogenide for Hydrogen Evolution Electrocatalysis, Adv. Sci., 2023, 10, 2204488 CrossRef CAS PubMed.
- Y.-W. Jun, Y.-Y. Jung and J. Cheon, Architectural Control of Magnetic Semiconductor Nanocrystals, J. Am. Chem. Soc., 2002, 124, 615–619 CrossRef CAS PubMed.
- Y. Yang, O. Chen, A. Angerhofer and C. Y. Cao, Radial-Position-Controlled Doping in CdS/ZnS Core/Shell Nanocrystals, J. Am. Chem. Soc., 2006, 128, 12428–12429 CrossRef CAS PubMed.
- Y. Zhang, R. Xu, W. Chen, O. Zhuo, Q. Wu, J. Cai, X. Wang and Z. Hu, Solution-solid-solid growth of metastable wurtzite γ-MnS nanowires with controlled length, J. Mater. Chem. C, 2017, 5, 6493–6496 RSC.
- Y. Zhang, Y. Xue, K. Qi, Z. Ru, J. Cai and W. Chen, Construction of Various One-Dimensional ZnS/MnS Hetero-nanostructures with Varied Diameters via the Multistep Solution-Solid-Solid Growth Method, Inorg. Chem., 2022, 61, 1152–1158 CrossRef CAS PubMed.
- H. B. Ferraz, P. H. Bertolucci, J. S. Pereira, J. G. Lima and L. A. Andrade, Chronic exposure to the fungicide maneb may produce symptoms and signs of CNS manganese intoxication, Neurology, 1988, 38, 550–553 CrossRef CAS PubMed.
- S. Costello, M. Cockburn, J. Bronstein, X. Zhang and B. Ritz, Parkinson's disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California, Am. J. Epidemiol., 2009, 169, 919–926 CrossRef PubMed.
- L. Kubens, K.-N. Truong, C. W. Lehmann, D. Luetzenkirchen-Hecht, J. Bornhorst and F. Mohr, The Structure of Maneb, An Important Manganese-Containing Bis(dithiocarbamate) Fungicide, Chem. – Eur. J., 2023, 29, e202301721 CrossRef CAS PubMed.
- J. G. Lozano, F. Dillon, A. J. Naylor, L. Y. Lee, C. Lippard, D. Johnstone, P. G. Bruce and N. Grobert, Single Source Precursor Route to Iron Sulfide Nanomaterials for Energy Storage, Chem. Phys. Lett., 2020, 739, 136993 CrossRef CAS.
- O. A. Ileperuma and R. D. Feltham, Crystal and molecular structure of iron(II) bis(diethyldithiocarbamate), Inorg. Chem., 1975, 14, 3042–3045 CrossRef CAS.
- J. Lu, C.-H. Lu, J.-H. Yu, J.-Q. Xu, Y. Li, X. Zhang, T.-G. Wang and Q.-X. Yang, Syntheses, structures and third-order nonlinear optical properties of heterometal and homometal clusters containing iron, Polyhedron, 2004, 23, 755–761 CrossRef CAS.
- L. F. Larkworthy, B. W. Fitzsimmons and R. R. Patel, Magnetic and Mossbauer investigation of N,N-disubstituted(dithiocarbamato)-iron(II) complexes, J. Chem. Soc., Chem. Commun., 1973, 902–903 RSC.
- B. W. Fitzsimmons, S. E. Al-Mukhtar, L. F. Larkworthy and R. R. Patel, Magnetic and Mössbauer investigations of N,N-disubstituted bis(dithiocarbamato)iron(II) complexes, J. Chem. Soc., Dalton Trans., 1975, 1969–1973 RSC.
- R. H. Holm, L. H. Pinolet and R. A. Lewis, Synthesis and stereochemical rearrangements of complexes containing the Fe-S6 core, J. Am. Chem. Soc., 1971, 93, 360–371 CrossRef CAS.
- J. L. K. F. De Vries, J. M. Trooster and E. De Boer, Iron(II) complexes with two and three dialkyldithiocarbamate ligands. Mössbauer and electronic spectra, Inorg. Chem., 1973, 12, 2730–2733 CrossRef CAS.
- Y. Deng, T. Wen, Q. Liu, H. Zhu, C. Chen and D. Wu, Structure and characterization of a mononuclear FeII dialkyldithiocarbamate complex Fe(OC4H8dtc)2(DMF), Inorg. Chim. Acta, 1999, 293, 95–99 CrossRef CAS.
- M. R. Houchin, Reactions of carbon monoxide with iron(II) diethyldithiocarbamate and iron(II) ethylxanthate, Inorg. Chim. Acta, 1984, 83, 103–110 CrossRef CAS.
- G. Hogarth, N. Hollingsworth, H. Islam, W. Bras, N. H. De Leeuw, A. Roffey and G. Sankar, Fe(II) and Fe(III) dithiocarbamate complexes as single source precursors to nanoscale iron sulfides: A combined synthetic and in situ XAS approach, Nanoscale Adv., 2019, 1, 2965–2978 RSC.
- G. R. Davies, J. A. J. Jarvis, B. T. Kilbourn, R. H. B. Mais and P. G. Owston, Crystal and molecular structure of bis-(N,N-dimethyldithiocarbamato)-nitrosyliron (at −80 deg.), J. Chem. Soc. A, 1970, 1275–1283 RSC.
- O. A. Ileperuma and R. D. Feltham, Iron-sulfur complexes of nitric oxide. 2. Synthesis and exchange studies of Fe(NO)X[S2CN(CH3)2]2. Crystal and molecular structure of cis-bis(diethyldithiocarbamato)(nitro)nitrosyliron, Inorg. Chem., 1977, 16, 1876–1883 CrossRef CAS.
- P. Mordvintcev, A. Muelsch, R. Busse and A. Vanin, On-line detection of nitric oxide formation in liquid aqueous phase by electron paramagnetic resonance spectroscopy, Anal. Biochem., 1991, 199, 142–146 CrossRef CAS PubMed.
- C. Csonka, T. Pali, P. Bencsik, A. Goerbe, P. Ferdinandy and T. Csont, Measurement of NO in biological samples, Br. J. Pharmacol., 2015, 172, 1620–1632 CrossRef CAS PubMed.
- S. Fujii and T. Yoshimura, A new trend in iron-dithiocarbamate complexes: as an endogenous NO trapping agent, Coord. Chem. Rev., 2000, 198, 89–99 CrossRef CAS.
- H. Kunkely and A. Vogler, Photochemistry of tris(diethyldithiocarbamato)iron(III). Reduction to a stable iron(II) complex induced by ligand-to-metal charge transfer excitation, Inorg. Chem. Commun., 2002, 5, 730–732 CrossRef CAS.
- W.-H. Leung, J. L. C. Chim, H. Hou, T. S. M. Hun, I. D. Williams and W.-T. Wong, Oxidation Reactions of Dithiocarbamate Complexes of Ruthenium(II), Inorg. Chem., 1997, 36, 4432–4437 CrossRef CAS PubMed.
- M. Md. Karim, N. Md. Abser, M. R. Hassan, N. Ghosh, H. G. Alt, I. Richards and G. Hogarth, Oxidative-addition of thiuram disulfides to osmium(0): Synthesis of cis-[Os(CO)2(S2CNR2)2] (R = Me, Et, Cy, CH2CH2OMe) and molecular structures of cis-[Os(CO)2(S2CNMe2)2] and [(MeOCH2CH2)2NCS]2, Polyhedron, 2012, 42, 84–88 CrossRef CAS.
