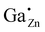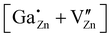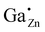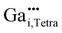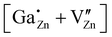Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Structural changes induced by the promoter Ga in nanocrystalline ZnO support used in methanol catalysis†
Jan Konrad
Wied‡
a,
Benjamin
Mockenhaupt‡
b,
Sebastian
Mangelsen
 c,
Ulrich
Schürmann
d,
Lorenz
Kienle
d,
Malte
Behrens
c,
Ulrich
Schürmann
d,
Lorenz
Kienle
d,
Malte
Behrens
 *c and
Jörn
Schmedt auf der Günne
*c and
Jörn
Schmedt auf der Günne
 *a
*a
aUniversity of Siegen, Faculty IV: School of Science and Technology, Department for Chemistry and Biology, Inorganic Materials Chemistry and Center of Micro- and Nanochemistry and (Bio)Technology (Cμ), Adolf-Reichwein Straße 2, 57076 Siegen, Germany. E-mail: gunnej@chemie.uni-siegen.de
bUniversity of Duisburg-Essen, Technical Chemistry 1, Universitätsstr. 7, 45141 Essen, Germany
cKiel University, Institute of Inorganic Chemistry, Max-Eyth-Straße 2, 24118 Kiel, Germany. E-mail: mbehrens@ac.uni-kiel.de
dKiel University, Department of Materials Science, Kaiserstraße 2, 24143 Kiel, Germany
First published on 30th June 2025
Abstract
Ga has an ionic radius fitting the radius of Zn much better than Al, which makes it an interesting candidate for doping of ZnO, which is relevant in context with Cu/ZnO-catalysts and with transparent conductive oxides. Here, the structural changes of Ga-doping of nano-scale ZnO, which is obtained via thermal decomposition of hydrozincite, are studied by a combination of X-ray diffraction, 71Ga/1H MAS NMR, quantum-chemical calculations and electron microscopy techniques. By quantum chemical calculations the NMR fingerprint of different Ga point defects is predicted, the calculations are validated against experimental data for different crystalline compounds. The relevant point defect in ZnO could be identified by the point symmetry of the isolated defect and comparison to the calculated values. The kinetic solubility limit for Ga in ZnO is determined by X-ray diffraction and NMR. It is shifted to higher values as compared to the Al variant. Finally, the distribution of Ga and H atoms within the nano-scale material is studied by “paramagnetically assisted surface peak assignment” (PASPA) NMR, REDOR and electron microscopy which shows that for Ga substitution ratios above the solubility limit the excess of Ga is incorporated into a heavily disordered or amorphous, hydrogen-rich surface-layer.
1. Introduction
Cu/ZnO is an industrial catalyst known for its efficiency in converting mixtures of CO/CO2 and H2 into methanol, which can also be used, with lower selectivity though, in converting pure streams of the greenhouse gas CO2 and H2 into methanol,1 which triggered the interest to find ways to further reduce costs for the catalyst2 and improve its catalytic activity.3 It has been observed that the substitution/doping of ZnO with other ions can improve the catalytic activity of the catalyst Cu/ZnO explaining the electronic promoter ion effect by Al3+ in the industrial catalyst.4,5 To the list of dopants which have been observed to promote the catalytic activity belong Al3+,6,7 Cr3+,8 Zr4+,9 Mg2+,10 Si4+ (ref. 11) and Ga3+.12,13 The doping induced defects of the host-structure are known to change the electronic band gap,14 the surface area of the particle15 and the catalytic selectivity.16 With the introduction of the Cu/ZnO:Al catalyst the temperature and pressure requirements for the catalytic process could be reduced to values of 490–560 K and 60–100 bar.17 In 2022 over 111 Mtons of methanol were produced by this method.18 A convenient way to introduce the promoter is by doping the ZnO support by co-precipitation with Al2O3, which is the technically employed method of catalyst manufacture.19,20Similar to Al2O3, Ga2O3 also promotes the performance of the binary Cu/Zn (CZ) catalyst (see Fig. 1), as previously demonstrated under industrially relevant conditions.5 Compared to the unpromoted Cu/Zn catalyst, the Ga-promoted Cu/Zn:Ga (CZG) catalyst exhibited enhanced activity (Fig. 1a) not only in CO hydrogenation but also in syngas mixtures containing both CO and CO2. The high methanol activity was attributed to the synergistic interaction between Cu and ZnO, with the Ga dopant improving ZnO reducibility. Moreover, the catalyst demonstrated stability for over 800 hours on stream (TOS), which was attributed to the structural robustness imparted by the coprecipitation synthesis method (see Fig. 1b).5
 | ||
Fig. 1 Illustration of the effect of Ga promotion on the performance of a Cu/ZnO catalyst in methanol synthesis: (a) enhanced activity in both CO hydrogenation and syngas (CO + CO2) conversion of Ga3+-promoted catalyst (CZG) compared with a binary unpromoted one (CZ), and (b) long-term stability of both catalysts in CO hydrogenation (TOS = time-on-stream). The reactions were carried out at 235 °C, 30 bar, with a gas hourly space velocity (GHSV) of 1700 h−1 and a COx![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 ratio of 2. This data was previously reported in ref. 5 and further experimental details can be found therein. H2 ratio of 2. This data was previously reported in ref. 5 and further experimental details can be found therein. | ||
It was recently shown that Al3+ can be replaced by Ga3+ and both showed a marked promoting effect compared to a binary unpromoted Cu/ZnO catalyst (CZ in Fig. 1) under industrially relevant conditions.5 A Ga3+-promoted Cu/ZnO:Ga (CZG in Fig. 1) exhibited enhanced activity (Fig. 1a) not only in CO hydrogenation but also in syngas mixtures containing both CO and CO2. The high methanol activity was attributed to the synergistic interaction between Cu and ZnO, while Ga was present as a dopant to ZnO improving its reducibility. In addition to its higher activity, the CZG catalyst demonstrated an interesting stability for over 800 hours on stream (TOS), which was higher than that of the unpromoted CZ catalyst (Fig. 1b). In the light of these promising catalytic properties, this present work now addresses fundamental questions such as the structural changes to coprecipitated zinc oxide supports induced by Ga3+ doping or the solubility limit.
Gallium also is in the focus of recent research by other groups and it was also reported to have a positive effect on the selectivity of Cu during methanol synthesis from CO2 if applied with proper loading.21 For incorporation into the ZnO component of Cu catalysts, Ga has an ionic radius better matching that of the host cation compared to Al (rAl3+ = 0.53 Å < rGa3+ = 0.61 Å < rZn2+ = 0.74 Å),22 potentially allowing a higher substitution ratio. It remains important to understand for the catalysts ZnO component is modified by the incorporation of promoter ions on different length scales. XANES measurements indicate, that Ga3+ occupies a fourfold coordinated position in ZnO in the Cu/ZnO:Ga catalyst and that it is not incorporated into metallic copper phase.4,5,23 This behavior is different from that of Al,21 which is also not expected to be incorporated into Cu, but not only exclusively found inside the ZnO phase and rather forms disordered and complex highly dispersed phases.4
Another aspect regarding the incorporation of promoter ions is the question up to which concentration ZnO takes up Ga3+.24,25 By the application of different techniques, solubility limits were determined ranging from 0.5 mol% by XRD and RAMAN,26 2 mol% by static solid-state NMR,27 2 mol% by resistivity,28 to 4 mol% by conductivity measurements.29 A reason for the difference in the reported solubility limits is that depending on sample synthesis the limit, that is set by thermodynamics, can be overcome by kinetic control for example by low-temperature decomposition reactions.30 The thermodynamic solubility limit is difficult to determine precisely, however, results from samples quenched from different temperatures can serve as a reference and show the thermodynamic limit at 1500 °C is below 0.1 (mol/mol)%.24 As for the case of ZnO:Al, the thermodynamic solubility limit is also lower than the solubility limit that can be achieved under kinetic control.31,32
Different low-temperature routes to nano-scale ZnO:Ga have been reported to achieve a high substitution ratio: nano-scale ZnO:Ga has been obtained similar to the industrially known low-temperature synthesis by decomposition of a hydroxycarbonate precursor.33 Other methods include sol–gel synthesis,34 Pecchini method,24 radio frequency magnetron sputtering35 or a solid-state reaction.29 Depending on the synthesis temperatures mixtures of ZnO and ZnGa2O4 spinel instead of ZnO:Ga are obtained.36,37 In case of Al, the spinel is not considered a promoting species for Cu/ZnO catalysts and its formation was associated to deactivation.38 For a catalyst in addition to the present phases also the morphology is relevant. So far films,39 platelets40 and rods41 of ZnO:Ga could be obtained, while the synthesis route via co-precipitated hydroxy carbonates usually leads to porous aggregates of doped ZnO nanoparticles that inherit their morphology from the crystalline precursor phases.31
For the optical and electrical materials properties, it matters how the Ga is incorporated into the host crystal structure of ZnO. To obtain site specific information about the Ga environments, solid-state NMR is a powerful method as gallium atoms have two NMR active nuclei with spin 3/2, namely 69Ga and 71Ga.42 Ga and Al are both group 13 elements and besides have good quadrupolar nuclei (27Al and 71/69Ga) suitable for NMR. Given the similarity in their chemistry, it is not surprising that correlations between the NMR observables of isostructural crystal structures have been reported for 27Al and 71Ga.43,44 Nano-scale ZnO:Ga did not get studied in detail by Ga NMR yet. What has been studied is a material synthesized at 1200 °C, most likely resulting in a composite of ZnO and ZnGa2O4 spinel instead of ZnO:Ga27 and in a different study static NMR experiments have been performed, which suffer from limited spectral resolution.13 Missing are NMR studies trying to identify the surface signals as in case of nano-scale ZnO:Al by 1H and 27Al NMR, using DNP-SENS45 or a combination of REDOR and “paramagnetically assisted surface peak assignment”.46 Also missing is the spectroscopic NMR finger-print for 69/71Ga defects by quantum chemical calculations similar to what is available for 27Al in ZnO:Al.47
The purpose of this contribution is to study how Ga3+ is incorporated into nano-scale ZnO at different substitution levels on the example of a material which was obtained under kinetic control using a synthetic protocol adopted from Cu/ZnO catalyst synthesis.
