Modulating the electronic interaction between Au and nitrogen-rich porous organic polymers for enhanced CO2 hydrogenation to formic acid†
Huixin
Yan
,
Xingyan
Wang
,
Xiaoyu
Liang
,
Xinxin
Zhang
,
LongFei
Liu
,
Min
Ji
 ,
Min
Wang
,
Min
Wang
 * and
Xinkui
Wang
* and
Xinkui
Wang
 *
*
School of Chemistry, Dalian University of Technology, Dalian 116024, Liaoning, China. E-mail: wangmin@dlut.edu.cn; wangxinkui@dlut.edu.cn
First published on 26th November 2024
Abstract
The regulation of the electronic state of catalytic sites is essential to improve the intrinsic activity of catalysts. Herein, we modulated the metal state of Au species by varying their particle size on nitrogen-rich triazine-based porous organic polymer supports. Due to the different interface percentages between Au and nitrogen species in selected supports, the electronic state of the metal can be modulated. The catalyst with the smallest Au particle size presented the most negative metallic state and the highest surface energy, thus exposing more sites for H2 activation and providing sufficient reactive H species to CO2 hydrogenation absorbed on the adjacent N. The designed Au/Trz-TETA (1.23 nm) exhibited high catalytic activity for the CO2 hydrogenation to formic acid and a turnover number (TON) up to 1687 over 10 h, which is higher than that of Au/Trz-DETA (2.24 nm) and Au/Trz-TEPA (1.96 nm) with a bigger metal particle size. This work shows a size-dependent CO2 hydrogenation for various sizes of Au metal catalysts and provides a new way for regulating the metal electronic state.
Introduction
The drastically increased CO2 emission attributed to the combustion of fossil fuels worldwide impacts the Earth's climatic conditions in an unprecedented fashion, leading to a realization that effective measures need to be taken to reduce CO2 emissions.1–4 In addition to CO2 capture and storage, catalytic reduction of CO2 into usable fuels and value-added chemicals has emerged as a crucial pathway to address the greenhouse effect and energy crisis. CO2 catalysis generating formic acid is highly attractive because formic acid is a green energy carrier with high density, low toxicity and high hydrogen content.5–8 Great efforts have been devoted in recent years to CO2 to formic acid catalytic conversion. However, due to the high chemical inertness of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and the difficulty of H2 activation, the conversion efficiency of CO2 cannot meet the industrial application requirement.9–11 Therefore, it is of great significance to develop novel catalysts and strategies to improve the catalytic efficiency of CO2 reduction to formic acid.
O and the difficulty of H2 activation, the conversion efficiency of CO2 cannot meet the industrial application requirement.9–11 Therefore, it is of great significance to develop novel catalysts and strategies to improve the catalytic efficiency of CO2 reduction to formic acid.
As for H2 activation, supported noble-metal catalysts such as Pd,12–14 Ru,15,16 Ir,17–19 and Au (ref. 20 and 21) have been used extensively in hydrogenation catalysis due to their high efficiency and selectivity. In most cases, only the atoms on the surface of the nanoparticles (NPs) act as the active sites, while those inside the nanoparticles are spectators, which leads to a waste of the noble metal.22 Hence, regulation of metal electronic states to possess high surface energy and synthesis of highly dispersed noble metals will be effective ways to improve the intrinsic activity and their atomic efficiency. The maximum limit of the reduction of the size of metal particles will increase the metal–support interface percentages, thus maximizing the utilization of noble metals and lowering the cost of the catalysts.23 However, when the particle size decreases, the surface energy will increase correspondingly, which leads to aggregation of the highly dispersed metal atoms. Due to thermodynamic instability and susceptibility to agglomeration, this phenomenon is particularly obvious for Au. Therefore, most of the typical supports have good catalytic activity with other noble metals such as Pd,24,25 but the results are not satisfactory when loaded with Au.
To improve the catalytic activity of supported noble-metal catalysts, it is necessary to consider the active species MNP and the support materials.26 Selecting suitable support materials and optimizing the pore size and surface properties of the support can improve the dispersion and utilization of noble metals, thus improving the cost-effectiveness of the catalysts. Given the high surface area and attractive chemical functionalization of porous organic polymers (POPs), it can be an excellent platform for anchoring and dispersing metals.27,28 MNP can be effectively stabilized on the polymer surface and resistant to aggregate.29 In particular, when organic amine functional groups are introduced into POPs, the abundant N atoms can be polydentate coordinated with metals, thus effectively solving the problem of poor metal dispersion.30 In addition, N atoms with strong electron-donating properties not only adjust the electronic state of the active metal components31,32 but also facilitate CO2 enrichment and activation,33–35 which is conducive to the follow-up of the catalytic reaction.
In this work, we achieved the modulation of the metal electronic state based on changing interface percentages between Au and nitrogen-rich triazine-based porous organic polymer supports. Specifically, three organic bases with different N contents were chosen for the synthesis of POPs to further achieve uniform dispersion of different metal sizes. The down-sized Au particles in Trz-DETA, Trz-TEPA, and Trz-TETA provided a model for us to study the size dependent CO2 hydrogenation, and the smallest Au particle size presented the most negative metallic state and the highest surface energy, thus effectively promoting the adsorption and activation of CO2 and the heterolytic dissociation of H2.
