Open Access Article
Open Access ArticleC(sp3)–H functionalization of N-protected dialkylpyrrole derivatives with azodicarboxylates
Jing
Guo†
 *a,
Maying
Yan†
*a,
Maying
Yan†
 a,
Lei
Xiao
a,
Lei
Xiao
 a,
Jiajie
Li
a,
Jiajie
Li
 a,
Zheng-wang
Qu
a,
Zheng-wang
Qu
 *b,
Stefan
Grimme
*b,
Stefan
Grimme
 b and
Douglas W.
Stephan
b and
Douglas W.
Stephan
 *c
*c
aInstitute of Drug Discovery Technology and Qian Xuesen Collaborative Research Center of Astrochemistry and Space Life Sciences, Ningbo University, Ningbo 315211, Zhejiang, China. E-mail: guojing@nbu.edu.cn
bMulliken Center for Theoretical Chemistry, Clausius Institut für Physikalische und Theoretische Chemie, Rheinische Friedrich-Wilhelms-Universität Bonn, Beringstrasse 4, 53115 Bonn, Germany. E-mail: qu@thch.uni-bonn.de
cDepartment of Chemistry, University of Toronto, Toronto, 80 St. George Street, Ontario M5S 3H6, Canada. E-mail: douglas.stephan@utoronto.ca
First published on 30th July 2025
Abstract
A metal-free, catalytic route to the activation of C(sp3)–H bonds in N-protected dialkylpyrroles to diazodicarboxylates is reported using HB(C6F5)2 as the optimized catalyst. These reactions tolerate aryl and alkyl substituents on the pyrrole N-atom as well as variation in the azodicarboxylates giving rise to 41 examples. These reactions were also performed on a gram scale and conversion to the corresponding amino-esters is demonstrated. A DFT computation study reveals that the Lewis acid adduct of azodicarboxylates generates a Lewis acidic N-atom capable of hydride abstraction from dimethylpyrrole, ultimately effecting C(sp3)–H functionalization.
Pyrrole is a privileged aromatic heterocycle that is found in chlorophyll, heme, vitamin B12, and bile acids. Naturally occurring and synthetic molecules incorporating pyrrole units have been shown to exhibit a broad range of biological and pharmacological activities.1 For example, over 20 synthetic pyrrole derivatives are commercially marketed drugs as such species exhibit anti-psychotic, anti-anxiolytic, anti-cancer, anti-bacterial, anti-fungal, anti-malarial, anti-inflammatory, and anti-hyperlipidemic behavior (Fig. 1a).1a,1b
Synthetic efforts to derivatize pyrroles have led to the development of a wide variety of transition metal catalyzed processes.2 These protocols allow the incorporation of a wide range of functional groups, typically leading to substitution at N or at the C(sp2) atoms at the C-2 or C-3 positions. These methods have been reviewed.2b–e Alternatively main group species are known to mediate the derivatization of N-protected pyrroles.3 For example, Lewis acid mediated Friedel–Crafts methods readily provide substitution again at sp2 carbons, where the steric demands of the N-protecting group can be used to direct substitution to either the C-2 or C-3 positions.3
In 2010, we showed that N-alkylpyrroles participate in frustrated Lewis pair (FLP) alkyne-addition reactions, leading to C–C bond formation at the C(sp2) atoms at the C-3 position.4 Several years later, in a seminal finding, Fontaine and coworkers5 exploited intramolecular N/B FLPs to effect borylation of N-methylpyrrole, thiophene and furan derivatives, again at the C-2 or C-3 positions depending on the other substituents (Fig. 1b). Subsequently, Shi and coworkers used BBr3 to direct C(sp2)–H borylation of indoles at the C-7 or C-4 positions and other (hetero)arenes.6 More recently, Tan et al. elegantly used chiral phosphoric acid catalysts to functionalize N-protected-pyrroles at the C-3 position, affording axially chiral arylpyrroles (Fig. 1b).7
In contemplating alternative strategies for the functionalization of pyrroles, we considered activation of the C(sp3)–H bonds of substituents at the C-2 position. While the majority of the activation strategies for unactivated C(sp3)–H bonds have been achieved using transition-metal catalysts,8 we noted that boranes have been used as catalysts to promote C–C and C–heteroatom bond formation.9 For example, Wang and coworkers achieved C(sp3)–H alkylation of tertiary amines with electron-deficient olefins using B(C6F5)3 as the catalyst.10 In another recent breakthrough, Lin et al.11 used frustrated radical pairs (FRPs) to functionalize the C(sp3)–H bonds of various organic substrates, affording aminoxylated products. Nonetheless, to our knowledge, C(sp3)–H bond activation of pyrrole derivatives is not known. Herein, we develop a protocol for the C(sp3)–H bond functionalization of the methyl groups of N-protected methylpyrroles with azodicarboxylates using Piers’ borane HB(C6F5)2 as the catalyst (Fig. 1c).