2. Experimental part
2.1 Precursor synthesis
The precursor of the zinc oxide sample was synthesized by co-precipitation (reagents Table S1†). The precipitation was carried out in an automated stirred tank reactor (OptiMax, Mettler Toledo) from 1 M metal salt nitrate solution at a temperature of 65 °C and at a constant pH of 6.5. As precipitating agent, 1.6 M sodium carbonate solution was used. Before starting precipitation, 200 ml deionized water was filled into the reactor, followed by pH-adjustment. The dosing rate of metal solution was adjusted to 4.2 g min−1. The setting of the synthesis parameter were taken from typical conditions of Cu/ZnO catalyst manufacture20 under the omission of Cu. After precipitation, the precipitate was aged for 10 minutes without further pH control in the mother liquor. The precursor was washed with deionized water 10 times, to reach a conductivity of the filtrate lower than 100 μS cm−1 and dried at 80 °C for 14 h in a heating cabinet.2.2 Zinc oxide synthesis
The zinc oxide was synthesized by calcination of the ground hydrozincite from co-precipitation. The calcination was performed at 320 °C with a heating ramp of 2 °C min−1 for 4 h in a Nabertherm (LE 6/11/B150) muffle furnace in static air (without any volume flow), which again resembles the typical conditions of catalyst synthesis.2.3 Sample labeling
The samples are labeled according to their nominal gallium content relative to the total amount of metal atoms either as molar percentage or as a value xGa representing the nominal metal-based ratio of gallium atoms calculated as shown in eqn (1). | (1) |
Here, ni is the molar amount of element i (i = Zn, Ga). It should be noted that the Ga content in reality is a bit lower as indicated by an analysis by atomic absorption spectroscopy (Fig. S10†). Those values in the following are referred to as xGa(AAS). Nevertheless the nominal values have been used throughout to be consistent with a previous study on ZnO:Al.
2.4 Characterization methods
 | (2) |
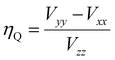 | (3) |
3. Results and discussion
First the materials synthesis of nano-scale ZnO:Ga is presented which is achieved via thermal decomposition of nano-scale Ga-doped precursors. The products are characterized for surface area, morphology and crystallinity. In the second part solid-state nuclear magnetic resonance (NMR) is used to obtain insight into the defect structure of ZnO. NMR fingerprint for various possible Ga site defects is predicted and the NMR resonances are localized in bulk or particle surface.3.2 Nano-scale gallium substituted precursors for ZnO
The precursor phase obtained under the co-precipitation conditions applied in this study is hydrozincite Zn5[(OH)6|(CO3)2]. In our previous study on Al,31 this phase was shown to be able to incorporate certain amounts of trivalent cations. We made an attempt to incorporate Ga3+ in hydrozincite, a compound structurally related to hydrotalcite or layered double hydroxides (LDH). As the latter ones are able to incorporate di- and trivalent cations, Powder X-ray diffraction (PXRD) reveals a similar behavior in the sense, that the samples up to a gallium to zinc substitution ratio xGa = 0.04 are crystalline and free of crystalline side phases (Fig. 2). The hydrozincite reflections become broader as the gallium content in the starting solution was increased, indicating that our attempt to incorporate Ga3+ in this phase was successful and that gallium doping leads to a reduction of the crystallite size. For a gallium content xGa > 0.04 an additional reflection of a side phase at around 2θ = 12° evolved, which is assigned to a hydrotalcite-type crystalline phase. The mineral of the analogous aluminum-rich hydrotalcite-type side-phase is called zaccagnaite with the composition: Zn4Al2(OH)12(CO3)·3H2O. This side phase was also found in a study about Al incorporation into hydrozincites and zinc oxides.31 Here, instead of aluminum gallium cations were incorporated, resulting in a Zn,Ga layered double hydroxide (Zn,Ga LDH) side-phase similar to zaccagnaite. With further increase of xGa further line broadening is observed. Additionally, new reflections at angles of 2θ = 6.45° and 19.49° evolved for xGa > 0.06. No matching entries could be found for these reflections in the ICSD and COD databases. The additional reflection at 2θ = 19.49° was also observed in a previous investigation with aluminum as trivalent cation and presumably corresponds to a defect enriched hydrozincite with broken symmetry.31 Interestingly for xGa = 0.15 all observed reflections aside the ones assigned to the gallium-containing zaccagnaite become sharper again, including both the additional ones as well as those assigned to hydrozincite. This further corroborates that all belong to a common phase.Further temperature resolved PXRD was carried out (Fig. S1†). Analogous to the Al-substituted hydrozincite,31 the gallium-doped precursor decomposes at around 220 °C to ZnO, which is in line with the results from thermogravimetric analysis (compare Fig. S2†). Contrarily, the zaccagnaite-like side phase reflections disappeared at a temperature of around 120 °C. The additional reflections at 2θ = 6.45° and 2θ = 19.49° vanish at virtually identical temperatures, further corroborating that these belong to a common hydrozincite phase modified by xGa > 0.06. The Ga-containing zaccagnaite side phase reflections disappear already at a temperature of around 120 °C, a much lower temperature despite the chemical and structural relationship to hydrozincite. In order to obtain further insight about the gallium incorporation into the hydrozincite host lattice 71Ga magic-angle spinning (MAS) NMR measurements were performed (Fig. 3).
In the hydrozincite crystal structure there are three different Zn sites, two of which show a six-fold coordination and one being four-fold coordinated, which can in principle all be substituted by Ga. Through 71Ga MAS NMR the presence of six-fold coordinated gallium cations in a potential gallium substituted hydrozincite Zn5−5xGa10/3·x(CO3)2(OH)6 could be confirmed (δexpobs = 37 ppm), indicating a preferred substitution of atoms on the six-fold coordinated Zn sites by Ga atoms. A similar preferred substitution of the atoms on the six-fold coordinated Zn sites has also been observed for Al-doped hydrozincite.31 The chemical shift and the quadrupolar lineshape differ slightly from the one observed for the octahedrally coordinated gallium atoms in a Ga-containing zaccagnaite. This peculiar lineshape was not observed in the spectra of the doped hydrozincite samples, indicating that the formation of zaccagnaite as observed by PXRD at xGa > 0.06 is only a minor impurity, while most gallium atoms are incorporated into the hydrozincite.
With the incorporation of Ga3+ ions in the hydrozincite precursor and the formation of potential side-phases at high concentrations characterized, the next subchapter focuses on the ZnO:Ga decomposition products. A deeper investigation of the precursors defect chemistry will be subject of a forthcoming study.
3.3 Formation and characterization of nano-scale ZnO:Ga
Nano-scale ZnO:Ga was formed by calcination of the Ga-doped hydrozincite at a temperature of 320 °C in static air, involving the elimination of H2O and CO2. PXRD analysis (Fig. 4) exhibits reflections of ZnO. Potential side phases like the spinel phase ZnGa2O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 or homologous phases of Ga2O3(ZnO)x
62 or homologous phases of Ga2O3(ZnO)x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 63,64 could not be observed, which also indicates that both hydrozincite and the zaccagnaite-like side phase have decomposed, which is also in agreement with the temperature-resolved PXRD investigation (Fig. S1†). The exclusive formation of ZnO is also supported by electron diffraction performed during the transmission electron microscopy (TEM) analysis (Fig. S3†). Herein samples with a Ga content of xGa = 0.4, and xGa = 0.15 were analyzed in the TEM with electron diffraction (ED) and energy dispersive X-Ray spectroscopy (EDX) with regard to phases and chemical composition. Electron diffraction of the ZnO:Ga sample with xGa = 0.04 (Fig. S3a and b†) exhibits a for ZnO typical d-spacing and a polycrystalline diffraction pattern. Fig. S3c† shows an overview image of the heavily doped ZnO (xGa = 0.15) sample with a big platelet having a diameter of about 200 nm, as well as an agglomeration comparable to that shown in Fig. S3a† with lower Ga concentration. The larger platelet shows a single-crystalline electron diffraction pattern (zone axis [001], Fig. S3d†) with a d-spacing slightly bigger than the one typical for ZnO (010 reflection 2.864 Å instead of 2.8145 Å; −120 reflection 1.669 Å instead of 1.6249 Å). Larger platelets are assumed to be a zaccagnaite-like side phase, as it was observed for the Al doped series, as well. Even, in case of Al as dopant, the decomposition of the side phase resulted in a highly defective ZnO. The main difference is, that in case of Ga as dopant, the side phase only evolved at higher doping levels in the precursor phase (xGa > 0.04). These results are also supported by EDX point measurements on the platelet and on the agglomerate shown in Fig. S3c.† As a result, the platelet has an amount of 33.3 (mol/mol) % Ga in contrast to the agglomerate where the amount of Ga is only slightly increased in comparison to the average composition (xGa = 0.15). The higher amount of Ga, as seen by EDX, in the big platelets can be explained by the high concentration of Ga in the zaccagnaite-like side phase which leads to a higher amount of amorphous/disordered side-phase covering the ZnO-platelets than in the rest of the sample.
63,64 could not be observed, which also indicates that both hydrozincite and the zaccagnaite-like side phase have decomposed, which is also in agreement with the temperature-resolved PXRD investigation (Fig. S1†). The exclusive formation of ZnO is also supported by electron diffraction performed during the transmission electron microscopy (TEM) analysis (Fig. S3†). Herein samples with a Ga content of xGa = 0.4, and xGa = 0.15 were analyzed in the TEM with electron diffraction (ED) and energy dispersive X-Ray spectroscopy (EDX) with regard to phases and chemical composition. Electron diffraction of the ZnO:Ga sample with xGa = 0.04 (Fig. S3a and b†) exhibits a for ZnO typical d-spacing and a polycrystalline diffraction pattern. Fig. S3c† shows an overview image of the heavily doped ZnO (xGa = 0.15) sample with a big platelet having a diameter of about 200 nm, as well as an agglomeration comparable to that shown in Fig. S3a† with lower Ga concentration. The larger platelet shows a single-crystalline electron diffraction pattern (zone axis [001], Fig. S3d†) with a d-spacing slightly bigger than the one typical for ZnO (010 reflection 2.864 Å instead of 2.8145 Å; −120 reflection 1.669 Å instead of 1.6249 Å). Larger platelets are assumed to be a zaccagnaite-like side phase, as it was observed for the Al doped series, as well. Even, in case of Al as dopant, the decomposition of the side phase resulted in a highly defective ZnO. The main difference is, that in case of Ga as dopant, the side phase only evolved at higher doping levels in the precursor phase (xGa > 0.04). These results are also supported by EDX point measurements on the platelet and on the agglomerate shown in Fig. S3c.† As a result, the platelet has an amount of 33.3 (mol/mol) % Ga in contrast to the agglomerate where the amount of Ga is only slightly increased in comparison to the average composition (xGa = 0.15). The higher amount of Ga, as seen by EDX, in the big platelets can be explained by the high concentration of Ga in the zaccagnaite-like side phase which leads to a higher amount of amorphous/disordered side-phase covering the ZnO-platelets than in the rest of the sample.
The visual inspection of the powder patterns in Fig. 4a shows that for all compositions studied, ZnO is observed as the only crystalline phase. The lattice parameters (Fig. 4b) a and c show for xGa = 0.01–0.04 a linear trend, which is corroborated by the fitted line. For a this trend appears to continue up to xGa = 0.1, while for c already at xGa = 0.06 a clear deviation from linearity is observed. Over the entire range of Ga-contents under study the reflections become broader. Further, the line broadening is anisotropic, i.e. more pronounced for specific crystallographic directions. The resulting crystallite sizes for h00 and 00l reflections are shown in Fig. 4c. A steady decline from above 10 nm down to 4–5 nm evolves. Further, the aspect ratio is inverted, which would correspond to a change in shape from slightly needle-like to platelets with increasing xGa. It must be mentioned that the line shape analysis is here limited to the assumption of pure size contribution for the sake of simplicity, neglecting possible strain broadening. Inclusion of strain contributions did not improve the fit significantly, but ZnO is well-known for its tendency to form stacking faults,65,66 which have an anisotropic line broadening effect as well (note the pronounced broadening of cross plane reflections like 102 and 103), but this analysis is beyond the scope of this work. The steady decrease of the crystallite size is in line with a continuous increase of the BET surface area (Fig. 4) with increasing xGa.
Regarding the question of the solubility limit of Ga3+ in ZnO the results are somewhat contradictory: a linear dependence of the lattice parameter on chemical composition may be stated for a up to xGa = 0.1, while c is only linear up to xGa = 0.04. The determination of the lattice parameters becomes increasingly unreliable due to the broad reflections and correlations with the specimen displacement. The crystallite size declines rather quickly up to xGa = 0.06 beyond which it rather converges. From these results a solubility limit of xGa = 0.06 or even lower may be inferred, requiring combination with results from complementary methods.
Further a brief comparison to the case of ZnO:Al from our previous study31 shall be drawn: the dependence of the crystallite size on the chemical composition was less pronounced, but still extended when exceeding the solubility limit determined by 27Al MAS NMR (∼1.3 mol%). Further the crystallite shape was needle-like as inferred from the aspect ratio of the anisotropic crystallite sizes. For ZnO:Ga this inverts around an nominal substitution ratio of xGa = 0.03 corresponding to a real value of xGa(AAS) = 0.023. This change in aspect ratio may be seen as further evidence for a substitution limit beyond xGa = 0.03 in ZnO:Ga.
Qualitatively, the increase in the surface area for ZnO:Ga with rising gallium concentration is associated with a change in the morphology as seen in SEM micrographs (Fig. 6).
While the overall platelet-like morphology from the hydrozincite is maintained during thermal decomposition (see Fig. S4†), with increasing Ga content a reduction in size of the ZnO:Ga aggregates is observed. Simultaneously, a reduction in the pore size and a rise of the numbers of pores occurs (see Fig. S5†), which is consistent with the increase in the surface area observed by BET (Fig. 5). Especially for the ZnO doped with xGa = 0.15 the pore size is reduced to the nano-scale. Furthermore, for this sample, additional platelets on top of the typical ZnO agglomerates are observed (marked with arrows in Fig. 6). Due to the typical shape for layered double hydroxide (LDH) crystallites and because of the observed side phase reflections of a zaccagnaite-like Zn,Ga LDH phase in PXRD in the precursor characterization, the platelets are expected to be the zaccagnaite-like side phase. This is in alignment with the earlier reported study of Al doped ZnO.31
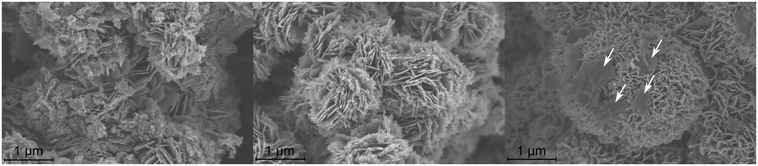 | ||
| Fig. 6 SEM micrographs of selected ZnO:Ga samples with xGa = 0.01 (left), 0.04 (center) and 0.15 (right). Larger platelets observed in the sample with xGa = 0.15 are marked with arrows. | ||
3.4 Ga-defects in ZnO
In order to study the incorporation of the Ga3+ ions into the ZnO crystal lattice 71Ga MAS NMR measurements were performed (Fig. 7) on a series of samples with different degree of substitution.In 71Ga MAS NMR spectra several gallium environments can be resolved: two peaks which can be assigned to 4-fold coordinated Ga atoms (δexpobs = 213 and ∼200 ppm) and one broad resonance corresponding to 5- and/or 6-fold coordinated gallium atoms (δexpobs ≈ 50 ppm) for samples with a high gallium concentration, xGa > 0.03. The overlap of peaks in 71Ga MAS NMR spectra is stronger than that in the corresponding 27Al MAS NMR spectra of ZnO:Al, which makes the distinction of different environments more difficult.31 Importantly, at low Ga-content only a single tetrahedrally coordinated gallium environment at δexpobs = 213 ppm could be observed. This 71Ga MAS NMR signal features a narrow linewidth, which indicates, that the Ga atom has a well-ordered environment and a small quadrupolar coupling because no second-order quadrupolar lineshape is observed, so that the observed chemical shift δexpobs equals the isotropic chemical shift δexpiso within error limits. This peak is assigned to Ga cations on a Zn-site in ZnO, i.e. , which needs to be confirmed by quantum-chemical calculations presented in the following.
, which needs to be confirmed by quantum-chemical calculations presented in the following.
3.5 NMR finger-print of different possible Ga defects
In principle, a number of different defects has been suggested previously for ZnO,24,29,47 besides the already mentioned defect. To obtain NMR fingerprint for the different point defects (see below), quantum chemical calculations can be applied. By comparison of chemical shift values and quadrupolar parameters to experimental values on known crystal structures, scales to translate calculated values on the experimental scale can empirically be determined and it is possible to estimate the error of prediction. To this end literature values were collected on 14 compounds yielding 18 individual, unambiguously assigned Ga peaks (Table S2†). Deliberately only non-metallic compounds were considered, which are chemically similar to the investigated system.Shielding calculations which are determined via density functional theory need to be converted from a shielding scale to a deshielding scale against a different standard which is required by the experimentalist. This not only requires a sign change and calculation of a reference compound but also needs a scaling of the shielding parameter, which is due to the imperfections of the theoretical method. Here, density functional theory under periodic boundary conditions using Kresse Joubert projector augmented wave (kjpaw) pseudopotentials,67 the Perdew–Burke–Ernzerhof (pbe) functional type and a cutoff energy of 120 Ry was applied. The shielding values were estimated using “Gauge Including Projector Augmented Waves” (GIPAW) as implemented in quantum espresso. The conversion formula to obtain from calculated isotropic shielding σcalciso predicted isotropic chemical shift values δprediso for 69/71Ga.
| δprediso = A·σcalciso + B |
The parameters A = −0.930 ± 0.014 and B = (1600 ± 22) ppm were obtained by a linear regression of experimental deshielding values δprediso against calculated shielding values σcalciso (Fig. 8a, Table S2†). The average error of prediction of shielding values δprediso amounts to roughly 6 ppm over a range from −98 to 333 ppm, which provides a good basis for predicting the hypothetical shift values for different defects.
 | ||
| Fig. 8 (a) Correlation plot of the experimentally determined isotropic chemical shifts δexpiso to the calculated isotropic chemical shielding values σcalciso for 71Ga. The gray line represents a linear regression with the δprediso = A·σcalciso + B conversion formula with A = −0.930 ± 0.014 and B = 1600 ± 22 ppm (R2 = 0.9965). The corresponding values were taken from Table S2.† (b) Correlation plot between the experimentally determined quadrupolar coupling CexpQ and the calculated quadrupolar coupling CcalcQ. The dotted line represents a linear regression with the CpredQ = C·CcalcQ prediction formula with C = 1.20 ± 0.07 (R2 = 0.9764). | ||
For the quadrupole interaction, quantum chemical calculations were used to get the electric field gradient tensor, whose eigenvalues Vxx, Vyy and Vzz are required to calculate the quadrupolar coupling CQ and the asymmetry parameter ηQ. The quadrupolar coupling CcalcQ in principle was obtained by a simple multiplication with the nuclear quadrupole moment Q for 71Ga as tabulated and the eigenvalue Vcalczz.68 The GIPAW method at this level of theory introduces an apparent scaling of the electric field gradients, which can be seen from the deviation of the slope parameter C from unity. The latter being determined via linear regression of the experimental quadrupole coupling CexpQ against calculated quadrupole couplings CcalcQ for of 16 individual Ga sites (Fig. 8b, and Table S2†), which leads to the conversion formula
| CpredQ = C·CcalcQ |
The calculated electric quadrupole couplings CcalcQ underestimate the experimental values CexpQ by about 20%. This discrepancy is partially related to the difference in temperatures between simulation and experiment but also to limitations of the theoretical method, for example using a pseudopotential for a nuclear property instead of an all-electron treatment.
The average error of prediction for the predicted quadrupolar couplings CpredQ is 6% in a range from 0 to 15 MHz. An especially drastic error in prediction was observed for ZnGa2O4, which could be a consequence of disorder and defects in the spinel structure.69 Nevertheless, the calculations in general provide a reliable basis to distinguish big from small quadrupolar couplings which is what is required for the identification of Ga defects.
For the following calculations different defect models were generated following literature and a similar study on ZnO:Al.24,29,47 The considered defect models are (a) the 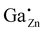 defect, in which a zinc atom is substituted by a gallium atom, (b) the
defect, in which a zinc atom is substituted by a gallium atom, (b) the  defect, in which a gallium atom is positioned on a tetrahedrally coordinated interstitial position, (c) the
defect, in which a gallium atom is positioned on a tetrahedrally coordinated interstitial position, (c) the  defect, in which a gallium atom is positioned on an octahedrally coordinated interstitial position and (d) a coupled defect of a gallium atom substituting a zinc atom neighboring to a zinc vacancy
defect, in which a gallium atom is positioned on an octahedrally coordinated interstitial position and (d) a coupled defect of a gallium atom substituting a zinc atom neighboring to a zinc vacancy  (Table 1). The defects are illustrated in Fig. 9. It should be noted that all models carry a non-zero formal charge.
(Table 1). The defects are illustrated in Fig. 9. It should be noted that all models carry a non-zero formal charge.
 ,
,  ,
,  and
and 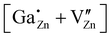 defects was obtained by conversion of the supercell size converged isotropic chemical shielding values σcalciso (see Fig. 10, S6, S7 and S8†)
defects was obtained by conversion of the supercell size converged isotropic chemical shielding values σcalciso (see Fig. 10, S6, S7 and S8†)
To calculate charged point-defects, an opposite jellium background charge is required.70 The interaction of charged defects in neighboring unit cells will influence the results, unlike when applying for example embedded point charge models, which allow to calculate defects at infinite dilution.71 To study the convergence behavior of the NMR parameters, the size of the supercells hosting a single defect were changed from 2 × 1 × 1 to 4 × 4 × 2, where the basic cell refers to the conventional unit cell of ZnO. In each case the structures were relaxed while keeping the cell parameters fixed. The values at infinite dilution were estimated by plotting the shielding values σcalciso as a function of shortest distance found between the charged defects in neighboring unit cells. In this way, results from a number of supercell combinations yield a plot which is dense enough to evaluate the convergence behavior. The converged value was estimated by fitting a decaying exponential function into the obtained curve (Fig. 10, S6–S8†). In all cases it was possible to obtain converged values with an additional estimated error of about 4 ppm for the predicted chemical shift δprediso. These values could then be converted using the above mentioned conversion formulas (Fig. 8, and Table S3†).
 | ||
Fig. 10 Isotropic chemical shielding σcalciso convergence plot for the  defect, resulting in an isotropic chemical shielding value of 1503.1 ± 3.9 ppm extrapolated to an infinitely large supercell. Plotted are the isotropic chemical shielding σcalciso values against the shortest Ga–Ga distance in the different supercell dGa–Ga. The dGa–Ga distance is illustrated on the example of a 2 × 1 × 2 supercell. The isotropic chemical shielding σcalciso values for the different sized supercells (points are labeled with the corresponding supercell) are shown in Table S3.† defect, resulting in an isotropic chemical shielding value of 1503.1 ± 3.9 ppm extrapolated to an infinitely large supercell. Plotted are the isotropic chemical shielding σcalciso values against the shortest Ga–Ga distance in the different supercell dGa–Ga. The dGa–Ga distance is illustrated on the example of a 2 × 1 × 2 supercell. The isotropic chemical shielding σcalciso values for the different sized supercells (points are labeled with the corresponding supercell) are shown in Table S3.† | ||
For all defect models the energy of the model was minimized by optimizing the positions of the atoms in a quantum-chemical calculation, which hereinafter is referred to as “relaxation”. By relaxation the structure of the defect in (a) the Ga–O bonds become shorter, forming an almost ideal tetrahedron, while the distances to the next environmental sphere increase.24 The relaxation conserves the point group symmetry C3v of the site. Based on the pseudo symmetry Td, a small quadrupolar coupling CQ is expected, while an asymmetry parameter ηpredQ of zero is the consequence of the C3v symmetry of the defect.
In (b) the gallium atom is originally located in a coordination with an Oh pseudosymmetry.47 By relaxation the gallium atom shifts along the c-axis, resulting in the formation of three shorter and three longer Ga–O bonds. The point group symmetry of this defect is C3v. The symmetry is consistent with a low quadrupolar coupling CpredQ and an asymmetry parameter ηpredQ of zero (Table 1).
The  defect in (c) exhibits a tetrahedral coordination in its unrelaxed state. During relaxation, due to electrostatic repulsion between the cations, the Zn2+ ion of the edge-sharing ZnO4 tetrahedron, is shifted onto an octahedrally coordinated interstitial position, similar to the one previously discussed for the
defect in (c) exhibits a tetrahedral coordination in its unrelaxed state. During relaxation, due to electrostatic repulsion between the cations, the Zn2+ ion of the edge-sharing ZnO4 tetrahedron, is shifted onto an octahedrally coordinated interstitial position, similar to the one previously discussed for the 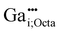 defect. Taking these changes into consideration, the relaxed
defect. Taking these changes into consideration, the relaxed  defect would need to be assessed as a
defect would need to be assessed as a  defect. Relaxation causes a reduction in the point group symmetry to Cs. Therefore, a high quadrupolar coupling CpredQ and an asymmetry parameter ηpredQ unequals zero are expected. This behavior differs from the one previously reported for the similar aluminium defect, in which no zinc vacancy is generated.47
defect. Relaxation causes a reduction in the point group symmetry to Cs. Therefore, a high quadrupolar coupling CpredQ and an asymmetry parameter ηpredQ unequals zero are expected. This behavior differs from the one previously reported for the similar aluminium defect, in which no zinc vacancy is generated.47
The coupled defect 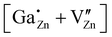 in (d) consists of a
in (d) consists of a  defect and a
defect and a  defect, located in the first coordination sphere of the
defect, located in the first coordination sphere of the  defect24 along the c-axis. During the relaxation process no significant change in the atomic coordinates occurred, resulting in a point group symmetry of Cs, giving rise to a considerable quadrupolar coupling CpredQ and an asymmetry parameter ηpredQ unequals zero.
defect24 along the c-axis. During the relaxation process no significant change in the atomic coordinates occurred, resulting in a point group symmetry of Cs, giving rise to a considerable quadrupolar coupling CpredQ and an asymmetry parameter ηpredQ unequals zero.
3.6 Point defects in nano-scale ZnO:Ga
In this subchapter the predicted finger-print for different point defects is compared with the experimental results. Experimentally, a 71Ga MAS NMR resonance with a chemical shift δexpiso of 213 ppm, a quadrupolar coupling with a magnitude |CexpQ| of roughly 3 MHz and a low quadrupolar asymmetry parameter ηexpQ could be determined for the signal occurring at low substitution ratios xGa. By exclusion principle it will be discussed which defects in principle are consistent with the experimental observations.Defects in (c) and (d) exhibit a large quadrupolar coupling CpredQ as well as a non-zero quadrupolar asymmetry parameter ηpredQ, which clearly rules out the defects 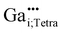 and
and  , because of the mismatch between expected and experimentally observed lineshape in 71Ga NMR (see Fig. S9†).
, because of the mismatch between expected and experimentally observed lineshape in 71Ga NMR (see Fig. S9†).
The remaining two defects (a) and (b) both would result in a relatively narrow lineshape matching the ones of the experimental spectra. However, the difference in coordination number of the  and the
and the  defect is expected to produce a significant change in isotropic chemical shift, which is consistent with predicted values from quantum chemical calculations. This results in an unambiguous assignment of the 71Ga signal at δexpiso = 213 ppm to the
defect is expected to produce a significant change in isotropic chemical shift, which is consistent with predicted values from quantum chemical calculations. This results in an unambiguous assignment of the 71Ga signal at δexpiso = 213 ppm to the  defect (see Fig. S9†). We note in passing, that the signal assignment is consistent with the shift of the
defect (see Fig. S9†). We note in passing, that the signal assignment is consistent with the shift of the 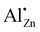 defect in ZnO of δexpiso = 82 ppm applying a correlation found between 27Al and 71Ga shift data established for isostructural solids, which suggests an isotropic chemical shift value of 227 ppm.43,44
defect in ZnO of δexpiso = 82 ppm applying a correlation found between 27Al and 71Ga shift data established for isostructural solids, which suggests an isotropic chemical shift value of 227 ppm.43,44
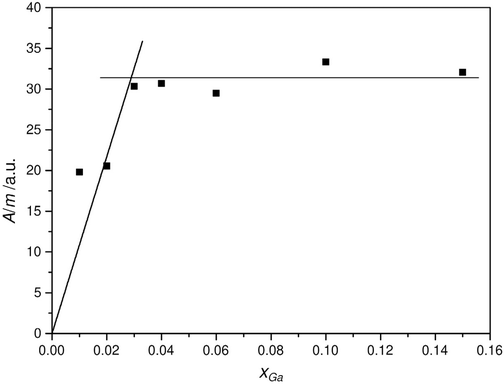
In order to access the solubility limit of the  species in ZnO by 71Ga MAS NMR, the peak area per mass was quantified as a function of the substitution ratio xGa. To this end the lineshape parameters, which were used to deconvolute of different resonances, are determined by fitting of the signals of the extreme substitution ratios. Then only the peak areas for the individual peaks were fitted which removes ambiguity in the fitting process introduced by the observed strong signal overlap (Fig. 11).
species in ZnO by 71Ga MAS NMR, the peak area per mass was quantified as a function of the substitution ratio xGa. To this end the lineshape parameters, which were used to deconvolute of different resonances, are determined by fitting of the signals of the extreme substitution ratios. Then only the peak areas for the individual peaks were fitted which removes ambiguity in the fitting process introduced by the observed strong signal overlap (Fig. 11).
An increase of the signal area per mass of the resonance at δexpiso = 213 ppm up to around xGa = 0.03 gallium concentration can be observed which then levels off to an almost constant value (Fig. 11). The solubility limit is estimated by fitting to linear functions into the rising part and the plateau, respectively and by determining their crossing point, which indicates a solubility limit for the  species of about nominal substitution ratio xGa = 0.029 (xGa(AAS) = 0.022). From PXRD the lattice parameter c showed marked deviations from a Vegard-like behavior for xGa > 0.04, which would roughly coincide with the limit found by 71Ga MAS NMR. Apparently, other factors influence the further evolution of lattice parameters and crystallite size beyond this limit. One may speculate that this could be the hindrance of crystallite growth by an amorphous, Ga-containing matrix or Ga3+ being attached to specific crystallite surfaces, giving rise to the observed change in the apparent crystallite shape.
species of about nominal substitution ratio xGa = 0.029 (xGa(AAS) = 0.022). From PXRD the lattice parameter c showed marked deviations from a Vegard-like behavior for xGa > 0.04, which would roughly coincide with the limit found by 71Ga MAS NMR. Apparently, other factors influence the further evolution of lattice parameters and crystallite size beyond this limit. One may speculate that this could be the hindrance of crystallite growth by an amorphous, Ga-containing matrix or Ga3+ being attached to specific crystallite surfaces, giving rise to the observed change in the apparent crystallite shape.
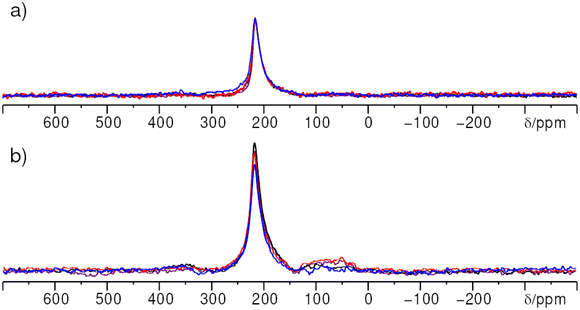
3.7 Surface characterization of nano-scale ZnO:Ga by NMR
A remaining question is if the different 71Ga resonances can be associated with the bulk of the ZnO particles or the surface. To this end two different experiments were applied: surface characterization of the nanoparticles by paramagnetic impregnation (PASPA) and 71Ga{1H} rotational-echo double resonance (REDOR) experiments.In order to distinguish the gallium signals arising from the inside of the ZnO:Ga particles from those from the particle surface, the surface of ZnO:Ga was impregnated with manganese(II)-acetylacetonate according to the procedure described in reference.4671Ga paramagnetically assisted surface peak assignment (PASPA) NMR measurements were performed on ZnO:Ga samples with xGa = 0.03 and xGa = 0.1 (Fig. 12). The condition, that the particles must be smaller than 20 nm for PASPA to cause big enough peak changes, is met (see results from PXRD above).
Acetonitrile was selected as solvent for the paramagnetic agent, as it has been shown to keep the surface of doped ZnO nanoparticles intact.46
Only a single 71Ga resonance at δexpiso = 213 ppm is observed corresponding to the 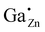 defect in case of ZnO:Ga with a gallium content of xGa = 0.03. By 71Ga PASPA a suppression ratio Rsup close to zero is determined for this species, as there is no significant change present between peak of the reference and the paramagnetically impregnated sample. This indicates that the
defect in case of ZnO:Ga with a gallium content of xGa = 0.03. By 71Ga PASPA a suppression ratio Rsup close to zero is determined for this species, as there is no significant change present between peak of the reference and the paramagnetically impregnated sample. This indicates that the  species is located in the particle bulk of the ZnO nanoparticles.
species is located in the particle bulk of the ZnO nanoparticles.
A suppression ratio Rsup of 0.23 is determined for the resonance at δexpiso = 213 ppm for the sample with a gallium content of xGa = 0.1 by 71Ga PASPA. This can be explained by an overlap of the NMR resonance of the 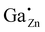 defect in ZnO with a second resonance of a fourfold coordinated Ga atom (“GaO4”) in an amorphous/disordered side phase, which is close to the particle surface (see Fig. 7). Additionally, a sixfold coordinated Ga species “GaO6” could be resolved for the sample with a gallium content of xGa = 0.1. This species also shows a high suppression ratio in 71Ga PASPA, which means that both species can be attributed to the surface. The presence of fivefold coordinate Ga atoms “GaO5” can neither be confirmed nor rejected because of the limited resolution in direct excitation 71Ga NMR. A MQMAS was impossible because of the limitations in signal to noise ratio at the given substitution ratios.
defect in ZnO with a second resonance of a fourfold coordinated Ga atom (“GaO4”) in an amorphous/disordered side phase, which is close to the particle surface (see Fig. 7). Additionally, a sixfold coordinated Ga species “GaO6” could be resolved for the sample with a gallium content of xGa = 0.1. This species also shows a high suppression ratio in 71Ga PASPA, which means that both species can be attributed to the surface. The presence of fivefold coordinate Ga atoms “GaO5” can neither be confirmed nor rejected because of the limited resolution in direct excitation 71Ga NMR. A MQMAS was impossible because of the limitations in signal to noise ratio at the given substitution ratios.
Because hydrogen atoms played an important role for surface defects of ZnO:Al,46 it was necessary to evaluate if this is also the case for the ZnO:Ga surface. To this end 1H PASPA experiments were performed (Fig. 13).
The as-made ZnO:Ga exhibits two different H environments similar to ZnO:Al,46 corresponding to a resonance at δexpiso = 2 ppm and one at δexpiso = 4.3 ppm, respectively. The latter signal can be assigned to H2O molecules on the particle surface, which are removed under vacuum. The other resonance is assigned to hydroxyl functions. Their peak shows a high suppression ratio in 1H PASPA, giving strong evidence for the claim that hydroxyl functions are located close-to or on the particle surface. The high suppression ratios observed in 1H PASPA indicate that the amount of hydrogen in the bulk of the ZnO particles is negligible.
This scenario can further be corroborated by 71Ga {1H} REDOR experiments (Fig. 14). In contrast to the signal of  in ZnO, the GaO6 signal exhibits a fast and complete dephasing, proving the presence of 1H in close proximity to all of the GaO6 groups. As the 1H species could be assigned to the particle surface, it can be concluded, GaO6 species can only be found at the surface of the nanoparticles.
in ZnO, the GaO6 signal exhibits a fast and complete dephasing, proving the presence of 1H in close proximity to all of the GaO6 groups. As the 1H species could be assigned to the particle surface, it can be concluded, GaO6 species can only be found at the surface of the nanoparticles.
 | ||
Fig. 14
71Ga {1H} REDOR NMR curves using the universal dephasing scale τuds![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 72,73 of the 71Ga MAS NMR signals at δexpiso = 213 ppm (left; 72,73 of the 71Ga MAS NMR signals at δexpiso = 213 ppm (left; 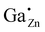 ) and δexpobs = 50 ppm (right; GaO6), of as-made ZnO:Ga with xGa = 0.03 for the ) and δexpobs = 50 ppm (right; GaO6), of as-made ZnO:Ga with xGa = 0.03 for the  defect and xGa = 0.15 for the GaO6 defect. The upper dotted line corresponds to the heterogeneous error and the lower dashed line corresponds to the homogeneous error.74 The measurements were performed at 20 kHz spinning frequency. defect and xGa = 0.15 for the GaO6 defect. The upper dotted line corresponds to the heterogeneous error and the lower dashed line corresponds to the homogeneous error.74 The measurements were performed at 20 kHz spinning frequency. | ||
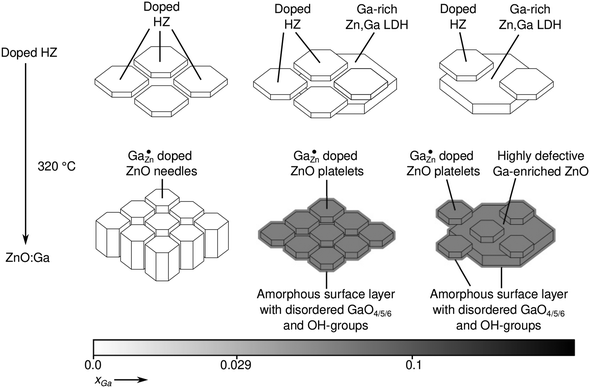
On the basis of the previous findings a model for the ZnO:Ga nanoparticle is suggested which is in agreement with all presented observations (Fig. 15). For low Ga substitution ratios xGa, Zn atoms in the crystalline particle core (δexpiso = 213 ppm) are substituted by gallium cations up to a solubility limit of about xGa = 0.029 (xGa(AAS) = 0.022). Additional gallium cations, exceeding this limit, are not incorporated into the crystal bulk anymore, but into an amorphous/disordered surface layer surrounding the crystalline ZnO core. GaO4, GaO6 and potentially GaO5 species are present in this surface shell. The amorphous shell further contains hydrogen atom containing defects in the form of water and hydroxyl functions. Given that the particles did not show a Knight-shift of 71Ga resonance, it can be concluded that the additional electrons introduced through Ga as an n-dopant are compensated for example by the formation of –O− groups in the surface of the particles or by color center in ZnO.47
4. Conclusions
The incorporation of Ga into nanocrystalline ZnO synthesized via the low-temperature decomposition of hydrozincite has been studied by PXRD, electron microscopy, gas adsorption and NMR. As expected from the ionic radii, a higher solubility limit of Ga in ZnO as compared to Al in ZnO could be achieved. The experiments provide evidence for a detailed model with a disordered/amorphous surface and a crystalline ZnO core of the nano-scale high-surface materials, which follows the example of ZnO:Al synthesized via the same route. The structural insight will help to improve the catalytic activity of ZnO support materials with different promoter ions.Besides conversion formula for computed shielding parameters and quadrupole parameters are provided, which could be applied in NMR studies of other gallium oxides.
Conflicts of interest
There are no conflicts to declare.Data availability
All experimental and computed data are made available if required.References
- E. Kunkes and M. Behrens, in Chemical Energy Storage, ed. R. Schlögl, De Gruyter, 2013, pp. 413–442. DOI:10.1515/9783110266320.413.
- D. Sheldon, Methanol Production - A Technical History, Johnson Matthey Technol. Rev., 2017, 61, 172–182, DOI:10.1595/205651317x695622.
- R. Guil-Lopez, N. Mota, J. Llorente, E. Millan, B. Pawelec, J. L. G. Fierro and R. M. Navarro, Methanol synthesis from CO2: a review of the latest developments in heterogeneous catalysis, Mater., 2019, 12, 3902, DOI:10.3390/ma12233902.
- M. Behrens, S. Zander, P. Kurr, N. Jacobsen, J. Senker, G. Koch, T. Ressler, R. W. Fischer and R. Schlögl, Performance Improvement of Nanocatalysts by Promoter-Induced Defects in the Support Material: Methanol Synthesis over Cu/ZnO:Al, J. Am. Chem. Soc., 2013, 135, 6061–6068, DOI:10.1021/ja310456f.
- B. Mockenhaupt, J. Gieser, S. Najafishirtari, L. Baumgarten, J. Jelic, T. Lunkenbein, E.-J. Ras, J.-D. Grunwaldt, F. Studt and M. Behrens, On the secondary promotion effect of Al and Ga on Cu/ZnO methanol synthesis catalysts, J. Catal., 2024, 439, 115785, DOI:10.1016/j.jcat.2024.115785.
- K. Hagedorn, W. Li, Q. Liang, S. Dilger, M. Noebels, M. R. Wagner, J. S. Reparaz, A. Dollinger, J. Schmedt auf der Günne, T. Dekorsy, L. Schmidt-Mende and S. Polarz, Catalytically Doped Semiconductors for Chemical Gas Sensing: Aerogel-Like Aluminum-Containing Zinc Oxide Materials Prepared in the Gas Phase, Adv. Funct. Mater., 2016, 26, 3424–3437, DOI:10.1002/adfm.201505355.
- T. Tsubota, M. Ohtaki, K. Eguchi and H. Arai, Thermoelectric properties of Al-doped ZnO as a promising oxide material for high-temperature thermoelectric conversion, J. Mater. Chem., 1997, 7, 85–90, 10.1039/a602506d.
- K.-D. Jung and O.-S. Joo, Preparation of Cu/ZnO/M2O3 (M = Al, Cr) catalyst to stabilize Cu/ZnO catalyst in methanol dehydrogenation, Catal. Lett., 2002, 84, 21–25 CrossRef CAS.
- G. Wang, D. Mao, X. Guo and J. Yu, Methanol synthesis from CO2 hydrogenation over CuO-ZnO-ZrO2-MxOy catalysts (M=Cr, Mo and W), Int. J. Hydrogen Energy, 2019, 44, 4197–4207, DOI:10.1016/j.ijhydene.2018.12.131.
- J. Schumann, M. Eichelbaum, T. Lunkenbein, N. Thomas, M. C. Álvarez Galván, R. Schlögl and M. Behrens, Promoting Strong Metal Support Interaction: Doping ZnO for Enhanced Activity of Cu/ZnO:M (M = Al, Ga, Mg) Catalysts, ACS Catal., 2015, 5, 3260–3270, DOI:10.1021/acscatal.5b00188.
- N. Barrow, J. Bradley, B. Corrie, Y. Cui, T. D. Tran, T. E. Erden, A. Fish, M. Garcia, P. Glen, N. Mistry, M. Nicholson, S. Roloff-Standring, D. Sheldon, T. Smith, A. Summer, K. U. Din and N. Macleod, Doubling the life of Cu/ZnO methanol synthesis catalysts via use of Si as a structural promoter to inhibit sintering, Sci. Adv., 2024, 10, eadk2081, DOI:10.1126/sciadv.adk2081.
- J. Toyir, P. R. De la Piscina, J. L. G. Fierro and N. Homs, Highly effective conversion of CO2 to methanol over supported and promoted copper-based catalysts: influence of support and promoter, Appl. Catal., B, 2001, 29, 207–215, DOI:10.1016/s0926-3373(00)00205-8.
- N. Roberts, R.-P. Wang, A. W. Sleight and W. W. Warren Jr., 27Al and 69Ga impurity nuclear magnetic resonance in ZnO:Al and ZnO:Ga., Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 5734–5741, DOI:10.1103/PhysRevB.57.5734.
- B. E. Sernelius, K. F. Berggren, Z. C. Jin, I. Hamberg and C. G. Granqvist, Band-gap tailoring of zinc oxide by means of heavy aluminum doping, Phys. Rev. B: Condens. Matter, 1988, 37, 10244 CrossRef CAS PubMed.
- S. Miao, R. N. d'Alnoncourt, T. Reinecke, I. Kasatkin, M. Behrens, R. Schlögl and M. Muhler, A study of the influence of composition on the microstructural properties of ZnO/Al2O3 mixed oxides, Eur. J. Inorg. Chem., 2009, 910–921, DOI:10.1002/ejic.200800987.
