Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Environment-sensitive fluorescent tryptophan analogues via indole C-2 alkenylation reactions†
Valeria K.
Burianova
,
Liyao
Zeng
,
Abigail W.
Thom
,
Niamh C.
Radcliffe-Kennedy
,
Steven W.
Magennis
 and
Andrew
Sutherland
and
Andrew
Sutherland
 *
*
School of Chemistry, The Joseph Black Building, University of Glasgow, Glasgow G12 8QQ, UK. E-mail: Andrew.Sutherland@glasgow.ac.uk
First published on 26th June 2025
Abstract
A modular synthetic strategy for the preparation of intrinsically fluorescent unnatural α-amino acids by C-2 oxidation and alkenylation of a tryptophan-derived tetrahydro-β-carboline is described. This approach yielded a novel tryptophan–coumarin hybrid with strong environmental sensitivity and compatibility with near-infrared two-photon excitation.
Unnatural α-amino acids are important targets for a range of applications. In synthesis, modified α-amino acids are widely used as chiral ligands, catalysts and starting materials.1,2 In medicinal chemistry and the life sciences, unnatural α-amino acids are used as enzyme inhibitors or as probes to study biological structure and function.3 In this regard, fluorescent unnatural α-amino acids in combination with fluorescence spectroscopy techniques have been used to study biological processes.4,5 This overcomes the limitations of large extrinsic fluorophores that require attachment by chemical spacers and are restricted to the peptide termini or amino acid sidechains. Fluorescent α-amino acids can be inserted into peptides at key positions using solid phase peptide synthesis (SPPS) or genetic encoding allowing more localised reporting.
One approach for the development of novel fluorescent α-amino acids is the modification of proteinogenic α-amino acids.5 With the strongest fluorescence, L-tryptophan (1) has been widely utilised for this purpose (Fig. 1a). In particular, extended conjugation of the indole sidechain has generated fluorophores with improved photophysical properties that have been used for various applications.6 For example, L-4-cyanotryptophan (2), which differs by only two atoms and has a long fluorescence lifetime and good photostability, has been used to visualise HEK cells and evaluate peptide-membrane interactions.7 Incorporation of a BODIPY motif at the C-2 position of L-tryptophan such as adduct 3 has produced compounds that have been used for imaging fungal infection in human tissue, as well as tumor-associated macrophages in aggressive carcinomas.8,9 Pyrazoloquinazoline α-amino acids 4 were designed as rigid, extended analogues of L-tryptophan.10 These were found to be compatible with two-photon excitation and incorporated into cell-penetrating peptides using SPPS. Carbazole α-amino acids 5 have also been reported as highly fluorescent tryptophan mimics and used as probes to measure protein–protein binding.11
 | ||
| Fig. 1 (a) L-Trp and fluorescent analogues. (b) Directed metal-catalysed alkenylation of Trp and associated peptides. (c) This work: alkenylation of a Trp analogue. | ||
As extended conjugation at the C-2 position of the indole ring has led to the discovery of tryptophan fluorophores with enhanced photophysical properties, we were interested in establishing a synthetic approach that would allow the preparation of C-2 alkenyl tryptophan analogues as charge transfer-based fluorophores for biological imaging. Tryptophan compounds bearing a C-2 alkenyl side chain have been reported. Wang and co-workers developed a palladium-catalysed C(sp2)–H olefination of tryptophan using the peptide backbone as a directing group.12 This methodology was used for macrocyclisation and the preparation of cyclic peptides with indole-alkene crosslinks. Zhu and co-workers used an N-heterocycle within the indole sidechain of tryptophan to direct rhodium- (Fig. 1b) or palladium-catalysed C(sp2)–H olefination.13 This reaction was also used as a stapling strategy for the preparation of cyclic peptides. To avoid the use of precious transition metals, the Vendrell and Ackerman groups developed a directed manganese-catalysed C(sp2)–H olefination of tryptophan (Fig. 1b).14 This chemistry was used for the incorporation of BODIPY and nitrobenzodiazole moieties, with the subsequent peptidic probes used for real-time imaging of live cells. To complement this previous methodology and avoid the need for directing groups, we proposed that new fluorescent C-2 alkenyl tryptophan analogues could be prepared using a novel two-step strategy from a readily available tryptophan-derived tetrahydro-β-carboline intermediate. Here, we report the synthesis of C-2 alkenyl tryptophan analogues by selenium dioxide oxidation of a tetrahydro-β-carboline intermediate, followed by alkenylation reactions (Fig. 1c). We also describe the preparation of a tryptophan–coumarin hybrid, which possesses bright environment-sensitive fluorescence and is compatible with two-photon excitation in the near-infrared.
The first stage of this project focused on a short, scalable synthesis of a 2-formyl-L-tryptophan analogue that could be used to investigate subsequent alkenylation reactions. 2-Formyl-tryptophan analogues have been reported via oxidation of the corresponding tetrahydro-β-carboline and thus, an efficient and scalable synthesis of compound 7 was developed (Scheme 1).15–17 Initially, a Pictet–Spengler reaction of L-tryptophan methyl ester (6) using paraformaldehyde and AcOH was performed.18 Following Boc-protection under standard conditions, this gave tetrahydro-β-carboline 7 in 69% overall yield. Oxidation of 7 was initially investigated using persulfate as the oxidant.16 At 40 °C and an overnight reaction, this gave 2-formyl-L-tryptophan 8 in 53% yield. Alternatively, oxidation of 7 using selenium oxide gave 2-formyl-L-tryptophan 8 after 1.5 h, and in an improved 68% yield.15
Following the scalable synthesis of 2-formyl-L-tryptophan 8 (> 2.5 mmol), this compound was examined as a substrate for C-2 olefination reactions (Scheme 1). Our aim was to prepare alkenes with electron-withdrawing groups, which in direct conjugation with the π-excessive indole ring would result in charge transfer-based fluorophores. To access a range of targets using an amino acid-derived aldehyde, the mild conditions of the Masamune–Roush version of the Horner–Wadsworth–Emmons (HWE) reaction was utilised.19 Reaction of 8 with ketone, ester and amide-substituted phosphonate esters, using lithium chloride and DBU gave the corresponding E-alkenes in 57–84% yield. NMR spectroscopy of the reaction mixtures confirmed the presence of only the E-isomers. Only the nitrile-substituted phosphonate ester gave a mixture of E and Z-isomers (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), respectively. These were separable by column chromatography, which afforded the major E-isomer 9e in 47% yield. 2-Formyl-L-tryptophan 8 was also compatible with the conditions of a Wittig reaction. Treatment of 2-formyl-L-tryptophan 8 with (triphenylphosphoranylidene)acetaldehyde at 50 °C gave 9f as the sole isomer in 49% yield.20
2), respectively. These were separable by column chromatography, which afforded the major E-isomer 9e in 47% yield. 2-Formyl-L-tryptophan 8 was also compatible with the conditions of a Wittig reaction. Treatment of 2-formyl-L-tryptophan 8 with (triphenylphosphoranylidene)acetaldehyde at 50 °C gave 9f as the sole isomer in 49% yield.20
On successful alkenylation of 8, it was proposed that incorporation of a more conjugated group via an olefination reaction would result in a target with enhanced fluorescent properties. The coumarin motif is widely utilised as a key component of fluorescent chemosensors, finding application in analytical and materials chemistry, as well as for biological imaging.21 Furthermore, coumarin analogues of tryptophan have been reported. In 2024, Ackermann and co-workers used their pyridine-directed manganese-catalysed C–H alkenylation for the preparation of tryptophan–coumarin conjugates.22 Although the coupling reactions were generally efficient, the alkene products were isolated as E/Z mixtures (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and attempted removal of the indole N-pyridine directing group led to compound decomposition. It was proposed that a Wittig reaction with 2-formyl-L-tryptophan 8 may overcome these issues, generating a single adduct that could be fully characterised as a fluorescent probe. Coumarin–tryptophan hybrid 12 was designed and subsequently prepared in two steps from 8 and the known coumarin phosphonium salt 10 (Scheme 2).23 Room temperature Wittig reaction of 8 with phosphonium salt 10 using K2CO3 and 18-crown-6 gave alkene 11.24 NMR spectroscopy of the crude reaction mixture showed that this process had formed a 1.5
1) and attempted removal of the indole N-pyridine directing group led to compound decomposition. It was proposed that a Wittig reaction with 2-formyl-L-tryptophan 8 may overcome these issues, generating a single adduct that could be fully characterised as a fluorescent probe. Coumarin–tryptophan hybrid 12 was designed and subsequently prepared in two steps from 8 and the known coumarin phosphonium salt 10 (Scheme 2).23 Room temperature Wittig reaction of 8 with phosphonium salt 10 using K2CO3 and 18-crown-6 gave alkene 11.24 NMR spectroscopy of the crude reaction mixture showed that this process had formed a 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of E- and Z-isomers, respectively. Without isolation, the mixture was treated with iodine (20 mol%) and this gave E-isomer 12 as the sole product in 82% yield over the two steps.25 Thus, the use of 8 and the combined olefination-isomerisation process allowed the efficient preparation of a single isomer, without requiring the use or removal of an indole directing group.
1 mixture of E- and Z-isomers, respectively. Without isolation, the mixture was treated with iodine (20 mol%) and this gave E-isomer 12 as the sole product in 82% yield over the two steps.25 Thus, the use of 8 and the combined olefination-isomerisation process allowed the efficient preparation of a single isomer, without requiring the use or removal of an indole directing group.
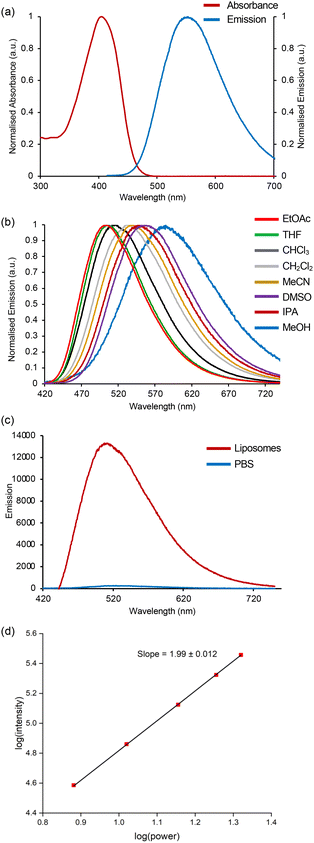
The photophysical properties of α-amino acids 9a–9f and 12 were then measured.26,27 The tryptophan analogues with ketone, aldehyde and ester alkenyl-substituents were found to possess weak emission and therefore not investigated further. Alkenyl tryptophan analogues with amide 9d and nitrile 9e groups were found to have red-shifted absorption and emission maxima compared to L-tryptophan (1), with large molar absorption coefficients and Stokes shifts (Table 1). However, with relatively low quantum yields (0.036–0.063), these compounds were only marginally brighter than tryptophan. In contrast, coumarin analogue 12 was found to possess significantly enhanced photophysical properties. In THF, absorption and emission maxima were found at 400 and 505 nm, respectively and with a quantum yield of 0.52, 12 was found to have a near 17-fold increase in brightness compared to tryptophan. Comparison of the fluorescence properties of 12 with amino acids bearing only coumarin side-chains highlights the advantage of using the coumarin motif in conjugation with the indole ring.28
| Amino acid | λ Abs (nm) | ε (× 104 cm−1 M−1) | λ Em (nm) | Φ F | Brightness (× 103 cm−1 M−1) |
|---|---|---|---|---|---|
| a Spectra were recorded in THF (5 μM). Quantum yields (ΦF) were determined using anthracene (9d and 9e) or diphenylanthracene (12) as the standard. b DMSO was used as the solvent (5 μM). | |||||
| 1 | 279 | 0.56 | 348 | 0.20 | 1.1 |
| 9d | 346 | 2.91 | 410 | 0.036 | 1.0 |
| 9e | 340 | 3.32 | 425 | 0.063 | 2.1 |
| 12 | 400 | 3.71 | 505 | 0.52 | 19 |
| 12 | 406 | 4.06 | 560 | 0.48 | 19 |
As these compounds were designed as charge transfer-based fluorophores, a solvatochromic study was conducted using amino acid 12. Across a range of solvents, the absorption spectra showed minimal variation, indicating negligible ground-state charge transfer between the indole and coumarin moieties. In contrast, the emission maxima were highly solvent dependent, with more polar environments inducing red-shifted emission. For example, the emission maximum was found at 505 nm in ethyl acetate, shifting to 560 nm in DMSO (Table 1 and Fig. 2a) and 585 nm in methanol (Fig. 2b). This pronounced solvatochromism confirms the charge-transfer character of the indole-coumarin system in the excited state, which is stabilised in polar solvents.
To further characterise the solvatochromic behaviour of amino acid 12, photophysical studies were performed in water. Interestingly, a significant suppression of emission was observed. This is noteworthy as amino acids that exhibit strong emission in lipophilic media but weak fluorescence in aqueous solutions have been developed as probes for imaging biological membranes.8,9,14a To evaluate the potential of 12 as a lipophilic probe, its emission in phosphate-buffered saline (PBS) was compared to phospholipid bilayer membranes designed to mimic biological environments (Fig. 2c). In PBS, 12 displayed weak emission, likely due to aggregate-induced quenching. In contrast, strong fluorescence was observed in liposomes (phosphatidylcholine/cholesterol, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1),8 with a 48-fold increase in intensity. These findings suggest that upon accumulation in lipid environments, 12 adopts a membrane-bound form that is highly emissive.
1),8 with a 48-fold increase in intensity. These findings suggest that upon accumulation in lipid environments, 12 adopts a membrane-bound form that is highly emissive.
Based on these interesting results, the photophysical properties of amino acid 12 were further investigated. A pH study demonstrated that 12 is relatively insensitive to pH changes with only a 20% reduction in emission intensity between neutral and basic pH.26 The time-resolved fluorescence in both THF and DMSO after one-photon excitation followed mono-exponential kinetics with lifetimes of 4.05 ns and 4.02 ns, respectively, mirroring the similar quantum yields in the same solvents (Table 1).26 The application of 12 in DMSO for two-photon excited fluorescence was then assessed. Two-photon fluorescence spectroscopy with near-infrared laser excitation has emerged as an important technique for biomedical research as it allows deeper tissue penetration, 3D control of excitation and a reduction in photobleaching.29 Two-photon excitation of amino acid 12 at 800 nm using a Ti:sapphire laser produced an emission spectrum with the same profile as obtained from one-photon excitation at 405 nm (see ESI†). Two-photon excitation was confirmed via a log–log plot of fluorescence intensity versus power, which exhibited a slope of 1.99 (Fig. 2d). The two-photon cross section of 12 was then measured at 800 nm and found to be 81 GM. This was used to calculate the two-photon brightness (σ2ΦF) for 12, which was determined as 39 GM. This represents a substantial increase in two-photon brightness compared to the recently reported thiazoloindole amino acids (σ2ΦF = 14–15 GM).6d This study represents a comprehensive analysis of the photophysical properties of amino acid 12 demonstrating its potential as a lipophilic sensitive, one- and two-photon fluorescent probe for biological imaging. Although, amino acids and peptides have previously been labelled with coumarin for imaging applications,3012 represents a rare example of the direct conjugation of a coumarin motif to an existing fluorescent natural side chain, with the combination producing enhanced optical properties.
In summary, we have developed a scalable synthesis of a 2-formyl-L-tryptophan intermediate, enabling the novel preparation of C-2 alkenyl tryptophans via olefination reactions. This modular strategy facilitated the discovery of new fluorescent probes, including a directly conjugated coumarin–tryptophan adduct. One- and two-photon fluorescence studies demonstrated the potential of amino acid 12 as a bright, solvatochromic probe suitable for biological imaging applications. Ongoing work is focused on assessing 12 for peptide synthesis and evaluating the resulting peptide-based probes for applications in analysing lipid-rich environments.
Financial support from EPSRC (EP/S029168/1, PhD studentships to V. K. B. and A. W. T., EP/T517895/1) and the University of Glasgow is gratefully acknowledged.
Conflicts of interest
There are no conflicts to declare.Data availability
Experimental procedures, characterisation and photophysical data, as well as NMR spectra are available in the ESI.†Notes and references
- (a) Chemistry and Biochemistry of the Amino Acids, ed. G. C. Barrett, Chapman and Hall, London, 1985 Search PubMed; (b) R. Breslow, J. Chmielewski, D. Foley, B. Johnson, N. Kumabe, M. Varney and R. Mehra, Tetrahedron, 1988, 44, 5515 CrossRef CAS.
- (a) C. Nájera and J. M. Sansano, Chem. Rev., 2007, 107, 4584 CrossRef PubMed; (b) R. Smits, C. D. Cadicamo, K. Burger and B. Koksch, Chem. Soc. Rev., 2008, 37, 1727 RSC; (c) M. Mortensen, R. Husmann, E. Veri and C. Bolm, Chem. Soc. Rev., 2009, 38, 1002 RSC; (d) A. F. M. Noisier and M. A. Brimble, Chem. Rev., 2014, 114, 8775 CrossRef CAS PubMed.
- (a) C. T. Walsh, R. V. O’Brien and C. Khosla, Angew. Chem., Int. Ed., 2013, 52, 7098 CrossRef CAS PubMed; (b) K. Lang and J. W. Chin, Chem. Rev., 2014, 114, 4764 CrossRef CAS PubMed.
- (a) R. W. Sinkeldam, N. J. Greco and Y. Tor, Chem. Rev., 2010, 110, 2579 CrossRef CAS PubMed; (b) H. Singh, K. Tiwari, R. Tiwari, S. K. Pramanik and A. Das, Chem. Rev., 2019, 119, 11718 CrossRef CAS PubMed.
- (a) A. T. Krueger and B. Imperiali, ChemBioChem, 2013, 14, 788 CrossRef CAS PubMed; (b) A. H. Harkiss and A. Sutherland, Org. Biomol. Chem., 2016, 14, 8911 RSC; (c) Z. Cheng, E. Kuru, A. Sachdeva and M. Vendrell, Nat. Rev., 2020, 4, 275 CAS.
- (a) J. Wen, L.-L. Zhu, Q.-W. Bi, Z.-Q. Shen, X.-X. Li, X. Li, Z. Wang and Z. Chen, Chem. – Eur. J., 2014, 20, 974 CrossRef CAS PubMed; (b) T. J. Williams, A. J. Reay, A. C. Whitwood and I. J. S. Fairlamb, Chem. Commun., 2014, 50, 3052 RSC; (c) F. Bartoccini, S. Bartolucci, M. Mari and G. Piersanti, Org. Biomol. Chem., 2016, 14, 10095 RSC; (d) A. C. Dodds, H. G. Sansom, S. W. Magennis and A. Sutherland, Org. Lett., 2023, 25, 8942 CrossRef CAS PubMed.
- (a) M. R. Hilaire, I. A. Ahmed, C.-W. Lin, H. Jo, W. F. DeGrado and F. Gai, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 6005 CrossRef CAS PubMed; (b) K. Zhang, I. A. Ahmed, H. T. Kratochvil, W. F. DeGrado, F. Gai and H. Jo, Chem. Commun., 2019, 55, 5095 RSC.
- L. Mendive-Tapia, C. Zhao, A. R. Akram, S. Preciado, F. Alberico, M. Lee, A. Serrels, N. Kielland, N. D. Read, R. Lavilla and M. Vendrell, Nat. Commun., 2016, 7, 10940 CrossRef CAS PubMed.
- R. Subiros-Funosas, V. C. L. Ho, N. D. Barth, L. Medive-Tapia, M. Pappalardo, X. Barril, R. Ma, C.-B. Zhang, B.-Z. Qian, M. Sintes, O. Ghashghaei, R. Lavilla and M. Vendrell, Chem. Sci., 2020, 11, 1368 RSC.
- J. D. Bell, A. H. Harkiss, D. Nobis, E. Malcolm, A. Knuhtsen, C. R. Wellaway, A. G. Jamieson, S. W. Magennis and A. Sutherland, Chem. Commun., 2020, 56, 1887 RSC.
- R. Clarke, L. Zeng, B. C. Atkinson, M. Kadodwala, A. R. Thomson and A. Sutherland, Chem. Sci., 2024, 15, 5944 RSC.
- Z. Bai, C. Cai, W. Sheng, Y. Ren and H. Wang, Angew. Chem., Int. Ed., 2020, 59, 14686 CrossRef CAS PubMed.
- (a) J. Liu, X. Liu, F. Zhang, J. Qu, H. Sun and Q. A. Zhu, Chem. – Eur. J., 2020, 26, 16122 CrossRef CAS PubMed; (b) J. Liu, P. Wang, W. Zeng, Q. Lu and Q. Zhu, Chem. Commun., 2021, 57, 11661 RSC.
- (a) N. Kaplaneris, J. Son, L. Mendive-Tapia, A. Kopp, N. D. Barth, I. Maksso, M. Vendrell and L. Ackermann, Nat. Commun., 2021, 12, 3389 CrossRef CAS PubMed; (b) T. Oyama, L. Mendive-Tapia, V. Cowell, A. Kopp, M. Vendrell and L. Ackermann, Chem. Sci., 2023, 14, 5728 RSC.
- K. Pulka, D. Feytens, I. Van den Eynde, R. De Wachter, P. Kosson, A. Misicka, A. Lipkowski, N. N. Chung, P. W. Schiller and D. Tourwé, Tetrahedron, 2007, 63, 1459 CrossRef CAS.
- H. Chen, F. Ye, J. Luo and Y. Gao, Org. Lett., 2019, 21, 7475 CrossRef CAS PubMed.
- Y. Ding, L. Shen, K. Liang and C. Xia, J. Org. Chem., 2022, 87, 16644 CrossRef CAS PubMed.
- J. Ye, J. Wu, T. Lv, G. Wu, Y. Gao and H. Chen, Angew. Chem., Int. Ed., 2017, 56, 14968 CrossRef CAS PubMed.
- M. A. Blanchette, W. Choy, J. T. Davis, A. P. Essenfeld, S. Masamune, W. R. Roush and T. Sakai, Tetrahedron Lett., 1984, 25, 2183 CrossRef CAS.
- An example of a Wittig reaction of a 2-formyl tryptophan has recently been reported: S. Cheng, Y. Liang, T. Zhang, M. Chen, J. Li, X. Zhang, S. Luo and Q. Zhu, Angew. Chem., Int. Ed., 2025, 64, e202501582 CrossRef CAS PubMed.
- (a) D. Cao, Z. Liu, P. Verwilst, S. Koo, P. Jangjili, J. S. Kim and W. Lin, Chem. Rev., 2019, 119, 10403 CrossRef CAS PubMed; (b) Y. Fan, Y. Wu, J. Hou, P. Wang, X. Peng and G. Ge, Coord. Chem. Rev., 2023, 480, 215020 CrossRef CAS.
- A. Kopp, T. Oyama and L. Ackermann, Chem. Commun., 2024, 60, 5423 RSC.
- D. Ivanova, V. Kolev, T. Lazarova and E. Padrós, Tetrahedron Lett., 1999, 40, 2645 CrossRef CAS.
- L. Wang, H. Zou, D. Ye and D. Cao, J. Heterocycl. Chem., 2013, 50, 551 CrossRef CAS.
- D. I. Ivanova, S. V. Eremin and V. I. Shvets, Tetrahedron, 1996, 52, 9581 CrossRef CAS.
- See ESI† for all photophysical measurements, data, and individual spectra.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, New York, 3rd edn, 2006 Search PubMed.
- M.-P. Brun, L. Bischoff and C. Garbay, Angew. Chem., Int. Ed., 2004, 43, 3432 CrossRef CAS PubMed.
- (a) H. M. Kim and B. R. Cho, Chem. Rev., 2015, 115, 5014 CrossRef CAS PubMed; (b) L. Wu, J. Liu, P. Li, B. Tang and T. D. James, Chem. Soc. Rev., 2021, 50, 702 RSC.
- J. Luo, R. Uprety, Y. Naro, C. Chou, D. P. Nguyen, J. W. Chin and A. Deiters, J. Am. Chem. Soc., 2014, 136, 15551 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterisation data, photophysical data, and NMR spectra. See DOI: https://doi.org/10.1039/d5cc02114f |
| This journal is © The Royal Society of Chemistry 2025 |