Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pseudorotaxane monolayers of pillar[5]arene and linear fatty acids at the air–water interface†
Masaki
Ishii
 *ab,
Yuto
Nakai
ab,
Yu
Yamashita
*ab,
Yuto
Nakai
ab,
Yu
Yamashita
 a,
Katsuto
Onishi
c,
Tan-hao
Shi
c,
Nobutaka
Shioya
d,
Hideki
Sakai
b,
Takeshi
Hasegawa
a,
Katsuto
Onishi
c,
Tan-hao
Shi
c,
Nobutaka
Shioya
d,
Hideki
Sakai
b,
Takeshi
Hasegawa
 d,
Tomoki
Ogoshi
ce,
Katsuhiko
Ariga
d,
Tomoki
Ogoshi
ce,
Katsuhiko
Ariga
 abf and
Taizo
Mori
d
abf and
Taizo
Mori
d
aResearch Center for Materials Nanoarchitectonics (MANA), National Institute for Materials Science (NIMS), Tsukuba, Ibaraki, Japan
bGraduate School of Science and Technology, Tokyo University of Science, Noda, Chiba, Japan. E-mail: 7221701@ed.tus.ac.jp
cGraduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, Japan
dInstitute for Chemical Research, Kyoto University, Uji, Kyoto, Japan
eWPI Nano Life Science Institute, Kanazawa University, Kakuma-machi, Kanazawa, Japan
fGraduate School of Frontier Science, The University of Tokyo, Kashiwa, Chiba, Japan
First published on 5th June 2025
Abstract
Pseudorotaxanes, which are formed by macrocyclic host molecules and linear guest molecules, show potential in molecular devices and surface applications. In this study, ethoxy-functionalized pillar[5]arene (P5A) and amphiphilic linear fatty acid guests were self-assembled into oriented monolayers at the air–water interface. The fatty acid structure dictates the monolayer formation: [2]pseudorotaxane-based, [3]pseudorotaxane-based and phase-separated monolayers. These findings provide insights into P5A-based pseudorotaxane monolayers, facilitating their integration into advanced functional materials.
Pseudorotaxanes are formed by a macrocyclic host molecule and a linear guest molecule that exhibits specific non-covalent interactions with the host.1 The tiling of oriented pseudorotaxanes particularly in two-dimensional planes is promising for applications such as molecular shuttles,2,3 surface wettability control,4 sensors,5 catalysts,6 and transistors.7 Compared with the commonly used self-assembled monolayer (SAM) method for surface modification, the Langmuir--Blodgett (LB) technique8 imposes significantly fewer material limitations because of the lack of chemical bonds between the pseudorotaxane terminal functional groups and the substrate surface. Additionally, this method allows for control of the density and orientation of pseudorotaxanes through in-plane mechanical compression.
Pillararenes, first developed in 2008,9 are a class of macrocyclic host molecules, with cyclopentamer (P5A) and cyclohexamer (P6A) derivatives being the most commonly used. Compared to other macrocyclic host molecules, such as crown ethers and calixarenes, pillararenes offer advantages in terms of easy synthesis, flexible functionality, and high symmetry.10 In particular, P5A forms pseudorotaxanes with saturated alkanes via C–H⋯π interactions.11,12 Recent studies have demonstrated that P5A-based pseudorotaxanes can exhibit bistable states,13,14 offering applications such as molecular machines, similar to those investigated in “blue box” systems.3 Accordingly, two-dimensional tiling of P5A-based pseudorotaxanes can maximize the expression of switchable surface functionalities. Therefore, understanding P5A-based pseudorotaxane monolayers at the air–water interface is expected to open new avenues for various applications.
In this study, we found that pseudorotaxanes, which is composed of ethoxy-functionalized pillar[5]arene (EtP5A) and linear fatty acid guests, were oriented and assembled in a monolayer at the air–water interface. The chemical structure of the fatty acid guest dictates the resulting monolayer structure; short-chain linear fatty acids lead to [2]pseudorotaxane monolayers, long-chain linear fatty acids form [3]pseudorotaxane monolayers, and a fatty acid guest unsuitable for EtP5A undergoes phase separation (Fig. 1a).
 | ||
| Fig. 1 Pseudorotaxane of EtP5A at the air–water interface. (a) Molecular structures and schematics of monolayer states for employed EtP5A host and guests. (b) π–A isotherms for pure EtP5A (black line), mixture with G5-18 (red line) and with G6 (blue line). (c) DFT-calculated IR spectrum of EtP5A with experimental one of the powder (Section 1.4, ESI†). MAIRS spectra of LB films for pure EtP5A, mixture with G5-18 and pure G5-18 in the 1550–1300 cm−1 (d) and 3000–2800 cm−1 (e) regions. The OP (pink line) and IP (light blue line) indicate the out-of-plane and in-plane directions of the LB films, respectively. | ||
To elucidate the monolayer state of Langmuir films at the air–water interface, we measured the surface pressure–area (π–A) isotherms (Fig. 1b). Pure EtP5A exhibited a lift-off of the surface pressure near 1.3 nm2 and monolayer collapse at 10.3 mN m−1. Interestingly, given the good agreement with the theoretical calculations (1.42 nm2), the pure EtP5A with simple ethoxy substitution was found to orient its cavities perpendicular to the water surface, as observed in previous studies using pillararene with longer and more complex substitution.15,16 The relatively low collapse pressure (πC) suggested weak intermolecular interactions within the plane. To obtain a stable and oriented pseudorotaxane monolayer, we mixed a chloroform solution with amphiphilic G5-18, which has been widely employed in insoluble monolayer studies.8,17 Mixing with G5-18 at a molar ratio of 1 : 1 decreased the limiting cross-sectional area (AL) per EtP5A molecule from 1.28 nm2 to 0.91 nm2, as calculated by extrapolating the π–A isotherms. The reduction in molecular area by as much as 0.37 nm2 does not occur if EtP5A and G5-18 form no complexes or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes, suggesting the formation of [3]pseudorotaxane structure at the air–water interface. Note that the AL per EtP5A molecule of 0.91 nm2 is close to the sum of one-half of the AL of 1.28 nm2 for a single PA molecule and the AL of 0.2 nm2 for an alkyl chain, which support [3]pseudorotaxane formation. Furthermore, the [3]pseudorotaxane structure might contribute to the formation of a more in-plane rigid film as confirmed by the significant increase in πC of 43.2 mN m−1 compared to 10.3 mN m−1 of pure EtP5A. On the other hand, when mixed with G6, a guest molecule incompatible with the cavity size of EtP5A, π–A isotherm shifted toward larger molecular areas corresponding to the cross-sectional area of G6, with minimal changes in πC compared to pure EtP5A. This behavior indicates phase separation within the two-dimensional film.18 Similar phase separation was observed when EtP5A and G5-18 were sequentially spread on the water surface (Fig. S6, ESI†). Note that host–guest chemistry strongly influences these phenomena, as demonstrated by the formation of pseudorotaxanes with G6 and phase separation with G5 when using EtP6A as the host (Fig. S7, ESI†).
1 complexes, suggesting the formation of [3]pseudorotaxane structure at the air–water interface. Note that the AL per EtP5A molecule of 0.91 nm2 is close to the sum of one-half of the AL of 1.28 nm2 for a single PA molecule and the AL of 0.2 nm2 for an alkyl chain, which support [3]pseudorotaxane formation. Furthermore, the [3]pseudorotaxane structure might contribute to the formation of a more in-plane rigid film as confirmed by the significant increase in πC of 43.2 mN m−1 compared to 10.3 mN m−1 of pure EtP5A. On the other hand, when mixed with G6, a guest molecule incompatible with the cavity size of EtP5A, π–A isotherm shifted toward larger molecular areas corresponding to the cross-sectional area of G6, with minimal changes in πC compared to pure EtP5A. This behavior indicates phase separation within the two-dimensional film.18 Similar phase separation was observed when EtP5A and G5-18 were sequentially spread on the water surface (Fig. S6, ESI†). Note that host–guest chemistry strongly influences these phenomena, as demonstrated by the formation of pseudorotaxanes with G6 and phase separation with G5 when using EtP6A as the host (Fig. S7, ESI†).
To investigate the molecular orientation and pseudorotaxane formation, we performed multiple-angle incidence resolution spectroscopy (MAIRS) measurements on LB films transferred from the water surface. This method involves tilting the thin film relative to the optical axis and analyzing the infrared absorption spectra acquired at varying polarization angles.19 The MAIRS method can separate the out-of-plane (OP) and in-plane (IP) absorption components of the film materials. Unlike traditional approaches that combine transmission and reflection methods,17 the MAIRS method offers the advantage of measuring one identical film with fewer substrate constraints. EtP5A exhibited similar absorption characteristics in both the powder and LB films (Fig. 1c and d). According to density functional theory (DFT) calculations, the most intense absorption bands at 1502 cm−1 and 1408 cm−1 were mainly consisted of aromatic C–H bending, aromatic C–H stretching, CH2 wagging, and CH3 wagging vibrations (Section 1.4, ESI†). The strong transition moments along the cavity direction of EtP5A can be useful for assessing the molecular orientation. For both LB films of pure EtP5A and the mixture of EtP5A and G5-18, these vibrational bands were more prominent in the OP spectrum than in the IP spectrum, indicating the vertical orientation of the EtP5A cavity, both at the air–water interface and after transfer to solid substrates. This vertical orientation of EtP5A corresponds to the fact that the AL obtained by π–A isotherm is close to the calculated cross-section value of 1.42 nm2 for pure EtP5A. Additionally, pure G5-18 exhibited characteristic vibrational peaks due to CH2trans-zigzag packing owing to its crystallinity, specifically CH2 antisymmetric stretching vibrations (2920 cm−1) and CH2 symmetric stretching vibrations (2850 cm−1) (Fig. 1e).20,21 Upon mixing with EtP5A, these peaks shifted to higher wavenumbers, indicating that the alkyl chains adopted a gauche conformation and supporting the formation of inclusion complexes with EtP5A.
Considering that the formation behavior of EtP5A-based pseudorotaxanes depends on the chain length of the guest molecules,12 the monolayer state was investigated using guests with carbon numbers ranging from 14 to 26, which can independently form insoluble monolayers. In this study, the molecules were mixed at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio before spreading to the air–water interface. The π–A isotherms for each system, guest-dependent AL, and guest-dependent collapse areas and πC are shown in Fig. 2a, b, and c, respectively. All the molecular areas were calculated per EtP5A molecule. Compared to pure EtP5A, the πC for all mixed systems increased, suggesting that pseudorotaxane formation and concomitant lateral hydrophobic interactions between alkyl chains improved monolayer rigidity. The constant AL and πC indicate that EtP5A forms a nearly identical two-dimensional film when mixed with guests having carbon numbers greater than 18, where [3]pseudorotaxane is stabilized as discussed later. In contrast, the mixed systems with G5-14 and G5-16 exhibited larger AL and lower πC than those with G5-18 and other guests. Note that a similar collapse area of 1.20 nm2 for pure EtP5A and the mixture with G5-14 implies that the mixed system mainly consists of a 1
1 molar ratio before spreading to the air–water interface. The π–A isotherms for each system, guest-dependent AL, and guest-dependent collapse areas and πC are shown in Fig. 2a, b, and c, respectively. All the molecular areas were calculated per EtP5A molecule. Compared to pure EtP5A, the πC for all mixed systems increased, suggesting that pseudorotaxane formation and concomitant lateral hydrophobic interactions between alkyl chains improved monolayer rigidity. The constant AL and πC indicate that EtP5A forms a nearly identical two-dimensional film when mixed with guests having carbon numbers greater than 18, where [3]pseudorotaxane is stabilized as discussed later. In contrast, the mixed systems with G5-14 and G5-16 exhibited larger AL and lower πC than those with G5-18 and other guests. Note that a similar collapse area of 1.20 nm2 for pure EtP5A and the mixture with G5-14 implies that the mixed system mainly consists of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex, that is, [2]pseudorotaxane. The gradual shift in the AL with increasing carbon number indicates that the stoichiometry of pseudorotaxane formation depends on the carbon number of the guest molecules.12 In other words, it is plausible that the composition of the monolayers—[2]pseudorotaxane, [3]pseudorotaxane, and uncomplexed molecules—varies depending on the type of guest molecule, given that the π–A isotherms provide average information about the Langmuir films on the water surface, which extend over an area of several hundred cm2.
1 complex, that is, [2]pseudorotaxane. The gradual shift in the AL with increasing carbon number indicates that the stoichiometry of pseudorotaxane formation depends on the carbon number of the guest molecules.12 In other words, it is plausible that the composition of the monolayers—[2]pseudorotaxane, [3]pseudorotaxane, and uncomplexed molecules—varies depending on the type of guest molecule, given that the π–A isotherms provide average information about the Langmuir films on the water surface, which extend over an area of several hundred cm2.
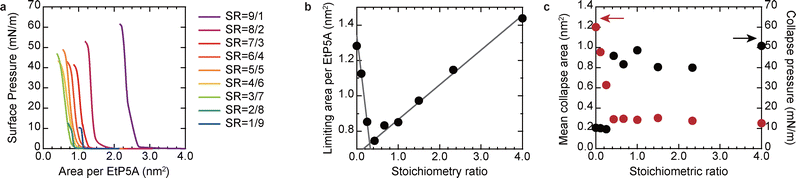
To assess the effect of the molecular mixing ratio, we analyzed π–A isotherms for a mixture with G5-20, which has a sufficiently long carbon chain to stabilize the monolayer state, as shown in Fig. 2b and c. Here, when nA represents the number of molecules of A, the host–guest stoichiometric ratio (SR) is defined as nG5/nEtP5A in the mixing solution. The horizontal axis in Fig. 3a and the AL in Fig. 3b are plotted against the area per EtP5A molecule. Given that the collapse behavior of Langmuir films depends on the compressibility of the entire film, the collapse area is represented as a mean value for all mixed molecules (Fig. 3c). As expected, π–A plots approached the characteristics of pure EtP5A (or pure G5-20) as the stoichiometric ratio approached zero (or infinity) (Fig. S9, ESI†). Interestingly, the AL per EtP5A molecule and the collapse behavior exhibited a clear bifurcation at a stoichiometric ratio of 3/7. Within each of the two groups, the AL can be approximated by a linear trend with an intersection of approximately 0.33. The decrease in the molecular area of binary Langmuir films is often indicative of specific attractive interactions.8,22 In this system, such a trend strongly suggests pseudorotaxane formation. Assuming that all G5-20 molecules participate in complex formation under excess conditions of EtP5A (e.g., SR = 1/9, 2/8, 3/7), the number of EtP5A molecules involved in each pseudorotaxane can be estimated from the AL. The results are 2.1, 2.3, and 2.0 EtP5A molecules, respectively, strongly indicating that [3]pseudorotaxane is the dominant species in the mixed monolayer films. Conversely, under conditions where G5-20 is in excess, the decrease in area surpasses the value expected from molecular interactions associated with pseudorotaxane formation, making it challenging to describe the behavior with a simple additivity rule. Further investigations are necessary to elucidate this phenomenon. Nonetheless, the identical collapse behavior implies that the monolayers include [3]pseudorotaxane.
In conclusion, we systematically investigated monolayers of ethoxy-substituted P5A and mixtures with fatty acid guests. Linear fatty acid guests lead to pseudorotaxane monolayers with P5A cavities oriented toward the water surface. The mixing ratio and chain length of the guests influenced the stoichiometry of pseudorotaxane formation and monolayer composition. This study provides insight into P5A-based pseudorotaxane monolayers for various applications. The simple procedure employed for monolayer formation also offers the possibility of introducing functional properties by appropriately selecting host and guest molecules. Furthermore, by removing guest molecules after the formation of aligned pseudorotaxane films, these materials can be explored for applications in sensors and filters.23–25
M. I. and Y. N. conducted comprehensive experiments and analyses, with the support of Y. Y. The synthesis of pillararenes was carried out by K. O. and T. S. under the supervision of T. O. The MAIRS measurements were overseen by T. H. and N. S. This study was conducted by K. A. and H. S., and T. M. M. I. was supported by JST, the establishment of university fellowships towards the creation of science technology innovation (Grant Number: JPMJFS2144). This work was also supported by KAKENHI (Grant Numbers: JP25H00898, JP23H05459 and JP23K04703). The calculations in this study were performed on the Numerical Materials Simulator at NIMS.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.References
- M. Xue, Y. Yang, X. Chi, X. Yan and F. Huang, Chem. Rev., 2015, 115, 7398–7501 CrossRef CAS.
- J. D. Badjic, V. Balzani, A. Credi, S. Silvi and J. F. Stoddart, Science, 2004, 303, 1845–1849 CrossRef CAS PubMed.
- Y. Liu, A. H. Flood, P. A. Bonvallet, S. A. Vignon, B. H. Northrop, H.-R. Tseng, J. O. Jeppesen, T. J. Huang, B. Brough, M. Baller, S. Magonov, S. D. Solares, W. A. Goddard, C.-M. Ho and J. F. Stoddart, J. Am. Chem. Soc., 2005, 127, 9745–9759 CrossRef CAS PubMed.
- E. Katz, O. Lioubashevsky and I. Willner, J. Am. Chem. Soc., 2004, 126, 15520–15532 CrossRef CAS PubMed.
- M. J. Langton and P. D. Beer, Acc. Chem. Res., 2014, 47, 1935–1949 CrossRef CAS PubMed.
- S.-Y. Chou, H. Masai, M. Otani, H. V. Miyagishi, G. Sakamoto, Y. Yamada, Y. Kinoshita, H. Tamiaki, T. Katase and H. Ohta, et al. , Appl. Catal., B, 2023, 327, 122373 CrossRef CAS.
- P. Wu, B. Dharmadhikari, P. Patra and X. Xiong, Nanoscale Adv., 2022, 4, 3418–3461 RSC.
- O. N. OliveiraJr, L. Caseli and K. Ariga, Chem. Rev., 2022, 122, 6459–6513 CrossRef CAS PubMed.
- T. Ogoshi, S. Kanai, S. Fujinami, T.-A. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022–5023 CrossRef CAS PubMed.
- T. Ogoshi, T.-a Yamagishi and Y. Nakamoto, Chem. Rev., 2016, 116, 7937–8002 CrossRef CAS PubMed.
- M. Xue, Y. Yang, X. Chi, Z. Zhang and F. Huang, Acc. Chem. Res., 2012, 45, 1294–1308 CrossRef CAS PubMed.
- T. Ogoshi, R. Sueto, Y. Hamada, K. Doitomi, H. Hirao, Y. Sakata, S. Akine, T. Kakuta and T.-A. Yamagishi, Chem. Commun., 2017, 53, 8577–8580 RSC.
- S. Dong, J. Yuan and F. Huang, Chem. Sci., 2014, 5, 247–252 RSC.
- X.-Q. Wang, W. Wang, W.-J. Li, L.-J. Chen, R. Yao, G.-Q. Yin, Y.-X. Wang, Y. Zhang, J. Huang and H. Tan, et al. , Nat. Commun., 2018, 9, 3190 CrossRef PubMed.
- A. N. Kursunlu, Y. Acikbas, M. Ozmen, M. Erdogan and R. Capan, Analyst, 2017, 142, 3689–3698 RSC.
- A. N. Kursunlu, Y. Acikbas, M. Ozmen, M. Erdogan and R. Capan, Colloids Surf., A, 2019, 565, 108–117 CrossRef CAS.
- J. Umemura, T. Kamata, T. Kawai and T. Takenaka, J. Phys. Chem., 1990, 94, 62–67 CrossRef CAS.
- A. Modlinska and D. Bauman, Int. J. Mol. Sci., 2011, 12, 4923–4945 CrossRef CAS PubMed.
- N. Shioya, T. Mori, K. Ariga and T. Hasegawa, Jpn. J. Appl. Phys., 2024, 63, 060102 CrossRef CAS.
- N. Shioya, M. Yoshida, M. Fujii, T. Shimoaka, R. Miura, S. Maruyama and T. Hasegawa, J. Phys. Chem. Lett., 2022, 13, 11918–11924 CrossRef CAS PubMed.
- N. Shioya, M. Yoshida, M. Fujii, K. Eda and T. Hasegawa, J. Am. Chem. Soc., 2024, 146, 32032–32039 CrossRef CAS PubMed.
- M. Ishii, T. Mori, W. Nakanishi, J. P. Hill, H. Sakai and K. Ariga, ACS Nano, 2020, 14, 13294–13303 CrossRef CAS PubMed.
- T. Ogoshi, S. Takashima and T.-A. Yamagishi, J. Am. Chem. Soc., 2015, 137, 10962–10964 CrossRef CAS PubMed.
- T. Ogoshi, K. Saito, R. Sueto, R. Kojima, Y. Hamada, S. Akine, A. M. P. Moeljadi, H. Hirao, T. Kakuta and T.-A. Yamagishi, Angew. Chem., Int. Ed., 2018, 57, 1592–1595 CrossRef CAS PubMed.
- S. Ohtani, K. Onishi, K. Wada, T. Hirohata, S. Inagi, J. Pirillo, Y. Hijikata, M. Mizuno, K. Kato and T. Ogoshi, Adv. Funct. Mater., 2024, 34, 2312304 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cc01040c |
| This journal is © The Royal Society of Chemistry 2025 |