Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ultrafast competition between CO release and spin crossover upon photoexcitation of a cyclopropenone-bipyridyl FeII complex†
Sebastian
Megow
 *a,
Annika
Prax
a,
Tjorge
Neumann
b,
Marc
Lehr
b,
Anna J.
McConnell
*a,
Annika
Prax
a,
Tjorge
Neumann
b,
Marc
Lehr
b,
Anna J.
McConnell
 *bc and
Friedrich
Temps
*bc and
Friedrich
Temps
 *a
*a
aInstitute of Physical Chemistry, Christian-Albrechts-University Kiel, Olshausen-straße 40, 24098 Kiel, Germany. E-mail: megow@phc.uni-kiel.de; temps@phc.uni-kiel.de
bOtto Diels-Institute of Organic Chemistry, Christian-Albrechts-University Kiel, Olshausenstraße 40, 24098 Kiel, Germany
cDepartment Chemie-Biologie, University of Siegen, Adolf-Reichwein-Straße 2, 57068 Siegen, Germany. E-mail: anna.mcconnell@uni-siegen.de
First published on 8th April 2025
Abstract
The photo-triggered release of carbon monoxide (CO) from a cyclopropenone-bipyridyl ligand and its iron(II) complex was investigated using a combination of stationary and ultrafast time-resolved spectroscopies. Upon continuous irradiation, the complex undergoes CO release and forms the corresponding acetylene photoproduct, albeit at a ∼ 3 times lower rate than the free cyclopropenone ligand, indicating a drastic reduction in quantum yield. We attribute this to the ultrafast dynamics revealed by femtosecond time-resolved electronic absorption spectroscopy, where photoexcitation of ligand-centered states of the complex is predominantely followed by energy-transfer, leading to spin-crossover of the FeII ion rather than CO release. This ultrafast response stands out as highly remarkable as it virtually outcompetes the photo-decarbonylation, which in the free ligand occurs in only ∼130 fs.
Introduction
The photo-initiated release of carbon monoxide (CO) from cyclic ketones has been a subject of ultrafast spectroscopic studies from the very beginnings of femtochemistry to the present.1,2 For the decarbonylation and formation of the respective acetylenes from cyclopropenones, for example, time scales of <200 fs and quantum yields near unity have been reported.3–5In a new development, McConnell and co-workers recently reported a promising new class of CO-releasing molecules, M-CPOnes, where 1 containing a cyclopropenone (CPOne) connected to a 2,2′-bipyridine functions as a ligand in a transition metal coordination complex (e.g., Fe-CPOne, Fe-1, Fig. 1).6 Using such scaffolds, varying the central metal ion and the second cyclopropenone substituent offers vast possibilities to access a broad range of M-CPOnes with attractive properties regarding stability, solubility, metal-ligand interactions and photochemical reactivity.6 Indeed, varying the transition metal from ZnII to CoII and ultimately FeII led to a significant increase in the time required for complete decarbonylation upon irradiation with a 365 nm LED in NMR studies.6 Furthermore, a colour change was observed upon photodecarbonylation of Fe-1 to Fe-2 (both diamagnetic low-spin FeII complexes) due to a hypsochromic shift of the MLCT band (Fig. 2B).
Here, we investigate how the coupling between cyclopropenone-bipyridyl ligands and the transition metal centre in Fe-1 impacts the photodynamics. To this end, we apply femtosecond time-resolved electronic absorption spectroscopy (fs-TEAS) to shed light on both the mechanism of CO release leading from the free cyclopropenone ligand 1 to its acetylene product 2, and on the evidently much more intricate photodynamics of the corresponding FeII complex Fe-1.6
Experimental details
Materials
The syntheses of compounds 1,62,6Fe-16 and Fe-26 are described in detail in Section S4 of the ESI.†Steady-state spectroscopy
Unless indicated otherwise, all stationary UV-vis spectra were recorded in spectroscopic grade acetonitrile (Uvasol®) on a Shimadzu UV-2410 desktop spectrometer in a 1 cm (for concentrations of 20 μM or less) or 1 mm quartz cuvette under air. For the stationary photodecarbonylation experiment, the absolute irradiation intensities of the high-power LED were determined behind the cuvettes with a calibrated power meter (Coherent PS19Q). As both irradiation experiments were conducted under identical conditions—including irradiation wavelength, geometry, intensity, and sample volume—and given that the chosen wavelength corresponds to an isosbestic point, the data allows for a meaningful comparison of the quantum yields for decarbonylation of both compounds.Femtosecond time-resolved electronic absorption spectroscopy (fs-TEAS)
The setup used for femtosecond time-resolved electronic absorption spectroscopy (fs-TEAS) has been described in some detail elsewhere.7–10 Briefly, it consists of a Ti:Sa regenerative amplifier (Clark MXR CPA 2001) delivering pulses of 120 fs FWHM (full width at half maximum) at 1 kHz repetition rate at a center wavelength of λ = 775 nm. The main part of the 775 nm beamline is used to run a home-built non-collinear optical parametric amplifier (NOPA) with a subsequent prism pulse compressor. Excitation pulses at 600 nm were provided directly by the compressed NOPA. Excitation pulses in the UV at 340 nm were obtained by sum-frequency generation (SFG) between the 600 nm NOPA output and the 775 nm laser fundamental in a type I BBO crystal. For broadband detection, a small fraction of the laser fundamental is sent through a CaF2 plate to generate supercontinuum probe pulses in the range 325 nm < λprobe < 750 nm. These are split into probe and reference beams, dispersed in a prism spectrograph and detected using fast frame-transfer CCD cameras. The pump and probe beams overlap in our sample flow cell of 1 mm optical pathlength which is connected to a reservoir with 100 ml volume. The focal size of the pump beams was ∼ 300 μm and the size of the probe beam at the same spot is ∼ 150 μm. The pump polarization was set to magic angle with respect to the probe polarization in all measurements. An optical chopper in the probe beam path, operated at half the repetition rate, blocks every second probe pulse, while another chopper in the pump beam path, operated at a quarter of the repetition rate, allows two consecutive pump pulses to pass while blocking the next two. In this way, it is possible to record transient absorption difference spectra background-corrected for scattered pump light. The sample was continuously recycled during the measurements and UV-vis spectra were taken before and after each measurement to ensure the integrity of the sample. These spectra are displayed in Fig. S4 (ESI†). To study the dynamics following ligand excitation 80 μM solutions of 1 and 2 as well as 58 μM solutions of Fe-1 and Fe-2 in acetonitrile were excited at λpump = 340 nm at fluence of 0.63 mJ cm−2, respectively. To study the dynamics following excitation of the MLCT bands 115 μM solutions of Fe-1 and Fe-2 were excited at λpump = 600 nm at a fluence of 1.41 mJ cm−2.Results and discussion
Steady-state spectroscopy
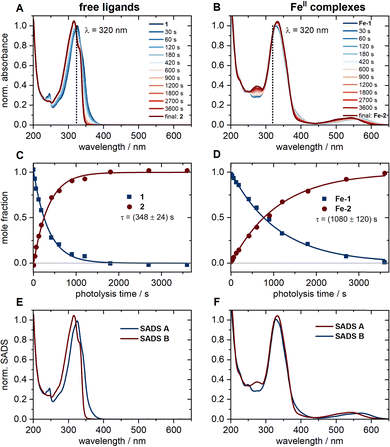
The UV-vis absorption spectra of the compounds are depicted in Fig. 2A and B. The respective spectra in units of molar extinction can be found in Fig. S2 in the ESI.† As can be seen, the two ligand spectra appear quite similar at first glance, owing to the related π-electron systems, but differ when it comes to some of their finer structures. The same applies to the two FeII complexes. The observed features were analysed by time-dependent density functional (TD-DFT) calculations11 to explore the nature of the first 20 (1 and 2) and 40 (Fe-1 and Fe-2) excited singlet states, respectively (Section S1, ESI†). Fig. S1 (ESI†) depicts the computed electronic transitions and spectra. Both 1 and 2 show the expected strong ππ* bands in the UV region at nearly identical wavelengths. For 1, the nπ* transition associated with the free electron pair of the carbonyl oxygen leads to an additional weak shoulder towards longer wavelengths.3,12Fe-1 and Fe-2 feature additional absorption bands in the vis region between ∼500 and 600 nm, and their intense UV bands appear slightly broadened. The TD-DFT calculations for both complexes predict a multitude of transitions in the respective wavelength ranges. The character of the vertical singlet transitions was derived by analysis of the transition density matrices13 (Fig. S1, ESI†). We conclude that the observed vis absorption band is governed by metal-to-ligand charge transfer (MLCT) transitions. The main UV absorption band still originates from a local ππ* transition within one ligand (ligand-centred, LC) and from ππ* transitions between ligands (ligand-to-ligand charge transfer, LLCT). The presence of some less intense MLCT and metal-centred (MC) transitions is noted as well.Photodecarbonylation of 1 to 2, and Fe-1 to Fe-2, respectively, was followed by UV-vis spectroscopy upon irradiation in the UV with a 320 nm light-emitting diode (LED). Fig. 2C and D show the spectral evolution and concentration profiles of the reactants and products during the photolysis and were obtained from a singular value decomposition (SVD) analysis applying a sequential kinetic model of type  . Note that the photodecarbonylation of Fe-1 to Fe-2 is a multistep process involving intermediate species with only one or two ligands in the acetylene form. The recorded UV-vis spectra show isosbestic points at all times, so that the assumption of such a model seems justified. Consequently, the obtained species-associated difference spectra (SADS) displayed in Fig. 2E and F reproduce the experimental absorption spectra. The results demonstrate that the photodecarbonylation process in Fe-1 is slower by about a factor of 3, indicating that the presence of the FeII ion leads to a decrease in the quantum yield of photodecarbonylation by a factor of about 10 (see ESI,† Section S2) compared to 1. Furthermore, irradiation of the MLCT band of Fe-1 in the vis spectral range does not cause any noticeable decarbonylation (Fig. S3, ESI†).
. Note that the photodecarbonylation of Fe-1 to Fe-2 is a multistep process involving intermediate species with only one or two ligands in the acetylene form. The recorded UV-vis spectra show isosbestic points at all times, so that the assumption of such a model seems justified. Consequently, the obtained species-associated difference spectra (SADS) displayed in Fig. 2E and F reproduce the experimental absorption spectra. The results demonstrate that the photodecarbonylation process in Fe-1 is slower by about a factor of 3, indicating that the presence of the FeII ion leads to a decrease in the quantum yield of photodecarbonylation by a factor of about 10 (see ESI,† Section S2) compared to 1. Furthermore, irradiation of the MLCT band of Fe-1 in the vis spectral range does not cause any noticeable decarbonylation (Fig. S3, ESI†).
Femtosecond time-resolved electronic absorption spectroscopy (fs-TEAS)
The measured femtosecond time-resolved electronic absorption maps uncovered the ultrafast photochemical dynamics of 1 and Fe-1 (Fig. 3). The experimental data were recorded after excitation of 1 and Fe-1 at λpump = 340 nm (Fig. 3A and B, respectively) and excitation of Fe-1 at λpump = 600 nm (Fig. 3C). Auxiliary measurements on the photodynamics of the acetylene ligand 2 and its complex Fe-2 are given in the ESI† (Fig. S6 and S9). Since the photodecarbonylation of cyclopropenone 1 is highly efficient, subsequent photoexcitation of the resulting acetylene product 2 is in practice unavoidable due to some accumulation in the sample reservoir, leading to artifacts in the recorded transient absorption map for 1. Consequently, the raw TEAS data for 1 (Fig. S6A, ESI†) were corrected for the contribution of the excited acetylene product to the transient absorption signal (see ESI,† Section S3 and Fig. S6 for a description of the correction procedure) and Fig. 3A depicts the corrected transient absorption results.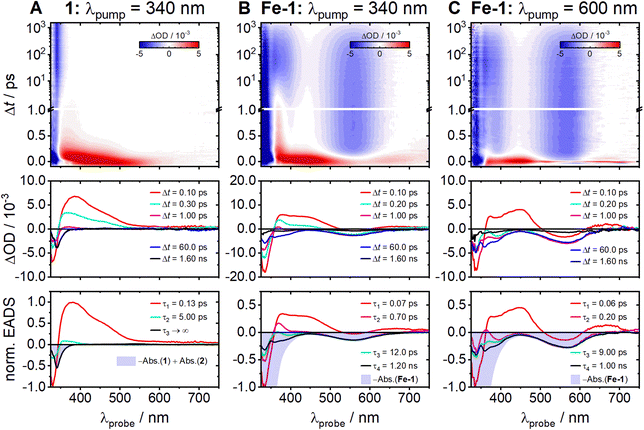 | ||
| Fig. 3 Two-dimensional time-resolved electronic absorption maps recorded in acetonitrile (top), transient absorption difference spectra at selected decay times (middle) and EADS with global time constants used for the modeling of the experimental spectra (bottom) for 1 and Fe-1 (columns A and B, respectively) after photoexcitation at λpump = 340 nm and for Fe-1 (C) after excitation at λpump = 600 nm. The data for 1 were corrected for contributions from the acetylene product 2 as described in the ESI.† | ||
The fs-TEAS results for the free cyclopropenone 1 reveal an initially broad excited-state absorption (ESA) that decays very rapidly in <1 ps, accompanied by a substantial blue-shift (Fig. 3A). The intense ground-state bleach (GSB) at λprobe < 350 nm, which appears immediately after photoexcitation, remains for the entire 1.6 ns experimental observation time. These data were successfully modelled using the python-based software package KiMoPack14 assuming a consecutive kinetic model. The resulting evolution-associated difference spectra (EADS) with their associated global time constants are given in the bottom row of Fig. 3. As can be seen, three spectral components were sufficient to describe the dynamics. The first EADS associated with time constant τ1 = 0.13 ± 0.02 ps describes the rapid decay of the broad initial ESA signal. The second decays with τ2 = 5.0 ± 3.0 ps and exhibits a similar shape but much smaller amplitude. The final component (τ3 → ∞) is negative in the region of the GSB and does not show any recognizable decay. Since it matches the scaled ground-state absorption difference spectrum –Abs(1) + Abs(2) (blue-shaded area in Fig. 3A bottom), it can be reasonably attributed to the formation of the acetylene photoproduct 2 from 1.
Turning to the cyclopropenone Fe-1 complex, the obtained fs-TEAS maps in Fig. 3B and C reveal very different pictures compared to free 1. At the two excitation wavelengths of 340 and 600 nm, however, the maps for Fe-1 appear rather similar. The observed broad, intense, initial ESA band from ∼370 to 540 nm undergoes a swift transformation into a distinct narrow peak at λprobe = 370 nm, which stands out especially after excitation in the UV (Fig. 3B). Shortly thereafter, the ESA transforms into negative GSB contributions, followed eventually by gradual, yet largely full recoveries towards the end of the observation time window (Δt ∼ 1.6 ns). The global analyses gave similar EADS and time constants at both excitation wavelengths. The first component describes the decay of the broad initial ESA and establishment at λprobe < 350 nm of the GSB. The associated time constant τ1 = 70 ± 15 fs (60 ± 25 fs for λpump = 600 nm) reflects dynamics occurring on time scales close to or even below our experimental time resolution, which in both measurements was of the order of Δt ∼ 50 fs. The following two EADS mainly show the loss of the spectral signatures at λprobe = 370 nm with τ2 = 0.7 ± 0.3 ps (0.2 ± 0.1 ps for λpump = 600 nm) and τ3 = 12.0 ± 5.0 ps (9.0 ± 6.0 ps). The final EADS carries mainly GSB contributions from the MLCT-related absorption features, as evidenced by the scaled negative ground state absorption spectrum (–Abs.(Fe-1), blue-shaded area). The associated time constants of τ4 = 1.2 ± 0.1 ns (1.0 ± 0.2 ns) describe the largely complete recovery of the initial ground state population. As can be seen from the last transient spectra, plotted at Δt = 1.6 ns after excitation (black lines in Fig. 3B and C), we observe little to virtually no final reaction product formation under the femtosecond experimental conditions at both excitation wavelengths, even after excitation at λpump = 340 nm. This is in strong contrast with the results for free 1 (Fig. 3A), and with the observed slow photodecarbonylation of Fe-1 to Fe-2 in the static photolysis experiment (cf.Fig. 2). We speculate—although this assertion requires further investigation—that the photodecarbonylation of Fe-1 in the static UV experiment may be due to partial dissociation and photolysis of the unbound ligand 1 in equilibrium. Experimental evidence for the presence of such an equilibrium can be found in the UV-vis spectra of Fe-1 at different concentrations (cf. Fig. S5, ESI†). Interestingly, the ultrafast dynamics of the cyclopropenone complex Fe-1 (Fig. 3B) and the acetylene complex Fe-2 (Fig. S9, ESI†) appear very similar.
The above results can be rationalized based on the relaxation schemes in Fig. 4A and B. According to Fig. 4A, initial excitation at λpump = 340 nm populates the lowest-lying 1ππ* state of cyclopropenone 1. In previous studies of related cyclopropenones, a rapid sub-200 fs decay of an initial ESA signal was assigned to the loss of CO from the photoproduced 1ππ* state and the formation of the acetylene either in the excited or in the ground state.3–5 In our case, therefore, we link the 130 fs time constant τ1 to this decarbonylation process giving 2 in its electronic ground state. We assign the second time constant τ2 = 5.0 ps to subsequent structural dynamics in the ground state of 2 from an assumed “cis-bent” intermediate.3 The formation of 2 was clearly identified from the persistent final TEA spectrum in Fig. 3A that matches the (negative) ground state absorption difference spectrum of 1 and 2. The ultrashort 130 fs time constant characterizing the CO elimination thus accounts for the observed remarkable decarbonylation efficiency of 1, as reported for other cyclopropenones,15 with a quantum yield likely close to unity.
This picture completely changes in the case of Fe-1 where cyclopropenone 1 is bound to the FeII centre as a ligand (Fig. 4B). In fact, the photodynamics of Fe-1 has to be discussed in the context of other FeII polypyridyl complexes such as the well-studied, structurally related [Fe(bpy)3]2+. Investigations of this16–24 and related25–30 complexes demonstrated that photoexcitation of the 1MLCT band in the vis spectrum results in the formation of an excited metal-centred (MC) state, in which the FeII centre ion adopts quintet spin multiplicity (5MC) and is thus in the FeII high-spin state. The ensuing spin crossover (SCO) after 1MLCT excitation has been found to occur extraordinarily fast, in < 50 fs, resulting in a quantum yield close to unity.31 This indicates that potential intermediates, such as 3MLCT and 1,3MC states, are traversed on even shorter time scales.21 Recent two-dimensional femtosecond spectroscopic measurements actually suggested parallel relaxation pathways via the 3MLCT state and directly to the MC states.23 In either case, these literature results suggest a blue-print regarding our observations for Fe-1 after MLCT excitation at λpump = 600 nm. The very broad observed initial ESA is a typical signature of the MLCT states.18,21,25 The rapid decay time of τ1 = 60 fs close to the instrument response function (IRF) of our experiment should in fact be viewed as upper limit for this process (i.e., τ1 ≤ 60 fs). The subsequent appearance of the mostly negative transient absorptions governed by the GSB of the MLCT states (cf.Fig. 3B) indicates the population of the 5MC state. This state is formed with some excess energy and undergoes vibrational relaxation with τ2 = 0.2 ps and τ3 = 9 ps as intermediate time constants.26,27 The reverse intersystem crossing leading to the recovery of the singlet ground state (1GS) eventually occurs within the typical 5MC lifetime of τ4 ∼ 1 ns in acetonitrile.28
Remarkably, similar dynamic processes are also seen for Fe-1 upon excitation at λpump = 340 nm, where the initial absorption of the 1GS is governed by the 1LC/LLCT bands of the ligands. However, the initially observed transient state that decays within near response-limited ≤ 60 fs is already associated with the MLCT states, suggesting that the 1LC/LLCT to MLCT transition is already virtually complete within the time resolution of our experiment. A similarly efficient funnelling of UV excitation in MC excited states has been implicitly observed in [Fe(bpy)3]2+ before.22 The intermediate time constants τ2 = 0.7 ps and τ3 = 12 ps can again be assigned to relaxation dynamics in the 5MC state. The slight differences in relaxation times between the measurements might be a result of the 5MC state being accessed at different crossing points from the MLCT manifold.19 Ultimately, full recovery of the 1GS state is observed with τ4 ∼ 1 ns, leaving little if any indication for release of CO from complex Fe-1. Attempts to study CO release in the absence of competing spin crossover using the related high-spin complex Fe-3 (Section S4, ESI†) were unfortunately not possible due to the formation of multiple metal complexes.
Conclusion
In conclusion, we showed that the irradiation time required for photodecarbonylation of cyclopropenone 1 to acetylene 2 in stationary experiments is significantly increased when the molecule coordinates as a ligand to an FeII centre ion as in the complex Fe-1. As revealed by our fs-TEAS measurements, this is due to an ultrafast SCO to the high-spin form of the Fe-1 complex in < 60 fs (possibly only ∼ 20 fs23). This behaviour is observed in many FeII polypyridyl complexes after excitation of their MLCT bands in the vis spectral range. We further demonstrated that excitation in the UV of the ligand absorption bands of the Fe-1 complex triggers a similar ultrafast process that outcompetes the expected photochemical CO release.These results reveal a number of interesting insights into the photodynamics as well as prospective applications of M–CPOnes. Firstly, while previous studies have shown that coordination to d6 ions can strongly suppress the trans–cis photo-isomerisation kinetics of azopyridines,32,33 it remains remarkable that the coordination of cyclopropenone 1 to FeII within Fe-1 is sufficient to almost completely eliminate photorelease of CO from this ligand, despite the fact that the latter process happens in only ∼ 130 fs in the free ligand 1 itself. Since the photodecarbonylation of Fe-1 can be achieved through continuous static irradiation—albeit with a significantly lower quantum yield than the free ligand—it remains unclear whether this process also occurs from coordinated ligands with low efficiency or only via photoexcitation of transiently de-coordinated ligands in equilibrium. Structure-sensitive ultrafast time-resolved vibrational spectroscopy in the carbonyl region could provide valuable insights, as the ground-state bleach (GSB) signals of the cyclopropenone species are less likely to interfere with potential product absorptions.
Secondly, the FeII metal centre is appealing for applications since there is the potential to extend the M-CPOne absorption to the vis and near-infrared (NIR) regions, outstripping the capabilities of other readily accessible M-CPOnes with first-row transition metals like ZnII and CoII that exhibit MLCT states at considerably higher energies.6 Moreover, the viability of inducing CO release from an initially excited MLCT state warrants exploration of systems featuring longer MLCT lifetimes. A multitude of strategies aiming at extended MLCT lifetimes in FeII complexes are already within reach.34 In addition, the RuII analogue, with its similar MLCT energy but lack of SCO due to its substantial ligand field splitting, might hold promise for expanding our understanding of the dynamics.35–38 Another interesting feature is the presence of LLCT states in these systems that may enable excitation of multiple ligands with a single photon, potentially achieving quantum yields greater than one. In this context, the ZnII analogue could be a viable candidate. Due to its closed-shell nature, ZnII acts as an electronic coupling centre for the ligands, while the complex itself is devoid of low-lying MLCT and MC states that could interfere with the photodecarbonylation. Lastly, the intriguing prospect of introducing other chromophores as the second cyclopropenone substituent may open diverse opportunities to control the excitation wavelength for CO release.6
Data availability
Data supporting this article have been included in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Deutsche Forschungsgemeinschaft (DFG, project number 413396832) for financial support and the spectroscopy department of the Otto Diels-Institute of Organic Chemistry for NMR and mass spectral data collection.Notes and references
- E. W.-G. Diau, J. L. Herek, Z. H. Kim and A. H. Zewail, Science, 1998, 279, 847 Search PubMed.
- M.-H. Kao, R. K. Venkatraman, M. N. R. Ashfold and A. J. Orr-Ewing, Chem. Sci., 2020, 11, 1991 RSC.
- S. Takeuchi and T. Tahara, J. Chem. Phys., 2004, 120, 4768 CrossRef CAS PubMed.
- A. Poloukhtine and V. V. Popik, J. Phys. Chem. A, 2006, 110, 1749 CrossRef CAS PubMed.
- S. C. Doan, G. Kuzmanich, M. N. Gard, M. A. Garcia-Garibay and B. J. Schwartz, J. Phys. Chem. Lett., 2012, 3, 81 CrossRef CAS.
- M. Lehr, T. Neumann, C. Näther and A. J. McConnell, Dalton Trans., 2022, 51, 6936 RSC.
- K. Röttger, S. Wang, F. Renth, J. Bahrenburg and F. Temps, Appl. Phys. B, 2015, 118, 185 CrossRef.
- S. Wang, M. Bohnsack, S. Megow, F. Renth and F. Temps, Phys. Chem. Chem. Phys., 2019, 21, 2080–2092 RSC.
- F. Renth, M. Foca, A. Petter and F. Temps, Chem. Phys. Lett., 2006, 428, 62–67 Search PubMed.
- K. Röttger, R. Siewertsen and F. Temps, Chem. Phys. Lett., 2012, 536, 140–146 CrossRef.
- C. Adamo and D. Jacquemin, Chem. Soc. Rev., 2013, 42, 845 RSC.
- Z. Yoshida and H. Miyahara, Bull. Chem. Soc. Jpn., 1972, 45, 1919 CrossRef CAS.
- F. Plasser, J. Chem. Phys., 2020, 152, 084108 CrossRef CAS PubMed.
- C. Müller, T. Pascher, A. Eriksson, P. Chabera and J. Uhlig, J. Phys. Chem. A, 2022, 126, 4087 CrossRef PubMed.
- R. W. Fessenden, P. M. Carton, H. Shimamori and J. C. Scalano, J. Phys. Chem., 1982, 86, 3803 CrossRef CAS.
- J. K. McCusker, K. N. Walda, R. C. Dunn, J. D. Simon, D. Magde and D. N. Hendrickson, J. Am. Chem. Soc., 1993, 115, 298 CrossRef CAS.
- J. K. McCusker, A. L. Rheingold and D. N. Hendrickson, Inorg. Chem., 1996, 35, 2100 CrossRef CAS.
- W. Gawelda, A. Cannizzo, V.-T. Pham, F. van Mourik, C. Bressler and M. Chergui, J. Am. Chem. Soc., 2007, 129, 8199 Search PubMed.
- C. De Graaf and C. Sousa, Chem. – Eur. J., 2010, 16, 4550 CrossRef CAS PubMed.
- M. Chergui, Acc. Chem. Res., 2015, 48, 801 Search PubMed.
- G. Auböck and M. Chergui, Nat. Chem., 2015, 7, 629 CrossRef PubMed.
- M. Fondell, S. Eckert, R. M. Jay, C. Weniger, W. Quevedo, J. Niskanen, B. Kennedy, F. Sorgenfrei, D. Schick, E. Giangrisostomi, R. Ovsyannikov, K. Adamczyk, N. Huse, P. Wernet, R. Mitzner and A. Föhlisch, Struct. Dyn., 2017, 4, 054902 Search PubMed.
- A. Lee, M. Son, M. Deegbey, M. D. Woodhouse, S. M. Hart, H. F. Beissel, P. T. Cesana, E. Jakubikova, J. K. McCusker and G. S. Schlau-Cohen, Chem. Sci., 2023, 14, 13140 RSC.
- C. Zahn, M. Pastore, J. L. P. Lustres, P. C. Gros, S. Haacke and K. Heyne, J. Am. Chem. Soc., 2024, 146, 9347 CrossRef CAS PubMed.
- J. E. Monat and J. K. McCusker, J. Am. Chem. Soc., 2000, 122, 4092 Search PubMed.
- C. Brady, P. L. Callaghan, Z. Ciunik, C. G. Coates, A. Døssing, A. Hazell, J. J. McGarvey, S. Schenker, H. Toftlund, A. X. Trautwein, H. Winkler and J. A. Wolny, Inorg. Chem., 2004, 43, 4289 CrossRef CAS PubMed.
- A. L. Smeigh, M. Creelman, R. A. Mathies and J. K. McCusker, J. Am. Chem. Soc., 2008, 130, 14105 CrossRef CAS PubMed.
- J. Tribollet, G. Galle, G. Jonusauskas, D. Deldicque, M. Tondusson, J. F. Letard and E. Freysz, Chem. Phys. Lett., 2011, 513, 42 CrossRef CAS.
- N. Huse, H. Cho, K. Hong, L. Jamula, F. M. F. De Groot, T. K. Kim, J. K. McCusker and R. W. Schoenlein, J. Phys. Chem. Lett., 2011, 2, 880 CrossRef CAS PubMed.
- C. Sousa, C. De Graaf, A. Rudavskyi and R. Broer, J. Phys. Chem. A, 2017, 121, 9720 CrossRef CAS PubMed.
- M. A. Bergkamp, C. K. Chang and T. L. Netzel, J. Phys. Chem., 1983, 87, 4441 CrossRef CAS.
- S. Megow, H. L. Fitschen, F. Tuczek and F. Temps, J. Phys. Chem. Lett., 2019, 10, 6048 CrossRef CAS PubMed.
- K. Yamaguchi, S. Kume, K. Namiki, M. Murata, N. Tamai and H. Nishihara, Inorg. Chem., 2005, 44, 9056 CrossRef CAS PubMed.
- O. S. Wenger, Chem. – Eur. J., 2019, 25, 6043 CrossRef CAS PubMed.
- A. Juris, V. Balzani, F. Barigelletti, S. Campagna, P. Belser and A. von Zelewsky, Coord. Chem. Rev., 1988, 84, 85 CrossRef CAS.
- N. H. Damrauer, G. Cerullo, A. Yeh, T. R. Boussie, C. V. Shank and J. K. McCusker, Science, 1997, 275, 54 CrossRef CAS PubMed.
- A. N. Tarnovsky, W. Gawelda, M. Johnson, C. Bressler and M. Chergui, J. Phys. Chem. B, 2006, 110, 26497 CrossRef CAS PubMed.
- A. Cannizzo, F. van Mourik, W. Gawelda, G. Zgrablic, C. Bressler and M. Chergui, Angew. Chem., Int. Ed., 2006, 45, 3174 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: DFT and TD-DFT calculations, additional stationary UV-vis spectroscopy results, additional fs time-resolved electronic absorption spectroscopy (fs-TEAS) results, syntheses of the sample compounds. See DOI: https://doi.org/10.1039/d5cp00019j |
| This journal is © the Owner Societies 2025 |