Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Late-stage lipidation of peptides via aqueous thiol-michael addition to dehydroalanine (Dha)†
Glenna
Swinand
 a,
Matthew
Rowe
a,
Katherine
Bowen
a,
Samir
Olatunji
b,
Martin
Caffrey
b and
Eoin M.
Scanlan
a,
Matthew
Rowe
a,
Katherine
Bowen
a,
Samir
Olatunji
b,
Martin
Caffrey
b and
Eoin M.
Scanlan
 *a
*a
aSchool of Chemistry, Trinity College Dublin, Trinity Biomedical Sciences Institute, Pearse St, Dublin 2, Ireland. E-mail: eoin.scanlan@tcd.ie
bSchool of Biochemistry and Immunology, Trinity College Dublin, Trinity Biomedical Sciences Institute, Pearse St, Dublin 2, Ireland
First published on 1st May 2025
Abstract
This work presents a late-stage aqueous peptide lipidation strategy via the thiol-Michael addition of thiolated lipids at dehydroalanine (Dha). This strategy was used to synthesise lipopeptides containing diacylglycerol (DAG), saturated, unsaturated and cholesterol lipid motifs. The DAG lipopeptide product was found to be a substrate for the lipoprotein processing enzyme, LspA.
Lipidation is a ubiquitous co- and post-translational modification (PTM) of peptides and proteins that regulates numerous biological pathways, including cell signalling, membrane trafficking and protein secretion.1 Typically, these lipids are fatty acyl or polyisoprenyl groups with modifications occurring at nucleophilic side chains, e.g., cysteine, serine, and lysine, as well as the N-termini of peptides and proteins. Given the prevalence and functional importance of peptide and protein lipidation, the development of chemical methods that enable facile access to these bioconjugates is of critical importance for expanding our understanding of their biological function. Thus, the introduction of novel synthetic and semisynthetic lipidation methods remains a topic of intense investigation with several efficient strategies being reported to date.1–3 These include in-line methods such as direct N- or S-lipidation during solid-phase peptide synthesis (SPPS),4,5 as well as the incorporation of a pre-lipidated amino acid building block during SPPS.6 Late-stage lipidation methods include direct alkylation,7 Cu click,8 thiol–ene click,9 and enzymatic lipidation,10 among others.11–13
DAG cysteine is a PTM found exclusively on bacterial lipoproteins with no eukaryotic orthologues.14 Perhaps unsurprisingly, lipopeptides containing a DAG Cys (also known as Pam2Cys) are well-known toll-like receptor agonists and therefore appear in many self-adjuvanting vaccine candidates.15,16 Synthetic lipopeptides displaying this DAG Cys motif have been used by our group and others as high-throughput probes for bacterial lipoprotein processing enzymes (Fig. 1B).17,18 However, late-stage chemical lipidation methods for installing DAG lipid groups onto peptides or proteins have not previously been reported.
Dehydroalanine (Dha) is naturally occurring in some peptides, the most common examples of which are the lanthipeptides.19 Dha is also frequently used as a reactive handle for peptide and protein modification in chemical synthesis. Following pioneering work by Davis and co-workers, Dha-mediated modification has been employed widely for the chemoselective installation of various functional groups onto peptides and proteins mimicking natural PTMs including glycosylation, phosphorylation, and lipidation (Fig. 1A).20,21 Furthermore, Dha has been used to install reactive functional groups and tags onto proteins and peptides.22–24 Dha is often generated in situ from a canonical amino acid and several methods for its formation have been reported.25
In this study, Dha is employed as a reactive intermediate under aqueous thiol-Michael addition conditions in a general approach to late-stage peptide lipidation (Fig. 1C). The strategy is applied to the synthesis of novel lipopeptides displaying DAG lipid motifs, including a Förster resonance energy transfer (FRET) probe. Chemical lipidation converts the peptide into a substrate for the bacterial enzyme lipoprotein signal peptidase II (LspA), highlighting the potential of this approach for the synthesis of functional peptides and proteins.
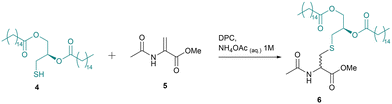
To begin our study, DAG thiol 4 was synthesised (Scheme 1). The initial two steps were informed by literature reports of a DAG Cys synthesis.17,18 A base-mediated, nucleophilic epoxide ring opening reaction furnished diol 2, followed by Steglich esterification with palmitic acid to furnish dipalmitoyl compound 3. The thiol moiety was deprotected upon treatment with BF3·OEt2 in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) as previously reported by Herczegh and co-workers.26
Next, optimisation studies of the thiol-Michael addition were performed using protected Dha amino acid 5 as a model system (Table 1). Inspired by the work of Boons and co-workers on the development of liposome-mediated native chemical ligation,27 we included the detergent dodecylphosphocholine (DPC) in our reaction mixture to aid the poor aqueous solubility of the DAG group. The critical micelle concentration of DPC is ∼1.1 mM.28 No product was formed in the absence of detergent (entry 1). When 1 equivalent of DAG thiol 4 was reacted with Dha amino acid 5 in the presence of DPC, a conversion of 95% to thioether 6 was determined after 18 h at rt (entry 2). Increasing the amount of thiol 4 to 2 equivalents resulted in a significant reduction in conversion however, this effect could be reversed by using a higher concentration of detergent, suggesting the potential disruption of micelle formation by the thiol when used in excess (entries 3 and 4). The reaction appeared tolerant of the higher concentration of detergent and this was maintained at 2.2 mM, even when the thiol concentration was reduced to 1.2 equivalents (entry 5).
This concentration was considered to be optimal and was retained for the remainder of Table 1. Next, the effects of varying pH were investigated with complete conversion of >99% observed under basic conditions (entry 7), whereas acidic conditions gave a reduced yield (entry 6). Basic conditions promote the formation of a more reactive thiolate and are well-known to catalyse thiol-Michael addition reactions.29 Indeed, it was determined that 86% and 98% conversion could be achieved in only 1 h and 3 h respectively, under basic conditions (entries 8 and 9). Anticipating potentially slower kinetics when this method was applied to more bulky peptide-based systems, we decided to proceed using the conditions which gave the highest yield overall (entry 7).
Using our optimised conditions (Table 1, entry 7), we next investigated a model peptide 7 with a series of lipid thiols (Fig. 2). The model peptide mimics the lipobox sequence in bacterial lipoproteins and can be found in activity probes for the bacterial lipoprotein processing enzyme, LspA.17 Dha was formed in situ from the corresponding cysteine derivative to furnish peptide 7, using conditions reported by Davis and co-workers (ESI†).25 The crude peptide was used directly in the thiol-Michael addition without purification. All reactions were monitored by HPLC and % conversion was determined after 18 h (Fig. 2).
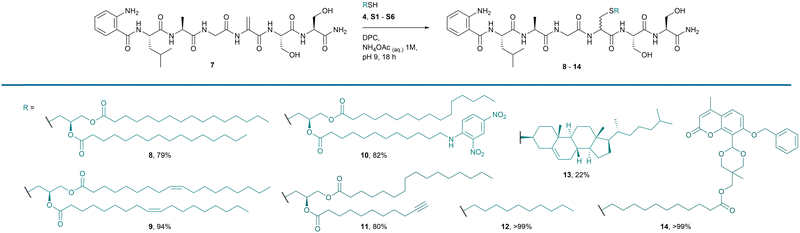 | ||
| Fig. 2 Scope of lipopeptides synthesised using optimised conditions. Percentages are conversion of starting material to product measured by HPLC after 18 h. | ||
Three additional DAG lipid thiols S1, S2 and S3 were accessed via modification of the synthesis of 4 as outlined in Scheme 1. Thiol S1 displays oleate esters, thiol S2 displays a quencher moiety commonly used in combination with aminobenzoic acid (Abz) as a FRET pair, and thiol S3 bears an alkyne functional group (Fig. S1, ESI†). Lipid thiols S4 and S5 were purchased from commercial suppliers and offer examples of both a simple monoalkyl chain lipid and a sterically challenging cholesterol derivative (Fig. S1, ESI†). Lipid thiol S6 displays an acid-labile acetal-linked coumarin fluorophore inspired by a probe published by Shao and co-workers (Fig. S1, ESI†).30 The highest conversions were achieved for lipopeptides 9, 12 and 14. The synthesis of lipopeptide 14 in high yield (>99%), with the retention of an acid-labile fluorescent tag, illustrates the advantage of the reported method over preparation by standard SPPS, which relies on a final acidic cleavage step. Good conversions were observed for 8 (79%), 10 (82%) and 11 (80%). This demonstrates a tolerance for the unnatural fluorophore structures in 10 and 14, which are key for the development of high-throughput probes, and the alkyne click-chemistry handle on peptide 11. Cholesterol derivative 13 reacted with lower conversion, likely due to the steric bulk and poor solubility of the substrate. As is common with addition reactions to Dha, no diastereoselectivity was observed in our reaction.31 It was shown by 1H NMR analysis of peptide 10 that the two diastereomers composing the lipopeptide product were present in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (ESI†). Importantly, these results demonstrate the broad scope of the methodology and its compatibility with saturated, unsaturated, and structurally complex lipids.
1 ratio (ESI†). Importantly, these results demonstrate the broad scope of the methodology and its compatibility with saturated, unsaturated, and structurally complex lipids.
To further investigate the scope of this reaction, the method was applied to two additional model peptides to assess the feasibility of the reaction in the presence of other potentially reactive canonical amino acid side chains. Peptide 15 is inspired by the sequence found at the lipidation site of the drug liraglutide. Dha was once again formed in situ from cysteine with an increase in the reaction time from 5 h to 8 h required for conversion of Cys to Dha on both peptides 15 and 17 (ESI†). The crude peptides were reacted with decane thiol S4 under the established conditions to furnish lipopeptides 16 and 18 in >99% conversion (Fig. 3). Glu underwent significant side reactions during the Dha-formation reaction when incorporated into a model peptide sequence. Thus Glu, Asp and Lys were also not used in the thiol-Michael addition reaction due to their nucleophilicity under basic conditions which could lead to inefficiency in the Dha-forming reaction step. These findings highlight the robustness of the method in the presence of the canonical amino acids.
To further validate this method, lipopeptide 10 was isolated and tested as a substrate and activity probe for the bacterial lipoprotein processing enzyme, LspA. The natural function of LspA is to cleave a signal peptide from the N-terminus of lipidated bacterial proteins.14 LspA cleaves the peptide bond between Cys and Gly in the highly conserved lipobox recognition sequence LAGC. Lipid S2 contains the LAGC sequence, together with a dinitrophenyl group that creates a FRET pair with the Abz residue on the N-terminus of peptide 10. Thus, as LspA cleaves the peptide bond between Cys and Gly, separating the FRET pair, the fluorescence of Abz increases. A similar FRET probe and the assay conditions were previously developed by our groups for a high-throughput drug discovery assay.17 Lipopeptide 10 was evaluated as a substrate for LspA from Pseudomonas aeruginosa (LspPae). Using our established method, time course assays measuring the increase in fluorescence at 420 nm, characteristic of Abz, were run at a series of substrate concentrations (Fig. S2, ESI†). Thus, using a Michaelis–Menten kinetics model, the kinetic parameters Vmax and Km for the enzymatic hydrolysis of peptide 10 by LspPae were determined to be 13.4 nM min−1 and 12.5 μM respectively (Fig. 4). These results establish the capability of our late-stage lipidation method in synthesising a novel activity probe for LspPae.
 | ||
| Fig. 4 Kinetic assay substrate saturation plot for the hydrolysis of peptide 10 by LspPae. Data points represent mean values ± SD (n = 3). | ||
In conclusion, a robust approach to late-stage peptide lipidation that combines Dha chemistry with mild, aqueous thiol-Michael addition has been developed. The protocol enables direct, chemoselective lipidation of peptides with the biologically important DAG lipid moiety to furnish functional peptides. The methodology is compatible with saturated, unsaturated and complex lipids as well as various peptide sequences. This method was employed for the synthesis of a novel FRET activity probe for the bacterial enzyme LspA, offering considerable potential for antibacterial drug discovery. The methodology is expected to find widespread application in furthering our understanding of bacterial lipoprotein biology and in the discovery of novel therapeutics.
This project was funded by the Irish Research Council project ID GOIPG/2020/312 and Science Foundation Ireland grants 19/FFP/6667 (E. M. S), 16/IA/4435 (M. C.), 22/FFP-A/10278 (M. C.), GOIPG/2020/312 (G. S.) and GOIPG/2023/3976 (M. R.)
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- C. C. Hanna, J. Kriegesmann, L. J. Dowman, C. F. W. Becker and R. J. Payne, Angew. Chem., Int. Ed., 2022, 61, e202111266 CrossRef CAS PubMed.
- W. Ma, H. Liu and X. Li, ChemBioChem, 2023, 24, e202300348 CrossRef CAS.
- T. Mejuch and H. Waldmann, Bioconjugate Chem., 2016, 27, 1771–1783 CrossRef CAS PubMed.
- R. D. Ballantine, K. Al Ayed, S. J. Bann, M. Hoekstra, N. I. Martin and S. A. Cochrane, RSC Med. Chem., 2022, 13, 1640–1643 RSC.
- B. Ludolph, F. Eisele and H. Waldmann, J. Am. Chem. Soc., 2002, 124, 5954–5955 Search PubMed.
- J. M. Palomo, M. Lumbierres and H. Waldmann, Angew. Chem., Int. Ed., 2006, 45, 477–481 CrossRef CAS PubMed.
- A. D. de Araujo, H. T. Nguyen and D. P. Fairlie, ChemBioChem, 2021, 22, 1784–1789 CrossRef CAS.
- L. Guo, C. Wang, J. Broos and O. P. Kuipers, J. Biol. Chem., 2023, 299, 104845 CrossRef CAS PubMed.
- S.-H. Yang, P. W. R. Harris, G. M. Williams and M. A. Brimble, Eur. J. Org. Chem., 2016, 2608–2616 CrossRef CAS.
- Y. Zheng, Y. Cong, E. W. Schmidt and S. K. Nair, Acc. Chem. Res., 2022, 55, 1313–1323 CrossRef CAS.
- D. Kobayashi, E. Kuraoka, J. Hayashi, T. Yasuda, Y. Kohmura, M. Denda, N. Harada, N. Inagaki and A. Otaka, ACS Med. Chem. Lett., 2022, 13, 1125–1130 CrossRef CAS PubMed.
- N. Nischan, M. A. Kasper, T. Mathew and C. P. R. Hackenberger, Org. Biomol. Chem., 2016, 14, 7500–7508 RSC.
- T. Schlatzer, J. Kriegesmann, H. Schroder, M. Trobe, C. Lembacher-Fadum, S. Santner, A. V. Kravchuk, C. F. W. Becker and R. Breinbauer, J. Am. Chem. Soc., 2019, 141, 14931–14937 CrossRef CAS.
- L. Smithers, S. Olatunji and M. Caffrey, Front. Microbiol., 2021, 12 Search PubMed.
- D. Ding, Y. Wen, C.-M. Liao, X.-G. Yin, R.-Y. Zhang, J. Wang, S.-H. Zhou, Z.-M. Zhang, Y.-K. Zou, X.-F. Gao, H.-W. Wei, G.-F. Yang and J. Guo, J. Med. Chem., 2023, 66, 1467–1483 CrossRef CAS.
- I. W. Hamley, Bioconjugate Chem., 2021, 32, 1472–1490 CrossRef CAS PubMed.
- S. Olatunji, X. Yu, J. Bailey, C.-Y. Huang, M. Zapotoczna, K. Bowen, M. Remškar, R. Müller, E. M. Scanlan, J. A. Geoghegan, V. Olieric and M. Caffrey, Nat. Commun., 2020, 11, 140 CrossRef CAS PubMed.
- S. Kitamura and D. W. Wolan, FEBS Lett., 2018, 592, 2289–2296 CrossRef CAS.
- S. Wang, K. Wu, Y.-J. Tang and H. Deng, Nat. Prod. Rep., 2024, 41, 273–297 RSC.
- G. J. Bernardes, J. M. Chalker, J. C. Errey and B. G. Davis, J. Am. Chem. Soc., 2008, 130, 5052–5053 CrossRef CAS.
- T. H. Wright, B. J. Bower, J. M. Chalker, G. J. L. Bernardes, R. Wiewiora, W.-L. Ng, R. Raj, S. Faulkner, M. R. J. Vallée, A. Phanumartwiwath, O. D. Coleman, M.-L. Thézénas, M. Khan, S. R. G. Galan, L. Lercher, M. W. Schombs, S. Gerstberger, M. E. Palm-Espling, A. J. Baldwin, B. M. Kessler, T. D. W. Claridge, S. Mohammed and B. G. Davis, Science, 2016, 354, aag1465 Search PubMed.
- B. Josephson, C. Fehl, P. G. Isenegger, S. Nadal, T. H. Wright, A. W. J. Poh, B. J. Bower, A. M. Giltrap, L. Chen, C. Batchelor-McAuley, G. Roper, O. Arisa, J. B. I. Sap, A. Kawamura, A. J. Baldwin, S. Mohammed, R. G. Compton, V. Gouverneur and B. G. Davis, Nature, 2020, 585, 530–537 CrossRef CAS.
- R. Petracca, K. A. Bowen, L. McSweeney, S. O’Flaherty, V. Genna, B. Twamley, M. Devocelle and E. M. Scanlan, Org. Lett., 2019, 21, 3281–3285 CrossRef CAS PubMed.
- G. Bao, P. Wang, X. Guo, Y. Li, Z. He, X. Song, T. Yu, J. Xie and W. Sun, Org. Lett., 2023, 25, 8338–8343 Search PubMed.
- J. M. Chalker, S. B. Gunnoo, O. Boutureira, S. C. Gerstberger, M. Fernández-González, G. J. L. Bernardes, L. Griffin, H. Hailu, C. J. Schofield and B. G. Davis, Chem. Sci., 2011, 2, 1666–1676 Search PubMed.
- M. Kicsák, M. Bege, I. Bereczki, M. Csávás, M. Herczeg, Z. Kupihár, L. Kovács, A. Borbás and P. Herczegh, Org. Biomol. Chem., 2016, 14, 3190–3192 RSC.
- S. Ingale, T. Buskas and G.-J. Boons, Org. Lett., 2006, 8, 5785–5788 CrossRef CAS.
- R. E. Stafford, T. Fanni and E. A. Dennis, Biochemistry, 1989, 28, 5113–5120 CrossRef CAS PubMed.
- D. Berne, V. Ladmiral, E. Leclerc and S. Caillol, Polymers, 2022, 14, 4457 CrossRef CAS PubMed.
- Y. Jiang, R. Li, F. Ren, S. Yang and A. Shao, Bioconjugate Chem., 2024, 35, 72–79 Search PubMed.
- J. Dadová, S. R. G. Galan and B. G. Davis, Curr. Opin. Chem. Biol., 2018, 46, 71–81 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cc01085c |
| This journal is © The Royal Society of Chemistry 2025 |