Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Au NHC complexes as anticancer agents: milestones, strategies and future developments
Melanie E.
Hoffmann
and
Fritz E.
Kühn
 *
*
Department of Chemistry and Catalysis Research Center, School of Natural Science, Molecular Catalysis, Technical University of Munich, Lichtenbergstraße 4, 85478 Garching, Germany. E-mail: fritz.kuehn@ch.tum.de; Tel: (+49)8928913096
First published on 7th October 2025
Abstract
The need for selective and efficient anticancer therapies drives the development of gold N-heterocyclic carbene (NHC) as efficient metallodrugs. Their stability, tunable electronics, and versatile steric features make NHCs ideal ligands, which, paired with an antiproliferating gold centre, form an exemplary metal complex for anticancer research. This review highlights the progress made in designing gold NHC complexes, emphasizing strategies to enhance cytotoxicity and selectivity towards cancer cells while minimizing toxicity to healthy tissues, emphasizing the crucial role of the NHC ligand. Furthermore, challenges concerning revealing the precise modes of action are discussed. Mechanistic pathways beyond the inhibition of thioredoxin reductase are highlighted. By underlining recent developments, this review aims to pave the way to a rational design of next-generation gold NHC complexes.
Key learning points1. Although a variety of Au NHC complexes inhibit TrxR, no distinct correlation between cytotoxicity and TrxR inhibition can be established.2. Au NHC complexes can interact with less commonly addressed biological targets: DNA, mitochondria, p53, and cysteine-containing enzymes. However, investigating these targets is often difficult due to cascade reactions happening in the cell. 3. Counter ion exchange, lipophilicity enhancement, addition of other metals, and auxiliaries are strategies to increase Au NHC complex cytotoxicity. 4. Au NHC complexes have been frequently tested against cell lines MCF-7 and HT-29. Based on these cell lines, the activity of Au NHC complexes can be compared. 5. Reports on NHC ligands other than imidazolylidene have been limited, as have investigations into the standalone cytotoxicity of NHCs. Novel NHC ligands can easily be facilitated with strong bases (K2CO3, KH, KHMDS, NaOH) or silver oxide to form Au NHC complexes. |
Introduction
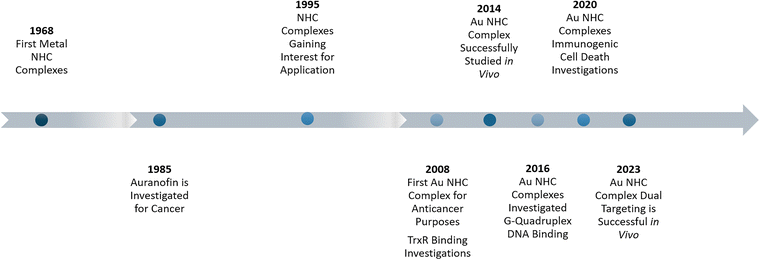
According to the World Health Organization, every sixth death worldwide is caused by cancer.1 In industrialised nations, the risk of developing cancer in one lifetime is around 38.48%.2 Therefore, billions of US dollars are invested yearly into finding new, selective, and efficient cancer treatments.3 Metalorganic compounds are seldom used in medicinal applications. However, one drug, a metal complex, is generally associated with chemotherapy: cisplatin.4 The modern interest in utilizing gold(I) compounds as potential anticancer agents has been sparked by the properties of auranofin.5,6 Auranofin was originally intended as a treatment for rheumatoid arthritis7 and was found to display cytotoxicity in human cervical cancer cells (HeLa). Due to gold's high affinity to sulphur and, therefore, cysteine, auranofin's stability in blood plasma is limited, as it reacts with sulphur-containing proteins through ligand dissociation.6 Auranofin's fragility in blood plasma encouraged the research on more stable gold systems. Berners-Price and coworkers first suggested gold(I) N-heterocyclic carbene (NHC) complexes as potential anticancer agents.8 NHC transition metal complexes, first reported in 1968,9,10 gained considerable attention when they found applications for catalysis, starting in 1995.11 Since then, they have been widely utilised and considerably modified,12,13 since they are generally stable under aerobic conditions.12 NHCs are strong sigma-donors and form strong metal–carbon bonds.12,13 The gold–NHC bond is one of the strongest carbene–metal bonds. Investigations have shown that in the presence of sulphur-containing compounds, the gold–NHC bond is stable for several hours, in contrast to, for example, NHC–ruthenium bonds.14 Berners-Price and coworkers were also the first to investigate the potential of Au(I) NHC complexes as anticancer agents.8,15–17 The high cytotoxicity of these compounds sparked the field to grow quickly.18,19Their versatility, together with their unique biological properties and stability, make Au NHC complexes ubiquitous in metal-based medicinal chemistry research (Chart 1). Au NHC complexes have shown outstanding anti-proliferation activity. The IC50 values, a given concentration that reduces the number of viable cells by 50%, of dozens Au(I) NHC complexes can reach the lower nanomolar range.20–24 Additionally, selectivity tests have, in various cases, shown a preference for cancerous cells over healthy cells, exhibiting higher IC50 values for healthy cells.21,25
Notably, Au NHC complexes also inhibit high cytotoxicity towards various drug-resistant cell lines.21,23,26 Most promising, in vivo (mice) studies of Au NHC complexes have shown tumour regression without observable damage to healthy liver, heart, and lung tissue.27,28
So far, hundreds of publications in the field have been published.19 This review will focus on current trends and successful strategies to make Au NHC complexes more selective, reach higher cytotoxicity, and explore new mechanistic pathways. The goal is to inspire researchers to find and close possible research gaps by giving a broad overview, highlighting best practices, and giving introductions to frequently addressed principles (Fig. 1).
NHCs as ligand building blocks
An important aspect of NHC complexes is the stable carbon–metal bond, formed due to their strong sigma-donating properties.12To form these bonds, the NHC-precursors, most commonly imidazolium salts, need to be deprotonated with bases (e.g. K2CO3, KH, KHMDS, NaOH) before ligand exchange reactions with gold precursors, such as [Au(tht)Cl] or [Au(Me2S)Cl], can take place. Besides commercially available bases, gold precursors with basic ligands can be used, such as [Au(N(SiMe3)2(tht))] and [Au(III)ac3].29,30 Another way of forming Au NHC complexes is the reaction of silver oxide with NHC-precursors to yield the corresponding silver complex.20 The obtained silver complex can be transmetalated with gold precursors.31 An advantage of the trans-metalation route is the possibility of isolating the silver complexes since the use of silver compounds as anticancer agents has also been explored successfully.32–34
When forming Au(I) NHC complexes, depending on the amount of ligand used in the reaction, ether Au(I) bis-NHC complexes (with two NHC ligands) or Au(I) mono-NHC complexes (containing one NHC ligand) are formed.31,35,36
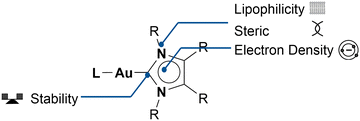
A great feature of NHCs is their high versatility. The classical imidazolium-based NHC can be modified in several different positions: the organic entities attached to the nitrogen atoms, technically termed wingtip ligands, and the residue-groups attached to the backbone (two carbons located opposite the carbene positions, ether connected by a double bond or by a single bond of the five-membered ring structure). Both backbone modification and wingtip modification can alter the electron-donating and steric properties of the NHC.12
In the field of Au NHC complexes as anticancer agents, wing-tip or backbone modifications are often used to alter the lipophilicity of the complex, as a direct correlation between higher antiproliferation and lipophilicity has been reported.20,37 The steric demand of the wingtip ligands has a direct influence on the accessibility of the gold atom, since these ligands can shield the gold atom from external influences. NHC modifications have been studied and described intensively, mainly for catalytic applications.12,13,38 The wingtips or backbone ligands can also be used to add moieties to enhance cytotoxicity: targeting entities, cytotoxic building blocks, or scaffolds with different biological properties are commonly used. In the section “Strategies for Boosting Au–NHC Complexes Cytotoxicity and Selectivity” this concept will be described in greater detail.
Cytotoxicity of NHC ligands
When designing a new ligand system, it is important to consider that various reported NHC ligands investigated for their anti-cancer effects have demonstrated cytotoxicity even in the absence of a metal.39–41 This can be expected since there are imidazole-based anticancer drugs approved by the US Federal Drug Administration (FDA): Dacarbazine, Mercaptopurine, Nilotinib, and Tipifarnib.42 In some reports, the NHC ligands show higher cytotoxicity than their corresponding gold complexes. Similar cytotoxicity for the ligand L1 and its corresponding Au(III) 1 complexes (Chart 2) has been reported. The IC50 values in HeLa (cervix cancer) cells are 72.2 ± 19 μM for ligand L1 and 85.7 ± 15 μM for complex 1.29 Rodríguez and coworkers synthesised ligands L2, which shows higher cytotoxicity in HT-29 (colon) and MDA-MB-231 (breast) cancer cells than the corresponding mono-NHC 2 and bis-NHC 3 gold(I) complexes. Only complex 2a exhibits similar cytotoxicity to its ligand L2a, with IC50 values being 3.10 ± 1.38 and 3.08 ± 0.25 μM, respectively. Therefore, Rodríguez and coworkers tested the TrxR inhibition capability of compounds L2a, 2a, and 3a. Among them, compound 2a is the only one showing significant TrxR inhibition (0.320 ± 0.040 μM). Au NHC complex 2a and L2a display no TrxR inhibition below a concentration of 10 μM.40The above-mentioned reports highlight the necessity of thoroughly evaluating both the ligands and their metal complexes individually to fully understand their biological activities and therapeutic potential.
NHC-ligand systems inspired by nature
A common strategy for ligand design is inspiration by nature, as approximately 60% of anticancer drugs are plant-derived.43 The possibility of utilizing L-histidine derivatives as NHC ligands for gold complexes has been explored. To use L-histidine as an NHC ligand, it needs to undergo metalation. The resulting bis-NHC complex was treated with trifluoroacetic acid to remove the BOC group, yielding Au(I) bis-NHC complex 4. Complex 4 shows only moderate cytotoxicity in MCF-7, PC3 (prostate), and A2780 (ovarian) cancer cell lines. The best result was obtained with MCF-7 cells (IC50: 4.6 ± 1.9 μM). The cellular uptake was assessed in PC3 cells after 2 h of incubation with complex 4. No gold has been detectable in the cells, complex 4 appeared to be weakly engaged at the cell surface, interacting with the membranal vital system (Chart 3).44The groups of Tamm and Ott investigated the marine natural product norzooanemonin (1,3-dimethyl-1H-imidazol-3-ium-5-carboxylate) as an NHC ligand for Au complexes. The carboxyl group, located at the backbone, can be utilised for functionalization. For this study, the carboxylate was esterified. The complexes 5a, 5b, 6a, 6b, 7a, and 7b, were tested against HT-29, A549 (lung cancer), breast cancer cell lines MCF-7 and MDA-MB-231. Additionally, the complexes were assessed against VeroE6, non-tumour cells isolated from the kidney of an African green monkey. Mostly, the complexes show a broad spectrum in cytotoxicity with IC50 values between 63 μM and 0.89 μM. From these complexes, complexes 6 have been reported as the most promising complexes for cell line A549 with IC50 values of 3.35 ± 0.89 μM (6a) and 0.89 ± 0.10 μM (6b). Additionally, complexes 6 shows almost no toxicity towards VeroE6 cells (kidney cells of the African green monkey), with IC50 values of >100 μM (6a) and 30.79 ± 0.49 μM (6b), which may indicate a selectivity towards cancerous cells over healthy cells.25 While natural product-inspired NHC ligands offer a promising foundation for developing novel Au complexes with anticancer potential, the wide variability in reported IC50 values indicates that this approach does not guarantee efficacy, highlighting the need for systematic optimization.
