Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis, structural characterization, and cytotoxic evaluation of monofunctional cis-[Pt(NH3)2(N7-guanosine/2′-deoxyguanosine)X] (X = Cl, Br, I) complexes with anticancer potential†
Asjad
Ali‡
 ,
Gianluca
Rovito‡
,
Erika
Stefàno
,
Federica
De Castro
,
Gianluca
Rovito‡
,
Erika
Stefàno
,
Federica
De Castro
 ,
Giuseppe
Ciccarella
,
Giuseppe
Ciccarella
 ,
Danilo
Migoni
,
Elisa
Panzarini
,
Antonella
Muscella
,
Santo
Marsigliante
,
Michele
Benedetti
,
Danilo
Migoni
,
Elisa
Panzarini
,
Antonella
Muscella
,
Santo
Marsigliante
,
Michele
Benedetti
 * and
Francesco Paolo
Fanizzi
* and
Francesco Paolo
Fanizzi

Department of Biological and Environmental Sciences and Technologies (DiSTeBA), University of Salento, Via Monteroni, I-73100 Lecce, Italy. E-mail: michele.benedetti@unisalento.it
First published on 28th April 2025
Abstract
A series of new monofunctional platinum(II) complexes of the type cis-[Pt(NH3)2(N7-guanosine/2′-deoxyguanosine)X] (X = Cl, Br, I) were synthesized and characterized using NMR spectroscopy, mass spectrometry, and ICP-atomic emission spectroscopy. These complexes are designed to address the limitations of conventional bifunctional platinum-based drugs, such as cisplatin, which include issues with cytotoxicity and selectivity towards cancer cells. By incorporating guanosine or 2′-deoxyguanosine ligands and varying halido substituents, the study investigated how structural modifications influence the selectivity and cytotoxicity of the different analogues. To evaluate the anticancer potential of the newly synthesized platinum derivatives, various cancer cell lines were tested, including renal (Caki-1), uterine cervix (HeLa), breast (MCF-7), lymphoma (Raji), and mesothelioma (ZL-34). Additionally, selectivity against tumor cells was assessed by comparing their cytotoxic effects to those in the healthy, immortalized HK-2 cell line, a proximal tubular cell line derived from a normal human adult male kidney. Cytotoxicity analysis revealed that bromido-substituted Pt(II) complexes exhibited superior cytotoxicity across several cancer cell lines, particularly in HeLa and Raji cells, compared to their chlorido- and iodido-substituted counterparts. The iodido complexes exhibited higher efficacy against MCF-7 breast cancer cells, suggesting tumor-specific selectivity. Notably, these complexes demonstrated lower cytotoxicity in healthy cells compared to most of the tested cancer cell lines, as reflected by generally favorable selectivity indices (SI) relative to cisplatin.
Introduction
Cancer is the leading cause of death worldwide, and the discovery of new, effective antitumor therapies is a primary objective of scientific research.1 Platinum-based anticancer drugs play a pivotal role in medical oncology, particularly due to the widespread use of drugs like cisplatin, oxaliplatin, and carboplatin.2–4 Approximately half of all patients undergoing anticancer chemotherapy are treated with a platinum-based drug.3 These drugs primarily function by forming bifunctional DNA adducts, which inhibit DNA replication and transcription, ultimately leading to cell death. However, the development of drug resistance can result in the rapid repair of DNA damage and reduced drug accumulation.3,5,6 Additionally, their non-specificity and lack of selectivity leads to undesirable side effects, highlighting the need to explore alternative platinum-based drugs with different mechanisms of action, cellular accumulation, and DNA-binding modes.7–9Cationic monofunctional platinum(II) complexes, characterized by a single labile ligand, present a promising alternative to conventional platinum-based therapies. These complexes deviate from the traditional bifunctional mechanism and exhibit unique interactions with biomolecules, potentially reducing side effects and drug resistance.8,10,11 Unlike cisplatin, each monofunctional platinum(II) complex can form only one covalent bond with DNA strands. Initially, monofunctional complexes similar to the inactive [Pt(dien)Cl]+ and [Pt(NH3)3Cl]+ complexes were also considered inactive against cancer cells, as it was believed that only cis-configured, square-planar, and neutral platinum(II) complexes could exhibit anticancer activity.12–15 This assumption was challenged when cationic monofunctional cis-[Pt(NH3)2(Am)Cl]+ complexes (where Am is an aromatic N-heterocyclic amine) were found to inhibit cancer cells in vitro and in mouse models of leukemia, forming stable DNA adducts and demonstrating intercalative effects.10,13,16,17 Recently, the discovery of the cationic monofunctional Pt(II) complex phenanthriplatin, cis-[Pt(NH3)2(phenanthridine)Cl]+, has provided new insights into the development of platinum-based antitumor agents as alternatives to existing clinical drugs.11,18,19 Due to the lipophilicity of its monodentate ligand, phenanthriplatin exhibits enhanced cellular uptake and greater cytotoxicity than cisplatin across various cancer cell lines.3,5,18,20,21 Further research has shown that organic cation transporters (OCTs) play a role in the cellular uptake and activity of this type of cationic platinum(II) complexes.22–24 Additionally, structure–activity relationship (SAR) analyses have demonstrated that steric hindrance of the adopted N-heterocyclic imine ligand is crucial. In fact, it can modulate the type of mono-adducts mainly formed with purines in DNA, and consequently the activity of RNA polymerase II.18,25 The improved aqueous solubility, monofunctionality, and cationic nature of these platinum(II) complexes offer advantages over cisplatin analogs.9
Nucleotide analogues (NAs) are a class of compounds that include various pyrimidine and purine derivatives, commonly used as antiviral and anticancer agents.26–30 NAs can function as antimetabolites, capable of interfering with nucleic acid synthesis or altering nucleoside metabolism.31,32 Platinum-linked nucleosides may act as biomimetic substrates, facilitating selective cellular uptake and processing, potentially following pathways similar to those of other nucleoside analogue-based drugs.27,33 This suggests that, once taken up by membrane transporters and phosphorylated, they may be recognized by nuclear or mitochondrial DNA polymerases and incorporated into newly synthesized DNA, leading to tumor cell death.27 Therefore, we propose that the development of novel drug species based on NAs and other chemotherapeutics could enhance the therapeutic efficacy against various types of cancer.34,35 In essence, these molecules could establish a new class of antitumor drugs by merging the properties of both nucleoside analogs and platinum-based drugs. The feasibility of this approach for anticancer applications of monofunctional cis-[Pt(NH3)2(Am)Cl]+ (Am = heterocyclic amine) complexes has been evaluated in many studies using different complexes and in vitro and/or in vivo experimental approaches.10,27,33,36–47
In this study, we synthesized and evaluated the anticancer potential of six platinated nucleoside-based complexes. These complexes, of the type cis-[Pt(NH3)2(N7-guanosine)X] (X = Cl (1), Br (2), I (3)) and cis-[Pt(NH3)2(N7-2′-deoxyguanosine)X] (X = Cl (d1), Br (d2), I (d3)), represent a promising class of antimetabolites for cancer treatment. The choice of halide (Cl, Br, I) influences both the reactivity and stability of the complexes, as well as their interaction with biological targets.48–50 By incorporating guanosine or 2′-deoxyguanosine ligands, which are intrinsically non-toxic and physiological, into the Pt(II) coordination sphere, we aim to enhance selectivity for cancer cells by mimicking natural nucleosides.27 We synthesized new monofunctional platinum(II) complexes and tested their anticancer activity against various cancer cell lines. Additionally, we explored how structural modifications affect the selectivity and cytotoxicity of the different analogues tested in this study.
