 Open Access Article
Open Access ArticleCrystal growth and magnetic properties of hexagonal Ba4CuNb3O12 single crystals†
Yuhu
Huang
a,
Wen
Xie
ab,
Fei
Zheng
c,
Chao
Zhang
*d and
Han-Shu
Xu
 *ab
*ab
aHefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei 230026, China. E-mail: xhs@ustc.edu.cn
bDepartment of Applied Physics, Anhui Medical University, Hefei 230032, China
cSchool of Earth and Space Sciences, University of Science and Technology of China, Hefei 230026, China
dInstrumentation and Service Center for Physical Science, Westlake University, Hangzhou 310024, China. E-mail: zhangchao68@westlake.edu.cn
First published on 19th March 2025
Abstract
We report a systematic investigation of the growth of high-quality hexagonal perovskite Ba4CuNb3O12 single crystals using the flux method. The optimal growth involved using the tetragonal phase BaCu0.33Nb0.67O3 as the precursor, with 20 g CuO as the flux, a cooling rate of 1 °C h−1 between 1350 °C and 1100 °C, and a cooling rate of 2 °C h−1 from 1100 °C to 930 °C. The magnetic properties along the ab-plane and the perpendicular direction are thoroughly studied. Fitting the inverse magnetic susceptibility data from 2 K to 300 K with the modified Curie–Weiss (CW) law yields a Weiss temperature (θCW) of −1.14 K, indicating the presence of antiferromagnetic correlations in the ground state between Cu2+ ions via superexchange interactions. Furthermore, the specific heat data of Ba4CuNb3O12 align well with the Debye–Einstein phonon model, allowing us to estimate the Debye temperature of 199 K and the average phonon velocity of 2014.98 m s−1.
1. Introduction
Hexagonal perovskite oxides have garnered increasing attention due to their rich physicochemical properties and potential applications.1–3 Their crystal structures are of the hexagonal perovskite type, characterized by the stacking of BO6 octahedra along the c-axis in different arrangements, with the B-site typically occupied by a transition metal. For instance, Xu et al. first reported the synthesis of two-dimensional triangular frustrated Ba3CoNb2O9 single crystals4 and quasi-one-dimensional Sr6Co5O15 single crystals doped with different amounts of Fe elements.5 Li et al. synthesized a hexagonal perovskite oxide (2H, 6H, and 10H)-BaCo0.9Ru0.1O3−δ with different [BaO3] layers in a unit cell and systematically explored its electrocatalytic oxygen evolution performance.6 Although 8H-hexagonal perovskite oxides are relatively common, as seen in systems like Ba8Ti3Ta4O24,7 Ba4LiM3O12 (M = Nb and Ta),8,9 Ba8Ti3Nb4O24,10,11 Ba4Na3RuO12,12 and Ba8MnNb6O24,13 8H-hexagonal perovskites containing copper atoms are very rare. It is also worth noting that, in addition to the detailed study of the magnetism of Ba8MnNb6O24, which confirmed a 120° ordering ground state below the antiferromagnetic transition temperature TN = 1.45 K,14 research on the other 8H-hexagonal perovskite materials often focuses primarily on their crystal structure, with limited studies on their magnetic properties.12The 8H-hexagonal perovskite structure requires a high coordination number between ions, with the size and charge of the A-site and B-site ions needing to meet specific conditions. Copper ions are prone to redox reactions, especially the conversion between Cu2+ (S = 1/2) and Cu+ (non-magnetic S = 0). This redox behavior can lead to structural instability or phase transition, further limiting the stability of copper-containing compounds in the 8H-hexagonal perovskite oxides. As a typical example of 8H-hexagonal perovskite oxides, Ba4CuNb3O12 crystallizes in the P63/mmc space group, and the Nb1O6 octahedron along the c-axis shares corners with the face-sharing (Nb2/Cu)O6 octahedron, as illustrated in Fig. 1(a). Furthermore, the structural projection onto the ab-plane reveals that Nb1 atoms form a honeycomb structure, while Nb2/Cu atoms arrange in a triangular lattice, as shown in Fig. 1(b). Since Kumada et al. first synthesized Ba4CuNb3O12 polycrystalline powders,15 there have been no reports on their physical properties, nor have any single crystal studies been published to date. If Cu forms a perfect triangular lattice system in this system, the presence of geometric frustration could lead to the system exhibiting a spin liquid or quantum spin liquid ground state.16 However, the random occupation of Nb2 and Cu atoms in Ba4CuNb3O12 disrupts the perfect triangular lattice structure, similar to the site mixing of Mg2+ and Ga3+ in YbMgGaO4,17,18 yet the magnetic ground state of Ba4CuNb3O12 remains an interesting subject for further exploration.
 | ||
| Fig. 1 (a) Crystal structure of Ba4CuNb3O12. (b) Projection of the crystal structure onto the ab-plane. | ||
In this study, we conducted a comprehensive investigation of the growth of Ba4CuNb3O12 single crystals using the flux method, thereby identifying the optimal synthesis route. We also examined the magnetism of the Ba4CuNb3O12 single crystal both along and perpendicular to the ab-plane. Our results confirm that its ground state is antiferromagnetic. The specific heat behavior of Ba4CuNb3O12 is in good agreement with that of the Debye–Einstein phonon model, and furthermore, it can be concluded that the average phonon velocity is 2014.98 m s−1.
2. Characterization section
The single crystal structure and elemental composition were characterized at 298 K using X-ray diffraction (XRD, Rigaku SmartLab SE diffractometer), energy-dispersive spectroscopy (EDS, JEOL JSM-6700F), X-ray fluorescence (XRF, XRF-1800), and X-ray photoelectron spectroscopy (XPS, ESCALAB250Xi). Magnetization measurements were performed with a magnetic property measurement system (MPMS3, Quantum Design), while the specific heat was measured using the relaxation method on a physical property measurement system (PPMS, Quantum Design). In particular, in the measurement process of the direct current magnetic susceptibility (χ) of field-cooling (FC) the temperature rise test is adopted, namely the field cooled warming (FCW) process. In the single crystal XRD characterization, the tested single crystal is millimeter-sized, and the sample used for magnetic and specific heat measurements is taken from the single crystal shown in the upper right corner of Fig. 5(a), with a mass of 6.52 mg.3. Synthesis steps of Ba4CuNb3O12 single crystals
3.1 Synthesis of polycrystalline precursors
We first fully ground BaCO3 (SCR, 99%), CuO (SCR, 99%), and Nb2O5 (SCR, 99%) in a molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and then transferred the mixture to an Al2O3 crucible and calcined it at 1200 °C for 48 hours. From the XRD patterns in Fig. 2, we can observe that the main phase of the product is BaCu0.33Nb0.67O3 with the P4/mmm space group, along with some impurity phase Ba5Nb4O15.33, unreacted Nb2O5 and BaO formed after the high-temperature decomposition of BaCO3. Our next step is to use these polycrystalline powders to prepare single crystals via the flux method. In addition, from the perspective of single crystal growth, a small amount of the impure phase in the polycrystalline powder does not significantly affect the final crystal quality, as the type and proportion of the flux, along with the reaction temperature, are the key factors.5
1, and then transferred the mixture to an Al2O3 crucible and calcined it at 1200 °C for 48 hours. From the XRD patterns in Fig. 2, we can observe that the main phase of the product is BaCu0.33Nb0.67O3 with the P4/mmm space group, along with some impurity phase Ba5Nb4O15.33, unreacted Nb2O5 and BaO formed after the high-temperature decomposition of BaCO3. Our next step is to use these polycrystalline powders to prepare single crystals via the flux method. In addition, from the perspective of single crystal growth, a small amount of the impure phase in the polycrystalline powder does not significantly affect the final crystal quality, as the type and proportion of the flux, along with the reaction temperature, are the key factors.5
3.2 Exploration of the best method for single crystal synthesis
 | ||
| Fig. 3 (a) The crucible and the product obtained using method I. (b) XRD results of the product after thorough grinding. | ||
The XRD results show that the synthesized product contains our target compound, the hexagonal perovskite Ba4CuNb3O12, along with an additional phase of BaNb2O6 with the Pmma space group. Moreover, the product also contains a small amount of BaNb3O6 with the R3m space group. This suggests that during the process of single crystal growth, the crystal system of the obtained product may differ from that of the raw material. At 1350 °C, which is above the melting point of CuO, the mixture must be in a molten state, and as the temperature gradually decreases, the different elements can recombine to form new phases. Although no single crystal was obtained via method I, the target hexagonal crystal system Ba4CuNb3O12 is synthesized, and additionally, the remaining product adhered to the crucible is very small, so in the improved method, the amount of the CuO flux should be increased.
Furthermore, to assess the quality of the crystals, we ground the obtained single crystals into powder and performed XRD characterization, as shown in Fig. 4(a). The results show that a small amount of BaNb3O6 is still in the Ba4CuNb3O12 single crystals, due to the gradual growth of the crystal nucleus during the crystallization process of Ba4CuNb3O12, which partially encapsulated and generated the formed BaNb3O6. Additionally, compared to method I, the product synthesized using method II does not contain the BaNb2O6 phase. Interestingly, by simply adjusting the content of the CuO flux, we can not only obtain single crystals but also further reduce the amount of the impurity phase.
| Parameter | Value | ||||
|---|---|---|---|---|---|
| Compound | Ba4CuNb3O12 | ||||
| Space group | P63/mmc | ||||
| a/b/Å | 5.7830(4) | ||||
| c/Å | 18.9289(6) | ||||
| Cell volume/Å3 | 548.23(5) | ||||
| R wp/% | 9.22 | ||||
| R p/% | 7.04 | ||||
| χ 2 | 5.01 | ||||
| Atom | Wyck. | Site | x/a | y/b | z/c |
| Ba1 | 2a | -3m. | 0 | 0 | 0 |
| Ba2 | 2d | -6m2 | 1/3 | 2/3 | 3/4 |
| Ba3 | 4f | 3m. | 1/3 | 2/3 | 0.13488 |
| Nb4 | 4f | 3m. | 1/3 | 2/3 | 9/16 |
| Nb5 | 4e | 3m. | 0 | 0 | 0.1829 |
| Cu6 | 4e | 3m. | 0 | 0 | 0.1829 |
| O7 | 6g | .2/m | 1/2 | 0 | 0 |
| O8 | 6h | mm2 | 0.161 | 0.322 | 1/4 |
| O9 | 12k | .m. | 0.82642 | 0.65284 | 0.1187 |
The EDS analysis clearly shows the presence of the elements Ba, Cu, Nb, and O, while the C element originates from the conductive carbon glue (Fig. 5(d)). Additionally, the EDS mapping reveals that Ba, Cu, Nb, and O are uniformly distributed across the tested area (inset of Fig. 5(d)). The EDS results indicate that the ratio of Cu and Nb elements is close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, but the Ba content is significantly higher, likely due to the presence of residual BaCO3 or BaO attached on the surface (Fig. S1†). Furthermore, we ground the Ba4CuNb3O12 single crystals into polycrystalline powder with a mass of about 25 mg and performed XRF testing (Fig. S1†). The results showed that the ratio of Ba, Cu and Nb is 4
3, but the Ba content is significantly higher, likely due to the presence of residual BaCO3 or BaO attached on the surface (Fig. S1†). Furthermore, we ground the Ba4CuNb3O12 single crystals into polycrystalline powder with a mass of about 25 mg and performed XRF testing (Fig. S1†). The results showed that the ratio of Ba, Cu and Nb is 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.85
1.85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.38, which is similar to the results given by Kumada et al.15 However, in order to maintain consistency with the previous work,15 we still used the expression form Ba4CuNb3O12 throughout the full text. In the chemical formula Ba4CuNb3O12, Ba ions have a +2 valence state, and Nb ions are +5. To ensure charge balance in the formula, Cu ions must be +1. However, under the high-temperature conditions of single crystal growth, some Cu+ will be converted into Cu2+. As shown in Fig. S2,† the XPS results show that the ratio of Cu+ to Cu2+ is approximately 1.12
2.38, which is similar to the results given by Kumada et al.15 However, in order to maintain consistency with the previous work,15 we still used the expression form Ba4CuNb3O12 throughout the full text. In the chemical formula Ba4CuNb3O12, Ba ions have a +2 valence state, and Nb ions are +5. To ensure charge balance in the formula, Cu ions must be +1. However, under the high-temperature conditions of single crystal growth, some Cu+ will be converted into Cu2+. As shown in Fig. S2,† the XPS results show that the ratio of Cu+ to Cu2+ is approximately 1.12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and it should be noted that Cu+ is non-magnetic (S = 0), while Cu2+ is magnetic (S = 1/2).
1, and it should be noted that Cu+ is non-magnetic (S = 0), while Cu2+ is magnetic (S = 1/2).
4. Magnetic properties
We systematically studied the magnetic properties of high-quality single crystals of Ba4CuNb3O12 synthesized using method III, as shown in Fig. 7. When the magnetic field is along the ab-plane, the zero-field-cooling (ZFC) and field-cooling (FC) magnetic susceptibility (χ) gradually increase with decreasing temperature, and there is no magnetic phase transition. In addition, the magnetic susceptibility curves for FC and ZFC almost overlap. When the magnetic field is perpendicular to the ab-plane, the magnetic susceptibility behavior is identical to that when the field is applied along the ab-plane (Fig. 7(b)), which is quite unusual in single crystal systems, as strong anisotropy is typically observed in such materials. In a single crystal system, the crystal structure exhibits anisotropy, and the magnetic ions located at the lattice points often also exhibit magnetic anisotropy. For example, in the triangular lattice systems such as Ba3CoSb2O9 (ref. 21 and 22) and Cs2CuCl4,23 magnetic susceptibility anisotropy is observed even within the paramagnetic temperature range. Furthermore, for the spin liquid candidate material YMgGaO4,24 the θCW values are approximately −1.47 K and −2.7 K when the magnetic field is applied parallel and perpendicular to the c-axis, respectively. The magnetism in Ba4CuNb3O12 originates from Cu2+, and the isotropic magnetism should be attributed to the randomness in atomic occupancy between Cu and Nb in the face-sharing CuO6 and NbO6 octahedra.The temperature-dependent 1/χ results indicate that the modified CW law, χ = χ0 + C/(T − θCW), is valid in the temperature range of 2 K to 300 K. Here, C is the Curie constant, and χ0 represents the temperature-independent contributions from the core-diamagnetic (originating from both the sample and the sample holder) and Van Vleck susceptibility (Fig. 7(c)).25,26 The effective magnetic moment (μeff) of Cu2+ is fitted to be 1.14 μB, with a θCW of −1.14 K and a χ0 value of −0.00012 emu mol−1 Oe−1. The negative θCW indicates the existence of antiferromagnetic correlation in the system. Importantly, in the ground state of Ba4CuNb3O12, magnetic Cu2+ ions can undergo superexchange interactions with the nearest Cu2+ ions via the Cu2+–O–O–(Nb5+/Cu+)–O–O–Cu2+ pathway, leading to the formation of antiferromagnetic correlations (Fig. S3†).27 Certainly, this represents only the most probable superexchange pathway, and due to the randomness of the Cu2+ position, other possible superexchange pathways are not discussed in detail.
Notably, the obtained μeff = 1.14 μB is lower than the theoretical value of g[S(S + 1)]1/2 = 1.73 μB, (assuming g = 2.0 and S = 1/2), where the spin–orbit coupling effect is ignored. In some spin-frustrated systems, the observed magnetic moment is lower than the theoretical value.28,29 In an antiferromagnetic system, the frustration parameter is given by f = |θCW|/TN. For the Ba4CuNb3O12 single crystal, since the TN value cannot be determined from magnetic susceptibility data, estimating the frustration parameter is challenging. This also requires testing of the magnetic properties at lower temperatures, such as 3He refrigeration or dilution refrigeration (3He–4He) equipment. Furthermore, it is important to note that if Cu atoms form a perfect triangular lattice, as seen in systems like Ba3CuSb2O9,29 spin frustration is highly likely to occur. In Ba4CuNb3O12, Cu and Nb atoms jointly form a triangular lattice, the random occupancy of Cu ions makes geometric frustration unlikely (Fig. 1(b)). However, the spin frustration may still arise due to competition between the nearest-neighbor and next-nearest-neighbor magnetic exchange interactions.30–32
To investigate the possible existence of a spin glass or cluster glass state in the Ba4CuNb3O12 single crystal, we conducted AC magnetic susceptibility tests (Fig. S4†). The results show that when the AC magnetic field is applied along the ab-plane with no DC magnetic field, the Ba4CuNb3O12 single crystal exhibits a peak at approximately 25 K with noticeable frequency dependence. However, since the peak position remains unchanged with frequency, the presence of a spin glass or cluster glass state can be ruled out. In fact, a similar frequency-dependent variation in the peak intensity has also been observed in La1.5Ca0.5(Co0.5Fe0.5)IrO6 (ref. 33) and Sr6(Co0.8Fe0.2)5O15,26 though the peak position remains unchanged, and the underlying physical origin of this behavior requires further investigation. Additionally, as for whether there is a short-range magnetic correlation, it is still difficult to give a clear conclusion based on the AC magnetic susceptibility.
The magnetization curves M(H) measured at 2 K in the field range of −7 to 7 T show similar behavior for both field orientations (Fig. 7(d)). At 7 T, the magnetic moments along the c-axis and the ab-plane are 0.38 μB and 0.35 μB, respectively, as shown in the inset of Fig. 7(d). However, the slight difference in magnetic moments between the two, within [(0.38 − 0.35)/0.38] × 100% = 7.89%, may be attributed to the fact that the spins of Cu ions tend to align in the ab-plane, indicating easy-plane anisotropy rather than easy-axis anisotropy.34 Furthermore, we obtained the angle-dependence of the magnetization curve for a magnetic field 0.1 T along the ab-plane at 10 K, as shown in Fig. 7(e) and (f). When the magnetic field rotates within the ab-plane, the magnetic moment remains constant, indicating the isotropic nature of the in-plane magnetism.
5. Specific heat
The specific heat results of the Ba4CuNb3O12 single crystal show no magnetic phase transition within the temperature range of 2 K to 200 K (Fig. 8), which is consistent with the magnetic behavior observed in the magnetic susceptibility curve. Importantly, our experimental data are well fitted by the Debye–Einstein model (red solid line in Fig. 8)which describes the phonon behavior (x = ℏω/kBT). Here, ω, R, ΘD, ΘEi, ND and NEi represent the phonon frequency, universal gas constant, Debye temperature, Einstein temperature, the number of acoustic phonon branches, and the number of optical branches with the same ΘEi, respectively.35,36 The best fitting parameters can be obtained as ΘD = 199 K, ΘE1 = 307 K, ΘE2 = 510 K, ΘE3 = 2100 K, ND = 3, NE1 = 2, NE2 = 3, and NE3 = 52. Furthermore, according to the formula
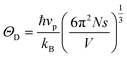 , where N is the number of molecules per mole, s is the number of atoms in each molecule, and V is the volume of the crystal, and the average velocity vp of phonons is calculated to be 2014.98 m s−1.
, where N is the number of molecules per mole, s is the number of atoms in each molecule, and V is the volume of the crystal, and the average velocity vp of phonons is calculated to be 2014.98 m s−1.
 | ||
| Fig. 8 Specific heat of the Ba4CuNb3O12 single crystal. The red solid line represents the phonon specific heat contribution fitted using the Debye–Einstein model. | ||
In addition, since the ratio of Nb2 to Cu atoms in the triangular lattice formed by Nb2/Cu is about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, this configuration effectively places two non-magnetic Nb2 atoms and one magnetic Cu atom at the triangular lattice sites. The spin exchange interaction is between Cu atoms via O atoms. The insufficient number of Cu atoms occupying the lattice sites increases the exchange path, which weakens the correlation between the Cu atoms (Fig. S3†). In geometrically frustrated systems, such as those with triangular, kagome, and honeycomb lattices, the introduction of non-magnetic atoms can often have a significant impact on the spin exchange interactions between magnetic atoms, thereby altering the ground state of the system.37,38
2, this configuration effectively places two non-magnetic Nb2 atoms and one magnetic Cu atom at the triangular lattice sites. The spin exchange interaction is between Cu atoms via O atoms. The insufficient number of Cu atoms occupying the lattice sites increases the exchange path, which weakens the correlation between the Cu atoms (Fig. S3†). In geometrically frustrated systems, such as those with triangular, kagome, and honeycomb lattices, the introduction of non-magnetic atoms can often have a significant impact on the spin exchange interactions between magnetic atoms, thereby altering the ground state of the system.37,38
Summary
In summary, we thoroughly explored the flux method for growing Ba4CuNb3O12 single crystals and identified the most effective synthesis approach. Additionally, we investigated the magnetic properties of the Ba4CuNb3O12 single crystal, both parallel and perpendicular to the ab-plane, with the findings confirming that the material exhibits an antiferromagnetic ground state. The reduced occupancy of Cu atoms at the triangular lattice sites extends the exchange path, weakening the interactions between the Cu atoms in Ba4CuNb3O12. Furthermore, the specific heat data of Ba4CuNb3O12 exhibit a strong correspondence with the Debye–Einstein phonon model, yielding estimates for the Debye temperature and the average phonon velocity as 199 K and 2014.98 m s−1, respectively.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
Yuhu Huang and Wen Xie: data curation, investigation, software, and validation. Fei Zheng: investigation, formal analysis, software, and reviewing. Chao Zhang: data curation, formal analysis, and supervision. Han-Shu Xu: methodology, writing – original draft, supervision, and review and editing, funding acquisition, formal analysis, and visualization.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We deeply appreciate Prof. Kaibin Tang (USTC) for his constructive comments on this work, and we are also grateful to Ivan Kostylev (OIST) for his technical support. This work is supported by the National Natural Science Foundation of China (Grant No. 12204452) and the Grants for Scientific Research of BSKY from Anhui Medical University.References
- L. T. Nguyen and R. J. Cava, Hexagonal perovskites as quantum materials, Chem. Rev., 2020, 121(5), 2935–2965 CrossRef PubMed.
- K. Matsuzaki, K. Saito, Y. Ikeda, Y. Nambu and M. Yashima, High proton conduction in the octahedral layers of fully hydrated hexagonal perovskite-related oxides, J. Am. Chem. Soc., 2024, 146(27), 18544–18555 CrossRef CAS PubMed.
- H.-C. zur Loye, Q. Zhao, D. E. Bugaris and W. M. Chance, 2 H-perovskite related oxides: Synthesis, structures, and predictions, CrystEngComm, 2012, 14(1), 23–39 RSC.
- H.-S. Xu, Y. Huang, J. Li and F. Zheng, Magnetic Properties and Specific Heat of Quasi-Two-Dimensional Triangular Lattice Antiferromagnet Ba3CoNb2O9 Single Crystals, J. Phys. Chem. C, 2025, 129(2), 1562–1567 CrossRef CAS.
- Y. Huang and H.-S. Xu, Growth of Fe-Substituted Sr6Co5O15 Hexagonal Perovskite Single Crystals by a Flux Method, Cryst. Growth Des., 2023, 23(5), 3747–3753 CrossRef CAS.
- J. Li, H.-S. Xu, J. Hu, Y. Huang, W. Xie, D. Yu and K. Tang, Comparative Study on the Electrocatalytic Performance of ABO3-Based Hexagonal Perovskite Oxides with Different [AO3] Layers, Inorg. Chem., 2024, 63(50), 23601–23613 CrossRef CAS PubMed.
- W. Cao, X. Yang, F. Lu, W. Zhu, L. Liu, X. Kuang and M. Allix, 8H–10H stacking periodicity control in twinned hexagonal perovskite dielectrics, Inorg. Chem., 2018, 57(7), 4117–4124 CrossRef CAS PubMed.
- E. F. Jendrek Jr., A. D. Potoff and L. Katz, A single-crystal study of eight-layer barium niobium lithium oxide, Ba4Nb3LiO12, J. Solid State Chem., 1974, 9(4), 375–379 CrossRef.
- T. Negas, R. Roth, H. Parker and W. Brower, Crystal chemistry of lithium in octahedrally coordinated structures. I. Synthesis of Ba8 (Me6Li2) O24 (Me = Nb or Ta) and Ba10 (W6Li4) O30. II. The tetragonal bronze phase in the system BaO Nb2O5 Li2O, J. Solid State Chem., 1973, 8(1), 1–13 CrossRef CAS.
- N. Teneze, P. Boullay, V. Petricek, G. Trolliard and D. Mercurio, Structural study of the cation ordering in the ternary oxide Ba8Ti3Nb4O24, Solid State Sci., 2002, 4(9), 1129–1136 CrossRef CAS.
- R. Rawal, A. Feteira, A. A. Flores, N. C. Hyatt, A. R. West, D. C. Sinclair, K. Sarma and N. M. Alford, Dielectric properties of the “twinned” 8H-hexagonal perovskite Ba8Nb4Ti3O24, J. Am. Ceram. Soc., 2006, 89(1), 336–339 CrossRef CAS.
- P. Battle, S. Kim and A. Powell, The crystal structure and electronic properties of Ba4Ru3MO12 (M = Li, Na, Mg, Zn), J. Solid State Chem., 1992, 101(1), 161–172 CrossRef CAS.
- F. Tao, C. Liang, X. Wang, X. Li, F. Porcher, M. Allix, F. Lu, H. Gong, L. Liu and X. Kuang, 8-layer shifted hexagonal perovskite Ba8MnNb6O24: long-range ordering of high-spin d5 Mn2+ layers and electronic structure, Inorg. Chem., 2018, 57(10), 5732–5742 CrossRef CAS PubMed.
- R. Rawl, L. Ge, Z. Lu, Z. Evenson, C. Dela Cruz, Q. Huang, M. Lee, E. Choi, M. Mourigal and H. Zhou, Ba8MnNb6O24: A model two-dimensional spin-5 2 triangular lattice antiferromagnet, Phys. Rev. Mater., 2019, 3(5), 054412 CrossRef CAS.
- N. Kumada, W. Zhang, Q. Dong, T. Mochizuki, Y. Yonesaki, T. Takei and N. Kinomura, Crystal structure and X-ray diffraction data of a new hexagonal perovskite compound, Ba4CuNb3O12, Powder Diffr., 2011, 26(3), 244–247 CAS.
- L. Balents, Spin liquids in frustrated magnets, Nature, 2010, 464(7286), 199–208 CAS.
- Z. Zhu, P. A. Maksimov, S. R. White and A. L. Chernyshev, Disorder-Induced Mimicry of a Spin Liquid in YbMgGaO4, Phys. Rev. Lett., 2017, 119(15), 157201 Search PubMed.
- Y. Li, D. Adroja, R. I. Bewley, D. Voneshen, A. A. Tsirlin, P. Gegenwart and Q. Zhang, Crystalline Electric-Field Randomness in the Triangular Lattice Spin-Liquid YbMgGaO4, Phys. Rev. Lett., 2017, 118(10), 107202 Search PubMed.
- B. Sun, R. Boutellier, P. Sciau, E. Burkhardt, V. Rodriguez and H. Schmid, High temperature solution growth of perovskite Pb2CoWO6 single crystals, J. Cryst. Growth, 1991, 112(1), 71–83 CAS.
- M. L. Mulvihill, S. E. Park, G. Risch, Z. Li, K. U. K. Uchino and T. R. S. T. R. Shrout, The role of processing variables in the flux growth of lead zinc niobate-lead titanate relaxor ferroelectric single crystals, Jpn. J. Appl. Phys., 1996, 35(7R), 3984 CAS.
- A. Sera, Y. Kousaka, J. Akimitsu, M. Sera, T. Kawamata, Y. Koike and K. Inoue, S = 1 2 triangular-lattice antiferromagnets Ba3CoSb2O9 and CsCuCl3: Role of spin-orbit coupling, crystalline electric field effect, and Dzyaloshinskii-Moriya interaction, Phys. Rev. B, 2016, 94(21), 214408 Search PubMed.
- H. D. Zhou, C. Xu, A. M. Hallas, H. J. Silverstein, C. R. Wiebe, I. Umegaki, J. Q. Yan, T. P. Murphy, J.-H. Park and Y. Qiu, Successive Phase Transitions and Extended Spin-Excitation Continuum in the S = ½ Triangular-Lattice Antiferromagnet Ba3CoSb2O9, Phys. Rev. Lett., 2012, 109(26), 267206 CAS.
- Y. Tokiwa, T. Radu, R. Coldea, H. Wilhelm, Z. Tylczynski and F. Steglich, Magnetic phase transitions in the two-dimensional frustrated quantum antiferromagnet Cs2CuCl4, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73(13), 134414 Search PubMed.
- Y. Li, G. Chen, W. Tong, L. Pi, J. Liu, Z. Yang, X. Wang and Q. Zhang, Rare-earth triangular lattice spin liquid: a single-crystal study of YbMgGaO4, Phys. Rev. Lett., 2015, 115(16), 167203 Search PubMed.
- S. Mugiraneza and A. M. Hallas, Tutorial: a beginner's guide to interpreting magnetic susceptibility data with the Curie-Weiss law, Commun. Phys., 2022, 5(1), 95 Search PubMed.
- H.-S. Xu, S. Zeng, S. Fang, Y. Huang, W. Xie, H. Huang, Y. Lu and K.-B. Tang, Tunability of magnetization in hexagonal perovskite Sr6Co5O15 ceramics through 20% Fe-substitution, J. Magn. Magn. Mater., 2023, 573, 170648 CAS.
- J. Cheng, W. Tian, J. Zhou, V. M. Lynch, H. Steinfink, A. Manthiram, A. F. May, V. O. Garlea, J. C. Neuefeind and J. Yan, Crystal and magnetic structures and physical properties of a new pyroxene NaMnGe2O6 synthesized under high pressure, J. Am. Chem. Soc., 2013, 135(7), 2776–2786 CrossRef CAS PubMed.
- H. D. Zhou, B. W. Vogt, J. A. Janik, Y. J. Jo, L. Balicas, Y. Qiu, J. R. D. Copley, J. S. Gardner and C. R. Wiebe, Partial Field-Induced Magnetic Order in the Spin-Liquid Kagome Nd3Ga5SiO14, Phys. Rev. Lett., 2007, 99(23), 236401 CrossRef CAS PubMed.
- J. E. Greedan, Geometrically frustrated magnetic materials, J. Mater. Chem., 2001, 11(1), 37–53 RSC.
- Y. Ishiguro, K. Kimura, S. Nakatsuji, S. Tsutsui, A. Q. Baron, T. Kimura and Y. Wakabayashi, Dynamical spin–orbital correlation in the frustrated magnet Ba3CuSb2O9, Nat. Commun., 2013, 4(1), 2022 CrossRef PubMed.
- Y. Shimizu, K. Miyagawa, K. Kanoda, M. Maesato and G. Saito, Spin liquid state in an organic Mott insulator with a triangular lattice, Phys. Rev. Lett., 2003, 91(10), 107001 CrossRef CAS PubMed.
- R. Coldea, D. Tennant, A. Tsvelik and Z. Tylczynski, Experimental realization of a 2D fractional quantum spin liquid, Phys. Rev. Lett., 2001, 86(7), 1335 CrossRef CAS PubMed.
- L. Bufaiçal, M. Heringer, J. Jesus, A. Caytuero, C. Macchiutti, E. Bittar and E. Baggio-Saitovitch, Structural, electronic and magnetic properties of La1.5Ca0.5(Co0.5Fe0.5) IrO6 double perovskite, J. Magn. Magn. Mater., 2022, 556, 169408 CrossRef.
- K. Y. Povarov, L. Facheris, S. Velja, D. Blosser, Z. Yan, S. Gvasaliya and A. Zheludev, et al. , Phys. Rev. Res., 2020, 2(4), 043384 CrossRef CAS.
- P. Svoboda, P. Javorský, M. Diviš, V. Sechovský, F. Honda, G. Oomi and A. A. Menovsky, Importance of anharmonic terms in the analysis of the specific heat of UNi2Si2, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63(21), 212408 CrossRef.
- F. W. de Wette and A. D. Kulkarni, Phonon dispersion, phonon specific heat, and Debye temperature of high-temperature superconductors, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46(22), 14922–14925 CrossRef CAS PubMed.
- Z. Zhang, D. Louca, A. Visinoiu, S. H. Lee, J. D. Thompson, T. Proffen, A. Llobet, Y. Qiu, S. Park and Y. Ueda, Local order and frustration in the geometrically frustrated spinels Cd1-xZnxV2O4, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74(1), 014108 Search PubMed.
- J. P. Sheckelton, J. R. Neilson and T. M. McQueen, Electronic tunability of the frustrated triangular-lattice cluster magnet LiZn2−xMo3O8, Mater. Horiz., 2015, 2(1), 76–80 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ce00085h |
| This journal is © The Royal Society of Chemistry 2025 |