- C. S. Santana, L. F. Rasteiro, F. C. F. Marcos, E. M. Assaf, J. F. Gomes and J. M. Assaf, Influence of Al, Cr, Ga, or Zr as promoters on the performance of Cu/ZnO catalyst for CO2 hydrogenation to methanol, Mol. Catal., 2022, 528, 112512, DOI:10.1016/j.mcat.2022.112512.
- A. Beck, M. A. Newton, L. G. A. van de Water and J. A. van Bokhoven, The Enigma of Methanol Synthesis by Cu/ZnO/Al2O3-Based Catalysts, Chem. Rev., 2024, 124, 4543–4678, DOI:10.1021/acs.chemrev.3c00148.
- G. Pacchioni, From CO2 to Methanol on Cu/ZnO/Al2O3 Industrial Catalyst. What Do We Know about the Active Phase and the Reaction Mechanism?, ACS Catal., 2024, 14, 2730–2745, DOI:10.1021/acscatal.3c05669.
- M. Behrens, G. Lolli, N. Muratova, I. Kasatkin, M. Hävecker, R. N. d'Alnoncourt, O. Storcheva, K. Köhler, M. Muhler and R. Schlögl, The effect of Al-doping on ZnO nanoparticles applied as catalyst support, Phys. Chem. Chem. Phys., 2013, 15, 1374–1381, 10.1039/c2cp41680h.
- M. Behrens and R. Schlögl, How to Prepare a Good Cu/ZnO Catalyst or the Role of Solid State Chemistry for the Synthesis of Nanostructured Catalysts, Z. Anorg. Allg. Chem., 2013, 639, 2683–2695, DOI:10.1002/zaac.201300356.
- J. L. Alfke, M. Tejeda-Serrano, S. Phadke, A. Tereshchenko, T. Z. H. Gani, L. Rochlitz, S. B. X. Y. Zhang, L. Lin, C. Copéret and O. V. Safonova, Boundary Conditions for Promotion versus Poisoning in Copper–Gallium-Based CO2-to-Methanol Hydrogenation Catalysts, ACS Catal., 2024, 14, 9166–9175, DOI:10.1021/acscatal.4c01985.
- R. D. Shannon and C. T. Prewitt, Effective ionic radii in oxide and fluorides, Acta Crystallogr., Sect. B, 1969, 25, 925, DOI:10.1107/s0567740869003220.
- L. Zhong-Lin, Z. Wen-Qin, X. Ming-Xiang, Z. Feng-Ming and D. You-Wei, Structural and Electrical Properties of Single Crystalline Ga-Doped ZnO Thin Films Grown by Molecular Beam Epitaxy, Chin. Phys. Lett., 2009, 26, 116102, DOI:10.1088/0256-307X/26/11/116102.
- H. Serier, A. Demourgues and M. Gaudon, Investigation of Ga Substitution in ZnO Powder and Opto-Electronic Properties, Inorg. Chem., 2010, 49, 6853–6858, DOI:10.1021/ic1000733.
- V. J. Norman, Diffusion of aluminum and gallium in zinc oxide, Aust. J. Chem., 1969, 22, 325, DOI:10.1071/ch9690325.
- M. H. Yoon, S. H. Lee, H. L. Park, H. K. Kim and M. S. Jang, Solid solubility limits of Ga and Al in ZnO, J. Mater. Sci. Lett., 2002, 21, 1703–1704, DOI:10.1023/A:1020841213266.
- W. W. Warren Jr., N. Roberts, R. P. Wang and A. W. Sleight, NMR study of carrier states and trapping complexes in the transparent conductor ZnO:MIII., Mater. Sci. Forum, 1997, 258–263, 1365–1370, DOI:10.4028/www.scientific.net/MSF.258-263.1365.
- G. K. Paul and S. K. Sen, Sol–gel preparation, characterization and studies on electrical and thermoelectrical properties of gallium doped zinc oxide films, Mater. Lett., 2002, 57, 742–746, DOI:10.1016/S0167-577X(02)00865-0.
- R. Wang, A. W. Sleight and D. Cleary, High Conductivity in Gallium-Doped Zinc Oxide Powders, Chem. Mater., 1996, 8, 433, DOI:10.1021/cm950372k.
- S. Lany and A. Zunger, Dopability, Intrinsic Conductivity, and Nonstoichiometry of Transparent Conducting Oxides, Phys. Rev. Lett., 2007, 98, 045501, DOI:10.1103/PhysRevLett.98.045501.
- B. Mockenhaupt, J. K. Wied, S. Mangelsen, U. Schürmann, L. Kienle, J. Schmedt auf der Günne and M. Behrens, Phase evolution, speciation and solubility limit of aluminium doping in zinc oxide catalyst supports synthesized via co-precipitated hydrozincite precursors, Dalton Trans., 2023, 52, 5321–5335, 10.1039/d3dt00253e.
- A. Walsh and A. Zunger, Instilling defect tolerance in new compounds, Nat. Mater., 2017, 16, 964–967, DOI:10.1038/nmat4973.
- D. Waller, D. Stirling, F. S. Stone and M. S. Spencer, Copper-zinc oxide catalysts: activity in relation to precursor structure and morphology, Faraday Discuss. Chem. Soc., 1989, 87, 107, 10.1039/dc9898700107.
- M. Hjiri, R. Dhahri, L. E. Mir, A. Bonavita, N. Donato, S. G. Leonardi and G. Neri, CO sensing properties of Ga-doped ZnO prepared by sol-gel route, J. Alloys Compd., 2015, 634, 187–192, DOI:10.1016/j.jallcom.2015.02.083.
- X. Yu, J. Ma, F. Ji, Y. Wang, X. Zhang, C. Cheng and H. Ma, Effects of sputtering power on the properties of ZnO:Ga films deposited by r.f. magnetron sputtering at low temperature, J. Cryst. Growth, 2005, 274, 474–479, DOI:10.1016/j.jcrysgro.2004.10.037.
- G. R. Patzke, S. Locmelis, R. Wartchow and M. Binnewies, Chemical transport phenomena in the ZnO–Ga2O3 system, J. Cryst. Growth, 1999, 203, 141–148, DOI:10.1016/S0022-0248(99)00083-4.
- G. B. Palmer and K. R. Poeppelmeier, Phase relations, transparency and conductivity in Ga2O3-SnO2-ZnO, Solid State Sci., 2002, 4, 317–322, DOI:10.1016/S1293-2558(01)01258-4.
- T. Lunkenbein, F. Girgsdies, T. Kandemir, N. Thomas, M. Behrens, R. Schlögl and E. Frei, Bridging the Time Gap: A Copper/Zinc Oxide/Aluminum Oxide Catalyst for Methanol Synthesis Studied under Industrially Relevant Conditions and Time Scales, Angew. Chem., Int. Ed., 2016, 55, 12708–12712, DOI:10.1002/anie.201603368.
- C.-Y. Tsay, K.-S. Fan and C.-M. Lei, Synthesis and characterization of sol-gel derived gallium-doped zinc oxide thin films, J. Alloys Compd., 2012, 512, 216–222, DOI:10.1016/j.jallcom.2011.09.066.
- M. S. Bernardo, P. G. Villanueva, T. Jardiel, D. G. Calatayud, M. Peiteado and A. C. Caballero, Ga-doped ZnO self-assembled nanostructures obtained by microwave-assisted hydrothermal synthesis: Effect on morphology and optical properties, J. Alloys Compd., 2017, 722, 920–927, DOI:10.1016/j.jallcom.2017.06.160.
- M. Yan, H. T. Zhang, E. J. Widjaja and R. P. H. Chang, Self-assembly of well-aligned gallium-doped zinc oxide nanorods, J. Appl. Phys., 2003, 94, 5240–5246, DOI:10.1063/1.1608473.
- K. J. D. MacKenzie and M. E. Smith, Multinuclear Solid-State Nuclear Magnetic Resonance of Inorganic Materials, Pergamon, 2002 Search PubMed.
- S. M. Bradley, R. F. Howe and R. A. Kydd, Correlation between aluminum-27 and gallium-71 NMR chemical shifts, Magn, Reson. Chem., 1993, 31, 883, DOI:10.1002/mrc.1260311002.
- D. Massiot, T. Vosegaard, N. Magneron, D. Trumeau, V. Montouillout, P. Berthet, T. Loiseau and B. Bujoli, 71Ga NMR of reference GaIV, GaV, and GaVI compounds by MAS and QPASS, extension of gallium/aluminum NMR parameter correlation, Solid State Nucl. Magn. Reson., 1999, 15, 159–169, DOI:10.1016/s0926-2040(99)00053-3.
- H. Nagashima, J. Trébosc, Y. Kon, K. Sato, O. Lafon and J.-P. Amoureux, Observation of Low-γ Quadrupolar Nuclei by Surface-Enhanced NMR Spectroscopy, J. Am. Chem. Soc., 2020, 142, 10659–10672, DOI:10.1021/jacs.9b13838.
- J. K. Wied, B. Mockenhaupt, U. Schürmann, L. Kienle, S. Mangelsen, J. Glänzer, V. R. Celinski, M. Behrens and J. Schmedt auf der Günne, Method for Surface Characterization Using Solid-State Nuclear Magnetic Resonance Spectroscopy Demonstrated on Nanocrystalline ZnO:Al, Anal. Chem., 2024, 96, 11290–11298, DOI:10.1021/acs.analchem.4c01170.
- Y. S. Avadhut, J. Weber, E. Hammarberg, C. Feldmann and J. Schmedt auf der Günne, Structural investigation of aluminium doped ZnO nanoparticles by solid-state NMR spectroscopy, Phys. Chem. Chem. Phys., 2012, 14, 11610–11625, 10.1039/c2cp41139c.
- A. A. Coelho, TOPAS and TOPAS-Academic: an optimization program integrating computer algebra and crystallographic objects written in C++, J. Appl. Crystallogr., 2018, 51, 210–218, DOI:10.1107/s1600576718000183.
- P. Thompson, D. E. Cox and J. B. Hastings, Rietveld refinement of Debye-Scherrer synchrotron X-ray data from alumina, J. Appl. Crystallogr., 1987, 20, 79–83, DOI:10.1107/s0021889887087090.
- R. K. Harris and E. D. Becker, NMR nomenclature: nuclear spin properties and conventions for chemical shifts - IUPAC recommendations, J. Magn. Reson., 2002, 156, 323–326, DOI:10.1006/jmre.2002.2554.
- R. K. Harris, E. D. Becker, S. M. Cabral De Menezes, P. Granger, R. E. Hoffman and K. W. Zilm, Further conventions for NMR shielding and chemical shifts: (IUPAC recommendations 2008), Pure Appl. Chem., 2008, 80, 59–84, DOI:10.1351/pac200880010059.
- D. Jardón-Álvarez and J. Schmedt auf der Günne, Reduction of the temperature gradients in laser assisted high temperature MAS NMR., Solid State Nucl. Magn. Reson., 2018, 94, 26–30, DOI:10.1016/j.ssnmr.2018.08.002.
- P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G. L. Chiarotti, M. Cococcioni, I. Dabo, A. Dal Corso, S. de Gironcoli, S. Fabris, G. Fratesi, R. Gebauer, U. Gerstmann, C. Gougoussis, A. Kokalj, M. Lazzeri, L. Martin-Samos, N. Marzari, F. Mauri, R. Mazzarello, S. Paolini, A. Pasquarello, L. Paulatto, C. Sbraccia, S. Scandolo, G. Sclauzero, A. P. Seitsonen, A. Smogunov, P. Umari and R. M. Wentzcovitch, QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials, J. Phys.: Condens. Matter, 2009, 21, 395502, DOI:10.1088/0953-8984/21/39/395502.
- P. Giannozzi, O. Andreussi, T. Brumme, O. Bunau, M. Buongiorno Nardelli, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, M. Cococcioni, N. Colonna, I. Carnimeo, A. Dal Corso, S. de Gironcoli, P. Delugas, R. A. Di Stasio Jr., A. Ferretti, A. Floris, G. Fratesi, G. Fugallo, R. Gebauer, U. Gerstmann, F. Giustino, T. Gorni, J. Jia, M. Kawamura, H.-Y. Ko, A. Kokalj, E. Kucukbenli, M. Lazzeri, M. Marsili, N. Marzari, F. Mauri, N. L. Nguyen, H.-V. Nguyen, A. Otero-de-la-Roza, L. Paulatto, S. Ponce, D. Rocca, R. Sabatini, B. Santra, M. Schlipf, A. P. Seitsonen, A. Smogunov, I. Timrov, T. Thonhauser, P. Umari, N. Vast, X. Wu and S. Baroni, Advanced capabilities for materials modelling with QUANTUM ESPRESSO, J. Phys.: Condens. Matter, 2017, 29, 465901, DOI:10.1088/1361-648x/aa8f79.
- H. J. Monkhorst and J. D. Pack, Special points for Brillouin-zone integrations, Phys. Rev. B:Condens. Matter Mater. Phys., 1976, 13, 5188–5192, DOI:10.1103/PhysRevB.13.5188.
- N. Troullier and J. L. Martins, Efficient pseudopotentials for plane-wave calculations, Phys. Rev. B:Condens. Matter Mater. Phys., 1991, 43, 1993–2006, DOI:10.1103/PhysRevB.43.1993.
- P. E. Blöchl, Projector augmented-wave method, Phys. Rev. B:Condens. Matter Mater. Phys., 1994, 50, 17953–17979, DOI:10.1103/PhysRevB.50.17953.
- K. Okhotnikov, T. Charpentier and S. Cadars, Supercell program: a combinatorial structure-generation approach for the local-level modeling of atomic substitutions and partial occupancies in crystals, J. Cheminf., 2016, 8, 17, DOI:10.1186/s13321-016-0129-3.
- T. Björkman, CIF2Cell: Generating geometries for electronic structure programs, Comput. Phys. Commun., 2011, 182, 1183–1186, DOI:10.1016/j.cpc.2011.01.013.
- C. J. Pickard and F. Mauri, All-electron magnetic response with pseudopotentials: NMR chemical shifts, Phys. Rev. B:Condens. Matter Mater. Phys., 2001, 63, 245101, DOI:10.1103/PhysRevB.63.245101.
- C. Gervais, L. Bonhomme-Coury, F. Mauri, F. Babonneau and C. Bonhomme, GIPAW (gauge including projected augmented wave) and local dynamics in 13C and 29Si solid state NMR: the study case of silsesquioxanes (RSiO1.5)8, Phys. Chem. Chem. Phys., 2009, 11, 6953–6961, 10.1039/B907450C.
- A. d. S. Goncalves, S. A. Marques de Lima, M. R. Davolos, S. G. Antonio and C. d. O. Paiva-Santos, The effects of ZnGa2O4 formation on structural and optical properties of ZnO:Ga powders, J. Solid State Chem., 2006, 179, 1330–1334, DOI:10.1016/j.jssc.2006.01.046.
- N. Kimizuka, M. Isobe and M. Nakamura, Syntheses and Single-Crystal Data of Homologous Compounds, In2O3(ZnO)m (m = 3, 4, and 5), InGaO3(ZnO)3, and Ga2O3(ZnO)m (m = 7, 8, 9, and 16) in the In2O3-ZnGa2O4-ZnO System, J. Solid State Chem., 1995, 116, 170–178, DOI:10.1006/jssc.1995.1198.
- M. Nakamura, N. Kimizuka and T. Mohri, The phase relations in the In2O3·Ga2ZnO4·ZnO system at 1350 °C, J. Solid State Chem., 1991, 93, 298–315, DOI:10.1016/0022-4596(91)90304-Z.
- J. P. Cline, M. Leoni, D. Black, A. Henins, J. E. Bonevich, P. S. Whitfield and P. Scardi, Crystalline domain size and faulting in the new NIST SRM 1979 zinc oxide, Powder Diffr., 2013, 28, S22, DOI:10.1017/s0885715613001188.
- J. I. Langford, A. Boultif, J. P. Auffrédic and D. Louër, The use of pattern decomposition to study the combined X-ray diffraction effects of crystallite size and stacking faults in ex-oxalate zinc oxide, J. Appl. Crystallogr., 1993, 26, 22–33, DOI:10.1107/S0021889892007684.
- G. Kresse and D. Joubert, From ultrasoft pseudopotentials to the projector augmented-wave method, Phys. Rev. B:Condens. Matter Mater. Phys., 1999, 59, 1758–1775, DOI:10.1103/PhysRevB.59.1758.
- P. Pyykkö, Year-2008 nuclear quadrupole moments, Mol. Phys., 2008, 106, 1965–1974, DOI:10.1080/00268970802018367.
- M. Allix, S. Chenu, E. Veron, T. Poumeyrol, E. A. Kouadri-Boudjelthia, S. Alahrache, F. Porcher, D. Massiot and F. Fayon, Considerable Improvement of Long-Persistent Luminescence in Germanium and Tin Substituted ZnGa2O4, Chem. Mater., 2013, 25, 1600–1606, DOI:10.1021/cm304101n.
- N. D. Lang and W. Kohn, Theory of Metal Surfaces: Charge Density and Surface Energy, Phys. Rev. B, 1970, 1, 4555–4568, DOI:10.1103/PhysRevB.1.4555.
- J. Weber and J. Schmedt auf der Günne, Calculation of NMR parameters in ionic solids by an improved self-consistent embedded cluster method, Phys. Chem. Chem. Phys., 2010, 12, 583–603, 10.1039/b909870d.
- T. Gullion and J. Schaefer, Rotational-echo double-resonance NMR, J. Magn. Reson., 1989, 81, 196–200, DOI:10.1016/0022-2364(89)90280-1.
- M. Roming, C. Feldmann, Y. S. Avadhut and J. Schmedt auf der Günne, Characterization of Noncrystalline Nanomaterials: NMR of Zinc Phosphate as a Case Study, Chem. Mater., 2008, 20, 5787–5795, DOI:10.1021/cm800805f.
- V. R. Celinski, J. Weber and J. Schmedt auf der Günne, C-REDOR curves of extended spin systems, Solid State Nucl. Magn. Reson., 2013, 49–50, 12–22, DOI:10.1016/j.ssnmr.2012.10.001.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5dt00928f |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |