 Open Access Article
Open Access ArticleHow the nitro group position determines the emission properties of π-expanded diketopyrrolopyrroles†
Kamil
Skonieczny‡
 a,
Francesco
Di Maiolo‡
a,
Francesco
Di Maiolo‡
 c,
Sara
Venturi‡
b,
Alessandro
Iagatti
c,
Sara
Venturi‡
b,
Alessandro
Iagatti
 bd,
Alessandro
Ricci
c,
Francesco
Bertocchi
c,
Daniel T.
Gryko
bd,
Alessandro
Ricci
c,
Francesco
Bertocchi
c,
Daniel T.
Gryko
 *a and
Andrea
Lapini
*a and
Andrea
Lapini
 *bcd
*bcd
aInstitute of Organic Chemistry of Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland. E-mail: dtgryko@icho.edu.pl
bLENS (European Laboratory for Non-Linear Spectroscopy), Via N. Carrara 1, 5001 Sesto Fiorentino, Italy. E-mail: andrea.lapini@unipr.it
cDepartment of Chemistry, Life Sciences and Environmental Sustainability, University of Parma, Parco Area delle Scienze 17/A, 43124 Parma, Italy
dIstituto Nazionale di ottica CNR-INO, Via Nello Carrara 1 50019 Sesto Fiorentino, Italy
First published on 28th February 2025
Abstract
Two complex π-expanded diketopyrrolopyrroles (EDPPs) have been prepared following a multistep but straightforward strategy. We discovered that the fate of these molecules in the excited state can be controlled by subtle differences in their structure. When NO2 groups are located at a distant position, the quadrupolar, centrosymmetric dye exhibits strong red emission across the solvents’ polarity scale. However, when NO2 groups are adjacent to the lactam moiety, the EDPPs exhibit negligible emission even in non-polar solvents. Density functional theory (DFT) calculations indicate that the primary distinction between the two molecules lies in the structural planarity. The molecule with NO2 groups adjacent to the lactam moiety exhibits a loss of planarity due to the coulombic repulsion between these groups. The calculations also suggest that the nitro group does not participate in the S0 → S1 excitation. Furthermore, for both compounds, the first two excited states (one bright and one dark) are found to be very close in energy. The change in molecular geometry affects the non-radiative deactivation of excited states, leading to the two distinct emission behaviors. Experiments in a glassy solvent at low temperatures reveal that at 77 K the photophysics of both dyes becomes the same, which proves that thermal activation is the key mechanism for the non-radiative decay of the excited state for non-emissive EDPPs.
Introduction
The design of organic analogs of n-doped semiconductors benefits from electron-deficient aromatic π-conjugated molecules.1 They can also serve as light sensitizers for photooxidation and for initiating charge transfer (CT) via hole transduction.2 The most typical approach for decreasing the electron density is to attach electron-withdrawing substituents, and the nitro group is one of the strongest of them (Hammett constants exceeding 0.7).3 Nitro-aromatics have been widely utilized in various applications involving intramolecular charge transfer (CT),4 fluorescent probes,5 and nonlinear optical materials.6 With a few exceptions, however, nitro-aromatics were historically regarded as non-emissive.7 The scattered examples of fluorescent nitroaromatics were regarded as a curiosity and hardly influenced the mainstream of functional dyes’ chemistry. The discovery of strongly emissive nitro-aromatics in the last decade enabled a partial change of this paradigm.8 Several quadrupolar, centrosymmetric nitro-aromatics were discovered and investigated.9 This in turn led to attempts to formulate some general rules governing their strong emission.10π-Expanded diketopyrrolopyrroles11 were thought to present an ideal synthetic platform for modular access to centrosymmetric nitroaromatics. We recently reported the synthesis of a new class of π-expanded diketopyrrolopyrroles (EDPPs), which exhibit strong fluorescence in various solvents despite the presence of NO2 groups in their structure.9a The target molecules were synthesized in a two-step process which involved the N-arylation of DPPs followed by an intramolecular direct arylation of the aromatic rings that are present in these building blocks. This established synthetic pathway prompted us to synthesize other pigments, especially those in which the NO2 group is in a different position. Earlier observations indicated that the position of the nitro group in relation to the π-system core has a profound influence on fluorescence.9b Here we report how competing excited state processes decide about the fate of centrosymmetric nitro-aromatics in the excited state, by determining their emissivity. To achieve this, we first designed a suitable DPP core containing alkylated fluorene, which ensured photophysical properties in the near-infrared region and provided appropriate solubility of the final dyes.
Results and discussion
Synthesis
At the outset we focused on the preparation of DPP 1 containing bromine atoms on the fluorene unit, which was achieved following the previously reported literature procedure (Scheme 1).11j The pigment was then modified by introducing an arylalkyne substituent. In order to enable the solubilization of the starting material in solvents commonly used in organic synthesis, the nitrogen atoms of the lactam core were protected with tert-butoxycarbonyl groups (Boc). The formed bis-Boc-DPP 2 was then subjected to Sonogashira coupling with 4-tert-butylphenylacetylene which resulted in the formation of DPP 3.It is important to note that the reaction must be carried out at a temperature equal to or below 80 °C, otherwise it leads to a mixture of products due to partial deprotection of the Boc groups of the substrate. Finally, the product of the double Sonogashira reaction i.e. DPP 3 was deprotected by adding TFA to the pigment solution in methylene chloride. This generated free DPP 4 in the quantitative reaction yield (Scheme 1). Subsequently, N-arylation of DPP 4 was carried out with electron-poor aryl fluorides namely methyl 3-bromo-2-fluoro-5-nitrobenzoate and methyl 3-bromo-4-fluoro-5-nitrobenzoate, in the presence of potassium carbonate in NMP (Scheme 1). The reaction was completed after 4 days, yielding 5 and 6, respectively. N-arylation of DPP 4 proceeded much slower compared to the previously described DPP bearing benzofuran units, where the reaction was completed after a few hours.9a In the next step, both 5 and 6 were subjected to Pd(PPh3)4 in order to perform intramolecular, direct arylation in our systems. As a result we obtained good yields of two π-expandable products: 7 and 8, both possessing 12 fused rings. The reaction was carried out overnight in dry toluene at 115![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. Both target pigments are soluble in chlorinated and aromatic solvents, which enabled their identification using 1H NMR and 13C NMR spectroscopies.
°C. Both target pigments are soluble in chlorinated and aromatic solvents, which enabled their identification using 1H NMR and 13C NMR spectroscopies.
Steady-state optical absorption and emission
Absorption, emission and excitation spectra of dyes 7 and 8 have been recorded in various solvents with different polarity properties (Table 1 and Fig. 1). These spectra are characterized by an absorption band in the 500–650 nm spectral range as well as through a pronounced vibronic progression (≈ 1520 cm−1); however, only a slight modification in the spectral bandwidth and the position of the absorption maximum has been observed as a function of solvent polarity. Excitation and fluorescence spectra are very poorly solvent-dependent as well.| 7 | 8 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| t (ns) | (kr + knr) (ns−1) | Φ fl | k r (ns−1) | k nr (ns−1) | t (ns) | (kr + knr) (ns−1) | Φ fl | k r (ns−1) | k nr (ns−1) | |
| CCl4 | 3.400 | 0.29 | 0.76 | 0.22 | 0.07 | 0.679 | 1.47 | 0.09 | 0.13 | 1.34 |
| Tol | 3.548 | 0.28 | 0.77 | 0.22 | 0.06 | 0.484 | 2.07 | 0.09 | 0.19 | 1.88 |
| CHCl3 | 0.340 | 1.59 | 0.09 | 0.26 | 2.60 | 0.032 | 31.25 | 0.004 | 0.13 | 31.1 |
| THF | 2.579 | 0.39 | 0.56 | 0.22 | 0.17 | 0.141 | 7.09 | 0.02 | 0.14 | 6.9 |
| CH2Cl2 | 0.158 | 6.33 | 0.04 | 0.25 | 6.08 | 0.022 | 45.5 | 0.003 | 0.14 | 45.3 |
| BZN | 0.863 | 1.16 | 0.16 | 0.19 | 0.97 | 0.067 | 14.9 | 0.03 | 0.45 | 14.5 |
 | ||
| Fig. 1 Absorption, emission and excitation spectra of dyes 7 and 8 in CHCl3 (left panels) and toluene (right panels). Spectra recorded in CCl4, THF, CH2Cl2 and benzonitrile are included in the ESI.† | ||
Fluorescence quantum yields (Φfl) reveal dramatically opposite behavior between dyes 7 (Φfl = 0.76) and 8 (Φfl = 0.09) in non-polar and slightly polar solvents (CCl4 and toluene). At the same time a decreasing trend in the fluorescence quantum yield is observed for both compounds as the polarity of the environment is increased (Φfl = 0.16 for dyes 7 and Φfl = 0.03 for 8 in BZN). The virtually absent response of the emission maximum (λmaxem) to solvent polarity for both dyes 7 and 8 indicates that the emissive excited state may be described as quadrupolar. However, the fluorescence emission quenching (for dyes 7 and 8) as the polarity of the solvent is increased from dye 7 to 8 suggests that an additional excited state plays a key role in the deactivation pathway. Given that molar absorption coefficients are ≈140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 × cm−1 in both cases, the optical brightness for 7 reaches almost 110
000 M−1 × cm−1 in both cases, the optical brightness for 7 reaches almost 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 × cm−1 in toluene. This value should be compared from one site with optical brightness ≈170
000 M−1 × cm−1 in toluene. This value should be compared from one site with optical brightness ≈170![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 × cm−1 recorded for somehow analogous π-expanded diketopyrrolopyrrole bearing fused fluorene units and flanking diarylamino groups.11j The presence of nitro groups does not increase the fluorescence quantum yield. On the other hand it slightly decreases the molar absorption coefficient. The optical brightness of EDPP 7 compared however with other described nitro-aromatics is one of the largest recorded.10
000 M−1 × cm−1 recorded for somehow analogous π-expanded diketopyrrolopyrrole bearing fused fluorene units and flanking diarylamino groups.11j The presence of nitro groups does not increase the fluorescence quantum yield. On the other hand it slightly decreases the molar absorption coefficient. The optical brightness of EDPP 7 compared however with other described nitro-aromatics is one of the largest recorded.10
Time resolved measurements
