 Open Access Article
Open Access ArticleZero-discharge, self-sustained 3D-printed microbial electrolysis cell for biohydrogen production: a review
Mandar S.
Bhagat
 *a,
Chirag
Mevada
*a,
Chirag
Mevada
 *b,
Jaini
Shah
a,
M. Abdul
Rasheed
a and
Matti
Mäntysalo
b
*b,
Jaini
Shah
a,
M. Abdul
Rasheed
a and
Matti
Mäntysalo
b
aDepartment of Environment Management, Gujarat Energy Research and Management Institute, Gandhinagar, Gujarat, India 382 007. E-mail: mandar.b@germi.res.in
bFaculty of Information Technology and Communication Sciences, Tampere University, Tampere, Finland. E-mail: chirag.mevada@tuni.fi
First published on 19th March 2025
Abstract
Microbial fuel cell (MFC) and microbial electrolysis cell (MEC) technologies have been used recently in bench-scale bioenergy (electricity) generation, biohydrogen (H2) production, biosensing, and wastewater treatment. There are still a lot of obstacles to overcome in terms of commercialization and industrial settling. These difficulties include lengthy start-up times, intricate reactor designs for managing large reaction volumes, and expensive and time-consuming large-scale system fabrication procedures. Interestingly, combining three-dimensional (3D) printing with MFC and MEC technology appears to be a workable and promising way to get past these obstacles. Moreover, a rapid start-up with no delays in the current generation using MFC and MEC is possible with 3D printed bio-anodes. Furthermore, H2 can be generated from wastewater by powering a stacked MFC and MEC-coupled with electrochemical capacitor (ECC) system using 3D printing technology. To the best of the author's knowledge, this review paper is the first to explicitly highlight the use of 3D printing in creating a stacked MFC–ECC–MEC system in conjunction with a photobioreactor (PBR) to produce significant quantities of H2 and carbon dioxide (CO2) can be utilized for algae production. A notable feature of 3D printing technology is its reliable production capabilities, enabling MFC–ECC–MEC–PBR systems to be expanded by setting up numerous stacks of MFC–ECC–MEC–PBR units devoid of material waste and human error. The present review attempts to provide an update on the current status of the 3D printing application, that is meant to propel the MFC–ECC–MEC–PBR system forward.
1. Introduction
Meeting the world's energy needs in the face of an ever-growing population has emerged as humanity's greatest challenge. As the population has increased over time, so need for energy, growing exponentially.1 Nevertheless, we rely primarily on fossil fuels to meet our energy needs. We use coal, oil, and other fossil fuels to meet our needs, that eventually leads to the release of pollutants into the environment and the need for additional remediation. Presently, the majority of international and national organizations have implemented policies designed to increase the total amount of “green” energy produced while reducing emissions of pollutants.2 Biogas or bio-hydrogen (CH4 or H2) is gaining more attention globally, as an alternative clean energy source that may help reduce the use of fossil fuels and greenhouse gas (GHG) emissions.3 Compared to hydrocarbon fuels, H2 has 2.75 times more energy content (122 kJ g−1).4 It is nontoxic, colourless, tasteless, odourless, light, and environmental friendly. Water is the only by-product of combustion when it is used as fuel. Biological processes such as microbial electrolysis cell (MEC), which operate at room temperature and pressure with little energy consumption (approx. practical voltage up to 1 V), are a viable and clean way to produce H2.5 The use of MEC, to treat wastewater, make chemicals or biofuels, and generate electricity with minimal energy input has gained increased attention lately as potentially green approaches.6,7In MEC, protons and electrons are produced by bacteria oxidizing organic matter in a microbial fuel cell (MFC).8 Protons move from the electrolyte toward the cathode by diffusing. Current is produced as the electrons move through a circuit to reach the cathode. A species, like oxygen, combines with the protons and electrons at the cathode to create a reduced compound, like water. By removing oxygen at the cathode and introducing a modest voltage (up to 1 V) to the circuit, H2 is produced at the cathode of a MEC.9 MEC can break down a wide range of complex substrates, including end products from fermentation, pig dung, and household and commercial wastewater. The performance of these substrates is less in MEC than that of simple substrates such as cellulose, acetate, and glucose due to the partially degraded substrates.5,6,9,10 Compared to alternative technologies like dark fermentation and photosynthesis, MEC are intriguing for producing H2 because (1) they require a significantly lower energy input than water electrolysis, that produces H2; and (2) they have a higher H2 yield.11 The schematic of the basic difference between MFC and MEC is shown in Fig. 1(a). On the contrary, the osmotic microbial fuel cell (OMFC) process that combines the benefits of both forward osmosis (FO) (transport of water molecules across a membrane from low to high osmotic pressure) and MFC to improve electricity performance. OMFC technology has drawn increasing attention owing to the growing demand for clean water and bioenergy sources. OMFC and MFC are novel approaches that combine electrochemistry and microbiological metabolism for a range of high-value applications.12,13
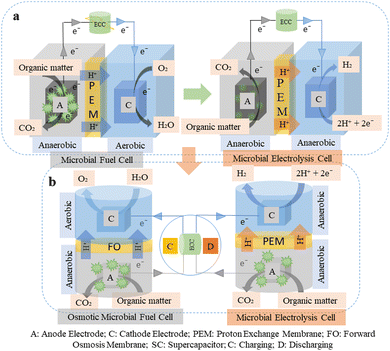 | ||
| Fig. 1 Schematic of the (a) basic difference between MFC and MEC coupled with ECC and (b) OMFC and MEC system coupled with ECC (charging and discharging). | ||
Electrochemical capacitors (ECCs) have attracted much attention lately as an incredibly affordable energy storage technology.14,15 They offer advantages like rapid charging, more power output, and longer lifespans by using fast oxidation–reduction and ion adsorption processes.2 The viability of combining ECC and MEC has recently been the subject of increasing research publications. Previously, it was proposed to use MFC to directly power MEC; however, the performance of the systems was limited by the low voltages produced when the two systems were directly connected.16,17 In an MFC–ECC–MEC circuit, adding ECC improved system performance in terms of energy recovery, MEC yield, and H2 production rates when the capacitance was adjusted to better match the charge generated by the MFCs. The ideal capacitance per MFC for the air-cathode, single chamber (SC) MFCs utilized here was 0.01 F. The capacitor circuit was added to the MFC–ECC–MEC system, increasing energy recoveries by 77%, MEC yields by 60%, and H2 production rates by 38%.17 A stacked MFCs used in parallel, or series allowed for quick charging of the ECC and efficient powering of the MEC.18,19 On the contrary, microalgae in photobioreactors (PBR), could just as easily convert CO2 (generated during microbial electrolysis in an anode chamber) into oxygen (O2).20 Carbon capture and wastewater treatment are currently possible with photobioreactor and MFC technologies.21,22 The MFC–ECC–MEC–PBR system combines zero emission (converting CO2 to O2) and self-sustained technology, eliminating the need for additional power sources to operate the system. ECC can be charged by MFC or OMFC and discharged by the MEC system to produce H2 through electrolysis, as shown in Fig. 1(b).