Experimental
Materials
Cyanuric chloride (99%), diethylenetriamine (DETA) (99%), triethylenetetramine (TETA) (70%), tetraethylenepentamine (TEPA) (95%), KHCO3 (99.5%) and NaHCO3 (99.5%) were purchased from Macklin Reagent Company. NaBH4 (98%), PdCl2 (Pd 59–60%), RuCl3·xH2O (Ru ≥ 37%) and D2O (99.9 atom% D) were purchased from Aladdin Corporation. Ethanol (≥99.7%) was obtained from Tianjin Fuyu Fine Chemical Co., Ltd. Methanol (99.5%), tetrahydrofuran (THF, 99.5%), triethylamine (TEA, 99%), dimethyl sulfoxide (DMSO, 99.5%) and 1,4-dioxane (99.5%) were purchased form Tianjin Kermel Chemical Reagent Development Center, China. HAuCl4 (Au ≥ 47.8%) was purchased from Sinopharm Chemical Reagent Co., Ltd. The deionized water was obtained from a Milli-Q system (Millipore). All reagents were used without further purification.Synthesis of the triazine-based N-rich porous organic polymers
The POPs were fabricated by reported methods with some modifications.36 In order to synthesize the polymer, first 8 mmol of cyanuric chloride was dissolved in 100 mL of 1,4-dioxane (solution 1) in a 250 mL round-bottomed flask. Then 64 mmol of triethylenetetramine (TETA) was added to 50 mL of 1,4-dioxane (solution 2). Under vigorous stirring, solution 2 was added dropwise to solution 1. The resulting mixture was stirred at ambient temperature for 1 h, followed by refluxing at 80 °C for 5 h. The mixture was cooled at room temperature and a white precipitate was appeared. Finally, the Trz-TETA material was obtained after the precipitate was washed several times with deionized water, 1,4-dioxane and ethanol at pH = 7 and dried at 100 °C for 10 h. The Trz-DETA and Trz-TEPA products were synthesized by a similar method as that of Trz-TETA, and were obtained using the polymerization of cyanuric chloride with diethylenetriamine (DETA) and tetraethylenepentamine (TEPA), respectively.Synthesis of Au/Trz-DETA, Au/Trz-TETA and Au/Trz-TEPA
The Au/Trz-TETA catalyst was prepared with a NaBH4 wet chemical reduction method. Typically, 0.3 g of Trz-TETA was dispersed in 100 mL of ethanol and sonicated for 15 min. Then a solution of aqueous HAuCl4 (0.9 g/50 mL, 0.35 mL) in 100 mL of ethanol was added rapidly to the above mixture at 80 °C, followed by stirring for 30 min. A 14-fold excess of NaBH4 was added and the mixture was stirred continuously for 2 h at 80 °C. After the reaction, the product was collected by filtration and washed several times with water and ethanol. Finally, Au/Trz-TETA was obtained by vacuum drying at 60 °C. Au/Trz-DETA and Au/Trz-TEPA catalysts were also prepared using the same procedure.General characterization
Powder X-ray diffraction (PXRD) was conducted on a Smartlab 9 kW (Rigaku Corporation) diffractometer at 240 kV and 50 mA Cu-Kα radiation with the scanning range was 5° to 80°. Thermogravimetric analysis (TGA) was performed using an STA 449F5 with a temperature range from 25 °C to 800 °C in a nitrogen atmosphere. Inductively coupled plasma optical emission spectrometry (ICP-OES) was performed using an Avio 220. Nitrogen adsorption–desorption isotherm and pore size distribution were taken using a BSD-PM1. Samples were treated at 150 °C for 4 h. X-ray photoelectron spectroscopy (XPS) analysis was conducted using an ESCALAB250Xi (Thermo, USA) with Al Kα (hv = 1486.6 eV) as the exciting source. The binding energies of N 1s and Au 4f can be corrected with C 1s (284.8 eV). Transmission electron microscopy (TEM) images were performed with a JEM-F200 microscope at 200 KV and an energy-dispersive X-ray spectrometer (EDX) attached to a FESEM instrument was used to analyze the compositions of the samples. Fourier transform infrared (FT-IR) spectroscopy and in situ Fourier transform infrared (in situ FT-IR) spectroscopy were conducted with a Nicolet iS10 IR spectrometer equipped with an MCT/A detector. After the sample was placed in a vacuum at 100 °C for 40 min and cooled to room temperature, the background spectra were recorded at a resolution of 4 cm−1, and then automatically subtracted from the subsequent spectra. Then the sample was exposed to CO2 gas for 30 min, after which the CO2 was desorbed. Finally, the sample was exposed to H2 gas and heated to 100 °C, recording the spectra at intervals. 1H NMR spectra were determined with a NEO 400 MHz NMR spectrometer.General procedure for CO2 hydrogenation to formic acid
