B2O3 supported La0.8Sr0.2FeO3 for direct ethane oxidation into ethylene and syngas for hydroformylation synthesis†
Shan
Hu
,
Yunfei
Gao
 *,
Lu
Ding
,
Xueli
Chen
,
Weitong
Pan
and
Fuchen
Wang
*,
Lu
Ding
,
Xueli
Chen
,
Weitong
Pan
and
Fuchen
Wang
Institute of Clean Coal Technology, East China University of Science and Technology, 200237 Shanghai, PR China
First published on 20th November 2024
Abstract
Hydroformylation is an important reaction for aldehyde synthesis and requires ethylene and syngas (CO + H2) with the feedstocks. While these feedstocks are typically synthesized separately, it would be highly desirable to directly convert abundant ethane into ethylene and syngas in a single step. In this work, we loaded B2O3 onto perovskite La0.8Sr0.2FeO3 (LSF) and formed a core–shell structured catalyst, with B2O3 and Sr3B2O6 being the shell and LSF being the core. The shell structure can substantially inhibit deep oxidation of ethane into CO2 and the optimized La0.8Sr0.2FeO3@20B2O3 can achieve 69.2% ethane conversion and 88.8% ethylene + syngas selectivity at 700 °C with good stability, with a ratio of CO/H2 close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The effects of reaction temperature, space velocity, B2O3 loading, and La/Sr were also investigated. This catalyst marks a promising progress for the direct oxidation of ethane and has good potential for industrial applications.
1. The effects of reaction temperature, space velocity, B2O3 loading, and La/Sr were also investigated. This catalyst marks a promising progress for the direct oxidation of ethane and has good potential for industrial applications.
1. Introduction
The hydroformylation of olefins is considered one of the pivotal technologies in the petrochemical industry, serving as an important method for the industrial synthesis of aldehydes and alcohols.1 The products of this reaction, along with their derivatives, are extensively utilized in the manufacture of plasticizers, fabric additives, surfactants, solvents, pharmaceutical intermediates, and fragrances.2 As a representative, ethylene hydroformylation reaction (C2H4 + CO + H2 = CH3CH2CHO) requires two feedstocks, namely ethylene and syngas (CO + H2). Typically, these two raw materials are prepared separately. Syngas is a promising substitute for fossil fuels and is produced mainly from coal gasification and natural gas conversion.3,4 The primary methods for ethylene production currently include the steam cracking of naphtha and ethane and the ODHE reaction.5Recent studies reported that as compared to the conventional steam cracking process, ODHE presents several advantages including a lower reaction temperature and reduced coke formation.6 As an exothermic reaction, ODHE was reported to exhibit potential to reduce the energy consumption per ton of ethylene by 35% as compared to steam cracking.7 ODHE can also eliminate the thermodynamic obstacles of ethane cracking and enhance the overall conversion of ethane. Given the advantages of ethylene production with ODHE, current catalysts are summarized in the following paragraph.
Over the past two decades, numerous catalysts have been demonstrated to be active for ODHE, including NiO-based catalysts,8,9 Cr-based catalysts,10 Fe-based catalysts11 and M1 phase-based catalysts with a common formula of (TeO)x(Mo, V, Nb)5O14 (ref. 12). The majority of ODHE reactions are conducted at temperatures ranging from 400 to 700 °C in the presence of gaseous oxygen over these catalysts. Among those, the M1-based catalysts were reported to be more promising for ODHE due to their lower reaction temperature and high ethylene yield. It was reported that the ethylene selectivity was 90% at an ethane conversion of 78% at a relatively low temperature of 400 °C and a short contact time of 5.5 s. It was reported that the VMoNbTe mixed oxide transformed into active catalytic phases under reaction atmospheres and conditions.12 However, it is noted that one of the main challenges of ODHE is still the excessive oxidation of ethylene, which results in a significant production of CO and CO2.13,14
Besides M1-based ODHE catalysts, perovskite-based metal oxide catalysts (ABO3) are also considered as promising due to their adjustable ethylene selectivity granted by different A/B element doping and surface modifications. ABO3 perovskite catalysts are typically composed of lanthanides or alkaline earth metals such as La and Sr in the A sites and transition metals such as Fe and Mn in the B sites. It has been well acknowledged that the unique structure and oxygen vacancies of perovskite make it an effective catalyst for redox reactions.15 Gao et al.16 reported that molten Li2CO3 covered LSF is beneficial for the ODHE. The molten layer facilitates the transport of reactive peroxide O22− species formed on the LSF while blocking the non-selective site. Dai et al.17 reported that halogen doping can further reduce the deep oxidation of C2H4 and thus enhance C2H4 selectivity and the resulting La1−xSrxFeO3−δXδ (X = F, Cl) can achieve an ethane conversion of 76.8% and an ethylene selectivity of 62.1% at 660 °C. However, the stability of these redox catalysts is questionable as halogens may be replaced by oxygen and eventually disappear. These studies demonstrated that the ODHE performances of perovskite catalysts can be improved by either doping or surface promotions.
In addition to conventional metal oxides mentioned above, boron-based non-metallic materials have also gained considerable attention as a novel catalytic material for oxidative dehydrogenation reactions. Studies have indicated that non-metallic B-based materials, such as BN,18–22 SiB6,23 and B2O3,21,24–26 are capable of catalyzing the oxidative dehydrogenation of C2–C4 alkanes into alkenes, with a notably low selectivity for CO2. Tian26 reported that B2O3-based catalysts are also selective in the direct conversion of methane to HCHO and CO with surface tri-coordinated BO3 units being the active sites for methane oxidation. Noting that the melting point of boron oxide is only 450 °C, it has been generally reported that a molten layer of B2O3 is present under working conditions (at 500–700 °C) for the enhanced ODHE selectivities. Metal oxides such Al2O3,27 ZrO2,28 TiO2,29 SiO2,24 and MgO have also been employed to support the molten B2O3. It was reported that the construction of a boron oxide-supported catalyst could accommodate the surface BOx species and contributes to the stability and dispersion of surface BOx.
Although ODHE and the effects of BOx in catalytic oxidation reactions have been well studied, there are very few studies that report the direct oxidative conversion of ethane into hydroformylation-ready products, namely ethylene and syngas with H2/CO = 1/1. We note that the direct conversion of ethane into ethylene + syngas would be very beneficial for the hydroformylation reaction with reduced product separation cost and process complexities. In this study, a core–shell structure catalyst, with B2O3 supported on the surface of La0.8Sr0.2FeO3 (LSF) was synthesized. B2O3 loading alters the oxygen species on the catalyst and inhibits the overoxidation of ethane into CO2, leading to the co-production of ethylene and syngas with H2/CO = 1/1. The catalyst structure of B2O3-loaded LSF was further explored using characterization methods such as TEM and XRD. The catalytic effects of different loadings of B2O3 in LSF and different ratios of La to Sr elements in LSF were also investigated and studied.
2. Experimental
2.1 Preparation of catalysts
All the chemical reagents, including La(NO3)2·6H2O, Fe(NO3)3·9H2O, Sr(NO3)2, H2BO3, citric acid, and polyethylene glycol, were analytical grade and purchased from Shanghai Aladdin Chemical Reagents Co. China. LSF was prepared by the sol–gel method. La(NO3)3·6H2O, Sr(NO3)2, Fe(NO3)3·9H2O, citric acid and ethylene glycol were used for the synthesis. La(NO3)3·6H2O, Sr(NO3)2 and Fe(NO3)3·9H2O with the required La/Sr/Fe molar ratio were dissolved in deionized water under continuous stirring to acquire a homogeneous solution. Then, citric acid was added to the solution, heated at 50 °C, and stirred for 30 min. Then, ethylene glycol was added into the solution, heated, and stirred at 80 °C until gel formation. The molar ratio of citric acid to metal ions (La3+ + Sr2+ + Fe3+) was kept at 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. And the molar ratio of ethylene glycol to citric acid was kept at 2
1. And the molar ratio of ethylene glycol to citric acid was kept at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. After drying at 105 °C for 24 h, the prepared sample was annealed at 950 °C for 12 h in air to obtain LSF.
1. After drying at 105 °C for 24 h, the prepared sample was annealed at 950 °C for 12 h in air to obtain LSF.
The B2O3-covered catalyst was prepared using the impregnation method, with LSF@B2O3 as an illustrative example. A quantitative amount of H3BO3 was dissolved in deionized water (10 mL), and the resulting aqueous solution was added dropwise to pure LSF (0.5 g). After vigorous stirring for 1 hour, the impregnated samples underwent multiple dips and were dried at 105 °C for 8 hours. The prepared product was then calcined at 650 °C in air for 5 hours. The B2O3-based LSF catalysts will be referred to as LSF@5B2O3, LSF@10B2O3 and LSF@20B2O3 based on different loadings of B2O3. LSF@20B2O3 indicates that LSF is loaded with B2O3 with a mass fraction of 20%.
2.2 Catalytic testing
The ODHE experiment was carried out under ambient pressure in a micro-vertical fixed-bed quartz U-tube reactor. The reactor system for catalyst testing is presented in Fig. 1. The reaction gases, namely C2H6, O2, and Ar, were mixed in the reactor and the flow rate was regulated through a flow meter, and then the gases were introduced from the top of the fixed-bed quartz tube reactor. The gas generated by the reaction was detected using a gas chromatograph/mass spectrometer. A redox catalyst weighing 0.7 g was loaded into a U-shaped tube with dimensions of 300 mm in length, 10 mm in outer diameter, and 3 mm in inner diameter. Quartz wool was loaded on the bottom of the catalyst bed to prevent catalyst particles from entering the gas chromatograph. In the beginning of the reaction, a 100% argon purge gas was introduced for 30 min with a flow rate of 50 mL min−1. The ratio of ethane to oxygen was determined to be 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by the previous literature and pre-experimentation. Then, a mixed gas of 22.2% ethane, 11.1% oxygen, and 66.7% argon were fed into the reactor with a flow rate of 33.75 to 73.75 mL min−1 depending on different space velocities. The reactor temperature was then raised to initiate the reaction. The optimal reaction conditions were studied using different reaction temperatures (500 °C, 550 °C, 600 °C, 650 °C, 700 °C) and different mass space velocities. The reaction gas products were passed directly into the gas chromatograph (GC) with a model of Clarus 690 GC (PerkinElmer) for measurements. The GC has two thermal conductivity detector (TCD) channels and a flame ionization detector (FID) for hydrocarbon analysis.
1 by the previous literature and pre-experimentation. Then, a mixed gas of 22.2% ethane, 11.1% oxygen, and 66.7% argon were fed into the reactor with a flow rate of 33.75 to 73.75 mL min−1 depending on different space velocities. The reactor temperature was then raised to initiate the reaction. The optimal reaction conditions were studied using different reaction temperatures (500 °C, 550 °C, 600 °C, 650 °C, 700 °C) and different mass space velocities. The reaction gas products were passed directly into the gas chromatograph (GC) with a model of Clarus 690 GC (PerkinElmer) for measurements. The GC has two thermal conductivity detector (TCD) channels and a flame ionization detector (FID) for hydrocarbon analysis.
Conversion and selectivity were calculated as follows. Conversion is defined as the moles of carbon converted divided by the total moles of carbon in the feed. The carbon balance is defined as the total moles of carbon at the reaction outlet divided by the total moles of carbon in the feed gas. The specific calculation formulas for the main reactant conversion rate and product selectivity are as follows:
 | (1.1) |
 | (1.2) |
 | (1.3) |
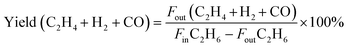 | (1.4) |
Here, Fin and Fout represent the volume contents (%) of the components at the inlet and outlet of the reactor.
2.3 Materials characterization
The composition and phase of the catalyst were characterized by X-ray powder diffraction (XRD). The XRD analysis was carried out on a Rigaku Smartlab diffractometer using a Co Kα radiation source at 40 kV and 40 mA. The 2θ value range was set between 10 and 80 degrees, with a scanning speed of 6 degrees per minute and a scanning step of 0.02 degrees. The crystallite size is acquired by the Scherrer equation. The study investigated the effects of active metal and B2O3 doping on the transformation of the catalyst's crystal form. X-ray photoelectron spectroscopy (XPS) was utilized to identify the elements in the catalyst and their contents (with a sensitivity of 0.1 at%), and the chemical state of the elements was further determined by the shift in photoelectron kinetic energy. XPS spectra were recorded using monochromatic Al X-rays on a Thermo Scientific K-Alpha ESCALAB 250XI instrument, and the binding energy scale was calibrated with C 1s (284.8 eV). Temperature-programmed hydrogen reduction (H2-TPR) was performed on a PX100A chemical absorption analyzer equipped with a U-shaped fixed bed reactor and a thermal conductivity detector. Typically, 50 mg of catalyst is calcined at 105 °C in a N2 atmosphere for 1 hour, then the reactor is cooled to 50 °C, and then the reactor is heated to 1000 °C at a heating rate of 10 °C min−1. The total gas flow rate of H2/N2 is 50 mL min−1. H2-TPR is used to detect the distribution and content of metal-loaded species in active metal oxide catalysts.3. Results and discussion
