Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Catalytic hydrodenitrogenation of primary, secondary, and tertiary C12-alkyl amines over a platinum on zirconia catalyst†
Leoni-Franziska
Klingelhöfer
ab,
Joakim
Kattelus
 *a,
Emma
Verkama‡
*a,
Emma
Verkama‡
 a,
Jorge A.
Velasco
a,
Jorge A.
Velasco
 a,
Leonhard
Iser
b,
Marcus
Rose
a,
Leonhard
Iser
b,
Marcus
Rose
 b,
Reetta
Karinen
b,
Reetta
Karinen
 a and
Riikka L.
Puurunen
a and
Riikka L.
Puurunen
 a
a
aSchool of Chemical Engineering, Aalto University, Finland. E-mail: joakim.kattelus@aalto.fi
bErnst-Berl-Institute of Technical and Macromolecular Chemistry, Department of Chemistry, Technische Universität Darmstadt, Germany
First published on 30th December 2024
Abstract
In this work, the hydrodenitrogenation (HDN) of a primary amine (dodecylamine), a secondary amine (didodecylamine), and a tertiary amine (tridodecylamine) over a Pt/ZrO2 catalyst was compared in a batch reactor. The main product of the amine hydrotreating was dodecane, but significant amounts of secondary amine were also formed as an intermediate during HDN of the primary and the tertiary amine. It was found that the primary amine is the only species for which direct HDN is possible; HDN of the secondary amine thus proceeds through a primary amine intermediate and HDN of the tertiary amine involves formation of the secondary amine, which decomposes to the primary amine. Consequently, HDN of the tertiary and secondary amines is slower than that of the primary amine. Kinetic modeling indicated that bimolecular condensation reactions of the primary amine, as well as potentially of the primary amine and the secondary amine, have a significant effect on the HDN process. Formation of the secondary amine from the primary amine increases the initial conversion and nitrogen removal rate but appeared to slow down the overall rate of nitrogen removal. The results thus demonstrate how condensation reactions affect amine HDN, which has implications for catalyst design for HDN of renewable feeds containing aliphatic amines.
Introduction
Replacing fossil fuels with sustainable, renewable options is an important strategy for lowering CO2 emissions and reducing the impact of the transportation sector on global warming. While electrification shows great promise in reducing emissions from the transportation sector in general, finding sustainable options for the aviation sector is particularly challenging, due to the need for fuels with high-energy density.1 Sustainable, bio-based fuels are thus a particularly promising option for the aviation sector.1 According to the International Air Transportation Association,2 bio-based fuels are estimated to contribute up to 65% to the reduction of greenhouse gas emissions in the aviation sector by 2050. High energy density drop-in fuels can be used in existing fleet, and thus make the transition towards a sustainable economy easier. The European Commission passed a mandate on 18 October 2023 for blending fossil based fuel with sustainable aviation fuel (SAF) gradually from 2% in 2025 to 63% in 2050.3 Estimates made by Klöwer et al.4 emphasize the importance of increasing the production of renewable fuels even faster, as an increase of bio-based fuels up to 90% by 2050 is needed to limit aviation transport effects on global warming.Hydrotreating is a commercially viable process to produce bio-based fuels from renewable feedstocks.5,6 For example, Neste Corporation and Eni S.p.A. commercially produce diesel and jet fuel from vegetable oils and fats via hydrotreating.6–8 Likewise, hydrotreating is a viable strategy to upgrade bio-based feedstocks produced through pyrolysis or liquefaction of biomass.5,9 During hydrotreating, heteroatoms, such as S, N, and O, are removed in the form of H2S, NH3, H2O, CO2 and CO by bringing the feedstock into contact with hydrogen in the presence of a catalyst.5,6 Oxygen-containing compounds, which are prevalent in renewable feedstocks, lower the pH value and cause corrosion, polymerization and fuel instability.10 Nitrogen-containing compounds can poison acidic catalysts in downstream processes such as reforming, hydrocracking and hydroisomerization, as well as impact the fuel stability negatively.5,11,12
With the change from fossil to bio-based feedstock, the composition of the hydrotreating feed differs, especially regarding the oxygen and nitrogen content but also the types of compounds present. For example, algae-based feeds can contain up to 35 wt% oxygen and up to 10 wt% nitrogen, while for bio-oils from pyrolysis and liquefaction, the heteroatom content can range between 9–38 wt% oxygen and 6–10 wt% nitrogen.5,6,9,13 In comparison, fossil-based feeds usually contain less than 2 wt% oxygen and less than 1 wt% nitrogen.9,14 This change in feed composition has led to increasing interest in hydrodeoxygenation (HDO).9,15–17 However, as removing nitrogen has been shown to be more difficult, studying hydrodenitrogenation (HDN) is of equal importance.18 In bio-based feedstocks like vegetable oils, animal fats, and algae, nitrogen is mostly found in the form of fatty amides, alkyl amines, cyclic amines, and amino acids.5,12,19–21
Supported noble metal catalysts, such as Ru, Rh, Pd, Pt, have been shown to be active in HDO and HDN of fatty acids, amides and amines.18,22–30 They show high HDO/HDN activity under mild conditions, with the metal component significantly impacting the product selectivity.11,22,29–31 As noble metal catalysts are active in their reduced state, they, in contrast to the commercially used transition metal sulfide catalysts, do not require sulfur additions to maintain their activity.5,9,11,18,22,24,31,32 For HDN of amines and amides, reduced noble metal catalysts display activity towards the formation of ammonia and paraffins.22,23,26 Secondary dialkylamines are also readily formed over most of the studied metals.18,22–24,26 Depending on the noble metal, the dialkylamine formation may exceed the paraffin formation.22 The formation of trialkylamines, imines, nitriles and olefins during amine HDN has also been reported, although in smaller quantities than the secondary amine formation.11,18,22,23
This study aims to compare the HDN reaction network and kinetics of primary, secondary, and tertiary alkyl amines over the Pt/ZrO2 catalyst. Furthermore, the goal is to investigate the role of the condensation products in the overall HDN reaction network. Therefore, dodecylamine (C12 amine), didodecylamine (C24 amine), and tridodecylamine (C36 amine) were hydrotreated at 80 bar H2 over Pt/ZrO2 at a reaction temperature of 300 °C in a batch reactor. The amines were chosen as model compounds for alkyl amines which are found in bio-based feeds and are also formed as intermediates during hydrotreating of nitrogen containing compounds.5,12,26 The experimental concentration–time data was used for kinetic modeling with an isothermal power law approach to gain further insight into the reactivity of the model compounds.
