Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Advances in oxychalcogenide materials for hydrogen evolution photocatalysis in aqueous media†
Sandy Al
Bacha
 *abc,
Emma E.
McCabe
*abc,
Emma E.
McCabe
 *c and
Houria
Kabbour
*c and
Houria
Kabbour
 *ad
*ad
aUniv. Lille, CNRS, Centrale Lille, ENSCL, Univ. Artois, UMR 8181 – UCCS – Unité de Catalyse et Chimie du Solide, F-59000 Lille, France. E-mail: sandy.albacha@univ-lille.fr
bSchool of Physical Sciences, University of Kent, Canterbury, Kent CT2 7NH, UK
cDepartment of Physics, Durham University, Durham DH1 3LE, UK. E-mail: emma.mccabe@durham.ac.uk
dCNRS, Institut des Matériaux de Nantes Jean Rouxel – UMR6502, Nantes Université, F-44000 Nantes, France. E-mail: houria.kabbour@cnrs-imn.fr
First published on 8th July 2025
Abstract
The growing demand for sustainable energy solutions has led to significant research in photocatalytic water splitting, a promising approach for clean hydrogen production. Oxychalcogenide materials have emerged as a compelling class of photocatalysts due to their tunable electronic structures, various architectures, and favorable band edge positions for solar water splitting. This review provides a comprehensive analysis of recent advances in oxychalcogenide photocatalysts, emphasizing their structural diversity, composition–property relationships, and key design strategies. We highlight the impact of anion selection, cation contributions, charge carrier dynamics, and material stability on photocatalytic performance. Furthermore, we discuss innovative experimental approaches, such as surface modifications that have been employed to enhance activity. By consolidating existing knowledge and identifying critical parameters for optimization, this review aims to shed light on this class of photocatalysts and help guide the rational design of next-generation oxychalcogenide photocatalysts for efficient and scalable solar hydrogen production.
1. Introduction
Solar energy, the most abundant energy source1–4 can be converted into chemical energy, for instance in the case of water-splitting photocatalysis,5 other photocatalytic reactions6 or photoelectrochemistry,7 and into electrical energy by means of the photovoltaic effect. Photocatalysis has been developing for a long time, with early work on oxides like ZnO that introduced the concept of photo-reduction.8 The observation that TiO2 could catalyse the decomposition of organic materials (under UV light),9 and then catalyse water splitting to give O2 and H210 has motivated research into new and efficient photocatalysts.11–13 A schematic illustration of the photocatalytic reaction process indicating factors that may affect photocatalytic activity is presented in Fig. 1. | ||
| Fig. 1 Schematic illustration of the entire photocatalytic reaction process in a semiconductor photocatalyst. | ||
For photocatalysis, first the semiconductor has to be irradiated by incident photons of energy greater than or equal to the bandgap energy of the material. The absorption of photons will cause the excitation of electrons (e−) from the valence band (VB) to the conduction band (CB) leaving a positive hole (h+) in the valence band. The e−/h+ pairs will separate and migrate to the reaction sites on the surface of the material or recombine. In an ideal photocatalytic reaction, after the adsorption of the molecules (H2O for water-splitting) involved in the reaction to be catalyzed, electrons and holes migrate to reaction sites and take part in reduction and oxidation reactions, respectively. In addition to the desired photocatalytic redox reactions, the e−/h+ pairs could recombine a short time after their separation, which is detrimental to the photocatalytic activity.14,15 This recombination can result from various phenomena and was documented in various reports.16,17 Some intrinsic properties of the photocatalysts such as built-in electrical field or high mobility charge carriers for instance are reported to enhance e−/h+ separation and rapid migration.18 On the other hand, approaches exploiting extrinsic factors, such as heterostructures and p–n junctions, can enhance this e−/h+ separation.
It is therefore, important to develop new and more sustainable strategies that can aid the future energy transition and maintain technological progress. In this context, fuel cells are one such example, as they use renewable sources (oxygen and hydrogen) to give electrical energy.19 Since oxygen is readily available, the challenge is to produce the required hydrogen. To this purpose, solar photocatalytic water splitting is very attractive due to its simplicity: if the photocatalyst in appropriate conditions (solution, co-catalyst) is directly active using only sunlight to efficiently produce renewable hydrogen. Fig. 1 illustrates the process of the water splitting reaction in a typical semiconductor material on the conduction and the valence band level, with a suitable bandgap for the solar spectrum.15,20
However, carrying out solar photocatalytic water splitting imposes constraints on the semiconductor, such as the magnitude of the bandgap (between 1.23 and 3.10 eV, where 1.23 eV is the theoretical minimum bandgap, and 3.10 eV corresponds to the wavelength of the visible light ranging from 400 nm to 700 nm). Secondly, the conduction band potential must be more negative than the reduction potential of H2O/H2 (0 V) and the valence band potential must be more positive potential than the oxidation potential of O2/H2O (1.23 V),21 to satisfy relations (1) and (2):
 | (1) |
| Reduction: 2H+ + 2e− → H2 | (2) |
Oxychalcogenide photocatalytic materials gained considerable attention in recent years.22,23 To understand their photocatalytic properties (and to design optimized oxychalcogenide photocatalysts), it is essential to understand their rich chemistry and structural diversity. Our review focuses on oxychalcogenide photocatalysts with bandgaps well-matched to the solar spectrum and with band edges of appropriate energies for photocatalytical water splitting. We explore compositions and structural features that play key roles in determining the photocatalytic activity of these oxychalcogenides. This understanding of structure–composition–property relationships allow us to propose some strategies to predict and design new oxychalcogenide photocatalysts.
2. Oxychalcogenides for photocatalysis
A mixed-anion compound is a material containing more than one anion in a single phase such as oxychalcogenides, oxypnictides,24 oxyhydrides25 and oxyhalides.26 The coexistence of more than one anion type in the material is a promising strategy to control various properties, giving them a range of applications (photocatalytic activity27–30 superconductivity,31–33 magnetic,34–36 non-linear optic (NLO),37 battery material,38 thermoelectricity39 and photoluminescence40).There are several reviews which highlight the importance of mixed-anion materials in general or for specific applications. Kageyama et al.41 reviewed the great achievements and potential of mixed-anion materials, emphasizing some important factors in designing new materials. The different characters of the anions, such as charge, ionic radii, electronegativity and bonding character, can give rise to anion-order/disorder giving access to new structural types.24 For example, oxynitrides often exhibit correlated disorder instead of a long-range one;42 oxyarsenides show a long-range order of O2− and As3− anions (e.g. iron-based superconductors with insulating oxide layers and more delocalized (even superconducting) iron arsenide layers).43,44
Among mixed-anion materials, layered oxychalcogenides are a promising family that has gained more attention recently. Oxychalcogenides contain an oxide as well as a chalcogenide (sulfide/selenide/telluride) anion. These materials adopt a diversity of structure types and show a range of properties related to the ordering of the small oxide and the larger chalcogenide, likely due to their different sizes and bonding characters.24
Several excellent reviews have explored the structural chemistry of oxychalcogenides and their broader applications. Clarke et al.24 examined the relationships between crystal structures and physical properties, while Orr et al.45 focused on the structural diversity of rare-earth oxychalcogenides. Additional studies have highlighted their thermoelectric, optoelectronic, and infrared nonlinear optical properties.46–48 Reviews on photocatalysis have also discussed mixed-anion materials, including oxychalcogenides, with key contributions from the Domen group49,50 and others covering water-splitting, CO2 reduction,51 and heteroanionic photocatalysts.52,53
While these reviews provide valuable insights, our work offers a timely and dedicated focus on photocatalytic oxychalcogenides for solar water splitting. We consolidate recent advances, highlight critical structure–property relationships, and explore emerging strategies to enhance their photocatalytic performance. To our knowledge, no prior review has specifically addressed this class of materials with such depth in the context of solar-driven hydrogen production. In particular, we emphasize their potential for direct H2 generation under sunlight, excluding more complex PEC systems and composite catalysts that combine sulfides and oxides.
2.1. Structures of oxychalcogenide materials
Oxychalcogenides, containing oxide anions as well as second larger and softer chalcogenide anion (e.g. S2−, Se2−, Te2−), tend to stabilize lower transition metal oxidation states and lower coordination numbers, compared with oxides. As mentioned before, this family of materials show structural diversity, but they often adopt layered structures due to anion order that can lead to segregation of the different anions into different layers.24 This layered arrangement of anions can depend on the relative electronegativities of the accompanying cations.45 For instance, in the case of a significant difference in hardness/softness of cations, stronger bonds can be formed with either hard oxide or soft chalcogenide anions and so layered structures with a greater proportion of homoleptic (single anion) coordinations are favoured;41 whilst in the case of similar hardness/softness of the cations, heteroleptic coordination (mixed anion) can be more favourable.54These oxychalcogenides present some common structural features such as the building blocks that they contain (Fig. 2). For example, OLn4 units in fluorite-like sheets, ribbons or chains (consisting of edge-linked units);45,55,56MQ4 tetrahedra edge-linked into chains or antifluorite-like sheets57 or square-based pyramid MQ5 or MOQ4 units for Bi, Sb with their 6s2 or 5s2 inert pairs.58
Having this layered aspect in most oxychalcogenides can give rise to a wide range of promising properties, due to the potential for chemical substitutions that can be done in both layers; such as highly anisotropic electronic structure and properties and high mobility semiconduction favored by the covalent aspect of the chalcogenide.59 For example, layered LaOCuS60 and BiCuOSe61 are known as important thermoelectrics, due to the presence of the insulating oxide layers and the semiconducting copper chalcogenide layers (see Fig. 2).46 As another example, Ln2O2M2OQ2, with M cations coordinated by both O2− and Q2− anions with long-range magnetic order, but electronically quite separated by the insulating [Ln2O2]2+ layers.62 These materials may have numerous properties such as magnetism,63–65 thermoelectricity48,66 IR-nonlinear optical materials,47,67–70 second harmonic generation (SHG),71–73 semiconductors,74–76 photocatalysts53 and piezoelectricity.77
2.2. Photocatalytic properties and band gap feature of oxychalcogenides
Several examples of oxychalcogenides for photocatalytic water splitting have been reported but to date there has not been a systematic exploration of their properties and features. A survey of the literature helped identify oxychalcogenides and their important characteristics (band gap, semiconduction type, cation choice, coordination environment, anion ratio and polarity); the band edge positions have also been estimated, which allowed the identification of some potential candidates for possible future characterizations (see ESI†). In the following section, the top materials with proven capacity to evolve H2 and/or O2 are discussed. These H2(g) and/or O2(g) evolution experiments have been reported for relatively few oxyselenides. | ||
| Fig. 3 (a) Structural representation of the Ruddlesden–Popper structure of the Ln2Ti2S2O5 series of I4/mmm symmetry; (b) shows apparent quantum yield (AQY) for overall water splitting and diffuse reflectance spectrum (DRS) measured for Cr2O3/Rh/IrO2-modified Y2Ti2O5S2. Reproduced from ref. 85 with permission from Springer Nature. | ||
| Compounds | Cocatalysts | Rate of gas evolution (μmol h−1) | |||
|---|---|---|---|---|---|
| H2 promotor | O2 promotor | H2 | O2 | ||
| Ln2Ti2S2O581 | Pr2Ti2S2O5 | Pt | — | 0 | 0 |
| Nd2Ti2S2O5 | 4 | 3 | |||
| Gd2Ti2S2O5 | 24 | 21 | |||
| Gd2Ti2O5S2 with atomically ordered surfaces88 | 3000 | ||||
| Tb2Ti2S2O5 | 19 | 20 | |||
| Dy2Ti2S2O5 | 10 | 9 | |||
| Ho2Ti2S2O5 | 22 | 5 | |||
| Er2Ti2S2O5 | 21 | 1 | |||
| Y2Ti2S2O585 | Cr2O3/Rh/IrO2 | ∼50 | ∼25 | ||
| Y2Ti2S2O5 nanosheets84 | Pt | IrO2 | 536 | ||
| Sc-doped Y2Ti2O5S2120 | Rh | 445 | |||
| Sm2Ti2S2O582,83 | Pt | IrO2 | 22 | 22 | |
| SrZn2S2O92 | Pt | IrO2 | 67.8 | 26.9 | |
| LaInOS2 | α-LaInOS294 | Pt | — | 5.1 | — |
| Layered-LaInOS295 | Pt | IrO2 | ∼25 | ∼6 | |
| AInOS296 | CeInOS2 | Pt | — | <2.5 | — |
| PrInOS2 | Pt | — | <2.5 | — | |
| La5In3S9O3100 | Pt | IrO2 | ∼50 | ∼12 | |
| La3GaS5O103 | RuCl3·3H2O | IrO2 | 80.7 | ∼12 | |
| La3NbS2O5112 | Pt (1 wt%) | — | 3–11 | — | |
| La5Ti2MS5O7105,106 | La5Ti2CuS5O7 | Pt | IrO2 | 110 | 24 |
| La5Ti2AgS5O7 | Pt | IrO2 | 220 | 12 | |
| La5Ti2Cu1−xAgxS5O7121 | Pt | — | ∼32 | — | |
| Ga doped – La5Ti2Cu0.9Ag0.1O7S5111 | Rh (imp + ph) | 464 μmol H2 during the first hour | |||
| La5Ti2Cu(S1−xSex)5O7110 | Pt/NiS | ||||
| BiOAgS113 | Ru | — | ∼10 | — | |
| ZnO0.6S0.4115 | |||||
| InMnO3−xSx122 | 158 (sacrificial S2−/SO32−) | 649 (with specific AgNO3 sacrificial agent) | |||
| Defect-(Bi,Ce)2(O,S)3−x nanorods118 | 526.5 | ||||
| Sn2Nb2O7−xSx (up to x = 0.6)114 | 6 | ||||
| Zn(Zn,Ni,In)2(O,S)4−x117 | 1700 | ||||
The fact of having suitable band gaps as well as a layered structure definitely contributed to the good activity demonstrated by this series. Another common factor is the presence of Ti 3d orbitals in the CBM; whilst for the VBM the lanthanide based phases have partially filled, highly localized, Ln 4f orbitals which are less effective in photoconductivity or photocatalysis and are usually ignored.82 Finally some of these phases exhibited lower activities than others which could be related to the larger amounts of sulfur defects or the thermodynamically less favorable VB position.81 More recently, Sc-doping of Y2Ti2O5S2 followed by an etching process to “clean” the surface, considerably increased the photocatalytic activity to 445 mmol h−1, which is 3.4 times higher than that for the pristine compound. The authors attribute this enhancement to a decrease of the defect density with Sc doping.86 In another article, Y2Ti2O5S2 nanosheets are synthesized by a flux-assisted solid-state reaction which exposed {101} and {001} facets that provides an anisotropic charge migration, which were found to be responsible for the reduction and oxidation of the water-splitting photocatalytic reaction, respectively. It is striking that selective photo-deposition of cocatalysts (on a particular facet) was possible and resulted in enhanced reactivity (H2 production, (536 μmol h−1)).84
Several studies focused on this particular Y2Ti2O5S2 photocatalyst, each of them bringing innovative approaches to design the particles, the surface or the contact with the co-catalyst. Another study focused on the solid-state reaction mechanism and intermediate.87 They thus found that from the traditional precursors Y2O3, TiO2 and Y2S3, the three intermediate phases Y2Ti2O7, Y2O2S and TiS2 appear during the formation of the final oxysulfide. The formation of agglomerates involving Y2Ti2O7 and Y2O2S which react with the third intermediate TiS2 (carried by the sulfur vapor) was a critical step impacting the morphology of the crystallites. With this knowledge, the authors could reduce the particle size by increasing the heating rate which resulted in improved photocatalytic performances.
These advances on those compositions show that the intrinsic properties of the crystalline phases are as important as the morphology (particle size and surface design). In the case of Ln = Gd, outstanding results were reported with recent advances.88 Gd2Ti2O5S2 with atomically ordered surfaces could be obtained using flux synthesis followed by acidic etching of the surface. The outstanding enhancement of the photocatalytic activity (3 mmol h−1 for H2 production) is attributed to the crystals shape (platelet) combined with the surface design and optimized contact between the particles and a composite cocatalyst (Cr2O3/Pt/IrO2).89
 | ||
| Fig. 4 (a) Structural representation of SrZn2S2O oxysulfide of Pmn21 symmetry; (b) oxygen evolution for SrZn2S2O and (c) hydrogen evolution for Pt/SrZn2S2O. Reproduced from ref. 92 with permission from the Royal Society of Chemistry. | ||
Compared to the Ln2Ti2S2O5 series discussed above, the O2 evolution rate is similar but the H2 evolution is slightly higher for SrZn2S2O (Table 1). This indicates the advantage of having the Zn orbitals contributing to the CB compared to the Ti orbitals. SrZn2S2O exhibits a structure with the presence of heteroleptic coordination environment (ZnS3O) connected three dimensionally unlike the layered Ln2Ti2S2O5, which could also have an impact on the transport properties of the charge carriers.
 | ||
| Fig. 5 Structural representation of LaOInS2 material with (a) alpha model of Pbcm symmetry and (b) metastable layered model of P21/m symmetry; below, the water splitting properties of the metastable layered phase of LaOInS2 are shown including (c) H2(g) evolution for 0.75 wt% Pt/LaOInS2 and (d) O2(g) evolution for 0.5 wt% IrO2/LaOInS2. Reproduced from ref. 95 with permission from the Royal Society of Chemistry. | ||
Then again, CeOInS2 and PrOInS2 phases were found to be isostructural to the monoclinic layered LaOInS2,95 except that the indium in these phases is on-center unlike in LaOInS2, which is off-center. They revealed direct band gaps of 2.41 and 2.43 eV, respectively. Compared to LaOInS2, these phases showed reduced H2 evolution (it decreased by 10% under visible light).96
The CBM in the La-based phases is mainly composed of In 5s/5p orbitals, whilst O 2p and S 3p contribute most to the VBM. The In states seem beneficial in that they contribute to the higher dispersion of the CBM (that should be associated with lower effective masses of the charge carriers and thus higher mobilities) and thus increase the capacity to evolve H2. However, the layered polymorph exhibited higher rates, probably related to the structure dimensionality (compared to the α-LaOInS2): the layered character gives efficient separation of the charge carriers and helps to avoid their recombination, and thus benefits to the photocatalytic reaction. However, measurements with the same conditions are needed to evaluate accurately the difference in H2 release rate for the two polymorphs.
As for CeOInS2 and PrOInS2 phases, the presence of the on-center indium decreased the hybridization of the In 5p–S 3p orbitals, which resulted in an increase in the energy difference of these hybridized orbitals, slightly shifting the optical absorption edge of these phases.96 Even though the 4f orbitals of Ln are negligible when highly localized, it seems that their presence might increase the electron–hole recombination rates.97,98
Similar to the LnOInS2 (Ln = La, Ce, Pr) series, the presence of the In 5s–5p orbitals contributes to the higher H2 evolution efficiency.82 This is similar to SrZn2S2O, with the presence of the Zn 4s–4p orbitals contributing to the enhanced H2 evolution.
LaGaS2O demonstrated a n-type semiconduction with an indirect band gap of 3 eV whilst La3GaS5O exhibited a n-type semiconduction with a direct band gap of 2.3 eV. The CBM level is similar in both phases, whereas the VBM in La3GaS5O is higher than LaGaS2O, due to the higher sulfur content. Both phases were found to be potential photocatalysts but only La3GaS5O was tested for water reduction and oxidation reactions under visible light, whilst LaGaS2O produced anodic photocurrent under ultraviolet light.103 The comparison of these phases highlights the importance of the chalcogen ratio in narrowing the band gaps.
 | ||
| Fig. 8 Structural representation of (a) La5Ti2MS5O7, (M = Cu, Ag) oxysulfides (Pnma symmetry) highlighting the different building blocks within the structure. | ||
Various synthesis methods to produce the photocathode La5Ti2CuS5O7 were investigated to further enhance its photocatalytic properties.107,108 The electronic band structures of these phases were found to be suitable for charge separation and revealed a CBM consisting of Ti 3d orbitals whilst the VBM was formed by hybridized Cu 3d and S 3p orbitals in La5Ti2CuS5O7, rather than just S 3p orbitals in La5Ti2AgS5O7, responsible for the longer absorption edge wavelength.106 The study of these materials highlights the importance of having disassociated paths for accelerating the charge separation while inhibiting the recombination process.109
Substituted phases were also investigated for their photocatalytic properties in this system such as La5Ti2Cu(S1−xSex)5O7 photocatalysts and their hydrogen evolution activity. The authors show that performances decrease with Se content due to excessive crystals growth which can be limited by controlling the calcination temperature.110
On the other hand, the experimental study of Ga doped-La5Ti2Cu0.9Ag0.1O7S5 synthesized using thermal sulfidation show that post annealing in sulfur vapor improved its properties (H2 production of 464 μmol h−1) by reducing the defect concentration at the surface and achieving smaller particle sizes. The best samples reached H2 production of 400 μmol after 4 hours in Na2S–Na2SO3 solution and with Rh as cocatalyst.111
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S ratios up to x = 0.6 and observe a gradual decrease of the band gap with increasing sulfur content (Fig. 10c). The resulting narrow band gap materials are active for water-splitting photocatalysis under visible light with highest amount of H2 obtained for x = 0.4 (6 μmol h−1), Fig. 10b.114
S ratios up to x = 0.6 and observe a gradual decrease of the band gap with increasing sulfur content (Fig. 10c). The resulting narrow band gap materials are active for water-splitting photocatalysis under visible light with highest amount of H2 obtained for x = 0.4 (6 μmol h−1), Fig. 10b.114
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 μmol g−1 h−1) was reached in a 50% ethanol solution.
800 μmol g−1 h−1) was reached in a 50% ethanol solution.
The spinel compound Zn(Zn,Ni,In)2(O,S)4−x is one of the few oxysulfides reported for this structure-type which have a complex non-stoichiometric composition. The later feature and its associated defect states are believed to enhance the photocatalytic H2 production under visible light, which has been reported as 1700 μmol g−1 h−1.117
Finally, we can cite another complex material which was designed with simple binary parent compounds as models. It is the defect-(Bi,Ce)2(O,S)3−x nanorods in which the control of oxygen vacancies led to rapid electron transport resulting in excellent photocatalytic properties (526.5 μmol h−1 for H2).118
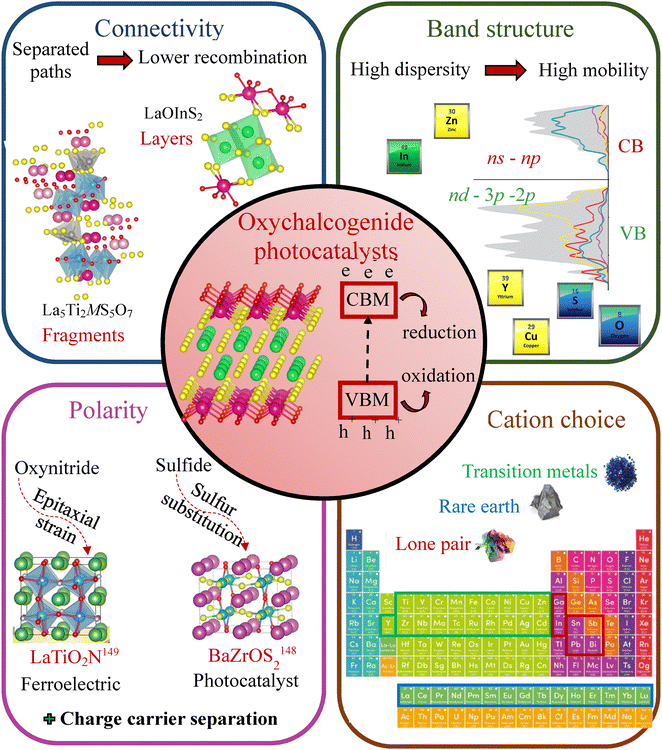
2.3. Summary of composition and structural features observed in photocatalytic oxychalcogenides
A range of factors can affect the outcome of the overall water splitting photocatalytic reaction: (i) morphology and microstructure such as defects and crystallinity (intrinsic features) and (ii) pH, temperature, concentration of the catalyst, type and amount of cocatalyst loading, incident light intensity (extrinsic features). The focus of this survey is to compare the structural (connectivity, polarity) and electronic (band gap magnitude and nature, density of states, charge carriers’ behaviour) properties, which allowed us to identify some key features/characteristics (including structural and relating to connectivity, and compositional) that contribute to the good activity of these materials:• Higher sulfur content can increase the VBM position (as illustrated by comparison of LaGaS2O with La3GaS5O, Section 2.2.5), more suitable for the oxygen evolution but this can create larger amounts of defects within the structure which can be a disadvantage for photocatalytic activity.
• The charge carriers’ behaviour is a very important factor and having a structure that enables disassociated paths for the photoexcited electrons and holes enhances their separation and decreases the rate of their recombination, as discussed for La5Ti2MS5O7 (Section 2.2.6).
• Cations with highly localized 4f orbitals, such as Sm, are less effective in photocatalysis and their contribution is usually ignored. On the other hand, cations with less localized 4f orbitals, such as Ce or Pr, can potentially increase the recombination rate of the photoexcited charges therefore decrease the photocatalytic activity, as suggested for the LnOInS2 series (Section 2.2.3).
• Highly dispersed conduction and valence bands are definitely an asset for better H2 and O2 evolution, respectively. Examples include the presence of ns–np orbitals (in In or Zn containing materials) highly hybridizing in the CBM, or the addition presence of 3d orbitals (in Y or Cu containing materials) strongly hybridizing with the S 3p and O 2p orbitals gives a better VBM position.
• A greater energy difference between cation and anion orbitals can reduce their hybridization, which in turn may lower photocatalytic activity. This effect can be influenced by structural factors, such as cation displacement, either on or off-center, particularly in the case of ns2 cations. The second-order Jahn–Teller effect plays a key role here, as the stereochemical activity of the lone pair can distort the local coordination environment, altering orbital overlap and hybridization strength.119 This strategy has not yet been fully explored and is discussed below (Section 3.1.2).
3. Proposed design strategy for active oxychalcogenide photocatalysts
3.1. Key structural and electronic structure features of oxychalcogenides for photocatalysis from experimental and theoretical work
Overall, this issue should be possible to address more accurately using specific theoretical calculations. For instance, in a recent study, the electron and hole transport properties could be investigated based on real-time time-dependent density functional theory (TDDFT) and nonadiabatic molecular dynamics (NAMD).140 Based on the excited-state dynamics, Y2Ti2O5S2 was found to exhibit a carrier lifetime much longer than that reported for lead-halide Perovskites. The authors identify a key factor underlying the high photocatalytic activity of this oxysulfide: the presence of distinct vibrational modes within different structural sublayers that contribute to non-adiabatic coupling (NAC). NAC describes how electronic transitions are influenced by atomic vibrations, and in this case, it facilitates efficient and spatially separated transport paths for electrons and holes, thereby enhancing charge separation and suppressing recombination (Fig. 11).
 | ||
| Fig. 11 The charge densities of the VBM (in purple) and CBM (in green) in (a) SrTiO3 and (b) Y2Ti2O5S2. Electrons and holes in bad edges are distributed around metallic and non-metallic atoms, respectively. In SrTiO3, the e–h only couple to Ti–O bonds, whereas the e–h couple to Ti–O and Y–S bonds in Y2Ti2O5S2, respectively. (c) The schematic drawing of the e–h separated distribution with a crowded transport channel in a crystal containing less than four elements, such as in SrTiO3 with relatively weak carrier transport ability. (d) The schematic drawing of e–h separate-path transport in Y2Ti2O5S2. The holes in Y2Ti2O5S2 mainly diffuse within the [Y2S2]2+ layer, and electrons mainly move within the [Ti2O5]2− layer. The blue and red balls represent the distribution of holes and electrons in real space, respectively. Reproduced from ref. 140 with permission from PCCP Owner Societies. | ||
3.2. Design strategy for oxychalcogenide photocatalysts
Based on the key features in oxychalcogenide photocatalysts discussed in the previous section, we will therefore propose a potential design strategy that could be serve as a useful tool for designing new efficient oxychalcogenide materials for photocatalysis specifically, solar water splitting photocatalysis. A schematic diagram of a potential design strategy for efficient oxychalcogenide materials is presented in Fig. 12.First, the nature of the bandgap (direct or indirect), the semiconduction type (n or p-type) are not of great influence on the properties of the photocatalysts as both types were exhibited by the materials discussed in Section 2.2. However, ensuring that the oxychalcogenide has a band gap well-matched in magnitude to the solar spectrum is essential. This can be achieved by selecting a softer chalcogenide anion Q2− and by tuning the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Q ratio. Additionally, incorporating chalcogenides can increase bandgaps due to enhanced covalency, resulting from their lower electronegativity compared to oxygen.145
Q ratio. Additionally, incorporating chalcogenides can increase bandgaps due to enhanced covalency, resulting from their lower electronegativity compared to oxygen.145
The structure and its connectivity are important for the transport properties of the photoexcited charges. Different pathways for the electrons and the holes help reduce the probability of recombination and this is often observed for layered or segmented structures. The band structure gives insight to the electronic properties of the material, in particular the effective masses of the charge carriers which describes their mobilities: higher dispersion of the bands close to the Fermi level indicate lower effective masses and hence higher carrier mobility and therefore enhanced transfer process. Recently, Li et al.148 reported that the recombination of photogenerated electrons and holes can be much faster than the transport from bulk to the surface reactive site and the catalytic reaction. Thus, fast mobility favours the charge migration to the surface of the photocatalyst to participate in the reaction, whilst slow mobility is more prone to result in charge recombination. Therefore, having a large mobility difference could be useful for the separation of e− and h+, reduction of the e− and h+ recombination rate, and improvement of the photocatalytic activity.
Cation choice is another important feature due to the contributions of their corresponding orbitals to the band edges; this can therefore tune the band gap as well as the charge carriers’ transport properties. Lastly, the polarity, is not widely studied as a design feature in photocatalysis, but it could enhance a catalyst's performance by improving charge carrier separation, as seen in ferroelectrics such as AB(OxS1−x)3146 and LaTiO2N.147 In this study they demonstrated, via density functional theory calculations, the potential of suppressing the recombination phenomena of the charge carriers due to the out-of-plane spontaneous polarization that has risen from the epitaxial strain on the anion order within the structure. Experimental studies on Sr6Cd2Sb6O7S10142 and Sr2Sb2O2Q3 (Q = S, Se)149 provide a direct comparison of polar vs. non-polar structures and different coordination environments for the same cation, supporting this theoretical proposal. Sr6Cd2Sb6O7S10, with its polar structure and zigzag [CdSb2OS5] layers, exhibits built-in polarization fields that enhance charge separation and suppress recombination, as confirmed by its strong photocurrent response. In contrast, Sr2Sb2O2Q3, despite its layered but non-centrosymmetric structure, lacks significant internal polarization and relies solely on heteroleptic SbOQ4 coordination, leading to high charge mobility but weaker polarization-driven separation. Thus, the presence of polar distortions, can indeed be a promising route to adopt for increasing the band gap and design new materials with efficient photocatalytic properties.
In this review, we have focused on structural and electronic features which may determine the water-splitting photocatalytic properties of active oxychalcogenides. However, for some of the oxysulfides discussed there are several further studies with drastic enhancement of the H2 production based on different strategies to those mentioned above including optimization of surface morphology (some of which is covered in specific reviews dealing with the photocatalytic measurement design and optimization of the photocatalysts/co-catalysts). The example of Gd2Ti2S2O5 or Y2Ti2S2O5 for instance, where the particle size and shape and the surface engineering allow drastic enhancement of H2 release, are very informative.85,88 The role of defects can also vary by being either counter-productive or helpful such as defect-(Bi,Ce)2(O,S)3−x nanorods118 where the multi-valence states and the surface defects facilitate the kinetic reactions, here the defects are controlled and induced during the synthetic process by reduction using hydrazine to incorporate oxygen vacancies. Therefore, beyond intrinsic properties, there is the synthesis design, the cocatalysts type and their interaction with the photocatalyst which play also a crucial role and should be fully considered when designing a photocatalyst. Depending on how the later aspects are handled, the activity might be hampered or improved.
While our review is centered on photocatalytic water splitting, a highly demanding reaction that requires precise band alignment, efficient charge separation, and strong stability under illumination and in aqueous conditions, other types of photocatalytic applications such as CO2 photoreduction demonstrate the versatility of these materials.150 However, these alternative photocatalytic processes remain relatively underexplored for oxychalcogenides and we hope our additions will also encourage further investigation beyond water splitting.
It is also interesting to consider the potential of hybrid compounds explicitly combining (oxy)chalcogenides with organic components which are extremely limited. There are a few instructive examples involving chalcogen elements in hybrid systems. For instance, metal–organic chalcogenide semiconductor nanocrystals have demonstrated the feasibility of incorporating soft chalcogen ligands into hybrid materials.151 The later study describes metal-chalcogenide layers stacked with organic ligand layers; both layers can be altered to tune the properties. Another example includes a cystamine-based hybrid perovskite, where noncovalent chalcogen bonding involving the cystamine disulfide bridges influences the structure and properties.152 These studies show that combining soft chalcogen chemistry with organic–inorganic frameworks is chemically viable and could inspire future exploration in oxychalcogenide-based hybrids that may be useful in the photocatalytic field.
A broader overview of these approaches is presented in a review about the current status and future of organic–inorganic hybrid perovskites for photoelectrocatalysis devices,153 which discusses both hybrid molecular systems and composite architectures used in water-splitting. It highlights how the integration of organic macromolecules (such as oligomers, polymers, or MOFs) with inorganic frameworks can provide synergistic benefits, including improved photocatalytic activity, enhanced stability, and interfacial tunability. On another hand, it is interesting to consider the design of hybrid (composite) photocatalysts that integrate oxychalcogenides with additional materials to form heterostructures or tandem systems. These systems can enhance light absorption, extend charge carrier lifetimes, or spatially separate redox reactions. For example, recent studies report hybrid nanostructures of PBA (Prussian blue analogues) nanocubes wrapped with transition-metal oxysulfides and all together grafted on CC (carbon cloth) substrate (PBA@Co–W–O–S/CC heterostructure), as efficient OER electrocatalysts.154 Besides, the dye modified Bi2O2S/In2O3 showed promise for the catalysis of water splitting thanks to the formation of a heterojunction between the oxide-chalcogenide and In2O3.155 Although such strategies are promising, our review deliberately focuses on single-phase oxychalcogenide materials, aiming to clarify intrinsic structure–composition–property relationships without convolution from multicomponent effects. Nonetheless, we now briefly acknowledge these composite designs as a complementary future strategy for improving photocatalytic performance. Lastly, it is interesting to mention theoretical prediction of fully inorganic, single-phase oxychalcogenides as a good alternative to hybrids and oxides in photovoltaics with an enhanced shift current. The authors put forward this strategy to reinforce the idea that simplifying material systems, rather than increasing complexity, can also be a viable and appealing route when structural and electronic requirements are met.156
4. Conclusion
In this review, we discussed the concept of photocatalytic water splitting alongside the necessary requirements for a successful reaction in the solar light with a specific focus on layered oxychalcogenides (oxysulfides and oxyselenides). We discussed several oxysulfide materials that are known for their capacity to split water, comparing their different structures and connectivities, band gaps (direct or indirect), semiconduction types (n or p-type), polarity as well as their photocatalytic activity (O2 and/or H2 evolution, solar or UV irradiation). This allowed us to extract some key features that can be beneficial for designing new photocatalysts and to propose a potential design strategy for photocatalytic oxychalcogenides consisting on several key points which are exploring the polarity, anion ratio, cation choice (transition metals, lone pairs cations) and connectivity regarding what was reported in the literature; and further work is needed for polarity as well as morphology.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
I-Site (ULNE), University of Lille and University of Kent are thanked for cotutelle funding (S. Al Bacha). Durham University is thanked for hosting research visit. Dr Donna Arnold is thanked for helpful discussions. This study was supported by the French government through the Programme Investissement d’Avenir (I-SITE ULNE/ANR-16-IDEX-0004 ULNE) managed by the Agence Nationale de la Recherche (Project ANION-COMBO).References
- A. Nzihou, G. Flamant and B. Stanmore, Synthetic Fuels from Biomass Using Concentrated Solar Energy–a Review, Energy, 2012, 42(1), 121–131 CrossRef CAS.
- Y. Yang, R. Zhao, T. Zhang, K. Zhao, P. Xiao, Y. Ma, P. M. Ajayan, G. Shi and Y. Chen, Graphene-Based Standalone Solar Energy Converter for Water Desalination and Purification, ACS Nano, 2018, 12(1), 829–835 CrossRef CAS PubMed.
- S. Mekhilef, R. Saidur and A. Safari, A Review on Solar Energy Use in Industries, Renewable Sustainable Energy Rev., 2011, 15(4), 1777–1790 CrossRef.
- B. Martinez, A. Roig, X. Obradors, E. Molins, A. Rouanet and C. Monty, Magnetic Properties of γ-Fe2O3 Nanoparticles Obtained by Vaporization Condensation in a Solar Furnace, J. Appl. Phys., 1996, 79(5), 2580–2586 CrossRef CAS.
- J. Qi, W. Zhang and R. Cao, Solar-to-hydrogen Energy Conversion Based on Water Splitting, Adv. Energy Mater., 2018, 8(5), 1701620 CrossRef.
- P. Zhang and X. W. Lou, Design of Heterostructured Hollow Photocatalysts for Solar-to-chemical Energy Conversion, Adv. Mater., 2019, 31(29), 1900281 CrossRef PubMed.
- T. Shiyani, S. Agrawal and I. Banerjee, Natural-Dye-Sensitized Photoelectrochemical Cells for Solar Energy Conversion, Nanomater. Energy, 2020, 9(2), 215–226 CrossRef.
- C. F. Goodeve and J. A. Kitchener, Photosensitisation by Titanium Dioxide, Trans. Faraday Soc., 1938, 34, 570–579 RSC.
- K. Hashimoto, H. Irie and A. Fujishima, TiO2 Photocatalysis: A Historical Overview and Future Prospects, Jpn. J. Appl. Phys., 2005, 44(12R), 8269 CrossRef CAS.
- A. Fujishima and K. Honda, Electrochemical Photolysis of Water at a Semiconductor Electrode, Nature, 1972, 238(5358), 37–38 CrossRef CAS PubMed.
- A. Fujishima, T. N. Rao and D. A. Tryk, Titanium Dioxide Photocatalysis, J. Photochem. Photobiol., C, 2000, 1(1), 1–21 CrossRef CAS.
- A. Fujishima, X. Zhang and D. A. Tryk, TiO2 Photocatalysis and Related Surface Phenomena, Surf. Sci. Rep., 2008, 63(12), 515–582 CrossRef CAS.
- K. Hashimoto, H. Irie and A. Fujishima, TiO2 Photocatalysis: A Historical Overview and Future Prospects, Jpn. J. Appl. Phys., 2005, 44(12R), 8269 CrossRef CAS.
- S. Leroy, Etude Des Propriétés Photocatalytiques et Photoélectriques Du Dititanate de Lanthane (La2Ti2O7) à Structure Pérovskite En Feuillets et Son Utilisation Dans Des Hétérojonctions Tout Oxyde Pour La Conversion d’énergie, Thèse de doctorat, Université d'Artois, 2020.
- T. Jafari, E. Moharreri, A. S. Amin, R. Miao, W. Song and S. L. Suib, Photocatalytic Water Splitting—the Untamed Dream: A Review of Recent Advances, Molecules, 2016, 21(7), 900 CrossRef PubMed.
- Z. Liang, C.-F. Yan, S. Rtimi and J. Bandara, Piezoelectric Materials for Catalytic/Photocatalytic Removal of Pollutants: Recent Advances and Outlook, Appl. Catal., B, 2019, 241, 256–269 CrossRef CAS.
- M. Wang, B. Wang, F. Huang and Z. Lin, Enabling PIEZOpotential in PIEZOelectric Semiconductors for Enhanced Catalytic Activities, Angew. Chem., Int. Ed., 2019, 58(23), 7526–7536, DOI:10.1002/anie.201811709.
- F. Chen, H. Huang, L. Guo, Y. Zhang and T. Ma, The Role of Polarization in Photocatalysis, Angew. Chem., Int. Ed., 2019, 58(30), 10061–10073 CrossRef CAS PubMed.
- E. D. Wachsman and K. T. Lee, Lowering the Temperature of Solid Oxide Fuel Cells, Science, 2011, 334(6058), 935–939, DOI:10.1126/science.1204090.
- A. Staykov; S. M. Lyth and M. Watanabe, Photocatalytic Water Splitting, Hydrogen Energy Engineering, Springer, 2016, pp. 159–174 Search PubMed.
- D. Kong, Y. Zheng, M. Kobielusz, Y. Wang, Z. Bai, W. Macyk, X. Wang and J. Tang, Recent Advances in Visible Light-Driven Water Oxidation and Reduction in Suspension Systems, Mater. Today, 2018, 21(8), 897–924 CrossRef CAS.
- Y. Luan, Y. Li, Z. Li, B. Y. Zhang and J. Z. Ou, Layered Anion-Mixed Oxycompounds: Synthesis, Properties, and Applications, Adv. Sci., 2025, 12, 2500477, DOI:10.1002/advs.202500477.
- M. Li, N. Wang, S. Zhang, J. Hu, H. Xiao, H. Gong, Z. Liu, L. Qiao and X. Zu, A Review of the Properties, Synthesis and Applications of Lanthanum Copper Oxychalcogenides, J. Phys. D:Appl. Phys., 2022, 55(27), 273002, DOI:10.1088/1361-6463/ac4b71.
- S. J. Clarke, P. Adamson, S. J. Herkelrath, O. J. Rutt, D. R. Parker, M. J. Pitcher and C. F. Smura, Structures, Physical Properties, and Chemistry of Layered Oxychalcogenides and Oxypnictides, Inorg. Chem., 2008, 47(19), 8473–8486 CrossRef CAS PubMed.
- Y. Kobayashi, O. Hernandez, C. Tassel and H. Kageyama, New Chemistry of Transition Metal Oxyhydrides, Sci. Technol. Adv. Mater., 2017, 18(1), 905–918 CrossRef CAS PubMed.
- L. Wang, L. Wang, Y. Du, X. Xu and S. X. Dou, Progress and Perspectives of Bismuth Oxyhalides in Catalytic Applications, Mater. Today Phys., 2021, 16, 100294 CrossRef CAS.
- A. Nakada, D. Kato, R. Nelson, H. Takahira, M. Yabuuchi, M. Higashi, H. Suzuki, M. Kirsanova, N. Kakudou and C. Tassel, Conduction Band Control of Oxyhalides with a Triple-Fluorite Layer for Visible Light Photocatalysis, J. Am. Chem. Soc., 2021, 143(6), 2491–2499 CrossRef CAS PubMed.
- Y. Li, H. Jiang, X. Wang, X. Hong and B. Liang, Recent Advances in Bismuth Oxyhalide Photocatalysts for Degradation of Organic Pollutants in Wastewater, RSC Adv., 2021, 11(43), 26855–26875 RSC.
- E. J. Cho, S.-J. Oh, H. Jo, J. Lee, T.-S. You and K. M. Ok, Layered Bismuth Oxyfluoride Nitrates Revealing Large Second-Harmonic Generation and Photocatalytic Properties, Inorg. Chem., 2019, 58(3), 2183–2190 CrossRef CAS PubMed.
- Y. Kobayashi, Y. Tang, T. Kageyama, H. Yamashita, N. Masuda, S. Hosokawa and H. Kageyama, Titanium-Based Hydrides as Heterogeneous Catalysts for Ammonia Synthesis, J. Am. Chem. Soc., 2017, 139(50), 18240–18246 CrossRef CAS PubMed.
- M. Ai-Mamouri, P. P. Edwards, C. Greaves and M. Slaski, Synthesis and Superconducting Properties of the Strontium Copper Oxy-Fluoride Sr2CuO2F2+δ, Nature, 1994, 369(6479), 382–384 Search PubMed.
- Y. Kamihara, H. Hiramatsu, M. Hirano, R. Kawamura, H. Yanagi, T. Kamiya and H. Hosono, Iron-Based Layered Superconductor: LaOFeP, J. Am. Chem. Soc., 2006, 128(31), 10012–10013 CrossRef CAS PubMed.
- Y. Kamihara, T. Watanabe, M. Hirano and H. Hosono, Iron-Based Layered Superconductor La [O1-x F x] FeAs (X = 0.05− 0.12) with T C = 26 K, J. Am. Chem. Soc., 2008, 130(11), 3296–3297 CrossRef CAS PubMed.
- R. K. Oogarah, E. Suard and E. E. McCabe, Magnetic Order and Phase Transition in the Iron Oxysulfide La2O2Fe2OS2, J. Magn. Magn. Mater., 2018, 446, 101–107 CrossRef CAS.
- S. Okada, M. Matoba, S. Fukumoto, S. Soyano, Y. Kamihara, T. Takeuchi, H. Yoshida, K. Ohoyama and Y. Yamaguchi, Magnetic Properties of Layered Oxysulfide Sr 2 Cu 2 (Co, Cu) O 2 S 2 with Cu-Doped CoO 2 Square Planes, J. Appl. Phys., 2002, 91(10), 8861–8863 CrossRef CAS.
- F. M. Ryan, E. W. Pugh and R. Smoluchowski, Superparamagnetism, Nonrandomness, and Irradiation Effects in Cu-Ni Alloys, Phys. Rev., 1959, 116(5), 1106 CrossRef CAS.
- Y.-Y. Li, W.-J. Wang, H. Wang, H. Lin and L.-M. Wu, Mixed-Anion Inorganic Compounds: A Favorable Candidate for Infrared Nonlinear Optical Materials, Cryst. Growth Des., 2019, 19(7), 4172–4192 CrossRef CAS.
- F. Sauvage, V. Bodenez, H. Vezin, T. A. Albrecht, J.-M. Tarascon and K. R. Poeppelmeier, Ag4V2O6F2 (SVOF): A High Silver Density Phase and Potential New Cathode Material for Implantable Cardioverter Defibrillators, Inorg. Chem., 2008, 47(19), 8464–8472 CrossRef CAS PubMed.
- J. Li, W. Zhai, C. Zhang, Y. Yan, P.-F. Liu and G. Yang, Anharmonicity and Ultralow Thermal Conductivity in Layered Oxychalcogenides BiAgOCh (Ch= S, Se, and Te), Mater. Adv., 2021, 2(14), 4876–4882 RSC.
- N. Hirosaki, R.-J. Xie, K. Kimoto, T. Sekiguchi, Y. Yamamoto, T. Suehiro and M. Mitomo, Characterization and Properties of Green-Emitting β-SiAlON: Eu 2+ Powder Phosphors for White Light-Emitting Diodes, Appl. Phys. Lett., 2005, 86(21), 211905 CrossRef.
- H. Kageyama, K. Hayashi, K. Maeda, J. P. Attfield, Z. Hiroi, J. M. Rondinelli and K. R. Poeppelmeier, Expanding Frontiers in Materials Chemistry and Physics with Multiple Anions, Nat. Commun., 2018, 9(1), 1–15 CrossRef CAS PubMed.
- J. P. Attfield, Principles and Applications of Anion Order in Solid Oxynitrides, Cryst. Growth Des., 2013, 13(10), 4623–4629 CrossRef CAS.
- G. Shi, Y., S. Yu, A. Belik, A., Y. Matsushita, M. Tanaka, Y. Katsuya, K. Kobayashi, K. Yamaura and E. Takayama-Muromachi, Synthesis and Superconducting Properties of the Iron Oxyarsenide TbFeAsO0.85, J. Phys. Soc. Jpn., 2008, 77(Suppl. C), 155–157 Search PubMed.
- S. V. Chong, T. Mochiji and K. Kadowaki, Superconductivity in Yttrium Iron Oxyarsenide System, Journal of Physics: Conference Series, IOP Publishing, 2009, vol. 150, p. 052036 Search PubMed.
- M. Orr, G. R. Hebberd, E. E. McCabe and R. T. Macaluso, Structural Diversity of Rare-Earth Oxychalcogenides, ACS Omega, 2022, 7(10), 8209–8218 CrossRef CAS PubMed.
- S. D. Luu and P. Vaqueiro, Layered Oxychalcogenides: Structural Chemistry and Thermoelectric Properties, J. Materiomics, 2016, 2(2), 131–140 CrossRef.
- Y.-F. Shi, W.-B. Wei, X.-T. Wu, H. Lin and Q.-L. Zhu, Recent Progress in Oxychalcogenides as IR Nonlinear Optical Materials, Dalton Trans., 2021, 50(12), 4112–4118 RSC.
- A. Parida, S. Senapati and R. Naik, Recent Developments on Bi-Based Oxychalcogenide Materials with Thermoelectric and Optoelectronic Applications: An Overview, Mater. Today Chem., 2022, 26, 101149 CrossRef CAS.
- K. Maeda and K. Domen, New Non-Oxide Photocatalysts Designed for Overall Water Splitting under Visible Light, J. Phys. Chem. C, 2007, 111(22), 7851–7861, DOI:10.1021/jp070911w.
- T. Takata and K. Domen, Development of Non-Oxide Semiconductors as Light Harvesting Materials in Photocatalytic and Photoelectrochemical Water Splitting, Dalton Trans., 2017, 46(32), 10529–10544 RSC.
- A. Miyoshi and K. Maeda, Recent Progress in Mixed-Anion Materials for Solar Fuel Production, Sol. RRL, 2021, 5(6), 2000521 CrossRef CAS.
- K. Chatterjee and S. E. Skrabalak, Durable Metal Heteroanionic Photocatalysts, ACS Appl. Mater. Interfaces, 2021, 13(31), 36670–36678, DOI:10.1021/acsami.1c09774.
- Y. Subramanian, A. Dhanasekaran, L. A. Omeiza, M. R. Somalu and A. K. Azad, A Review on Heteroanionic-Based Materials for Photocatalysis Applications, Catalysts, 2023, 13(1), 173 CrossRef CAS.
- E. J. Salter, J. N. Blandy and S. J. Clarke, Crystal and Magnetic Structures of the Oxide Sulfides CaCoSO and BaCoSO, Inorg. Chem., 2016, 55(4), 1697–1701 CrossRef CAS PubMed.
- K. Shah, A. Ćirić, K. V. R. Murthy and B. S. Chakrabarty, Investigation of a New Way of Synthesis for Nano Crystallites of La2O2S & 1% Ln3+ (Ln = Pr, Eu, Tb, Dy, Er) Doped La2O2S and Study Their Structural and Optical Properties, J. Alloys Compd., 2021, 851, 156725 CrossRef CAS.
- S. Strobel, A. Choudhury, P. K. Dorhout, C. Lipp and T. Schleid, Rare-Earth Metal (III) Oxide Selenides M4O4Se [Se2](M = La, Ce, Pr, Nd, Sm) with Discrete Diselenide Units: Crystal Structures, Magnetic Frustration, and Other Properties, Inorg. Chem., 2008, 47(11), 4936–4944 CrossRef CAS PubMed.
- V. Johnson and W. Jeitschko, ZrCuSiAs: A “Filled” PbFCl Type, J. Solid State Chem., 1974, 11(2), 161–166 CrossRef CAS.
- W. A. Phelan, D. C. Wallace, K. E. Arpino, J. R. Neilson, K. J. Livi, C. R. Seabourne, A. J. Scott and T. M. McQueen, Stacking Variants and Superconductivity in the Bi–O–S System, J. Am. Chem. Soc., 2013, 135(14), 5372–5374 CrossRef CAS PubMed.
- K. Ueda, H. Hiramatsu, M. Hirano, T. Kamiya and H. Hosono, Wide-Gap Layered Oxychalcogenide Semiconductors: Materials, Electronic Structures and Optoelectronic Properties, Thin Solid Films, 2006, 496(1), 8–15 CrossRef CAS.
- M. Palazzi, C. Carcaly, P. Laruelle and J. Flahaut, Crystal Structure and Properties of (LaO) CuS and (LaO) AgS, The Rare Earths in Modern Science and Technology, Springer, 1982, pp. 347–350 Search PubMed.
- L.-D. Zhao, J. He, D. Berardan, Y. Lin, J.-F. Li, C.-W. Nan and N. Dragoe, BiCuSeO Oxyselenides: New Promising Thermoelectric Materials, Energy Environ. Sci., 2014, 7(9), 2900–2924 RSC.
- J.-X. Zhu, R. Yu, H. Wang, L. L. Zhao, M. D. Jones, J. Dai, E. Abrahams, E. Morosan, M. Fang and Q. Si, Band Narrowing and Mott Localization in Iron Oxychalcogenides La2O2Fe2OSeS2, Phys. Rev. Lett., 2010, 104(21), 216405, DOI:10.1103/PhysRevLett.104.216405.
- M. Lü, O. Mentré, E. E. Gordon, M.-H. Whangbo, A. Wattiaux, M. Duttine, N. Tiercelin and H. Kabbour, A Comprehensive Study of Magnetic Exchanges in the Layered Oxychalcogenides Sr3Fe2O5Cu2Q2 (Q = S, Se), J. Magn. Magn. Mater., 2017, 444, 147–153, DOI:10.1016/j.jmmm.2017.07.026.
- E. E. McCabe, D. G. Free, B. G. Mendis, J. S. Higgins and J. S. Evans, Preparation, Characterization, and Structural Phase Transitions in a New Family of Semiconducting Transition Metal Oxychalcogenides β-La2O2 M Se2 (M = Mn, Fe), Chem. Mater., 2010, 22(22), 6171–6182 CrossRef CAS.
- Z. A. Gál, O. J. Rutt, C. F. Smura, T. P. Overton, N. Barrier, S. J. Clarke and J. Hadermann, Structural Chemistry and Metamagnetism of an Homologous Series of Layered Manganese Oxysulfides, J. Am. Chem. Soc., 2006, 128(26), 8530–8540 CrossRef PubMed.
- A. H. Reshak, CaCoSO Diluted Magnetic Antiferromagnet Semiconductor as Efficient Thermoelectric Materials, Mater. Res. Bull., 2017, 94, 22–30 CrossRef CAS.
- Y.-F. Shi, W.-B. Wei, X.-T. Wu, H. Lin and Q.-L. Zhu, Recent Progress in Oxychalcogenides as IR Nonlinear Optical Materials, Dalton Trans., 2021, 50(12), 4112–4118 RSC.
- B.-W. Liu, X.-M. Jiang, G.-E. Wang, H.-Y. Zeng, M.-J. Zhang, S.-F. Li, W.-H. Guo and G.-C. Guo, Oxychalcogenide BaGeOSe2: Highly Distorted Mixed-Anion Building Units Leading to a Large Second-Harmonic Generation Response, Chem. Mater., 2015, 27(24), 8189–8192 CrossRef CAS.
- W. Xing, P. Fang, N. Wang, Z. Li, Z. Lin, J. Yao, W. Yin and B. Kang, Two Mixed-Anion Units of [GeOSe3] and [GeO3S] Originating from Partial Isovalent Anion Substitution and Inducing Moderate Second Harmonic Generation Response and Large Birefringence, Inorg. Chem., 2020, 59(22), 16716–16724 CrossRef CAS PubMed.
- B.-W. Liu, X.-M. Jiang, G.-E. Wang, H.-Y. Zeng, M.-J. Zhang, S.-F. Li, W.-H. Guo and G.-C. Guo, Oxychalcogenide BaGeOSe2: Highly Distorted Mixed-Anion Building Units Leading to a Large Second-Harmonic Generation Response, Chem. Mater., 2015, 27(24), 8189–8192 CrossRef CAS.
- R. Wang, F. Liang, F. Wang, Y. Guo, X. Zhang, Y. Xiao, K. Bu, Z. Lin, J. Yao and T. Zhai, Sr6Cd2Sb6O7S10: Strong SHG Response Activated by Highly Polarizable Sb/O/S Groups, Angew. Chem., Int. Ed., 2019, 58(24), 8078–8081 CrossRef CAS PubMed.
- Y. Wang, M. Luo, P. Zhao, X. Che, Y. Cao and F. Huang, Sr 4 Pb 1.5 Sb 5 O 5 Se 8: A New Mid-Infrared Nonlinear Optical Material with a Moderate SHG Response, CrystEngComm, 2020, 22(20), 3526–3530 RSC.
- R. Wang, F. Wang, X. Zhang, X. Feng, C. Zhao, K. Bu, Z. Zhang, T. Zhai and F. Huang, Improved Polarization in the Sr6Cd2Sb6O7Se10 Oxyselenide through Design of Lateral Sublattices for Efficient Photoelectric Conversion, Angew. Chem., 2022, 61(33), e202206816, DOI:10.1002/anie.202206816.
- W. J. Zhu and P. H. Hor, Unusual Layered Transition-Metal Oxysulfides: Sr2Cu2MO2S2 (M = Mn, Zn), J. Solid State Chem., 1997, 130(2), 319–321 CrossRef CAS.
- W. J. Zhu and P. H. Hor, Sr2CuGaO3S, a Rare Example of Square Pyramidal Gallium, Inorg. Chem., 1997, 36(17), 3576–3577 CrossRef CAS PubMed.
- M. Guittard, S. Benazeth, J. Dugue, S. Jaulmes, M. Palazzi, P. Laruelle and J. Flahaut, Oxysulfides and Oxyselenides in Sheets, Formed by a Rare Earth Element and a Second Metal, J. Solid State Chem., 1984, 51(2), 227–238 Search PubMed.
- T. Sambrook, C. F. Smura, S. J. Clarke, K. M. Ok and P. S. Halasyamani, Structure and Physical Properties of the Polar Oxysulfide CaZnOS, Inorg. Chem., 2007, 46(7), 2571–2574 CrossRef CAS PubMed.
- C. Boyer, C. Deudon and A. Meerschaut, Synthesis and Structure Determination of the New Sm2Ti2O5S2 Compound, C. R. Acad. Sci., Ser. IIc: Chim., 1999, 2(2), 93–99, DOI:10.1016/S1387-1609(99)80007-3.
- M. Goga, R. Seshadri, V. Ksenofontov, P. Gütlich and W. Tremel, Ln 2 Ti 2 S 2 O 5 (Ln = Nd, Pr, Sm): A Novel Series of Defective Ruddlesden–Popper Phases, Chem. Commun., 1999, 979–980 RSC.
- C. Boyer-Candalen, J. Derouet, P. Porcher, Y. Moëlo and A. Meerschaut, The Family of Ln2Ti2S2O5 Compounds (Ln = Nd, Sm, Gd, Tb, Dy, Ho, Er, and Y): Optical Properties, J. Solid State Chem., 2002, 165(2), 228–237 CrossRef CAS.
- A. Ishikawa, T. Takata, T. Matsumura, J. N. Kondo, M. Hara, H. Kobayashi and K. Domen, Oxysulfides Ln2Ti2S2O5 as Stable Photocatalysts for Water Oxidation and Reduction under Visible-Light Irradiation, J. Phys. Chem. B, 2004, 108(8), 2637–2642 CrossRef CAS.
- A. Ishikawa, T. Takata, J. N. Kondo, M. Hara, H. Kobayashi and K. Domen, Oxysulfide Sm2Ti2S2O5 as a Stable Photocatalyst for Water Oxidation and Reduction under Visible Light Irradiation (Λ ≤ 650 Nm), J. Am. Chem. Soc., 2002, 124(45), 13547–13553 CrossRef CAS PubMed.
- A. Ishikawa, Y. Yamada, T. Takata, J. N. Kondo, M. Hara, H. Kobayashi and K. Domen, Novel Synthesis and Photocatalytic Activity of Oxysulfide Sm2Ti2S2O5, Chem. Mater., 2003, 15(23), 4442–4446 CrossRef CAS.
- J. Zhang, K. Liu, B. Zhang, J. Zhang, M. Liu, Y. Xu, K. Shi, H. Wang, Z. Zhang, P. Zhou and G. Ma, Anisotropic Charge Migration on Perovskite Oxysulfide for Boosting Photocatalytic Overall Water Splitting, J. Am. Chem. Soc., 2024, 146(6), 4068–4077, DOI:10.1021/jacs.3c12417.
- Q. Wang, M. Nakabayashi, T. Hisatomi, S. Sun, S. Akiyama, Z. Wang, Z. Pan, X. Xiao, T. Watanabe and T. Yamada, Oxysulfide Photocatalyst for Visible-Light-Driven Overall Water Splitting, Nat. Mater., 2019, 18(8), 827–832 CrossRef CAS PubMed.
- H. Yoshida, Z. Pan, R. Shoji, V. Nandal, H. Matsuzaki, K. Seki, T. Hisatomi and K. Domen, Heterogeneous Doping of Visible-Light-Responsive Y2Ti2O5S2 for Enhanced Hydrogen Evolution, J. Mater. Chem. A, 2022, 10(46), 24552–24560, 10.1039/D2TA06895H.
- L. Lin, M. Nakabayashi, D. Lu, T. Hisatomi, T. Takata and K. Domen, Formation Mechanism for Particulate Y2Ti2O5S2 Photocatalyst by the Solid-State Reaction, Chem. Mater., 2024, 36(3), 1612–1620, DOI:10.1021/acs.chemmater.3c02929.
- H. Yoshida, Z. Pan, R. Shoji, V. Nandal, H. Matsuzaki, K. Seki, L. Lin, M. Kaneko, T. Fukui, K. Yamashita, T. Takata, T. Hisatomi and K. Domen, An Oxysulfide Photocatalyst Evolving Hydrogen with an Apparent Quantum Efficiency of 30% under Visible Light, Angew. Chem., 2023, 135(46), e202312938, DOI:10.1002/ange.202312938.
- H. Yoshida, Z. Pan, R. Shoji, V. Nandal, H. Matsuzaki, K. Seki, L. Lin, M. Kaneko, T. Fukui, K. Yamashita, T. Takata, T. Hisatomi and K. Domen, An Oxysulfide Photocatalyst Evolving Hydrogen with an Apparent Quantum Efficiency of 30% under Visible Light, Angew. Chem., 2023, 135(46), e202312938, DOI:10.1002/ange.202312938.
- Y. Tsujimoto, C. A. Juillerat, W. Zhang, K. Fujii, M. Yashima, P. S. Halasyamani and H.-C. zur Loye, Function of Tetrahedral ZnS3O Building Blocks in the Formation of SrZn2S2O: A Phase Matchable Polar Oxysulfide with a Large Second Harmonic Generation Response, Chem. Mater., 2018, 30(18), 6486–6493 CrossRef CAS.
- A. H. Reshak, Sulfide Oxide XZnSO (X = Ca or Sr) as Novel Active Photocatalytic Water Splitting Solar-to-Hydrogen Energy Conversion, Appl. Catal., B, 2018, 225, 273–283 CrossRef CAS.
- S. Nishioka, T. Kanazawa, K. Shibata, Y. Tsujimoto, H.-C. Zur Loye and K. Maeda, A Zinc-Based Oxysulfide Photocatalyst SrZn 2 S 2 O Capable of Reducing and Oxidizing Water, Dalton Trans., 2019, 48(42), 15778–15781 RSC.
- H. Kabbour, L. Cario, Y. Moëlo and A. Meerschaut, Synthesis, X-Ray and Optical Characterizations of Two New Oxysulfides: LaInS2O and La5In3S9O3, J. Solid State Chem., 2004, 177(4–5), 1053–1059 CrossRef CAS.
- H. Kabbour, A. Sayede, S. Saitzek, G. Lefevre, L. Cario, M. Trentesaux and P. Roussel, Structure of the Water-Splitting Photocatalyst Oxysulfide α-LaOInS 2 and Ab Initio Prediction of New Polymorphs, Chem. Commun., 2020, 56(11), 1645–1648 RSC.
- A. Miura, T. Oshima, K. Maeda, Y. Mizuguchi, C. Moriyoshi, Y. Kuroiwa, Y. Meng, X.-D. Wen, M. Nagao and M. Higuchi, Synthesis, Structure and Photocatalytic Activity of Layered LaOInS 2, J. Mater. Chem. A, 2017, 5(27), 14270–14277 RSC.
- H. Ito, A. Miura, Y. Goto, Y. Mizuguchi, C. Moriyoshi, Y. Kuroiwa, M. Azuma, J. Liu, X.-D. Wen and S. Nishioka, An Electronic Structure Governed by the Displacement of the Indium Site in In–S 6 Octahedra: LnOInS 2 (Ln = La, Ce, and Pr), Dalton Trans., 2019, 48(32), 12272–12278 Search PubMed.
- M. Machida, S. Murakami, T. Kijima, S. Matsushima and M. Arai, Photocatalytic Property and Electronic Structure of Lanthanide Tantalates, LnTaO4 (Ln = La, Ce, Pr, Nd, and Sm), J. Phys. Chem. B, 2001, 105(16), 3289–3294 Search PubMed.
- H. Wakayama, K. Hibino, K. Fujii, T. Oshima, K. Yanagisawa, Y. Kobayashi, K. Kimoto, M. Yashima and K. Maeda, Synthesis of a Layered Niobium Oxynitride, Rb2NdNb2O6N· H2O, Showing Visible-Light Photocatalytic Activity for H2 Evolution, Inorg. Chem., 2019, 58(9), 6161–6166 CrossRef CAS PubMed.
- L. Gastaldi, D. Carre and M. P. Pardo, Structure de l’oxysulfure d’indium et de Lanthane In6La10O6S17, Acta Crystallogr., Sect. B, 1982, 38(9), 2365–2367 CrossRef.
- K. Ogisu, A. Ishikawa, K. Teramura, K. Toda, M. Hara and K. Domen, Lanthanum–Indium Oxysulfide as a Visible Light Driven Photocatalyst for Water Splitting, Chem. Lett., 2007, 36(7), 854–855 CrossRef CAS.
- S. Jaulmes, Oxysulfure de Gallium et de Lanthane LaGaOS2, Acta Crystallogr., Sect. B, 1978, 34(8), 2610–2612 CrossRef.
- S. Jaulmes, A. Mazurier and M. Guittard, Structure de l’oxypentasulfure de Gallium et de Trilanthane, GaLa3OS5, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1983, 39(12), 1594–1597 CrossRef.
- K. Ogisu, A. Ishikawa, Y. Shimodaira, T. Takata, H. Kobayashi and K. Domen, Electronic Band Structures and Photochemical Properties of La−Ga-Based Oxysulfides, J. Phys. Chem. C, 2008, 112(31), 11978–11984 Search PubMed.
- V. Meignen, L. Cario, A. Lafond, Y. Moëlo, C. Guillot-Deudon and A. Meerschaut, Crystal Structures of Two New Oxysulfides La5Ti2MS5O7 (M = Cu, Ag): Evidence of Anionic Segregation, J. Solid State Chem., 2004, 177(8), 2810–2817 Search PubMed.
- M. Katayama, D. Yokoyama, Y. Maeda, Y. Ozaki, M. Tabata, Y. Matsumoto, A. Ishikawa, J. Kubota and K. Domen, Fabrication and Photoelectrochemical Properties of La5Ti2MS5O7 (M= Ag, Cu) Electrodes, Mater. Sci. Eng., B, 2010, 173(1–3), 275–278 Search PubMed.
- T. Suzuki, T. Hisatomi, K. Teramura, Y. Shimodaira, H. Kobayashi and K. Domen, A Titanium-Based Oxysulfide Photocatalyst: La 5 Ti 2 MS 5 O 7 (M= Ag, Cu) for Water Reduction and Oxidation, Phys. Chem. Chem. Phys., 2012, 14(44), 15475–15481 Search PubMed.
- J. Liu, T. Hisatomi, G. Ma, A. Iwanaga, T. Minegishi, Y. Moriya, M. Katayama, J. Kubota and K. Domen, Improving the Photoelectrochemical Activity of La5Ti2CuS5O7 for Hydrogen Evolution by Particle Transfer and Doping, Energy Environ. Sci., 2014, 7(7), 2239–2242 RSC.
- G. Ma, Y. Suzuki, R. B. Singh, A. Iwanaga, Y. Moriya, T. Minegishi, J. Liu, T. Hisatomi, H. Nishiyama and M. Katayama, Photoanodic and Photocathodic Behaviour of La 5 Ti 2 CuS 5 O 7 Electrodes in the Water Splitting Reaction, Chem. Sci., 2015, 6(8), 4513–4518 RSC.
- H. Hirose, K. Ueda, H. Kawazoe and H. Hosono, Electronic Structure of Sr2Cu2ZnO2S2 Layered Oxysulfide with CuS Layers, Chem. Mater., 2002, 14(3), 1037–1041 CrossRef CAS.
- S. Nandy, T. Hisatomi, M. Katayama, T. Minegishi and K. Domen, Effects of Calcination Temperature on the Physical Properties and Hydrogen Evolution Activities of La5Ti2Cu(S1-Se)5O7 Photocatalysts, Part. Part. Syst. Charact., 2018, 35(1), 1700275, DOI:10.1002/ppsc.201700275.
- Z. Pan, Q. Xiao, S. Chen, Z. Wang, L. Lin, M. Nakabayashi, N. Shibata, T. Takata, T. Hisatomi and K. Domen, Synthesis of a Ga-Doped La5Ti2Cu0.9Ag0.1O7S5 Photocatalyst by Thermal Sulfidation for Hydrogen Evolution under Visible Light, J. Catal., 2021, 399, 230–236, DOI:10.1016/j.jcat.2021.05.015.
- X. Tang, H. Ye and H. Hu, Sulfurization Synthesis and Photocatalytic Activity of Oxysulfide La3NbS2O5, Trans. Nonferrous Met. Soc. China, 2013, 23(9), 2644–2649, DOI:10.1016/S1003-6326(13)62780-6.
- A. BaQais, A. Curutchet, A. Ziani, H. Ait Ahsaine, P. Sautet, K. Takanabe and T. Le Bahers, Bismuth Silver Oxysulfide for Photoconversion Applications: Structural and Optoelectronic Properties, Chem. Mater., 2017, 29(20), 8679–8689, DOI:10.1021/acs.chemmater.7b02664.
- M. Ogawa, H. Suzuki, O. Tomita, A. Nakada and R. Abe, Sn2+-Based Pyrochlore Oxysulfides with Narrow Band Gaps for Photocatalytic Water Splitting, J. Photochem. Photobiol., A, 2023, 444, 114895, DOI:10.1016/j.jphotochem.2023.114895.
- K. H. Chu, L. Ye, W. Wang, D. Wu, D. K. L. Chan, C. Zeng, H. Y. Yip, J. C. Yu and P. K. Wong, Enhanced Photocatalytic Hydrogen Production from Aqueous Sulfide/Sulfite Solution by ZnO0.6S0.4 with Simultaneous Dye Degradation under Visible-Light Irradiation, Chemosphere, 2017, 183, 219–228, DOI:10.1016/j.chemosphere.2017.05.112.
- N. S. Gultom, H. Abdullah and D.-H. Kuo, Enhanced Photocatalytic Hydrogen Production of Noble-Metal Free Ni-Doped Zn(O,S) in Ethanol Solution, Int. J. Hydrogen Energy, 2017, 42(41), 25891–25902, DOI:10.1016/j.ijhydene.2017.08.198.
- N. S. Gultom, H. Abdullah, J.-C. Xie, H. Shuwanto and D.-H. Kuo, Improved Hydrogen Production Rate of a Nickel-Doped Zinc Indium Oxysulfide Visible-Light Catalyst: Comparative Study of Stoichiometric and Nonstoichiometric Compounds, ACS Appl. Energy Mater., 2022, 5(2), 1755–1766, DOI:10.1021/acsaem.1c03200.
- X. Chen, Q. Wu, D.-H. Kuo, A. B. Abdeta, H. Zhang, P. Li, T. Huang, O. A. Zelekew and J. Lin, Initiating Highly Efficient (Bi,Ce)2(O,S)3−x Oxysulfide Catalysts with Rich Oxygen Vacancies for Hydrogen Evolution via Adjusting Valence Band Configuration, J. Mater. Chem. A, 2023, 11(8), 4126–4141, 10.1039/D2TA09780J.
- S. Mallick, F. Orlandi, P. Manuel, W. Zhang, P. S. Halasyamani and M. A. Hayward, MnCaTa2O7—A Magnetically Ordered Polar Phase Prepared via Cation Exchange, Chem. Mater., 2023, 35(18), 7839–7846, DOI:10.1021/acs.chemmater.3c01850.
- H. Yoshida, P. Zhenhua, S. Ryota, M. Vikas, M. Hiroyuki, S. Kazuhiko, H. Takashi and D. Kazunari, Heterogeneous doping of visible-light-responsive Y2Ti2O5S2 for enhanced hydrogen evolution, J. Mater. Chem. A, 2022, 10(46), 24552–24560, 10.1039/D2TA06895H.
- T. Hisatomi, S. Okamura, J. Liu, Y. Shinohara, K. Ueda, T. Higashi, M. Katayama, T. Minegishi and K. Domen, La5Ti2Cu1−xAgxS5O7 Photocathodes Operating at Positive Potentials during Photoelectrochemical Hydrogen Evolution under Irradiation of up to 710 Nm, Energy Environ. Sci., 2015, 8(11), 3354–3362 RSC.
- H. Liang, H. Sun, S. Jiang, S. Cui, F. Song, L. Fan and Q. Xu, Two-Dimensional (2D) Oxysulfide Nanosheets with Sulfur-Rich Vacancy as an Visible-Light-Driven Difunctional Photocatalyst for Hydrogen and Oxygen Evolution, J. Alloys Compd., 2024, 1004, 175898, DOI:10.1016/j.jallcom.2024.175898.