The CO2 hydrogenation to formic acid reaction was carried out using a 30 mL stainless steel autoclave reactor. In a typical experiment, 10 mg of catalyst was added to aqueous triethylamine solution, after which the reactor was sealed and the air was replaced three times with pure CO2. The reactor was pressurized with CO2 to 2.0 MPa and then pressurized with H2 to 5 MPa, and subsequently heated to 100 °C and stirred for 10 h. When the reaction was finished, the reactor was cooled to room temperature and the pressure was slowly released. The catalyst was separated from the reaction solution by centrifugation. Formic acid concentrations were measured via the internal standard method of 1H NMR, using DMSO as the internal standard and D2O as the solvent.Results and discussion
Synthesis and characterization of the catalysts
To adjust the particle size of Au, three organic bases DETA, TETA, and TEPA with different N contents were selected to synthesize POPs and coordinate with metals. The typical synthetic scheme was illustrated in Fig. 1a; three secondary amine-rich triazine-based porous organic polymer supports were synthesized by nucleophilic substitution reaction of cyanuric chloride with organic bases. Then, a simple impregnation method was used to deposit Au on the supports, and the composites were obtained by NaBH4 reduced. The composites were named Au/Trz-TETA, Au/Trz-TEPA, and Au/Trz-DETA, respectively. The metal loadings were measured by inductively coupled plasma optical emission spectrometry (ICP-OES) analysis, and the results were similar to the theoretical value at ca.1 wt% (Table S1†).The successful synthesis of the porous organic polymers was confirmed by the Fourier transform infrared spectroscopy (FT-IR) results. In the FT-IR spectrum of POPs (Fig. 1b), the peak at 3360 cm−1 corresponded to the N–H stretching vibration. Notably, the vibrational bands at 2939, 2832, and 1424 cm−1 were associated with the stretching and bending vibrations of the aliphatic C–H. Focusing on the region 1600–800 cm−1, the peaks at 1540 and 1507 cm−1 belonged to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N of the triazine ring, which appeared as double peaks due to the presence of tautomeric forms (Fig. S1†). The vibrational band ascribed to the out-of-plane vibration of the triazine ring was also clearly observed at 810 cm−1.37 Besides, the C–N stretching vibration that was not inside the ring can be seen at 1130 cm−.1 However, the absence of a peak related to the C–Cl stretching at 850 cm−1 was accordant with the fact that the chlorine of cyanuric chloride was completely substituted by the amino groups of the aminating agents.38,39 The PXRD patterns (Fig. 1c) showed that the three samples had a broad peak spanning a 2θ range of ca. 21°, confirming that all the POP-based catalysts were amorphous. It is worth noting that due to the low content and high dispersity, there were no detectable peaks corresponding to Au nanoparticles. Thermogravimetric analysis (TGA) results (Fig. 1d) demonstrated the similar thermal stability of the three samples in a nitrogen atmosphere. The first weight loss before 100 °C was related to the loss of water from the materials. The second and third weight loss at around 400 and 500 °C were associated with the degradation of the amino groups and the decomposition of the triazine rings, respectively. Since the CO2 hydrogenation reaction was carried out at 100 °C, the composites were able to maintain the structure for our subsequent catalyst-performance relationships analysis.
N of the triazine ring, which appeared as double peaks due to the presence of tautomeric forms (Fig. S1†). The vibrational band ascribed to the out-of-plane vibration of the triazine ring was also clearly observed at 810 cm−1.37 Besides, the C–N stretching vibration that was not inside the ring can be seen at 1130 cm−.1 However, the absence of a peak related to the C–Cl stretching at 850 cm−1 was accordant with the fact that the chlorine of cyanuric chloride was completely substituted by the amino groups of the aminating agents.38,39 The PXRD patterns (Fig. 1c) showed that the three samples had a broad peak spanning a 2θ range of ca. 21°, confirming that all the POP-based catalysts were amorphous. It is worth noting that due to the low content and high dispersity, there were no detectable peaks corresponding to Au nanoparticles. Thermogravimetric analysis (TGA) results (Fig. 1d) demonstrated the similar thermal stability of the three samples in a nitrogen atmosphere. The first weight loss before 100 °C was related to the loss of water from the materials. The second and third weight loss at around 400 and 500 °C were associated with the degradation of the amino groups and the decomposition of the triazine rings, respectively. Since the CO2 hydrogenation reaction was carried out at 100 °C, the composites were able to maintain the structure for our subsequent catalyst-performance relationships analysis.
To reveal the morphological and structural features of the POP-based catalysts, transmission electron microscopy (TEM) was performed. It can be seen from Fig. 2a–c that all the catalysts exhibited porous morphology, which was favorable to the exposure of active sites. To measure the size of Au nanoparticles (NPs), aberration-corrected high-angle annular dark-field STEM (HAADF-STEM) was conducted (Fig. 2d–f). The Au NPs were uniformly distributed on the surface and in the pores of the POPs, with an average particle size of 1.23 nm, 1.96 nm, and 2.24 nm for Trz-TETA, Trz-TEPA and Trz-DETA respectively. It is noteworthy that the particle size of Au on Trz-TEPA was mainly concentrated at 1–2 nm, but a few Au particles larger than 4 nm appeared. This is because TEPA contains more N atoms compared with TETA and DETA; a large number of amine groups contained in the Trz-TEPA support have inherent weak reducibility. In the process of preparing Au/Trz-TEPA, the reducing property of Trz-TEPA can reduce some of the Au precursors to obtain the larger-sized Au particles before the addition of NaBH4.40 The EDS mapping image (Fig. 2g, S2†) clearly shows the presence of C, N, and Au atoms, further confirming the existence and uniform distribution of Au NPs. Based on the above results, we can conclude that both components and structural parameters in all composites were fixed to be similar, with the particle size of Au NPs being the only difference. Obviously, because of the downsize of Au NPs, the interface percentage between Au and the support will increase, thus modulating the electronic state of the metal by metal–support interactions, which may significantly improve catalytic performance.41
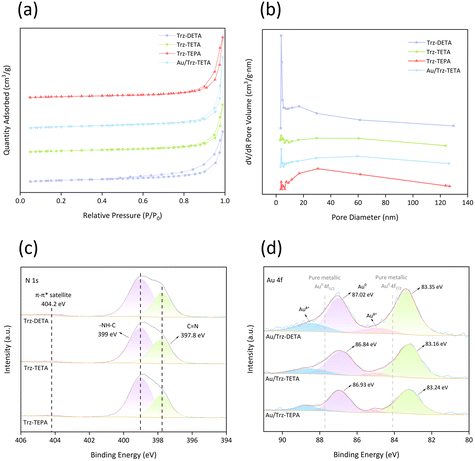
Even if Au was loaded in the POPs, the porous property of Trz-TETA was still retained, as revealed by nitrogen sorption isotherms. As shown in Fig. 3a, all sorption isotherm materials can be categorized as type I and IV according to the IUPAC,42 suggesting the existence of microporous and mesoporous structures, which is essential for CO2 adsorption and activation. Notably, there was no significant change in the specific surface area and pore size between Au/Trz-TETA and Trz-TETA (Fig. 3a, Table S2†), indicating that Au NPs were uniformly dispersed in the catalyst support without blocking the pores, which was in agreement with the observation of TEM. However, the larger pore size distribution (Fig. 3b, Table S2†) observed for Trz-TEPA compared to that for Trz-TETA and Trz-DETA may also account for the bigger particle size of Au NPs. Besides, the appropriate specific surface area and pore size of the catalyst support can increase the loading points of the active components and expose more active sites to increase the reactivity, but too large pore sizes may lead to the inability of reactant molecules to remain on the inner surface of the catalyst for a long time.
To gain the electronic state of Au NPs and to understand the metal–support interactions, X-ray photoelectron spectroscopy (XPS) analysis was performed (Fig. S3b†). In the N 1s spectra (Fig. 3c), in addition to the two peaks at 399 eV (–NH–) and 397.8 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N of the triazine ring), the weak characteristic peak of the π–π* satellite at 404.2 eV suggested the formation of nitrogen-containing aromatic polymers, which may provide delocalized electrons for metal.43 As expected, the values of the binding energy for the Au 4f line were found to decrease to different degrees compared to metallic Au (87.7 eV (4f5/2) and 84.1 eV (4f7/2)),44,45 ascribing to the abundant N species on the POP surface (Fig. 3d). Among them, Au/Trz-TETA exhibited the most negative metallic state, because with decreasing Au particle size, the number of Au coordination atoms decreases significantly, and Au atoms with higher interfacial free energy can have stronger electronic interactions with amine groups and thus show stronger electronegativity. This electron-rich active center was beneficial for the heterolytic dissociation of H2 and provides sufficient active H species for subsequent intermediate hydrogenation.
N of the triazine ring), the weak characteristic peak of the π–π* satellite at 404.2 eV suggested the formation of nitrogen-containing aromatic polymers, which may provide delocalized electrons for metal.43 As expected, the values of the binding energy for the Au 4f line were found to decrease to different degrees compared to metallic Au (87.7 eV (4f5/2) and 84.1 eV (4f7/2)),44,45 ascribing to the abundant N species on the POP surface (Fig. 3d). Among them, Au/Trz-TETA exhibited the most negative metallic state, because with decreasing Au particle size, the number of Au coordination atoms decreases significantly, and Au atoms with higher interfacial free energy can have stronger electronic interactions with amine groups and thus show stronger electronegativity. This electron-rich active center was beneficial for the heterolytic dissociation of H2 and provides sufficient active H species for subsequent intermediate hydrogenation.
Catalytic CO2 hydrogenation to formic acid performance
Given the property of CO2 absorption and H2 dissociation of catalysts, the catalytic performance was investigated for the preparation of formic acid by the CO2 hydrogenation reaction. To explore the intrinsic activity of the metal and to circumvent the effect of particle size, we screened for optimal active sites by loading different metals on Trz-DETA (Fig. S5a†). The catalytic activity was conducted in triethylamine aqueous solution under a total pressure of 5.0 MPa (CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 = 2
H2 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) at 100 °C for 10 h. Compared with other metals, the POPs with Au NPs exhibited the highest catalytic activity and the TON achieved can be up to 502, and the catalytic activity of the support without metals loaded was negligible. Afterward, different Au loading amounts were also studied in Au/Trz-DETA. As shown in Fig. S5b,† the TON value of the CO2 hydrogenation reached a maximum of 736 at 1.0 wt% Au, but decreased with further increase in metal content due to the Au agglomeration. The characteristic peak belonging to Au (111) that can be seen at 2θ = 38° in the PXRD pattern of Au/Trz-DETA at 2.0 wt% metal loading confirms the above conjecture (Fig. S3a†). Then, to explore the effect of the electronic state on CO2 hydrogenation, the catalytic activity of different supports with 1.0 wt% Au was investigated. As revealed in Fig. 4a, the TON of Au/Trz-DETA and Au/Trz-TEPA with Au sizes of 2.24 nm and 1.96 nm was 736 and 978 respectively. Significantly, Au/Trz-TETA exhibited the highest catalytic activity (TON = 1687), which is 2.29 and 1.72 times as high as that of Au/Trz-DETA and Au/Trz-TEPA with a bigger metal particle size. Formic acid was obtained with >99% selectivity, and other products were not observed (Fig. S4†). Considering that the gap between the specific surface areas was much smaller than the gap between their activities, we can determine that the performance disparity stemmed from the intrinsic catalytic ability of different catalytic sites, in the sense that the high hydrogenation activity of Au/Trz-TETA was attributed to the more negative electronic state of Au. Moreover, the trend of the performance in CO2 hydrogenation increases as the electron density in Au increases (observed in XPS), as well as the opposite tendency for the particle size (observed in TEM) confirm above results. Then, we performed cycling stability experiments on Au/Trz-TETA for further testing. From Fig. S6a,† we can see that the stability of Au/Trz-TETA was poor and the activity no longer decreased after four cycles, which could be attributed to the sintering of the Au nanoparticles. The growth of Au NPs from 1.23 nm to 3.94 nm can be seen in the HAADF-STEM image after Au/Trz-TETA cycling (Fig. S6b†), which is consistent with the observation in the above relationship between the performance and Au particle size. In conclusion, the smallest size and the most negative metallic state of Au in Au/Trz-TETA was beneficial for CO2 reduction, which may be achieved by promoting H2 heterolytic dissociation, the decisive step of the whole hydrogenation reaction (discussed later).
3) at 100 °C for 10 h. Compared with other metals, the POPs with Au NPs exhibited the highest catalytic activity and the TON achieved can be up to 502, and the catalytic activity of the support without metals loaded was negligible. Afterward, different Au loading amounts were also studied in Au/Trz-DETA. As shown in Fig. S5b,† the TON value of the CO2 hydrogenation reached a maximum of 736 at 1.0 wt% Au, but decreased with further increase in metal content due to the Au agglomeration. The characteristic peak belonging to Au (111) that can be seen at 2θ = 38° in the PXRD pattern of Au/Trz-DETA at 2.0 wt% metal loading confirms the above conjecture (Fig. S3a†). Then, to explore the effect of the electronic state on CO2 hydrogenation, the catalytic activity of different supports with 1.0 wt% Au was investigated. As revealed in Fig. 4a, the TON of Au/Trz-DETA and Au/Trz-TEPA with Au sizes of 2.24 nm and 1.96 nm was 736 and 978 respectively. Significantly, Au/Trz-TETA exhibited the highest catalytic activity (TON = 1687), which is 2.29 and 1.72 times as high as that of Au/Trz-DETA and Au/Trz-TEPA with a bigger metal particle size. Formic acid was obtained with >99% selectivity, and other products were not observed (Fig. S4†). Considering that the gap between the specific surface areas was much smaller than the gap between their activities, we can determine that the performance disparity stemmed from the intrinsic catalytic ability of different catalytic sites, in the sense that the high hydrogenation activity of Au/Trz-TETA was attributed to the more negative electronic state of Au. Moreover, the trend of the performance in CO2 hydrogenation increases as the electron density in Au increases (observed in XPS), as well as the opposite tendency for the particle size (observed in TEM) confirm above results. Then, we performed cycling stability experiments on Au/Trz-TETA for further testing. From Fig. S6a,† we can see that the stability of Au/Trz-TETA was poor and the activity no longer decreased after four cycles, which could be attributed to the sintering of the Au nanoparticles. The growth of Au NPs from 1.23 nm to 3.94 nm can be seen in the HAADF-STEM image after Au/Trz-TETA cycling (Fig. S6b†), which is consistent with the observation in the above relationship between the performance and Au particle size. In conclusion, the smallest size and the most negative metallic state of Au in Au/Trz-TETA was beneficial for CO2 reduction, which may be achieved by promoting H2 heterolytic dissociation, the decisive step of the whole hydrogenation reaction (discussed later).
To further optimize the reaction conditions, control experiments were conducted. It has been demonstrated that the protonated solvent can facilitate the CO2 reduction to formic acid. Solvents act as H-transfer reagents and participate in the reaction by transferring H radicals to CO2, thus achieving the hydrogenation process.46 Based on this (Fig. 4b), we investigated the effect of solvent polarity on the catalytic performance of Au/Trz-TETA. The results showed that with the increase of solvent polarity, the catalytic activity of the catalyst in THF, ethanol, methanol, and water increased successively, and similar patterns were also observed for Au/Trz-DETA and Au/Trz-TEPA (Fig. S7†). The higher the polarity of the solvent, the higher the yield of CO2 hydrogenation to formic acid or formate.20 This is due to the fact that the reaction intermediates generated by the interaction of CO2 with nitrogen species on the catalyst surface have high polarity, so the polar solvent has a good stabilizing effect on these intermediate species.47 However, the non-protonic solvent DMSO showed poor CO2 hydrogenation activity despite its high polarity, which emphasizes the importance of the H-transfer solvent in our system. In addition, the vital role of H2 pressure was also suggested by the observation of a decreased trend in formic acid yield as the H2 partial pressure decreases (Fig. 4c).
Thermodynamic and kinetic studies were carried out to gain further insight into the catalytic performance of the catalyst. Based on Arrhenius plots as depicted in Fig. 4d, the apparent activation energy (Ea) of Au/Trz-TETA in CO2 hydrogenation to formic acid was determined and the Ea value was 26.8 kJ mol−1. Furthermore, the reaction kinetics of Au/Trz-TETA was also illustrated. The reaction rate (v) was expressed by v = k PH2m PCO2n, where m and n were the reaction orders of PCO2 and PH2, respectively. It can be seen from Fig. 4e that the reaction orders of H2 and CO2 were 0.92 and 0.44, respectively, which indicates that H2 is involved in the reaction and is the rate-controlling step of the reaction.
Possible reaction mechanism for the CO2 hydrogenation
To further understand the reaction mechanism, we compared the catalytic performance of Au/Trz-DETA using KHCO3 and NaHCO3 as carbon sources, respectively. In Fig. 4f, the catalytic ability of the catalyst for hydrogenation of bicarbonate species was very weak, but the catalytic performance was greatly improved when 2 MPa CO2 was added. Therefore, the bicarbonates in the system only act as base additives to promote the formation of formic acid. This fully indicated that Au/Trz-TETA catalyzed the direct hydrogenation of CO2 to formic acid, which was different from the bicarbonate hydrogenation mechanism in conventional catalytic systems.48 Then, the specific reaction mechanism of CO2 hydrogenation to formic acid on Au/Trz-TETA was investigated by in situ Fourier-transform infrared (in situ FT-IR) experiments under the reaction conditions (Fig. 5a). Upon the introduction of CO2 and H2 on Au/Trz-TETA, the characteristic peaks of NH2+ species (1633 and 1504 cm−1),49 COO− species (symmetric stretching vibration peak at 1426 cm−1 and asymmetric stretching vibration peak at 1565 cm−1),50,51 carbamate species (1666 cm−1)52 and formate species (1393 and 1605 cm−1)53,54 can be observed. With increasing reaction time, the peak intensity of COO− and carbamate species decreased continuously, while that of formic acid increased, proving that the hydrogenation reaction of CO2 on the catalyst followed the path of carbamate hydrogenation. | ||
| Fig. 5 (a) In situ FT-IR spectra of Au/Trz-TETA. (b) Possible mechanism for the CO2 hydrogenation to formic acid over Au/Trz-TETA. | ||
Based on the above results, we propose a possible reaction mechanism of CO2 hydrogenation on Au/Trz-TETA. As depicted in Fig. 5b, H2 is activated and dissociated on Au NPs, resulting in the generation of reactive Au hydride species. At the same time, CO2 is adsorbed and activated by the amine sites of Trz-TETA in the form of carbamate zwitterion species (RR′N+H⋯COO−) and concentrated near Au NPs. In this alkaline environment, the H atom of the unstable intermediate is immediately transferred to hydroxyl or neighboring amine groups to form carbamate species (RR'NCOO− N+H2RR′) (Fig. S8†). Then, the Au hydride species perform a nucleophilic attack to the C atom of the carbamate intermediate to yield a formate intermediate. In particular, due to the strong electron-donating ability of the abundant N atoms on Trz-TETA, the electron-rich Au NPs offer more negative hydride species, which is conducive to the nucleophilic attack to the C atom. Finally, formic acid is formed by the acid–base neutralization reaction, and the original Au/Trz-TETA catalyst is regenerated for the next catalytic cycle reaction.
Conclusions
In conclusion, we achieved the regulation of the metal electronic state by selecting different N contents of organic bases in the synthesized POPs to disperse Au in different sizes. The catalyst with the smallest Au particle size exhibited the highest performance of CO2 hydrogenation due to the most negative metallic state and the highest surface energy. The abundant amine functional groups on the POPs not only facilitated the Au NPs to obtain more electrons for H2 activation but also promoted the adsorption and activation of CO2 in a near position of reactive H species, thus favoring efficient CO2 hydrogenation. This work provides a simple and effective strategy for the modulation of the metal electronic state and offers insight into the construction of supported Au catalysts in the field of CO2 hydrogenation catalysis.Data availability
All relevant data are within the manuscript and its additional files.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21978032, 21676045, 22372023, and 22178040) and the Natural Science Foundation of Liaoning Province (2022-MS-142). The authors acknowledge the assistance of DUT Instrumental Analysis Center.Notes and references
- R. Fouquet and R. Hippe, Energy Res. Soc. Sci., 2022, 91, 102736 CrossRef.
- S. Li, J. Zhang, H. Lin, Y. Ding, Y. Bai, Y. Zhou, B. Zhu and Z. Dai, Coal Sci. Technol., 2024, 52, 138–153 CAS.
- Z. Zhang, T. Wang, M. J. Blunt, E. J. Anthony, A.-H. A. Park, R. W. Hughes, P. A. Webley and J. Yan, Appl. Energy, 2020, 278, 394–427 Search PubMed.
- S. Kar, M. Rahaman, V. Andrei, S. Bhattacharjee, S. Roy and E. Reisner, Joule, 2023, 7, 1496–1514 CrossRef CAS.
- K. Park, K. R. Lee, S. Ahn, C. Van Nguyen and K.-D. Jung, Appl. Catal., B, 2024, 346, 123751 CrossRef CAS.
- R. Sun, Y. Liao, S.-T. Bai, M. Zheng, C. Zhou, T. Zhang and B. F. Sels, Energy Environ. Sci., 2021, 14, 1247–1285 RSC.
- H. Chai, C. Zhou, S. Li, R. Zhang, J. Yuan, J. Hu, Z. Liu, A. Duan, C. Xu and X. Wang, Fuel, 2024, 371, 131908 CrossRef CAS.
- Z. D. Feng, C. Z. Tang, P. F. Zhang, K. Li, G. N. Li, J. J. Wang, Z. C. Feng and C. Li, J. Am. Chem. Soc., 2023, 145, 12663–12672 CrossRef CAS PubMed.
- X. San, X. Li, L. Zhang, D. Meng, X. Chang and J. Qi, Process Saf. Environ. Prot., 2024, 187, 320–331 CrossRef CAS.
- W. Lyu, Y. Liu, J. Zhou, D. Chen, X. Zhao, R. Fang, F. Wang and Y. Li, Angew. Chem., Int. Ed., 2023, 62, e202310733 CrossRef CAS PubMed.
- H. Zhou, H. Zhang, S. Mu, W.-Z. Zhang, W.-M. Ren and X.-B. Lu, Green Chem., 2019, 21, 6335–6341 RSC.
- P. Verma, S. Zhang, S. Song, K. Mori, Y. Kuwahara, M. Wen, H. Yamashita and T. An, J. CO2 Util., 2021, 54, 101765 CrossRef CAS.
- G. Ren, J. Sun, S. Zhai, L. Yang, T. Yu, L. Sun and W. Deng, Cell Rep. Phys. Sci., 2022, 3, 100705 CrossRef CAS.
- Z. Wang, D. Ren, Y. He, M. Hong, Y. Bai, A. Jia, X. Liu, C. Tang, P. Gong, X. Liu, W. Huang and Z. Zhang, ACS Catal., 2023, 13, 10056–10064 CrossRef CAS.
- J. Zhao, J. Wang, Y. Bai, H. Du, J. Yang, B. Yin, B. Jiang and H. Li, J. CO2 Util., 2023, 74, 102528 CrossRef CAS.
- K. R. Lee, A. Masudi, K. Park, S. Ahn, J. S. Lee, S. J. Sim and K.-D. Jung, Chem. Eng. J., 2024, 494, 152922 CrossRef CAS.
- X. Shao, X. Yang, J. Xu, S. Liu, S. Miao, X. Liu, X. Su, H. Duan, Y. Huang and T. Zhang, Chem, 2019, 5, 693–705 CAS.
- R. Kanega, M. Z. Ertem, N. Onishi, D. J. Szalda, E. Fujita and Y. Himeda, Organometallics, 2020, 39, 1519–1531 CrossRef CAS.
- B. D. Bankar, K. Ravi, R. J. Tayade and A. V. Biradar, J. CO2 Util., 2023, 67, 102315 CrossRef CAS.
- G. A. Filonenko, W. L. Vrijburg, E. J. M. Hensen and E. A. Pidko, J. Catal., 2016, 343, 97–105 CrossRef CAS.
- Q. Liu, X. Yang, L. Li, S. Miao, Y. Li, Y. Li, X. Wang, Y. Huang and T. Zhang, Nat. Commun., 2017, 8, 1407 CrossRef PubMed.
- K. Murata, E. Eleeda, J. Ohyama, Y. Yamamoto, S. Arai and A. Satsuma, Phys. Chem. Chem. Phys., 2019, 21, 18128–18137 RSC.
- X. Sui, L. Zhang, J. Li, K. Doyle-Davis, R. Li, Z. Wang and X. Sun, Adv. Energy Mater., 2022, 12, 2102556 CrossRef CAS.
- K. Mori, H. Hata and H. Yamashita, Appl. Catal., B, 2023, 320, 122022 CrossRef CAS.
- S. Ali, R. Iqbal, A. Khan, S. U. Rehman, M. Haneef and L. Yin, ACS Appl. Nano Mater., 2021, 4, 6893–6902 CrossRef CAS.
- T. Parandhaman, M. D. Dey and S. K. Das, Green Chem., 2019, 21, 5469–5500 RSC.
- K. S. Song, P. W. Fritz and A. Coskun, Chem. Soc. Rev., 2022, 51, 9831–9852 RSC.
- B. Dziejarski, J. Serafin, K. Andersson and R. Krzyzynska, Mater. Today Sustain., 2023, 24, 100483 Search PubMed.
- S. Aggarwal and S. K. Awasthi, Dalton Trans., 2024, 53, 11601–11643 RSC.
- D. Yadav, Subodh and S. K. Awasthi, Mater. Chem. Front., 2022, 6, 1574–1605 RSC.
- B. Panic, T. Frey, M. Borovina, K. Konopka, M. Sambolec, I. Kodrin and I. Biljan, Polymers, 2023, 15, 229 CrossRef CAS PubMed.
- N. Gemo, F. Menegazzo, P. Biasi, A. Sarkar, A. Samikannu, D. G. Raut, K. Kordas, A.-R. Rautio, M. Mohl, D. Bostroem, A. Shchukarev and J.-P. Mikkola, RSC Adv., 2016, 6, 103311–103319 RSC.
- M. Zheng, J. Yang, W. Fan and X. Zhao, Phys. Chem. Chem. Phys., 2021, 23, 24801–24813 RSC.
- Y. Li, T. Ding, X. Xu, S. Chakir, Y. Ding, S. Zhu, J. Mei, H. Wang and X. Wang, J. Environ. Chem. Eng., 2024, 12, 113197 CrossRef CAS.
- T. Guo, Y. Zhang, Y. Geng, J. Chen, Z. Zhu, A. H. Bedane and Y. Du, Ind. Crops Prod., 2023, 206, 117582 CrossRef CAS.
- M. Niksefat, J. Rahimi, A. Maleki and A. S. Nia, Algal Res., 2023, 70, 103003 CrossRef.
- M. J. Bojdys, J. Jeromenok, A. Thomas and M. Antonietti, Adv. Mater., 2010, 22, 2202–2205 CrossRef CAS PubMed.
- P. Puthiaraj and K. Pitchumani, Green Chem., 2014, 16, 4223–4233 RSC.
- M. Sayin, M. Can, M. Imamoglu and M. Arslan, React. Funct. Polym., 2015, 88, 31–38 CrossRef CAS.
- Q. Liu, X. Wang, Y. Ren, X. Yang, Z. Wu, X. Liu, L. Li, S. Miao, Y. Su, Y. Li, C. Liang and Y. Huang, Chin. J. Chem., 2018, 36, 329–332 CrossRef CAS.
- W. Zhan, Y. Shu, Y. Sheng, H. Zhu, Y. Guo, L. Wang, Y. Guo, J. Zhang, G. Lu and S. Dai, Angew. Chem., Int. Ed., 2017, 56, 4494–4498 CrossRef CAS PubMed.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87, 1051–1069 CrossRef CAS.
- J. A. Gardella, S. A. Ferguson and R. L. Chin, Appl. Spectrosc., 1986, 40, 224–232 CrossRef CAS.
- C. J. Powell, N. E. Erickson and T. Jach, J. Vac. Sci. Technol., 1982, 20, 625–625 CrossRef.
- T. D. Thomas and P. Weightman, Phys. Rev. B: Condens. Matter Mater. Phys., 1986, 33, 5406–5413 CrossRef CAS PubMed.
- Z. Wang, Y. Kang, J. Hu, Q. Ji, Z. Lu, G. Xu, Y. Qi, M. Zhang, W. Zhang, R. Huang, L. Yu, Z.-q. Tian and D. Deng, Angew. Chem., Int. Ed., 2023, 62, e202307086 CrossRef CAS PubMed.
- W.-H. Wang, J. T. Muckerman, E. Fujita and Y. Himeda, ACS Catal., 2013, 3, 856–860 CrossRef CAS.
- K. Mori, T. Sano, H. Kobayashi and H. Yamashita, J. Am. Chem. Soc., 2018, 140, 8902–8909 CrossRef CAS PubMed.
- F. Baitalow, J. Baumann, G. Wolf, K. Jaenicke-Rößler and G. Leitner, Thermochim. Acta, 2002, 391, 159–168 CrossRef CAS.
- G. S. Foo, J. J. Lee, C.-H. Chen, S. E. Hayes, C. Sievers and C. W. Jones, ChemSusChem, 2017, 10, 266–276 CrossRef CAS PubMed.
- M. W. Hahn, J. Jelic, E. Berger, K. Reuter, A. Jentys and J. A. Lercher, J. Phys. Chem. B, 2016, 120, 1988–1995 CrossRef CAS PubMed.
- Y. Kuwahara, Y. Fujie, T. Mihogi and H. Yamashita, ACS Catal., 2020, 10, 6356–6366 CrossRef CAS.
- A. Karelovic and P. Ruiz, ACS Catal., 2013, 3, 2799–2812 CrossRef CAS.
- L. Fan, J. Zhang, K. Ma, Y. Zhang, Y.-M. Hu, L. Kong, A.-p. Jia, Z. Zhang, W. Huang and J.-Q. Lu, J. Catal., 2021, 397, 116–127 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cy01151a |
| This journal is © The Royal Society of Chemistry 2025 |