Experimental
Materials
For the catalyst preparation platinum(IV) nitrate (15 wt% Pt) from Alfa Aesar was used as a metal precursor. For the support, monoclinic zirconia (ZrO2) from Saint-Gobain NorPro (SZ 31164) was used. The following chemicals were used for the reactor experiments without further purification: dodecylamine (>99.0%, Sigma Aldrich), didodecylamine (>97.0%, Sigma Aldrich), tridodecylamine (>97.0%, Sigma Aldrich), decalin (decahydronaphthalene, mixture of cis and trans, >99%, Sigma Aldrich), nitrogen (99.999%, Woikoski), and hydrogen (99.999%, Woikoski). The same hydrogen gas was also used for the analytics. Furthermore, synthetic air (99.999%), helium (99.999%), argon (99.999%) and oxygen (99.999%) from Woikoski were used. n-pentadecane (>99%, Sigma Aldrich) was used as an internal standard and propan-2-ol (>99.5%, Fisher Chemicals) as a second solvent for the GC analytics.Catalyst preparation
The Pt/ZrO2 catalyst was synthesized with a vacuum impregnation method according to Verkama et al.18 The support (ZrO2) was crushed, sieved (particle size of sieve 0.25–0.45 mm), and calcined for 10 h at 600 °C in a static muffle furnace. For the impregnation, 2.5 g of ZrO2 was dried in a 100 mL round-bottom flask at 60 °C for 90 min under vacuum in a rotary evaporator. The metal precursor was dissolved in type 1 ultra-pure water with approximately four times the pore volume of the support. The precursor solution was added to the support at room temperature, under vacuum and stirring. The solution was stirred for 2 h under vacuum, allowing the excess impregnation solution to slowly evaporate, and the catalyst precursor was dried the following day under vacuum for 60 min at 40 °C and 30 min at 60 °C. The Pt catalyst was calcined in a flow through calcination oven at 100 ml min−1 in synthetic air at 450 °C for 1 h with a heating ramp of 2 °C min−1.Catalyst characterization
Carbon analysis
A Thermo Flash Smart CHNSO Elemental Analyzer was used to determine the amount of carbon present in the spent catalysts. 2,5-Bis(5-tert-butyl-benzoxazol-2-yl)thiophene (BBOT) was used as a calibration standard. The temperature of the (left) furnace was 950 °C, the temperature of the oven was 65 °C, the carrier gas flow was 140 ml min−1, the reference gas flow was 100 ml min−1, the oxygen flow was 250 ml min−1, the oxygen injection end time was 4 s, the sampling delay time was 12 s and the run time was 750 s.Catalytic activity tests
For the experiments a 100 mL Hastelloy batch reactor from Parr Instrument Company was used. For the amine HDN experiments, 20 mg of the Pt/ZrO2 catalyst was dried in situ at 180 °C and 10 bar N2 for 60 min. The catalyst was then reduced in situ at 350 °C and 20 bar H2 for 60 min while stirring with 100 rpm.The reaction mixture was prepared as a solution of the respective alkyl amine in 27.8 g decalin, targeting a total initial nitrogen concentration of 100 ppm (mg L−1). Therefore, the mass of the alkyl amines varied accordingly, with 41.0 mg for dodecylamine, 78.3 mg for didodecylamine, and 115.5 mg for tridodecylamine. The reaction mixture was heated (to approximately 80 °C) under constant stirring to dissolve the amine in the solvent. Before the reaction, 1 mL of the reaction mixture (zero-sample) was taken to quantify the reactants. The reaction mixture was inserted into the pre-heated feed vessel (heater set to 100 °C) and released into the reactor, which had been heated to the reaction temperature of 300 °C. For the reaction, the reactor was pressurized at 80 bar H2 and the stirring set to 600 rpm, marking the start of the reaction. The reaction time varied between 15 min and 300 min, corresponding to a batch residence time τ of 0–500 gcat h molN, feed−1. The stirring was stopped, and the reaction was quenched with an ice bath after the reaction time elapsed. Finally, 1 mL of sample (reaction-sample) was taken for analysis.
The 60 min reaction for dodecylamine was repeated three times as a control experiment. To test for thermal activity of each amine, the procedure was repeated without the catalyst, the drying and reduction, and with a reaction time of 60 min. The activity of the ZrO2 support was tested with the same procedure as for the amine HDN experiments using ZrO2 instead of the catalyst with a reaction time of 60 min.
Product analysis
To avoid precipitation of the products and reactants in the 1 mL analysis samples, 0.18 mL propan-2-ol was added as a second solvent before the analysis. As an internal standard (ISTD) 6 μL n-pentadecane was also added beforehand.Qualitative analysis of reaction products
The samples were analyzed with gas chromatography with a mass spectrometer (GC–MS) using Shimadzu's GCMS-QP2010 SE equipped with a HP-5MS column (30 m × 0.250 mm × 0.25 μm) by Agilent J&W GC Columns to identify reactants and products using multiple programs.Quantification of liquid products
The reaction products were quantified with gas chromatography (GC), using an Agilent Technologies 7890B GC System with a flame ionization detector (FID) and a nitrogen–phosphorus detector (NPD) and using an Agilent J&W HP1-MS column (60 m × 0.25 mm × 0.25 μm). Two GC-FID methods were used for the product quantification, and details on these methods can be found in the ESI.†For determining the reactant and product concentrations with the FID, the relative response factors (RRF) of dodecylamine, didodecylamine, n-dodecane, 1-dodecan-ol and isopropyl–dodecylamine were estimated based on their combustion enthalpy and weight, using a method developed by de Saint Laumer et al.35,36 For tridodecylamine, an experimental calibration for the RRF was made, and the results can be found in the ESI.†
The carbon balance closure BC (%) for each amine HDN experiment was calculated using eqn (1)
 | (1) |
Carbon-based yields YC (%) for each of each product species were calculated using eqn (2)
 | (2) |
Total nitrogen content analysis
The nitrogen content (ppm) of the samples was measured with a P422022 ElemeNts nitrogen analyzer from PAC L.P. The injection volume for the analysis was 20 μL and each sample was measured three times.The nitrogen removal Nremoval (%) was calculated from both the GC-FID and N-analyzer results using eqn (3)
 | (3) |
Kinetic modeling
Kinetic modeling of the amine HDN batch reactions was done using Jupyter Notebook 6.5.4 from Python 3.11. The concentration/time profiles of the reactants were mathematically modeled assuming power law kinetics. The optimal set of kinetic parameters was obtained using the scipy.optimize.least_squares37,38 solver. The parameter bounds for all reaction rate constants were set to 0–1. Several different initial values for the parameters were tested, with no significant effect on the fitting results.Results & discussion
Catalyst preparation and characterization
A summary of the catalyst characterization results is shown in Table 1. The data shows that the Pt/ZrO2 catalyst has the targeted metal loading, the pore volume was 0.25 cm3 g−1 and the BET surface area was 38.9 m2 g−1. The surface area and pore volume of the support did not change significantly during impregnation, calcination, and reduction of the catalyst. Based on the CO chemisorption results, the Pt particle size was 3.8 nm and the dispersion was 29%. X-ray diffraction showed only the peaks associated with the m-ZrO2 support (ICDD 00-065-0687) (see the ESI†). This implies that no large, XRD-visible Pt crystallites were present, which was in good agreement with the CO chemisorption results. Thus, it appears that the catalyst synthesis was successful, and the obtained Pt/ZrO2 catalyst was well-dispersed and had the correct metal loading.| Sample | Pore volume (cm3 g−1) | BET surface area (m2 g−1) | Pt (wt%) | Pt particle size (nm) | Dispersion |
|---|---|---|---|---|---|
| m-ZrO2 | 0.26 | 38 | — | — | — |
| Pt/ZrO2 | 0.25 | 39 | 1.0 | 3.8 | 29% |
HDN experiments
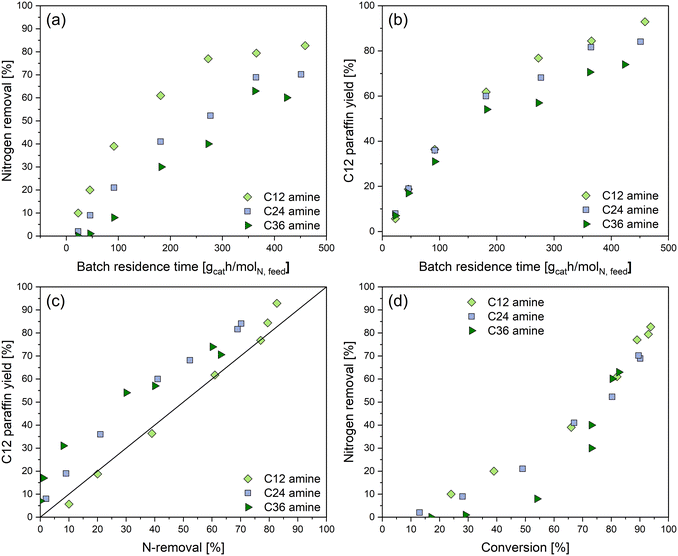
Fig. 1 shows the composition of the product samples and the nitrogen removal as a function of batch residence time for the primary, secondary, and tertiary amines' hydrotreating experiments. The data shows that the Pt/ZrO2 catalyst is active for the HDN of dodecylamine (C12 amine), didodecylamine (C24 amine), and tridodecylamine (C36 amine). The final product for hydrotreating of all three amines is dodecane (C12 paraffin). The secondary amine appears to be a major intermediate during HDN of both primary and tertiary amines, while the primary amine is formed as an intermediate during HDN of the secondary and tertiary amines. Small amounts of the tertiary amine and dodecan-1-ol (C12 alcohol) were also present as intermediates. In addition to these compounds, small amounts of an acetone-derived side product were detected (N-isopropyldodecan-1-amine). It is proposed to have formed by carbonyl–amine condensation of dodecylamine and acetone. Acetone was a residue from cleaning the feed vessel and reactor, thus it does not belong to the HDN network of the primary, secondary, and tertiary alkylamines. The carbon balance closure of all amine HDN experiments over Pt/ZrO2 ranged from 92–105%, indicating that there were no major unaccounted products. Details on all the experiments, including the carbon balances and data for three repeat experiments performed under the same conditions, are given in the ESI.†Based on the data shown in Fig. 1, there are clear differences in the reactivity of the primary, secondary, and tertiary amines. The low batch residence time data in Fig. 1a–c indicates that the secondary amine is an initial product during hydrotreating of both primary amine and tertiary amines. For the secondary amine hydrotreating, less than 5% of primary amine is present as an intermediate. It is also clear that the secondary amine can be formed from a condensation reaction between two molecules of the primary amine, as has been reported before.22,23,29,39 However, only trace amounts of tertiary amine were detected during the primary and secondary amine experiments. This may indicate that a condensation reaction between the primary and secondary amine is not favored, or that the tertiary amine formed in such a reaction is rapidly converted to other products.
Fig. 1 also shows that at higher batch residence times, the conversion rate of the amines slows down, and the intermediates are consumed as dodecane, the final product, is formed. Generally, it appears that amine HDN occurs much slower at higher batch residence times, which can be explained by a lower concentration of reactants. However, deactivation cannot be ruled out based on the experimental data. A CHNS analysis of the spent catalyst showed no evidence for deactivation by coking, as the carbon content of the spent catalyst used in the secondary amine experiments did not appear to increase at higher batch residence times (see the ESI†).