Results and discussion
Synthesis of cis-[Pt(NH3)2(N7-guanosine)X], X = Cl (1), Br (2), I (3), and cis-[Pt(NH3)2(N7-2′-deoxyguanosine)X], X = Cl (d1), Br (d2), I (d3), complexes
In this study, we present a new synthetic pathway for the production of a whole set of monofunctional, water-soluble platinum(II) complexes as possible promising candidates for anticancer applications. By varying the halido ligands in these monofunctional complexes, incorporating N7-guanosine (N7-Guo) and N7-2′-deoxyguanosine (N7-dGuo) ligands, we aimed to enhance their selectivity and efficacy in targeting cancer cells, already observed for the chlorido species.10,36 As reported, besides the selection of other ligands, the halides choice is critical in determining the stability and biological activity of the resulting complexes.51–53 Indeed, our findings indicate that substituting different halides is an effective strategy to modify the biological activities of these complexes while preserving their molecular structure.The synthesis of cis-[Pt(NH3)2(Am)X]NO3, where Am represents guanosine (N7-Guo) or 2′-deoxyguanosine (N7-dGuo) and X is either Cl or Br, follows a previously reported two-step method.10,36,54 In the first step, cis-[Pt(NH3)2X2] (X = Cl or Br) reacts with one equivalent of AgNO3 in DMF, resulting in the formation of the monohalido species cis-[Pt(NH3)2(NO3)X] and/or cis-[Pt(NH3)2(DMF)X](NO3) (X = Cl or Br). These species contain oxygen-bound ligands, which act as superior leaving groups compared to chloride and bromide, thereby enhancing the Am coordination kinetics. In the subsequent step, these intermediates react with the guanosine (Guo) or 2′-deoxyguanosine (dGuo) ligands to yield the desired platinum complexes (1–2 and d1–d2). The iodido complexes cis-[Pt(NH3)2(N7-Guo)I]+ (3) and cis-[Pt(NH3)2(N7-dGuo)I]+ (d3) were synthesized from the corresponding chlorido species (1 or d1) by reaction with one equivalent of AgNO3 in water and further addition of one equivalent of KI, after AgCl removal (Fig. 1).
 | ||
| Fig. 1 Synthesis of platinum(II) nucleoside monoadducts: cis-[Pt(NH3)2(N7-Guo)X]+ (1–3) and cis-[Pt(NH3)2(N7-dGuo)X]+ (d1–d3) complexes, where X = Cl (1), Br (2), I (3). | ||
The structural characterization of the resulting complexes was performed using a combination of techniques, including nuclear magnetic resonance (NMR) spectroscopy, mass spectrometry, and inductively coupled plasma atomic emission spectroscopy (ICP-AES).
NMR spectroscopy
The 1H NMR spectra for all six platinum complexes (1–3, d1–d3) were collected and analyzed confirming the structure of the compounds. The spectra confirmed the nucleoside coordination in the metal complexes with chemical shifts variations according to the halido ligands (Cl, Br, I) attached to the platinum center. Key proton signals, including those for H8, NH2 (of guanine), H1′, H2′, H2′′, H3′, H4′, H5′, and H5′′ (of the sugar moiety), and NH3 (platinum-bound ammonia groups), were clearly assigned (Fig. 2 and S1†). The absence of the H2′ signal of compounds (1–3) and the H3′ signal of compounds (d1–d3) in 1H NMR spectra is due to the overlapping with H2O signal. Moreover, the variation in the total proton integration arises due to the presence of labile protons that undergo rapid exchange with the solvent. These exchangeable protons are often not observable (e.g., –OH and –NH groups) or appear with reduced intensity (e.g., –NH2 and –NH3 groups) under the conditions used. For each compound (1–3, d1–d3), the expected correlations between nucleoside protons coupled to each other within the complexes was confirmed using the [1H–1H]-COSY experiment, as reported in the literature.55,56 The non-exchangeable protons of the sugar moiety resonate between 2.45 and 4.60 ppm. The NH3 signals, appearing around 3.90–4.25 ppm, exhibit slight shifts across the spectra because of the different halido ligands bound to the platinum center. The observed chemical shifts variation for the NH3 signals suggests that the halido ligands modulate the electronic structure of the platinum-nucleoside complex, affecting the chemical environment of the surrounding protons. This structural information is crucial for understanding how these different halido ligands influence the properties of the platinum complexes, potentially impacting their biological activity.Comparison of the H8 proton chemical shifts of N7-Guo and N7-dGuo in platinum complexes also reveals distinct shifts depending on the halido ligands (Cl, Br, I). In all newly synthesized platinum complexes, the H8 proton resonates in the 8.36–8.46 ppm range. The H8 signals display a significant separation in chemical shifts, with the iodido complexes showing the highest downfield shifts and higher frequencies, followed by the bromido (2 and d2), and then the chlorido complexes (1 and d1). In contrast, all other peaks, including those of the sugar moiety and NH3 protons, tend to shift slightly upfield (lower frequencies) by increasing the halogen size, with the effect most pronounced in the iodido complexes (3 and d3). The observed trend can be attributed to the varying electronegativities and polarizabilities of the halido ligands, as well as the relative positions of the observed nuclei in relation to the occurring NMR magnetic shielding (Fig. 2 and S1†).57
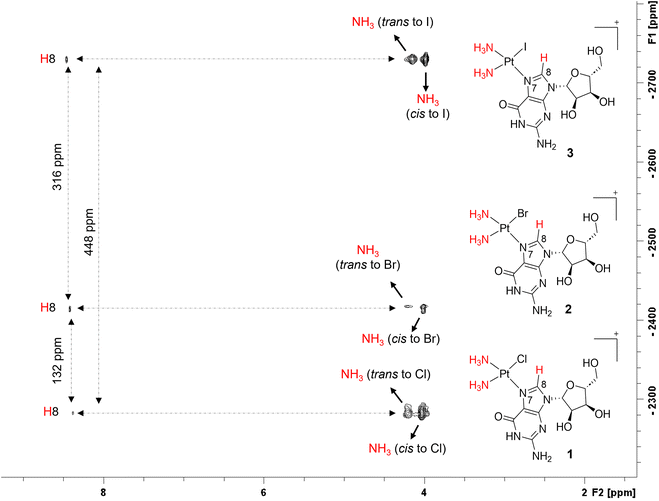
To observe the direct coupling between the platinum center and nearby protons, such as those on the N7-Guo/N7-dGuo base and the ammonia ligands, 2D heteronuclear [1H,195Pt] NMR spectra were acquired (Fig. 3 and S2†). The bidimensional spectra display three cross-peaks corresponding to NH3 (cis to halido), NH3 (trans to halido), and the H8 proton of the nucleobase moiety, all coupled with the central 195Pt. Each platinum-nucleoside complex exhibits unique chemical shifts for the platinum center, influenced by the electronic properties of the halido ligands. Complexes with iodido ligands show the most shielded 195Pt signals (3 = δ(195Pt) −2730 ppm, d3 = δ(195Pt) −2729 ppm), followed by bromido (2 = δ(195Pt) −2413 ppm, d2 = δ(195Pt) −2413 ppm) and chlorido (1 = δ(195Pt) −2282 ppm, d1 = δ(195Pt) −2282 ppm), indicating that bulkier halido ligands lead to a more shielded platinum environment (Fig. 3). These findings align with previous studies57–59 that indicate an inverse relationship between 195Pt NMR chemical shifts and the cumulative ionic radii of halides.57,60 The 2D NMR spectra also reveal different cis and trans NH3 ligands. Where, coordinated NH3's trans to halido ligand, show less intense cross peaks with 195Pt, if compared to the corresponding signal for cis NH3. This is because of the higher trans influence of the halido ligand, with respect to the N7-donor, reducing NMR coupling with 195Pt, as previously reported.10,36
Mass spectrometry
The mass spectrometric analysis of the newly synthesized complexes was performed by dissolving them in water and using a HPLC-FT/MS Thermo Fischer Scientific Q-Exactive HPLC/HRMS instrument. The results were analyzed and compared with theoretical spectra61 to assess the accuracy of the predicted mass-to-charge (m/z) ratios and isotopic distributions. The experimental spectra (Fig. S3†) revealed similar isotopic patterns for each complex, with peaks corresponding to the expected mass values, although slight deviations in m/z and relative abundances were observed. These deviations are expected in complexes containing platinum and halogens due to their inherently complex isotopic signatures. The experimental pattern, obtained directly from a high-resolution mass spectrometer, reflects the actual isotope ratios present in the sample, which may slightly differ from standard theoretical models assuming idealized natural abundances. For instance, platinum has multiple stable isotopes, and halogens contribute further complexity with their own isotope pairs. The observed discrepancies are likely due to slight variations in isotope ratios, instrument-specific response factors, or local isotope effects during ionization. The presence of the molecular ion peaks for the synthesized complexes at m/z [M + H]+ 548.0776 (1), [M + H]+ 532.0840 (d1), [M + H]+ 592.0282 (2), [M + H]+ 576.0326 (d2), [M]+ 639.0171 (3), and [M]+ 623.0216 (d3) is consistent with the proposed formulas of the corresponding complexes. In this context, “M” represents the corresponding platinum compound without NO3. These results provide strong evidence for the successful synthesis of these target complexes.Cytotoxicity studies
Fig. 4 and S10–S11† illustrate the cytotoxic effects of cisplatin and the platinum(II) nucleoside complexes cis-[Pt(NH3)2(N7-Guo)X]+ (X = Cl, 1; Br, 2; I, 3) and cis-[Pt(NH3)2(N7-dGuo)X]+ (X = Cl, d1; Br, d2; I, d3). Cytotoxicity assays were performed using the SRB method for renal (Caki-1), uterine cervix (HeLa), breast (MCF-7), mesothelioma (ZL-34) and normal human kidney (HK-2) cells. However, for lymphoma (Raji) cells, which grow in suspension, the MTT assay was used, as it is more suitable for non-adherent cell cultures. Cytotoxic effects were evaluated over 24–72 hours of exposure. At 24 hours, cisplatin displayed greater cytotoxicity than most of the newly synthesized complexes in all tested cell lines, except for the Raji cells, where complex 2 was more cytotoxic. The highest cytotoxicity in every investigated cell line was exhibited by cisplatin for longer exposures (48 and 72 hours). Notably, at a concentration of 50 μM, cisplatin caused complete cell death in the pleural mesothelioma cell line (ZL-34) within 48 hours (Fig. S10†).Among the new synthesized complexes, cis-[Pt(NH3)2(N7-Guo)Br]+ (2) and cis-[Pt(NH3)2(dGuo)Br]+ (d2) generally exhibited greater cytotoxicity than their chlorido (1, d1) and iodido (3, d3) counterparts, particularly in the HeLa cell line (see IC50 values in Table 1 and Fig. 4). Consistently, complexes 1, d1, 3, and d3 were overall the least cytotoxic of the new synthesized series. In the case of the chlorido species (1, d1), their relatively higher propensity to halido ligand exchange with sulfur-containing biomolecules likely could hinder DNA targeting.62 On the other hand, for the iodido complexes (3, d3), reduced aqueous stability and greater steric hindrance from the iodido ligand may reduce in some cases cytotoxic efficacy with respect to the other halido species. Nonetheless, it is noteworthy that after 24 hours of exposure, iodido complex 3 showed higher potency against MCF-7 cells than the other Guo-based complexes. This trend was also observed for the iodinated dGuo derivative d3, which proved to be the most cytotoxic compound against MCF-7 cells among all tested Guo and dGuo derivatives in the 24–72-hour timeframe (see IC50 values in Table 1).
Overall, the new Guo complexes were less toxic in renal (Caki-1) and mesothelioma (ZL-34) cancer cells, where the IC50 could often be determined only after 48–72 hours (Fig. S10 and S11;†Table 1). For instance, in Caki-1 cells, the IC50 for the iodido complexes 3 and d3 exceeded 500 μM and could only be measured after 72 hours (Fig. S11;†Table 1). After 72 hours, ZL-34 cells remained relatively resistant to the chlorido derivatives (IC50 > 500 μM), whereas cell viability decreased upon exposure to the more cytotoxic bromido complexes 2 and d2 (Fig. S10;†Table 2).
| a IC50(HK-2) or IC50(tumor cell line) not measurable, since higher of maximum tested concentration (1000 μM). In this case, the not measurable IC50 value is assumed equal to 1000 μM for the estimation of the possible indicated minimum or maximum SI value. |
|---|

|
To further evaluate the antitumor potential of the cis-[Pt(NH3)2(N7-Guo)X]+ (X = Cl, 1; Br, 2; I, 3) and cis-[Pt(NH3)2(N7-dGuo)X]+ (X = Cl, d1; Br, d2; I, d3) complexes, we calculated their selectivity index (SI), defined as the ratio of the IC50 in a normal immortalized proximal tubule epithelial cell line (HK-2) to that in each immortalized cancer cell line (HeLa, MCF-7, Raji, Caki-1, ZL-34) (Fig. 4, S10, S11;†Table 2). A value of SI greater than 1 indicates a higher specificity for cancer cells compared to healthy cells, while SI values above 4 classify the compound as highly selective (Table 2).63 Several of the nucleoside-based complexes studied in this work exhibited SI values that exceeded those of cisplatin. To better identify the most selective complexes against each tumor cell line, we calculated an average SI across the 24-, 48-, and 72-hour intervals that we will indicate as SIAV (Table 2). The principal factors affecting the SIAV appear to be the type of cancer cell line and the identity of the halido ligand in the considered nucleoside derivatives. A secondary, yet still noteworthy, effect could be attributed to the presence of either Guo or dGuo.
In HeLa cancer cells, the bromo complex d2 yielded the highest SIAV, surpassing also its structural analog 2 (Table 2). Both complexes were more selective than cisplatin. The order of decreasing SIAV for HeLa cells was d2 > 2 > cisplatin > 1 > d1 > d3 > 3. Interestingly, d2 was also the most cytotoxic complex in HeLa cells among the tested nucleobase derivatives, followed by 2. For this reason, both complexes d2, 2 appear promising antitumor drugs for HeLa cells related tumors.
In contrast, the iodido derivative d3 (dGuo), followed by the iodido derivative 3 (Guo) showed higher selectivity for MCF-7 cells where cisplatin appeared the least selective. The order of decreasing selectivity for MCF-7 cells was d3 ≫ 3 > 2 > d1 > d2 > 1 > cisplatin. Notably, d3 was also the most cytotoxic complex for the cisplatin resistant MCF-7 tumor cells among tested nucleobase derivatives, followed by 3. For this reason, both complexes seem promising antitumor drugs for tumor types related to MCF-7 cells.
In the Raji cells the bromido derivative 2 was the most selective, followed by the chlorido complex 1, both showing higher selectivity than cisplatin (Table 2). The descending order of selectivity for Raji cells was 2 > 1 > d2 > d1 > cisplatin > d3 > 3. Complex 2 was also the most cytotoxic in Raji cells among the tested nucleobase derivatives, followed by d2 and 1. For this reason, both complexes could be considered promising antitumor drugs for tumor types related to Raji cells.
In Caki-1 the chlorido complexes 1 and d1 were most selective, both exceeding cisplatin (Table 2). The descending order of SIAV for Caki-1 was 1 > d1 > d2 > 2 > d3 > cisplatin > 3. Meanwhile, the significant cytotoxicity of 1 and d2 for Caki-1 cells indicates that both complexes could be considered promising antitumor drugs for tumor types related to Caki-1 cells.
Lastly, in ZL-34 the iodido derivative d3 exhibited the highest SIAV, followed by the chlorido derivative d1, both surpassing cisplatin (Table 2). The descending order of SIAV for ZL-34 was d3 > d1 > 2 > 3 > d2 = cisplatin. On the other hand, d3 showed relatively moderate cytotoxicity (IC50 after 72 hours ≈ 505 μM), especially if compared to complex 2 (IC50 ≈ 183 μM at 72 hours) and d2 (IC50 ≈ 189 μM at 72 hours). Therefore 2 and d2 appear the most promising compounds for tumor types related to ZL-34 cells as the only nucleoside-bound platinum agents among those tested to exhibit a significant cytotoxicity and a selectivity similar or slightly higher than that of cisplatin. This suggests the need for further studies on complexes 2 and d2 to maximize both selectivity and potency when targeting the ZL-34 and other related tumor types, for which cisplatin remains more immediately cytotoxic but less selective overall.
Considering together, these comparative results (Table 2) indicate substantial potential for the here reported platinated nucleoside derivatives in designing possible innovative therapeutic strategies. By selecting halido ligands (Cl, Br, I) and nucleobase moieties (Guo or dGuo), one could fine-tune both cytotoxic potency and tumor selectivity, guiding future development and further preclinical evaluation of these promising anticancer agents.