- Md. N. Huda, J. C. Sarker, V. N. Nesterov, G. Hogarth and S. E. Kabir, Oxidative-addition of tetramethylthiram disulfide (Me4TDS) and monosulfide (Me4TMS) at a triosmium centre: Generation of dithiocarbamate, trithiocarbamate, thiocarboxamide and amino-carbyne ligands, J. Organomet. Chem., 2024, 1016, 123252 CrossRef CAS.
- N. C. Bhoumik, Md. N. Huda, V. N. Nesterov, G. Hogarth, S. E. Kabir and J. C. Sarker, Reactivity of Labile Triosmium Complexes, [Os3(CO)10(MeCN)2] and [Os3(CO)10(μ-H)2] with Tetraethylthiuram Disulfide (Disulfiram), J. Cluster Sci., 2025, 36, 34 CrossRef CAS.
- R. M. Golding, P. C. Healy, P. W. G. Newman, E. Sinn and A. H. White, Temperature dependence of the proton nuclear magnetic resonance spectra of some diamagnetic N,N-dialkyldithiocarbamate complexes of transition metals, Inorg. Chem., 1972, 11, 2435–2440 CrossRef CAS.
- P. C. Healy, J. W. Connor, B. W. Skelton and A. H. White, Alkyl substituent effects in diamagnetic dithiocarbamate cobalt(III) and nickel(II) complexes, Aust. J. Chem., 1990, 43, 1083–1095 CrossRef CAS.
- F. Jian, F. Bei, P. Zhao, X. Wang, H. Fun and K. Chinnakali, Synthesis, crystal structure and stability studies of dithiocarbamate complexes of some transition elements (M = Co, Ni, Pd), J. Coord. Chem., 2002, 55, 429–437 CrossRef CAS.
- N. V. Khitrich, V. G. Vlasenko, I. I. Seifullina, Ya. V. Zubavichus, S. I. Levchenkov and L. S. Skorokhod, Local surrounding of cobalt(II) in dithiocarbamate complexes, their magnetic and spectral properties, Russ. J. Gen. Chem., 2014, 84, 555–561 CrossRef CAS.
- A. M. Bond, A. R. Hendrickson, R. L. Martin, J. E. Moir and D. R. Page, Electrochemical reduction and oxidation of cobalt(III) dithiocarbamates, Inorg. Chem., 1983, 22, 3440–3446 CrossRef CAS.
- G. Hogarth, K. T. Holman, A. Pateman, A. Sella, J. W. Steed and I. Richards, Multiple nitrene insertions into metal-sulfur bonds of dithiocarbamate complexes: synthesis of sulfido-amido and zwitterionic tetraamido complexes, Dalton Trans., 2005, 2688–2695 RSC.
- K. K. Pandey, D. T. Nehete and R. B. Sharma, Square planar and square pyramidal complexes of rhodium(II), Polyhedron, 1990, 9, 2013–2018 CrossRef CAS.
- N. M. Sosibo and N. Revaprasadu, Synthesis and characterization of rhodium sulfide nanoparticles and thin films, Mater. Sci. Eng., B, 2008, 150, 111–115 CrossRef CAS.
- C. L. Raston and A. H. White, Crystal structures of tris(diethyldithiocarbamato)rhodium(III) and -arsenic(III), J. Chem. Soc., Dalton Trans., 1975, 2425–2429 RSC.
- C. G. Sceney and R. J. Magee, Iridium(III) dithiocarbamate complexes, Inorg. Nucl. Chem. Lett., 1973, 9, 595–597 CrossRef CAS.
- A. M. Bond, R. Colton and D. R. Mann, Electrochemical investigation of kinetic and thermodynamic aspects of oxidation and reduction of mononuclear and binuclear rhodium dithiocarbamate and diselenocarbamate complexes, Inorg. Chem., 1989, 28, 54–59 CrossRef CAS.
- S. Lahiry and V. K. Anand, Manganese(II) complex in a pure spin quartet state, J. Chem. Soc., Chem. Commun., 1971, 1111–1112 RSC.
- S. Tellez, C. A. Costa Jr., M. A. Mondragon, G. B. Ferreira, O. Versiane, J. L. Rangel, G. Lima and M. A. A. Muller, Molecular structure, natural bond analysis, vibrational and electronic spectra, surface enhanced Raman scattering and Mulliken atomic charges of the normal modes of [Mn(DDTC)2] complex, Spectrochim. Acta, Part A, 2016, 169, 95–107 CrossRef PubMed.
- C. A. T. Zepeda, A. Coelho, O. Versiane, M. A. Mondragon, R. S. Pessoa and C. A. Téllez Soto, Synthesis, structure determination, NBO analysis and vibrational/electronic spectroscopic study of Iron(II) Bis(diethyldithiocarbamate) [Fe(DDTC)2], J. Mol. Struct., 2023, 1287, 135618 CrossRef CAS.
- A. C. Costa Jr., O. Versiane, O. G. Faget, J. M. Ramos, G. B. Ferreira, A. A. Martin and C. A. Téllez Soto, An experimental and theoretical approach of spectroscopic and structural properties of the bis(diethyldithiocarbamate)-cobalt(II), J. Mol. Struct., 2012, 1029, 119–134 CrossRef.
- R. R. Eley, R. R. Myers and N. V. Duffy, Electron spin crossover in iron(III) dithiocarbamates, Inorg. Chem., 1972, 11, 1128–1130 CrossRef CAS.
- H. L. Nigam, K. B. Pandeya and R. Singh, 6A1g⇌2T2g spin-crossover in iron(III) dithiocarbamates, J. Indian Chem. Soc., 2001, 78, 525–532 CAS.
- A. H. Ewald, R. L. Martin, E. Sinn and A. H. White, Electronic equilibrium between the 6A1 and 2T2 states in iron(III) dithio chelates, Inorg. Chem., 1969, 8, 1837–1846 CrossRef CAS.
- C. Milsmann, S. Sproules, E. Bill, T. Weyhermueller, S. D. George and K. Wieghardt, Stabilization of High-Valent FeIVS6-Cores by Dithiocarbamate(1-) and 1,2-Dithiolate(2-) Ligands in Octahedral [FeIV(Et2dtc)3(-n(mnt)n](n−1)− Complexes (n = 0, 1, 2, 3): A Spectroscopic and Density Functional Theory Computational Study, Chem. – Eur. J., 2010, 16, 3628–3645 CrossRef CAS PubMed.
- L. H. Pignolet, Dynamic stereochemistry of tris-chelate complexes. IV. Crystal structure of tris(N,N-diethyldithiocarbamato)ruthenium(III), Inorg. Chem., 1974, 13, 2051–2055 CrossRef CAS.
- R. E. DeSimone, Electron paramagnetic resonance studies of low-spin d5 complexes. Trisbidentate complexes of iron(III), ruthenium(III), and osmium(III) with sulfur-donor ligands, J. Am. Chem. Soc., 1973, 95, 6238–6244 CrossRef CAS.
- G. R. Hall and D. N. Hendrickson, Ferric tris(dithiocarbamate) spin equilibrium revisited. Variable-temperature (4.2–296 deg K) magnetic susceptibility, (30–300 deg K) infrared, and (4.2–85 deg K) electron paramagnetic resonance data, Inorg. Chem., 1976, 15, 607–618 CrossRef CAS.
- A. M. Paca, M. Singh and P. A. Ajibade, Synthesis, characterization and in vitro anticancer studies of Ru(III) dithiocarbamate complexes, J. Coord. Chem., 2022, 75, 2923–2932 CrossRef CAS.
- J. Z. Mbese and P. A. Ajibade, Synthesis, spectroscopic, structural and optical studies of Ru2S3 nanoparticles prepared from single-source molecular precursors, J. Mol. Struct., 2017, 1143, 274–281 CrossRef CAS.
- J. Z. Mbese and P. A. Ajibade, Homonuclear tris-dithiocarbamato ruthenium(III) complexes as single-molecule precursors for the synthesis of ruthenium(III) sulfide nanoparticles, J. Sulfur Chem., 2017, 38, 173–187 CrossRef CAS.
- L. Giovagnini, S. Sitran, I. Castagliuolo, P. Brun, M. Corsini, P. Zanello, A. Zoleo, A. Maniero, B. Biondi and D. Fregona, Ru(III)-based compounds with sulfur donor ligands: synthesis, characterization, electrochemical behaviour and anticancer activity, Dalton Trans., 2008, 6699–6708 RSC.
- E. M. Nagy, C. Nardon, L. Giovagnini, L. Marchio, A. Trevisan and D. Fregona, Promising anticancer mono- and dinuclear ruthenium(III) dithiocarbamato complexes: systematic solution studies, Dalton Trans., 2011, 40, 11885–11895 RSC.
- L. Brustolin, C. Nardon, N. Pettenuzzo, N. Zuin Fantoni, S. Quarta, F. Chiara, A. Gambalunga, A. Trevisan, L. Marchio and P. Pontisso, et al., Synthesis, chemical characterization and cancer cell growth-inhibitory activities of Cu(II) and Ru(III) aliphatic and aromatic dithiocarbamato complexes, Dalton Trans., 2018, 47, 15477–15486 RSC.
- A. R. Hendrickson, J. M. Hope and R. L. Martin, Tris- and pentakis-dialkyldithiocarbamates of ruthenium, [Ru(S2CNR2)3]n and [Ru2(S2CNR2)5]n (n = +1, 0, and −1): chemical and electrochemical interrelations, J. Chem. Soc., Dalton Trans., 1976, 2032 RSC.
- K. W. Given, S. H. Wheeler, B. S. Jick, L. J. Maheu and L. H. Pignolet, Synthesis, characterization, and electrochemical properties of dithiocarbamato complexes of osmium(III) and -(IV), Inorg. Chem., 1979, 18, 1261–1266 CrossRef CAS.
- E. R. T. Tiekink and I. Haiduc, Stereochemical aspects of metal xanthate complexes: Molecular structures and supramolecular self-assembly, Prog. Inorg. Chem., 2005, 54, 127–319 CAS.
- P. J. Heard, K. Kite, J. S. Nielsen and D. A. Tocher, Trimethylplatinum(IV) complexes of dithiocarbamato ligands: an experimental NMR study on the barrier to C-N bond rotation, Dalton Trans., 2000, 1349–1356 RSC.
- M. Moriyasu, Y. Hashimoto and M. Endo, Kinetic studies of fast equilibrium by means of high-performance liquid chromatography. IV. Separation of rotamers of palladium(II) dithiocarbamates, Bull. Chem. Soc. Jpn., 1983, 56, 1972–1977 CrossRef CAS.
- W. H. Pan, T. R. Halbert, L. L. Hutchings and E. I. Stiefel, Ligand and induced internal electron transfer pathways to new molybdenum-sulfur and tungsten-sulfur dithiocarbamate complexes, J. Chem. Soc., Chem. Commun., 1985, 927–929 RSC.
- M. A. Harmer, T. R. Halbert, W. H. Pan, C. L. Coyle, S. A. Cohen and E. I. Stiefel, Ligand and induced internal redox processes in molybdenum- and tungsten-sulfur systems, Polyhedron, 1986, 5, 341–347 CrossRef CAS.
- T. R. Halbert, L. L. Hutchings, R. Rhodes and E. I. Stiefel, Induced redox reactivity of tetrathiovanadate(V): synthesis of the vanadium(IV) dimer V2(μ-S2)2(iso-Bu2NCS2)4 and its structural relationship to the V/S mineral patronite, J. Am. Chem. Soc., 1986, 108, 6437–6438 CrossRef CAS.
- L. Wei, T. R. Halbert, H. H. Murray III and E. I. Stiefel, Induced internal electron transfer reactivity of tetrathioperrhenate(VII): synthesis of the interconvertible dimers Re2(μ-S)2(S2CNR2)4 and [Re2(μ-SS2CNR2)2(S2CNR2)3][O3SCF3] (R = Me, iso-Bu), J. Am. Chem. Soc., 1990, 112, 6431–6433 CrossRef CAS.
- Y. Gea, M. A. Greaney, C. I. Coyle and E. I. Stiefel, Analogous reactivity of MoS42− and WSe42: preparation of WSe2(iso-Bu2NCS2)3 by an induced internal redox reaction, J. Chem. Soc., Chem. Commun., 1992, 160–161 RSC.
- J. M. Hope, R. L. Martin, D. Taylor and A. H. White, Ring expansion in a metal-dithiocarbamate complex by oxygen insertion; synthesis and properties of [Cr(S2CNR2)2(OS2CNR2)]. The X-ray structure of bis[NN-diethyl(dithiocarbamato-SS’)][NN-diethyl(dithioperoxycarbamato-OS)]chromium(III), J. Chem. Soc., Chem. Commun., 1977, 99–100 RSC.
- S. Ng, J. W. Ziller and P. J. Farmer, Multiple Pathways for the Oxygenation of a Ruthenium(II) Dithiocarbamate Complex: S-Oxygenation and S-Extrusion, Inorg. Chem., 2004, 43, 8301–8309 CrossRef CAS PubMed.
- D. F. Brayton, K. Tanabe, M. Khiterer, K. Kolahi, J. Ziller, J. Greaves and P. J. Farmer, Oxygenation of Zinc Dialkyldithiocarbamate Complexes: Isolation, Characterization, and Reactivity of the Stoichiometric Oxygenates, Inorg. Chem., 2006, 45, 6064–6072 CrossRef CAS PubMed.
- E. Eijarvi, L. H. J. Lajunen and M. Heikka, Simultaneous determination of chromium(III) and chromium(VI) by reversed-phase high performance liquid chromatography with UV detection, Finn. Chem. Lett., 1985, 225–230 CAS.
- Y. Honma, Formation of two chromium(III) dithiocarbamates from Cr(VI) in solvent extraction system and origin of oxygen atom in bis(1-pyrrolidinecarbodithioato)[1-pyrrolidinecarbothio (thioperoxoato)]chromium(III), Bull. Chem. Soc. Jpn., 2002, 75, 2415–2421 CrossRef CAS.
- M. Safari, S. Nojavan, S. S. H. Davarani and A. Morteza-Najarian, Speciation of chromium in environmental samples by dual electromembrane extraction system followed by high performance liquid chromatography, Anal. Chim. Acta, 2013, 789, 58–64 CrossRef CAS PubMed.
- L. F. Shvydka, Yu. I. Usatenko and F. M. Tulyupa, Strength of osmium(VI) dithiocarbamates, Zh. Neorg. Khim., 1973, 18, 756–761 CAS.
- W. P. Griffith and J. M. Jolliffe, Studies on transition-metal nitrido and oxo complexes. Part 14. Carboxylato oxo-osmium(VI) and -ruthenium(VI) complexes and their reactions, J. Chem. Soc., Dalton Trans., 1992, 3483–3488 RSC.
- C. Nardon, S. M. Schmitt, H. Yang, J. Zuo, D. Fregona and Q. P. Dou, Gold(III)-Dithiocarbamato Peptidomimetics in the Forefront of the Targeted Anticancer Therapy: Preclinical Studies against Human Breast Neoplasia, PLoS One, 2014, 9, e84248 CrossRef PubMed.
- H. J. A. Blaauw, R. J. F. Nivard and G. J. M. van der Kerk, Chemistry of organogold compounds: I. Syntheses and properties of dihalogold(III) N,N-dialkyldithiocarbamates and dialkylgold(III) N,N-dialkyldithiocarbamates, J. Organomet. Chem., 1964, 2, 236–244 CrossRef CAS.
- R. K. Brown, J. N. Bunyan, A. Agrawal, G. Li, D. Dautoras, J. C. Sarker, T. T. Keat, T. Hicks, G. Hogarth and D. Pugh, A Revised Understanding of the Speciation of Gold(III) Dithiocarbamate Complexes in Solution, Dalton Trans., 2025, 54, 7627–7640 RSC.
- J. Cordón, G. Jiménez-Osés, J. M. López-de-Luzuriaga, M. Monge, M. E. Olmos and D. Pascual, Experimental and Theoretical Study of Gold(III)-Catalyzed Hydration of Alkynes, Organometallics, 2014, 33, 3823–3830 CrossRef.
- L. Giovagnini, L. Ronconi, D. Aldinucci, D. Lorenzon, S. Sitran and D. Fregona, Synthesis, Characterization, and Comparative in Vitro Cytotoxicity Studies of Platinum(II), Palladium(II), and Gold(III) Methylsarcosinedithiocarbamate Complexes, J. Med. Chem., 2005, 48, 1588–1595 CrossRef CAS PubMed.
- M. Altaf, A. A. Isab, J. Vančo, Z. Dvorák, Z. Trávníček and H. Stoeckli-Evans, Synthesis, characterization and in vitro cytotoxicity of gold(III) dialkyl/diaryldithiocarbamato complexes, RSC Adv., 2015, 5, 81599–81607 RSC.
- O. Loseva , Private communication to the CCDC, deposition number 1977816.
- A. Angeloski, K. Flower-Donaldson, F. Matar, D. Hayes, M. Duman, D. Oldfield, M. Westerhausen and A. McDonagh, Gold Microstructures by Thermolysis of Gold(III) Di-isopropyldithiocarbamate Complexes, ChemNanoMat, 2024, 10, e202300514 CrossRef CAS.
- M. Mäkelä, T. HatanpäÄ, K. Mizohata, J. Räisänen, M. Ritala and M. Leskelä, Thermal Atomic Layer Deposition of Continuous and Highly Conducting Gold Thin Films, Chem. Mater., 2017, 29, 6130–6136 CrossRef.
- M. Contel, A. J. Edwards, J. Garrido, M. B. Hursthouse, M. Laguna and R. Terroba, Mesityl gold(III) complexes. X-ray structure of mononuclear [Au(mes)2Cl(PPh3)] and the dimer [Au(mes)2Cl]2, J. Organomet. Chem., 2000, 607, 129–136 CrossRef CAS.
- J. Quero, S. Cabello, T. Fuertes, I. Mármol, R. Laplaza, V. Polo, M. C. Gimeno, J. Rodriguez-Yoldi and E. Cerrada, Proteasome versus Thioredoxin Reductase Competition as Possible Biological Targets in Antitumor Mixed Thiolate-Dithiocarbamate Gold(III) Complexes, Inorg. Chem., 2018, 57, 10832–10845 CrossRef CAS PubMed.
- A. Pettenuzzo, D. Montagner, P. McArdle and L. Ronconi, An innovative and efficient route to the synthesis of metal-based glycoconjugates: proof-of-concept and potential applications, Dalton Trans., 2018, 47, 10721–10736 RSC.
- C. Nardon, D. Fregona, L. Brustolin and N. Pettenuzzo, Coordination compounds, syntheses, nanoformulation and use thereof in oncology, World Intellectual Property Organization, WO2018100561, 2017 Search PubMed.
- S. C. Bajia and A. Mishra, Synthesis and spectroscopic characterization of bis(N-alkyldithiocarbamato)nickel(II) complexes: crystal structures of [Ni(S2CNH(n-Pr))2] and [Ni(S2CNH(i-Pr))2], J. Coord. Chem., 2011, 64, 2727–2734 CrossRef CAS.
- A. Z. Halimehjani, K. Marjani, A. Ashouri and V. Amani, Synthesis and characterization of transition metal dithiocarbamate derivatives of 1-aminoadamantane: Crystal structure of (N-adamantyldithiocarbamato)nickel(II), Inorg. Chim. Acta, 2011, 373, 282–285 CrossRef CAS.
- F. F. Bobinihi, D. C. Onwudiwe, A. C. Ekennia, O. C. Okpareke, C. Arderne and J. R. Lane, Group 10 metal complexes of dithiocarbamates derived from primary anilines: Synthesis, characterization, computational and antimicrobial studies, Polyhedron, 2019, 158, 296–310 CrossRef CAS.
- C. E. Morrison, F. Wang, N. P. Rath, B. M. Wieliczka, R. A. Loomis and W. E. Buhro, Cadmium Bis(phenyldithiocarbamate) as a Nanocrystal Shell-Growth Precursor, Inorg. Chem., 2017, 56, 12920–12929 CrossRef CAS PubMed.
- L. H. van Poppel, T. L. Groy and M. T. Caudle, Carbon-Sulfur Bond Cleavage in Bis(N-alkyldithiocarbamato)cadmium(II) Complexes: Heterolytic Desulfurization Coupled to Topochemical Proton Transfer, Inorg. Chem., 2004, 43, 3180–3188 CrossRef CAS PubMed.
- A. A. Memon, M. Afzaal, M. A. Malik, C. Q. Nguyen, P. O'Brien and J. Raftery, The N-alkyldithiocarbamato complexes [M(S2CNHR)2] (M = Cd(II) Zn(II); R = C2H5, C4H9, C6H13, C12H25); their synthesis, thermal decomposition and use to prepare of nanoparticles and nanorods of CdS, Dalton Trans., 2006, 4499–4505 RSC.
- D. C. Onwudiwe, T. Arfin, C. A. Strydom and R. J. Kriek, A study of the thermal and AC impedance properties of N-phenyldithiocarbamate complexes of Zn(II), Electrochim. Acta, 2013, 109, 809–817 CrossRef CAS.
- B. B. Kaul and K. B. Pandeya, N-Monoaryldithiocarbamatecobalt(III) complexes, Transition Met. Chem., 1979, 4, 112–114 CrossRef CAS.
- G. L. Zhang, Y. T. Li and Z. Y. Wu, Tris(ethyldithiocarbamato-κ2S,S′)cobalt(III), Acta Crystallogr., Sect. E: Struct. Rep. Online, 2006, 62, m350–m351 CrossRef CAS.
- E. Tejeria, J. Giglio, L. Fernandez and A. Rey, Development and evaluation of a 99mTc(V)-nitrido complex derived from estradiol for breast cancer imaging, Appl. Radiat. Isot., 2019, 154, 108854 CrossRef CAS PubMed.
- X. Song, Y. Wang, J. Zhang, Z. Jin, W. Zhang and Y. Zhang, Synthesis and evaluation of a novel 99mTc nitrido radiopharmaceutical with alendronate dithiocarbamate as a potential bone-imaging agent, Chem. Biol. Drug Des., 2018, 91, 545–551 CrossRef CAS PubMed.
- Y. Chen, H. Guo, F. Xie and J. Lu, Preparation and biological evaluation of 99mTcN-labeled pteroyl-lys derivative as a potential folate receptor imaging agent, J. Labelled Compd. Radiopharm., 2014, 57, 12–17 CrossRef CAS PubMed.
- N. Hollingsworth, A. Roffey, H. U. Islam, M. Mercy, A. Roldan, W. Bras, M. Wolthers, C. R. A. Catlow, G. Sankar, G. Hogarth and N. H. De Leeuw, Active Nature of Primary Amines during Thermal Decomposition of Nickel Dithiocarbamates to Nickel Sulfide Nanoparticles, Chem. Mater., 2014, 26, 6281–6292 CrossRef CAS.
- R. Wong and S. J. Dolman, Isothiocyanates from Tosyl Chloride Mediated Decomposition of in Situ Generated Dithiocarbamic Acid Salts, J. Org. Chem., 2007, 72, 3969–3971 CrossRef CAS PubMed.
- N. Sun, B. Li, J. Shao, W. Mo, B. Hu, Z. Shen and X. Hu, A general and facile one-pot process of isothiocyanates from amines under aqueous conditions, Beilstein J. Org. Chem., 2012, 8, 61–70 CrossRef CAS PubMed.
- Z. Fu, W. Yuan, N. Chen, Z. Yang and J. Xu, Na2S2O8-mediated efficient synthesis of isothiocyanates from primary amines in water, Green Chem., 2018, 20, 4484–4491 RSC.
- M. A. Agoro and E. L. Meyer, FeS/FeS2 nanoscale structures synthesized in one step from Fe(ll) dithiocarbamate complexes as a single source precursor, Front. Chem., 2022, 10, 1035594 CrossRef CAS PubMed.
- A. M. Paca and P. A. Ajibade, Synthesis and structural studies of iron sulphide nanocomposites prepared from Fe(III) dithiocarbamates single source precursors, Mater. Chem. Phys., 2017, 202, 143–150 CrossRef CAS.
- M. Tarique and M. Aslam, Synthesis and characterization of some transition metal complexes of dithiocarbamate ligand derived from p-toluidine, Asian J. Chem., 2010, 22, 2031–2034 CAS.
- M. A. Agoro and E. L. Meyer, Influence of a One-Pot Approach on a Prepared CuS Macro/Nanostructure from Various Molecular Precursors, Inorganics, 2023, 11, 266 CrossRef CAS.
- E. I. Duran-Garcia, J. Martinez-Santana, N. Torres-Gomez, A. R. Vilchis-Nestor and I. Garcia-Orozco, Copper sulfide nanoparticles produced by the reaction of N-alkyldithiocarbamatecopper(II) complexes with sodium borohydride, Mater. Chem. Phys., 2021, 269, 124743 CrossRef CAS.
- N. L. Botha and P. A. Ajibade, Effect of temperature on crystallite sizes of copper sulfide nanocrystals prepared from copper(II) dithiocarbamate single source precursor, Mater. Sci. Semicond. Process., 2016, 43, 149–154 CrossRef CAS.
- C. Chen, K.-W. Yang, L. Zhai, H.-H. Ding and J.-Z. Chigan, Dithiocarbamates combined with copper for revitalizing meropenem efficacy against NDM-1-producing Carbapenem-resistant Enterobacteriaceae, Bioorg. Chem., 2022, 118, 105474 CrossRef CAS PubMed.
- S. Huang, X. Xu, J. C. Sarker, D. Pugh and G. Hogarth, Primary-amine-derived Cu(I)-dithiocarbamate complexes and their use as low temperature single source precursors to CuS (covellite) nanomaterials, Dalton Trans., 2024, 53, 17140–17145 RSC.
- C. Bianchini, C. A. Ghilardi, A. Meli, S. Midollini and A. Orlandini, Reactivity of copper(I) tetrahydroborates toward carbon disulfide and phenyl isothiocyanate. Structures of (PPh3)2Cu(μ-S2CSCH2SCS2)Cu(PPh3)2, (PPh3)2Cu(S2COEt), and (PPh3)2Cu(S2CNHPh), Inorg. Chem., 1985, 24, 932–939 CrossRef CAS.
- Q. Gaydon and S. D. Bohle, Coordination Chemistry of the Parent Dithiocarbamate H2NCS2−: Organometallic Chemistry and Tris-Chelates of Group 9 Metals, Inorg. Chem., 2022, 61, 4660–4672 CrossRef CAS PubMed.
- C. L. Teske and W. Bensch, On Crystal Structure Investigations of α- and β-Ammoniumdithiocarbamate NH4CS2NH2 and the Role of Hydrogen Bonding, Z. Anorg. Allg. Chem., 2010, 636, 356–362 CrossRef CAS.
- L. Capacchi, M. Nardelli and A. Villa, The crystal structure of nickel(II) bis(dithiocarbamate), Chem. Commun., 1966, 441 Search PubMed.
- M. A. Bernard, M. M. Borel and J. Gallay, Metal dithiocarbamates. III. Crystallographic study of zinc dithiocarbamate, Bull. Soc. Chim. Fr., 1969, 9, 3069 CAS.
- C. L. Teske and W. Bensch, On Tris(dithiocarbamato)-Chromium(III), Cr(S2CNH2)3, Z. Anorg. Allg. Chem., 2012, 638, 2093–2097 CrossRef CAS.
- C. L. Raston, A. H. White and A. C. Willis, Crystal structure of tris(dithiocarbamato)cobalt(III), J. Chem. Soc., Dalton Trans., 1975, 2429–2432 RSC.
- C. L. Teske and W. Bensch, On Mono(dithiocarbamato)-silver(I), AgS2CNH2, Z. Anorg. Allg. Chem., 2015, 641, 1031–1035 CrossRef CAS.
- C. L. Teske, H. Reinsch, H. Terraschke and W. Bensch, Synthesis, Crystal Structure and Selected Properties of Mono(dithiocarbamato)-gold(I), AuS2CNH2, Z. Anorg. Allg. Chem., 2017, 643, 466–470 CrossRef CAS.
- C. L. Teske, A.-L. Hansen, R. Weihrich, L. Kienle, M. Kamp, K. P. van der Zwan, J. Senker, C. Dosche, G. Wittstock and W. Bensch, Synthesis, Crystal Structure, and Selected Properties of [Au(S2CNH2)2]SCN: A Precursor for Gold Macro-Needles Consisting of Gold Nanoparticles Glued by Graphitic Carbon Nitride, Chem. – Eur. J., 2019, 25, 6763–6772 CrossRef CAS PubMed.
- C. L. Teske, On Ammonium-bis(dithiocarbamato)-copper(I)-monohydrate and Mono(dithiocarbamato)-copper(I), Z. Anorg. Allg. Chem., 2013, 639, 2767–2773 CrossRef CAS.
- M. A. Agoro and E. L. Meyer, Roles of TOPO Coordinating Solvent on Prepared Nano-Flower/Star and Nano-Rods Nickel Sulfides for Solar Cells Applications, Nanomaterials, 2022, 12, 3409 CrossRef CAS PubMed.
- M. A. Agoro and E. L. Meyer, Influence of a One-Pot Approach on a Prepared CuS Macro/Nanostructure from Various Molecular Precursors, Inorganics, 2023, 11, 266 CrossRef CAS.
- M. A. Agoro, E. L. Meyer and O. I. Olayiwola, Assemble of porous heterostructure thin film through CuS passivation for efficient electron transport in dye-sensitized solar cells, Discover Nano, 2024, 19, 130 CrossRef CAS PubMed.
- M. A. Agoro, E. L. Meyer, J. Z. Mbese and K. Manu, Electrochemical fingerprint of CuS-hexagonal chemistry from (Bis(N-1,4-Phenyl-N-(4-morpholinedithiocarbamato) copper(II) complexes) as photon absorber in quantum-dot/Dye-sensitised solar cells, Catalysts, 2020, 10, 300 CrossRef CAS.
- M. M. Salman, A. A. Al-Dulaimi, A. S. M. Al-Janabi and M. A. Alheety, Novel dithiocabamate nano Zn(II), Cd(II) and Hg(II) complexes with pyrrolidinedithiocarbamate and N,N-diethyldithiocarbamate, Mater. Today: Proc., 2021, 43, 863–868 CAS.
- P. A. Ajibade, D. C. Onwudiwe and M. J. Moloto, Synthesis of hexadecylamine capped nanoparticles using group 12 complexes of N-alkyl-N-phenyl dithiocarbamate as single-source precursors, Polyhedron, 2011, 30, 246–252 CrossRef CAS.
- D. C. Onwudiwe and P. A. Ajibade, Synthesis and Crystal Structure of Bis(N-alkyl-N-phenyl dithiocarbamato)mercury(II), J. Chem. Crystallogr., 2011, 41, 980–985 CrossRef CAS.
- D. C. Onwudiwe, Y. B. Nthwane, A. C. Ekennia and E. Hosten, Synthesis, characterization and antimicrobial properties of some mixed ligand complexes of Zn(II) dithiocarbamate with different N-donor ligands, Inorg. Chim. Acta, 2016, 447, 134–141 CrossRef CAS.
- P. A. Ajibade and D. C. Onwudiwe, Synthesis and characterization of group 12 complexes of N,N-methyl phenyl-N,N-butyl phenyl dithiocarbamate, J. Coord. Chem., 2011, 64, 2963–2973 CrossRef CAS.
- E. L. Meyer and M. A. Agoro, Impact of FeS on the TiO2 Layer As Support System in QDSCs, ACS Omega, 2024, 9, 37891–37900 CrossRef CAS PubMed.
- M. A. Agoro and E. L. Meyer, FeS/FeS2 nanoscale structures synthesized in one step from Fe(ll) dithiocarbamate complexes as a single source precursor, Front. Chem., 2022, 10, 1035594 CrossRef CAS PubMed.
- M. A. Agoro and E. L. Meyer, Coating of CoS on hybrid anode electrode with enhance performance in hybrid dye-sensitized solar cells, Electrochim. Acta, 2024, 502, 144877 CrossRef CAS.
- M. A. Agoro, J. Z. Mbese and E. L. Meyer, Inorganic Pb(II)-P and Pb(II)-S Complexes as Photosensitizers from Primary and Secondary Amines in Dyes-Sensitized Solar Cells, ACS Omega, 2021, 6, 23700–23709 CrossRef CAS PubMed.
- M. A. Agoro, J. Z. Mbese and E. L. Meyer, Electrochemistry of Inorganic OCT-PbS/HDA and OCT-PbS Photosensitizers Thermalized from Bis(N-diisopropyl-N-octyldithiocarbamato) Pb(II) Molecular Precursors, Molecules, 2020, 25, 1919 CrossRef CAS PubMed.
- J. Z. Mbese, E. L. Meyer and M. A. Agoro, Electrochemical performance of photovoltaic cells using HDA capped-SnS nanocrystal from bis (N-1,4-phenyl-N-morpho-dithiocarbamato) Sn(II) complexes, Nanomaterials, 2020, 10, 414 CrossRef CAS PubMed.
- E. L. Meyer, J. Z. Mbese, M. A. Agoro and R. Taziwa, Optical and structural-chemistry of SnS nanocrystals prepared by thermal decomposition of bis(N-di-isopropyl-N-octyl dithiocarbamato)tin(II) complex for promising materials in solar cell applications, Opt. Quantum Electron., 2020, 52, 90 CrossRef CAS.
- J. Z. Mbese, E. L. Meyer and M. A. Agoro, Electrocatalytic properties of PbS nanocrystals structured from (bis(N-1,4-phenyl-N-(4-morpholine)dithiocarbamato Pb(II) complexes) as photosensitizer for quantum-dots-sensitized solar cells, Mater. Lett., 2020, 271, 127770 CrossRef CAS.
- M. A. Agoro, E. L. Meyer, J. Z. Mbese, X. Fuku and C. C. Ahia, Aliphatic mixed ligands Sn(II) complexes as photon absorbers in quantum dots sensitized solar cell, J. Solid State Chem., 2022, 308, 122890 CrossRef CAS.
- L. K. Macreadie, C. M. Forsyth, D. R. Turner and A. S. R. Chesman, Cadmium tris(dithiocarbamate) ionic liquids as single source, solvent-free cadmium sulfide precursors, Chem. Commun., 2018, 54, 8925–8928 RSC.
- H. L. M. Van Gaal, J. W. Diesveld, F. W. Pijpers and J. G. M. Van der Linden, Carbon-13 NMR spectra of dithiocarbamates. Chemical shifts, carbon-nitrogen stretching vibration frequencies and π-bonding in the NCS2 fragment, Inorg. Chem., 1979, 18, 3251–3260 CrossRef CAS.
- M. Moriyasu and Y. Hashimoto, Kinetic studies of fast equilibrium by high-performance liquid chromatography. I. Ternary complex formation of N,N-disubstituted dithiocarbamate chelates of nickel(II) and copper(II), Bull. Chem. Soc. Jpn., 1980, 53, 3590–3595 CrossRef CAS.
- M. Moriyasu and Y. Hashimoto, Kinetic studies on the labile ternary nickel(II) chelates of N-disubstituted dithiocarbamic acids by high-performance liquid chromatography, Chem. Lett., 1980, 117–120 CrossRef CAS.
- M. Moriyasu and Y. Hashimoto, Kinetic studies of fast equilibrium by means of high-performance liquid chromatography. II. Ligand exchange of N,N-disubstituted dithiocarbamate chelates of Ni(II), Bull. Chem. Soc. Jpn., 1981, 54, 2470–2474 CrossRef CAS.
- O. Liska, G. Guiochon and H. Colin, Liquid chromatography of metal complexes of N-disubstituted dithiocarbamic acids. I. High-performance liquid chromatography of nickel(II) bisdialkyldithiocarbamates, J. Chromatogr., 1979, 171, 145–151 CrossRef CAS.
- O. Liska, J. Lehotay, E. Brandsteterova and G. Guiochon, Liquid chromatography of metal complexes of N-disubstituted dithiocarbamic acids. II. Identification of nickel(II) bisdialkyldithiocarbamate mixed-ligand complexes, J. Chromatogr., 1979, 171, 153–159 CrossRef CAS.
- S. Dilli and P. Tong, Liquid chromatography of metal chelates. Chromatographic studies of homologous dialkyldithiocarbamates, Anal. Chim. Acta, 1999, 395, 101–112 CrossRef CAS.
- E. Beinrohr and J. Garaj, NMR study of ligand exchange in some metal dithiocarbamates, Collect. Czech. Chem. Commun., 1980, 45, 1785–1792 CrossRef CAS.
- M. Moriyasu and Y. Hashimoto, Kinetic studies of fast equilibrium by means of high-performance liquid chromatography. III. Ternary complex formation between nickel(II) diethyldithiocarbamate and other unlike nickel(II) chelates, Bull. Chem. Soc. Jpn., 1981, 54, 3374–3378 CrossRef CAS.
- N. V. Duffy, Ligand exchange in tris(diorganodithiocarbamato)iron(III) complexes, Inorg. Chim. Acta, 1981, 47, 31–35 CrossRef CAS.
- K. Drabent and L. Latos-Grazynski, NMR studies of mixed-ligand iron(III) dithiocarbamates, Polyhedron, 1985, 4, 1637–1641 CrossRef CAS.
- R. Chant, A. R. Hendrickson, R. L. Martin and N. M. Rohde, Tris(dithiocarbamato) complexes of iron(II), iron(III), and iron(IV). Electrochemical study, Inorg. Chem., 1975, 14, 1894–1902 CrossRef CAS.
- A. M. Bond, R. Colton, D. Dakternieks, M. L. Dillon, J. Hauenstein and J. E. Moir, Phosphorus-31, cadmium-113 and mercury-199 NMR studies on dithiolate complexes of cadmium and mercury and their phosphine adducts, Aust. J. Chem., 1981, 34, 1393–1400 CrossRef CAS.
- A. M. Bond, R. Colton, M. L. Dillon, J. E. Moir and D. R. Page, Investigation of exchange and redox reactions of mercury dithiocarbamate complexes by electrochemical techniques at mercury electrodes, mercury-199 nuclear magnetic resonance spectrometry and mass spectrometry, Inorg. Chem., 1984, 23, 2883–2891 CrossRef CAS.
- M. C. Palazzotto, D. J. Duffy, B. L. Edgar, L. Que Jr and L. H. Pignolet, Dynamic stereochemistry of tris(chelate) complexes. I. Tris(dithiocarbamato) complexes of iron, cobalt, and rhodium, J. Am. Chem. Soc., 1973, 95, 4537–4545 CrossRef CAS.
- A. R. Hendrickson, R. L. Martin and D. Taylor, Synthesis and properties of dimeric cobalt(III) dithiocarbamate complexes [Co2(R2dtc)5]+. X-ray structural analysis of pentakis(diethyldithiocarbamato)dicobalt(III) tetrafluoroborate, J. Chem. Soc., Dalton Trans., 1975, 2182–2188 RSC.
- A. M. Bond, R. Colton, J. E. Moir and D. R. Page, Investigations of mixed-ligand cobalt dithiocarbamate complexes by cobalt-59 nuclear magnetic resonance spectroscopy, mass spectrometry, and electrochemistry, Inorg. Chem., 1985, 24, 1298–1302 CrossRef CAS.
- F. Artizzu, L. Marchio, L. Pilia, A. Serpe and P. Deplano, Heteroleptic Co(III) bisdithiocarbamato-dithione complexes: Synthesis, structure and bonding of [Co(Et2dtc)2(R2pipdt)]BF4 (R = Me, 1; Ph, 2; pipdt = piperazin-2,3-dithione) complexes, J. Coord. Chem., 2022, 75, 2434–2447 CrossRef CAS.
- A. A. Olanrewaju, F. S. Fabiyi, C. U. Ibeji, E. G. Kolawole and R. Gupta, Synthesis, spectral, structure and computational studies of novel transition Metal(II) complexes of (Z)-((dimethylcarbamothioyl)thio) ((1,1,1-trifluoro-4-(naphthalen-2-yl)-4-oxobut-2-en-2-yl)oxy), J. Mol. Struct., 2020, 1211, 128057 CrossRef CAS.
- A. A. Olanrewaju, C. U. Ibeji and O. E. Oyeneyin, Biological evaluation and molecular docking of some newly synthesized 3d-series metal(II) mixed-ligand complexes of fluoro-naphthyl diketone and dithiocarbamate, SN Appl. Sci., 2020, 2, 678 CrossRef CAS.
- A. C. Ekennia, D. C. Onwudiwe, L. O. Olasunkanmi, A. A. Osowole and E. E. Ebenso, Synthesis, DFT calculation, and antimicrobial studies of novel Zn(II), Co(II), Cu(II), and Mn(II) heteroleptic complexes containing benzoylacetone and dithiocarbamate, Bioinorg. Chem. Appl., 2015, 1–13 Search PubMed.
- A. C. Ekennia, D. C. Onwudiwe, A. A. Osowole, L. O. Olasunkanmi and E. E. Ebenso, J. Chem., 2016, 5129010 Search PubMed.
- E. V. Ignatov, L. E. Zelenkov, S. V. Baykov, M. K. Shurikov, A. V. Semenov, N. A. Bokach, P. S. Postnikov and V. Yu. Kukushkin, Controlled Mono- and Double-Insertion of Sulfonamide Fragments into Ni–S Bonds of Nickel(II) Dithiocarbamate Complexes via Sulfonyl Azide Reactivity, Inorg. Chem., 2025, 64, 11867–11879 CrossRef CAS PubMed.
- G. Exarchos and S. D. Robinson, Constitution of some “dithiocarbamato-bridged heterobimetallic complexes”, Polyhedron, 1997, 16, 1573–1576 CrossRef CAS.
- G. Exarchos, S. C. Nyburg and S. D. Robinson, The synthesis and characterization of [Pd(S2CNEt2)(Ph2PCH2CH2PPh2)]+ salts of some chloro- and bromo-metalate anions- X-ray crystal structures of [Pd(S2CNEt2)(Ph2PCH2CH2PPh2)]+[MCl2]−(M = Cu, Ag), Polyhedron, 1998, 17, 1257–1266 CrossRef CAS.
- G. Exarchos, S. D. Robinson and J. W. Steed, The synthesis and characterisation of [Pd(S2CNEt2){Ph2P(CH2)nPPh2}]+ salts of some chlorometallate anions (n = 1, 3, 4): X-ray crystal structures of the salts [Pd(S2CNEt2){Ph2P(CH2)nPPh2}]2[MCl4] (n = 1, 4; M = Cd; n = 3; M = Hg), Polyhedron, 2000, 19, 1511–1517 CrossRef CAS.
- G. Exarchos, S. D. Robinson and J. W. Steed, The synthesis of new bimetallic complex salts by halide/sulfur chelate cross transfer: X-ray crystal structures of the salts [Ni(S2CNEt2)(dppe)]2[HgBr4], [Pt(S2CNEt2)(dppe)]2[CdCl4], [Co(S2CNEt2)2(dppe)]2 [Cl3ZnO:(Ph)2PCH2CH2P(Ph)2:OZnCl3] and [Pd(S2CNnBu2)(bipy)]2[CdCl4], Polyhedron, 2001, 20, 2951–2963 CrossRef CAS.
- T. Torimoto, T. Adachi, K.-I. Okazaki, M. Sakuraoka, T. Shibayama, B. Ohtani, A. Kudo and S. Kuwabata, Facile Synthesis of ZnS-AgInS2 Solid Solution Nanoparticles for a Color-Adjustable Luminophore, J. Am. Chem. Soc., 2007, 129, 12388–12389 CrossRef CAS PubMed.
- T. Kameyama, T. Takahashi, T. Machida, Y. Kamiya, T. Yamamoto, S. Kuwabata and T. Torimoto, Controlling the Electronic Energy Structure of ZnS-AgInS2 Solid Solution Nanocrystals for Photoluminescence and Photocatalytic Hydrogen Evolution, J. Phys. Chem. C, 2015, 119, 24740–24749 CrossRef CAS.
- W. Hoisang, T. Uematsu, T. Torimoto and S. Kuwabata, Luminescent Quaternary Ag(InxGa1(-x)S2/GaSy Core/Shell Quantum Dots Prepared Using Dithiocarbamate Compounds and Photoluminescence Recovery via Post Treatment, Inorg. Chem., 2021, 60, 13101–13109 CrossRef CAS PubMed.
- T. Uematsu, M. Tepakidareekul, T. Hirano, T. Torimoto and S. Kuwabata, Facile High-Yield Synthesis of Ag-In-Ga-S Quaternary Quantum Dots and Coating with Gallium Sulfide Shells for Narrow Band-Edge Emission, Chem. Mater., 2023, 35, 1094–1106 CrossRef CAS.
- E. J. Mensforth, M. R. Hill and S. R. Batten, Coordination polymers of sulphur-donor ligands, Inorg. Chim. Acta, 2013, 403, 9–24 CrossRef CAS.
- M. Ebihara, K. Tokoro, M. Maeda, M. Ogami, K. Imaeda, K. Sakurai, H. Masuda and T. Kawamura, Bonding interaction between group 10 and 11 metals. Synthesis, structure and properties of [M3(S2CNR2)6M’2]2+ (M = Pt or Pd; M′ = Ag or Cu; R = Et, Pri, Prn, Bun or C6H11), J. Chem. Soc., Dalton Trans., 1994, 3621–3635 RSC.
- J. Fornies, A. Martin, R. Navarro, V. Sicilia, P. Villarroya and A. G. Orpen, Nucleophilic behavior of the neutral complexes [M(C ^P)(S2CNMe2)] [M = Pd, Pt; C ^P = CH2C6H4P(C6H4Me-o)2-κ-C,P ] towards Ag(I) and Au(I) compounds. Synthesis (M = Pd, Pt) and molecular structures (M = Pt) of polynuclear complexes containing M-Ag and M-S bonds, J. Chem. Soc., Dalton Trans., 1998, 3721–3726 RSC.
- K. Himoto, T. Horii, S. Oda, S. Suzuki, K. Sugimoto, T. Okubo, M. Maekawa and T. Kuroda-Sowa, Crystal structure of a new mixed-metal coordination polymer consisting of NiII piperidine-dithiocarbamate and pentanuclear CuI-I cluster units, Acta Crystallogr., Sect. E:Crystallogr. Commun., 2018, 74, 233–236 CrossRef CAS PubMed.
- T. Ikada, S. Kuwata, Y. Mizobe and M. Hidai, Syntheses and Structures of Mixed-Metal Sulfido Clusters Containing Incomplete Cubane-Type M2M′S4 and Cubane-Type M2M’2S4 Cores (M = Mo, W; M′ = Rh, Ir), Inorg. Chem., 1999, 38, 64–69 CrossRef CAS.
- H. Brunner, A. Hollman, M. Zabel and B. Nuber, The ligand [Cp2MoH2] in complexes with Ag-S bonds, J. Organomet. Chem., 2000, 609, 44–52 CrossRef CAS.
- K. Ramasamy, M. A. Malik, N. Revaprasadu and P. O'Brien, Routes to Nanostructured Inorganic Materials with Potential for Solar Energy Applications, Chem. Mater., 2013, 25, 3551–3569 CrossRef CAS.
- K. Ramasamy, M. A. Malik and P. O'Brien, Routes to Copper Zinc Tin Sulfide Cu2ZnSnS4 a Potential Material for Solar Cells, Chem. Commun., 2012, 48, 5703–5714 RSC.
- J. A. O. Hill and R. J. Magee, R. J. The Thermochemistry of the Metal Dithiocarbamate and Xanthate Complexes, Rev. Inorg. Chem., 1981, 4, 141–197 Search PubMed.
- J. O. Hill, J. P. Murray and K. C. Patel, The Thermochemistry of the Metal Dithiocarbamate and Xanthate Complexes - A Review Up-date, Rev. Inorg. Chem., 1994, 14, 363–387 CAS.
- A. K. Sharma, Thermal Behaviour of Metal-Dithiocarbamates, Thermochim. Acta, 1986, 104, 339–372 CrossRef CAS.
- S. K. Sengupta and S. Kumar, Thermal studies on metal dithiocarbamato complexes. A review, Thermochim. Acta, 1984, 72, 349–361 CrossRef CAS.
- S. T. Breviglieri, E. T. G. Cavalheiro and G. O. Chierice, Correlation Between Ionic Radius and Thermal Decomposition of Fe(II), Co(II), Ni(II), Cu(II) And Zn(II) Diethanoldithiocarbamates, Thermochim. Acta, 2000, 356, 79–84 CrossRef CAS.
- L. Xi, D. Y. Cho, M. Duchamp, C. B. Boothroyd, J. Y. Lek, A. Besmehn, R. Waser, Y. M. Lam and B. Kardynal, Understanding the Role of Single Molecular ZnS Precursors in the Synthesis of In(Zn)P/ZnS Nanocrystals, ACS Appl. Mater. Interfaces, 2014, 6, 18233–18242 CrossRef CAS PubMed.
- P. B. Mann, I. J. McGregor, S. Bourke, M. Burkitt-Gray, S. Fairclough, M. T. Ma, G. Hogarth, M. Thanou, N. Long and M. Green, An Atom Efficient, Single source Precursor Route to Plasmonic CuS Nanocrystals, Nanoscale Adv., 2019, 1, 522–526 RSC.
- K. Ramasamy, M. A. Malik and P. O'Brien, The Chemical Vapor Deposition of Cu2ZnSnS4 Thin Films, Chem. Sci., 2011, 2, 1170–1172 RSC.
- P. Kevin, M. A. Malik, P. O'Brien, J. Cameron, R. G. Taylor, N. J. Findlay, A. R. Inigo and P. J. Skabara, Nanoparticles of Cu2ZnSnS4 as Performance Enhancing Additives for Organic Field-Effect Transistors, J. Mater. Chem. C, 2016, 4, 5109–5115 RSC.
- T. Uematsu, M. Tepakidareekul, T. Hirano, T. Torimoto and S. Kuwabata, Facile High-Yield Synthesis of Ag-In-Ga-S Quaternary Quantum Dots and Coating with Gallium Sulfide Shells for Narrow Band-Edge Emission, Chem. Mater., 2023, 35, 1094–1106 CrossRef CAS.
- W. Hoisang, T. Uematsu, T. Torimoto and S. Kuwabata, Luminescent Quaternary Ag(InxGa1(-x)S2/GaSy Core/Shell Quantum Dots Prepared Using Dithiocarbamate Compounds and Photoluminescence Recovery via Post Treatment, Inorg. Chem., 2021, 60, 13101–13109 CrossRef CAS PubMed.
- K. S. Ahmad, S. B. Jaffri, H. Panchal, R. K. Gupta, M. A. Abdel-Maksoud, A. Malik and W. H. Al-Qahtani, Advancing energy storage and producing potential with a single source driven semiconducting BaS3:Cs2S:La2S3 chalcogenide prepared via a sustainable mode, New J. Chem., 2025, 49, 2291–2307 RSC.
- K. S. Ahmad, S. B. Jaffri, K. Chaudhary, R. K. Gupta, G. A. Ashraf and N. H. Alotaibi, Harnessing energy sustainability: synthesis of BaS2:CoS:MnS semiconductor trichotomous chalcogenide for superior supercapacitor device operation, Ionics, 2025, 31, 2109–2122 CrossRef CAS.
- K. S. Ahmad, S. B. Jaffri, B. Makawana, R. K. Gupta, G. A. Ashraf and M. Altaf, Sustainable Semiconducting BaS3:Sb2S3:LaS2 Filiform Rods: Revolutionizing Energy Storage and Production With Mixed Metal Chalcogenides, Appl. Organomet. Chem., 2025, 39, e7880 CrossRef CAS.
- S. B. Jaffri, K. S. Ahmad, J. S. Al-Hawadi, B. Makawana, R. K. Gupta, G. A. Ashraf and M. K. Okla, Revolutionizing energy storage and electro-catalysis: unleashing electrode power with novel BaS3:La2S3:Ho2S3 synthesized from single-source precursors for enhanced electrochemical functionality, J. Sol-Gel Sci. Technol., 2025, 113, 197–212 CrossRef CAS.
- S. B. Jaffri, K. S. Ahmad, B. Makawana, R. K. Gupta, M. A. Abdel-Maksoud, A. Malik and W. H. Al-Qahtani, Amplifying energy storage and production efficiency: Utilizing BaS3: Ni2S3: Sb2S3 synthesized from dithiocarbamate precursors for enhanced and sustainable energy solutions, J. Phys. Chem. Solids, 2025, 196, 112394 CrossRef CAS.
- R. Yang, J. Nelson, C. Fai, H. A. Yetkin, C. Werner, M. Tervil, A. D. Jess, P. J. Dale and C. J. Hages, A Low-Temperature Growth Mechanism for Chalcogenide Perovskites, Chem. Mater., 2023, 35, 4743–4750 CrossRef CAS.
- A. E. Dixon, New benzylic derivatives of thiocarbamide, J. Chem. Soc., Dalton Trans., 1891, 59, 551–569 RSC.
| This journal is © The Royal Society of Chemistry 2025 |