The field of MFC or MEC could undergo a revolutionary shift due to the potential for flexible and intricate design of electrochemically active materials, made possible by additive manufacturing, also referred to as 3-dimensional (3D) printing.23–25 This technology has been a major driver of innovation in industrial manufacturing. Recent successful applications of the technology include enhanced electrochemical CO2 reduction and the quick development and testing of customized electrode and reactor prototypes.26,27 Apart from producing reactor parts, 3D printing holds great potential for creating next-generation electrodes since it allows for the production of materials with a large surface area and the ability to structure them at the nanoscale level through post-processing techniques.26,28,29 It is possible to realize complex custom designs, such as the addition of flow channels to provide a high surface area per volume and the addition of a metallic core to provide high conductivity over large electrodes. Printing metal ink is one way to create conductive metal substrates. Furthermore, there are plans to 3D print metallic materials directly via electrochemical reduction of metal ions from solutions onto conductive substrates.30,31
The most recent Scopus data shows that, to date, ∼2 and ∼1216 research or review articles with the keywords 3D printed MEC and MEC for H2 recovery, respectively, have been published.32 These articles cover a range of MEC characteristics and operating conditions. In a recent study, the spiral computer-aided design (CAD) model is created with four counter-clockwise revolutions and a 0.5 cm pitch.33 Selective laser sintering (SLS) 3D printing techniques are used to produce spiral electrodes with controlled porosity as well as interdigitated electrodes in desired electrode forms. As standard laser melting materials, stainless steel and commercially available Al–Si10–Mg alloy materials are used to test materials for electrode printing. The highest H2-production rate achieved using SC-MEC is 2.89 m3 d−1 at an applied voltage (AV) of 0.9 V and a current density (CD) of 322.1 A m−3. The superior catalytic properties of platinum-coated carbon cloth and Ni alloy-based cathodes allow them to outperform their stainless-steel cathode counterparts in terms of cathodic H2 recovery values. On the other hand, better gas production is possible with the porous anode (Al-based) because it has a higher surface area-to-volume ratio than the carbon cloth anode. In another study, 3D printed electrodes with different geometries were prepared using a copper-based electrify filament to enhance mass transfer within the cell.29 According to the study, one of the spiral geometries produced H2 at a high rate quickly among all other spiral geometries, with a potential and current density of 2 V and 1.5 mA cm−2 respectively by using cheese whey wastewater. An additional research piece illustrates the application of Ni Mo-alloyed, 3D printed carbon aerogel cathodes to facilitate the production of extremely conductive using a fused filament 3D printer. The MEC can produce H2 at a rate of between 0.3 and 0.4 mL min−1.31 Recently, a novel three-chambered non-3D printed MEC design was fabricated consisting of a membrane-separated common anodic chamber and two cathodic chambers at either end of the anodic chamber.34 Together, the MFC and MEC produce H2 to help recover electricity produced by the MFC. The MFC-coupled MEC system's cathodic H2 recovery is 4.81 mL L−1 and 8.89 mL L−1 per day. The amount of H2 that can be produced is directly influenced by the content of organic waste because it contains carbon, hydrogen, and oxygen (C, H, and O).29 Numerous studies have looked into the possibility of organic wastes producing H2, with cheese whey wastewater having the highest volume of H2 production among organic wastes. High concentrations of lactose and lactic acid (C12H22O11 and C3H6O3) are produced by the use of cheese whey substrate, that yields H2 of 290 mL g−1. Similarly, of all organic sugars, lactose has the highest H2 content.35,36 On the contrary, the material, geometry, physical structures, and active surface area of the electrodes are the main parameters that affect the MEC performance. The most popular types of electrodes used in MEC are ceramic-based, nickel (Ni), platinum (Pt), carbon-based, stainless steel (SS), bio-electrodes (living cell), nickel (Ni), etc. can be made by using 3D printing technology.36–38
In order to develop a self-sustained, zero-discharge H2 production system, our research integrates MEC, MFC, ECC, and a PBR. Although the MEC needs an external power source in order to operate effectively. This is resolved by adding an MFC to generate electricity from the same wastewater feedstock, that enhances system sustainability and helps to partially offset the MEC energy needs. However, the MFC uneven voltage output could make it more difficult for stable MEC operation as discussed above. This is avoided by using an ECC, which stabilizes and controls the voltage applied to the MEC while reducing the amount of external energy needed. This guarantees efficient and ongoing H2 production. Furthermore, one important by-product of MEC-based H2 generation is CO2. In a PBR, it is captured and utilized to grow algae instead of being released to atmosphere. This integration ensures zero discharge by reducing reliance on freshwater, recycling treated wastewater for future use. The MFC–ECC–MEC–PBR combination creates a scalable, waste-free, and energy-efficient decentralized H2 production system that adheres to the principles of the circular economy. Reuse (electricity), regeneration (H2), and recycling (wastewater) of resources are made possible by energy recovery from waste, that is consistent with the ideas of the circular economy. This strategy encourages resource sustainability, lessens reliance on fossil fuels, and has a minimal negative impact on the environment. Innovative methods for regenerating H2 from waste guarantee a closed-loop system, improving energy security and supporting a robust and sustainable circular economy. In this review article, scientific discussions have been presented on the following points: (i) the performance of the 3D printed stacked MFC–ECC power the MEC–ECC system; (ii) the performance of series and parallel 3D printed stacked MEC–ECC system; (iii) insights on the development of a prototype stacked MFC–ECC–MEC–PBR (self-sustained and zero discharge) system that delivers H2 (bioenergy); (iv) strategies to improve the performance of stacked MFC–ECC–MEC–PBR systems using other techniques like use of 3D printed bio-anode. In addition, suggestions and possible future applications of stacked MFC–ECC–MEC–PBR have been presented with the possible future of H2 energy as a fuel source.
2. Types of MEC system
Fig. 2(a) and (b) represent, SC and dual-chamber (DC) MEC systems are classified according to their configurations. In SC-MEC, both electrodes are positioned in a SC in a SC-MEC, neglecting the need for a membrane.39–41 However, in DC-MEC, the cathode and anode are divided by a membrane. Anion and cation exchange membrane (AEM and CEM) help obtain pure H2, prevent microbial H2 consumption, minimize fuel and bacterial crossover from anode to cathode, and prevent short circuits.42–44 In addition to reducing bio-contaminations of cathode metal catalysts, the DC arrangement allows control over distinct microbial species in the cathode and anode. On the other hand, high current densities can be achieved because the SC cell minimizes potential loss brought on by the membrane. | ||
| Fig. 2 A schematic and real images of MEC system forms categorized by application and compartment (a) SC-MEC and SC-MEC with sandwich C-electrode8 (b) DC-MEC with PEM and DC-MEC with AEM8,45 (c) three-chamber MEC46 and (d) four chambers MEC.47,48 | ||
2.1 SC-MEC system
In MEC, a variation of MFC, anaerobic bacteria on the anode are utilized to break down organic matter and produce H2 through the process of electrohydrogenesis.39 Protons and electrons from the anode are converted to H2 at the cathode. Since electrohydrogenesis uses less energy and is more environmental friendly than thermochemical or biological methods, it is a promising technology to produce H2 at a higher yield.49 Thus, MEC has been extensively studied for improved renewable H2 production from biomass and wastewater. Recently, the performance of the SC-MEC was assessed at applied voltages ranging from 0.8 to 2.2 V during a six-week operating period.50 The MEC generated 0.258 m3 m−2 of H2 over ten cycles at applied voltage of 2.0 V. The MEC demonstrated a constant operating current density of 27.8 A m−2 in contrast to the control MEC. An applied voltage increase to 2.2 V results in a decrease in the rate of H2 production. Another study recovered H2 using food waste as the substrate, this study combined anaerobic digestion (AD) with SC-MEC treatment using an integrated reactor.51 The quantity of H2 produced is 511.02 mL g−1 during continuous AD-MEC operation was more than that produced by AD (i.e., 49.39 mL g−1). A different study focuses on a semi-SC-MEC that uses a cellulose dialysis bag to enclose a combined-material anode composed of stainless steel and carbon cloth.52 The anode was encapsulated to promote the acclimation of bacteria and the formation of biofilms on the anode material. The study of dialysis bags with varying molecular weight cut-offs revealed that the current density was only 12.4 A m−2, and the H2 production rate at 50 kDa was 0.160 m3 m−2 d−1. In another study, researchers looked at the production of H2 using a SC-MEC fed with high-strength wastewater and varying electrode materials (metal and carbon), configurations of the reactor.53 The maximum H2 production rate of 314 m3 m−3 d−1 was recorded in MEC using metal electrodes.Recently, MEC treatment of combined leachate and dairy wastewater at two days hydraulic retention time (HRT) and an applied voltage of 0.8 V were studied.54 H2 can be produced with a power density and a current density of 15 mL L−1 d−1 and 80 mW cm−2 10 A m−2 respectively. In another study source-separated urine could be used to stop H2 loss in the MEC; the primary cause of the H2 loss was total ammonium nitrogen from urea hydrolysis.41 The optimal range for total ammonium nitrogen concentration was 1.17 g N L−1 to 1.75 g N L−1. In this range, the rate of H2 production increased dramatically, from less than 100 L m−3 d−1 to 520 L m−3 d−1. In another study using a nickel electrode, the H2 production rate in a SC-MEC produced 2.8 mL L d−1 and 17.7 mW m−2 of power density at pH 7.55 According to another study, a carbon electrode coated with nano-porous oxide and TiO2 produced the highest H2 yield of 0.064![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m3 m−3 d−1.56 In place of more expensive metallic electrodes, carbon electrodes with more-porous coatings may prove to be a viable and cost-effective cathode in the MEC for the production of H2. A similar study explored that photo-assisted SC-MEC with ZnFe2O4/g-C3N4 cathodes can produce H2 up to 1.70 m3 m−3 d−1.57 Another study examined the effects of applied voltages on the performance of the MEC and the anodic biofilm viability during the anaerobic biotransformation of straw waste biomass into green energy.58 The best H2 yield was found at 0.8 V after the study investigated applied voltages between 0.5 and 1.0 V, indicating that the highly potential-sensitive mixed electroactive consortia. Another research investigation uses anode modification and biomass enzymatic pre-treatment.59 In MEC with the coated anode (graphene and iron oxide nanoparticles) at an applied voltage of 0.8 V and moderate mixing, the maximum H2 production of 224 mL g−1 of biomass was achieved. Similar research employs SC-MEC fed with high-strength wastewater to demonstrate electrocatalytic activities and H2 production.53 Various electrode configurations, materials (metal and carbon), and electrode distances are used. Using the electrode configuration of stainless steel 304 flat mesh 60 as a cathode and pleated mesh 60 as an anode at a distance of 4 cm from the electrode, the maximum H2 production rate (HPR) of 314 m3 m−3 d−1 with an overall H2 recovery of 340% was achieved. Another research explored MEC components that were set up in a concentric configuration: a cylindrical graphite felt anode and a platinum-coated titanium mesh cathode. At 0.8 V, the fermentation effluent produced a high H2 production rate of 6.26
m3 m−3 d−1.56 In place of more expensive metallic electrodes, carbon electrodes with more-porous coatings may prove to be a viable and cost-effective cathode in the MEC for the production of H2. A similar study explored that photo-assisted SC-MEC with ZnFe2O4/g-C3N4 cathodes can produce H2 up to 1.70 m3 m−3 d−1.57 Another study examined the effects of applied voltages on the performance of the MEC and the anodic biofilm viability during the anaerobic biotransformation of straw waste biomass into green energy.58 The best H2 yield was found at 0.8 V after the study investigated applied voltages between 0.5 and 1.0 V, indicating that the highly potential-sensitive mixed electroactive consortia. Another research investigation uses anode modification and biomass enzymatic pre-treatment.59 In MEC with the coated anode (graphene and iron oxide nanoparticles) at an applied voltage of 0.8 V and moderate mixing, the maximum H2 production of 224 mL g−1 of biomass was achieved. Similar research employs SC-MEC fed with high-strength wastewater to demonstrate electrocatalytic activities and H2 production.53 Various electrode configurations, materials (metal and carbon), and electrode distances are used. Using the electrode configuration of stainless steel 304 flat mesh 60 as a cathode and pleated mesh 60 as an anode at a distance of 4 cm from the electrode, the maximum H2 production rate (HPR) of 314 m3 m−3 d−1 with an overall H2 recovery of 340% was achieved. Another research explored MEC components that were set up in a concentric configuration: a cylindrical graphite felt anode and a platinum-coated titanium mesh cathode. At 0.8 V, the fermentation effluent produced a high H2 production rate of 6.26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ±
± ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.23 L L−1 d−1.60 Another study on H2 production from glucose was enhanced by adding a specific methane inhibitor (such as chloroform) to inhibit the activity of methanogens in an SC-MEC with a double anode configuration.61 The maximum H2 production at 0.8 V is 2.31 and 2.81 m3 m−3 d−1 with glucose concentrations of 3 g L−1 and 4 g L−1, respectively. The H2 production increases as the glucose concentration increases. Table 1 shows the overall performance of the SC-MEC system.
0.23 L L−1 d−1.60 Another study on H2 production from glucose was enhanced by adding a specific methane inhibitor (such as chloroform) to inhibit the activity of methanogens in an SC-MEC with a double anode configuration.61 The maximum H2 production at 0.8 V is 2.31 and 2.81 m3 m−3 d−1 with glucose concentrations of 3 g L−1 and 4 g L−1, respectively. The H2 production increases as the glucose concentration increases. Table 1 shows the overall performance of the SC-MEC system.
| Type of system | CD (A m−2) | AV (V) | CE (%) | H2 production (m3 m−3 d−1) | CHR (%) | Ref. |
|---|---|---|---|---|---|---|
| Note: SC-MEC: single chamber MECa Unit of H2 production/yield and CD: m3 m−2 d−1 and A m−3 respectively.b Unit of H2 production/yield and CD: L L−1 d−1 and mA m−2 respectively.c Unit of H2 production/yield: mmole of H2 g−1 of COD.d H2 production/yield: mL g−1.e H2 production/yield: mL L−1 d−1. | ||||||
| SC-MEC (dual anode) | 286–316 | 0.8 | 64–61 | 2.31–2.81 | 81–77 | 61 |
| SC-MEC | 27.8 | 2.0 | 99.0 | 1.29 | — | 50 |
| Semi SC-MEC | 15.7 | — | — | 0.160a | — | 52 |
| SC-MEC | 362.0b | 0.6 | 23.0 | 0.053 | 81.0 | 62 |
| SC-MEC | — | 0.8 | — | 6.26b | — | 60 |
| SC-MEC | — | — | — | 1.70 | — | 57 |
| SC-MEC | — | — | — | 314.0 | 340.0 | 53 |
| SC-MEC | — | 0.8 | 68.4 | 6.017c | 460.4 | 58 |
| SC-MEC | — | 0.8 | — | 224d | — | 59 |
| SC-MEC | — | 0.8 | — | 0.06 | — | 63 |
| AD-MEC | 9.87 | 0.8 | — | 4.86b | — | 51 |
| SC-MEC | 10 | 0.8 | — | 15e | — | 54 |
| SC-MEC (dual anode) | 1355.0a | 0.8–1.5 | — | 5.56–10.88 | 118.0 | 64 |
The reaction occurs at the anode side:
| CH3COO− + 2H2O → 2CO2 + 7H+ + 8e− (E° = −0.296 V at pH = 7) | (1) |
The reaction occurs at the cathode side:
| 2H2O → 2e− + H2 + 2OH− (E° = −0.414 V at pH = 7) | (2) |
| E0cell = E0cathode − E0anode = −0.118 V | (3) |
2.2 DC-MEC system
Fig. 2(b), DC-MEC system, membranes are used to keep H2 and oxygen gases apart and from reacting. Membranes have also been used in MEC, most likely to guarantee high H2 concentrations and prevent bacteria in the anode chamber from utilizing H2. However, even when a membrane is employed, some researchers have found that the H2 generated at the cathode is contaminated by other gases (like CO2) generated at the anode. Additionally, the membrane's presence does not prevent H2 from diffusing back into the anode chamber. Moreover, methanogenic bacterial degradation and membrane diffusion cause H2 loss in SC-MEC and DC-MEC, respectively. It is therefore essential to find a barrier to protect the bacterial anode using 3D printed modified AEM or PEM.45,63Recently, a new separator-electrode assembly configuration was created, the cathode was positioned between a hydrophobic polytetrafluoroethylene–(PTFE) membrane and a hydrophilic polyvinylidene difluoride (PVDF) membrane.65 A low H2 crossover between the cathode and the anode decreased methanobrevibacter enrichment within anode biofilms in MEC, resulting in an H2 production rate of 4.53–5.02 m3 m−3 d−1 that was significantly higher than that of conventional SC-MEC. Another investigation is the DC-MEC performance with fermentation effluent and AEM or CEM.66 At 0.8 V applied voltage, the highest yield of H2 in DC-MEC (1.26 L g−1) by using AEM compared to CEM. In contrast to PEM–MEC, AEM-MECs performed significantly better due to their much higher cathodic H2 recovery (i.e., a higher proportion of cathodic electrons toward H2 evolution) and COD removal efficiency (i.e., more electrons to be transferred to the anodes). The observed results may be attributed to the pH imbalance between the anode and cathode sides in PEM-MEC, as opposed to AEM-MEC.43,67,68 Another study investigated DC-MEC that was operated with a starch-based medium as the anolyte and rice paddy field soil as the anode inoculum.69 The anode chamber was bio-augmented with Geobacter sulfurreducens strain (YM18) to increase current generation and H2 production. The highest H2 production was achieved by using the above stain up to 0.8 L L−1 d−1. Another study used a reduction deposition method to create a hybrid containing ruthenium nanoparticles loaded on conductive carbon nanotubes (Ru/CNTs).70
Adopting a cathode electrode coated with Ru/CNTs can produce H2 at a rate of 0.167 m3 m−2 d−1. A different study demonstrates that using MEC in a fuel cell sensor can be an inexpensive online tool for monitoring H2 production. The H2 generated in the MEC was oxidized at the fuel cell's anode to produce easily monitored electricity.71Table 2 shows the overall performance of the DC-MEC system.
| Type of system | CD (A m−3) | AV (V) | CE (%) | H2 production/yield (m3 m−3 d−1) | CHR (%) | Ref. |
|---|---|---|---|---|---|---|
| Note: DC-MEC: double chamber MEC.a Unit of H2 production/yield and CD: m3 m−2 d−1 and A m−2 respectively.b Unit of H2 production/yield and CD: L L−1 d−1 and mA respectively.c Unit of H2 production/yield and CD: L g−1.d Unit of H2 production/yield and CD: mg H2 g−1 of COD.e Unit of H2 production/yield: L d−1. | ||||||
| DC-MEC | — | 0.8 | — | 5.31 | — | 45 |
| DC-MEC (a novel separator) | 482.5–515.0 | 0.8 | — | 4.53–5.02 | 90.0 | 65 |
| DC-MEC | 17.13a | — | — | 0.167a | — | 70 |
| DC-MEC (AEM) | 5.10a | 0.8 | 96.0 | 1.26c | 85.0 | 66 |
| DC-MEC | 13–14b | — | — | 0.7–0.8b | — | 69 |
| DC-MEC | — | 0.8 | — | 10.3d | — | 42 |
| DC-MEC | 0.632a | 0.8 | 50.0 | 0.066e | — | 72 |
2.3 Three-chamber MEC
An alternate, successful, and economical method for achieving simultaneous biogas upgrading, CO2 recovery, and wastewater treatment is the novel microbial electrolytic capture, separation, and regeneration cell.7 A bipolar membrane (BPM) and an AEM, respectively, divide the anode chamber, generation chamber, and cathode chamber that make up this system as shown in Fig. 2(c). If an external voltage is applied, electrons from the oxidation of organic matter by bacteria move from the anode to the cathode, where they undergo H2 evolution and OH accumulation. Water dissociation in the BPM produces H+ and OH−, which leads to an acidic regeneration chamber and neutralization of OH− with H+ released from substrate oxidation in the anode chamber. In addition to preventing a sharp drop in pH in the anode, BPM has the demonstrated ability to produce acid and alkali in (bio)electrolytic processes more efficiently and economically than traditional AEM and CEM.73,74 Recently, the alkali solution generated in the cathode first absorbs CO2 from the biogas input as HCO3− and CO32−.7 Owing to the potential differential between the cathode and middle chambers, HCO3− and CO32− then move via AEM to the regeneration chamber, where they recombine with H+ to generate CO2. Thus, it is possible to simultaneously achieve biogas upgrading, CO2 recovery, wastewater treatment, and H2 production through such a process. In another similar study, the combination of microbial desalination cell (MDC) and MEC has been implemented to improve desalination efficiency and H2 recovery in three chambers of microbial electrolysis desalination cell (MEDC).46 The possible reaction that occurs in the three-chamber MEC system is as follows.Possible reaction at the cathode side:
| CO2(aq) + H2O → H+ + HCO3− | (4) |
| HCO3− + OH− → H2O + CO32− | (5) |
Possible reaction at the middle chamber:
| CO32− + H+ → HCO3− | (6) |
| HCO3− + H+ → H2CO3 ↔ CO2(aq) + H2O | (7) |
2.4 Four-chamber MEC
The microbial electrolysis desalination and chemical production cell (MEDCC) is a novel desalination device for wastewater water treatment that combines the MEC and the MDC.47 The water dissociation reaction takes place between the two layers when there is an electric field in the BPM. H+ migrates through the cation exchange layer and OH− migrates through the anion exchange layer. We propose a new device using BPM to control pH changes in the anode chamber, eliminate Cl− and phosphate's detrimental effects, and recover Na+ in the cell. Fig. 2(d), MEDCC is the name of the apparatus. An anode chamber, an acid-producing chamber, a desalination chamber, and a cathode chamber (the alkali-producing chamber) make up the device's four chambers.47,483. Additive manufacturing
3D printing, also known as additive manufacturing (AM), has grown rapidly over the last few years. Summarily, 3D printing produces structures in three dimensions by layering materials onto computer-aided design (CAD) models.25,75,76 This process minimizes human intervention and enables the swift and accurate creation of intricate devices and structures with complicated geometries.77,78 These encouraging characteristics have led to the application of 3D printing in several industries, including arts, aerospace, medical implants, industrial prototype printing, and aerospace industries. Furthermore, 3D printing has been used in various energy-producing devices, including batteries and solar cells. Recently, 3D printing has been used to fabricate various parts of metal-oxide thermocouples, including reactor bodies, electrodes, and membranes, owing to its growing popularity and applicability.78–823D printing offers more than fifty different technologies to design and make different materials or objects.83 ASTM F2792-12a divides these AM technologies into seven process categories: materials extrusion, powder bed fusion, sheet lamination, vat photopolymerization, direct energy deposition, binder jetting, and jetting.83,84 These technologies can be grouped simply as solid, liquid, and powder-based printers depending on the type of feed material (Fig. 3).78 FDM involves heating the tip of a non-brittle filament to create a semi-liquid state i.e., subsequently laid down for bonding and cooling. An ultraviolet (UV) lamp or UV laser is used in the stereolithography apparatus (SLA), DLP, continuous liquid interface production (CLIP), and two-photon polymerization (2PP) processes to cure a liquid photopolymer in a vat to solidify it. A part of the 3D printing solution is solidified layer by layer, with each subsequent layer bonded to the preceding layer after a small amount of over-curing.78,85 Similarly, polyjet technology uses the UV curing principle. The polyjet technique offers higher resolution and can print multiple materials simultaneously except for 2PP. For example, it can print a combination of soft rubber and rigid plastic. Superfine inkjet technology can produce ultrafine droplets with a diameter of less than 1 μm, i.e., 10 times smaller than droplets ejected by traditional inkjet printers. Notably, superfine inkjet technology offers the second-best resolution after the 2PP process.78 Solid-state sintering involves the use of selective laser sintering (SLS) scans followed by heating powder particles at a temperature close to the melting point so that the powder particles’ surfaces soften and fuse.76,78
4. Components for MFC–MEC–ECC–PBR system
4.1 Membranes
AEM or CEM is mostly used in the DC, three and four-chamber MEC system along with BPM (mostly three and four-chamber systems).48 Two distinct layers make up a BPM: one is anion- and cation-selective. Once applied under reverse potential bias, it exhibits its usual function. In this scenario, the transition region will experience a depletion of electrolyte ions, and solvent dissociation will produce the current carriers. Currently, only two types of solvents have been found to dissociate in BPM: methanol (CH3OH) and water (H2O). They are divided into H+ and CH3O− and H+ and OH−. When it comes to water splitting, it explains the majority of the uses for BPM processes that have been used till now. In several chemical processes, including the extraction of ions from their corresponding salts, electroextraction, back-extraction, purification of acids and bases, and the synthesis of organic acids and soy protein isolates, BPM has proven to be effective.47,48,734.2 Electrodes
In MEC, electroactive microbes are important components that impact the performance of H2 production, apart from the electrodes and other physical components. The anode or cathode biofilms’ slow growth kinetics can make complete enrichment difficult, that in turn prolongs the reactor's start-up time. Several studies have used anaerobic sewage sludge as an inoculum source to speed up the enrichment of electroactive biofilms. However, a novel strategy is required to quickly form electroactive biofilms on the electrode surface. Thus, the production of these bio-anodes from 3D printing techniques opens up new possibilities for high-performance MFC–ECC–MEC system.864.3 Reactor body
In designing MEC, biocompatible materials are favoured because they provide better surfaces on which biofilms can adhere and proliferate. MFC reactors were precisely constructed with FDM using biocompatible and nontoxic PLA. Similarly, compared to conventional acrylic fiber material, the quality of the single-chamber MFC reactor made using 3D printing was the same. Furthermore, reactor shapes can be printed again to construct a MEC–ECC system, demonstrating the numerous opportunities that 3D printing has brought about for MEC–ECC design stacking and reactor upscaling.24,87 The materials used in 3D printing techniques are shown in Fig. 4.5. Energy storage technology
Various electrical energy storage technologies have been developed over time. Batteries and fuel cells are commonly found in everyday use, while others, such as pumped hydro, flywheels, compressed air, superconducting magnetic systems, and supercapacitors, are geared more toward industrial applications. Among these, batteries are the most extensively used across a wide range of applications, largely due to their exceptional performance.88,89 Depending on their chemical composition, batteries can be either rechargeable or non-rechargeable. Both types convert chemical energy into electrical energy through redox reactions that occur at the anode and cathode. Rechargeable batteries, however, can reverse this process a limited number of times. Since the 20th century, batteries have become an essential part of modern life, but they are not without limitations.88–91 Some of the key challenges include: (i) low power density, which restricts their effectiveness in applications that require rapid charge or discharge, (ii) heat generation, which can arise from redox reactions and lead to overheating, thermal runaway, or even fire if not managed properly, and (iii) limited cycle life, as most batteries cannot fully reverse redox reactions during repeated charge and discharge cycles, ultimately reducing their longevity.88,89,92,93 Due to the challenges mentioned earlier, batteries on their own cannot offer a complete solution for energy storage. An energy storage device that combines durability, safety, and high power or energy capacity would revolutionize the way electric power is generated, distributed, and utilized. Moreover, with growing demands from consumers, industries, and military sectors for more compact and dependable power systems, advancing such technologies remains a key priority in the field of energy storage.92,94,95Electrochemical capacitors (ECCs), supercapacitors, also known as ultracapacitors, offer exceptional power performance, high reversibility, and an impressively long lifespan (exceeding 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles). Their simple operating mechanism and ease of integration into electronic systems make them highly attractive.96,97 Additionally, ECCs produce less thermochemical heat due to their straightforward charge storage process. As a result, they are widely used in consumer electronics, memory backup systems, and industrial power and energy management applications. Their presence is also expected to expand into more specialized markets in the near future with increase energy density and low cost. ECCs are classified via two primary charge storage mechanisms. The first involves electrostatic charge accumulation at the electrode interface, known as electric double-layer capacitors (EDLCs).98,99
000 cycles). Their simple operating mechanism and ease of integration into electronic systems make them highly attractive.96,97 Additionally, ECCs produce less thermochemical heat due to their straightforward charge storage process. As a result, they are widely used in consumer electronics, memory backup systems, and industrial power and energy management applications. Their presence is also expected to expand into more specialized markets in the near future with increase energy density and low cost. ECCs are classified via two primary charge storage mechanisms. The first involves electrostatic charge accumulation at the electrode interface, known as electric double-layer capacitors (EDLCs).98,99
The second mechanism, called pseudocapacitance, involves the faradaic process where charges are stored via redox reactions on the surface of the electrode. Activated carbon, known for its extensive specific surface area (≥2000 m2 g−1), is commonly utilized as the active material in these systems. Due to this large surface area, EDLCs have a significantly higher capacity to store energy, often measured in Farads (F). In contrast, traditional dielectric and electrolytic capacitors are measured in much smaller units such as picofarads (pF) and microfarads (μF). Pseudocapacitance occurs at the electrode surfaces where charge storage takes place through faradaic processes. During charging, redox-active materials like RuO2, MoxN, or MnO2 undergo reduction to lower oxidation states, accompanied by the adsorption or insertion of cations from the electrolyte at or near the electrode surfaces. When the device is discharged, this process is nearly fully reversible. Additionally, printed electronics represent a major advancement in the fabrication of ECCs by offering a range of simple, affordable, and efficient production techniques.99,100 These methods are versatile, environmental friendly, and allow for the development of cutting-edge ECC designs, including micro, asymmetric, and flexible configurations. This approach enhances the potential of ECCs for use in next-generation electronic devices. Recently, various fabrication techniques for ECCs have been investigated, including electroless plating, thermal evaporation, and electrodeposition. However, these methods can often be intricate and expensive, posing significant challenges for their practical application in commercial settings. 3D printing technology offers a cost-effective, straightforward, and efficient approach that maximizes the capabilities of ECCs. This technique allows for the attainment of desired capacitance at high mass loadings, the creation of complex structures, and the direct fabrication of integrated systems-on-chip. Additional studies on 3D-printed ECCs have explored a variety of materials for their primary electrodes, including activated carbon, graphite, graphene, carbon nanotubes, poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT), polyaniline, and MXenes.14,91,101 In summary, 3D-printed ECCs are a good choice among all electrical energy storage technologies because they offer rapid charging capabilities and enhanced reliability. They have a virtually unlimited lifespan, resist temperature fluctuations, and are non-toxic.
6. PBR technology
The benefits of microalgae include their high photosynthetic efficiency, quick growth rate, strong environmental adaptability, and ability to combine with other methods (e.g., wastewater treatment, CO2 bio-fixation). Microalgae can be grown in a PBR, a specialized device that regulates variables like light, temperature, CO2, pH, and nutrients.102,103 The main obstacles to the large-scale planting of microalgae are the design, optimization, and scaling-up of high-efficiency PBR. PBR typically comes in two varieties: open-type and closed-type. The uneven mixing and cell settling in corners are two major problems with using flat panel photobioreactors for algal growth, particularly when bubbling is the only method of mixing biomass.102,104 Recently, the combination of MFC and PBR systems was used to improve the performance of MFC by utilizing algae as an oxygen source.20,22,105 In our system, by capturing CO2 released from the MEC process (from an anode chamber) or other industrial and residential vehicle sources, the combination of MEC technology with a microalgal-based process in MEC-PBR systems could lower GHG from wastewater treatment and H2 production.7. Stacked 3D printed MFC–ECC–MEC–PBR system
Researchers predicted that the developments would make it possible to upgrade biogas using CO2 capture-assisted (PBR) systems in conjunction with MEC.106 The schematic prediction of a stacked 3D-printed MEC–ECC–PBR system is shown in Fig. 5. The performance of a stacked MEC system also heavily depends on the cathode surface area. Larger surface area cathodes have been shown to increase current density and H2 production, and the H2 evolution reaction is frequently the limiting factor in MEC performance. Additionally, the MFC and MEC tests have shown that a reduction in the distance between the electrodes can increase the maximum power output.8,107,108 Therefore using MEC technology for wastewater treatment, more research is needed to improve the design and develop low-cost electrode materials with a high specific surface area, good conductivity, and high stability. Recently, several anode materials, such as graphite granules, carbon foam, reticulate vitreous carbon, and graphite brush electrodes, have been tested in MEC.8,44,109A recent study shows that six different MEC cell cassettes, each acting as a single electrolysis cell, were placed in a 120-liter polypropylene tank to make up the MEC reactor setup.110 The cells were arranged in series inside the wastewater tank. An average of 0.6 L of H2 per day was produced by a 100 L MEC that was run for a year on raw household wastewater fed at temperatures between 1 °C and 22 °C. Even though it gradually decreased, gas production continued. The applied voltage was gradually increased from 0.6 V to 0.9 V and finally, 1.1 V to acclimate the reactor setup. In another study, electroactive cathodic biofilms were enriched for H2 evolution reaction in a 10-L single chamber MEC (multi-electrodes system) with a high electrode surface area to volume ratio.111 The feed was made with lignocellulosic hydrolysate at an organic loading rate of 0.4 g d−1 and H2 production rate of 0.71 L L−1 d−1. Based on microbial community analysis, Enterococcus species were identified as the main electroactive bacteria in both the anodic and cathodic groups. Similar work designs the multi-anode and cathode system in the MEC system to produce H2.112 The stacked MEC system achieved H2 flow rate of up to 272 mL d−1. Table 3 shows the overall performance of the stacked MEC system.
| Type of system | Current (A) | AV (V) | CE (%) | H2 production (L d−1) | CHR (%) | Ref. |
|---|---|---|---|---|---|---|
| a Note: unit of H2 production and CD: L L−1 d−1 and A m−2 respectively. b Unit of H2 Production and current: L CH4 and mA respectively. c Unit of H2 production: mL d−1; S-MEC and P-MEC: MEC in series and parallel stacked respectively. | ||||||
| S-MEC | 1.1 | 0.1 | 41.2 | 0.6 | — | 110 |
| P-MEC | 13.7–16.5a | 1.36 | — | 0.71a | 30–56 | 111 |
| Sandwich design | — | 1.1 | 66 | — | — | 113 |
| 3DC-MEC | 50 to 80b | — | 99 | 2.2b | — | 31 |
| Stacked MEC | 49.8b | 1.0 | 86.6 | 258c | — | 112 |
8. Applications and future prospect
Recent developments in this technology have led to an increase in the use of 3D printing in MFC–ECC–MEC. Several MFC–ECC–MEC components can be precisely and swiftly fabricated. Interestingly, 3D printing can improve the complex geometrical structures of electrodes with particular properties (like porosity and roughness) to make them more efficient than traditional electrodes. Despite encouraging results, the application of 3D printing for MEC may be limited due to several drawbacks, such as the high cost, low durability, biocompatibility, and electrochemical properties of 3D printed components. Thus, the search for inexpensive yet functional 3D printing materials for MEC components should be continued in future research.82,1148.1 Benefits of MFC–ECC–MEC over solar power systems
The ability of MFC–ECC–MEC to continuously produce capture and utilize bioenergy for the production of H2 in the absence of solar power is one of its greatest advantages. Sea habitats and polar regions, where sunlight is either rare or non-existent for long periods, can benefit immensely from MFC–ECC–MEC technology. These MFC–ECC–MEC systems use the energy produced by microorganisms’ metabolic processes, that continue regardless of light's presence. Therefore, in locations previously limited by solar dependence, MFC–ECC–MEC technology offers a dependable and renewable energy solution, extending the reach of sustainable energy solutions to some of the world's most remote and energy-poor areas. Through the use of photosynthetic organisms, the integration of photobioreactors with MEC presents a novel approach to utilizing CO2 for algal growth.8.2 Commercial application for MFC–ECC–MEC–PBR system
There is tremendous potential for increasing the production of H2 through the combination of 3D printing and biodegradable materials. Through the advancement of technology, 3D printing will enable more affordable mass production and customization of MFC–ECC–MEC–PBR components. Because they produce less long-term waste, biodegradable materials guarantee environmental sustainability. Large-scale uses of this technology have the potential to replace conventional H2 production methods, that are frequently energy-intensive and dependent on fossil fuels, with ongoing advancements.8.3 Contribution to the treatment of wastewater
The MFC–ECC–MEC–PBR system provides significant environmental benefits including solutions related to wastewater treatment. Conventional treatment techniques frequently produce large volumes of sludge that adds to environmental pollution and must be disposed of.115 Conversely, MFC–ECC–MEC–PBR reduces sludge production by using microorganisms to break down organic matter efficiently.116,117 In addition to raising the water quality, it also reduces the carbon footprint caused by sludge disposal. Therefore, MFC–ECC–MEC–PBR is an important technology for sustainable development because it combines H2 production with wastewater treatment. Furthermore, by turning waste streams into useful resources, this process improves sustainability in a variety of industries, that is in line with the principles of the circular economy.8.4 Enhanced manufacturing with 3D printing
The fabrication of a stacked MFC–ECC–MEC system using 3D printing technology is a major and innovative advancement in the technology's affordability and scalability. 3D printing makes it possible to produce MFC–ECC–MEC components precisely, quickly, and with customization, that maximizes the use of available resources and time. This invention lowers production costs and makes it easier to create intricate, highly efficient designs that would be hard or impossible to accomplish using conventional manufacturing methods. The end product is a more widely available and scalable technology that can be used for decentralized energy production, especially in isolated or underserved areas. Additionally, local MFC–ECC–MEC–PBR production promotes resilience and energy independence at the community level.9. Prediction of future H2 energy regeneration system
Sustainable H2 production is essential for decarbonizing industries, lowering reliance on fossil fuels, and promoting clean energy transitions in light of the escalating global energy crisis, freshwater shortage, and climate change concerns. By turning wastewater into H2 though reducing energy consumption and environmental impact, this research directly addresses these issues. Large-scale deployment in wastewater treatment facilities and renewable energy systems is made possible by the integration of circular economy principles, that encourage resource regeneration, waste minimization, and economic viability. The H2 fuel cell is a device that converts H2 energy into electrical energy simultaneously producing heat and water as by-products as per eqn (7) and (8).118,119Fig. 6, H2 cannot be recovered from this system alone; researchers need to combine it with another biological or any other system (e.g., utilizing solar or wind energy) that can do so. If this device or system could be made, it would definitely help to reduce the world's energy, water crisis (use of fossil fuel and pure water for the production of H2) and carbon impact (GHG). On the other hand, generated H2 that can be used right away is a suitable solution because H2 storage and transportation are highly expensive.120–122Moreover, extreme heat waves endanger social and economic activities by raising the demand for electricity and water, that leads to crop losses and forest fires. Due to these gases, solar heat is also retained in the Earth's atmosphere. The basic idea behind the mechanism is that visible light, which is the primary form of solar radiation, travels through the atmosphere and reaches the Earth's surface. Longer-wave infrared radiation is the heat that is released after being absorbed by the land, water, and vegetation. Space would normally be the escape route for this radiation. CO2, methane (CH4), nitrous oxide (N2O), and chlorofluorocarbon (CCl3F and CCl2F2) can absorb some of the infrared radiation that the Earth emits. These pollutants trap heat and can remain in the atmosphere for years or even centuries. This heat no longer radiates into space, this directly leads to the greenhouse effect.123,124 Nevertheless, any effort to reduce these GHG emissions will undoubtedly support later campaigns to reduce energy consumption. Table 4 tabulate the economic analysis of MEC w.r.t HFC system.
| System | Energy input (kW h kg−1 of H2) | H2 yield range (L min−1) | H2 flow versus output power in HFC | Cost ($ per kg) | Ref. | |
|---|---|---|---|---|---|---|
| (L min−1) | (W) | |||||
| WE – water electrolysis, BG – biomass gasification, SMR – steam methane reforming.a (kW h m−3 of H2).b (m3 m−3 d−1).c Carbon tax: 52 to 77 $ per tCO2.d 9 kg distilled water: ∼1 to 2 $ per kg. | ||||||
| MEC | 1.0–2.2a | 0.01–3.12b | 0.1 | 8–10 | 4–8 | 29,33,111,113,125 |
| WE | 50–65c | 1–12c | 10 | 800–1000 | 3–6d | 126–129 |
| BG | 100–111c | 0.01–0.2c | 0.2 | 15–20 | 2–3c | 130–133 |
| SMR | 3.5–45c (depends on feed volume) | 5.0–10.0c | 10 | 800–1000 | 0.2–0.7c | 131,134–136 |
Possible reaction at the anode side:
| 2H2(Pt catalyst) → 4H+ + 4e− | (8) |
Possible reaction at the cathode side:
| O2 + 4H+ + 4e− → 2H2O | (9) |
Wastewater, agricultural residues, and organic waste are examples of feedstocks that are plentiful and renewable resources for MEC and MFC in a circular economy framework. The availability of essential resources like membranes and 3D printed biodegradable materials affects scalability. In order to lower material costs and increase energy efficiency, MEC need to be further optimized in comparison to traditional H2 production techniques like gasification, electrolysis and steam methane reforming. Government subsidies, carbon credits, and incentives for H2 and circular economy projects can also improve affordability. As per Table 4, combining 3D-printed MEC with MFC and ECC offers a scalable and affordable way to produce H2. This system drastically lowers operating costs by reducing energy input by up to 50–60% (predicted value) when compared to traditional electrolysis. Through removing the need for pricey raw materials and offering an efficient wastewater treatment solution, the use of wastewater as a feedstock further improves economic viability. This system is expected to have a levelized cost of H2i.e., 30–40% (predicted value) lower than that of conventional H2 derived from fossil fuels, making it competitive in the expanding green H2 market. Through the production of H2, carbon capture, and resource recovery, this technology not only improves sustainability but also generates economic opportunities by advancing the principles of the circular economy.
10. Conclusion
An increase in global warming and the depletion of natural resources are being caused by the overuse of fossil fuels, including wood, coal, diesel, gasoline, and other petrochemical products. In order to replace the current natural resources, it is crucial to develop environmental friendly and sustainable technology. Additionally, the detrimental effects of wastewater on the environment make treatment necessary. Therefore, the application of 3D printing in MEC has increased as a result of recent developments in the technology. Several MEC components can be fabricated more efficiently using 3D printing, a rapid and accurate fabrication process that has been shown to have promise in the literature. ECC are a simple, efficient, and cost-effective way to extract and utilize energy in the 3D-printed stacked MFC–ECC–MEC–PBR system without the need for additional use of energy. Hence, more research should look into such possibilities for the best possible design and manufacture of 3D printed parts, particularly for stacked MFC–ECC–MEC–PBR systems. Also, by accelerating the process of optimizing custom designs and new materials for the entire system, 3D printing can be a great tool for the commercialization of MFC–ECC–MEC–PBR technology. It is beneficial in markets such as the H2 energy and desalination sector, with a strong need for precision, customization, flexibility, and complex designs. Customized 3D-printed reactors are crucial to the development of H2 production because they increase design flexibility, cost effectiveness, and scalability. Their low cost of fabrication, precise structural control, and waste minimization make them ideal for both industrial applications and basic research. For a self-sufficient, zero-discharge system, 3D printing's modular design enables efficient scale-up and integration with MECs, MFCs, and ECCs. Future research should focus on material optimization, durability, and techno-economic viability to speed up commercialization and develop a cost-effective, sustainable H2 production pathway.Author contributions
Conceptualization: Mandar S. Bhagat and Chirag Mevada; methodology: Mandar S. Bhagat, Chirag Mevada and Jaini Shah; writing – original draft: Mandar S. Bhagat, Chirag Mevada and Jaini Shah; writing – review and editing: Mandar S. Bhagat, Chirag Mevada, and Matti Mäntysalo; supervision: M. Abdul Rasheed and Matti Mäntysalo; funding acquisition: Matti Mäntysalo.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the Research Council of Finland (grant no. 350309) as part of the CHIST-ERA Transient Electronics for Sustainable ICT in Digital Agriculture (TESLA) Project.Notes and references
- B. Hüner, M. Klstl, S. Uysal, İ. N. Uzgören, E. Özdoǧan, Y. O. Süzen, N. Demir and M. F. Kaya, ACS Omega, 2022, 7, 40638–40658 CrossRef PubMed.
- K. Fic, A. Platek, J. Piwek and E. Frackowiak, Mater. Today, 2018, 21, 437–454 CrossRef CAS.
- Y. Koul, V. Devda, S. Varjani, W. Guo, H. H. Ngo, M. J. Taherzadeh, J. S. Chang, J. W. C. Wong, M. Bilal, S. H. Kim, X. T. Bui and R. Parra-Saldívar, Bioengineered, 2022, 13, 8115–8134 CrossRef CAS PubMed.
- K. T. Møller, T. R. Jensen, E. Akiba and H. Wen Li, Prog. Nat. Sci.: Mater. Int., 2017, 27, 34–40 CrossRef.
- Y. H. Jia, J. Y. Choi, J. H. Ryu, C. H. Kim, W. K. Lee, H. T. Tran, R. H. Zhang and D. H. Ahn, Korean J. Chem. Eng., 2010, 27, 1854–1859 CrossRef CAS.
- Y. Zhang and I. Angelidaki, Water Res., 2014, 56, 11–25 CrossRef CAS PubMed.
- X. Jin, Y. Zhang, X. Li, N. Zhao and I. Angelidaki, Environ. Sci. Technol., 2017, 51, 9371–9378 CrossRef CAS PubMed.
- B. E. Logan, Microbial Fuel Cells, John Wiley & Sons, Inc., New Jersey, 2007 Search PubMed.
- J. Ditzig, H. Liu and B. E. Logan, Int. J. Hydrogen Energy, 2007, 32, 2296–2304 CrossRef CAS.
- A. Wang, D. Sun, G. Cao, H. Wang, N. Ren, W. M. Wu and B. E. Logan, Bioresour. Technol., 2011, 102, 4137–4143 CrossRef CAS PubMed.
- N. Montpart, L. Rago, J. A. Baeza and A. Guisasola, Water Res., 2015, 68, 601–615 CrossRef CAS PubMed.
- M. S. Bhagat, A. K. Mungray and A. A. Mungray, J. Indian Chem. Soc., 2022, 99, 100552 CrossRef CAS.
- M. S. Bhagat, A. K. Mungray and A. A. Mungray, Water-Energy Nexus, 2021, 4, 113–122 CrossRef CAS.
- C. Mevada, J. Tissari, V. S. Parihar, A. Tewari, J. Keskinen, M. Kellomaki and M. Mantysalo, J. Mater. Chem. A, 2024, 12, 24357–24369 RSC.
- C. Mevada, V. S. Parihar, J. Keskinen, M. Kellomaki and M. Mantysalo, in FLEPS 2024 – IEEE Inter. Conf. on Flex. and Print. Sen. and Sys., Proceed., Institute of Electrical and Electronics Engineers Inc., 2024.
- M. Sun, G.-P. Sheng, L. Zhang, C.-R. Xia, Z.-X. Mu, X.-W. Liu, H.-L. Wang, H.-Q. Yu, R. Qi, T. Yu and M. Yang, Environ. Sci. Technol., 2008, 42, 8095–8100 CrossRef CAS PubMed.
- M. C. Hatzell, Y. Kim and B. E. Logan, J. Power Sources, 2013, 229, 198–202 CrossRef CAS.
- M. C. Hatzell, Y. Kim and B. E. Logan, J. Power Sources, 2013, 229, 198–202 CrossRef CAS.
- S. Fan, Y. Song, D. Zheng, X. Peng, S. Li, P. Gao and D. Li, J. Environ. Sci., 2025, 152, 87–98 CrossRef CAS PubMed.
- S. Bolognesi, D. Cecconet, A. Callegari and A. G. Capodaglio, Environ. Res., 2021, 192, 110263 CrossRef CAS PubMed.
- H. T. Tse, S. Luo, J. Li and Z. He, Bioprocess Biosyst. Eng., 2016, 39, 1703–1710 CrossRef CAS PubMed.
- H. M. Jiang, S. J. Luo, X. S. Shi, M. Dai and R. B. Guo, J. Cent. South Univ., 2013, 20, 488–494 CrossRef CAS.
- A. Hornes, A. Pesce, H. Afonso, A. Morata, M. Torrell and A. Tarancon, 3D Print. Energy Appl., 2021, 1–34 Search PubMed.
- Y. Z. Y. Ryan, K. Balachandran, I. Ibrahim, M. Hani Abu Bakar, M. Ismail, W. Lun Ang, E. Hao Yu and S. Su Lim, Energy Revolution, 2023, 9, 1004053 Search PubMed.
- A. Sachdeva and B. Vidyapeeth, Intagr. Dist. Comput., 2020, 13, 1899–1936 Search PubMed.
- C. Y. Lee, A. C. Taylor, A. Nattestad, S. Beirne and G. G. Wallace, Joule, 2019, 3, 1835–1849 CrossRef CAS.
- D. Corral, J. T. Feaster, S. Sobhani, J. R. DeOtte, D. U. Lee, A. A. Wong, J. Hamilton, V. A. Beck, A. Sarkar, C. Hahn, T. F. Jaramillo, S. E. Baker and E. B. Duoss, Energy Environ. Sci., 2021, 14, 3064–3074 RSC.
- K. Kassym and A. Perveen, Mater. Today: Proc., 2020, 26, 1727–1733 CAS.
- F. Baş and M. F. Kaya, Fuel, 2022, 317, 123560 CrossRef.
- X. Chen, X. Liu, P. Childs, N. Brandon and B. Wu, Adv. Mater. Technol., 2017, 2, 1700148 CrossRef.
- F. Kracke, J. S. Deutzmann, B. S. Jayathilake, S. H. Pang, S. Chandrasekaran, S. E. Baker and A. M. Spormann, Front. Microbiol., 2021, 12, 696473 CrossRef.
- Scopus Data (20th Feb, 2025) from https://www.scopus.com.
- V. Kumar, M. Prasad Behera, Y. Lv, B. Pradheepa Kamarajan and S. Singamneni, Mater. Des., 2024, 245, 113237 CrossRef CAS.
- D. A. Jadhav, G. Kumar, J. K. Jang and K.-J. Chae, J. Environ. Manage., 2024, 368, 122209 CrossRef CAS PubMed.
- F. Carvalho, A. R. Prazeres and J. Rivas, Sci. Total Environ., 2013, 445–446, 385–396 CrossRef CAS PubMed.
- I. K. Kapdan and F. Kargi, Enzyme Microb. Technol., 2006, 38, 569–582 CrossRef CAS.
- J. Ren, H. Li, N. Li, Y. Song, J. Chen and L. Zhao, RSC Adv., 2020, 10, 17163–17170 RSC.
- L. Pötschke, P. Huber, S. Schriever, V. Rizzotto, T. Gries, L. M. Blank and M. A. Rosenbaum, Front. Energy Res., 2019, 7, 100 CrossRef.
- D. Call and B. E. Logan, Environ. Sci. Technol., 2008, 42, 3401–3406 CrossRef CAS PubMed.
- H. Hu, Y. Fan and H. Liu, Water Res., 2008, 42, 4172–4178 CrossRef CAS PubMed.
- B. Wang, Y. Liu, X. Wang and P. Sun, Water Res., 2023, 247, 120755 CrossRef CAS PubMed.
- R. A. Chacón-Carrera, A. López-Ortiz, V. Collins-Martínez, M. J. Meléndez-Zaragoza, J. Salinas-Gutiérrez, J. C. Espinoza-Hicks and V. H. Ramos-Sánchez, Int. J. Hydrogen Energy, 2019, 44, 12339–12345 CrossRef.
- T. H. J. A. Sleutels, H. V. M. Hamelers, R. A. Rozendal and C. J. N. Buisman, Int. J. Hydrogen Energy, 2009, 34, 3612–3620 CrossRef CAS.
- Y. Zuo, S. Cheng, D. Call and B. E. Logan, Environ. Sci. Technol., 2007, 41, 3347–3353 CrossRef CAS PubMed.
- S. He, S. Chen, X. Li and Y. Fan, Chin. J. Environ. Eng., 2019, 13, 1441–1448 Search PubMed.
- M. Mehanna, P. D. Kiely, D. F. Call and B. E. Logan, Environ. Sci. Technol., 2010, 44, 9578–9583 CrossRef CAS PubMed.
- S. Chen, G. Liu, R. Zhang, B. Qin and Y. Luo, Environ. Sci. Technol., 2012, 46, 2467–2472 CrossRef CAS PubMed.
- A. Kadier, Y. Simayi, P. Abdeshahian, N. F. Azman, K. Chandrasekhar and M. S. Kalil, Alexandria Eng. J., 2016, 55, 427–443 CrossRef.
- N. Wrana, R. Sparling, N. Cicek and D. B. Levin, J. Cleaner Prod., 2010, 18, S105–S111 CrossRef CAS.
- W. Cui, Y. Lu, C. Zeng, J. Yao, G. Liu, H. Luo and R. Zhang, Sci. Total Environ., 2021, 780, 146597 CrossRef CAS PubMed.
- J. Huang, H. Feng, L. Huang, X. Ying, D. Shen, T. Chen, X. Shen, Y. Zhou and Y. Xu, Waste Manage., 2020, 103, 61–66 CrossRef CAS PubMed.
- S. Rozenfeld, B. Gandu, L. O. Hirsch, I. Dubrovin, A. Schechter and R. Cahan, Int. J. Energy Res., 2021, 45, 19074–19088 CrossRef CAS.
- M. Posadas-Hernández, J. L. García-Rojas, S. Khamkure, L. García-Sánchez, T. Gutierrez-Macías, C. Morales-Morales and E. B. Estrada-Arriaga, Int. J. Hydrogen Energy, 2023, 48, 495–513 CrossRef.
- G. Rani, Z. Nabi, J. Rajesh Banu and K. N. Yogalakshmi, Renewable Energy, 2020, 153, 168–174 CrossRef CAS.
- C. Mumtha, J. Kabiriyel and P. U. Mahalingam, Wast. Dispos. Sustain. Energy, 2023, 5, 511–524 CrossRef.
- P. Srivastava, C. González, J. Palma and E. Garcia-Quismondo, Catal. Today, 2023, 422, 114246 CrossRef CAS.
- S. Song, L. Huang and P. Zhou, Appl. Microbiol. Biotechnol., 2023, 107, 391–404 CrossRef CAS PubMed.
- F. Ndayisenga, Z. Yu, B. Wang and D. Zhou, Chem. Eng. J., 2023, 469, 144002 Search PubMed.
- S. Chavan and A. Gaikwad, Biochem. Eng. J., 2023, 193, 108853 Search PubMed.
- J. Zhang, H. Chang, X. Li, B. Jiang, T. Wei, X. Sun and D. Liang, Environ. Sci. Pollut. Res., 2022, 29, 89727–89737 CrossRef CAS PubMed.
- J. Zhang, Y. Bai, Y. Fan and H. Hou, J. Biosci. Bioeng., 2016, 122, 488–493 CrossRef CAS PubMed.
- A. Askari, M. Taherkhani and F. Vahabzadeh, Korean J. Chem. Eng., 2022, 39, 2148–2155 Search PubMed.
- S. Luo, F. Liu, B. Fu, K. He, H. Yang, X. Zhang, P. Liang and X. Huang, Water Res., 2021, 201, 117326 CrossRef CAS PubMed.
- D. W. Liang, S. K. Peng, S. F. Lu, Y. Y. Liu, F. Lan and Y. Xiang, Bioresour. Technol., 2011, 102, 10881–10885 CrossRef CAS PubMed.
- N. Zhao, D. Liang, H. Liu, S. Meng and X. Li, Chem. – Eng. J., 2023, 451, 138561 CrossRef CAS.
- Y. Choi, D. Kim, H. Choi, J. Cha, G. Baek and C. Lee, Bioengineered, 2023, 14, 2244759 CrossRef PubMed.
- B. R. Dhar and H. S. Lee, Environ. Technol., 2013, 34, 1751–1764 CrossRef CAS PubMed.
- G. Zhen, X. Lu, G. Kumar, P. Bakonyi, K. Xu and Y. Zhao, Prog. Energy Combust. Sci., 2017, 63, 119–145 CrossRef.
- I. Ochiai, T. Harada, S. Jomori, A. Kouzuma and K. Watanabe, Bioresour. Technol., 2023, 386, 129508 CrossRef CAS PubMed.
- L. Dai, L. Xiang, M. Zhang, Z. Wen, Q. Xu, K. Chen, Z. Zhao and S. Ci, ChemElectroChem, 2022, 9, e202101584 CrossRef CAS.
- N. Montpart, M. Baeza, J. A. Baeza and A. Guisasola, Int. J. Hydrogen Energy, 2016, 41, 20465–20472 CrossRef CAS.
- M. A. M. Asrul, M. F. Atan, H. A. H. Yun, N. A. Wahab, H. H. Hung, J. C. H. Lai and I. A. W. Tan, ASEAN Eng. J., 2024, 14, 7–17 CrossRef.
- X. Zhu and B. E. Logan, Bioresour. Technol., 2014, 159, 24–29 CrossRef CAS PubMed.
- C. Huang and T. Xu, Environ. Sci. Technol., 2006, 40, 5233–5243 CrossRef CAS PubMed.
- A. Nazir, A. Azhar, U. Nazir, Y. F. Liu, W. S. Qureshi, J. E. Chen and E. Alanazi, J. Manuf. Syst., 2021, 60, 774–786 CrossRef PubMed.
- T. D. Ngo, A. Kashani, G. Imbalzano, K. T. Q. Nguyen and D. Hui, Composites, Part B, 2018, 143, 172–196 CrossRef CAS.
- K. C. Obileke, H. Onyeaka, E. L. Meyer and N. Nwokolo, Electrochem. Commun., 2021, 125, 107003 CrossRef CAS.
- J. Y. Lee, W. S. Tan, J. An, C. K. Chua, C. Y. Tang, A. G. Fane and T. H. Chong, J. Membr. Sci., 2016, 499, 480–490 CrossRef CAS.
- M. P. Browne, F. Novotný, Z. Sofer and M. Pumera, ACS Appl. Mater. Interfaces, 2018, 10, 40294–40301 CrossRef CAS PubMed.
- B. Bian, D. Shi, X. Cai, M. Hu, Q. Guo, C. Zhang, Q. Wang, A. X. Sun and J. Yang, Nano Energy, 2018, 44, 174–180 CrossRef CAS.
- J. You, R. J. Preen, L. Bull, J. Greenman and I. Ieropoulos, Sustain. Energy Technol. Assess., 2017, 19, 94–101 Search PubMed.
- T. H. Chung and B. R. Dhar, Front. Energy Res., 2021, 9, 679061 CrossRef.
- S. Saleh Alghamdi, S. John, N. Roy Choudhury and N. K. Dutta, Polymer, 2021, 13, 299 Search PubMed.
- A. Zhakeyev, P. Wang, L. Zhang, W. Shu, H. Wang and J. Xuan, Adv. Sci., 2017, 4, 1700187 CrossRef PubMed.
- S. Scott Crump, US Pat., 5121329, 1992, 1–15 Search PubMed.
- M. C. Freyman, T. Kou, S. Wang and Y. Li, Nano Res., 2020, 13, 1318–1323 CrossRef CAS.
- E. Jannelli, P. Di Trolio, F. Flagiello and M. Minutillo, Energy Proc., 2018, 148, 1135–1142 CrossRef CAS.
- C. Arbizzani, M. Mastragostino and F. Soavi, J. Power Sources, 2001, 100, 164–170 CrossRef CAS.
- S. Zhang and N. Pan, Adv. Energy Mater., 2015, 5, 1401401 CrossRef.
- C. Santoro, F. Soavi, A. Serov, C. Arbizzani and P. Atanassov, Biosens. Bioelectron., 2016, 78, 229–235 CrossRef CAS PubMed.
- Z. Han, R. Fang, D. Chu, D. W. Wang and K. Ostrikov, Nanoscal. Adv., 2023, 5, 4015–4017 RSC.
- M. Arulepp, J. Leis, M. Lätt, F. Miller, K. Rumma, E. Lust and A. F. Burke, J. Power Sources, 2006, 162, 1460–1466 CrossRef CAS.
- F. Mastragostino Marina and A. C. Soavi, in Advances in Lithium-Ion Batteries, ed. B. van Schalkwijk Walter and A. Scrosati, Springer, US, Boston, MA, 2002, pp. 481–505 Search PubMed.
- M. A. Guerrero-Martinez, M. I. Milanes-Montero, F. Barrero-Gonzalez, V. M. Miñambres-Marcos, E. Romero-Cadaval and E. Gonzalez-Romera, Sensors, 2017, 17, 1217 CrossRef PubMed.
- S. Dhanraj Nehate, S. Sundaresh, A. Kumar Saikumar, J. Catanzarite and M. Shao, J. Electrochem. Soc., 1991, 138, 1539–1548 CrossRef.
- M. A. Dar, S. R. Majid, M. Satgunam, C. Siva, S. Ansari, P. Arusalan and S. Rafi Ahamed, Int. J. Hydrogen Energy, 2024, 70, 10–28 CrossRef CAS.
- H. A. Khan, M. Tawalbeh, B. Aljawrneh, W. Abuwatfa, A. Al-Othman, H. Sadeghifar and A. G. Olabi, Energy, 2024, 295, 131043 CrossRef CAS.
- S. Sahani, H. Mahajan and S. S. Han, J. Energy Storage, 2024, 90, 111808 CrossRef.
- C. Mevada and M. Mukhopadhyay, in Handbook of Nanocomposite Supercapacitor Materials IV: Next-Generation Supercapacitors, ed. K. K. Kar, Springer International Publishing, Cham, 2023, pp. 1–17 Search PubMed.
- C. Mevada and M. Mukhopadhyay, Ind. Eng. Chem. Res., 2021, 60, 1096–1111 CrossRef CAS.
- C. Mevada, J. Tissari, V. S. Parihar, A. Tewari, J. Keskinen and M. Mäntysalo, J. Power Sources, 2024, 624, 235529 CrossRef CAS.
- L. Zhao, G. Zeng, Y. Gu, Z. Tang, G. Wang, T. Tang, Y. Shan and Y. Sun, Chem. Eng. Sci., 2019, 193, 6–14 CrossRef CAS.
- N. Castro, J. M. Queirós, D. C. Alves, M. M. M. Fernandes, S. Lanceros-Méndez and P. M. Martins, Nanomaterials, 2024, 14, 525 CrossRef CAS PubMed.
- M. Meagher, J. Tamburro and N. R. Boyle, Biotechnol. Prog., 2024, 40, e3430 CrossRef CAS PubMed.
- T. Ngoc Dan Cao, T. H. Wang, Y. Peng, H.-Y. Hsu, H. Mukhtar and C.-P. Yu, Crit. Rev. Biotechnol., 2024, 44, 31–46 CrossRef PubMed.
- T. Gao, H. Zhang, X. Xu and J. Teng, Water Res., 2021, 190, 116679 CrossRef CAS PubMed.
- M. M. Ghangrekar and V. B. Shinde, Bioresour. Technol., 2007, 98, 2879–2885 CrossRef CAS PubMed.
- H. Liu, S. Cheng and B. E. Logan, Environ. Sci. Technol., 2005, 39, 5488–5493 CrossRef CAS PubMed.
- S. K. Chaudhuri and D. R. Lovley, Nat. Biotechnol., 2003, 21, 1229–1232 CrossRef CAS PubMed.
- E. S. Heidrich, S. R. Edwards, J. Dolfing, S. E. Cotterill and T. P. Curtis, Performance of a pilot scale microbial electrolysis cell fed on domestic wastewater at ambient temperatures for a 12 month period, Bioresour. Technol., 2014, 173, 87–95 CrossRef CAS PubMed.
- L. Wang, F. Long, D. Liang, X. Xiao and H. Liu, Bioresour. Technol., 2021, 320, 124314 CrossRef CAS PubMed.
- L. Gil-Carrera, P. Mehta, A. Escapa, A. Morán, V. García, S. R. Guiot and B. Tartakovsky, Bioresour. Technol., 2011, 102, 9593–9598 CrossRef CAS PubMed.
- H. Guo and Y. Kim, Biotechnol. Biofuels, 2019, 12, 1–23 CrossRef PubMed.
- B. Bian, C. Wang, M. Hu, Z. Yang, X. Cai, D. Shi and J. Yang, Front. Energy Res., 2018, 6, 50 CrossRef.
- A. Demirbas, V. Coban, O. Taylan and M. Kabli, Energy Sources, Part A, 2017, 39, 1056–1062 CrossRef CAS.
- F. Zhang, K. S. Brastad and Z. He, Environ. Sci. Technol., 2011, 45, 6690–6696 CrossRef CAS PubMed.
- C. M. Werner, B. E. Logan, P. E. Saikaly and G. L. Amy, J. Membr. Sci., 2013, 428, 116–122 CrossRef CAS.
- T. Kulkarni and G. Slaughter, J. Biochips Tissue Chips, 2015, 5, 1 Search PubMed.
- D. Choi, I. Jang, T. Lee, Y. S. Kang and S. J. Yoo, J. Mater. Sci. Technol., 2025, 207, 308–316 CrossRef CAS.
- T. Galimova, M. Fasihi, D. Bogdanov and C. Breyer, Appl. Energy, 2023, 347, 121369 CrossRef.
- J. Collis and R. Schomäcker, Front. Energy Res., 2022, 10, 909298 CrossRef.
- R. Hren, A. Vujanović, Y. Van Fan, J. J. Klemeš, D. Krajnc and L. Čuček, Renewable Sustainable Energy Rev., 2023, 173, 113113 CrossRef CAS.
- T. S. Ledley, E. T. Sundquist, S. E. Schwartz, D. K. Hall, J. D. Fellows and T. L. Killeen, Eos, 1999, 80, 453–458 CrossRef.
- M. Filonchyk, M. P. Peterson, L. Zhang, V. Hurynovich and Y. He, Sci. Total Environ., 2024, 935, 173359 CrossRef CAS PubMed.
- B. E. Logan, D. Call, S. Cheng, H. V. M. Hamelers, T. H. J. A. Sleutels, A. W. Jeremiasse and R. A. Rozendal, Environ. Sci. Technol., 2008, 42, 8630–8640 CrossRef CAS PubMed.
- M. M. Rashid, M. K. Al Mesfer, H. Naseem and M. Danish, Int. J. Eng. Adv. Technol., 2015, 4, 2249–8958 Search PubMed.
- L. Wang, T. Weissbach, R. Reissner, A. Ansar, A. S. Gago, S. Holdcroft and K. A. Friedrich, ACS Appl. Energy Mater., 2019, 2, 7903–7912 CrossRef CAS.
- I. Vincent, E. C. Lee and H. M. Kim, Sci. Rep., 2021, 11, 1–12 CrossRef PubMed.
- F. P. Lohmann-Richters, S. Renz, W. Lehnert, M. Müller and M. Carmo, J. Electrochem. Soc., 2021, 168, 114501 CrossRef CAS.
- N. K. Obiora, C. O. Ujah, C. O. Asadu, F. O. Kolawole and B. N. Ekwueme, Green Technol. Sustainability, 2024, 2, 100100 CrossRef.
- M. Katebah, M. Al-Rawashdeh and P. Linke, Clean Eng. Technol., 2022, 10, 100552 CrossRef.
- J. J. Alvarado-Flores, J. V. Alcaraz-Vera, M. L. Ávalos-Rodríguez, E. Guzmán-Mejía, J. G. Rutiaga-Quiñones, L. F. Pintor-Ibarra and S. J. Guevara-Martínez, Energy, 2024, 17, 537 CAS.
- N. K. Obiora, C. O. Ujah, C. O. Asadu, F. O. Kolawole and B. N. Ekwueme, Green Technol. Sustainability, 2024, 2, 100100 CrossRef.
- X. Sun, G. Nocton and P. W. Roesky, Chem. Commun., 2025, 61, 1761–1772 RSC.
- A. Magnino, P. Marocco, M. Santarelli and M. Gandiglio, Adv. Appl. Energy, 2025, 17, 100204 CrossRef CAS.
- A. Di Nardo, M. Portarapillo, D. Russo and A. Di Benedetto, Int. J. Hydrogen Energy, 2024, 55, 1143–1160 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |