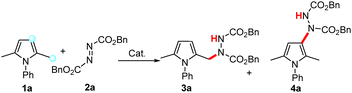
Our investigation began with the reaction of N-phenylpyrrole 1a and commercially available dibenzyl azodicarboxylate 2a in toluene. In the presence of 10 mol% Al(C6F5)3, this gave mixtures of C(sp2)–H and C(sp3)–H activation products, with poor regioselectivity at 60 °C (Table 1, entry 1). In the presence of B(C6F5)3 and HB(C6F5)2, the selectivity for the C(sp3)–H functionalization product 3a improved (Table 1, entries 2 and 3). Using HB(C6F5)2 as the catalyst, product 3a was obtained in 47% yield at 60 °C. Altering the reactant ratio of 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a to 1.5
2a to 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 afforded product 3a in 61% yield with a 13% yield of the C(sp2)–H functionalization product 4a (Table 1, entries 3–6), while further variations of the solvent, temperature and catalyst loading did not increase the yield (Table 1, entries 7–12).
1 afforded product 3a in 61% yield with a 13% yield of the C(sp2)–H functionalization product 4a (Table 1, entries 3–6), while further variations of the solvent, temperature and catalyst loading did not increase the yield (Table 1, entries 7–12).
| Entrya | Cat. |
1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a 2a |
Solvent | T (°C) | Yield (%) 3ab | Yield (%) 4ab |
|---|---|---|---|---|---|---|
| a Unless otherwise noted, all reactions were performed using 10 mol% catalyst, 1a and 2a in solvent (2.0 mL) under argon for 12 h. b Isolated yield after chromatography. c 5 mol% catalyst. | ||||||
| 1 | Al(C6F5)3 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
Toluene | 60 | 21 | 22 |
| 2 | B(C6F5)3 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
Toluene | 60 | 43 | 6 |
| 3 | HB(C6F5)2 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
Toluene | 60 | 47 | 6 |
| 4 | HB(C6F5)2 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
Toluene | 60 | 47 | 12 |
| 5 | HB(C6F5)2 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
Toluene | 60 | 61 | 13 |
| 6 | HB(C6F5)2 | 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
Toluene | 60 | 55 | 13 |
| 7 | HB(C6F5)2 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
Benzene | 60 | 57 | 14 |
| 8 | HB(C6F5)2 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
p-Xylene | 60 | 52 | 10 |
| 9 | HB(C6F5)2 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
PhF | 60 | 39 | 19 |
| 10 | HB(C6F5)2 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
Toluene | 45 | 47 | 11 |
| 11 | HB(C6F5)2 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
Toluene | 80 | 52 | 12 |
| 12c | HB(C6F5)2 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
Toluene | 60 | 23 | 26 |
Using the optimized reaction conditions, the substrate scope for C(sp3)–H functionalization of N-protected dialkylpyrroles with azodicarboxylates was examined. Firstly, the reactions of a series of N-arylpyrroles with 2a in toluene were investigated. In the presence of 10 mol% HB(C6F5)2, para-substituted N-arylpyrroles with electron-donating or electron-withdrawing substituents on the phenyl ring (Fig. 2, R = C6H51a, 4-FC6H41b, 4-ClC6H41c, 4-BrC6H41d, 4-IC6H41e, 4-CF3C6H41f, 4-MeOC6H41g, 4-MeC6H41h) reacted smoothly with 2a to provide the corresponding products 3a–3h in 49–68% yields. Similarly, meta-substituted N-arylpyrroles bearing differing functional groups (Fig. 2, R = 3-FC6H41i, 3-MeOC6H41j, 3-MeC6H41k) were tolerated, affording 3i–3k in 48–65% yields. ortho-Substituted N-arylpyrroles (Fig. 2, R = 2-FC6H41l, 2-ClC6H41m, 2-BrC6H41n, 2-IC6H41o, 2-CF3C6H41p, 2-MeC6H41q, 2-iPrC6H41r, 2-tBuC6H41s) were also suitable substrates, affording the C(sp3)–H functionalization products 3l–3s in 30–64% yields. The identity of 3s was confirmed by X-ray crystallography (see the SI).12
In addition, N-arylpyrroles with 2 or 3 substituents on the phenyl ring (Fig. 2, R = 2-I,3-ClC6H31t, 2-I,4-ClC6H31u, 2-I,5-ClC6H31v, 2-I,5-FC6H31w, 2-I,5-BrC6H31x, 2,5-(CF3)2C6H31y, 3,5-(CF3)2C6H31z, 3,5-Cl2C6H31aa, 3,5-Br2C6H31ab, 3,4,5-Cl3C6H21ac) were also successfully converted to the C(sp3)–H functionalized products 3t–3ac in 56–82% yields. The reactions of N-benzylpyrroles (Fig. 2, R = CH2Ph 1ad, CH2C6H4Me 1ae, CH2C6H4Cl 1af) provided products 3ad–3af in 51–60% yields. The nature of 3ad was also confirmed by X-ray analysis (see the SI).12 Moreover, alkylpyrroles (Fig. 2, R = Me 1ag, C3H51ah, C6H111ai) also reacted with 2a, giving the products 3ag–3ai, albeit in somewhat reduced yields of 27–35%. Furthermore, changing the pyrrole substituents to Et groups, as in 1aj, afforded 3aj in 50% yield, while the reaction of the dissymmetric pyrrole 2-Me-5-Ph-N-(C6H2Cl3)-pyrrole 1ak with 2a gave the C(sp2)–H amination product 4ak in 55% yield (see the SI).
Efforts to identify by-products were undertaken. Even on doubling the reaction scale for all reactions, most by-products were not unambiguously identifiable although the C(sp2)–H amination products 4ah and 4ai were observed in 10 and 11% yields, respectively, while the double C(sp2)–H amination product 4ag′ was observed in 9% yield (see the SI).
The reaction also tolerated variations in the azodicarboxylates. Thus, the reaction of the commercially available (RO2CN)2 (Fig. 3, R = CH2C6H4Cl 2b, Et 2c, iPr 2d) with 1ac proceeded smoothly to give products 3ak–3am in 43–83% yields. In contrast, the use of 2e (R = tBu) gave only a 19% yield of 3an (Fig. 3). We note that this diazo-species is known to react with boranes to liberate CO2 and isobutylene.13 Notably, 4-phenyl-1,2,4-triazoline-3,5-dione 2f also reacted smoothly with 1ac, affording the desired product 3ao in 40% yield.
The scalability of this protocol to gram-scale reactions was demonstrated. Thus, using over 2 grams of 1ac with 2a in the presence of 10 mol% HB(C6F5)2 afforded 2.35 g of 3ac in an overall yield of 82% (Fig. 4). In addition, 3ac was reacted with bromoacetate and Cs2CO3 using the method of Magnus et al.,14 affording C4H2Me(CH2NH(CO2CH2Ph)N(C6H2Cl3) 5 in 70% yield (Fig. 4). This carbamate ester was characterized by X-ray crystallography (see the SI).12
The mechanism of these reactions was established via a computational study using density functional theory (DFT) computations at the PW6B95-D3/def2-QZVP + COSMO-RS//TPSS-D3/def2-TZVP + COSMO level of theory.15 As B(C6F5)3 and HB(C6H5)2 showed similar reactivity, B(C6F5)3 was used in the calculations to avoid the complexity associated with the dimerization equilibrium of Piers’ borane in solution. The initial interaction of B(C6F5)3 with the carbonyl fragment of azodicarboxylate 2c enhances the Lewis acidity of the remote N-atom, allowing it to abstract hydride from methylpyrrole 1a over a free energy barrier of 22.5 kcal mol−1 (TS1). This generates the transient ion pair 1a+ and 2cBH− (Fig. 5), which reacts exothermically to form a new C–N bond. The release of borane is slightly endergonic, allowing the catalytic reaction to continue, consistent with both experimental conditions and the improved catalysis for (C6F5)2BH, where the slightly reduced Lewis acidity presumably accelerates the Lewis acid release. Regarding the role of the N-Lewis acid, we note that such species have been pioneered by Gandelman,16 although we17 and others18 have described related diazo-derived Lewis acid systems.
 | ||
| Fig. 5 DFT computed mechanism (in kcal mol−1, at 298 K and 1 M concentration) for C(sp3)–H functionalization of N-Ph-dimethylpyrrole to azodicarboxylates. | ||
In conclusion, we have reported a metal-free, catalytic and scalable protocol for the functionalization of C(sp3)–H bonds in N-protected dimethylpyrroles with azodicarboxylates. The resulting diazo-pyrrole derivatives can be converted to the corresponding amino-esters. A mechanistic study showed that the Lewis acid adduct of the azodicarboxylate generates a Lewis acidic N-atom capable of hydride abstraction from the C(sp3) carbon on the pyrrole. We are continuing to study metal-free avenues for C–H functionalization and Lewis acid applications in organic synthesis.
The authors thank the Ningbo Natural Science Foundation (No. 2023J379) and the Ningbo Top Talent Project (No. 215-432094250) for financial support. Z.-W. Q. and S. G. are grateful to the Deutsche Forschungsgemeinschaft (project 490737079). We also acknowledge the Analysis Center of Institute of Drug Discovery Technology for collecting spectral data.
Conflicts of interest
There are no conflicts to declare.Data availability
The data that support this study are available in the SI of this article.Experimental and spectral data are available as SI See DOI: https://doi.org/10.1039/d5cc03254g
3s: CCDC 2434837; 3ad: CCDC 2434838 and 5: CCDC 2434839 contain the supplementary crystallographic data for this paper.19–21
Notes and references
- (a) B. H. Ganesh, A. G. Raj, B. Aruchamy, P. Nanjan, C. Drago and P. Ramani, ChemMedChem, 2024, 19, e202300447 CrossRef PubMed; (b) T. Biswas, R. K. Mittal, V. Sharma, Kanupriya and I. Mishra, Med. Chem., 2024, 20, 369–384 CrossRef PubMed; (c) B. S. Manya, M. R. P. Kumar, K. Rajagopal, A. M. Hassan, S. O. Rab, M. A. Alshehri and T. B. Emran, Chem. Biodiversity, 2024, 21, e202400534 CrossRef PubMed.
- (a) A. M. Wagner and M. S. Sanford, Org. Lett., 2011, 13, 288–291 Search PubMed; (b) M. K. Hunjan, S. Panday, A. Gupta, J. Bhaumik, P. Das and J. K. Laha, Chem. Rec., 2021, 21, 715–780 Search PubMed; (c) K. Pedretty, K. Tillett, W. Tsuei and J. M. Lopchuk, Prog. Heterocycl. Chem., 2021, vol. 32, pp. 193–240 Search PubMed; (d) B. Prabagar and Z. Shi, Recent Advances in C–H Functionalization of Five-Membered Heterocycles with Single Heteroatoms, John Wiley & Sons, Inc., 2023 Search PubMed; (e) J. W. Campbell, M. J. Cotnam, F. R. Annan, J. W. Hilborn and A. Thompson, Chem. Commun., 2024, 60, 11385–11414 Search PubMed.
- S. Rej and N. Chatani, Angew. Chem., Int. Ed., 2022, 61, e202209539 CrossRef PubMed.
- M. A. Dureen, C. C. Brown and D. W. Stephan, Organometallics, 2010, 29, 6422–6432 CrossRef.
- (a) M. A. Legare, M. A. Courtemanche, E. Rochette and F. G. Fontaine, Science, 2015, 349, 513–516 CrossRef CAS PubMed; (b) S. K. Bose and T. B. Marder, Science, 2015, 349, 473–474 CrossRef CAS PubMed; (c) J. Legare Lavergne, A. Jayaraman, L. C. Misal Castro, E. Rochette and F.-G. Fontaine, J. Am. Chem. Soc., 2017, 139, 14714–14723 CrossRef CAS PubMed.
- J. Lv, X. Chen, X.-S. Xue, B. Zhao, Y. Liang, M. Wang, L. Jin, Y. Yuan, Y. Han, Y. Zhao, Y. Lu, J. Zhao, W.-Y. Sun, K. N. Houk and Z. Shi, Nature, 2019, 575, 336–340 CrossRef CAS.
- L. Zhang, S. H. Xiang, J. J. Wang, J. Xiao, J. Q. Wang and B. Tan, Nat. Commun., 2019, 10, 566 CrossRef PubMed.
- (a) J. He, M. Wasa, K. S. L. Chan, Q. Shao and J.-Q. Yu, Chem. Rev., 2016, 117, 8754–8786 CrossRef PubMed; (b) B. Liu, A. M. Romine, C. Z. Rubel, K. M. Engle and B. F. Shi, Chem. Rev., 2021, 121, 14957–15074 CrossRef CAS PubMed; (c) Y. Yang, W. Gao, Y. Wang, X. Wang, F. Cao, T. Shi and Z. Wang, ACS Catal., 2021, 11, 967–984 CrossRef CAS; (d) K. P. Bryliakov, ACS Catal., 2023, 13, 10770–10795 CrossRef CAS; (e) M. Sadeghi, ACS Catal., 2024, 14, 15356–15373 CrossRef CAS.
- (a) S. Basak, L. Winfrey, B. A. Kustiana, R. L. Melen, L. C. Morrill and A. P. Pulis, Chem. Soc. Rev., 2021, 50, 3720–3737 RSC; (b) G. Kumar, S. Roy and I. Chatterjee, Org. Biomol. Chem., 2021, 19, 1230–1267 RSC; (c) A. Dasgupta, E. Richards and R. L. Melen, ACS Catal., 2021, 12, 442–452 CrossRef PubMed; (d) J. Guo, M. Yan and D. W. Stephan, Org. Chem. Front., 2024, 11, 2375–2396 RSC; (e) T. Liu, Org. Chem. Front., 2025, 12, 2481–2498 RSC; (f) B. Rao and R. Kinjo, Chem. – Asian J., 2018, 13, 1279–1292 CrossRef CAS PubMed.
- X.-Y. Zhou, Y.-B. Shao, R.-T. Guo, Y.-L. Zhang, X.-S. Xue and X.-C. Wang, ACS Catal., 2024, 14, 8041–8049 CrossRef CAS.
- Z. Lu, M. Ju, Y. Wang, J. M. Meinhardt, J. I. Martinez Alvarado, E. Villemure, J. A. Terrett and S. Lin, Nature, 2023, 619, 514–520 CrossRef CAS PubMed.
- The X-ray data have been deposited in the CCDC 2434837 (3s), 2434838 (3ad) and 2434839 (5).
- Z. Hussain, Y. A. Luo, Y. Wu, Z. W. Qu, S. Grimme and D. W. Stephan, J. Am. Chem. Soc., 2023, 145, 7101–7106 CrossRef CAS PubMed.
- P. Magnus, N. Garizi, K. A. Seibert and A. Ornholt, Org. Lett., 2009, 11, 5646–5648 CrossRef CAS PubMed.
- (a) A. Klamt and G. Schüürmann, J. Chem. Soc. Perkin Trans. 2, 1993, 799–805 RSC; (b) K. Eichkorn, F. Weigend, O. Treutler and R. Ahlrichs, Theor. Chem. Acc., 1997, 97, 119–124 Search PubMed; (c) F. Weigend, M. Häser, H. Patzelt and R. Ahlrichs, Chem. Phys. Lett., 1998, 294, 143–152 CrossRef CAS; (d) F. Eckert and A. Klamt, AIChE J., 2002, 48, 369–385 CrossRef CAS; (e) J. Tao, J. P. Perdew, V. N. Staroverov and G. E. Scuseria, Phys. Rev. Lett., 2003, 91, 146401 CrossRef PubMed; (f) F. Weigend, F. Furche and R. Ahlrichs, J. Chem. Phys., 2003, 119, 12753–12762 CrossRef CAS; (g) P. Deglmann, K. May, F. Furche and R. Ahlrichs, Chem. Phys. Lett., 2004, 384, 103–107 CrossRef CAS; (h) F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC; (i) Y. Zhao and D. G. Truhlar, J. Phys. Chem. A, 2005, 109, 5656–5667 CrossRef CAS PubMed; (j) F. Weigend, Phys. Chem. Chem. Phys., 2006, 8, 1057–1065 RSC; (k) S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104–154119 CrossRef PubMed; (l) S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed; (m) S. Grimme, Chem. – Eur. J., 2012, 18, 9955–9964 CrossRef CAS PubMed; (n) F. Eckert and A. Klamt, COSMOtherm, Version C3.0, Release 16.01, COSMOlogic GmbH & Co., Leverkusen, Germany, 2015; (o) TURBOMOLE V7.4, 2019, a development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989-2007, TURBOMOLE GmbH, since 2007; available from https://www.turbomole.com., 2019 Search PubMed.
- (a) Y. Tulchinsky, S. Kozuch, P. Saha, M. Botoshansky, L. J. W. Shimon and M. Gandelman, Chem. Sci., 2014, 5, 1305–1311 RSC; (b) Y. Tulchinsky, S. Kozuch, P. Saha, A. Mauda, G. Nisnevich, M. Botoshansky, L. J. W. Shimon and M. Gandelman, Chem. – Eur. J., 2015, 21, 7099–7110 CrossRef CAS PubMed; (c) A. Pogoreltsev, Y. Tulchinsky, N. Fridman and M. Gandelman, J. Am. Chem. Soc., 2017, 139, 4062–4067 CrossRef CAS PubMed; (d) I. Avigdori, A. Pogoreltsev, A. Kaushanski, N. Fridman and M. Gandelman, Angew. Chem., Int. Ed., 2020, 59, 23476–23479 CrossRef CAS PubMed.
- (a) A. E. Waked, R. O. Memar and D. W. Stephan, Angew. Chem., Int. Ed., 2018, 57, 11934–11938 CrossRef CAS PubMed; (b) J. Zhou, L. L. Liu, L. Cao and D. W. Stephan, Chem. Commun., 2018, 54, 4390–4393 RSC; (c) J. L. Zhou, L. L. Liu, L. L. Cao and D. W. Stephan, Angew. Chem., Int. Ed., 2018, 57, 3322–3326 CrossRef CAS PubMed.
- E. Habraken, A. Jupp and J. Slootweg, Synlett, 2019, 875–884 CAS.
- J. Guo, M. Yan, L. Xiao, J. Li, Z.-W. Qu, S. Grimme and D. W. Stephan, CCDC 2434837: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2mqn45.
- J. Guo, M. Yan, L. Xiao, J. Li, Z.-W. Qu, S. Grimme and D. W. Stephan, CCDC 2434838: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2mqn56.
- J. Guo, M. Yan, L. Xiao, J. Li, Z.-W. Qu, S. Grimme and D. W. Stephan, CCDC 2434839: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2mqn67.
Footnote |
| † J. Guo and M. Yan contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |