Open Access Article
Open Access ArticleHydrochloric acid-mediated mechanical synthesis of red-emitting all-inorganic zinc halides†
Siyu
Li
 a,
Jiali
Yao
a,
Dayang
Wang
a,
Jiali
Yao
a,
Dayang
Wang
 a,
Keke
Huang
a,
Wensheng
Yang
a,
Keke
Huang
a,
Wensheng
Yang
 ab and
Renguo
Xie
ab and
Renguo
Xie
 *a
*a
aState Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry Jilin University, Changchun 130012, China. E-mail: renguoxie@jlu.edu.cn
bEngineering Center for Nanomaterials, Henan University, Kaifeng, 475004, China
First published on 11th February 2025
Abstract
Metal halides (MHs), specifically CsPbX3 (where X = Cl and Br), have garnered significant research interest due to excellent optoelectronic properties. However, most of these metal halides exhibit no red emission in the visible region. In this study, Cs2ZnCl4 powders in the presence of hydrochloric acid were synthesized through mechanical milling. X-ray diffraction (XRD) analyses revealed no detectable diffraction signals before or after acid treatment, suggesting a uniform crystal structure. The initial Cs2ZnCl4 powders displayed intrinsic blue emission centered at 486 nm, with a Photoluminescence Quantum Yield (PLQY) of approximately 5.07%. In contrast, the hydrochloric acid-treated Cs2ZnCl4 powder exhibited bright red emission centered at 645 nm, with a PLQY of about 12.78%. The broad emission, characterized by a full width at half maximum (FWHM) of 135 nm, was attributed to self-trapped exciton (STE) emission. X-ray photoelectron spectroscopy (XPS) analysis of Cs2ZnCl4 before and after acid treatment suggests that hydrochloric acid introduction may have induced distortions or defects in the coordination environment of Zn2+ ions. This provides a valuable route for further investigation into the optical properties of other metal halides.
Introduction
As emerging semiconductor materials, metal halides not only have excellent light absorption, tunable bandgaps and long carrier lifetimes, but also exhibit rich photovoltaic properties due to their structural diversity and tunability, which have attracted great attention in the fields of solar cells, photodetectors, light-emitting diodes (LEDs) and lasers.1–8 Pb-based metal halide perovskites, such as CsPbX3 (X = Cl, Br, and I) nanocrystals, are more advantageous in terms of luminescence performance than conventional quantum dot materials because they exhibit high fluorescence monochromaticity (FWHM less than 20 nm) and a high PLQY.9–14 In addition, the PLQY of Pb-based perovskite materials can be significantly enhanced by optimizing the synthesis conditions, metal-ion doping or surface passivation.15–18In contrast to the narrow-band emission exhibited by free excitons in lead-based perovskites, low-dimensional metal halides typically produce broadband emission resulting from self-trapped domain excitons (STEs), a characteristic attributed to their soft lattice properties. This phenomenon has generated significant interest in these luminescent materials, which offer promising applications.19–24 Notably, lead-free zero-dimensional (0D) metal halides, with their isolated polyhedral structures, facilitate multi-exciton emission, leading to robust electron–phonon interactions and photoluminescence efficiencies of nearly 100%.25–28 For instance, copper halides exhibiting emission within the range of 400–560 nm have been reported extensively. Silver-based halides demonstrate photoluminescence across the entire visible spectrum. Recently, fully inorganic Zn-based metal halides, such as Cs2ZnCl4 and Cs3ZnCl5 ternary halide crystals, have been synthesized and reported to exhibit excellent X-ray detectivity.29–31 Moreover, Cr3+-doped Cs2ZnCl4 microcrystals have achieved near-infrared (NIR) emission, opening new avenues for NIR blood vessel visualization.32 Additionally, the blue light emission of Cs2ZnCl4 powder has been enhanced by doping with ions such as Cu2+, Bi3+, Zr4+, Sb3+, Mn2+ or Te4+, which significantly increased the PLQY.33–39 Nevertheless, few metal halides, particularly zinc-based halides, exhibit red emission.