3.1 Determination of the core–shell structure
XRD was first conducted to determine the crystal structures of LSF and B2O3 supported LSF. Fig. 2 shows the XRD patterns of LSF, LSF@5B2O3, LSF@10B2O3 and LSF@20B2O3. Based on the standard database (ICDD File No. 00-035-1480), it was confirmed that the desired LSF perovskite structure was formed in all the samples. The characteristic peak of the parent LSF phase was gradually widened with the increasing amount of B2O3 loadings, indicating a decrease of grain size after supporting with B2O3. The grain sizes of LSF with different B2O3 loadings were calculated via the Scherrer equation, and the common Scherrer formula is:D = Kλ/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) θ) |
| Catalysts | LSF | LSF@5B2O3 | LSF@10B2O3 | LSF@20B2O3 |
|---|---|---|---|---|
| D (crystallite size) (nm) | 97.52 | 50.69 | 7.32 | 5.16 |
To further determine the surface compositions of LSF and B2O3 covered LSF, the XPS spectra of the LSF, LSF@10B2O3 and LSF@20B2O3 catalysts are collected and illustrated in Fig. 3. The surface atomic concentrations, obtained from XPS (Fig. 3a), were analyzed to determine the surface element composition. The metal element compositions on the surface of LSF are mainly Fe, La and Sr. After the impregnation of B2O3, the surface of the LSF@10B2O3 and LSF@20B2O3 samples is enriched with B while Fe and La are substantially suppressed.27 This indicates a surface coverage of mainly B after the B2O3 impregnation. We also note that the content of Sr on the surface remained basically unchanged. As discussed above in the XRD, Sr may react with B2O3 and form a minor Sr3B2O6 phase. Thus, the stable presence of Sr may indicate a sub-layer of Sr3B2O6 between the B2O3 coverage and the LSF surface. The surface of LSF, LSF@10B2O3, and LSF@20B2O3 were further analyzed by detailed scanning of O 1s. As shown in Fig. 3b, the O 1s peak could be fitted with three peaks. These include the lattice oxygen peak OL (O2−) at a lower binding energy,30 the adsorption peak OA (O22−/O−) at a medium binding energy, the higher lattice oxygen peak on the catalyst surface, and hydroxide and/or carbonate oxygen (OH−/CO32−) at the high binding energy peak.31 The OL/OA in LSF is calculated to be 0.77. After 10 wt% B2O3 loading, this ratio decreased substantially to 0.30. With the B2O3 loading increased to 20 wt%, only the OA peak could be observed. This indicates that only surface-absorbed oxygen species are dominant with an increased amount of B2O3, and this result is consistent with other studies.20,23,32,33 Simultaneously, the surface-absorbed oxygen species shifted from 531.1 eV in LSF and LSF@10B2O3 to a higher binding energy of 531.9 eV in LSF@20B2O3, indicating that surface-absorbed oxygen in LSF@10B2O3 is an intermediate state between LSF and LSF@20B2O3. Fig. 3c further displays the B 1s spectrum of the reduction catalyst using Al2O3@20B2O3 as a comparison. As can be seen, Al2O3@20B2O3 shows a B 1s peak near 192 eV. The peak of B at 192 eV is mainly from B2O3. Besides the 192 eV peak, B 1s peaks at 190 eV, 195 eV and 198 eV were also observed for LSF@10B2O3 and LSF@20B2O3.34 Although there are no other reports in the literature, we believe that these peaks should be assigned to the sublayer minor Sr3B2O6 phase based on the XRD and elemental compositions in Fig. 3(a). As the B2O3 loading increased from 10 wt% to 20 wt%, the peaks at 190 eV, 195 eV and 198 eV were all suppressed, indicating the thickening of the B2O3 layer. Based on these results, a schematic drawing of B2O3 covered LSF is shown in Fig. 3(d), where B2O3 covers the outer layer and a sub-layer of Sr3B2O6 exists between B2O3 and LSF.
In-depth characterization of the B2O3-loaded LSF structure was further conducted via high-resolution TEM to confirm the schematic structure in Fig. 3d. Fig. 4(a–d) depict the TEM and elemental mapping images of the catalysts. Although the B element was observed to be spreading all over the particles and did not show a clear edge, this may be attributed to the less sensitivity of B in EDS. As compared, the edges of the LSF particles are clearly more populated with Sr. This is consistent with the sub-layer of Sr3B2O6 on top of LSF, as suggested by XRD and XPS and illustrated in Fig. 3(d).
3.2 ODHE performances of B2O3 covered LSF
The ODHE performances of LSF, LSF@5B2O3, LSF@10B2O3 and LSF@20B2O3 were further tested. Fig. 5a and b illustrate the change of ethane conversion and the yield of ethylene + syngas with temperature for different B2O3 loadings. As can be seen in Fig. 5a, the ethane conversion of standalone LSF was the highest with 17.5% at 500 °C, while all other B2O3 loaded samples exhibited minimal ethane conversions below 3%. With the temperature increasing from 500 to 700 °C, the ethane conversion of LSF slightly increased to 29.66%. All B2O3 loaded samples exhibited a sharper increase of ethane conversion with respect to temperature. Moreover, the increase of B2O3 loading also leads to the increase of ethane conversion, and the ethane conversion of LSF@20B2O3 is at 62.33% in 700 °C. The yield of ethylene + syngas followed a similar trend with the ethane conversion as shown in Fig. 5b. As can be seen, the ethylene + syngas yield of LSF@20B2O3 sharply increased from 16.65% at 650 °C to 55.37% at 700 °C, which is the highest yield among all samples. The detailed product selectivity distribution obtained at 700 °C is displayed in Fig. 5c. As can be seen, there is a similar product selectivity distribution between standalone LSF, LSF@5B2O3 and LSF@10B2O3, where there is a significant amount of CO2 formed with the selectivity between 30 to 40% and the ethylene + syngas selectivity remained at around 56%. As the B2O3 loading increased to 20 wt%, the ethylene + syngas selectivity sharply increased to 88.8% and the CO2 selectivity drops to only 3.03%. Moreover, the H2/CO ratio obtained with LSF@20B2O3 was 0.89/1, very close to the H2/CO = 1/1 that is required for the hydroformylation reaction, indicating that fewer downstream cost would be needed in the case of direct ethane oxidation for the hydroformylation reaction. The effects of WHSV were also investigated. As shown in Fig. 5(d), with the increase of WHSV, the selectivity to ethylene + syngas only increased slightly, but the conversion rate of ethane decreased significantly. Given that the maximum ethylene + syngas yield occurs at 700 °C and 4.8 h−1, this temperature and WHSV will be used for testing in the subsequent section unless otherwise specified.To validate the influence of B2O3 on the reduction performance of the catalyst, the reducibility of LSF and 20% B2O3 supported catalysts was examined by H2-TPR. The results, depicted in Fig. 6, showed three prominent reduction peaks for LSF at 632 °C, 680 °C, and 833 °C, respectively. Redox catalysts employ lattice oxygen to convert hydrocarbons and the first peak could be ascribed to the reduction of Fe4+ to Fe3+, while the latter peak may be associated with the reduction of Fe3+ to Fe2+.16,35,36 For the B2O3 supported LSF catalyst, The reduction peaks at 632 °C and 680 °C have disappeared, leaving only the reduction peaks at around 833 °C. The introduction of boron moves the reduction peak to a higher temperature and concurrently decreases the number of reduction peaks. The XPS peak of Fe is shown in Fig. S2,† in which an increase in Fe3+ can be observed, which also confirms the enhancement of the signal of the reduction peak at high temperatures, which plays a facilitating role in the reaction at high temperatures. Thus, it can be seen that the presence of boron inhibits the reduction of LSF at low temperatures and is consistent with the activity trend as shown in Fig. 5.
The stability of the B2O3 supported LSF catalyst for direct oxidation of ethane was demonstrated by conducting a 400 min test at 700 °C. The experimental conditions were C2H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2
O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar = 1
Ar = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, with a WHSV of 643 mL h−1 gcat−1. The ethane conversion and product selectivity with respect to reaction time are shown in Fig. 7a. As the reaction time increases from 10 min to 60 min, the C2H6 conversion gradually decreases from 60% to 57% and was stabilized after 60 min, while the ethylene + syngas selectivity remains around 90%. It was noted that the ratio of H2/CO is also almost 1/1. This again indicates that the direct ethane oxidation can produce ethylene + syngas that is beneficial for industrial processes.
3, with a WHSV of 643 mL h−1 gcat−1. The ethane conversion and product selectivity with respect to reaction time are shown in Fig. 7a. As the reaction time increases from 10 min to 60 min, the C2H6 conversion gradually decreases from 60% to 57% and was stabilized after 60 min, while the ethylene + syngas selectivity remains around 90%. It was noted that the ratio of H2/CO is also almost 1/1. This again indicates that the direct ethane oxidation can produce ethylene + syngas that is beneficial for industrial processes.
The catalyst after 400 min of ethane direct oxidation experiment was labeled as used-LSF@20B2O3. The XPS scans of O and B elements before and after the reaction are shown in Fig. 7b. It could be seen that the XPS of O on the surface of used-LSF@20B2O3 and fresh-LSF@20B2O3 is generally consistent. As shown in Fig. 7c, the B peaks with signals of 196.5 eV and 199 eV increased slightly on used-LSF@20B2O3. From the previous analysis, it could be seen that the peaks at these two positions correspond to Sr3B2O6. The relative increase of the Sr3B2O6 characteristic peaks suggested that B2O3 is gradually vaporized from the catalyst surface during prolonged catalytic reactions, but the catalyst activity is still steady within the time of investigation. Fig. 7d presents the XRD patterns of the samples before and after the reaction. Comparison of these patterns shows only minor changes in the positions and intensities of the characteristic XRD peaks, further demonstrating the robust stability of the catalyst.
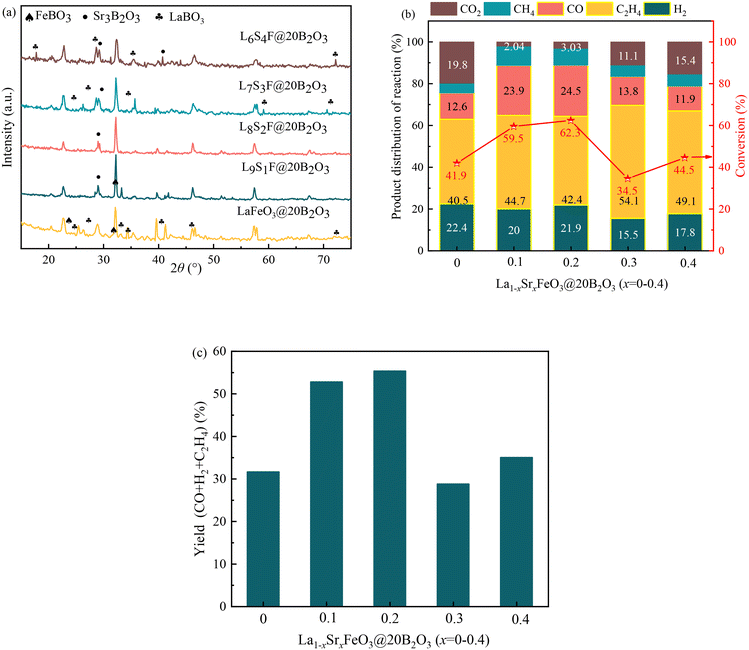
To further explore the effect of the La/Sr ratio on the catalytic performance, we have also prepared and tested LSF@20B2O3 with La/Sr = 1, 9/1, 8/2, 7/3, and 6/4. The XRD results obtained for these catalysts are illustrated in Fig. 8a. When there is no Sr in the LSF, the B2O3 loaded on the catalyst reacts with La and Fe and forms a substantial amount of LaBO3 and FeBO3. With Sr doping into the perovskite, B2O3 more preferentially reacts with Sr and forms Sr3B2O6. Fig. 8b and c show the trend of the catalytic performance with the La/Sr ratios at 700 °C. As shown in Fig. 8b, increasing the Sr content from 0 to 20% increases the ethylene + syngas selectivity from 76.9% to 88.8% and the ethane conversion from 41.9% to 62.3%. And the highest ethylene + syngas yield of 55.3% could be obtained with La0.8Sr0.2FeO3@20B2O3. Further increasing the Sr content from 20% to 40% decreases the ethylene + syngas selectivity from 88.8% to 78.8% and the ethane conversion from 62.3% to 44.5%, and the CO/H2 ratio also decreases significantly from 1.12 to 0.67.
B2O3 preferentially forms Sr3B2O6 with Sr. When the Sr content is greater than 0.2, B2O3 starts to form LaBO3 with La. The formation sequence of MexByOz is FeBO3–Sr3B2O6–LaBO3. Meanwhile, it can be seen that the catalytic effect of Sr3B2O6 is the most significant. The catalytic effect of Sr3B2O6 is the most significant, which is related to the type of metal. Therefore, a decrease in ethane conversion and ethylene + syngas yield was observed when the Sr content exceeded 0.2.
Conclusion
In this work, we have developed a B2O3 loaded La1−xSrxFeO3 catalyst for the direct oxidation of ethane into a hydroformylation-ready gas, namely ethylene and syngas with H2/CO close to 1/1. As confirmed by XRD and TEM, the catalyst has a core–shell structure with a B2O3 and Sr3B2O6 layer encapsulating a solid LSF substrate. The B2O3 loading can substantially inhibit the formation of CO2 as an overoxidized product. At a reaction temperature of 700 °C and a B2O3 loading of 20 wt%, the catalyst demonstrated excellent catalytic activity, achieving an ethane conversion rate of 62.3% and an ethylene + syngas selectivity of 88.6% with a H2/CO ratio close to 1/1. The effects of the B2O3 loading and La/Sr ratio on catalyst performance were further investigated and optimized. The optimized La0.8Sr0.2FeO3@20B2O3 catalyst showed good stability in terms of catalytic performance and material structure in the long-term test. Overall, the LSF@B2O3 catalyst can convert ethane into ethylene and syngas in a single step and is promising in terms of industrial applications.Data availability
The authors confirm that the data supporting the findings of this study are available within the article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key Research and Development Program of China (2022YFA1504701 and 2022YFB4101900), the National Natural Science Foundation of China (22208104), the Shanghai Yangfan Program (22YF1410300) and the Shanghai Chenguang Program (21CGA35).References
- K. Zhao, et al. Recent development towards alkene hydroformylation catalysts integrating traditional homo- and heterogeneous catalysis, Catal. Sci. Technol., 2022, 12, 4962–4982 RSC.
- J. Motte, et al. Developing circularity, renewability and efficiency indicators for sustainable resource management: Propanol production as a showcase, J. Cleaner Prod., 2022, 379, 134843 CrossRef CAS.
- E. Kasai, T. Kitajima, T. Akiyama, J. Yagi and F. Saito, Rate of Methane-steam Reforming Reaction on the Surface of Molten BF Slag. For Heat Recovery from Molten Slag by Using a Chemical Reaction, ISIJ Int., 1997, 37, 1031–1036 CrossRef CAS.
- P. Suparmin, N. Purwanti, L. O. Nelwan and A. H. Tambunan, Syngas production by biomass gasification: A meta-analysis, Renewable Sustainable Energy Rev., 2024, 206, 114824 CrossRef CAS.
- Y. Chen, M. J. Kuo, R. Lobo and M. Ierapetritou, Ethylene production: process design, techno- economic and life-cycle assessments, Green Chem., 2024, 26(5), 2903–2911 RSC.
- S. Dong, N. R. Altvater, L. O. Mark and I. Hermans, Assessment and comparison of ordered & non-ordered supported metal oxide catalysts for upgrading propane to propylene, Appl. Catal., A, 2021, 617, 118121 CrossRef CAS.
- T. Kahn, J. Bosch, M. F. Levitt and M. H. Goldstein, Effect of sodium nitrate loading on electrolyte transport by the renal tubule, Am. J. Physiol., 1975, 229, 746–753 CrossRef CAS PubMed.
- X. Zhang, Y. Gong, G. Yu and Y. Xie, Oxygen species on NiO/Al2O3 and their reactivities, J. Mol. Catal. A:Chem., 2002, 180, 293–298 CrossRef CAS.
- H. Zhu, et al. Metal oxides modified NiO catalysts for oxidative dehydrogenation of ethane to ethylene, Catal. Today, 2014, 228, 58–64 CrossRef CAS.
- A. Talati, M. Haghighi and F. Rahmani, Oxidative dehydrogenation of ethane to ethylene by carbon dioxide over Cr/TiO2–ZrO2 nanocatalyst: Effect of active phase and support composition on catalytic properties and performance, Adv. Powder Technol., 2016, 27, 1195–1206 CrossRef CAS.
- M. Rydén, et al. Novel oxygen-carrier materials for chemical-looping combustion and chemical-looping reforming; LaxSr1−xFeyCo1−yO3−δ perovskites and mixed-metal oxides of NiO, Fe2O3 and Mn3O4, Int. J. Greenhouse Gas Control, 2008, 2, 21–36 CrossRef.
- T. Y. Kardash, et al. The evolution of the M1 local structure during preparation of VMoNbTeO catalysts for ethane oxidative dehydrogenation to ethylene, RSC Adv., 2018, 8, 35903–35916 RSC.
- X. Tian, C. Zheng and H. Zhao, Ce-modified SrFeO3- for ethane oxidative dehydrogenation coupled with CO2 splitting via a chemical looping scheme, Appl. Catal., B, 2022, 303, 120894 CrossRef CAS.
- L. Leveles, Oxidative conversion of propane over lithium-promoted magnesia catalyst I. Kinetics and mechanism, J. Catal., 2003, 218, 296–306 CrossRef CAS.
- F. Martinovic, C. Galletti, S. Bensaid, R. Pirone and F. A. Deorsola, Soot oxidation in low-O2 and O2-free environments by lanthanum-based perovskites: structural changes and the effect of Ag doping, Catal. Sci. Technol., 2022, 12, 5453–5464 RSC.
- Y. Gao, et al. A molten carbonate shell modified perovskite redox catalyst for anaerobic oxidative dehydrogenation of ethane, Sci. Adv., 2020, 6, eaaz9339 CrossRef CAS PubMed.
- H. X. Dai, C. F. Ng and C. T. Au, Perovskite-Type Halo-oxide La1−xSrxFeO3−δXσ (X=F, Cl) Catalysts Selective for the Oxidation of Ethane to Ethene, J. Catal., 2000, 189, 52–62 CrossRef CAS.
- R. Huang, et al. Direct Insight into Ethane Oxidative Dehydrogenation over Boron Nitrides, ChemCatChem, 2017, 9, 3293–3297 CrossRef CAS.
- J. Tian, et al. Propane oxidative dehydrogenation over highly selective hexagonal boron nitride catalysts: The role of oxidative coupling of methyl, Sci. Adv., 2019, 5, eaav8063 CrossRef CAS PubMed.
- L. Shi, et al. Edge-hydroxylated Boron Nitride for Oxidative Dehydrogenation of Propane to Propylene, ChemCatChem, 2017, 9, 1788–1793 CrossRef CAS.
- Z. Liu, et al. Understanding the Unique Antioxidation Property of Boron-Based Catalysts during Oxidative Dehydrogenation of Alkanes, J. Phys. Chem. Lett., 2021, 12, 8770–8776 CrossRef CAS PubMed.
- F. Jin, et al. The role of modified manganese perovskite oxide for selective oxidative dehydrogenation of ethane: Not only selective H2 combustion but also ethane activation, Catal. Commun., 2022, 172, 106531 CrossRef CAS.
- B. Yan, W.-C. Li and A.-H. Lu, Metal-free silicon boride catalyst for oxidative dehydrogenation of light alkanes to olefins with high selectivity and stability, J. Catal., 2019, 369, 296–301 CrossRef CAS.
- W.-D. Lu, et al. Supported Boron Oxide Catalysts for Selective and Low-Temperature Oxidative Dehydrogenation of Propane, ACS Catal., 2019, 9, 8263–8270 CrossRef CAS.
- Q. Liu, et al. B2O3@BPO4 sandwich-like hollow spheres as metal-free supported liquid-phase catalysts, J. Catal., 2020, 381, 599–607 CrossRef CAS.
- J. Tian, et al. Direct conversion of methane to formaldehyde and CO on B2O3 catalysts, Nat. Commun., 2020, 11, 5693 CrossRef CAS PubMed.
- G. Colorio, J. C. Védrine, A. Auroux and B. Bonnetot, Partial oxidation of ethane over alumina-boria catalysts, Appl. Catal., A, 1996, 137, 55–68 CrossRef CAS.
- X. Hou, et al. Superiority of ZrO2 surface enrichment on ZSM-5 zeolites in n-pentane catalytic cracking to produce light olefins, Microporous Mesoporous Mater., 2019, 276, 41–51 CrossRef CAS.
- O. V. Buyevskaya, D. Müller, I. Pitsch and M. Baerns, Selective Oxidative Conversion of Propane to Olefins and Oxygenates on Boria-Containing Catalysts, in Studies in Surface Science and Catalysis, Elsevier, 1998, vol. 119, pp. 671–676 Search PubMed.
- D. Chen, et al. Investigation of the role of surface lattice oxygen and bulk lattice oxygen migration of cerium-based oxygen carriers: XPS and designed H2-TPR characterization, Appl. Catal., B, 2017, 218, 249–259 CrossRef CAS.
- N. A. Merino, B. P. Barbero, P. Eloy and L. E. Cadús, La−xCaxCoO3 perovskite-type oxides: Identification of the surface oxygen species by XPS, Appl. Surf. Sci., 2006, 253, 1489–1493 CrossRef CAS.
- J. T. Grant, et al. Boron and Boron-Containing Catalysts for the Oxidative Dehydrogenation of Propane, ChemCatChem, 2017, 9, 3622 CrossRef CAS.
- C. Xin and G. Q. Xu, Boron-doped nanocarbon catalysts for oxidative dehydrogenation of ethane to ethylene, Carbon, 2022, 193, 381–393 CrossRef CAS.
- J. Sheng, et al. Oxidative dehydrogenation of light alkanes to olefins on metal-free catalysts, Chem. Soc. Rev., 2021, 50, 1438–1468 RSC.
- Y. Gao, F. Haeri, F. He and F. Li, Alkali Metal-Promoted LaxSr2−xFeO4−δ Redox Catalysts for Chemical Looping Oxidative Dehydrogenation of Ethane, ACS Catal., 2018, 8, 1757–1766 CrossRef CAS.
- Y. Gao, L. M. Neal and F. Li, Li-Promoted LaxSr2−xFeO4−δ Core–Shell Redox Catalysts for Oxidative Dehydrogenation of Ethane under a Cyclic Redox Scheme, ACS Catal., 2016, 6, 7293–7302 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cy01268b |
| This journal is © The Royal Society of Chemistry 2025 |