 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Turning over a new leaf: innovative pest control from a materials science perspective
Abinaya
Arunachalam
 a,
Maria
Perraki
a,
Maria
Perraki
 a,
Bram
Knegt
a,
Bram
Knegt
 b,
Mirka
Macel
b,
Mirka
Macel
 b,
Dagmar
Voigt
b,
Dagmar
Voigt
 c and
Marleen
Kamperman
c and
Marleen
Kamperman
 *a
*a
aPolymer Science, Zernike Institute for Advanced Materials, University of Groningen, Nijenborgh 3, 9747 AG Groningen, The Netherlands. E-mail: marleen.kamperman@rug.nl
bAeres University of Applied Sciences, Arboretum West 98, 1325 WB, Almere, The Netherlands
cBotany, Faculty of Biology, Technische Universität Dresden, 01062 Dresden, Germany
First published on 9th June 2025
Abstract
The growing demand for food due to a global population increase has made the use of pesticides in agriculture unavoidable despite their various harmful side effects. Driven by stricter legislation, nations are now compelled to find alternatives. This situation led to accelerated research around the world, focusing on developing new chemistries to enhance the environmental safety of pesticides. In recent years, bioinspired strategies of pest control have emerged as alternatives to the development of new synthetic pesticides. In order to design innovative eco-friendly pest management techniques, a thorough understanding of naturally existing physical and chemical defences in plants is needed. Building upon this knowledge, material science provides innovative strategies for designing physical barriers, biomimetic adhesives, and targeted delivery systems that go beyond traditional chemical approaches. This tutorial review explores the intricate relationships between plants and insects, focusing on natural defence mechanisms such as plant cuticles, trichomes, and thigmonasty. We also review advances in synthetic pesticide use, including enhanced adhesion and controlled release formulations. In addition, we delve into advances in other integrated pest management domains, discussing the potential of bioinspired surfaces and biological control methods. This overview aims to foster comprehensive understanding and interdisciplinary approaches, highlighting the pivotal role of material science in improving sustainable pest control for the future.
1. Pesticides through the ages: historical developments and emerging alternatives
Synthetic pesticides emerged after World War II as a vital means to control the spread of harmful diseases via insect vectors. Among the initial insecticides to be investigated, dichloro-diphenyl-trichloroethane (DDT), developed by Paul Mueller, proved highly toxic to a wide spectrum of insects while exhibiting relatively low acute toxicity to mammals at typical usage concentrations. This discovery further led to the development of various organophosphates and carbamates.1,2 Although the initial need for insecticides was to target vectors of human diseases, their potential to control a wide range of pests led to their rapid expansion into the fields of agriculture as described by Flint and van den Bosch in 1981: “Their success was immediate. They were cheap, effective in small quantities, easy to apply, and widely toxic. They seemed to be truly ‘miracle’ insecticides”.2Today, the term ‘pesticides’ describes a vast group of substances used as insecticides, acaricides, herbicides, bactericides, fungicides, rodenticides, nematicides, molluscicides, and growth regulators.3 These substances may act (1) protectively or curatively, (2) selectively or broadly, and (3) via contact or via uptake. Pesticide toxicity must be clearly labeled in accordance with internationally recognized hazard classifications, as either toxic, corrosive, irritating, or harmful.
Pesticides play a key role in the process of agricultural intensification – increasing yield, productivity, and quality on existing agricultural fields. Agriculture today relies heavily on pesticides to minimise plant pests and diseases, and meet the growing food demands of the ever-increasing human population.4 In fact, there has been a surge in the worldwide use of pesticides over the last couple of decades, resulting in several positive and negative effects as summarised in Fig. 1.
 | ||
| Fig. 1 Overview of desirable (green/blue panels) and undesirable (grey/red panels) consequences of synthetic pesticides on insects, environment, and humans. | ||
Among these negative effects, pesticides were identified as the root cause of numerous health effects in humans. These include diseases of the skin, nerves, lungs, reproductive organs, and the endocrine system.5–11 Pesticides may also threaten the ecosystem by harming aquatic and terrestrial life. Although soil can act as a large reservoir for storing pesticides, contamination of water bodies is often inevitable due to the close relationship of soil with surface and ground water sources.12 Pesticide residues are commonly found in water bodies as a result of various processes like leaching, run-off, soil erosion, and drift.13,14 Even trace residues of pesticides in these water sources can accumulate to harmful levels as the substances travel through the food chain, a process called biomagnification. In addition, pesticides can disrupt the ecological balance by harming non-target organisms in different classes like soil invertebrates (earthworms and predatory bugs), microorganisms, and pollinators (bees).12 Further, undesirable consequences such as the development of pesticide resistance in target pests, can render certain pesticides ineffective, leading to the need for harsher chemicals or higher doses to kill pesticide-resistant species.15
The various harmful side effects of pesticides that emerged due to this increased usage urged researchers worldwide to search for alternatives. In the European Union, pesticides must undergo a strict admission procedure for an indication license, i.e., they can be applied only to certain plant species under certain conditions. In addition, the admission period and multiple uses are very limited, to ensure rotation of different active chemical substances, thus preventing pest resistance. However, current political strategies lead to a restricted spectrum of available active chemical substances, resulting in repeated use and, thus, increased pest resistance against pesticides. Even sublethal doses of agrochemicals along with changes in environmental temperatures contribute to the global decline of insect populations.16 These trends may have severe economic consequences, particularly considering the challenges posed by invasive pests in a warming world.
A transition towards minimising and replacing toxic chemicals is not immediately feasible. For example, Aldicarb, a carbamate pesticide used on citrus plants and potatoes, was banned in the United States a decade ago because it posed “unacceptable dietary risks”. In the present day, despite being banned in over 100 countries and classified as “extremely hazardous” by the World Health Organization (WHO), Aldicarb is again being considered for use by the U.S. Environmental Protection Agency (EPA) as part of its routine pesticide review process.17–19
This highlights a pressing need to switch to more environmentally friendly pest management techniques to prevent the immediate and long-term negative effects of pesticide exposure. To develop such techniques, it is essential to recognise that there is potential not only in new chemistry but also in improving our understanding of the innate physical and chemical defences employed by plants. For instance, surface patterning observed in plant structures can effectively deter pests, inspiring material scientists to create innovative solutions.20 Approaches that plants have evolved to defend themselves may be synthetically mimicked, enhanced, or reproduced.
In nature, typically, a combination of multiple defence strategies is employed. This principle is also embodied in integrated pest management (IPM), a sustainable and large-scale approach that has been in place for about 45 years. Integrated crop management (ICM) is a broader approach that combines physical, cultural, biological, and chemical management strategies of IPM along with crop-specific strategies to grow healthy plants while minimising pesticide use.21,22 Physical control considers, e.g., mechanical devices that can prevent the pest from accessing the plant, such as barriers and traps. Cultural control refers to agricultural practices undertaken by farmers to make crops less favourable for pests, such as crop rotation, the use of sacrificial crops and resistant genotypes. Biological control relies on natural antagonists, such as predatory insects and microorganisms preying on target insects. Considering the current state of science and technological advances, there is still ample scope for improvement in all individual IPM domains as well as the integration of these different domains to enhance their overall effectiveness while minimising the effect on the environment. Therefore, plant biologists, agricultural scientists, microbiologists, entomologists, environmental scientists, chemists, materials scientists, and potentially other disciplines, have to combine expertise from their diverse fields.
This review aims to provide a comprehensive understanding of natural plant defence strategies and how material science can contribute to developing collaborative pest management solutions. We will discuss recent IPM developments with a particular focus on how material science can enhance chemical control, for instance, by improving the adhesion of pesticides to plants, thereby ensuring that pesticide loss and pollution is reduced. Our primary focus lies in advancing IPM, particularly against pest insects, but we will also cover some interesting examples against fungi and bacteria. This is not meant to be an exhaustive overview since there are already several specialised reviews such as on the use of nanoparticles in agriculture,23–26 controlled release pesticide formulations27–29 and currently employed techniques in agriculture.30,31 Starting from interactions observed in nature and the resulting defence mechanisms evolved by plants to protect themselves (Sections 2 and 3), our review delves deeper into developments to improve the eco-friendliness and reduce the toxicity of chemical pesticides (Section 4). Moreover, we highlight promising strategies to control the release of synthetic pesticides (Section 5). We also draw attention to the fact that many natural defence mechanisms explored in Sections 2 and 3 remain underutilised in synthetic approaches (Sections 4 and 5), suggesting several promising, nature-inspired solutions that may be interesting to explore. Lastly, we offer insights into the combination of these emerging strategies into novel IPM approaches, suggesting synergistic combinations for enhanced effectiveness.
2. Interactions between plants and insects
Interactions between species, be it direct or indirect, form the basis for all processes in an ecosystem. Darwin already emphasised the coevolution process between insect and plant communities.32 Here, we discuss two commonly occurring types of interactions between different organisms and their relevance to plant defence and herbivorous insects. These types are mutualism and antagonism. A third type, commensalism, is not easily identifiable between plants and insects and hence, is not explored further here.2.1 Mutualism
Plants and insects have mutualistic relationships mainly for pollination, seed dispersal, and protection purposes.33 Mutualism, a type of symbiosis, is a cooperative interaction between two or more species, where each species benefits from the relationship. It also acts as a line of defence for plants. Ants are widely reported to have a mutualistic relationship with different plants.12,34,35 For example, Cecropia plants36 have hollow internodes which Azteca ants use for nesting. The internal spongy tissues lining the internodes act as a food source for the cohabitant ants (Fig. 2a).37Cecropia plants also produce structures called pearl bodies rich in fats to provide the ants with a balanced diet. These evolutionary features are suggested to have been solely developed by the Cecropia plant to provide food and shelter to Azteca ants. In return, the ants protect the plants against attacking insects and sometimes even slightly larger herbivores owing to their sharp mandibles (Fig. 2b).38 The hook-shaped claws on the ants’ feet allow them to hold on to the plant while capturing large insects (Fig. 2c), weighing over 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times a single ant's body weight. Apart from protecting the plant against herbivores, these ants also prevent competing plants to grow nearby (Fig. 2d), by chewing and killing the shoot tips and tendrils of encroaching vines.39 Such forms of defence provided by the ants help plants direct their energy towards growth rather than defence. These ants were also found to excrete nitrogenous wastes that act as rich nitrogen sources for the plant, thus fertilising the plant.40–43 However, such mutualistic relationships are sensitive to environmental shifts. Janzen et al. documented how the absence of herbivores on the Caribbean islands removed the need for Cecropia plants to attract Azteca ants, which eventually led to a dissociation of their mutualistic relationship.41
000 times a single ant's body weight. Apart from protecting the plant against herbivores, these ants also prevent competing plants to grow nearby (Fig. 2d), by chewing and killing the shoot tips and tendrils of encroaching vines.39 Such forms of defence provided by the ants help plants direct their energy towards growth rather than defence. These ants were also found to excrete nitrogenous wastes that act as rich nitrogen sources for the plant, thus fertilising the plant.40–43 However, such mutualistic relationships are sensitive to environmental shifts. Janzen et al. documented how the absence of herbivores on the Caribbean islands removed the need for Cecropia plants to attract Azteca ants, which eventually led to a dissociation of their mutualistic relationship.41
 | ||
| Fig. 2 Mutualistic relationship between Azteca ants and Cecropia plants. Photographs of (a) Azteca ants feeding on white Müllerian bodies, which are glycogen-rich food bodies located on fuzzy pads (trichilia) at the base of the plant's petiole, (b) ants hiding on the underside of the leaf with open mandibles for an ambush, (c) sphingid moth captured by the army of ants at the leaf margin, and (d) ants defending the Cecropia plant against an encroaching vine. (a) and (d) Reproduced under the terms of a Creative Commons Attribution License.36 Copyright 2018, Springer Nature. (b) and (c) Reproduced under the terms of a Creative Commons Attribution License.38 Copyright 2010, Dejean et al. | ||
2.2 Antagonism
Antagonistic interactions between plants and insects usually result in the insect benefitting at the expense of the plant. Unlike commensalism and mutualism, where interactions are cooperative, antagonistic relationships such as predation, parasitism, and herbivory are characterised by competition and harm to the organisms involved.Predation refers to one organism (predator) killing and consuming another organism (prey) for food. For example, carnivorous plants have evolved various attributes that help them capture and digest prey insects to supplement their nutrient needs.44 What differentiates carnivorous plants from each other are their specialised active or passive traps to attract and capture prey.45,46 Some plants employ active moving traps that shut quickly, capture and absorb the prey upon contact such as that of bladderworts.47 A typical example of snap traps is the Venus flytrap (Dionaea muscipula) (Fig. 3a), which emits over 60 volatile compounds to attract prey insects. When the prey insect explores the trap, their movement can set off the sensory hairs that trigger the closure of the trap, thus ensnaring the prey.48 In contrast, plants employing passive traps do not rely on moving plant parts but on innate features such as slippery and sticky surfaces and their hierarchical cascade arrangement.49–52 For example, pitfall traps employed by different families of pitcher plants (Fig. 3b) attract prey through various means, including secretion of sweet-smelling nectar, appealing patterns, and coloured veins. Once attracted, the prey loses its grip on the rim and inner pitcher wall as it encounters slippery surfaces arising from anisotropic epidermal cell curvatures and two layers of epicuticular wax crystals.50,53–56 The pitcher also contains a pool of viscoelastic biopolymer digestive fluids at the base, that break down the prey's soft tissues, releasing essential nutrients like nitrogen and phosphorus for the plant.57
 | ||
| Fig. 3 Antagonistic plant-insect relationships. Photographs of (a) snap trap of the Venus flytrap, (b) pitfall trap of the pitcher plant, (c) galls of two different species of Cynipidae wasps on the underside of an oak leaf, and (d) an aphid feeding by penetrating its slender mouthparts (stylets) into the plant tissues. (a) Reproduced with permission from National Institute for Basic Biology.58 Copyright 2020, Mitsuyasu Hasebe. (b)–(d) Reproduced under the terms of a Creative Commons Attribution License.59–61 Copyright 2020, Schwallier et al., Copyright 2021, Jankiewicz et al., and Copyright 2014, Lohaus and Schwerdtfeger respectively. | ||
Parasitism, in contrast, does not kill the host immediately. Instead, the parasitic organism draws nutrients from the host and slowly weakens it over time.62,63 For example, the adult females of gall-forming insects belonging to the Cynipidae (gall wasps, order Hymenoptera) and Cecidomyiidae (gall midges, order Diptera) families (Fig. 3c) inject eggs along with a secretion into their host plant tissues. This results in the death of the plant cells adjacent to the eggs, causing neighbouring cells to create a growing tumour-like mass called gall. After the larvae emerge, their feeding activity, along with oral secretions they produce, stimulate further gall growth, thus draining energy from the plant.64
Herbivory concerns insects that feed on plants by chewing on plant parts like roots, stems, and leaves, sucking out the plants’ cell contents, injecting fluids into the plant cells, or transmitting diseases.65 For example, aphids have slender specialised mouthparts called stylets, which they use to penetrate plant tissues, after which they secrete digestive enzymes directly into the tissue and suck out the sugary phloem sap (Fig. 3d). Aphids can also inflict damage on plants by the sticky waste they secrete called honeydew, which causes leaf loss.66
To defend themselves against attacking species, plants have evolved a number of morphological and chemical defence mechanisms, leading to an ongoing co-evolutionary race between plants and herbivores. Although in this review we focus on interactions between plants and insects, antagonistic relationships between different insects, mainly herbivore pests and their natural enemies, have also been exploited in recent years in the form of biological control with beneficial insects in IPM.67–69
3. Structural natural defence in plants
Plant defence mechanisms can be classified into different categories, such as constitutive vs. induced. Constitutive defence refers to preformed plant traits that are always present in the plant irrespective of the presence of a pest,70 while induced defence refers to temporary plant traits that develop rapidly in response to an attack, for example, when foreign molecules or damaged cells activate an immune response.71,72 Since most attacks on plants are unforeseeable and quite unpredictable to an extent, many plants express low levels of constitutive defence to conserve energy and repurpose these resources during an infestation as a form of induced defence.73Another classification uses the distinction between direct and indirect defence mechanisms. Direct defence refers to plant attributes that negatively affect the attacking pest, including morphological characters like thorns, spines, trichomes, etc., and chemical compounds such as toxins that interfere with the pest's metabolic activities.74,75 Conversely, indirect defence acts on undesirable pests by involving other organisms that can attack and remove plant enemies. Since a defence mechanism can fall under multiple categories of constitutive vs. induced, direct vs. indirect, or morphological vs. chemical attributes, we discuss the mechanisms individually in this section, focusing on morphological features. Natural chemical defence mechanisms involving secondary metabolites, toxins, digestive inhibitors, and recruitment of predatory insects with volatiles also play an equally important part and have been reviewed in detail elsewhere.76–79
3.1 Plant cell attributes
The plant cell wall typically contains a complex mixture of components like cellulose, hemicellulose, lignin, and pectin, which may play essential roles in cell wall fortification as a response to external stresses (induced defence).80,81 In addition, plants absorb dissolved calcium and silicon from the soil via the root system.82–85 Coordinated mineralisation processes lead to the deposition of microscopic solids such as cystoliths (calcium carbonate), idioblasts (calcium oxalate), and phytoliths (silicon), that act as toxic antinutrients and physical deterrents against herbivores:.83,86,87Calcium oxalate crystals are the most widespread solid minerals across the plant kingdom, reaching 3–80% plant dry mass mineral content. They perform a number of functions, such as providing structural support for tissues, storing essential substances, and regulating mineral content within the plant.88 This type of plant defence is typically more effective against chewing insects compared to sucking insects. When offered a calcium oxalate-rich wild type and a mutant calcium-oxalate defective line of the legume species Medicago truncatula (Fig. 4a), the beet armyworm Spodoptera exigua displayed clear feeding preferences for the mutant. The larval teeth remained sharply pointed along with a slightly serrated edge while feeding on the mutant, similar to the control group fed with an artificial diet. In contrast, larvae that fed on the wild type ended up with smoothened teeth and a blunt point displaying the abrasive effects of these crystal idioblasts (Fig. 4b).83,89
 | ||
| Fig. 4 Plant cell attributes acting as a natural defence including (a) calcium oxalate crystal idioblast visible in the leaf cross-section of a mature Claoxylon sandwicense plant. Scale bar = 400 μm, (b) scanning electron microscopy (SEM) images showing smoothening of a Spodoptera exigua larvae tooth (bottom) due to abrasive effects from calcium oxalate crystals in Medicago truncatula in comparison to a sharp tooth from larvae on an artificial diet (top). Scale bar = 10 μm, (c) callose deposition highlighted using epifluorescence microscopy with wounded (middle) and bacterial peptide treated (bottom) Arabidopsis thaliana cotyledons in comparison to unwounded control seedlings (top). (d) Monarch caterpillar succumbed after contact with released latex after initial larval feeding on Asclepias syriaca leaves. (a) Adapted with permission.90 Copyright 2001, Elsevier Science Ltd. (b) Reproduced with permission.89 Copyright 2006, American Society of Plant Biologists (c) Adapted under the terms of a Creative Commons Attribution License.91 Copyright 2018, Keppler et al. (d) Reproduced with permission.92 Copyright 2019, Springer Science + Business Media, LLC, part of Springer Nature 2019. | ||
High silicon contents (>4%) have been observed in grasses (Poaceae), horsetails (Equisetae), and sedges (Cyperaceae), where they frequently strengthen plant cell walls.83,84 Rice, for example, stores up to 10–15% of its dry mass in silicon. Apart from acting as a structural support for these plants, silicified cell walls are closely associated with reduced blast disease since they protect the plant cells from being penetrated by attacking fungi.93,94 Phytoliths occur within and between the plant cells and wear down the mandibles of herbivorous insects due to their abrasive nature, impeding feeding.95 The African armyworm Spodoptera exempta fed with silica-rich grasses (Deschampsia caespitosa, Festuca ovina, Lolium perenne) struggled to digest the leaves and showed rapid mandible wear. It could not easily adapt to the physical defence and lost fitness.96 In addition to a physical barrier, silicon can trigger systemic plant defence mechanisms by inducing defensive enzymes such as peroxidases and polyphenol oxidases, as observed after adding silicon to soil or nutrient solution.94,97,98 The exact mechanism of how silicon triggers the activation of defensive enzymes is still unclear. However, there is evidence that silicon boosts activity of various antioxidant enzymes like peroxidases, which help mitigate the cytotoxic effects of excess reactive oxygen species, typically generated during herbivore attacks.98,99
Callose also plays a role in the induced fortification of cell walls, as shown in Fig. 4c. In contrast to silicon, callose is a linear β-glucan polysaccharide synthesised by the plant itself. When a plant faces a pathogen attack, it responds by depositing callose between the plasma membrane and the cell wall at the locations where the pathogen has struck.100 Callose is also an important defence against larger insects, making it more difficult for them to penetrate the plant tissues.101 After herbivory attacks, the deposition of callose can help seal plant wounds, restricting further access to feed from other plant parts and preventing infections with microorganisms.102
Similarly, laticifers contain a sticky viscous emulsion called latex, serving as a defence in most plants.103 When herbivores damage plant parts, the tubular network of laticifers release latex locally at the site of attack thereby sealing the wound.104 This exudate contains secondary metabolites like proteins, alkaloids, flavonoids, and terpenoids that act as toxins for attacking pests, as observed in milkweeds (Asclepias spp.) containing cardiac glycosides, toxic to many herbivores.105 After monarch butterflies, Danaus plexippus, attach their eggs reliably on the underside of the milkweed leaves, the hatched larvae start feeding on leaves for survival. While chewing the plant tissues, latex gets released and solidified in the presence of air, forming a sticky glue hindering the feeding process and potentially killing the caterpillar (Fig. 4d).92
A similar plant defence is the presence of resin ducts, which are intercellular spaces filled with resin under internal pressure. Typically present in most conifers, resins are a mixture of monoterpenes, sesquiterpenes, and diterpene resin acids. When stem-boring insects break into these networks of ducts, they get expelled out due to the pressure from the resin flow. Upon contact with air, volatile compounds in the resin mixture evaporate, resulting in the insects getting solidified in resin acids. Further, this reaction upon exposure with air also ensures that the wounded site in the plant is sealed off by solid resin. The breached resin duct system is subsequently fixed by the formation of new resin ducts (induced plant response).106
3.2 Plant cuticle
Plant surfaces above the ground have a hydrophobic covering composed of cutin and intracuticular wax, referred to as the cuticle, acting as an extracellular interface with the environment.107–109 This biopolymer has important mechanical properties for the plant, providing structural stability and maintenance of its physiological health.108 The chemical composition and microstructure of the cuticle may differ profoundly between plant species and developmental stages.110 This is important since cuticular folds can influence the leaf blade and flower petal stability, as well as plant surfaces' optical and tribological properties.111 For example, it has been shown that leaf beetles and pollinators slip away when walking on flat substrates with nm-sized folds, while μm-sized folds on epidermal convexities support insect foothold.112Being the interface for biotic interactions, the cuticle can affect adhesion, host recognition, and mechanical prevention of microbes and arthropods.109 Apart from acting as a first line of defence for most plants against various pests and pathogens, the cuticle also plays a significant role in protecting against UV radiation, high temperatures, and water loss.113
Effects of epicuticular plant waxes on insect feeding and behaviour, especially for insect-host selection, have been reported extensively.115 Alkanes of epicuticular waxes were identified to serve as insect deterrents in sorghum and maize; wax esters increased aphids resistance in alfalfa, and wax alcohols in concert with reduced hydrocarbon chain length on sugarcane reduced the sugarcane stalk bore larvae survival. Moreover, aromatic wax constituents were found to promote resistance of various plants such as the Rhododendron species to the azalea lace bug.116
Wax crystals on plant surfaces have been studied for over a century and were found to hinder insect attachment by influencing factors such as their composition, structure, size, abundance, and distribution.117 Gorb and Gorb proposed and tested four possible mechanisms involved in weak insect attachment on plant surfaces covered with crystalline epicuticular wax: (1) roughness, (2) contamination, (3) fluid-adsorption, and (4) wax-dissolution.117,118 The roughness mechanism refers to the nanoscopic to microscopic roughness caused by wax crystals decreasing the real contact area between the plant surface and the insect attachment pad. The contamination mechanism causes insects to lose foothold due to wax crystals detaching from plant surfaces. The fluid-adsorption mechanism refers to highly porous, lipophilic plant epicuticular wax coverage absorbing the fluid responsible for adhesion released by the insect attachment pads. Finally, the wax-dissolution mechanism involves plant epicuticular wax crystals dissolving by lipids and lipophilic substances in the adhesion-mediating fluid of insect attachment pads. However, this mechanism is based on indirect evidence and has not been experimentally proven.117,118
3.3 Trichomes
Trichomes are hair-like structures present naturally as an extension of the epidermis on some plants.119 They perform several functions in plants, such as protecting against UV radiation, preventing excess water loss from transpiration, regulating temperature, facilitating gas exchange, and acting as a natural defence.119 Most trichomes are inherently present, meaning they are a type of constitutive defence. However, it is also very likely that some plants can induce them by increasing the density of trichomes defending against predator attacks.120–122Sticky exudates of glandular trichomes can trap or hamper the movement of pests (physical defence), or produce antagonising volatiles or toxins that can deter and harm the insect when detected, contacted or ingested (chemical defence).123 For example, the wild potato Solanum berthaultii resists the attack of pest beetles, leafhoppers, and aphids owing to the presence of two types of glandular trichomes; elongated cone-shaped with non-covered glandular heads (type B) and short capitate cutin-covered four-celled glandular heads (type A). When insects attack plants, they usually encounter type-B trichomes first, which continuously release an adhesive viscoelastic exudate, thereby coating the body of the pests. The insect struggles to free itself and gets in contact with the shorter, stiffer type-A trichomes, which release a two-compound adhesive consisting of polyphenol oxidase enzymes and a phenolic substance, chlorogenic acid. Their reaction produces a quinone-based brown polymer, which hardens and immobilises the pest. Additionally, the exudate from type-B trichomes contains sesquiterpenes, repellent to pests settling on the plants.124,125 A similar mechanism is observed in the trichomes of Sicana odorifera where aphids rupture the heads, leading to the deposition of a sticky exudate on the insect.126
Murungi et al. studied different African nightshades (Solanum sp.) in which glandular trichomes prevented oviposition by tomato spider mites Tetranychus evansi (Acari, Tetranychidae). This was attributed to the unsaturated fatty acids present in these exudates, interfering oviposition through volatile chemical signals.127 Transferring the exudate of the glandular trichomes from Solanum sarrachoides onto Solanum scabrum, void of these trichomes, exhibited a 70% reduction of placed mite eggs. Similarly, potato tuber moths laid 97% fewer eggs on potato crop Solanum tuberosum, when exudates of the wild potato Solanum berthaultii were manually transferred to the susceptible cultivar.128
Non-glandular trichomes are mainly physical barriers, preventing pests from reaching the leaf surface.129 For example, field beans Phaseolus vulgaris are equipped with tapered hook-shaped trichomes, impaling, e.g., potato leafhoppers Empoasca fabae and leafminers Liriomyza trifolii, particularly on the lower leaf side.130 Once trapped, the leafhoppers immediately try to free themselves and get stabbed by neighbouring trichomes. The trapped leafhoppers cannot feed and die from starvation and/or dehydration.130,131 Similar effects have been observed with hook-shaped non-glandular trichomes in prickled herbs, belonging to the family Loasaceae (Fig. 5a–c), which are known for reinforced trichomes with incorporated biominerals (calcium phosphate, silica) in tips and barbs, providing mechanical defence.132
 | ||
| Fig. 5 Trichomes acting as a natural defence mechanism: SEM images of (a) hook-shaped, (b) tapered conical-shaped and barbed, and (c) cylindrically shaped and barbed trichomes of Mentzelia pumila. Photographs showcasing (d) an adult mirid bug Pameridea roridulae in motion on the surfaces of carnivorous Roridula gorgonias leaves covered with glandular trichomes and (e) a trapped fly on R. gorgonias trichomes. (f) A thick, greasy epicuticular lipid layer equipping P. roridulae with sloughing-off surface properties and enabling the bug to navigate across the sticky plant surface unlike the stuck prey insects. (a)–(c) Reproduced with permission.133 Copyright 1998, The National Academy of Sciences. (d)–(f) Reproduced under the terms of The Company of Biologists Publication Agreement.134 Copyright 2008, The Company of Biologists Limited. | ||
Trichomes can also have a detrimental effect on non-target organisms, including predatory insects.133,135 The hook-shaped field bean trichomes of the previous example are also known to impale and kill acariphagous ladybird beetles.136 Similarly, the long erect tomato trichomes have been observed to impale larvae of the two-spotted lady beetle attempting to forage green peach aphids.137 In addition, trichome exudates on glandular hairy tomato plants also hindered omnivorous bugs, reducing their prey searches and forcing them to groom more often.138
Some insects have evolved morphological and behavioural characteristics that help them to overcome trichome defences.139 For example, while resins secreted by the glandular trichomes of the carnivorous Roridula gorgonias plants normally capture insects, mirid bugs (Pameridea roridulae) use their body strength and a thick lipoid greasy layer on the integument to prevent getting stuck (Fig. 5f).134,140 Other mirid bugs living on pubescent plants use long slender legs to cling to trichomes while keeping their body away from the plant surface.140 On other glandular plants, adhering prey insect corpses are known to attract predatory insects, thus indirectly defending by herbivore suppression.141
3.4 Spines, thorns, and prickles
Robust, sharp structures like spines, thorns, and prickles are physical deterrents against large herbivores.142,143 Spines (Fig. 6a) contain vascular bundles and are a modified form of leaf tissues. They are stiff and slender and typically replace photosynthetic leaves in plants to prevent water loss and protect them from herbivores. Cactus spines further aid in directing water drops toward the plant body.144 Thorns (Fig. 6b) also contain vascular bundles but are a modified form of the stem and are quite tough and woody. Prickles are not vascularised and are breakable, short extensions of the epidermis (Fig. 6c).145,146 | ||
| Fig. 6 Natural defences, including (a) spines on California barrel cactus Ferocactus cylindraceus, (b) thorns on honey locust Gleditsia triacanthos, and (c) prickles on rose Rosa floribunda stems. (d) Camouflage in Mimosa pudica before mechanical stimulation and (e) after mechanical stimulation leading to folded leaves. (f) Yellow stipules on Passiflora sp. resembling eggs of Heliconius butterflies. (a)–(c) Adapted with permission.146 Copyright 2013, Nature Education. (d) and (e) Reproduced under the terms of a Creative Commons Attribution License.147 Copyright 2020, Hagihara and Toyota. (f) Reproduced with permission.148 Copyright 2017, Cambridge Philosophical Society. | ||
3.5 Thigmonasty
Thigmonastic defence refers to non-directional plant movements in response to an external stimulus.149 When Mimosa pudica (‘touch-me-not’ plant) leaves were disturbed by an external trigger, the water-filled cells were observed to loose turgor pressure due to the redistribution of potassium, sodium, and calcium ions.150–152 Leaflets almost instantly folded up and petioles dropped (Fig. 6d and e). In this way, the plant reduces the visible amount of foliage and exposes its thorny stem to deter herbivores.153 Specialised swollen structures, called pulvini, present at the base of the leaflets and along the stems function as rigid supports to maintain the structure and hold the mass of the leaflets.3.6 Camouflage
Camouflage as a natural defence is observed in passion flowers Passiflora sp. to deter Heliconiine butterflies.148 Female Heliconiine butterflies avoid laying eggs on passion flowers Passiflora sp. plants covered with existing butterfly eggs to reduce competition, because certain Heliconius sp. larvae exhibit cannibalistic behaviour.148,154Passiflora plants defend themselves from being damaged by these caterpillars by having structures mimicking the yellow eggs of butterflies on their leaves, which are swollen ends of stipules (Fig. 6f). Visual cues arising from the yellow-coloured eggs or egg-mimicking stipules are critical for butterflies' oviposition site decision.155 When artificially coloured green eggs were introduced on the plant, butterflies laid eggs nearby, yet yellow eggs resulted in a drastic reduction in ovipositional frequency.1553.7 Morphological features contributing to indirect defence
Plant morphology has evolved to support indirect defences by attracting and hosting predatory insects that help control pests. For example, hollow internodes in Cecropia plants house Azteca ants to protect the plant (see Section 2.1). Similarly, domatia, small cavities found on the underside of leaves, provide shelter for beneficial arthropods like predatory mites.156 In return, they protect the plant from herbivores and pathogens.157 Another morphological feature contributing to indirect defence are extrafloral nectaries on various plant parts. They secrete a sweet nectar solely employed to attract predatory insects like ants and lady beetles that can defend the plant against herbivores.158In summary, nature employs a range of structural natural defences in plants to protect them against pests, including both constitutive and induced mechanisms. The natural plant defences discussed in this review and their classification are summarised in Table 1. Specialised structures like calcium oxalate crystals, silicon-rich cell walls, and callose act as morphological barriers, while plant cuticle and epicuticular waxes prevent pest attachment. Other defence mechanisms like trichomes play a dual role by acting as both physical and chemical defences. These mechanisms, which have evolved to counteract various environmental threats, are foundational in exploring sustainable pest control alternatives.