Ligand scaffold: beyond (benz-)imidazole
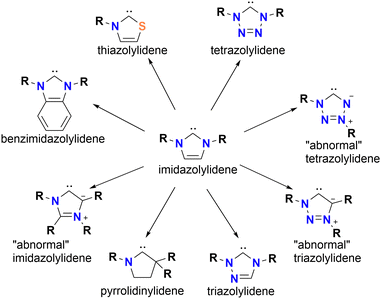
Most reports about metal NHC complexes, in medicinal and catalytic applications, focus on imidazole and benzimidazole ligand scaffolds. However, there are several reports about Au NHC complexes with scaffolds beyond imidazole, which show remarkable anti-proliferating properties.20,21,30,45,46NHC ligands are classified as heterocyclic ring systems containing at least one nitrogen atom, which helps stabilise the adjacent carbene position (Fig. 2). The nature of the ring scaffold, including its size and heteroatom composition, has a significant impact on the electronic donating properties of the resulting NHC. Therefore, the ranking of electron-donating probabilities must be done carefully. While increasing the number of nitrogen atoms in the ring often correlates with reduced σ-donor strength, structural differences can lead to deviations. For instance, 1,3,4-triazolylidene NHCs are weaker electron donors than imidazolylidenes, but 1,2,3-triazolylidenes are stronger electron donors than imidazolylidenes. This discrepancy arises from the distinction between “abnormal”, in which the carbene is located adjacent to only one nitrogen, and “normal” NHCs, where the carbene is flanked by two heteroatoms. Besides nitrogen, other heteroatoms in the scaffold play a significant role in the electron-donating properties. Among the scaffolds discussed here, thiazole-based NHCs are the least donating.12,13,38
Metzler-Nolte and coworkers were the first (and only to this date) to investigate Au(I) NHC complexes facilitating thiazole-based NHCs for their anticancer properties. Inspired by bioorganic thiazole derivatives such as vitamin B1, Metzler-Nolte and coworkers synthesised an L-thiazolyl alanine-containing dipeptide. This ligand, together with [Au(HMDS)(tht)], forms the desired iodine NHC gold complex 8. The IC50 values for 8 in A549 cells (lung carcinoma) are in the high nanomolar range (IC50 = 0.4 ± 0.01 μM) (Chart 4).30 These results are very interesting since there are fewer than a dozen results about gold mono-NHC complexes reaching cytotoxicity up to the nanomolar range.19 It is not clear if the high cytotoxicity stems from the low donating probability, as other NHCs with low electron-donating probabilities do not show consistent cytotoxicity in the nanomolar range.
For example, the first gold NHC complex bearing a normal triazolylidene scaffold tested against cancerous cells did not reach IC50 values in the nanomolar range. Additionally, to being active toward breast cancer cells (MDA-MB-231) (IC50 = 1.0 ± 0.0 μM) and colon cancer cells (HT-29) (IC50 = 2.1 ± 0.0 μM), complex 9 was found to accumulate fast, and efficiently in HT-29 cells.46 Shortly after, another example of 1,2,4-triazolylidene-based NHC gold complexes was published. Also, complexes 10a, 10b, and 10c display IC50 values in the lower micromolar range in HeLa-S3 (cervical) and HL-60 (leukemia) cells.45
Furthermore, the anticancer activity of gold complexes bearing strongly σ-donating 1,2,3-triazole-based NHCs was explored. The 1,2,3-triazolylidene complex incorporating isopropyl wingtips 11a displays IC50 values in the lower micromolar range in various cell lines. Remarkably, 1,2,3-triazolylidene NHC gold complexes with mesityl wingtips 11b achieve IC50 values in the nanomolar range and perform best in MCF-7 (IC50 = 0.084 ± 0.016 μM) and MDA-MB-231 (IC50 = 0.063 ± 0.02 μM) cell lines.20 Interestingly, compound 11a, even though showing lower cytotoxicity, shows higher inhibition of TrxR than compound 11b. Nevertheless, the inhibition values of TrxR are low compared to 11's high cytotoxicity. Suggesting that 11 initiates apoptosis over a different pathway than TrxR.
The performance of these complexes can be partially ascribed to the influence of the mesityl groups located at the NHC. Other groups, such as Ott and coworkers, have indeed reported that an increase in phenyl groups can lead to an increase in cytotoxicity in different cell lines, but prominently in MCF-7 cells.22,25
Raubenheimer and coworkers were the first to investigate tetrazole-based NHC gold complexes for their anticancer properties. The cationic gold complex 12 contains one PPh3 moiety and one tetrazole-NHC ring. 12 shows cytotoxicity activity against HeLa cells (IC50 = 1.67 ± 0.20 μM).47
In 2024, the synthesis of a 1-mesityl-3-mesityl-tetrazole NHC ligand was reported. This ligand forms the cationic Au(I) bis-NHC complex 13. Complex 13 achieves high cytotoxicity with IC50 values in the low nanomolar range for leukaemia (Nalm6) cells (IC50 = 0.014 μM). Interestingly, even at 10 times higher potency (0.1 μM) 13 does not show any activity towards healthy human leukocytes, testifying some degree of selectivity.21
Since the reports of Au NHC Complexes with ligand scaffolds besides imidazole are sparely, it is difficult to determine any general trends between cytotoxicity and scaffolds. However, the activity of complexes 11b and 13, suggest that an increase in nitrogen atoms for abnormal NHC, and therefore a decrease in donating probabilities and steric hindrance, does yield more active complexes. While compound 11b caused 10.98% of Nalm6 cells to be apoptotic at concentrations of 0.05 μM after 72 h, compound 13 at the same concentrations and time caused over 75% of the cells to be apoptotic.
Especially, the low IC50 values reported for 11b, 13, and 8, which are among the lowest reported for Au NHC complexes, make exploration of new NHC ligands beyond imidazole scaffolds attractive.20,21,30 There are many yet unexplored NHC ligands for gold complexes as potential anticancer agents available.13 Investigating a variety of new scaffolds, investigating the backbone and wingtip influence in these systems, the cytotoxicity, and uptake can give interesting new insights into the field of Au NHC complexes.
Potential targets of Au–NHC complexes in cancer cells
Understanding Au NHC complex's mode of action is crucial for designing highly cytotoxic complexes. Most Au complexes induce apoptosis, the primary form of controlled cell death. Intrinsic apoptosis operates mainly through the mitochondrial and endoplasmic reticulum pathways and is a response to stimuli such as DNA damage, growth factor withdrawal, or oxidative stress.48Prominent examples of apoptosis via DNA damage are platinum(II) complexes (such as the long known cisplatin).4 Au(III) NHC gold complexes have been developed based on their isoelectronic structure to platinum(II) complexes. However, most Au(III) complexes tend to be unstable in the reducing intercellular environment and are reduced to Au(I) or Au(0). Since Au(III) complexes tend to be reduced to Au(I) complexes, it is assumed that Au(III) complexes follow a similar mode of action as Au(I) complexes.49,50
As a prominent example of Au(I) complexes, auranofin causes an imbalance in the intercellular redox system and oxidative stress. Auranofin strongly binds with thiol- and seleno-species in cells. The inhibition of the seleno-enzyme thioredoxin reductase (TrxR) by auranofin is assumed to be the leading cause of apoptosis.5 However, other modes of action have also been reported, such as the inhibition of glutathione S-transferase (GST) P1-1.51
Thioredoxin reductase as main inhibitor of Au(I) NHC complexes
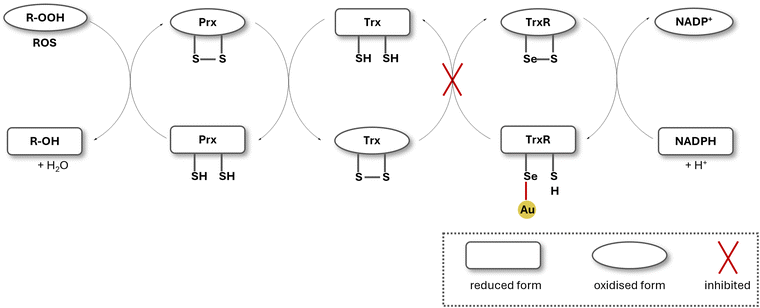
Thioredoxin reductase is part of the thioredoxin system, which includes thioredoxin (Trx) and NADPH. The thioredoxin system is involved in maintaining cellular redox homeostasis: the ability to maintain a stable internal environment in the cell despite external changes, such as oxidative stress. Thioredoxin reductase primarily reduces thioredoxin after its oxidation, caused by oxidants. Trx actively stops the accumulation of H2O2 in the cell, by converting generated H2O2 to water. The reduction of Trx via TrxR takes place at the cysteine–selenocysteine active site. Disruption of the thioredoxin system results in the accumulation of reactive oxygen species (ROS), ultimately triggering apoptosis and cell death (Fig. 3).52There is a significant amount of reports on Au(I) NHC complexes with high TrxR inhibition, making TrxR inhibition the most commonly proposed mode of action for Au(I) NHC complexes.36,40,46,53–57 Gold complexes are assumed to coordinate to the thiolate group of the cysteine or the selenolate group of the selenocysteine located on the active site of TrxR. This interaction blocks the electron transfer within the enzyme, thus disrupting the thioredoxin system and henceforth triggering the accumulation of reactive oxygen species (ROS) in the cell.58
To investigate the role of TrxR on the cytotoxicity of Au(I/III) NHC complexes, in the following paragraphs, the cytotoxicity data reported in connection to the corresponding TrxR inhibition studies are examined. In 2012, Gust and coworkers studied the correlation between TrxR inhibition and cytotoxicity, investigating bis[1,3-diethyl-4,5-diarylimidazole-2-ylidiene] gold complexes. Besides the NHC, the examined complexes contain either a bromide, a phosphine, or a thioglucose derivative-ligand. Additionally, Au bis-NHC complexes and their corresponding Au(III) complexes were tested. A comparison between the activity in HT-29 and TrxR inhibition did not yield a comprehensive correlation between cytotoxicity and TrxR inhibition (Table 1).54
| Complex | HT-29 | TrxR | Complex | HT-29 | TrxR |
|---|---|---|---|---|---|
| 6 46 | 2.1 ± 0.00 | 1200 ± 600 | 38 56 | 17.0 ± 1.8 | 4371.3 ± 322.2 |
| 14a 54 | 0.36 ± 0.01 | 3430.6 ± 249.2 | 39 56 | 4.3 ± 0.1 | 802.7 ± 68.1 |
| 14b 54 | 0.62 ± 0.02 | 6786.2 ± 616.5 | 40 56 | 3.2 ± 0.3 | 374.4 ± 9 |
| 15a 54 | 0.43 ± 0.01 | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
41 56 | 7.7 ± 0.8 | 379.8 ± 105.1 |
| 15b 54 | 0.23 ± 0.01 | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
42 56 | 6.2 ± 1.0 | 621.6 ± 51.3 |
| 16 54 | 17 ± 2.8 | 4371.3 ± 322.1 | 43 56 | 7.5 ± 2.9 | 903.6 ± 1.1 |
| 17 54 | 2.9 ± 0.1 | 1505 ± 27.1 | 44 57 | 4.0 ± 0.7 | 280 ± 120 |
| 18 40 | 3.08 ± 0.25 | 320 ± 40 | 45 57 | 1.9 ± 0.3 | 400 ± 130 |
| 19 54 | 0.47 ± 0.01 | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
46 57 | 7.0 ± 3.5 | 420 ± 120 |
| 20 54 | 0.42 ± 0.04 | 700.1 ± 40.2 | 47 57 | 4.9 ± 0.1 | 320 ± 70 |
| 21 54 | 0.37 ± 0.02 | 424.3 ± 50.4 | 48 54 | 7.6 ± 0.3 | 330 ± 90 |
| 22 54 | 3.19 ± 0.21 | 784.1 ± 155.2 | 49 36 | 5.2 ± 0.6 | 430 ± 30 |
| 23 54 | 3.22 ± 0.02 | 527.7 ± 33.5 | 50 36 | 12.1 ± 2.4 | 490 ± 150 |
| 24 54 | 0.32 ± 0.01 | 4028.7 ± 374.1 | 51 36 | 4.2 ± 0.2 | 1100 ± 300 |
| 25 54 | 0.26 ± 0.03 | 2521.5 ± 84.5 | 52 36 | 63.8 ± 8.7 | 2950 ± 700 |
| 26 54 | 0.3 ± 0.01 | 2388.3 ± 96.1 | 53 36 | 125.8 ± 49.7 | 6890 ± 1000 |
| 27 55 | 0.89 ± 0.4 | 660 ± 20 | 54 36 | 2.8 ± 1.7 | 5080 ± 1000 |
| 28 55 | 5.98 ± 1.78 | 110 ± 60 | 55 36 | 12.7 ± 1.2 | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| 29 55 | 8.85 ± 2.1 | 30 ± 0 | 56 36 | 10.5 ± 1.9 | 7230 ± 1670 |
| 30 55 | 1.46 ± 0.19 | 30 ± 10 | 57 36 | 45.3 ± 0.12 | 360 ± 200 |
| 31 56 | 3.1 ± 0.4 | 597.5 ± 89.6 | 58 36 | 7.1 ± 2.4 | 1200 ± 400 |
| 32 56 | 4.2 ± 1.0 | 668.3 ± 38.1 | 59 36 | 0.26 ± 0.06 | 6400 ± 1400 |
| 33 56 | 2.9 ± 0.1 | 1505.5 ± 27.3 | 60 36 | 5.1 ± 1.1 | 470 ± 140 |
| 34 56 | 3.3 ± 1.0 | 703.9 ± 61.5 | 61 40 | 38.6 ± 2.76 | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| 35 56 | 4.2 ± 0.3 | 1202 ± 110.3 | 62 54 | 0.33 ± 0.01 | 5163 ± 104.1 |
| 36 56 | 2.3 ± 0.1 | 815.4 ± 74.1 | 63 54 | 2.3 ± 0.1 | 815.4 ± 74.3 |
| 37 56 | 3.3 ± 0.7 | 1036.1 ± 40.4 |
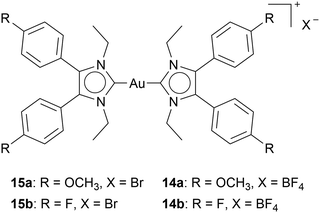
It is noteworthy that four cationic Au(I) bis-NHC complexes only differed in their anion: two containing Br−15, and the other two BF4−14, inhibit TrxR differently. While all complexes exhibit similar cytotoxicity in HT-29 cells, the BF4− containing complexes inhibit TrxR, while complexes 15a and 15b do not inhibit TrxR in the examined concentrations (Chart 5).54 These results highlight the influence of different anions in cationic Au(I) NHC complexes. Moreover, they show the sensitivity of biological activity to seemingly small changes.
 | ||
| Chart 5 Anion-dependent TrxR inhibition: BF4 – containing complexes inhibit TrxR moderately. Bromide-containing complexes are TrxR inactive. | ||
In 2013, Ott and coworkers executed a similar study, expanding the list of ligand systems: thiophenolate ligands and different wing-tip substituents. They did not find a direct correlation between TrxR results and cytotoxicity (Table 1). However, some trends can be observed: non-steric thiophenolates lead to potent TrxR inhibition, and Au(I) complexes exhibit stronger TrxR inhibition than Au(III) complexes.36
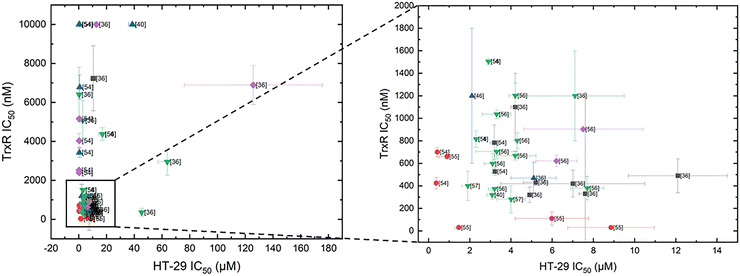
Inspired by the findings of Ott's and Gust's work, we extended their table of compounds to include data from more recent published TrxR inhibition studies, which are given in Table 1. The cytotoxicity in HT-29 cells was, therefore, compared against TrxR inhibition of different complexes.36,40,46,53–57 Just as with Ott's and Gust's results, no direct correlation can be observed (Fig. 4). Of the 53 considered compounds, 14 show high cytotoxicity in HT-29 cells, with IC50 values in the nanomolar range, eight display moderate to non-existent TrxR inhibition. However, Au NHC complexes with phosphine ligands are potent TrxR inhibitors, consistently showing TrxR inhibition with IC50 values in the nanomolar range.
Fig. 4 indicates that Au(I) bis-NHC complexes exhibit significantly weaker TrxR inhibition compared to other Au NHC complexes. While chloride-containing Au(I) NHC complexes achieve high TrxR inhibition, Au(I) bis-NHC complexes have repeatedly achieved higher cytotoxicity than their halide counterparts.22,33,54 This phenomenon is often explained by the high uptake of gold(I) bis-NHC complexes into the cell. Due to their lipophilicity and charge, Au(I) bis-NHC complexes and Au(I) NHC phosphine complexes are called delocalised lipophilic cations (DLCs). The mitochondria of cancer cells have an elevated transmembrane potential (Δψ). DLCs can utilise this potential to accumulate in mitochondria.59 One example is given in the work of Ott and coworkers: the synthesised Au(I) NHC complexes show low efficiency against TrxR, but higher uptake.22 However, this discrepancy between TrxR inhibition and cytotoxicity is hard to explain in other cases.
This inconsistency may be explained by the requirement of ligand dissociation for an effective TrxR inhibition by Au(I) bis-NHC complexes. Ligands, as mentioned above, can inhibit cytotoxicity of their own.29,39–41 The resulting Au(I) mono-NHC complex and the dissociated ligand may both contribute to the cytotoxicity, thereby accounting for the observed effect. Alternatively, the Au(I) bis-NHC complexes may exert their cytotoxic effects through a TrxR-independent mechanism entirely, as other researchers in the field of Au(I) NHC complexes have stated.20,46,54,60,61
Nevertheless, the TrxR inhibition results summarised in Fig. 4, together with previous reports,20,46,54,60,61 indicate that other pathways besides TrxR inhibition seem to play a role for various Au(I/III) NHC complexes.
Pathways beyond thioredoxin reductase
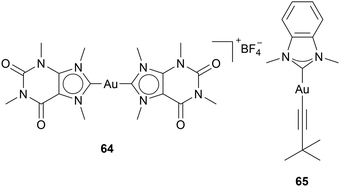
Yet, the question remains: how do Au(I) NHC complexes interact in the cell besides TrxR inhibition? The reports of other mechanistic pathways are scarce, due to various reasons: for one, the expectation of TrxR being the main pathway discourages researchers from finding other paths, but most importantly, identifying pathways is difficult and costly. Nevertheless, there have been reports on different targets besides TrxR for Au NHC complexes.Casini and coworkers developed a caffeine-based NHC ligand, which can bind to gold to form an Au(I) bis-NHC complex 64 (Chart 6). Investigations of 64 show a strong binding ability to the G-quadruplexes structure of DNA. G-Quadruplexes consist of four-stranded nucleic acid structures formed by guanine-rich sequences that form hydrogen bonds. These noncanonical structures play a critical role in gene expression, telomere maintenance, and genome stability.62 Molecular docking simulations showed that three molecules of 64 can bind to one quadruplex due to: the planar structure of compound 64, the positive charge enhancing the affinity to the negatively charged DNA, and the two aromatic caffeine ligands, able to interact with the guanine moieties through π-stacking.63 This coordination stabilises the G4-structure, therefore interfering with DNA replication and transcription. Some other complexes have since shown G-quadruplex DNA coordination. The groups of Casini and Kühn also tested the ability of imidazole- and benzimidazole-based Au(I) NHC alkynyl complexes to bind to G-quadruplex DNA. The FRET DNA melting analysis indicates that compound 65 significantly stabilises G-quadruplex DNA.64 The benzimidazole-based complex 65 highlights the ability of benzimidazole moieties to interact with G-quadruplex DNA (Chart 6).64
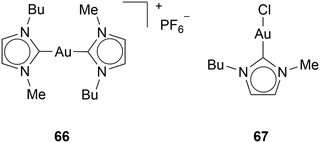
Magherini and coworkers suggested that Au(I) NHC complexes inhibit the electron transport chain. The disruption of the respiratory complex, a series of protein complexes located in the inner membrane of the mitochondria that are essential for energy production through oxidative phosphorylation, leads to ROS and apoptosis.65 Magherini and coworkers showed that complex 66 inhibits the respiratory complex (Chart 7). Moreover, they have shown that halide Au(I) NHC complex 67 can interact with other cysteine-containing enzymes, such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which is a crucial glycolytic enzyme that catalyses the sixth step in glycolysis.66 However, the inhibition of GAPDH outside of the cell is weak. Only 15–20% of GAPDH is inhibited. Therefore, Magherini and coworkers suggest that GAPDH downregulation might be a result of ROS.67
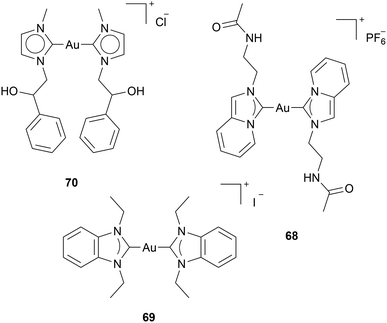
Some reports have been focusing on the effect of Au(I) NHC on p53. p53 is a tumour suppression protein that detects cellular stresses and induces adequate responses, such as ROS-associated apoptosis.68 In almost all human cancer cells, wild-type p53 activity is disrupted. Reports about Au(I) complexes inducing P53-dependent apoptosis have been published.34,69,70 However, in some cases, it is unclear if ROS is caused by TrxR inhibition, inducing p53 activity, or if Au(I) NHC complexes directly activate p53. Dinda and coworkers found that tumour death by complex 68 occurs by activation of p53 and inhibition of anti-apoptotic NF-κB transcription factor and metastatic markers: VEGF and MMP-9 (Chart 8). However, the authors assume that ROS is induced upstream of p53, and the anticancer activity of complex 68 results from TrxR inhibition.70 Ott and coworkers investigated complex 69 against different cell lines with wild-type p53 and p53 mutations. 69 induces high ROS levels regardless of their p53 mutations; however, the pro-apoptotic response occurs in a p53-dependent manner. The authors suggest that 69 can exhibit anticancer effects both dependent and independent from p53.69 Saturnino et al. assessed complex 70 concerning the expression levels of p53 and p21. P21, a cyclin-dependent kinase inhibitor, plays a crucial role in cell cycle regulation, particularly in halting cell division in response to DNA damage or other stress signals.71 The levels of p53 and p21 increase depending on the anticancer activity. Molecular docking simulation illustrated the possibility of complex 70 binding to the zinc finger domain of Sp1, which is assumed to be a co-activator in p53-mediated gene regulation.34
The investigations of p53 and GAPDH show the difficulty of detecting new biological targets for Au NHC complexes. Down- or up-regulation of biological targets could be directly or indirectly caused by Au NHC complexes.
