 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
α-Ketoglutaric acid as a promising platform chemical for sustainable bio-based industries
Louis-Thibault J. D.
Opsommer†
 a,
Thomas
Schalck†
b,
Sasha
Yogiswara†
c,
Kevin J.
Verstrepen
*c,
Jan
Michiels
*b and
Bert F.
Sels
a,
Thomas
Schalck†
b,
Sasha
Yogiswara†
c,
Kevin J.
Verstrepen
*c,
Jan
Michiels
*b and
Bert F.
Sels
 *a
*a
aCentre for Sustainable Catalysis and Engineering (CSCE), Department of Microbial and Molecular Systems (M2S), KU Leuven, Celestijnenlaan 200F, Leuven, 3001, Belgium. E-mail: bert.sels@kuleuven.be
bVIB-KU Leuven Center for Microbiology & Centre of Microbial and Plant Genetics, Department of Microbial and Molecular Systems (M2S), KU Leuven, Kasteelpark Arenberg 20, Leuven, 3001, Belgium. E-mail: jan.michiels@kuleuven.be
cLaboratory for Systems Biology, VIB-KU Leuven Center for Microbiology & Laboratory for Genetics and Genomics, Department of Microbial and Molecular Systems (M2S), KU Leuven, Gaston Geenslaan 1, Leuven, 3001, Belgium. E-mail: kevin.verstrepen@kuleuven.be
First published on 1st September 2025
Abstract
The chemical industry is gradually shifting from fossil-derived resources to more sustainable bio-based processes. Natural bio-molecules such as succinic, lactic, and itaconic acid are promising platform chemicals for this green chemistry transition because they can be produced from biomass and converted into various products that are currently produced through fossil-based processes, or they can replace these fossil-based products. One specific bio-molecule, α-ketoglutaric acid (α-KGA), is particularly interesting because it can be directly applied in certain nutrition and healthcare applications, and also serves as a precursor for other commodity and fine chemicals. This review examines the unique chemical properties and application potential of α-KGA and summarises the current state-of-the-art in chemical synthesis and microbial production of α-KGA. Specifically, we discuss how recent advances in precision fermentation, microbial metabolic engineering, and downstream purification are opening new avenues towards sustainable α-KGA production from renewable feedstocks such as sugars, glycerol, fatty acids, alkanes, and alcohols, with titres reaching up to 195 g L−1 and productivity up to 1.75 g L−1 h−1. Finally, we critically assess the future potential and remaining challenges to implement a cost-competitive industrial bio-based α-KGA chemistry.
1. Introduction
During the 19th century Industrial Revolution, linear economies emerged that relied heavily on fossil resources to produce energy, fuels, and key chemicals. These relatively cheap and efficient fossil-based industries provided the goods and energy to support a rapidly growing global population. However, the finite reserves of fossil crude oil, coal, and natural gas – along with their severe environmental and climate impacts – necessitate a fundamental shift in industrial processes. The underlying issue is the emission of carbon dioxide (CO2)1 and other harmful substances2,3 (e.g., SOx, NOx, and volatile organic compounds) emitted by fossil-based industries, contributing to environmental degradation and public health problems. Therefore, replacing fossil-based feedstocks with renewable biomass that does not increase net atmospheric CO2 levels is generally considered a logical and crucial shift.As a result, exploiting biomass to produce value-added products has gained significant attention over the past decade.4–9 One of the most promising routes is using microorganisms as cell factories to produce chemical compounds or polymer precursors. Bacteria, yeasts, or fungi can be cultivated on sustainable resources, such as plant biomass or waste streams, while producing value-added metabolites that can be used directly or can serve as building blocks for further processing. Developing such processes is complex and interdisciplinary, involving synthetic–catalytic chemistry, biochemistry, and molecular (micro)biology.
Microbes catabolise biomass-derived carbohydrates, fats, and/or proteins, amino acids, and other nitrogen-containing compounds into cellular metabolites. Many of these metabolites can be applied directly in food, feed, fuel, pharmaceutical, or chemical sectors. This approach has been indispensable for centuries in the fermentation of ethanol in alcoholic beverages, acetic acid, and lactic acid. Recent advances in biotechnology, including genetic and evolutionary engineering and fermentation process control, have enabled rewiring of microbes' metabolism. As a result, living cells were reprogrammed as microbial cell factories, expediting the production of precise and valuable molecules, including drop-in biofuels (e.g., isoprene)10 and specific pharmaceuticals (e.g., insulin, vitamins, and vaccines).11–13 This strategy is called “precision fermentation,” hinting that it allows the production of specific, precise molecules with high yields and purity.
Innovative green chemistry methods can further refine fermentation-derived products into more advanced and diverse derivatives.14,15 For instance, microbial intermediate compounds like succinic or adipic acid have been explored as novel chemical building blocks through specific catalytic processes.8,16–21 Together, these dual biochemical and chemocatalytic approaches offer sustainable alternatives to traditional fossil-based manufacturing.
Popular guidelines of priority bio-based chemicals include the US Department of Energy's (DOE) 2004 and 2010 Platform Chemical Lists.22,23 This list contains chemicals that can be produced from biomass with strong commercial potential and environmental benefits. Considering both bio-production and catalytic valorisation, this list has been instrumental for guiding research priorities to advance bio-industries.
α-Ketoglutaric acid (α-KGA) is one such promising molecule that can be produced through precision fermentation for applications in nutrition, pharmaceuticals, and particularly in chemical manufacturing, where it can be catalytically converted into higher-value derivatives. Currently, α-KGA is produced de novo using chemical synthesis to meet an annual demand of 100 tonnes in the European Economic Area.24 However, as α-KGA is a key tricarboxylic acid cycle (TCAc) intermediate in living organisms, many studies have proposed microbial fermentation as a potential source of α-KGA.25–28
In this review, we provide a comprehensive overview of sustainable α-KGA production and its potential in various industrial applications. First, we discuss α-KGA's chemical structure and properties. Second, we outline the applications of α-KGA across traditional and emerging fields, ranging from medicine to chemistry. Third, we provide a complete overview of the production strategies of α-KGA through chemical synthesis, biocatalysis, and microbial fermentation. This includes a summary of the strategies for optimising fermentation conditions and strain engineering, as well as the production parameters, reported titres, and productivity. Lastly, we discuss the recovery methods of α-KGA from fermentation broths. In short, this review aims to highlight the immense potential of α-KGA in bio-based industries, even though it has not yet been included in DOE's priority bio-based platform chemical list.22,29
2. Structure, properties, and applications of α-KGA
2.1. Structure and properties
The molecular structure of α-KGA, also known as 2-oxopentanedioic acid or 2-oxoglutaric acid, consists of two carboxylic acids and a ketone moiety at the α-position. This conformation is linked to multiple favourable chemical properties and reactivity profiles. First, the position and nature of the functional groups are ideal for cross-linking with other molecules such as dialcohols or diamines, which enables the formation of polymeric networks that could lead to interesting novel materials. Second, the polar groups of α-KGA make it highly soluble in water and non-volatile, enabling high titres in bio-based processes (Table 1). Third, α-KGA exceeds water's boiling point, allowing easy separation from aqueous solutions. Finally, α-KGA does not show any toxic properties.30| Property (unit) | α-KGA |
|---|---|
| CAS | 328-50-7 |
| Molecular mass (g mol−1) | 146.1 |
| Melting point (°C) | 115 |
| Boiling point (°C) | 345.6 |
| Decomposition point (T10%, °C) | 173 |
| Solubility (25 °C, aqueous, unbuffered solution) | 1.9 M (278 g L−1) |
| pKa1 | 2.35 |
| pKa2 | 4.85 |
In aqueous solution, α-KGA can adopt multiple conformations in equilibrium, depending on the environmental pH. This concept was introduced in 1975 by Arthur Cooper and Alfred Redfield with a 1H-NMR study of α-ketoacids35 and has since been supported by experimental evidence. The main structure at neutral pH is the α-ketone over the α-geminal-diol that shifts towards the diol structure when the pH drops below pKa1 (cf.Table 1). Additionally, the α-ketone may convert into the cyclic lactol structure at an even lower pH due to the nucleophilic attack of the γ-carboxylic acid on the carbonyl group. Such intramolecular reaction is enhanced by the electron-withdrawing effect of the α-carboxylic acid group which is mostly present in acidic solutions. In basic solution, however, deprotonation of β-methylene occurs and results in an enolate conformation (Fig. 1). Besides the acidity, temperature and α-KGA concentration can also influence the equilibrium structure ratio. Eventually, the ratio lactol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) keto
keto![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diol was typically found to be 16
diol was typically found to be 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53 at pH 0.5 and 25 °C, although the values differ among reported studies.32,35–38 At room temperature and pH 7, α-KGA is mainly in the ketone form with neglectable lactol concentrations (keto
53 at pH 0.5 and 25 °C, although the values differ among reported studies.32,35–38 At room temperature and pH 7, α-KGA is mainly in the ketone form with neglectable lactol concentrations (keto![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diol 93
diol 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7).38
7).38
 | ||
| Fig. 1 The equilibrium molecular structures of α-KGA in accordance with the pH of the aqueous solution.32,35–38 | ||
Understanding the structure of α-KGA under different pH conditions could also clarify its reactivity when exploring new reaction schemes and products (cf. Section 2.2).
2.2. Applications
Ketoacids or oxo-carboxylic acids are a broad class of organic acids where the carbonyl group is positioned in the α, β, γ, or δ position relative to the carboxylic acid group. This wide range of keto acids originate from various metabolic pathways, and have applications in various industries, serving as food additives, flavours, feed, medicines, cosmetics, precursors for synthesis, and intermediates in fine chemistry.39 An extensive overview of the bio-based production and applications of these ketoacids (Fig. 2) has already been published in previous reviews.40–42 | ||
| Fig. 2 Overview of relevant ketoacids used in bio-based and chemical industries.40 | ||
The α- and γ-ketoacids (and their esters or salts) are relatively stable. The α-ketoacids, such as oxaloacetic acid, pyruvic acid, and α-KGA, often serve as metabolic links between the amino acid, carbohydrate, and fatty acid metabolic pathways in living organisms. In contrast, β-ketoacids are not often found in nature and are less chemically resistant when not esterified (i.e., susceptible to thermal decarboxylation). α-Ketoacids are relatively more stable to thermal decarboxylation (even at 100 °C in diluted HCl solution), although they can undergo catalytic (oxidative) decarboxylation. An example is the enzymatic conversion of α-KGA into succinic acid in living cells.37,39,43,44 Recent research has also shown the use of α-ketoacids in the synthesis of enolates, Strecker aldehydes, unsaturated carbonyls, pyridoxamines, pyrroles, amides, and furanones.45–53 Furthermore, the superelectrophilic activation of α-ketoacids upon condensation with weak (aromatic) nucleophiles has also been described for the synthesis of geminal-diphenyl compounds (e.g., 1-tetralone derivatives).54 Lastly, α-ketoacids may also act as green acylating agents in organic chemistry, in which only CO2 is released as the sole by-product.55 Because of their structure, α-ketoacids such as α-KGA or α-ketoadipic acid (α-KAA) offer the advantage of a more controlled reactivity compared to oxaloacetic acid, which easily decomposes into pyruvic acid and CO2 at 25 °C and pH 7, or mesoxalic acid, which readily forms its hydrate form. Thus, working with α-KGA reduces the need for very strict control of reaction conditions such as temperature and pH needed to reduce the decomposition of α-KGA or α-KAA.37,56
Numerous applications of α-KGA have been described, including the production of building blocks for polymers and the synthesis of more complex chemical compounds used as pharmaceuticals, food supplements or cosmetics. Each of these applications is discussed in more detail in the following sections.
| Monomer | Structure | Material | Applications | Ref. |
|---|---|---|---|---|
| Tri-alcohols |

|
Polyketoesters | Tissue engineering, drug delivery | 57 |
| Diethylene glycol |

|
Polyketoesters-oximes | Cell scaffolds, adhesive elastomers | 58 |
| OEG-BGE |

|
Polyesters | Thermoresponsive, degradable materials | 59 |
| Isosorbide |

|
Polyol (for PUA synthesis) | Thermally stable, UV-curable coatings | 60 |
| diTBA |

|
PAA | Dispersing agents, adsorbents, additives | 61 and 62 |
| HMDA |

|
Nylon | Nylon 66 substitution | 63 |
One such innovative biomaterial is poly(triol α-KGA) which is synthesised from the thermal condensation of α-KGA with triols like glycerol, 1,2,4-butanetriol, or 1,2,6-hexanetriol.57 The abundance of ketone groups in the polymer backbone allows for post-polymerisation modifications to expand the polymer's functionalities and to further modulate its mechanical properties and degradation rates.57 Owing to these features, this polyketoester holds potential for tissue engineering and drug delivery. It can also serve as a cell scaffolding system, when oxime linkers are integrated into the α-KGA-diethylene glycol copolymer structure.58 Finally, researchers succeeded in developing a novel type of thermoresponsive polyester that consists of hexa(ethylene glycol) or ethylene glycol-bis(glycidyl ether), two modified di-alcohols. The resulting material is especially suitable for medical applications as it spontaneously degrades over time through self-hydrolysis.59
Besides polyesters, α-KGA can also be incorporated into the backbone of polyurethane acrylate materials (PUAs). For example, a bio-based polyurethane was synthesised from hexamethylene diisocyanate and a novel polyol comprising α-KGA and isosorbide. The PUA eventually functioned as a thermally stable, UV-curable coating.60 Polyacrylic acids (PAAs) are another class of polymers in which α-KGA plays a key role. Di-tert-butyl acrylate (diTBA) was fabricated from α-KGA and subsequently polymerised into PAA, which served as a dispersing agent and adsorbent,61 or as a polymer additive for personal healthcare products and water treatments.62 More recently, α-KGA was investigated as a commercial building block together with glutaric acid in the melt polymerisation for bio-based nylon 56 and nylon 66 analogue blends. The resulting structures showed interesting thermal properties, including melt temperatures in a range close to that of commercial nylon 66 (250–300 °C).63
The utility of the carboxylic acid groups on α-KGA to form polyesters and polyamides has been well investigated. Future research could focus on exploring the chemical versatility of the ketone group, which could serve as an interesting moiety to explore degradability of novel materials by means of micro-organisms, enzymes, or catalytic hydrolysis.
Glutamic acid (Glu) is mainly produced from glucose via microbial fermentation using the bacteria Corynebacterium.64 Such fermentation processes, however, can have several drawbacks such as high energy and time requirements, low efficiency, and low purity of the product.65 Although Glu fermentation has been commercially established,66 researchers have investigated whether chemical transformations from the readily available α-KGA are also feasible (Table 3).
| Reaction | Reagent | Product | Catalyst | Ref. |
|---|---|---|---|---|
| Reductive amination (biocatalytic) | PXBr | Glu | Semi-synthetic enzyme (papain-PX) | 71 |
| NH4+HCOO− | D-Glu | E. coli (pFADA) | 68 | |
| NADH/NAD+ | ||||
| Urea | L-Glu | Artificial cells | 69 | |
| Dextran-NAD+ | Multienzyme system | |||
| NH4+HCOO− | D-Glu | AA aminotransferase | 70 | |
| NADH/NAD+ | Alanine racemase | |||
| L-Alanine dehydrogenase | ||||
| Formate dehydrogenase | ||||
| Catalytic | NH2OH | Glu | TiO2 | 65 |
| NH4Cl | Glu | FeS | 72 | |
| NH4Cl | Glu | ZnS | 73 | |
| Benzylamine | N-Benzyl-Glu | Rh complex | 74 | |
| Alkylation transamination | Cysteine sulfinic acid | (4R)-4-Methyl-L-Glu | LHMDS, lipases, transaminases | 75 |
| Transamination | Pyridoxamine | L-Glu | ALBP pyridoxamine cofactor (ALBP-PX) | 76–78 |
Glu can be made via reductive amination from α-KGA, catalyzed by the enzyme Glu dehydrogenase (GDH), with NADH cofactor as the electron donor67 by using re-engineered cells or their extracted enzymes68–70 in combination with various nitrogen sources (cf.Table 3). The reductive amination can also be performed using inorganic compounds as catalysts, such as TiO2, FeS, and ZnS.65,71–73 Glu-analogues can also be synthesized from α-KGA using benzylamine as a reagent and a Rh-based catalyst,74 or by means of a two-step alkylation-transaminase approach.75 The transamination of L-Glu can also occur using semi-synthetic enzymes.76–78 The β-cyclopropane analog of Glu, in turn, was synthesised from α-KGA and used in vitamin K-dependent carboxylase studies.79 Furthermore, the reductive amination of α-KGA has been frequently related to light-induced NADH-regeneration systems for enzymatic reactions.80–87
α-KGA is frequently used to synthesize benzimidazole-type compounds by reacting with o-phenylenediamines. These benzimidazoles serve as core intermediates or final products in the synthesis of potential anticancer agents.88–94 A typical example is bendamustine, a benzimidazole with a glutaric moiety, which has been used to tackle leukemia, multiple myeloma, and non-Hodgkin's lymphoma.88 2-alkylamino-1-aminobenzimidazoles in turn react with α-KGA to produce benzimidazolones.95 Similarly, α-KGA can also react with o-phenylenediamines to form quinoxalines, another class of heterocyclic components that have widespread therapeutical applications, such as to treat Chagas disease,96 HIV,97,98 cancer,98–102 and inflammation.103 Benzoxazinones constitute an alternative category of heterocycles that are formed upon reaction of α-KGA with o-aminophenols and display antibacterial104,105 or fluorescence activity.106
α-KGA also acts as a precursor for indoles, a group of compounds with promising therapeutic potential. Members of the indole class target diseases such as leukemia,107,108 gastroenterological cancer,109 and bone malignancies,110 and could serve as antitumour compounds.110,111 Indoles are also considered important intermediates for the synthesis of β-carbolinium salts, which were tested as antifungal agents112,113 and novel acetylcholinesterase inhibitors.114 Similar indole-type structures are involved in the synthesis of quinoline-6-alkanamides, known as melatonin analog drugs for various clinical applications.115 An overview of these compounds can be found in Fig. 3.
α-KGA can also be used as a building block for novel peptide structures. In this synthesis pathway, oximes are implemented to protect the ketoacid moiety in the so-called annulation reaction. The protected structure is subsequently used in chemoselective peptide synthesis in which a final deprotection step with Zn recovers the ketoacid moiety.116 These novel peptides could serve as biologically active compounds117–119 or for drug release strategies.120 Their novelty lies in preserving the ketoacid or ketoamide moiety along or at the end of the polymer chain. Another strategy to form the amide bond is the decarboxylative acylation of amines, for which tert-butyl hydroperoxide is typically used.121 The structure of α-KGA has a tendency to coordinate with metal centers. As such, it could serve as an ideal bidentate ligand in transition metal complexes (TMCs), based on Ni and Rh,122,123 that display antibacterial and antitumour properties.122 Moreover, α-KGA was also used for the synthesis of novel ligands in various TMCs124–126 with antioxidant characteristics.124
The carbonyl group of α-KGA readily reacts with hydrazine (H2N–NH2) to produce hydrazone, which subsequently converts into the versatile compound 1,4,5,6-tetrahydro-6-oxo-3-pyridazinecarboxylic acid (THOPCA).127 For instance, THOPCA was transformed into glutamine (Gln) as an alternative for the natural L-isomer using catalytic hydrogenation. In this two-step process, a 5% Pd/C catalyst in water was first used to hydrogenate the imine bond followed by hydrogenolysis of the N–N bond, or vice versa.128 Furthermore, THOPCA was applied as an intermediate for pyridazine-3-carboxamide or pyridazinone synthesis, two promising pharmaceuticals for various diseases.129–131 Lastly, THOPCA plays a key role in the fabrication of 3,3′-dipyridazinyl disulfide, a molecule that enables the elucidation of active site mechanisms in enzymes.132
α-KGA has also been used in combination with various hydrazines (R2N–NH2) to form novel hydrazones. For instance, isonicotinyl hydrazide can be applied to produce a Schiff base-type ligand, which can be used for organotin complexes in homogeneous catalysis.133 Similarly, methyl carbazate was employed to form (2-(methoxycarbonyl-hydrazono)-pentanedioic acid), a ligand for Ag, Co, and Zn complexes with antimicrobial and/or anticancer properties.134,135 Other closely related hydrazine structures are thiosemicarbazides that can react with α-KGA to form thiosemicarbazones. These sulphur-containing compounds are able to form a complex with metals, like Zn,136 Cu137 or rare-earth metals,138 either in an open or in a closed form, to fight leukemia or to develop contrasting compounds for MRI diagnosis. Analogously, cyclic isothiosemicarbazones were prepared from α-KGA as potential novel antibiotics.139
Another broad class of antibacterial agents encompasses the canthin-6-one analogs.140–143 These compounds are made in a multi-step synthesis starting from tryptamine, where α-KGA is employed in the so-called Pictet–Spengler condensation step.144 β-Carbolines are derived from similar condensation reactions of α-KGA with tryptamine-2-carboxylic acids and may serve as valuable pharmaceutical building blocks.145,146
α-KGA as a di-acid precursor can be combined with heterocyclic anilines to yield quinazoline derivatives. Such structures have a broad range of applications in medicine and pharmaceutics as biologically active compounds. Therefore, numerous synthesis procedures for a broad class of quinazolines have been published over the years.147–150 An overview of some of the earlier-mentioned compounds is given in Fig. 4.
 | ||
| Fig. 4 Synthesis routes from α-KGA (part 2). The α-KGA moiety is highlighted in blue, and the reagent for each reaction is provided in a box. | ||
When the decarboxylating enzymes MenD or SucA from Escherichia coli or Kgd from Mycobacterium tuberculosis are used, α-KGA is an ideal substrate for the biocatalytic two-step decarboxylation-addition reaction with aldehydes to produce vicinal-hydroxyketone adducts.151 The resulting hydroxyketone products, obtained in high enantiomeric excess, are important structures for pharmaceutical compounds.152–155 When pyruvic acid is used instead of aldehydes, the groups (i.e., ketone and alcohol) switch on the vicinal positions.156
In the pursuit of a synthetic route to produce industrially relevant α-hydroxy butyrolactones, the inclusion of α-KGA as a potential substrate has been considered. These lactone building blocks are prepared by mixing the α-keto acid with an olefin in the presence of a Lewis acid.157 When the so-called superacids like trifluoromethanesulfonic acid are used, α-KGA reacts with weak(er) nucleophiles such as benzene to form diphenyl 1-tetralone, owing to the superelectrophilic nature of protonated α-KGA.54 In addition, α-KGA plays a key role in the synthesis of lactivicin, a lactone-derived antibiotic.158 Furthermore, homocitric acid lactone can be created from α-KGA in a multistep process, yielding homologs for studying biological nitrogen fixation.159 α-KGA is also a precursor to produce 2-allyl-5-oxo-tetrahydrofuran-2-carboxylic acids, which can be further converted to form nonanes, which are present in natural products.160 Finally, chiral 5-oxo-tetrahydrofuran-2-carboxylic acids can be obtained from the hydrogenation reaction of α-KGA using a Pt/alumina catalyst with cinchona alkaloid modifiers. The resulting lactones are in turn used as chiral building blocks or derivatisation agents.161 An overview of the adducts and lactones is given in Fig. 5.
Lastly, α-KGA is also involved in the synthesis of various other compounds, spanning a wide range of applications, including fluorescent162,163 and enzyme activity probes,164 blue pigments,165 the dihydro-2H-pyran-3(4H)-one chemical precursor,166 the vitamin menaquinone,167,168 the 2-hydroxy-3-oxoadipate metabolite,169 thioacetal antidotes,170 biologically active isoxazolylpyrrolones,171 enzyme-inhibiting sulfoxide analogs,172 thiadiazole β-peptides,173 hydantoin-derivatized pharmaceuticals,174 and finally, antitumour agents, such as hadacidin analogs,175 isoxazoleacetic acid,176 and thiazolidinones.177
In this section, we provided extensive insights into the potential of α-KGA as a substrate or intermediate in chemical synthesis. The (m)ethyl esters of α-KGA have also been discussed in the same manner in a recent review paper.42
Treating patients with α-KGA has been demonstrated to improve (muscle) recovery after invasive traumas, such as surgical interventions.183–185 Studies have shown that administering α-KGA during heart surgeries can prevent muscle degradation and ensure proper blood and oxygen flow to vital organs such as the heart and kidneys. This intervention decreases the likelihood of heart or kidney dysfunction following surgery.186–189 Although these reports suggest that supplementing α-KGA improves patient recovery and surgical outcomes, this practice is not yet applied in standard clinical procedures due to the lack of large-scale supporting evidence. In addition, a recent study corroborated that (dietary) α-KGA suppresses blood clots, also called thrombosis (Fig. 6,  f).190 In this respect, administration of α-KGA to type 2 diabetes (T2D) patients makes sense as they often suffer from thromboinflammation (Fig. 6,
f).190 In this respect, administration of α-KGA to type 2 diabetes (T2D) patients makes sense as they often suffer from thromboinflammation (Fig. 6,  d and f) that may cause organ damage, pneumonia, asthma, and fibrosis.191 Besides ameliorating cardiovascular conditions associated with diabetes, α-KGA directly prevents obesity in T2D by improving glucose homeostasis through lowering blood glucose levels, suppressing hepatic gluconeogenesis, and stimulating insulin secretion.192 Next, α-KGA has been suggested to be effective in treating bone, breast, and skin cancer.193–195 α-KGA inhibits the proliferation of malicious cancer cells by inducing cell death,194 attenuating tumour-induced blood vessel growth – as a result of reduced levels of erythropoietin and growth factors (e.g., HIF-1 and VEGF)196 – and by suppressing tumour cell migration (metastasis) (Fig. 6,
d and f) that may cause organ damage, pneumonia, asthma, and fibrosis.191 Besides ameliorating cardiovascular conditions associated with diabetes, α-KGA directly prevents obesity in T2D by improving glucose homeostasis through lowering blood glucose levels, suppressing hepatic gluconeogenesis, and stimulating insulin secretion.192 Next, α-KGA has been suggested to be effective in treating bone, breast, and skin cancer.193–195 α-KGA inhibits the proliferation of malicious cancer cells by inducing cell death,194 attenuating tumour-induced blood vessel growth – as a result of reduced levels of erythropoietin and growth factors (e.g., HIF-1 and VEGF)196 – and by suppressing tumour cell migration (metastasis) (Fig. 6, 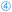 b).193 Consequently, combining α-KGA with other cancer treatments significantly improves the efficacy of anticancer drugs, such as 5-fluorouracil, and immunotherapy.195,196 Similarly, an anti-cancer mixture, composed of B87 and dimethyl-α-KGA, has been proposed to kill tumour cells by shutting down respiration and glycolysis simultaneously.197 Moreover, α-KGA could alleviate osteopenia, a condition that weakens skeletal bones, by concurrently inhibiting degradation and stimulating mineralisation of bone tissue.198 Finally, α-KGA can act as an antidote for cyanides, a toxin with detrimental effects on the liver, kidneys, and nervous system, by rapidly forming a complex with the cyanide moiety called cyanohydrin.30,199–202 As such, α-KGA has been shown to mitigate the toxic effects of sodium nitroprusside in fruit flies.203 Lastly, α-KGA also serves as a biomarker for the diagnosis of hyperinsulinism-hyperammonemia syndrome204 and Rey's syndrome.205
b).193 Consequently, combining α-KGA with other cancer treatments significantly improves the efficacy of anticancer drugs, such as 5-fluorouracil, and immunotherapy.195,196 Similarly, an anti-cancer mixture, composed of B87 and dimethyl-α-KGA, has been proposed to kill tumour cells by shutting down respiration and glycolysis simultaneously.197 Moreover, α-KGA could alleviate osteopenia, a condition that weakens skeletal bones, by concurrently inhibiting degradation and stimulating mineralisation of bone tissue.198 Finally, α-KGA can act as an antidote for cyanides, a toxin with detrimental effects on the liver, kidneys, and nervous system, by rapidly forming a complex with the cyanide moiety called cyanohydrin.30,199–202 As such, α-KGA has been shown to mitigate the toxic effects of sodium nitroprusside in fruit flies.203 Lastly, α-KGA also serves as a biomarker for the diagnosis of hyperinsulinism-hyperammonemia syndrome204 and Rey's syndrome.205
 | ||
| Fig. 6 The application of α-KGA in the healthcare context. α-KGA can bind to chromaffin cells to stimulate the release of epinephrine (1), which, in turn, promotes muscle growth (2a) and lipolysis of adipose tissue (2b). α-KGA drives OGDD-mediated hydroxylation of cytosine base pairs (3a), demethylation of lysine residues at histones (3b), and hydroxylation of key transcription factors (such as Akt and HIF1α) (3c). Since these nucleotide or protein targets of OGDDs are implied in multiple disease states, α-KGA has an indirect, profound impact on stem cell development (4a), carcinogenesis and metastasis (4b), inflammation (4d), the outgrowth and morphology of the vasculature (4e), and blood clot formation (i.e., thrombosis, 4f). Lastly, α-KGA may serve as an antioxidant (5) and, therefore, prolongs the lifespan in (model) organisms (6a) and attenuates inflammation responses (4b). Abbreviations: OGDD, 2-oxoglutarate dependent dioxygenase; OH, hydroxyl; TF, transcription factor; and ROS, reactive oxygen species. The figure is created based on ref. 182, 206, 208, 230, 233 and 240. | ||
One of the reasons why α-KGA can effectively treat pathologies is because it is strongly connected to the superfamily of 2-oxoglutarate-dependent dioxygenases (2-OGDDs, Fig. 6). These universal enzymes consume α-KGA and oxygen to produce carbon dioxide and succinate while catalysing hydroxylation-initiated oxidation or demethylation of proteins, nucleic acids, or lipids.206 Particularly, prolyl hydroxylases and histone demethylases, both belonging to 2-OGDDs, are being studied intensively as they are implicated in cancer and diabetes.207,208 Prolyl hydroxylases (PHDs) are known to hydroxylate proline residues in several regulatory proteins, suppressing their activities. These proteins include Akt, a regulator involved in blood clot formation and inflammation, and HIF1α, a factor promoting blood vessel growth (Fig. 6,  c).209 The hydroxyl groups either mask the phosphorylation sites in Akt and increase the affinity of phosphatases for Akt209,210 or promote proteasomal degradation of HIF1α.209,211 Since α-KGA drives the hydroxylation reaction, it can be applied as a “PHD booster” to inactivate Akt or HIFα. α-KGA was shown to help prevent thrombosis and inflammation due to reduced platelet aggregation and monocyte activation in COVID-19-infected murine models (Fig. 6,
c).209 The hydroxyl groups either mask the phosphorylation sites in Akt and increase the affinity of phosphatases for Akt209,210 or promote proteasomal degradation of HIF1α.209,211 Since α-KGA drives the hydroxylation reaction, it can be applied as a “PHD booster” to inactivate Akt or HIFα. α-KGA was shown to help prevent thrombosis and inflammation due to reduced platelet aggregation and monocyte activation in COVID-19-infected murine models (Fig. 6,  d and f).190 Alternatively, treatment of an oncogenic rat model with octyl-α-KGA diminished the blood vessel density surrounding the tumour due to decreased HIF1α levels (Fig. 6,
d and f).190 Alternatively, treatment of an oncogenic rat model with octyl-α-KGA diminished the blood vessel density surrounding the tumour due to decreased HIF1α levels (Fig. 6,  e).212,213 HIF1α-targeting strategies have been prioritised in recent years since this regulator is considered a “hallmark” in cancer biology and its activation is linked to cell proliferation, metastasis, and tumour resistance to chemo- and radiotherapy.214
e).212,213 HIF1α-targeting strategies have been prioritised in recent years since this regulator is considered a “hallmark” in cancer biology and its activation is linked to cell proliferation, metastasis, and tumour resistance to chemo- and radiotherapy.214
Although this anti-angiogenesis strategy improves the efficacy of cancer therapies,215 patients should be administered α-KGA with caution. Recent reports have indicated that tumour cells, relying heavily on glutamine consumption (i.e., glutaminolysis), exploit the released α-KGA as a messenger that triggers the central cascade pathways (NF-κB216 and TOR217). Subsequent pathway activation promotes cancer cell immortality, medicinally known as anoikis resistance, during circulation in the bloodstream (i.e., metastasis)218 and accelerates tumour development.219
Finally, apart from targeting central regulators, 2-OGDDs are also involved in the demethylation of histones at lysine residues (Fig. 6,  b)208 or DNA at cytosine positions (Fig. 6,
b)208 or DNA at cytosine positions (Fig. 6,  a).220 Attaching covalent groups, such as methyl moieties, to histones alters the spatial organisation of the chromosome and gene expression patterns, whereas DNA (de)methylation often occurs during cell differentiation and development.221,222 These epigenetic modifications, at both the histone and DNA level, can cause cancer and neurodevelopmental or autoimmune disorders when they occur aberrantly.221 Particularly, the nucleotide derivatives methylcytosine (5-mC) and hydroxymethylcytosine (5-hmC) are considered strong determinants of tumour fate and are believed to be associated with heart-related complications in diabetes patients.223,224 Indeed, the TET (Ten-Eleven Translocation) 2-OGDD superfamily mediates hydroxylation of 5-mC and the resulting increase in 5-hmC inhibits tumour progression (Fig. 6,
a).220 Attaching covalent groups, such as methyl moieties, to histones alters the spatial organisation of the chromosome and gene expression patterns, whereas DNA (de)methylation often occurs during cell differentiation and development.221,222 These epigenetic modifications, at both the histone and DNA level, can cause cancer and neurodevelopmental or autoimmune disorders when they occur aberrantly.221 Particularly, the nucleotide derivatives methylcytosine (5-mC) and hydroxymethylcytosine (5-hmC) are considered strong determinants of tumour fate and are believed to be associated with heart-related complications in diabetes patients.223,224 Indeed, the TET (Ten-Eleven Translocation) 2-OGDD superfamily mediates hydroxylation of 5-mC and the resulting increase in 5-hmC inhibits tumour progression (Fig. 6,  b) or restores cardiac function.224–227 At the histone level, supplementing α-KGA to mice, suffering from colorectal cancer, drives lysine demethylation by stimulating the Jumonji C-domain containing histone demethylase (JHDM), which belongs to the 2-OGDD superfamily. As a result, this treatment arrested the tumours in a terminally differentiated and non-proliferative state.228 A similar instance of epigenetic-induced cell reprogramming, linked to α-KGA, has also been observed in pluripotent stem cells. However, in this case, α-KGA seems to exert a divergent effect on stem cell fate as, depending on their state, this ketoacid may either accelerate differentiation or favour pluripotency in stem cells through the activity of the TET and JHDM demethylating enzymes (Fig. 6,
b) or restores cardiac function.224–227 At the histone level, supplementing α-KGA to mice, suffering from colorectal cancer, drives lysine demethylation by stimulating the Jumonji C-domain containing histone demethylase (JHDM), which belongs to the 2-OGDD superfamily. As a result, this treatment arrested the tumours in a terminally differentiated and non-proliferative state.228 A similar instance of epigenetic-induced cell reprogramming, linked to α-KGA, has also been observed in pluripotent stem cells. However, in this case, α-KGA seems to exert a divergent effect on stem cell fate as, depending on their state, this ketoacid may either accelerate differentiation or favour pluripotency in stem cells through the activity of the TET and JHDM demethylating enzymes (Fig. 6,  a).229,230 Moreover, supplementation of α-KGA attenuates differentiation of immunological T-cells through a complex interplay of altering the epigenetic profile and promoting triacylglyceride synthesis as well as oxidative phosphorylation in mitochondria.231 The latter provides an excellent illustration showcasing the broad-range impact of the “epigenetic modifier” α-KGA.
a).229,230 Moreover, supplementation of α-KGA attenuates differentiation of immunological T-cells through a complex interplay of altering the epigenetic profile and promoting triacylglyceride synthesis as well as oxidative phosphorylation in mitochondria.231 The latter provides an excellent illustration showcasing the broad-range impact of the “epigenetic modifier” α-KGA.
Besides being prescribed to treat diseases, α-KGA can also be administered to healthy individuals for its anti-aging benefits,232 its capacity to induce metabolic effects similar to those attained through intense physical training,233,234 or its role in procollagen production.235 The anti-aging benefits were first demonstrated in a mouse model that had improved survival and suppressed morbidity, shown as a reduction in frailty, colour loss, dermatitis, etc. (Fig. 6,  a).236 Moreover, α-KGA promotes longevity in C. elegans worms since this molecule binds and inactivates the ATP synthase, resulting in decreased oxygen consumption.237 Restraining the activity of the ATP synthase also increases autophagy, which prolongs lifespan (Fig. 6,
a).236 Moreover, α-KGA promotes longevity in C. elegans worms since this molecule binds and inactivates the ATP synthase, resulting in decreased oxygen consumption.237 Restraining the activity of the ATP synthase also increases autophagy, which prolongs lifespan (Fig. 6,  a and b).237
a and b).237
Related to the link between exercise and α-KGA, high intensity and short duration (resistance) training induces α-KGA synthesis in muscles, causing higher blood α-KGA levels.233 Circulatory α-KGA molecules bind to the OXGR1AG receptor of chromaffin cells in the adrenal gland to induce the release of epinephrine through the NF-κB signaling cascade (Fig. 6, 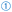 ).233 Eventually, elevated epinephrine concentrations enlarge skeletal muscles and promote the breakdown of fat tissue (Fig. 6,
).233 Eventually, elevated epinephrine concentrations enlarge skeletal muscles and promote the breakdown of fat tissue (Fig. 6,  a and b).233,238 Therefore, the authors hinted towards the use of α-KGA as an anti-obesity therapeutic.233
a and b).233,238 Therefore, the authors hinted towards the use of α-KGA as an anti-obesity therapeutic.233
Finally, α-KGA not only targets receptors and protein complexes but is also involved in mitigating oxidative stress. The antioxidising properties of α-KGA result from its direct scavenging function towards toxic reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), its role in the cellular synthesis of glutathione, a well-known antioxidant, or its ability to trigger and modulate ROS-dedicated stress response pathways (Fig. 6,  ). The H2O2-scavenging function of α-KGA is a result of a spontaneous conversion of α-KGA into succinate in the presence of H2O2, thereby releasing the harmless products H2O and CO2.239,240 Moreover, α-KGA serves as an indirect precursor for glutathione synthesis, as this tripeptide is produced from glutamic acid—the transamination product of α-KGA—along with cysteine and glycine.241 Alternatively, α-KGA may also act as a signaling molecule that engages the constitutive-androstane-receptor (CAR) pathway and as a result, promotes the expression of ROS detoxifying enzymes, including superoxide dismutases.242 In hospital settings, administering α-KGA as an antioxidising agent to patients showed improved recovery after lung surgery and reduced myocardial injury in pressure-overloaded heart.243,244 Furthermore, in combination with 5-hydroxymethylfurfural (5-HMF), α-KGA has also been patented as an antioxidant agent for humans and animals.245
). The H2O2-scavenging function of α-KGA is a result of a spontaneous conversion of α-KGA into succinate in the presence of H2O2, thereby releasing the harmless products H2O and CO2.239,240 Moreover, α-KGA serves as an indirect precursor for glutathione synthesis, as this tripeptide is produced from glutamic acid—the transamination product of α-KGA—along with cysteine and glycine.241 Alternatively, α-KGA may also act as a signaling molecule that engages the constitutive-androstane-receptor (CAR) pathway and as a result, promotes the expression of ROS detoxifying enzymes, including superoxide dismutases.242 In hospital settings, administering α-KGA as an antioxidising agent to patients showed improved recovery after lung surgery and reduced myocardial injury in pressure-overloaded heart.243,244 Furthermore, in combination with 5-hydroxymethylfurfural (5-HMF), α-KGA has also been patented as an antioxidant agent for humans and animals.245
Next to its antioxidant activity, α-KGA supplementation improves nitrogen metabolism and has a positive effect on the intestinal microbiota of pigs. Therefore, α-KGA could be potentially applied as a growth-promoting factor for enhancing pork production in livestock farming.246
3. Chemical synthesis routes for the production of α-KGA
α-KGA can be produced from a condensation reaction of diethyl succinate (DES) with diethyl oxalate (DEO), followed by hydrolysis of the obtained triethyl oxalyl succinate (Fig. 7). The first precursor, DES, is obtained by the oxidation of butane into maleic anhydride, then the ring-opening hydrogenation of maleic anhydride to succinic acid using a Ni- or Pd-catalyst, followed by an esterification of succinic acid into DES.247,248 Alternatively, succinic acid can also be obtained from fermentation using glucose as a substrate.249 The second α-KGA precursor, DEO, is typically generated from the reaction of glucose with nitric acid using V–Fe catalysts to yield oxalic acid, which is then esterified into DEO. Other routes towards oxalic acid include the propylene and ethylene glycol oxidation.250–252 The two-step condensation process of DES with DEO, despite its overall yield of 63–76%,39,253,254 is far from ideal as it involves dangerous chemicals, such as sodium or potassium ethoxides, toluene, and diethyl ether. Other compounds involved are considered as acute toxic according to ECHA (including maleic anhydride and oxalic acid with LD50 values of 1090 and 375 mg kg−1 for oral uptake, respectively).255,256 Moreover, the reaction chemistry leads to environmental issues due to the partial use of fossil resources and the emission of nitric oxides during synthesis.Anther chemical route to synthesise α-KGA or its salts is via the intermediate adduct dimethyl 2,2-dichloroglutarate (DMDG). The reaction between methyl dichloroacetate (MDA) and methyl acrylate (MA) yields DMDG, which is subsequently transformed into an aqueous α-KGA solution using a hydroxide medium.257 The precursor MDA is derived from alcoholysis of trichloroethylene-originated dichloroacetyl chloride.258,259 The precursor MA is derived from the liquid-phase methyl esterification of acrylic acid that arises from the vapor-phase catalytic oxidation of propylene.260 Again, these compounds are considered toxic.258–260 Under mild temperatures, α-KGA can be produced through the transamination reaction between glutamate and glyoxylate, with Cu, Ni, Co, or V metal salts as a catalyst, and with glycine as a by-product.261
Another possible chemical pathway is the oxidation of aqueous 5-hydroxymethyl furfural (5-HMF) to α-KGA on an Amberlyst®-15 catalyst with H2O2. However, only a yield of 31% was achieved at 75 °C after 24 h, with formic acid and succinic acid as primary by-products.262
More recently, a heterogeneously catalysed route towards α-KGA has been developed via an aldol condensation between pyruvic acid and glyoxylic acid. This reaction resulted in 2-hydroxy-4-oxoglutaric acid, which formed α-KGA upon dehydration and hydrogenation using a Pd/TiO2 catalyst. The overall yield of this reaction was 85%, with a volumetric productivity of 50 g L−1 h−1, and could therefore offer a valuable green alternative to the classic industrial production routes.263 Moreover, pyruvic acid can be obtained from consecutive dehydration-decarboxylation chemistry from tartaric acid, or can be sourced from microbial fermentation.39,264 Similarly, glyoxylic acid can be obtained from the oxidation of glyoxal, derived from the oxidation of ethylene glycol,265 or can be produced through enzymatic oxidation of bio-based glycolic acid.266,267
4. Cell-free biocatalytic pathways towards α-KGA
Apart from chemical synthesis, α-KGA can also be produced through in vitro metabolic pathways comprising a few enzymatic steps that are applied in a cell-free system. This approach is considered safe and sustainable since generally high selectivity and yield of the desired end-product are achieved without the burden of toxic catalysts or reagents. Additionally, this strategy requires only mild reaction temperatures (4–60 °C).268 The enzyme's reactivity and selectivity are also tuneable through protein engineering.269 However, producing α-KGA using this strategy may require isolation of enzymes from the microbial cells that produce them, therefore potentially increasing production costs.270Different enzymes can be applied to produce α-KGA in a cell-free biocatalytic system. Glutamic acid (Glu) or its cyclic version, 2-pyrrolidone-5-carboxylic acid, can be transformed into α-KGA with the oxygen-dependent Streptomyces ghanaensisL-glutamate oxidase (L-GOX) (Fig. 8,  ),271 an engineered variant of the Proteus mirabilisL-amino acid deaminase (L-AAD)272 (Fig. 8,
),271 an engineered variant of the Proteus mirabilisL-amino acid deaminase (L-AAD)272 (Fig. 8,  ) or the NAD+-dependent Clostridium symbiosum glutamate dehydrogenase (L-GDH) (Fig. 8,
) or the NAD+-dependent Clostridium symbiosum glutamate dehydrogenase (L-GDH) (Fig. 8,  ).273 Often, the primary Glu-converting enzymes are combined with either a NADH oxidase or a catalase to regenerate NAD+ or to detoxify the released hydrogen peroxide, respectively.271,273 Applying immobilised L-GOX in combination with catalase in a 1 L reactor yielded the highest α-KGA production metrics in a cell-free system, reaching a titre of 71 g L−1 and a spatiotemporal yield of 14.2 g L−1 h−1.271
).273 Often, the primary Glu-converting enzymes are combined with either a NADH oxidase or a catalase to regenerate NAD+ or to detoxify the released hydrogen peroxide, respectively.271,273 Applying immobilised L-GOX in combination with catalase in a 1 L reactor yielded the highest α-KGA production metrics in a cell-free system, reaching a titre of 71 g L−1 and a spatiotemporal yield of 14.2 g L−1 h−1.271
 | ||
Fig. 8
In vitro biocatalytic conversion of L-glutamic and 2-pyrrolidone 5-carboxylic acid (or their corresponding salts) into α-KGA (salt). Three enzyme-based strategies can be distinguished using L-glutamate oxidase (L-GOX)  , L-amino acid deaminase (L-AAD) , L-amino acid deaminase (L-AAD)  , and L-glutamate dehydrogenase (L-GDH) , and L-glutamate dehydrogenase (L-GDH)  . Furthermore, the NADH oxidase (NOX) is responsible for NAD+ regeneration. M+ represents either a proton, in the case of an acid, or an alkali metal cation (e.g., Na+), in the case of a salt. The figure is based on ref. 273, 338 and 614. . Furthermore, the NADH oxidase (NOX) is responsible for NAD+ regeneration. M+ represents either a proton, in the case of an acid, or an alkali metal cation (e.g., Na+), in the case of a salt. The figure is based on ref. 273, 338 and 614. | ||
More elaborate enzyme cascades have been designed to use other sugar(-derived) substrates for the production of α-KGA. For example, D-glucuronic acid can be converted into α-KGA in a four-step, redox-balanced pathway with a yield of 92% and with H2O and CO2 as primary side-products (Fig. 9A).274 Recently, a similar approach has been proposed using D-xylose or L-arabinose as feedstocks while simultaneously co-producing green hydrogen gas (Fig. 9B).275,276 This setup enabled the production of 41.6 g L−1 α-KGA, corresponding to a theoretical yield of at least 99%, with a spatiotemporal yield of 4.6 g L−1 h−1.
 | ||
| Fig. 9 In vitro enzyme cascades for the production of α-KGA from the hexose D-glucuronate (A) or pentoses D-xylose or L-arabinose (B). Enzyme abbreviations: UDH, uronate dehydrogenase; GlucD, glucarate dehydratase; KdgD; 5-keto-4-deoxyglucarate dehydratase; KgsaDH; α-ketoglutaric semialdehyde dehydrogenase; XylDH, xylose dehydrogenase; Lac, lactonase; DHT, dehydratase; NOX, NADH oxidase; SH, soluble hydrogenase; and KdpD, 2-keto-3-deoxy-D-xylonate or arabonate dehydratase. The figure is based on ref. 274–276. | ||
Producing α-KGA using cell-free biocatalysis seems to be a promising strategy, primarily due to the high yields and product purity. The substrate for biocatalytic production of α-KGA, mainly glutamic acid, has been industrially produced on a large scale (over 2 million tons per year) using the bacterium Corynebacterium glutamicum.277 Substrate availability is thus not an issue.
Currently, cell-free biocatalysis for α-KGA production primarily relies on purified enzymes, contributing to higher overall process costs. Although not yet applied to α-KGA synthesis, the use of crude cell extracts could offer a more cost-effective alternative. A second approach is whole-cell biocatalysis, where intact living cells serve as catalysts, which do not require enzyme purification as well. However, this may compromise product purity due to metabolic side reactions within the host organism. The specific advantages and limitations of whole-cell biocatalysis for α-KGA production are discussed in Section 5.2.
5. Producing α-KGA using microbial cell factories
5.1. The biochemistry of α-KGA in microorganisms
(Micro)organisms typically synthesise α-KGA as part of aerobic fatty acid and carbohydrate catabolism. Specifically, hexose or pentose sugars are first metabolised through the Emden Meyerhof pathway (glycolysis) or pentose phosphate pathway to yield phosphoenolpyruvate or pyruvate. These central C3 intermediates are converted into either oxaloacetate or acetyl-CoA through a carboxylation or decarboxylation reaction, respectively. The resulting products serve as key precursors for the tricarboxylic acid cycle (TCAc; also known as the Krebs cycle) within the cytoplasm of bacteria or the mitochondria of yeast (Fig. 10 and Table 4).278–280 Fatty acids are first degraded through the β-oxidation pathway into acetyl-CoA, which then enters the TCAc (Fig. 10 and Table 4).281–285 When the TCA cycle operates in its oxidative mode (“clockwise” orientation in Fig. 10), acetyl-CoA combines with oxaloacetate to form citrate. Citrate then undergoes a series of (de)hydration, decarboxylation, acetyltransferase, and oxidation reactions, sequentially generating (iso)citrate (C6), α-KGA (C5), and C4 intermediates. This oxidative TCAc (oTCAc) produces reducing equivalents (NADH, FADH2), which are crucial for multiple cellular reactions and also fuel the respiratory chain to yield energy in the form of ATP.278,286 Under anaerobic conditions, the TCAc can also function in reductive mode to convert oxaloacetate, originating from (phosphoenol)pyruvate carboxylation, into succinic acid (Fig. 10).287–289 Combined with the oTCAc, the reductive cycle prevents accumulation of reduced coenzymes (NADH, FADH2) that cannot be readily re-oxidised in the absence of oxygen or other electron acceptors, while still ensuring the formation of intermediary TCA products that are essential for the cell's anabolism. In most model microorganisms, including Escherichia coli and Saccharomyces cerevisiae, succinic acid is the end point of the reductive TCAc (rTCAc) because the reaction catalysed by α-KGA dehydrogenase (KGDH, step 10 in Fig. 10) is irreversible in the clockwise orientation. However, in (thermophilic) green sulphur (proteo)bacteria, a fully functional reverse TCA cycle (rTCAc) enables CO2 fixation into TCA cycle intermediates, catalysed by the alternative enzymes 2-oxoglutarate synthase and aconitate hydratase.290–292 | ||
| Fig. 10 The microbial metabolism of sugars and fats towards α-KGA. Yellow shading refers to the glycolysis and pentose phosphate pathways, green to the TCAc, blue to the fatty acid (β-oxidation) degradation pathway, and fuchsia to the Weimberg pathway. The purple arrows indicate heterologous enzymes that cannot be natively found in E. coli, C. glutamicum, and Y. lipolytica. The solid lines highlight the oxidative reactions in the TCAc and Weimberg pathway, whereas the dotted lines represent reductive reactions. The numbers and the associated reaction chemistry are explained in more detail in Table 4. The figure is created based on ref. 296, 410, 615 and 616. | ||
Apart from being an indispensable intermediate of TCA cycles, α-KGA serves as the main precursor for the de novo synthesis of glutamate, which, in turn, plays a key role as a nitrogen donor in transamination reactions.293–295 Furthermore, Pseudomonas fluorescens exploits α-KGA as an antioxidant and possesses a dedicated pathway to “recycle” α-KGA from succinate based on the spontaneous reaction between α-KGA and reactive oxygen species.296–298
While the TCAc is the most universal pathway leading to α-KGA biosynthesis, several other pathways can also contribute to α-KGA formation, including oxidative glutamate deamination, the Weimberg pathway, and galacturonic acid degradation. Firstly, oxidative deamination of glutamate may proceed in a cofactor-dependent or -independent way. The former reaction is performed by glutamate dehydrogenase, which also converts α-KGA into glutamate in the opposite direction, and requires NAD(P)+.299 As discussed previously, L-amino acid deaminase (L-AAD)300,301 or L-glutamate oxidase (L-GOX)302 solely requires oxygen and releases hydrogen peroxide while deaminating glutamate. Finally, the xylose oxidative (Weimberg) pathway is a thermodynamically more favourable route that converts xylose into α-KGA in only five consecutive steps (Fig. 14).303 This atypical sugar metabolism can be found in hyperthermophilic Archaea from the Sulfolobus genus,304 the bacterial Pseudomonas303,305,306 or Caulobacter crescentus307 species, and the filamentous fungus Myceliophthora thermophila.308
5.2. L-Glutamic acid to α-KGA via whole-cell biocatalysis
Whole-cell biocatalysis (or biotransformation) leverages living microbial cells as natural biocatalysts to convert precursor molecules into α-KGA. In this approach, the entire microbial cell is utilised rather than purified or extracted enzymes as employed in the cell-free biocatalytic approach discussed in the previous chapter (Fig. 8). These cells contain the desired enzymes and biochemical pathways to catalyse a small number of key reactions without the need to disrupt the cells or purify individual enzymes, thereby reducing costs.327Specifically for α-KGA production, whole-cell biocatalysis has been explored to deaminate the substrate L-glutamic acid or its cyclic derivative, 2-pyrrolidone-5 carboxylic acid. In this case, the L-amino acid oxidase (L-AAO) or L-AAD from Proteus species328,329 and L-GOX from Streptomyces321,330–336 are heterologously expressed in a microbial host, such as B. subtilis or E. coli (Table 5). Importantly, when using oxidases (either L-AAO or L-GOX) as a catalyst, the reaction generates hydrogen peroxide, a toxic by-product for the microbial host cell. Hence, a catalase is often co-expressed alongside L-GOX to convert the released hydrogen peroxide into water and oxygen.323,337 Protein engineering can be applied to enhance the enzyme activity, resulting in a higher conversion efficiency. For instance, L-GOX from Streptomyces mobaraensis was subjected to two rounds of site-saturation mutagenesis, which increased the enzyme activity 1.9-fold due to two combinatorial amino acid substitutions (S280T and H533L). When the engineered L-GOX mutant was introduced together with the KatE catalase in E. coli, this whole-cell biocatalysis system was able to reach an average spatiotemporal yield of 15.2 g L−1 h−1 α-KGA over a 12 h period and a molar conversion efficiency of 86.3% from L-glutamic acid.337
 , introduction and heterologous expression; *, mutant; Δ, gene deletion/knock-out;
, introduction and heterologous expression; *, mutant; Δ, gene deletion/knock-out;  , increase; and
, increase; and  , inhibition. Strain designation: Ec, E. coli; Bs, Bacillus subtilis; Sg, Streptomyces ghanaensis; Sm, Streptomyces mobaraensis; Sp, Streptomyces platensis; Sl, Streptomyces lividans; Sv, Streptomyces viridosporus; and Pm, Proteus mirabilis. Abbreviations: L-Glu, L-glutamic acid
, inhibition. Strain designation: Ec, E. coli; Bs, Bacillus subtilis; Sg, Streptomyces ghanaensis; Sm, Streptomyces mobaraensis; Sp, Streptomyces platensis; Sl, Streptomyces lividans; Sv, Streptomyces viridosporus; and Pm, Proteus mirabilis. Abbreviations: L-Glu, L-glutamic acid
Due to the different enzyme activities of L-GOX and catalase, and to reduce the burden of expressing multiple heterologous enzymes simultaneously, fine-tuning their expression ratio can be crucial. One study adjusted the co-expression of L-GOX from Streptomyces ghanaensis and the native KatG catalase in E. coli using promoter and ribosome binding site engineering to obtain an LGOX![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KatG activity ratio of 2
KatG activity ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1185. This resulted in a spatiotemporal yield of 13.25 g L−1 h−1 with a conversion efficiency of 96% over an 8 h period.338 Another solution exploited a double-strain setup in which one of the E. coli strains expressed the enzyme L-GOX from Streptomyces platensis and the other the enzyme catalase from Streptomyces lividans. Through fine-tuning the inoculum ratios of the two E. coli strains, α-KGA productivity was increased by 97% compared to the single-strain system. Moreover, a scale-up strategy brought the spatiotemporal yield to 15.9 g L−1 h−1, the highest reported value to date.339
1185. This resulted in a spatiotemporal yield of 13.25 g L−1 h−1 with a conversion efficiency of 96% over an 8 h period.338 Another solution exploited a double-strain setup in which one of the E. coli strains expressed the enzyme L-GOX from Streptomyces platensis and the other the enzyme catalase from Streptomyces lividans. Through fine-tuning the inoculum ratios of the two E. coli strains, α-KGA productivity was increased by 97% compared to the single-strain system. Moreover, a scale-up strategy brought the spatiotemporal yield to 15.9 g L−1 h−1, the highest reported value to date.339
To further improve productivity and reduce operational costs of whole-cell α-KGA synthesis, cell immobilisation has been explored.324,325,340 Here, microbial cells are attached to a solid support (i.e., alginate beads or metal–organic frameworks) within the reaction vessels, allowing higher cell densities and easier cell recycling and separation from the final product. However, conversion efficiencies are often lower in immobilised compared to planktonic cells, and enzymatic activity decreases by 20–30% after each cycle.325,340 Hence, it is not entirely clear whether cell immobilisation is truly a viable strategy.
Whole-cell α-KGA production has gained considerable interest, achieving higher titres and productivity than cell-free systems (see Section 4). Advances in metabolic engineering and process scale-up have contributed to improved yields. In addition, whole-cell biocatalysis can make costly enzyme purification redundant and even allows reusing cells across multiple reaction cycles, potentially further improving cost-efficiency.327
5.3. Native α-KGA-producing microbial candidates for industrial settings
Over the years, several candidate microbial producers have been discovered that naturally produce high levels of α-KGA. In the bacterial kingdom, Escherichia coli,341Pseudomonas fluorescens,342–344Corynebacterium glutamicum,345,346Micrococcus paraffinolyticus, Arthrobacter paraffineus or hydrocarboglutamicus347,348 have been reported to natively secrete the ketoacid of interest. In the case of yeasts, natural α-KGA production occurs in Candida glabrata,349–352Yarrowia lipolytica,353 and several Pichia species.354,355 Although the above-mentioned microbial species all show potential as microbial α-KGA cell factories, most research thus far has focused on E. coli, C. glutamicum, or Y. lipolytica for metabolic or process engineering to enhance α-KGA biosynthesis.Since its discovery in the mid-1950s in Japan, C. glutamicum has also received considerable interest as a potential chassis strain for producing various value-added compounds.368–370 Originally, this non-spore-forming Gram-positive soil species was appreciated as an excellent glutamate producer but later on it was also exploited for industrial-scale lysine production.370–372 Today, the list of C. glutamicum-derived products has gradually extended towards other proteogenic (e.g., L-methionine and L-isoleucine) and non-proteogenic (e.g., ϒ-aminobutyric acid (GABA) and ectoine) amino acids, including their less common D-isoforms.373,374 Furthermore, C. glutamicum has been successfully applied for the synthesis of TCAc-derived acids including itaconic,375 glutaric,376 ornithine,377 and 5-aminolevulinic acid378 as well as for the production of aromatic amino acid-derived compounds such as muconic acid.379,380 While E. coli is well known for its broad carbon utilisation spectrum, C. glutamicum has a narrow substrate range, which is an important limitation. Specifically, C. glutamicum lacks the key pathways and enzymes for xylose, arabinose, lactose, galactose, and glycerol consumption.345 This limitation can, however, be overcome by introducing the corresponding carbon catabolism pathways from E. coli to enable fermentation using less expensive carbon substrates (see further).381
Although the microorganisms discussed above already produce α-KGA to some extent, achieving economically sustainable production rates requires process optimisation, i.e., creating the ideal production environment, and strain improvement through metabolic engineering. Hence, the next sections elaborate on these two aspects. Although the focus is primarily on α-KGA (Table 6), publications on α-KGA-derivatives (including glutamic, glutaric, mesaconic, ϒ-aminobutyric acid, arginine, ornithine, and 1,4-butanediol) (Table 7) and succinic/malic acid (Table 8) are also covered. These citations often mention similar or overlapping optimisation approaches to those commonly pursued for α-KGA and can therefore serve as a valuable source of inspiration.
 , introduction of non-native genes;
, introduction of non-native genes;  , expression optimisation;
, expression optimisation;  , increase;
, increase;  , decrease;
, decrease;  , disruption/deactivation. E following a numerical value indicates that the substrate concentration was maintained around that value, once the initial substrate concentration i was consumed. Strain designation: Ec, E. coli; Cg, C. glutamicum; Cc, C. crescentus; Tg, T. glabrata; Yl, Y. lipolytica; Pk, Pichia kudriavzevii; Km, Kluyveromyces marxianus; Sc, Saccharomyces cerevisiae, and Af, Aspergillus flavus. Enzyme abbreviations: KGDH, α-ketoglutarate dehydrogenase (complex)
, disruption/deactivation. E following a numerical value indicates that the substrate concentration was maintained around that value, once the initial substrate concentration i was consumed. Strain designation: Ec, E. coli; Cg, C. glutamicum; Cc, C. crescentus; Tg, T. glabrata; Yl, Y. lipolytica; Pk, Pichia kudriavzevii; Km, Kluyveromyces marxianus; Sc, Saccharomyces cerevisiae, and Af, Aspergillus flavus. Enzyme abbreviations: KGDH, α-ketoglutarate dehydrogenase (complex)
5.4. Optimising fermentation conditions
This section discusses the efforts invested in identifying fermentation conditions, such as nutrients, additives, temperature, aeration, and pH, that can be regulated and controlled within bioproduction units and positively influence α-KGA production titres. Profound knowledge of the optimal conditions for a given bioproduction process is key to achieving economic sustainability in fermentation activities.In the case of Y. lipolytica, this yeast species takes up thiamine from the growth broth as it is an essential cofactor for the KGDH complex.388 Hence, limiting thiamine levels is the key strategy to reduce the KGDH complex activity without harming cell growth (Fig. 11,  ).388–391 Indeed, several studies have demonstrated that thiamine concentrations between 0.15 and 4 μg L−1 are optimal for α-KGA production.388,390,391 However, when thiamine is too low, α-KGA production drops because the pyruvate dehydrogenase complex (PDH) is also thiamine-dependent. As a result, low PDH activity causes accumulation of pyruvate as a major by-product.390,392–394 Generally, the optimal thiamine concentration depends on the strain, carbon substrate, and culture conditions. However, the common trend is that low thiamine enhances α-KGA but limits biomass production, and vice versa. As such, the initial thiamine concentration is typically set higher than the abovementioned optimal concentrations to support biomass growth before transitioning into α-KGA production under thiamine-limited conditions.
).388–391 Indeed, several studies have demonstrated that thiamine concentrations between 0.15 and 4 μg L−1 are optimal for α-KGA production.388,390,391 However, when thiamine is too low, α-KGA production drops because the pyruvate dehydrogenase complex (PDH) is also thiamine-dependent. As a result, low PDH activity causes accumulation of pyruvate as a major by-product.390,392–394 Generally, the optimal thiamine concentration depends on the strain, carbon substrate, and culture conditions. However, the common trend is that low thiamine enhances α-KGA but limits biomass production, and vice versa. As such, the initial thiamine concentration is typically set higher than the abovementioned optimal concentrations to support biomass growth before transitioning into α-KGA production under thiamine-limited conditions.
 | ||
Fig. 11 The impact of optimizing fermentation conditions on the α-KGA-oriented metabolism in E. coli (Ec), C. glutamicum (Cg), and Y. lipolytica (Yl).  The activity of the α-ketoglutarate dehydrogenase (KGDH) complex can be attenuated in C. glutamicum by treating with xenobiotics (such as penicillin and methotrexate) or in Y. lipolytica by reducing the thiamine concentration in the medium. The activity of the α-ketoglutarate dehydrogenase (KGDH) complex can be attenuated in C. glutamicum by treating with xenobiotics (such as penicillin and methotrexate) or in Y. lipolytica by reducing the thiamine concentration in the medium.  Limiting biotin levels decreases the activity of the acyl-CoA carboxylase complex in C. glutamicum, which indirectly represses the KGDH complex as well. Limiting biotin levels decreases the activity of the acyl-CoA carboxylase complex in C. glutamicum, which indirectly represses the KGDH complex as well.  Constraining nitrogen levels prevents the glutamate dehydrogenase (GDH)-mediated amination reaction and therefore blocks the transformation of α-KGA into glutamic acid. Constraining nitrogen levels prevents the glutamate dehydrogenase (GDH)-mediated amination reaction and therefore blocks the transformation of α-KGA into glutamic acid.  Supplementing metal ions (such as calcium) stimulates pyruvate carboxylase in Y. lipolytica which promotes the conversion of pyruvic acid into oxaloacetic acid. Supplementing metal ions (such as calcium) stimulates pyruvate carboxylase in Y. lipolytica which promotes the conversion of pyruvic acid into oxaloacetic acid.  Improving oxygen supply (i.e., aeration) shuts down anaerobic pathways and avoids accumulation of organic acids and alcohols. In E. coli, oxygen interrupts ArcA phosphorylation which diminishes the activity of the anaerobic pathways and relieves the repression of the oTCAc. The figure is created based on ref. 396, 403, 431 and 615. Improving oxygen supply (i.e., aeration) shuts down anaerobic pathways and avoids accumulation of organic acids and alcohols. In E. coli, oxygen interrupts ArcA phosphorylation which diminishes the activity of the anaerobic pathways and relieves the repression of the oTCAc. The figure is created based on ref. 396, 403, 431 and 615. | ||
In C. glutamicum, biotin limitation, supplementation of Tween 40 and 60 detergents and copper,395 and sublethal penicillin doses are known to trigger α-KGA and glutamate production (Fig. 11,  ).396 Regarding exposure to Tween and penicillin, this observation can be partially attributed to an increase in pyruvate carboxylase activity, an enzyme that is involved in the synthesis of oxaloacetate, an α-KGA precursor.397 Moreover, Tween and penicillin might also affect the flux from α-KGA to succinyl-CoA through KGDH, thereby promoting α-KGA accumulation.
).396 Regarding exposure to Tween and penicillin, this observation can be partially attributed to an increase in pyruvate carboxylase activity, an enzyme that is involved in the synthesis of oxaloacetate, an α-KGA precursor.397 Moreover, Tween and penicillin might also affect the flux from α-KGA to succinyl-CoA through KGDH, thereby promoting α-KGA accumulation.
Alternatively, medium supplements can also modulate the activity of other key enzymes. Indeed, exposure to surfactants destabilises DtsR, a critical component of the Acyl-CoA carboxylase complex. Instead, depriving cells of biotin also attenuates the activity of the complex because this vitamin is essential for the enzyme's function. Both strategies compromise the flux of acetyl-CoA towards fatty acids and consequently promote α-KGA accumulation as inhibition of fatty acid synthesis indirectly reduces KGDH activity.396,398 Practically, adjusting the biotin concentration in the medium to about 7 μg L−1 proved most efficient in ensuring normal growth of C. glutamicum while stimulating α-KGA production (25% more α-KGA than with 9 μg L−1 biotin).399 Penicillin's mode-of-action is more transcription-oriented since this antibiotic decreases odhA expression, encoding the catalytic subunit of KGDH, and increases the transcript levels of its corresponding repressor, odhI.400 Another strategy to disrupt KGDH activity is by specifically targeting the complex using the dehydrogenase inhibitor methotrexate (also called Rheumatrex), a medicine to cure rheumatoid arthritis and an anticancer agent. Indeed, treatment with this pharmaceutical improved α-KGA titres in both C. glutamicum401 and the yeast T. glabrata.402
Finally, besides their influence on enzyme activity and transcription, biotin limitation and Tween 40 or penicillin treatment may have an even higher impact on glutamate synthesis than on α-KGA production. It has been demonstrated that the induced fatty acid synthesis defect elicits membrane tension, which in turn triggers the mechanosensitive and major glutamate-exporting MscCG channel in C. glutamicum.403
 ). In Y. lipolytica, a carbon-to-nitrogen ratio of 17.4
). In Y. lipolytica, a carbon-to-nitrogen ratio of 17.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 29
1 to 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is shown to promote α-KGA production, regardless of the carbon substrate used.388,390,404 Below or above this level, α-KGA production decreases, and this effect is even more pronounced under extremely limiting nitrogen conditions. These results contradict the consensus that nitrogen deficiency would stop the amination of α-KGA to glutamate, allowing more α-KGA accumulation. However, in Y. lipolytica, nitrogen limitation triggers lipid production and the storage response, diverting carbon catabolites away from the TCAc, causing a reduction in α-KGA.405
1 is shown to promote α-KGA production, regardless of the carbon substrate used.388,390,404 Below or above this level, α-KGA production decreases, and this effect is even more pronounced under extremely limiting nitrogen conditions. These results contradict the consensus that nitrogen deficiency would stop the amination of α-KGA to glutamate, allowing more α-KGA accumulation. However, in Y. lipolytica, nitrogen limitation triggers lipid production and the storage response, diverting carbon catabolites away from the TCAc, causing a reduction in α-KGA.405
Similarly, limiting the nitrogen supply to C. glutamicum improves α-KGA productivity, because nitrogen starvation prevents the α-KGA end-product from being transformed into glutamate. In practice, a pH-neutralising, ammonium-rich base such as ammonium hydroxide is added to the fermentation broth during the growth stage and exchanged for an ammonium-free base such as sodium hydroxide during the stationary phase to switch the cells into production mode.399,406 Adjusting the ammonium concentration results in a 24% increase in α-KGA, while the glutamate titre drops by ca. 80%.406 Apart from reducing the ammonium content, the conversion of α-KGA into glutamate can be selectively blocked by adding methionine sulfoximine, an inhibitor of the ammonium assimilating glutamine synthetase (GS)–glutamine 2-oxoglutarate amidotransferase (GOGAT) pathway.407,408 These enzymes convert α-KGA biochemically into glutamate, and consecutively into glutamine.409
Optimising the level of micronutrients such as calcium or metal ions can also improve α-KGA production in Y. lipolytica.390,414 Elevated calcium ion concentrations (e.g., from using calcium carbonate as a buffering agent in the fermentation broth) lower pyruvate by-product levels, as calcium activates pyruvate carboxylase which converts pyruvate into oxaloacetate (Fig. 11,  ).350,414 Higher concentrations of other metal ions, including zinc, iron, copper, or manganese, were shown to be favourable, but the mechanism remains to be elucidated in Y. lipolytica.390 In the baker's yeast S. cerevisiae, the role of metal ions in its metabolism has been extensively investigated,418,419 providing a foundational framework for the research on metal utilisation in Y. lipolytica.
).350,414 Higher concentrations of other metal ions, including zinc, iron, copper, or manganese, were shown to be favourable, but the mechanism remains to be elucidated in Y. lipolytica.390 In the baker's yeast S. cerevisiae, the role of metal ions in its metabolism has been extensively investigated,418,419 providing a foundational framework for the research on metal utilisation in Y. lipolytica.
Similar to pH levels, oxygen levels in the broth also increase α-KGA accumulation by influencing the cells’ metabolism. Dissolved oxygen (DO) alters the activity of aerobic and anaerobic pathways, which in turn affects growth kinetics and the formation of metabolites (Fig. 11,  ). Since Y. lipolytica is an obligate aerobic yeast, sufficient aeration of the culture medium is required to ensure cell growth.26 Additionally, α-KGA production via the oTCAc necessitates oxygen as the electron acceptor because the cycle is intertwined with the electron transport chain, which recycles cofactors (i.e., NADH) and generates ATP. Indeed, multiple studies showed that a 50–60% DO saturation is necessary for α-KGA production regardless of the carbon substrate.390,420,424
). Since Y. lipolytica is an obligate aerobic yeast, sufficient aeration of the culture medium is required to ensure cell growth.26 Additionally, α-KGA production via the oTCAc necessitates oxygen as the electron acceptor because the cycle is intertwined with the electron transport chain, which recycles cofactors (i.e., NADH) and generates ATP. Indeed, multiple studies showed that a 50–60% DO saturation is necessary for α-KGA production regardless of the carbon substrate.390,420,424
In bacterial cells, low DO levels are less appropriate for α-KGA production as oxygen limitation triggers the secretion of organic acid by-products. Insufficient oxygen availability predominantly promotes lactate accumulation followed by succinate and acetate in C. glutamicum425–428 due to expression induction of ldhA, gapA, tpi, pgk, ppc, and mdh.429 Similarly, reduced DO concentrations trigger the production of acetate, formate, succinate, and a little ethanol in E. coli430 because the redox state sensor, ArcAB, induces fermentative pathways and represses the expression of oxidative TCAc enzymes simultaneously.431 Hence, ensuring appropriate oxygen availability throughout the entire fermentation vessel is essential to prevent accumulation of undesired acid by-products (Fig. 11,  ).425–427
).425–427
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with a minimal pyruvate concentration of 2.9 g L−1.394 In the case of a nitrogen-feeding fed-batch setup, a 20% increase in α-KGA production is obtained when nitrogen levels are maintained above 1 g L−1.388 The results, however, contradict the nitrogen limitation (high C
1 with a minimal pyruvate concentration of 2.9 g L−1.394 In the case of a nitrogen-feeding fed-batch setup, a 20% increase in α-KGA production is obtained when nitrogen levels are maintained above 1 g L−1.388 The results, however, contradict the nitrogen limitation (high C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N ratio) rule-of-thumb for high α-KGA production that was previously discussed. Apparently, restricting thiamine and maintaining a constant feeding rate of ammonium sulphate can force cells to convert the nitrogen source into amino acids to alleviate 'ammonium stress’. In turn, the activation of enzymes like glutamate dehydrogenase (GDH) impels an increased flow towards α-KGA, which serves as the primary substrate of GDH.388
N ratio) rule-of-thumb for high α-KGA production that was previously discussed. Apparently, restricting thiamine and maintaining a constant feeding rate of ammonium sulphate can force cells to convert the nitrogen source into amino acids to alleviate 'ammonium stress’. In turn, the activation of enzymes like glutamate dehydrogenase (GDH) impels an increased flow towards α-KGA, which serves as the primary substrate of GDH.388
5.5. Metabolic strain engineering
Apart from optimising production culture conditions, the fluxes within microbial cells can also be re-routed through genetic engineering to maximise α-KGA yields. Regardless of the selected α-KGA-producing route (oTCA, rTCA, Glu-based oxidation, and Weimberg pathway), most strain engineering strategies pursued so far can be categorised into (i) replenishing the key precursor pools for α-KGA, (ii) maximally directing key precursors (such as acetyl-CoA and (phosphoenol)pyruvate) into the α-KGA-biosynthesis cascade, and (iii) diminishing accumulation of side-products (Fig. 12). These approaches are expected to enhance α-KGA production, if they do not cause any growth defects. Next to improving the efficiency of α-KGA biosynthesis, the industrial potential of the cell factories can be further enhanced by stimulating the α-KGA efflux (to aid product recovery), and by expanding the microbe's substrate utilisation range. | ||
Fig. 12 Metabolic engineering strategies to improve α-KGA production in the bacteria E. coli and C. glutamicum and the yeast Y. lipolytica. Encircled alphanumeric codes represent the different approaches that have been pursued to enhance α-KGA titres in the aforementioned species:  improved replenishment of the (phosphoenol)pyruvate pool and α-KGA is also shown to inhibit the PTS system and reduce malate and acetate formation. improved replenishment of the (phosphoenol)pyruvate pool and α-KGA is also shown to inhibit the PTS system and reduce malate and acetate formation.  maximising conversion of (phosphoenol)pyruvate into acetyl-CoA and oxaloacetate, maximising conversion of (phosphoenol)pyruvate into acetyl-CoA and oxaloacetate,  improving the flux through the oxidative and reductive TCAc branches, improving the flux through the oxidative and reductive TCAc branches,  suppressing degradation of α-KGA, suppressing degradation of α-KGA,  preventing other by-product accumulation, and preventing other by-product accumulation, and  enhancing product export. orTCAc refers to pathways that are shared among the oTCAc and rTCAc. Each strategy is more elaborately discussed in the main text. Abbreviation: α-KGAex, exported α-KGA towards the growth broth. The figure is based on ref. 27, 309, 311, 410, 468, 477, 479 and 615. enhancing product export. orTCAc refers to pathways that are shared among the oTCAc and rTCAc. Each strategy is more elaborately discussed in the main text. Abbreviation: α-KGAex, exported α-KGA towards the growth broth. The figure is based on ref. 27, 309, 311, 410, 468, 477, 479 and 615. | ||
Since PEP is also the direct precursor for pyruvate, high PEP levels enhance the flux throughout the TCAc in bacteria and therefore stimulate α-KGA production. Cellular processes that do not contribute to α-KGA synthesis and drain the PEP-pool should thus be blocked. The sugar phosphotransferase system (PTS) is the dominant PEP-consuming process in bacteria and constitutes a mechanism in which sugars such as glucose or trehalose are simultaneously imported and phosphorylated.453–455 Hence, inactivating this sugar transport system is a well-established strategy in the case of rTCAc-based succinic acid production to ensure that PEP is routed toward the desired product instead of pyruvate (Fig. 12,  ). Therefore, the glucose-specific permease, encoded by ptsG, is often deleted in E. coli444,456–458 and C. glutamicum.459–461 Similarly, disrupting the EI subunit (encoded by ptsI)462,463 and the histidine protein (encoded by ptsH)464 also abolishes PTS-mediated glucose import, but this strategy is less frequently applied. In the specific case of α-KGA production, the α-keto acid renders the native glucose PTS inactive through inhibition of the PTS enzyme I (E1) subunit.465,466 To compensate for the deliberate loss or α-KGA-mediated inactivation of the glucose transport system, the galactose permease (galP)-glucokinase (glk)463,467,468 or the recently discovered ExuT transporter469 are often recruited in E. coli to rescue glucose uptake. Combined with a mutant version of phosphoenolpyruvate carboxykinase (pck*), disrupting the PTS in E. coli can improve the succinic acid production titres up to 4-fold.462 In C. glutamicum, a similar approach has been pursued in which the myo-inositol transporter (iolT1), linked to (polyphosphate) glucokinases (ppgk or glk), serves as the substitute for the inactivated PTS.461 This strategy increases the succinic acid titres in this species by ca. 12%.461
). Therefore, the glucose-specific permease, encoded by ptsG, is often deleted in E. coli444,456–458 and C. glutamicum.459–461 Similarly, disrupting the EI subunit (encoded by ptsI)462,463 and the histidine protein (encoded by ptsH)464 also abolishes PTS-mediated glucose import, but this strategy is less frequently applied. In the specific case of α-KGA production, the α-keto acid renders the native glucose PTS inactive through inhibition of the PTS enzyme I (E1) subunit.465,466 To compensate for the deliberate loss or α-KGA-mediated inactivation of the glucose transport system, the galactose permease (galP)-glucokinase (glk)463,467,468 or the recently discovered ExuT transporter469 are often recruited in E. coli to rescue glucose uptake. Combined with a mutant version of phosphoenolpyruvate carboxykinase (pck*), disrupting the PTS in E. coli can improve the succinic acid production titres up to 4-fold.462 In C. glutamicum, a similar approach has been pursued in which the myo-inositol transporter (iolT1), linked to (polyphosphate) glucokinases (ppgk or glk), serves as the substitute for the inactivated PTS.461 This strategy increases the succinic acid titres in this species by ca. 12%.461
 a). One strategy is to express the heterologous ATP-citrate lyase (ACL1) from Mus musculus in Y. lipolytica to release more acetyl-CoA from citrate in the cytosol.414 Alternatively, the native PDA1 gene, encoding the E1 α-subunit of the PDH complex,315 and the acetyl-CoA synthetase (ACS1) from S. cerevisiae can be induced to provide more acetyl-CoA for α-KGA production.414 When the three genes are separately overexpressed in Y. lipolytica, the improvements in α-KGA titres are comparable, at around 20–30% increase (Table 6), indicating that there might be a pathway bottleneck elsewhere.
a). One strategy is to express the heterologous ATP-citrate lyase (ACL1) from Mus musculus in Y. lipolytica to release more acetyl-CoA from citrate in the cytosol.414 Alternatively, the native PDA1 gene, encoding the E1 α-subunit of the PDH complex,315 and the acetyl-CoA synthetase (ACS1) from S. cerevisiae can be induced to provide more acetyl-CoA for α-KGA production.414 When the three genes are separately overexpressed in Y. lipolytica, the improvements in α-KGA titres are comparable, at around 20–30% increase (Table 6), indicating that there might be a pathway bottleneck elsewhere.
As is the case in yeast, enhancing the activity of the PDH complex in E. coli promotes the conversion of pyruvate to acetyl-CoA (Fig. 12,  a). Hereto, the aceE and aceF genes, encoding the E1 and E2 subunits of PDH, respectively, can be overexpressed.410 Furthermore, introducing the E354K substitution into the NAD+-interacting lipoamide dehydrogenase, LpdA, renders the PDH less sensitive to NADH feedback inhibition. This increases PDH activity by 80%, contributing to an increment of ca. 12% in succinic acid titres.470 Alternatively, induction of the entire aceEF-lpd operon, encoding all subunits of the PDH complex, can be achieved by eliminating the dual transcription regulator PdhR.471
a). Hereto, the aceE and aceF genes, encoding the E1 and E2 subunits of PDH, respectively, can be overexpressed.410 Furthermore, introducing the E354K substitution into the NAD+-interacting lipoamide dehydrogenase, LpdA, renders the PDH less sensitive to NADH feedback inhibition. This increases PDH activity by 80%, contributing to an increment of ca. 12% in succinic acid titres.470 Alternatively, induction of the entire aceEF-lpd operon, encoding all subunits of the PDH complex, can be achieved by eliminating the dual transcription regulator PdhR.471
Apart from acetyl-CoA, oxaloacetate, which originates from the carboxylation of pyruvate, is the other prime precursor for initiating the TCAc (Fig. 12,  b). In Y. lipolytica, this reaction can be accelerated by expressing heterologous pyruvate carboxylases from either S. cerevisiae or Rhizopus oryzae.392,481,482 Surprisingly, overexpressing the native pyruvate carboxylase (PYC1) in Y. lipolytica does not improve α-KGA levels but instead improves the levels of other TCAc intermediates. This may be due to the imbalance of pyruvate carboxylase activity and the unchanged activities of downstream enzymes.483 However, when PYC1 is overexpressed together with the NADP+-dependent isocitrate dehydrogenase (IDP1), α-KGA titres increase by 19% and pyruvate accumulation is neglectable. This approach, combined with limited thiamine and fed-batch cultivation, yielded the highest reported α-KGA titre (186 g L−1) in Y. lipolytica when glycerol was used as the feedstock (Table 6).415
b). In Y. lipolytica, this reaction can be accelerated by expressing heterologous pyruvate carboxylases from either S. cerevisiae or Rhizopus oryzae.392,481,482 Surprisingly, overexpressing the native pyruvate carboxylase (PYC1) in Y. lipolytica does not improve α-KGA levels but instead improves the levels of other TCAc intermediates. This may be due to the imbalance of pyruvate carboxylase activity and the unchanged activities of downstream enzymes.483 However, when PYC1 is overexpressed together with the NADP+-dependent isocitrate dehydrogenase (IDP1), α-KGA titres increase by 19% and pyruvate accumulation is neglectable. This approach, combined with limited thiamine and fed-batch cultivation, yielded the highest reported α-KGA titre (186 g L−1) in Y. lipolytica when glycerol was used as the feedstock (Table 6).415
In bacteria, oxaloacetate is uniquely derived from PEP, as in E. coli, or also from pyruvate, as in C. glutamicum. The key enzymes involved in these reaction pathways are called (phosphoenol)pyruvate carboxylases and phosphoenolpyruvate carboxykinases (Fig. 12,  b).432,433 Natively, PEP acts as the direct precursor for oxaloacetate in E. coli and therefore overexpressing the endogenous phosphoenolpyruvate carboxylase gene (ppc) or a heterologous copy from the cyanobacterium Anabaena sp. 7120 increases the production of α-KGA or its derived products such as mesaconate and succinic acid.444,457 Additionally, base substitutions in the upstream region of phosphoenolpyruvate carboxykinase (pck) enhance gene expression levels and, consequently, stimulate succinic acid productivity in E. coli by 30–600% (in terms of spatiotemporal yield).462,463 In C. glutamicum, oxaloacetate can originate from either pyruvate or PEP. Therefore, enhancing the expression of the native phosphoenolpyruvate or pyruvate carboxylase genes, called ppc or pyc respectively, results in higher titres of succinic acid460,478–480 and the α-KGA-mediated hydroxylation product hydroxy-isoleucine.484 Regarding pyc, the P458S mutant is often exploited to further improve the conversion into oxaloacetate as this substitution renders the pyruvate carboxylase insensitive for feedback inhibition.478 Combined with overexpressing ppc, the pyc mutant improved the succinate production titres in C. glutamicum by 24% under aerobic conditions.479
b).432,433 Natively, PEP acts as the direct precursor for oxaloacetate in E. coli and therefore overexpressing the endogenous phosphoenolpyruvate carboxylase gene (ppc) or a heterologous copy from the cyanobacterium Anabaena sp. 7120 increases the production of α-KGA or its derived products such as mesaconate and succinic acid.444,457 Additionally, base substitutions in the upstream region of phosphoenolpyruvate carboxykinase (pck) enhance gene expression levels and, consequently, stimulate succinic acid productivity in E. coli by 30–600% (in terms of spatiotemporal yield).462,463 In C. glutamicum, oxaloacetate can originate from either pyruvate or PEP. Therefore, enhancing the expression of the native phosphoenolpyruvate or pyruvate carboxylase genes, called ppc or pyc respectively, results in higher titres of succinic acid460,478–480 and the α-KGA-mediated hydroxylation product hydroxy-isoleucine.484 Regarding pyc, the P458S mutant is often exploited to further improve the conversion into oxaloacetate as this substitution renders the pyruvate carboxylase insensitive for feedback inhibition.478 Combined with overexpressing ppc, the pyc mutant improved the succinate production titres in C. glutamicum by 24% under aerobic conditions.479
 a). At the same time, the fumarases (fumABC) and fumarate reductases (frdBC) are often knocked-out to improve the α-KGA titre even further, i.e., by ca. 47% (Fig. 12,
a). At the same time, the fumarases (fumABC) and fumarate reductases (frdBC) are often knocked-out to improve the α-KGA titre even further, i.e., by ca. 47% (Fig. 12,  b).410 This strategy directs the carbon flux through isocitrate, α-KGA's direct precursor, towards α-KGA410 or, with more extensive engineering, towards its derivatives mesaconate,443trans-4-hydroxyproline,471 and hydroxyleucine.484 Additionally, the isocitrate lyase (aceA) in E. coli and C. glutamicum can be disrupted to prevent isocitrate from bypassing the oTCAc through the glyoxylate shunt, thus skipping the α-KGA node (Fig. 12,
b).410 This strategy directs the carbon flux through isocitrate, α-KGA's direct precursor, towards α-KGA410 or, with more extensive engineering, towards its derivatives mesaconate,443trans-4-hydroxyproline,471 and hydroxyleucine.484 Additionally, the isocitrate lyase (aceA) in E. coli and C. glutamicum can be disrupted to prevent isocitrate from bypassing the oTCAc through the glyoxylate shunt, thus skipping the α-KGA node (Fig. 12,  c).406,485 Deleting aceA in C. glutamicum improves the α-KGA production titre by 67%.406
c).406,485 Deleting aceA in C. glutamicum improves the α-KGA production titre by 67%.406
In P. putida, α-KGA may also arise from succinic acid through the unique enzyme tandem, comprising succinate semialdehyde dehydrogenase (SSADH) and α-KGA decarboxylase (KGDC) (Fig. 12,  d).296–298 Although the potential of these enzymes has not yet been extensively explored for α-KGA production, their implementation could unlock the possibility of producing α-KGA through the carbon-assimilating rTCAc. As such, CO2 (in the form of bicarbonate) can be consumed, in contrast to the oTCAc280 where CO2 is released. This succinic acid-producing rTCAc requires pathway optimisation, which mainly focuses on replenishing NADH levels to drive the reduction reactions, implementing non-PTS systems, increasing the oxaloacetate precursor concentration, and eliminating by-product formation (i.e., mixed organic acids).475,486 NADH regeneration is accomplished by diverting the carbon flux towards the pentose phosphate pathway (PPP), which is more efficient in generating NADPH,475,486 or by introducing one-carbon (C1)-consuming modules from methanotrophs, such as Bacillus methanolicus476,477 or Mycobacterium vaccae478 (Fig. 12,
d).296–298 Although the potential of these enzymes has not yet been extensively explored for α-KGA production, their implementation could unlock the possibility of producing α-KGA through the carbon-assimilating rTCAc. As such, CO2 (in the form of bicarbonate) can be consumed, in contrast to the oTCAc280 where CO2 is released. This succinic acid-producing rTCAc requires pathway optimisation, which mainly focuses on replenishing NADH levels to drive the reduction reactions, implementing non-PTS systems, increasing the oxaloacetate precursor concentration, and eliminating by-product formation (i.e., mixed organic acids).475,486 NADH regeneration is accomplished by diverting the carbon flux towards the pentose phosphate pathway (PPP), which is more efficient in generating NADPH,475,486 or by introducing one-carbon (C1)-consuming modules from methanotrophs, such as Bacillus methanolicus476,477 or Mycobacterium vaccae478 (Fig. 12,  d). To favour the PPP over glycolysis, the expression of glucose-6-phosphate dehydrogenase (zwf) and 6-phosphogluconate dehydrogenase (gnd) can be enhanced through ribosomal binding site engineering,475 or their enzyme activities can be stimulated by relieving feedback regulation, resulting from amino acid substitutions in key residues.486 The PPP-derived NADPH is converted into NADH using the soluble transhydrogenase (sthA) to support the rTCAc activity.475,486 As a result, the succinate titre can be improved by about 27%.486 Alternatively, heterologous methanol and formate dehydrogenases can be exploited to completely reduce C1 substrates like methanol or formic acid into carbon dioxide, releasing NADH.476–478 Moreover, the released CO2 may be fixed into oxaloacetate or α-KGA in reactions catalysed by pyruvate carboxylase or KGDC from P. putida,476,477 respectively.
d). To favour the PPP over glycolysis, the expression of glucose-6-phosphate dehydrogenase (zwf) and 6-phosphogluconate dehydrogenase (gnd) can be enhanced through ribosomal binding site engineering,475 or their enzyme activities can be stimulated by relieving feedback regulation, resulting from amino acid substitutions in key residues.486 The PPP-derived NADPH is converted into NADH using the soluble transhydrogenase (sthA) to support the rTCAc activity.475,486 As a result, the succinate titre can be improved by about 27%.486 Alternatively, heterologous methanol and formate dehydrogenases can be exploited to completely reduce C1 substrates like methanol or formic acid into carbon dioxide, releasing NADH.476–478 Moreover, the released CO2 may be fixed into oxaloacetate or α-KGA in reactions catalysed by pyruvate carboxylase or KGDC from P. putida,476,477 respectively.
Modeling metabolic fluxes can accelerate the identification of less obvious gene targets for optimising α-KGA production. For example, a flux balance using a genome-scale model of Y. lipolytica metabolism identified metabolic levers associated with citrate production. This computational approach revealed a correlation between high citrate production and the inactivity of the mitochondrial respiratory chain via the alternative oxidase, or AOX (Fig. 12,  a). The AOX enzyme is involved in the cyanide resistance pathway in plants and fungi. Based on this insight, targeted inhibition of the endogenous AOX enzyme led to a two-fold increase in citric acid production in Y. lipolytica.487 Given that α-KGA is only two enzymatic steps downstream of citrate, leveraging similar strategies could be promising for enhanced α-KGA yields.
a). The AOX enzyme is involved in the cyanide resistance pathway in plants and fungi. Based on this insight, targeted inhibition of the endogenous AOX enzyme led to a two-fold increase in citric acid production in Y. lipolytica.487 Given that α-KGA is only two enzymatic steps downstream of citrate, leveraging similar strategies could be promising for enhanced α-KGA yields.
Conversion of α-KGA into succinyl-CoA throughout the oTCAc can be blocked by knocking-out the decarboxylating moiety of the KGDH complex (Fig. 13), encoded by odhA in C. glutamicum490 or sucA in E. coli (Fig. 12,  a).491 These KGDH-inactivated mutants accumulate higher α-KGA levels,406,490,492 which can also be exploited to overproduce α-KGA-derived compounds, such as glutamate,436 GABA,440,441 and mesaconate.444 However, shutting down the KGDH complex perturbs the TCAc and therefore slows down microbial growth in the yeast Y. lipolytica493 and in bacteria E. coli492 and C. glutamicum.490 An alternative approach involves attenuating or temporarily suppressing KGDH activity rather than outrightly disrupting its function. This strategy reduces excessive α-KGA degradation while concurrently mitigating adverse growth effects. In Y. lipolytica, the amino acid substitution of two active site residues (His419 and Asp423) in the dihydrolipoamide succinyltransferase subunit (KGD2) decreases the catalytic activity of the KGDH complex, conferring a 40% increase in α-KGA production (Fig. 12,
a).491 These KGDH-inactivated mutants accumulate higher α-KGA levels,406,490,492 which can also be exploited to overproduce α-KGA-derived compounds, such as glutamate,436 GABA,440,441 and mesaconate.444 However, shutting down the KGDH complex perturbs the TCAc and therefore slows down microbial growth in the yeast Y. lipolytica493 and in bacteria E. coli492 and C. glutamicum.490 An alternative approach involves attenuating or temporarily suppressing KGDH activity rather than outrightly disrupting its function. This strategy reduces excessive α-KGA degradation while concurrently mitigating adverse growth effects. In Y. lipolytica, the amino acid substitution of two active site residues (His419 and Asp423) in the dihydrolipoamide succinyltransferase subunit (KGD2) decreases the catalytic activity of the KGDH complex, conferring a 40% increase in α-KGA production (Fig. 12,  a).494 However, growth defects were not mitigated because the growth rate of the Kgd2Asp423 mutant was reduced by half compared to the wild-type growth rate. In bacterial producers, several other options have been explored, especially in the natural glutamate producer C. glutamicum. Like all actinomycetes, this microbe has a single, hybrid PDH-KGDH supercomplex (Fig. 13) that predominantly localises at the cell poles and performs the dehydrogenase reaction on both pyruvate and α-KGA.314 In addition, this hybrid complex is tightly regulated by a serine/threonine protein kinase G (PknG) that phosphorylates the PDH-KGDH inhibitor, OdhI (Fig. 12,
a).494 However, growth defects were not mitigated because the growth rate of the Kgd2Asp423 mutant was reduced by half compared to the wild-type growth rate. In bacterial producers, several other options have been explored, especially in the natural glutamate producer C. glutamicum. Like all actinomycetes, this microbe has a single, hybrid PDH-KGDH supercomplex (Fig. 13) that predominantly localises at the cell poles and performs the dehydrogenase reaction on both pyruvate and α-KGA.314 In addition, this hybrid complex is tightly regulated by a serine/threonine protein kinase G (PknG) that phosphorylates the PDH-KGDH inhibitor, OdhI (Fig. 12,  a).495 When PknG is active, it phosphorylates OdhI which causes the inhibitor to be released from the OdhA subunit.496 Presumably, the activation state of PknG is regulated through the GlnH- and GlnX-mediated signaling cascade. Extracellular aspartate and glutamate bind onto the GlnH receptor which causes PknG to dissociate from GlnX and, in turn, the unleashed PknG prevents OdhI from binding to OdhA through phosphorylation (Fig. 13).497 Interfering with this regulatory pathway provides an alternative strategy to increase α-KGA production in C. glutamicum. Indeed, deleting pknG keeps OdhI in an unphosphorylated state, allowing the inhibitor to suppress KGDH activity and thereby leading to higher α-KGA accumulation. Together with overexpressing glutamate decarboxylase (gadB), the pknG-engineered strain produces more than twice the amount of GABA than its parent, in which pknG was still intact.439 Replacing the native ribosomal binding site of odhA with a weaker variant, or exchanging the consensus start codon for a less efficient one are other successful approaches that favour α-KGA accumulation and enhance the titres of α-KGA-derived products, such as ornithine (+16.7%),449 arginine (+12%),446 and putrescine (+15%)498, in corynebacterial producers. Finally, redesigning regulatory circuits with promoters that are induced during the exponential phase or by quorum allows for the dynamic expression of the KGDH complex.442,471 This strategy ensures proper functioning of the TCAc during the growth phase, but bypasses the α-KGA dehydrogenase reaction during the stationary phase to maximally produce α-KGA as a precursor for the production of GABA (+77% in titre) in C. glutamicum442 or trans-4-hydroxyproline (+14.7% in titre) in E. coli.471 As an alternative, a metabolite (e.g., isoleucine)-responsive feedback loop can also be implemented to dynamically repress odhA in order to favour the α-KGA-dependent production route of 4-hydroxy isoleucine in C. glutamicum.484
a).495 When PknG is active, it phosphorylates OdhI which causes the inhibitor to be released from the OdhA subunit.496 Presumably, the activation state of PknG is regulated through the GlnH- and GlnX-mediated signaling cascade. Extracellular aspartate and glutamate bind onto the GlnH receptor which causes PknG to dissociate from GlnX and, in turn, the unleashed PknG prevents OdhI from binding to OdhA through phosphorylation (Fig. 13).497 Interfering with this regulatory pathway provides an alternative strategy to increase α-KGA production in C. glutamicum. Indeed, deleting pknG keeps OdhI in an unphosphorylated state, allowing the inhibitor to suppress KGDH activity and thereby leading to higher α-KGA accumulation. Together with overexpressing glutamate decarboxylase (gadB), the pknG-engineered strain produces more than twice the amount of GABA than its parent, in which pknG was still intact.439 Replacing the native ribosomal binding site of odhA with a weaker variant, or exchanging the consensus start codon for a less efficient one are other successful approaches that favour α-KGA accumulation and enhance the titres of α-KGA-derived products, such as ornithine (+16.7%),449 arginine (+12%),446 and putrescine (+15%)498, in corynebacterial producers. Finally, redesigning regulatory circuits with promoters that are induced during the exponential phase or by quorum allows for the dynamic expression of the KGDH complex.442,471 This strategy ensures proper functioning of the TCAc during the growth phase, but bypasses the α-KGA dehydrogenase reaction during the stationary phase to maximally produce α-KGA as a precursor for the production of GABA (+77% in titre) in C. glutamicum442 or trans-4-hydroxyproline (+14.7% in titre) in E. coli.471 As an alternative, a metabolite (e.g., isoleucine)-responsive feedback loop can also be implemented to dynamically repress odhA in order to favour the α-KGA-dependent production route of 4-hydroxy isoleucine in C. glutamicum.484
 | ||
Fig. 13 The biochemistry behind the KGDH complex, its regulation, and its preferred engineering targets. (Left) The KGDH complex in the context of the oTCAc. The KGDH complex in Y. lipolytica and E. coli consists of three domains: E1, α-KGA decarboxylase, E2, lipoylated succinyl transferase, and E3, dihydrolipoyl dehydrogenase (right).617 In contrast, C. glutamicum has a PDH-KGDH supercomplex, which, besides the pyruvate dehydrogenase domains (AceEF), also includes E3 and OdhA. OdhA acts as both E2 and E1 domains and assembles in vivo into a hexameric, three-blade propeller shape.618 When OdhI is unphosphorylated, the inhibitor can bind to one of the OdhA domains to keep it inactive. The potential of OdhI to act as an inhibitor depends on its phosphorylation state, which, in turn, is influenced by the GlnH–GlnX–PknG signaling cascade as explained in the main text.619 Symbols refer to the KGDH-related engineering strategies,  refers to the gene knock-out strategy. The colors of the symbols refer to the microorganism, with dark red representing Y. lipolytica, orange E. coli, and dark blue C. glutamicum. The figure is based on ref. 617–619. refers to the gene knock-out strategy. The colors of the symbols refer to the microorganism, with dark red representing Y. lipolytica, orange E. coli, and dark blue C. glutamicum. The figure is based on ref. 617–619. | ||
Aside from being converted into succinyl-CoA, α-KGA can also be transformed into glutamate by the GS-GOGAT pathway. Disrupting glutamate dehydrogenase (gdh)499 and the β-subunit of glutamate synthase (gltB)500 prevents the transamination in C. glutamicum, resulting in a 9-fold increase of α-KGA titres (Fig. 12,  b).399,406 This approach has never been attempted in Y. lipolytica. However, the function and cofactor utilisation of the Y. lipolytica glutamate dehydrogenase genes (GDH1 and GDH2) has been characterised recently. GDH2 was found to be the primary dehydrogenase for glutamate assimilation. Deleting this gene did not alter growth rates in glucose media with ammonia or glutamate as nitrogen sources.320 If Y. lipolytica's GDH2 also performs the reversible glutamate dehydrogenation (α-KGA ↔ glutamate) as in the model yeast S. cerevisiae, GDH2 deletion could be an interesting gene target to promote α-KGA accumulation (Fig. 12,
b).399,406 This approach has never been attempted in Y. lipolytica. However, the function and cofactor utilisation of the Y. lipolytica glutamate dehydrogenase genes (GDH1 and GDH2) has been characterised recently. GDH2 was found to be the primary dehydrogenase for glutamate assimilation. Deleting this gene did not alter growth rates in glucose media with ammonia or glutamate as nitrogen sources.320 If Y. lipolytica's GDH2 also performs the reversible glutamate dehydrogenation (α-KGA ↔ glutamate) as in the model yeast S. cerevisiae, GDH2 deletion could be an interesting gene target to promote α-KGA accumulation (Fig. 12,  b).
b).
Since excessive by-product formation drains the pyruvate, PEP, and acetyl-CoA pools and therefore competes with α-KGA production, the mixed organic acid pathways are often eliminated in re-engineered bacterial producers to enhance the yields of α-KGA (Fig. 12,  ) (Table 6), succinic acid (Table 8), and α-KGA- or glutamate-derived chemicals (Table 7).410,457,463,470,472,478,505–509
) (Table 6), succinic acid (Table 8), and α-KGA- or glutamate-derived chemicals (Table 7).410,457,463,470,472,478,505–509
In addition to the fatty acid biosynthesis pathway, other competing acetyl-CoA-consuming pathways can be inhibited to leave more acetyl-CoA for the TCAc. For example, deleting the Y. lipolytica CoA-transferase gene ACH1 eliminated acetic acid by-product formation from acetyl-CoA and, therefore, improved succinic acid production by 27% (Fig. 12,  ).510 While these strategies have thus far been mainly explored for other TCA-derived carboxylic acids in Y. lipolytica, they may also help boost α-KGA production.
).510 While these strategies have thus far been mainly explored for other TCA-derived carboxylic acids in Y. lipolytica, they may also help boost α-KGA production.
 ).416 In addition to cell-membrane transporters, five mitochondrial carboxylic acid transporters were identified. Overexpression of all transporter genes led to improvements in extracellular succinic acid production, except for gene YALI0E34672g.512 The YALI0E34672g mitochondrial transporter was then tested for α-KGA, where overexpression led to a 20% increase in α-KGA production in Y. lipolytica (Fig. 12,
).416 In addition to cell-membrane transporters, five mitochondrial carboxylic acid transporters were identified. Overexpression of all transporter genes led to improvements in extracellular succinic acid production, except for gene YALI0E34672g.512 The YALI0E34672g mitochondrial transporter was then tested for α-KGA, where overexpression led to a 20% increase in α-KGA production in Y. lipolytica (Fig. 12,  ).404
).404
 | ||
| Fig. 14 Different substrate utilisation routes contribute to α-KGA production. The numbers refer to the reaction chemistry as explained in Table 9. Abbreviations: G, glucose; G6P, glucose-6-phosphate; F6P, fructose-6-phosphate; DHAP, dihydroxyacetone phosphate; G3P, glyceraldehyde-3-phosphate; PEP, phosphoenol pyruvate; PYR, pyruvate; Gly, glycerol; Gly3P, glycerol-3-phosphate; EtOH, ethanol; MeOH, methanol; FHO, formaldehyde; AcHO, acetaldehyde; AcOOH, acetic acid; X, xylose; A, arabinose; GA, galacturonate; 6PGL, 6-phosphogluconolactone; 6PG, 6-phosphogluconate; Ru, ribulose; Xu, xylulose; Ru5P, ribulose-5-phosphate; Xu5P, xylulose-5-phosphate; R5P, ribose-5-phosphate; S7P, sedoheptulose-7-phosphate; E4P, erythrose-4-phosphate; Hu6P, hexulose-6-phosphate; ALK, n-alkane; ALKOH, alkanol; ALKHO, alkanal; ALKOOH, alkanoic acid; AcCoA, acyl-coenzyme A; ActCoA, acetyl-coenzyme A; XL, xylonolactone; AL, arabinolactone; GL, galactarolactone; XA, xylonic acid; AA, arabonic acid; GAA, galactaric acid; KDX, 2-keto-3-deoxy-xylonic acid; KDA, 2-keto-3-deoxy-arabonic acid; KDG, 5-keto-4-deoxy-glucaric acid; DPA, 2,5-dioxopentanoic acid; and β, fatty acid degradation (β-oxidation) pathway. Structures in blue boxes represent the primary carbon sources that originate from conventional (e.g., starch-based) and alternative feedstocks (e.g., vegetable oils, lignocellulose, and alcohols). The colors of the arrows correspond to the ones used in Fig. 10. The figure is based on ref. 438, 452, 530, 531, 552 and 616. | ||
5.5.8.1. Alkanes. Alkanes are abundantly present in petroleum, generally reaching up to 45 (v/v)%.526 Their biodegradation is often restricted to free-living off-shore microorganisms, which remain largely unexplored as microbial cell factories.527,528 One exception is the yeast Y. lipolytica, which can degrade and thrive on n-alkanes as the sole carbon source owing to the secretion of emulsifiers and the activity of a cytochrome P450-based hydroxylation cascade (Fig. 14 and Table 9, reactions (25)–(28)).529–531 Uptake and alkane catabolism in Y. lipolytica ultimately results in acetyl-CoA, bypassing the thiamine-dependent pyruvate dehydrogenase (PDH) that would normally replenish acetyl-CoA from glucose or glycerol.530 Hence, even at low thiamine concentrations, a high α-KGA output can be achieved without the issue of pyruvate accumulation. The highest α-KGA production level yet (196 g L−1, 0.94 g g−1) was reached on a mixture of C12-C18 alkanes by Y. lipolytica.413
While n-alkanes are an efficient substrate for α-KGA production, sustainability concerns are often raised when using petroleum-derived hydrocarbons as a substrate for fermentation.26 However, n-alkanes can be sourced in a more sustainable way, such as through chemical recycling of plastic waste. Upon thermal pyrolysis of polyolefin plastics, one of the primary products obtained is high molecular weight hydrocarbons (≥C18).532 Not only are these hydrocarbons suitable substrates for α-KGA production by Y. lipolytica, but they also hold promise in the transition towards a circular economy. Still, the feasibility of α-KGA production from the products of plastic pyrolysis is yet to be evaluated.
5.5.8.2. Glycerol. The use of alternative renewable carbon feedstocks that originate from the main by-product of the biodiesel industry, raw glycerol, has been explored.26 In biodiesel production, the triglycerides in vegetable or animal fats are saponified or transesterified, releasing fatty acids (biodiesel) and glycerol as by-products. Glycerol is an attractive carbon substrate for α-KGA production in Y. lipolytica because this yeast grows faster on this triol (and lipid-rich substrates) than on glucose.384,533,534 This preference for glycerol may be linked to Y. lipolytica's six glycerol transporters (STL2, STL3, STL6, STL8, FPS1, and FPS2)535 in combination with a weak catabolite repression system.384,536 Accordingly, glycerol serves as the primary carbon substrate in most strain engineering and process optimisation studies on Y. lipolytica discussed in this review.
Apart from Y. lipolytica, E. coli is also a suitable microbial producer for glycerol-based bioproduction processes owing to its native glycerol metabolism. This bacterium possesses the aerobic GlpK–GldD and anaerobic GlpK–GlpABC respiratory pathways that employ ubiquinol as an electron acceptor. Alternatively, it can also use the microaerobic NAD+-mediated GldA–DhaKLM fermentative route to transform the triol into dihydroxyacetone-phosphate (Fig. 14 and Table 9, reactions (5) and (6)).474,537,538 Although E. coli is well equipped for dissimilating glycerol, growth rates on glycerol do not match those obtained with glucose-rich media.539 However, this limitation can be overcome through ALE by evolving cells in a minimal medium supplemented with glycerol as the sole carbon source.540 The fittest clone, isolated from the evolution experiment, consumed glycerol 46% faster than its parental strain, which improved the growth rate by nearly 40% in a glycerol-rich minimal medium. The authors identified a missense mutation in the fructose-1,6-bisphosphate allosteric inhibitor binding site of GlpK as the prime driver for enhanced glycerol uptake in the adapted clone. Furthermore, this study also demonstrated that the superior glycerol consumption phenotype can be exploited for GABA production from the α-KGA/glutamate precursor, when gabT, ackA, and mgsA, encoding GABA aminotransferase, acetate kinase, and methylglyoxal synthase, respectively, are also inactivated. C. glutamicum lacks a functional glycerol metabolism, but this limitation can be solved by introducing glycerol kinase (glpK) and dehydrogenase (glpD) from E. coli.541 Interestingly, although these engineered strains showed lower substrate uptake rates on a glucose–glycerol mixture compared to growth on the corresponding single carbon sources, no diauxic growth behaviour was observed. This improved glycerol utilisation phenotype has been successfully exploited to enhance the production of the α-KGA derivatives, such as glutamate,541 GABA,442 and putrescine,542 in C. glutamicum.
5.5.8.3. Fatty acids. In addition to its ability to accumulate lipids in the cell, Y. lipolytica can also consume lipids or aliphatic fatty acids as a carbon source (Fig. 14). Similar to alkane degradation, fatty acid catabolism in Y. lipolytica also connects with the TCAc via acetyl-CoA, bypassing the thiamine-dependent PDH. Several studies have tested growth substrates with renewable fatty acid sources, such as rapeseed oil, sunflower oil, and olive oil,385,424 and as a mixture with glycerol.393,394,404 At low thiamine concentrations, Y. lipolytica grows better on oil than on glycerol, and it produces more α-KGA with trace amounts of pyruvate as a by-product.394 However, while fatty acids are generally a better substrate for α-KGA production in Y. lipolytica, glycerol is less expensive than vegetable oil. Additionally, mixing oil with a water-soluble substrate like glycerol can facilitate industrial processes due to easier mixing and reduced need for emulsifiers.393
5.5.8.4. Alcohols. Mono-alcohols, such as ethanol or methanol, can also be applied as feedstock for microbial production processes. For example, Y. lipolytica can be cultured on ethanol for the production of α-KGA.388,543 Similarly to fatty acids and alkanes, ethanol also enters the TCAc via acetyl-CoA which bypasses the pyruvate intermediate (Fig. 14 and Table 9, reactions (7)–(9)). However, ethanol is partly oxidised into acetic acid when pH drops to 3 or lower, a condition that occurs commonly during carboxylic acid production which may limit the applicability of ethanol as feed.388 Overall, α-KGA production titres in Y. lipolytica are higher when glycerol or oil is used as a substrate instead of ethanol (Table 7).
Methanol has also drawn the attention of the scientific and industrial community as this alcohol can be obtained sustainably from carbon dioxide hydrogenation and biogas-based steam reforming.544 From a biological perspective, methanol consumption is far from a universal trait and is mostly restricted to methylotrophic bacteria such as Bacillus, Burkholderia, Methylomonas, Methylobacter, and Methylosarcina species, and the Candida and Pichia yeasts.545,546 Recent research efforts have focused on integrating these natural methanol assimilation pathways into industrial microbial chassis strains like E. coli and C. glutamicum. As discussed earlier, introducing methanol and formate dehydrogenases in E. coli considerably enhances succinate production in glucose–methanol co-feeding regimes because metabolising methanol provides both reducing equivalents and extra carbon that can be assimilated in the final product.476,477 Furthermore, the co-consumption of xylose and methanol can be stimulated in C. glutamicum by introducing a methanol dehydrogenase and a synthetic ribulose monophosphate pathway.438 This phenotype was artificially engineered by disrupting the pentose metabolism at the ribose phosphate isomerase (rpiB) node and transferring the 3-hexulose-6-phosphate synthase (hps) and 6-phospho-3-hexuloisomerase (phi) from Bacillus methanolicus to complement the anticipated defect in the ribose metabolism (Fig. 14 and Table 9, reactions (22)–(24)). Methanol utilisation was further optimised in the methanol-consuming C. glutamicum strain through fourteen ALE passages in methanol- and xylose-based defined medium, resulting in a 20-fold growth rate increase and a final glutamate titre of 90 mg L−1.437
5.5.8.5. The C5 (hemi)cellulose sugars: xylose and arabinose. In the EU, ca. 53 million tons of cellulose and ca. 36 million tons of hemicellulose are harvested annually from agricultural and forestry activities.547 Although these biomass fractions are unsuitable for human consumption, they can serve as a carbohydrate source to support microbial growth. Alongside glucose, pretreatment of (hemi)cellulose also releases xylose and arabinose monomers that cannot be efficiently catabolised by C. glutamicum548,549 and Y. lipolytica.524 While C. glutamicum only expresses the xylulokinase (xylB) gene at low levels,548Y. lipolytica harbors a complete but dormant PPP, resulting in poor xylose consumption rates.550,551 Many other microorganisms, however, are capable of converting xylose and arabinose into D-xylulose-5-phosphate through a series of oxo-reductive or isomerisation reactions that are found in fungi or (eu)bacteria such as E. coli, respectively (Fig. 14 and Table 9, reactions highlighted in orange). The resulting D-xylulose-5-phosphate is further metabolised throughout the non-oxidative PPP before entering the TCA at the citrate synthase node.552–554 To enable C. glutamicum to consume pentose sugars, the missing enzymes from closely related species have been sourced for integration in the producer's genome. Co-overexpressing the xylose isomerase (xylA) from Xanthomonas campestris in combination with either the xylulokinase from the same foreign species or the native xylB facilitates pentose consumption and production of α-KGA-derived metabolites (lysine, glutamate, and diamines).480,555 Furthermore, most C. glutamicum strains, except for ATCC 31831, lack the araBAD operon that is crucial for arabinose utilisation and encodes ribulokinase, arabinose isomerase and ribulose 5-phosphate 4-epimerase.556 In these bacterial cells, the arabinose consumption deficiency can be resolved by introducing the E. coli-originated araBAD operon, which enables the production of α-KGA-derived amino acids, such as glutamate, ornithine, and arginine, from mixtures of glucose and arabinose.557,558 Apart from the general phosphorylating pathway (discussed above), two other alternative pentose-metabolising and PPP-independent pathways exist in which either xylose or arabinose is converted into 2-keto-3-D-deoxy-xylonate or -arabonate, respectively.303,554,559 These last intermediates are finally transformed into ethylene glycol or glycolic acid through the Dahms pathway560 or, alternatively, converted via 2,5-dioxopentanoate into α-KGA through the Weimberg route (Fig. 14 and Table 9, reactions highlighted in purple).305,554 Evidently, the Weimberg pathway is most relevant for this review and is often encountered in Archaebacteria as well as in certain bacterial species, including C. crescentus and Burkholderia multivorans.303 Compared to the combination of glycolysis or PPP with TCAc, the Weimberg pathway requires fewer reaction steps to reach α-KGA and is inherently carbon-conservative (i.e. no emission of CO2).561 Besides these intrinsic biochemical advantages, introducing the foreign Weimberg pathway in E. coli and C. glutamicum circumvents carbon catabolite repression,562–564 paving the path for the simultaneous consumption of pentose and hexose sugars when feeding lignocellulose-derived substrates.565 Integrating the Weimberg enzyme cascade from C. crescentus or B. multivorans enables E. coli to efficiently produce α-KGA-derived chemicals like mesaconate445 and butanediol566 from xylose and/or arabinose. In addition, the Weimberg pathway can be implemented to relieve the glutamate auxotrophy that may arise during pathway optimisation of another TCAc-derived target chemical, such as itaconic acid567 or even α-KGA. In other bacterial species, including C. glutamicum and Bacillus subtilis, the Weimberg metabolic route has been employed to produce succinic acid568,569 or the biopolymer poly-γ-glutamic acid,570 respectively, in a carbon-neutral way from xylose. In yeast, the Weimberg pathway has only been tested in S. cerevisiae, but its introduction resulted in poor xylose utilisation efficiency.571
6. Recovery and purification of α-KGA from the fermentation broth
During fermentation, α-KGA accumulates in the fermentation broth alongside microbial by-products, with the residual substrate and nutrients potentially still present. These impurities need to be removed in the downstream processing step, to obtain α-KGA in high yield and purity. The required purification process is far from trivial and can account for up to 40% of the total production expenses.572,573Generally, (di)carboxylic acids are recovered from the fermentation broth in several steps. In the first pretreatment stage, biomass (cells and proteins) is eliminated by centrifugation and filtration. Next, the supernatant is decoloured and purified by active carbon treatment, and salts (and water) are withdrawn using ion exchange resins (Fig. 15A). Finally, the pure end-product is obtained by crystallisation (Fig. 15B).573 Recuperating α-KGA from concentrated fermentation solutions typically involves ethyl acetate as the extraction solvent and sulphuric acid as an acidification agent to convert potassium or sodium salts of α-KGA to organic acids (besides the use of ion exchange resins).342,421,574 Apart from acidification, a liquid–liquid extraction system can also be used to recover α-KGA, such as an acetone/(NH4)2SO4-solvent system, followed by crystallisation.575,576 These methods have been industrially implemented to purify other microbially produced carboxylic acids, such as succinic acid, lactic acid, and itaconic acid,572 whereas they have only been tested at the laboratory scale for α-KGA recovery.
 | ||
| Fig. 15 Recovery strategies for purification of α-KGA from microbial fermentation. Inside the fermentation broth, α-ketoglutarate usually exists in its salt form, bound to sodium, potassium, or calcium cations. (A) Pre-treatment of the fermentation broth by centrifugation and ultra- or nanofiltration using ceramic membranes (CM) to separate the biomass (microbial cells, cell debris, and proteins) from the supernatant. Acidification is often implemented to convert the α-KG salt into α-KGA. (B) Traditionally, α-KGA is isolated and enriched from the complex fermentation broth using an ethyl acetate (EtAc)-based extraction protocol, followed by distillation. The distillate is vacuum evaporated, facilitating crystallisation to yield solid α-KGA (salt) crystals. Alternatively, α-KGA (salts) can be recovered using membrane-based setups. One approach is called bipolar membrane electrodialysis which uses a combination of an electric field and multiple membranes, both bipolar and cation exchange membranes that are organised in a stack (C). Another approach, called forward osmosis, exploits a high salt draw solution to create an osmotic gradient that withdraws water from the pre-treated fermentation broth (D). Abbreviations: EtAc, ethyl acetate; BPM, bipolar membrane; CEM, cation exchange membrane; and FOM, forward osmosis membrane. The figure is created based on ref. 574, 578 and 581. | ||
Although high purity (99%) can be achieved through both methods described above, using concentrated mineral acids (e.g., sulphuric acid) and organic solvents may generate considerable waste and harm the environment.574 To provide a more sustainable alternative, solvent-free setups such as bipolar membrane electrodialysis or forward osmosis can be used.577–581 The advantage of electrodialysis is that multiple bipolar membranes can be stacked in combination with anion and/or cation exchange membranes to concentrate α-KGA progressively. The application of electric power is necessary to generate an electric field that drives the purification process (Fig. 15C).580 In the case of forward osmosis, water is withdrawn from the feed (i.e., preprocessed fermentation broth) using an osmotic gradient imposed by a draw solution, rich in salts. Since establishing an osmotic gradient in this recovery method requires no energy input other than the circulation of feed and draw solutions, it may be more cost-effective (Fig. 15D). However, although high α-KGA recovery was observed (94%), the efficiency of forward osmosis depends greatly on the composition of the fermentation broth. Indeed, excessive concentrations of impurities like proteins and inorganic cations (Ca2+ and Mg2+) are notorious for causing membrane fouling.581 Therefore, extracting α-KGA from these complex media requires an extra pretreatment step before the fermentation broth can be fed over the membranes. This operation substantially increases the overall purification cost.580,581
In summary, the recovery efficiency of α-KGA and organic acids in general from fermentation broths can vary significantly, depending on the purification strategy applied, the choice of producer strain, and the type of feedstock.582–584 Although the methods discussed above show potential, α-KGA recovery is relatively new and hence underexplored. As such, established or even recently emerged methods, such as electrodeionisation,585 for purifying other carboxylic acids (e.g., succinic acid and lactic acid) can serve as a stepping stone for developing improved purification processes for α-KGA. Overall, improved downstream processes should aim for industrial-scale recovery yield and product purity (i.e., >90% and >98%, respectively).572
7. Concluding remarks and future perspectives
α-KGA is a key platform chemical that enables a more bio-based and sustainable production of a diverse range of products that are traditionally derived from (petro)chemical processes. The combination of an α-keto and di-acidic moiety makes α-KGA an interesting building block, either in its original form or as a precursor for chemocatalytic conversion into novel intermediates and polymers. For example, implementing α-KGA directly in copolymer synthesis opens new avenues toward fabrication of innovative polyamides and poly(keto)esters with applications in tissue engineering, drug delivery, and surface coating. Alternatively, α-KGA can be converted into highly reactive, ring-shaped building blocks (e.g., lactones, carbazones, hydrazones, carbolines, and quinazolines) that serve as precursors for bioactive and pharmaceutical compounds. In medicine, α-KGA is an important intermediate of the central metabolism in living cells and plays a pivotal role in multiple cellular processes, including energy and fat metabolism, aging, and inflammation. Consequently, α-KGA has been evaluated in several studies as a nutraceutical or pharmaceutical to attenuate inflammation, accelerate muscle recovery, promote longevity, and for treating obesity, cancer, and cardiovascular pathologies. Despite the vast range of potential applications, industrial exploitation of α-KGA is still in its infancy. Specifically, the industrial use of α-KGA as a precursor for polymerisation or catalytic conversion to other derivatives and secondary chemicals remains underexplored.Today's traditional fossil-based chemical industry will eventually need to be replaced by a circular bio-based economy. This transition will likely put α-KGA in the spotlight as one of the most promising platform chemicals because it can be produced through precision fermentation starting from sustainable plant-based biomass rich in sugars and/or fatty acids, and even from waste streams such as alcohols, alkanes, and glycerol. Moreover, recent advances in molecular biology, metabolic engineering, and process optimisation in organisms like Y. lipolytica, E. coli, and C. glutamicum have already enabled α-KGA production with yields up to 0.94 g g−1, titres as high as 195 g L−1, and spatiotemporal yields up to 1.75 g L−1 h−1. These values are especially promising since these numbers surpass the commonly considered rule-of-thumb references of 100 g L−1 and 1 g L−1 h−1. The structure of α-KGA bears resemblance to some of the highly researched organic acids included in the DOE's bio-based chemical list, such as lactic acid (C3), succinic acid (C4), fumaric acid (C4), malic acid, levulinic acid (C4), itaconic acid (C5), and the aspartic (C4) and glutamic (C5) (amino)acids.23,586–588 However, compared to these organic acids, α-KGA is perhaps more interesting owing to its unique and versatile chemical properties, broad application potential, and the progress that has been made towards its sustainable production via precision fermentation. In the following paragraphs, we elaborate further on these advantages, as well as the main challenges that have to be addressed to establish α-KGA as a commonly adopted platform chemical.
7.1. α-KGA as a versatile building block for the bioeconomy model
Like most other DOE-listed chemicals, α-KGA is not considered acutely toxic which limits hazards during production, storage, and transport. Owing to its safety profile and pharmacological benefits, α-KGA, either as acid or salt, is currently tested for treating severe human diseases (e.g., cardiovascular diseases,190 cancer,194–197 and obesity191,233), even in clinical trial settings.589 To the best of our knowledge, none of the other DOE-listed chemicals has a comparable broad-range medical use to α-KGA. Only for succinic and itaconic acid, evidence suggests that both DOE molecules may be applied as potential pharmaceuticals or cosmetics in some limited cases. Succinic acid is linked to skincare590 applications or to relieve menopausal symptoms in women,591,592 whereas itaconic acid reduces inflammatory responses.593,594 Because α-KGA has been demonstrated to improve the physiological state of healthy individuals and patients suffering from human pathologies, we expect that this biochemical might trickle down into pharma- or nutriceutical applications.In addition, α-KGA can serve as a precursor or additive in various chemical synthesis pathways, making it particularly suitable as a platform chemical to produce other valuable chemicals. This versatility also opens a wealth of opportunities for novel chemical derivatives and intermediates for polymer synthesis that could be derived from α-KGA (Fig. 16). While α-KGA exhibits the same di-acidic structure as most other DOE-prioritised chemicals, such as succinic, fumaric, and malic acid, it also carries a unique carbonyl group on its backbone that can be targeted for further chemical modification.23 Moreover, the α-carboxylic acid group can positively affect the adjacent carbonyl group (through electronic effects and proton shuttle), which results in an intramolecular activation of the carbonyl group when producing novel derivatives from the α-KGA substrate (i.e., electrophilic ketones; cf. Section 2 and Fig. 16).54,595 The electrophilic nature of the α-carbonyl group can be completely reversed (i.e., umpolung) when α-carboxylate salts are used instead. Subsequent decarboxylation results in a nucleophilic acyl anion which is very suitable for acylation or, in general, cross-coupling reactions, while the α-carbonyl and γ-carboxyl(ate) groups are retained.596 Similarly, incorporating α-KGA as a co-monomer into the backbone of novel materials can expand its application potential in material engineering even more owing to the unique chemical properties of the carbonyl group. Additionally, the immediate use of the non-toxic, polar α-KGA turns the resulting polymers also more safe and environmentally friendly.597
 | ||
| Fig. 16 Potential platform of α-KGA towards secondary derivatives for chemical/polymer, green solvent, and detergent applications. Abbreviation: Pd/Cu, palladium–copper bimetallic catalyst. | ||
The examples of novel, valuable chemicals derived from α-KGA (Fig. 16), along with the in-depth overview provided in Section 2.2, can inspire chemists and polymer scientists to explore new applications of α-KGA in the synthesis of specialised derivatives and polymers. Its potential as a polymer building block opens opportunities for entry into the plastics industry beyond its current and modest market, which is primarily focused on lactones, pharmaceuticals, and other fine chemicals.
When exploring new valorisation routes, it is of utmost importance to prioritise the use of inexpensive resources (such as hydrogen, oxygen, (di)amines, and (di)alcohols) and catalysts, while hazardous reagents for humans and the environment should be avoided. In the case of novel polymer materials, the biodegradability or recyclability aspect should also be considered. Several review papers have discussed this topic in more detail, from the different formulations for biodegradable plastics598 to the end-of-life treatments to recycle plastics.599 Concerning the end-of-life considerations, we have briefly discussed in this review about using pyrolysis to degrade less-recyclable plastics and implementing this mixture as feedstocks for Y. lipolytica. Although this field is still in its infancy, it could in time offer a sustainable upcycling opportunity in which α-KGA-based polymers are manufactured from old plastic waste.
7.2. Microbial α-KGA production guarantees sustainable production with satisfactory yields
α-KGA can be produced through microbial cell factories fed with biomass, renewable carbon substrates, or hydrocarbons from the chemical recycling of plastic, offering a sustainable alternative to the current catalytic process that relies on crude oil and toxic (chlorinated) reagents (cf.Fig. 7). One exception is the heterogeneously catalysed, but experimental, aldol condensation route starting from pyruvic acid and glyoxylic acid,263 two building blocks derived from glucose metabolism, which might offer an alternative for current industrial practices.α-KGA is an intrinsic intermediate of the oTCAc respiratory pathway, a central metabolic route that is present throughout the tree of life. This implies that most microbes possess all key enzymes for α-KGA production. However, because α-KGA is an intermediate of an essential metabolic pathway, attenuating the oTCAc to accumulate α-KGA became one of the key challenges. In this review, we have summarised all strategies for high α-KGA accumulation in bacteria and yeast, from fermentation condition optimisation to metabolic pathway engineering. Many targets to manipulate the cells’ metabolism towards higher α-KGA production have been tested, including engineering of genes directly linked to the TCAc. In addition, different parameters in fermentation, from pH and aeration to substrate-feeding strategies, have also been explored. We found several strategies that are most effective for α-KGA production.
The bacterium C. glutamicum and the yeast Y. lipolytica are some of the most promising microbial hosts for α-KGA production. In the case of C. glutamicum, disrupting the KGDH complex and the α-KGA amination systems while keeping ammonium concentrations low provides a product concentration of ca. 50 g L−1 with a yield of 0.42 g g−1 substrate.406 In Y. lipolytica, limiting thiamine in a fed-batch cultivation system generally results in high titres (up to 195 g L−1) and yields (up to 0.94 g g−1 substrate), regardless of the strain's genotype.393,413,415 These values are comparable to those reported for other DOE bio-produced chemicals (Table 10), suggesting that it could become technically and economically realistic to implement α-KGA production in commercial industrial processes. Additionally, implementing a fed-batch setup for substrate feeding generally increased α-KGA production, as observed in many other industrial bioprocesses.
| Compound/structure | Microbial producer | Titre [g L−1] | Productivity [g L−1 h−1] | Global production [tonnes per year] | Manufacturers |
|---|---|---|---|---|---|
| a The current production volume of bio-based succinic acid is not available and is expected to be lower than the one provided in the table (corresponding to 2015). This decrease is due to the suspension of activities by BioAmber in 2018 and its successor, LCY Biosciences, in 2023. | |||||

|
LAB | 207604 | 3–9604 | 750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
NatureWorks (US) |
| Bacillus sp. | 225604 | Corbion (NL) | |||
| Rhizopus sp. | 231604 | Galactic (BE) | |||
| Futerro (BE) | |||||

|
Actinobacillus | 96605 | 1.99605 | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000a 000a |
GC Innovation America (US) |
| succinogenes | Reverdia (NL) | ||||
| Y. lipolytica | 160606 | 0.40606 | Succinity (NL, GE) | ||

|
Aspergillus niger | 201607 | 0.93607 | 80–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000608 000608 |
Bartek (CAN) |
| Trichoderma resei | 220607 | 1.15607 | Mitsubishi Corporation Life Sciences (JP) | ||
| Anhui Sealong Biotechnology (CN) | |||||

|
E. coli | 47609 | 0.39609 | 40–70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Qingdao Kehai Biochemistry (CN) |
| Aspergillus terreus | 78609 | 1.07609 | Cargill (US) | ||
| Ustilago maydis | 220610 | 0.74610 | Citrique Belge (BE) | ||

|
C. glutamicum | 120611 | ∼4.6611 | ∼4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000612 000612 |
Ajinomoto (JP) |
| Hefei TNJ Chemical Industry (CN) | |||||
| Evonik (GE) | |||||
Based on the literature, there are three aspects that caught our attention. First, the research for microbial α-KGA production is still mostly limited to the laboratory scale. Scaling up to larger volumes often changes fermentation performance, so estimating economic viability using current parameters is difficult. Consequently, process optimisation and scale-up across the entire production chain, including the pretreatment process, bioreactor processing, and product recovery (cf. next subsection) at industrial biorefinery scales, should be intensively revisited.
Second, the use of inexpensive and non-edible biomass (such as lignocellulosic agricultural residues) and waste streams (e.g., plastic pyrolysis or wasted vegetable oil, and crude glycerol fractions from biodiesel industries) for α-KGA production is still underexplored. The utilisation of these feedstocks can be applied in synergy in both C. glutamicum, which prefers hexose sugars present in plant biomass, and Y. lipolytica, which prefers fatty acids, alkanes, and glycerol as substrates (see Table 6). However, feeding non-edible lignocellulosic biomass remains particularly challenging since the high producers are incapable of utilising pentose sugars (i.e., arabinose and xylose) natively. Therefore, one can transition to E. coli, a bacterium that naturally consumes pentose and hexose sugars, yet produces lower α-KGA yields. Alternatively, expanding the carbon utilisation range of C. glutamicum towards xylose and arabinose, which are abundantly present in hemicellulose, becomes feasible through engineering. Indeed, recently a C5-consuming C. glutamicum strain was constructed that produced 0.40 g g−1 glutamic acid (up to 62 g L−1 final titre) from straw hydrolysate, approaching the metrics when glucose is used alone (0.42 g g−1).600 Although a similar example for α-KGA has not yet been reported, the authors’ approach is expected to be applicable to α-KGA production.
The ability of the microbial host to metabolise lignocellulose-derived carbohydrates, however, is not the only factor that needs consideration. Lignocellulosic feedstocks are more recalcitrant and composition-wise more complex, therefore complicating the pretreatment schemes that convert hemicellulose into microbe-consumable monomers. This increases production cost and may thus threaten the profitability of the end product. Applying reductive catalytic fractionation (RCF) on lignocellulose, however, effectively separates this complex biomass into lignin oil and pure carbohydrate-rich pulps, which can be more readily converted into C5 and C6 sugar monomers through enzymatic hydrolysis.601,602 This integrated chemoenzymatic strategy could enhance economic and environmental sustainability of existing (α-KGA) fermentation protocols.
The last point of attention is the carbon conservation during the microbial conversion processes. Although the oTCAc is the route with the highest α-KGA yield, one carbon dioxide molecule is intrinsically lost per molecule of α-KGA due to the activity of isocitrate dehydrogenase. To avoid this carbon loss, two alternative pathways have been described in the literature.
The first one encompasses the anaerobic conversion of sugars and, optionally, C1 substrates (including methanol or formic acid) into succinic acid, following the rTCAc, and subsequent transformation of succinic acid into α-KGA using a dedicated dehydrogenase–decarboxylase cascade from P. putida. Whereas the first segment of the pathway, leading to succinic acid, has been intensively investigated, the ‘bridging’ reaction from succinic acid into α-KGA has never been demonstrated in a production setting. Yet, this strategy is particularly interesting in valorising CO2, alongside the principal carbohydrate-rich substrates, into biomolecules since two moles of carbon dioxide will be sequestered for every mole of α-KGA produced. A second alternative is the carbon-neutral Weimberg pathway, which has been recently explored for α-KGA (Fig. 14 and Table 9). With a yield of ca. 10 g L−1, this metabolism is inferior to the traditional oTCAc in terms of total yields, but carbon dioxide emission can be considerably reduced, leading to higher efficiencies. Moreover, the reaction pathway is shorter than the oTCAc pathway and is less connected to the central carbohydrate metabolism of the production host since the entire cascade is sourced from C. crescentus or Burkholderia species. In the short term, we argue that even a suboptimal Weimberg pathway is suited for converting the C5-fraction of lignocellulose substrates into α-KGA in a carbon-neutral way, whereas the glycolysis and oTCAc deal with the hexose sugars.
7.3. Prospects and challenges related to recovering α-KGA from complex fermentation broths
We have summarised various options for purifying α-KGA from the fermentation broth. The approaches range from conventional methods involving inorganic acids and solvents to more innovative membrane-based systems. These methods have their own advantages and disadvantages, depending on the fermentation feedstock used, the microbial species and its metabolic by-products. The purification processes discussed are relatively simple, typically involving five main steps (filtration, ion-exchange, decolouring, dehydration, and crystallisation), and are adapted from protocols used for other bio-based carboxylic acids, such as succinic acid, whose feasibility at the industrial scale has already been demonstrated.Despite the promising production titres, current studies on α-KGA purification were conducted on a small scale, mostly benchmarked using a simplified feed composition (glucose with α-KGA and other metabolites in MQ water, with NaOH as the pH regulator, and cations) instead of a post-fermentation broth. Hence, further research is required to investigate purification from other, more realistic fermented matrices, containing by-products and impurities. Of course, the choice of the purification method may depend on the intended application domain of α-KGA: more expensive methods yielding higher purity may be suitable for nutraceutical or pharmaceutical uses, while the opposite may be true for bulk production of α-KGA for plastic polymer manufacturing or further catalytic derivatisation into novel building blocks—particularly when water is used as the solvent, allowing the dewatering step to be omitted.
Furthermore, research and development efforts in fermentation process optimisation should be conducted with downstream processing in mind as fine-tuning fermentation parameters could reduce microbial by-product levels and potentially simplify or eliminate complex purification steps. Finally, a holistic techno-economic analysis could be performed to assess economic viability and identify steps that require further optimisation.
7.4. The future of α-KGA as a potential platform chemical
Our review shows that α-KGA has received increasing attention as a compound for pharmaceutical and chemical applications, and that advances in both fermentation and downstream recovery support the emergence of an α-KGA-based bioprocess industry (see also Table 11). We therefore believe that α-KGA is a particularly promising platform chemical, with some key advantages over more known compounds like succinic acid, due to its versatile chemical reactivity and the potential for a microbial-based sustainable production route. Unfortunately, because microbial α-KGA production is not fully matured yet, it is difficult to estimate the production costs at the industrial scale for evaluating economic viability. Moreover, volatile fossil fuel prices and possible governmental (tax) incentives also greatly influence the economic perspective of a bio-based α-KGA industry. Nonetheless, we have presented an indicative overview of how the metrics of the microbial-based production route compare to those of the (bio)catalytic production schemes (Table 12).| Aspect | Criteria | For α-KGA |
|---|---|---|
| Feedstock | Scope | Broad (waste and lignocellulosic biomass) |
| Cost | Cheap (n-alkanes, glycerol, pulps) | |
| Processing | Feasible (direct feed into bio-process) | |
| Bio-process | Performance | Good production parameters |
| Estimated costs | No TEA has been performed yet | |
| Downstream | Optimisation required at industrial levels | |
| Technical complexity | Straightforward and covered in extensive literature | |
| Integration with bio-refinery | Yes (downstream of lignin processing, saccharification, or pyrolysis) | |
| Scale-up | Not initiated yet | |
| Economics | Price | Estimated in 2019: $12–15 per kg613 |
| Market volume | Low (<kt scale, $100 million market value) | |
| Market potential | Chemical, pharmaceutical, and nutritional | |
| Market potential | Chemical functionality | Three: 2 acids, 1 carbonyl |
| Drop-in | Petrochemical α-KGA, intermediate for fine chemicals | |
| Building block as such | Possible, several novel materials reported | |
| Intermediate to derivatives | Broad product portfolio, but underexplored | |
| Platform | New valorisation schemes → could increase market volume | |
| Others | C1–C6 monomer | Yes: C5 |
| No aromatic structure | Yes: linear monomer | |
| No super commodity | Indeed | |
| Parameter | Chemical synthesisc | Cell-free and whole-cell biocatalysis | Microbial-based production |
|---|---|---|---|
| a Feedstock parameter considers both the range of substrates, compatible for a given approach, and the cost of the substrates. b Scalability refers to the maximum working volume of tests that have been conducted, which could indicate how attractive or how easily the technology could be scaled-up for commercial use. c For chemical synthesis, both the classic condensation step between DEO and DES and the novel aldol condensation step between glyoxylic acid and pyruvic acid are considered. Legend: ++, + (advantage), +/− (neutral), −, −− (disadvantage); DEO, diethyl oxalate; DES, diethyl succinate, 1G, first generation. | |||
| Feedstocka | • Range: limited | • Range: limited | • Range: broad |
| • Cost: cheap-moderate | • Cost: variable | Cost: cheap, but processing required | |
| +/−, chemical building blocks such as DEO, DES, glyoxylic acid, and pyruvic acid from well-established processes | −, glutamate, pyrrolidone carboxylic acid | ++, broad: waste streams and lignocellulose | |
| −−, glucuronic acid | +, 1G biomass | ||
| Energy | −, high temperatures required for synthesis of building blocks (DEO, DES) or during reaction (110 °C for glyoxylic acid and pyruvic acid) | +, minimal product purification required compared to microbial-based production | +, 1G biomass (hydrolysis of sugars or no pretreatment required for ethanol and fatty acids) |
| +, energy control possible during reaction | −, waste and lignocellulose (requires pretreatment) | ||
| Environmental impact | −, emission of NOx, use of strong bases, non-green solvents and toxic reagents | +/−, containment needed for genetically modified whole-cells | ++, 1G biomass with limited pretreatment/hydrolysis and non-engineered microorganisms (solely optimising fermentation conditions) |
| +, waste streams and lignocellulose with extensive pretreatment/hydrolysis and non-engineered microorganisms (solely optimising fermentation conditions) | |||
| +/−, containment needed for genetically modified microorganisms | |||
| Scalabilityb | +, established industrial processes (for DEO and DES) | −, tests limited to max 3 L | ++, tests up to 1500 L |
| −, lab scale tested up to 50 mL | |||
| Production metrics (spatiotemporal yield) | ++, aldol condensation up 50 g L−1 h−1 | +, up to 14-16 g L−1 h−1 | +, up to 1.75 g L−1 h−1, with a portion of the substrate lost for cell metabolism |
This review covered the state-of-the-art in α-KGA from a combined chemical-biological perspective and emphasised the application potential and the progress in terms of fermentation, downstream processing, and chemical valorisation. We hope that this review may serve as a reference and convince chemists, microbiologists, and engineers to build further on the building blocks, polymer, and therapeutic applications provided here and translate the learnings into a demo α-KGA plant as a step towards full-scale commercialisation.
Author contributions
Conceptualisation, L.-T. O. and B. F. S.; methodology (literature survey), investigation, and writing – original draft, L.-T. O., T. S., and S. Y.; writing – review & editing, L.-T. O., T. S., S. Y., K. J. V., J. M., and B. F. S.; visualisation, T. S.; supervision, project administration, and funding acquisition, K. J. V., J. M., and B. F. S.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
This work was performed within SBO StrainDrops project S001421N from Research Foundation Flanders (FWO Vlaanderen) and Hybrid Moonshot project HBC.2023.0550 from Flanders Innovation & Entrepreneurship (VLAIO). L. T. O. and B. F. S. acknowledge support from FWO (S001421N) and VLAIO (HBC.2023.0550). T. S. and J. M. acknowledge support from FWO (12O1922N, 12O1917N, S001421N, G0B0420N, G0C4322N), FWO-SBO (S001421N), EOS (40007496), internal KUL funding (928 C16/17/006), and VIB. S. Y. is supported by a PhD fellowship from FWO (1S22422N). Research in the lab of K. J. V. is supported by KU Leuven, VIB, VLAIO, FWO (S001421N) and iBOF (IBOF/21/092).References
- S. R. Nicholson, N. A. Rorrer, T. Uekert, G. Avery, A. C. Carpenter and G. T. Beckham, ACS Sustainable Chem. Eng., 2023, 11, 2198–2208 CrossRef
.
- F. M. Adebiyi, Environ. Chem. Ecotoxicol., 2022, 4, 89–96 CrossRef
.
- H. Rajabi, M. Hadi Mosleh, P. Mandal, A. Lea-Langton and M. Sedighi, Sci. Total Environ., 2020, 727, 138654 CrossRef
.
-
A. Juneja and V. Singh, in Green Energy to Sustainability, ed. A. A. Vertès, N. Qureshi, H. P. Blaschek and H. Yukawa, John Wiley & Sons, Inc., 2020, pp. 157–184 Search PubMed
.
- P. Sudarsanam, R. Zhong, S. Van den Bosch, S. M. Coman, V. I. Parvulescu and B. F. Sels, Chem. Soc. Rev., 2018, 47, 8349–8402 RSC
.
- P. Sudarsanam, E. Peeters, E. V. Makshina, V. I. Parvulescu and B. F. Sels, Chem. Soc. Rev., 2019, 48, 2366–2421 RSC
.
- L. T. Mika, E. Cséfalvay and Á. Németh, Chem. Rev., 2018, 118, 505–613 CrossRef
.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef PubMed
.
- J. M. Francois, C. Alkim and N. Morin, Biotechnol. Biofuels, 2020, 13, 118 CrossRef
.
- J. Keasling, H. Garcia Martin, T. S. Lee, A. Mukhopadhyay, S. W. Singer and E. Sundstrom, Nat. Rev. Microbiol., 2021, 19, 701–715 CrossRef PubMed
.
- C. Gomes, A. C. Silva, A. C. Marques, J. S. Lobo and M. H. Amaral, Cosmetics, 2020, 7, 1–14 CrossRef
.
- C. G. Acevedo-Rocha, L. S. Gronenberg, M. Mack, F. M. Commichau and H. J. Genee, Curr. Opin. Biotechnol., 2019, 56, 18–29 CrossRef
.
- M. T. de Pinho Favaro, J. Atienza-Garriga, C. Martínez-Torró, E. Parladé, E. Vázquez, J. L. Corchero, N. Ferrer-Miralles and A. Villaverde, Microb. Cell Fact., 2022, 21, 1–17 CrossRef PubMed
.
- L. Sun, M. Gong, X. Lv, Z. Huang, Y. Gu, J. Li, G. Du and L. Liu, Appl. Microbiol. Biotechnol., 2020, 104, 9109–9124 CrossRef CAS PubMed
.
- M. J. Volk, V. G. Tran, S.-I. Tan, S. Mishra, Z. Fatma, A. Boob, H. Li, P. Xue, T. A. Martin and H. Zhao, Chem. Rev., 2023, 123, 5521–5570 CrossRef CAS
.
- S. Y. Lee, H. U. Kim, T. U. Chae, J. S. Cho, J. W. Kim, J. H. Shin, D. I. Kim, Y. S. Ko, W. D. Jang and Y. S. Jang, Nat. Catal., 2019, 2, 18–33 CrossRef CAS
.
- I. Khalil, G. Quintens, T. Junkers and M. Dusselier, Green Chem., 2020, 22, 1517–1541 RSC
.
- M. Ventura, A. Marinas and M. E. Domine, Top. Catal., 2020, 63, 846–865 CrossRef CAS
.
- M. Dusselier, P. Van Wouwe, A. Dewaele, E. Makshina and B. F. Sels, Energy Environ. Sci., 2013, 6, 1415–1442 RSC
.
- J. S. Cho, G. B. Kim, H. Eun, C. W. Moon and S. Y. Lee, JACS Au, 2022, 2, 1781–1799 CrossRef CAS PubMed
.
- L. T. Mika, E. Cséfalvay and Á. Németh, Chem. Rev., 2018, 118, 505–613 CrossRef CAS PubMed
.
-
T. Werpy and G. Petersen, Top Value Added Chemicals from Biomass: Results of Screening for Potential Candidates from Sugars and Synthesis Gas, U.S. Department of Energy, 2004, vol. I Search PubMed
.
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539–555 RSC
.
- Registered Substances: 2-oxoglutaric acid, https://echa.europa.eu/, (accessed 21 September 2023).
- B. Zdzisińska, A. Żurek and M. Kandefer-Szerszeń, Arch. Immunol. Ther. Exp., 2017, 65, 21–36 CrossRef
.
- H. Guo, S. Su, C. Madzak, J. Zhou, H. Chen and G. Chen, Appl. Microbiol. Biotechnol., 2016, 100, 9875–9884 CrossRef CAS PubMed
.
- F. Legendre, A. MacLean, V. P. Appanna and V. D. Appanna, World J. Microbiol. Biotechnol., 2020, 36, 123 CrossRef
.
- C. Otto, V. Yovkova and G. Barth, Appl. Microbiol. Biotechnol., 2011, 92, 689–695 CrossRef PubMed
.
-
T. Ronzon, T. Lammens, J. Spekreijse, M. Vis and C. Parisi, Insights into the European market for bio-based chemicals: analysis based on 10 key product categories, Publications Office, European Commission, Joint Research Centre, 2019 Search PubMed
.
- R. Bhattacharya, D. Kumar, K. Sugendran, S. C. Pant, R. K. Tulsawani and R. Vijayaraghavan, J. Appl. Toxicol., 2001, 21, 495–499 CrossRef
.
- Chemical Abstracts Service: Columbus; OH, SciFinder, https://scifinder-n.cas.org/, (accessed 31 May 2023).
- C. Dudás, B. Kutus, G. Peintler, I. Pálinkó and P. Sipos, Polyhedron, 2018, 156, 89–97 CrossRef
.
- I. Hevus, N. G. Ricapito, S. Tymoshenko, S. N. Raja and D. C. Webster, ACS Sustainable Chem. Eng., 2020, 8, 5750–5762 CrossRef
.
- M. Voges, F. Schmidt, D. Wolff, G. Sadowski and C. Held, Fluid Phase Equilib., 2016, 422, 87–98 CrossRef
.
- A. J. Copper and A. G. Redfield, J. Biol. Chem., 1975, 250, 527–532 CrossRef PubMed
.
- T. S. Viswanathan, R. E. Johnson and H. F. Fisher, Biochemistry, 1982, 21, 339–345 CrossRef PubMed
.
- A. J. L. Cooper, J. Z. Ginos and A. Meister, Chem. Rev., 1983, 83, 321–358 CrossRef
.
- R. C. Kerber and M. S. Fernando, J. Chem. Educ., 2010, 87, 1079–1084 CrossRef
.
-
F. D. Klingler and W. Ebertz, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2000 Search PubMed
.
- Z. Luo, S. Yu, W. Zeng and J. Zhou, Biotechnol. Adv., 2021, 47, 107706 CrossRef PubMed
.
- Y. Song, J. Li, H. Shin, L. Liu, G. Du and J. Chen, Bioresour. Technol., 2016, 219, 716–724 CrossRef PubMed
.
- U. Stottmeister, A. Aurich, H. Wilde, J. Andersch, S. Schmidt and D. Sicker, J. Ind. Microbiol. Biotechnol., 2005, 32, 651–664 CrossRef CAS PubMed
.
- R. W. Hanson, J. Chem. Educ., 1987, 64, 591 Search PubMed
.
- A. Ahmad and I. D. Spenser, Can. J. Chem., 1961, 39, 1340–1359 CrossRef CAS
.
- S. L. Bartlett, Y. Sohtome, D. Hashizume, P. S. White, M. Sawamura, J. S. Johnson and M. Sodeoka, J. Am. Chem. Soc., 2017, 139, 8661–8666 CrossRef CAS
.
- F. J. Hidalgo, R. M. Delgado and R. Zamora, Food Chem., 2013, 141, 1140–1146 CrossRef CAS PubMed
.
- Q. Jiang, J. Jia, B. Xu, A. Zhao and C.-C. Guo, J. Org. Chem., 2015, 80, 3586–3596 CrossRef CAS PubMed
.
- X. Lan, C. Tao, X. Liu, A. Zhang and B. Zhao, Org. Lett., 2016, 18, 3658–3661 CrossRef CAS
.
- B. Lee, P. Kang, K. H. Lee, J. Cho, W. Nam, W. K. Lee and N. H. Hur, Tetrahedron Lett., 2013, 54, 1384–1388 CrossRef CAS
.
- T. Nanjo, N. Kato and Y. Takemoto, Org. Lett., 2018, 20, 5766–5769 CrossRef CAS PubMed
.
- J. Spengler, S. N. Osipov, E. Heistracher, A. Haas and K. Burger, J. Fluorine Chem., 2004, 125, 1019–1023 CrossRef CAS
.
- W.-T. Xu, B. Huang, J.-J. Dai, J. Xu and H.-J. Xu, Org. Lett., 2016, 18, 3114–3117 CrossRef CAS
.
- C. Zhou and D. Ma, Chem. Commun., 2014, 50, 3085–3088 RSC
.
- D. A. Klumpp, S. Lau, M. Garza, B. Schick and K. Kantardjieff, J. Org. Chem., 1999, 64, 7635–7637 CrossRef CAS
.
- F. Penteado, E. F. Lopes, D. Alves, G. Perin, R. G. Jacob and E. J. Lenardão, Chem. Rev., 2019, 119, 7113–7278 CrossRef
.
- A. Drif, A. Pineda, D. Morvan, V. Belliere-Baca, K. De Oliveira Vigier and F. Jérôme, Green Chem., 2019, 21, 4604–4608 RSC
.
- D. G. Barrett and M. N. Yousaf, Macromolecules, 2008, 41, 6347–6352 CrossRef
.
- D. G. Barrett and M. N. Yousaf, Biomacromolecules, 2008, 9, 2029–2035 CrossRef PubMed
.
- H. Chao, G. Li, J. Yu, Z. Liu, Z.-W. Liu and J. Jiang, Macromol. Chem. Phys., 2019, 220, 1900004 CrossRef
.
- A. A. Gadgeel and S. T. Mhaske, Prog. Org. Coat., 2021, 150, 105983 CrossRef
.
- Z. A. Page, R. Bou Zerdan, W. R. Gutekunst, A. Anastasaki, S. Seo, A. J. McGrath, D. J. Lunn, P. G. Clark and C. J. Hawker, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 801–807 CrossRef
.
- D. J. Lunn, S. Seo, S.-H. Lee, R. B. Zerdan, K. M. Mattson, N. J. Treat, A. J. McGrath, W. R. Gutekunst, J. Lawrence, A. Abdilla, A. Anastasaki, A. S. Knight, B. V. K. J. Schmidt, M. W. Bates, P. G. Clark, J. P. DeRocher, A. K. Van Dyk and C. J. Hawker, J. Polym. Sci., Part A: Polym. Chem., 2019, 57, 716–725 CrossRef
.
- N. A. Rorrer, S. F. Notonier, B. C. Knott, B. A. Black, A. Singh, S. R. Nicholson, C. P. Kinchin, G. P. Schmidt, A. C. Carpenter, K. J. Ramirez, C. W. Johnson, D. Salvachúa, M. F. Crowley and G. T. Beckham, Cell Rep. Phys. Sci., 2022, 3, 100840 CrossRef
.
-
S. Hashimoto, in Amino Acid Fermentation, ed. A. Yokota and M. Ikeda, Springer, Japan, Tokyo, 2017, pp. 15–34 Search PubMed
.
- T. Fukushima and M. Yamauchi, Chem. Commun., 2019, 55, 14721–14724 RSC
.
-
J. A. Kent, in Riegel's Handbook of Industrial Chemistry, ed. J. A. Kent, Springer US, Boston, MA, 2003, pp. 963–1045 Search PubMed
.
- H. Lin, Y. Liu, C. Yang, G. Zhao, J. Song, T. Zhang and X. Huang, Catal. Sci. Technol., 2022, 12, 4057–4065 RSC
.
- A. Galkin, L. Kulakova, T. Yoshimura, K. Soda and N. Esaki, Appl. Environ. Microbiol., 1997, 63, 4651–4656 CrossRef CAS
.
- K. F. Gu and T. M. S. Chang, Biotechnol. Bioeng., 1988, 32, 363–368 CrossRef CAS
.
- A. Galkin, L. Kulakova, H. Yamamoto, K. Tanizawa, H. Tanaka, N. Esaki and K. Soda, J. Ferment. Bioeng., 1997, 83, 299–300 CrossRef CAS
.
- C.-X. Chen, B. Jiang, C. Branford-White and L.-M. Zhu, Biochem., 2009, 74, 36–40 CAS
.
- C. Huber and G. Wächtershäuser, Tetrahedron Lett., 2003, 44, 1695–1697 CrossRef CAS
.
- W. Wang, X. Liu, Y. Yang and W. Su, Int. J. Astrobiol., 2013, 12, 69–77 CrossRef CAS
.
- R. Kadyrov, T. H. Riermeier, U. Dingerdissen, V. Tararov and A. Börner, J. Org. Chem., 2003, 68, 4067–4070 Search PubMed
.
- V. Helaine and J. Bolte, Eur. J. Org. Chem., 1999, 3403–3406 CrossRef CAS
.
- H. Kuang, M. L. Brown, R. R. Davies, E. C. Young and M. D. Distefano, J. Am. Chem. Soc., 1996, 118, 10702–10706 Search PubMed
.
- H. Kuang, R. R. Davies and M. D. Distefano, Bioorg. Med. Chem. Lett., 1997, 7, 2055–2060 Search PubMed
.
- H. Kuang and M. D. Distefano, J. Am. Chem. Soc., 1998, 120, 1072–1073 CrossRef CAS
.
- J. T. Slama, R. K. Satsangi, A. Simmons, V. Lynch, R. E. Bolger and J. Suttie, J. Med. Chem., 1990, 33, 824–832 CrossRef CAS
.
- J. H. Kim, S. H. Lee, J. S. Lee, M. Lee and C. B. Park, Chem. Commun., 2011, 47, 10227–10229 RSC
.
- D. Mandler and I. Willner, J. Chem. Soc., Chem. Commun., 1986, 851–853 RSC
.
- D. H. Nam and C. B. Park, ChemBioChem, 2012, 13, 1278–1282 CrossRef CAS PubMed
.
- E. Siu, K. Won and C. B. Park, Biotechnol. Prog., 2007, 23, 293–296 CrossRef CAS
.
- K. Tao, B. Xue, S. Frere, I. Slutsky, Y. Cao, W. Wang and E. Gazit, Chem. Mater., 2017, 29, 4454–4460 CrossRef CAS
.
- C. H. Wong and G. M. Whitesides, J. Org. Chem., 1982, 47, 2816–2818 CrossRef CAS
.
- Y. Zhao, H. Liu, C. Wu, Z. Zhang, Q. Pan, F. Hu, R. Wang, P. Li, X. Huang and Z. Li, Angew. Chem., Int. Ed., 2019, 58, 5376–5381 CrossRef CAS
.
- M. Zheng, S. Zhang, G. Ma and P. Wang, J. Biotechnol., 2011, 154, 274–280 CrossRef CAS PubMed
.
- H. A. Ibrahim, F. M. Awadallah, H. M. Refaat and K. M. Amin, Bioorg. Chem., 2018, 77, 457–470 Search PubMed
.
- T. Panneerselvam, S. Arumugam, M. A. Ali, K. Selvaraj, M. Indhumathy, A. Sivakumar and S. D. Joshi, ChemistrySelect, 2017, 2, 2341–2347 CrossRef CAS
.
- M. Rashid, Bioorg. Chem., 2020, 96, 103576 CrossRef CAS PubMed
.
- M. Rashid, A. Husain, R. Mishra, S. Karim, S. Khan, M. Ahmad, N. Al-wabel, A. Husain, A. Ahmad and S. A. Khan, Arabian J. Chem., 2019, 12, 3202–3224 CrossRef CAS
.
- M. Rashid, A. Husain and R. Mishra, Eur. J. Med. Chem., 2012, 54, 855–866 CrossRef PubMed
.
- Z. Song, X. Wang, Y. Zhang, W. Gu, A. Shen, C. Ding, H. Li, R. Xiao, M. Geng, Z. Xie and A. Zhang, J. Med. Chem., 2021, 64, 1649–1669 CrossRef
.
- A. Husain, M. Rashid, R. Mishra, S. Parveen, D.-S. Shin and D. Kumar, Bioorg. Med. Chem. Lett., 2012, 22, 5438–5444 CrossRef PubMed
.
- T. A. Kuz’menko, V. V. Kuz’menko, A. S. Morkovnik and L. N. Divaeva, Chem. Heterocycl. Compd., 2006, 42, 648–656 CrossRef
.
- L. Fabian, M. F. Martini, E. S. Sarduy, D. A. Estrin and A. G. Moglioni, Bioorg. Med. Chem. Lett., 2019, 29, 2197–2202 CrossRef PubMed
.
- L. Fabian, M. Taverna Porro, N. Gómez, M. Salvatori, G. Turk, D. Estrin and A. Moglioni, Eur. J. Med. Chem., 2020, 188, 111987 CrossRef PubMed
.
- J. Gris, R. Glisoni, L. Fabian, B. Fernández and A. G. Moglioni, Tetrahedron Lett., 2008, 49, 1053–1056 CrossRef
.
- E. A. Fayed, Y. A. Ammar, M. A. Saleh, A. H. Bayoumi, A. Belal, A. B. M. Mehany and A. Ragab, J. Mol. Struct., 2021, 1236, 130317 CrossRef
.
- R. Ferreira de Freitas, R. J. Harding, I. Franzoni, M. Ravichandran, M. K. Mann, H. Ouyang, M. Lautens, V. Santhakumar, C. H. Arrowsmith and M. Schapira, J. Med. Chem., 2018, 61, 4517–4527 CrossRef PubMed
.
- R. J. Harding, R. Ferreira de Freitas, P. Collins, I. Franzoni, M. Ravichandran, H. Ouyang, K. A. Juarez-Ornelas, M. Lautens, M. Schapira, F. von Delft, V. Santhakumar and C. H. Arrowsmith, J. Med. Chem., 2017, 60, 9090–9096 CrossRef PubMed
.
- M. S. Kazunin, O. Y. Voskoboynik, O. M. Shatalova, L. N. Maloshtan and S. I. Kovalenko, Chem. Heterocycl. Compd., 2019, 55, 408–415 CrossRef CAS
.
- S. Tariq, O. Alam and M. Amir, Bioorg. Chem., 2018, 76, 343–358 CrossRef CAS
.
- V. L. Gein, N. A. Rassudikhina, N. V. Shepelina, M. I. Vakhrin, E. B. Babushkina and E. V. Voronina, Pharm. Chem. J., 2008, 42, 529–532 CrossRef CAS
.
- K. Hachama, M. Khodja, S. Moulay, H. Boutoumi, L. Hennig and D. Sicker, J. Heterocycl. Chem., 2013, 50, 413–416 CrossRef CAS
.
- M.-T. Le Bris, J. Heterocycl. Chem., 1985, 22, 1275–1280 CrossRef CAS
.
- J. P. Burke, Z. Bian, S. Shaw, B. Zhao, C. M. Goodwin, J. Belmar, C. F. Browning, D. Vigil, A. Friberg, D. V. Camper, O. W. Rossanese, T. Lee, E. T. Olejniczak and S. W. Fesik, J. Med. Chem., 2015, 58, 3794–3805 CrossRef CAS
.
- T. D. Kapti Cagatay and M. Balci, Synthesis, 2017, 1898–1904 Search PubMed
.
- H. Yang, M. Poznik, S. Tang, P. Xue, L. Du, C. Liu, X. Chen and J. J. Chruma, ACS Omega, 2021, 6, 19291–19303 CrossRef CAS PubMed
.
- W. Jahnke, J.-M. Rondeau, S. Cotesta, A. Marzinzik, X. Pellé, M. Geiser, A. Strauss, M. Götte, F. Bitsch, R. Hemmig, C. Henry, S. Lehmann, J. F. Glickman, T. P. Roddy, S. J. Stout and J. R. Green, Nat. Chem. Biol., 2010, 6, 660–666 CrossRef CAS PubMed
.
- P. Yu, T. Wang, J. Li and J. M. Cook, J. Org. Chem., 2000, 65, 3173–3191 CrossRef CAS PubMed
.
- Z. Hou, L.-F. Zhu, X. Yu, M.-Q. Sun, F. Miao and L. Zhou, J. Agric. Food Chem., 2016, 64, 2847–2854 CrossRef CAS PubMed
.
- X. Li, B. Zhang, W. Zhao, S. Yang, X. Yang and L. Zhou, Sci. Rep., 2019, 9, 1941 CrossRef PubMed
.
- B. Zhou, B. Zhang, X. Li, X. Liu, H. Li, D. Li, Z. Cui, H. Geng and L. Zhou, Sci. Rep., 2018, 8, 1559 CrossRef
.
- A. Tsotinis, M. Panoussopoulou, A. Eleutheriades, K. Davidson and D. Sugden, Eur. J. Med. Chem., 2007, 42, 1004–1013 CrossRef CAS
.
- M. A. Flores and J. W. Bode, Org. Lett., 2010, 12, 1924–1927 CrossRef CAS
.
- Y.-L. Huang and J. W. Bode, Nat. Chem., 2014, 6, 877–884 CrossRef CAS PubMed
.
- M. M. Sheha, N. M. Mahfouz, H. Y. Hassan, A. F. Youssef, T. Mimoto and Y. Kiso, Eur. J. Med. Chem., 2000, 35, 887–894 CrossRef CAS
.
- T. G. Wucherpfennig, F. Rohrbacher, V. R. Pattabiraman and J. W. Bode, Angew. Chem., Int. Ed., 2014, 53, 12244–12247 CrossRef CAS
.
- C. E. Murar, F. Thuaud and J. W. Bode, J. Am. Chem. Soc., 2014, 136, 18140–18148 CrossRef CAS PubMed
.
- T. Nanjo, N. Kato, X. Zhang and Y. Takemoto, Chem. – Eur. J., 2019, 25, 15504–15507 CrossRef CAS PubMed
.
- M. Mihalache, O. Oprea, C. Guran and A. M. Holban, C. R. Chim., 2018, 21, 32–40 CrossRef CAS
.
- R. Müller, E. Hübner and N. Burzlaff, Eur. J. Inorg. Chem., 2004, 2151–2159 CrossRef
.
- S. Parveen, T. Premkumar, H. H. Nguyen and S. Govindarajan, New J. Chem., 2019, 43, 13371–13380 RSC
.
- A. Tavman, Main Group Met. Chem., 2012, 35, 81–89 Search PubMed
.
- Z.-H. Zhou, H. Wang, P. Yu, M. M. Olmstead and S. P. Cramer, J. Inorg. Biochem., 2013, 118, 100–106 CrossRef PubMed
.
- R. C. Evans and F. Y. Wiselogle, J. Am. Chem. Soc., 1945, 67, 60–62 CrossRef
.
- G. B. Kline and S. H. Cox, J. Org. Chem., 1961, 26, 1854–1856 CrossRef
.
- H.-Y. Qian, Z.-L. Wang, X.-Y. Xie, Y.-L. Pan, G.-J. Li, X. Xie and J.-Z. Chen, Eur. J. Med. Chem., 2017, 137, 598–611 CrossRef PubMed
.
- M. P. Giovannoni, I. A. Schepetkin, A. Cilibrizzi, L. Crocetti, A. I. Khlebnikov, C. Dahlgren, A. Graziano, V. Dal Piaz, L. N. Kirpotina, S. Zerbinati, C. Vergelli and M. T. Quinn, Eur. J. Med. Chem., 2013, 64, 512–528 CrossRef PubMed
.
- A. Katrusiak, P. Piechowiak and A. Katrusiak, J. Mol. Struct., 2011, 998, 84–90 CrossRef
.
- S. Hussain, A. Khan, S. Gul, M. Resmini, C. S. Verma, E. W. Thomas and K. Brocklehurst, Biochemistry, 2011, 50, 10732–10742 CrossRef
.
- J. Li, H. Yin and M. Hong, Z. Anorg. Allg. Chem., 2012, 638, 387–391 CrossRef
.
- S. Parveen, S. Govindarajan, H. Puschmann and R. Revathi, Inorg. Chim. Acta, 2018, 477, 66–74 CrossRef
.
- S. Parveen, T. Premkumar, H.-H. Nguyen, S. Govindarajan, D. Manikandan, S. K. Anandasadagopan and E. Vijayakumar, Inorg. Chim. Acta, 2021, 516, 120142 CrossRef
.
- M. Baldini, M. Belicchi-Ferrari, F. Bisceglie, S. Capacchi, G. Pelosi and P. Tarasconi, J. Inorg. Biochem., 2005, 99, 1504–1513 CrossRef
.
- M. Baldini, M. Belicchi-Ferrari, F. Bisceglie, P. P. Dall’Aglio, G. Pelosi, S. Pinelli and P. Tarasconi, Inorg. Chem., 2004, 43, 7170–7179 CrossRef
.
- Z.-Y. Yang and R.-D. Yang, Synth. React. Inorg. Met.-Org. Chem., 2002, 32, 1059–1069 CrossRef
.
- E. Novotná, K. Waisser, J. Kuneš, K. Palát, L. Skálová, B. Szotáková, V. Buchta, J. Stolaříková, V. Ulmann, M. Pávová, J. Weber, J. Komrsková, P. Hašková, I. Vokřál and V. Wsól, Arch. Pharm., 2017, 350, 1700020 CrossRef
.
- J.-K. Dai, W.-J. Dan, N. Li, H.-T. Du, J.-W. Zhang and J.-R. Wang, Bioorg. Med. Chem. Lett., 2016, 26, 580–583 CrossRef PubMed
.
- J. Dai, W. Dan, Y. Zhang, M. He and J. Wang, Bioorg. Med. Chem. Lett., 2018, 28, 3123–3128 CrossRef PubMed
.
- J. Dai, W. Dan, N. Li, R. Wang, Y. Zhang, N. Li, R. Wang and J. Wang, Food Chem., 2018, 253, 211–220 CrossRef
.
- F. Zhao, J.-K. Dai, D. Liu, S.-J. Wang and J.-R. Wang, Molecules, 2016, 21, 390 CrossRef PubMed
.
- K. M. Czerwinski, C. A. Zificsak, J. Stevens, M. Oberbeck, C. Randlett, M. King and S. Mennen, Synth. Commun., 2003, 33, 1225–1231 CrossRef
.
- K. Narayanan and J. M. Cook, Tetrahedron Lett., 1990, 31, 3397–3400 CrossRef
.
- S. V. Ryabukhin, D. M. Panov, A. S. Plaskon, A. A. Tolmachev and R. V. Smaliy, Monatsh. Chem., 2012, 143, 1507–1517 CrossRef
.
- V. Stavytskyi, O. Voskoboinik, O. Antypenko, N. Krasovska, K. Shabelnyk, I. Konovalova, S. Shishkyna, S. Kholodniak and S. Kovalenko, J. Heterocycl. Chem., 2020, 57, 1249–1260 CrossRef
.
- Y.-P. Wang, X.-R. Ou, Y. Wang, J.-Q. Liu and X.-S. Wang, J. Heterocycl. Chem., 2018, 55, 2325–2333 CrossRef
.
- M.-M. Zhang, L. Lu, Y.-J. Zhou and X.-S. Wang, Res. Chem. Intermed., 2013, 39, 3327–3335 CrossRef
.
- D. Zicāne, Z. Tetere, I. Rāviņa and M. Turks, Chem. Heterocycl. Compd., 2013, 49, 310–316 CrossRef
.
- M. Beigi, S. Waltzer, A. Fries, L. Eggeling, G. A. Sprenger and M. Müller, Org. Lett., 2013, 15, 452–455 CrossRef
.
- A. Kurutsch, M. Richter, V. Brecht, G. A. Sprenger and M. Müller, J. Mol. Catal. B: Enzym., 2009, 61, 56–66 CrossRef
.
- R. Westphal, S. Waltzer, U. Mackfeld, M. Widmann, J. Pleiss, M. Beigi, M. Müller, D. Rother and M. Pohl, Chem. Commun., 2013, 49, 2061–2063 RSC
.
- R. Westphal, S. Jansen, C. Vogel, J. Pleiss, M. Müller, D. Rother and M. Pohl, ChemCatChem, 2014, 6, 1082–1088 CrossRef
.
- R. Westphal, D. Hahn, U. Mackfeld, S. Waltzer, M. Beigi, M. Widmann, C. Vogel, J. Pleiss, M. Müller, D. Rother and M. Pohl, ChemCatChem, 2013, 5, 3587–3594 CrossRef
.
- M. Beigi, S. Loschonsky, P. Lehwald, V. Brecht, S. L. A. Andrade, F. J. Leeper, W. Hummel and M. Müller, Org. Biomol. Chem., 2013, 11, 252–256 RSC
.
- B. B. Jarvis, K. M. Wells and T. Kaufmann, Synthesis, 1990, 1079–1082 CrossRef
.
- H. Natsugari, Y. Kawano, A. Morimoto, K. Yoshioka and M. Ochiai, J. Chem. Soc., Chem. Commun., 1987, 62–63 RSC
.
- H.-B. Chen, L.-Y. Chen, P.-Q. Huang, H.-K. Zhang, Z.-H. Zhou and K.-R. Tsai, Tetrahedron, 2007, 63, 2148–2152 CrossRef
.
- P. Singh, A. Mittal, P. Kaur and S. Kumar, Tetrahedron, 2006, 62, 1063–1068 CrossRef
.
- K. Felföldi, K. Szöri and M. Bartók, Appl. Catal., A, 2003, 251, 457–460 CrossRef
.
- Y. Laras, V. Hugues, Y. Chandrasekaran, M. Blanchard-Desce, F. C. Acher and N. Pietrancosta, J. Org. Chem., 2012, 77, 8294–8302 CrossRef CAS PubMed
.
- S. Sasaki, H. Suzuki, H. Ouchi, T. Asakawa, M. Inai, R. Sakai, K. Shimamoto, Y. Hamashima and T. Kan, Org. Lett., 2014, 16, 564–567 CrossRef CAS PubMed
.
- N. Ohgami, S. Upadhyay, A. Kabata, K. Morimoto, H. Kusakabe and H. Suzuki, Biosens. Bioelectron., 2007, 22, 1330–1336 CrossRef CAS PubMed
.
- H. Irikawa, S. Ooe and Y. Okumura, Bull. Chem. Soc. Jpn., 1988, 61, 3365–3367 CrossRef CAS
.
- A. P. Mityuk, A. V. Denisenko, O. O. Grygorenko and A. A. Tolmachev, ARKIVOC, 2012, 2012, 226–230 Search PubMed
.
- M. Jiang, M. Chen, Y. Cao, Y. Yang, K. H. Sze, X. Chen and Z. Guo, Org. Lett., 2007, 9, 4765–4767 CrossRef CAS
.
- M. Chen, M. Jiang and Z. Guo, Sci. China: Chem., 2013, 56, 312–320 CrossRef CAS
.
- A. Balakrishnan, F. Jordan and C. F. Nathan, J. Biol. Chem., 2013, 288, 21688–21702 CrossRef CAS PubMed
.
- Y. A. Davidovich and S. V. Kozlov, Pharm. Chem. J., 2021, 55, 506–509 CrossRef CAS
.
- Y. I. Sakhno, O. V. Radchenko, E. A. Muravyova, S. M. Sirko, S. V. Shishkina, V. I. Musatov, S. M. Desenko and V. A. Chebanov, Chem. Heterocycl. Compd., 2021, 57, 261–265 CrossRef CAS
.
- J. H. Bushweller and P. A. Bartlett, J. Org. Chem., 1989, 54, 2404–2409 CrossRef CAS
.
- J. G. Hubert, I. A. Stepek, H. Noda and J. W. Bode, Chem. Sci., 2018, 9, 2159–2167 RSC
.
- V. A. Kozlov, K. V. Novikov, T. G. Mokeeva and S. A. Kuz’mina, Russ. J. Gen. Chem., 2013, 83, 1467–1468 CrossRef CAS
.
- N. Tibrewal and G. I. Elliott, Bioorg. Med. Chem. Lett., 2011, 21, 517–519 CrossRef CAS PubMed
.
- J. E. Baldwin, J. K. Cha and L. I. Kruse, Tetrahedron, 1985, 41, 5241–5260 CrossRef CAS
.
- M. A. Iqbal, A. Husain, O. Alam, S. A. Khan, A. Ahmad, M. R. Haider and M. A. Alam, Arch. Pharm., 2020, 353, 2000071 CrossRef CAS
.
- 2-oxoglutaric acid, https://chem.echa.europa.eu, (accessed 15 May 2024).
- L. A. Cynober, Curr. Opin. Clin. Nutr. Metab. Care, 1999, 2, 33–37 CrossRef CAS
.
- J. E. Abusalim, K. Yamamoto, N. Miura, B. Blackman, J. R. Brender, C. Mushti, T. Seki, K. A. Camphausen, R. E. Swenson, M. C. Krishna and A. H. Kesarwala, ACS Chem. Biol., 2021, 16, 2144–2150 CrossRef CAS PubMed
.
- H. Abla, M. Sollazzo, G. Gasparre, L. Iommarini and A. M. Porcelli, Semin. Cell Dev. Biol., 2020, 98, 26–33 CrossRef
.
- B. Gyanwali, Z. X. Lim, J. Soh, C. Lim, S. P. Guan, J. Goh, A. B. Maier and B. K. Kennedy, Trends Endocrinol. Metab., 2022, 33, 136–146 CrossRef PubMed
.
- F. Hammarqvist, J. Wernerman, A. von der Decken and E. Vinnars, Surgery, 1991, 109, 28–36 Search PubMed
.
- M. Wirén, J. Permert and J. Larsson, Nutrition, 2002, 18, 725–728 CrossRef PubMed
.
- P. Iwaniak, E. Tomaszewska, S. Muszyński, M. Marszałek-Grabska, S. G. Pierzynowski and P. Dobrowolski, Nutrients, 2022, 14, 2062 CrossRef PubMed
.
- U. Kjellman, K. Björk, R. Ekroth, H. Karlsson, R. Jagenburg, F. Nilsson, G. Svensson and J. Wernerman, Lancet, 1995, 345, 553–555 CrossRef PubMed
.
- U. W. Kjellman, K. Björk, R. Ekroth, H. Karlsson, R. Jagenburg, F. N. Nilsson, G. Svensson and J. Wernerman, Ann. Thorac. Surg., 1997, 63, 1625–1633 CrossRef PubMed
.
- J. Wernerman, F. Hammarqvist and E. Vinnars, Lancet, 1990, 335, 701–703 CrossRef PubMed
.
- A. Jeppsson, R. Ekroth, P. Friberg, K. Kirnö, I. Milocco, F. N. Nilsson, S. Svensson and J. Wernerman, Ann. Thorac. Surg., 1998, 65, 684–690 CrossRef PubMed
.
- N. M. Shrimali, S. Agarwal, S. Kaur, S. Bhattacharya, S. Bhattacharyya, J. T. Prchal and P. Guchhait, eBioMedicine, 2021, 73, 103672 CrossRef PubMed
.
- S. Agarwal, R. Ghosh, G. Verma, R. Khadgawat and P. Guchhait, Clin. Exp. Immunol., 2023, XX, 12 Search PubMed
.
- Y. Yuan, C. Zhu, Y. Wang, J. Sun, J. Feng, Z. Ma, P. Li, W. Peng, C. Yin, G. Xu, P. Xu, Y. Jiang, Q. Jiang and G. Shu, Sci. Adv., 2022, 8, eabn2879 CrossRef CAS PubMed
.
- C. W. Tseng, W. H. Kuo, S. H. Chan, H. L. Chan, K. J. Chang and L. H. Wang, Cancer Res., 2018, 78, 2799–2812 CrossRef CAS PubMed
.
- K. Kaławaj, A. Sławińska-Brych, M. Mizerska-Kowalska, A. Żurek, A. Bojarska-Junak, M. Kandefer-Szerszeń and B. Zdzisińska, Int. J. Mol. Sci., 2020, 21, 1–21 Search PubMed
.
- N. Liu, J. Zhang, M. Yan, L. Chen, J. Wu, Q. Tao, B. Yan, X. Chen and C. Peng, Cell Death Dis., 2023, 14, 170 CrossRef CAS PubMed
.
- K. Matsumoto, N. Obara, M. Ema, M. Horie, A. Naka, S. Takahashi and S. Imagawa, Cancer Sci., 2009, 100, 1639–1647 CrossRef CAS PubMed
.
- V. Sica, J. M. Bravo-San Pedro, V. Izzo, J. Pol, S. Pierredon, D. Enot, S. Durand, N. Bossut, A. Chery, S. Souquere, G. Pierron, E. Vartholomaiou, N. Zamzami, T. Soussi, A. Sauvat, L. Mondragón, O. Kepp, L. Galluzzi, J. C. Martinou, H. Hess-Stumpp, K. Ziegelbauer, G. Kroemer and M. C. Maiuri, Cell Rep., 2019, 27, 820–834.e9 CrossRef CAS
.
- R. P. Radzki, M. Bienko and S. G. Pierzynowski, J. Bone Miner. Metab., 2012, 30, 651–659 CrossRef CAS
.
- R. Bhattacharya, J. Hariharakrishnan, R. M. Satpute and R. Tulsawani, Mol. Cell. Toxicol., 2012, 8, 83–93 CrossRef CAS
.
- R. Ali, G. Mittal, S. Sultana and A. Bhatnagar, Exp. Lung Res., 2012, 38, 435–444 CrossRef CAS
.
- D. C. Mathangi, R. Shyamala, R. Vijayashree, K. R. Rao, A. Ruckmani, R. Vijayaraghavan and R. Bhattacharya, Neurochem. Res., 2011, 36, 540–548 CrossRef CAS
.
- J. C. Norris, W. A. Utley and A. S. Hume, Toxicology, 1990, 62, 275–283 CrossRef CAS PubMed
.
- M. M. Bayliak, H. V. Shmihel, M. P. Lylyk, O. M. Vytvytska, J. M. Storey, K. B. Storey and V. I. Lushchak, Environ. Toxicol. Pharmacol., 2015, 40, 650–659 CrossRef CAS PubMed
.
- T. Meissner, E. Mayatepek, M. Kinner and R. Santer, Clin. Chim. Acta, 2004, 341, 23–26 CrossRef CAS
.
- W. Harrington, A. Liu, D. Lonsdale and D. Igou, Clin. Chim. Acta, 1977, 74, 247–254 CrossRef CAS PubMed
.
- S. Islam, T. M. Leissing, R. Chowdhury, R. J. Hopkinson and C. J. Schofield, Annu. Rev. Biochem., 2018, 87, 585–620 CrossRef
.
- H. L. H. Green and A. C. Brewer, Clin. Epigenet., 2020, 12, 1–15 CrossRef
.
- J. A. Losman, P. Koivunen and W. G. Kaelin, Nat. Rev. Cancer, 2020, 20, 710–726 CrossRef CAS PubMed
.
- G. Zurlo, J. Guo, M. Takada, W. Wei and Q. Zhang, Biochim. Biophys. Acta, Rev. Cancer, 2016, 1866, 208–220 CrossRef CAS
.
- J. Guo, A. A. Chakraborty, P. Liu, W. Gan, X. Zheng, H. Inuzuka, B. Wang, J. Zhang, L. Zhang, M. Yuan, J. Novak, J. Q. Cheng, A. Toker, S. Signoretti, Q. Zhang, J. M. Asara, W. G. K. Jr and W. Wei, Science, 2016, 353, 929–932 CrossRef CAS
.
- B. W. Wong, A. Kuchnio, U. Bruning and P. Carmeliet, Trends Biochem. Sci., 2013, 38, 3–11 CrossRef CAS PubMed
.
- G. Sutendra, P. Dromparis, A. Kinnaird, T. H. Stenson, A. Haromy, J. M. R. Parker, M. S. Mcmurtry and E. D. Michelakis, Oncogene, 2013, 32, 1638–1650 CrossRef CAS PubMed
.
- D. A. Tennant and E. Gottlieb, J. Mol. Med., 2010, 88, 839–849 CrossRef CAS
.
- A. Sharma, S. Sinha and N. Shrivastava, Front. Genet., 2022, 13, 1–10 Search PubMed
.
- B. Wang, Q. Zhao, Y. Zhang, Z. Liu, Z. Zheng, S. Liu, L. Meng, Y. Xin and X. Jiang, J. Exp. Clin. Cancer Res., 2021, 40, 1–16 CrossRef
.
- L. Jin, J. Chun, C. Pan, A. Kumar, G. Zhang, Y. Ha, D. Li, G. N. Alesi, Y. Kang, L. Zhou, W. M. Yu, K. R. Magliocca, F. R. Khuri, C. K. Qu, C. Metallo, T. K. Owonikoko and S. Kang, Mol. Cell, 2018, 69, 87–99.e7 CrossRef CAS
.
- X. Wang, R. Liu, X. Qu, H. Yu, H. Chu, Y. Zhang, W. Zhu, X. Wu, H. Gao, B. Tao, W. Li, J. Liang, G. Li and W. Yang, Mol. Cell, 2019, 76, 148–162.e7 CrossRef CAS PubMed
.
- L. Jin, J. Chun, C. Pan, A. Kumar, G. Zhang, Y. Ha, D. Li, G. N. Alesi, Y. Kang, L. Zhou, W. M. Yu, K. R. Magliocca, F. R. Khuri, C. K. Qu, C. Metallo, T. K. Owonikoko and S. Kang, Mol. Cell, 2018, 69, 87–99.e7 CrossRef CAS
.
- X. Wang, R. Liu, X. Qu, H. Yu, H. Chu, Y. Zhang, W. Zhu, X. Wu, H. Gao, B. Tao, W. Li, J. Liang, G. Li and W. Yang, Mol. Cell, 2019, 76, 148–162.e7 CrossRef CAS
.
- X. Wu and Y. Zhang, Nat. Rev. Genet., 2017, 18, 517–534 CrossRef CAS
.
- A. Portela and M. Esteller, Nat. Biotechnol., 2010, 28, 1057–1068 CrossRef CAS
.
- X. Wu and Y. Zhang, Nat. Rev. Genet., 2017, 18, 517–534 CrossRef CAS
.
- W. Li, X. Zhang, X. Lu, L. You, Y. Song, Z. Luo, J. Zhang, J. Nie, W. Zheng, D. Xu, Y. Wang, Y. Dong, S. Yu, J. Hong, J. Shi, H. Hao, F. Luo, L. Hua, P. Wang, X. Qian, F. Yuan, L. Wei, M. Cui, T. Zhang, Q. Liao, M. Dai, Z. Liu, G. Chen, K. Meckel, S. Adhikari, G. Jia, M. B. Bissonnette, X. Zhang, Y. Zhao, W. Zhang, C. He and J. Liu, Cell Res., 2017, 27, 1243–1257 CrossRef CAS PubMed
.
- R. Dhat, D. Mongad, S. Raji, S. Arkat, N. R. Mahapatra, N. Singhal and S. L. Sitasawad, Epigenet. Chromatin, 2023, 16, 12 CrossRef CAS
.
- F. Neri, D. Dettori, D. Incarnato, A. Krepelova, S. Rapelli, M. Maldotti, C. Parlato, P. Paliogiannis and S. Oliviero, Oncogene, 2015, 34, 4168–4176 CrossRef CAS
.
- L. Cimmino, M. M. Dawlaty, D. Ndiaye-Lobry, Y. S. Yap, S. Bakogianni, Y. Yu, S. Bhattacharyya, R. Shaknovich, H. Geng, C. Lobry, J. Mullenders, B. King, T. Trimarchi, B. Aranda-Orgilles, C. Liu, S. Shen, A. K. Verma, R. Jaenisch and I. Aifantis, Nat. Immunol., 2015, 16, 653–662 CrossRef CAS PubMed
.
- J. P. Morris, J.
J. Yashinskie, R. Koche, R. Chandwani, S. Tian, C. C. Chen, T. Baslan, Z. S. Marinkovic, F. J. Sánchez-Rivera, S. D. Leach, C. Carmona-Fontaine, C. B. Thompson, L. W. S. Finley and S. W. Lowe, Nature, 2019, 573, 595–599 CrossRef
.
- T. Q. Tran, E. A. Hanse, A. N. Habowski, H. Li, M. B. I. Gabra, Y. Yang, X. H. Lowman, A. M. Ooi, S. Y. Liao, R. A. Edwards, M. L. Waterman and M. Kong, Nat. Cancer, 2020, 1, 345–358 CrossRef CAS PubMed
.
- T. TeSlaa, A. C. Chaikovsky, I. Lipchina, S. L. Escobar, K. Hochedlinger, J. Huang, T. G. Graeber, D. Braas and M. A. Teitell, Cell Metab., 2016, 24, 485–493 CrossRef CAS PubMed
.
- B. W. Carey, L. W. S. Finley, J. R. Cross, C. D. Allis and C. B. Thompson, Nature, 2015, 518, 413–416 CrossRef CAS
.
- M. I. Matias, C. S. Yong, A. Foroushani, C. Goldsmith, C. Mongellaz, E. Sezgin, K. R. Levental, A. Talebi, J. Perrault, A. Rivière, J. Dehairs, O. Delos, J. Bertand-Michel, J. C. Portais, M. Wong, J. C. Marie, A. Kelekar, S. Kinet, V. S. Zimmermann, I. Levental, L. Yvan-Charvet, J. V. Swinnen, S. A. Muljo, H. Hernandez-Vargas, S. Tardito, N. Taylor and V. Dardalhon, Cell Rep., 2021, 37, 109911 CrossRef CAS PubMed
.
- S. H. Naeini, L. Mavaddatiyan, Z. R. Kalkhoran, S. Taherkhani and M. Talkhabi, Exp. Gerontol., 2023, 175, 112154 CrossRef CAS PubMed
.
- Y. Yuan, P. Xu, Q. Jiang, X. Cai, T. Wang, W. Peng, J. Sun, C. Zhu, C. Zhang, D. Yue, Z. He, J. Yang, Y. Zeng, M. Du, F. Zhang, L. Ibrahimi, S. Schaul, Y. Jiang, J. Wang, J. Sun, Q. Wang, L. Liu, S. Wang, L. Wang, X. Zhu, P. Gao, Q. Xi, C. Yin, F. Li, G. Xu, Y. Zhang and G. Shu, EMBO J., 2021, 40, 1–30 Search PubMed
.
- B. Campbell, M. Roberts, C. Kerksick, C. Wilborn, B. Marcello, L. Taylor, E. Nassar, B. Leutholtz, R. Bowden, C. Rasmussen, M. Greenwood and R. Kreider, Nutrition, 2006, 22, 872–881 CrossRef CAS
.
- E. D. Son, G. H. Choi, H. Kim, B. Lee, I. S. Chang and J. S. Hwang, Biol. Pharm. Bull., 2007, 30, 1395–1399 CrossRef CAS PubMed
.
- A. Asadi Shahmirzadi, D. Edgar, C. Y. Liao, Y. M. Hsu, M. Lucanic, A. Asadi Shahmirzadi, C. D. Wiley, G. Gan, D. E. Kim, H. G. Kasler, C. Kuehnemann, B. Kaplowitz, D. Bhaumik, R. R. Riley, B. K. Kennedy and G. J. Lithgow, Cell Metab., 2020, 32, 447–456.e6 CrossRef CAS PubMed
.
- R. M. Chin, X. Fu, M. Y. Pai, L. Vergnes, H. Hwang, G. Deng, S. Diep, B. Lomenick, V. S. Meli, G. C. Monsalve, E. Hu, S. A. Whelan, J. X. Wang, G. Jung, G. M. Solis, F. Fazlollahi, C. Kaweeteerawat, A. Quach, M. Nili, A. S. Krall, H. A. Godwin, H. R. Chang, K. F. Faull, F. Guo, M. Jiang, S. A. Trauger, A. Saghatelian, D. Braas, H. R. Christofk, C. F. Clarke, M. A. Teitell, M. Petrascheck, K. Reue, M. E. Jung, A. R. Frand and J. Huang, Nature, 2014, 510, 397–401 CrossRef CAS
.
- X. Cai, Y. Yuan, Z. Liao, K. Xing, C. Zhu, Y. Xu, L. Yu, L. Wang, S. Wang, X. Zhu, P. Gao, Y. Zhang, Q. Jiang, P. Xu and G. Shu, FASEB J., 2018, 32, 488–499 CrossRef CAS PubMed
.
- M. M. Bayliak, M. P. Lylyk, O. M. Vytvytska and V. I. Lushchak, Eur. Food Res. Technol., 2016, 242, 179–188 CrossRef CAS
.
- S. Liu, L. He and K. Yao, Biomed. Res. Int., 2018, 2018, 3408467 Search PubMed
.
- S. Whillier, B. Garcia, B. E. Chapman, P. W. Kuchel and J. E. Raftos, FEBS J., 2011, 278, 3152–3163 CrossRef CAS
.
- L. He, J. Wu, W. Tang, X. Zhou, Q. Lin, F. Luo, Y. Yin and T. Li, J. Agric. Food Chem., 2018, 66, 11273–11283 CrossRef CAS
.
- D. An, Q. Zeng, P. Zhang, Z. Ma, H. Zhang, Z. Liu, J. Li, H. Ren and D. Xu, Redox Biol., 2021, 46, 1–14 Search PubMed
.
- V. Matzi, J. Lindenmann, A. Muench, J. Greilberger, H. Juan, R. Wintersteiger, A. Maier and F. M. Smolle-Juettner, Eur. J. Cardio-Thoracic Surg., 2007, 32, 776–782 CrossRef PubMed
.
- CYL Pharmazeutika GmbH, EP1842536A1, 2007.
- B. Tugnoli, G. Giovagnoni, A. Piva and E. Grilli, Animals, 2020, 10, 134 CrossRef
.
-
K. Lohbeck, H. Haferkorn, W. Fuhrmann and N. Fedtke, Ullmann's Encyclopedia of Industrial Chemistry, John Wiley & Sons, Inc., 2000 Search PubMed
.
-
B. Cornils, P. Lappe and U. Staff, Ullmann's Encyclopedia of Industrial Chemistry, John Wiley & Sons, Inc., 2014, pp. 1–18 Search PubMed
.
- J. M. Pinazo, M. E. Domine, V. Parvulescu and F. Petru, Catal. Today, 2015, 239, 17–24 CrossRef CAS
.
-
W. Riemenschneider and M. Tanifuji, Ullmann's Encyclopedia of Industrial Chemistry, John Wiley & Sons, Inc., 2011 Search PubMed
.
- E. Schuler, M. Demetriou, N. R. Shiju and G.-J. M. Gruter, ChemSusChem, 2021, 14, 3636–3664 CrossRef CAS PubMed
.
- C. L. Mehltretter and C. E. Rist, J. Agric. Food Chem., 1953, 1, 779–783 CrossRef CAS
.
- E. M. Bottorff and L. L. Moore, Org. Synth., 1964, 44, 67 CrossRef CAS
.
- L. Friedman and E. Kosower, Org. Synth., 1946, 26, 42 CrossRef CAS
.
- Maleic anhydride, https://chem.echa.europa.eu, (accessed 15 May 2024).
- Oxalic acid, https://chem.echa.europa.eu, (accessed 15 May 2024).
- Tianjin Tiancheng Pharmaceutical Co., Ltd., US8680329B2, 2014.
-
G. Koenig, E. Lohmar, N. Rupprich, M. Lison and A. Gnass, Ullmann's Encyclopedia of Industrial Chemistry, John Wiley & Sons, Inc., 2012 Search PubMed
.
-
E.-L. Dreher, K. K. Beutel, J. D. Myers, T. Lübbe, S. Krieger and L. H. Pottenger, Ullmann's Encyclopedia of Industrial Chemistry, John Wiley & Sons, Inc., 2014, pp. 1–81 Search PubMed
.
-
T. Ohara, T. Sato, N. Shimizu, G. Prescher, H. Schwind, O. Weiberg, K. Marten, H. Greim, T. D. Shaffer and P. Nandi, Ullmann's Encyclopedia of Industrial Chemistry, John Wiley & Sons, Inc., 2020, pp. 1–21 Search PubMed
.
- R. J. Mayer, H. Kaur, S. A. Rauscher and J. Moran, J. Am. Chem. Soc., 2021, 143, 19099–19111 CrossRef CAS PubMed
.
- H. Choudhary, S. Nishimura and K. Ebitani, Appl. Catal., A, 2013, 458, 55–62 CrossRef CAS
.
- C.-B. Hong, W. Hua, L. Liu and H. Liu, Nat. Commun., 2025, 16, 1245 CrossRef CAS
.
- W. Yuan, Y. Du, K. Yu, S. Xu, M. Liu, S. Wang, Y. Yang, Y. Zhang and J. Sun, Microorganisms, 2022, 10(12) DOI:10.3390/microorganisms10122454
.
-
A. Karpov, C. Walsdorff, M. Siemer, G. Mattioda and A. Blanc, Ullmann's Encyclopedia of Industrial Chemistry, 2021, pp. 1–11 Search PubMed
.
- H. Li, P. S. Bhadury, A. Riisager and S. Yang, Catal. Sci. Technol., 2014, 4, 4138–4168 RSC
.
- University of Iowa Research Foundation UIRF, US5221621A, 1993.
- E. M. M. Abdelraheem, H. Busch, U. Hanefeld and F. Tonin, React. Chem. Eng., 2019, 4, 1878–1894 RSC
.
- J. B. Pyser, S. Chakrabarty, E. O. Romero and A. R. H. Narayan, ACS Cent. Sci., 2021, 7, 1105–1116 CrossRef CAS
.
- B. Beer, A. Pick and V. Sieber, Metab. Eng., 2017, 40, 5–13 CrossRef CAS
.
- F. Busch, J. Brummund, E. Calderini, M. Schürmann and R. Kourist, ACS Sustainable Chem. Eng., 2020, 8, 8604–8612 CrossRef CAS PubMed
.
- L. Liu, G. Sakir, H. Shin, J. Li, G. Du and J. Chen, J. Biotechnol., 2013, 164, 97–104 CrossRef CAS PubMed
.
- P. Ödman, W. B. Wellborn and A. S. Bommarius, Tetrahedron: Asymmetry, 2004, 15, 2933–2937 CrossRef
.
- B. Beer, A. Pick and V. Sieber, Metab. Eng., 2017, 40, 5–13 CrossRef CAS PubMed
.
- A. Al-Shameri, D. L. Siebert, S. Sutiono, L. Lauterbach and V. Sieber, Nat. Commun., 2023, 14, 2693 CrossRef CAS PubMed
.
- S. Sutiono, A. Pick and V. Sieber, Green Chem., 2021, 23, 3656–3663 RSC
.
- T. Hermann, J. Biotechnol., 2003, 104, 155–172 CrossRef CAS PubMed
.
- J. E. Cronan and D. Laporte, EcoSal Plus, 2005, 24 Search PubMed
.
- C. Malina, C. Larsson and J. Nielsen, FEMS Yeast Res., 2018, 18, foy040 CrossRef CAS
.
- H. L. Kornberg and H. A. Krebs, Nature, 1957, 179, 988–991 CrossRef CAS
.
-
L. Jimenez-Diaz, A. Caballero and A. Segura, in Aerobic Utilization of Hydrocarbons, Oils, and Lipids, ed. K. N. Timmis, M. Boll, O. Geiger, H. Goldfine, T. Krell, S. Y. Lee, J. M. Terry, F. Rojo, D. Z. Sousa, A. J. M. Stams, R. Steffan, H. Wilkes and O. Geiger, Springer, Cham, 2019, pp. 291–325 Search PubMed
.
- R. L. Anderson and W. A. Wood, Annu. Rev. Microbiol., 1969, 23, 539–578 CrossRef CAS
.
- A. Beopoulos, J. M. Nicaud and C. Gaillardin, Appl. Microbiol. Biotechnol., 2011, 90, 1193–1206 CrossRef CAS PubMed
.
-
B. H. Kim and G. M. Gadd, Bacterial Physiology and Metabolism, Cambridge University Press, 2nd edn, 2019, pp. 85–125 Search PubMed
.
- M. Inigo, S. Deja and S. C. Burgess, Annu. Rev. Nutr., 2021, 41, 19–47 CrossRef CAS
.
- B. Bergdahl, D. Heer, U. Sauer, B. Hahn-hägerdal and E. W. J. Van Niel, Biotechnol. Biofuels, 2012, 5, 1–19 CrossRef
.
- C. Thakker, I. Martínez, K. San and G. N. Bennett, Biotechnol. J., 2012, 7, 213–224 CrossRef CAS PubMed
.
- A. M. Raab and C. Lang, Bioeng. Bugs, 2011, 2, 120–123 CrossRef PubMed
.
- K. S. Vuoristo, A. E. Mars, J. P. M. Sanders, G. Eggink and R. A. Weusthuis, Trends Biotechnol., 2016, 34, 191–197 CrossRef CAS
.
-
J. C. Bergmann and L. P. Sallet, Advances in Sugarcane Biorefinery, eds. C. Anuj and M. H. Luciano Silveira, Elsevier Inc., 2018, pp. 73–95 Search PubMed
.
- L. Steffens, E. Pettinato, T. M. Steiner, A. Mall, S. König, W. Eisenreich and I. A. Berg, Nature, 2021, 592, 784–788 CrossRef CAS PubMed
.
- A. Mall, J. Sobotta, C. Huber, C. Tschirner, S. Kowarschik, K. Ba, M. Mergelsberg, M. Boll, M. Hügler, W. Eisenreich and I. A. Berg, Science, 2018, 359(6375), 563–567 CrossRef CAS PubMed
.
- P. Mara, G. S. Fragiadakis, F. Gkountromichos and D. Alexandraki, Microb. Cell Fact., 2018, 17, 1–13 CrossRef
.
- R. B. Helling, J. Bacteriol., 1994, 176, 4664–4668 CrossRef CAS PubMed
.
-
L. Reitzer, in EcoSal Plus, ed. V. Stewart, American Society for Microbiology, 2004, p. 18 Search PubMed
.
- A. A. Alhasawi, S. C. Thomas, T. Sujeethar, F. Legendre and V. D. Appanna, Front. Microbiol., 2019, 10, 1–13 CrossRef
.
- A. Maclean, S. Tharmalingam and V. D. Appanna, Antioxidants, 2022, 11, 14 Search PubMed
.
- R. J. Mailloux, R. Singh, G. Brewer, C. Auger, J. Lemire and V. D. Appanna, J. Bacteriol., 2009, 191(12), 3804–3810 CrossRef CAS
.
- F. Oliveira, P. C. Engel and A. R. Khan, FEBS J., 2013, 280, 4681–4692 CrossRef PubMed
.
- G. Massad, H. U. I. Zhao and H. L. T. Mobley, J. Bacteriol., 1995, 177, 5878–5883 CrossRef CAS
.
- H. Drechsel, A. Thieken, R. Reissbrodt, G. Jung, R. K. Institut and O. Chemie, J. Bacteriol., 1993, 175, 2727–2733 CrossRef CAS PubMed
.
- H. Kusakabe, Y. Midorikawa, T. Fujishlma, A. Kuninaka and H. Yoshino, Agric. Biol. Chem., 1983, 47, 1323–1328 CAS
.
- K. N. G. Valdehuesa, K. R. M. Ramos, G. M. Nisola, A. B. Bañares, R. B. Cabulong, W. K. Lee, H. Liu and W. J. Chung, Appl. Microbiol. Biotechnol., 2018, 102, 7703–7716 CrossRef CAS PubMed
.
- C. E. M. Nunn, U. Johnsen, P. Scho, T. Fuhrer, U. Sauer, D. W. Hough and M. J. Danson, J. Biol. Chem., 2010, 285, 33701–33709 CrossRef CAS
.
- R. Weimberg, J. Biol. Chem., 1961, 236, 629–635 CrossRef CAS
.
- K. A. K. Köhler, L. M. Blank, O. Frick and A. Schmid, Environ. Microbiol., 2015, 17, 156–170 CrossRef
.
- C. Stephens, B. Christen, T. Fuchs, V. Sundaram, K. Watanabe and U. Jenal, J. Bacteriol., 2007, 189, 2181–2185 CrossRef CAS PubMed
.
- D. Liu, Y. Zhang, J. Li, W. Sun, Y. Yao and C. Tian, Biotechnol. Biofuels Bioprod., 2023, 16, 1–16 CrossRef
.
- P. D. Karp, S. Paley, R. Caspi, A. Kothari, M. Krummenacker, P. E. Midford, L. R. Moore, P. Subhraveti, S. Gama-Castro, V. H. Tierrafria, P. Lara, L. Muñiz-Rascado, C. Bonavides-Martinez, A. Santos-Zavaleta, A. Mackie, G. Sun, T. A. Ahn-Horst, H. Choi, M. W. Covert, J. Collado-Vides and I. Paulsen, EcoSal Plus, 2023, 11(1) DOI:10.1128/ecosalplus.esp-0002-2023eesp-0002-2023
.
- G. L. Winsor, D. K. W. Lam, L. Fleming, R. Lo, M. D. Whiteside, N. Y. Yu, R. E. W. Hancock and F. S. L. Brinkman, Nucleic Acids Res., 2011, 39, D596–D600 CrossRef CAS PubMed
.
- M. Kanehisa, Y. Sato, M. Kawashima, M. Furumichi and M. Tanabe, Nucleic Acids Res., 2016, 44, D457–D462 CrossRef CAS
.
- L. Sundermeyer, J.-G. Folkerts, B. Lückel, C. Mack, M. Baumgart and M. Bott, Microbiol. Spectrosc., 2023, 11, e02668–23 Search PubMed
.
- T. Haas, M. Graf, A. Nieß, T. Busche, J. Kalinowski, B. Blombach and R. Takors, Front. Microbiol., 2019, 10, 1–14 CrossRef
.
- L. Sundermeyer, J.-G. Folkerts, B. Lückel, C. Mack, M. Baumgart and M. Bott, Microbiol. Spectrosc., 2023, 11, e02668–23 Search PubMed
.
- H. Guo, C. Madzak, G. Du, J. Zhou and J. Chen, Appl. Microbiol. Biotechnol., 2014, 98, 7003–7012 CrossRef CAS
.
- Y. Guo, L. Su, Q. Liu, Y. Zhu, Z. Dai and Q. Wang, Comput. Struct. Biotechnol. J., 2022, 20, 2503–2511 CrossRef CAS PubMed
.
- M. Bussmann, D. Emer, S. Hasenbein, S. Degraf, B. J. Eikmanns and M. Bott, J. Biotechnol., 2009, 143, 173–182 CrossRef CAS
.
- Z. Jiang, Z. Cui, Z. Zhu, Y. Liu, Y. Jie Tang, J. Hou and Q. Qi, Biotechnol. Biofuels, 2021, 14, 1–10 CrossRef PubMed
.
- D. Molenaar, M. E. Van der Rest, A. Drysch and R. Yüchel, J. Bacteriol., 2000, 182, 6892–6899 CrossRef
.
- P. J. Trotter, K. Juco, H. T. Le, K. Nelson, L. I. Tamayo, J. M. Nicaud and Y. K. Park, Yeast, 2020, 37, 103–115 CrossRef CAS PubMed
.
- Q. Liu, X. Ma, H. Cheng, N. Xu, J. Liu and Y. Ma, Biotechnol. Lett., 2017, 39, 913–919 CrossRef CAS
.
- X. Zhang, N. Xu, J. Li, Z. Ma, L. Wei, Q. Liu and J. Liu, Enzyme Microb. Technol., 2020, 136, 109530 CrossRef CAS PubMed
.
- K. Liu, Y. Liu, X. Li, X. Zhang, Z. Xue and M. Zhao, Appl. Microbiol. Biotechnol., 2023, 107, 6497–6506 CrossRef CAS
.
- S. Niu, F. Liu, Y. Wang, B. Rao and Y. Wang, Molecules, 2024, 29(8), 1861 CrossRef CAS
.
- G. S. Hossain, J. Li, H. Dong Shin, R. R. Chen, G. Du, L. Liu and J. Chen, J. Biotechnol., 2014, 169, 112–120 CrossRef CAS PubMed
.
- G. S. Hossain, J. Li, H. D. Shin, L. Liu, M. Wang, G. Du and J. Chen, J. Biotechnol., 2014, 187, 71–77 CrossRef CAS
.
- B. Lin and Y. Tao, Microb. Cell Fact., 2017, 16, 1–12 CrossRef
.
- UNIV JIANGNAN OP – CN 201410132063 A, 2014.
- LUOYANG HUARONG BIOTECHNOLOGY CO LTD OP - CN 201410372262 A, 2014.
- UNIV JIANGNAN OP - CN 201510179796 A, 2015.
- HENAN JULONG BIO ENG CO LTD OP - CN 201810257243 A, 2018.
- TIANJIN INST IND BIOTECHNOLOGY CAS OP - CN 201610637736 A, 2018.
- UNIV JIANGNAN OP - CN 201610363824 A, 2016.
- UNIV JIANGNAN OP - CN 201310309739 A, 2013.
- SICHUAN JISHENG BIOPHARMACEUTICAL CO LTD OP - CN 201910030458 A, 2019.
- INST MICROBIOLOGY CAS OP - CN 201510923517 A, 2017.
- X. Zhang, N. Xu, J. Li, Z. Ma, L. Wei, Q. Liu and J. Liu, Enzyme Microb. Technol., 2020, 136, 109530 CrossRef CAS
.
- J. Wu, X. Fan, J. Liu, Q. Luo, J. Xu and X. Chen, Appl. Microbiol. Biotechnol., 2018, 102, 4755–4764 CrossRef CAS PubMed
.
- K. Liu, Y. Liu, X. Li, X. Zhang, Z. Xue and M. Zhao, Appl. Microbiol. Biotechnol., 2023, 107, 6497–6506 CrossRef CAS PubMed
.
- F. Busch, F. Busch, J. Brummund, E. Calderini, M. Schürmann and R. Kourist, ACS Sustainable Chem. Eng., 2020, 8, 8604–8612 CrossRef CAS
.
- K. Hideo, I. Kazutami and T. Tatsurokuro, US2786799A, 1954.
- H. J. Koepsell, F. H. Stodola and E. S. Sharpe, US2724680A, 1952.
- H. J. Koepsell, F. H. Stodola and E. S. Sharpe, J. Am. Chem. Soc., 1952, 74, 5142–5144 CrossRef CAS
.
- L. B. Lockwood and F. H. Stodola, J. Biol. Chem., 1946, 164, 81–83 CrossRef CAS
.
- K. A. G. Baritugo, H. T. Kim, Y. C. David, J. H. Choi, J. il Choi, T. W. Kim, C. Park, S. H. Hong, J. G. Na, K. J. Jeong, J. C. Joo and S. J. Park, Biofuels, Bioprod. Biorefining, 2018, 12, 899–925 CrossRef CAS
.
- Tianjin University, Science and Technology, CN103194496A, 2013.
- KH Neochem Co Ltd, US3450599A, 1968.
- KH Neochem Co Ltd, US3450599A, 1967.
- H. J. Huang, L. M. Liu, Y. Li, G. C. Du and J. Chen, Biotechnol. Lett., 2006, 28, 95–98 CrossRef CAS PubMed
.
- L. Liu, Y. Li, Y. Zhu, G. Du and J. Chen, Metab. Eng., 2007, 9, 21–29 CrossRef CAS PubMed
.
- D. Zhang, N. Liang, Z. Shi, L. Liu, J. Chen and G. Du, Biotechnol. Bioprocess Eng., 2009, 14, 134–139 CrossRef CAS
.
- Jiangnan University, CN101153295A, 2007.
- Qingdao University of Science and Technology, CN104195184A, 2014.
- O. G. Chernyavskaya, N. V. Shishkanova and T. V. Finogenova, Appl. Biochem. Microbiol., 1997, 33, 300 Search PubMed
.
- Daziran Biological Group Co Ltd, CN114507611A, 2022.
- Z. D. Blount, eLife, 2015, 4, 1–12 CrossRef
.
- F. R. Blattner, G. Plunkett, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau and Y. Shao, Science, 1997, 277, 1453–1462 CrossRef CAS
.
- M. H. Serres, S. Gopal, L. A. Nahum, P. Liang and M. Riley, Genome Biol., 2001, 2, 1–7 CrossRef
.
- E. Tantoso, B. Eisenhaber, S. Sinha, L. J. Jensen and F. Eisenhaber, Biol. Direct, 2023, 18, 1–17 CrossRef
.
- S. Pontrelli, T. Y. Chiu, E. I. Lan, F. Y. H. Chen, P. Chang and J. C. Liao, Metab. Eng., 2018, 50, 16–46 CrossRef CAS
.
- S. Ghatak, Z. A. King, A. Sastry and B. O. Palsson, Nucleic Acids Res., 2019, 47, 2446–2454 CrossRef CAS PubMed
.
- J. Jeong, N. Cho, D. Jung and D. Bang, Biotechnol. Adv., 2013, 31, 804–810 CrossRef CAS
.
- D. Yang, C. P. S. Prabowo, H. Eun, S. Y. Park, I. J. Cho, S. Jiao and S. Y. Lee, Essays Biochem., 2021, 65, 225–246 CrossRef CAS
.
- C. Zhao, Y. Zhang and Y. Li, Biotechnol. Adv., 2019, 37, 107402 CrossRef CAS PubMed
.
- C. Wang, B. F. Pfleger and S. W. Kim, Curr. Opin. Biotechnol, 2017, 45, 92–103 CrossRef CAS PubMed
.
- X. Chen, L. Zhou, K. Tian, A. Kumar, S. Singh, B. A. Prior and Z. Wang, Biotechnol. Adv., 2013, 31, 1200–1223 CrossRef CAS PubMed
.
-
T. N. B. T. Ibrahim, A. Bin Abas and N. F. A. Razak, in Biomanufacturing for Sustainable Production of Biomolecules, ed. V. Singh and P. Loke, Springer Nature, Singapore, 2023, pp. 141–163 Search PubMed
.
- J. Yang and S. Yang, BMC Genomics, 2017, 18, 940 CrossRef PubMed
.
-
J. Kalinowski, in Handbook of Corynebacterium glutamicum, ed. L. Eggeling and M. Bott, CRC Press, Boca Raton, 1st edn, 2005, pp. 37–56 Search PubMed
.
-
J. Becker and C. Wittmann, in Industrial Biotechnology: Microorganisms, ed. C. Wittmann and J. C. Liao, Wiley-VCH Verlag GmbH & Co, 1st edn, 2017, pp. 183–219 Search PubMed
.
- L. Eggeling and M. Bott, Appl. Microbiol. Biotechnol., 2015, 99, 3387–3394 CrossRef CAS PubMed
.
- D. Ray, U. Anand, N. K. Jha, E. Korzeniewska, E. Bontempi, J. Proćków and A. Dey, Environ. Res., 2022, 213, 113622 CrossRef CAS PubMed
.
- N. Stäbler, T. Oikawa, M. Bott and L. Eggeling, J. Bacteriol., 2011, 193, 1702–1709 CrossRef
.
- J. Becker, C. M. Rohles and C. Wittmann, Metab. Eng., 2018, 50, 122–141 CrossRef CAS
.
- M. Merkel, D. Kiefer, M. Schmollack, B. Blombach, L. Lilge, M. Henkel and R. Hausmann, Bioresour. Technol., 2022, 351, 126994 CrossRef CAS
.
- T. Han, G. B. Kim and S. Y. Lee, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 30328–30334 CrossRef CAS PubMed
.
- X. Y. Wu, X. Y. Guo, B. Zhang, Y. Jiang and B. C. Ye, Front. Bioeng. Biotechnol., 2020, 7, 440 CrossRef PubMed
.
- L. Feng, Y. Zhang, J. Fu, Y. Mao, T. Chen, X. Zhao and Z. Wang, Biotechnol. Bioeng., 2016, 113, 1284–1293 CrossRef CAS
.
- T. Kogure and M. Inui, Appl. Microbiol. Biotechnol., 2018, 102, 8685–8705 CrossRef CAS
.
- H. N. Lee, W. S. Shin, S. Y. Seo, S. S. Choi, J. Soo Song, J. Yeon Kim, J. H. Park, D. Lee, S. Y. Kim, S. J. Lee, G. T. Chun and E. S. Kim, Sci. Rep., 2018, 8, 1–12 Search PubMed
.
- V. F. Wendisch, L. F. Brito, M. Gil Lopez, G. Hennig, J. Pfeifenschneider, E. Sgobba and K. H. Veldmann, J. Biotechnol., 2016, 234, 139–157 CrossRef CAS PubMed
.
- H. H. Liu, X. J. Ji and H. Huang, Biotechnol. Adv., 2015, 33, 1522–1546 CrossRef CAS
.
- K. K. Miller and H. S. Alper, Appl. Microbiol. Biotechnol., 2019, 103, 9251–9262 CrossRef CAS
.
- M. Workman, P. Holt and J. Thykaer, AMB Express, 2013, 3, 1–9 CrossRef
.
-
A. Aurich, R. Specht, R. A. Muller, U. Stottmeister, V. Yovkova, C. Otto, M. Holz, G. Barth, P. Heretsch, F. A. Thomas, D. Sicker and A. Giannis, Monitoring microbial diversity of bioreactors using metagenomic approaches, 2012, pp. 391–423 Search PubMed
.
- M. Gatter, S. Ottlik, Z. Kövesi, B. Bauer, F. Matthäus and G. Barth, Fungal Genet. Biol., 2016, 95, 30–38 CrossRef CAS
.
- IFP Energies Nouvelles IFPEN, US3930946A, 1972.
- O. G. Chernyavskaya, N. V. Shishkanova, A. P. Il’chenko and T. V. Finogenova, Appl. Microbiol. Biotechnol., 2000, 53, 152–158 CrossRef CAS
.
- Ajinomoto Co Inc, US3616213A, 1969.
- S. V. Kamzolova and I. G. Morgunov, Appl. Microbiol. Biotechnol., 2020, 104, 7979–7989 CrossRef CAS PubMed
.
- J. Zhou, H. Zhou, G. Du, L. Liu and J. Chen, Lett. Appl. Microbiol., 2010, 51, 264–271 CrossRef CAS
.
- X. Yin, C. Madzak, G. Du, J. Zhou and J. Chen, Appl. Microbiol. Biotechnol., 2012, 96, 1527–1537 CrossRef CAS PubMed
.
- L. Tomaszewska-Hetman, A. Rywińska, Z. Lazar and W. Rymowicz, Catalysts, 2023, 13, 14 CrossRef CAS
.
- A. Rywińska, L. Tomaszewska-Hetman, M. Rakicka-Pustułka, P. Juszczyk and W. Rymowicz, Sustainability, 2020, 12, 6109 CrossRef
.
- S. Ogata and T. Hirasawa, Appl. Microbiol. Biotechnol., 2021, 105, 6909–6920 CrossRef CAS
.
- E. Kimura, J. Biosci. Bioeng., 2002, 94, 545–551 CrossRef CAS PubMed
.
- T. Hasegawa, K. I. Hashimoto, H. Kawasaki and T. Nakamatsu, J. Biosci. Bioeng., 2008, 105, 12–19 CrossRef CAS PubMed
.
- E. Kimura, C. Yagoshi, Y. Kawahara, T. Ohsumi, T. Nakamatsu and H. Tokuda, Biosci., Biotechnol., Biochem., 1999, 63, 1274–1278 CrossRef CAS PubMed
.
- Y. Li, L. Sun, J. Feng, R. Wu, Q. Xu, C. Zhang, N. Chen and X. Xie, Bioprocess Biosyst. Eng., 2016, 39, 967–976 CrossRef CAS
.
- T. Hirasawa, M. Saito, K. Yoshikawa, C. Furusawa and H. Shmizu, Biotechnol. J., 2018, 13, 1700612 CrossRef
.
- Jiangsu Intelligent Workshop Technology Research Institute Co Ltd, CN102391977A, 2011.
- Guangdong Huanxi Biotechnology Co Ltd, CN101250563B, 2008.
- Y. Nakayama, K. Ichi Hashimoto, Y. Sawada, M. Sokabe, H. Kawasaki and B. Martinac, Biophys. Rev., 2018, 10, 1359–1369 CrossRef CAS
.
- L. Tomaszewska-Hetman, A. Rywińska, Z. Lazar, P. Juszczyk, M. Rakicka-Pustułka, T. Janek, M. Kuźmińska-Bajor and W. Rymowicz, Int. J. Mol. Sci., 2021, 22, 7577 CrossRef CAS
.
- K. R. Pomraning, Y. M. Kim, C. D. Nicora, R. K. Chu, E. L. Bredeweg, S. O. Purvine, D. Hu, T. O. Metz and S. E. Baker, BMC Genomics, 2016, 17, 1–18 CrossRef
.
- Y. B. Lee, J. H. Jo, M. H. Kim, H. H. Lee and H. H. Hyun, Biotechnol. Bioprocess Eng., 2013, 18, 770–777 CrossRef CAS
.
- W. C. van Heeswijk, H. V. Westerhoff and F. C. Boogerd, Microbiol. Mol. Biol. Rev., 2013, 77, 628–695 CrossRef
.
- Evonik Degussa Gmbh, WO2009053489A1, 2008.
- M. Tesch, A. A. De Graaf and H. Sahm, Appl. Environ. Microbiol., 1999, 65, 1099–1109 CrossRef CAS PubMed
.
- X. Chen, X. Dong, J. Liu, Q. Luo and L. Liu, Biotechnol. Bioeng., 2020, 117, 2791–2801 CrossRef CAS PubMed
.
- N. Tenhaef, J. Kappelmann, A. Eich, M. Weiske, L. Brieß, C. Brüsseler, J. Marienhagen, W. Wiechert and S. Noack, Biotechnol. J., 2021, 16, 2100043 CrossRef CAS PubMed
.
- R. Tsugawa and S. Okumura, Agric. Biol. Chem., 1969, 33, 676–682 CAS
.
- Akad Wissenschaften Ddr, DD267999B5, 1988.
- J. Zhou, X. Yin, C. Madzak, G. Du and J. Chen, J. Biotechnol., 2012, 161, 257–264 CrossRef CAS
.
- V. Yovkova, C. Otto, A. Aurich, S. Mauersberger and G. Barth, Appl. Microbiol. Biotechnol., 2014, 98, 2003–2013 CrossRef CAS PubMed
.
- H. Guo, P. Liu, C. Madzak, G. Du, J. Zhou and J. Chen, Sci. Rep., 2015, 5, 1–10 CAS
.
- W. Zeng, G. Du, J. Chen, J. Li and J. Zhou, Process Biochem., 2015, 50, 1516–1522 CrossRef CAS
.
- Y. Chen, F. Li, J. Mao, Y. Chen and J. Nielsen, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2020154118 CrossRef CAS
.
- M. S. Cyert and C. C. Philpott, Genetics, 2013, 193, 677–713 CrossRef CAS
.
- S. V. Kamzolova, M. N. Chiglintseva, J. N. Lunina and I. G. Morgunov, Appl. Microbiol. Biotechnol., 2012, 96, 783–791 CrossRef CAS PubMed
.
- I. G. Morgunov, S. V. Kamzolova and V. A. Samoilenko, Appl. Microbiol. Biotechnol., 2013, 97, 8711–8718 CrossRef CAS
.
- Z. Yu, G. Du, J. Zhou and J. Chen, Bioresour. Technol., 2012, 114, 597–602 CrossRef CAS PubMed
.
- K. Cybulski, L. Tomaszewska-Hetman, M. Rakicka, W. Łaba, W. Rymowicz and A. Rywińska, Ind. Crops Prod., 2018, 119, 102–110 CrossRef
.
- S. V. Kamzolova and I. G. Morgunov, Appl. Microbiol. Biotechnol., 2013, 97, 5517–5525 CrossRef CAS
.
- H. Dominguez, C. Nezondet, N. D. Lindley and M. Cocaign, Biotechnol. Lett., 1993, 15, 449–454 CrossRef CAS
.
- F. Käβ, S. Junne, P. Neubauer, W. Wiechert and M. Oldiges, Microb. Cell Fact., 2014, 13, 6 CrossRef
.
- A. Lemoine, N. Maya Martnez-Iturralde, R. Spann, P. Neubauer and S. Junne, Biotechnol. Bioeng., 2015, 112, 1220–1231 CrossRef CAS PubMed
.
- H. Xu, W. Dou, H. Xu, X. Zhang, Z. Rao, Z. Shi and Z. Xu, Biochem. Eng. J., 2009, 43, 41–51 CrossRef CAS
.
- M. Inui, M. Suda, S. Okino, H. Nonaka, L. G. Puskás, A. A. Vertès and H. Yukawa, Microbiology, 2007, 153, 2491–2504 CrossRef CAS PubMed
.
- A. R. Lara, H. Taymaz-Nikerel, M. R. Mashego, W. M. Van Gulik, J. J. Heijnen, O. T. Ramírez and W. A. Van Winden, Biotechnol. Bioeng., 2009, 104, 1153–1161 CrossRef CAS
.
- A. N. Brown, M. T. Anderson, M. A. Bachman and H. L. T. Mobley, Microbiol. Mol. Biol. Rev., 2022, 86, e00110–21 CrossRef
.
- J. G. Koendjbiharie, R. Van Kranenburg and M. Kengen, FEMS Microbiol. Rev., 2021, 1–19 Search PubMed
.
- U. Sauer and B. J. Eikmanns, FEMS Microbiol. Lett., 2005, 29, 765–794 CrossRef CAS PubMed
.
- G. Wiegand and S. J. Remington, Annu. Rev. Biophys. Biophys. Chem., 1986, 15, 97–117 CrossRef CAS
.
- X. Zhang, X. Wang, K. T. Shanmugam and L. O. Ingram, Appl. Environ. Microbiol., 2011, 77, 427–434 CrossRef CAS PubMed
.
- Y. Asakura, E. Kimura, Y. Usuda, Y. Kawahara, K. Matsui, T. Osumi and T. Nakamatsu, Appl. Environ. Microbiol., 2007, 73, 1308–1319 CrossRef CAS
.
- Y. Wang, L. Fan, P. Tuyishime, J. Liu, K. Zhang, N. Gao, Z. Zhang, X. Ni, J. Feng, Q. Yuan, H. Ma, P. Zheng, J. Sun and Y. Ma, Commun. Biol., 2020, 3, 217 CrossRef CAS
.
- P. Tuyishime, Y. Wang, L. Fan, Q. Zhang, Q. Li, P. Zheng, J. Sun and Y. Ma, Metab. Eng., 2018, 49, 220–231 CrossRef CAS PubMed
.
- N. Okai, C. Takahashi, K. Hatada, C. Ogino and A. Kondo, AMB Express, 2014, 4, 1–8 CrossRef CAS
.
- N. Wang, Y. Ni and F. Shi, Biotechnol. Lett., 2015, 37, 1473–1481 CrossRef CAS PubMed
.
- A. Zhao, X. Hu and X. Wang, Appl. Microbiol. Biotechnol., 2017, 101, 3587–3603 CrossRef CAS
.
- L. Wei, J. Zhao, Y. Wang, J. Gao, M. Du, Y. Zhang, N. Xu, H. Du, J. Ju, Q. Liu and J. Liu, Metab. Eng., 2022, 69, 134–146 CrossRef CAS
.
- J. Wang, X. Shen, Y. Lin, Z. Chen, Y. Yang, Q. Yuan and Y. Yan, ACS Synth. Biol., 2018, 7, 24–29 CrossRef CAS PubMed
.
- J. Wang, J. Wang, Y. Shu Tai, Q. Zhang, W. Bai and K. Zhang, Appl. Microbiol. Biotechnol., 2018, 102, 7377–7388 CrossRef CAS
.
- W. Bai, Y. S. Tai, J. Wang, J. Wang, P. Jambunathan, K. J. Fox and K. Zhang, Metab. Eng., 2016, 38, 285–292 CrossRef CAS PubMed
.
- Z. Man, M. Xu, Z. Rao, J. Guo, T. Yang, X. Zhang and Z. Xu, Sci. Rep., 2016, 6, 1–10 CrossRef
.
- S. H. Park, H. U. Kim, T. Y. Kim, J. S. Park, S. S. Kim and S. Y. Lee, Nat. Commun., 2014, 5, 4618 CrossRef CAS
.
- S. Y. Kim, J. Lee and S. Y. Lee, Biotechnol. Bioeng., 2015, 112, 416–421 CrossRef CAS PubMed
.
- B. Zhang, M. Yu, Y. Zhou, Y. Li and B. C. Ye, Microb. Cell Fact., 2017, 16, 158 CrossRef
.
- B. Zhang, M. Yu, W. P. Wei and B. C. Ye, Microb. Cell Fact., 2018, 17, 91 CrossRef
.
- B. Zhang, G. Gao, X. H. Chu and B. C. Ye, Bioresour. Technol., 2019, 284, 204–213 CrossRef CAS
.
- Y. S. Tai, M. Xiong, P. Jambunathan, J. Wang, J. Wang, C. Stapleton and K. Zhang, Nat. Chem. Biol., 2016, 12, 247–253 CrossRef CAS
.
- A. Alva, A. Sabido-Ramos, A. Escalante and F. Bolívar, Appl. Microbiol. Biotechnol., 2020, 104, 1463–1479 CrossRef CAS PubMed
.
- K. Jahreis, E. F. Pimentel-Schmitt, R. Brückner and F. Titgemeyer, FEMS Microbiol. Rev., 2008, 32, 891–907 CrossRef CAS PubMed
.
- A. Escalante, A. S. Cervantes, G. Gosset and F. Bolívar, Appl. Microbiol. Biotechnol., 2012, 94, 1483–1494 CrossRef CAS PubMed
.
- R. Chatterjee, C. S. Millard, K. Champion, D. P. Clark and M. I. Donnelly, Appl. Environ. Microbiol., 2001, 67, 148–154 CrossRef CAS
.
- D. Wang, Q. Li, Z. Song, W. Zhou, Z. Su and J. Xing, J. Chem. Technol. Biotechnol., 2011, 86, 512–518 CrossRef CAS
.
- H. Lin, G. N. Bennett and K. Y. San, Metab. Eng., 2005, 7, 116–127 CrossRef CAS
.
- J. Z. Xu, H. B. Yu, M. Han, L. M. Liu and W. G. Zhang, J. Ind. Microbiol. Biotechnol., 2019, 46, 937–949 CrossRef
.
- S. C. Chung, J. S. Park, J. Yun and J. H. Park, Metab. Eng., 2017, 40, 157–164 CrossRef CAS
.
- Z. Zhou, C. Wang, H. Xu, Z. Chen and H. Cai, J. Ind. Microbiol. Biotechnol., 2015, 42, 1073–1082 CrossRef CAS
.
- X. Zhang, K. Jantama, K. T. Shanmugam and L. O. Ingram, Appl. Environ. Microbiol., 2009, 75, 7807–7813 CrossRef CAS
.
- X. Zhang, K. Jantama, J. C. Moore, L. R. Jarboea, K. T. Shanmugam and L. O. Ingram, Proc. Natl. Acad. Sci. U. S. A., 2009, 106(48), 20180–20185 CrossRef CAS
.
- Q. Liang, F. Zhang, Y. Li, X. Zhang, J. Li, P. Yang and Q. Qi, Sci. Rep., 2015, 5, 1–10 Search PubMed
.
- L. F. Huergo and R. Dixon, Microbiol. Mol. Biol. Rev., 2015, 79, 419–435 CrossRef CAS PubMed
.
- V. Chubukov, J. J. Desmarais, G. Wang, L. J. G. Chan, E. E. K. Baidoo, C. J. Petzold, J. D. Keasling and A. Mukhopadhyay, npj Syst. Biol. Appl., 2017, 3, 16035 CrossRef
.
- J. Tang, X. Zhu, J. Lu, P. Liu, H. Xu, Z. Tan and X. Zhang, Appl. Microbiol. Biotechnol., 2013, 97, 2513–2520 CrossRef CAS PubMed
.
- J. Meng, B. Wang, D. Liu, T. Chen, Z. Wang and X. Zhao, Microb. Cell Fact., 2016, 15, 141 CrossRef
.
- H. J. Kim, H. Jeong and S. J. Lee, Front. Microbiol., 2020, 11, 27 CrossRef
.
- X. Zhu, Z. Tan, H. Xu, J. Chen, J. Tang and X. Zhang, Metab. Eng., 2014, 24, 87–96 CrossRef CAS PubMed
.
- M. Long, M. Xu, Z. Ma, X. Pan, J. You, M. Hu, Y. Shao, T. Yang, X. Zhang and Z. Rao, Sci. Adv., 2020, 6, 1–11 Search PubMed
.
- K. Jantama, X. Zhang, J. C. Moore, K. T. Shanmugam, S. A. Svoronos and L. O. Ingram, Biotechnol. Bioeng., 2008, 101, 881–893 CrossRef CAS PubMed
.
- K. Jantama, M. J. Haupt, S. A. Svoronos, X. Zhang, J. C. Moore, K. T. Shanmugam and L. O. Ingram, Biotechnol. Bioeng., 2008, 99, 1140–1153 CrossRef CAS
.
- X. Zhang, K. T. Shanmugam and L. O. Ingram, Appl. Environ. Microbiol., 2010, 76, 2397–2401 CrossRef CAS
.
- Z. Tan, J. Chen and X. Zhang, Biotechnol. Biofuels, 2016, 9, 1–13 CrossRef PubMed
.
- W. Zhang, T. Zhang, M. Song, Z. Dai, S. Zhang, F. Xin, W. Dong, J. Ma and M. Jiang, ACS Synth. Biol., 2018, 7, 2803–2811 CrossRef CAS
.
- F. Guo, M. Wu, S. Zhang, Y. Feng, Y. Jiang, W. Jiang and F. Xin, Bioresour. Bioprocess., 2022, 9, 13 CrossRef
.
- B. Litsanov, M. Brocker and M. Bott, Appl. Environ. Microbiol., 2012, 78, 3325–3337 CrossRef CAS PubMed
.
- B. Litsanov, A. Kabus, M. Brocker and M. Bott, Microb. Biotechnol., 2012, 5, 116–128 CrossRef CAS PubMed
.
- Y. Mao, G. Li, Z. Chang, R. Tao, Z. Cui, Z. Wang, Y. J. Tang, T. Chen and X. Zhao, Biotechnol. Biofuels, 2018, 11, 1–17 CrossRef CAS
.
- Z. Cui, C. Gao, J. Li, J. Hou, C. S. K. Lin and Q. Qi, Metab. Eng., 2017, 42, 126–133 CrossRef CAS
.
- Q. Yu, Z. Cui, Y. Zheng, H. Huo, L. Meng, J. Xu and C. Gao, Biochem. Eng. J., 2018, 139, 51–56 CrossRef CAS
.
- C. Otto, V. Yovkova, A. Aurich, S. Mauersberger and G. Barth, Appl. Microbiol. Biotechnol., 2012, 95, 905–917 CrossRef CAS
.
- C. Zhang, Y. Li, J. Ma, Y. Liu, J. He, Y. Li, F. Zhu, J. Meng, J. Zhan, Z. Li, L. Zhao, Q. Ma, X. Fan, Q. Xu, X. Xie and N. Chen, Metab. Eng., 2018, 49, 287–298 CrossRef CAS
.
- E. Theodosiou, M. Breisch, M. K. Julsing, F. Falcioni, B. Bühler and A. Schmid, Biotechnol. Bioeng., 2017, 114, 1511–1520 CrossRef CAS
.
- J. Meng, B. Wang, D. Liu, T. Chen, Z. Wang and X. Zhao, Microb. Cell Fact., 2016, 15, 141 CrossRef PubMed
.
- J. da Veiga Moreira, M. Jolicoeur, L. Schwartz and S. Peres, Sci. Rep., 2021, 11, 1–11 CrossRef PubMed
.
- K. Thongbhubate, K. Irie, Y. Sakai, A. Itoh and H. Suzuki, AMB Express, 2021, 11, 168 CrossRef CAS PubMed
.
- Z. Zhang, P. Liu, W. Su, H. Zhang, W. Xu and X. Chu, Microb. Cell Fact., 2021, 20, 1–15 CrossRef
.
- M. Hoffelder, K. Raasch, J. Van Ooyen and L. Eggeling, J. Bacteriol., 2010, 192, 5203–5211 CrossRef CAS
.
- C. A. Steginsky, K. J. Gruys and P. A. Frey, J. Biol. Chem., 1985, 260, 13690–13693 CrossRef CAS
.
- M. Li, P. Y. Ho, S. Yao and K. Shimizu, Biochem. Eng. J., 2006, 30, 286–296 CrossRef CAS
.
- T. V. Yuzbashev, E. Y. Yuzbasheva, T. I. Sobolevskaya, I. A. Laptev, T. V. Vybornaya, A. S. Larina, K. Matsui, K. Fukui and S. P. Sineoky, Biotechnol. Bioeng., 2010, 107, 673–682 CrossRef CAS
.
- H. Guo, C. Madzak, G. Du and J. Zhou, Appl. Microbiol. Biotechnol., 2016, 100, 649–659 CrossRef CAS
.
- A. Niebisch, A. Kabus, C. Schultz, B. Weil and M. Bott, J. Biol. Chem., 2006, 281, 12300–12307 CrossRef CAS PubMed
.
- M. Bott, Trends Microbiol., 2007, 15, 417–425 CrossRef CAS
.
- L. Sundermeyer, G. Bosco, S. Gujar, M. Brocker, M. Baumgart, D. Willbold, O. H. Weiergräber, M. Bellinzoni and M. Bott, Microbiol. Spectrosc., 2022, 10, e02677–22 Search PubMed
.
- A. Q. D. Nguyen, J. Schneider, G. K. Reddy and V. F. Wendisch, Metabolites, 2015, 5, 211–231 CrossRef CAS PubMed
.
- E. R. B. E. Kholy, B. J. Eikmanns, M. Gutmann and H. Sahm, Appl. Environ. Microbiol., 1993, 59, 2329–2331 CrossRef CAS PubMed
.
- G. Beckers, L. Nolden and A. Burkovski, Microbiology, 2001, 147, 2961–2970 CrossRef CAS
.
- A. Michel, A. Koch-Koerfges, K. Krumbach, M. Brocker and M. Bott, Appl. Environ. Microbiol., 2015, 81, 7496–7508 CrossRef CAS
.
- S. Okino, M. Inui and H. Yukawa, Appl. Microbiol. Biotechnol., 2005, 68, 475–480 CrossRef CAS
.
- D. P. Clark, FEMS Microbiol. Lett., 1989, 63, 223–234 CrossRef CAS
.
- N. Zhu, H. Xia, Z. Wang, X. Zhao and T. Chen, PLoS One, 2013, 8, e60659 CrossRef CAS PubMed
.
- L. Jiang, J. Pang, L. Yang, W. Li, L. Duan, G. Zhang and Y. Luo, J. Biotechnol., 2021, 329, 104–117 CrossRef CAS PubMed
.
- S. Okino, R. Noburyu, M. Suda, T. Jojima, M. Inui and H. Yukawa, Appl. Microbiol. Biotechnol., 2008, 81, 459–464 CrossRef CAS
.
- Y. Mao, G. Li, Z. Chang, R. Tao, Z. Cui, Z. Wang, Y. J. Tang, T. Chen and X. Zhao, Biotechnol. Biofuels, 2018, 11, 1–17 CrossRef CAS
.
- H. Lin, G. N. Bennett and K. Y. San, Biotechnol. Bioeng., 2005, 89, 148–156 CrossRef CAS PubMed
.
- A. M. Sánchez, G. N. Bennett and K. Y. San, Metab. Eng., 2006, 8, 209–226 CrossRef PubMed
.
- Z. Cui, C. Gao, J. Li, J. Hou, C. S. K. Lin and Q. Qi, Metab. Eng., 2017, 42, 126–133 CrossRef CAS
.
- M. S. Ahmed, K. J. Lauersen, S. Ikram and C. Li, ACS Synth. Biol., 2021, 10, 646–669 CrossRef CAS
.
- Z. Jiang, Z. Cui, Z. Zhu, Y. Liu, Y. Jie Tang, J. Hou and Q. Qi, Biotechnol. Biofuels, 2021, 14, 1–10 CrossRef PubMed
.
-
S. K. Mohanty and M. R. Swain, in Bioethanol Production from Food Crops, ed. R. C. Ray and S. Ramachandran, Elsevier Inc., 2019, pp. 45–59 Search PubMed
.
-
R. C. Ray, K. B. Uppuluri, C. Trilokesh and C. Lareo, in Bioethanol Production from Food Crops, ed. R. C. Ray and S. Ramachandran, Elsevier Inc., 2019, pp. 81–100 Search PubMed
.
-
C. Laluce, G. R. Leite, B. Z. Zavitoski, T. T. Zamai and R. Ventura, Sugarcane-based Biofuels and Bioproducts, 2016, pp. 53–86 Search PubMed
.
-
C. Marzo, A. B. Díaz and I. Caro, in Bioethanol Production from Food Crops, ed. R. C. Ray and S. Ramachandran, Elsevier Inc., 2019, pp. 61–79 Search PubMed
.
- J. Valentine, J. Clifton-Brown, A. Hastings, P. Robson, G. Allison and P. Smith, GCB Bioenergy, 2012, 4, 1–19 CrossRef
.
- T. Arpit Singh, M. Sharma, M. Sharma, G. Dutt Sharma, A. Kumar Passari and S. Bhasin, Fuel, 2022, 322, 124284 CrossRef CAS
.
- T. Raj, K. Chandrasekhar, R. Morya, A. Kumar Pandey, J. H. Jung, D. Kumar, R. R. Singhania and S. H. Kim, Bioresour. Technol., 2022, 360, 127512 CrossRef CAS PubMed
.
- S. Periyasamy, J. Beula Isabel, S. Kavitha, V. Karthik, B. A. Mohamed, D. G. Gizaw, P. Sivashanmugam and T. M. Aminabhavi, Chem. Eng. J., 2023, 453, 139783 CrossRef CAS
.
- S. Rezania, B. Oryani, J. Cho, A. Talaiekhozani, F. Sabbagh, B. Hashemi, P. F. Rupani and A. A. Mohammadi, Energy, 2020, 199, 117457 CrossRef CAS
.
- L. R. Kumar, S. K. Yellapu, R. D. Tyagi and X. Zhang, Bioresour. Technol., 2019, 293, 122155 CrossRef CAS
.
- N. Zhao, L. Qian, G. Luo and S. Zheng, Appl. Microbiol. Biotechnol., 2018, 102, 9517–9529 CrossRef CAS PubMed
.
- R. Ledesma-Amaro and J. M. Nicaud, Trends Biotechnol., 2016, 34, 798–809 CrossRef CAS PubMed
.
- C. Zhang, C. Ottenheim, M. Weingarten and L. H. Ji, Front. Bioeng. Biotechnol., 2022, 10, 1–22 Search PubMed
.
- F. D. Rossinil, J. Chem. Educ., 1960, 37, 554–561 CrossRef
.
- J. Son, S. H. Lim, Y. J. Kim, H. J. Lim, J. Y. Lee, S. Jeong, C. Park and S. J. Park, Bioresour. Technol., 2023, 371, 128607 CrossRef CAS PubMed
.
- M. Ganesan, R. Mani, S. Sai, G. Kasivelu, M. K. Awasthi, R. Rajagopal, N. I. Wan Azelee, P. K. Selvi, S. W. Chang and B. Ravindran, Chemosphere, 2022, 303, 134956 CrossRef CAS PubMed
.
-
R. Fukuda and A. Ohta, Aerobic Utilization of Hydrocarbons, Oils and Lipids, 2017, pp. 1–14 Search PubMed
.
- P. Fickers, Y. Waché, A. Marty, S. Mauersberger, M. S. Smit, P.-H. Benetti and J.-M. Nicaud, FEMS Yeast Res., 2005, 5, 527–543 CrossRef CAS PubMed
.
- R. Fukuda, World J. Microbiol. Biotechnol., 2023, 39, 1–11 CrossRef PubMed
.
- J. Van Waeyenberg, K. Vikanova, B. Smeyers, T. Van Vaerenbergh, M. Aerts, Z. Zhang, S. Sivanandan, H. Van Leuven, X. Wu and B. Sels, ACS Sustainable Chem. Eng., 2024, 12, 11074–11092 CrossRef CAS
.
- P. Mishra, N. R. Lee, M. Lakshmanan, M. Kim, B. G. Kim and D. Y. Lee, BMC Syst. Biol., 2018, 12, 12 CrossRef PubMed
.
- M. Klein, S. Swinnen, J. M. Thevelein and E. Nevoigt, Environ. Microbiol., 2017, 19, 878–893 CrossRef CAS PubMed
.
- A. M. Erian, M. Egermeier, H. Marx and M. Sauer, Yeast, 2022, 39, 323–336 CrossRef CAS PubMed
.
- P. Hapeta, P. Szczepańska, C. Neuvéglise and Z. Lazar, Sci. Rep., 2021, 1–14 Search PubMed
.
- G. Durnin, J. Clomburg, Z. Yeates, P. J. J. Alvarez, K. Zygourakis, P. Campbell and R. Gonzalez, Biotechnol. Bioeng., 2009, 103, 148–161 CrossRef CAS PubMed
.
- M. D. Blankschien, J. M. Clomburg and R. Gonzalez, Metab. Eng., 2010, 12, 409–419 CrossRef CAS PubMed
.
- K. B. Andersen and K. V. O. N. Meyenburg, J. Bacteriol., 1980, 144, 114–123 CrossRef CAS PubMed
.
- K. Kim, C. Y. Hou, D. Choe, M. Kang, S. Cho, B. H. Sung, D. H. Lee, S. G. Lee, T. J. Kang and B. K. Cho, Metab. Eng., 2022, 69, 59–72 CrossRef CAS PubMed
.
- D. Rittmann, S. N. Lindner and V. F. Wendisch, Appl. Environ. Microbiol., 2008, 74, 6216–6222 CrossRef CAS PubMed
.
- T. M. Meiswinkel, D. Rittmann, S. N. Lindner and V. F. Wendisch, Bioresour. Technol., 2013, 145, 254–258 CrossRef CAS PubMed
.
- A. P. Il’chenko, O. G. Cherniavskaia, N. V. Shishkanova and T. V. Finogenova, Mikrobiologiia, 2002, 71, 316–322 Search PubMed
.
- E. Delikonstantis, E. Igos, S. A. Theofanidis, E. Benetto, G. B. Marin, K. Van Geem and G. D. Stefanidis, Green Chem., 2021, 23, 7243–7258 RSC
.
- A. Gęsicka, P. Oleskowicz-Popiel and M. Łężyk, Biotechnol. Adv., 2021, 53, 107861 CrossRef PubMed
.
- V. Wegat, J. T. Fabarius and V. Sieber, Biotechnol. Biofuels Bioprod., 2022, 15, 1–19 CrossRef PubMed
.
- A. Thorenz, L. Wietschel, D. Stindt and A. Tuma, J. Cleaner Prod., 2018, 176, 348–359 CrossRef PubMed
.
- J. W. Choi, E. J. Jeon and K. J. Jeong, Curr. Opin. Biotechnol, 2019, 57, 17–24 CrossRef CAS PubMed
.
- B. Blombach and G. M. Seibold, Appl. Microbiol. Biotechnol., 2010, 86, 1313–1322 CrossRef CAS PubMed
.
- S. Ryu, J. Hipp and C. T. Trinh, Appl. Environ. Microbiol., 2016, 52, 621–631 Search PubMed
.
- S. Ryu and C. T. Trinh, Appl. Environ. Microbiol., 2018, 84, e02146–17 CrossRef PubMed
.
- G. A. Sprenger, Arch. Microbiol., 1995, 164, 324–330 CrossRef CAS PubMed
.
- A. Masi, R. L. Mach and A. R. Mach-Aigner, Appl. Microbiol. Biotechnol., 2021, 105, 4017–4031 CrossRef CAS PubMed
.
- J. M. Francois, C. Alkim and N. Morin, Biotechnol. Biofuels, 2020, 13, 118 CrossRef CAS PubMed
.
- T. M. Meiswinkel, V. Gopinath, S. N. Lindner, K. M. Nampoothiri and V. F. Wendisch, Microb. Biotechnol., 2013, 6, 131–140 CrossRef
.
- H. Kawaguchi, M. Sasaki, A. A. Vertès, M. Inui and H. Yukawa, Appl. Environ. Microbiol., 2009, 75, 3419–3429 CrossRef CAS PubMed
.
- H. Kawaguchi, M. Sasaki, A. A. Vertès, M. Inui and H. Yukawa, Appl. Microbiol. Biotechnol., 2008, 77, 1053–1062 CrossRef CAS PubMed
.
- J. Schneider, K. Niermann and V. F. Wendisch, J. Biotechnol., 2011, 154, 191–198 CrossRef CAS
.
- J. Kim, S. Hwang and S. Lee, Metab. Eng., 2022, 71, 2–12 CrossRef CAS
.
- S. A. Dahms, Biochem. Biophys. Res. Commun., 1974, 60, 1433–1439 CrossRef
.
- L. Shen, M. Kohlhaas, J. Enoki, R. Meier, B. Schönenberger, R. Wohlgemuth, R. Kourist, F. Niemeyer, D. van Niekerk, C. Bräsen, J. Niemeyer, J. Snoep and B. Siebers, Nat. Commun., 2020, 11, 1098 CrossRef CAS PubMed
.
- A. Nair and S. J. Sarma, Microbiol. Res., 2021, 251, 9 CrossRef PubMed
.
- J. M. Gancedo, Microbiol. Mol. Biol. Rev., 1998, 62, 334–361 CrossRef CAS PubMed
.
- B. Görke and J. Stülke, Nat. Rev. Microbiol., 2008, 6, 613–624 CrossRef
.
- V. Narisetty, R. Cox, R. Bommareddy and D. Agrawal, Sustainable Energy Fuels, 2022, 6, 29–65 RSC
.
- Y.-S. Tai, M. Xiong, P. Jambunathan, J. Wang, J. Wang, C. Stapleton and K. Zhang, Nat. Chem. Biol., 2016, 12, 247–253 CrossRef CAS PubMed
.
- K. W. Lu, C. T. Wang, H. Chang, R. S. Wang and C. R. Shen, Metab. Eng. Commun., 2021, 13, 9 Search PubMed
.
- C. Brüsseler, A. Sp, S. Sokolowsky and J. Marienhagen, Metab. Eng. Commun., 2019, 9, 1–7 Search PubMed
.
- K. Li, C. Li, X. Zhao, C. Liu and F. Bai, Bioresour. Technol., 2023, 378, 128991 CrossRef CAS PubMed
.
- B. Halmschlag, K. Hoffmann, R. Hanke, S. P. Putri, E. Fukusaki, J. Büchs and L. M. Blank, Front. Bioeng. Biotechnol., 2020, 7, 476 CrossRef PubMed
.
- C. Borgström, L. Wasserstrom, H. Almqvist, K. Broberg, B. Klein, S. Noack, G. Lidén and M. F. Gorwa-Grauslund, Metab. Eng., 2019, 55, 1–11 CrossRef
.
- C. S. López-Garzón and A. J. J. Straathof, Biotechnol. Adv., 2014, 32, 873–904 CrossRef
.
- E. M. Karp, R. M. Cywar, L. P. Manker, P. O. Saboe, C. T. Nimlos, D. Salvachúa, X. Wang, B. A. Black, M. L. Reed, W. E. Michener, N. A. Rorrer and G. T. Beckham, ACS Sustainable Chem. Eng., 2018, 6, 15273–15283 CrossRef CAS
.
- W. Zeng, S. Xu, G. Du, S. Liu and J. Zhou, Bioprocess Biosyst. Eng., 2018, 41, 1519–1527 CrossRef CAS
.
- X. Shi, W. Su, H. Zhang, J. Fang, N. Xu, Y. Jiang and H. Li, J. Ind. Eng. Chem., 2022, 115, 528–536 CrossRef CAS
.
- X. Shi, M. Yang, A. Chu, F. Zhang, J. Fang, N. Xu, Y. Jiang and H. Li, AIChE J., 2022, 68, e17814 CrossRef CAS
.
- M. Szczygiełda and K. Prochaska, J. Membr. Sci., 2017, 536, 37–43 CrossRef
.
- M. Szczygiełda and K. Prochaska, Process Biochem., 2020, 96, 38–48 CrossRef
.
- M. Szczygiełda and K. Prochaska, Sep. Sci. Technol., 2020, 55, 165–175 CrossRef
.
- M. Szczygiełda and K. Prochaska, Biochem. Eng. J., 2021, 166, 1–10 CrossRef
.
- M. Szczygiełda, M. Krajewska, L. Zheng, L. D. Nghiem and K. Prochaska, J. Membr. Sci., 2021, 637, 119593 CrossRef
.
- W. Dessie, X. Luo, G. J. Duns, M. Wang and Z. Qin, Environ. Technol. Innovation, 2023, 32, 103243 CrossRef CAS
.
- C. S. López-Garzón and A. J. J. Straathof, Biotechnol. Adv., 2014, 32, 873–904 CrossRef
.
- C. Chen, X. Zhang, C. Liu, Y. Wu, G. Zheng and Y. Chen, Bioresour. Technol., 2022, 346, 126609 CrossRef CAS
.
- B. Rukowicz, ACS Sustainable Chem. Eng., 2023, 11, 11459–11469 CrossRef CAS
.
- S. Choi, C. W. Song, J. H. Shin and S. Y. Lee, Metab. Eng., 2015, 28, 223–239 CrossRef CAS PubMed
.
- E. de Jong, H. Stichnothe, G. Bell and H. Jørgensen, IEA Bioenergy, 2020, 79 Search PubMed
.
- L. Winstel, Top Value Added Chemicals: The Biobased Economy 12 Years Later, https://communities.acs.org/t5/GCI-Nexus-Blog/Top-Value-Added-Chemicals-The-Biobased-Economy-12-Years-Later/ba-p/15759, (accessed 21 May 2024).
- S.N., No Title, https://clinicaltrials.gov/search?term=alpha-ketoglutarate, (accessed 15 May 2024).
- T. Papurina, O. Barsukov, O. Zabuga, D. Krasnienkov and E. Denis, Biochem. Biophys. Rep., 2023, 33, 101429 CAS
.
- V. E. Radzinsky, Y. Uspenskaya, L. P. Shulman and I. V. Kuznetsova, Obstet. Gynecol. Int., 2019, 2019, 1572196 Search PubMed
.
- V. E. Radzinskii, I. V. Kuznetsova, Y. B. Uspenskaya, N. B. Repina, Y. K. Gusak, O. M. Zubova, D. I. Burchakov and A. A. Osmakova, Gynecol. Endocrinol., 2016, 32, 64–68 CrossRef CAS PubMed
.
- X. Zhu, Y. Guo, Z. Liu, J. Yang, H. Tang and Y. Wang, Sci. Rep., 2021, 11, 1–9 CrossRef PubMed
.
- E. L. Mills, D. G. Ryan, H. A. Prag, D. Dikovskaya, D. Menon, Z. Zaslona, M. P. Jedrychowski, A. S. H. Costa, M. Higgins, E. Hams, J. Szpyt, M. C. Runtsch, M. S. King, J. F. McGouran, R. Fischer, B. M. Kessler, A. F. McGettrick, M. M. Hughes, R. G. Carroll, L. M. Booty, E. V. Knatko, P. J. Meakin, M. L. J. Ashford, L. K. Modis, G. Brunori, D. C. Sévin, P. G. Fallon, S. T. Caldwell, E. R. S. Kunji, E. T. Chouchani, C. Frezza, A. T. Dinkova-Kostova, R. C. Hartley, M. P. Murphy and L. A. O’Neill, Nature, 2018, 556, 113–117 CrossRef CAS
.
-
J. Clayden, N. Greeves and S. G. Warren, Organic Chemistry, Oxford University Press, Oxford, 2nd edn, 2012 Search PubMed
.
- L. J. Gooßen, F. Rudolphi, C. Oppel and N. Rodríguez, Angew. Chem., Int. Ed., 2008, 47, 3043–3045 CrossRef PubMed
.
- S. Tian, Q. Yue, C. Liu, M. Li, M. Yin, Y. Gao, F. Meng, B. Z. Tang and L. Luo, J. Am. Chem. Soc., 2021, 143, 10054–10058 CrossRef CAS
.
- M. S. Kim, H. Chang, L. Zheng, Q. Yan, B. F. Pfleger, J. Klier, K. Nelson, E. L. W. Majumder and G. W. Huber, Chem. Rev., 2023, 123, 9915–9939 CrossRef CAS
.
- J. G. Rosenboom, R. Langer and G. Traverso, Nat. Rev. Mater., 2022, 7, 117–137 CrossRef
.
- C. Jin, Z. Huang and J. Bao, ACS Sustainable Chem. Eng., 2020, 8, 6315–6322 CrossRef CAS
.
- T. Nicolaï, W. Arts, S. Calderon-Ardila, R. Smets, M. Van Der Borght, J. M. Thevelein and B. F. Sels, ACS Sustainable Chem. Eng., 2024, 12, 8353–8365 CrossRef
.
- S. Van den Bosch, T. Renders, S. Kennis, S.-F. Koelewijn, G. Van den Bossche, T. Vangeel, A. Deneyer, D. Depuydt, C. M. Courtin, J. M. Thevelein, W. Schutyser and B. F. Sels, Green Chem., 2017, 19, 3313–3326 RSC
.
- T. A. Ewing, N. Nouse, M. van Lint, J. van Haveren, J. Hugenholtz and D. S. van Es, Green Chem., 2022, 24, 6373–6405 RSC
.
- E. Abedi and S. M. B. Hashemi, Heliyon, 2020, 6, e04974 CrossRef CAS
.
- P. Zheng, K. Zhang, Q. Yan, Y. Xu and Z. Sun, J. Ind. Microbiol. Biotechnol., 2013, 40, 831–840 CrossRef CAS
.
- L. Mitrea, B. E. Teleky, S. A. Nemes, D. Plamada, R. A. Varvara, M. S. Pascuta, C. Ciont, A. M. Cocean, M. Medeleanu, A. Nistor, A. M. Rotar, C. R. Pop and D. C. Vodnar, Heliyon, 2024, 10, 1–12 CrossRef
.
- Y. Xi, F. Fan and X. Zhang, Green Carbon, 2023, 1, 118–132 CrossRef CAS
.
- A. Kövilein, C. Kubisch, L. Cai and K. Ochsenreither, J. Chem. Technol. Biotechnol., 2020, 95, 513–526 CrossRef
.
- R. Zhang, H. Liu, Y. Ning, Y. Yu, L. Deng and F. Wang, Fermentation, 2023, 9, 71 CrossRef CAS
.
- H. Hosseinpour Tehrani, J. Becker, I. Bator, K. Saur, S. Meyer, A. C. Rodrigues Lóia, L. M. Blank and N. Wierckx, Biotechnol. Biofuels, 2019, 12, 1–11 CrossRef CAS
.
- D. Zhang, D. Guan, J. Liang, C. Guo, X. Xie, C. Zhang, Q. Xu and N. Chen, Braz. J. Microbiol., 2014, 45, 1477–1483 CrossRef CAS PubMed
.
- D. Yang, H. Li, X. Jia, F. Yu, G. Wang, Y. Zhang, W. Wang, L. Zang and F. Shi, Chem. Eng. Trans., 2023, 103, 739–744 Search PubMed
.
- Q. Lei, W. Zeng, J. Zhou and G. Du, Bioresour. Technol., 2019, 292, 121897 CrossRef CAS
.
- R. Li, H. G. Sakir, J. Li, H. D. Shin, G. Du, J. Chen and L. Liu, RSC Adv., 2017, 7, 6615–6621 RSC
.
- C. Otto, V. Yovkova and G. Barth, Appl. Microbiol. Biotechnol., 2011, 92, 689–695 CrossRef CAS
.
- P. D. Karp, R. Billington, R. Caspi, C. A. Fulcher, M. Latendresse, A. Kothari, I. M. Keseler, M. Krummenacker, P. E. Midford, Q. Ong, W. K. Ong, S. M. Paley and P. Subhraveti, Briefings Bioinf., 2018, 20, 1085–1093 CrossRef
.
- R. A. W. Frank, A. J. Price, F. D. Northrop, R. N. Perham and B. F. Luisi, J. Mol. Biol., 2007, 368, 639–651 CrossRef CAS
.
- L. Yang, T. Wagner, A. Mechaly, A. Boyko, E. M. Bruch, D. Megrian, F. Gubellini, P. M. Alzari and M. Bellinzoni, Nat. Commun., 2023, 14, 4851 CrossRef CAS PubMed
.
- L. Sundermeyer, G. Bosco, S. Gujar, M. Brocker, M. Baumgart, D. Willbold, O. H. Weiergräber, M. Bellinzoni and M. Bott, Microbiol. Spectrosc., 2022, 10, e02677–22 Search PubMed
.
Footnote |
| † Authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |















