Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis, structure, and stability of a novel 2H-azirine under pressure†
Alexa
Cabrera
 a,
Fatemah
Safari
a,
Fatemah
Safari
 b,
Ravhi
Kumar
b,
Ravhi
Kumar
 b,
Haoran
Zhu
b,
Haoran
Zhu
 a,
Muhtar
Ahart
a,
Muhtar
Ahart
 b,
Zhenxian
Liu
b,
Zhenxian
Liu
 b,
Tom G.
Driver
b,
Tom G.
Driver
 a and
Russell J.
Hemley
a and
Russell J.
Hemley
 abc
abc
aDepartment of Chemistry, University of Illinois Chicago, Chicago, IL 60607, USA. E-mail: rhemley@uic.edu
bDepartment of Physics, University of Illinois Chicago, Chicago, IL 60607, USA
cDepartment of Earth and Environmental Sciences, University of Illinois Chicago, Chicago, IL 60607, USA
First published on 5th February 2025
Abstract
We have synthesized 2,3-diphenyl-2H-azirine, a strained unsaturated heterocyclic compound, and examined its high-pressure behavior to above 10 GPa using diamond anvil cell techniques. Single crystal X-ray diffraction at ambient conditions reveals that the crystal structure is hexagonal and consists of interesting helices surrounding voids in the structure. A continuous shift of the Raman and infrared vibrational spectra with increasing pressure is observed, indicating that the molecular structure is preserved to at least 8 GPa at room temperature. High-pressure synchrotron powder X-ray diffraction shows that the hexagonal structure persists, with a smooth compression of the a and c parameters to 10 GPa. The structural stability under pressure is attributed to the reduction in void size within the helical framework despite the inherent strain and reactivity of the molecule. The channels in the structure could encapsulate small molecules for gas or energy storage applications.
Introduction
2H-Azirines represent a unique class of organic compounds characterized by their remarkable strain and unsaturated three-membered ring structure housing a pivotal nitrogen atom. The ring strain of 2H-azirines imbues them with significant reactivity (ring strain calculated to be 44–47 kcal mol−1).1,2 The inherent strain in 2H-azirines renders them intriguing subjects of inquiry and endows them with exceptional reactivity, facilitating diverse transformations that potentially lead to complex organic architectures.3Scheme 1 shows the changes 2,3-diphenyl-2H-azirine could undergo. Photolysis of 2,3-diphenyl-2H-azirine 1 produces an irreversible ring opening through cleavage of the C–C bond to generate a nitrile ylide 2, and the direct observation of this reactive intermediate has been reported.4–6 The resulting nitrile ylide can dimerize to afford tetraphenylpyrazine 3 or undergo cycloaddition with a range of dipolarophiles (X![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Y) to afford pyrroline derivatives 4.6–8 In contrast, thermolysis of 2H-azirines produces vinyl nitrene 5 through heterolysis of the C–N bond. This intermediate reacts with proximal C–H bonds to produce 2-substituted indoles 6.9–11 This reactivity enables them to be positioned strategically as synthetic intermediates to facilitate the efficient production of a wide range of organic compounds with diverse applications across pharmaceuticals, agrochemicals, materials, and fine chemicals industries.3,9,12–21
Y) to afford pyrroline derivatives 4.6–8 In contrast, thermolysis of 2H-azirines produces vinyl nitrene 5 through heterolysis of the C–N bond. This intermediate reacts with proximal C–H bonds to produce 2-substituted indoles 6.9–11 This reactivity enables them to be positioned strategically as synthetic intermediates to facilitate the efficient production of a wide range of organic compounds with diverse applications across pharmaceuticals, agrochemicals, materials, and fine chemicals industries.3,9,12–21
 | ||
| Scheme 1 Potential reactivity of 2H-azirines induced by light, temperature, and high pressure, the latter leading to possible high-energy density materials (HEDMs). | ||
Armed with new high-pressure tools, we wished to examine the stability and potential reactivity of 2,3-diphenyl-2H-azirine under pressure to determine at what/if any pressure triggers C–N or C–C bond cleavage and if this reactivity could be harnessed to generate novel nitrogen-rich high energy density materials (HEDMs).
Investigating the crystal and molecular structure of organic compounds over a range of thermodynamic conditions helps gain a deeper insight into their physical and chemical properties.22,23 Subjecting molecular crystals to high pressures can dramatically affect crystal structures, reactivity, solubility, and melting behavior, even over relatively modest conditions up to several to tens of gigapascals.24–26 The materials can be probed in situ with diamond-anvil cells using various techniques, most notably single-crystal and powder diffraction coupled with vibrational spectroscopies.27–35 Furthermore, accurately determining pressure–volume (P–V) equations of state (EOS) can provide detailed information on compression mechanisms and thermodynamic properties.36,37
Despite this variety of organic compounds that have been examined under pressure, the behavior of 2H-azirines has yet to be examined. In this study, we present an investigation of compound 2,3-diphenyl-2H-azirine, which features a strained nitrogen-ring structure. We synthesized the 2H-azirine, determined its structure under ambient conditions, and assessed its high-pressure stability, structure, and vibrational properties using Raman spectroscopy, infrared spectroscopy, and X-ray diffraction up to 20 GPa. Rather than reacting or breaking down to known more stable molecules or transforming denser HEDMs, the structure persists to remarkably high compression. We attribute the stability of the material under pressure to the unusual spiral structure with voids that collapse. The findings have important implications for the high-pressure synthesis and properties of other HEDMs.
Results
Synthesis and structure
2,3-Diphenyl-2H-azirine was synthesized from phenyl benzyl ketone and sodium acetate (Fig. S1†).38 Pressure was calibrated using the ruby-fluorescence method.39 Single-crystal X-ray diffraction techniques at ambient conditions were used to reveal the atomic position of the compound. Crystal structure refinement yielded a hexagonal structure with lattice parameters a = 18.596(5) Å, c = 5.704(2) Å. 2,3-Diphenyl-2H-azirine is a nonlinear molecule with C1 point group symmetry (Table 1). Due to the molecule's chirality, the crystal structure has a 6-fold translational symmetry that lacks a mirror plane, inversion center, and no rotational or reflection symmetry (P65 space group). The stacked crystallographic layers can be visualized by plotting the unit cell. The strained molecules show the interaction of the hydrogen bonds as the diphenyls are in close proximity. Surprisingly, the helices of 2,3 diphenyl-2H-azirine molecules are not connected by hydrogen bonds, and voids are formed in the corners of the unit cell (Fig. 1 and S2†).Raman spectroscopy
Raman spectra were measured as a function of pressure to examine compressional effects on the molecular stability, lattice and internal molecular vibrations, and possible pressure-induced phase transitions or chemical reactions in the solid state. The 2,3-diphenyl-2H-azirine molecule consists of 26 atoms, which gives rise to 72 vibrational modes that correspond to its C1 point group, all of which are Raman-active. The P65 space group of the crystal structure gives rise to 432 zone-center vibrations, of which 387 are Raman-active (77A + 77E1 + 78E2).Raman spectra were measured under pressure in several different runs. A 785 nm laser was used for these measurements because of the intense fluorescence of the sample with shorter wavelength laser lines (Fig. S3†).40,41 A gradual increase in background fluorescence was observed with pressure using the 785 nm laser excitation (Fig. S4†). Nevertheless, well-defined Raman bands could be measured up to 8.3 GPa (Fig. 2a), including prominent modes initially at 149 and 615 cm−1 (azirine group twist and anti-symmetric stretch), ∼997 and 1602 cm−1 (phenyl stretch and scissoring), 1747 cm−1 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch). Peaks at lower frequencies were also observed but broadened on compression (Fig. S5†). Quantum calculations of the vibrational modes of the isolated molecules were used to guide the analysis. Specifically, we used the B3LYP/6-31G method in Gaussian 09 (ref. 42) to calculate the frequencies and displacements of the vibrational modes of the isolated molecule (Fig. S6†). From the predicted intensities, the 26 observed bands were tentatively assigned (Fig. 2b).
C stretch). Peaks at lower frequencies were also observed but broadened on compression (Fig. S5†). Quantum calculations of the vibrational modes of the isolated molecules were used to guide the analysis. Specifically, we used the B3LYP/6-31G method in Gaussian 09 (ref. 42) to calculate the frequencies and displacements of the vibrational modes of the isolated molecule (Fig. S6†). From the predicted intensities, the 26 observed bands were tentatively assigned (Fig. 2b).
Infrared spectroscopy
High-pressure infrared spectroscopy was also used to provide information on molecular stability and possible pressure-induced changes in the material. In particular, both far-IR (50–700 cm−1) and mid- to near-IR (700–5000 cm−1) spectra were measured using synchrotron infrared techniques up to 20 GPa. Given the C1 point group symmetry, all vibrational modes are both IR and Raman-active for the isolated molecule. To measure the 0.1 MPa spectrum, the sample was compressed as a thin pellet in which all absorption peaks could be identified without interference from diamond absorption (Fig. S7†). The high-pressure spectra reveal a substantial number of peaks indicating stability of the molecule up to at least 10 GPa (Fig. 3a). High-frequency peaks are observed that are readily identified as combination bands arising from the excitation of IR- and Raman-active modes at 4042 cm−1, 4343 cm−1, 4443 cm−1, 4527 cm−1, 4613 cm−1, and 4657 cm−1 (Table S1†). For example, the IR-active ν2 vibration is observed at 3083 cm−1, and ν4 appears in Raman at 1268 cm−1. The 4343 cm−1 peak can thus be identified as a combination band of the ν2 and ν4 fundamentals (Table S1†). These positions of the combination bands confirm the identification and assignments of lower frequency Raman and IR modes.High-pressure X-ray diffraction
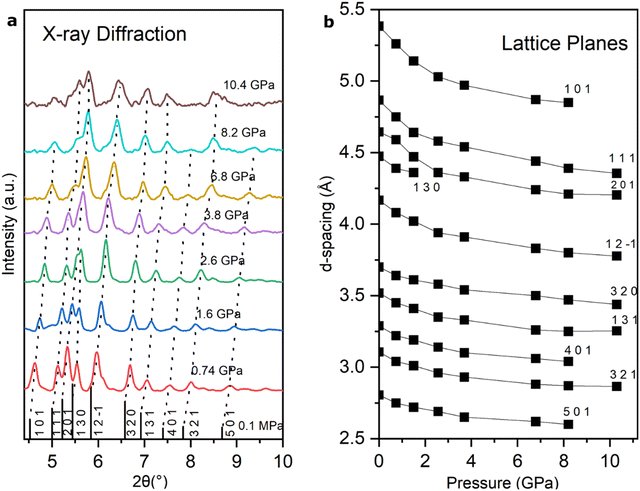
High-pressure powder X-ray diffraction was also measured to further elucidate the diffraction and spectroscopic changes discussed above. The 2,3-diphenyl-2H-azirine was compressed up to 10.4 GPa with neon as a medium to investigate possible pressure-induced structural and chemical changes directly (Fig. S8 and S9†). The unit cell parameters obtained from the powder diffraction data agree with those obtained from the single crystal refinements. The evolution of X-ray diffraction patterns at various pressures is shown in Fig. 4a. The observed diffraction patterns were readily indexed to a hexagonal unit cell at each pressure. The diffraction peaks systematically shift with pressure with no evident splitting but some peak broadening is observed at the highest pressures. The d-spacings determined from the X-ray patterns are shown in Fig. 4b.The hexagonal unit-cell parameters a and c obtained from the analysis are shown in Fig. 5. There is a smooth compression of the hexagonal unit cell up to 10 GPa with no evidence of pressure-induced phase transitions or chemical reactions over the range of conditions explored.
 | ||
| Fig. 5 Unit-cell parameters as a function of pressure for 2,3-diphenyl-2H-azirine measured by X-ray diffraction. The error bars are within the size of the data points. | ||
Equation of state
The pressure–volume (P–V) data obtained from the X-ray diffraction were fitted to a third-order Vinet EOS (Fig. 6).43 A Vinet equation of state fit of the entire pressure–volume data with a zero-pressure reference volume V0 = 1708 Å3 gives a bulk modulus K0 and pressure derivative of 6.2(1.6) GPa and 15.6(2.4), respectively. However, a single EOS fit does not accurately fit the data at lower pressure, introducing too much curvature at low compression. A superior fit of the data was obtained by dividing the data into two EOS regimes. A fit to the data from ambient pressure to 37 GPa gave K0 = 10.6(1.2) GPa and
of 6.2(1.6) GPa and 15.6(2.4), respectively. However, a single EOS fit does not accurately fit the data at lower pressure, introducing too much curvature at low compression. A superior fit of the data was obtained by dividing the data into two EOS regimes. A fit to the data from ambient pressure to 37 GPa gave K0 = 10.6(1.2) GPa and  . This analysis provides a much better fit to the lower pressure data as well as a more reasonable
. This analysis provides a much better fit to the lower pressure data as well as a more reasonable  . The comparison suggests a slight change in the compression mechanism near 3.7 GPa. The fit to the higher-pressure data from 3.7 to 10.5 GPa gave K0 = 3.9(2.4) GPa and
. The comparison suggests a slight change in the compression mechanism near 3.7 GPa. The fit to the higher-pressure data from 3.7 to 10.5 GPa gave K0 = 3.9(2.4) GPa and  . Despite the possible change in compression mechanism, the X-ray diffraction data reveal the persistence of hexagonal unit cell over the entire pressure range explored.
. Despite the possible change in compression mechanism, the X-ray diffraction data reveal the persistence of hexagonal unit cell over the entire pressure range explored.
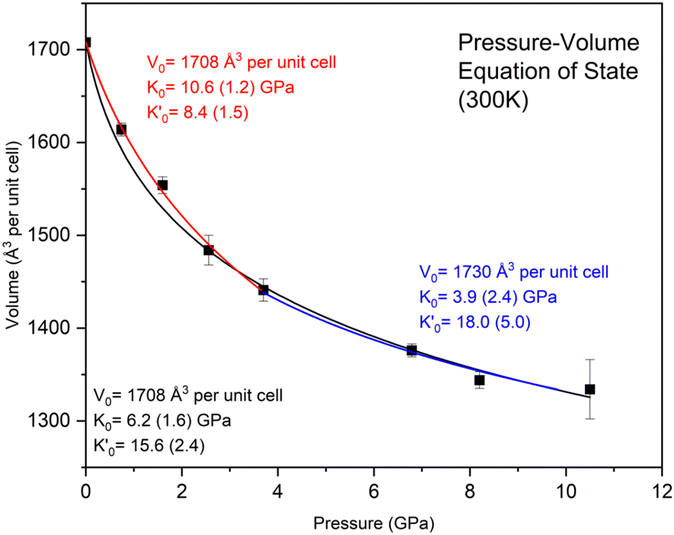 | ||
| Fig. 6 P–V relations of 2,3-diphenyl-2H-azirine obtained from the X-ray diffraction. The black solid line shows a Vinet EOS fit of the entire data set, while the red line indicates the fit from ambient to 3.7 GPa, and the blue line shows the fit from 3.7 GPa to 10.5 GPa. The P–V data are listed in Table S2.† | ||
Discussion
We first discuss the stability of the molecule and molecular crystal structure on compression, beginning with the crucial evidence provided by the high-pressure Raman and IR spectra. Distinct features characteristic of intact azirine molecules persist to comparable pressures using both techniques; i.e., at least 8 GPa in Raman and 9.2 GPa in IR. Measurements of the Raman spectra were complicated at higher pressure by background fluorescence, such that only faint vibrational peaks could be observed beginning at 12 GPa. In the IR, vibrational peaks were apparent at 20 GPa (although broadened at higher pressures). Spectra were also measured on decompression of the samples. Decompression from 12 GPa to ambient pressure revealed no visible azirine peaks due to the persistence of fluorescence (Fig. S10†). On the other hand, measurement of the IR spectrum on decompression from these pressures shows the persistence of azirine vibrations (Fig. S11†).The in situ high-pressure X-ray diffraction measurements show that the compound persists in a hexagonal structure up to 10 GPa, the maximum pressure of these experiments. At that pressure, the unit-cell volume is reduced by 22%, which is a notable degree of compression. The ambient pressure structure refined by single-crystal X-ray diffraction is characterized by the presence of voids within the structure, as noted above. We attribute the reduction in the volume under pressure without a phase transformation or chemical reaction to the shrinkage of the voids during compression.
Additional insight is gained by further examination of the crystal structure. The molecular arrangements in the unit cell show that adjacent molecules are perpendicular to each other, with C4B–H4B on top of C3A–H3A at a distance of 3.16 Å (Fig. 1b). Vibrational modes associated with the short C4B–H4B and C3A–H3A distances are expected to have a strong pressure dependence. We suggest that the vibrational bands with stronger pressure shifts are associated with displacements of the atoms mentioned above.
We compare our results with those of related molecular crystals under pressure. Comparing the parameters to other similar aromatic compounds can serve as an observation for compression behavior when analyzing the potential changes in the crystal structure.44,45 The aromatic heterocyclic compound 2-phenylindole contains a pyrrole ring fused to a benzene ring.46 The vibrational spectrum of indole was examined (Fig. S12b†) to compare with our results for the azirine (Fig. S12a†) and, in particular, to examine the evidence for azirine chemically transforming under pressure (Fig. S12c†). Notably, the azirine and indole have distinct spectra at ambient pressure. With increasing pressure, none of the peaks measured for the azirine matched those of the indole.
We also compare our results with those obtained for 4-hydroxycyanobenzene (4HCB), which has one phenyl group and thus potentially similar π–π and H-bonding interactions.47 Notably the bulk moduli are close, with K0 = 9.7(2) GPa for 4HCB compared to the best-fit K0 = 10.6(1.2) GPa for the 2H-azirine. On the other hand, 4HCB undergoes two phase transitions over a similar pressure range, in contrast to the 2H-azirine, perhaps associated with the additional diphenyl group in the latter. Another related compound is paracetamol, which contains a phenyl group; it has two known polymorphs, monoclinic (form I) and orthorhombic (form II), with similar bulk moduli.48 Like the azirine, no phase transition or chemical transformation was observed on compression of paracetamol (up to 4 GPa). These organic compounds give insight into the stability of related molecular structures on compression, with one remaining at its original structure and the other that did not. High-pressure study of such compounds and comparing their EOS allows a better understanding of the crystal packing in these and other compounds.49–54
Conclusions
2,3-Diphenyl-2H-azirine has been successfully synthesized and found to crystallize in a novel hexagonal structure at ambient conditions. High-pressure Raman and infrared spectroscopy, together with high-pressure X-ray diffraction, reveal that the molecules and crystal structure remain stable up to the 10 GPa pressure range at room temperature. Despite the compound's inherent reactivity and molecular instability, this unexpected stability can be attributed to the unique crystal structure, which accommodates compression by reducing voids around the helical units in the hexagonal lattice. On the other hand, analysis of the P–V relation suggests a change in compression mechanism within the pressure range studied. Further study, including measurements at variable temperatures and high pressures, would provide additional information on the interatomic and intermolecular interactions as well as chemical reactions of the compound under varying thermodynamic conditions. In addition, the voids in the novel structure of the azirine could encapsulate small molecules for gas or energy storage applications. The results highlight how specific crystal structures can allow enhanced stability under pressure of molecules that may otherwise be considered to be highly chemically unstable.Data availability
Crystallographic data supporting this article have been uploaded to CCDC as deposition #2380981.Author contributions
T. D. and H. Z. synthesized the 2,3-diphenyl-2H-azirine and the 2-phenylindole. A. C. and F. S. performed the single-crystal XRD. A. C. and R. K. conducted the high-pressure XRD experiments. A. C. measured all the Raman spectra with the help of M. A. A. C. and Z. L. performed the IR measurements. R. J. H. supervised the project. All authors contributed to the preparation of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Extreme EnErgy Density (EXEED), an Army HBCU/MI Center of Excellence at the University of Illinois Chicago, under grant #W911NF2110275 from the Army Research Office, and by the U.S. Department of Energy-National Nuclear Security Administration (DOE-NNSA) cooperative agreement DE-NA-0004153 (Chicago/DOE Alliance Center, CDAC). Synchrotron X-ray experiments were performed at HPCAT (Sector 16) Advanced Photon Source (APS), Argonne National Laboratory (ANL). HPCAT operations are supported by DOE-NNSA's Office of Experimental Sciences. The APS is a DOE Office of Science User Facility operated for the DOE Office of Science by ANL under contract DE-AC02-06CH11357. Synchrotron infrared experiments were performed at the Frontier Infrared Spectroscopy (FIS) at the National Synchrotron Light Source II (NSLS-II), Brookhaven National Laboratory (BNL). FIS is supported by DOE-NNSA (CDAC) and NSF cooperative agreement EAR-2223273 (Synchrotron Earth and Environmental Science, SEES). The NSLS-II is a DOE Office of Science User Facility operated by the DOE Office of Science by BNL under contract DE-SC0012704.Notes and references
- S. Calvo-Losada, J. Comput. Chem., 1998, 19, 912–922 CrossRef CAS.
- A. Rey Planells and A. Espinosa Ferao, Inorg. Chem., 2022, 61, 6459–6468 CrossRef CAS PubMed.
- A. F. Khlebnikov, M. S. Novikov and N. V. Rostovskii, Tetrahedron, 2019, 75, 2555–2624 CrossRef CAS.
- A. Padwa, M. Dharan, J. Smolanoff and S. Wetmore, Pure Appl. Chem., 1973, 33, 269–284 CrossRef CAS.
- P. Claus, Th. Doppler, N. Gakis, M. Georgarakis, H. Giezendanner, P. Gilgen, H. Heimgartner, B. Jackson, M. Märky, N. S. Narasimhan, H. J. Rosenkranz, A. Wunderli, H. Hanse and H. Schmid, Pure Appl. Chem., 1973, 33, 339–362 CrossRef CAS.
- A. Padwa, S. Clough, M. Dharan, J. Smolanoff and S. I. Wetmore, J. Am. Chem. Soc., 1972, 94, 1395–1397 CrossRef CAS.
- A. Padwa, M. Dharan, J. Smolanoff and S. Wetmore, J. Am. Chem. Soc., 1973, 95, 1954–1961 CrossRef CAS.
- A. Padwa, J. Smolanoff and A. Tremper, J. Am. Chem. Soc., 1975, 97, 4682–4691 CrossRef CAS.
- L. A. Wendling and R. G. Bergman, J. Org. Chem., 1976, 41, 831–836 CrossRef CAS.
- D. F. Taber and W. Tian, J. Am. Chem. Soc., 2006, 128, 1058–1059 CrossRef CAS PubMed.
- X. Li, Y. Du, Z. Liang, Y. Pan and K. Zhao, Org. Lett., 2009, 11, 2643–2646 CrossRef CAS PubMed.
- P. A. Sakharov, M. S. Novikov and N. V. Rostovskii, Chem. Heterocycl. Comp., 2021, 57, 512–521 CrossRef CAS.
- E. Babaoglu and G. Hilt, Chem. – Eur. J., 2020, 26, 8879–8884 CrossRef CAS PubMed.
- C. K. Skipper, D. S. Dalisa and T. F. Molinski, Bioorg. Med. Chem. Lett., 2010, 20, 2029–2032 CrossRef PubMed.
- C. K. Skepper, D. S. Dalisay and T. F. Molinski, Org. Lett., 2008, 10, 5269–5271 CrossRef CAS.
- J. L. Keffer, A. Plaza and C. A. Bewley, Org. Lett., 2009, 11, 1087–1090 CrossRef CAS.
- A. Padwa, Adv. Heterocycl. Chem., 2010, 99, 1–31 CrossRef CAS.
- E. Orton, S. T. Collins and G. C. Pimentel, J. Phys. Chem., 1986, 90, 6139–6143 CrossRef CAS.
- L. A. Wendling and R. G. Bergman, J. Am. Chem. Soc., 1974, 96, 308–309 CrossRef CAS.
- M. J. Alves and F. Texeira e Costa, in Heterocyclic Targets in Advanced Organic Synthesis, ed. M. Carreiras and J. Marco-Contelles, Research Signpost, Kerala, India, 2011, vol. 37(661), pp. 145–172 Search PubMed.
- G. S. Singh, M. D'hooghe and N. De Kimpe, Chem. Rev., 2007, 107, 2080–2135 CrossRef CAS.
- G. R. Desiraju, J. Am. Chem. Soc., 2013, 135, 9952–9967 CrossRef CAS PubMed.
- S. J. Maginn and G. R. Desiraju, J. Appl. Crystallogr., 1991, 24, 265 CrossRef.
- E. Boldyreva, Z. Kristallogr. - Cryst. Mater., 2014, 229, 236–245 CAS.
- A. Healy, Z. Worku, D. Kumar and A. Madi, Adv. Drug Delivery Rev., 2017, 117, 25–46 CrossRef CAS PubMed.
- J. A. Ciezak, T. A. Jenkins, Z. Liu and R. J. Hemley, J. Phys. Chem. A, 2007, 111, 59–63 CrossRef CAS PubMed.
- E. V. Boldyreva, J. Mol. Struct., 2003, 647, 159–179 CrossRef CAS.
- E. V. Boldyreva, Cryst. Eng., 2003, 6, 235–254 CrossRef CAS.
- E. V. Boldyreva, H. Ahsbahs and H. P. Weber, Z. Kristallogr. - Cryst. Mater., 2003, 218, 231–236 CrossRef CAS.
- E. V. Boldyreva, T. P. Shakhtshneider, H. Ahsbahs, H. Sowa and H. Uchtmann, J. Therm. Anal. Calorim., 2002, 68, 437–452 CrossRef CAS.
- E. V. Boldyreva, V. A. Drebushchak, I. E. Paukov, Y. A. Kovalevskaya and T. N. Drebushchak, J. Therm. Anal. Calorim., 2004, 77, 607–623 CrossRef CAS.
- A. Katrusiak, Acta Crystallogr., Sect. B, 1995, 51, 873–879 CrossRef.
- A. Katrusiak, Acta Crystallogr., Sect. B, 1990, 46, 246–256 CrossRef.
- A. Katrusiak, High Pressure Res., 1991, 6, 265–275 CrossRef.
- A. Botteon, M. Vermeulen, L. Cristina, S. Bruni, P. Matousek, C. Miliani, M. Realini, L. Angelova and C. Conti, Anal. Chem., 2024, 96, 4535–4543 CrossRef CAS.
- E. Stavrou, M. R. Manaa, J. M. Zaug, I. Kuo, P. F. Pagoria, B. Kalkan, J. C. Crowhurst and M. R. Armstrong, J. Chem. Phys., 2015, 143, 144506 CrossRef PubMed.
- F. J. Zerilli and M. M. Kuklja, J. Phys. Chem. A, 2007, 111, 1721–1725 CrossRef CAS PubMed.
- Y. Wang, X. Lei and Y. Tang, Chem. Commun., 2015, 51, 4507–4510 RSC.
- H. Mao, J. Xu and P. M. Bell, J. Geophys. Res., B, 1986, 91, 4673–4676 CrossRef CAS.
- E. P. Huang, E. Huang, S. Yu, Y. Chen and J. Lee, Mater. Lett., 2010, 64, 580–582 CrossRef CAS.
- G. Qi, K. Wang, K. Yang and B. Zou, J. Phys. Chem. C, 2016, 120, 21293–21298 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- P. Vinet, J. Ferrante, J. H. Rose and J. R. Smith, J. Geophys. Res., B, 1987, 92, 9319–9325 CrossRef CAS.
- O. Franco, G. Reck, I. Orgzall and B. Schulz, J. Phys. Chem. Solids, 2002, 63, 1805–1813 CrossRef CAS.
- C. Weidenthaler, T. J. Frankcombe and M. Felderhoff, Inorg. Chem., 2006, 45, 3849–3851 CrossRef CAS.
- S. S. El-Nakkady, M. M. Hanna, H. M. Roaiah and I. A. Ghannam, Eur. J. Med. Chem., 2012, 47, 387–398 CrossRef CAS PubMed.
- I. E. Collings and M. Hanfland, Molecules, 2019, 24, 1759 CrossRef.
- E. V. Boldyreva, H. Sowa, H. Ahsbahs, S. V. Goryainov, V. V. Chernyshev, V. P. Dmitriev, Y. V. Seryotkin, E. N. Kolesnik, T. P. Shakhtshneide, S. N. Ivashevskaya and T. N. Drebushchak, J. Phys.: Conf. Ser., 2008, 121, 022023 CrossRef.
- Agilent, CrysAlisPro Software System (1.171.36.28), Agilent Technologies UK Ltd, Oxford, UK, 2013 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- C. F. Macrae, P. R. Edgington, P. McCabe, E. Pidcock, G. P. Shields, R. Taylor, M. Towler and J. Van de Streek, J. Appl. Crystallogr., 2006, 39, 453–457 CrossRef CAS.
- J. Gonzalez-Platas, M. Alvaro, F. Nestola and R. Angel, J. Appl. Crystallogr., 2016, 49, 1377–1382 CrossRef.
- G. Novak and A. Colville, Am. Mineral., 1989, 74, 488–490 Search PubMed.
- R. J. Hemley and P. Dera, Rev. Mineral. Geochem., 2001, 41, 335–419 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: CCDC 2380981. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ce00937a |
| This journal is © The Royal Society of Chemistry 2025 |