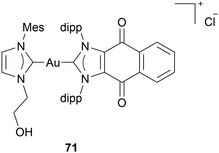
Another novel anticancer approach in Au NHC complex research is the work of Sen et al. They assessed Au(I) bis-NHC complexes' ability to induce immunogenic cell death (ICD). Unlike conventional apoptosis, which often evades immune detection, ICD not only eliminates cancer cells but also activates the host immune system to mount a sustained antitumor response. When cancer cells undergo ICD, damage-associated molecular patterns signal to activate antigen-presenting cells (ACPs). These APCS then process tumour antigens and present them to T cells. This process transforms a local tumour cell death event into a systemic immune response, enhancing the elimination of residual tumour cells and potentially establishing immunological memory against cancer recurrence.72,73 Sen et al. suggest that a double-enhanced ROS mechanism can induce ICD. Therefore, they developed Au(I) NHC complex 71 containing a quinone moiety (Chart 9). Complex 71 is very active in human A549, HCCT-116 cells, and mouse colon carcinoma CT-26. MTT assays indicate IC50 values in the nanomolar range after 72 h incubation. Afterward, complex 71 was assessed in vivo. Mice were injected with 71-pretreated CT-26 on their right flank; 6 days later, they were injected with untreated CT-26 cells. Some mice that were injected with pretreated cells showed delayed or no tumour development. Therefore, complex 71 is assumed to be able to induce ICD.74
Che and coworkers detected vimentin, nucleophosmin, HSP60 and YB-1 to play a potential role in the mechanism of Au(III) NHC complex 72. Vimentin is an intermediate filament protein whose primary function is to provide structural support to the cell, maintaining the integrity of organelles, such as mitochondria, inside the cell.75 Nucleophosmin is a nucleolar phosphoprotein released from the nucleolus in response to cellular stress. It interacts with p53, and is crucial for maintaining genomic stability.76 HSP60 is a heat shock protein, found primarily in mitochondria, where it is essential for maintaining the integrity and homeostasis of the mitochondrial proteome, especially under cellular stress.77 Che and coworkers were able to detect these proteins as targets for 72, due to a combination of simulation and photo-active modification of 72. They attached two clickable photoaffinity probes to identify multiple targets for Au(III) NHC complex 72, bearing pincer ligands (Chart 10). The photo-active diazirine group located on the wingtip of complex 73 can interact with proteins upon light irradiation. The alkynyl group on the second wingtip can be clicked to azido-biotin or dyes. After treating HeLa cells with complex 73, UV light, copper and azido-biotin were added. Gel electrophoresis and MALDI-TOF mass spectrometry identified cellular proteins of HeLa cells: mitochondrial HSP60, nucleoside diphosphate kinase A (NKDA), nucleophosmin (NPM), vimentin (VIM) and peroxiredoxin 1(PrDX1), nuclease-sensitive element binding protein (Y box binding protein YB-1). Subsequently, the subcellular distribution of complex 73 was investigated. Again, HeLa cells were incubated with 73 before irradiation with UV light, and a click reaction with Alexa Fluor 488 took place. Complex 73 is mainly detected in the cytoplasm, with a small portion present in the nucleus. Analysis of complex 72, such as western blot analysis, molecular docking, and hybrid quantum mechanics/molecular mechanics (QM/MM), affirmed the possible binding of complex 72 to vimentin, nucleophosmin, HSP60, and YB-1.78
 | ||
| Chart 10 Complex 73 bearing a photo-sensitive azido group and an alkynyl group and the corresponding complex 72. | ||
A deeper understanding of the mechanisms of Au(I) NHC complexes is essential for their rational design and future applications. The presented investigations all detected targets attributed to a mitochondria-based pathway. More studies in this field are needed to give a clearer understanding of the mechanisms. While TrxR as a target and apoptosis as a mechanism are established, other targets and regulated cell death pathways, such as ICD, have emerged as a compelling mechanism of action. Additional regulated cell death pathways, such as ferroptosis (typically initiated by excessive ROS accumulation), remain underexplored in the context of gold-based therapeutics. Investigating these mechanisms requires precise tools to study cellular uptake, distribution, and target engagement. Labelling strategies play a central role in these investigations. In cell modification, like in Che's work,78 offers a unique alternative to traditional pre-labelling techniques, which may interfere with compounds' bioavailability or intracellular behaviours. In the context of the next chapter, other reports of labelling Au NHC complexes will be described.
Strategies in the field of Au–NHC complexes: cytotoxicity, labelling, selectivity and drug-resistance
In this chapter, a brief overview of different fields of investigation concerning Au NHC complexes in anticancer research is provided. Established procedures and reports, having gained considerable attention, will be elaborated.Tracing Au NHC complexes in vitro and in vivo
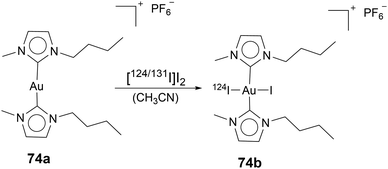
As mentioned in the previous section, labelling Au(I) NHC compounds is a necessary tool for gaining insights into mechanisms. Au(I) NHC complexes can be labelled with fluorescence, luminescence, or radioactive markers. While all three labelling types can be used for in vivo and in vitro studies, luminescence and fluorescence markers are commonly used to track Au(I) NHC in vitro, for in vivo experiments, radiolabelling is a standard procedure, as sacrificing the animal is not necessary. Salassa and coworkers used radioactive iodine 124I to oxidise Au(I) bis-NHC complex 74a to Au(III) complex 74b (Scheme 1). As previously mentioned, most Au(III) complexes are reduced to Au(I) under cellular environment,49,50 which explains their similar cytotoxicity and mode of action compared to their Au(I) counterparts.54,56,79,80 After 74b admission to the rat, the compounds' distribution can be traced by positron emission tomography (PET). To determine if the radiation was attributed to free radioactive iodine, arising due to reduction in the cell to 74a, inductively coupled plasma mass spectrometry (ICP-MS) analysis was used (Fig. 5). ICP-MS can be used to detect metals in very low concentrations and is often used for uptake studies in cells.81 The ICP-MS data, together with the PET images, determined localisation in the liver, kidneys, and lungs.82 Therefore, this simple radiolabelling of complex 74a to compound 74b can give good information about the distribution of complex 74ain vivo.82 Making radiolabelling due to radioactive iodine oxidation a great tool to analyse the distribution of Au(I) NHC complexes in vivo.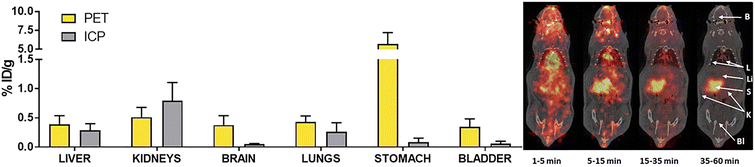 | ||
| Fig. 5 Concentration of radioactivity (as determined by PET) of 74b and concentration of Au (as determined by ICP-MS) of 74a in different organs, together with the PET images (maximum intensity projections) obtained at different time intervals after intravenous administration of 74b.82 | ||
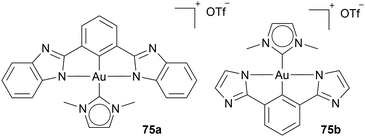
Additionally, Che and coworkers use Au(III) reduction probability as an advantage by adding fluorescence active ligands (2,6-bis(benzimidazol-2-yl)pyridine) or 2,6-bis(imidazol-2-yl)pyridine to an Au NHC complex 75a, 75b (Chart 11). In the vicinity of thiol-containing compounds, the ligand detaches and reveals its fluorescence activity, while an Au(I) NHC complex is formed. The complexes 75a, 75b are utilised as a prodrug for Au(I) NHC complexes and function as a fluorescence marker.78,83
Hussaini and Razali give a good overview of Au(I) NHC complexes showing photoluminescence,84 which serves as a good inspiration for which Au(I) complexes to investigate for anticancer activity. Besides organic modification and d10–d10 interaction, adding metalorganic moieties with luminescent properties to form a hetero-bimetallic system has also been investigated. Hemmert and coworkers added a [Ru(bipy)3] building block to an Au(I) mono-NHC complex through wingtip modification of the NHC. Unfortunately, the resulting complex showed moderate to absent activity towards human hepatocellular carcinoma (Hep3B). The authors concluded that the [Ru(bipy)3] was responsible for the deactivation of the compound.85
This stands in contrast to various other reports of heterobimetallic systems that show enhanced cytotoxicity.86–89
Multitargeting moieties
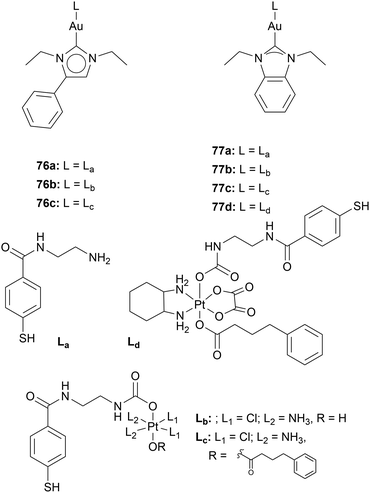
Adding a moiety facilitating regulated cell death through a mechanism other than gold complexes can increase the cytotoxicity. Moreover, drug resistance can be prevented or overcome since drug resistance merely occurs for single-target anticancer agents caused by genetic mutations.90 Multitargeting due to hetero-bimetallic systems is highly reported in the field of Au NHC complexes. Reports about Ru–Au, Fe–Au, Cu–Au, Ti–Au, and Pt–Au complexes have been published.91 Especially, Contel and coworkers have thoroughly investigated Ru–Au and Ti–Au complexes.86–89Ott and coworkers synthesised five compounds containing a Pt(IV) complex that is linked to an Au(I) NHC moiety via an ethylenediamine-derivatised mercaptobenzoic spacer 76b,c, 77c-d (Chart 12). Compounds against 76b,c, 77c-d were assessed together with compounds 76a and 77a, containing no Pt(IV) moiety. The stability of the compounds was tested in cell medium. Compounds 76b and 77b show a half-life of 19 and 15.8 h, respectively, while the other platinum-containing compounds are stable for several days. To mimic the reducing environment of the cell, compound 77c was monitored in a solution of ascorbic acid. HPLC and mass spectroscopy analysis showed complex 77a, 77c, and free ligand. TrxR inhibition tests of all compounds were conducted against isolated TrxR. The platinum-containing compounds show higher TrxR inhibition than the Au(I) compounds 76a and 77a. However, when tested towards cysteine- and selenocysteine-containing peptides, compounds 76a and 77a react immediately with the peptides while compounds 76b,c, 77c-d only convert to 15–20%. This indicates that the Pt(IV)–Au(I) compounds might interact with TrxR in another way besides the direct binding of the Au(I) to the selenocysteine moiety.
The compounds were also assessed against A2780 cells and A2780 cells resistant against cisplatin (A2780cis). Compound 76c is the most active compound in both cell lines (IC50 = 0.08 ± 0.001 μM (A2780) and IC50 = 0.15 ± 0.01 μM (A2780cis)), even though compound 76c contains a platinum moiety. However, when platinum-free Au–NHC complex is incubated together with cisplatin in A2780cis cells, significantly higher IC50 values are reached (compound 76c: IC50 = 0.15 ± 0.01 μM; Au NHC + cisplatin: IC50 = 1.41 ± 0.01 μM). These results highlight that gold complexes can overcome cisplatin resistance and show a critical difference between multi-targeting (one drug) and multiple-targeting (several drugs) therapy.26
The combination of Au NHC complexes with ferrocene is a prominent example.92–95 Several examinations show that Au NHC complexes cause apoptosis due to reactive oxygen species (ROS) formation.
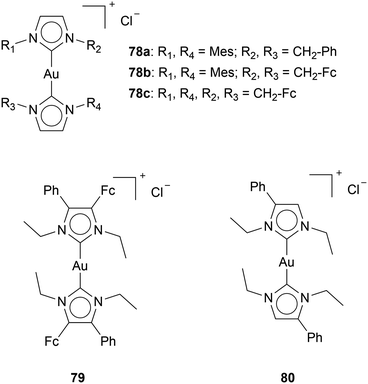
The synergy of Au NHC complexes with a redox-active species, Ferrocene, should increase apoptosis by increasing the formation of ROS. Although Arumbula and Arumugam show enhanced cytotoxicity with an increase of ferrocene moieties on cationic Au(I) bis-NHC complexes (78a,b,c), which could indicate a synergetic effect of ferrocene with Au complexes,96 some other reports are not so comprehensive (Chart 13). Muenzner et al. reported an Au bis-NHC complex 79 with one phenyl group and one ferrocene moiety on the backbone of the NHC.93 Complex 79 shows IC50 values of 0.13 ± 0.001 μM for HT-29 cell lines. A year later, Ott's group reported a similar complex: Au bis-NHC complex 80 with one phenyl group located on the backbone of the ligand.22 The IC50 value of complex 80 for HT-29 cells is 0.15 ± 0.001 μM. Comparison of cytotoxicity between reports has to be done carefully; however, in this case, the difference in cytotoxicity seems negligible. One would assume a redox-active species to directly influence and significantly impact the cytotoxicity. However, the cytotoxicity of complex 79 and complex 80 at the same incubation times is very similar. Since ferrocenes are aromatic moieties, the enhanced cytotoxicity of ferrocene-containing complexes might be attributed to aromatic-moiety addition.
Besides, hetero-bimetallic systems, dual-targeting approaches with organic compounds in combination with Au(I) NHC complexes have also been reported.
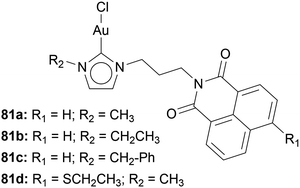
Ott and coworkers implemented 1,8-naphthalimide moieties into Au(I) NHC halide complexes 81 by modifying the side arm of the imidazole-based NHC (Chart 14). The incorporation of 1,8-naphthalimide does not hinder the TrxR inhibition. IC50 values of 0.28 ± 0.12 μM and 0.40 ± 0.13 μM are comparable to the inhibition of [AuCl(PR3)]. The gold moiety does not inhibit the intercalation of 1,8-naphthalimide into DNA. Via circular dichroism spectroscopy, the structural changes of circulating tumour DNA incubated with compounds 81 have been observed.57
Liu and coworkers investigated the combination of a selective oestrogen receptor degrader (SERD) with Au(I) NHC complexes.28 Endocrine therapy, which uses SERDs as treatment, is the conventional approach for advanced oestrogen receptor (ER) positive breast cancer. SERDs are destroying the oestrogen receptor in the cell, which can stop tumour growth in ER-positive cell lines,97 such as MCF-7. Since there is evidence that Trx and TrxR are regulated by oestrogen and ER,98 Liu and coworkers investigated if a combination of TrxR inhibitor with SERDs may have a positive synergic effect against malignant cells. The newly formed complex 82, incorporating G1T48, was intensively studied in vitro: 82 showed anti-proliferation properties in the lower micromolar range in MCF-7 cells, TrxR inhibition, and maximum degradation efficacy is reached at 1 μM. Lastly, in vivo studies in mice were performed. Complex 82 exhibits a higher antitumor activity than auranofin and fulvestrant and stimulates the immune response. During the experiment, the mice did not suffer from significant body weight loss. No significant morphologic or structural changes were observed in the heart, liver, spleen, and kidney tissues of the mice.28 Besides these remarkable results in vitro and in vivo, the investigations lack a control study of G1T48 given together with Au(I) bis-NHC complexes. Synthesis of dual-targeting compounds is often costly, highly difficult, and time-intensive. These syntheses should take place if a direct bond between the two moieties is beneficial.
Overcoming drug-resistance
One major obstacle in anticancer therapy is the development of drug resistance during chemotherapy. Drug resistance, the unresponsiveness towards drugs, can happen in every drug application. There are several known drug-resistance mechanistic pathways, while individual drugs can trigger specific pathways or several pathways at once; naming all drug-resistant pathways known would expand the scope of this review.99 Notably, in the case of gold(I) compounds, a drug-resistance mechanism involving TrxR may be hypothesised.100Especially in chemotherapy, where drug resistance can occur towards standard chemotherapeutics, designing new compounds that can overcome drug resistance is essential, as overcoming drug resistance can improve the chances of patient recovery.101 In chemotherapy, cytostatic drugs are often given in combination.102 Therefore, Au(I) complexes are frequently tested towards cell lines with drug resistance of common cytostatic drugs, such as cisplatin or daunorubicin, and multiple drug-resistant cell lines.20,21,23
Also, the ability to overcome cisplatin resistance of A2780 cells with Au(I) bis-NHC complexes has been assessed often. For example, Auranofin and complex 69 were tested against A2780 cells and A2780cis. The A2780cis cells were obtained by treating A2780 cells with sub-toxic concentrations of cisplatin. The activity of complex 69 against resistant and non-resistant cell lines is the same, with IC50 values being 0.055 ± 0.017 μM (A2780) and 0.071 ± 0.047 (A2780cis). Additionally, the A2780cis cells were treated with sub-toxic levels of complex 69. Afterward, cisplatin was assessed against this cell line, and the cytotoxicity of cisplatin towards A2780cis cells is not enhanced.23
The previously mentioned complex 13 was examined against chronic myeloid leukemia (K562), B-cell leukaemia (Nalm6), and burkitt-lymphoma (BJAB) cells. Complex 13 displays IC50 values in the nanomolar range. When tested against a daunorubicin-resistant K562 cell line NiWi-Dau, complex 13 shows even more potent cytotoxicity (0.16 μM K562; 0.14 μM Niwi-Dau).
Complex 13 causes a dose-dependent decrease in mitochondrial outer membrane potential (MOMP). Oligomerization of the BCL-2 can reduce the MOMP. Moreover, BCL-2 is overexpressed in Niwi-Dau cells, which is an indication that complex 13 has an impact on the BCL-2 pathway.21 Complex 13 was assessed against leukaemia and lymphoma cells, which is unusual for Au NHC complexes. Au NHC complexes are often tested against solid tumours commonly known, easily accessible, and inexpensive cancerous cell lines: HeLa (cervical), HT-29 (colon), MCF-7 (breast), A2780 (ovarian), A549 (lung), PC3 (prostate).
Even though auranofin is currently only applied in clinical trials, some studies have investigated resistance in auranofin-treated cells. Landini et al. made A2780 cells resistant to auranofin and tested their characteristics. Interestingly, they tested the activity of two NHC containing Au(I) complexes towards the drug-resistant cell line A2780/AF-R. Complex 74a and 74a show highly different activity toward A270/AF-R. While the mono-NHC Au(I) complex 74a shows cross-resistance, complex 74a completely circumvents resistance to auranofin.103 These findings appear to support the previously proposed possibility of a different mechanistic pathway for Au(I) bis-NHC complexes compared to auranofin and Au(I) mono-NHC complexes. It also highlights that Au(I) bis-NHC complexes may help to overcome drug resistance, underscoring their promise as effective candidates for next-generation chemotherapeutic agents.
Enhancing selectivity towards malignant cells
MCF-7 cell lines are commonly used since Au(I) NHC complexes have consistently shown high cytotoxicity against this cell line.20,22,54,55 Therefore, high selectivity towards MCF-7 is strongly desirable.To enhance selectivity towards cancer cells and target specific cell lines, targeting vectors can be employed. These vectors can specifically recognise and bind to cancer cell lines that exclusively overexpress certain receptors.104,105 Alternatively, a more general targeting approach is also possible, as many cancer cells, due to their high proliferation rate, commonly overexpress particular receptors.105–107
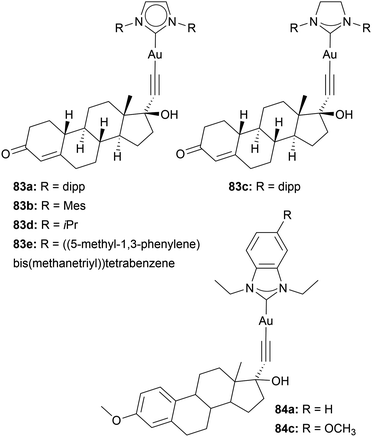
MCF-7 bears α-positive oestrogen receptors. Therefore, the groups of Ott and Nolan bound oethisterone to Au imidazole-based NHC complexes 83 (Chart 15). They thereby facilitated gold's ability to bind to alkynyl moieties. The resulting compounds were tested against A549, HT-29, MDA-MB-231, and MCF-7 cells. Complex 83c is the least active, with IC50 values of 13.6 to 25.7 μM. Complex 83a displays the most effect with IC50 values of 3.7 ± 0.3 μM in MDA-MB-231 cells. Interestingly, for all examined complexes, the cytotoxicity is the highest in triple-negative MDA-MB-231 cell lines, despite the lack of oestrogen receptors. However, 83a achieves high cellular uptake compared to the complex without oethisterone 83a in MCF-7 cells.108 In 2024, Ott and coworkers again utilised gold's affinity towards alkynyl and added mestranol to gold benzimidazole-based NHC complexes 84. However, based on their anti-proliferating activity, the mestranol-containing complexes perform similarly to Au(I) NHC complexes without mestranol moiety.109 The inconclusiveness of these studies may be attributed to the instability of the alkynyl–gold bond in the presence of thiol-containing molecules.64 Attaching oestrogen to the backbone or wing-tip of the NHC may improve complex stability and lead to more conclusive results.
Proliferating cells, such as cancer cells, use a glycolytic metabolism, thereby stimulating a higher sugar intake. This effect, called the Warburg effect, is used in cancer therapy by using glycolysis inhibitors or glucose derivatives as targets.107 Auranofin itself also possesses a thioglucose moiety. Inspired by the Warburg effect and auranofin, several Au(I) NHC complexes bear glucose derivatives as a second ligand53 or on the wingtips.33
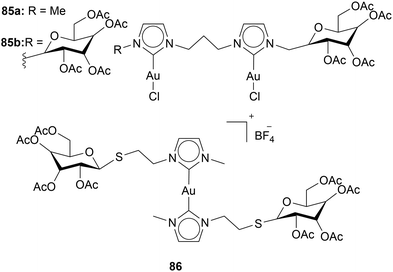
Tubaro and coworkers investigated the influence of acetylated glucopyranose moieties on the cytotoxicity of Au(I) NHC complexes. Therefore, the acetylated glucopyranose was incorporated into the wingtip of the NHC. Compounds with one and two acetylated glycopyranose were assessed (Chart 16). With an increase of glycopyranose moieties, an increase in cytotoxicity is observed. In all cell lines 85a and 85b were tested against (SVT2, BALB/c3T3 and A431), 85b is more effective than 85a.110
Pratesi and coworkers synthesised an Au(I) NHC complex 86 with one acetylated glycopyranose on the wingtip. The activity against A2780 cells is only moderate (IC50 = 13 ± 1 μM). However, 86 shows remarkable selectivity. In the healthy cell line, HSkMC (human skeletal muscle cells), toxicity is low (IC50 = 280 ± 18 μM).33
Furthermore, Kühn and coworkers added a sugar derivative to triazole-based Au(I) NHC complex 11b. Here, the sugar derivative was clicked to an azido moiety located on the backbone of the triazole 87a (Chart 17). The azido group allows the incorporation of biologically active species via click chemistry. 2-Propinyl-tetra-O-acetyl-β-D-glucopyranoside via the CuAAC reaction and bicyclononyne via the SPAAC reaction was added, as a proof of concept. Both reactions resulted in the desired compound. It is further planned to investigate modifying complex 87a with biologically active species. Moreover, the azido group can be used for fluorescence labelling, as Che et al. have reported.78 Investigations of cytotoxicity of the complexes 87a, 87b, and 87c display interesting IC50 values. All complexes 87 perform better in A2780 cells than in MCF-7 cells. The methyl-substituted complex 11b shows the opposite trend. The IC50 values of complex 11b are 360 ± 90 nM (A2780) and 84 ± 16 nM (MCF-7), while complex 87a shows higher efficiency for A2780 cells (26.6 ± 1.3 nM) and higher IC50 values for MCF-7 cells (261 ± 75 nM). This trend highlights the influence linkers can play on cytotoxicity.24
 | ||
| Chart 17 Au(I) bis-NHC complexes bearing an azido moiety at the backbone, which can be utilised via click chemistry. | ||
Tacke and coworkers explored direct binding between the Au complex and vectors, by metal–sulphur coordination. Direct bioconjugation between the vector and the complex comes with advantages: no linker influence can occur, while the synthetic expense is significantly decreased.
Tacke and coworkers bioconjugated Au NHC complexes with Human serum albumin – recombumin (rHSA), complex 88a. In rats, radiolabeled rHSA accumulated in tumours; therefore, Tacke and coworkers tested rHSA ability as target drug delivery. By conjugating rHSA to gold via the free cysteine moiety of rHSA, the ability of thiol–gold linked protein metal complexes is tested as well. The complex 88a remains stable for 48 h in human plasma and retains its ability to bind to neonatal FcRn receptors.
Moreover, based on their previous results, Tacke and coworkers utilised the engineered monoclonal antibody (Thiomab LC-V205C). Antibodies can be used as drug carriers since they are able to recognise antigens overexpressed in cancer cells. Tacke and coworkers chose Thiomab LC-V205C, which possesses an additional free cysteine per light chain, where the Au(I) NHC complex can bind. Thiomab can bind to the Her2 antigen in SKBR3 breast cancer cells that overexpress Her2. Complex 88b (Chart 18) was tested against three different cell lines: SKBR3, MDA-MB-231 (breast cancer), and MCF10A (non-tumorigenic breast cells). MDA-MB-231 and MCF10A do not express HER2 receptors. The cell growth (GI50) is reduced by adding antibodies (GI50 = 13.64 μM 88Cl; GI50 = 9.85 μM 88b) in SKBR3. However, the GI50 values towards the Her2 antigen-negative cell lines are also reduced. The GI50 between 88Cl and 88b differed around 3 μM in all cell lines independent of Her2 expression.104,111 Even though the Thiomab LC-V205C moiety made the complexes more cytotoxic, it failed to make compound 88 more selective. However, targeted therapy with antibodies has been successfully applied in medicine106 and should be investigated further for Au(I) NHC complexes.
 | ||
| Chart 18 Functionalisation of chloride Au(I) NHC complexes with modified antigens via a sulphur–gold-bond. | ||
Based on the above-discussed reports, modifying the NHC ligand at the backbone or wingtip to attach targeting vectors appears to be a more favourable strategy than direct coordination to the gold centre.
Conclusions and outlook
Au NHC complexes appear to have the potential to be a new generation of anti-tumour drugs and are promising candidates for clinical trials. Several complexes have shown remarkable in vitro results: cytotoxicity reaching IC50 values in the nanomolar range,20–24 overcoming drug resistance against cisplatin or daunorubicin-resistant cell lines, or, even more impressively, against multidrug-resistant cells.21,26 Some Au NHC complexes were able to show selectivity towards malignant cells over healthy cells without intensive modifications.21,25In vivo studies of Au NHC complexes have demonstrated tumour regression, while the vitality of the mice was maintained.27,28However, extensive research is still required before Au NHC complexes can be utilised in clinical trials.
A deeper understanding of structure–activity relationships and cellular mechanisms is essential to optimise therapeutic performance and selectivity. While inhibition of TrxR remains the most cited mode of action, the lack of a clear correlation between TrxR inhibition and cytotoxicity in several studies indicates that Au NHC complexes may exert their anticancer effect through multifactorial or other regulated cell death pathways.20,46,54,60,61 Especially, Au bis-NHC complexes show remarkable cytotoxicity, despite low inhibition of TrxR. Au bis-NHC complexes may act over an intrinsically different pathway, such as G-quadruplex DNA interaction,63,64 or over several pathways. The remarkable ability of NHC complexes to overcome drug resistance might be an indication of their multi-targeting pathway. This review sheds light on the cytotoxicity of the ligand system, which is often overlooked, and in a few cases, even exceeds the cytotoxicity of the corresponding gold compounds.29,40 The high cytotoxicity of Au bis-NHC may derive from ligand dissociation, which is needed for TrxR inhibition. Therefore, more ligands should be investigated for their cytotoxicity and their mechanisms in the cell.
Investigations of other mechanisms of Au NHC complexes all point to a mitochondrial-based pathway. This is in alignment with the high number of reports suggesting apoptosis through the mitochondrial-based pathway as a regulated cell death mechanism.20,70,74,83 However, the discovery of Au(I) NHC complexes inducing immunogenic cell death not only makes Au(I) NHC complexes an attractive candidate for immune-oncology applications but should also spark interest in investigating different regulated cell death mechanisms.74
Nevertheless, investigations of biological targets are challenging to approach. Au NHC complexes might directly or indirectly influence the down or up-regulation of a biological target. Therefore, finding the right tools for labelling Au(I) NHC complexes, identification without loss of activity, is highly important. Even though great tools have been reported,82,83 they need to be tested out for various types of Au(I) NHC to see if they are applicable in the great scheme.
To reduce side effects and achieve success in clinical trials, the complexes need to be selective towards cancer cells.
Targeted therapy is a good approach to achieve high selectivity. The targeting approaches for Au NHC complexes are leading to varying results. Attaching the targeting vectors via covalent bonding on the NHC is preferred to ligand coordination via sulphur or alkyl bonds, as these bonds tend to break in the presence of thiol-containing molecules.24,108,109,112 There is great potential for targeted therapy in the field of Au NHC complexes as anticancer agents to gain selectivity.
Some Au(I) NHC complexes have shown selectivity without modification. Especially abnormal coordinating NHCs have shown high cytotoxicity to cancer cells, while showing low toxicity to healthy cells.20,21 While imidazole and benzimidazole-based NHCs have been intensively published in the field of Au NHC complexes as anticancer agents, other scaffolds, like abnormal NHCs, have not been widely investigated. Therefore, novel NHC scaffolds could be explored for their cytotoxicity and selectivity.
Accordingly, Au NHC complexes hold significant potential as anticancer agents. Their structural tunability, multifaceted mechanisms of action, and demonstrated ability to overcome drug resistance make them compelling as next-generation chemotherapeutics. Systematic exploration of ligand properties, target engagement, and in vivo behaviour will be crucial for advancing these compounds to reach clinical trials.
Conflicts of interest
There are no conflicts of interest.Data availability
This tutorial review article does not include primary research results, software, or code. No new data was generated or analysed.Acknowledgements
Melanie E. Hoffmann gratefully acknowledges the financial and academic support provided by the Hans-Böckler Foundation through her doctoral scholarship (416387).References
- J. Ferlay, M. Colombet, I. Soerjomataram, D. M. Parkin, M. Piñeros, A. Znaor and F. Bray, Int. J. Cancer, 2021, 149, 778–789 CrossRef CAS.
- R. Zheng, S. Wang, S. Zhang, H. Zeng, R. Chen, K. Sun, L. Li, F. Bray and W. Wei, Sci. Bull., 2023, 68, 2620–2628 CrossRef.
- A. J. R. Carter and C. N. Nguyen, BMC Public Health, 2012, 12, 526–537 CrossRef PubMed.
- A. W. Prestayko, J. C. D'Aoust, B. F. Issell and S. T. Crooke, Cancer Treat. Rev., 1979, 6, 17–39 CrossRef CAS.
- T. Onodera, I. Momose and M. Kawada, Chem. Pham. Bull., 2019, 67, 186–191 CrossRef CAS.
- F. H. Abdalbari and C. M. Telleria, Discover Oncol., 2021, 12, 42 CrossRef CAS.
- B. M. Sutton, E. McGusty, D. T. Walz and M. J. DiMartino, J. Med. Chem., 1972, 15, 1095–1098 CrossRef CAS.
- P. J. Barnard, M. V. Baker, S. J. Berners-Price and D. A. Day, J. Inorg. Biochem., 2004, 98, 1642–1647 CrossRef CAS PubMed.
- H. W. Wanzlick and H. J. Schönherr, Angew. Chem., Int. Ed. Engl., 1968, 7, 141–142 CrossRef CAS.
- K. Öfele, J. Organomet. Chem., 1968, 12, 42–43 CrossRef.
- W. A. Herrmann, M. Elison, J. Fischer, C. Köcher and G. R. J. Artus, Angew. Chem., 1995, 107, 2602–2605 CrossRef.
- T. Droge and F. Glorius, Angew. Chem., Int. Ed., 2010, 49, 6940–6952 CrossRef.
- H. V. Huynh, Chem. Rev., 2018, 118, 9457–9492 CrossRef CAS PubMed.
- N. Segaud, C. Johnson, A. Farre and M. Albrecht, Chem. Commun., 2021, 57, 10600–10603 RSC.
- M. V. Baker, P. J. Barnard, S. J. Berners-Price, S. K. Brayshaw, J. L. Hickey, B. W. Skelton and A. H. White, Dalton Trans., 2006, 3708–3715 RSC.
- M. V. Baker, P. J. Barnard, S. J. Berners-Price, S. K. Brayshaw, J. L. Hickey, B. W. Skelton and A. H. White, J. Organomet. Chem., 2005, 690, 5625–5635 CrossRef CAS.
- P. J. Barnard, M. V. Baker, S. J. Berners-Price, B. W. Skelton and A. H. White, Dalton Trans., 2004, 1038–1047 RSC.
- M. Mora, M. C. Gimeno and R. Visbal, Chem. Soc. Rev., 2019, 48, 447–462 RSC.
- M. Porchia, M. Pellei, M. Marinelli, F. Tisato, F. Del Bello and C. Santini, Eur. J. Med. Chem., 2018, 146, 709–746 CrossRef CAS.
- J. F. Schlagintweit, C. H. G. Jakob, N. L. Wilke, M. Ahrweiler, C. Frias, J. Frias, M. König, E.-M. H. J. Esslinger, F. Marques, J. F. Machado, R. M. Reich, T. S. Morais, J. D. G. Correia, A. Prokop and F. E. Kühn, J. Med. Chem., 2021, 64, 15747–15757 CrossRef CAS PubMed.
- F. Bannwart, L. F. Richter, S. Stifel, J. Rueter, H. N. Lode, J. D. G. Correia, F. E. Kühn and A. Prokop, J. Med. Chem., 2024, 67, 15494–15508 CrossRef CAS PubMed.
- C. Schmidt, B. Karge, R. Misgeld, A. Prokop, M. Bronstrup and I. Ott, MedChemComm, 2017, 8, 1681–1689 RSC.
- P. König, R. Zhulenko, E. Suparman, H. Hoffmeister, N. Buckreiss, I. Ott and G. Bendas, Cancer Chemother. Pharmacol., 2023, 92, 57–69 CrossRef.
- L. F. Richter, F. Marques, J. D. G. Correia, A. Pothig and F. E. Kühn, Dalton Trans., 2023, 52, 17185–17192 RSC.
- S. M. Mahdavi, D. Bockfeld, I. V. Esarev, P. Lippmann, R. Frank, M. Bronstrup, I. Ott and M. Tamm, RSC Med. Chem., 2024, 15, 3248–3255 RSC.
- T. Babu, H. Ghareeb, U. Basu, H. Schueffl, S. Theiner, P. Heffeter, G. Koellensperger, N. Metanis, V. Gandin, I. Ott, C. Schmidt and D. Gibson, Angew. Chem., Int. Ed., 2023, 62, e202217233 CrossRef CAS PubMed.
- M. Bian, R. Fan, G. Jiang, Y. Wang, Y. Lu and W. Liu, J. Med. Chem., 2020, 63, 9197–9211 CrossRef CAS PubMed.
- Y. Lu, X. Sheng, C. Liu, Z. Liang, X. Wang, L. Liu, Z. Wen, Z. Yang, Q. Du and W. Liu, Pharmacol. Res., 2023, 190, 106731 CrossRef CAS.
- E. B. Bauer, M. A. Bernd, M. Schutz, J. Oberkofler, A. Pothig, R. M. Reich and F. E. Kühn, Dalton Trans., 2019, 48, 16615–16625 RSC.
- A. Gutiérrez, M. C. Gimeno, I. Marzo and N. Metzler-Nolte, Eur. J. Inorg. Chem., 2014, 2512–2519 CrossRef.
- F. Nahra, N. V. Tzouras, A. Collado and S. P. Nolan, Nat. Protoc., 2021, 16, 1476–1493 CrossRef CAS.
- A. Mariconda, D. Iacopetta, M. Sirignano, J. Ceramella, A. D'Amato, M. Marra, M. Pellegrino, M. S. Sinicropi, S. Aquaro and P. Longo, Int. J. Mol. Sci., 2024, 25, 2599–2630 CrossRef CAS PubMed.
- V. Ceccherini, E. Giorgi, M. Mannelli, D. Cirri, T. Gamberi, C. Gabbiani and A. Pratesi, Inorg. Chem., 2024, 63, 16949–16963 CrossRef CAS PubMed.
- C. Saturnino, I. Barone, D. Iacopetta, A. Mariconda, M. S. Sinicropi, C. Rosano, A. Campana, S. Catalano, P. Longo and S. Ando, Future Med. Chem., 2016, 8, 2213–2229 CrossRef CAS.
- D. Curran, H. Muller-Bunz, S. I. Bar, R. Schobert, X. Zhu and M. Tacke, Molecules, 2020, 25, 3474–3481 CrossRef CAS PubMed.
- R. Rubbiani, E. Schuh, A. Meyer, J. Lemke, J. Wimberg, N. Metzler-Nolte, F. Meyer, F. Mohr and I. Ott, MedChemComm, 2013, 4, 942–948 RSC.
- C. Zhang, M. L. Maddelein, R. Wai-Yin Sun, H. Gornitzka, O. Cuvillier and C. Hemmert, Eur. J. Med. Chem., 2018, 157, 320–332 CrossRef CAS PubMed.
- M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485–496 CrossRef CAS PubMed.
- M. Rodrigues, L. Russo, E. Aguiló, L. Rodríguez, I. Ott and L. Pérez-García, RSC Adv., 2016, 6, 2202–2209 RSC.
- J. Arcau, V. Andermark, M. Rodrigues, I. Giannicchi, L. Pérez-Garcia, I. Ott and L. Rodríguez, Eur. J. Inorg. Chem., 2014, 6117–6125 CrossRef CAS.
- M. Pellei, V. Gandin, M. Marinelli, C. Marzano, M. Yousufuddin, H. V. Dias and C. Santini, Inorg. Chem., 2012, 51, 9873–9882 CrossRef CAS.
- M. Ghorbanpour and B. Soltani, Coord. Chem. Rev., 2025, 523, 216233–216286 CrossRef CAS.
- F. A. Sofi and N. Tabassum, J. Biomol. Struct. Dyn., 2023, 41, 8605–8628 CrossRef CAS.
- C. H. G. Jakob, B. Dominelli, E. M. Hahn, T. O. Berghausen, T. Pinheiro, F. Marques, R. M. Reich, J. D. G. Correia and F. E. Kühn, Chem. – Asian J., 2020, 15, 2754–2762 CrossRef CAS PubMed.
- J. Turek, Z. Růžičková, E. Tloušťová, H. Mertlíková-Kaiserová, J. Günterová, L. Rulíšek and A. Růžička, Appl. Organomet. Chem., 2016, 30, 318–322 CrossRef CAS.
- T. V. Serebryanskaya, A. A. Zolotarev and I. Ott, MedChemComm, 2015, 6, 1186–1189 RSC.
- W. F. Gabrielli, S. D. Nogai, M. Nell, S. Cronje and H. G. Raubenheimer, Polyhedron, 2012, 34, 188–197 CrossRef CAS.
- S. Fulda, Semin. Cancer Biol., 2015, 31, 84–88 CrossRef CAS PubMed.
- A. Nandy, T. Samanta, S. Mallick, P. Mitra, S. K. Seth, K. D. Saha, S. S. Al-Deyab and J. Dinda, New J. Chem., 2016, 40, 6289–6298 RSC.
- T. Zou, C. T. Lum, C. N. Lok, J. J. Zhang and C. M. Che, Chem. Soc. Rev., 2015, 44, 8786–8801 RSC.
- A. De Luca, C. G. Hartinger, P. J. Dyson, M. Lo Bello and A. Casini, J. Inorg. Biochem., 2013, 119, 38–42 CrossRef CAS PubMed.
- L. Zhong, E. S. J. Arnér and A. Holmgren, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 5854–5859 CrossRef CAS PubMed.
- J. L. Hickey, R. A. Ruhayel, P. J. Barnard, M. V. Baker, S. J. Berners-Price and A. Filipovska, J. Am. Chem. Soc., 2008, 130, 12570–12571 CrossRef CAS.
- W. Liu, K. Bensdorf, M. Proetto, A. Hagenbach, U. Abram and R. Gust, J. Med. Chem., 2012, 55, 3713–3724 CrossRef CAS.
- R. Rubbiani, L. Salassa, A. de Almeida, A. Casini and I. Ott, ChemMedChem, 2014, 9, 1205–1210 CrossRef CAS.
- W. Liu, K. Bensdorf, M. Proetto, U. Abram, A. Hagenbach and R. Gust, J. Med. Chem., 2011, 54, 8605–8615 CrossRef CAS.
- A. Meyer, L. Oehninger, Y. Geldmacher, H. Alborzinia, S. Wolfl, W. S. Sheldrick and I. Ott, ChemMedChem, 2014, 9, 1794–1800 CrossRef CAS PubMed.
- T. C. Karlenius and K. F. Tonissen, Cancers, 2010, 2, 209–232 CrossRef CAS PubMed.
- J. S. Modica-Napolitano and J. R. Aprille, Drug Delivery, 2001, 49, 63–70 CrossRef CAS.
- Z. Trávníček, J. Vančo, M. Čajan, J. Belza, I. Popa, J. Hošek, R. Lenobel and Z. Dvořák, Appl. Organomet. Chem., 2024, 38, e7401 CrossRef.
- G. Augello, A. Azzolina, F. Rossi, F. Prencipe, G. F. Mangiatordi, M. Saviano, L. Ronga, M. Cervello and D. Tesauro, Pharmaceutics, 2023, 15, 466–477 CrossRef CAS PubMed.
- D. Varshney, J. Spiegel, K. Zyner, D. Tannahill and S. Balasubramanian, Nat. Rev. Mol. Cell Biol., 2020, 21, 459–474 CrossRef CAS PubMed.
- D. Wragg, A. de Almeida, R. Bonsignore, F. E. Kühn, S. Leoni and A. Casini, Angew. Chem., Int. Ed., 2018, 57, 14524–14528 CrossRef CAS.
- J. Oberkofler, B. Aikman, R. Bonsignore, A. Pöthig, J. Platts, A. Casini and F. E. Kühn, Eur. J. Inorg. Chem., 2020, 1040–1051 CrossRef CAS.
- L. K. Sharma, J. Lu and Y. Bai, Curr. Med. Chem., 2009, 16, 1266–1277 CrossRef CAS PubMed.
- M. A. Sirover, Int. J. Biochem. Cell Biol., 2014, 57, 20–26 CrossRef CAS.
- L. Massai, L. Messori, A. Carpentieri, A. Amoresano, C. Melchiorre, T. Fiaschi, A. Modesti, T. Gamberi and F. Magherini, Cancer Chemother. Pharmacol., 2022, 89, 809–823 CrossRef CAS.
- A. Rufini, P. Tucci, I. Celardo and G. Melino, Oncogene, 2013, 32, 5129–5143 CrossRef CAS.
- Y. Dabiri, M. A. Abu El Maaty, H. Y. Chan, J. Wolker, I. Ott, S. Wolfl and X. Cheng, Front. Oncol., 2019, 9, 438 CrossRef.
- A. Nandy, S. K. Dey, S. Das, R. N. Munda, J. Dinda and K. D. Saha, Mol. Cancer, 2014, 13, 57–71 CrossRef.
- S. Al Bitar and H. Gali-Muhtasib, Cancers, 2019, 11, 1475–1496 CrossRef CAS PubMed.
- L. Dou, Y. Fang, H. Yang, G. Ai and N. Shen, Hum. Vaccinines Immunother., 2024, 20, 2437918 CrossRef.
- K. I. Arimoto, S. Miyauchi, M. Liu and D. E. Zhang, Front. Immunol., 2024, 15, 1390263 CrossRef CAS.
- S. Sen, S. Hufnagel, E. Y. Maier, I. Aguilar, J. Selvakumar, J. E. DeVore, V. M. Lynch, K. Arumugam, Z. Cui, J. L. Sessler and J. F. Arambula, J. Am. Chem. Soc., 2020, 142, 20536–20541 CrossRef CAS.
- J. Arrindell and B. Desnues, Front. Immunol., 2023, 14, 1224352 CrossRef CAS.
- M. S. Taha and M. R. Ahmadian, Cells, 2024, 13, 1266–1280 CrossRef CAS.
- M. K. Singh, Y. Shin, S. Han, J. Ha, P. K. Tiwari, S. S. Kim and I. Kang, Int. J. Mol. Sci., 2024, 25, 5483–5508 CrossRef CAS.
- S. K. Fung, T. Zou, B. Cao, P. Y. Lee, Y. M. Fung, D. Hu, C. N. Lok and C. M. Che, Angew. Chem., Int. Ed., 2017, 56, 3892–3896 CrossRef CAS PubMed.
- A. M. Al-Majid, M. I. Choudhary, S. Yousuf, A. Jabeen, R. Imad, K. Javeed, N. N. Shaikh, A. Collado, E. Sioriki, F. Nahra and S. P. Nolan, ChemistrySelect, 2017, 2, 5316–5320 CrossRef CAS.
- R. Rubbiani, S. Can, I. Kitanovic, H. Alborzinia, M. Stefanopoulou, M. Kokoschka, S. Monchgesang, W. S. Sheldrick, S. Wolfl and I. Ott, J. Med. Chem., 2011, 54, 8646–8657 CrossRef CAS.
- B. Dominelli, C. H. G. Jakob, J. Oberkofler, P. J. Fischer, E. M. Esslinger, R. M. Reich, F. Marques, T. Pinheiro, J. D. G. Correia and F. E. Kühn, Eur. J. Med. Chem., 2020, 203, 112576 CrossRef CAS PubMed.
- F. Guarra, A. Terenzi, C. Pirker, R. Passannante, D. Baier, E. Zangrando, V. Gomez-Vallejo, T. Biver, C. Gabbiani, W. Berger, J. Llop and L. Salassa, Angew. Chem., Int. Ed., 2020, 59, 17130–17136 CrossRef CAS PubMed.
- T. Zou, C. T. Lum, S. S. Chui and C. M. Che, Angew. Chem., Int. Ed., 2013, 52, 2930–2933 CrossRef CAS PubMed.
- S. Y. Hussaini and M. R. Razali, Mol. Struct., 2025, 1322, 140614–140627 CrossRef CAS.
- L. Boselli, M. Carraz, S. Mazères, L. Paloque, G. González, F. Benoit-Vical, A. Valentin, C. Hemmert and H. Gornitzka, Organometallics, 2015, 34, 1046–1055 CrossRef CAS.
- B. T. Elie, Y. Pechenyy, F. Uddin and M. Contel, J. Biol. Inorg. Chem., 2018, 23, 399–411 CrossRef CAS.
- L. Massai, J. Fernandez-Gallardo, A. Guerri, A. Arcangeli, S. Pillozzi, M. Contel and L. Messori, Dalton Trans., 2015, 44, 11067–11076 RSC.
- Y. F. Mui, J. Fernandez-Gallardo, B. T. Elie, A. Gubran, I. Maluenda, M. Sanau, O. Navarro and M. Contel, Organometallics, 2016, 35, 1218–1227 CrossRef CAS PubMed.
- J. Fernandez-Gallardo, B. T. Elie, M. Sanau and M. Contel, Chem. Commun., 2016, 52, 3155–3158 RSC.
- C. Holohan, S. Van Schaeybroeck, D. B. Longley and P. G. Johnston, Nat. Rev. Cancer, 2013, 13, 714–726 CrossRef CAS.
- A. van Niekerk, P. Chellan and S. F. Mapolie, Eur. J. Inorg. Chem., 2019, 3432–3455 CrossRef CAS.
- U. E. I. Horvath, G. Bentivoglio, M. Hummel, H. Schottenberger, K. Wurst, M. J. Nell, C. E. J. van Rensburg, S. Cronje and H. G. Raubenheimer, New J. Chem., 2008, 32, 533–539 RSC.
- J. K. Muenzner, B. Biersack, A. Albrecht, T. Rehm, U. Lacher, W. Milius, A. Casini, J. J. Zhang, I. Ott, V. Brabec, O. Stuchlikova, I. C. Andronache, L. Kaps, D. Schuppan and R. Schobert, Chemistry, 2016, 22, 18953–18962 CrossRef CAS PubMed.
- S. Vanicek, M. Podewitz, J. Stubbe, D. Schulze, H. Kopacka, K. Wurst, T. Muller, P. Lippmann, S. Haslinger, H. Schottenberger, K. R. Liedl, I. Ott, B. Sarkar and B. Bildstein, Chemistry, 2018, 24, 3742–3753 CrossRef CAS.
- D. Aucamp, S. V. Kumar, D. C. Liles, M. A. Fernandes, L. Harmse and D. I. Bezuidenhout, Dalton Trans., 2018, 47, 16072–16081 RSC.
- J. F. Arambula, R. McCall, K. J. Sidoran, D. Magda, N. A. Mitchell, C. W. Bielawski, V. M. Lynch, J. L. Sessler and K. Arumugam, Chem. Sci., 2016, 7, 1245–1256 RSC.
- N. Bhatia, S. Hazra and S. Thareja, Eur. J. Med. Chem., 2023, 256, 115422 CrossRef CAS.
- B. J. Deroo, S. C. Hewitt, S. D. Peddada and K. S. Korach, Endocrinology, 2004, 145, 5485–5492 CrossRef CAS.
- Y. A. Luqmani, Med. Princ. Pract., 2005, 14, 35–48 CrossRef PubMed.
- X. Liu, Y. Zhang, W. Lu, Y. Han, J. Yang, W. Jiang, X. You, Y. Luo, S. Wen, Y. Hu and P. Huang, Redox. Biol., 2020, 36, 101652 CrossRef CAS.
- G. Housman, S. Byler, S. Heerboth, K. Lapinska, M. Longacre, N. Snyder and S. Sarkar, Cancers, 2014, 6, 1769–1792 CrossRef PubMed.
- R. B. Mokhtari, T. S. Homayouni, N. Baluch, E. Morgatskaya, S. Kumar, B. Das and H. Yeger, Oncotarget, 2017, 8, 38022–38043 CrossRef.
- I. Landini, A. Lapucci, A. Pratesi, L. Massai, C. Napoli, G. Perrone, P. Pinzani, L. Messori, E. Mini, S. Nobili, E. Mini, L. Messori, A. Modesti and T. Gamberi, Oncotarget, 2017, 8, 96062–96078 CrossRef.
- M. Tacke, Presented in part at the ICCBIC2019, Bratislava, 2019.
- R. L. Eckert, A. Mullick, E. A. Roske and B. S. Katzenellenbogen, Endocrinology, 1984, 114, 629–637 CrossRef CAS.
- C. Zhuang, X. Guan, H. Ma, H. Cong, W. Zhang and Z. Miao, Eur. J. Med. Chem., 2019, 163, 883–895 CrossRef CAS PubMed.
- M. G. Vander Heiden, L. C. Cantley and C. B. Thompson, Science, 2009, 324, 1029–1033 CrossRef CAS.
- T. Scattolin, P. Lippmann, M. Beliš, K. van Hecke, I. Ott and S. P. Nolan, Appl. Organomet. Chem., 2022, e6624 Search PubMed.
- A. Varchmin, A. Muñoz-Castro and I. Ott, J. Organomet. Chem., 2024, 1012, 123148 CrossRef CAS.
- F. Tresin, V. Stoppa, M. Baron, A. Biffis, A. Annunziata, L. D'Elia, D. M. Monti, F. Ruffo, M. Roverso, P. Sgarbossa, S. Bogialli and C. Tubaro, Molecules, 2020, 25, 3850–3863 CrossRef CAS.
- M. J. Matos, C. Labao-Almeida, C. Sayers, O. Dada, M. Tacke and G. J. L. Bernardes, Chemistry, 2018, 24, 12250–12253 CrossRef CAS.
- S. Sen, M. W. Perrin, A. C. Sedgwick, V. M. Lynch, J. L. Sessler and J. F. Arambula, Chem. Sci., 2021, 12, 7547–7553 RSC.
| This journal is © The Royal Society of Chemistry 2025 |