Experimental section
Reagents and methods
All commercially available reagents and solvents were obtained from Sigma-Aldrich and used as received, without further purification. All reactions were performed under ambient conditions. NMR spectra were acquired using a Bruker Avance III 400 NMR spectrometer, equipped with inverse detection probes and z-gradient capabilities for gradient-enhanced NMR spectroscopy. Two-dimensional [1H,195Pt]-HETCOR and 1H NMR spectra were acquired using H2O/D2O (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as the solvent. The 1H NMR spectra were calibrated against tetramethylsilane (TMS), using the residual proton signal from H2O/D2O (90
10) as the solvent. The 1H NMR spectra were calibrated against tetramethylsilane (TMS), using the residual proton signal from H2O/D2O (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) (δ(1H) = 4.7 ppm) as the internal reference. 195Pt NMR chemical shifts were referenced to H2[PtCl6] [δ(195Pt) = 0 ppm] in H2O/D2O (90
10) (δ(1H) = 4.7 ppm) as the internal reference. 195Pt NMR chemical shifts were referenced to H2[PtCl6] [δ(195Pt) = 0 ppm] in H2O/D2O (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as the external reference. The use of H2O/D2O (90
10) as the external reference. The use of H2O/D2O (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as the solvent ensures the detection of exchangeable protons (e.g., –NH3 and –NH2 groups). In pure D2O, these labile protons would rapidly undergo deuterium exchange, effectively replacing protons with deuterons and making them undetectable in the 1H NMR spectrum. By maintaining a 90% H2O environment, the solvent acts as a reservoir for protons, preserving the protonation state of exchangeable groups and enabling their observation. Additionally, a small fraction of D2O (10%) provides a sufficient deuterium signal for the NMR spectrometer's “lock system”, which stabilizes the magnetic field during data acquisition. Coupling constants (J values) are given in Hertz, and the NMR signal multiplicities are noted as follows: s (singlet), d (doublet), dd (doublet of doublets), t (triplet), m (multiplet), and b (broad). Mass spectrometry (MS) analysis was conducted by dissolving complexes (1–3, d1–d3) in water, with sample injections handled via a Chemyx Inc. Model Fusion 101 syringe pump. Electrospray ionization (ESI) and high-resolution mass spectrometry (HRMS) spectra were collected using a Thermo Fisher Scientific Q-Exactive HPLC-FT/MS system under standard conditions: positive ion mode, flow rate of 0.200 mL min−1, sheath gas at 5.0 L min−1, capillary temperature at 320 °C, spray voltage at 4500 V, and a mass range of 3
10) as the solvent ensures the detection of exchangeable protons (e.g., –NH3 and –NH2 groups). In pure D2O, these labile protons would rapidly undergo deuterium exchange, effectively replacing protons with deuterons and making them undetectable in the 1H NMR spectrum. By maintaining a 90% H2O environment, the solvent acts as a reservoir for protons, preserving the protonation state of exchangeable groups and enabling their observation. Additionally, a small fraction of D2O (10%) provides a sufficient deuterium signal for the NMR spectrometer's “lock system”, which stabilizes the magnetic field during data acquisition. Coupling constants (J values) are given in Hertz, and the NMR signal multiplicities are noted as follows: s (singlet), d (doublet), dd (doublet of doublets), t (triplet), m (multiplet), and b (broad). Mass spectrometry (MS) analysis was conducted by dissolving complexes (1–3, d1–d3) in water, with sample injections handled via a Chemyx Inc. Model Fusion 101 syringe pump. Electrospray ionization (ESI) and high-resolution mass spectrometry (HRMS) spectra were collected using a Thermo Fisher Scientific Q-Exactive HPLC-FT/MS system under standard conditions: positive ion mode, flow rate of 0.200 mL min−1, sheath gas at 5.0 L min−1, capillary temperature at 320 °C, spray voltage at 4500 V, and a mass range of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–8
000–8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m/z. Platinum concentration was measured by Inductively Coupled Plasma – Atomic Emission Spectroscopy (ICP-AES) using a Thermo iCAP 6000 spectrometer. To quantify platinum levels, the samples were subjected to acid digestion using 10 mL of ultrapure nitric acid (2%) for 24 hours at room temperature. Before analysis, the samples were filtered to remove any particulate matter that could potentially interfere with the spectrometer. Calibration was carried out using a four-point standard curve (1, 10, 100, and 1000 μg L−1) to ensure analytical precision.64
000 m/z. Platinum concentration was measured by Inductively Coupled Plasma – Atomic Emission Spectroscopy (ICP-AES) using a Thermo iCAP 6000 spectrometer. To quantify platinum levels, the samples were subjected to acid digestion using 10 mL of ultrapure nitric acid (2%) for 24 hours at room temperature. Before analysis, the samples were filtered to remove any particulate matter that could potentially interfere with the spectrometer. Calibration was carried out using a four-point standard curve (1, 10, 100, and 1000 μg L−1) to ensure analytical precision.64
Synthesis of platinum complexes
Platinum complexes cis-[Pt(NH3)2(N7-Guo/dGuo)Cl] (1 and d1) and cis-[Pt(NH3)2(N7-Guo/dGuo)Br] (2 and d2) were synthesized following a previously reported method10,36,54 with minor modifications. Briefly, cis-[Pt(NH3)2Cl2] (for complexes 1 and d1) (100 mg, 0.332 mmol) or cis-[Pt(NH3)2Br2] (for complexes 2 and d2) (100 mg, 0.257 mmol) and an equimolar amount of AgNO3 were dissolved in 5 mL of N,N-dimethylformamide (DMF) and stirred at room temperature for 24 hours. The resulting AgX (X = Cl or Br) precipitate was then removed by centrifugation. The clear supernatant was further reacted with an equimolar amount of guanosine (for complexes 1 and 2) or 2′-deoxyguanosine monohydrate (for complexes d1 and d2) and stirred for an additional 24 hours. Upon completion of the reaction, the DMF solvent was evaporated under reduced pressure, leaving a solid residue. This residue was washed three times with dichloromethane (CH2Cl2), with stirring each time, and then dried. Finally, the crude product was purified by recrystallization from hot water, repeating the process three times, and then dried in air.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 300 K) δ: 8.41 (s, 1H, H-8), 6.48 (s, 2H, NH2), 5.90 (d, 1H, H-1′, J = 4.82 Hz), 4.34 (t, 1H, H-3′, J = 4.96 Hz), 4.24 (s, 3H, NH3), 4.17 (q, 1H, H-4′, J = 3.35 Hz), 4.06 (s, 3H, NH3), 3.88–3.75 (m, 2H, H-5′ & H-5′′), δ(195Pt) −2282 ppm.
10, 300 K) δ: 8.41 (s, 1H, H-8), 6.48 (s, 2H, NH2), 5.90 (d, 1H, H-1′, J = 4.82 Hz), 4.34 (t, 1H, H-3′, J = 4.96 Hz), 4.24 (s, 3H, NH3), 4.17 (q, 1H, H-4′, J = 3.35 Hz), 4.06 (s, 3H, NH3), 3.88–3.75 (m, 2H, H-5′ & H-5′′), δ(195Pt) −2282 ppm.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 300 K) δ: 8.44 (s, 1H, H-8), 6.47 (s, 2H, NH2), 5.89 (d, 1H, H-1′, J = 4.70 Hz), 4.33 (t, 1H, H-3′, J = 4.99 Hz), 4.23 (s, 3H, NH3), 4.16 (q, 1H, H-4′, J = 3.81 Hz), 4.04 (s, 3H, NH3), 3.88–3.75 (m, 2H, H-5′ & H-5′′), δ(195Pt) −2414 ppm.
10, 300 K) δ: 8.44 (s, 1H, H-8), 6.47 (s, 2H, NH2), 5.89 (d, 1H, H-1′, J = 4.70 Hz), 4.33 (t, 1H, H-3′, J = 4.99 Hz), 4.23 (s, 3H, NH3), 4.16 (q, 1H, H-4′, J = 3.81 Hz), 4.04 (s, 3H, NH3), 3.88–3.75 (m, 2H, H-5′ & H-5′′), δ(195Pt) −2414 ppm.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 300 K) δ: 8.36 (s, 1H, H-8), 6.45 (s, 2H, NH2), 6.27 (t, 1H, H-1′, J = 6.50 Hz), 4.23 (s, 3H, NH3), 4.07 (q, 1H, H-4′, J = 4.08 Hz), 4.06 (s, 3H, NH3), 3.79–3.69 (m, 2H, H-5′ & H-5′′), 2.69 (m, 1H, H-2′), 2.49 (m, 1H, H-2′′), δ(195Pt) −2282 ppm.
10, 300 K) δ: 8.36 (s, 1H, H-8), 6.45 (s, 2H, NH2), 6.27 (t, 1H, H-1′, J = 6.50 Hz), 4.23 (s, 3H, NH3), 4.07 (q, 1H, H-4′, J = 4.08 Hz), 4.06 (s, 3H, NH3), 3.79–3.69 (m, 2H, H-5′ & H-5′′), 2.69 (m, 1H, H-2′), 2.49 (m, 1H, H-2′′), δ(195Pt) −2282 ppm.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 300 K) δ: 8.39 (s, 1H, H-8), 6.44 (s, 2H, NH2), 6.26 (t, 1H, H-1′, J = 6.48 Hz), 4.22 (s, 3H, NH3), 4.07 (q, 1H, H-4′, J = 4.08 Hz), 4.04 (s, 3H, NH3), 3.79–3.68 (m, 2H, H-5′ & H-5′′), 2.69 (m, 1H, H-2′), 2.49 (m, 1H, H-2′′), δ(195Pt) −2413 ppm.
10, 300 K) δ: 8.39 (s, 1H, H-8), 6.44 (s, 2H, NH2), 6.26 (t, 1H, H-1′, J = 6.48 Hz), 4.22 (s, 3H, NH3), 4.07 (q, 1H, H-4′, J = 4.08 Hz), 4.04 (s, 3H, NH3), 3.79–3.68 (m, 2H, H-5′ & H-5′′), 2.69 (m, 1H, H-2′), 2.49 (m, 1H, H-2′′), δ(195Pt) −2413 ppm.
Synthesis of the platinum complexes 3 and d3
The aqueous solution of chlorido precursors (1 or d1) was reacted with an equimolar aqueous solution of AgNO3. After the AgCl was removed, one equivalent of KI was added, initiating an immediate reaction. The solvent was then removed under reduced pressure. The resulting solid was washed thoroughly with cold water three times and dried to yield the pure iodido complexes cis-[Pt(NH3)2(N7-Guo/dGuo)I]NO3 (3 and d3).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 300 K) δ: 8.46 (s, 1H, H-8), 6.45 (s, 2H, NH2), 5.84 (d, 1H, H-1′, J = 4.66 Hz), 4.29 (t, 1H, H-3′, J = 4.96 Hz), 4.18 (s, 3H, NH3), 4.13 (q, 1H, H-4′, J = 3.92 Hz), 3.99 (s, 3H, NH3), 3.84–3.71 (m, 2H, H-5′ & H-5′′), δ(195Pt) −2730 ppm.
10, 300 K) δ: 8.46 (s, 1H, H-8), 6.45 (s, 2H, NH2), 5.84 (d, 1H, H-1′, J = 4.66 Hz), 4.29 (t, 1H, H-3′, J = 4.96 Hz), 4.18 (s, 3H, NH3), 4.13 (q, 1H, H-4′, J = 3.92 Hz), 3.99 (s, 3H, NH3), 3.84–3.71 (m, 2H, H-5′ & H-5′′), δ(195Pt) −2730 ppm.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 300 K) δ: 8.41 (s, 1H, H-8), 6.43 (s, 2H, NH2), 6.21 (t, 1H, H-1′, J = 6.43 Hz), 4.18 (s, 3H, NH3), 4.02 (q, 1H, H-4′, J = 4.12 Hz), 3.98 (s, 3H, NH3), 3.74–3.64 (m, 2H, H-5′ & H-5′′), 2.65 (m, 1H, H-2′), 2.44 (m, 1H, H-2′′), δ(195Pt) −2729 ppm.
10, 300 K) δ: 8.41 (s, 1H, H-8), 6.43 (s, 2H, NH2), 6.21 (t, 1H, H-1′, J = 6.43 Hz), 4.18 (s, 3H, NH3), 4.02 (q, 1H, H-4′, J = 4.12 Hz), 3.98 (s, 3H, NH3), 3.74–3.64 (m, 2H, H-5′ & H-5′′), 2.65 (m, 1H, H-2′), 2.44 (m, 1H, H-2′′), δ(195Pt) −2729 ppm.
Cell cultures
The following cell lines derived from frozen stocks MCF-7 (human breast adenocarcinoma): HTB-22™, HeLa (human cervical adenocarcinoma): CRM-CCL-2™, Caki-1 (human renal carcinoma): HTB-46™, HK-2 (human kidney proximal tubule epithelial cells): CRL-2190™, Raji (human Burkitt's lymphoma): CCL-86™ were purchased from the American Type Cell Culture collection (ATCC), Manassas, VA, USA. ZL-34 (human pleural mesothelioma) cell line (cat. no. 11120713) was purchased from Sigma-Aldrich, St Louis, MO, USA.ZL-34, Raji and MCF-7 cells were cultured in RPMI 1640 medium (EuroClone, Pero, MI) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS), 2 mM L-glutamine, penicillin (100 U mL−1), and streptomycin (100 μg mL−1). HeLa, Caki-1, and HK-2 cells were cultured in Dulbecco's Modified Eagle's medium (DMEM) with 4.5 g L−1 glucose (EuroClone, Pero, MI), supplemented with 10% (v/v) heat-inactivated FBS, 2 mM L-glutamine, penicillin (100 U mL−1), and streptomycin (100 μg mL−1). Cells were grown in a humidified incubator with 5% CO2 in air at 37 °C and were used for biological assays upon reaching 70–80% confluence. Cells were sub-cultured for further study after reaching 80% confluence.
Cell viability assays
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per ml). For cell treatments, the Pt-compounds were dissolved in Dulbecco's phosphate-buffered saline (PBS) to prepare a stock solution (1 mM), from which subsequent dilutions ranging from 1 to 1000 μM were prepared. After overnight incubation, the cells were treated with various concentrations (0, 1, 10, 50, 100, 200, 500, and 1000 μM) of cisplatin and platinum(II) nucleoside complexes for 24, 48 and 72 h. At the end of each treatment period, 100 μL of ice-cold 10% (w/v) trichloroacetic acid was added to each well and incubated for 30 min at 4 °C. The plates were then washed five times with double-distilled water and allowed to air-dry overnight. Subsequently, 60 μL of 0.4% (w/v) SRB solution was added to each well and incubated for 20 min, followed by four washes with 1% (v/v) acidic acetic acid. Finally, the SRB was solubilized in 200 μL of 10 mM unbuffered Tris-base solution, and the absorbance was measured at 560 nm using a spectrophotometer.67 The percentage of cell survival was calculated as the ratio of the absorbance of treated cells to that of vehicle-treated control cells.
000 cells per ml). For cell treatments, the Pt-compounds were dissolved in Dulbecco's phosphate-buffered saline (PBS) to prepare a stock solution (1 mM), from which subsequent dilutions ranging from 1 to 1000 μM were prepared. After overnight incubation, the cells were treated with various concentrations (0, 1, 10, 50, 100, 200, 500, and 1000 μM) of cisplatin and platinum(II) nucleoside complexes for 24, 48 and 72 h. At the end of each treatment period, 100 μL of ice-cold 10% (w/v) trichloroacetic acid was added to each well and incubated for 30 min at 4 °C. The plates were then washed five times with double-distilled water and allowed to air-dry overnight. Subsequently, 60 μL of 0.4% (w/v) SRB solution was added to each well and incubated for 20 min, followed by four washes with 1% (v/v) acidic acetic acid. Finally, the SRB was solubilized in 200 μL of 10 mM unbuffered Tris-base solution, and the absorbance was measured at 560 nm using a spectrophotometer.67 The percentage of cell survival was calculated as the ratio of the absorbance of treated cells to that of vehicle-treated control cells.
Conclusions
In this study, we synthesized and characterized six Pt(II) complexes of the form cis-[Pt(NH3)2(N7-Guo)X]+ (X = Cl, 1; Br, 2; I, 3) and cis-[Pt(NH3)2(N7-dGuo)X]+ (X = Cl, d1; Br, d2; I, d3). We also assessed their effects in multiple cancer cell lines (HeLa, MCF-7, Raji, Caki-1, ZL-34) as well as a proximal tubule epithelial cell line (HK-2). The impact of different coordinated halido ligands on cytotoxicity of the examined six monofunctional platinated nucleosides resulted significant. Our finding confirmed various recent investigations on metal complexes showing how the halido ligand modulates both the reactivity and stability of these metal-drugs, thereby altering their cytotoxic profiles against diverse cancer cell lines.49,69,70In the HeLa cells, the bromido-complex d2 displayed the highest cytotoxicity and selectivity among the tested nucleobase derivatives, followed closely by complex 2, which was slightly less potent yet still more selective than cisplatin (Tables 1 and 2). A similar pattern emerged in MCF-7 breast cancer cells but shifted toward the iodido species, where d3 outperformed the other nucleobase derivatives in both cytotoxicity and selectivity. Complex 3 was moderately effective in these cells but remained less selective than d3, although both complexes surpassed cisplatin in terms of selectivity.
In Raji cells, complex 2 emerged as the most cytotoxic agent among the nucleobase derivatives, followed by d2. Notably, 1, d1 and 2, d2 all demonstrated superior selectivity relative to cisplatin, suggesting further potential in the lymphoma treatment. In Caki-1 cells, d2 was the most cytotoxic among the nucleobase derivatives; however, complexes 1 and d1 exhibited the highest selectivity, again surpassing cisplatin (Table 2).
With ZL-34, the iodido derivative d3 presented the greatest selectivity but only moderate cytotoxicity, while complex 2 and d2 achieved stronger cytotoxic effects (albeit with slightly reduced selectivity), remaining more selective than cisplatin overall.
Although these newly synthesized complexes generally showed lower absolute cytotoxicity than cisplatin, their structural features, particularly the identity of the halido ligand (Cl, Br, or I), strongly influence their biological activity and selectivity profiles. Bromido-substituted species (2, d2) often exhibited elevated potency, underscoring the prospect of nucleoside-based platinum agents in the treatment of lymphoid and myeloid malignancies.27,71 Likewise, iodido complexes (3, d3) demonstrate marked cytotoxicity and selectivity in MCF-7 cells, illustrating that the type of halido ligand can tailor activity in a tumor-selective manner.
Importantly, all six Pt(II) nucleoside complexes displayed significantly reduced cytotoxicity in the healthy immortalized cell line (HK-2), frequently showing IC50 values beyond the maximum tested concentration. While they were not as potent as cisplatin in most investigated tumor models, their higher Selectivity Indexes suggest possible available therapeutic windows in which cancer cells are more strongly affected than healthy cells. Overall, the halido substituent emerges as a crucial factor in tuning both cytotoxic properties and overall selectivity, offering a strategic route to develop Pt(II)-based agents with improved therapeutic and fewer off-target effects. According to the here reported results for Guo and dGuo platinated derivatives, this is particularly true in the following tumor cell lines, showing the better compromise between cytotoxicity and selectivity for each of the selected tumors: HeLa (2, d2); MCF-7 (3, d3); Raji (2); Caki-1 (1, d2); ZL-34 (2, d2).
Author contributions
Asjad Ali: conceptualization, software, data curation, formal analysis, investigation, and writing–original draft. Gianluca Rovito: software, data curation, formal analysis, investigation, and writing–original draft. Erika Stefàno: data curation, formal analysis, investigation, and writing–original draft. Federica De Castro: validation, visualization, and writing–review and editing. Giuseppe Ciccarella: data curation, investigation and visualization. Danilo Migoni: formal analysis. Elisa Panzarini: validation and visualization. Antonella Muscella: validation and visualization. Santo Marsigliante: supervision, validation, and visualization. Michele Benedetti: supervision, validation, visualization, and writing–review and editing. Francesco Paolo Fanizzi: visualization, and writing–review and editing.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.References
- R. L. Siegel, A. N. Giaquinto and A. Jemal, Cancer statistics, 2024, CA-Cancer J. Clin., 2024, 74, 12–49 CrossRef PubMed.
- E. J. Anthony, E. M. Bolitho, H. E. Bridgewater, O. W. L. Carter, J. M. Donnelly, C. Imberti, E. C. Lant, F. Lermyte, R. J. Needham, M. Palau, P. J. Sadler, H. Shi, F.-X. Wang, W.-Y. Zhang and Z. Zhang, Metallodrugs are unique: opportunities and challenges of discovery and development, Chem. Sci., 2020, 11, 12888–12917 RSC.
- T. C. Johnstone, G. Y. Park and S. J. Lippard, Understanding and improving platinum anticancer drugs–phenanthriplatin, Anticancer Res., 2014, 34, 471–476 CAS.
- T. C. Johnstone, K. Suntharalingam and S. J. Lippard, The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs, Chem. Rev., 2016, 116, 3436–3486 CrossRef CAS PubMed.
- I. A. Riddell, T. C. Johnstone, G. Y. Park and S. J. Lippard, Nucleotide Binding Preference of the Monofunctional Platinum Anticancer-Agent Phenanthriplatin, Chem. – Eur. J., 2016, 22, 7574–7581 CrossRef CAS PubMed.
- J. Zhou, Y. Kang, L. Chen, H. Wang, J. Liu, S. Zeng and L. Yu, The Drug-Resistance Mechanisms of Five Platinum-Based Antitumor Agents, Front. Pharmacol., 2020, 11, 343 CrossRef CAS PubMed.
- A. M. P. Romani, Cisplatin in cancer treatment, Biochem. Pharmacol., 2022, 206, 115323 CrossRef CAS PubMed.
- G.-B. Liang, Y.-C. Yu, J.-H. Wei, W.-B. Kuang, Z.-F. Chen and Y. Zhang, Design, synthesis and biological evaluation of naphthalenebenzimidizole platinum(II) complexes as potential antitumor agents, Eur. J. Med. Chem., 2020, 188, 112033 CrossRef CAS PubMed.
- A. Ali, E. Stefàno, F. De Castro, G. Ciccarella, G. Rovito, S. Marsigliante, A. Muscella, M. Benedetti and F. P. Fanizzi, Synthesis, Characterization, and Cytotoxicity Evaluation of Novel Water-Soluble Cationic Platinum(II) Organometallic Complexes with Phenanthroline and Imidazolic Ligands, Chem. – Eur. J., 2024, 30, e202401064 CrossRef CAS PubMed.
- L. S. Hollis, W. I. Sundquist, J. N. Burstyn, W. J. Heiger-Bernays, S. F. Bellon, K. J. Ahmed, A. R. Amundsen, E. W. Stern and S. J. Lippard, Mechanistic Studies of a Novel Class of Trisubstituted Platinum(II) Antitumor Agents, Cancer Res., 1991, 51, 1866–1875 CAS.
- A. A. Almaqwashi, W. Zhou, M. N. Naufer, I. A. Riddell, Ö. H. Yilmaz, S. J. Lippard and M. C. Williams, DNA Intercalation Facilitates Efficient DNA-Targeted Covalent Binding of Phenanthriplatin, J. Am. Chem. Soc., 2019, 141, 1537–1545 CrossRef CAS PubMed.
- J.-P. Macquet and J.-L. Butour, Platinum-Amine Compounds: Importance of the Labile and Inert Ligands for Their Pharmacological Activities Toward L1210 Leukemia Cells, JNCI, J. Natl. Cancer Inst., 1983, 70, 899–905 CAS.
- S. Dilruba and G. V. Kalayda, Platinum-based drugs: past, present and future, Cancer Chemother. Pharmacol., 2016, 77, 1103–1124 CrossRef CAS PubMed.
- M. J. Cleare and J. D. Hoeschele, Studies on the antitumor activity of group VIII transition metal complexes. Part I. Platinum(II) complexes, Bioinorg. Chem., 1973, 2, 187–210 CrossRef CAS.
- M. J. Cleare and J. D. Hoeschele, Anti-tumour Platinum Compounds, Platinum Met. Rev., 1973, 17, 2–13 CrossRef CAS.
- W. I. Sundquist, D. P. Bancroft and S. J. Lippard, Synthesis, characterization, and biological activity of cis-diammineplatinum(II) complexes of the DNA intercalators 9-aminoacridine and chloroquine, J. Am. Chem. Soc., 1990, 112, 1590–1596 CrossRef CAS.
- S. Jin, Y. Guo, Z. Guo and X. Wang, Monofunctional platinum(II) anticancer agents, Pharmaceuticals, 2021, 14, 133 CrossRef CAS PubMed.
- G. Y. Park, J. J. Wilson, Y. Song and S. J. Lippard, Phenanthriplatin, a monofunctional DNA-binding platinum anticancer drug candidate with unusual potency and cellular activity profile, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 11987–11992 CrossRef CAS PubMed.
- M. W. Kellinger, G. Y. Park, J. Chong, S. J. Lippard and D. Wang, Effect of a Monofunctional Phenanthriplatin-DNA Adduct on RNA Polymerase II Transcriptional Fidelity and Translesion Synthesis, J. Am. Chem. Soc., 2013, 135, 13054–13061 CrossRef CAS PubMed.
- T. C. Johnstone and S. J. Lippard, The Chiral Potential of Phenanthriplatin and Its Influence on Guanine Binding, J. Am. Chem. Soc., 2014, 136, 2126–2134 CrossRef CAS PubMed.
- D. Veclani, A. Melchior, M. Tolazzi and J. P. Cerón-Carrasco, Using Theory To Reinterpret the Kinetics of Monofunctional Platinum Anticancer Drugs: Stacking Matters, J. Am. Chem. Soc., 2018, 140, 14024–14027 Search PubMed.
- S. Zhang, K. S. Lovejoy, J. E. Shima, L. L. Lagpacan, Y. Shu, A. Lapuk, Y. Chen, T. Komori, J. W. Gray, X. Chen, S. J. Lippard and K. M. Giacomini, Organic Cation Transporters Are Determinants of Oxaliplatin Cytotoxicity, Cancer Res., 2006, 66, 8847–8857 Search PubMed.
- K. S. Lovejoy, R. C. Todd, S. Zhang, M. S. McCormick, J. A. D'Aquino, J. T. Reardon, A. Sancar, K. M. Giacomini and S. J. Lippard, cis-Diammine(pyridine)chloroplatinum(II), a monofunctional platinum(II) antitumor agent: Uptake, structure, function, and prospects, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 8902–8907 CrossRef CAS PubMed.
- S. Soodvilai, P. Meetam, L. Siangjong, R. Chokchaisiri, A. Suksamrarn and S. Soodvilai, Germacrone Reduces Cisplatin-Induced Toxicity of Renal Proximal Tubular Cells via Inhibition of Organic Cation Transporter, Biol. Pharm. Bull., 2020, 43, 1693–1698 CrossRef CAS PubMed.
- D. Wang, G. Zhu, X. Huang and S. J. Lippard, X-ray structure and mechanism of RNA polymerase II stalled at an antineoplastic monofunctional platinum-DNA adduct, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 9584–9589 CrossRef CAS PubMed.
- C. M. Galmarini, J. R. Mackey and C. Dumontet, Nucleoside analogues and nucleobases in cancer treatment, Lancet Oncol., 2002, 3, 415–424 CrossRef CAS PubMed.
- F. De Castro, E. Stefàno, E. De Luca, M. Benedetti and F. P. Fanizzi, Platinum-Nucleos(t)ide Compounds as Possible Antimetabolites for Antitumor/Antiviral Therapy: Properties and Perspectives, Pharmaceutics, 2023, 15, 941 CrossRef CAS PubMed.
- E. De Clercq, New Nucleoside Analogues for the Treatment of Hemorrhagic Fever Virus Infections, Chem. – Asian J., 2019, 14, 3962–3968 CrossRef CAS PubMed.
- N. Tsesmetzis, C. B. J. Paulin, S. G. Rudd and N. Herold, Nucleobase and Nucleoside Analogues: Resistance and Re-Sensitisation at the Level of Pharmacokinetics, Pharmacodynamics and Metabolism, Cancers, 2018, 10, 240 CrossRef PubMed.
- L. P. Jordheim, D. Durantel, F. Zoulim and C. Dumontet, Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases, Nat. Rev. Drug Discovery, 2013, 12, 447–464 CrossRef CAS PubMed.
- P. B. Matthew, M. B. Kayla and A. L. Vladislav, Base-Modified Nucleosides as Chemotherapeutic Agents: Past and Future, Curr. Top. Med. Chem., 2016, 16, 1231–1241 CrossRef PubMed.
- A. Abdullah Al Awadh, Nucleotide and nucleoside-based drugs: past, present, and future, Saudi J. Biol. Sci., 2022, 29, 103481 CrossRef CAS PubMed.
- P. Lunetti, A. Romano, C. Carrisi, D. Antonucci, T. Verri, G. E. De Benedetto, V. Dolce, F. P. Fanizzi, M. Benedetti and L. Capobianco, Platinated Nucleotides are Substrates for the Human Mitochondrial Deoxynucleotide Carrier (DNC) and DNA Polymerase γ: Relevance for the Development of New Platinum-Based Drugs, ChemistrySelect, 2016, 1, 4633–4637 CrossRef CAS.
- Q. Yang, Y. H. Nie, M. B. Cai, Z. M. Li, H. B. Zhu and Y. R. Tan, Gemcitabine Combined with Cisplatin Has a Better Effect in the Treatment of Recurrent/Metastatic Advanced Nasopharyngeal Carcinoma, Drug Des., Dev. Ther., 2023, 16, 1191–1198 CrossRef PubMed.
- A. M. Mosconi, L. Crinò and M. Tonato, Combination therapy with gemcitabine in non-small cell lung cancer, Eur. J. Cancer, 1997, 33, S14–S17 CrossRef CAS PubMed.
- L. S. Hollis, A. R. Amundsen and E. W. Stern, Chemical and biological properties of a new series of cis-diammineplatinum(II) antitumor agents containing three nitrogen donors: cis-[Pt(NH3)2(N-donor)Cl]+, J. Med. Chem., 1989, 32, 128–136 CrossRef CAS PubMed.
- F. De Castro, E. De Luca, M. Benedetti and F. P. Fanizzi, Platinum compounds as potential antiviral agents, Coord. Chem. Rev., 2022, 451, 214276 CrossRef CAS.
- K. K. Nayak, R. Bhattacharyya and P. Maity, Synthesis, characterization, and in vitro cytotoxic effects of K4[PtCl2ATP], J. Inorg. Biochem., 1991, 41, 293–298 CrossRef CAS PubMed.
- S. Kirschner, Y.-K. Wei, D. Francis and J. G. Bergman, Anticancer and potential antiviral activity of complex inorganic compounds, J. Med. Chem., 1966, 9, 369–372 CrossRef CAS PubMed.
- M. S. Ali, S. R. Ali Khan, H. Ojima, I. Y. Guzman, K. H. Whitmire, Z. H. Siddik and A. R. Khokhar, Model platinum nucleobase and nucleoside complexes and antitumor activity: X-ray crystal structure of [PtIV(trans-1R,2R-diaminocyclohexane)trans-(acetate)2(9-ethylguanine)Cl]NO3·H2O, J. Inorg. Biochem., 2005, 99, 795–804 CrossRef CAS PubMed.
- R. D. Kuchta, Nucleotide Analogues as Probes for DNA and RNA Polymerases, Curr. Protoc. Chem. Biol., 2010, 2, 111–124 CrossRef PubMed.
- D. A. Sartori, B. Miller, U. Bierbach and N. Farrell, Modulation of the chemical and biological properties of trans platinum complexes: monofunctional platinum complexes containing one nucleobase as potential antiviral chemotypes, JBIC, J. Biol. Inorg. Chem., 2000, 5, 575–583 CrossRef CAS PubMed.
- N. Margiotta, A. Bergamo, G. Sava, G. Padovano, E. de Clercq and G. Natile, Antiviral properties and cytotoxic activity of platinum(II) complexes with 1,10-phenanthrolines and acyclovir or penciclovir, J. Inorg. Biochem., 2004, 98, 1385–1390 CrossRef CAS PubMed.
- F. De Castro, E. De Luca, C. R. Girelli, A. Barca, A. Romano, D. Migoni, T. Verri, M. Benedetti and F. P. Fanizzi, First evidence for N7-Platinated Guanosine derivatives cell uptake mediated by plasma membrane transport processes, J. Inorg. Biochem., 2022, 226, 111660 CrossRef CAS PubMed.
- C. Carrisi, D. Antonucci, P. Lunetti, D. Migoni, C. R. Girelli, V. Dolce, F. P. Fanizzi, M. Benedetti and L. Capobianco, Transport of platinum bonded nucleotides into proteoliposomes, mediated by Drosophila melanogaster thiamine pyrophosphate carrier protein (DmTpc1), J. Inorg. Biochem., 2014, 130, 28–31 CrossRef CAS PubMed.
- M. Benedetti, C. Ducani, D. Migoni, D. Antonucci, V. M. Vecchio, A. Ciccarese, A. Romano, T. Verri, G. Ciccarella and F. P. Fanizzi, Experimental evidence that a DNA polymerase can incorporate N7-platinated guanines to give platinated DNA, Angew. Chem., Int. Ed. Engl., 2008, 47, 507 CrossRef CAS PubMed.
- B. Lippert and P. J. Sanz Miguel, Beyond sole models for the first steps of Pt-DNA interactions: Fundamental properties of mono(nucleobase) adducts of PtII coordination compounds, Coord. Chem. Rev., 2022, 465, 214566 CrossRef CAS.
- Y. Lu, Y. Liu, Z. Xu, H. Li, H. Liu and W. Zhu, Halogen bonding for rational drug design and new drug discovery, Expert Opin. Drug Discovery, 2012, 7, 375–383 CrossRef CAS PubMed.
- A. Sarkar, S. Acharya, K. Khushvant, K. Purkait and A. Mukherjee, Cytotoxic RuII-p-cymene complexes of an anthraimidazoledione: halide dependent solution stability, reactivity and resistance to hypoxia deactivation, Dalton Trans., 2019, 48, 7187–7197 RSC.
- S. K. Goetzfried, P. Kapitza, C. M. Gallati, A. Nindl, M. Cziferszky, M. Hermann, K. Wurst, B. Kircher and R. Gust, Investigations of the reactivity, stability and biological activity of halido (NHC)gold(I) complexes, Dalton Trans., 2022, 51, 1395–1406 RSC.
- J. J. Wilson and S. J. Lippard, Synthetic Methods for the Preparation of Platinum Anticancer Complexes, Chem. Rev., 2014, 114, 4470–4495 CrossRef CAS PubMed.
- M. Fanelli, M. Formica, V. Fusi, L. Giorgi, M. Micheloni and P. Paoli, New trends in platinum and palladium complexes as antineoplastic agents, Coord. Chem. Rev., 2016, 310, 41–79 CrossRef CAS.
- C. Mügge, T. Marzo, L. Massai, J. Hildebrandt, G. Ferraro, P. Rivera-Fuentes, N. Metzler-Nolte, A. Merlino, L. Messori and W. Weigand, Platinum(II) Complexes with O,S Bidentate Ligands: Biophysical Characterization, Antiproliferative Activity, and Crystallographic Evidence of Protein Binding, Inorg. Chem., 2015, 54, 8560–8570 CrossRef PubMed.
- B. Lippert, R. Pfab and D. Neugebauer, The role of N(1) coordianted thynine in ‘platinum thymine blue’, Inorg. Chim. Acta, 1979, 37, L495–L497 CrossRef CAS.
- V. X. Jin, S. I. Tan and J. D. Ranford, Platinum(II) triammine antitumour complexes: structure–activity relationship with guanosine 5′-monophosphate (5′-GMP), Inorg. Chim. Acta, 2005, 358, 677–686 CrossRef CAS.
- M. Masuda, T. Suzuki, M. D. Friesen, J.-L. Ravanat, J. Cadet, B. Pignatelli, H. Nishino and H. Ohshima, Chlorination of Guanosine and Other Nucleosides by Hypochlorous Acid and Myeloperoxidase of Activated Human Neutrophils: Catalysis by Nicotine and Trimethylamine, J. Biol. Chem., 2001, 276, 40486–40496 CrossRef CAS PubMed.
- M. Benedetti, F. De Castro, P. Papadia, D. Antonucci and F. P. Fanizzi, 195Pt and 15N NMR Data in Square Planar Platinum(II) Complexes of the Type [Pt(NH3)X] (X = Combination of Halides): “NMR Effective Molecular Radius” of Coordinated Ammonia, Eur. J. Inorg. Chem., 2020, 2020, 3395–3401 CrossRef CAS.
- M. Benedetti, F. de Castro, D. Antonucci, P. Papadia and F. P. Fanizzi, General cooperative effects of single atom ligands on a metal: a 195Pt NMR chemical shift as a function of coordinated halido ligands’ ionic radii overall sum, Dalton Trans., 2015, 44, 15377–15381 RSC.
- M. Benedetti, F. De Castro and F. P. Fanizzi, Square-Planar PtII versus Octahedral PtIV Halido Complexes: 195Pt NMR Explained by a Simple Empirical Approach, Eur. J. Inorg. Chem., 2016, 2016, 3957–3962 CrossRef CAS.
- T. G. Appleton, J. R. Hall and S. F. Ralph, Nitrogen-15 and platinum-195 NMR spectra of platinum ammine complexes: trans- and cis-influence series based on platinum-195-nitrogen-15 coupling constants and nitrogen-15 chemical shifts, Inorg. Chem., 1985, 24, 4685–4693 CrossRef CAS.
- L. Patiny and A. Borel, ChemCalc: A Building Block for Tomorrow's Chemical Infrastructure, J. Chem. Inf. Model., 2013, 53, 1223–1228 CrossRef CAS PubMed.
- S. Scoditti, V. Vigna, E. Dabbish and E. Sicilia, Iodido equatorial ligands influence on the mechanism of action of Pt(IV) and Pt(II) anti-cancer complexes: A DFT computational study, J. Comput. Chem., 2021, 42, 608–619 CrossRef CAS PubMed.
- X. Wu, L. Liu, Q. Wang, H. Wang, X. Zhao, X. Lin, W. Lv, Y. Niu, T. Lu and Q. Mei, Antitumor Activity and Mechanism Study of Riluzole and Its Derivatives, Iran. J. Pharm. Res., 2020, 19, e124401 Search PubMed.
- F. De Castro, E. Stefàno, D. Migoni, G. N. Iaconisi, A. Muscella, S. Marsigliante, M. Benedetti and F. P. Fanizzi, Synthesis and Evaluation of the Cytotoxic Activity of Water-Soluble Cationic Organometallic Complexes of the Type [Pt(η1-C2H4OMe)(L)(Phen)]+ (L = NH3, DMSO; Phen = 1,10-Phenanthroline), Pharmaceutics, 2021, 13, 642 CrossRef CAS PubMed.
- P. Skehan, R. Storeng, D. Scudiero, A. Monks, J. McMahon, D. Vistica, J. T. Warren, H. Bokesch, S. Kenney and M. R. Boyd, New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening, JNCI, J. Natl. Cancer Inst., 1990, 82, 1107–1112 CrossRef CAS PubMed.
- M. C. Alley, D. A. Scudiero, A. Monks, M. L. Hursey, M. J. Czerwinski, D. L. Fine, B. J. Abbott, J. G. Mayo, R. H. Shoemaker and M. R. Boyd, Feasibility of Drug Screening with Panels of Human Tumor Cell Lines Using a Microculture Tetrazolium Assay, Cancer Res., 1988, 48, 589–601 CAS.
- E. Stefàno, L. G. Cossa, F. De Castro, E. De Luca, V. Vergaro, G. My, G. Rovito, D. Migoni, A. Muscella, S. Marsigliante, M. Benedetti and F. P. Fanizzi, Evaluation of the Antitumor Effects of Platinum-Based [Pt(η1-C2H4-OR)(DMSO)(phen)]+ (R = Me, Et) Cationic Organometallic Complexes on Chemoresistant Pancreatic Cancer Cell Lines, Bioinorg. Chem. Appl., 2023, 2023, 5564624 Search PubMed.
- F. De Castro, M. Benedetti, G. Antonaci, L. Del Coco, S. A. De Pascali, A. Muscella, S. Marsigliante and F. P. Fanizzi, Response of Cisplatin Resistant Skov-3 Cells to [Pt(O,O′-Acac)(γ-Acac)(DMS)] Treatment Revealed by a Metabolomic 1H-NMR Study, Molecules, 2018, 23, 2301 CrossRef PubMed.
- L. G. Lavrenova, T. A. Kuz'menko, A. D. Ivanova, A. I. Smolentsev, V. Y. Komarov, A. S. Bogomyakov, L. A. Sheludyakova and E. V. Vorontsova, Synthesis and magnetic and cytotoxic properties of copper(II) halide complexes with 1,2,4-triazolo[1,5-a] benzimidazoles, New J. Chem., 2017, 41, 4341–4347 RSC.
- O. Sánchez-Guadarrama, H. López-Sandoval, F. Sánchez-Bartéz, I. Gracia-Mora, H. Höpfl and N. Barba-Behrens, Cytotoxic activity, X-ray crystal structures and spectroscopic characterization of cobalt(II), copper(II) and zinc(II) coordination compounds with 2-substituted benzimidazoles, J. Inorg. Biochem., 2009, 103, 1204–1213 CrossRef PubMed.
- T. Robak, New nucleoside analogs for patients with hematological malignancies, Expert Opin. Invest. Drugs, 2011, 20, 343–359 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5dt00616c |
| ‡ These authors contributed equally to this paper. |
| This journal is © The Royal Society of Chemistry 2025 |