Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The influence of the crystal sponge framework on guest molecule conformation†
Eleanor M.
Soper
 ,
Simon J.
Coles
,
Simon J.
Coles
 and
Graeme M.
Day
and
Graeme M.
Day
 *
*
School of Chemistry, University of Southampton, Southampton, SO17 1BJ, UK. E-mail: e.soper@soton.ac.uk; s.j.coles@soton.ac.uk; g.m.day@soton.ac.uk
First published on 31st January 2025
Abstract
The crystalline sponge (CS) method has become an important technique for structural elucidation of compounds that are challenging to crystallise. The impact of the CS environment on guest molecule conformations has not been systematically studied. We present a computational investigation of the conformations of organic molecules of varying flexibility in a set of experimentally determined CS structures, comparing them to gas phase conformers and, where available, pure and co-crystal structures. Via solid state and molecular density functional theory calculations, we quantify the total relative energy, conformational energy, and intramolecular strain of guest molecules, as well as framework strain. Our results show that while CS structures induce distortion in guest geometries (total relative energies up to 41 kJ mol−1), they generally adopt low-energy conformations, often within 2 kJ mol−1 of the global energy minimum. Intramolecular strain in CS structures is often lower than in conventional crystal structures, suggesting a more neutral packing environment where molecules are closer to their favoured isolated-molecule geometries. We also observe that multiple guests can influence each other's geometries, even in the absence of direct guest–guest interactions. These findings provide a quantification of conformational distortion that can form the basis for interpreting molecular geometries obtained from CS structures.
1 Introduction
The accurate determination of molecular structure is of critical importance in chemical research. Single crystal X-ray diffraction (SCXRD) is considered to be one of the most powerful methods of structure elucidation, giving the ability to determine the absolute configuration and stereochemistry of molecules with relative ease. A major drawback of the technique is that, as the name suggests, a good-quality single crystal needs to be produced. Many synthetic organic or natural products can be difficult to crystallise, produced in minute quantities, or are liquid at ambient temperatures, and as a result are often unsuitable for SCXRD analysis.The crystal sponge (CS) method, first reported by Fujita and coworkers1 in 2013, has become an important technique in the structural elucidation of compounds considered “uncrystallisable”. The technique removes the necessity of sample crystallisation by utilising the porosity of crystalline metal–organic frameworks (MOFs) to form host–guest complexes with the desired compound, which can then be analysed by SCXRD.2 The CS method is particularly useful in pharmaceutical research, where knowing the exact structure and stereochemical information of a molecule is of critical importance.
The most successful and generally applicable MOF used for the CS method is the [((ZnX2)3(tpt)2·x(solvent))n] system, (tpt = 2,4,6-tris(4-pyridyl)-1,3,5-triazine ligand; X = I, Br, Cl). The system has several advantageous properties including large, hydrophobic pores (5 × 8 Å) with electron-deficient binding sites offered by the tpt ligand. The ZnX2 components and pyridyl protons of the tpt ligands provide hydrogen-bond donor and acceptor sites, respectively, which enables reproducible and long-range ordering of the guests within the MOF pores and absorption of organic and aromatic molecules of a range of sizes. The interpenetrated framework allows for some flexibility and expansion of the pore size to accommodate larger guests.3,4
The study of MOFs and host–guest complexes by computational methods is a growing area of research, dominated by studies aiming to model the uptake of gases, drug delivery, and calculation of binding energies and interaction strengths of small molecules in the pores of frameworks by molecular dynamics, Monte Carlo simulations, and DFT methods.5–9 There have been fewer studies directed at MOFs containing flexible molecules, and specifically CS structures. There are certain challenges that arise due to the size and complexity of these systems, especially when considering the presence of large guest molecules and solvent(s); however, the development of molecular simulation methods and access to more powerful high performance computing has meant that modelling such systems is more accessible, and has led to an increasing number of MOFs being studied computationally.
While there are many examples of computational approaches being applied to MOFs, the (ZnX2)3(tpt)2 structures most commonly used for the CS method are not well-studied from a computational perspective, despite the large subset of >500 CS structures available in the Cambridge Structural Database10 (CSD). Investigation into the CS method has primarily focused on the determination of novel, otherwise intractable, molecular structures and the improvement of experimental procedures.3,11–16 At the time of writing, the only study of CS structures using computational methods that the authors are aware of is the work of Cardenal and Ramadhar,17 who demonstrated the use of dispersion-corrected density functional theory (DFT) to analyse a set of previously-published experimental crystalline sponge structures and obtained binding energies of guests within the pores of the framework.
This study seeks to provide a fundamental understanding of the extent to which the CS structure impacts the geometry of guest molecules and the type of environment that is present in the pores of the CS. The geometries of guest molecule(s) from the optimised CS structures are compared to the DFT conformer landscape to quantify the intramolecular strain and conformational energetics of the distortion induced by the pore environment. Comparison to pure and cocrystal structures, where available, aids further understanding of the exact nature of the pore, defining if the environment of the CS is similar or different from typical, purely organic crystal structures.
2 Methods
2.1 Geometry optimisations
The first step is to obtain geometric and energetic information for the conformations of a molecule in its observed crystal structures. The observed molecular geometries and energies are evaluated after optimisation of the experimentally determined crystal structures, which removes any experimental artefacts and errors in atomic positions remaining after structure refinement.For consistency, the same level of theory and calculation settings are used for all crystal structure and single molecule energy calculations. All density functional theory (DFT) calculations were performed in the CRYSTAL17 (ref. 18) software using the POB-TZVP basis set19,20 and B3LYP functional21 in combination with the D3 dispersion correction.22 To account for the basis set superposition error (BSSE) that arises in finite basis sets, the geometric counterpoise (gCP) correction23 was implemented in all calculations.
The symmetry-independent (unique) guest molecules were extracted from the DFT optimised experimental crystal structures. Single-point energy calculations were performed to obtain the energies of the isolated molecules from their crystalline state, Emolcrystal (see red point in Fig. 1). The crystalline molecular geometry was also used to obtain the associated local energy minimum on the conformational energy surface, Eopt,mollocal, by running unconstrained geometry optimisations to relax the molecule to the nearest local energy minimum (blue point marked LM in Fig. 1). The energy difference between the single-point and relaxed molecular geometries of the unique molecules within the crystal structures is the intramolecular strain energy, ΔEstrain (see Fig. 1 and eqn (1)):
| ΔEstrain = Emolcrystal − Eopt,mollocal | (1) |
2.2 Conformational searches
The CREST conformer search24 method was applied to generate complete sets of conformers for each guest molecule studied. To ensure thorough sampling, we generated multiple CREST searches from diverse starting structures to fully search the conformational landscape. Duplicates from the combined searches are removed by clustering based on torsion angles within a maximum energy window of 2 kJ mol−1 and torsion angle tolerances were set to 10.0° and 5.0° for the single angle maximum and the maximum RMS angle, respectively.All unique conformers resulting from the initial search were re-optimised with DFT-D3, applying the same computational parameters as described in section 2.1. The result should be a complete set of low energy conformers for each molecule. The DFT-optimised conformers were re-clustered to remove duplicates, resulting in the final DFT conformer landscape.
To identify the local conformer of the isolated molecule from the observed crystal structures in the conformational landscape, geometric comparisons with the sets of conformers were performed. The difference in energy between the lowest energy generated conformer (the global energy minimum conformer), EGM, and the energy of the local conformer corresponding to the crystalline molecular geometry, Eopt,mollocal, is referred to as the conformational energy, ΔEconf (see Fig. 1 and eqn (2)):
| ΔEconf = Eopt,mollocal − EGM | (2) |
| ΔEtotal = ΔEstrain + ΔEconf = Emolcrystal − EGM | (3) |
2.3 Framework strain
The strain energy of the MOF was also quantified to provide insight into the distortion of the framework that occurs on uptake of the guest molecule. All guest and solvent molecules were removed from the optimised CS structure, leaving the empty (ZnX2)3(tpt)2 framework. The energy of the MOF in the optimised CS structure EcrystalMOF was calculated by running a single point energy calculation of the empty framework. An unconstrained geometry optimisation was also performed on the empty MOF structure, applying the same calculation settings as described above. The energy difference between the single-point and relaxed empty MOF structures (Eopt,MOFlocal) is the intramolecular strain energy of the framework, ΔEMOF (eqn (4)):| ΔEMOF = EMOFcrystal − Eopt,MOFlocal | (4) |
2.4 Selection criteria for crystalline sponge structures
In order to gain insights into the effect of the CS framework on the conformation of guest molecules within the pore, it is important to choose a set of experimental structures that reflect the range of flexibility of guests that have been soaked into CS. While this set must be representative of the full range of different types of analytes, it must also comprise of experimental structures that are of a suitable quality to be a starting point for computational analysis. Making decisions solely based on individual quantitative crystallographic statistics, such as the R-factor, cannot be relied upon as there are many factors, including some qualitative ones, which contribute to the completeness of a CS structure. Therefore, the selection process requires manual filtering to sort through the CS candidates and produce a subset for study. The considerations that have been used in this process, and their justification, are provided in the ESI.†To identify CS structures for this study, the CSD was searched based on the ZnX2 (X = Cl, Br, I) and tpt ligand features of the (ZnX2)3(tpt)2 framework. The subset of CS structures were chosen from the resulting CSD hits and selected based on the following criteria: (1) the guest molecule satisfies Lipinski's rule of five, (2) no solvent masking methods have been used in the structure refinement, and (3) there is minimal residual solvent accessible void space. The number of rotatable bonds, excluding methyl groups, was also considered to ensure the set of molecules included the full range of possible guest flexibility.
2.5 Preparation of the dataset
CS structures, i.e. those with isolated molecules residing in framework pores, typically have more disorder than conventional organic crystal structures. This can be present in both the framework and also the guest molecules in the pore, which can comprise both the analyte of interest and solvent molecules. The guest molecules can be situated over a symmetry site, or due to low occupancy i.e. not being present in exactly the same location in every pore, found in multiple positions, orientations, or disordered with solvent. Consequently, it is important to consider how disorder affects experimental structures on a case-by-case basis to ensure that the structure taken forward for computational study is as realistic as possible. Full details for dealing with framework, guest and solvent disorder is described in the ESI.†2.6 Structures chosen for study
Beyond filtering on quality, the 17 CS structures used in this study were selected primarily to reflect the range of conformation flexibility of guest molecules that have successfully been soaked into this CS framework. The 16 unique guests are detailed in Fig. 2. The flexibility distribution, based on the number of rotatable bonds (excluding methyl groups), can be found in the ESI.† An additional, guest-free (solvent-only), structure (1, LABMOT) was also included in the structure set for the purpose of framework strain analysis. If available, the single-component (pure) crystal structures and any co-crystal and/or solvate structures of the guest molecules were also studied to provide comparison.3 Results and discussion
As many of the chosen structures contain some guest and/or solvent disorder, multiple configurations have been optimised to consider any effects of positional changes in the pores of the CS. The resulting values of ΔEstrain and ΔEconf vary by <1 kJ mol−1 between configurations; this small energy difference is consistent with the presence of disorder. For discussion, in all further analysis we only include the CS structure configurations with the lowest energy of the guest molecule. These results are summarised in Table 1. The full results are reported in Table S2.†| MOF type | CSD Refcode | Config.a | Guest | ΔEstrain (kJ mol−1) | RMSD (Å) | ΔEconf (kJ mol−1) | ΔEtotal (kJ mol−1) | ΔEMOFb (kJ mol−1) | |
|---|---|---|---|---|---|---|---|---|---|
| a For disordered structures where multiple configurations have been considered, the lowest energy configuration is reported. For the complete set of results see the ESI.† b The value of ΔEMOF is quoted per minimum framework unit, i.e. (ZnX2)3(tpt)2, or 81 atoms, rather than per unit cell as quoted in the calculation output, to make the values comparable. c It is important to note here that although 1R-(−)-methyl acetate and vanillin have a non-zero ΔEconf, geometric comparison with the set of generated conformers shows that the conformer in the experimental structure matches the global minimum energy conformer, and therefore the non-zero energy values are indicative of calculation errors resulting from the convergence tolerances. d These data values are included to show that calculations on this structure have been carried out, however they will not be discussed as the calculated energies are unusually high and may have resulted from the use of restraints/constraints on the disordered molecule. | |||||||||
| ZnI2 | 1 | LABMOT3 | 1 | — | — | — | — | 16.06 | |
| 2 | XAZPEW14 | 1 | 3.99 | 0.042 | 0.00 | 3.99 | 4.53 | ||
| 3 | XAZNUK14 | 1 | A | 9.87 | 0.060 | 4.24 | 14.10 | 19.83 | |
| B | 3.31 | 0.071 | 0.01c | 3.33 | |||||
| C | 2.41 | 0.034 | 0.01c | 2.42 | |||||
| D | 7.81 | 0.124 | 0.01c | 7.81 | |||||
| 4 | ZOQSUV12 | 1 | A | 4.09 | 0.127 | 0.17 | 4.26 | 8.79 | |
| B | 3.37 | 0.129 | 0.16 | 3.53 | |||||
| C | 6.73 | 0.101 | 0.18 | 6.90 | |||||
| 5 | XAZPAS14 | 1 | 2.50 | 0.094 | 0.00 | 2.50 | 7.19 | ||
| 6 | CENBOP25 | 1 | 17.79 | 0.463 | 8.74 | 26.53 | 8.49 | ||
| 7 | GARLAQ26 | 1 | 18.16 | 0.185 | 0.00 | 18.16 | 16.82 | ||
| 8 | LIRLIL27 | 2 | A | 23.39 | 0.577 | 5.13 | 28.52 | 9.98 | |
| B | 6.75 | 0.102 | 21.78 | 28.53 | |||||
| 9 | LIRLEH27 | 1 | A | 19.93 | 0.423 | 3.81 | 23.76 | 9.10 | |
| B | 5.23 | 0.085 | 21.05 | 26.28 | |||||
| ZnCl2 | 10 | CENBUV25 | 3 | 13.52 | 0.384 | 1.79 | 15.31 | 100.95 | |
| 11 | RECJAN25 | 1 | 25.91 | 0.651 | 6.74 | 32.65 | 16.89 | ||
| 12 | EZEWUE28 | 3 | 10.99 | 0.385 | 0.57 | 11.56 | 14.26 | ||
| 13 | COQBIW29 | 1 | A | 12.56 | 0.404 | 28.45 | 41.01 | 17.49 | |
| B | 16.24 | 0.481 | 19.98 | 36.22 | |||||
| 14 | EZEXAL28 | 6 | A | 12.46 | 0.432 | 6.82 | 19.28 | 18.07 | |
| B | 18.39 | 0.965 | 5.03 | 23.42 | |||||
| 15 | EZEVUD28 | 1 | 6.90 | 0.112 | 1.59 | 8.50 | 13.02 | ||
| 16 | EZEWOY28 | 2 | 40.80d | 0.609d | 79.75d | 120.55d | 13.28d | ||
| 17 | VUKYUX11 | 1 | A | 4.66 | 0.195 | 0.45c | 5.11 | 20.70 | |
| B | 4.61 | 0.143 | 0.02c | 4.63 | |||||
| ZnBr2 | 18 | VUKYOR11 | 1 | A | 3.08 | 0.060 | 0.63c | 3.71 | 3.41 |
| B | 3.41 | 0.110 | 0.05c | 3.46 | |||||
The experimental CS structure 10 (CENBUV) contains one dapsone molecule, two disordered n-hexane solvent molecules and two water molecules in the asymmetric unit. Both n-hexane solvent molecules are 2-part disordered with equal occupancies, so four configurations of the structure have been considered to account for all combinations of solvent location. The ΔEtotal of the dapsone guest in each configuration are within a 2 kJ mol−1 window of each other (see Table S2†). Breaking down the ΔEtotal term into its components, we can see this is solely resulting from variation in intramolecular strain energy ΔEstrain, as all configurations share the same conformation, with a calculated ΔEconf = 1.8 kJ mol−1. Although it is not large, the difference in energies between configurations shows the impact of the proximity of solvent, and therefore sterics, on the total energy of guest molecules.
In the following sections, the impact of the CS environment on the energy and conformation of the guest molecules will be discussed. Any analysis of interactions between the guest and the framework or other guests will be commented on from a qualitative perspective; detailed quantitative discussion, similar to recent work by Carroll,27 would be required to fully understand the strength of the interactions.
The structure of doxorubicinone (16, EZEWOY) has unphysically high ΔEstrain and ΔEconf energies. The host framework is well-resolved, however the guest model required a considerable number of restraints and constraints to impose reliable geometry and crystallographic parameters. There is a high likelihood that these have significantly distorted the molecular geometry and resulted in these artificially high calculated energies. For this reason this structure is an outlier in the group and has not been included further in this analysis. All remaining discussion includes only the remaining 16 CS structures.
3.1 Total relative energy
The distortion of any molecule away from its gas phase geometry involves an increase in the intramolecular energy ΔEstrain and possibly of the conformational energy ΔEconf, if the molecule adopts a conformer other than its most favourable gas phase conformation within the CS. Firstly the total relative energy ΔEtotal of a molecule in a CS crystal structure from its gas phase geometry is analysed. This is calculated as the difference between the energy of the isolated molecule in the geometry from its DFT optimised crystal structure and the gas phase (global minimum) energy, according to eqn (3).The distribution of ΔEtotal is detailed in Fig. 3 for the 27 distinct guest geometries exhibited in the 16 optimised experimental CS structures (excluding structure 16) outlined in Fig. 2. The distribution includes the total relative energies of each independent molecule found within each crystalline sponge structure.
Molecules which would typically be considered rigid are slightly distorted and therefore have a non-zero total relative energy: we calculate values of ΔEtotal = 2.50 kJ mol−1 for 4-trifluoromethylphenyl azide (5, XAZPAS), and 3.99 kJ mol−1 for (+)-artemisinin (2, XAZPEW), which has zero rotatable bonds, excluding terminal methyl groups (Fig. 4a). Although the geometric difference is small, this demonstrates the crystal packing effects present in CS structures induce distortions of even the most rigid of molecules. The lowest calculated ΔEtotal is 2.41 kJ mol−1 for one of the four vanillin guest molecules in 3 (XAZNUK), and one third (9 of 27) of molecules studied have a calculated ΔEtotal less than 5 kJ mol−1 (approximately 2RT at room temperature). These mostly correspond to the smaller molecules with few rotatable bonds (Table 1): structures 2, 3, 4 (ZOQSUV), 5, 17 (VUKYUX), and 18 (VUKYOR).
The conformations of larger molecules with more flexible degrees of freedom are more substantially influenced by the CS environment. Ignoring the outlier, doxorubicinone (16), calculated ΔEtotal of guests in CS structures range up to 41 kJ mol−1. Examples of the highest energy guests include nifedipine (11 RECJAN, ΔEtotal = 32.7 kJ mol−1), fluoxetine (6 CENBOP, ΔEtotal = 26.5 kJ mol−1), and tenebrathin (13 COQBIW, ΔEtotal = 41.0 and 36.2 kJ mol−1 in the two distinct guest sites), which all show significant geometric distortion from their gas phase conformation in the CS (see Fig. 4b, c and 5).
 | ||
| Fig. 5 The two unique crystalline molecular geometries of tenebrathin in the pores of the optimised CS structure 13 (left) and the gas phase (global energy minimum) structure of tenebrathin (right). | ||
From the perspective of understanding the impact of the CS on the conformation of a guest molecule, these results demonstrate that the interactions with guest molecules in the pore environment often play a role in dictating their molecular conformation: there is always some distortion of molecular structures determined using the CS method away from the intrinsically most stable geometry of the isolated molecule. While this can result in small energetic changes in the structures of small molecules, the effect is often large and should be considered when interpreting CS structures.
We analyse ΔEtotal in more depth in the following sections, where the distortion is broken down into conformational change ΔEconf and intramolecular strain ΔEstrain.
3.2 Conformational energy: which conformation is adopted in CS structures?
During crystallisation, molecules are not restricted to their lowest energy conformer, and the balance of inter- and intramolecular interactions can sometimes lead to higher energy conformers.30 We quantify this with ΔEconf, the difference in energy between the conformer that relates to the crystalline molecular geometry and the global minimum energy conformer found by the conformational search process (see Fig. 1). The distribution of conformational energies of guest molecules in CS structures, and comparison to those in pure and cocrystal structures, is detailed in Fig. 6.For all molecules, a corresponding conformer to that obtained from the experimental crystal structure is found in the generated DFT conformer landscape. Most guest molecules do not adopt the global minimum energy conformer in their CS structures: only 6 out of 16 CS structures contain a guest molecule which adopts the global energy minimum conformer. (+)-artemisinin (2), 4-trifluoromethylphenyl azide (5), and the dithiine guest (7, GARLAQ), all of which have ΔEconf = 0 kJ mol−1, are reasonably rigid molecules. Three further structures containing vanillin (3) and (1R)-(−)-menthyl acetate (17 and 18) adopt the global minimum conformer in their experimental CS crystal structures; however, these have small, non-zero (up to 0.6 kJ mol−1) calculated ΔEconf, which is considered negligible once a visual comparison is made with the corresponding global minimum energy conformer. These small energetic discrepancies provide a measure of the numerical noise in the molecular optimisations applied in this work.
Although it is rare for molecules to adopt their lowest energy conformer in CS structures, for over half of the unique molecules (59%, 16 out of 27 molecules in all structures) the crystalline geometry remains close to the global minimum (below 2.0 kJ mol−1). In 23 out of 27 unique guest molecules, the conformational energy lies below 10 kJ mol−1: molecules usually adopt low energy conformers in CS structures.
For the remaining molecules, their geometry in the CS structures corresponds to a conformer that is energetically far above the gas phase global energy minimum. Structures 8 (LIRLIL) and 9 (LIRLEH) each contain a biaryl guest with high ΔEconf, at 21.78 and 21.05 kJ mol−1, respectively; both CS structures also contain a second guest with much lower ΔEconf (see Table 1). In the gas phase, the global minimum conformer for the guest molecules in both 8 and 9 adopt a geometry stabilised by intramolecular hydrogen bonding. The biaryl guests which adopt a local conformation with no intramolecular hydrogen bonds have a high ΔEconf, due to the high energetic barrier for geometric distortion/destabilisation of the molecule. The biaryl guests with low ΔEconf adopt geometries with intramolecular hydrogen bonding intact after relaxation to their local conformation (see Fig. 7). Both guest molecules of tenebrathin (13), as mentioned previously in discussion of ΔEtotal, also adopt relatively high energy conformers in their CS structure, with ΔEconf = 19.98 and 28.45 kJ mol−1.
Previous work by Thompson30 showed that conformers with large surface areas are favoured in the crystal structures of flexible molecules, as it provides greater available surface area for stabilising intermolecular interactions, while lower energy conformers typically display relatively compact geometries to enable intramolecular stabilisation. This is what we observe for tenebrathin, which adopts extended conformers in its CS structure, compared to the folded global energy minimum conformer (Fig. 5), that could be stabilised by general van der Waals interactions with solvent and the framework. This is not the case for high ΔEconf values in 8 and 9, however, where the driving force for these molecules comes from breaking intramolecular hydrogen bonding stabilised by strong, specific interactions with the framework (Fig. 8).
3.3 Intramolecular strain energy
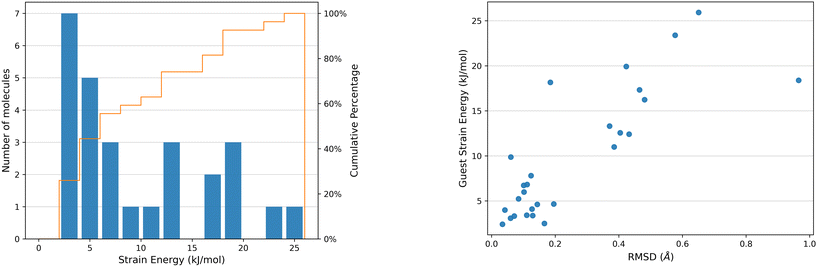
The distortion of any molecule in a crystal structure away from the relaxed isolated molecular geometry involves an increase in the intramolecular energy, Estrain. This energy is calculated as the difference in energy of the molecule in the optimised crystal structure from its local energy minimum on the conformational energy surface (Fig. 1).The distribution of ΔEstrain is detailed in Fig. 9 for the 27 distinct guest geometries in the 16 optimised experimental CS structures outlined in Fig. 2. The distribution includes the strain energies of each independent molecule found within each CS structure configuration. Calculated CS strain energies range up to 26 kJ mol−1 and are concentrated in the lower end of the distribution: over a third (10 out of 26 guest geometries) have calculated ΔEstrain < 5 kJ mol−1 and 59% of guest molecules (16 out of 26 guest geometries) have ΔEstrain < 10 kJ mol−1. These molecules adopt geometries energetically near to a gas phase optimised conformer in the CS structures.
There is a correlation between the intramolecular strain energy ΔEstrain of the guest within the CS pores and the RMSD in atomic positions between the crystalline guest molecular geometry and the optimised isolated guest (see Fig. 9), confirming that large distortions in the molecular geometry are usually associated with a relatively large energetic cost. There are a few cases where significant geometry changes have a relatively low energetic cost, such as risperidone (14, EZEXAL) which has an extended structure; or where a small change in molecular distortion leads to a high strain energy, such as the dithiine guest (7). The latter example contains two highly-strained 4-membered rings in the molecular structure, for which there is a large energetic penalty for making relatively small geometric changes.
There are several instances of surprisingly high strain energies above 20 kJ mol−1. The highest values calculated from the guest molecules studied here are 23.39 kJ mol−1 in 8 and 25.9 kJ mol−1 in 11. High strain energies are not restricted to the most flexible molecules; two molecules with relatively few flexible degrees of freedom both show a relatively large strain energy in their CS structures: 7 (ΔEstrain = 18.16 kJ mol−1, 2 rotatable bonds) and 10 structure 1 (see ESI,† ΔEstrain =15.50 kJ mol−1, 4 rotatable bonds).
3.4 Pure and cocrystal structures
To understand how the CS pore environment differs from typical crystal packing, the conformational energetics of molecules in their pure and cocrystal structures have been compared to those calculated for the CS structures. The CS method is used primarily for structure elucidation of substances that do not crystallise or are produced in minute quantities, so several guest molecules in this study do not have any non-CS crystal structures which can be studied. Of those that do have crystal structures, there are 10 molecules with pure (single-component) structures and 7 molecules with cocrystal structures, including 6 which have both pure and cocrystal structures. The complete set included in this study is detailed in Tables S3 and S4.†The distributions of ΔEtotal in conformers present in CS structures are compared to those in pure and cocrystal structures in Fig. 10. Molecules typically have a lower total relative energy in the CS compared to both their pure and cocrystal structure counterparts. The ΔEtotal distribution for molecules in their pure and cocrystal structures ranges up to 45 kJ mol−1, higher than those observed in the CS at 34 kJ mol−1. For pure and cocrystal structures the distributions peak between 12–14 and 10–12 kJ mol−1, respectively, whereas for CS structures this is observed between 2–4 kJ mol−1. For the subset of CS structures where the guest has pure and/or cocrystal structures, 47% of unique molecules have a calculated ΔEtotal below 10 kJ mol−1. In comparison, the proportion of unique molecules with ΔEtotal below 10 kJ mol−1 is much lower in pure and cocrystal structures: 12.5% (5 out of 40) of molecules in pure crystal structures and 20% (23 out of 114) of molecules in cocrystal structures.
The shift to higher ΔEtotal for cocrystals could likely be a consequence of the strong interactions between coformers, which will have an impact on molecular geometry and ΔEtotal. The distribution also has a larger ΔEtotal range, suggesting that the intermolecular interactions with the pore environment of the CS are weaker than in typical crystal structures. The ΔEtotal distribution of pure and cocrystal structures in Fig. 10 is also skewed by unequal proportions of cocrystal structures: some molecules have many more structures than others and if the number of structures per molecule of interest were equal, the distribution would be flatter. Fig. S2† shows the ΔEtotal distribution for cocrystal structures, broken down by the molecule of interest: there are significantly more cocrystal structures than pure crystal and CS structures, providing greater sampling of cocrystal structures and resulting in an inconsistent comparison.
As previously described, a large proportion of calculated ΔEconf is limited to <2 kJ mol−1 and consequently ΔEstrain has the biggest contribution to ΔEtotal of guest molecules. The distribution of ΔEstrain for both pure and cocrystal structures is detailed in Fig. S3b.† Calculated strain energies range up to 28 kJ mol−1 and peak between 10–12 kJ mol−1. Previous conformational studies of single-component organic crystal structures has shown that strain energies range up to 20 kJ mol−1 and the majority (75%) of strain energies lie below 10 kJ mol−1.30 Comparison to the ΔEstrain distribution of the subset of 10 CS guest molecules that have pure and/or cocrystal shows that the distribution peaks in the 2–4 kJ mol−1 bin (Fig. S3a†) and only 61% have a strain energy below 10 kJ mol−1 (11 out of 18 unique molecules). Similarly to CS structures, there is a positive correlation between ΔEstrain and RMS deviation of atomic positions between the experimental and gas phase local conformer molecular geometries, for both pure and cocrystal structures (Fig. S4†).
The increased probability of a higher ΔEstrain in pure and cocrystal structures suggests that the close-packed nature of typical crystal packing provides greater stability of molecular distortion than interactions in the pores of the CS framework. It should be noted that Thompson's study30 excluded molecules with potential for intramolecular hydrogen bonding, while the molecules in this study have greater potential for hydrogen bonding. These strong interactions could also lead to a higher ΔEstrain.
3.5 Framework strain
Quantifying the strain of the CS framework gives insight into the breathing behaviour of the interpenetrated structure and how it is affected upon uptake and exchange of guest molecules. All guest and solvent molecules were removed from the DFT optimised CS structures before running single-point energy and geometry optimisation calculations to find the intramolecular strain energy of the framework, ΔEMOF, as described in section 2.3. Calculated ΔEMOF values are reported per minimum framework unit ((ZnX2)3(tpt)2, 81 atoms) and can be found in Table 1. Structure information and cell volume changes are found in Table S5.†A CS structure containing only solvent was included to understand the impact of a non-interacting guest on the framework. The ZnI2 structure (1, LABMOT) was chosen due to the inclusion of cyclohexane, present in many structures in this study. The structure has a calculated ΔEMOF of 16.06 kJ mol−1 with only a slight increase in cell volume of 0.62% after all solvent molecules are removed, demonstrating that weakly interacting solvent in the pores accounts for some of the induced framework strain, but do not significantly distort the structure.
The CS frameworks have a calculated ΔEMOF of up to 21 kJ mol−1 per minimum unit, and only 7 out of 16 structures studied have a framework strain higher than the solvent-only structure. ΔEMOF is on the same order of magnitude as for single molecules, indicating that breathing of the framework does not come at a large energetic penalty. Furthermore, guest exchange does not cause significant amounts of distortion in the framework: the change in cell volume between the optimised experimental structure and the optimised empty framework is typically less than 3%. This exhibits the breathing capabilities of the framework that occurs during the thermodynamic guest exchange process, and does not appear to correlate with framework strain (Fig. S6†).
The system including dapsone in the ZnI2 framework (10) is an outlier in the subset. The guest molecule in the experimental structure is clearly defined, with no constraints or restraints applied, and there is no mention of unusual observations relating to the framework by the authors.28 The framework, however, has a calculated ΔEMOF = 100.95 kJ mol−1, an order of magnitude greater than typical values. In addition, the cell volume contracts by 15.51%, much greater than all other structures. A typical CS structure has a unit cell volume of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–16
000–16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Å3 whereas 10 has a volume of 12
000 Å3 whereas 10 has a volume of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Å3. The high framework strain could result from strong interactions between the guest molecule and the framework, but would require separate detailed studies to understand fully. Comparisons of the structure before and after re-optimisation without guests and solvent can be found in Fig. S6–S8 in the ESI.†
000 Å3. The high framework strain could result from strong interactions between the guest molecule and the framework, but would require separate detailed studies to understand fully. Comparisons of the structure before and after re-optimisation without guests and solvent can be found in Fig. S6–S8 in the ESI.†
Insight into the effect of changing the halide ion within the framework can be gained from comparison of ZnCl2 and ZnBr2 analogues containing the same guest molecule. The experimental structures 17 (ZnCl2) and 18 (ZnBr2) containing 1R-(−)-menthyl acetate are included in this study. The 1R-(−)-menthyl acetate guest molecules are present in the same exchange sites in both structures, with additional presence of one and two CHCl3 solvent molecules, respectively. Calculated framework strain values show a large difference between each system: ΔEavMOF = 3.41 kJ mol−1 for ZnBr2, with an overall cell volume increase of 0.05%, and ΔEavMOF = 20.7 kJ mol−1 for ZnCl2, with an overall cell volume reduction of 6.27%. The ZnCl2 structure also has an overall smaller experimental unit cell size than the ZnBr2 structure (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 720.4 Å3versus 16
720.4 Å3versus 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 176.6 Å3). It is clear that guest exchange causes greater distortion of the ZnCl2 framework due to smaller overall pore volumes, resulting in higher framework strain.
176.6 Å3). It is clear that guest exchange causes greater distortion of the ZnCl2 framework due to smaller overall pore volumes, resulting in higher framework strain.
The average framework strain for the ZnCl2 systems (ΔEavMOF = 16.74 kJ mol−1) is larger than for the ZnI2 systems (ΔEavMOF = 11.41 kJ mol−1).‡ There are several factors which could have caused this: the size of the guest molecules in the pores, and the overall unit cell and therefore pore size. In this study, the average molecular weight of guest molecules in ZnCl2 structures (296.8 g mol−1) is larger than in ZnI2 structures (245.0 g mol−1). The average unit cell sizes of ZnCl2 systems is also larger than ZnI2 systems, at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 335.1 Å3 and 15
335.1 Å3 and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 793.9 Å3, respectively. While the increased strain in the ZnCl2 frameworks could simply be due to the larger molecules present in the pores, accounting for the average unit cell sizes of the ZnCl2 and ZnI2 structures indicates that the higher ΔEavMOF of ZnCl2 systems is affected by a combination of factors. The reduced pore size of the ZnCl2 system results in greater distortion of the framework to accommodate the overall larger guest molecule size, ultimately leading to higher framework strain.
793.9 Å3, respectively. While the increased strain in the ZnCl2 frameworks could simply be due to the larger molecules present in the pores, accounting for the average unit cell sizes of the ZnCl2 and ZnI2 structures indicates that the higher ΔEavMOF of ZnCl2 systems is affected by a combination of factors. The reduced pore size of the ZnCl2 system results in greater distortion of the framework to accommodate the overall larger guest molecule size, ultimately leading to higher framework strain.
3.6 What is the effect of multiple guests in the pore?
It is not uncommon for guest molecules to soak into several exchange sites within the pores of the CS. There are 8 CS structures in this study which feature multiple guests in the asymmetric unit of the unit cell. Energetic differences between guest molecules in the same CS structure are observed, resulting from molecules experiencing different interactions in each exchange site. For all but one CS structure with multiple guests, ΔΔEtotal is below 5 kJ mol−1 (Table 1), but is much larger, ΔΔEtotal = 11.68 kJ mol−1, in the CS structure of vanillin (3).The intermolecular interactions experienced by the molecule, resulting from differences in pore environment, can have a profound effect on ΔEstrain and ΔEconf. To understand how molecular geometry is affected by multiple guests present in the pore, we can look at structures 8 and 9. Each structure has two guest molecules: guest A with high ΔEstrain and low ΔEconf, and guest B with low ΔEstrain and high ΔEconf (see Table 1). The high ΔEstrain of guest A and high ΔEconf of guest B results from distortion that breaks the intramolecular hydrogen bond within the molecule; in the case of guest A, it is simply intramolecular strain/distortion, whereas for guest B, the complete change in geometry to a conformation with no intramolecular hydrogen bonding is energetically costly (see Fig. 7). The guest with high ΔEstrain in each structure relaxes to a local conformational minimum stabilised by intramolecular hydrogen bonding. The number and type of interactions present in these two structures were recently discussed in a systematic study.27 In structure 8, interactions between guest A and the framework seem to be dominated by polar O–Ar interactions, while guest B experiences mostly aromatic Ar–Ar, in addition to the long-range dispersion interactions present in the pore environment. Guest–guest interactions between A and B are also observed, which will contribute to the overall molecular strain and conformational changes from the lowest energy conformer.
The experimental CS structure of risperidone in the ZnI2 framework (14) contains two guest molecules in the asymmetric unit and enables a quantification of the effect of multiple guests in the pore. Guest B has an increased induced intramolecular strain compared to guest A (ΔEstrain = 18.39 kJ mol−1 and 12.46 kJ mol−1, respectively), which is most likely due to experiencing stronger interactions in its exchange site. Configurations where only one molecule of risperidone is present have also been considered to investigate the interactions between guests. The calculated ΔEconf of guest A and B is the same in all configurations, regardless of the total number of guests present. When considering only guest B in the pore, the strain energy is unaffected: the calculated ΔEstrain of 18.13 kJ mol−1 in configuration 3 (see Table S2†) is just 0.26 kJ mol−1 less than configuration 6 (Table 1), where both guests A and B are present, corresponding to a small RMS deviation in atomic positions of 0.505 Å. When considering only guest A in the pore, however, the conformer in configuration 2 has a calculated ΔEstrain of 8.26 kJ mol−1. Compared to configurations where both guests are present the RMS deviation of atomic positions is small (0.166 Å), but an increase in ΔEstrain of 4.20 kJ mol−1 is observed. While there are no guest–guest close contacts in the experimental and optimised structures, the increase in intramolecular strain energy of guest A when guest B is present demonstrates the influence of long-range interactions between molecules in the pores, which could be direct long-ranged interactions (e.g. electrostatics) between guests, or indirect interactions via the influence of guest molecules on solvent molecules.
It is clear from these examples that the number of interactions a guest experiences can have a pronounced effect on the intramolecular strain energy of the guest molecules when there are multiple guests in the pore.
4 Conclusions
This computational study provides important insights into the influence of the crystalline sponge (CS) environment on guest molecule conformation, which should inform the interpretation and use of molecular geometries that are obtained from CS structures.Guest molecules in CS structures, particularly larger molecules with several rotatable bonds, almost always experience some distortion away from their most stable gas phase geometries; total relative energies, ΔEtotal, calculated as the difference between the geometry in the CS structure and the global energy minimum of the isolated molecule, are frequently small (below 5 kJ mol−1 in a third of structures studied here), but range up to 41 kJ mol−1. Partitioning these conformational energetics into the conformer energy of the nearest gas phase conformer to the geometry in the CS structure, and strain energy of molecule (distortion away from the nearest local minimum), provides additional insight into the conformational effects of the CS environment on guest molecules.
The largest energetic effects (highest ΔEtotal) usually correspond to breaking of strong non-bonded intramolecular interactions, such as intramolecular hydrogen bonds or stabilising interactions from folding of long, flexible molecules, that are present in the most stable geometry of the isolated molecule. In the absence of such effects, molecules in CS structures usually adopt a geometry that is energetically close to one of their lowest energy conformers, although this is often not the lowest possible conformer of the isolated molecule.
By comparing the conformations of guest molecules in CS structures with those in pure, close-packed crystal structures and co-crystals of the same molecules, we find that the molecular geometries from CS structures have a lower total relative energy, and lower molecular strain, than those found in conventional crystal structures. These results suggest that CS structures represent a more “neutral” environment for studying the geometry of organic molecules than conventional crystal structures, providing a closer representation of molecules' intrinsically preferred geometries.
Data availability
The data supporting this article have been included as part of the ESI.† Further data, including optimised versions of crystal structures and conformers are available at https://www.pure.soton.ac.uk at [https://doi.org/10.5258/SOTON/D3370].Author contributions
E. M. Soper: methodology, investigation, analysis, writing. S. J. Coles: supervision, analysis, writing. G. M. Day: conceptualisation, supervision, methodology, analysis, writing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank R. C. Carroll for his help with interpreting experimental CS structures. We thank the EPSRC for studentship funding (EP/T157859/1). The authors acknowledge the use of the IRIDIS High Performance Computing Facility, and associated support services at the University of Southampton, in the completion of this work. Via our membership of the UK's HEC Materials Chemistry Consortium, which is funded by EPSRC (EP/R029431), this work also used the ARCHER2 UK National Supercomputing Service (https://www.archer2.ac.uk).References
- Y. Inokuma, S. Yoshioka, J. Ariyoshi, T. Arai, Y. Hitora, K. Takada, S. Matsunaga, K. Rissanen and M. Fujita, Nature, 2013, 495, 461–466 CrossRef CAS PubMed.
- N. Zigon, V. Duplan, N. Wada and M. Fujita, Angew. Chem., Int. Ed., 2021, 60, 25204–25222 CrossRef CAS PubMed.
- M. Hoshino, A. Khutia, H. Xing, Y. Inokuma and M. Fujita, IUCrJ, 2016, 3, 139–151 CrossRef CAS PubMed.
- J. P. Metherall, R. C. Carroll, S. J. Coles, M. J. Hall and M. R. Probert, Chem. Soc. Rev., 2023, 52, 1995–2010 RSC.
- M. Ernst, J. D. Evans and G. Gryn'ova, Chem. Phys. Rev., 2023, 4, 041303 CrossRef.
- F.-X. Coudert and A. H. Fuchs, Coord. Chem. Rev., 2016, 307, 211–236 CrossRef CAS.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC.
- M. Ernst and G. Gryn'ova, ChemPhysChem, 2022, 23, e202200098 CrossRef CAS PubMed.
- M. Ernst and G. Gryn'ova, Helv. Chim. Acta, 2023, 106, e202300013 CrossRef CAS.
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Crystallogr., Sect. B, 2016, 72, 171–179 CrossRef CAS PubMed.
- T. R. Ramadhar, S.-L. Zheng, Y.-S. Chen and J. Clardy, Chem. Commun., 2015, 51, 11252–11255 RSC.
- T. R. Ramadhar, S.-L. Zheng, Y.-S. Chen and J. Clardy, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 46–58 CrossRef CAS.
- L. M. Hayes, N. J. Press, D. A. Tocher and C. J. Carmalt, Cryst. Growth Des., 2017, 17, 858–863 CrossRef CAS.
- T. R. Ramadhar, S.-L. Zheng, Y.-S. Chen and J. Clardy, CrystEngComm, 2017, 19, 4528–4534 RSC.
- G. W. Waldhart, N. P. Mankad and B. D. Santarsiero, Org. Lett., 2016, 18, 6112–6115 CrossRef CAS PubMed.
- F. Habib, D. A. Tocher and C. J. Carmalt, Mater. Today: Proc., 2022, 56, 3766–3773 Search PubMed.
- A. D. Cardenal and T. R. Ramadhar, CrystEngComm, 2021, 23, 7570–7575 RSC.
- R. Dovesi, A. Erba, R. Orlando, C. M. Zicovich-Wilson, B. Civalleri, L. Maschio, M. Rérat, S. Casassa, J. Baima, S. Salustro and B. Kirtman, WIREs Comput. Mol. Sci., 2018, 8, e1360 CrossRef.
- J. Laun, D. V. Oliveira and T. Bredow, J. Comput. Chem., 2018, 39, 1285–1290 CrossRef CAS PubMed.
- M. F. Peintinger, D. V. Oliveira and T. Bredow, J. Comput. Chem., 2013, 34, 451–459 CrossRef CAS PubMed.
- B. Civalleri, C. M. Zicovich-Wilson, L. Valenzano and P. Ugliengo, CrystEngComm, 2008, 10, 405–410 RSC.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- J. G. Brandenburg, M. Alessio, B. Civalleri, M. F. Peintinger, T. Bredow and S. Grimme, J. Phys. Chem. A, 2013, 117, 9282–9292 CrossRef CAS PubMed.
- P. Pracht, F. Bohle and S. Grimme, Phys. Chem. Chem. Phys., 2020, 22, 7169–7192 RSC.
- F. Sakurai, A. Khutia, T. Kikuchi and M. Fujita, Chem. – Eur. J., 2017, 23, 15035–15040 CrossRef CAS PubMed.
- P. Chauhan, S. Javed, D. C. Levendis and M. Fernandes, J. Mol. Struct., 2022, 1251, 132054 CrossRef CAS.
- R. C. Carroll, D. C. Harrowven, J. E. Pearce and S. J. Coles, IUCrJ, 2023, 10, 497–508 CrossRef CAS PubMed.
- L. Rosenberger, C. von Essen, A. Khutia, C. Kühn, K. Georgi, A. K. Hirsch, R. W. Hartmann and L. Badolo, Eur. J. Pharm. Sci., 2021, 164, 105884 CrossRef CAS PubMed.
- S. Hoshino, T. Mitsuhashi, T. Kikuchi, C. P. Wong, H. Morita, T. Awakawa, M. Fujita and I. Abe, Org. Lett., 2019, 21, 6519–6522 CrossRef CAS PubMed.
- H. P. G. Thompson and G. M. Day, Chem. Sci., 2014, 5, 3173–3182 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of crystal and molecular geometry optimisations, conformational searches, calculated strain, conformational, and total relative energies for all molecules, correlation between strain energy and molecular geometry changes. See DOI: https://doi.org/10.1039/d4ce00699b |
| ‡ We have excluded structures 10 and 16 from averaging values due to their unusually high respective framework and guest energies. |
| This journal is © The Royal Society of Chemistry 2025 |