In this study, we synthesized Cs2ZnCl4 powder using a solid-phase method. Subsequent treatment with hydrochloric acid transformed the originally blue-light-emitting Cs2ZnCl4 powder into one that exhibits bright red emission with a peak of 645 nm. The X-ray diffraction (XRD) measurement shows the same diffraction characterization, and no detectable change was observed before and after acid treatment. This red emissive characterization might be attributed to the introduction of hydrochloric acid, as determined by X-ray photoelectron spectroscopy (XPS), where hydrochloride was detected in the sample treated with acid. Accordingly, the broad emission should have originated from STEs as confirmed by the result from variable temperature spectroscopy measurements. This red emissive characterization in zinc-based halides would promote their further applications in the optoelectronic field.
Experimental
Materials
ZnCl2 (98.0%) was purchased from Alfa Aesar. CsCl (99.9%) was purchased from Aladdin Industrial Corporation. All powder materials were stored in a nitrogen-filled glovebox. HCl (36–38 wt% in H2O) was purchased from Beijing Chemical Reagent Ltd. All the chemicals and materials were used without any further purification.Synthesis of Cs2ZnCl4 MHs
To produce Cs2ZnCl4, typically, CsCl (1 mmol) and ZnCl2 (0.5 mmol) were put together into a 25 mL zirconia jar with about 25–35 zirconia balls, and all operations were carried out under nitrogen protection in a glovebox. Subsequently, the jar was closed and transferred to a ball-milling grinder (QM-3SP04). Ball milling was carried out at a frequency of 35 Hz for 4 h to produce Cs2ZnCl4 as a white powder with weak blue luminescence under 254 nm UV irradiation.Acid treatment of Cs2ZnCl4 MHs
After adequate grinding of all MHs, 200 μL of HCl was added to Cs2ZnCl4. Subsequently, the grinding was continued for 30 min.pH measurements
All samples were dried in a vacuum drying oven at 100° for 2 h. Then, 0.2 mmol of as-prepared and acid-treated Cs2ZnCl4 were added to 2 mL of H2O, respectively. All samples were sonicated for 5 min until they were fully dissolved in water, followed by centrifugation at 1000 rpm for 20 minutes, and the supernatant was tested for pH.Characterization method
X-ray photoelectron spectroscopy (XPS) was conducted with a Thermo ESCALAB 250 Xi instrument. Powder wide-angle X-ray diffraction (XRD) patterns were measured with a Philips PW1830 X-ray diffractometer with Cu Kα radiation (λ = 1.54056 Å) in a 2q (q: diffraction angle) range from 10° to 70°. UV-visible absorption spectra were obtained with a U4100 UV-visible spectrophotometer (Shimadzu). Photoluminescence (PL) spectra and time-resolved and temperature-dependent PL spectra were acquired on a steady-state fluorescence spectrometer (Edinburgh FLS920). Mean fluorescence lifetimes 〈t〉 for multi-exponential iterative fitting were calculated from the decay time and the pre-exponential factors. Fluorescence quantum yields were estimated using an FLS920 fluorescence spectrometer with a xenon lamp and Quanta-φ integrating sphere.Results and discussion
In our previous work, a green and efficient solid-state synthesis method was used for the synthesis of various metal halides. Here, we directly synthesized pure-phase Cs2ZnCl4 powder through directly milling a CsCl and ZnCl2 mixture at a frequency of 35 Hz for 4 h. Subsequently, 200 μL of hydrochloric acid (HCl) was rapidly added to an agate jar containing the pure-phase Cs2ZnCl4, and milling was continued for 30 minutes. The resulting pure-phase Cs2ZnCl4 powder exhibited blue light emission under a 254 nm UV lamp, indicating the intrinsic luminescence of Cs2ZnCl4. In contrast, the as-prepared powder further ground for 30 min in the presence of chloride acid demonstrated bright red emission under the 254 nm UV lamp, as illustrated in Fig. 1a. Fig. 1b demonstrates X-ray diffraction (XRD) patterns of the samples before and after acid treatment. The crystal structures of the resulting zinc halides align with standard powder diffraction files (PDFs). The corresponding generic crystal structures viewed down the a-axis are provided in Fig. S1† where Cs2ZnCl4 crystallizes in an orthorhombic structure with the space group Pnma, where the disconnected [ZnCl4]2− tetrahedra are separated by Cs+ cations, forming a soft lattice that facilitates local structural deformation under thermal stimulation, thus enabling optical modulation.40,41 By comparison, no detectable diffraction signals were observable for the sample with acid treatment, suggesting that the samples before and after the introduction of the acid have a uniform crystal structure. Nevertheless, the diffraction peaks of the acid-treated samples shifted slightly toward smaller angles, suggesting that parameters such as the Zn–Cl bond length and the Cl–Zn–Cl bond angle of the acid-treated [ZnCl4]2− tetrahedra should be altered, subsequently affecting the degree of distortion of the tetrahedra. Fig. 1c and d exhibit the absorption and steady-state photoluminescence (PL) spectra of Cs2ZnCl4 powders before and after the introduction of hydrochloric acid. The UV-vis absorption spectra of both samples almost have the same optical profile in which a weak absorption peak at approximately 390 nm was observed, although the sample treated with the acid exhibits stronger absorption properties. The untreated sample exhibits a weak PL peak centered at 486 nm with a FWHM of 82 nm under an excitation wavelength of 270 nm. This optical property is similar to that reported previously in our work. In contrast, the Cs2ZnCl4 sample treated with hydrochloric acid displays a significant emission peak centered at 645 nm with an FWHM of 135 nm and a large Stokes shift under the same excitation wavelength. To the best of our knowledge, this is the first report on red-emissive all-inorganic zinc halides.To further investigate the origin of the red emission from hydrochloric acid-treated Cs2ZnCl4, we conducted X-ray photoelectron spectroscopy (XPS) analysis on the samples both before and after acid treatment. Fig. 2a presents the XPS full-scan spectra of untreated and hydrochloric acid-treated Cs2ZnCl4. The observed peak positions align with the expected chemical states of the elements (Cs+, Zn2+, and Cl−). Subsequently, split peaks were fitted to the fine XPS spectra of Cl 2p, Zn 2p, and Cs 3d. The bimodal peaks for Cl 2p3/2 and Cl 2p1/2 in untreated Cs2ZnCl4 were identified at 198.54 eV and 200.15 eV, respectively, whereas those in the hydrochloric acid-treated sample were found at 198.43 eV and 200.03 eV (Fig. 2b). Additionally, the peaks for Zn 2p3/2 and Zn 2p1/2 were located at 1023.42 eV and 1046.31 eV in untreated Cs2ZnCl4, compared to 1023.13 eV and 1046.02 eV in the hydrochloric acid-treated sample (Fig. 2c), while the peaks for Cs 3d3/2 and Cs 3d5/2 show minor shifts from 724.6 eV and 738.5 eV to 725.4.42 eV and 738.91 eV (Fig. 2d), respectively, with and without the presence of acid. The positions of these peaks shifted slightly toward a lower binding energy, indicating that hydrochloric acid treatment may have modified the coordination environment of the Zn2+ ion or distorted the [ZnCl4]2− tetrahedron. This observation corresponds to the shift in the XRD peaks of Cs2ZnCl4, both with and without acid treatment. By integrating the areas of the corresponding signal peaks for various elements, we compared the Cl/Zn molar ratios in Cs2ZnCl4 before and after hydrochloric acid treatment, yielding values of 4.15 and 4.48, respectively. This change suggests that additional Cl− was introduced into the samples during the hydrochloric acid treatment, resulting in a slight increase in the Cl/Zn molar ratio. The deviation of the Cl/Zn molar ratio in untreated Cs2ZnCl4 from the theoretical value of 4 may arise from variations in the electron detection efficiency among different elements in the XPS analyses.
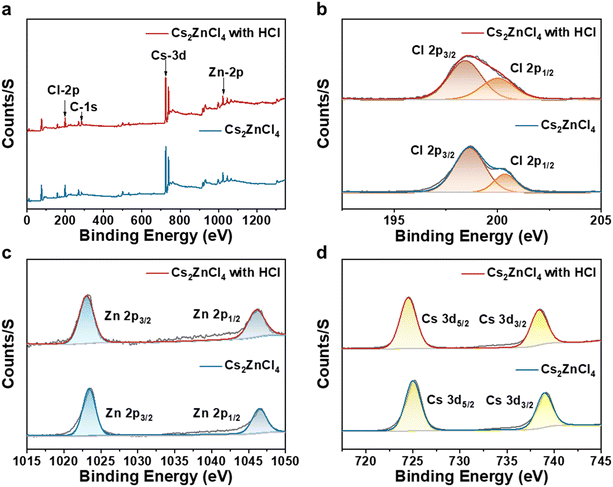 | ||
| Fig. 2 (a) XPS full spectrum analysis and XPS split-fit spectra of (b) Cl 2p, (c) Zn 2p and (d) Cs 3d of Cs2ZnCl4 before and after hydrochloric acid treatment. | ||
In addition to determining Cl− ions in the samples, we also conducted H+ ion measurements for samples with and without hydrochloric acid-treated Cs2ZnCl4. To remove the excess HCl completely from the samples, two specimens were dried in a vacuum drying oven at 100 °C for 2 hours. The resulting powders (0.2 mmol each) were subsequently placed in individual 2 mL volumes of deionized water, treated with ultrasound for 20 minutes, and the pH of the supernatant was then measured using a calibrated electrode. Following the acid treatment, the pH decreased from 3.13 to 1.21, and the concentration of H+ increased from 1.48 × 10−3 mmol to 6.16 × 10−2 mmol (Table 1). This study confirms the presence of H+ ions in Cs2ZnCl4. For comparison, we also estimated the excess Cl− in Cs2ZnCl4 (0.2 mmol) before and after the acid treatment to be approximately 0.066 mmol based on the atomic ratio of Cl/Zn determined by X-ray photoelectron spectroscopy (XPS). The amount of excess H+ determined from the pH test was approximately 0.060 mmol, which is nearly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This finding suggests that acid-treated Cs2ZnCl4 should contain hydrochloric acid, which could be integral to the observed red emission.
1. This finding suggests that acid-treated Cs2ZnCl4 should contain hydrochloric acid, which could be integral to the observed red emission.
| Material | pH | n(H+)/mmol |
|---|---|---|
| Cs2ZnCl4 | 3.13 | 1.48 × 10−3 |
| Cs2ZnCl4 with HCl | 1.21 | 6.16 × 10−2 |
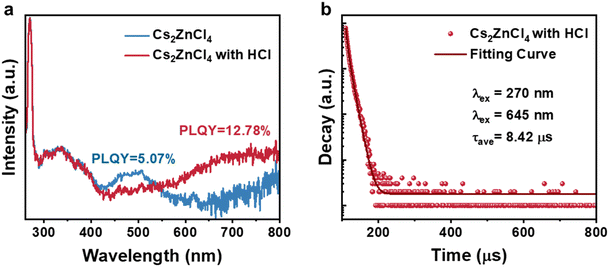
We characterized the PLQY of the Cs2ZnCl4 powder before and after the treatment with hydrochloric acid, as illustrated in Fig. 3a. The sample without the acid exhibits a PLQY of 5.07%, while the hydrochloric acid-treated Cs2ZnCl4 powder demonstrated a significant increase in red light emission, achieving a PLQY of 12.78%. Furthermore, additional efforts were undertaken to vary the concentration of hydrochloric acid. However, the results indicated that the PLQY of Cs2ZnCl4 powders treated with different hydrochloric acid concentrations remained approximately constant. The decay curves of the hydrochloric acid-treated Cs2ZnCl4 were accurately fitted using a biexponential function, as shown in Fig. 3b and detailed in Table S1.† The average lifetime of the hydrochloric acid-treated Cs2ZnCl4 was found to be approximately 8.42 μs.
The intrinsic emission mechanism of the HCl-treated Cs2ZnCl4 was further characterized by the emission wavelength-dependent PLE spectra and excitation wavelength-dependent and temperature-dependent PL spectra. As shown in Fig. 4a and b, when the monitoring emission was changed from 630 nm to 670 nm, the PLE spectra remained unchanged at various excitation wavelengths. Similarly, when the excitation was varied from 250 nm to 270 nm, the PL spectra exhibited the same features as well. Such identical features of the PLE and PL spectra at different wavelengths indicate that the red emission originates from the recombination of the same excited state instead of the defect emission.42 Temperature-dependent PL spectra were measured for HCl-treated Cs2ZnCl4 in the range of 138 K to 318 K (Fig. 5a). The intensity of the emission peaks gradually decreases as the temperature gradually increases from 138 K to 318 K, while the FWHM broadens. This result indicates that more photons couple with excitons at higher temperatures, thus accelerating the nonradiative irradiation process. In addition, a slight blue shift of the PL peak position occurs with increasing temperature, which is due to the weakening of the crystal field strength and the narrowing of the splitting energy of the excited state as a result of thermal expansion.43,44Fig. 5b shows the integral PL intensity (I) of acid-treated Cs2ZnCl4 as a function of the reciprocal of temperature (1/T). The exciton binding energy (Eb) can be derived from the following equation:45
By fitting the temperature-dependent full width at half maximum (FWHM) curve, the S factor was determined to be 18.68. The relatively high Huang–Rhys factor and phonon energy indicate strong electron–phonon coupling in acid-treated Cs2ZnCl4. Combined with a large Stokes shift and significant Huang–Rhys factor, as well as phonon frequency, the emission mechanism of HCl-treated Cs2ZnCl4 is likely attributed to the formation of self-trapped excitons (STEs).48 The exciton self-trapping process of HCl-treated Cs2ZnCl4 is schematically illustrated in Fig. 5d. Upon photoexcitation, the electrons are promoted from the ground state to a high-energy free-exciton excited state. This transition induces the formation of a self-trapped excited state, which is attributed to lattice deformation driven by strong electron–phonon coupling. Subsequently, the excited electrons undergo rapid relaxation and intersystem crossing, transitioning from the free-exciton to self-trapped excited states. Finally, broadband red emission with a large Stokes shift is observed.
 | ||
| Fig. 4 (a) PLE spectra and the (b) PL spectra of hydrochloric acid-treated Cs2ZnCl4 measured under different excitations and different emissions, respectively. | ||
Self-trapped excitons are consistently observed in metal halide crystals. Previous studies have indicated that Cs2ZnCl4 powder exhibits only a weak blue emission, suggesting the presence of a single self-trapped state for intrinsic STEs (Fig. S2†). Furthermore, the PL emission of intrinsic STEs decreases with increasing temperature. This reduction may be attributed to several factors, including the abundance of structural defects within the Cs2ZnCl4 powder, thermal dissociation of excitons, and enhanced electron–phonon coupling associated with intrinsic STEs, which collectively contribute to non-radiative decay and diminished emission.33 Following hydrochloric acid treatment, additional extrinsic STE states are observed. The radiative transitions from the intrinsic STE and extrinsic STE to the ground state correspond to PL bands at wavelengths of 486 nm and 645 nm, respectively. The emergence of extrinsic STE states may be attributed to the distortion of the coordination environment of Zn2+ ions or the introduction of defects resulting from hydrochloric acid treatment.49 Acid-treated Cs2ZnCl4 also demonstrates good environmental stability, as evidenced by its sustained bright red emission under a 254 nm UV lamp after 15 days of exposure (Fig. S3†).
Conclusions
In summary, “red-emissive” zinc-based halide powders were synthesized through a mechanical milling strategy in the presence of hydrochloric acid. XRD analyses revealed no detectable diffraction signals in the samples before or after acid treatment, indicating that the synthesized samples maintained a consistent crystal structure. The acid-treated Cs2ZnCl4 exhibited bright red emission centered at 645 nm, with a PLQY of approximately 12.78%. XPS analysis confirmed the Cl and H ions in the hydrochloric acid-treated Cs2ZnCl4 samples. Therefore, the nature of the red emission can be attributed to the presence of HCl in the sample. Experimental studies reveal that the intrinsic photoluminescence nature of the sample originates from STEs. The red-emissive properties of zinc-based halides not only enhance their potential applications in the optoelectronic field but also provide a valuable pathway for further investigations into the optical properties of other metal halides.Data availability
The data supporting this article are available from the corresponding author, Renguo Xie, upon reasonable request.Author contributions
Siyu Li carried out the experiments and wrote the manuscript. Jiali Yao aided with testing and characterization. Jiali Yao, Dayang Wang, Keke Huang, and Wensheng Yang helped with the analysis of the experimental data. Renguo Xie designed the organization of the manuscript and revised it. All authors contributed to the general discussion.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (Grant No. 51872114, 21932003, 22371090 and 22161132009).References
- H. P. Zhou, Q. Chen, G. Li, S. Luo, T. B. Song, H. S. Duan, Z. R. Hong, J. B. You, Y. S. Liu and Y. Yang, Science, 2014, 345, 542–546 CrossRef PubMed.
- N. J. Jeon, J. H. Noh, Y. C. Kim, W. S. Yang, S. Ryu and S. I. Seok, Nat. Mater., 2014, 13, 897–903 CrossRef PubMed.
- L. T. Dou, Y. Yang, J. B. You, Z. R. Hong, W. H. Chang, G. Li and Y. Yang, Nat. Commun., 2014, 5, 6 Search PubMed.
- P. Ramasamy, D. H. Lim, B. Kim, S. H. Lee, M. S. Lee and J. S. Lee, Chem. Commun., 2016, 52, 2067–2070 RSC.
- J. Byun, H. Cho, C. Wolf, M. Jang, A. Sadhanala, R. H. Friend, H. Yang and T. W. Lee, Adv. Mater., 2016, 28, 7515–7520 CrossRef PubMed.
- Z. G. Xiao, R. A. Kerner, L. F. Zhao, N. L. Tran, K. M. Lee, T. W. Koh, G. D. Scholes and B. P. Rand, Nat. Photonics, 2017, 11, 108–115 CrossRef.
- Q. Zhang, R. Su, W. N. Du, X. F. Liu, L. Y. Zhao, S. T. Ha and Q. H. Xiong, Small Methods, 2017, 1, 12 Search PubMed.
- Q. Zhang, R. Su, X. F. Liu, J. Xing, T. C. Sum and Q. H. Xiong, Adv. Funct. Mater., 2016, 26, 6238–6245 CrossRef.
- X. Lu, Y. Hu, J. Z. Guo, C. F. Wang and S. Chen, Adv. Sci., 2019, 6, 9 Search PubMed.
- Y. W. Pan, Y. F. Zhang, W. M. Kang, N. P. Deng, Z. R. Yan, W. Sun, X. Y. Kang and J. Ni, Mater. Adv, 2022, 3, 4053–4068 RSC.
- J. De Roo, M. Ibáñez, P. Geiregat, G. Nedelcu, W. Walravens, J. Maes, J. C. Martins, I. Van Driessche, M. V. Koyalenko and Z. Hens, ACS Nano, 2016, 10, 2071–2081 CrossRef CAS.
- Y. B. Zhao, C. Xie, X. Zhang and P. Yang, ACS Appl. Nano Mater., 2021, 4, 5478–5485 CrossRef CAS.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692–3696 CrossRef CAS.
- S. N. Guo, H. Wu, D. Wang and J. X. Wang, Langmuir, 2021, 37, 11520–11525 CrossRef CAS PubMed.
- J. D. Lin, Y. X. Lu, X. Y. Li, F. Huang, C. B. Yang, M. L. Liu, N. Z. Jiang and D. Q. Chen, ACS Energy Lett., 2021, 6, 519–528 CrossRef CAS.
- F. Di Stasio, S. Christodoulou, N. J. Huo and G. Konstantatos, Chem. Mater., 2017, 29, 7663–7667 CrossRef.
- V. G. V. Dutt, S. Akhil and N. Mishra, Nanoscale, 2021, 13, 14442–14449 RSC.
- F. Liu, Y. H. Zhang, C. Ding, S. Kobayashi, T. Izuishi, N. Nakazawa, T. Toyoda, T. Ohta, S. Hayase, T. Minemoto, K. Yoshino, S. Y. Dai and Q. Shen, ACS Nano, 2017, 11, 10373–10383 CrossRef PubMed.
- Y. Y. Wu, W. B. Fan, Z. R. Gao, Z. Tang, L. Lei, X. F. Sun, Y. L. Li, H. L. Cai and X. S. Wu, Nano Energy, 2020, 77, 11 Search PubMed.
- T. M. Jiang, W. B. Ma, H. Zhang, Y. Tian, G. Lin, W. G. Xiao, X. Yu, J. B. Qiu, X. H. Xu, Y. Yang and D. X. Ju, Adv. Funct. Mater., 2021, 31, 9 Search PubMed.
- T. Hu, M. D. Smith, E. R. Dohner, M. J. Sher, X. X. Wu, M. T. Trinh, A. Fisher, J. Corbett, X. Y. Zhu, H. I. Karunadasa and A. M. Lindenberg, J. Phys. Chem. Lett., 2016, 7, 2258–2263 CrossRef.
- M. D. Smith and H. I. Karunadasa, Acc. Chem. Res., 2018, 51, 619–627 CrossRef.
- Z. Yuan, C. K. Zhou, Y. Tian, Y. Shu, J. Messier, J. C. Wang, L. J. van de Burgt, K. Kountouriotis, Y. Xin, E. Holt, K. Schanze, R. Clark, T. Siegrist and B. W. Ma, Nat. Commun., 2017, 8, 7 CrossRef.
- L. L. Mao, P. J. Guo, M. Kepenekian, I. Hadar, C. Katan, J. Even, R. D. Schaller, C. C. Stoumpos and M. G. Kanatzidis, J. Am. Chem. Soc., 2018, 140, 13078–13088 CrossRef.
- B. Park, H. Yang, T. H. Ha, H. S. Park, S. J. Oh, Y. S. Ryu, Y. Cho, H. S. Kim, J. Oh, D. K. Lee, C. Kim, T. Lee, M. Seo, J. Choi, Y. M. Jhon, D. H. Woo, S. Lee, S. H. Kim, H. J. Lee, S. C. Jun, H. S. Song, T. H. Park and J. H. Kim, Adv. Mater., 2018, 30, 8 Search PubMed.
- L. Zhou, J. F. Liao, Z. G. Huang, J. H. Wei, X. D. Wang, W. G. Li, H. Y. Chen, D. B. Kuang and C. Y. Su, Angew. Chem., Int. Ed., 2019, 58, 5277–5281 CrossRef PubMed.
- B. M. Benin, D. N. Dirin, V. Morad, M. Wörle, S. Yakunin, G. Rainò, O. Nazarenko, M. Fischer, I. Infante and M. V. Kovalenko, Angew. Chem., Int. Ed., 2018, 57, 11329–11333 CrossRef PubMed.
- M. Chen, M. G. Ju, A. D. Carl, Y. X. Zong, R. L. Grimm, J. J. Gu, X. C. Zeng, Y. Y. Zhou and N. P. Padture, Joule, 2018, 2, 558–570 CrossRef CAS.
- K. Takahashi, M. Arai, M. Koshimizu, Y. Fujimoto, T. Yanagida and K. Asai, Jpn. J. Appl. Phys., 2020, 59, 5 Search PubMed.
- N. Yahaba, M. Koshimizu, Y. Sun, T. Yanagida, Y. Fujimoto, R. Haruki, F. Nishikido, S. Kishimoto and K. Asai, Appl. Phys. Express, 2014, 7, 4 Search PubMed.
- K. Sugawara, M. Koshimizu, T. Yanagida, Y. Fujimoto, R. Haruki, F. Nishikido, S. Kishimoto and K. Asai, Opt. Mater., 2015, 41, 53–57 CrossRef.
- T. C. Zheng, H. X. Yang, H. L. Lu, Y. L. Liu, Y. Li, C. X. Peng, L. B. Zhang and X. Y. Li, J. Lumin., 2024, 271, 8 CrossRef.
- X. M. Dong and R. K. Pan, Phys. B, 2024, 676, 5 CrossRef.
- D. X. Zhu, M. L. Zaffalon, V. Pinchetti, R. Brescia, F. Moro, M. Fasoli, M. Fanciulli, A. Tang, I. Infante, L. De Trizio, S. Brovelli and L. Manna, Chem. Mater., 2020, 32, 5897–5903 CrossRef PubMed.
- X. Y. Zhao, X. S. Zhang, X. K. Gong, X. R. Yuan, M. X. Chen, S. W. Huang, B. Z. Zhou, J. P. Xu and L. Li, Inorg. Chem. Front., 2023, 11, 71–84 RSC.
- Y. Tian, Q. Wei, L. Duan and C. Peng, Molecules, 2024, 29, 1651 CrossRef PubMed.
- Y. Guo, J. K. Chen, B. Chen, W. L. Zheng, X. Zhang, H. Suo, F. J. Chun, X. H. Wei and F. Wang, Mater. Today Phys., 2023, 35, 101111 CrossRef.
- T. C. Zheng, H. X. Yang, Y. L. Liu, Y. Li, Q. Huang, L. B. Zhang and X. Y. Li, Inorg. Chem., 2023, 62, 17352–17361 CrossRef PubMed.
- X. X. Liu, C. D. Peng, L. J. Zhang, D. Y. Guo and Y. X. Pan, J. Mater. Chem. C, 2022, 10, 204–209 RSC.
- X. X. Liu, C. D. Peng, L. J. Zhang, D. Y. Guo and Y. X. Pan, J. Mater. Chem. C, 2021, 10, 204–209 RSC.
- I. Romdhane, A. Ajmi, M. Ben Bechir, R. Barille and A. Ben Rhaiem, RSC Adv., 2024, 14, 36253–36263 RSC.
- W. R. Gao, G. D. Niu, L. X. Yin, B. Yang, J. H. Yuan, D. D. Zhang, K. H. Xue, X. S. Miao, Q. S. Hu, X. Y. Du and J. Tang, ACS Appl. Electron. Mater., 2020, 2, 2242–2249 CrossRef CAS.
- Z. N. Zhang, H. M. Cheng, S. Y. Teng, K. K. Huang, D. Y. Wang, W. S. Yang and R. G. Xie, Inorg. Chem., 2022, 61, 20552–20560 CrossRef CAS.
- L. Y. Lian, P. Zhang, J. B. Gao, D. L. Zhang and J. B. Zhang, Chem. Mater., 2023, 35, 9339–9345 CrossRef CAS.
- M. Baranowski and P. Plochocka, Adv. Energy Mater., 2020, 10, 15 Search PubMed.
- S. M. Lee, C. J. Moon, H. Lim, Y. Lee, M. Y. Choi and J. Bang, J. Phys. Chem. C, 2017, 121, 26054–26062 CrossRef.
- M. Y. Jin, W. Zheng, Z. L. Gong, P. Huang, R. F. Li, J. Xu, X. W. Cheng, W. Zhang and X. Y. Chen, Nano Res., 2022, 15, 6422–6429 CrossRef.
- F. Jiang, Z. N. Wu, M. Lu, Y. B. Gao, X. Li, X. Bai, Y. Ji and Y. Zhang, Adv. Mater., 2023, 35, 13 Search PubMed.
- M. Watanabe, H. Takahashi, K. Uematsu, M. Sato, T. Masaki, D. H. Yoon and K. Toda, J. Ceram. Soc. Jpn., 2022, 130, 458–463 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ce01243g |
| This journal is © The Royal Society of Chemistry 2025 |