Fig. 2 shows nitrogen removal (a) and paraffin yield (b) for the three amines as a function of batch residence time, as well as the paraffin yield as a function of nitrogen removal (c) and nitrogen removal as a function of conversion (d). On the one hand, as shown in Fig. 1 and 2a, conversion of primary amine is slightly faster than conversion of the tertiary and secondary amines, while the nitrogen removal decreases in the order primary amine > secondary amine > tertiary amine. On the other hand, the paraffin yield is essentially the same for all three amines at batch residence times below 100 gcat h molN, feed−1 (see Fig. 2b), although at higher batch residence time the tertiary amine has a lower paraffin yield than the secondary and primary amines.
Thus, it appears that for the primary amine, the paraffin is formed concurrently with nitrogen removal, while for both secondary and tertiary amines, paraffin is formed initially, before nitrogen removal takes place. This is supported by Fig. 2c, which shows the paraffin yield as a function of the nitrogen removal. For the primary amine, the paraffin yield initially has a linear relationship with the nitrogen removal, showing that both paraffin formation and nitrogen removal initially take place. However, for secondary and tertiary amines the increase in paraffin yield, as nitrogen removal increases, is steeper, indicating that paraffin is formed before nitrogen removal takes place. Indeed, as shown in Fig. 2d, nitrogen removal of the tertiary amine increases slowly as the conversion increases, showing that the initial tertiary amine conversion step does not involve nitrogen removal. Thus, the data presented in Fig. 1 and 2 indicates that there are clear differences in how HDN proceeds for the primary, secondary, and tertiary amines.
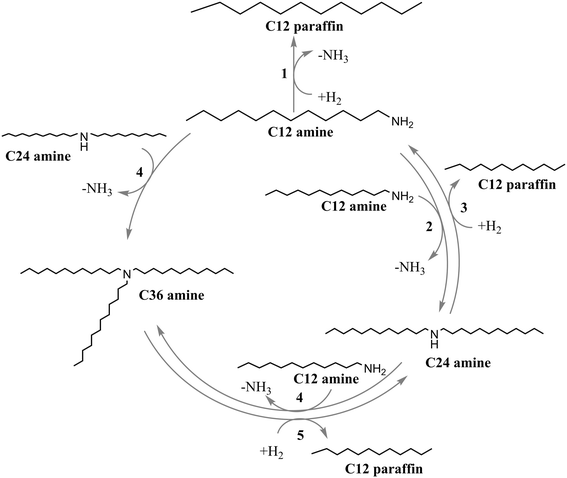
Based on the data shown in Fig. 1, it appears that the primary and secondary amines are always present as intermediates during HDN of primary, secondary, and tertiary amines, while the tertiary amine is not formed in significant amounts. As indicated by the conversion, nitrogen removal and paraffin yield data, HDN is fastest for the primary amine, while the tertiary amine and the secondary amine form paraffins faster than they undergo HDN. Based on these observations, it is deemed likely that the HDN of the tertiary amine occurs through the secondary amine, while the HDN of the secondary amine involves the primary amine as an intermediate. Thus, the reaction network shown in Fig. 3 is proposed.
In the proposed reaction network (Fig. 3), the primary amine can react directly through hydrodenitrogenation (1) or undergo a condensation reaction (2) to form the secondary amine. The secondary amine decomposes via hydrogenolysis into dodecane and primary amine (3). While a condensation reaction between the primary amine and secondary amine (4) may seem feasible, no evidence for formation of significant amounts of tertiary amine was detected in the primary and secondary amine experiments. This may be due to this condensation reaction not being favored, or due to the tertiary amine decomposing via hydrogenolysis into dodecane and secondary amine (5) as quickly as it is formed.
The proposed reaction network shown in Fig. 3 is well in line with the observed conversion trend. Conversion of the primary amine can take place through one of two mechanisms: direct HDN (1) and condensation (2), while conversion of secondary and tertiary amines takes place through a C–N bond hydrogenolysis step. The trend in the nitrogen removal can also be explained: all initial reaction steps for the primary amine (1, 2, 4) involve the loss of nitrogen, while nitrogen removal with the secondary amine first involves formation of the primary amine (3), and nitrogen removal with the tertiary amine occurs through secondary amine and primary amine intermediates (5, 3). Indeed, nitrogen removal occurs only through the primary amine, either through condensation reactions (2, 4) or through direct HDN (1).
The proposed reaction network is well in line with previous studies investigating the reactions of amines over noble metals. Condensation reactions between primary amines, forming secondary amines, have long been known to take place in the presence of hydrogen and a metal catalyst. For example, in 1932 Winans and Adkins39 reported the formation of dipentylamine from pentylamine using nickel catalysts, and in 1986 Meitzner et al.22 studied the reactions of methylamine on Pt/SiO2, and found that significant amounts of dimethylamine were formed. More recent work by Verkama et al.29 confirmed that such condensation reactions also can take place on Pt/ZrO2 for tetradecylamine and hexadecylamine, which have carbon chain lengths more relevant for industrial HDN. The hydrotreating reactions of secondary and tertiary amines have also been studied by Sivasankar et al.,23 who showed that on Pd/Al2O3, dipentylamine may undergo hydrogenolysis to form pentylamine and pentane/pentene, as well as also undergo a bimolecular reaction to form a primary amine and tertiary amine. In this study, we found that the hydrogenolysis of didodecylamine takes place on Pt/ZrO2 but found no evidence for a bimolecular reaction between two molecules of didodecylamine. Sivasankar et al.23 also found that tri-pentylamine reacted exclusively through hydrogenolysis to dipentylamine and olefin/paraffin,23 which matches well the results obtained in this study for hydrotreating of tridodecylamine.
Kinetic modeling
Kinetic modeling was carried out with the aim to quantitatively describe the data with a suitable physical kinetic model based on the proposed reaction mechanism. For the kinetic modeling, power law kinetics were assumed. The model assumed constant concentration of hydrogen in the reaction mixture, since hydrogen was present in large excess. The power law model was isothermal and did not consider mass transfer or diffusion limitations. The kinetic modeling was done based on the reaction network shown in Fig. 3, and also includes the condensation (6) of two molecules of secondary amine to a primary amine and tertiary amine as described by Sivasankar et al.23 The reaction rate equations are shown Table 2 and the molar amount changing rates in Table 3.Fig. 4 shows the experimentally determined concentrations of the reactants and products, as well as the concentrations predicted by the (optimized) kinetic model. Overall, the model appears to match the experimental data well, although it overestimates the tertiary amine formation during the primary and secondary amine experiments. For further comparison of predictive and experimental values, refer to the parity plots given in the ESI.† The fitted reaction rate constants are reported in Table 4.
The rate constants for direct HDN of dodecylamine (1), hydrogenolysis of didodecylamine (3) and hydrogenolysis of tridodecylamine (5) shown in Table 4 have similar values (0.012–0.016 min−1). This is reasonable, since all three reactions involve the cleavage of a C–N bond. The reaction rate constant for the reaction between two didodecylamine molecules is practically zero, and we thus have not found any evidence that this reaction takes place for didodecylamine on Pt/ZrO2. Thus, our decision to not include it in the reaction network in Fig. 3 appears justified.
The reaction rate constants were used to calculate the reaction rates r1–r6 and the nitrogen removal rate, using the rate equations in Table 1. The predicted nitrogen removal rate was calculated as a sum of all reaction rates involving nitrogen removal, namely, r1, r2, r4 and r6. These rates, the nitrogen removal predicted by the kinetic model and the experimental nitrogen removal (GC-FID based), are plotted in Fig. 5.
As can be seen in Fig. 5a, in the primary amine experiments, nitrogen removal starts immediately and slows down at longer reaction times. The direct HDN (1, r1) and the condensation (2, r2) of two primary amines make up most of the overall nitrogen removal for the primary amine experiment, with r1 (direct HDN) being the fastest. The simulated nitrogen removal rate for the secondary and tertiary amine experiments goes through a maximum at 30 min for the secondary amine and 100 min for the tertiary amine. As the primary amine is formed through secondary amine hydrogenolysis, the nitrogen removal rate increases, but when the primary amine is later decomposed, the nitrogen removal rate decreases again. In other words, the nitrogen removal rate correlates with the concentration of the primary amine, which indicates that the primary amine is involved in all nitrogen removal pathways. This is further supported by the nitrogen removal rate maximum occurring at longer reaction times for the tertiary amine, which first forms the secondary amine and then the primary amine. Even though the tertiary amine is equally reactive as the secondary amine in terms of conversion (see Fig. 1d) the maximum nitrogen removal rate of the secondary amine comes sooner.
Effect of the condensation reactions on amine HDN
The formation of significant amounts of tridodecylamine was not detected during the primary and secondary amine experiments. However, the modeling results indicate that the condensation reaction (4) between primary and secondary amines, forming the tertiary amine, appears to significantly contribute to the nitrogen removal in the secondary and tertiary amine experiments. It should be noted that the experimental concentration of tridodecylamine was very low in the primary and secondary amine experiments, and that the GC-FID based tertiary amine concentrations thus likely have large uncertainties associated with them. The amount of tertiary amine formed in the secondary and primary amine experiments was also overestimated by the kinetic model. Therefore, to check the fitting results related to this condensation reaction (4), a fit without this reaction was done (r4 was set to zero). These results can be found in the ESI.† Without the condensation reaction between primary and secondary amines, the dodecylamine concentration was underestimated by the model in the primary amine experiments and overestimated in the secondary amine experiments. Furthermore, the nitrogen removal in the secondary and tertiary amine experiments was underestimated, indicating that the condensation (4) does play a role in the overall nitrogen removal, even though this was not apparent from the raw experimental data. This would furthermore show the overall importance of the condensation reactions in the amine HDN reaction network.The low amount of tertiary amine detected in the liquid phase may be due to the tertiary amine forming in higher concentrations in the catalyst pore system, but, because of diffusion limitations, being unable to leave the pores before it reacts further via hydrogenolysis (5). It should be noted that due to its size, pore diffusion of the tertiary amine is expected to be slower than that of the primary and secondary amines. This may also be why the reaction (6) involving two molecules of secondary amine does not take place for didodecylamine on Pt/ZrO2. We hypothesize that this reaction cannot take place inside the catalyst pore structure due to steric hindrance, which prevents two molecules of secondary amine from approaching each other inside the pores. It is noteworthy that the primary amine is involved in both condensation reactions we have found evidence for; either two molecules of primary amine react, or one molecule of primary amine and one molecule of secondary amine react. We thus suspect that mass transfer limitations involving the larger secondary and tertiary amines could play a significant role during amine hydrotreating. By choosing the correct pore size distribution for the catalyst, it may even be feasible to develop shape-selective HDN catalysts, which suppress the formation of condensation products from long-chained primary amines.
The effect of the condensation reaction pathways (2, 4) on the overall amine HDN rate can be deduced from the data and the proposed reaction network. Initially, nitrogen removal of the primary amine can take place both through direct HDN (1) and through condensation (2). As seen in Fig. 1a, at low batch residence times the condensation reaction pathway is significant and the secondary amine is formed faster than it decomposes. Based on the kinetic modeling results shown in Fig. 5, the rate of condensation reaction (2) is approximately half of the rate of primary amine direct HDN (1). In this condensation reaction, two molecules of primary amine are consumed, and one molecule of ammonia is formed. This thus explains why conversion and nitrogen removal of the primary amine is faster than that of the secondary and tertiary amines (see Fig. 1d and 2a).
However, the secondary amine formed in the condensation reaction cannot undergo direct HDN, but instead undergoes hydrogenolysis to reform one molecule of the primary amine. The condensation reaction thus momentarily consumes two molecules of primary amine, but only liberates one molecule of ammonia. The other N-atom is “trapped” in the secondary amine intermediate, until this intermediate decomposes to form the primary amine, which can undergo HDN. As the kinetic data in Table 4 shows that C–N bond splitting steps have similar rate constants for primary and secondary amines, nitrogen removal in two steps (through the condensation pathway) is overall slower than nitrogen removal through direct HDN. Thus, we propose that the condensation reactions increase the initial nitrogen removal rate, but that at higher reaction times the condensation reactions instead may start slowing down the overall HDN as part of the nitrogen is present as secondary and tertiary amine. To highlight this effect of the condensation reactions on primary amine HDN, we compared the results calculated using the kinetic parameters fitted to the experimental data with the results calculated using the same parameters, but without any condensation reactions taking place (k2, k4, k6 set to zero). The results are shown in the ESI† and are in good agreement with the above discussion. The nitrogen removal initially increases faster for the model which includes the condensation reactions, but at high reaction times nitrogen removal is slightly higher for the model for which condensation reactions do not take place.
Conclusions
In this study, the HDN of primary, secondary, and tertiary alkyl amines, namely dodecylamine, didodecylamine, and tridodecylamine, over a Pt/ZrO2 catalyst was examined. Based on the results, all three amines were found to share the same reaction network. The nitrogen removal increased in the order primary amine > secondary amine > tertiary amine. This is in good agreement with the proposed reaction network, in which the initial reactions of the secondary and tertiary amines mainly involved hydrogenolysis into paraffin as well as primary and secondary amines, respectively. Indeed, all steps involving nitrogen removal involve the primary amine, which can either undergo direct HDN through hydrogenolysis, or undergo a condensation reaction with another molecule of primary or secondary amine. The kinetic modeling results showed that the reaction rate constants for the hydrogenolysis reactions of the primary, secondary, and tertiary amines were similar, which is not unexpected since all three reactions involve the C–N bond hydrogenolysis of a saturated amine.This study thus emphasizes the connection of the reaction networks of primary, secondary, and tertiary alkyl amines in the HDN over a noble metal catalyst (Pt/ZrO2). While the model compounds share a reaction network, the conversion and nitrogen removal considerably depend on the reactant, as the initial reaction pathways varied accordingly. As the HDN of secondary and tertiary amines was found to be slower than HDN of primary amines, HDN of feedstock containing more highly substituted amines is likely to be more difficult. We also suggest that condensation reactions involving primary amines initially increased nitrogen removal, but at longer reaction times may slightly decrease nitrogen removal, as nitrogen is present in the form of secondary and tertiary amines, which cannot undergo direct HDN. This has implications for both the catalyst and process design for HDN of feeds containing primary aliphatic amines. If a lower degree of nitrogen removal is acceptable, it may be beneficial to develop catalysts or processes which promote the formation of condensation products since these reactions contribute to nitrogen removal at low reaction times. However, if a high degree of nitrogen removal is required, the formation of condensation products may need to be avoided.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
E. V. prepared the catalyst and developed the batch reactor experiment methodology. The reactor experiments were planned by E. V., L. F. K., R. K. and R. L. P. Batch reactor experiments and analysis of the reaction products were carried out by L. F. K., J. K. and E. V. Catalyst characterization was performed by J. K., E. V. and J. V. The kinetic modeling was performed by L. F. K., and supervised by L. I. and M. R. The first version of the manuscript was written by J. K. and L. F. K. All authors contributed to the final version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We thank Sylvia Albersberger and all other members of the Neste-Aalto HDN catalyst development project group for useful discussions and insights. The experimental work for this study was funded by Neste Corporation (Neste-Aalto HDN catalyst development project). J. K. also acknowledges The Finnish Foundation for Technology Promotion for additional funding during the writing process. The Bioeconomy and Raw materials research infrastructures at Aalto University were used for the experimental work in this study. This work was done in collaboration between researchers at Aalto University and Technische Universität Darmstadt. This collaboration was carried out within the UNITE! University Network for Innovation, Technology and Engineering.References
- C. Bergero, G. Gosnell, D. Gielen, S. Kang, M. Bazilian and S. J. Davis, Pathways to Net-Zero Emissions from Aviation, Nat. Sustain., 2023, 6(4), 404–414, DOI:10.1038/s41893-022-01046-9.
- International Air Transport Association, Developing Sustainable Aviation Fuel, 2022, https://www.iata.org/en/programs/environment/sustainable-aviation-fuels/, retrieved 10.09.2024 Search PubMed.
- ReFuelAviation Initiative: Sustainable Avaiation Fuels and the ‘fit for 55’package, 2022, https://www.europarl.europa.eu/thinktank/en/document/EPRS_BRI(2022)698900, retrieved 10.09.2024 Search PubMed.
- M. Klöwer, M. R. Allen, D. S. Lee, S. R. Proud, L. Gallagher and A. Skowron, Quantifying Aviation's Contribution to Global Warming, Environ. Res. Lett., 2021, 16(10), 104027, DOI:10.1088/1748-9326/ac286e.
- E. Furimsky, Hydroprocessing Challenges in Biofuels Production, Catal. Today, 2013, 217, 13–56, DOI:10.1016/j.cattod.2012.11.008.
- F. Cavani, S. Albonetti, F. Basile and A. Gandini, Chemicals and Fuels from Bio-Based Building Blocks, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2016, DOI:10.1002/9783527698202.
- Neste MY Renewable Diesel Lowers Your CO2 Emissions, https://www.neste.us/neste-my-renewable-diesel, retrieved 10.09.2024 Search PubMed.
- Ecofining, https://www.eni.com/en-IT/actions/energy-transition-technologies/biofuels/biomass-ecofining.html, retrieved 10.09.2024 Search PubMed.
- E. Furimsky, Catalytic Hydrodeoxygenation, Appl. Catal., A, 2000, 199(2), 147–190, DOI:10.1016/S0926-860X(99)00555-4.
- C. Gutiérrez-Antonio, F. I. Gómez-Castro, J. A. de Lira-Flores and S. Hernández, A Review on the Production Processes of Renewable Jet Fuel, Renewable Sustainable Energy Rev., 2017, 79, 709–729, DOI:10.1016/j.rser.2017.05.108.
- E. Furimsky and F. E. Massoth, Hydrodenitrogenation of Petroleum, Catal. Rev.: Sci. Eng., 2005, 47(3), 297–489, DOI:10.1081/CR-200057492.
- W.-T. Chen, L. Tang, W. Qian, K. Scheppe, K. Nair, Z. Wu, C. Gai, P. Zhang and Y. Zhang, Extract Nitrogen-Containing Compounds in Biocrude Oil Converted from Wet Biowaste via Hydrothermal Liquefaction, ACS Sustainable Chem. Eng., 2016, 4(4), 2182–2190, DOI:10.1021/acssuschemeng.5b01645.
- L. Leng, W. Zhang, H. Peng, H. Li, S. Jiang and H. Huang, Nitrogen in Bio-Oil Produced from Hydrothermal Liquefaction of Biomass: A Review, Chem. Eng. J., 2020, 401, 126030, DOI:10.1016/j.cej.2020.126030.
- G. H. C. Prado, Y. Rao and A. de Klerk, Nitrogen Removal from Oil: A Review, Energy Fuels, 2017, 31(1), 14–36, DOI:10.1021/acs.energyfuels.6b02779.
- G. W. Huber, P. O'Connor and A. Corma, Processing Biomass in Conventional Oil Refineries: Production of High Quality Diesel by Hydrotreating Vegetable Oils in Heavy Vacuum Oil Mixtures, Appl. Catal., A, 2007, 329, 120–129, DOI:10.1016/j.apcata.2007.07.002.
- P. M. Mortensen, J.-D. Grunwaldt, P. A. Jensen, K. G. Knudsen and A. D. Jensen, A Review of Catalytic Upgrading of Bio-Oil to Engine Fuels, Appl. Catal., A, 2011, 407(1–2), 1–19, DOI:10.1016/j.apcata.2011.08.046.
- Q. Bu, H. Lei, A. H. Zacher, L. Wang, S. Ren, J. Liang, Y. Wei, Y. Liu, J. Tang, Q. Zhang and R. Ruan, A Review of Catalytic Hydrodeoxygenation of Lignin-Derived Phenols from Biomass Pyrolysis, Bioresour. Technol., 2012, 124, 470–477, DOI:10.1016/j.biortech.2012.08.089.
- E. Verkama, P. Auvinen, S. Albersberger, M. Tiitta, R. Karinen and R. L. Puurunen, Competitive Hydrodeoxygenation and Hydrodenitrogenation Reactions in the Hydrotreatment of Fatty Acid and Amine Mixtures, Top. Catal., 2023, 66, 1353–1368, DOI:10.1007/s11244-023-01784-w.
- A. C. Badari, Sz Harnos, F. Lónyi, Gy Onyestyák, M. Štolcová, A. Kaszonyi and J. Valyon, A Study of the Selective Catalytic Hydroconversion of Biomass-Derived Pyrolysis or Fermentation Liquids Using Propylamine and Acetic Acid as Model Reactants, Catal. Commun., 2015, 58, 1–5, DOI:10.1016/j.catcom.2014.07.041.
- O. Palardy, C. Behnke and L. M. L. Laurens, Fatty Amide Determination in Neutral Molecular Fractions of Green Crude Hydrothermal Liquefaction Oils From Algal Biomass, Energy Fuels, 2017, 31(8), 8275–8282, DOI:10.1021/acs.energyfuels.7b01175.
- C. Zhu, O. Y. Gutiérrez, D. M. Santosa, M. Flake, R. Weindl, I. Kutnyakov, H. Shi and H. Wang, Kinetics of Nitrogen-, Oxygen- and Sulfur-Containing Compounds Hydrotreating during Co-Processing of Bio-Crude with Petroleum Stream, Appl. Catal., B, 2022, 307, 121197, DOI:10.1016/j.apcatb.2022.121197.
- G. Meitzner, W. J. Mykytka and H. Sinfelt, Metal-Catalyzed Reactions of Methylamine in the Presence of Hydrogen, J. Catal., 1986, 98, 513–521 CrossRef.
- N. Sivasankar and R. Prins, Reactions of Mixed Dialkyl- and Trialkylamines over Pd/\textgreekg-Al2O3, J. Catal., 2006, 241(2), 342–355, DOI:10.1016/j.jcat.2006.04.032.
- L. Di, S. Yao, M. Li, G. Wu, W. Dai, G. Wang, L. Li and N. Guan, Selective Catalytic Hydrogenolysis of Carbon–Carbon \textgreeks Bonds in Primary Aliphatic Alcohols over Supported Metals, ACS Catal., 2015, 5(12), 7199–7207, DOI:10.1021/acscatal.5b02180.
- M. Cattenot, J.-L. Portefaix, J. Afonso, M. Breysse, M. Lacroix and G. Perot, Mechanism of Carbon–Nitrogen Bond Scission on Unsupported Transition Metal Sulfides, J. Catal., 1998, 173(2), 366–373, DOI:10.1006/jcat.1997.1929.
- E. Verkama, S. Albersberger, A. Arandia, K. Meinander, M. Tiitta, R. Karinen and R. L. Puurunen, Hydrodeoxygenation and Hydrodenitrogenation of n -Hexadecanamide over Pt Catalysts: Effect of the Support, Catal. Sci. Technol., 2024, 14(2), 431–448, 10.1039/D3CY01480K.
- M. Snåre, I. Kubičková, P. Mäki-Arvela, K. Eränen and D. Yu. Murzin, Heterogeneous Catalytic Deoxygenation of Stearic Acid for Production of Biodiesel, Ind. Eng. Chem. Res., 2006, 45(16), 5708–5715, DOI:10.1021/ie060334i.
- K. W. Cheah, S. Yusup, A. C. M. Loy, B. S. How, V. Skoulou and M. J. Taylor, Recent Advances in the Catalytic Deoxygenation of Plant Oils and Prototypical Fatty Acid Models Compounds: Catalysis, Process, and Kinetics, Mol. Catal., 2022, 523, 111469, DOI:10.1016/j.mcat.2021.111469.
- E. Verkama, S. Albersberger, K. Meinander, M. Tiitta, R. Karinen and R. L. Puurunen, Zirconia-Supported Pt, Pd, Rh, Ru, and Ni Catalysts in the Hydrotreatment of Fatty Amides and Amines, Energy Fuels, 2024, 38(5), 4464–4479, DOI:10.1021/acs.energyfuels.3c04372.
- E. Verkama, E. Järvinen, S. Albersberger, K. Meinander, H. Jiang, M. Tiitta, R. Karinen and R. L. Puurunen, Hydrodeoxygenation and Hydrodenitrogenation of N-Hexadecanamide to n-Paraffins: Bimetallic Catalysts Supported on Ceria-Zirconia, Appl. Catal., A, 2024, 676, 119602, DOI:10.1016/j.apcata.2024.119602.
- M. Marafi and E. Furimsky, Hydroprocessing Catalysts Containing Noble Metals: Deactivation, Regeneration, Metals Reclamation, and Environment and Safety, Energy Fuels, 2017, 31(6), 5711–5750, DOI:10.1021/acs.energyfuels.7b00471.
- A. M. Robinson, J. E. Hensley and J. W. Medlin, Bifunctional Catalysts for Upgrading of Biomass-Derived Oxygenates: A Review, ACS Catal., 2016, 6(8), 5026–5043, DOI:10.1021/acscatal.6b00923.
- S. Brunauer, P. H. Emmett and E. Teller, Adsorption of Gases in Multimolecular Layers, J. Am. Chem. Soc., 1938, 60(2), 309–319, DOI:10.1021/ja01269a023.
- B. C. Lippens, B. G. Linsen and J. H. de Boer, Studies on Pore Systems in Catalysts I. The Adsorption of Nitrogen; Apparatus and Calculation, J. Catal., 1964, 3(1), 32–37, DOI:10.1016/0021-9517(64)90089-2.
- J.-Y. de Saint Laumer, S. Leocata, E. Tissot, L. Baroux, D. M. Kampf, P. Merle, A. Boschung, M. Seyfried and A. Chaintreau, Prediction of Response Factors for Gas Chromatography with Flame Ionization Detection: Algorithm Improvement, Extension to Silylated Compounds, and Application to the Quantification of Metabolites, J. Sep. Sci., 2015, 38(18), 3209–3217, DOI:10.1002/jssc.201500106.
- J.-Y. de Saint Laumer, E. Cicchetti, P. Merle, J. Egger and A. Chaintreau, Quantification in Gas Chromatography: Prediction of Flame Ionization Detector Response Factors from Combustion Enthalpies and Molecular Structures, Anal. Chem., 2010, 82(15), 6457–6462, DOI:10.1021/ac1006574.
- M. A. Branch, T. F. Coleman and Y. Li, A Subspace, Interior, and Conjugate Gradient Method for Large-Scale Bound-Constrained Minimization Problems, SIAM J. Sci. Comput., 1999, 21(1), 1–23, DOI:10.1137/S1064827595289108.
- P. Virtanen, R. Gommers, T. E. Oliphant, M. Haberland, T. Reddy, D. Cournapeau, E. Burovski, P. Peterson, W. Weckesser, J. Bright, S. J. Van Der Walt, M. Brett, J. Wilson, K. J. Millman, N. Mayorov, A. R. J. Nelson, E. Jones, R. Kern, E. Larson, C. J. Carey, İ. Polat, Y. Feng, E. W. Moore, J. VanderPlas, D. Laxalde, J. Perktold, R. Cimrman, I. Henriksen, E. A. Quintero, C. R. Harris, A. M. Archibald, A. H. Ribeiro, F. Pedregosa, P. Van Mulbregt, SciPy 1.0 Contributors, A. Vijaykumar, A. P. Bardelli, A. Rothberg, A. Hilboll, A. Kloeckner, A. Scopatz, A. Lee, A. Rokem, C. N. Woods, C. Fulton, C. Masson, C. Häggström, C. Fitzgerald, D. A. Nicholson, D. R. Hagen, D. V. Pasechnik, E. Olivetti, E. Martin, E. Wieser, F. Silva, F. Lenders, F. Wilhelm, G. Young, G. A. Price, G.-L. Ingold, G. E. Allen, G. R. Lee, H. Audren, I. Probst, J. P. Dietrich, J. Silterra, J. T. Webber, J. Slavič, J. Nothman, J. Buchner, J. Kulick, J. L. Schönberger, J. V. De Miranda Cardoso, J. Reimer, J. Harrington, J. L. C. Rodríguez, J. Nunez-Iglesias, J. Kuczynski, K. Tritz, M. Thoma, M. Newville, M. Kümmerer, M. Bolingbroke, M. Tartre, M. Pak, N. J. Smith, N. Nowaczyk, N. Shebanov, O. Pavlyk, P. A. Brodtkorb, P. Lee, R. T. McGibbon, R. Feldbauer, S. Lewis, S. Tygier, S. Sievert, S. Vigna, S. Peterson, S. More, T. Pudlik, T. Oshima, T. J. Pingel, T. P. Robitaille, T. Spura, T. R. Jones, T. Cera, T. Leslie, T. Zito, T. Krauss, U. Upadhyay, Y. O. Halchenko and Y. Vázquez-Baeza, SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python, Nat. Methods, 2020, 17(3), 261–272, DOI:10.1038/s41592-019-0686-2.
- C. F. Winans and H. Adkins, The Alkylation Of Amines As Catalyzed By Nickel, J. Am. Chem. Soc., 1932, 54(1), 306–312, DOI:10.1021/ja01340a046.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cy01099j |
| ‡ Present address: Fraunhofer Institute for Solar Energy Systems ISE, Heidenhofstr. 2, 79110 Freiburg, Germany. |
| This journal is © The Royal Society of Chemistry 2025 |