| Category | Types | Constitutive vs. induced | Direct vs. indirect | Morphological vs. chemical | Ref. |
|---|---|---|---|---|---|
| Plant cell attributes | Phytoliths and cell wall fortification (silicon-related) | The cell wall is a first line of constitutive defence, while fortification is mostly induced. | Direct defence against pests. | Physical barriers against pests accessing the plant cell contents. | 83,84,94,95 |
| Idioblasts (calcium-related) | Constitutive defence. | Direct defence against herbivores. | Morphological features acting as an irritant for chewing insects. | 82,83,89 | |
| Laticifers | Constitutive defence. | Latex acts as a direct defence due to its toxins and sticky nature. | Latex can act as a physical and chemical defence. The sticky fluid can seal wounds and the mouthparts of feeding pests. It also contains several toxins that are toxic for insects once ingested. | 92,103–105 | |
| Resin ducts | Typically preformed and part of the constitutive defence. However, breached resin ducts can be replenished with the formation of new ones as an induced response to wounding by the plant. | Resins add to direct defence. | Resin flows act against stem-boring insects by physically expelling them. Further solidification of the resin occurs upon being exposed to air. | 106 | |
| Plant cuticle | Epicuticular wax crystals | Constitutive defence. | Direct defence. | Physical barrier against invading pathogens and parasites. | 114,117 |
| Trichomes | Glandular and non-glandular, etc. | Most trichomes are a part of the constitutive defence. Some plants can increase their density and/or glandular activity during an attack. | Glandular trichomes directly affect the pest owing to mechanical robustness or the sticky secretions that trap them or toxins that can be ingested. Some trichomes can also secrete volatile compounds to attract predators of the target pests, acting as an indirect defence mechanism. | Physical barriers for pests, restricting access to the plant surface. They also contain chemical secretions or biominerals aiding the defence mechanisms. | 120–123 |
| Spines, thorns, and prickles | Constitutive defence. | Affect the herbivore directly. | Morphological and mechanical defence. | 142–146 | |
| Thigmonasty (plant movements) | Typically induced by an external stimulus such as touch or feeding. | Rapid movements act as a direct defence against target pests. | Plant movements like folding or drooping constitute a morphological defence. | 149,153 | |
| Camouflage | Constitutive defence. | Camouflage could be classified as an indirect defence since it reduces the likelihood of being discovered by herbivores. | Primarily morphological since it uses pigments or patterns to deceive the target organism. | 148 | |
| Morphological features contributing to indirect defence | Hollow internodes, extrafloral nectaries, domatia | Although most form a constitutive defence mechanism, extrafloral nectaries are mostly induced in response to herbivory. | Indirect defence by providing incentives for predatory insects to visit the plant for pest control. | Morphological defence structures. | 157–159 |
4. Advances in the usage of synthetic pesticides
Currently used commercial pesticides predominantly work as neurotoxins by interfering with the insect's nervous system after ingestion, inhalation, or upon contact. They are categorised based on their chemistry as detailed in Table 2.| Group | Examples | Composition | Mode of pesticide action | Side effects on humans and animals | EU legislation |
|---|---|---|---|---|---|
| Chlorinated hydrocarbons | DDT, lindane, aldrin, endosulfane, methoxychlor and heptachlor | Non-polar and lipophilic molecules containing C, H, and Cl atoms. | They bind to sodium ion channels, inhibiting the flow of sodium ions and thus, impeding the nerve impulse transmission. | Potentially carcinogenic, affecting the kidney, liver, immune system, and reproductive organs over long-term exposure. | Several, such as DDT, lindane, and aldrin, are banned. |
| Organophosphates | Parathion, malathion, chlorpyrifos | Alkyl or aromatic phosphoric acid esters. | Acetylcholinesterase is inhibited, which leads to an accumulation of the nerve-stimulating chemical, acetycholine in insects. | Acute exposure can cause poisoning and neurological effects. | Several, such as parathion and dimethoate, are banned, while malathion is one of the oldest and most widely used. |
| Carbamates | Carbaryl, aldicarb, carbofuran | N-Methyl carbamates are derived from carbamic acid and are mechanistically similar to organophosphates. | They function similar to organophosphates by (reversible) inhibition of acetylcholinesterase. | Similar to, but considered less toxic than organophosphates. | Several, such as aldicarb, carbaryl, and carbofuran, are banned. |
| Pyrethroids | Permethrin, bifenthrin, cypermethrin | Pyrethroids are the more light-stable synthetic analogues of the naturally occurring pyrethrins from plant sources (Chrysanthemum cinerariefolium). | Once ingested, they attach to the sodium channel, inducing overstimulation of the nervous system, which causes pests to lose their coordination (paralysis). | Comparatively low mammalian toxicity, although acute exposure to high doses can induce adverse effects on the nervous system and potential allergic reactions. Also particularly toxic to aquatic organisms. | Permethrin, bifenthrin, and resmethrin are banned from agricultural use. |
| Neonicotinoids | Imidacloprid, acetamiprid | Compounds containing a negatively charged cyano or nitro group chemically similar to nicotine. | They bind to the nicotinic acetylcholine receptor in insects, blocking them and, thus, causing paralysis. | It is highly toxic to bees and other pollinators since they can accumulate in pollen and nectar. | Clothianidin, imidacloprid, and thiamethoxam are restricted to indoor use. |
Current economic, social, and technical circumstances necessitate the continued use of these pesticides, despite their impact to the environment and human health. However, there appears to be ample room to reduce their use. It is estimated that about 99.9% of the total pesticides applied on plants are wasted due to various processes like evaporation, degradation, and surface run-off, resulting in about 0.1% of the initial volume being effective against target pests (Fig. 7).165–167
 | ||
| Fig. 7 Illustration depicting various pathways by which pesticides get lost from the target plants and infiltrate the surrounding environment. | ||
Hence, the efficacy of synthetic chemical pesticides needs to be optimised through approaches such as enhancing their retention and using controlled-release formulations.
4.1 Improving pesticide adhesion on plants
Pesticide application procedures comprise spraying, fogging (creating a fine mist), pouring, powder release, fumigation (using gaseous pesticides), and seed dressing.168 Accordingly, synthetic pesticides are available in different formulations, ranging from solid powders to liquid solutions. Solid formulations include ready-to-use varieties such as granules (e.g., aldicarb from the carbamates group) and pellets that need to be sprinkled on target areas, and powders (e.g., DDT from chlorinated hydrocarbons) that should be mixed in water before application. Liquid formulations are available as (1) aqueous solutions of hydrophilic active substances (e.g., imidacloprid from neonicotinoids), (2) concentrates of oil-soluble (i.e., hydrophobic) active substances that emulsify when mixed in water (e.g., chlorpyrifos from organophosphates), and (3) suspension concentrates that have to be diluted with water before application (e.g., carbaryl from the carbamates group).169 Spraying is the most practical technique used commercially to apply these pesticide formulations on crops since it can quickly and easily cover a large area. However, this technique often results in a large amount of chemicals ending up around the plants, thus contaminating the environment.The wetting of plants is challenging because of their diverse three-dimensional, hierarchical anatomy, structures, and low cuticle surface energy.170 Several superhydrophobic plant species are naturally self-cleaning due to their waxy nature and roughness arising from ordered micro- and nanoscale cell and cuticle sculptures.114,170,171 Consequently, droplets tend to bounce and splash upon application, while also drifting or leaching from the plant due to environmental forces like wind and rain. A droplet hitting a hydrophobic plant surface goes through four distinct phases: (i) initial impact, (ii) spreading, controlled by inertial forces, (iii) receding, controlled by surface tension, and (iv) final state, which can either be a complete detachment from the plant, or the drop sitting on the non-wetting leaf surface with a high contact angle (Fig. 8a).172–175
 | ||
| Fig. 8 (a) Four distinct phases of a droplet impinging on a hydrophobic leaf surface. (b) SEM images of droplets with glycyrrhizic acid after impact show filamentous residues, indicating one-dimensional nanofibres pinning the droplet to a polytetrafluoroethylene (PTFE) surface. (c) Snapshots of negatively (polyanions) and positively (polycations) charged polymers impacting a superhydrophobic surface, indicating high retention when they combine and form precipitates directly on the surface (bottom panel). (d) Illustration of soybean leaves functionalised with dermaseptin–thanatin dipeptide, showing the potential of anchoring antimicrobial peptides on the leaf surface. (a) Adapted with permission.176 Copyright 2023, Elsevier B.V. (b) Adapted under the terms of a Creative Commons Attribution License.177 Copyright 2020, American Chemical Society. (c) Adapted under the terms of a Creative Commons Attribution License.178 Copyright 2016, Springer Nature. (d) Reproduced under the terms of a Creative Commons Attribution License.179 Copyright 2019, The Royal Society of Chemistry. | ||
Whether a drop bounces off or not depends on parameters such as the plant's surface energy and texture, but also the volume, viscosity, and surface tension of the drop. Plant surface wetting is often inhomogeneous or affected by aggregating droplets on trichomes and other structures, and/or domains of varying surface properties on the same plant.180
Most common attempts to enhance droplet adhesion to foliar surfaces rely on additives that alter the fluid properties of the spray product. For example, surfactants reduce the surface tension by the formation of micelles.181,182 Li et al. studied the properties of the cationic surfactant didecyldimethylammonium bromide (DDAB),175 showing that it migrates rapidly from the bulk to the gas–liquid interface, thus reducing the surface tension. Apart from solely increasing retention, this also improves spreading on a hydrophobic leaf surface due to the fast adsorption of the DDAB molecules at the interface. Field experiments using the herbicide glyphosate IPA to control weeds support this hypothesis, revealing improved inhibitory herbicide effects at increased concentrations of DDAB (0.01–0.1%). Another additive used in pesticide applications is glycyrrhizic acid, a surfactant with low surface activity and viscosity.183 Its ability to delay the bounce-off of pesticide-containing watery drops is due to surfactant molecules self-assembling into one-dimensional nanofibres that pin the droplets to a rough leaf surface (Fig. 8b).177
In general, however, a drawback for all surfactants is that they can lead to smaller droplets, which are more susceptible to evaporation and wind drift.184,185 Surfactants may also induce changes in leaf micromorphology. For example, a rapid deterioration was observed for cabbage Brassica oleracea epicuticular wax crystals, which could impact plant fitness.186
Damak et al. described the potential incorporation of polyelectrolytes in chemical pesticides to increase retention on hydrophobic surfaces.178 In their proof-of-concept study, two oppositely charged polyelectrolyte solutions were sprayed simultaneously on a superhydrophobic surface, forming solid precipitates of polyelectrolyte complexes, which led to a 10-fold increased retention in comparison to pure water. In contrast, spraying just one of these solutions results in droplets rolling off the leaf surface similar to water (Fig. 8c). Presumably, the combination of two oppositely charged precipitates causes surface defects that pin the drops to the hydrophobic substrate.178
The rheological properties of spray products can also be altered using flexible polymers. Bergeron and coworkers showed that the addition of a small quantity of the hydrophilic polyethylene oxide (PEO) substantially increased retention of sprayed solutions on (super)hydrophobic surfaces, even without considerably changing their surface tension and viscosity under shear flow.187 The suppression of droplet rebound was due to the significant reduction of the retraction velocity, attributed to non-Newtonian properties like the extensional viscosity (i.e., resistance to stretching) of the polymer solutions.178,187,188 In particular, the authors proposed that this effect resulted from the significant elongational characteristics of the fluid inside the receding droplet. As the fluid in the droplets undergoes expansion and retraction, high-molecular-weight polymers stretch out due to a velocity gradient, resulting in energy dissipation, which can effectively inhibit droplet rebound. While some authors supported this interpretation,189,190 others remain unconvinced. For example, Smith et al. argued that the anti-rebound effect is due to an additional force at the contact line. This force, known as contact line friction, occurs when the polymer chains stretch as the droplet moves, resisting its retraction.191 Evidently, the effects of polymer additives require further study, also concerning their environmental and food safety. Nevertheless, the positive effect of these additives is undeniable, with proof-of-concept studies demonstrating that they enhance fluid retention by over tenfold compared to water alone, achieving a plant surface coverage up to 80%.178
Taking a completely different path to increased pesticide efficacy, Schwinges et al. functionalised soybean plant leaves to curb the spread of the fungi Phakopsora pachyrhizi, which causes the Asian soybean rust (Fig. 8d).179 A bifunctional peptide composed of an antimicrobial and anchoring part was employed for this purpose. Since the outer membrane of microbes is composed of lipids, certain peptides can destabilise this lipid membrane, thereby killing the microbes.192,193 Thanatin, an amphiphilic peptide, was used to adhere dermaseptins, a class of antimicrobial peptides, on soybean leaves. Although the exact chemical compounds that participate in the binding are unknown, hydrophobic interactions between the lipophilic epicuticular leaf waxes and the peptides were thought to be responsible for the strong adhesion on the leaf surface. The bifunctional peptide combining thanatin and dermaseptins reduced rust infection symptoms in soybean by up to 30% compared to separate application of thanatin and dermaseptin. Presumably, the dipeptides on the leaf surface protruded from the epicuticular wax rosettes, which ensured peptide interaction with the fungi at very early developmental stages, preventing the fungi from infecting the plant cells.179,192,193
4.2 Controlled release
Controlled-release formulations involve the customised release of compounds (e.g., drugs, proteins, fertilisers, nutrients, and other biologically active agents) after a certain time or in response to external stimuli (e.g., pH, temperature, enzymes, UV light, magnetic fields, osmosis).194 By controlling the pesticide release in this way, less organic solvents and dispersing agents may be needed, and leaching of the sprayed product may be reduced.29 Controlled-release formulations are widely researched in the medical field to facilitate targeted drug delivery. Similar formulations are being developed to enhance the efficacy of pesticide delivery in agricultural settings. These formulations serve to keep the concentration of the active pesticide ingredient above a critical effective level for a longer period of time (Fig. 9a). This reduces the number of applications needed, preventing side effects from excessive concentrations, and overall, minimising waste and toxicity.29 These delivery systems can be formed from organic, inorganic, or hybrid compositions and vary from the more straightforward cases of (nano)particles, capsules, emulsions, and porous beads to more complex and extended configurations such as fibres, films, hydrogels, layered and framework materials (Fig. 9b).27 Controlled-release systems can be distinguished based on whether their performance is a passive or dynamic process. | ||
| Fig. 9 (a) Pesticide release concentration for conventional pesticides (blue line) and controlled-release formulations (purple line) as a function of time after application. The dashed line indicates the minimum level for effectiveness. (b) Different types of controlled-release pesticide systems. (c) Various sustained-release mechanisms, namely diffusion through water-filled pores or directly through the polymer, release due to erosion/degradation of the polymer carrier, and release due to differences in osmotic pressures. (d) Various ‘smart release’ mechanisms triggered by external stimuli, namely pH, temperature, enzymes, and light. (b) and (d) Adapted with permission.29 Copyright 2021, American Chemical Society. (c) Adapted with permission.27 Copyright 2020, Elsevier B.V. | ||
Traditional controlled-release formulations are typically passive or sustained-release systems where a continuous, stable release of the loaded pesticide concentration is achieved through inherent processes. It is predominantly a consequence of passive diffusion and osmotic pressure or gradual carrier breakdown without external triggers (Fig. 9c).195 The controlled-release formulations belonging to this category are advantageous in situations where pesticide action is required equally throughout a large part of the crop growth cycle. Examples of such passive delivery systems are sorption-based materials characterised by high porosity.196 For example, metal–organic frameworks (MOFs) are crystalline materials composed of metal ions and organic ligands, forming a highly porous three-dimensional network. In 2017, Yang et al. first incorporated biodegradable MOFs in agricultural contexts by preparing an eco-friendly MOF that exhibited good uptake and release kinetics of the pesticide cis-1,3-dichloropropene. These MOFs consisted of non-toxic Ca2+ ions bridged by naturally occurring L-lactate and acetate linkers. The pesticide-loaded MOFs achieved a 100-fold increase of the time the pesticide is present compared to the plain, volatile liquid product, as well as good degradability, illustrating the potential of MOFs as sustained-release carriers in agriculture.196 Following this invention, many more sustained-release porous framework materials have been reported,197–199 and similar performance has been reported for other material designs (Fig. 9b).29,195
Controlled release systems relying on dynamic, stimulus-responsive processes have been established by drawing inspiration and using theoretical foundations from biomedicine.200,201 These ‘smart-release’ systems respond to external signals and undergo physicochemical changes, resulting in the release of the loaded pesticides. These signals include changes in pH, temperature, presence of specific enzymes, or light irradiation (Fig. 9d). This approach aims to make the application of pesticides more targeted, safe, and efficient.195,202 For example, the commercially available pesticide, Seltima, uses an encapsulation technology that allows for the fungicide, pyraclostrobin, to be released onto rice crops while simultaneously restricting it from being released into groundwater.203 The key to this double action is the sensitivity of the microcapsule wall to humidity, which cause the capsules to release the fungicide when applied on rice leaves. If the microcapsules fail to adhere to the crop and end up into nearby water bodies, they sink to the bottom while remaining intact having the toxic ingredient trapped.203 The microbes also present inside are then expected to degrade the active ingredients.
4.3 Smart solutions
Synergistically combining the passive and active types of controlled-release systems results in innovative formulations that enhance adhesion to leaf surfaces and release the active chemicals in a controlled fashion, thus minimising environmental impact without compromising efficacy.Song et al. explored one such system by using folate/zinc supramolecular hydrogels loaded with a herbicide called dicamba.185 Folic acid and zinc, which are important elements of plant growth activities, form a hydrogel with a nanofibre network, which aids in the encapsulation of dicamba. The folate/zinc supramolecular hydrogels display apparent shear-thinning properties, indicating that they can be sprayed through a small nozzle, after which the nanofibre network is quickly re-established. Since the surface tension of these hydrogels is not very different from that of water, a retention mechanism similar to the one discussed in Section 4.1 with PEO addition is hypothesised (Fig. 10a). All liquids spread instantly upon impact due to inertial forces, up to a maximum spreading diameter.7,8 Once the surface energy starts coming into play, the droplet tries to retract. While liquids like water experience a large surface energy when impacting a hydrophobic surface, causing them to bounce off, the folate/zinc supramolecular hydrogels, akin to PEO as an additive, can securely hold on to the surface owing to the dynamic nanofibre network, which initially spreads up to the maximum diameter, dissipating most of its energy due to its viscous nature and from surface friction losses. Although the focus of the study by Song et al. was solely on increasing retention, these hydrogels may also influence the release kinetics.
 | ||
| Fig. 10 (a) Schematic mechanism of the dicamba-in-hydrogel droplet forming a cross-linked nanofibre network when it impacts a super-hydrophobic leaf. (b) Nanoemulsion droplets containing castor oil-based polyurethane (CO-PU) and lambda-cyhalothrin (LC) spread well due to their low surface tension followed by strong adhesion due to the coalescence of the nanoparticles and hydrogen bonding between nanoemulsion and leaf surface. (c) Mechanism of pesticide release and leaf surface adhesion of pH-responsive adhesive nanocapsules. (d) Formation and retention mechanism of catechol-modified hat-shaped carriers loaded with pesticides. (e) Schematic of loaded microgels whose outer surface is decorated with anchor peptides to facilitate strong binding to the leaf surface. (a) Adapted under the terms of a Creative Commons Attribution License.185 Copyright 2020, American Chemical Society. (b) Adapted under the terms of a Creative Commons Attribution License.165 Copyright 2017, The Royal Society of Chemistry. (c) Adapted with permission.204 Copyright 2021, The Royal Society of Chemistry. (d) Reproduced with permission.205 Copyright 2020, Elsevier B.V. (e) Reproduced with permission.206 Copyright 2020, Wiley-VCH Verlag GmbH & Co. | ||
Nanocapsules also offer a smart solution by combining controlled release, improved retention, and enhanced efficiency. Zhu et al. studied the retention of nanocapsules loaded with β-cypermethrin, which is a hydrophobic pesticide.207 The nanocapsules were synthesised using complexes of oppositely charged biopolymers, i.e. gelatin and acacia gum, in an oil-in-water microemulsion. The resulting system is hypothesised to increase deposition on a leaf surface owing to its small size, although small droplets may also increase loss through pesticide drift.207
Zhang et al. used a similar formulation and combined it with the concept of spraying oppositely charged polymers discussed earlier.178 Azadirachtin, a biopesticide, was incorporated into the core of anionic and cationic polyurethane core–shell structures synthesised from castor oil.208 The increased retention of the solutions on the hydrophobic surface is attributed to surface precipitation arising from simultaneously spraying oppositely charged particles, as discussed earlier, in addition to reduced surface tension effects from the added Tween-80 surfactant. This formulation includes controlled release and the added advantages of heat and light protection for sensitive encapsulated pesticides.
Qin et al. explored a castor oil-based polyurethane carrier system by incorporating lambda-cyhalothrin (LC) in a nanoemulsion formulation without surfactants.165 The LC nanoemulsions adhered much better to leaf surfaces than a commercial emulsifiable concentrate and wettable powder formulation after washing with water. However, all three systems showed similar deposition behaviour before washing. After spraying these nanoemulsions, the liquids spread on the leaf surface owing to their surface tension (34.9–40.3 mN m−1) being significantly lower than the critical surface tension of the tested crops (cotton: 63.3–71.8 mN m−1, corn: 47.4–58.7 mN m−1), without the use of any surfactants or emulsifiers (Fig. 10b). Subsequently, the droplets of the nanoemulsion coalesced, and evaporation of water led to the formation of solid films on the leaf surface, increasing contact between the LC residues and the plant surface. Better adhesion to plants was also attributed to hydrogen bonding between urea and urethane groups of pesticide emulsions and the leaf surface functional groups, like hydroxyl and aldehyde.165
Chen et al. combined the concept of hydrogen bonding for increased retention with controlled-release nanocapsules (Fig. 10c).204 They utilised catechol compounds, which are known for facilitating strong underwater adhesion in mussels.209 Acidity-controlled nanocapsules were covered with catechol groups in order to form hydrogen bonds with the alcohols, acids, and aldehydes present on a leaf's waxy surface, thereby increasing pesticide retention on the plant. Under acidic conditions (pH < 6.5), protonation of amino groups from NH2 to NH3+ in the nanocapsules led to an increase in the positive charge, which resulted in swelling due to electrostatic repulsion and swelling of the channels, thus promoting diffusion and release of the encapsulated pesticide. Although such pH-responsive materials are promising, most of these systems are still in the proof-of-principle stage, and not yet optimised for real-world settings. For example, a more plausible trigger was proposed with an alkaline pH-responsive pesticide system, developed by encapsulating the insecticide LC in O-carboxymethyl chitosan (O-CMCS) and covering it with polyurethane (PU).210 This system was designed to release the active ingredient in an alkaline environment (pH 8.0–9.5), which is the pH condition found inside the gut of some pests. When taken up by dew drops present on the plant, the acidic nature of these drops (pH 5.3–6.8) significantly slowed down the release of LC, thereby ensuring that the pesticide is active mostly when ingested by the pest.210
While most techniques for improving retention focus on the properties of the incoming droplets for better adhesion, as discussed up until now, one can also consider the leaf surface. Leaves can have a number of structures on their surface, such as nanosplinters and micropapillae. Zhao et al. were among the first to create pesticide carriers that took into account both surface modification of the incoming particles and the leaf surface topology.188,205 Emulsion interfacial polymerisation allowed the preparation of hat-shaped carriers (HSCs) loaded with pesticide, which have a complementary shape to the micropapillae on the leaf surface, provoking a hanger-hat effect to enable better adhesion (Fig. 10d). So-called Janus particles were used for this purpose. In their follow-up work, adding catechol compounds improved this hanger-hat effect. Comparing HSCs with and without catechol groups, the retention of the fungicide on the leaf surface improved when catechol groups were added, which was attributed to a synergistic effect of the hanger-hat topology and non-covalent interactions arising from the catechol groups. An added value of functionalising the HSCs with catechol groups is the faster pesticide release for a prolonged period of 15 days compared to the unmodified HSCs. This acceleration was attributed to the difference in synthesis techniques where the modified particles were loaded with pesticide on a fully swollen polymer, facilitating quick release. In contrast, the unmodified particles were synthesised along with the presence of the pesticide, resulting in a tightly bound structure and thus, slower release rates.
Antimicrobial peptides, as discussed in Section 4.2, are of particular interest due to their ability to interact with microbial membranes. They have been previously attached to leaf surfaces using adhering peptides.179 Similarly, Meurer et al. used these peptides to attach hydrophilic microgels loaded with nutrients to leaf surfaces (Fig. 10e).206 Poly(allylamine) microgels were synthesised using a water-in-oil emulsion polymerisation reaction, and modified with a catechol derivative, 2,3-dihydroxybenzoicacid (DHBA) to enhance the uptake of Fe3+ micronutrients. The adhering peptide lantaricin A was used to ensure reliable retention on hydrophobic plant surfaces. The peptides adhere on small islands of hydrophobic wax-like regions on the leaf surface. These Fe3+-loaded microgels were tested on iron-deficient cucumber plants, and results indicated positive re-greening of the application sites in contrast to the chlorotic spots with iron deficiency in the leaves. Although this system has been designed to deliver micronutrients to targeted locations by increasing retention and reducing wastage on the leaves, it may be extended into the fields of pesticides and insecticides.211 Research along similar lines was conducted by Mai et al. who developed an alginate gel modified by catechol-based compounds that could form strong non-covalent interactions with the waxy leaf surface.212 Subsequently, Fe3+ ions were introduced into the polymer network to form a dense 3D crosslinked network with a high resistance to washing off. This significantly improves pesticide retention on leaf surfaces and enhances resistance to rainwater while facilitating controlled release of the active ingredients when irradiated by light.
In summary, recent advances in synthetic pesticides focus on improving pesticide adhesion on plant surfaces, aiming to prevent premature wash-off and thereby increase effectiveness. This has led to the development of surfactants and polyelectrolyte additives that help maintain pesticide retention. In parallel, controlled-release systems have gained attention, as they can regulate the pesticide release over time, minimising environmental impact and reducing toxicity. Convergence of innovations in these areas have resulted in smart delivery solutions, using systems based on hydrogels, antimicrobial peptides, and nanocapsules that combine both improved adhesion and controlled release to create more efficient and sustainable pest control strategies.
5. Integrated pest management domains beyond chemical control
While significant advances in improving the efficacy of chemical control strategies in IPM are underway, as discussed in the previous sections, efforts to entirely eliminate synthetic chemical pesticides from contemporary agriculture are still scarce. In the following sections, we highlight some ideas in the physical, cultural, and biological control domains of IPM, offering glimpses of a possible future where agriculture is completely free from synthetic chemical interventions.5.1 Mineral powders
In olden times, stored grains were commercially mixed with minerals like silica to prevent infestation of the common grain weevil.213 One of the first non-chemical pest management strategies to be explored by scientists was the use of dust particles. Since the 1930s, research has been conducted to study the exact mechanism of action of these inert dusts. Although it was initially hypothesised that weevils died after ingesting the dust, it was later confirmed that the main cause of death was increased rates of water loss due to friction between their cuticle and the dust particles.213 Abrasion is necessary for hard, non-sorptive particles to remove the insect's epicuticular grease, leading to death by dessication.214 Other small inorganic particles made from materials like clay and silica interfere with target pests in different ways. Spraying kaolin (clay mineral) shields plant surfaces from aphid attacks since these phloem-feeders tend to settle on foliage based on a sensitivity to colour. Spraying kaolin as a film on citrus plants led to a whitish appearance, thus affecting the host-finding abilities of aphids, reducing pest colonisation.215,216 Glenn et al. first studied insect and disease suppression by applying a porous and hydrophobic layer of kaolin mineral particles.217 The kaolin particles were modified to become hydrophobic by coating them with an undisclosed synthetic hydrocarbon. Although kaolin is relatively soft and non-abrasive compared to other commonly studied particle layers, pear psylla Psylla pyri feeding and ovipositional activities were found to be affected. Adults constantly cleaned themselves to remove the tiny kaolin particles adhered to their bodies, neglecting their regular activities and eventually dying due to starvation. In addition to these effects, minerals also affect pests by means of physical hindrance and their repelling hydrophobic nature. For example, several bacteria and fungi require water to propagate their species. The hydrophobic mineral particles ensure that water is not available for this purpose, and also prevents the propagules from coming into contact with the plant surface (Fig. 11a). An unexpected advantage of these particle films is the improvement in plant photosynthesis by reducing the heat stress on the leaf surface and allowing photosynthesis for a longer part of the day.218 However, the most recognisable disadvantage of such particle films comes from the application point of view, where a perfect coverage of foliage is required for the particles to curb the spread of an infestation effectively. This is restricted by the fact that these particles cannot adhere strongly to the leaf surface and drift could lead to reduced effectiveness. Also, the particles are not as effective with rainfall in open fields. More recently, researchers studied these particle films with different pests, proposing strategies to improve their functionality.219–222 Faliagka et al. tried to utilise the same concept of desiccation caused by abrasion of the insect cuticles with a textile impregnated with silica dust.223 Although the dust-impregnated fabric nets effectively restricted the entry of aphids, concerns regarding reduced ventilation inside a greenhouse persist. Further discussion is omitted since such nets are not directly applied to plants and, hence, are out of the scope of this review.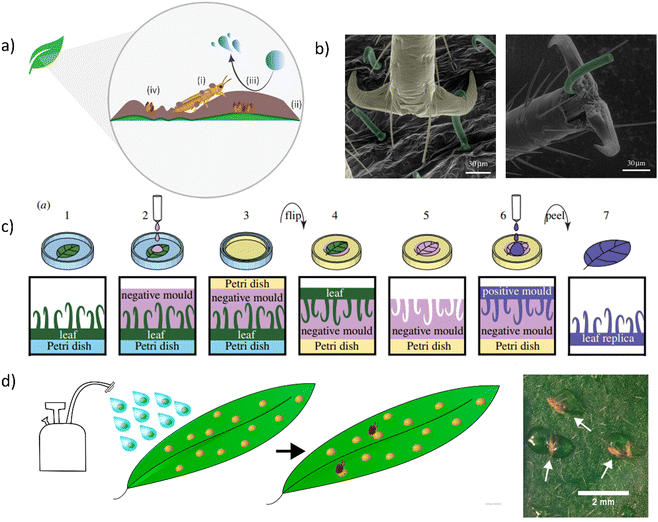 | ||
| Fig. 11 (a) Mechanisms of action of hydrophobic particle films, namely (i) adhering to the insect to hinder feeding and locomotion, (ii) interrupting colour signalling and presenting a physical barrier to feeding from the leaf surface, (iii) preventing water access for propagation, and (iv) preventing propagules from reaching the plant surface. (b) SEM images of hook-shaped trichomes of bean leaves interlocking with bed bug's claws (left) and impaling the pretarsus (right). (c) Double-moulding microfabrication of biomimetic leaf surfaces. (d) Physical pesticide consisting of a sprayable aqueous suspension of adhesive particles to immobilise target pests upon contact. White arrows indicate Western flower thrips trapped in the trichome mimic particles on a detached leaf. (b) and (c) Adapted with permission.224 Copyright 2017, The Royal Society. (d) Adapted under the terms of a Creative Commons Attribution License.225 Copyright 2024, National Academy of Sciences. | ||
It is also interesting to note that the mechanism of action of these mineral powders closely resemble the roughness and contamination hypotheses of epicuticular wax crystals described in Section 3.2.1 highlighting the promise that bioinspired strategies hold to change the future of pest management, as discussed in the next section.
5.2 Bioinspired alternatives
Nature's vast array of defence mechanisms could serve as valuable inspiration for creating synthetic pesticides that are both effective and environmentally friendly. Developing bioinspired pesticides could also potentially achieve targeted pest control methods that minimise harm to non-target organisms in concert with nature's own strategies.226Taking a step in this direction, Schifani et al. described the potential of artificial nectaries designed to mimic extrafloral nectaries in order to attract predatory ants as discussed in Section 3.7.227 By simply using a sugar solution of sucrose in water, they demonstrated a significant reduction in typical pear orchard pests, along with an increase in predatory arthropods. This relatively simple strategy highlights the potential of leveraging natural plant defence mechanisms for developing bioinspired alternatives.
Similarly, Szyndler et al. studied the hook-shaped trichomes of bean leaves to fabricate surface replicas that hinder bed bugs.224 Their inspiration came from a historical practice where bean leaves trapped bed bugs owing to a physical entanglement mechanism between the trichomes and the legs of the bed bugs (Fig. 11b).228 To fabricate synthetic trichomes, a double moulding process was used where a negative mould was applied to replicate the leaf surface, followed by a polymeric positive mould to create the test substrate (Fig. 11c). For comparison, they also fabricated hybrid trichomes, by breaking off the tips of natural trichomes in the negative mould, leading to a hybrid variation with natural tips and synthetic stalks. The synthetic trichomes were observed not to pierce any of the bed bugs in the study, while the natural trichomes trapped 90% of the bed bugs within seconds on average. The hybrid trichomes, although expected to function similar to natural trichomes, also performed poorly in terms of piercing the insect feet.
The reason for this result was attributed to the differences in terms of the material's flexural and torsional stiffness. For synthetic trichomes, the entire structure (tip, stalk, and base) is composed of a solid material, while natural trichomes consist of solid tips and hollow cylindrical stalks set on flexible bases. Thus, the hollowness of the stalk could give the natural trichomes enough flexibility to graze along the cuticle of the bed bug till it gets stuck eventually piercing the feet, whereas synthetic and hybrid trichomes might end up bending instead. Such replication approaches and synthetic surfaces serves as excellent models to study biological surfaces via comparative persistent models.229,230
Exploring the potential behind mimicking trichomes further, an innovative physical pesticide was developed by Zwieten et al. whereby adhesive particles made from oxidised vegetable oils imitated the defence mechanism arising from glandular trichomes. An aqueous suspension of these adhesive particles was sprayed on plants, leaving the sticky particles behind after water evaporation. They acted as miniature glue traps, physically immobilising target pests similar to the sticky exudates of glandular trichomes as discussed earlier (Fig. 11d).225,231 Being trapped, mortality of western flower thrips Frankliniella occidentalis increased approximately three-fold due to lack of food supply as compared to the pure control.232 A water-based variation of this strategy was undertaken in our own work, where Drosera trichomes were mimicked using sugar-based natural deep eutectic solvents.233 These recent developments highlight the potential of the diverse defences found in nature to pave the way for bioinspired eco-friendly pest management solutions. It is important to note that such advances still require significant research to study their impact in real-world settings, including any side effects arising from their accumulation in the environment.
5.3 Pest repellence
Physical pest control can be through mechanical, thermal, optical, and acoustic procedures, mainly repelling herbivores. One well-known strategy is to manipulate pest behaviours such as host feeding, host plant detection, and mating.234,235 In the ‘push–pull’ strategy introduced by Pyke et al., pests are ‘pushed’ away from the host plant and ‘pulled’ towards an alternate attractive source to entice them away from the target field/crops. This strategy was tested with neem tree extracts which repelled, while sacrificial crops attracted Heliothis away from cotton plants.236 Various kinds of push–pull strategies, such as visual cues, pheromones, and trap cropping, have been summarised in detail elsewhere.237Plants that secrete sticky fluids have been experimentally shown to act as dead-end trap crops to protect nearby plants. Unlike traditional trap crops that simply attract pests away from the plants of interest, dead-end trap crops lure pests towards them but do not allow them to reproduce or survive long-term.238,239 For example, a recent greenhouse trial found that a special laboratory strain of tobacco (Nicotiana benthamiana) was as effective as commercial sticky traps in killing whitefly and thrips. Such dead-end trap crops offer a sustainable alternative to sticky traps as they do not rely on non-biodegradable plastics and provide the added advantage of minimal negative effects to predatory insects.238,240
Experimenting with another type of behavioural manipulation technique, Polajnar et al. used mechanical vibrations to cause mating disruptions in the invasive leafhopper pest Scaphoideus titanus. In these insects, the mating sequence involves a series of pulses from the male with short occasional pulses as a reply from the female, resulting in a characteristic duet between the two. The team used continuous mechanical vibrations that acted as noise, to block the communication between the male and the female leafhoppers with a disruption efficiency of around 90% male-female pairs for one day. To save energy, the mechanical vibrations can also be tuned down during specific time periods, where the target pests are unlikely to mate.241 This behavioural manipulation technique is part of the field of bioacoustics or applied biotremology, which is attracting increasing attention in phytomedical research. Species-specific ‘semiophysicals’ (physical cues) can be generated to interfere with Aleyrodidae white flies (Hemiptera), Mycetophilidae fungus gnats (Diptera), Pentatomidae true bugs (Heteroptera), moths (Lepidoptera), and various beetles (Coleoptera) in greenhouses, fields, orchards, vineyards, and forests, reducing the pest population densities.242,243
5.4 Biopesticides
Another approach to pest control involves the exploitation of natural pest control measures such as releasing the natural predators of target pests. This type of biocontrol is summarised in numerous overviews.69,244,245 Here, we review pesticides isolated from natural sources, such as microorganisms, animals, and plants.246,247 These biopesticides have emerged as viable alternatives to synthetic pesticides and, in recent years, comprise approximately 10% of the international pesticide market.248 They match to an extended degree the profile of an ‘ideal pesticide’ due to their biodegradability, frequent target-specific action, and high efficiency even at low concentrations.246,249 Additionally, reports have shown that using some natural pesticides not only affects pests but also promotes soil biodiversity and helps plants build up tolerance to environmental conditions such as droughts.248,250 Biopesticides are classified into three main categories by EPA: (1) microbial pesticides, (2) biochemical pesticides, and (3) plant-incorporated protectants (PIPs) (Fig. 12).247,251 | ||
| Fig. 12 Overview of the three different biopesticide categories that are recognised by the U.S. Environmental Protection Agency (EPA). | ||
Microbial pesticides are pesticides consisting of microorganisms, including viruses, bacteria, protozoa, and fungi that enter the host through ingestion or contact, and kill the host by multiplying and releasing toxins. Bacillus thuringiensis (Bt) has been credited as the most successful microbial pesticide in the market owing to its fast and host-specific action that leaves other organisms mainly unaffected. The bacterium produces parasporal crystal proteins called δ-endotoxins, also known as ‘Cry’ and ‘Cyt’ proteins, that exhibit toxicity towards a broad spectrum of insect pests, including Lepidoptera, Coleoptera, and Diptera. Around one thousand toxin genes encoding entomopathogenic protein toxins have been discovered and studied in Bt strains collected from various geographic areas.252
Another important example of microbial pesticides, also approved by the EPA, is the entomopathogenic fungus Metarhizium anisopliae.249 It has a broad spectrum of activity against arthropod hosts while remaining safe for the crops and the environment.253,254 A wide range of strains and isolates of M. anisopliae can be found in moist soil environments that promote filamentous growth and the generation of infectious spores known as conidia.254 The infection begins with the adherence of conidia to the insects residing in the soil upon direct contact, followed by conidia germination. Subsequently, the generated germ tubes differentiate to form the appressorium (highly specialised infection cell), used to penetrate the host cuticle. Once the fungus overcomes the epidermis, it colonises the insect's hemolymph (body fluids), extrudes hyphae, and sporulates, killing the host. This whole process is completed in about five days under optimal conditions.255,256
Biochemical pesticides are mostly designed to work through non-toxic mechanisms, although this category can include natural toxins. In contrast to conventional pesticides, this type of pest control does not rely on harming the pests but rather on repelling or attracting them into traps. For example, more than 1600 insect pheromones are currently used as ‘semiochemicals’ (chemical signals used between organisms), to affect the mating patterns of insects, either by disrupting them or by triggering them to search for a sexual partner instead of laying eggs and aggregating.249,257 This mode of diverting insects away from crops has several benefits, like lower costs, ease of use, and high selectivity and sensitivity.254
Essential and vegetable oils sourced from plants have been used as insect repellents, attractants, and contact pesticides since ancient times because they are widely accessible, affordable, rapidly biodegradable, and safe for mammals.258 However, their mode of action is not fully understood. For example, eggs of the two-spotted spider mite Tetranychus urticae, when covered with vegetable oil, did not suffocate as commonly supposed, but hatching larvae were hampered.259
Commercially available botanical pesticides are mainly isolated from neem Azadirachta indica, chrysanthemum Tanacetum cinerariifolium, tobacco Nicotiana tabacum, ryania Ryania speciosa, and sabadilla Schoenocaulon officinale. They primarily target insects and plant parasites like nematodes, fungi, bacteria, and viruses.258
Plant-incorporated protectants (PIPs) are classified as the third category of biopesticides by the EPA, but remain unrecognised by most international authorities.251 PIPs refer, in particular, to genetically modified (GM) crops that can produce pesticide substances by themselves through rDNA technology. This method relies on the insertion of genetic material endowed with the capability to introduce substances for natural defence into the designated plant's genome. Dating back to the 1980s, the first reported PIPs were Cry proteins expressed in GM crops containing Bt transgenes. Ever since, many Bt crops have been established, the majority of which are cotton and corn genotypes.249,260 Additionally, a new generation of PIPs has recently been registered, consisting of GM crops that protect themselves by expressing double-stranded RNA (dsRNA).261 This method is called host-induced gene silencing (HIGS) and is based on RNA interference (RNAi), a form of post-transcriptional gene silencing described initially by Fire et al. in 1998.262 The mode of action is as follows: dsRNA molecules enter the insect's body via the plant, where an endoribonuclease enzyme, Dicer, cleaves the dsRNA into smaller RNA fragments. These fragments are then incorporated into the RNA-induced silencing complex (RISC) by the protein Argonaute. RISC is directly responsible for silencing essential genes for pest survival.261,263 HIGS is a transgenic delivery system that can also improve inherent plant defence qualities.264 The first RNAi crop that was approved by EPA, in 2017, SmartStax PRO by Bayer AG, targets the corn rootworm Diabrotica virgifera by expressing dsRNA that interferes with the synthesis of one of its essential vacuolar sorting proteins.260,265
RNAi-based pest control was first studied in beetles and moths, where the genes responsible for insect development and survival were targeted for suppression (Fig. 13).266,267 However, most earlier experiments injected dsRNA into insects to study the resulting gene expression.268–271 In terms of commercial pest control, it is more practical if the pest takes up the dsRNA autonomously.272 Spray-induced gene silencing (SIGS) is a non-transgenic strategy for delivering dsRNA via a foliar spray, root irrigation, trunk injections, or seed dressings, whereby insects acquire it through plant feeding and chewing.264,273 Oral delivery of dsRNA has been successful in a number of organisms, such as the western corn rootworm, striped flea beetle, and cotton bollworm.272 Although uptake by oral ingestion shows great potential, the efficiency of RNAi may be heavily influenced by the environment it has to travel through, such as the pH and the presence of enzymes in the insect gut region, depending on the insect species.274 In a study with locusts and small mottled willow moths, dsRNA strands were incubated in the digestive fluids. While the dsRNA degraded quickly in the fluid from locusts, it was successfully incorporated in moths fluid. Although the pH of both fluids varies in locusts and moths (pH 6.8 vs. 8.8), it was hypothesised that the degradation of the dsRNA strands is due to the nuclease enzymes present in the locust fluids. A deactivation of these enzymes by heating resulted in a significant reduction in the degradation of dsRNA.274 In the case of lepidopteran insects where RNAi oral ingestion was previously unsuccessful due to the gut enzymatic activity, Parsons et al. developed inter-polyelectrolyte polymer-dsRNA complexes to protect the dsRNA strands in the gut.275 Poly-[N-(3-guanidinopropyl)methacrylamide] (pGPMA), a synthetic cationic polymer, was shown to form complexes through electrostatic interactions with the negatively charged RNA backbone. Apart from enhancing the stability of the RNA strands, these complexes also protect them from enzymatic degradation since pGPMA is a weak acid, which prevents dissociation of the complexes in an alkaline gut environment (pH 10–11) of the insects.275
 | ||
| Fig. 13 The steps involved in RNAi-based pest control. (a) Ingestion of dsRNA by insects. (b) Uptake of dsRNA by the microvilli of the columnar cells (MCC) in the insect midgut. (c) Mechanism of gene silencing. Reproduced under the terms of a Creative Commons Attribution License.276 Copyright 2016, Joga et al. | ||
Further research in RNAi has revealed many other novel dsRNA delivery strategies, such as the usage of modified viruses (virus-induced gene silencing: VIGS), or via modified microorganisms like bacteria, yeast, and fungi.276–278 A bottleneck regarding such pest control techniques is the necessity of substantial quantities of dsRNA for field applications. Being an evolving field of research, an engineered bacterium was developed by biotechnology company RNAgri (formerly Apse), called RNA Containers TM (ARCs) which can mass produce dsRNA for target applications.276
In summary, there is a wide range of non-chemical methods of pest control, such as mineral powders like kaolin, which work by desiccating pests, and bioinspired surfaces that mimic natural plant defences, like trichomes, to trap pests. Pest repellence strategies include mechanical, thermal, and acoustic methods, while biopesticides can be derived from natural sources like microorganisms, plants, and animals. All these innovations contribute to environmentally friendly and bioinspired pest control approaches, offering potential strategies to replace traditional chemical solutions.
6. Future perspectives
While navigating the complexities of modern agriculture, it is evident that substantial efforts are necessary to transition towards optimised innovative pest management to ensure food safety and compliance with stricter legislation surrounding the currently used synthetic pesticides. IPM (and ICM) are key concepts towards creating a sustainable agriculture. Despite much progress, more remains to be done.The future of chemical strategies in IPM looks promising as researchers continue to explore environmentally friendly solutions. There has been notable research and advances to reduce the loss of synthetic pesticides and to control release rates over time. For instance, the humidity-responsive pesticide Seltima demonstrates how controlled-release formulations can improve efficacy while minimising environmental impact.
Physical, cultural, and biological control strategies are also being judiciously explored. Traditional methods continue to evolve alongside novel techniques. New research domains are focusing on developing pesticides that are selectively toxic to target pests. One such promising concept is chemical genetics, where small molecules are used to elicit biological action. After this concept initially emerged in biology and therapeutics, it is now explored in targeted pesticide development.279,280 It is known that one of the various mechanisms that pesticides use to control pathogens is the disruption of key proteins that are vital for their growth, reproduction, or metabolism.281 Hence, investigating ligand binding and other active sites of these essential proteins is an important step toward understanding the common molecular target structures that could be exploited when designing new pesticides.280 Computational modelling is also an upcoming field in agriculture, with potential for predicting environmental impact and toxicity of organic pesticides include pesticide loss and drift.282 However, its use in material design for plant protection, such as for screening novel compounds, is still in its early stages.283
In parallel, there are several promising commercial products entering the market which have similarities to natural plant defence mechanisms. For example, Eradicoat, a maltodextrin-based contact pesticide, suffocates pests by forming a coating around them, upon spraying and drying.284 This mode of action closely resembles how resin ducts and trichome exudates trap and suffocate pests. Agrical Pro is another such mechanical pesticide, based on clay, which creates a white inert layer on plants that acts as a visual barrier to insects, thereby using camouflage as a defence strategy.285 Dezone, a diatomaceous earth-based mechanical pesticide, dehydrates insects by sticking to their cuticle and absorbing their protective lipid layer.286 This mechanism mirrors the defence strategy provided by plant epicuticular wax crystals. Given their mechanical modes of action, such innovations reduce the risk of pesticide resistance and pose minimal risks to human health and the environment.
Despite these advances, there remains a gap between the wealth of knowledge about pest control in nature and the development of bioinspired techniques, which could aid in advancing other IPM domains. While some biochemical strategies are exploited, many others remain underexplored. The primary challenge in developing practical and effective synthetic chemical-free methods is their limited suitability for commercial applications due to being overly time or labour-intensive. Most mechanical pesticides also still require further research to effectively target specific pests without adverse effects to beneficial insects. Adhesive pesticides that mimic trichomes, for example, could potentially achieve this specificity by tuning the size and adhesive strength of the particles, much like how predatory insects navigate around trichomes in nature. It can also be inferred from our review that plant structures may play a role in shaping such pesticide innovations. While not many direct studies have explored linking plant morphology to improved adhesion or alternative delivery systems, this presents an intriguing research opportunity. For instance, thorns on the stem could potentially puncture vesicles, triggering the release of active compounds. We believe there lies an opportunity to draw inspiration from nature and introduce biomimetic approaches to enhance IPM strategies further.
Moving forward, efforts to address this challenge should focus on bridging the gap between plant biologists, agricultural scientists, chemists, material scientists, farmers, and policymakers to foster innovative, interdisciplinary solutions. Furthermore, commercialisation prospects and potential challenges must be critically assessed including the cost-effectiveness of new materials, the feasibility of large-scale manufacturing, and the long-term impact on the environment and human health. Regulatory frameworks also play a pivotal role in shaping the adoption of innovative pest control solutions. Currently, pesticides have to undergo a rigorous authorisation process in the EU before being used commercially.287 Policymakers have the power to facilitate the transition to sustainable IPM by restricting the usage of non-biodegradable chemicals while supporting research initiatives, streamlining approval processes for safer alternatives, and promoting farmer education programmes to encourage widespread adoption. For example, Switzerland's IP Suisse initiative introduced a voluntary, pesticide-free production scheme for cereals, like wheat, spelt, and rye. It combines both private market incentives such as a 30% price-mark up by bakeries, with government support such as direct payments to farmers for adopting pesticide-free practices. This programme is part of Switzerland's broader efforts to balance environmental and economic interests in farming.288
Ultimately, the future of IPM may be in creating non-toxic strategies to human health, specific to target pests that cause no harm to non-target organisms, do not contribute to pesticide resistance over time, and are both environmentally friendly and cost-effective. They should complement and be easily incorporated into existing IPM strategies. By embracing collaboration, innovation, and a commitment to sustainability, we can pave the way for a future where agricultural pest control harmonises with nature and contributes to a healthier and more resilient food system.
Abbreviations
| Bt | Bacillus thuringiensis |
| Cat | Catechol |
| CO | Castor oil |
| Cry protein | Crystal protein |
| Cyt protein | Cytolytic protein |
| DDAB | Didecyldimethylammonium bromide |
| DDT | Dichloro-diphenyl-trichloroethane |
| DHBA | 2,3-Dihydroxybenzoic acid |
| dsRNA | Double-stranded RNA |
| EPA | U.S. Environmental Protection Agency |
| Glyphosate IPA | Glyphosate-isopropylammonium |
| GM | Genetically modified |
| HIGS | Host-induced gene silencing |
| HSCs | Hat-shaped carriers |
| ICM | Integrated crop management |
| IPM | Integrated pest management |
| LC | Lambda-cyhalothrin |
| MCC | Microvilli of the columnar cells |
| MOF | Metal–organic framework |
| mRNA | Messenger RNA |
| O-CMCS | O-Carboxymethyl chitosan |
| PEO | Polyethylene oxide |
| pGPMA | Poly-[N-(3-guanidinopropyl)methacrylamide] |
| PIPs | Plant incorporated protectants |
| PTFE | Polytetrafluoroethylene |
| PU | Polyurethane |
| RISC | RNA-induced silencing complex |
| RNAi | RNA interference |
| SEM | Scanning electron microscopy |
| SIGS | Spray-induced gene silencing |
| VIGS | Virus-induced gene silencing |
Author contributions
Abinaya Arunachalam: conceptualisation, visualisation, writing – original draft, writing – review & editing; Maria Perraki: writing – original draft; Bram Knegt: writing – review & editing; Mirka Macel: writing – review & editing; Dagmar Voigt: writing – original draft, writing – review & editing; Marleen Kamperman: conceptualisation, visualisation, writing – review & editing, supervision, funding acquisition. All authors have given approval to the final version of the manuscript.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review. Any cited data can be found in the relevant citations.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the Dutch Research Council (NWA.1160.18.071) for their financial support. Dagmar Voigt's contribution was funded by the German BMEL project LEADER (FKZ 28A8706B19). The authors also thank Katarzyna Heilman for immense support in creating the graphical abstract and Fig. 1, 7 and 12. Special thanks to Diederik Rep for his editorial support.Notes and references
- C. Rachel, Silent spring, Penguin Books, London, 1962 Search PubMed.
- M. L. Flint and R. van den Bosch, Introduction to Integrated Pest Management, Springer US, Boston, MA, 1981, pp. 51–81 Search PubMed.
- M. Tudi, H. D. Ruan, L. Wang, J. Lyu, R. Sadler, D. Connell, C. Chu and D. T. Phung, Int. J. Environ. Res. Public Health, 2021, 18, 1–24 CrossRef PubMed.
- J. Popp, K. Pető and J. Nagy, Agron. Sustainable Dev., 2013, 33, 243–255 Search PubMed.
- R. W. Bretveld, C. M. G. Thomas, P. T. J. Scheepers, G. A. Zielhuis and N. Roeleveld, Reprod. Biol. Endocrinol., 2006, 4, 30 CrossRef PubMed.
- P. Nicolopoulou-Stamati, S. Maipas, C. Kotampasi, P. Stamatis and L. Hens, Front. Public Health, 2016, 4, 148 CrossRef PubMed.
- P. Salameh, M. Waked, I. Baldi, P. Brochard and B. A. Saleh, J. Epidemiol. Community Health, 2006, 60, 256–261 CrossRef PubMed.
- S. M. Bradberry, A. T. Proudfoot and J. A. Vale, Toxicol. Rev., 2004, 23, 159–167 CrossRef CAS PubMed.
- N. Roeleveld and R. Bretveld, Curr. Opin. Obstet. Gynecol., 2008, 20, 229–233 CrossRef PubMed.
- J. A. Hoppin, D. M. Umbach, S. J. London, P. K. Henneberger, G. J. Kullman, M. C. R. Alavanja and D. P. Sandler, Am. J. Respir. Crit. Care Med., 2008, 177, 11–18 Search PubMed.
- K. L. Bassil, C. Vakil, M. Sanborn, D. C. Cole, J. S. Kaur and K. J. Kerr, Cancer health effects of pesticides: Systematic review, 2007, vol. 53 Search PubMed.
- T. T. Iyaniwura, Rev. Environ. Health, 1991, 9, 161–176 CAS.
- G. Wang, J. Li, N. Xue, A. Abdulkreem AL-Huqail, H. S. Majdi, E. Darvishmoghaddam, H. Assilzadeh, M. A. Khadimallah and H. E. Ali, Chemosphere, 2022, 307, 135632 CrossRef CAS PubMed.
- M. Syafrudin, R. A. Kristanti, A. Yuniarto, T. Hadibarata, J. Rhee, W. A. Al-Onazi, T. S. Algarni, A. H. Almarri and A. M. Al-Mohaimeed, Int. J. Environ. Res. Public Health, 2021, 18, 1–15 Search PubMed.
- N. J. Hawkins, C. Bass, A. Dixon and P. Neve, Biol. Rev., 2019, 94, 135–155 Search PubMed.
- L. Gandara, R. Jacoby, F. Laurent, M. Spatuzzi, N. Vlachopoulos, N. O. Borst, G. Ekmen, C. M. Potel, M. Garrido-Rodriguez, A. L. Böhmert, N. Misunou, B. J. Bartmanski, X. C. Li, D. Kutra, J.-K. Hériché, C. Tischer, M. Zimmermann-Kogadeeva, V. A. Ingham, M. M. Savitski, J.-B. Masson, M. Zimmermann and J. Crocker, Science, 2024, 386, 446–453 CrossRef CAS PubMed.
- The Guardian, EPA considers approving fruit pesticide despite risks to children, records show, https://www.theguardian.com/environment/2023/nov/21/epa-fruit-pesticide-risk-children-aldicarb, (accessed 14 February 2024).
- M. Cone, Toxic Pesticide Banned after Decades of Use, https://www.scientificamerican.com/article/toxic-pesticide-banned-after-decades-of-use/, (accessed 19 October 2022) Search PubMed.
- FDACS, FDACS Issues Denial of Aldicarb Pesticide Usage Application in Florida, https://www.fdacs.gov/News-Events/Press-Releases/2021-Press-Releases/FDACS-Issues-Denial-of-Aldicarb-Pesticide-Usage-Application-in-Florida#, (accessed 19 October 2022).
- K. Koch, B. Bhushan and W. Barthlott, Prog. Mater. Sci., 2009, 54, 137–178 Search PubMed.
- European Commission, Integrated Pest Management (IPM), https://food.ec.europa.eu/plants/pesticides/sustainable-use-pesticides/integrated-pest-management-ipm_en, (accessed 5 August 2024).
- M. Jeger, R. Beresford, C. Bock, N. Brown, A. Fox, A. Newton, A. Vicent, X. Xu and J. Yuen, CABI Agric. Biosci., 2021, 2, 1–18 CrossRef.
- M. Nuruzzaman, M. M. Rahman, Y. Liu and R. Naidu, J. Agric. Food Chem., 2016, 64, 1447–1483 CrossRef CAS PubMed.
- I. F. Mustafa and M. Z. Hussein, Nanomaterials, 2020, 10, 1–26 CrossRef PubMed.
- X. Jiang, F. Yang, W. Jia, Y. Jiang, X. Wu, S. Song, H. Shen and J. Shen, Langmuir, 2024, 40, 18806–18820 Search PubMed.
- H. Wang, M. Jafir, M. Irfan, T. Ahmad, M. Zia-ur-Rehman, M. Usman, M. Rizwan, Y. A. Hamoud and H. Shaghaleh, J. Environ. Manage., 2024, 360, 121178 Search PubMed.
- A. Singh, N. Dhiman, A. K. Kar, D. Singh, M. P. Purohit, D. Ghosh and S. Patnaik, J. Hazard. Mater., 2020, 385, 121525 CrossRef CAS PubMed.
- A. Roy, S. K. Singh, J. Bajpai and A. K. Bajpai, Cent. Eur. J. Chem., 2014, 12, 453–469 CAS.
- N. Li, C. Sun, J. Jiang, A. Wang, C. Wang, Y. Shen, B. Huang, C. An, B. Cui, X. Zhao, C. Wang, F. Gao, S. Zhan, L. Guo, Z. Zeng, L. Zhang, H. Cui and Y. Wang, J. Agric. Food Chem., 2021, 69, 12579–12597 CrossRef CAS PubMed.
- S. K. Dara, J. Integr. Pest Manage., 2019, 10, 12 Search PubMed.
- S. Mouden, K. F. Sarmiento, P. G. Klinkhamer and K. A. Leiss, Pest Manage. Sci., 2017, 73, 813–822 CrossRef CAS PubMed.
- C. Darwin, The various contrivances by which orchids are fertilised by insects, John Murray, 1877 Search PubMed.
- J. L. Bronstein, R. Alarcón and M. Geber, New Phytol., 2006, 172, 412–428 CrossRef PubMed.
- C. V. Tillberg, Oecologia, 2004, 140, 506–515 CrossRef PubMed.
- A. G. Stephenson, Ecology, 1982, 63, 663–669 CrossRef.
- P. R. Marting, N. M. Kallman, W. T. Wcislo and S. C. Pratt, Sci. Rep., 2018, 8, 1–15 Search PubMed.
- F. R. Rickson, Science, 1971, 173, 344–347 CrossRef CAS PubMed.
- A. Dejean, C. Leroy, B. Corbara, O. Roux, R. Céréghino, J. Orivel and R. Boulay, PLoS One, 2010, 5, e11331 Search PubMed.
- D. H. Janzen, Ecology, 1969, 50, 147–153 CrossRef.
- K. N. Oliveira, P. D. Coley, T. A. Kursar, L. A. Kaminski, M. Z. Moreira and R. I. Campos, PLoS One, 2015, 10, 1–13 Search PubMed.
- D. H. Janzen, Biotropica, 1973, 5, 15–28 CrossRef.
- C. L. Sagers, S. M. Ginger and R. D. Evans, Oecologia, 2000, 123, 582–586 Search PubMed.
- M. Nepel, J. Pfeifer, F. B. Oberhauser, A. Richter, D. Woebken and V. E. Mayer, BMC Biol., 2022, 20, 135 CrossRef CAS PubMed.
- A. Mithöfer, J. Plant Interact., 2022, 17, 333–343 CrossRef.
- Y. Heslop-Harrison, Sci. Am., 1978, 238, 104–116 CrossRef.
- A. Slack, Carnivorous plants, MIT Press, 1979 Search PubMed.
- S. Poppinga, T. Masselter and T. Speck, BioEssays, 2013, 35, 649–657 CrossRef PubMed.
- R. Hedrich and E. Neher, Trends Plant Sci., 2018, 23, 220–234 CrossRef CAS PubMed.
- U. Bauer, M. Paulin, D. Robert and G. P. Sutton, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 13384–13389 CrossRef CAS PubMed.
- L. Gaume, S. Gorb and N. Rowe, New Phytol., 2002, 156, 479–489 CrossRef PubMed.
- E. Gorb, K. Haas, A. Henrich, S. Enders, N. Barbakadze and S. Gorb, J. Exp. Biol., 2005, 208, 4651–4662 CrossRef CAS PubMed.
- D. Voigt, E. Gorb and S. Gorb, J. Exp. Biol., 2009, 212, 3184–3191 CrossRef PubMed.
- E. V. Gorb and S. N. Gorb, Beilstein J. Nanotechnol., 2011, 2, 302–310 Search PubMed.
- E. V. Gorb and S. N. Gorb, Functional Surfaces in Biology: Adhesion Related Phenomena Volume 2, Springer, 2009, pp. 205–238 Search PubMed.
- U. Bauer and W. Federle, Plant Signaling Behav., 2009, 4, 1019–1023 CrossRef PubMed.
- I. Scholz, M. Bückins, L. Dolge, T. Erlinghagen, A. Weth, F. Hischen, J. Mayer, S. Hoffmann, M. Riederer, M. Riedel and W. Baumgartner, J. Exp. Biol., 2010, 213, 1115–1125 CrossRef CAS PubMed.
- L. Gaume and Y. Forterre, PLoS One, 2007, 2, e1185 CrossRef PubMed.
- National Institute for Basic Biology, Memory of the Venus flytrap, https://www.nibb.ac.jp/en/pressroom/news/2020/10/06.html, 2020.
- R. Schwallier, V. van Wely, M. Baak, R. Vos, B. J. van Heuven, E. Smets, R. R. van Vugt and B. Gravendeel, Plants, 2020, 9, 1603 CrossRef CAS PubMed.
- L. S. Jankiewicz, M. Guzicka and A. Marasek-Ciolakowska, Insects, 2021, 12, 850 CrossRef PubMed.
- G. Lohaus and M. Schwerdtfeger, PLoS One, 2014, 9, e87689 Search PubMed.
- J. T. Lill, R. J. Marquis and R. E. Ricklefs, Nature, 2002, 417, 170–173 CrossRef CAS PubMed.
- P. N. Windle and E. H. Franz, Ecology, 1979, 60, 521–529 CrossRef.
- S. P. Egan, G. R. Hood, E. O. Martinson and J. R. Ott, Curr. Biol., 2018, 28, R1370–R1374 Search PubMed.
- The Connecticut Agricultural Experiment Station, Insects and their Injury to Plants, https://portal.ct.gov/CAES/Plant-Pest-Handbook/pph-Introductory/Insects-and-their-Injury-to-Plants, (accessed 14 February 2024).
- E. Guerrieri and M. C. Digilio, J. Plant Interact., 2008, 3, 223–232 Search PubMed.
- L. S. Thomashow, Curr. Opin. Biotechnol, 1996, 7, 343–347 CrossRef CAS PubMed.
- D. Palmieri, G. Ianiri, C. Del Grosso, G. Barone, F. De Curtis, R. Castoria and G. Lima, Horticulturae, 2022, 8, 577 CrossRef.
- M. A. Jervis, Insect natural enemies: practical approaches to their study and evaluation, Springer Science & Business Media, 2012 Search PubMed.
- D. V. Savatin, G. Gramegna, V. Modesti and F. Cervone, Front. Plant Sci., 2014, 5, 470 Search PubMed.
- A. S. Parmagnani and M. E. Maffei, Plants, 2022, 11, 2689 CrossRef CAS PubMed.
- P. A. Divekar, S. Narayana, B. A. Divekar, R. Kumar, B. G. Gadratagi, A. Ray, A. K. Singh, V. Rani, V. Singh and A. K. Singh, Int. J. Mol. Sci., 2022, 23, 2690 CrossRef CAS PubMed.
- J. Koricheva, H. Nykänen and E. Gianoli, Am. Nat., 2004, 163, E64–E75 CrossRef PubMed.
- R. Gols, Plant, Cell Environ., 2014, 37, 1741–1752 CrossRef PubMed.
- W. Abebe, Review on Plant Defense Mechanisms Against Insect Pests, 2021, vol. 8 Search PubMed.
- G. A. Howe and G. Jander, Annu. Rev. Plant Biol., 2008, 59, 41–66 CrossRef CAS PubMed.
- A. Mithöfer and W. Boland, Annu. Rev. Plant Biol., 2012, 63, 431–450 CrossRef PubMed.
- R. N. Bennett and R. M. Wallsgrove, New Phytol., 1994, 127, 617–633 CrossRef CAS PubMed.
- J. B. Harborne, Ciba Found. Symp., 1990, 154, 126–129 CAS.
- J. Wan, M. He, Q. Hou, L. Zou, Y. Yang, Y. Wei and X. Chen, Stress Biol., 2021, 1, 3 CrossRef CAS PubMed.
- F. G. Malinovsky, J. U. Fangel and W. G. T. Willats, Front. Plant Sci., 2014, 5, 178 Search PubMed.
- P. J. White and M. R. Broadley, Ann. Bot., 2003, 92, 487–511 CrossRef CAS PubMed.
- P. Bauer, R. Elbaum and I. M. Weiss, Plant Sci., 2011, 180, 746–756 CrossRef CAS PubMed.
- S. Kumar, M. Soukup and R. Elbaum, Front. Plant Sci., 2017, 8, 438 Search PubMed.
- N. Zexer, S. Kumar and R. Elbaum, Ann. Bot., 2023, 131, 897–908 CrossRef CAS PubMed.
- H. A. Currie and C. C. Perry, Ann. Bot., 2007, 100, 1383–1389 CrossRef CAS PubMed.
- G. Karabourniotis, H. T. Horner, P. Bresta, D. Nikolopoulos and G. Liakopoulos, New Phytol., 2020, 228, 845–854 CrossRef CAS PubMed.
- G. G. Coté, Am. J. Bot., 2009, 96, 1245–1254 CrossRef PubMed.
- K. L. Korth, S. J. Doege, S.-H. Park, F. L. Goggin, Q. Wang, S. K. Gomez, G. Liu, L. Jia and P. A. Nakata, Plant Physiol., 2006, 141, 188–195 CrossRef CAS PubMed.
- V. Franceschi, Trends Plant Sci., 2001, 6, 331 CrossRef CAS PubMed.
- B. D. Keppler, J. Song, J. Nyman, C. A. Voigt and A. F. Bent, Front. Plant Sci., 2018, 9, 1907 CrossRef PubMed.
- A. A. Agrawal and A. P. Hastings, J. Chem. Ecol., 2019, 45, 1004–1018 CrossRef CAS PubMed.
- S. G. Kim, K. W. Kim, E. W. Park and D. Choi, Phytopathology, 2002, 92, 1095–1103 CrossRef PubMed.
- K. Cai, D. Gao, S. Luo, R. Zeng, J. Yang and X. Zhu, Physiol. Plant., 2008, 134, 324–333 CrossRef CAS PubMed.
- C. A. E. Strömberg, V. S. Di Stilio and Z. Song, Funct. Ecol., 2016, 30, 1286–1297 CrossRef.
- F. P. Massey and S. E. Hartley, J. Anim. Ecol., 2009, 78, 281–291 CrossRef PubMed.
- A. Rahman, C. M. Wallis and W. Uddin, Phytopathology®, 2015, 105, 748–757 CrossRef CAS PubMed.
- R. Karthik, M. K. Deka, S. Ajith, S. Kalita and N. B. Prakash, Int. J. Trop. Insect Sci., 2024, 44, 2685–2694 CrossRef.
- K. M. Hassan, R. Ajaj, A. N. Abdelhamid, M. Ebrahim, I. F. Hassan, F. A. S. Hassan, S. M. Alam-Eldein and M. A. A. Ali, Horticulturae, 2024, 10, 806 CrossRef.
- D. Ellinger and C. A. Voigt, Ann. Bot., 2014, 114, 1349–1358 CrossRef CAS PubMed.
- X.-Y. Chen and J.-Y. Kim, Plant Signaling Behav., 2009, 4, 489–492 CrossRef CAS PubMed.
- I. Vega-Muñoz, D. Duran-Flores, Á. D. Fernández-Fernández, J. Heyman, A. Ritter and S. Stael, Front. Plant Sci., 2020, 11, 610445 CrossRef PubMed.
- D. E. Dussourd, Ann. Entomol. Soc. Am., 1995, 88, 163–172 CrossRef.
- M. V. Ramos, D. Demarco, I. C. da Costa Souza and C. D. T. de Freitas, Trends Plant Sci., 2019, 24, 553–567 CrossRef CAS PubMed.
- J. Gracz-Bernaciak, O. Mazur and R. Nawrot, Int. J. Mol. Sci., 2021, 22, 12427 CrossRef CAS PubMed.
- A. Schaller, Induced Plant Resistance To Herbivory, Springer, 2008 Search PubMed.
- G. Kerstiens, J. Exp. Bot., 1996, 47, 50–60 CrossRef.
- H. Bargel, K. Koch, Z. Cerman and C. Neinhuis, Funct. Plant Biol., 2006, 33, 893–910 CrossRef CAS PubMed.
- M. Riederer and C. Muller, Annual plant reviews, biology of the plant cuticle, John Wiley & Sons, 2008 Search PubMed.
- W. Barthlott, M. Mail, B. Bhushan and K. Koch, Nano-Micro Lett., 2017, 9, 1–40 CrossRef PubMed.
- J. Skrzydeł, D. Borowska-Wykręt and D. Kwiatkowska, Int. J. Mol. Sci., 2021, 22, 4160 CrossRef PubMed.
- D. Voigt, A. Schweikart, A. Fery and S. Gorb, J. Exp. Biol., 2012, 215, 1975–1982 CrossRef PubMed.
- V. Bhanot, S. V. Fadanavis and J. Panwar, Environ. Exp. Bot., 2021, 183, 104364 CrossRef CAS.
- C. Neinhuis and W. Barthlott, Ann. Bot., 1997, 79, 667–677 CrossRef.
- I. Kaur, S. Watts, C. Raya, J. Raya and R. Kariyat, Caterpillars in the middle: tritrophic interactions in a changing world, Springer International Publishing, 2022, pp. 65–92 Search PubMed.
- M. A. Jenks, E. N. Ashworth and J. Janick, Hortic. Rev., 1999, 23, 1–54 CAS.
- E. V. Gorb and S. N. Gorb, J. Exp. Bot., 2017, 68, 5323–5337 CrossRef CAS PubMed.
- E. V. Gorb and S. N. Gorb, Entomol. Exp. Appl., 2002, 105, 13–28 CrossRef.
- D. A. Levin, Q. Rev. Biol., 1973, 48, 3–15 CrossRef.
- M. Chen, J. Wu and G. Zhang, Recent Advances in Entomological Research: From Molecular Biology to Pest Management, Springer Berlin Heidelberg, 2011, pp. 49–72 Search PubMed.
- M. B. Traw and J. Bergelson, Plant Physiol., 2003, 133, 1367–1375 CrossRef CAS PubMed.
- M. B. Traw and T. E. Dawson, Oecologia, 2002, 131, 526–532 CrossRef PubMed.
- J. J. Glas, B. C. J. Schimmel, J. M. Alba, R. Escobar-Bravo, R. C. Schuurink and M. R. Kant, Int. J. Mol. Sci., 2012, 13, 17077–17103 CrossRef CAS PubMed.
- R. L. Vallejo, W. W. Collins and R. H. Moll, J. Am. Soc. Hortic. Sci., 1994, 119, 829–832 CAS.
- P. Gregory, W. M. Tingey, D. A. Ave and P. Y. Bouthyette, Natural Resistance of Plants to Pests, 1986, 160–167 CAS.
- M. Krings, D. W. Kellogg, H. Kerp and T. N. Taylor, Bot. J. Linn. Soc., 2003, 141, 133–149 CrossRef.
- M. Lucy Kananu, H. Kirwa, D. Salifu and B. Torto, PLoS One, 2016, 11, 1–20 Search PubMed.
- R. Malakar and W. M. Tingey, Entomol. Exp. Appl., 2000, 94, 249–257 CrossRef.
- H. Liu, S. Liu, J. Jiao, T. J. Lu and F. Xu, Soft Matter, 2017, 13, 5096–5106 RSC.
- Z. Xing, Y. Liu, W. Cai, X. Huang, S. Wu and Z. Lei, Front. Plant Sci., 2017, 8, 2006 CrossRef PubMed.
- E. A. Pillemer and W. M. Tingey, Science, 1976, 193, 482–484 CrossRef CAS PubMed.
- H. Ensikat, A. Mustafa and M. Weigend, Am. J. Bot., 2017, 104, 195–206 CrossRef CAS PubMed.
- T. Eisner, M. Eisner and E. R. Hoebeke, When defense backfires: Detrimental effect of a plant's protective trichomes on an insect beneficial to the plant, 1998, vol. 95 Search PubMed.
- D. Voigt and S. Gorb, J. Exp. Biol., 2008, 211, 2647–2657 CrossRef PubMed.
- E. W. Riddick and A. M. Simmons, Pest Manage. Sci., 2014, 70, 1655–1665 CrossRef CAS PubMed.
- E. W. Riddick and Z. Wu, BioControl, 2011, 56, 55–63 CrossRef.
- M. A. Shah, Entomol. Exp. Appl., 1982, 31, 377–380 CrossRef.
- L. P. Economou, D. P. Lykouressis and A. E. Barbetaki, Environ. Entomol., 2006, 35, 387–393 CrossRef.
- B. A. Krimmel, Pest Manage. Sci., 2014, 70, 1666–1667 CrossRef CAS PubMed.
- D. Voigt and S. Gorb, Arthropod-Plant Interact., 2010, 4, 69–79 CrossRef.
- B. A. Krimmel and I. S. Pearse, Ecol. Lett., 2013, 16, 219–224 CrossRef CAS PubMed.
- M. E. Hanley, B. B. Lamont, M. M. Fairbanks and C. M. Rafferty, Perspect. Plant Ecol. Evol. Syst., 2007, 8, 157–178 CrossRef.
- G. E. Belovsky, O. J. Schmitz, J. B. Slade and T. J. Dawson, Oecologia, 1991, 88, 521–528 CrossRef PubMed.
- F. T. Malik, R. M. Clement, D. T. Gethin, M. Kiernan, T. Goral, P. Griffiths, D. Beynon and A. R. Parker, Philos. Trans. R. Soc., A, 2016, 374, 20160110 CrossRef PubMed.
- Z. Ningning, S. Fabienne, T. Tatiana, O. L. Hibrand-Saint and F. Fabrice, Hortic. Res., 2021, 8, 221 CrossRef PubMed.
- B. Mortensen, Nat. Educ. Knowl., 2013, 4, 5 Search PubMed.
- T. Hagihara and M. Toyota, Plants, 2020, 9, 587 CrossRef CAS PubMed.
- É. C. P. de Castro, M. Zagrobelny, M. Z. Cardoso and S. Bak, Biol. Rev., 2018, 93, 555–573 CrossRef PubMed.
- J. Braam, New Phytol., 2005, 165, 373–389 CrossRef PubMed.
- A. G. Volkov, J. C. Foster, T. A. Ashby, R. K. Walker, J. A. Johnson and V. S. Markin, Plant, Cell Environ., 2010, 33, 163–173 CrossRef PubMed.
- D. Tran, H. Petitjean, Y. Chebli, A. Geitmann and R. Sharif-Naeini, Plant Physiol., 2021, 187, 1704–1712 CrossRef CAS PubMed.
- D. A. Sleboda, Integr. Comp. Biol., 2023, 63, 1–9 Search PubMed.
- S. K. Sopory, Sensory Biology of Plants, Springer, 2019 Search PubMed.
- M. D. Rausher, Anim. Behav., 1979, 27, 1034–1040 CrossRef.
- K. S. Williams and L. E. Gilbert, Science, 1981, 212, 467–469 CrossRef CAS PubMed.
- D. E. Walter, Annu. Rev. Entomol., 1996, 41, 101–114 CrossRef CAS PubMed.
- M. Biddick, Biol. Lett., 2023, 19, 4 Search PubMed.
- R. W. Pemberton and J. Lee, Am. J. Bot., 1996, 83, 1187–1194 CrossRef.
- B. Marazzi, J. L. Bronstein and S. Koptur, Ann. Bot., 2013, 111, 1243–1250 CrossRef PubMed.
- M. Laura, E. Snchez-Salinas, E. Dantn Gonzlez and M. Luisa, in Biodegradation – Life of Science, ed. R. Chamy and F. Rosenkranz, IntechOpen, Rijeka, 2013 Search PubMed.
- J. Silberman and A. Taylor, Carbamate Toxicity, https://www.ncbi.nlm.nih.gov/books/NBK482183/, (accessed 27 September 2022) Search PubMed.
- C. Costas-Ferreira and L. R. F. Faro, Int. J. Mol. Sci., 2021, 22, 8413 Search PubMed.
- V. Verebová and J. Staničová, Insecticides; impact and benefits of its use for humanity, IntechOpen, Rijeka, 2021 Search PubMed.
- European Commission, Active substances, safeners and synergists, https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/active-substances, (accessed 1 May 2024).
- H. Qin, H. Zhang, L. Li, X. Zhou, J. Li and C. Kan, RSC Adv., 2017, 7, 52684–52693 RSC.
- B. Liu, Y. Fan, H. Li, W. Zhao, S. Luo, H. Wang, B. Guan, Q. Li, J. Yue, Z. Dong, Y. Wang and L. Jiang, Adv. Funct. Mater., 2021, 31, 1–11 Search PubMed.
- M. Vickneswaran, J. C. Carolan, M. Saunders and B. White, Heliyon, 2023, 9, e19416 Search PubMed.
- G. M. Hoffmann, F. Nienhaus, F. Schönbeck, H. C. Weltzien and H. Wilbert, Textbook of phytomedicine, 1985 Search PubMed.
- V. Stejskal, T. Vendl, R. Aulicky and C. Athanassiou, Insects, 2021, 12, 590 Search PubMed.
- T. H. Yeats and J. K. C. Rose, Plant Physiol., 2013, 163, 5–20 Search PubMed.
- S. S. Latthe, C. Terashima, K. Nakata and A. Fujishima, Molecules, 2014, 19, 4256–4283 CrossRef PubMed.
- U. Mock, T. Michel, C. Tropea, I. Roisman and J. Rühe, J. Phys.: Condens. Matter, 2005, 17, S595 CrossRef CAS.
- J. C. Bird, R. Dhiman, H.-M. Kwon and K. K. Varanasi, Nature, 2013, 503, 385–388 CrossRef CAS PubMed.
- M. A. Quetzeri-Santiago, A. A. Castrejón-Pita and J. R. Castrejón-Pita, Sci. Rep., 2019, 9, 15030 CrossRef PubMed.
- Z. Li, Y. Ma, K. Zhao, C. Zhang, Y. Gao and F. Du, ACS Sustainable Chem. Eng., 2021, 9, 2891–2901 CrossRef CAS.
- R. Zhao, M. Yu, Z. Sun, L. Jie Li, X. Yu Guo, Y. Xu and X. Min Wu, J. Colloid Interface Sci., 2023, 629, 926–937 CrossRef CAS PubMed.
- Y. Ma, Y. Gao, K. Zhao, H. Zhang, Z. Li, F. Du and J. Hu, ACS Appl. Mater. Interfaces, 2020, 12, 50126–50134 CrossRef CAS PubMed.
- M. Damak, M. N. Hyder and K. K. Varanasi, Nat. Commun., 2016, 7, 1–9 Search PubMed.
- P. Schwinges, S. Pariyar, F. Jakob, M. Rahimi, L. Apitius, M. Hunsche, L. Schmitt, G. Noga, C. Langenbach, U. Schwaneberg and U. Conrath, Green Chem., 2019, 21, 2316–2325 RSC.
- T. Thieme, D. Schenke, D. Voigt, E. Götte, P. Detzel, G. Köhler, R. Schmidt and S. Lorenz, J. Kult., 2024, 76, 85–97 Search PubMed.
- I. Cantat, S. Cohen-Addad, F. Elias, F. Graner, R. Höhler, O. Pitois, F. Rouyer and A. Saint-Jalmes, Foams: structure and dynamics, OUP, Oxford, 2013 Search PubMed.
- C. Hill and J. Eastoe, Adv. Colloid Interface Sci., 2017, 247, 496–513 CrossRef CAS PubMed.
- K. Wei, Z. Li, Z. Zheng, Y. Gao, Q. Huang, M. Li and J. Hu, Adv. Funct. Mater., 2024, 34, 2315493 CrossRef CAS.
- M. C. Butler Ellis, C. R. Tuck and P. C. H. Miller, Colloids Surf., A, 2001, 180, 267–276 CrossRef CAS.
- Y. Song, C. Cao, K. Liu, J. Huang, L. Zheng, L. Cao, F. Li, P. Zhao and Q. Huang, ACS Sustainable Chem. Eng., 2020, 8, 12911–12919 Search PubMed.
- G. J. Noga, M. Knoche, M. Wolter and W. Barthlott, Angew. Bot., 1988, 61, 521–528 Search PubMed.
- V. Bergeron, D. Bonn, J. Y. Martin and L. Vovelle, Nature, 2000, 405, 772–775 CrossRef CAS PubMed.
- K. Zhao, J. Hu, Y. Ma, T. Wu, Y. Gao and F. Du, ACS Sustainable Chem. Eng., 2019, 7, 13148–13156 CrossRef CAS.
- L. Chen, Y. Wang, X. Peng, Q. Zhu and K. Zhang, Macromolecules, 2018, 51, 7817–7827 CrossRef CAS.
- M. Y. Pack, A. Yang, A. Perazzo, B. Qin and H. A. Stone, Phys. Rev. Fluids, 2019, 4, 123603 CrossRef.
- M. Smith and V. Bertola, in Proceedings of 23rd annual conference liquid atomization spray systems Europe (ILASS-Europe), 2010, vol. 124.
- V. Teixeira, M. J. Feio and M. Bastos, Prog. Lipid Res., 2012, 51, 149–177 CrossRef CAS PubMed.
- H. Leontiadou, A. E. Mark and S. J. Marrink, J. Am. Chem. Soc., 2006, 128, 12156–12161 CrossRef CAS PubMed.
- C. T. Huynh and D. S. Lee, in Encyclopedia of Polymeric Nanomaterials, ed. S. Kobayashi and K. Müllen, Springer Berlin Heidelberg, 2015, pp. 439–449 Search PubMed.
- D. Xiao, H. Wu, Y. Zhang, J. Kang, A. Dong and W. Liang, J. Controlled Release, 2022, 352, 288–312 CrossRef CAS PubMed.
- J. Yang, C. A. Trickett, S. B. Alahmadi, A. S. Alshammari and O. M. Yaghi, J. Am. Chem. Soc., 2017, 139, 8118–8121 CrossRef CAS PubMed.
- G. Huang, Y. Deng, Y. Zhang, P. Feng, C. Xu, L. Fu and B. Lin, Chem. Eng. J., 2021, 403, 126342 CrossRef CAS.
- X. Deng, P. Zhao, X. Zhou and L. Bai, Chem. Eng. J., 2021, 405, 126979 CrossRef CAS.
- Y. Shan, Y. Ma, C. Wu, X. Ren, X. Song, D. Wang, H. Hu, X. Ma and Y. Ma, J. Environ. Chem. Eng., 2023, 11, 109967 CrossRef CAS.
- S. Chand Mali, S. Raj and R. Trivedi, Biochem. Biophys. Rep., 2020, 24, 100821 Search PubMed.
- X. Zhao, H. Cui, Y. Wang, C. Sun, B. Cui and Z. Zeng, J. Agric. Food Chem., 2017, 66, 6504–6512 CrossRef PubMed.
- M. C. Camara, E. V. R. Campos, R. A. Monteiro, A. do Espirito Santo Pereira, P. L. de Freitas Proença and L. F. Fraceto, J. Nanobiotechnol., 2019, 17, 100 CrossRef PubMed.
- B. Huang, F. Chen, Y. Shen, K. Qian, Y. Wang, C. Sun, X. Zhao, B. Cui, F. Gao and Z. Zeng, Nanomaterials, 2018, 8, 102 CrossRef PubMed.
- H. Chen, H. Zhi, J. Liang, M. Yu, B. Cui, X. Zhao, C. Sun, Y. Wang, H. Cui and Z. Zeng, J. Mater. Chem. B, 2021, 9, 783–792 RSC.
- K. Zhao, B. Wang, C. Zhang, Y. Guo, Y. Ma, Z. Li, T. Wu, Z. Bao, Y. Gao and F. Du, Chem. Eng. J., 2021, 420, 127689 CrossRef CAS.
- R. A. Meurer, S. Kemper, S. Knopp, T. Eichert, F. Jakob, H. E. Goldbach, U. Schwaneberg and A. Pich, Angew. Chem., Int. Ed., 2017, 56, 7380–7386 CrossRef CAS PubMed.
- Y. Zhu, X. An, S. Li and S. Yu, J. Surfactants Deterg., 2009, 12, 305–311 CrossRef CAS.
- Y. Zhang, B. Liu, K. Huang, S. Wang, R. L. Quirino, Z. X. Zhang and C. Zhang, ACS Appl. Mater. Interfaces, 2020, 12, 37607–37618 CrossRef CAS PubMed.
- J. Yang, M. A. Cohen Stuart and M. Kamperman, Chem. Soc. Rev., 2014, 43, 8271–8298 RSC.
- R. Hou, J. Zhou, Z. Song, N. Zhang, S. Huang, A. E. Kaziem, C. Zhao and Z. Zhang, Carbohydr. Polym., 2023, 302, 120373 CrossRef CAS PubMed.
- S. Johann, F. G. Weichert, L. Schröer, L. Stratemann, C. Kämpfer, T.-B. Seiler, S. Heger, T. Töpel Alexanderand Sassmann, A. Pich, F. Jakob, U. Schwaneberg, P. Stoffels, M. Philipp, M. Terfrüchte, A. Loeschcke, K. Schipper, M. Feldbrügge, N. Ihling, J. Büchs, I. Bator, T. Tiso, L. M. Blank, M. Roß-Nickoll and H. Hollert, J. Hazard. Mater., 2022, 426, 127800 CrossRef CAS PubMed.
- K. Mai, S. Yang, X. Zhao, R. Huang, S. Huang, C. Xu, G. Yu, Y. Feng and J. Li, Chem. Eng. J., 2024, 479, 147357 CrossRef CAS.
- P. Alexander, J. A. Kitchener and H. V. A. Briscoe, Ann. Appl. Biol., 1944, 31, 143–149 CrossRef CAS.
- V. B. Wigglesworth, Nature, 1944, 153, 493–494 CrossRef.
- J. S. Kennedy, C. O. Booth and W. J. S. Kershaw, Ann. Appl. Biol., 1961, 49, 1–21 CrossRef.
- M. Bar-Joseph and H. Frenkel, Crop Prot., 1983, 2, 371–374 CrossRef CAS.
- D. M. Glenn, G. J. Puterka, T. Vanderzwet, R. E. Byers and C. Feldhake, J. Econ. Entomol., 1999, 92, 759–771 CrossRef.
- R. R. Sharma, S. Vijay Rakesh Reddy and S. C. Datta, Appl. Clay Sci., 2015, 116–117, 54–68 CrossRef CAS.
- G. J. Puterka, D. M. Glenn, D. G. Sekutowski, T. R. Unruh and S. K. Jones, Environ. Entomol., 2000, 29, 329–339 CrossRef.
- S. O. Duke and S. B. Powles, Pest Manage. Sci., 2008, 63, 1100–1106 Search PubMed.
- L. Peng, J. T. Trumble, J. E. Munyaneza and T. X. Liu, Pest Manage. Sci., 2011, 67, 815–824 CrossRef CAS PubMed.
- E. Larentzaki, A. M. Shelton and J. Plate, Crop Prot., 2008, 27, 727–734 CrossRef CAS.
- S. Faliagka, P. Agrafioti, E. Lampiri, N. Katsoulas and C. G. Athanassiou, Crop Prot., 2020, 137, 105312 CrossRef CAS.
- M. W. Szyndler, K. F. Haynes, M. F. Potter, R. M. Corn and C. Loudon, J. R. Soc., Interface, 2013, 10, 1–9 CrossRef PubMed.
- R. van Zwieten, T. V. Bierman, P. G. L. Klinkhamer, T. M. Bezemer, K. Vrieling and T. E. Kodger, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2321565121 CrossRef CAS PubMed.
- K. A. G. Wyckhuys, X.-W. Wang and M. Elkahky, J. Biosci., 2024, 49, 1–11 Search PubMed.
- E. Schifani, C. Castracani, D. Giannetti, F. A. Spotti, R. Reggiani, S. Leonardi, A. Mori and D. A. Grasso, Insects, 2020, 11, 129 CrossRef PubMed.
- H. H. Richardson, J. Econ. Entomol., 1943, 36, 543–545 CrossRef.
- D. Voigt, S. Gorb and J.-L. Boevé, Zoology, 2011, 114, 265–271 CrossRef PubMed.
- K. Koch, A. J. Schulte, A. Fischer, S. N. Gorb and W. Barthlott, Bioinspiration Biomimetics, 2008, 3, 46002 Search PubMed.
- T. V. Bierman, K. Vrieling, R. van Zwieten, T. E. Kodger, M. Macel and T. M. Bezemer, J. Pest Sci., 2024, 97, 2175–2186 CrossRef CAS.
- T. V. Bierman, K. Vrieling, R. van Zwieten, T. E. Kodger, M. Macel and T. Bezemer, J. Pest Sci., 2024, 97, 2175–2186 CrossRef CAS.
- A. Arunachalam, T. Oosterhoff, I. Breet, P. Dijkstra, R. A. Mohamed Yunus, D. Parisi, B. Knegt, M. Macel and M. Kamperman, Commun. Mater., 2025, 6, 101 CrossRef PubMed.
- S. P. Foster and M. O. Harris, Annu. Rev. Entomol., 1997, 42, 123–146 CrossRef CAS PubMed.
- A. Hassanali, H. Herren, Z. R. Khan, J. A. Pickett and C. M. Woodcock, Philos. Trans. R. Soc., B, 2008, 363, 611–621 CrossRef PubMed.
- B. Pyke, M. Rice, B. Sabine and M. Zalucki, Aust. Cott. Grow., 1987, 9, 7–9 Search PubMed.
- S. M. Cook, Z. R. Khan and J. A. Pickett, Annu. Rev. Entomol., 2007, 52, 375–400 CrossRef CAS PubMed.
- W. Han, J. Wang, F. Zhang, S. Ji, Y. Zhong, Y. Liu, S. Liu and X. Wang, Plants, People, Planet, 2024, 6, 743–759 CrossRef.
- S. C. Sarkar, E. Wang, S. Wu and Z. Lei, Insects, 2018, 9, 128 CrossRef PubMed.
- J.-X. Wang, W.-H. Han, R. Xie, F.-B. Zhang, Z.-W. Ge, S.-X. Ji, S.-S. Liu and X.-W. Wang, Plant, Cell Environ., 2025, 48, 387–405 CrossRef CAS PubMed.
- J. Polajnar, A. Eriksson, M. Virant-Doberlet and V. Mazzoni, J. Pest Sci., 2016, 89, 909–921 CrossRef.
- A. Pekas, V. Mazzoni, H. Appel, R. Cocroft and M. Dicke, Trends Plant Sci., 2024, 32–39 CrossRef CAS PubMed.
- R. Yanagisawa, H. Tatsuta, T. Sekine, T. Oe, H. Mukai, N. Uechi, T. Koike, R. Onodera, R. Suwa and T. Takanashi, Entomol. Exp. Appl., 2024, 172, 1113–1216 CrossRef.
- J. K. Waage and D. J. Greathead, Philos. Trans. R. Soc., B, 1988, 318, 111–128 Search PubMed.
- P. A. O’Brien, Australas. Plant Pathol., 2017, 46, 293–304 CrossRef.
- A. Sharma, A. Shukla, K. Attri, M. Kumar, P. Kumar, A. Suttee, G. Singh, R. P. Barnwal and N. Singla, Ecotoxicol. Environ. Saf., 2020, 201, 110812 CrossRef CAS PubMed.
- D. Abdollahdokht, Y. Gao, S. Faramarz, A. Poustforoosh, M. Abbasi, G. Asadikaram and M. H. Nematollahi, Chem. Biol. Technol. Agric., 2022, 9, 13 CrossRef.
- P. G. Marrone, Pest Manage. Sci., 2024, 80, 81–86 CrossRef CAS PubMed.
- H. E. Hezakiel, M. Thampi, S. Rebello and J. M. Sheikhmoideen, Appl. Biochem. Biotechnol., 2024, 196, 5533–5562 CrossRef CAS PubMed.
- R. Marasco, E. Rolli, B. Ettoumi, G. Vigani, F. Mapelli, S. Borin, A. F. Abou-Hadid, U. A. El-Behairy, C. Sorlini, A. Cherif, G. Zocchi and D. Daffonchio, PLoS One, 2012, 7, e48479 CrossRef CAS PubMed.
- J. N. Seiber, J. Coats, S. O. Duke and A. D. Gross, J. Agric. Food Chem., 2014, 62, 11613–11619 CrossRef CAS PubMed.
- G. S. Jouzani, E. Valijanian and R. Sharafi, Appl. Microbiol. Biotechnol., 2017, 101, 2691–2711 CrossRef CAS PubMed.
- F. F. Umaru and K. Simarani, Insects, 2020, 11, 277 CrossRef PubMed.
- A. R. Agboola, C. O. Okonkwo, E. I. Agwupuye and G. Mbeh, AROC Agric., 2022, 1, 14–32 Search PubMed.
- Z.-Y. Peng, S.-T. Huang, J.-T. Chen, N. Li, Y. Wei, A. Nawaz and S.-Q. Deng, All Life, 2022, 15, 1141–1159 Search PubMed.
- W. Fang, Y. Pei and M. J. Bidochka, Microbiology, 2007, 153, 1017–1025 CrossRef CAS PubMed.
- J. Kumar, A. Ramlal, D. Mallick and V. Mishra, Plants, 2021, 10, 1185 CrossRef CAS PubMed.
- P. M. Ngegba, G. Cui, M. Z. Khalid and G. Zhong, Agriculture, 2022, 12, 600 CrossRef CAS.
- N. Takeda, A. Takata, Y. Arai, K. Sasaya, S. Noyama, S. Wakisaka, N. A. Ghazy, D. Voigt and T. Suzuki, Eng. Life Sci., 2020, 20, 525–534 Search PubMed.
- K. M. Parker and M. Sander, Environ. Sci. Technol., 2017, 51, 12049–12057 Search PubMed.
- J. Willow and G. Smagghe, Curr. Opin. Environ. Sci. Health, 2025, 100612 CrossRef.
- A. Fire, S. Xu, M. K. Montgomery, S. A. Kostas, S. E. Driver and C. C. Mello, Nature, 1998, 391, 806–811 CrossRef CAS PubMed.
- J. Niu, R. Chen and J. J. Wang, Insect Sci., 2024, 31, 2–12 CrossRef CAS PubMed.
- O. Christiaens, S. Whyard, A. M. Vélez and G. Smagghe, Front. Plant Sci., 2020, 11, 451 CrossRef PubMed.
- US EPA, Notice of Conditional Pesticide Registration and Product Label for MON 89034 × TC1507 × MON 87411 × DAS-59122-7, 2017.
- J. A. Baum, T. Bogaert, W. Clinton, G. R. Heck, P. Feldmann, O. Ilagan, S. Johnson, G. Plaetinck, T. Munyikwa, M. Pleau, T. Vaughn and J. Roberts, Nat. Biotechnol., 2007, 25, 1322–1326 CrossRef CAS PubMed.
- Y. B. Mao, W. J. Cai, J. W. Wang, G. J. Hong, X. Y. Tao, L. J. Wang, Y. P. Huang and X. Y. Chen, Nat. Biotechnol., 2007, 25, 1307–1313 CrossRef CAS PubMed.
- Y. Tomoyasu and R. E. Denell, Dev. Genes Evol., 2004, 214, 575–578 CrossRef CAS PubMed.
- V. Bischoff, C. Vignal, B. Duvic, I. G. Boneca, J. A. Hoffmann and J. Royet, PLoS Pathog., 2006, 2, e14 CrossRef PubMed.
- Y. Y. Zhao, F. Liu, G. Yang and M. S. You, Insect Mol. Biol., 2011, 20, 97–104 CrossRef CAS PubMed.
- I. E. Badillo-Vargas, D. Rotenberg, B. A. Schneweis and A. E. Whitfield, J. Insect Physiol., 2015, 76, 36–46 CrossRef CAS PubMed.
- H. Huvenne and G. Smagghe, J. Insect Physiol., 2010, 56, 227–235 CrossRef CAS PubMed.
- X. Li, X. Liu, W. Lu, X. Yin and S. An, Front. Bioeng. Biotechnol., 2022, 10, 1–12 Search PubMed.
- Y. Luo, X. Wang, X. Wang, D. Yu, B. Chen and L. Kang, Insect Mol. Biol., 2013, 22, 574–583 CrossRef CAS PubMed.
- K. H. Parsons, M. H. Mondal, C. L. McCormick and A. S. Flynt, Biomacromolecules, 2018, 19, 1111–1117 CrossRef CAS PubMed.
- M. R. Joga, M. J. Zotti, G. Smagghe and O. Christiaens, Front. Physiol., 2016, 7, 553 Search PubMed.
- A. Kolliopoulou, C. N. T. Taning, G. Smagghe and L. Swevers, Front. Physiol., 2017, 8, 399 CrossRef PubMed.
- M. Zotti, E. A. dos Santos, D. Cagliari, O. Christiaens, C. N. T. Taning and G. Smagghe, Pest Manage. Sci., 2018, 74, 1239–1250 CrossRef CAS PubMed.
- T. A. Walsh, Pest Manage. Sci., 2007, 63, 1165–1171 CrossRef CAS PubMed.
- X. Li, X. Yang, X. Zheng, M. Bai and D. Hu, Int. J. Mol. Sci., 2020, 21, 7144 CrossRef CAS PubMed.
- E. Ward and P. Bernasconi, Nat. Biotechnol., 1999, 17, 618–619 CrossRef CAS PubMed.
- S. G. Costa, T. S. Lima, L. Codognoto and H. P. M. de Oliveira, J. Solid State Electrochem., 2024, 1–11 CAS.
- J.-L. Cui, H. Li, Q. He, B.-Y. Jin, Z. Liu, X.-M. Zhang and L. Zhang, Comput. Biol. Chem., 2024, 112, 108113 CrossRef CAS PubMed.
- Certis Belchim, Eradicoat Max, https://certisbelchim.nl/producten/eradicoat-max/, (accessed 30 March 2025).
- Imerys, ArgicalTM Pro, https://www.imerys.com/product-ranges/argical-pro, (accessed 30 March 2025).
- Imerys, DezoneTM, https://www.imerys.com/product-ranges/dezone, (accessed 30 March 2025).
- V. Storck, D. G. Karpouzas and F. Martin-Laurent, Sci. Total Environ., 2017, 575, 1027–1033 Search PubMed.
- R. Finger and N. Möhring, Nat. Plants, 2024, 10, 360–366 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |






