Open Access Article
Open Access ArticleSynthesis innovations for crystallizing covalent organic framework thin films on biological and non-biological substrates
Ashok Kumar
Mahato
ab,
Satyadip
Paul
ab and
Rahul
Banerjee
 *ab
*ab
aDepartment of Chemical Sciences, Indian Institute of Science Education and Research, Kolkata, Mohanpur 741246, India. E-mail: r.banerjee@iiserkol.ac.in
bCentre for Advanced Functional Materials, Indian Institute of Science Education and Research, Kolkata, Mohanpur 741246, India
First published on 5th March 2025
Abstract
Thin film technology has emerged as a pivotal field with numerous industrial applications. Depending on their properties—such as magnetic characteristics, conductivity, architectural structure, stability, and functional backbones—thin films are widely utilized in optoelectronics, thin-film coatings, solar cells, energy storage devices, semiconductors, and separation applications. However, for all these applications, thin films must be securely attached to specific substrates, and substrate compatibility with both the thin film and the film-growth process is crucial for optimal performance. In this review, we emphasize the significance of growing thin films, particularly covalent organic framework (COF) thin films, on suitable substrates tailored for various applications. For separation technologies, polymer thin films are commonly fabricated on porous polymeric or metal-based membranes. In contrast, thin films of metals and metal oxides are typically deposited on conducting substrates, serving as current collectors for energy storage devices. Semiconductor thin films, on the other hand, are often grown on silicon or glass substrates for transistor applications. Emerging COF thin films, with their tunable properties, well-defined pore channels, and versatile functional backbones, have demonstrated exceptional potential in separation, energy storage, and electronic and optoelectronic applications. However, the interplay between COF thin films and the substrates, as well as the compatibility of growth conditions, remains underexplored. Studies investigating COF thin film growth on substrates such as metals, metal oxides, glass, silicon, polymers, ITO, and FTO have provided insights into substrate properties that promote superior film growth. The quality of the film formed on these substrates significantly influences performance in applications. Additionally, we discuss the stabilization of biological substrates, like peptide-based biomimetic catalysts and enzymes, which often suffer from instability in non-aqueous environments, limiting their industrial use. Growing COF membranes on these biological substrates can enhance their stability under harsh conditions. We also highlight techniques for growing COF membranes on biological substrates, ensuring the preservation of their structural integrity and functional properties.
1. Introduction
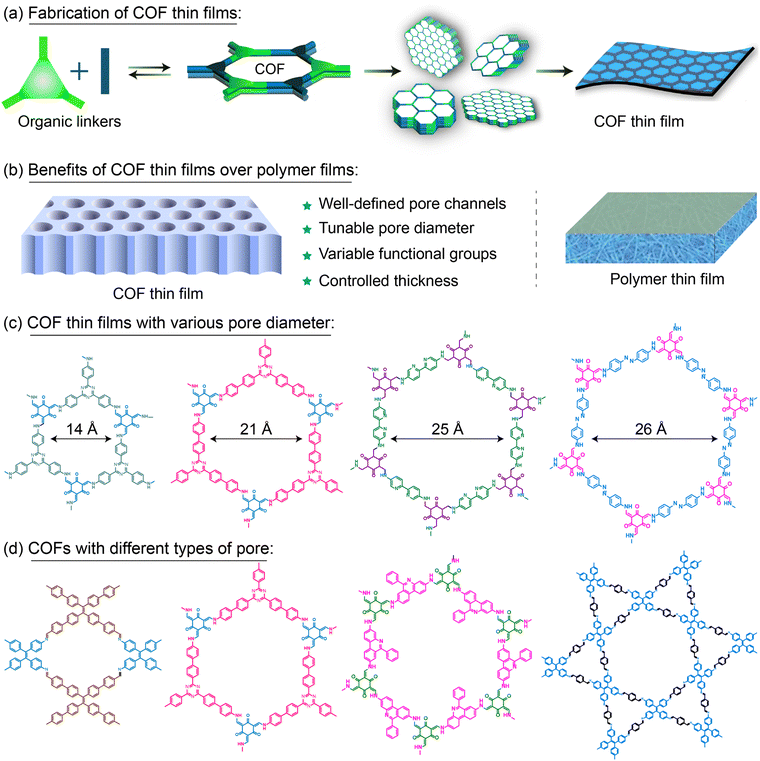
Thin films known for their exceptional functional diversity, including polymers, metals, metal oxides, organic and metal–organic materials, are extensively used in electronics, optoelectronics, electrochemical devices, and separation applications.1–9 Covalent organic framework (COF) thin films represent an exciting advancement in thin film technology, exhibiting remarkable versatility and high-performance potential across diverse industrial sectors.10–16 These films are characterized by their high crystallinity, tunable pore sizes, and customizable functional groups, which distinguish them from conventional thin films.17–19 The unique structures of COF thin films set them apart from traditional thin films made from polymers, organic molecules, metals, and metal oxides (Fig. 1(a) and (b)). Their distinct crystalline architecture and modular design enable precise control over pore channels, redox-active sites, conductive pathways, and catalytic centres, making them well-suited for targeted applications such as selective ion transport, molecular sieving, electronic devices, and energy storage systems.20,21In any thin film-related applications, integration of the film with a suitable substrate is crucial.22,23 Substrates provide essential structural support, preserve film integrity, enhance electron and mass transport, and stabilize performance under various operating conditions. The interaction between the film and substrate influences the crystallization and self-assembly of crystallites during film growth, affecting critical performance metrics, such as mechanical strength and operational durability.24–27 This is particularly vital for direct film growth, as it reduces macroscopic defects and improves adhesion, enhancing film functionality and operational durability. Various synthetic techniques, including drop casting, spin coating, vapor deposition, solvothermal, atomic layer deposition and electrochemical deposition, have been employed to grow COF films on substrates (Fig. 1(c)).28,29 Substrates are chosen based on their properties such as porosity, conductivity, magnetic properties, wettability and surface functionalities as well as their stability under growth conditions. Common substrates include polymers, metals, metal oxides, carbon-based materials, silicon wafers, and glass. Furthermore, techniques like interfacial crystallization enable COF thin film growth at liquid–liquid or liquid–air interfaces. However, transferring such thin films to substrates often leads to cracks and defects, limiting their practical usability. As a result, the direct growth of COF films on appropriate substrates has become a preferred approach over traditional film transfer methods, which often introduce micro-cracks, result in poor adherence, and reduce overall film quality. Therefore, it is crucial to ensure the substrate compatibility of a thin film and also an optimal film growth process tailored to the desired substrate.
This review explores recent advancements in COF thin film growth directly on various substrates, focusing on how substrate–film interactions, synthesis techniques, and substrate selection contribute to producing high-quality, durable films. It also discusses the use of COF thin films on biological substrates.30–35 By cladding COF films on biocatalysts such as enzymes, we can overcome the stability challenges these biological materials face in non-aqueous environments. This innovation expands the operational stability of biocatalysts, opening up new possibilities for stable, functional biocatalytic systems in industrial settings. We aim to demonstrate how COF thin films can potentially transform industrial applications, offering customized solutions that address the functional requirements of next-generation technologies.
2. Thin films grown on substrates
This review focuses on the synthesis and applications of COF thin films grown on or adhered to various substrates. Highlighting the importance of substrate compatibility and growth processes, we focus on the applications of COF thin films on various substrates, and the advantages and disadvantages of different synthesis methods. Although numerous methods exist for synthesizing COF thin films on substrates challenges persist, including suboptimal film quality, limited material diversity, and complex experimental setups. This review aims to provide insights into synthesis techniques for COF thin films on substrates to achieve optimal performance.2.1 Primary applications of various thin films on substrates
Polymer thin films are grown on different substrates to serve a variety of applications, including membrane separation, electronic and optoelectronic devices, packaging, electrochemical devices and protective coatings.36–40 Polyamide thin films, for instance, are often fabricated on microporous substrates such as polyethersulfone and polytetrafluoroethylene (PTFE) for separation applications (Fig. 2(a)).23,41,42 Organic thin-film transistors (OTFTs) frequently utilize polythiophene-based polymers, where thin polythiophene layers are deposited on silicon wafers and glass substrates to create the active semiconductor layer for transistor applications.43–45 This approach highlights the adaptability of polymer thin films across a spectrum of substrates, enabling advancements in electronics, environmental protection, and energy storage solutions.Metals and metal oxides are deposited as thin films to form the active functional layers in energy storage systems, electronic devices, optoelectronic devices, and fuel cells as catalyst layers.46–58 Commonly used materials for these applications include lithium, zinc, nickel, platinum, lithium cobalt oxides–(LiCoO2), lithium manganese oxides–(LiMn2O4), LiFePO4, and LiNiPO4, and zinc oxide (ZnO) typically deposited as thin films onto diverse substrates.59–68 Since the commercialization of Li batteries, lithium cobalt oxide, with a theoretical capacity of 274 mA h g−1, has long been a popular cathode material in energy storage devices, particularly lithium-ion batteries (Fig. 2(b)).69,70 Methods like vapor deposition, atomic layer deposition, and electrodeposition are often employed for these coatings, primarily on copper and aluminium foils. The vapor deposition technique is versatile for growing uniform thin films with good adhesion with substrates. However, this method is often limited to types of substrates. Atomic layer deposition and electrodeposition can be performed at lower temperatures with good control over film thickness. These methods are also limited to materials compatibility. The selection of deposition techniques mostly depends on the stability and properties of the materials and the substrates.
Organic molecules are extensively applied in thin film synthesis for applications in electronic and energy storage devices. Organic molecules are widely utilized in organic field effect transistors because of efficient charge carrier mobility and desired semiconducting properties.71,72 Pentacene, a well-known organic molecule, is widely used to fabricate OFETs (Fig. 2(c)).73 These small molecules are typically deposited as thin films on substrates like SiO2 and glass through spin coating, which provides a uniform layer essential for electronic and optoelectronic applications. Redox-active organic molecules are also deposited as thin films to fabricate the electrodes in batteries.74–76
2.2 COF thin films on substrates
Current research efforts are increasingly focused on the sustainable development of metal-free active materials for various applications. Researchers have made significant advances in creating metal-free systems, such as organocatalysts and organophotocatalysts, as well as small and complex molecules for energy storage and optoelectronics. Among these materials, crystalline polymers stand out due to their unique properties conferred by crystallinity, often resulting in novel functions.77–79 COFs comprise symmetric organic linkers covalently connected to form extended two-dimensional (2D) or three-dimensional (3D) structures, adopting various geometries including hexagonal, tetragonal, rhombic, kagome, and triangular arrangements.80–85 The physicochemical properties, catalytic activities, and morphologies of COFs are further tuned by selecting appropriate linkers, synthesis methods, and post-synthesis modifications. Interestingly, many such applications, including separation and energy storage, specifically require porous crystalline films (Fig. 2(d)). The insolubility and limited processability of bulk COF powders present significant obstacles to membrane fabrication, restricting their practical use.15 For instance, anthraquinone-based COF powder blended with carbon black demonstrated improved electrode performance; however, calculations revealed that only 2.5% of the anthraquinone moieties were electrochemically accessible due to the random orientation of the COF powder.86 Consequently, crystalline and oriented COF thin films have emerged as highly promising materials, offering tunable properties suitable for practical applications.The synthesis technique is crucial for producing high-quality COF thin films, as it directly impacts their properties and performance.87–92 The growth and assembly of COF crystallites depend heavily on the chosen method (Fig. 3(a)). Unlike polymer thin films, which often exhibit random orientations, COF thin films have well-defined pore channels with adjustable pore diameters (Fig. 3(b)). This tunability enables molecular sieving, allowing for the selective separation of molecules based on size.93–99 Furthermore, COF thin films can exhibit different pore structures, depending on their geometric configuration—whether tetragonal, hexagonal, kagome, rhombic, or trigonal—which can be tailored for specific separation and other applications (Fig. 3(c) and (d)). Typically, COF thin films possess semiconducting properties, with band gaps ranging from 0.5 eV to 2.8 eV. The band gap can be easily adjusted to meet the requirements of various applications by modifying the functional backbone structures.100–103 This level of control over structural and electronic properties positions COF thin films as versatile and valuable materials for a wide array of technological advancements.
To examine the growth of COF films on substrates, we utilized residual crystallization processes to synthesize COF thin films (TpAzo and TpDPP) on several substrates with different wettabilities (ITO, FTO, borosilicate glass, silicon wafer, metallic copper and polytetrafluoroethylene).26 Under the same reaction conditions for all substrates, we observed a superior film growth of TpAzo and TpDPP COFs on a glass substrate. Crystallinity and porosity of the thin films grown on hydrophilic substrates are much better than the thin films grown on hydrophobic substrates. We anticipate that the hydrophilic amine and aldehyde functional groups on the crystallite surface favour the assembly and crystallization on hydrophilic surfaces (like glass) during the reaction. Therefore, it is crucial to realize the nature of substrates and COF crystallites to obtain the optimal film growth on substrates. Moreover, it is well known that the crystallinity and porosity are the important parameters for the performances in membrane-based applications. A β-ketoenamine-based crystalline COF (TFP-DHF) thin film was fabricated on a porous anodic aluminum oxide substrate, which exhibited improved organic solvent permeability by 100 times compared to the amorphous membrane synthesized by the same process.104 This suggests that the crystallinity plays an important role in separation applications. Therefore, the substrate selection is crucial as it greatly influences the crystallinity, porosity, and, eventually, the performance of the thin films. Furthermore, the substrates are often modified by introducing several functionalized molecules to react with the precursor molecules and then drive the crystallization for a better-quality film growth. For example, aldehyde-modified and dual-amino-modified Al2O3 supports have been used to grow ACOF-1 and COF-LZU1-ACOF-1 composite membranes for improved gas separation efficiency.105,106
Stability of substrates is another important parameter to be considered under the film growth conditions. Fragile substrates especially biological substrates are not stable under harsh reaction conditions like solvothermal and other methods involving high temperature and organic solvents. For such fragile substrates, it is highly desirable to design the reaction system associated with aqueous media. For example, we developed a cladding technique to grow a COF layer on peptide-based biomimetic catalysts.27 During the reaction we take the biomimetic catalysts in the water layer to preserve their structure and activity.
 | ||
| Fig. 4 Schematic representation of (a) the growth mechanism on supports, (b) solvothermal synthesis of COF thin films on single layer graphene, (c) vapour assisted synthesis on a glass substrate, (d) electrophoretic deposition on conducting supports, (e) solvent evaporation technique for film synthesis on a glass support. Fig. 4(a) is adapted from ref. 24 with permission from the Royal Society of Chemistry, Copyright 2017. | ||
While the solvothermal method facilitates easy film synthesis on various supports, it often leads to issues like bulk powder contamination and inhomogeneous film growth. To address these challenges, various techniques have been employed for COF thin film synthesis. The vapor-assisted conversion process, used to grow BDT-COF, COF-5, and pyrene-COF thin films at room temperature (Fig. 4(c)), involves drop-casting dissolved precursor solutions onto a glass substrate and placing it in a desiccator with an open solvent vial containing mesitylene and dioxane. This method produces thick films ranging from 300 nm to 7.5 μm after 72 hours of crystallization, which is beneficial for substrates unable to endure the harsh conditions of solvothermal synthesis.109 However, achieving thin films using this approach remains challenging and restricts specific applications. Electrophoretic deposition is another technique for fabricating COF films and coatings on conducting surfaces (Fig. 4(d)). Using this method, COF-5, COF-300, and BDT-ETTA COFs with different functional groups (imine and boronate ester bonds) are synthesized on metal and metal oxide substrates. Electrodes are immersed in nanoparticle suspensions of bulk COFs, with an applied bias ranging from 100 to 900 V cm−1. This technique enables rapid, large-area film growth (up to 25 cm2) within just 2 minutes, with thicknesses varying from 400 nm to 24 μm. The film thickness can be precisely controlled by adjusting parameters such as electrode potential, deposition time, and particle concentration.110
Despite the advantages of quick and extensive film growth, the requirement for high voltage is a limitation.
Another well-known COF thin film fabrication method is the direct solvent evaporation of a COF solution or slurry (Fig. 4(e)). Preparing a highly processable COF particle solution is essential to ensure film quality. This method involves creating stable colloidal suspensions of COF-5 using co-solvents, achieving a stable suspension that remains homogeneous for weeks without precipitation, even when cooled. Solvent evaporation of the COF-5 colloidal solution at 90 °C results in film growth on glass substrates, producing films with thicknesses exceeding 10 μm.111 However, this method requires the substrate to be compatible with the conditions of colloidal synthesis, underscoring the importance of substrate selection.
A modified solvothermal approach was utilized to synthesize crystalline, oriented thin films on an Au-working electrode, while the conventional solvothermal process provided only nondiffracting thin films (Fig. 5(a)).112 This method provides excellent improvement in capacitance when thin films are used as electrode materials compared to the randomly oriented powdered COFs. Slow addition of a trialdehyde precursor solution (TFP in DMF) into the diamine (DAAQ) solution of DMF at 90 °C was performed to avoid the rapid crystallization and produce a crystalline, oriented DAAQ–TFP thin film on an Au-substrate. Such oriented thin film growth on the Au electrode helped in accessing the large percentage of redox-active functional groups electrochemically, providing an areal capacitance of 3.0 mF cm−2. In contrast, the COF powder-modified electrodes showed only 0.4 mF cm−2. Such research findings of oriented film growth on relevant substrates and improved capacitance could be potentially applicable in optoelectronics and energy storage devices.
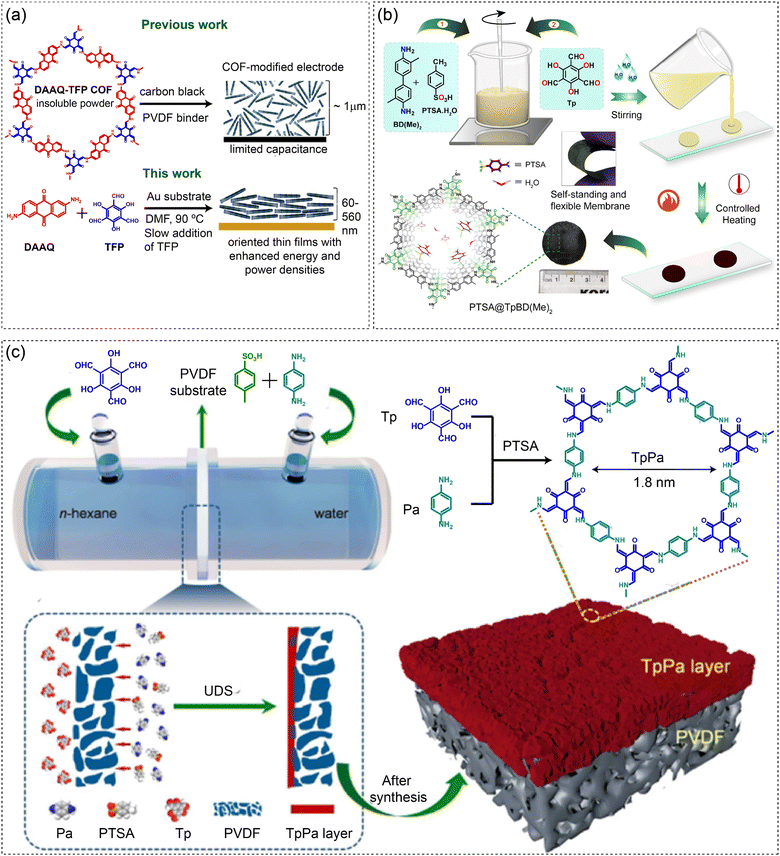 | ||
| Fig. 5 Synthesis of COF films on substrates by (a) a modified solvothermal approach, (b) drop casting and slow crystallization processes and (c) unidirectional diffusion. Fig. 5(a) is reproduced with permission from ref. 112; Copyright 2015, American Chemical Society. Fig. 5(b) is reproduced with permission from ref. 113; Copyright 2018, Wiley. Fig. 5(c) is reproduced with permission from ref. 114; Copyright 2019, Elsevier. | ||
Furthermore, we reported a strategic synthesis approach to fabricate self-standing and highly flexible covalent organic framework membranes (COFMs) with superior proton conductivity (Fig. 5(b)).113 This slow crystallization approach involves the reactions of amine-p-toluene sulfonic acid (PTSA·H2O) salt with a trialdehyde to synthesize three COF membranes, followed by the impregnation of PTSA·H2O as a proton carrier within the COFM pore. In brief, the diamines are homogeneously mixed with PTSA·H2O and subsequently, the trialdehyde (Tp) was mixed. Then water was added to prepare a viscous slurry, which was cast into the molds of a glass substrate, followed by slow baking at 50–90 °C for 3–4 days. The impregnation of PTSA·H2O within the COFM pore provides three COFMs PTSA@TpBD(Me)2, PTSA@TpAzo and PTSA@TpBpy. The COFMs prepared by this method showed the highest reported proton conductivity amongst all porous crystalline polymeric materials. The membranes are operated under fuel cell proton exchange membrane operating conditions to confirm their practical utilization.
Unidirectional diffusion synthesis (UDS) is another technique to grow a COF layer on substrates at a liquid–liquid interface (Fig. 5(c)).114 The TpPa COF was grown on a polyvinylidene fluoride (PVDF) substrate at room temperature to prepare a COF-based composite membrane for efficient dye separation and water permeance. Immiscible solutions of an aldehyde (Tp) in hexane and an amine (Pa) in water were separately introduced into two sides of a diffusion cell. The Pa molecules pass through the PVDF membrane to react with the Tp aldehyde molecules at the interface, producing the TpPa COF layer on the PVDF substrate within 24 h of reaction. The composite membrane is highly efficient in dye separation. These techniques illustrate the versatility and challenges in fabricating COF thin films, highlighting the need for continued optimization to achieve high-quality, application-specific films.
 | ||
| Fig. 6 Schematic representation of (a) transferring films onto supports, (b) interfacial synthesis of COF thin films then transferred to a polyester substrate, (c) synthesis of pristine COF films from CONs, (d) synthesis of pristine COF films at a liquid–air interface, and (e) surfactant assisted synthesis of crystalline polymer thin films. Fig. 6(b) is adapted with permission from ref. 26, Copyright 2021, American Chemical Society. Fig. 6(d) is adapted with permission from ref. 120, Copyright 2018, American Chemical Society. | ||
Additionally, we have reported the synthesis of pristine COF thin films from covalent organic nanosheets (CONs), which are obtained by chemically exfoliating a 2D COF (DaTp).121 This process involves attaching N-hexylmaleimide molecules to the 9- and 10-positions of anthracene units in DaTp COF through a [4+2] cycloaddition reaction. The incorporation of N-hexylmaleimide disrupts the π–π interactions and the planarity of the COF layers, resulting in exfoliation and the formation of CONs. These exfoliated CONs are then assembled into pristine DaTp-CON thin films at an air/water interface (Fig. 6(c)), which can be transferred to substrates like silicon wafers, glass slides, or even loops. Furthermore, photon-assisted synthesis has been utilized to create polyimine-based 2D COF thin films (pi-COF) on a water surface via Schiff-base condensation of terephthalaldehyde (PDA) and 1,3,5-tris(4-aminophenyl)-benzene (TAPB) (Fig. 6(d)).120,122 This method allows for the rapid (approximately 1 hour) and scalable synthesis of pi-COF films, with diameters of up to ∼4 inches and precise control over the number of layers. The resulting films can be transferred to substrates such as silicon oxide, silicon, quartz, and mica, making them suitable for applications in field-effect transistors, sensors, and photodetector devices.
Surfactant-monolayer-assisted synthesis is another reported method for creating a few layers of crystalline polymer thin films on water surfaces at room temperature (Fig. 6(e)).123 Surfactant monolayers, such as sodium (9Z)-octadec-9-en-1-yl sulfate, guide the arrangement and polymerization of monomers, forming 2D polyimide (2DPI) films. These films are synthesized by the condensation of isochromeno[4′,5′,6:6,5,10]anthra[2,1,9-def]isochromene-1,3,8,10-tetrone and 4,4′,4′′,4′′′-(porphyrin-5,10,15,20-tetrayl)tetraaniline. The resulting thin films, with thicknesses of around 2 nm, can be transferred to substrates such as SiO2/Si and holey copper grids for characterization. This suite of synthesis and transfer techniques underscore the versatility and adaptability of COF thin films, paving the way for their integration into a range of advanced applications.
The solvothermal process is the most effective approach for film growth on substrates. The simple and straightforward method is versatile for growing different types of thin films on various substrates. However, the method still suffers the prevailing challenge of thin film contamination with bulk powder as the method is dedicated to powdered COF synthesis. Furthermore, the vapor-assisted synthesis, solvent evaporation, and various solution casting methods are beneficial for thicker film synthesis and user-friendly, but the limitations of thinner and smooth film synthesis are yet to overcome. Rapid film synthesis by electrophoretic deposition demands the use of very high voltage (up to 900 V) and also this method is limited to conducting substrates.
Various modified approaches of the solvothermal process have been utilized to grow COF thin films on substrates and to avoid bulk powder contamination. For example, the synthesis of redox active DAQ-TFP oriented COF films involves a modified solvothermal approach using slow addition of one precursor solution to another.112 This method produces oriented and crystalline COF films to access more redox active functional groups for enhanced areal capacitance over the randomly oriented COF powder. However, it is difficult to completely avoid film contamination with powder using such a direct precursor mixing approach.
Furthermore, film thickness is also critical for applications in membrane separation and electronic devices. Thinner films can provide higher solvent flux, enhance separation performance, and increase conductivity. However, an optimal thickness is necessary to maintain the film's mechanical strength and uniformity. Thus, there is a pressing need to develop protocols for synthesizing high-quality thin films on various substrates, with stringent control over crystallization and thickness. We developed a unique concomitant crystallization methodology to address these challenges to synthesize large-area, continuous, and crack-free COF thin films on different supports. Our approach combines the benefits of interfacial crystallization and modified solvothermal synthesis, utilizing the interfacial crystallization process to initiate and regulate crystallization in a new method we call residual crystallization (Fig. 7(c)). Conventional methods like solvothermal processes often lead to uncontrolled precursor mixing and precipitation without the interfacial crystallization component. However, the residual crystallization (RC) method enables the formation of smooth, homogeneous, and crack-free COF thin films with high crystallinity and permanent porosity on various substrates. Additionally, this method facilitates the fabrication of large-area sub-10 nm thin films.
It is crucial to incorporate these powders into other processes during film formation to prevent contamination from bulk COF powders. Our residual crystallization process effectively grows COF thin films on substrates with varying wettability, including metals, metal oxides, glass, silicon, ITO, and FTO. We observed superior film quality, particularly on hydrophilic substrates, with enhanced crystallinity and porosity for specific COFs. This advancement underscores the importance of substrate compatibility and precise control in synthesizing high-performance COF thin films (Table 1).
| COFs | Methods used | Advantages | Disadvantages | Stability | Surface area | Applications and potential uses | Ref. |
|---|---|---|---|---|---|---|---|
| COF-5, TP-COF and NiPc-PBBA COF on SLG/Cu, SLG/SiO2, and SLG/SiC | Solvothermal synthesis | Versatile for synthesis of different COF films | Thin film contamination with COF powder | Organic photovoltaic | 107,135 | ||
| Scalable technique | Not suitable for substrates sensitive to solvents and high temperature | ||||||
| Straightforward process | |||||||
| Strong attachment with substrates | |||||||
| HHTP-DPB COF on SLG/SiO2 | Solvothermal synthesis | 930 m2 g−1 (powder) | Fluorescent sensors | 134 | |||
| BDT-COF on ITO-coated glass | Solvothermal synthesis | 175 cm2 cm−2 | 108 | ||||
| TAPB-PDA on h-BN | Dynamic liquid/solid interfacial growth | Avoid film contamination | Limited COF synthesis | Optical applications | 136 | ||
| Exceptional surface quality | Complex reaction set-up | ||||||
| Adjustable thickness | |||||||
| BDT-ETTA COF and BDT-ETTA COF/Pt on FTO coated glass | Electrophoretic deposition | Large-area films | High electric voltage (up to 900 V) | Photoelectrochemical water reduction | 110 | ||
| Very less synthesis time (2 min) | |||||||
| BDT-COF and COF-5 on a glass substrate | Vapor-assisted conversion | Room temperature synthesis | Difficult to synthesize thinner films | 990 m2 g−1 (BDT-COF film) | 109 | ||
| Beneficial for COF film synthesis using fragile precursors and on sensitive substrates | Rough surface | ||||||
| Limited scope of COFs | |||||||
| TpBD(Me)2. TpAzo and TpBpy | Solution casting | Self-standing COF film | Difficult to synthesize thinner films | 2583–2933 m2 g−1 | Fuel cells | 113 | |
| Straightforward process | Rough surface | ||||||
| COF-5, DPB-COF, TP-COF and COF-10 on Pt QCM | Synthesis under continuous flow | Avoid film contamination | Monomer waste | 137 | |||
| Controlled thickness | |||||||
| TpAzo and TpDPP on glass, FTO, ITO, Si, and Cu | Residual crystallization | Avoid film contamination | Long reaction time (4–5 days) | 2093 and 1649 m2 g−1 on glass | 26 | ||
| Controlled thickness | |||||||
| Strong attachment | |||||||
| COF-BTDH with pectinase | Adsorption | Straightforward process | Weak interaction | Ginsenoside hydrolysis | 138 | ||
| COF1 with AChE | Adsorption | High loading efficiency | Leaching | Sensing | 139 | ||
| Ni-TpBpy with enzymes (BGL, CBH and EG) and proteins (GFP and BG Rho) | Adsorption | Generality | Long-term stability of enzymes at room temperature | 58 m2 g−1 (BGL-COF) | Purification of enzymes and proteins | 33 | |
| 73 m2 g−1 (CBH-COF) | Enzyme catalysiss | ||||||
| 41 m2 g−1 (EG-COF) | |||||||
| H-COF-OMe with laccase | Covalent linkage | Strong interactions | Limited to specialized functional groups | Enhanced pH, thermal, and storage stability | Tetracycline degradation | 140 | |
| Reusability | Complex process | ||||||
| Prevents enzyme leakage | |||||||
| MNPs@TTB-DHBD and Tz-Da with OPH,GOx | Covalent linkage | Improved stability | 549 m2 g−1 (d@Tz-Da@OPH) | Enzyme catalysis | 141 | ||
| NKCOF-98 with lipase | In situ encapsulation | Straightforward approach | Limited reaction environments (like aqueous solution) | Improved stability | 142 | ||
| Large scale fabrication | |||||||
| COF-42-B with Cyt c | Sacrificial templating | High loading | Difficulty in COF synthesis | Stable at high temperature and in organic solvents | 143 | ||
| Sufficient protection | |||||||
| Confined microenvironment | |||||||
| TpAzo and TpDPP with C10FFVK assembly | Cladding | Excellent protection | COF cladding on enzymes is yet to be explored | Excellent stability in organic solvents | 708 & 504 m2 g−1 | Biocatalysis | 27 |
| Prevents enzyme leakage | |||||||
| High loading |
Renewable energy sources and storage devices are critical for sustaining modern society's technological demands. COF thin films, with their customizable redox-active functional groups and controlled thickness, have shown promise as electrode materials in energy storage devices like lithium-ion batteries, zinc-ion batteries, and supercapacitors. Quinone-based systems, such as those incorporating anthraquinone and hydroquinone units, have attracted significant interest. For example, an anthraquinone-based COF (DAAQ–ECOF) was mixed with conductive carbon black and a polyvinylidene fluoride binder in N-methyl-2-pyrrolidinone and drop-cast onto an alumina plate to form a cathode for lithium-ion batteries. Exfoliating the DAAQ COF to 3–5 nm thickness reduced the lithium-ion diffusion length, improving charge transfer and overall energy storage performance.124 Hydroquinone-based COFs, such as HqTp COF, have also been used as cathodes for rechargeable aqueous zinc-ion batteries, where they are combined with conducting carbon nanofibers and a Nafion binder and coated onto modified carbon fiber paper (Fig. 8(a)).125
 | ||
| Fig. 8 Schematic representation of (a) a Zn-ion battery with a deposited COF layer as a cathode, (b) COF thin film as a separator in a battery, (c) COF membrane as a supercapacitor electrode material, (d) COF thin film as a protection layer for metal anodes in batteries, (e) flexible COF membranes for fuel cell applications and (f) field effect transistor with COF film as an active semiconducting layer. Fig. 8(c) is reproduced with permission from ref. 126; Copyright 2018, American Chemical Society. Fig. 8(e) is reproduced with permission from ref. 113; Copyright 2018, Wiley. | ||
COF thin films also act as separators in energy storage devices, using their ordered pore channels to block undesired molecules while allowing necessary ion transport. In sodium–sulfur (Na–S) batteries, azobenzene-functionalized COF films with dual-purpose narrow pores inhibit the polysulfide shuttle effect and provide sites for sodium ion migration, thereby extending the battery's lifespan and boosting capacity (Fig. 8(b)).127
Furthermore, anthraquinone-based COFs have been employed as electrode materials for capacitors. DAAQ-BTA COF films were synthesized on 3D graphene using interfacial polymerization and subsequently attached to nickel foam as current collectors for electrochemical analysis.128 We synthesized a redox active TpOMe-DAQ COF having an interlayer hydrogen bonding structure as a free-standing supercapacitor electrode material (Fig. 8(c)).126 The pristine COF membrane exhibited an excellent areal capacitance of 1600 mF cm−2 with very high cycling stability of more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The expected energy storage mechanism is attributed to both EDLC and pseudocapacitance due to its high porosity and redox active groups present in the backbone.
000 cycles. The expected energy storage mechanism is attributed to both EDLC and pseudocapacitance due to its high porosity and redox active groups present in the backbone.
Li-metal and aqueous Zn-ion batteries face challenges such as dendrite growth, unstable solid–electrolyte interfaces (SEI), and electrolyte corrosion, leading to poor reversibility (Fig. 8(d)). COF thin films serve as effective protective layers, suppressing dendrite formation and minimizing undesirable reactions on metal anodes.129 Due to their high mechanical strength, ordered pore channels, and controlled thickness, COF thin films facilitate ion transport while preventing dendrite growth. For example, ultrathin fluorinated FCOF films have been used to protect zinc surfaces, improving corrosion resistance and enhancing ion transport through fluorine-containing pore channels.
Proton exchange membrane (PEM) fuel cells are a promising platform for clean power generation especially in transportation power systems. COFs with high proton conductivity, exceptional stability and desired functional groups have become the potential materials as proton exchange membranes in fuel cells. We have developed a synthetic strategy to prepare various self-standing and flexible COF membranes (COFMs) with impregnated p-toluene sulfonic acid (PTSA·H2O) as a proton carrier within COFM pores (Fig. 8(e)).113 The COFMs exhibited highest proton conductivity of 7.8 × 10−2 S cm−1 compared to all other porous crystalline organic materials. Moreover, we tested their practical utilization under real PEM operating conditions and achieved the highest power density of 24 mW cm−2 amongst COFs and MOFs.
Field-effect transistors (FETs) are fundamental components of modern electronics, including amplifiers, sensors, memory chips, and more. Smooth and homogeneous COF thin films are crucial for effective electronic connectivity between the substrate and the semiconductor layer. The polyTB COF thin film, with its imine-based conjugated structure, has demonstrated effectiveness as an active semiconducting layer in FETs (Fig. 8(f)). Previously reported boronate ester-based COFs, which require an edge-on orientation for charge transport, often pose fabrication challenges. In contrast, the polyTB COF thin film features in-plane charge transport with a highly smooth surface (an average roughness of 0.2 nm) and reduced thickness (1.8–29 nm), making it a strong candidate for semiconductor applications.130 This film, synthesized at the solution/air interface, can be transferred onto silicon substrates for device fabrication.
COFs have been utilized as multifunctional materials for separation applications due to their intrinsic porous, crystalline structures with tunable pore size and structure, large surface area, tailored functionality and low density. COFs are not only utilized as continuous membranes but also as thin film nanocomposite and mixed matrix membranes. Randomly oriented amorphous polymer membranes lack ordered pore channels, uniform pore sizes and porosity, which creates difficulties in achieving high selectivity. The COF-based membranes are designed considering some crucial factors such as pore size, surface charge, wettability and stability for separation applications.
Gas separation is a promising application of COF-based membranes owing to their intrinsic porosity, high stability, and low transport resistance and adjustable pore size. A highly stable azine linked ACOF-1 membrane was grown on an aldehyde-modified Al2O3 support for CO2/CH4 gas separation.105 The continuous COF membrane, with a narrow pore size of 0.94 nm, exhibits a high selectivity of 86.3 with a reasonable CO2 permeance of 9.9 × 10−9 mol−2 s−1 Pa−1. The overall performance also exceeds the CO2/CH4 Robeson upper bound. The excellent performance is attributed to the high CO2 adsorption capacity and very narrow pore diameter of the COF (Fig. 9(a)).
 | ||
| Fig. 9 Applications of COF-based membranes in (a) gas separation, (b) water treatment and (c) organic solvent nanofiltration. Fig. 9(c) is reproduced with permission from ref. 131; Copyright 2024, Elsevier. | ||
Furthermore, to overcome the difficulties in synthesizing COF membranes with a very narrow pore aperture close to the size of gas molecules, a bilayer COF membrane (COF-LZU1–ACOF-1) was synthesized on a dual-amino-functionalized Al2O3 substrate for H2 separation from other gases.106 A temperature-swing solvothermal process was utilized to grow the imine-based COF-LZU1 and ACOF-1 layers on the porous alumina substrate. The interlaced pore size of the COF–COF composite membrane (pore sizes of ∼1.8 nm for COF-LZU1 and ∼0.94 nm for ACOF1) is expected to be in the range of kinetic diameter of common gas molecules. The bilayer COF membrane exhibits a superior separation selectivity for H2/CH4, H2/CO2 and H2/N2 gas mixtures compared to the individual COF membranes. The high separation performances of the COF–COF composite membrane exceed the Robeson upper bounds for the gas mixtures.
Membrane separation is an energy efficient, environment-friendly, and sustainable process producing for clean water. Desalination and waste-water recycling require COF membranes with appropriate pore size, surface charge, and functional backbones for nanofiltration of salts, dyes, and various organic molecules.
Industrial wastewater is mostly contaminated with carcinogenic and hazardous organic dyes, which are difficult to remove because they are highly resistant to heat and light. To overcome this issue, we developed the interfacial crystallization process to synthesize COF thin films (Tp-Tta, TpTtba, TpBpy and Tp-Azo) for separation of various dyes.119 The COF films showed excellent dye rejection performances, with the Tp-Bpy film showing 94% (BB, brilliant blue-G), 80% (CR, Congo red), 97% (AF, acid fuchsin), and 98% (RH, rhodamine B) and the Tp-Azo film showing 90% (BB), 79% (CR), 99% (AF), and 99% (RH). Furthermore, Tp-Bpy exhibits selective separation for AF when subjected to a mixed feed of AF and p-nitrophenol (NP). These highly stable β-ketoenamine COFs demonstrate the dye rejection efficiency over five cycles.
Technologies based on seawater desalination and wastewater treatment demand membranes with high ion sieving performance. A negatively charged COF-based composite nanofiltration membrane (SCOF/PA) was synthesized with pendant sulfonic acid groups on the COF backbone.132 The membrane, with remarkable hydrophilicity and substantial negative zeta potential (−57 mV), showed an excellent rejection of Na2SO4 (99.6%), a high single salt selectivity (NaCl/Na2SO4) of 178.5 and precise mono/divalent ion separation with a selectivity of up to 312.6 for Cl−/SO42− (Fig. 9(b)).
In the pharmaceutical and chemical industries, organic solvent nanofiltration (OSN) has gained significant attention for solvent recycling and purification. Such applications necessitate membranes with high stability and solvent resistance as they are exposed to organic solvents. Therefore, the COFs with β-ketoenamine, hydrazine and imine linkages are more preferable.
A β-ketoenamine-based COF (TFP-DHF) thin film was fabricated on a porous anodic aluminium oxide substrate using the Langmuir–Blodgett (LB) technique.104 The membrane showed excellent permeabilities for polar and nonpolar organic solvents because of the very low membrane thickness of the four-unit-cell and the crystalline nature of the COF. The ordered pore channels in the crystalline COF membrane improved the permeability by 100 times compared to the amorphous membrane synthesized by the same process. This suggests that the crystallinity plays an important role in separation applications, which signifies the practical use of COF-based membranes over polymeric membranes. Furthermore, the membrane showed excellent dye rejection ability with a molecular-weight-cut-off of approximately 900 Da.
Another COF (TpPa) was constructed as a separation layer by interfacial polymerization on a polyimide (PI) substrate for enhanced solvent transport and selective molecular sieving. Excellent solvent resistance of the TpPa/PI membrane makes it appropriate for separation and purification of organic solvents. The membrane showed high performance in ethanol permeance (58.0 LMH/MPa), fast green FCF rejection (96.2%) and n-hexane permeance (102.0 LMH/MPa).133
Another example describes the synthesis of TraHz and TpaHz COF-based composite membranes by swift interfacial polymerization on a polyacrylonitrile (PAN) substrate.131 The highly stable membranes exhibit excellent separation performance even at higher temperatures in polar aprotic solvents (up to 80 °C). The membranes showed a molecular-weight cut-off of ∼650 g mol−1 with an excellent dye rejection of more than 98% for the Chicago blue and Congo red dyes in THF, water, and ethanol. Also, the Rhodanile blue rejection is higher than 98% in toluene with remarkable permeances of 47 and 33 L m−2 h−1 bar−1 for toluene using TraHz-30 and TpaHz-30, respectively (Fig. 9(c)).
3. Future directions
Biocatalysts and biomimetic catalysts are known for their remarkable efficiency and selectivity in performing various organic transformations (Fig. 10(a)). However, their inherent fragility in non-aqueous media significantly limits their industrial applicability. To address this challenge, researchers have explored porous, inert matrices to encapsulate biocatalysts, which has allowed for some degree of stabilization during reactions (Fig. 10(b)). Despite these advancements, such encapsulation methods—often based on noncovalent interactions—suffer from leaching and poor recyclability under operational conditions. A more sustainable and robust approach involves cladding or armouring biocatalysts with porous, crystalline membranes that feature well-defined pore channels. Similar to how a cell membrane facilitates the selective transport of molecules, ions, and metabolic products, COF membranes with precisely structured pores can provide access to active sites for catalytic transformations while ensuring stability. These well-defined pore channels allow organic molecules to transit efficiently, thus preserving the catalytic activity of the biocatalysts. We recently developed a novel cladding technique to armour peptide-based biomimetic catalysts with COF membranes, enabling them to perform retro-aldol catalysis in various non-aqueous media, including acetone, acetonitrile, and dichloromethane. This approach demonstrates that COF cladding can offer structural protection and functional access, effectively stabilizing biocatalysts in environments where they typically degrade (Fig. 10(c)). We believe this method can be extended to enzyme-based biocatalysts, using noncovalent interactions to armour them with COF membranes without compromising their structural integrity and catalytic functions. Such COF cladding represents a significant advancement in the field by stabilizing biocatalysts and preventing leaching in non-aqueous media. The ability to grow COF membranes directly on biological substrates holds immense potential for enabling various organic transformations in both aqueous and non-aqueous environments, thus broadening the industrial applicability of biocatalysts.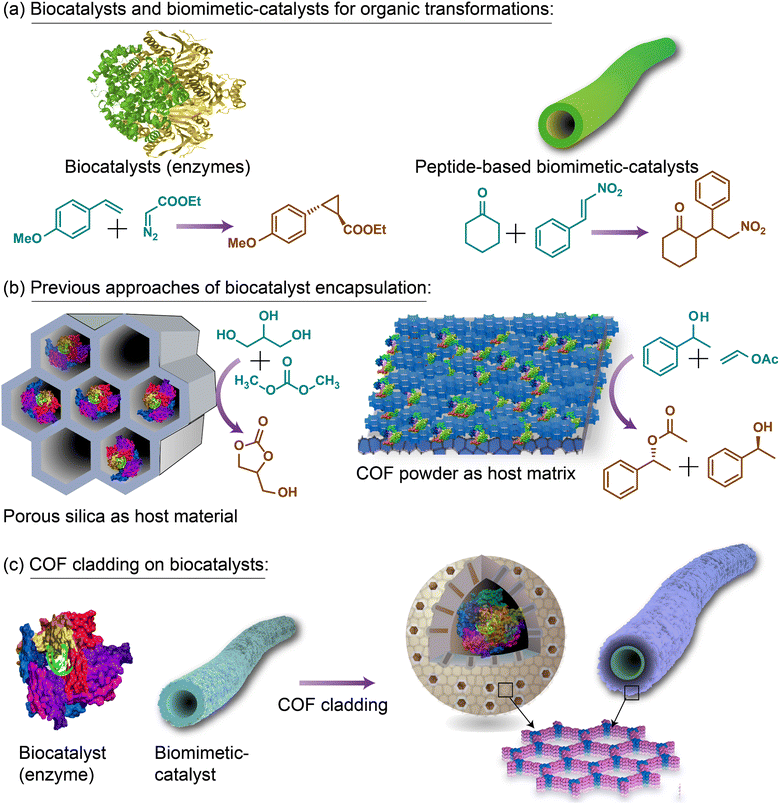 | ||
| Fig. 10 Schematic representation of (a) biocatalysis with enzyme and biomimetic catalysts, (b) encapsulation of biocatalysts in porous matrixes, and (c) cladding COF membranes on biocatalysts. Fig. 10(c) is adapted with permission from ref. 27; Copyright 2023, American Chemical Society. | ||
4. Conclusions and overview
This review emphasizes the critical role of substrate compatibility and innovative synthesis methods in successfully fabricating and applying covalent organic framework (COF) thin films. Directly growing COF thin films on suitable substrates is essential to ensure structural integrity, optimal performance, and strong film–substrate adhesion. The versatility of COF thin films, with properties such as high crystallinity, tunable pore sizes and customizable functional groups, has made them invaluable for separation processes, energy storage, and electronic and optoelectronic device applications. We have highlighted various strategies for synthesizing COF thin films on diverse substrates, including metals, metal oxides, glass, and polymers, and discussed the benefits and challenges associated with each method. This review also explores the critical parameters influencing film quality, such as reaction rate control, film thickness, and substrate compatibility, which are pivotal for advancing COF thin-film technologies. Moreover, developing a cladding technique to armour biocatalysts with COF membranes marks a significant breakthrough. This approach offers a sustainable solution to the instability of biocatalysts in non-aqueous environments, ensuring robust performance and preventing leaching while preserving the catalytic function. By extending the COF cladding technique to biological substrates, this method can revolutionize biocatalytic processes in both aqueous and non-aqueous media. This review provides a comprehensive understanding of COF thin film fabrication, highlighting innovative strategies to enhance film quality and functionality across various applications. The insights presented here pave the way for future research and development in COF thin films, offering tailored solutions for next-generation industrial and technological advancements.Author contributions
Ashok Kumar Mahato: conceptualization, writing – original draft, writing – reviewing and editing; Satyadip Paul: global checking; and Rahul Banerjee: conceptualization, writing – reviewing and editing.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
A. K. M. and S. P. acknowledge CSIR for Research fellowship. R. B. acknowledges SERB SUPRA (SPR/2021/000020) for funding.References
- A. W. Feinberg, A. Feigel, S. S. Shevkoplyas, S. Sheehy, G. M. Whitesides and K. K. Parker, Science, 2007, 317, 1366–1370 CrossRef CAS PubMed.
- D. Gao, B. Li, Q. Liu, C. Zhang, Z. Yu, S. Li, J. Gong, L. Qian, F. Vanin, K. Schutt, M. A. Davis, A. F. Palmstrom, S. P. Harvey, N. J. Long, J. M. Luther, X. C. Zeng and Z. Zhu, Science, 2024, 386, 187–192 CrossRef CAS PubMed.
- L. Ge, H. Song, J. Zhu, Y. Zhang, Z. Zhou and B. Van der Bruggen, J. Mater. Chem. A, 2024, 12, 7975–8013 RSC.
- A. Gumyusenge, D. T. Tran, X. Luo, G. M. Pitch, Y. Zhao, K. A. Jenkins, T. J. Dunn, A. L. Ayzner, B. M. Savoie and J. Mei, Science, 2018, 362, 1131–1134 CrossRef CAS PubMed.
- K. Hikima, K. Shimizu, H. Kiuchi, Y. Hinuma, K. Suzuki, M. Hirayama, E. Matsubara and R. Kanno, J. Am. Chem. Soc., 2022, 144, 236–247 CrossRef CAS PubMed.
- X. Luo, M. Zhang, Y. Hu, Y. Xu, H. Zhou, Z. Xu, Y. Hao, S. Chen, S. Chen, Y. Luo, Y. Lin and J. Zhao, Science, 2024, 385, 647–651 CrossRef CAS PubMed.
- M. Sandru, E. M. Sandru, W. F. Ingram, J. Deng, P. M. Stenstad, L. Deng and R. J. Spontak, Science, 2022, 376, 90–94 CrossRef CAS PubMed.
- H. Tsai, F. Liu, S. Shrestha, K. Fernando, S. Tretiak, B. Scott, D. T. Vo, J. Strzalka and W. Nie, Sci. Adv., 2020, 6, eaay0815 CrossRef CAS PubMed.
- Z. Zhang, Y. Li, R. Xu, W. Zhou, Y. Li, S. T. Oyakhire, Y. Wu, J. Xu, H. Wang, Z. Yu, D. T. Boyle, W. Huang, Y. Ye, H. Chen, J. Wan, Z. Bao, W. Chiu and Y. Cui, Science, 2022, 375, 66–70 CrossRef CAS PubMed.
- A. P. Côté, A. I. Benin, N. W. Ockwig, M. O’Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef PubMed.
- C. S. Diercks and O. M. Yaghi, Science, 2017, 355, eaal1585 CrossRef CAS PubMed; L. Ge, H. Song, J. Zhu, Y. Zhang, Z. Zhou and B. Van der Bruggen, J. Mater. Chem. A, 2024, 12, 7975–8013 RSC.
- K. Geng, T. He, R. Liu, S. Dalapati, K. T. Tan, Z. Li, S. Tao, Y. Gong, Q. Jiang and D. Jiang, Chem. Rev., 2020, 120, 8814–8933 CrossRef CAS PubMed.
- S. Kandambeth, K. Dey and R. Banerjee, J. Am. Chem. Soc., 2019, 141, 1807–1822 CrossRef CAS PubMed.
- P. Shao, J. Li, F. Chen, L. Ma, Q. Li, M. Zhang, J. Zhou, A. Yin, X. Feng and B. Wang, Angew. Chem., Int. Ed., 2018, 57, 16501–16505 CrossRef CAS PubMed.
- H. Wang, Z. Zeng, P. Xu, L. Li, G. Zeng, R. Xiao, Z. Tang, D. Huang, L. Tang, C. Lai, D. Jiang, Y. Liu, H. Yi, L. Qin, S. Ye, X. Ren and W. Tang, Chem. Soc. Rev., 2019, 48, 488–516 RSC.
- X. Zhao, P. Pachfule and A. Thomas, Chem. Soc. Rev., 2021, 50, 6871–6913 RSC.
- L. K. Beagle, Q. Fang, L. D. Tran, L. A. Baldwin, C. Muratore, J. Lou and N. R. Glavin, Mater. Today, 2021, 51, 427–448 CrossRef CAS.
- S. Kandambeth, B. P. Biswal, H. D. Chaudhari, K. C. Rout, S. Kunjattu H, S. Mitra, S. Karak, A. Das, R. Mukherjee, U. K. Kharul and R. Banerjee, Adv. Mater., 2017, 29, 1603945 CrossRef PubMed.
- H. S. Sasmal, A. Kumar Mahato, P. Majumder and R. Banerjee, J. Am. Chem. Soc., 2022, 144, 11482–11498 CrossRef CAS PubMed.
- S. Bag, H. S. Sasmal, S. P. Chaudhary, K. Dey, D. Blätte, R. Guntermann, Y. Zhang, M. Položij, A. Kuc, A. Shelke, R. K. Vijayaraghavan, T. G. Ajithkumar, S. Bhattacharyya, T. Heine, T. Bein and R. Banerjee, J. Am. Chem. Soc., 2023, 145, 1649–1659 CrossRef CAS PubMed.
- J. I. Feldblyum, C. H. McCreery, S. C. Andrews, T. Kurosawa, E. J. G. Santos, V. Duong, L. Fang, A. L. Ayzner and Z. Bao, Chem. Commun., 2015, 51, 13894–13897 Search PubMed.
- A. Gangopadhyay, B. J. Nablo, M. V. Rao and D. R. Reyes, Adv. Eng. Mater., 2017, 19, 1600592 CrossRef PubMed.
- P. Sarkar, C. Wu, Z. Yang and C. Y. Tang, Chem. Soc. Rev., 2024, 53, 4374–4399 RSC.
- H. Wang, B. He, F. Liu, C. Stevens, M. A. Brady, S. Cai, C. Wang, T. P. Russell, T.-W. Tan and Y. Liu, J. Mater. Chem. C, 2017, 5, 5090–5095 RSC.
- D. Hou, G. Dai, J. Wang, H. Fan, L. Zhang and Z. Luan, Sep. Purif. Technol., 2012, 101, 1–10 CrossRef CAS.
- A. Kumar Mahato, S. Bag, H. S. Sasmal, K. Dey, I. Giri, M. Linares-Moreau, C. Carbonell, P. Falcaro, E. B. Gowd, R. K. Vijayaraghavan and R. Banerjee, J. Am. Chem. Soc., 2021, 143, 20916–20926 CrossRef CAS PubMed.
- A. Kumar Mahato, S. Pal, K. Dey, A. Reja, S. Paul, A. Shelke, T. G. Ajithkumar, D. Das and R. Banerjee, J. Am. Chem. Soc., 2023, 145, 12793–12801 CrossRef CAS PubMed.
- S. Hameed, A. Hayat, E. A. Alghamdi, N. Bano, F. Jubeen and A. A. Khurram, J. Sci.: Adv. Mater. Devices, 2023, 8, 100632 CAS.
- Y. Jin, Y. Hu, M. Ortiz, S. Huang, Y. Ge and W. Zhang, Chem. Soc. Rev., 2020, 49, 4637–4666 RSC.
- X. Fan, S. Zhai, S. Xue and L. Zhi, ACS Appl. Mater. Interfaces, 2024, 16, 40371–40390 CrossRef CAS PubMed.
- R. Gao, X. Kou, S. Huang, G. Chen and G. Ouyang, ChemBioChem, 2024, 25, e202400339 CrossRef CAS PubMed.
- S. Paul, M. Gupta, K. Dey, A. K. Mahato, S. Bag, A. Torris, E. B. Gowd, H. Sajid, M. A. Addicoat, S. Datta and R. Banerjee, Chem. Sci., 2023, 14, 6643–6653 RSC.
- S. Paul, M. Gupta, A. Kumar Mahato, S. Karak, A. Basak, S. Datta and R. Banerjee, J. Am. Chem. Soc., 2024, 146, 858–867 CrossRef CAS PubMed.
- D. Zheng, Y. Zheng, J. Tan, Z. Zhang, H. Huang and Y. Chen, Nat. Commun., 2024, 15, 5510 CrossRef CAS PubMed.
- Q. Zhu, Y. Zheng, Z. Zhang and Y. Chen, Nat. Protoc., 2023, 18, 3080–3125 CrossRef CAS PubMed.
- H. Yan, X. Miao, J. Xu, G. Pan, Y. Zhang, Y. Shi, M. Guo and Y. Liu, J. Membr. Sci., 2015, 475, 504–510 CrossRef CAS.
- J. Li, J.-L. Gong, G.-M. Zeng, B. Song, W.-C. Cao, S.-Y. Fang, S.-Q. Tang, Y. Guan, Z.-K. Tan, Z.-P. Chen, X.-Q. Mao and R.-L. Zhu, Sep. Purif. Technol., 2021, 276, 119352 CrossRef CAS.
- S. Peng, Y.-X. Fang, Z.-L. Xu, R. Jia, R. Han, L. Yu and S.-J. Xu, Sep. Purif. Technol., 2025, 355, 129633 CrossRef CAS.
- H. Herman, R. C. T. Slade and J. R. Varcoe, J. Membr. Sci., 2003, 218, 147–163 CrossRef CAS.
- A. A. Oun, G. H. Shin, J.-W. Rhim and J. T. Kim, Food Packag. Shelf Life, 2022, 34, 100991 CrossRef CAS.
- N. Zhang, Z. Huang, N. Yang, L. Zhang, B. Jiang, Y. Sun and J. Ma, Sep. Purif. Technol., 2020, 232, 115964 CrossRef.
- Y. Lu, Z. Wang, W. Fang, Y. Zhu, Y. Zhang and J. Jin, Ind. Eng. Chem. Res., 2020, 59, 22533–22540 CrossRef CAS.
- D. C. Borrelli, S. Lee and K. K. Gleason, J. Mater. Chem. C, 2014, 2, 7223–7231 RSC.
- M.-K. Dai, J.-T. Lian, T.-Y. Lin, M.-C. Chen, Y. Tai and Y.-F. Chen, Org. Electron., 2012, 13, 2229–2234 CrossRef CAS.
- P. Liu, Y. Wu, H. Pan, B. S. Ong and S. Zhu, Macromolecules, 2010, 43, 6368–6373 CrossRef CAS.
- M.-T. Lee, J.-K. Chang, Y.-T. Hsieh, W.-T. Tsai and C.-K. Lin, J. Solid State Electrochem., 2010, 14, 1697–1703 CrossRef CAS.
- M. Nakayama, A. Tanaka, Y. Sato, T. Tonosaki and K. Ogura, Langmuir, 2005, 21, 5907–5913 CrossRef CAS PubMed.
- M. S. Çögenli, S. Mukerjee and A. B. Yurtcan, Fuel Cells, 2015, 15, 288–297 CrossRef.
- S. Cuynet, A. Caillard, T. Lecas, J. Bigarré, P. Buvat and P. Brault, J. Phys. D: Appl. Phys., 2014, 47, 272001 CrossRef.
- M. M. Rahman, K. Inaba, G. Batnyagt, M. Saikawa, Y. Kato, R. Awata, B. Delgertsetsega, Y. Kaneta, K. Higashi, T. Uruga, Y. Iwasawa, K. Ui and T. Takeguchi, RSC Adv., 2021, 11, 20601–20611 RSC.
- Y.-J. Wang, N. Zhao, B. Fang, H. Li, X. T. Bi and H. Wang, Chem. Rev., 2015, 115, 3433–3467 CrossRef CAS PubMed.
- K. Liang, Y. Wang, S. Shao, M. Luo, V. Pecunia, L. Shao, J. Zhao, Z. Chen, L. Mo and Z. Cui, J. Mater. Chem. C, 2019, 7, 6169–6177 RSC.
- B. K. Yap, Z. Zhang, G. S. H. Thien, K.-Y. Chan and C. Y. Tan, Appl. Surf. Sci., 2023, 16, 100423 CrossRef.
- Z. Chen, D. Han, X. Zhang and Y. Wang, Sci. Rep., 2019, 9, 17175 CrossRef PubMed.
- Y. Zhao, Z. Wang, G. Xu, L. Cai, T.-H. Han, A. Zhang, Q. Wu, R. Wang, T. Huang, P. Cheng, S.-Y. Chang, D. Bao, Z. Zhao, M. Wang, Y. Huang and Y. Yang, Adv. Funct. Mater., 2020, 30, 2003285 CrossRef CAS.
- Y. Zhu, Y. He, S. Jiang, L. Zhu, C. Chen and Q. Wan, J. Semicond., 2021, 42, 031101 CrossRef CAS.
- J. Park, S. Go, W. Chae, C. I. Ryoo, C. Kim, H. Noh, S. Kim, B. Du Ahn, I.-T. Cho, P. S. Yun, J. U. Bae, Y. S. Park, S. Kim and D. H. Kim, Sci. Rep., 2024, 14, 10067 CrossRef CAS PubMed.
- Y. Shin, S. T. Kim, K. Kim, M. Y. Kim, S. Oh and J. K. Jeong, Sci. Rep., 2017, 7, 10885 CrossRef PubMed.
- K. Banerjee, A. Roy, S. Ghosh, H. R. Inta, A. Mondal, S. Ghosh, A. Mitra, A. K. Mahato and V. Mahalingam, ACS Appl. Energy Mater., 2024, 7, 7745–7758 CrossRef CAS.
- G. Fang, J. Zhou, A. Pan and S. Liang, ACS Energy Lett., 2018, 3, 2480–2501 CrossRef CAS.
- X. Jia, C. Liu, Z. G. Neale, J. Yang and G. Cao, Chem. Rev., 2020, 120, 7795–7866 CrossRef CAS PubMed.
- A. Khayum M, M. Ghosh, V. Vijayakumar, A. Halder, M. Nurhuda, S. Kumar, M. Addicoat, S. Kurungot and R. Banerjee, Chem. Sci., 2019, 10, 8889–8894 RSC.
- W. Kim, C. Hwang, Y. M. Kim, J.-S. Yu, Y.-J. Kim, K. J. Kim and H.-S. Kim, J. Mater. Chem. A, 2024, 12, 14786–14791 RSC.
- A. Manthiram, ACS Cent. Sci., 2017, 3, 1063–1069 CrossRef CAS PubMed.
- A. Mondal, H. R. Inta, A. Roy, A. Kumar Mahato and V. Mahalingam, ACS Appl. Nano Mater., 2023, 6, 12040–12049 CrossRef CAS.
- G. Tudu, S. Ghosh, H. R. Inta, H. V. S. R. M. Koppisetti, S. Ganguli, A. K. Mahato and V. Mahalingam, ChemNanoMat, 2022, 8, e202200199 CrossRef CAS.
- P. Vanaphuti, K. Scanlan and A. Manthiram, RSC Sustainability, 2024, 2, 1969–1978 RSC.
- B. Zhang, X. Wang, S. Wang, Y. Li, L. Chen, H. Jiao, Z. Yu and J. Tu, J. Energy Chem., 2025, 100, 1–17 CrossRef CAS.
- Z. Duan, Y. Wu, J. Lin, L. Wang and D.-L. Peng, Energies, 2022, 15, 8980 CrossRef CAS.
- A. O’Donoghue, M. Shine, I. M. Povey and J. F. Rohan, Int. J. Mol. Sci., 2023, 24, 11207 CrossRef PubMed.
- N. An, F. Zhang, Z. Hu, Z. Li, L. Li, Y. Yang, B. Guo and Z. Lei, RSC Adv., 2015, 5, 23942–23951 RSC.
- N. J. Hestand and F. C. Spano, Acc. Chem. Res., 2017, 50, 341–350 CrossRef CAS PubMed.
- T.-H. Chao, M.-J. Chang, M. Watanabe, M.-H. Luo, Y. J. Chang, T.-C. Fang, K.-Y. Chen and T. J. Chow, Chem. Commun., 2012, 48, 6148–6150 RSC.
- R. A. Jonson, V. S. Battaglia and M. C. Tucker, ACS Appl. Energy Mater., 2023, 6, 745–752 CrossRef CAS.
- E. J. Son, J. H. Kim, K. Kim and C. B. Park, J. Mater. Chem. A, 2016, 4, 11179–11202 RSC.
- N. An, F. Zhang, Z. Hu, Z. Li, L. Li, Y. Yang, B. Guo and Z. Lei, RSC Adv., 2015, 5, 23942–23951 RSC.
- K. M. Blackwood, Science, 1996, 273, 909–912 CrossRef CAS PubMed.
- A. K. Rylski, H. L. Cater, K. S. Mason, M. J. Allen, A. J. Arrowood, B. D. Freeman, G. E. Sanoja and Z. A. Page, Science, 2022, 378, 211–215 CrossRef CAS PubMed.
- Y. Zhang, K. Zhou, S. Su, J. Gao, J. Liu and L. Jiang, Sci. Adv., 2024, 10, eadm9620 CrossRef CAS PubMed.
- J. Liu, G. Han, D. Zhao, K. Lu, J. Gao and T.-S. Chung, Sci. Adv., 2020, 6, eabb1110 CrossRef CAS PubMed.
- Q.-W. Meng, S. Wu, M. Liu, Q. Guo, W. Xian, X. Zuo, S. Wang, H. Yin, S. Ma and Q. Sun, Sci. Adv., 2023, 9, eadh0207 CrossRef CAS PubMed.
- Q. Zhang, S. Dong, P. Shao, Y. Zhu, Z. Mu, D. Sheng, T. Zhang, X. Jiang, R. Shao, Z. Ren, J. Xie, X. Feng and B. Wang, Science, 2022, 378, 181–186 CrossRef CAS PubMed.
- E. Zhou, X. Zhang, L. Zhu, E. Chai, J. Chen, J. Li, D. Yuan, L. Kang, Q. Sun and Y. Wang, Sci. Adv., 2024, 10, eadk8564 CrossRef CAS PubMed.
- X. Wang, M. Liu, Y. Liu, S. Shang, C. Du, J. Hong, W. Gao, C. Hua, H. Xu, Z. You, J. Chen and Y. Liu, J. Am. Chem. Soc., 2023, 145, 26900–26907 CrossRef CAS PubMed.
- D. Zhu, Y. Zhu, Y. Chen, Q. Yan, H. Wu, C.-Y. Liu, X. Wang, L. B. Alemany, G. Gao, T. P. Senftle, Y. Peng, X. Wu and R. Verduzco, Nat. Commun., 2023, 14, 2865 CrossRef CAS PubMed.
- C. R. DeBlase, K. E. Silberstein, T.-T. Truong, H. D. Abruña and W. R. Dichtel, J. Am. Chem. Soc., 2013, 135, 16821–16824 CrossRef CAS PubMed.
- L. K. Beagle, Q. Fang, L. D. Tran, L. A. Baldwin, C. Muratore, J. Lou and N. R. Glavin, Mater. Today, 2021, 51, 427–448 CrossRef CAS.
- J. Fan, Z. Wang, H. Cheng, D. Wang and A. T. Wee, Electron. Mater., 2023, 4, 49–61 CrossRef.
- I. Gadwal, G. Sheng, R. L. Thankamony, Y. Liu, H. Li and Z. Lai, ACS Appl. Mater. Interfaces, 2018, 10, 12295–12299 CrossRef CAS PubMed.
- T. Kim, J. Oh, S. C. Kim, J.-G. Ahn, S. Kim, Y. Y. Kim and H. Lim, Small Methods, 2024, 2400063 CrossRef CAS PubMed.
- S. Park, Z. Liao, B. Ibarlucea, H. Qi, H.-H. Lin, D. Becker, J. Melidonie, T. Zhang, H. Sahabudeen, L. Baraban, C.-K. Baek, Z. Zheng, E. Zschech, A. Fery, T. Heine, U. Kaiser, G. Cuniberti, R. Dong and X. Feng, Angew. Chem., Int. Ed., 2020, 59, 8218–8224 CrossRef CAS PubMed.
- D. B. Shinde, L. Cao, A. D. D. Wonanke, X. Li, S. Kumar, X. Liu, M. N. Hedhili, A.-H. Emwas, M. Addicoat, K.-W. Huang and Z. Lai, Chem. Sci., 2020, 11, 5434–5440 RSC.
- L. A. Baldwin, J. W. Crowe, D. A. Pyles and P. L. McGrier, J. Am. Chem. Soc., 2016, 138, 15134–15137 CrossRef CAS PubMed.
- G. Chen, T. Wang, G. Zhang, G. Liu and W. Jin, Adv. Membr., 2022, 2, 100025 CrossRef.
- C. Gong, H. Wang, G. Sheng, X. Wang, X. Xu, J. Wang, X. Miao, Y. Liu, Y. Zhang, F. Dai, L. Chen, N. Li, G. Xu, J. Jia, Y. Zhu and Y. Peng, Angew. Chem., Int. Ed., 2022, 61, e202204899 CrossRef CAS PubMed.
- B. Gui, X. Liu, Y. Cheng, Y. Zhang, P. Chen, M. He, J. Sun and C. Wang, Angew. Chem., Int. Ed., 2022, 61, e202113852 CrossRef CAS PubMed.
- G. Lin, H. Ding, D. Yuan, B. Wang and C. Wang, J. Am. Chem. Soc., 2016, 138, 3302–3305 CrossRef CAS PubMed.
- Y. Qu, Y. Liu, H. Jia, S. Xu, M. Zhang, T. Xiao and X. Du, J. Environ. Chem. Eng., 2024, 12, 112711 CrossRef CAS.
- K. Sheng, J. Zhu, L. Ge, J. Du, T. Luo, L. Jiang, W. Jing, S.-P. Sun, Y. Zhang and B. Van der Bruggen, Adv. Membr., 2024, 4, 100109 CrossRef.
- Z. Chen, J. Wang, M. Hao, Y. Xie, X. Liu, H. Yang, G. I. N. Waterhouse, X. Wang and S. Ma, Nat. Commun., 2023, 14, 1106 CrossRef CAS PubMed.
- A. Jati, A. K. Mahato, D. Chanda, P. Kumar, R. Banerjee and B. Maji, J. Am. Chem. Soc., 2024, 146, 23923–23932 CrossRef CAS PubMed.
- T. Skorjanc, D. Shetty, M. E. Mahmoud, F. Gándara, J. I. Martinez, A. K. Mohammed, S. Boutros, A. Merhi, E. O. Shehayeb, C. A. Sharabati, P. Damacet, J. Raya, S. Gardonio, M. Hmadeh, B. R. Kaafarani and A. Trabolsi, ACS Appl. Mater. Interfaces, 2022, 14, 2015–2022 CrossRef CAS PubMed.
- L. Stegbauer, K. Schwinghammer and B. V. Lotsch, Chem. Sci., 2014, 5, 2789–2793 RSC.
- D. B. Shinde, G. Sheng, X. Li, M. Ostwal, A. H. Emwas, K. W. Huang and Z. Lai, J. Am. Chem. Soc., 2018, 140, 14342–14349 CrossRef CAS PubMed.
- H. Fan, A. Mundstock, J. Gu, H. Meng and J. Caro, J. Mater. Chem. A, 2018, 6, 16849 RSC.
- H. Fan, A. Mundstock, A. Feldhoff, A. Knebel, J. Gu, H. Meng and J. Caro, J. Am. Chem. Soc., 2018, 140, 10094–10098 CrossRef CAS PubMed.
- J. W. Colson, A. R. Woll, A. Mukherjee, M. P. Levendorf, E. L. Spitler, V. B. Shields, M. G. Spencer, J. Park and W. R. Dichtel, Science, 2011, 332, 228–231 CrossRef CAS PubMed.
- D. D. Medina, V. Werner, F. Auras, R. Tautz, M. Dogru, J. Schuster, S. Linke, M. Döblinger, J. Feldmann, P. Knochel and T. Bein, ACS Nano, 2014, 8, 4042–4052 CrossRef CAS PubMed.
- D. D. Medina, J. M. Rotter, Y. Hu, M. Dogru, V. Werner, F. Auras, J. T. Markiewicz, P. Knochel and T. Bein, J. Am. Chem. Soc., 2015, 137, 1016–1019 CrossRef CAS PubMed.
- J. M. Rotter, S. Weinberger, J. Kampmann, T. Sick, M. Shalom, T. Bein and D. D. Medina, Chem. Mater., 2019, 31, 10008–10016 CrossRef CAS.
- B. J. Smith, L. R. Parent, A. C. Overholts, P. A. Beaucage, R. P. Bisbey, A. D. Chavez, N. Hwang, C. Park, A. M. Evans, N. C. Gianneschi and W. R. Dichtel, ACS Cent. Sci., 2017, 3, 58–65 CrossRef CAS PubMed.
- C. R. DeBlase, K. Herndez-Burgos, K. E. Silberstein, G. G. R. guez-Calero, R. P. Bisbey, H. D. Abruna and W. R. Dichtel, ACS Nano, 2015, 9(3), 3178–3183 CrossRef CAS PubMed.
- H. S. Sasmal, H. B. Aiyappa, S. N. Bhange, S. Karak, A. Halder, S. Kurungot and R. Banerjee, Angew. Chem., Int. Ed., 2018, 57(34), 10894–10898 CrossRef CAS PubMed.
- R. Wang, X. Shi, Z. Zhang, A. Xiao, S. Sun, Z. Cui and Y. Wang, J. Membr. Sci., 2019, 586, 274–280 CrossRef CAS.
- S. Karan, Z. Jiang and A. G. Livingston, Science, 2015, 348, 1347–1351 CrossRef CAS PubMed.
- F. Radmanesh, A. Tena, E. J. R. Sudhölter, M. A. Hempenius and N. E. Benes, ACS Appl. Polym. Mater., 2023, 5, 1955–1964 CrossRef CAS PubMed.
- Y. Song, J.-B. Fan and S. Wang, Mater. Chem. Front., 2017, 1, 1028–1040 RSC.
- F. Zhang, J.-b Fan and S. Wang, Angew. Chem., Int. Ed., 2020, 59, 21840–21856 CrossRef CAS PubMed.
- K. Dey, M. Pal, K. C. Rout, S. Kunjattu H, A. Das, R. Mukherjee, U. K. Kharul and R. Banerjee, J. Am. Chem. Soc., 2017, 139, 13083–13091 CrossRef CAS PubMed.
- S. Kim, H. Lim, J. Lee and H. C. Choi, Langmuir, 2018, 34, 8731–8738 CrossRef CAS PubMed.
- M. A. Khayum, S. Kandambeth, S. Mitra, S. B. Nair, A. Das, S. S. Nagane, R. Mukherjee and R. Banerjee, Angew. Chem., Int. Ed., 2016, 55, 15604–15608 CrossRef CAS PubMed.
- F. Shao, W. Dai, Y. Zhang, W. Zhang, A. D. Schlüter and R. Zenobi, ACS Nano, 2018, 12(5), 5021–5029 CrossRef CAS PubMed.
- K. Liu, H. Qi, R. Dong, R. Shivhare, M. Addicoat, T. Zhang, H. Sahabudeen, T. Heine, S. Mannsfeld, U. Kaiser, Z. Zheng and X. Feng, Nat. Chem., 2019, 11, 994–1000 CrossRef CAS PubMed.
- S. Wang, Q. Wang, P. Shao, Y. Han, X. Gao, L. Ma, S. Yuan, X. Ma, J. Zhou, X. Feng and B. Wang, J. Am. Chem. Soc., 2017, 139, 4258–4261 CrossRef CAS PubMed.
- A. Khayum M, M. Ghosh, V. Vijayakumar, A. Halder, M. Nurhuda, S. Kumar, M. Addicoat, S. Kurungot and R. Banerjee, Chem. Sci., 2019, 10, 8889–8894 RSC.
- A. Halder, M. Ghosh, A. Khayum M, S. Bera, M. Addicoat, H. S. Sasmal, S. Karak, S. Kurungot and R. Banerjee, J. Am. Chem. Soc., 2018, 140, 10941–10945 CrossRef CAS PubMed.
- C. Yin, Z. Li, D. Zhao, J. Yang, Y. Zhang, Y. Du and Y. Wang, ACS Nano, 2022, 16, 14178–14187 CrossRef CAS PubMed.
- Z. Zha, L. Xu, Z. Wang, X. Li, Q. Pan, P. Hu and S. Lei, ACS Appl. Mater. Interfaces, 2015, 7, 17837–17843 CrossRef CAS PubMed.
- Z. Zhao, R. Wang, C. Peng, W. Chen, T. Wu, B. Hu, W. Weng, Y. Yao, J. Zeng, Z. Chen, P. Liu, Y. Liu, G. Li, J. Guo, H. Lu and Z. Guo, Nat. Commun., 2021, 12, 6606 CrossRef CAS PubMed.
- J. I. Feldblyum, C. H. McCreery, S. C. Andrews, T. Kurosawa, E. J. G. Santos, V. Duong, L. Fang, A. L. Ayzner and Z. Bao, Chem. Commun., 2015, 51, 13894–13897 RSC.
- S. Kumar, N. Alqadhi, J. Hu and G. Szekely, J. Membr. Sci., 2024, 691, 122257 CrossRef CAS.
- S. Xua, H. Lina, G. Lia, J. Wanga, Q. Hana and F. Liu, Chem. Eng. J., 2022, 427, 132009 CrossRef.
- F. Xu, S. Zhao, J. Song, Y. Peng and B. Su, Membranes, 2024, 14, 234 CrossRef CAS PubMed.
- E. L. Spitler, B. T. Koo, J. L. Novotney, J. W. Colson, F. J. Uribe-Romo, G. D. Gutierrez, P. Clancy and W. R. Dichtel, J. Am. Chem. Soc., 2011, 133, 19416–19421 CrossRef CAS PubMed.
- C. Poelking, M. Tietze, C. Elschner, S. Olthof, D. Hertel, B. Baumeier, F. Wurthner, K. Meerholz, K. Leo and D. Andrienko, Nat. Mater., 2015, 14, 434–439 CrossRef CAS PubMed.
- T. Kim, J. Oh, S. C. Kim, J. Ahn, S. Kim, Y. Y. Kim and H. Lim, Small Methods, 2024, 8, 2400063 CrossRef CAS PubMed.
- R. P. Bisbey, C. R. DeBlase, B. J. Smith and W. R. Dichtel, J. Am. Chem. Soc., 2016, 138(36), 11433–11436 CrossRef CAS PubMed.
- H. Cheng, Y. Wei, J. Han, X. Wang, W. Ji and X. Jin, Process Biochem., 2022, 118, 317–322 CrossRef CAS.
- K. Niu, Y. Zhang, J. Chen and X. Lu, ACS Sens., 2022, 7, 3551–3559 CrossRef CAS PubMed.
- Y. Tang, W. Li, Y. Muhammad, S. Jiang, M. Huang, H. Zhang, Z. Zhao and Z. Zhao, Chem. Eng. J., 2021, 421, 129743 CrossRef CAS.
- H. Zhao, G. Liu, Y. Liu, X. Liu, H. Wang, H. Chen, J. Gao and Y. Jiang, ACS Appl. Mater. Interfaces, 2022, 14, 2881–2892 CrossRef CAS PubMed.
- Y. Zheng, S. Zhang, J. Guo, R. Shi, J. Yu, K. Li, N. Li, Z. Zhang and Y. Chen, Angew. Chem., Int. Ed., 2022, 61, e202208744 CrossRef CAS PubMed.
- S. Qiao, M. Li, J. Yu, S. Zhang, D. Yan, Z. Zhang and Y. Chen, Particuology, 2022, 64, 140–144 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |