Open Access Article
Open Access ArticleDecorated high-dispersity Fe(OH)3 nanoparticles on NiZn LDH nanosheets towards enhanced alkaline oxygen evolution reaction†
Tianyi
Yang
a,
Jianfeng
Huang
 a,
Zhaohui
Liu
*a and
Cailing
Chen
a,
Zhaohui
Liu
*a and
Cailing
Chen
 *b
*b
aState Key Laboratory of Coal Mine Disaster Dynamics and Control, Institute of Advanced Interdisciplinary Studies, School of Chemistry and Chemical Engineering, Chongqing University, Chongqing 400044, China. E-mail: zhaohui.liu@cqu.edu.cn
bAdvanced Membranes and Porous Materials (AMPM) Center, Physical Science and Engineering Division, King Abdullah University of Science and Technology (KAUST), Thuwal, 23955, Saudi Arabia. E-mail: cailing.chen@kaust.edu.sa
First published on 1st July 2025
Abstract
The creation of efficient, earth-abundant electrocatalysts is crucial to address the sluggish kinetics of the oxygen evolution reaction (OER). In this study, a straightforward immersion technique is employed to fabricate a NiZnFex LDH composite by embedding Fe(OH)3 nanoparticles onto NiZn LDH nanosheets. The optimized NiZnFe12.9 LDH catalyst exhibits an overpotential of just 191 mV at 10 mA cm−2, significantly outperforming traditional OER catalysts. Additionally, it demonstrates exceptional long-term stability, maintaining a current density of 120 mA cm−2 for more than 125 hours. This approach fosters the advancement of materials for water splitting and hydrogen production, which are crucial for sustainable energy solutions.
Hydrogen is increasingly recognized as a clean alternative to fossil fuels, offering a promising solution for sustainable energy systems. Electrochemical water electrolysis, which produces high-purity hydrogen, plays a key role in this field.1–3 The OER is a critical yet energy-intensive half-reaction in water electrolysis, involving complex electron transfer steps that create significant energy barriers. As a result, developing highly efficient OER electrocatalysts is essential for improving the efficiency of water splitting and enabling scalable hydrogen production.4,5 Research into OER electrocatalysts has prioritized materials that reduce overpotentials to lower energy demands. Despite the superior activity of IrO2 and RuO2, their scarcity and cost limit large-scale use.6 Consequently, efforts now focus on developing efficient, durable catalysts from earth-abundant metals to enable sustainable and scalable OER technologies.
Layered double hydroxides (LDHs) offer significant potential in electrocatalysis due to their low cost, abundance, and compositional flexibility.7,8 However, challenges such as poor conductivity and limited active site exposure hinder their OER efficiency. Strategies like exfoliation,9,10 etching,11,12 decoration,13,14 and doping15,16 have been employed to enhance active site accessibility, conductivity, and surface area. These advancements not only improve intrinsic properties but also optimize the electrochemical performance, making it a promising electrocatalyst.
Nickel (Ni), cobalt (Co), and zinc (Zn)-based LDHs have emerged as promising catalysts for the OER, owing to their favorable catalytic properties. Incorporating iron (Fe) into these materials, such as in Fe–Ni and Fe–Co LDHs, has been shown to significantly enhance catalytic performance compared to their iron-free counterparts, like Ni(OH)2 and Co(OH)2.17–19 This enhancement is attributed to Fe's ability to generate additional active sites, improving catalyst efficiency. Furthermore, combining Fe with other materials, such as metal–organic frameworks (MOFs) or metal oxides, can increase active sites and boost electrochemical properties, enhancing OER effectiveness.20,21 This strategy not only improves activity but also enhances the stability of the catalysts, which is essential for their practical use in renewable energy applications.
Among conventional transition metal (Fe, Co, Ni) hydroxides used in the OER, Fe hydroxide stands out due to two critical merits. First, the crustal abundance of iron surpasses other transition metals, which offers economic advantages for scalable electrocatalyst development. Secondly, Fe hydroxide outperforms Co hydroxide and Ni hydroxide in single-component OER activity (FeOxHy > CoOxHy > NiOxHy), as experimentally validated.22 Recent works have also demonstrated that immobilizing iron hydroxide on other substrates can significantly enhance the OER activity, due to the abundant active surfaces/interfaces generated.23,24
Based on the aforementioned considerations, Fe(OH)3-decorated composites have been synthesized by anchoring highly dispersed Fe(OH)3 nanoparticles onto NiZn LDH nanosheets. This approach effectively combines the electrochemical advantages of both Fe and NiZn, resulting in a trimetallic hydroxide structure. The resulting 2D trimetallic nanosheets demonstrate both high activity and long-term stability under alkaline OER conditions, which are typically challenging for many catalysts. The synergistic interaction between Fe, Ni, and Zn within the structure not only enhances the accessibility of the active sites but also improves conductivity, thereby facilitating efficient electron transfer during the OER process.
As shown in Fig. 1a, trimetallic NiZnFe LDH nanosheets were successfully synthesized on nickel foam using a simple one-pot solution method at a moderate temperature. Inspired by the imidazole ligand assisted approach conducted at ambient temperature and pressure,25 this process involved reacting Ni/Zn/Fe precursors with 2-methylimidazole. By regulating the Fe dosage, a series of NiZnFex LDH samples (where x = 2.6, 8.9, 12.9, 23.7 represents the Fe content by weight (wt%), as determined by ICP-OES) were obtained. TEM and SEM observations confirmed that NiZn LDH and NiZnFex LDH exhibited an ultrathin nanosheet structure with a height of 3–5 nm (Fig. 1b and Fig. S1–S4, ESI†). Interestingly, according to the HRTEM image, highly dispersed small Fe(OH)3 nanoparticles can be observed on the nanosheet substance, with an average size of 2.68 ± 0.64 nm. The increasing Fe content led to larger Fe(OH)3 nanoparticles with an average size of 7.41 ± 1.64 nm (NiZnFe12.9 LDH, Fig. S4b, ESI†). The HRTEM image (Fig. 1c) reveals a lattice spacing of 0.15 nm on the NiZnFe LDH nanosheets, which well matched the (110) facet of Ni(Zn)-LDH. Additionally, the lattice spacing of the smaller nanoparticles was measured to be 0.25 nm, aligning with the (111) facet of Fe(OH)3 (Fig. 1c). The thickness of NiZnFe12.9 LDH was determined to be approximately 4 nm (Fig. 1d), further confirming its ultrathin nature. The Fe proportion in the NiZnFe12.9 LDH sample was determined to be 12.9% and 12.3%, as determined by ICP and EDS results (Fig. S5, ESI†). Elemental mapping of the NiZnFe LDH confirmed the uniform distribution of Ni, Zn, Fe, and O, providing evidence for the successful formation of the NiZnFe LDH composite (Fig. 1e).
The crystal structure of the different samples was analyzed using X-ray powder diffraction (XRD) measurements. The XRD patterns of NiZn LDH and NiZnFex LDH exhibited the characteristic peaks of layered double hydroxides (Fig. 2a).26 The peaks at 11.3° can be indexed as the (003) facet of typical Ni based LDH (PDF #38-0715). In comparison, when the Fe content was increased from 2.6 wt% to 23.7 wt%, the peak at 21.5° gradually increased, indicating the formation of Fe(OH)3 phase. Besides, from the enlarged XRD patterns, we noticed a negative shift of the LDH (003) facet for all the Fe-containing catalysts compared to NiZn LDH. The results indicated that the Fe doping may cause the expansion of the NiZn LDH interlayer. The XRD results confirmed that the Fe species were both doped in the LDH lattice and formed in the Fe(OH)3 phase, which is aligned with the HRTEM analysis. The FT-IR spectra of NiZn LDH and NiZnFe LDH are displayed in Fig. 2b. The absorption band located at 3435 cm−1 corresponds to the stretching vibrations of interlayer H2O molecules and hydroxyl groups (–OH) on the surface. The band at around 1000 cm−1 is attributed to the vibration signal of –OH coordinated with the metal cations (Ni2+, Zn2+, Fe3+) within the layers. Additionally, the peaks observed in the range of 400 to 800 cm−1 are attributed to the characteristic vibrations of metal–oxygen (M–O) and oxygen-bridged metal–oxygen–metal (O–M–O) bonds, where M represents Ni, Zn, and Fe, confirming the successful formation of the LDH structure.27 To further investigate the elemental composition, the XPS spectra of both the NiZn LDH and NiZnFe12.9 LDH composites were measured, revealing the presence of Ni, Zn, Fe, and O elements (Fig. S6, ESI†). These findings were consistent with the EDX analysis results, confirming the successful incorporation of Fe into the NiZn LDH structure.
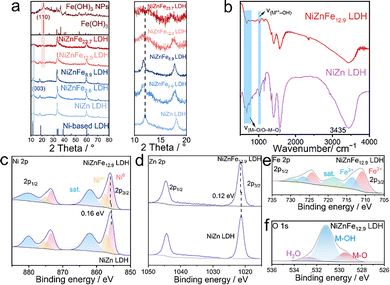 | ||
| Fig. 2 (a) XRD patterns and (b) FT-IR spectrum of NiZn LDH and NiZnFe12.9 LDH. The high-resolution Ni 2p (c), Zn 2p (d), Fe 2p (e) and O 1s (f) spectrum of different samples. | ||
The high-resolution XPS spectra of Ni 2p, Zn 2p, Fe 2p, and O 1s are displayed in Fig. 2c–f, providing detailed insights into the electronic states and chemical environments within the catalysts. The Ni 2p spectrum (Fig. 2c) is deconvoluted into four distinct peaks corresponding to Ni 2p3/2, Ni 2p1/2, and two satellite peaks. The primary binding energy for Ni 2p3/2 is located at 856.1 eV, indicating the dominance of Ni2+ in both NiZn LDH and NiZnFe12.9 LDH.20 In the Zn 2p spectrum (Fig. 2d), two peaks are observed at 1021.2 eV and 1044.1 eV, attributed to Zn 2p3/2 and Zn 2p1/2, respectively. A slight shift of 0.16 eV and 0.12 eV toward higher binding energies for the Ni 2p3/2 and Zn 2p3/2 peaks in NiZnFe12.9 LDH, compared to NiZn LDH, suggests strong electronic interactions among Ni, Zn, and Fe within the LDH structure. These interactions are facilitated by the ligand (2-methylimidazole), highlighting partial substitution of Ni and Zn by Fe atoms. The Fe 2p spectrum (Fig. 2e) further confirms the incorporation of Fe, showing peaks at 710.6 eV (Fe2+ 2p3/2), 713.5 eV (Fe3+ 2p3/2), 723.8 eV (Fe2+ 2p1/2), and 726.6 eV (Fe3+ 2p1/2). This substantiates the successful introduction of Fe ions into the NiZnFe12.9 LDH. Additionally, the O 1s spectrum (Fig. 2f) can be deconvoluted into three peaks: 529.7 eV (lattice oxygen from M–O bonding), 531.5 eV (oxygen species in M–OH), and 532.5 eV (adsorbed oxygen), collectively confirming the formation of the LDH structure.28
To further evaluate the hydrophilicity of the catalysts, contact angle tests were applied (Fig. S7, ESI†). The contact angles measured for clean Ni foam, NiZn LDH on Ni foam, and NiZnFe12.9 LDH on Ni foam were 33.8°, 15.0°, and 6.9°, respectively. The significantly reduced contact angle for NiZnFe12.9 LDH indicates a highly hydrophilic surface, which is a critical factor in enhancing water-splitting efficiency by facilitating water adsorption and reaction kinetics.
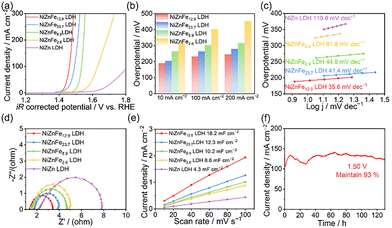
Based on the previous discussion, NiZnFe12.9 LDH exhibits compositional and structural advantages, which are expected to contribute to improved electrochemical performance. The OER catalytic performance of different catalysts was acquired in a three-electrode setup with 1.0 M KOH as the electrolyte. Fig. 3a and b represent the linear sweep voltammetry (LSV) polarization curves for the OER at 5 mV s−1 with 95% iR-compensation and the comparison of the overpotential in 1 M KOH under an O2 atmosphere. Using NiZnFe2.6 LDH, NiZnFe8.9 LDH, NiZnFe12.9 LDH, NiZnFe23.7 LDH and NiZn LDH as OER catalysts, all the Fe-containing catalysts display more effective OER performance than NiZn LDH. To be specific, the corresponding overpotential of NiZnFe12.9 LDH at 10, 100 and 200 mA cm−2 is 191/232/246 mV, superior to NiZnFe23.7 LDH (205/264/280 mV), NiZnFe8.9 LDH (265/303/316 mV) and NiZnFe2.6 LDH (313/401/453 mV) (Fig. 3b). The NiZnFe12.9 LDH catalyst also demonstrates the lowest Tafel slope among all samples (35.6 mV dec−1), which indicates the fastest reaction kinetics (Fig. 3c). Furthermore, the overpotential and Tafel slope of NiZnFe12.9 LDH are even lower than those of previously reported Fe-containing materials in 1 M KOH (Table S1, ESI†).
To further examine the charge transfer properties of different catalysts, electrochemical impedance spectroscopy (EIS) was performed. The Nyquist plots for the various samples are presented in Fig. 3d. The electrocatalytic activity and conductivity were also reflected by the solution resistance (Rs) and charge transfer resistance (Rct), which are summarized in Table S2 (ESI†). The lowest Rs and Rct values of NiZnFe12.9 LDH among the tested samples indicated a highest conductivity and a fastest electron transfer ability, and then boosted its OER kinetics as a result, which was consistent with the Tafel analysis. The number of active sites is a critical factor influencing the catalytic performance of the electrocatalysts. To evaluate the electrochemical properties of the NiZnFe12.9 LDH catalyst, CV curves in the non-faradaic region (Fig. S8, ESI†) were recorded to determine the double-layer capacitance (Cdl). According to Fig. 3e, the Cdl value of NiZnFe12.9 LDH was measured to be 18.2 mF cm−2, which is significantly higher than that of other comparable catalysts. This increased capacitance further confirms the presence of a high density of active sites within the NiZnFe12.9 LDH material. These findings validate that the moderate incorporation of Fe into the NiZn LDH structure effectively enhances its OER activity by increasing the number of active sites.
In order to investigate the interaction between the NiZn nanosheet support and the Fe(OH)3 nanoparticles, we synthesized pure Fe(OH)3 nanoparticles on Ni foam using a similar method, but without adding the Ni and Zn precursors. The corresponding SEM and TEM images are displayed in Fig. S9 (ESI†), which showed a severe aggregation. The LSV curves of Fe(OH)3 nanoparticles and NiZnFe12.9 LDH are shown in Fig. S10 (ESI†). The overpotential of Fe(OH)3 nanoparticles at 10 mA cm−2 is 296 mV, much higher than the NiZnFe12.9 LDH catalyst. Besides, we prepared Fe(OH)3 nanoparticles and NiZn LDH separately. Followed by a physical mixing process of these samples through ultrasonication and grinding, and the Fe content was determined to be 13.8 wt%. Its STEM images and electrochemical performances are shown in Fig. S11 (ESI†). The Fe(OH)3 particles show obvious aggregation and separation from the NiZn LDH nanosheets. The overpotential of Fe(OH)3 on the NiZn LDH catalyst at 10 and 100 mA cm−2 was tested to be 262 mV and 296 mV, and the Tafel slope was 66.39 mV dec−1, inferior to the NiZnFe12.6 LDH. These results further confirmed the interaction between Fe(OH)3 and NiZn LDH in our one-pot synthesized samples.
The long-term stability of NiZnFe12.9 LDH was evaluated via chronoamperometric measurements (Fig. 3f). The current density remained above 93% of its initial value over 125 hours at 120 mA cm−2 and 1.50 V, demonstrating excellent stability. A stability test was also performed for NiZn LDH for comparison (Fig. S12, ESI†), and the current density only displayed 50% retention after 6 hours. These results further highlight that the incorporation of Fe plays a crucial role in enhancing the catalytic stability of NiZnFe12.9 LDH. To evaluate this, SEM and TEM images of the catalyst after the long-term stability test were analyzed (Fig. S13, ESI†). The images reveal that the nanosheet structure of NiZnFe12.9 LDH remained largely unchanged, even after extended exposure to oxidation conditions. The Fe(OH)3 nanoparticles retained a consistent size distribution with a mean size of 2.51 ± 0.62 nm, further indicating the stability of the material. Moreover, the Fe content was found to be approximately 10% after the stability test (Fig. S14, ESI†), suggesting that the Fe(OH)3 nanoparticles exhibited negligible leaching during the reaction, thus confirming the robustness of the catalysts.
In conclusion, a highly dispersed Fe(OH)3-decorated ultrathin NiZn LDH catalyst was successfully synthesized via a straightforward co-precipitation method. The unique ultrathin nanosheet structure maximizes the exposure of high-activity sites, significantly enhancing catalytic efficiency. The incorporation of Fe(OH)3 nanoparticles introduces additional active sites and promotes efficient electron transfer, leading to optimized reaction kinetics and improved charge transfer dynamics. The optimized NiZnFe12.9 LDH catalyst demonstrated exceptional OER performance, achieving a low overpotential of 191 mV at 10 mA cm−2 and exhibiting outstanding durability, maintaining consistent catalytic activity for over 120 hours at a current density of 120 mA cm−2 under alkaline conditions. These results underscore the effectiveness of this synthetic approach in creating high-performance LDH-based electrocatalysts and highlight its potential for advancing energy-related technologies.
The financial support for this work was provided by the National Key Research and Development Project of China (2022YFE0113800), National Natural Science Foundation of China (22102013), Natural Science Foundation of Chongqing (CSTB2024NSCQ-MSX0413) and Baseline Funds (BAS/1/1372-01-01) & Research Translation Funds (REI/1/4220-01-01) from King Abdullah University of Science and Technology (KAUST).
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.References
- N. T. Suen, S. Fu., H. Q. Quan, N. Zhang, Y. J. Xu and H. M. Chen, Chem. Soc. Rev., 2017, 46, 337–3365 RSC.
- Y. M. Shi, M. Y. Li, Y. F. Yu and B. Zhang, Energy Environ. Sci., 2020, 13, 4564–4582 RSC.
- J. C. Li, C. Zhang, T. Zhang, Z. H. Shen, Q. W. Zhou, J. Pu, H. J. Ma, T. H. Wang, H. G. Zhang, H. M. Fan, Y. Y. Wang and H. X. Ma, Chem. Eng. J., 2020, 397, 11 Search PubMed.
- Z. Wang, J. Xu, J. H. Yang, Y. H. Xue and L. M. Dai, Chem. Eng. J., 2022, 427, 131498 CrossRef CAS.
- J. Y. Tian, F. L. Jiang, D. Q. Yuan, L. J. Zhang, Q. H. Chen and M. C. Hong, Angew. Chem., Int. Ed., 2020, 59, 13101–13108 CrossRef CAS.
- W. X. Ning, R. Wang, X. X. Li, M. H. Wang, H. G. Xu, H. Y. Lin, X. P. Fu, M. M. Wang, P. F. Liu and H. G. Yang, Chem. Commun., 2023, 59, 11803–11806 RSC.
- M. Berger, A. Markus, S. Palkovits and R. Palkovits, ChemElectroChem, 2024, 11, e202400457 CrossRef CAS.
- A. S. Darsan, P. Devi, P. Murugan and A. Pandikumar, ACS Appl. Mater. Interfaces, 2024, 16(39), 52515–52528 CrossRef CAS.
- Y. Wei, F. C. Li and L. Liu, RSC Adv., 2014, 4, 18044–18051 RSC.
- S. Wang, S. Qin, G. L. Yang, Y. C. Liu, T. R. Yang, Z. Y. Wang, X. H. Li, D. Liu and W. W. Lei, ACS Appl. Mater. Interfaces, 2024, 16, 69725–69732 CrossRef CAS.
- P. Zhou, Y. Y. Wang, C. Xie, C. Chen, H. W. Liu, R. Chen, J. Huo and S. Y. Wang, Chem. Commun., 2017, 53, 11778–11781 RSC.
- Y. X. Zhao, L. R. Zheng, R. Shi, S. Zhang, X. A. Bian, F. Wu, X. Z. Cao, G. I. N. Waterhouse and T. R. Zhang, Adv. Energy Mater., 2020, 10, 2002199 CrossRef CAS.
- D. P. Liu, Y. Du, T. Z. Li, H. H. Zhang, D. D. Liu, W. N. Zhang, H. Tang, Y. H. Hou, J. S. Li, S. C. Yan, T. Yu and Z. G. Zou, Chem. Commun., 2020, 56, 11465 RSC.
- T. T. Sun, Z. F. Zhao, Z. K. Xu and S. Y. Lin, Rare Met., 2023, 42, 1453–1459 CrossRef CAS.
- D. J. Zhou, Z. Cai, Y. Jia, X. Y. Xiong, Q. X. Xie, S. Y. Wang, Y. Zhang, W. Liu, H. H. Duan and X. M. Sun, Nanoscale Horiz., 2018, 3, 532–537 RSC.
- X. B. Hu, L. D. Zhang, S. Y. Li, J. Y. Chen, B. Y. Zhang, Z. Y. Zheng, H. Y. He, S. P. Luo and A. J. Xie, New J. Chem., 2022, 46, 19491–19500 RSC.
- C. Feng, M. B. Faheem, J. Fu, Y. Q. Xiao, C. L. Li and Y. B. Li, ACS Catal., 2020, 10(7), 4019–4047 Search PubMed.
- J. D. Chen, F. Zheng, S. J. Zhang, A. Fisher, Y. Zhou, Z. Y. Wang, Y. Y. Li, B. B. Xu, J. T. Li and S. G. Sun, ACS Catal., 2018, 8(12), 11342–11351 CrossRef CAS.
- P. M. Bodhankar, P. B. Sarawade, G. Singh, A. Vinu and D. S. Dhawale, J. Mater. Chem. A, 2021, 9, 3180–3208 Search PubMed.
- Q. Y. He, L. Han and K. Tao, Chem. Commun., 2024, 60, 1116 RSC.
- W. Q. Li, H. Zhang, K. Zhang, Z. Z. Cheng, H. P. Chen, G. Tan, X. Feng, L. Y. Wang and S. C. Mu, Chem. Commun., 2023, 59, 4750 RSC.
- B. R. Guo, H. H. Huo, Q. X. Zhuang, X. Q. Ren, X. X. Wen, B. L. Yang, X. X. Huang, Q. W. Chang and S. W. Li, Adv. Funct. Mater., 2023, 33, 2300557 CrossRef CAS.
- L. Trotochaud, S. L. Young, J. K. Ranney and S. W. Boettcher, J. Am. Chem. Soc., 2014, 136, 6744–6753 CrossRef CAS.
- W. Zhang, Y. Wang, H. Zheng, R. Li, Y. Tang, B. Li, C. Zhu, L. You, M. R. Gao, Z. Liu, S. H. Yu and K. Zhou, ACS Nano, 2020, 14, 1971 CrossRef CAS.
- J. Zhao, Y. Guo, Z. Q. Zhang, X. L. Zhang, Q. Q. Ji, H. Zhang, Z. Q. Song, D. Q. Liu, J. R. Zeng, C. H. Chuang, E. H. Zhang, Y. H. Wang, G. Z. Hu, M. A. Mushtaq, W. Raza, X. K. Cai and F. Ciucci, Nat. Nanotechnol., 2025, 20, 57–66 CrossRef CAS.
- Z. W. Cai, J. Liang, Z. X. Li, T. Y. Yan, C. X. Yang, S. J. Sun, M. Yue, X. W. Liu, T. Xie, Y. Wang, T. S. Li, Y. S. Luo, D. D. Zheng, Q. Liu, J. X. Zhao, X. P. Sun and B. Tang, Nat. Commun., 2024, 15, 6624 CrossRef CAS.
- Z. L. Hsieh, M. C. Lin and J. Y. Uan, J. Mater. Chem. A, 2011, 21, 1880 RSC.
- J. D. Chen, F. Zheng, S. J. Zhang, A. Fisher, Y. Zhou, Z. Y. Wang, Y. Y. Li, B. B. Xu, J. T. Li and S. G. Sun, ACS Catal., 2018, 8, 11342–11351 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cc01008j |
| This journal is © The Royal Society of Chemistry 2025 |