- J. Cui, C. Li and F. Zhang, Development of Mixed-Anion Photocatalysts with Wide Visible-Light Absorption Bands for Solar Water Splitting, ChemSusChem, 2019, 12(9), 1872–1888 CrossRef CAS PubMed.
- Y. Goto, J. Seo, K. Kumamoto, T. Hisatomi, Y. Mizuguchi, Y. Kamihara, M. Katayama, T. Minegishi and K. Domen, Crystal Structure, Electronic Structure, and Photocatalytic Activity of Oxysulfides: La2Ta2ZrS2O8, La2Ta2TiS2O8, and La2Nb2TiS2O8, Inorg. Chem., 2016, 55(7), 3674–3679 CrossRef CAS PubMed.
- J. P. Allen, J. J. Carey, A. Walsh, D. O. Scanlon and G. W. Watson, Electronic Structures of Antimony Oxides, J. Phys. Chem. C, 2013, 117(28), 14759–14769 CrossRef CAS.
- R. Vadapoo, S. Krishnan, H. Yilmaz and C. Marin, Self-Standing Nanoribbons of Antimony Selenide and Antimony Sulfide with Well-Defined Size and Band Gap, Nanotechnology, 2011, 22(17), 175705 CrossRef PubMed.
- F. Liang, L. Kang, Z. Lin and Y. Wu, Mid-Infrared Nonlinear Optical Materials Based on Metal Chalcogenides: Structure–Property Relationship, Cryst. Growth Des., 2017, 17(4), 2254–2289 CrossRef CAS.
- K. Sayama, A. Nomura, Z. Zou, R. Abe, Y. Abe and H. Arakawa, Photoelectrochemical Decomposition of Water on Nanocrystalline BiVO 4 Film Electrodes under Visible Light, Chem. Commun., 2003, 2908–2909 RSC.
- S. R. Kavanagh, C. N. Savory, D. O. Scanlon and A. Walsh, Hidden Spontaneous Polarisation in the Chalcohalide Photovoltaic Absorber Sn2SbS2I3, Mater. Horiz., 2021, 8(10), 2709–2716 RSC.
- X. Wang, Z. Li, S. R. Kavanagh, A. M. Ganose and A. Walsh, Lone Pair Driven Anisotropy in Antimony Chalcogenide Semiconductors, Phys. Chem. Chem. Phys., 2022, 24(12), 7195–7202 RSC.
- K. M. Ok, Functional Layered Materials with Heavy Metal Lone Pair Cations, Pb2+, Bi3+, and Te4+, Chem. Commun., 2019, 55(85), 12737–12748 RSC.
- J. V. Handy, W. Zaheer, A. R. Rothfuss, C. R. McGranahan, G. Agbeworvi, J. L. Andrews, K. E. García-Pedraza, J. D. Ponis, J. R. Ayala and Y. Ding, Lone but Not Alone: Precise Positioning of Lone Pairs for the Design of Photocatalytic Architectures, Chem. Mater., 2022, 34(4), 1439–1458 CrossRef CAS.
- H. Suzuki, H. Kunioku, M. Higashi, O. Tomita, D. Kato, H. Kageyama and R. Abe, Lead Bismuth Oxyhalides PbBiO2X (X = Cl, Br) as Visible-Light-Responsive Photocatalysts for Water Oxidation: Role of Lone-Pair Electrons in Valence Band Engineering, Chem. Mater., 2018, 30(17), 5862–5869 CrossRef CAS.
- S. Al Bacha, S. Saitzek, H. Kabbour and E. E. McCabe, Iron Oxychalcogenides and Their Photocurrent Responses, Inorg. Chem., 2024, 63(7), 3292–3302, DOI:10.1021/acs.inorgchem.3c03672.
- J. Cui, C. Li and F. Zhang, Development of Mixed-Anion Photocatalysts with Wide Visible-Light Absorption Bands for Solar Water Splitting, ChemSusChem, 2019, 12(9), 1872–1888, DOI:10.1002/cssc.201801829.
- H. Zhang, L. Liu and Z. Zhou, Towards Better Photocatalysts: First-Principles Studies of the Alloying Effects on the Photocatalytic Activities of Bismuth Oxyhalides under Visible Light, Phys. Chem. Chem. Phys., 2012, 14(3), 1286–1292 RSC.
- J. Zhang, P. Zhou, J. Liu and J. Yu, New Understanding of the Difference of Photocatalytic Activity among Anatase, Rutile and Brookite TiO2, Phys. Chem. Chem. Phys., 2014, 16(38), 20382–20386 RSC.
- J. Zhang, W. Yu, J. Liu and B. Liu, Illustration of High-Active Ag2CrO4 Photocatalyst from the First-Principle Calculation of Electronic Structures and Carrier Effective Mass, Appl. Surf. Sci., 2015, 358, 457–462 CrossRef CAS.
- J. Yang, P. Jiang, M. Yue, D. Yang, R. Cong, W. Gao and T. Yang, Bi2Ga4O9: An Undoped Single-Phase Photocatalyst for Overall Water Splitting under Visible Light, J. Catal., 2017, 345, 236–244 CrossRef CAS.
- Y. Yao, Q. Li, W. Chu, Y. Ding, L. Yan, Y. Gao, A. Neogi, A. Govorov, L. Zhou and Z. Wang, Exploration of the Origin of the Excellent Charge-Carrier Dynamics in Ruddlesden–Popper Oxysulfide Perovskite Y2Ti2O5S2, Phys. Chem. Chem. Phys., 2023, 25(48), 32875–32882, 10.1039/D3CP02860G.
- N. Vonrüti, Ferroelectricity and Metastability in (Mixed-Anion) Perovskite Oxides for Improved Solar Water Splitting, 2019 Search PubMed.
- S. Al Bacha, S. Saitzek, E. E. McCabe and H. Kabbour, Photocatalytic and Photocurrent Responses to Visible Light of the Lone-Pair-Based Oxysulfide Sr6Cd2Sb6S10O7, Inorg. Chem., 2022, 61(46), 18611–18621, DOI:10.1021/acs.inorgchem.2c03040.
- N. Vonrüti and U. Aschauer, Catalysis on Oxidized Ferroelectric Surfaces—Epitaxially Strained LaTiO2N and BaTiO3 for Photocatalytic Water Splitting, J. Chem. Phys., 2020, 152(2), 024701 CrossRef PubMed.
- R. F. Berger, C. J. Fennie and J. B. Neaton, Band Gap and Edge Engineering via Ferroic Distortion and Anisotropic Strain: The Case of SrTiO3, Phys. Rev. Lett., 2011, 107(14), 146804 CrossRef PubMed.
- N. Vonrüti and U. Aschauer, Epitaxial Strain Dependence of Band Gaps in Perovskite Oxynitrides Compared to Perovskite Oxides, Phys. Rev. Mater., 2018, 2(10), 105401 CrossRef.
- N. Vonrüti and U. Aschauer, Band-Gap Engineering in AB (O x S 1− x) 3 Perovskite Oxysulfides: A Route to Strongly Polar Materials for Photocatalytic Water Splitting, J. Mater. Chem. A, 2019, 7(26), 15741–15748 RSC.
- N. Vonrüti and U. Aschauer, Anion Order and Spontaneous Polarization in LaTiO 2 N Oxynitride Thin Films, Phys. Rev. Lett., 2018, 120(4), 046001 CrossRef PubMed.
- J. Li, L. Cai, J. Shang, Y. Yu and L. Zhang, Giant Enhancement of Internal Electric Field Boosting Bulk Charge Separation for Photocatalysis, Adv. Mater., 2016, 28(21), 4059–4064 CrossRef CAS PubMed.
- S. Al Bacha, S. Saitzek, P. Roussel, M. Huvé, E. E. McCabe and H. Kabbour, Low Carrier Effective Masses in Photoactive Sr2Sb2O2Q3 (Q = S, Se): The Role of the Lone Pair, Chem. Mater., 2023, 35(22), 9528–9541, DOI:10.1021/acs.chemmater.3c01298.
- F. Xu, Z. Li, R. Zhu, Y. Chu, Z. Pan, S. Xia, J. Fu, Z. Xiao, X. Ji, M. Liu and B. Weng, Narrow Band-Gapped Perovskite Oxysulfide for CO2 Photoreduction towards Ethane, Appl. Catal., B, 2022, 316, 121615, DOI:10.1016/j.apcatb.2022.121615.
- A. C. Hernandez Oendra, M. A. Aspect, J. L. Jaeggi, J. Baumann, C. R. Lightner, A. B. Pun and D. J. Norris, Tunable Synthesis of Metal–Organic Chalcogenide Semiconductor Nanocrystals, Chem. Mater., 2023, 35(21), 9390–9398, DOI:10.1021/acs.chemmater.3c02275.
- N. Louvain, G. Frison, J. Dittmer, C. Legein and N. Mercier, Noncovalent Chalcogen Bonds and Disulfide Conformational Change in the Cystamine-Based Hybrid Perovskite [H3N(CH2)2SS(CH2)2NH3]PbIII4, Eur. J. Inorg. Chem., 2014,(2), 364–376, DOI:10.1002/ejic.201301017.
- A. Kumar, D. W. Chang and J.-B. Baek, Current Status and Future of Organic–Inorganic Hybrid Perovskites for Photoelectrocatalysis Devices, Energy Fuels, 2023, 37(23), 17782–17802, DOI:10.1021/acs.energyfuels.3c02680.
- P. Mukherjee, K. Sathiyan, A. K. Vijay, R. Bar-Ziv and T. Zidki, Hybrid Nanostructure of Mixed Transition Metal Oxysulfides Supported by Porous PBA as Efficient Electrocatalysts for the Oxygen Evolution Reaction, Isr. J. Chem., 2022, 62(3–4), e202100110, DOI:10.1002/ijch.202100110.
- A. L. Pacquette, H. Hagiwara, T. Ishihara and A. A. Gewirth, Fabrication of an Oxysulfide of Bismuth Bi2O2S and Its Photocatalytic Activity in a Bi2O2S/In2O3 Composite, J. Photochem. Photobiol., A, 2014, 277, 27–36, DOI:10.1016/j.jphotochem.2013.12.007.
- R. P. Tiwari, Visible-Light-Activated Enhanced Shift Current Bulk Photovoltaic Effect in Lead-Free Oxychalcogenide Perovskites: Emergence of Fully Inorganic Photovoltaic Materials, J. Phys. Chem. C, 2022, 126(25), 10258–10265, DOI:10.1021/acs.jpcc.2c01848.
Footnote |
| † Electronic supplementary information (ESI) available: Comparison tables of the structural and physical properties of oxysulfide, oxyselenide and oxytelluride materials. Estimated band edge positions using the empirical method of different oxychalcogenides. See DOI: https://doi.org/10.1039/d5cc01448d |
| This journal is © The Royal Society of Chemistry 2025 |