Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Electrochemical nitrogen fixation and utilization: theories, advanced catalyst materials and system design
Wenhan
Guo
a,
Kexin
Zhang
a,
Zibin
Liang
a,
Ruqiang
Zou
*a and
Qiang
Xu
*bc
aBeijing Key Laboratory for Theory and Technology of Advanced Battery Materials, Department of Materials Science and Engineering, College of Engineering, Peking University, Beijing 100871, P. R. China. E-mail: rzou@pku.edu.cn
bAIST-Kyoto University Chemical Energy Materials Open Innovation Laboratory (ChEM-OIL), National Institute of Advanced Industrial Science and Technology (AIST), Sakyo-ku, Kyoto 606-8501, Japan. E-mail: q.xu@aist.go.jp
cSchool of Chemistry & Chemical Engineering, Yangzhou University, Yangzhou 225009, P. R. China. E-mail: qxuchem@yzu.edu.cn
First published on 19th May 2025
Abstract
Correction for ‘Electrochemical nitrogen fixation and utilization: theories, advanced catalyst materials and system design’ by Wenhan Guo et al., Chem. Soc. Rev., 2019, 48, 5658–5716, https://doi.org/10.1039/C9CS00159J.
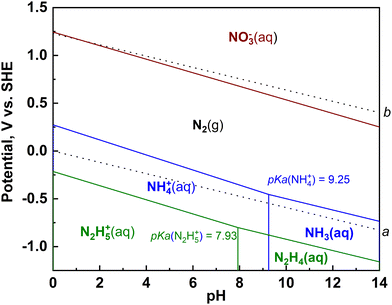
The authors regret that the pKa of hydrazine was incorrectly given in the original article. The correct value should be 7.93. This means that eqn (8) and (9) and Fig. 3 were incorrect. The correct versions are shown below. This also applies to the sentence beginning “For hydrazine, the case is similar…”, where the boundary should be given as 7.93.
| N2(g) + 5H+ + 4e− ⇌ N2H5+(aq.) (pH < 7.93) E0 = −0.214 V vs. RHE at pH = 0 | (8) |
| N2(g) + 4H2O(l) + 4e− ⇌ N2H4(aq.) + 4OH−(aq.) (pH ≥ 7.93) E0 = −0.332 V vs. RHE | (9) |
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2025 |