To disentangle the mechanism leading to the fluorescence emission properties of dyes 7 and 8, we characterized the relaxation dynamics with pump–probe spectroscopy in the UV-vis, NIR and Mid-IR spectral range.UV-vis pump–probe spectra in the 400–750 nm spectral range have been recorded with an excitation wavelength range of 525–530 nm for various solvents using the transient absorption setup described in the ESI.† All spectra show ground state bleaching plus stimulated emission signals and an excited state absorption located at 470 nm. Spectra as a function of time have been analyzed with a global fitting approach using the Glotaran program package9 and the unidirectional sequential model as the kinetic decaying scheme. The analysis showed that both dyes 7 and 8 contain only one main spectral component that relaxes back to the ground state (some representative transient absorption spectra are reported in Fig. S7 and S8 in the ESI†); the additional stimulated emission increases and slight red-shift (for the ≈700 nm band) observed in the first tens of picoseconds (Fig. S8a and b, ESI†) have been ascribed to vibrational and structural relaxation following the new charge distribution in the excited state. The time constants for ground state recovery are strongly solvent dependent, as suggested by fluorescence quantum yields, and are reported in Table 1.
Fluorescence lifetimes were determined by time correlated single photon counting and supported by comparison to the decay kinetics of the bleaching signal acquired with pump–probe. The agreement of the two techniques allowed the exploitation of the latter to determine the fast ground state relaxation observed for EDPP 8. Ground state recovery dynamics allowed us to highlight that radiative decay constants are, as expected, solvent independent and very much similar for dyes 7 and 8 (see Table 1); in contrast, non-radiative processes are strongly influenced by solvent environment and by the position of nitro-groups. In more detail, the non-radiative constant increases by increasing the solvent polarity and even higher non-radiative constant (one order of magnitude larger on average) are observed when moving from compound 7 to compound 8, suggesting that in both cases a second non-radiative decay channel is opened. UV-vis pump–probe data are fully in agreement with the presence of an additional excited state with a more pronounced charge transfer (CT) character in the relaxation pathway. The fluorescence quantum yields measured in CHCl3 and CH2Cl2 do not follow the solvent polarity trend response observed for other solvents.
Pump–probe measurements evidence that ground state recovery take place at a few hundred picoseconds for dye 7 and tens of picoseconds for dye 8.12 This evidence suggests that both solvents may come into specific molecular interactions with dyes 7 and 8 as a result of their low but not negligible capability, to enhance the non-radiative decay13 and form hydrogen-bonding with molecules in the excited electronic state. It is worth mentioning that among the various solvents used to characterize the properties of excited state compounds 7 and 8, chloroform and dichloromethane are the only ones having hydrogen bonding donor properties and are the ones where both compounds have the shortest lifetimes (no evidence of triplet state formation from pump–probe data). Due to the extended delocalization of the electron density for both dyes 7 and 8, we expected, in analogy with formerly reported quadrupolar molecules,9a NIR excited state absorptions to take place. Pump–probe measurements in the 800–910 nm spectral range have been performed for both compounds in toluene and chloroform solutions using the US-pp setup15 described in the ESI† and the global analysis results have been reported as shown in Fig. 2. In chloroform, both dyes 7 and 8 showed a broader spectral shape than in toluene as well as a slight time-dependent blue shift of the band maximum; which is practically absent in toluene. Dynamical spectral shift and spectral broadening are very common markers for the stabilization of excited states. This is due to polar and slightly polar solvents, strongly suggesting that excited state absorption involves a state characterized by a certain degree of charge separation.
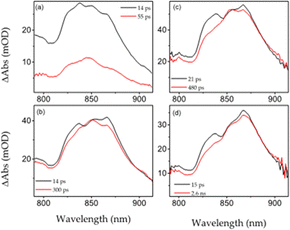 | ||
| Fig. 2 Evolution associated decay spectra (EADS) of transient absorption measurements in the NIR spectral region for: 8 in CHCl3 (a), 7 in CHCl3 (b), 8 in toluene (c) and 7 in toluene (d). | ||
Global analysis, within a two-component sequential model scheme, revealed that one main spectral component relaxes back to the ground state, with the time constant of this process being in very good agreement with that of the ground state bleaching decay. The first picosecond component has been ascribed to intramolecular structural relaxation. Transient infrared spectra (Fig. S13, ESI†) are in good agreement with the UV-vis pump–probe, showing ground state recovery on the hundred's picosecond timescale for EDPP 8 in THF.14 Additional evidence that the presence of a CT state plays the key role in determining fluorescence properties of dyes 7 and 8 has been obtained from temperature-dependent fluorescence lifetime measurements in 2Me-THF. The data are reported as shown in Fig. 3 and Fig. S10 (ESI†). Dye 7 shows no temperature dependence from 298 K to 77 K, while EDPP 8 evidences a clear increase in fluorescence lifetimes upon cooling down the solution. Broadband pump–probe measurements in the visible spectral range have been also performed in a frozen solvent and at room temperature. Results, reported in Fig. 3 and Fig. S11 and S12 of the ESI,† show no spectral shift in the first 200 ps, in net contrast to room temperature, demonstrating that in a glassy solvent, the structural relaxation is blocked.
These results suggest that both thermal activation and structural relaxation play key roles in the radiative and non-radiative decay mechanisms of excited states EDPPs 7 and 8.
First principle calculations and modeling
We have performed quantum chemistry calculations for both dyes 7 and 8. Ground state (S0) optimized geometries have been obtained at the DFT level (B3LYP/6-31+G(d,p)), whereas the first few excited states have been calculated relying on the time-dependent DFT (TD-DFT) approach (B3LYP/6-31+G(d,p)). All the calculations have been performed in the gas phase using the Gaussian16 (Revision B.01) package.16 A fairly notable difference between the two molecules occurs already at the level of the ground state geometry, with dye 7 being planar and EDPP 8 showing a bent geometry (≈160°, see Fig. S4 in the ESI†). TD-DFT calculations show that in dyes 7 and 8 the bright, lowest, excited state S1 corresponds to a π–π* (HOMO → LUMO) transition, which involves two fluorenes and the DPP core, with almost no contribution coming from the nitro-groups (see Fig. 4). The relevant oscillator strengths are high, ranging from 1.54 for dye 8 to 1.6 for EDPP 7. Steady-state absorption and emission spectra in Fig. 1 correspond to transitions that mainly involve S1. On the other hand, in both dyes 7 and 8, the second singlet excited state S2 corresponds to a HOMO → LUMO+1 transition with a large involvement of the nitro-groups and a strong quadrupolar charge-transfer (CT) character (see Fig. 3). As a result, S2 is optically dark and has no influence in the steady state spectra (see Fig. 1). At the same time, being very close in energy to S1 in the Franck–Condon region, S2 is involved in the solvent-dependent non-radiative processes discussed in the previous section (Fig. 5). | ||
| Fig. 5 Excited state energy levels obtained from TDDFT calculations in the gas phase for compounds EDPP 7 and EDPP 8; oscillators’ strength is reported in brackets. | ||
Our recent analysis of the literature10 on fluorescent nitro-aromatics has shown that there are two major scenarios of the dependence of fluorescence intensity on solvent polarity, which originates from the interplay between ISC and CT once the molecules reach their S1 states. Indeed in most cases, the fluorescent nitroaromatics’ emission strength decreases as the medium polarity increases. In the case of nitro-stilbenes however, fluorescence intensity is low in non-polar solvents and it increases in media of intermediate polarity or strong polarity.4,7i,j This is related to suppressing intersystem crossing in moderately polar solvents. Interestingly in the case of nitro-1,4-distyrylbenzenes Spalletti and co-workers claim that the structure of emissive 1ICT* state does not lose planarity even in very polar solvents and the fluorescence is not quenched in e.g. DMF.7i,j In other words excited state dynamics in polar media facilitates the population of a planar 1ICT* state resulting in strong emission. The emphasis on the importance of planarity bears a strong resemblance to our findings.
Conclusions
By implementing very subtle changes in the structures of two regioisomeric, quadrupolar, centrosymmetric, π-expanded diketopyrrolopyrroles, it is possible to completely alter their emissive properties. Moving nitro groups from a distant position to the sp2 carbon atom close to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond decreases the fluorescence intensity by a factor of 10. In contrast, placing the nitro group at a distant position leads to a super strong emitter with optical brightness ≈ 110
O bond decreases the fluorescence intensity by a factor of 10. In contrast, placing the nitro group at a distant position leads to a super strong emitter with optical brightness ≈ 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 × cm−1 at 635 nm. TD-DFT computations for dyes 7 and 8, indicate, that upon excitation, these molecules reach a bright S1 state characterized by ππ* nature, with the energy of the dark S2 state being only slightly higher than that of S1 (Fig. 5). Unlike dye 7, EDPP 8, once in the bright S1 state, undergoes a rapid internal conversion to the dark S2 state through thermal activation.17 Photophysical studies in frozen glass provide convincing evidence that the rate constant knr for the non-emissive EDPP reaches values comparable to those of the emissive DPP below 120 K. Broadband pump–probe suggests that the thermally activated internal conversion through the CT S2 state is mediated by intramolecular structural relaxation. The fundamental difference between dyes 7 and 8 arises from the fact that the presence of the nitro group at the ortho position relative to the bridge with the DPP core, is sufficient to induce non-planarity in both the ground state and excited state conformations of dye 8. In this context, the presence of the nitro group plays a decisive role, as the coulombic repulsion between the negative charges (with the second being the oxygen of the C
000 M−1 × cm−1 at 635 nm. TD-DFT computations for dyes 7 and 8, indicate, that upon excitation, these molecules reach a bright S1 state characterized by ππ* nature, with the energy of the dark S2 state being only slightly higher than that of S1 (Fig. 5). Unlike dye 7, EDPP 8, once in the bright S1 state, undergoes a rapid internal conversion to the dark S2 state through thermal activation.17 Photophysical studies in frozen glass provide convincing evidence that the rate constant knr for the non-emissive EDPP reaches values comparable to those of the emissive DPP below 120 K. Broadband pump–probe suggests that the thermally activated internal conversion through the CT S2 state is mediated by intramolecular structural relaxation. The fundamental difference between dyes 7 and 8 arises from the fact that the presence of the nitro group at the ortho position relative to the bridge with the DPP core, is sufficient to induce non-planarity in both the ground state and excited state conformations of dye 8. In this context, the presence of the nitro group plays a decisive role, as the coulombic repulsion between the negative charges (with the second being the oxygen of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group) results in a non-planar conformation. In the broader context, a comparison of these findings with some earlier results9b points out the following: in the case of larger polarized dyes, the molecules which possess nitro groups in positions that enable a maintaining the planarity of the π-system tend to show strong fluorescence. Their excellent properties such as large optical brightness open the door for future applications in optoelectronics. Given the recent renaissance of interest in nitroaromatics this work should inspire the future design of strongly emitting fluorophores bearing NO2 groups, with distinctive π-expanded architectures.
O group) results in a non-planar conformation. In the broader context, a comparison of these findings with some earlier results9b points out the following: in the case of larger polarized dyes, the molecules which possess nitro groups in positions that enable a maintaining the planarity of the π-system tend to show strong fluorescence. Their excellent properties such as large optical brightness open the door for future applications in optoelectronics. Given the recent renaissance of interest in nitroaromatics this work should inspire the future design of strongly emitting fluorophores bearing NO2 groups, with distinctive π-expanded architectures.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Data availability
All experimental details and data supporting this article are available in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was financially supported by the Polish National Science Center, Poland (OPUS 2020/37/B/ST4/00017) and the Foundation for Polish Science (TEAM POIR.04.04.00-00-3CF4/16-00). The support from the Italian Ministry of Research through the project 20225NPY8P (LANTERN), through the project P2022ALSMP_002 (VISIO) and the IPHOQS project is acknowledged. The support from Parma University through the project SFM-CARS is also gratefully acknowledged. F. D. M. acknowledges financial support from Bando di ateneo per la ricerca 2023 – azione B. This work benefited from the support of the HPC (High Performance Computing) facility at the University of Parma. F. D. M. acknowledges the CINECA award under the ISCRA initiative, for the availability of high-performance computing resources and support (project IsCb8 InveST (HP10CIDH42)). F. D. M. position was co-funded by the European Union – PON Research and Innovation 2014-2020. AL would like to acknowledge Alessio Montori from LENS Electronic Workshop for his contribution to the development of the experimental signal acquisition. We thank N. D. G. for amending the manuscript.Notes and references
- A. Nowak-Król, K. Shoyama, M. Stolte and F. Würthner, Naphthalene and perylene diimides – better alternatives to fullerenes for organic electronics?, Chem. Commun., 2018, 54, 13763–13772 RSC.
- S.-H. Wen, A. Li, J. Song, W.-Q. Deng, K.-L. Han and W. A. Goddard, III, First-principles investigation of anistropic hole mobilities in organic semiconductors, J. Phys. Chem. B, 2009, 113, 8813–8819 CrossRef CAS.
- C. Hansch, A. Leo and R. W. Taft, A survey of Hammett substituent constants and resonance and field parameters, Chem. Rev., 1991, 91, 165–195 CrossRef CAS.
- (a) H. Görner and D. Schulte-Frohlinde, Study of the trans → cis photoisomerization of 4-nitro-4'- dimethylaminostilbene in toluene solutions, J. Mol. Struct., 1982, 84, 227–236 CrossRef; (b) H. Görner, Photophysics and photochemistry of trans-4-nitrostilbenes and trans-2,4-dinitrostilbenes: Effect of intramolecular charge transfer, Ber. Bunsenges. Phys. Chem., 1998, 102, 726–737 CrossRef.
- T. Ueno, Y. Urano, H. Kojima and T. Nagano, Mechanism-Based Molecular Design of Highly Selective Fluorescence Probes for Nitrative Stress, J. Am. Chem. Soc., 2006, 128, 10640–10641 CrossRef CAS.
- C.-K. Wang, P. Macak, Y. Luo and H. Ågren, Effects of π centers and symmetry on two-photon absorption cross sections of organic chromophores, J. Chem. Phys., 2001, 114, 9813–9820 CrossRef CAS.
- (a) M.-C. Chen, D.-G. Chen and P.-T. Chou, Fluorescent Chromophores Containing the Nitro Group: Relatively Unexplored Emissive Properties, ChemPlusChem, 2021, 86, 11–27 CrossRef CAS; (b) D. J. Cowley, Triplet-Triplet Absorption Spectroscopy of Some Highly Dipolar Unsaturated Nitro Compounds, Helv. Chim. Acta, 1978, 61, 184–197 CrossRef CAS; (c) B. Bursa, D. Wróbel, B. Barszcz, M. Kotkowiak, O. Vakuliuk, D. T. Gryko, Ł. Kolanowski, M. Baraniak and G. Lota, The impact of solvents on the singlet and triplet states of selected fluorine corroles – absorption, fluorescence, and optoacoustic studies, Phys. Chem. Chem. Phys., 2016, 18, 7216–7228 RSC; (d) E. F. Plaza-Medina, W. Rodríguez-Córdoba and J. Peon, Role of Upper Triplet States on the Photophysics of Nitrated Polyaromatic Compounds: S-1 Lifetimes of Singly Nitrated Pyrenes, J. Phys. Chem. A, 2011, 115, 9782–9789 CrossRef CAS PubMed; (e) J.-M. Mewes, V. Jovanović, C. M. Marian and A. Dreuw, On the molecular mechanism of non-radiative decay of nitrobenzene and the unforeseen challenges this simple molecule holds for electronic structure theory, Phys. Chem. Chem. Phys., 2014, 16, 12393–12406 RSC; (f) C. E. Crespo-Hernández, G. Burdzinski and R. Arce, Environmental Photochemistry of Nitro-PAHs: Direct Observation of Ultrafast Intersystem Crossing in 1-Nitropyrene, J. Phys. Chem. A, 2008, 112, 6313–6319 CrossRef; (g) E. M. Espinoza, B. Xia, N. Darabedian, J. M. Larsen, V. Nuñez, D. Bao, J. T. Mac, F. Botero, M. Wurch, F. Zhou and V. I. Vullev, Nitropyrene Photoprobes: Making Them, and What Are They Good for?, Eur. J. Org. Chem., 2016, 343–356 CrossRef CAS; (h) K. Rybicka-Jasińska, E. M. Espinoza, J. A. Clark, J. B. Derr, G. Carlos, M. Morales, M. K. Billones, O. O’Mari, H. Ågren, G. V. Baryshnikov and V. I. Vullev, Making Nitronaphthalene Fluoresce, J. Phys. Chem. Lett., 2021, 12, 10295–10303 CrossRef PubMed; (i) B. Carlotti, F. Elisei, U. Mazzucato and A. Spalletti, Unusual high fluorescence of two nitro-distyrylbenzene-like compounds induced by CT processes affecting the fluorescence/intersystem-crossing competition, Phys. Chem. Chem. Phys., 2015, 17, 14740–14749 RSC; (j) S. Ciorba, G. Galiazzo, U. Mazzucato and A. Spalletti, Photobehavior of the Geometrical Isomers of Two 1,4-Distyrylbenzene Analogues with Side Groups of Different Electron Donor/Acceptor Character, J. Phys. Chem. A, 2010, 114, 10761–10768 CrossRef CAS PubMed; (k) P.-Y. Wang, Y.-C. Hsu, P. H. Chen, G.-Y. Chen, Y.-K. Liao and P.-Y. Cheng, Solvent-polarity dependence of ultrafast excited-state dynamics of trans-4-nitrostilbene, Phys. Chem. Chem. Phys., 2024, 26, 788–807 RSC; (l) B. Sadowski, M. Kaliszewska, G. Clermont, Y. M. Poronik, M. Blanchard-Desce, P. Piątkowski and D. T. Gryko, Realization of nitroaromatic chromophores with intense two-photon brightness, Chem. Commun., 2023, 59, 11708–11711 RSC; (m) Q. He, F. Dong, L. Xing, H. He, X. Chen, H. Wang, S. Ji and Y. Huo, The effects of 1-and 3-positions substitutions on the photophysical properties of perylene and its application in thiol fluorescent probes, Tetrahedron, 2022, 104, 132565 CrossRef CAS.
- (a) P. B. Ghosh and M. W. Whitehouse, 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole: a new fluorigenic reagent for amino acids and other amines, Biochem. J., 1968, 108, 155–156 CrossRef CAS; (b) S. Benson, A. Fernandez, N. D. Barth, F. De Moliner, M. H. Horrocks, C. S. Herrington, J. L. Abad, A. Delgado, L. Kelly, Z. Chang, Y. Feng, M. Nishiura, Y. Hori, K. Kikuchi and M. Vendrell, SCOTfluors: Small, Conjugatable, Orthogonal, and Tunable Fluorophores for In Vivo Imaging of Cell Metabolism, Angew. Chem., Int. Ed., 2019, 58, 6911–6915 CrossRef CAS PubMed; (c) M.-C. Chen, Y.-L. Lee, Z.-X. Huang, D.-G. Chen and P.-T. Chou, Tuning Electron Withdrawing Strength on Phenothiazine Derivatives: Achieving 100% Photoluminescence Quantum Yield by NO2 Substitution, Chem. – Eur. J., 2020, 26, 7124–7130 CrossRef CAS PubMed.
- (a) K. Skonieczny, I. Papadopoulos, D. Thiel, K. Gutkowski, P. Haines, P. M. McCosker, A. D. Laurent, P. A. Keller, T. Clark, D. Jacquemin, D. M. Guldi and D. T. Gryko, How To Make Nitroaromatic Compounds Glow: Next-Generation Large X-Shaped, Centrosymmetric Diketopyrrolopyrroles, Angew. Chem., Int. Ed., 2020, 59, 16104–16113 CrossRef CAS; (b) Y. M. Poronik, G. V. Baryshnikov, I. Deperasińska, E. M. Espinoza, J. A. Clark, H. Ågren, D. T. Gryko and V. I. Vullev, Deciphering the unusual fluorescence in weakly coupled bis-nitro-pyrrolo[3,2-b]pyrroles, Commun. Chem., 2020, 3, 190 CrossRef CAS; (c) B. Sadowski, M. Kaliszewska, Y. P. Poronik, M. Czichy, P. Janasik, M. Banasiewicz, D. Mierzwa, W. Gadomski, T. D. Lohrey, J. A. Clark, M. Łapkowski, B. Kozankiewicz, V. I. Vullev, A. L. Sobolewski, P. Piątkowski and D. T. Gryko, Strategy Towards Strongly Emissive Nitroaromatics Through a Weakly Electron-Deficient Core, Chem. Sci., 2021, 12, 14039–14049 RSC.
- Y. M. Poronik, B. Sadowski, K. Szychta, F. H. Quina, V. I. Vullev and D. T. Gryko, Revisiting the non-fluorescence of nitroaromatics: presumption versus reality, J. Mater. Chem. C, 2022, 10, 2870–2904 RSC.
- (a) G. M. Fischer, A. P. Ehlers, A. Zumbusch and E. Daltrozzo, Near-Infrared Dyes and Fluorophores Based on Diketopyrrolopyrroles, Angew. Chem., Int. Ed., 2007, 46, 3750–3753 CrossRef CAS PubMed; (b) S. Shimizu, T. Iino, Y. Araki and N. Kobayashi, Pyrrolopyrrole aza-BODIPY analogues: A facile synthesis and intense fluorescence, Chem. Commun., 2013, 49, 1621–1623 RSC; (c) S. Shimizu, T. Iino, A. Saeki, S. Seki and N. Kobayashi, Rational Molecular Design towards Vis/NIR Absorption and Fluorescence by using Pyrrolopyrrole aza-BODIPY and its Highly Conjugated Structures for Organic Photovoltaics, Chem. – Eur. J., 2015, 21, 2893–2904 CrossRef CAS PubMed; (d) S. Shimizu, Aza-BODIPY synthesis towards vis/NIR functional chromophores based on a Schiff base forming reaction protocol using lactams and heteroaromatic amines, Chem. Commun., 2019, 55, 8722–8743 RSC; (e) Y. Kage, S. Mori, M. Ide, A. Saeki, H. Furuta and S. Shimizu, Blackening of aza-BODIPY analogues by simple dimerization: panchromatic absorption of a pyrrolopyrrole aza-BODIPY dimer, Mater. Chem. Front., 2018, 2, 112–120 RSC; (f) W. Yue, S.-L. Suraru, D. Bialas, M. Müller and F. Würthner, Synthesis and Properties of a New Class of Fully Conjugated Azahexacene Analogues, Angew. Chem., Int. Ed., 2014, 53, 6159–6162 CrossRef CAS PubMed; (g) M. Grzybowski, E. Glodkowska-Mrowka, T. Stoklosa and D. T. Gryko, Bright, Color-Tunable Fluorescent Dyes Based on π-Expanded Diketopyrrolopyrroles, Org. Lett., 2012, 14, 2670–2673 CrossRef CAS PubMed; (h) M. Grzybowski and D. T. Gryko, US Pat., US20140357869A1, 2015 Search PubMed; (i) D. T. Gryko, M. Grzybowski, P. Hayoz and A. Jeżewski, US Pat., US9698348B2, 2017 Search PubMed; (j) M. Grzybowski, V. Hugues, M. Blanchard-Desce and D. T. Gryko, Two-Photon-Induced Fluorescence in New π-Expanded Diketopyrrolopyrroles, Chem. – Eur. J., 2014, 20, 12493–12501 CrossRef CAS; (k) F. Trilling, O. Sachnik and U. Scherf, π-Expanded diketopyrrolopyrroles as acceptor building blocks for the formation of novel donor–acceptor copolymers, Polym. Chem., 2019, 10, 627–632 RSC; (l) L. Ma, B. Chen, Y. Guo, Y. Liang, D. Zeng, X. Zhan, Y. Liu and X. Chen, NIR polymers and phototransistors, J. Mater. Chem. C, 2018, 6, 13049–13058 RSC; (m) B. Chen, Y. Yang, P. Cheng, X. Chen, X. Zhan and J. Qin, designing a thiophene-fused DPP unit to build an A–D–A molecule for solution-processed solar cells, J. Mater. Chem. A, 2015, 3, 6894–6900 RSC; (n) E. Gońka, L. Yang, R. Steinbock, F. Pesciaioli, R. Kuniyil and L. Ackermann, π-Extended Polyaromatic Hydrocarbons by Sustainable Alkyne Annulations through Double C−H/N−H Activation, Chem. – Eur. J., 2019, 25, 16246–16250 CrossRef PubMed; (o) A. Minotto, P. A. Haigh, G. Łukasiewicz, E. Lunedei, D. T. Gryko, I. Darwazeh and F. Cacialli, Visible light communication with efficient far-red/near-infrared polymer light-emitting diodes, Light: Sci. Appl., 2020, 9, 70 CrossRef; (p) L. Wang, W. Jiang, S. Guo, S. Wang, M. Zhang, Z. Liu, G. Wang, Y. Miao, L. Yan, Y.-Y. Shao, Y.-W. Zhong, Z. Liu, D. Zhang, H. Fu and Y. Yao, Robust singlet fission process in strong absorption π-expanded diketopyrrolopyrroles, Chem. Sci., 2022, 13, 13907–13913 RSC.
- J. J. Snellenburg, S. Laptenok, R. Seger, K. M. Mullen and I. H. M. van Stokkum, Glotaran: A Java-Based Graphical User Interface for the R Package TIMP, J. Stat. Softw., 2012, 49, 1–22 Search PubMed.
- (a) B. Valeur and M. N. Berberan-Santos, Molecular Fluorescence: Principles and Applications, Wiley-VCH, 2012, pp. 1–592 CrossRef; (b) J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, 2006, pp. 1–954 CrossRef; (c) J. B. Birks, Photophysics of Aromatic Molecules, Wiley-Interscience, 1970, pp. 1–704 Search PubMed; (d) J. N. Demas, Excited State Lifetime Measurements, Academic Press, 1983, pp. 1–273 Search PubMed; (e) C. Reichardt, Solvents and Solvent Effects in Organic Chemistry, Wiley-VCH, 2006, pp. 1–629 Search PubMed.
- (a) M. Di Donato, M. S. Centellas, A. Lapini, M. Lima, F. Avila, F. Santoro, C. Cappelli and R. Righini, Combination of Transient 2D-IR Experiments and Ab Initio Computations Sheds Light on the Formation of the Charge-Transfer State in Photoexcited Carbonyl Carotenoids, J. Phys. Chem. B, 2014, 118, 9613–9630 CrossRef CAS PubMed; (b) M. Di Donato, E. Ragnoni, A. Lapini, P. Foggi, R. G. Hiller and R. Righini, Femtosecond transient infrared and stimulated Raman spectroscopy shed light on the relaxation mechanisms of photo-excited peridinin, J. Chem. Phys., 2015, 142, 212409 CrossRef PubMed.
- S. Doria, M. Di Donato, R. Borrelli, M. F. Gelin, J. Caram, M. Pagliai, P. Foggi and A. Lapini, Vibronic coherences in light harvesting nanotubes: unravelling the role of dark states, J. Mater. Chem. C, 2022, 10, 7216–7226 RSC.
- M. J. Frisch, et al., Gaussian 16 Revision B.01, Gaussian Inc., Wallingford CT, 2016 Search PubMed.
- (a) S. I. Druzhinin, A. Demeter, V. A. Galievsky, T. Yoshihara and K. A. Zachariasse, Thermally Activated Internal Conversion with 4-(Dimethylamino)benzonitrile, 4-(Methylamino)benzonitrile, and 4-Aminobenzonitrile in Alkane Solvents. No Correlation with Intramolecular Charge Transfer, J. Phys. Chem. A, 2003, 107, 8075–8085 CrossRef CAS; (b) K. Suzuki, A. Demeter, W. Kühnle, E. Tauer, K. A. Zachariasse, S. Tobita and H. Shizuka, Internal conversion in 4-substituted 1-naphthylamines. Influence of the electron donor/acceptor substituent character, Phys. Chem. Chem. Phys., 2000, 2, 981–991 RSC; (c) J. Hoche, A. Schulz, L. M. Dietrich, A. Humeniuk, M. Stolte, D. Schmidt, T. Brixner, F. Wurthner and R. Mitric, The origin of the solvent dependence of f luorescence quantum yields in dipolar merocyanine dyes, Chem. Sci., 2019, 10, 11013–11022 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of the synthesis, spectroscopic characterisation, and crystallographic and computational data. See DOI: https://doi.org/10.1039/d4cp04689g |
| ‡ These authors contributed equally. |
| This journal is © the Owner Societies 2025 |



